Abstract
Messenger RNA (mRNA) vaccination was developed to mitigate the coronavirus disease 2019 pandemic. However, data on antibody kinetics and factors influencing these vaccines’ immunogenicity are limited. We conducted a prospective study on healthy young adults who received two doses of the mRNA-1273 vaccine at 28-day intervals. After each dose, adverse events were prospectively evaluated, and blood samples were collected. The correlation between humoral immune response and reactogenicity after vaccination was determined. In 177 participants (19–55 years), the geometric mean titers of anti-S IgG antibody were 178.07 and 4409.61 U/mL, while those of 50% neutralizing titers were 479.95 and 2851.67 U/mL four weeks after the first and second vaccine doses, respectively. Anti-S IgG antibody titers were not associated with local reactogenicity but were higher in participants who experienced systemic adverse events (headache and muscle pain). Antipyretic use was an independent predictive factor of a robust anti-SARS-CoV-2 antibody response after receiving both vaccine doses. Systemic reactogenicity after the first dose influenced antibody response after the second dose. In conclusion, mRNA-1273 induced a robust antibody response in healthy young adults. Antipyretic use did not decrease the anti-SARS-CoV-2 antibody response after mRNA-1273 vaccination.
Keywords: COVID-19, SARS-CoV-2, vaccine, reactogenicity, immunogenicity
1. Introduction
As the coronavirus disease 2019 (COVID-19) pandemic continues, vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been rapidly developed to control the disease spread [1]. In South Korea, the national COVID-19 vaccination program has been gradually expanded to include the messenger RNA (mRNA)-1273 vaccine (Spikevax®, ModernaTX, Inc., Cambridge, UK), the fourth vaccine authorized as of May 2021 [2]. mRNA-1273 is a lipid nanoparticle-encapsulated mRNA vaccine that elicits an antibody response against the spike protein of SARS-CoV-2 and has shown favorable results in both clinical trials and real-world studies [3,4,5]. Nevertheless, data on the kinetics of antibodies after vaccination with mRNA-1273 are currently limited, particularly in Asian countries [6,7,8,9,10,11].
In clinical trials, mRNA-1273 proved more immunogenic and more likely to cause adverse events (AEs) than BNT162b2 [12]. Moreover, among those vaccinated with mRNA-1273, certain groups (e.g., younger population) showed a higher immune response accompanied by frequent AEs [6,13,14]. These synchronous associations may support the hypothesis that strong reactogenicity is associated with better immunogenicity. This correlation has been reported in previous studies, but the results have been inconsistent depending on the vaccine platform, vaccine type (antigen and adjuvant) and study population [11,15,16,17,18,19,20,21]. In this study, to shed light on these uncertainties, we evaluated the immunogenicity of mRNA-1273 and its correlation with AEs in healthy young adults.
2. Methods
2.1. Study Participants
This prospective cohort study was conducted in June 2021 at four university hospitals. Healthy young adults between the ages of 19 and 55 years, who were willing to receive the mRNA-1273 vaccine, were enrolled in the study; the participants provided written informed consent (Clinical Trial Number—NCT05258708). Individuals were excluded from the study if they were previously diagnosed with laboratory-confirmed COVID-19, had a history of autoimmune disease, or were immunocompromised, pregnant, or breastfeeding. Demographic information and data regarding the presence of comorbidities were collected from each participant. This study was approved by the institutional review board of each participating hospital.
2.2. Immunogenicity Assessment
Blood samples were collected at baseline (T0), 28 ± 7 days after the first dose but before the second dose (T1), and 28 ± 7 days after the second dose (T2). Two doses of mRNA-1273 were injected into the deltoid muscle of the upper arm at an interval of 28 days. To analyze immunogenicity, the anti-S antibody was measured using SARS-CoV-2 immunoglobulin G (IgG) (Elecsys anti-SARS-CoV-2 spike ECLIA, Roche Diagnostics, Pleasanton, CA, USA) according to the manufacturer’s protocol. For the analysis of factors influencing humoral immune response, the strong antibody response was defined as IgG antibody titers ≥250 U/mL (the threshold for further dilution) at T1 and ≥5400 U/mL (four-fold higher titer than that correlated with viral neutralization titer ≥160) at T2 [22]. The plaque reduction neutralization test was performed using the wild-type SARS-CoV-2 virus (BetaCoV/Korea/KCDC03/2020) [23]. The median neutralizing titer (ND50) was defined as the concentration of the antibodies that reduced the number of viruses by 50%, and a threshold ≥1:20 was considered positive.
2.3. Adverse Event Assessment
At 7 days after each vaccine dose, the participants were requested to record the occurrence, severity, and duration of solicited AEs through a standardized electronic questionnaire. Information on the use of antipyretics was collected after each vaccination dose. A standard scale was used to grade the severity of AEs [24]. The overall severity of AEs was evaluated in the following three ways: (1) the highest level of severity of the AEs reported by the subjects, (2) the sum of the severity scores (SUM) for each AE, and (3) the sum of multiplying each symptoms’ severity by the duration (days) of symptoms (SoM) for each AE.
2.4. Statistical Analysis
We analyzed the differences in AEs occurring after the first and second doses using McNemar’s and Wilcoxon signed-rank tests for paired dichotomous and continuous variables, respectively. A repeated-measures analysis of variance (ANOVA) was used to determine the changes in antibody titers by time points (T0–T2) within the group of participants. Log-transformed data were used to calculate the geometric mean titers (GMTs) with 95% confidence intervals (CI). Either the χ2 test or Fisher’s exact test was used for categorical variables, whereas Student’s t-test or one-way ANOVA was used to compare the continuous variables, followed by Scheffé’s test for multiple comparisons. Multivariate logistic regression analysis was performed to identify the factors predictive of a strong antibody response. For the correlation analysis, Spearman’s rank correlation coefficient was calculated. Statistical significance was set at p < 0.05. All statistical tests were performed using SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Baseline Characteristics of the Study Participants
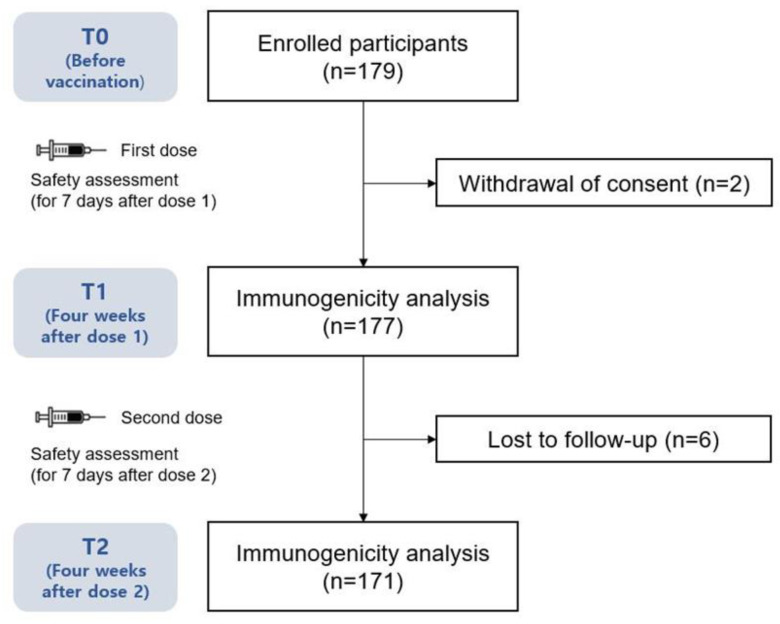
A total of 179 adult volunteers who were scheduled to receive the two doses of the mRNA-1273 vaccine participated in this study (Figure 1). Blood samples were obtained from 171 (95.5%) participants at all three time points (T0, T1, and T2) and 177 (98.9%) at two time points (T0 and T1). The baseline demographics are summarized in Table 1. The mean age of the patients was 25 ± 3.8 (range, 20–55) years, and 70% of the participants were women. All participants were healthy with no comorbidities. The mean body mass index (BMI) was 21.5 ± 2.8 kg/m2.
