Abstract
Clinical practice guidelines support resilience training and exercise for patients with Parkinson’s disease (PD). This assessor-blinded, randomized clinical trial aimed to compare the effects of a modified mindfulness meditation program versus stretching and resistance training exercise (SRTE) in patients with mild-to-moderate PD. A total of 126 potential participants were enrolled via convenience sampling, of which 68 eligible participants were randomized 1:1 to receive eight weekly 90-min sessions of mindfulness meditation or SRTE. Compared to the SRTE group, generalized estimating equation analyses revealed that the mindfulness group had significantly better improvement in outcomes, particularly for improving depressive symptoms (d, −1.66; 95% CI, −3.31 to −0.02) at week 8 and maintaining emotional non-reactivity at week 20 (d, 2.08; 95% CI, 0.59 to 3.56). Both groups demonstrated significant immediate, small-moderate effects on cognition (effect size [d] = 0.36–0.37, p = 0.006–0.011). Compared with the SRTE, mindfulness meditation appeared to be a feasible and promising strategy for managing depressive symptoms and maintaining emotional stability, with comparable benefits on cognitive performance. To combat the psychospiritual and cognitive sequelae of social unrest and COVID-19 pandemic, the integration of mindfulness training into motor-oriented PD rehabilitation protocols is recommended to strengthen the resilience and minimize the psycho-cognitive comorbidities among patients with mild-to-moderate PD.
Trial Registration: HKU Clinical Trials Registry identifier: HKUCTR-2681.
Subject terms: Parkinson's disease, Human behaviour
Introduction
Parkinson’s disease (PD) is the second-most common neurodegenerative disorder worldwide, which severely limits patients’ functional mobility, independence, and health-related quality of life (HRQOL)1–3. Pharmacotherapy provides symptomatic relief, however, it is often limited by response fluctuations as the disease progresses. The non-pharmacological, lifestyle approaches including physical activity, stress management, and social support are recommended by clinical guidelines to provide additional symptomatic relief in the illness trajectory1. However, maintaining a healthy lifestyle might be difficult in times of adversities such as the 2019–2020 social unrest in Hong Kong and the ongoing coronavirus disease 2019 (COVID-19) pandemic, which are major stressful life events that recently occur4,5. Take for example the 2019–2020 Hong Kong social unrest, a series of large-scale demonstrations and protests throughout the city have escalated into conflicting scenes among protestors and police on the streets and other venues such as shopping malls, restaurants, and public transportations6. PD patients cannot avoid these scenes when going out or traveling. These unexpected scenes were not only highly stressful, but also exacerbated various motor symptoms including tremors and freezing of gait, which markedly hampered their functional mobility and placed them at high risk of falls and/or injuries. In addition, the different political views among families and friends further shrunken PD patients’ social roles and support network. Although the outbreak of the COVID-19 pandemic in early 2020 largely silenced the protests, the mental health crisis continued due to the fear of infection. Helmich and Bloem7 highlighted that successful coping with drastic changes is a dopamine-dependent cognitive process. Owing to the PD-related nigrostriatal dopamine depletion, patients are prone to experience emotional, cognitive, and motor inflexibility, and thus, particularly vulnerable to stressful life events jeopardizing their disease management and prognosis. Hence, stress management and resilience training have emerged as an important topic in contemporary PD care2.
Emerging empirical evidence suggests socially engaging activities such as mindfulness and physical exercise can facilitate the psycho-cognitive process of coping and reduce stress8–10. A recent survey interviewing 5000 PD patients in the United States found that physical exercise was identified as the most frequently used method (83.1%), while 38.7% of PD respondents who practiced mindfulness noticed improvement in both motor and non-motor symptoms11. With the increasing attention of mindfulness in wellness promotion, its research evidence on PD population was just recently evolving. Indeed, mindfulness encompasses a family of practices, including meditation and yoga which are commonly used in healthcare. A clinical trial of yoga involving 138 PD patients demonstrated its significant superior therapeutic effects on alleviating anxiety and depression compared to active exercise control (stretching and resistance training exercises), with similar physical benefits12. However, some may argue such positive effects may be contributed by the physical pathways of yoga exercise. Meanwhile, a 2017 systematic review concluded the effects of mindfulness meditation remained inconclusive in PD patients13. Hence, this proposed study aimed to further examine the effects of the relatively static, mentally focused mindfulness practice – mindfulness meditation – versus conventional physical exercise on psychological distress (primary outcomes: anxiety and depression), and cognition, mindfulness, physical health, and HRQOL (secondary outcomes) among patients with mild-to-moderate PD, compared to stretching and resistance training exercise. This study provided valuable evidence examining these behavioral approaches and their effects in protecting PD patients against stressors owing to major social, political, and public adversities during the 2019–2020 social unrest in Hong Kong. We hypothesized that patients with PD randomly assigned to receive mindfulness meditation would show a greater improvement in psychological distress and the listed health outcomes than patients receiving physical exercises.