Figure 1.
Study diagram.
Table 1.
Baseline characteristics and solicited adverse events after each vaccine dose.
| Baseline Characteristics | Dose 1 (n = 177) |
Dose 2 (n = 171) |
p-Value |
|---|---|---|---|
| Age, mean ± SD (range) | 25.4 ± 3.83 (20–55) | 25.4 ± 3.88 (20–55) | |
| 20–29 years | 171 | 165 | |
| 30–39 years | 4 | 4 | |
| 40–49 years | 1 | 1 | |
| 50–54 years | 1 | 1 | |
| Male, number (%) | 53 (29.9%) | 53 (31.0%) | |
| BMI, mean ± SD | 21.50 ± 2.87 | 21.58 ± 2.88 | |
| AE experienced after each dose | |||
| Any AE, number (%) | 176 (99.4%) | 168 (98.2%) | 0.625 |
| Any systemic AE, number (%) | 155 (87.6%) | 163 (95.3%) | 0.007 |
| fever | 38 (21.5%) | 125 (73.1%) | <0.001 |
| chills | 37 (20.9%) | 121 (70.8%) | <0.001 |
| headache | 56 (31.6%) | 120 (70.2%) | <0.001 |
| muscle pain | 130 (73.4%) | 141 (82.5%) | 0.038 |
| fatigue | 104 (58.8%) | 134 (78.4%) | <0.001 |
| joint pain | 17 (9.6%) | 45 (26.3%) | <0.001 |
| vomiting | 3 (1.7%) | 16 (9.4%) | 0.002 |
| rash | 13 (7.3%) | 11 (6.4%) | 0.815 |
| dyspnea | 5 (2.8%) | 6 (3.5%) | 1.000 |
| flushing/lip swelling | 5 (2.8%) | 1 (0.6%) | 0.219 |
| facial palsy | 5 (2.8%) | 1 (0.6%) | 0.219 |
| paresthesia | 15 (8.5%) | 8 (4.7%) | 0.189 |
| Any local AE, number (%) | 174 (98.3%) | 164 (95.9%) | 0.289 |
| injection site pain | 170 (96.0%) | 161 (94.2%) | 0.549 |
| injection site redness/swelling | 27 (15.3%) | 38 (22.2%) | 0.080 |
| limited motion | 158 (89.3%) | 152 (88.9%) | 1.000 |
| Severity of AE, mean ± SD a | |||
| maximum severity | 1.94 ± 0.75 | 2.29 ± 0.76 | <0.001 |
| highest grade of systemic AE | 1.25 ± 0.77 | 1.98 ± 0.88 | <0.001 |
| highest grade of local AE | 1.86 ± 0.74 | 1.87 ± 0.79 | 0.770 |
| systemic SUM | 2.99 ± 2.95 | 6.71 ± 4.06 | <0.001 |
| systemic SoM | 4.71 ± 5.31 | 10.55 ± 8.00 | <0.001 |
| localized SUM | 3.28 ± 1.53 | 3.40 ± 1.69 | 0.295 |
| localized SoM | 4.51 ± 2.34 | 4.74 ± 2.62 | 0.214 |
| Antipyretic use, number (%) | 107 (60.5%) | 155 (90.6%) | <0.001 |
a Severity was calculated by considering only some AEs (local AEs including pain, redness/swelling, motion limitation, and systemic AEs including fever, chills, headache, muscle pain, fatigue, joint pain, and vomiting). AE, adverse event; SUM, sum of each symptoms’ severity score; SoM, sum of multiplying each symptom severity by duration (days).
The mean interval between vaccine dose 1 and dose 2 was 28.9 ± 2.3 (range 26–43) days. The mean intervals from dose 1 to follow-up time points were 23.7 ± 3.3 (20–42) days to T1 and 56.8 ± 1.8 (54–63) days to T2. The mean interval from dose 2 to T2 was 27.9 ± 3.0 (14–35) days.
3.2. Adverse Events
The AEs after each vaccine dose are summarized in Table 1 and Supplementary Figure S1. After the first dose, nearly all participants (99.4%) reported at least one AE. The most common AE was pain at the injection site (96%), followed by the limitation of movement at the injection site (89.3%), muscle pain (73.4%), and fatigue (58.8%). Fever and chills were reported in > 20% of the participants. Most AEs were grade 1 or 2 in terms of intensity. However, 6.8% of participants reported grade 3 or higher fatigue and redness/swelling at the injection site, and 14.1% reported grade 3 or higher limitation of movement at the injection site. After the second dose, 98.2% of the participants reported at least one AE. The most common AE was pain at the injection site (94.2%), followed by limitation of movement (88.9%), muscle pain (82.5%), and fatigue (78.4%). More than 70% of participants reported fever, chills, and headaches. Overall, systemic AEs were more common and severe after the second dose than after the first dose of the vaccine. A total of five participants complained of grade 4 AEs (emergency room visits or hospitalizations). Among them, one patient developed the AEs after the first dose of the vaccine (fatigue, myalgia, and chills), whereas the other four patients developed the AEs after the second dose; one complained of vomiting, one complained of myalgia with chills, and the last complained of fatigue, myalgia, headache, and chills. All patients recovered without sequelae.
3.3. Antibody Immune Response after Vaccination
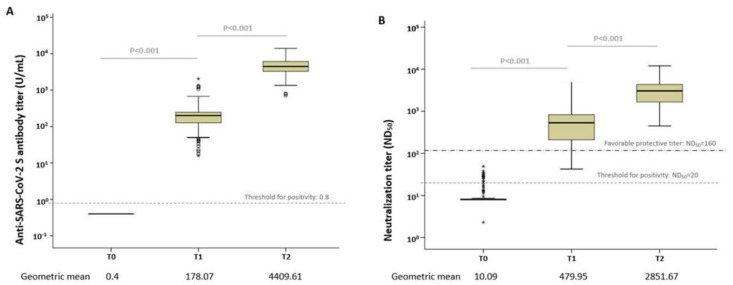
The GMTs of anti-S IgG antibodies at each time point are presented in Figure 2. The anti-S IgG antibody titer was 0.4 U/mL in all volunteers before vaccination. The GMTs were 178.07 U/mL (95% CI, 159.00–199.48) and 4409.61 U/mL (95% CI, 4082.25–4762.12) after the first and second doses, respectively, which showed a statistically significant increase over time (p < 0.001) (Figure 2A); the antibody titers at T1 showed a significant correlation with those at T2 (r = 0.547, p < 0.001).
Figure 2.
Box plots of the anti-SARS-CoV-2 antibody levels. (A) Anti-S IgG antibody and (B) median neutralizing titer (ND50) at each time point. The dotted line shows the threshold for positivity. Open circles depict outliers. Extreme values marked with a star.
Neutralizing antibody response was tested in 100 participants using the plaque reduction neutralization test. Before vaccination, the GMT of ND50 was 10.09 (95% CI, 9.13–11.16). At 4 weeks after the first dose (T1), GMT increased to 479.95 (95% CI, 394.37–583.98); 85% (85/100) of them showed ND50 titers exceeding 160. At 8 weeks after the first dose (4 weeks after the second dose; T2), all of them showed ND50 titers exceeding 160, with a GMT of 2851.67 (95% CI, 2481.99–3276.42). The neutralizing antibody titers increased significantly with the first and second doses from baseline to 8 weeks post-vaccination (p < 0.001; Figure 2B). Neutralizing antibody titers at T1 showed a significant positive correlation with those at T2 (r = 0.396, p < 0.001). The titers of anti-S IgG and neutralizing antibodies showed a strong positive correlation at each time point after vaccination (Supplementary Figure S2).
3.4. Association between Antibody Response and Adverse Events
The temporal changes in antibody immune responses were compared between those with and without AEs up to 8 weeks after the first dose (Table 2). No significant difference was found in the IgG antibody titers between those with and without at least one AE (local or systemic) after the first dose. As for the individual AEs, participants with a headache after the first dose showed significantly higher antibody titers after 8 weeks than those without (p = 0.034). Although statistically insignificant, participants with a fever after the first dose had higher IgG antibody titers at 8 weeks post-vaccination (first dose) than those without (p = 0.056). A comparison of anti-S IgG antibody responses between those with and without any AEs after the second dose at T2 showed no significant difference in IgG antibody titers between them (local or systemic). Although statistically insignificant, participants with a fever (≥37.5 °C) showed higher IgG antibody titers than those without (p = 0.056).
Table 2.
Relationship between reactogenicity and antibody response after the mRNA-1273 vaccine.
| Type of AE | SARS-CoV-2 Antibody Assay | Participants with AEs after 1st Dose | p-Value | Participants with AEs after 2nd Dose | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | |||||
| Any local AE | Anti-S IgG (U/mL) | T1 | N = 3 | N = 174 | 0.332 | |||
| 274.92 (48.00–1575.07) | 176.77 (157.65–198.20) | |||||||
| T2 | N = 3 | N = 168 | 0.394 | N = 7 | N = 164 | 0.658 | ||
| 3435.58 (1395.40–8460.58) | 4428.94 (4096.38–4788.51) | 4054.15 | 4424.86 (4090.72–4787.40) | |||||
| ND50 | T1 | N = 2 | N = 98 | 0.66 | ||||
| 652.38 (0.001–534195223) | 476.87 (391.29–581.17) | |||||||
| T2 | N = 2 | N = 98 | 0.938 | N = 1 | N = 99 | 0.967 | ||
| 2962.78 (24.46–358921.93) | 2849.71 (2474.00–3282.46) | 2934.95 | 2851.02 (2477.42–3434.79) | |||||
| Injection site pain | Anti-S IgG (U/mL) | T1 | N = 7 | N = 170 | 0.263 | |||
| 244.74 (145.64–411.24) | 175.75 (156.42–197.51) | |||||||
| T2 | N = 7 | N = 164 | 0.765 | N = 10 | N = 161 | 0.836 | ||
| 4166.77 (3113.15–5576-99) | 4419.77 (4081.31–4787.40) | 4267.76 (3067.61–5937.45) | 4418.76 (4078.50–4786.30) | |||||
| ND50 | T1 | N = 4 | N = 96 | 0.373 | ||||
| 310.89 (44.33–2184.74) | 488.65 (400.50–596.21) | |||||||
| T2 | N = 4 | N = 96 | 0.957 | N = 2 | N = 98 | 0.912 | ||
| 2798.98 (1629.67–4807.29) | 2854.30 (2470.59–3296.86) | 3011.62 (2171.20–4177.34) | 2848.39 (2472.29–3282.46) | |||||
| Injection site redness/ swelling | Anti-S IgG (U/mL) | T1 | N = 150 | N = 27 | 0.206 | |||
| 181.97 (159.81–207.25) | 157.91 (131.46–189.71) | |||||||
| T2 | N = 144 | N = 27 | 0.762 | N = 133 | N = 38 | 0.213 | ||
| 4432.00 (4088.96–4807.29) | 4290.42 (3377.54–5450.04) | 4296.35 (3946.39–4676.27) | 4830.59 (4018.83–5804.97) | |||||
| ND50 | T1 | N = 80 | N = 20 | 0.454 | ||||
| 462.38 (367.45–581.70) | 557.06 (382.65–810.96) | |||||||
| T2 | N = 80 | N = 20 | 0.664 | N = 75 | N = 25 | 0.153 | ||
| 2808.67 (2386.16–3305.98) | 3031.80 (2329.16–3947.30) | 3022.04 (2569.21–3553.86) | 2397.18 (1818.86–3158.64) | |||||
| Injection site motion limitation | Anti-S IgG (U/mL) | T1 | N = 19 | N = 158 | 0.758 | |||
| 187.46 (131.95–266.32) | 177.01 (156.82–199.76) | |||||||
| T2 | N = 19 | N = 152 | 0.138 | N = 19 | N = 152 | 0.535 | ||
| 3743.69 (3111.72–4504.02) | 4500.91 (4140.00–4892.15) | 4116.23 (3269.64–5183.22) | 4447.34 (4095.43–4829.48) | |||||
| ND50 | T1 | N = 8 | N = 92 | 0.696 | ||||
| 420.53 (154.06–1147.63) | 485.51 (396.64–594.16) | |||||||
| T2 | N = 8 | N = 92 | 0.919 | N = 4 | N = 96 | 0.891 | ||
| 2783.56 (1886.69–4105.82) | 2857.59 (2462.63–3316.65) | 2990.20 (659.63–13551.89) | 2846.43 (2472.86–3276.42) | |||||
| Any systemic AE | Anti-S IgG (U/mL) | T1 | N = 22 | N = 155 | 0.769 | |||
| 186.29 (132.65–261.64) | 176.97 (156.71–199.80) | |||||||
| T2 | N = 22 | N = 149 | 0.758 | N = 8 | N = 163 | 0.46 | ||
| 4273.66 (3506.71–5208.35) | 4429.96 (4071.93–4819.48) | 3869.90 (2622.41–5710.84) | 4438.13 (4099.21–4803.97) | |||||