Results
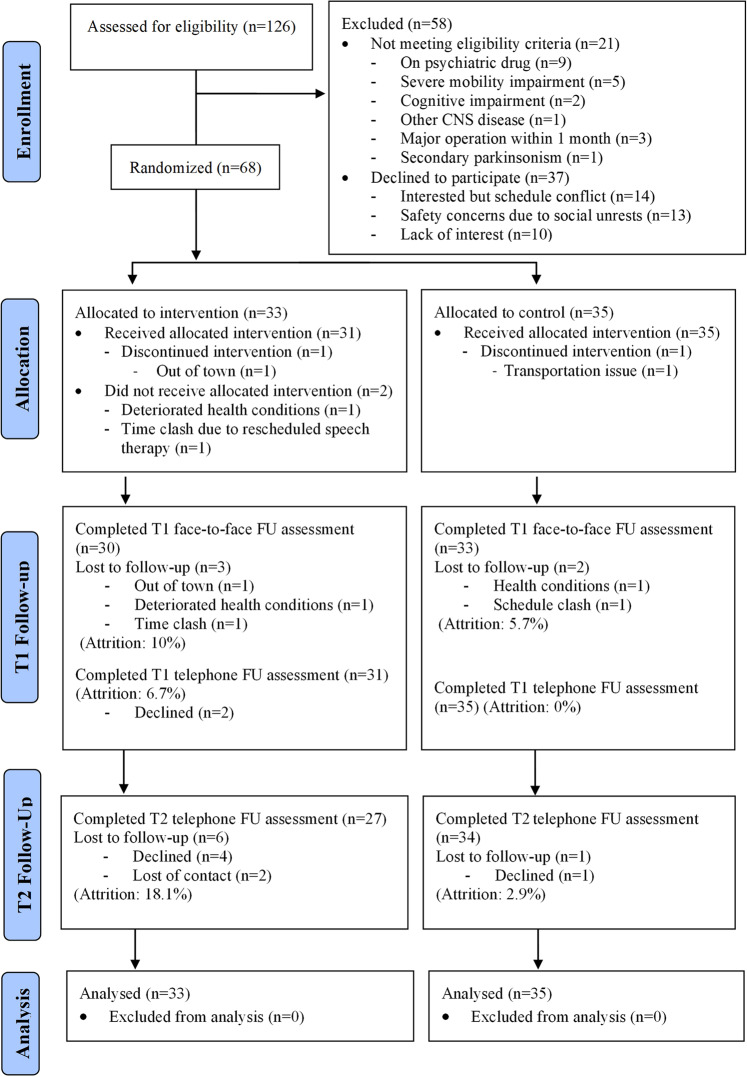
Of 126 potential participants screened, 21 did not meet eligibility criteria and 37 declined to participate because of schedule conflicts (n = 14) (Fig. 1). Transportation and safety concerns are also related to the recent outbreak of social movement (n = 13) and lack of interest (n = 10) (enrollment rate: 54.0%) (Fig. 1). Of the 68 randomized participants, 33 were in the experimental group (MM) and 35 were in the active control group (SRTE). Two participants randomized to the mindfulness group did not attend any sessions, whereas participants randomized to the SRTE group attended at least two sessions. The mean (SD) attendance rates were 6.03 (1.9) for the mindfulness group and 7.14 (1.29) sessions for the SRTE group; 24 out of 33 participants (72.7%) attended at least six sessions of mindfulness meditation, and 31 out of 35 participants (88.6%) attended at least six sessions of SRTE.