| ND50 | T1 | N = 12 | N = 88 | 0.417 | ||||
| 385.57 (175.15–848.79) | 494.42 (403.92–605.34) | |||||||
| T2 | N = 12 | N = 88 | 0.06 | N = 2 | N = 98 | 0.912 | ||
| 1998.02 (1256.90–3176.14) | 2993.64 (2588.81–3461.78) | 3011.62 (2171.20–4177.34) | 2848.39 (2472.29–3282.46) | |||||
| Fever (≥37.5 °C) | Anti-S IgG (U/mL) | T1 | N = 139 | N = 38 | 0.764 | |||
| 179.72 (157.58–204.97) | 172.31 (137.09–216.52) | |||||||
| T2 | N = 136 | N = 35 | 0.056 | N = 46 | N = 125 | 0.056 | ||
| 4245.22 (3900.32–4621.68) | 5108.58 (4259.91–6124.91) | 3899.42 (3316.65–4584.58) | 4613.18 (4228.63–5032.69) | |||||
| ND50 | T1 | N = 79 | N = 21 | 0.227 | ||||
| 451.13 (360.08–565.20) | 605.62 (400.50–916.01) | |||||||
| T2 | N = 79 | N = 21 | 0.866 | N = 22 | N = 78 | 0.842 | ||
| 2834.00 (2414.90–3326.60) | 2918.77 (2162.72–3939.13) | 2777.15 (2071.57–3723.92) | 2873.43 (2445.68–3375.20) | |||||
| Chills | Anti-S IgG (U/mL) | T1 | N = 140 | N = 37 | 0.309 | |||
| 183.53 (161.36–208.74) | 158.93 (124.17–203.38) | |||||||
| T2 | N = 137 | N = 34 | 0.658 | N = 50 | N = 121 | 0.117 | ||
| 4371.19 (4002.21–4775.29) | 4565.62 (3885.08–5365.37) | 4008.67 (3434.00–4679.51) | 4586.70 (4197.59–5011.87) | |||||
| ND50 | T1 | N = 79 | N = 21 | 0.267 | ||||
| 453.42 (362.41–567.28) | 594.43 (387.61–911.59) | |||||||
| T2 | N = 79 | N = 21 | 0.698 | N = 24 | N = 76 | 0.731 | ||
| 2811.90 (2407.13–3284.73) | 3007.46 (2148.33–4210.17) | 2731.49 (2128.63–3505.10) | 2890.68 (2444.56–3419.01) | |||||
| Headache | Anti-S IgG (U/mL) | T1 | N = 121 | N = 56 | 0.266 | |||
| 170.49 (148.15–196.25) | 195.66 (161.18–237.52) | |||||||
| T2 | N = 117 | N = 54 | 0.034 | N = 51 | N = 120 | 0.212 | ||
| 4168.69 (3796.65–4577.20) | 4979.66 (4353.11–5696.39) | 4091.66 (3483.37–4805.07) | 4551.98 (4173.50–4964.78) | |||||
| ND50 | T1 | N = 72 | N = 28 | 0.399 | ||||
| 455.41 (358.53–581.70) | 549.16 (396.10–761.20) | |||||||
| T2 | N = 72 | N = 28 | 0.399 | N = 25 | N = 75 | 0.99 | ||
| 2747.89 (2329.7–3241.90) | 3136.90 (2395.52–4106.77) | 2847.08 (2099.91–3861.00) | 2852.99 (2433.32–3345.80) | |||||
| Muscle pain | Anti-S IgG (U/mL) | T1 | N = 47 | N = 130 | 0.401 | |||
| 193.02 (160.10–232.65) | 172.98 (150.42–198.93) | |||||||
| T2 | N = 46 | N = 125 | 0.676 | N = 30 | N = 141 | 0.958 | ||
| 4308.24 (3837.96–4837.27) | 4447.34 (4035.52–4901.17) | 4425.88 (3809.78–5141.62) | 4405.55 (4032.74–4813.93) | |||||
| ND50 | T1 | N = 26 | N = 74 | 0.162 | ||||
| 379.75 (237.41–607.30) | 521.07 (421.50–644.17) | |||||||
| T2 | N = 26 | N = 74 | 0.018 | N = 9 | N = 91 | 0.949 | ||
| 2159.73 (1715.54–2718.94) | 3144.13 (2661.95–3713.64) | 2870.12 (2396.07–3438.75) | 2849.71 (2447.37–3318.18) | |||||
| Fatigue | Anti-S IgG (U/mL) | T1 | N = 73 | N = 104 | 0.576 | |||
| 185.05 (157.80–217.02) | 173.34 (147.84–203.24) | |||||||
| T2 | N = 72 | N = 99 | 0.593 | N = 37 | N = 134 | 0.885 | ||
| 4519.60 (4017.91–5082.76) | 4331.12 (3905.71–4803.97) | 4362.14 (3634.96–5234.80) | 4422.83 (4058.82–4818.37) | |||||
| ND50 | T1 | N = 39 | N = 61 | 0.902 | ||||
| 472.61 (356.78–626.04) | 484.62 (368.81–636.94) | |||||||
| T2 | N = 39 | N = 61 | 0.69 | N = 18 | N = 82 | 0.868 | ||
| 2752.96 (2195.84–3452.23) | 2916.76 (2433.88–3494.62) | 2780.99 (1957.49–3951.85) | 2867.48 (2456.97–3346.57) | |||||
| Joint pain | Anti-S IgG (U/mL) | T1 | N = 160 | N = 17 | 0.46 | |||
| 175.63 (155.52–198.34) | 202.91 (147.84–278.48) | |||||||
| T2 | N = 155 | N = 16 | 0.298 | N = 126 | N = 45 | 0.481 | ||
| 4352.11 (4015.13–4717.37) | 5004.95 (3778.33–6629.79) | 4337.11 (3948.21–4765.41) | 4617.43 (4045.76–5271.08) | |||||
| ND50 | T1 | N = 92 | N = 8 | 0.352 | ||||
| 466.98 (380.72–572.80) | 657.05 (277.27–1557.04) | |||||||
| T2 | N = 92 | N = 8 | 0.886 | N = 72 | N = 28 | 0.639 | ||
| 2843.15 (2456.97–3290.03) | 2951.89 (1661.50–5243.24) | 2793.83 (2365.92–3298.37) | 3006.77 (2298.79–3932.78) | |||||
| Vomiting | Anti-S IgG (U/mL) | T1 | N = 174 | N = 3 | 0.267 | |||
| 179.60 (160.10–201.42) | 109.42 (43.590 274.73) | |||||||
| T2 | N = 168 | N = 3 | 0.646 | N = 155 | N = 16 | 0.386 | ||
| 4419.77 (4086.96–4779.69) | 3853.90 (2535.71–5858.68) | 4457.59 (4105.82–4839.49) | 3967.35 (3168.11–4967.07) | |||||
| ND50 | T1 | N = 98 | N = 2 | 0.649 | ||||
| 476.87 (390.30–582.51) | 659.02 (97.54–4451.43) | |||||||
| T2 | N = 98 | N = 2 | 0.321 | N = 90 | N = 10 | 0.549 | ||
| 2880.71 (2506.11–3310.55) | 1748.24 (0.07–41409501) | 2811.90 (2426.61–3258.37) | 3236.68 (1976.97–5299.07) | |||||
| Rash | Anti-S IgG (U/mL) | T1 | N = 164 | N = 13 | 0.872 | |||
| 178.57 (158.60–201.00) | 172.31 (110.71–268.16) | |||||||
| T2 | N = 158 | N = 13 | 0.688 | N = 160 | N = 11 | 0.548 | ||
| 4428.94 (4099.21–4786.30) | 4173.50 (2720.19–6404.72) | 4382.28 (4045.76–4746.79) | 4822.81 (3450.64–6742.17) | |||||
| ND50 | T1 | N = 92 | N = 8 | 0.16 | ||||
| 460.57 (375.06–565.46) | 770.19 (365.76–1622.18) | |||||||
| T2 | N = 92 | N = 8 | 0.558 | N = 91 | N = 9 | 0.516 | ||
| 2886.69 (2494.59–3340.41) | 2479.70 (1410.26–4360.14) | 2893.34 (2490.00–3362.02) | 2465.47 (1758.73–3455.41) | |||||
Data are presented as geometric mean titers (95% confidence interval). AE, adverse event; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IgG, immunoglobulin G; ND50, median neutralizing titer.
Table 2 summarize the association between AEs after each dose of vaccine and neutralizing antibody titers. Participants with at least one AE (local or systemic) after the first dose did not show a significant difference in neutralizing antibody responses from those without AE. However, those with at least one systemic AE had higher neutralizing antibody titers over time (p = 0.06 at T2). Among individual AEs, only muscle pain after the first dose was significantly associated with higher neutralizing antibody levels at T2 (p = 0.018). After the second dose, participants with at least one AE (local or systemic) showed a neutralizing antibody response similar to those without. No significant difference was found in neutralizing antibody titers after the second dose with respect to individual AEs.
3.5. Association between Antibody Response and the Severity of Adverse Events
The correlation between AE severity and immunogenicity was evaluated using the following three parameters: the highest level of severity, SUM, and SoM. Overall, the highest level of severity of local or systemic AEs was not predictive of higher antibody titers in the short-term (<8 weeks) follow-up period (Supplementary Figure S3). Similarly, no significant difference was found in the antibody responses according to the increase in SUM or SoM after any vaccine dose (Supplementary Figures S4 and S5).
3.6. Association between Antibody Response and Antipyretic Use
Antipyretic use was more frequently observed after the second dose of the vaccine. A total of 107 (60.5%) and 155 (90.6%) participants reported the use of antipyretics after the first and second doses, respectively (Table 1). All of them took acetaminophen; 4 (3.7%) and 11 (7.1%) participants additionally took nonsteroidal anti-inflammatory drugs (NSAIDs) after the 1st and 2nd dose, respectively. Of the 100 participants with neutralizing antibody results, 60 (60%) and 94 (94%) took antipyretics after the first and second doses, respectively. At each time point, both IgG and neutralizing antibody titers were higher in the antipyretic group (Table 3). After the first dose, antipyretic users showed higher antibody titers than non-users over time (p = 0.022 for anti-S IgG antibody titers and p = 0.124 for neutralizing antibody titers at T2). After the second dose, anti-S IgG antibody titers were higher in antipyretic users at T2 than in non-users (p = 0.010); however, the results were statistically insignificant for neutralizing antibody titers (p = 0.753). Antipyretic use was significantly associated with systemic (p < 0.001 after dose 1 and p = 0.028 after dose 2) and local AEs (p = 0.060 after dose 1 and p = 0.019 after dose 2).
Table 3.
Relationship between the use of antipyretics and antibody response.
| After Dose 1 | Antipyretic Use | p-Value | ||
|---|---|---|---|---|
| No | Yes | |||
| Anti-S IgG (U/mL) | T1 (N = 177) | 176.13 (148.56–208.82) | 179.38 (153.93–209.03) | 0.877 |
| T2 (N = 171) | 3962.57 (3560.72–4409.77) | 4748.31 (4269.28–5281.08) | 0.022 | |
| ND50 | T1 (N = 100) | 411.50 (311.06–544.38) | 531.72 (405.05–698.02) | 0.206 |
| T2 (N = 100) | 2498.72 (2028.41–3078.08) | 3114.46 (2584.41–3753.21) | 0.124 | |
| After dose 2 | ||||
| Anti-S IgG (U/mL) | T2 (N = 171) | 3234.86 (2427.75–4310.29) | 4552.66 (4206.72–4927.06) | 0.010 |
| ND50 | T2 (N = 100) | 2611.77 (1803.82–3781.61) | 2867.83 (2476.01–3321.66) | 0.753 |
| After either dose | ||||
| Anti-S IgG (U/mL) | T2 (N = 171) | 2932.21 (2063.26–4167.13) | 4547.28 (4207.95–4913.97) | 0.004 |
| ND50 | T2 (N = 100) | 2611.77 (1803.82–3781.61) | 2867.83 (2476.01–3321.66) | 0.753 |
Data are presented as geometric mean titers (95% confidence interval). IgG, immunoglobulin G; ND50, median neutralizing titer.
3.7. Multivariate Analysis
In 177 participants, four weeks after the first dose (T1), anti-S IgG antibody titers were 250 U/mL or higher in 43 patients (24.3%). A strong anti-S IgG antibody response (≥250 U/mL) was not significantly associated with age, sex, BMI, or individual AEs (Supplementary Table S1). At 4 weeks after the second dose (T2), the anti-S IgG antibody titer was ≥5400 U/mL in 60 patients among the 171 participants (35.1%). A strong antibody response (≥5400 U/mL) after the second dose was not associated with age, sex, or BMI. Among individual AEs, chills, and fever after the second dose was related to a strong antibody response (p = 0.051 and 0.063, respectively). Notably, antipyretic use after any vaccine dose was significantly associated with a strong antibody response at T2. In the multivariate analysis adjusted for age, sex, BMI, pain at the site of injection, and antipyretic use at each vaccine dose, antipyretic use at any vaccine dose was a significant predictive factor of the strong antibody response at T2 (Table 4).
Table 4.
Multivariate analysis for predictive factors of strong antibody response four weeks after administration of the second dose of mRNA-1273 vaccine.
| Variables | AEs after Dose 1 | AEs after Dose 2 | ||
|---|---|---|---|---|
| Odds Ratio, 95% CI | p-Value | Odds Ratio, 95% CI | p-Value | |
| Age | 1.009 (0.931–1.094) | 0.822 | 1.023 (0.944–1.110) | 0.577 |
| Male | 1.525 (0.674–3.449) | 0.311 | 1.716 (0.718–4.102) | 0.224 |
| BMI | 0.969 (0.854–1.100) | 0.626 | 0.982 (0.863–1.116) | 0.777 |
| Injection site pain after dose 1 | 2.638 (0.297–23.399) | 0.384 | ||
| Antipyretic use after dose 1 | 2.202 (1.110–4.367) | 0.024 | ||
| Injection site pain after dose 2 | 0.511 (0.098–2.652) | 0.424 | ||
| Antipyretic use after dose 2 | 10.033 (1.185–84.924) | 0.034 | ||
AE, adverse event; CI, confidence interval; BMI, body mass index.