Fig. 1. CONSORT 2010 flow diagram.
A flow diagram showing the enrolment, allocation, follow-up and analysis of subjects involved in the RCT.
In view of the safety concerns related to ongoing protests during the intervention period (Oct 2019), our team has adopted additional strategies to maintain the safety for participants while accessing to the community centers, including (i) real-time monitoring of the protests situations, and a summary of affected areas/disturbed transportation were sent to the participants via WhatsApp 1.5 h before the intervention sessions, (ii) if there were any protests nearby the community centers, we would reschedule the intervention sessions and make the announcement at least 1.5 h before the intervention sessions, and (iii) all participants were reassured that their safety was the top priority and they could decide to join/skip any sessions without any consequences. Owing to the sudden onset of protests and affected MTR transportation services in areas nearby the community centers, we had to reschedule about 10% of the intervention sessions to other timeslots. For the rescheduled sessions, a lower attendance has been recorded compared to the prescheduled sessions (56% vs 88%). The crude attendance rates were 80.2% for the mindfulness group and 89.3% for the SRTE group. Excluding rescheduled sessions, the attendance rates were 85.3% for the mindfulness group and 89.7% sessions for the SRTE group.
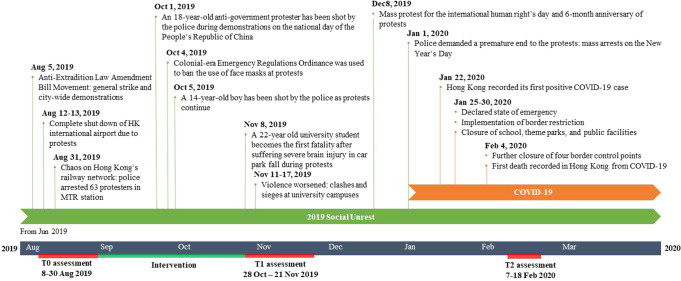
Figure 2 shows the timeline of major adverse events in Hong Kong during the study was implemented. 68 participants were recruited and randomized for the first batch of interventions. All self-reported and clinical outcomes were administered in Aug 2019 (T0: baseline) and Nov 2019 (T1: immediate post-intervention). Given the local enforcement of stringent public health measures toward the COVID-19 pandemic since Jan 2020 (including the suspension of all research and non-clinical services), we were not able to conduct any face-to-face T2 assessment in Feb 2020. Only self-reported outcomes were collected via telephone calls at T2. The overall drop-out rates were 5 of 68 (7.4%) at T1 (MM: 10%; SRTE: 5.7%) and 7 of 68 (10.3%) at T2 (MM: 18.1%; SRTE: 2.9%). With the mandatory closing of all community-based leisure and recreational facilities and suspending research and non-clinical services since Jan 2020, all recruitment and community group-based rehabilitation services were suspended since Jan 2020 and till the project end date (August 2020).
Fig. 2.
Major events in Hong Kong during the study implementation from Aug, 2019 to Feb, 2020.
Baseline characteristics of participants
Experimental and control groups were similar in sociodemographic and clinical characteristics at baseline, respectively, except more participants from the mindfulness group were females (Table 1). The mean (SD) age of participants was 64.5 (8.4) years, ranging from 40–88 years old, and 39 out of 68 were females (57.4%). Of 68 participants, mild PD (Hoehn and Yahr scale, stage 1–2) was seen in 27 participants (39.7%), and most (60.3%) had moderate PD (Hoehn and Yahr scale, stage 3). The mean (SD) MDS-UPDRS-III score was 34.8 (12.7) among all participants. At baseline, 21 out of 68 (30.9%) and 20 out of 68 (23.5%) presented with clinically significant anxiety symptoms and depressive symptoms, respectively, with a mean (SD) HADS score of 11.94 (6.5). Drop- (n = 7) and non-drop-out cases (n = 61) were similar in sociodemographic and clinical characteristics at baseline. Except that drop-out participants had a higher levodopa equivalent dose and better MoCA scores in naming and abstraction subscales (SupplementaryTable 1. Characteristics of drop-out and non-drop-out cases).