4. Discussion
In the present study, we evaluated short-term humoral immune response in mRNA-1273 recipients up to 8 weeks after vaccination. The GMTs of anti-S IgG antibody were 178.07 and 4409.61 U/mL, and those of 50% neutralizing titers (ND50) were 479.95 and 2851.67 U/mL at 4 weeks after the first and second doses, respectively. Anti-SARS-CoV-2 antibody response after the second dose was positively correlated with systemic AEs (headache and muscle pain). Antipyretic use was an independent predictive factor for a strong antibody response after both the first and second doses.
Even low levels of neutralizing antibodies have been found to protect against SARS-CoV-2 [25]. However, the immune correlates of protection for antibody levels have not yet been established. Data from an efficacy trial of the ChAdOx1 nCoV-19 vaccine showed 50%–80% vaccine efficacy against symptomatic infections, with live virus neutralization titers of 68–247 [26]. The United States Food and Drug Administration (FDA) guideline for convalescent plasma initially recommended a target antibody titer of 160 [27]. In this study, the neutralizing antibody levels reached a titer level considered positive (≥160) based on the FDA recommendations in the majority of participants (85%) after only a single dose and all participants (100%) after the second dose. This finding highlighted robust immune response induction by the mRNA-1273 vaccine among young adult participants.
In this study, systemic AEs were more frequent after the second dose than after the first dose; more than 70% of the participants experienced fever, chills, headache, muscle pain, and fatigue after the second dose. These findings are consistent with the reports of a phase 3 clinical trial, but the frequency of AEs was higher in this study; this difference might be related to the study design and characteristics of the participants [3]. Assuming that antigenic priming of the immune system after the first vaccine dose might contribute to increased reactogenicity following the subsequent antigenic exposure [28], we hypothesize that increased reactogenicity after mRNA-1273 vaccination, especially the occurrence of systemic AEs after the second dose, is strongly associated with higher immunogenicity.
Vaccine antigens are recognized as potential pathogens by the pattern recognition receptors of the innate immune system, which results in the release of pyrogenic cytokines (interleukin [IL]-1, IL-6, tumor necrosis factor-α, and prostaglandin E2) and the subsequent cascade of immune responses [29]. Such post-vaccination immune responses may be accompanied by local or systemic AEs in vaccinated individuals. However, studies on the immunological correlates of reactogenicity in humans are limited, and they have reported inconsistent results depending on the vaccine type [11,15,16,17,18,19,20,21]. Both mRNA-1273 and BNT162b2 vaccines are the first human mRNA vaccines, which raised concerns about their immunological correlates with reactogenicity [3,30]. After the introduction of mRNA vaccines, several studies have investigated the correlation between immunogenicity and reactogenicity, with inconsistent results [11,18,20,28,31,32,33,34,35,36,37,38,39,40,41,42]. Some studies did not show a significant association, but those studies had limitations. In those studies, AEs were assessed as a total score or severity level, and more than half of the participants were elderly individuals (aged ≥ 80 years) [18,31,32,33,41]. Although the statistical significance of such an association was variable in other studies, systemic reactogenicity was related to a higher immune response, and the correlation was more prominent after the second dose [11,12,20,28,34,35,36,37,38,39,40,42,43]. Up to now, the association of mRNA-1273 vaccine immunogenicity with reactogenicity has been investigated in only three studies. Two of them included both kinds of mRNA vaccines (mRNA-1273 and BNT162b2) [12,40], while Coronavirus Efficacy (COVE) trial in the United States just focused on the mRNA-1273 vaccine [11]. According to the results of COVE trial, several systemic symptoms after the second dose were significantly associated with both the neutralizing and binding antibody titers at day 28 after the second dose. The lack of significant association between systemic AEs and neutralizing antibody titers in our study might be related to the limited number of cases. Nevertheless, the multivariate analysis of our study showed that anti-S IgG titer was significantly higher in antipyretics users at any vaccine dose. The antipyretics use would reflect the systemic reactogenicity, which might be associated with the immunogenicity of mRNA vaccine. In the situation that repeated mRNA vaccination would be anticipated, the findings in this study might provide a useful message that systemic reactogenicity might be a good sign of immune response, and it is not necessary to avoid taking antipyretics. However, most participants only took acetaminophen, so further research is needed to investigate the influence of NSAIDs.
Immunological correlation with reactogenicity can be explained as follows. (1) Similar to the mechanism proposed in the observational study of the influenza vaccine [44], which described a significant correlation between post-vaccination fever and immune response, a systemic reaction after vaccination may be an indicator of a healthy innate immune response. A systemic febrile response could be related to the activation of the innate immune system, which facilitates adaptive immune engagement and the subsequent antibody response [44,45]. (2) The inflammatory nature of lipid nanoparticles in mRNA vaccines, as a potential adjuvant, can be partially responsible for AEs and related to the intensity of eliciting protective immunity [46]. (3) The positive effect of the post-first-dose systemic reactogenicity on the antibody response after the second dose can reflect an association with memory B cell production. (4) Higher reactogenicity, particularly after the second dose, can be partially explained by the stronger anamnestic cytokine response with repeated vaccinations. To better understand and verify the observed immunological correlates with reactogenicity, it would be helpful to include the cytokines and other quantifiable factors in the future studies.
This study had some limitations. First, only short-term immune responses were investigated in mRNA-1273 recipients. A longitudinal follow-up should be planned to ensure that the relationship becomes clearer over time. Second, neutralization titers were measured in only half of the participants in our study; thus, the small number of patients could have yielded a statistically not significant correlation between AEs and neutralizing antibody responses. Finally, in this study, we focused on healthy young adults. Further studies, particularly in the elderly population, are required.
5. Conclusions
In conclusion, mRNA-1273 induced a robust humoral immune response in healthy young adults and the anti-SARS-CoV-2 antibody response was significantly stronger in participants who experienced systemic AEs and used antipyretics. Antipyretic use might be an objective indicator of systemic reactogenicity after vaccination. The use of antipyretics did not decrease the anti-SARS-CoV-2 antibody response after mRNA-1273 vaccination.
Acknowledgments
We would like to thank all the participants who volunteered to participate in the study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines11010120/s1, Table S1: relationship between adverse events experienced after either dose (first or second dose) and immune response eight weeks after the first dose (approximately four weeks after the second dose; T2); Table S2: univariate and multivariate analyses for predictive factors of strong antibody response at each time point; Figure S1: solicited adverse events after each dose; Figure S2: correlation between anti-SARS-CoV-2 S IgG and neutralizing antibody titer at each time point; Figure S3: relationship of immune response with (A) highest severity of all adverse events, (B) highest severity of local adverse events, and (C) highest severity of systemic adverse events at each time point. Immune responses are presented as geometric mean titer; Figure S4: relationship of anti-SARS-CoV-2 S antibody response with (A) systemic SUM and SOM, and (B) localized SUM and SOM at each time point; Figure S5: relationship of neutralizing antibody response (PRNT ND50) with (A) systemic SUM and SOM, and (B) localized SUM and SOM at each time point. PRNT: plaque reduction neutralization test.