Table 1.
Baseline socio-demographic characteristics of the participants (n = 68).
| Characteristics | Participants, No. (%) | P-value | ||
|---|---|---|---|---|
| All (n = 68) | MM (n = 33) | SRTE (n = 35) | ||
| Age, mean (SD), years | 64.5 (8.4) | 62.7 (7.7) | 66.1 (8.9) | 0.094 |
| Gender | ||||
| Male | 29 (42.6) | 10 (30.3) | 19 (54.3) | 0.046* |
| Female | 39 (57.4) | 23 (69.7) | 16 (45.7) | |
| Marital status | ||||
| Single, separated, divorced, or widowed | 17 (25%) | 11 (33.3) | 6 (17.1) | 0.123 |
| Married | 51 (75%) | 22 (66.7) | 29 (82.9) | |
| BMI | 22.8 (4.1) | 35.2 (23.6) | 30.9 (21.9) | 0.089 |
| Education | ||||
| Illiterate/Primary | 14 (20.6) | 7 (21.2) | 7 (20) | 0.902 |
| Secondary or above | 54 (79.4) | 26 (78.9) | 28 (80) | |
| Religion | ||||
| Christianity | 10 (14.7) | 4 (12.1) | 6 (17.1) | 0.767 |
| Catholicity | 6 (8.8) | 4 (12.1) | 2 (5.7) | |
| Buddhism | 9 (13.2) | 4 (12.1) | 5 (14.3) | |
| No religion | 43 (63.2) | 21 (63.6) | 22 (62.9) | |
| Living status | ||||
| Alone | 10 (14.7) | 5 (15.2) | 5 (14.3) | 0.920 |
| With spouse, family, or friends | 58 (85.3) | 28 (84.8) | 30 (85.7) | |
| Social security allowance | 36 (52.9) | 21 (63.6) | 15 (42.9) | 0.219 |
| Disease staging | ||||
| Mild PD, H&Y stage I or II | 27 (39.7) | 17 (51.5) | 10 (28.6) | 0.053 |
| Moderate PD, H&Y stage III | 41 (60.3) | 16 (48.5) | 25 (71.4) | |
| Levodopa equivalent dose, mean (SD) | 638.8 (593.4) | 766.4 (607.2) | 518.5 (562.3) | 0.085 |
| Charlson Comorbidity Index ≥1 | 8 | 4 | 4 | 1.0 |
| Myocardial infarction | 1 (1.5) | 0 (0) | 1 (2.9) | 0.515 |
| Congestive heart failure | 2 (2.9) | 1 (3.0) | 1 (2.9) | 0.739 |
| Peripheral vascular disease | 1 (1.5) | 1 (3.0) | 0 (0) | 0.485 |
| Mild liver disease | 1 (1.5) | 0 (0) | 1 (2.9) | 0.515 |
| Diabetes without chronic complications | 3 (4.4) | 2 (6.1) | 1 (2.9) | 0.478 |
| Tumor without metastases | 2 (2.9) | 1 (3.0) | 1 (2.9) | 0.739 |
| Schwab and England Scale | 8.8 (1.9) | 8.8 (2.0) | 8.9 (1.8) | 0.965 |
Categorical and continuous variables were compared between the two groups using chi-square test, fisher’s exact test, and Independent t-test, respectively. Data are presented as mean (standard deviation), or n (%).
Comorbid conditions were assessed using Charlson Comorbidity Index.
H&Y stage Hoehn and Yahr stage, MM mindfulness meditation, SRTE stretching and resistance training exercise.
Co-primary outcomes
For depressive symptoms, there was a significant difference between groups at T1 end point (p = 0.019) (Table 2). Compared with the SRTE group, the mindfulness group demonstrated significantly greater improvement in depressive symptoms at T1 with a small-to-moderate effect size of 0.37 (time-by-group interaction, T1: β, −1.13 [95% CI, −2.06, −0.19; p = 0.019]), whereas no significant difference between groups at T2 (T2: β, 0.83 [95% CI, −0.42, 2.06; p = 0.191]). In the mindfulness group, significant improvement within the group in depressive symptoms (T1: d = −0.48, p = 0.002) was found with a mean (SD) difference of −1.36 (0.37), whereas no significant difference within the SRTE group at either end points (T1: d = 0.07, p = 0.388; T2: d = 0.03, p = 0.71).
Table 2.
Analysis of GEE for the comparison of outcomes (n = 68).
| Outcomes | Mean (SD) | Effect Size (within group change from baseline) | Group × Time Interaction Effecta | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MM | STRE | MM | p | STRE | p | β (95% CI) | Effect size | p | |
| HADS-anxiety | |||||||||
| T0 | 6.24 (3.46) | 5.51 (3.80) | |||||||
| T1 | 6.12 (3.14) | 5.54 (3.81) | −0.04 | 0.734 | 0.01 | 0.941 | −0.15 (−1.19, 0.88) | 0.17 | 0.77 |
| T2 | 6.66 (3.80) | 6.28 (3.82) | 0.12 | 0.602 | 0.20 | 0.098 | −0.35 (−1.56, 0.87) | 0.10 | 0.58 |
| HADS-depression | |||||||||
| T0 | 5.97 (2.95) | 6.17 (3.57) | |||||||
| T1 | 4.59 (2.75) | 5.91 (4.18) | −0.48 | 0.002** | 0.07 | 0.388 | −1.13 (−2.06, −0.19) | 0.37 | 0.019* |
| T2 | 6.91 (3.26) | 6.29 (3.45) | 0.30 | 0.110 | 0.03 | 0.710 | 0.83 (−0.42, 2.06) | 0.18 | 0.191 |
| FFMQ-observing | |||||||||
| T0 | 12.97 (2.84) | 12.57 (3.37) | |||||||
| T1 | 13.52 (3.37) | 12.54 (3.47) | 0.18 | 0.122 | −0.01 | 0.958 | 0.08 (−1.63, 1.78) | 0.29 | 0.381 |
| T2 | 12.52 (3.31) | 12.04 (3.71) | −0.15 | 0.601 | −0.15 | 0.446 | 0.58 (−0.72, 1.89) | 0.14 | 0.929 |
| FFMQ-describing | |||||||||
| T0 | 13.39 (3.26) | 13.11 (3.47) | |||||||
| T1 | 13.72 (3.56) | 12.77 (3.29) | 0.09 | 0.332 | −0.10 | 0.472 | 0.67 (−0.52, 1.85) | 0.28 | 0.271 |
| T2 | 13.45 (3.64) | 12.24 (3.35) | −0.02 | 0.795 | −0.26 | 0.092 | 0.94 (−0.50, 2.38) | 0.35 | 0.202 |
| FFMQ-acting with awareness | |||||||||
| T0 | 12.45 (3.85) | 14.03 (2.93) | |||||||
| T1 | 13.37 (3.65) | 13.97 (3.03) | 0.25 | 0.163 | −0.02 | 0.899 | 0.98 (−0.48, 2.43) | 0.18 | 0.190 |
| T2 | 12.76 (3.44) | 13.97 (3.21) | 0.08 | 0.377 | −0.02 | 0.781 | 0.37 (−0.84, 1.57) | 0.36 | 0.551 |
| FFMQ-non-judging | |||||||||
| T0 | 11.70 (2.53) | 11.71 (3.12) | |||||||
| T1 | 12.37 (2.55) | 12.60 (2.74) | 0.26 | 0.213 | 0.30 | 0.121 | −0.22 (−1.63, 1.20) | 0.90 | 0.765 |
| T2 | 12.91 (2.58) | 12.38 (2.63) | 0.48 | 0.022* | 0.23 | 0.177 | 0.54 (−0.75, 0.69) | 0.20 | 0.407 |
| FFMQ-non-reacting | |||||||||
| T0 | 12.97 (2.17) | 12.80 (2.97) | |||||||
| T1 | 13.21 (2.85) | 12.23 (2.99) | 0.09 | 0.598 | −0.19 | 0.084 | 0.81 (−0.31, 1.93) | 0.34 | 0.156 |
| T2 | 13.26 (3.28) | 11.18 (2.96) | 0.10 | 0.793 | −0.55 | 0.001*** | 1.91 (0.54, 3.27) | 0.67 | 0.006** |