Author Contributions
Conceptualization, M.J.C., B.K. and J.Y.S.; methodology, M.J.C., B.K. and J.Y.S.; software, M.J.C.; formal analysis and investigation, M.J.C., J.Y.H., Y.B.S., Y.K.Y., J.W.S., J.Y.N., H.J.C., W.J.K., J.-y.C., Y.J.L., H.W.L., S.S.K., B.K. and J.Y.S.; resources, M.J.C., J.Y.H., Y.B.S. and J.Y.S.; writing—original draft preparation, M.J.C. and J.Y.S.; writing—review and editing, all authors; supervision, J.Y.S. and B.K.; funding acquisition, J.Y.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the ethics committees of the Korea University Guro Hospital (2021GR0099), Ajou University Hospital (AJIRB-BMR-SMP-21-267), Kangnam Sacred Hallym University Hospital (HKS 2021-05-023), and International St. Mary’s Hospital (S21MIME0045).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available within the article or Supplementary Material here.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Korea National Institute of Health, Korea Disease Control and Prevention Agency [2021ER260300].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.WHO COVID-19 Vaccine Tracker and Landscape. 21 January 2022. [(accessed on 24 January 2022)]. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 2.Safety M.o.F.a.D. ‘Moderna COVID-19 Vaccine’ Product Approval. [(accessed on 24 January 2022)]; Available online: https://www.mfds.go.kr/brd/m_99/view.do?seq=45366&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1.
- 3.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickerman B.A., Gerlovin H., Madenci A.L., Kurgansky K.E., Ferolito B.R., Figueroa Muñiz M.J., Gagnon D.R., Gaziano J.M., Cho K., Casas J.P. Comparative Effectiveness of BNT162b2 and mRNA-1273 Vaccines in US Veterans. N. Engl. J. Med. 2021;386:105–115. doi: 10.1056/NEJMoa2115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruxvoort K.J., Sy L.S., Qian L., Ackerson B.K., Luo Y., Lee G.S., Tian Y., Florea A., Takhar H.S., Tubert J.E. Real-world effectiveness of the mRNA-1273 vaccine against COVID-19: Interim results from a prospective observational cohort study. Lancet Reg. Health-Am. 2021;6:100134. doi: 10.2139/ssrn.3916094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doria-Rose N., Suthar M.S., Makowski M., O’Connell S., McDermott A.B., Flach B., Ledgerwood J.E., Mascola J.R., Graham B.S., Lin B.C. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for COVID-19. N. Engl. J. Med. 2021;384:2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tré-Hardy M., Cupaiolo R., Wilmet A., Beukinga I., Blairon L. Waning antibodies in SARS-CoV-2 naïve vaccinees: Results of a three-month interim analysis of ongoing immunogenicity and efficacy surveillance of the mRNA-1273 vaccine in healthcare workers. J. Infect. 2021;83:381–412. doi: 10.1016/j.jinf.2021.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tré-Hardy M., Cupaiolo R., Papleux E., Wilmet A., Horeanga A., Antoine-Moussiaux T., Della Vecchia A., Beukinga I., Vekemans M., Blairon L. Reactogenicity, safety and antibody response, after one and two doses of mRNA-1273 in seronegative and seropositive healthcare workers. J. Infect. 2021;83:237–279. doi: 10.1016/j.jinf.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tré-Hardy M., Cupaiolo R., Wilmet A., Antoine-Moussiaux T., Della Vecchia A., Horeanga A., Papleux E., Vekemans M., Beukinga I., Blairon L. Six-month interim analysis of ongoing immunogenicity surveillance of the mRNA-1273 vaccine in healthcare workers: A third dose is expected. J. Infect. 2021;83:559–564. doi: 10.1016/j.jinf.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siangphoe U., Baden L.R., El Sahly H.M., Essink B., Ali K., Berman G., Tomassini J.E., Deng W., Pajon R., McPhee R. Associations of Immunogenicity and Reactogenicity After Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) mRNA-1273 Vaccine in the COVE and TeenCOVE Trials. Clin. Infect. Dis. 2022:ciac780. doi: 10.1093/cid/ciac780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debes A.K., Xiao S., Colantuoni E., Egbert E.R., Caturegli P., Gadala A., Milstone A.M. Association of vaccine type and prior SARS-CoV-2 infection with symptoms and antibody measurements following vaccination among health care workers. JAMA Intern. Med. 2021;181:1660–1662. doi: 10.1001/jamainternmed.2021.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J. An mRNA vaccine against SARS-CoV-2—Preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widge A.T., Rouphael N.G., Jackson L.A., Anderson E.J., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 2021;384:80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhuang C.-L., Lin Z.-J., Bi Z.-F., Qiu L.-X., Hu F.-F., Liu X.-H., Lin B.-Z., Su Y.-Y., Pan H.-R., Zhang T.-Y. Inflammation-related adverse reactions following vaccination potentially indicate a stronger immune response. Emerg. Microbes Infect. 2021;10:365–375. doi: 10.1080/22221751.2021.1891002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell T.C., Casella C.R. No pain no gain? Adjuvant effects of alum and monophosphoryl lipid A in pertussis and HPV vaccines. Curr. Opin. Immunol. 2017;47:17–25. doi: 10.1016/j.coi.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burny W., Marchant A., Hervé C., Callegaro A., Caubet M., Fissette L., Gheyle L., Legrand C., Ndour C., Da Silva F.T. Inflammatory parameters associated with systemic reactogenicity following vaccination with adjuvanted hepatitis B vaccines in humans. Vaccine. 2019;37:2004–2015. doi: 10.1016/j.vaccine.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Hwang Y.H., Song K.-H., Choi Y., Go S., Choi S.-J., Jung J., Kang C.K., Choe P.G., Kim N.-J., Park W.B. Can reactogenicity predict immunogenicity after COVID-19 vaccination? Korean J. Intern. Med. 2021;36:1486–1491. doi: 10.3904/kjim.2021.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi H., Lee S.-M., Lim S., Shin K.-H., Kim T., Kim W.-j., Yun M., Oh S.-H. Immunogenicity after Second ChAdOx1 nCoV-19 (AZD1222) Vaccination According to the Individual Reactogenicity, Health Status and Lifestyle. Vaccines. 2021;9:1473. doi: 10.3390/vaccines9121473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy I., Levin E.G., Olmer L., Regev-Yochay G., Agmon-Levin N., Wieder-Finesod A., Indenbaum V., Herzog K., Doolman R., Asraf K. Correlation between Adverse Events and Antibody Titers among Healthcare Workers Vaccinated with BNT162b2 mRNA COVID-19 Vaccine. Vaccines. 2022;10:1220. doi: 10.3390/vaccines10081220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyun J., Park Y., Song Y.G., Han S.H., Park S.Y., Kim S.H., Park J.S., Jeon S.Y., Lee H.S., Lee K.H. Reactogenicity and Immunogenicity of the ChAdOx1 nCOV-19 Coronavirus Disease 2019 Vaccine in South Korean Healthcare Workers. Yonsei Med. J. 2022;63:1078–1087. doi: 10.3349/ymj.2022.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salazar E., Kuchipudi S.V., Christensen P.A., Eagar T.N., Yi X., Zhao P., Jin Z., Long S.W., Olsen R.J., Chen J. Relationship between anti-spike protein antibody titers and SARS-CoV-2 in vitro virus neutralization in convalescent plasma. biorxiv. 2020 doi: 10.1101/2020.06.08.138990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noh J.Y., Kwak J.-E., Yang J.-S., Hwang S.Y., Yoon J.G., Seong H., Hyun H., Lim C.S., Yoon S.-Y., Ryou J. Longitudinal Assessment of Antisevere Acute Respiratory Syndrome Coronavirus 2 Immune Responses for Six Months Based on the Clinical Severity of Coronavirus Disease 2019. J. Infect. Dis. 2021;224:754–763. doi: 10.1093/infdis/jiab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FDA Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. [(accessed on 27 November 2021)]; doi: 10.1016/j.vaccine.2023.07.072. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical. [DOI] [PubMed]
- 25.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 26.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., Dold C., Fuskova M., Gilbert S.C., Hirsch I. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Food & Drug Administration . Investigational COVID-19 Convalescent Plasma Guidance for Industry. Center for Biologics Evaluation and Research; Silver Spring, MD, USA: 2020. [Google Scholar]
- 28.Oyebanji O.A., Wilson B., Keresztesy D., Carias L., Wilk D., Payne M., Aung H., Denis K.S., Lam E.C., Rowley C.F. Does a lack of vaccine side effects correlate with reduced BNT162b2 mRNA vaccine response among healthcare workers and nursing home residents? Aging Clin. Exp. Res. 2021;33:3151–3160. doi: 10.1007/s40520-021-01987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hervé C., Laupèze B., Del Giudice G., Didierlaurent A.M., Da Silva F.T. The how’s and what’s of vaccine reactogenicity. npj Vaccines. 2019;4:1–11. doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G.P., Moreira E.D., Zerbini C. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauernfeind S., Salzberger B., Hitzenbichler F., Scigala K., Einhauser S., Wagner R., Gessner A., Koestler J., Peterhoff D. Association between Reactogenicity and Immunogenicity after Vaccination with BNT162b2. Vaccines. 2021;9:1089. doi: 10.3390/vaccines9101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müller L., Andrée M., Moskorz W., Drexler I., Walotka L., Grothmann R., Ptok J., Hillebrandt J., Ritchie A., Rabl D. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. medRxiv. 2021 doi: 10.1101/2021.03.03.21251066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Si'Ana A.C., Laing E.D., Olsen C.H., Goguet E., Moser M., Jackson-Thompson B.M., Samuels E.C., Pollett S.D., Tribble D.R., Davies J. Adverse effects and antibody titers in response to the BNT162b2 mRNA COVID-19 vaccine in a prospective study of healthcare workers. medRxiv. 2021 doi: 10.1101/2021.06.25.21259544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lapić I., Rogić D., Šegulja D., Zaninović L. Antibody response and self-reported adverse reactions following vaccination with Comirnaty: A pilot study from a Croatian university hospital. J. Clin. Pathol. 2021;75:782–786. doi: 10.1136/jclinpath-2021-207572. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi M., Higa Y., Esaki A., Nabeshima Y., Nakazono A. Does reactogenicity after a second injection of the BNT162b2 vaccine predict spike IgG antibody levels in healthy Japanese subjects? medRxiv. 2021 doi: 10.1371/journal.pone.0257668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeda K., Amano M., Uemura Y., Tsuchiya K., Matsushima T., Noda K., Shimizu Y., Fujiwara A., Takamatsu Y., Ichikawa Y. Correlates of neutralizing/SARS-CoV-2-S1-binding antibody response with adverse effects and immune kinetics in BNT162b2-vaccinated individuals. Sci. Rep. 2021;11:1–10. doi: 10.1038/s41598-021-01930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naaber P., Tserel L., Kangro K., Sepp E., Jürjenson V., Adamson A., Haljasmägi L., Rumm P., Maruste R., Kärner J. Declined Antibody Responses to COVID-19 mRNA Vaccine within First Three Months. medRxiv. 2021 doi: 10.2139/ssrn.3854683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto S., Fukunaga A., Tanaka A., Takeuchi J.S., Inoue Y., Kimura M., Maeda K., Ueda G., Mizoue T., Ujiie M. Association between reactogenicity and SARS-CoV-2 antibodies after the second dose of the BNT162b2 COVID-19 vaccine. Vaccine. 2022;40:1924–1927. doi: 10.1016/j.vaccine.2022.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jubishi D., Okamoto K., Hamada K., Ishii T., Hashimoto H., Shinohara T., Yamashita M., Wakimoto Y., Otani A., Hisasue N. The association between adverse reactions and immune response against SARS-CoV-2 spike protein after vaccination with BNT162b2 among healthcare workers in a single healthcare system: A prospective observational cohort study. Hum. Vaccines Immunother. 2022;18 doi: 10.1080/21645515.2022.2048559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams E., Kizhner A., Stark V.S., Nawab A., Muniz D.D., Tribin F.E., Carreño J.M., Bielak D., Singh G., Hoffer M.E. Predictors for Reactogenicity and Humoral Immunity to SARS-CoV-2 Following Infection and mRNA Vaccination: A Regularized Mixed-Effects Modelling Approach. medRxiv. 2022 doi: 10.1101/2022.04.05.22273450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim S.Y., Kim J.Y., Park S., Kwon J.-S., Park J.Y., Cha H.H., Suh M.H., Lee H.J., Lim J.S., Bae S. Correlation between Reactogenicity and Immunogenicity after the ChAdOx1 nCoV-19 and BNT162b2 mRNA Vaccination. Immune Netw. 2021;21:e41. doi: 10.4110/in.2021.21.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi W., Mizuno T., Hara K., Ara Y., Hurutani R., Agatsuma T., Fujimori M. Association of Systemic Adverse Reactions and Serum SARS-CoV-2 Spike Protein Antibody Levels after Administration of BNT162b2 mRNA COVID-19 Vaccine. Intern. Med. 2022;61:3205–3210. doi: 10.2169/internalmedicine.9699-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Held J., Esse J., Tascilar K., Steininger P., Schober K., Irrgang P., Alsalameh R., Tenbusch M., Seggewies C., Bogdan C. Reactogenicity Correlates Only Weakly with Humoral Immunogenicity after COVID-19 Vaccination with BNT162b2 mRNA (Comirnaty®) Vaccines. 2021;9:1063. doi: 10.3390/vaccines9101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowling B.J., Thompson M.G., Ng T.W., Fang V.J., Perera R.A., Leung N.H., Chen Y., So H.C., Ip D.K., Iuliano A.D. Comparative reactogenicity of enhanced influenza vaccines in older adults. J. Infect. Dis. 2020;222:1383–1391. doi: 10.1093/infdis/jiaa255. [DOI] [PubMed] [Google Scholar]
- 45.Li-Kim-Moy J., Wood N., Jones C., Macartney K., Booy R. Impact of fever and antipyretic use on influenza vaccine immune reponses in children. Pediatr. Infect. Dis. J. 2018;37:971–975. doi: 10.1097/INF.0000000000001949. [DOI] [PubMed] [Google Scholar]
- 46.Ndeupen S., Qin Z., Jacobsen S., Bouteau A., Estanbouli H., Igyártó B.Z. The mRNA-LNP platformߣs lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. Iscience. 2021;24:103479. doi: 10.1016/j.isci.2021.103479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available within the article or Supplementary Material here.