| FFMQ-total | |||||||||
| T0 | 63.48 (1.53) | 64.23 (1.30) | |||||||
| T1 | 66.00 (1.76) | 64.11 (1.33) | 1.53 | 0.010** | 0.09 | 0.074 | 2.62 (−0.79, 6.05) | 2.44 | 0.132 |
| T2 | 66.33 (1.69) | 61.79 (1.69) | 1.78 | 0.026 | 1.62 | 0.582 | 3.28 (−0.42, 6.98) | 2.67 | 0.082 |
| MDS-UPDRS-III | |||||||||
| T0 | 34.76 (11.83) | 34.74 (13.29) | |||||||
| T1 | 32.51 (11.44) | 32.36 (11.91) | −0.19 | 0.095 | −0.19 | 0.010** | 0.132 (-2.86, 3.12) | 0.01 | 0.931 |
| TUG | |||||||||
| T0 | 14.29 (11.16) | 12.94 (3.79) | |||||||
| T1 | 12.61 (6.63) | 12.28 (3.83) | −0.18 | 0.207 | −0.17 | 0.123 | −1.02 (−3.12, 1.07) | 0.06 | 0.338 |
| HK-MoCA-visuospatial | |||||||||
| T0 | 3.82 (1.19) | 4.14 (1.02) | |||||||
| T1 | 4.11 (1.01) | 4.30 (1.21) | 0.27 | 0.199 | 0.14 | 0.419 | 0.14 (−0.35, 0.63) | 0.17 | 0.575 |
| HK-MoCA-naming | |||||||||
| T0 | 2.85 (0.36) | 2.89 (0.40) | |||||||
| T1 | 2.85 (0.37) | 2.85 (0.50) | 0.01 | 1.000 | 0.07 | 0.572 | 0.03 (−0.11, 0.17) | 0 | 0.680 |
| HK-MoCA-attention | |||||||||
| T0 | 5.27 (0.93) | 5.37 (0.64) | |||||||
| T1 | 5.45 (0.64) | 5.52 (0.63) | 0.22 | 0.442 | 0.23 | 0.282 | 0.03 (−0.38, 0.44) | 0.11 | 0.887 |
| HK-MoCA-language | |||||||||
| T0 | 2.82 (0.39) | 2.77 (0.48) | |||||||
| T1 | 2.80 (0.41) | 2.83 (0.46) | −0.04 | 0.662 | 0.12 | 0.325 | −0.07 (−0.26, 0.12) | 0.07 | 0.461 |
| HK-MoCA-abstraction | |||||||||
| T0 | 1.45 (0.56) | 1.43 (0.60) | |||||||
| T1 | 1.43 (0.58) | 1.34 (0.60) | 0.05 | 1.000 | −0.16 | 0.374 | 0.067 (−0.21, 0.35) | 0.15 | 0.640 |
| HK-MoCA-delayed recall | |||||||||
| T0 | 3.58 (1.46) | 3.06 (1.84) | |||||||
| T1 | 4.10 (1.30) | 3.77 (1.25) | 0.36 | 0.045 | 0.45 | 0.008** | −0.19 (−0.81, 0.43) | 0.26 | 0.547 |
| HK-MoCA-orientation | |||||||||
| T0 | 5.73 (0.62) | 5.63 (0.59) | |||||||
| T1 | 5.96 (0.19) | 5.88 (0.42) | 0.52 | 0.103 | 0.49 | 0.037 | −0.01 (−0.34, 0.31) | 0.25 | 0.936 |
| HK-MoCA-total | |||||||||
| T0 | 25.73 (3.06) | 25.49 (3.32) | |||||||
| T1 | 26.77 (2.61) | 26.70 (3.33) | 0.37 | 0.006** | 0.36 | 0.011* | −0.17 (−1.22, 0.89) | 0.02 | 0.757 |
| PDQ-8 summary index | |||||||||
| T0 | 33.43 (15.76) | 26.70 (18.44) | |||||||
| T1 | 31.73 (15.19) | 29.11 (17.58) | −0.11 | 0.284 | 0.13 | 0.136 | −4.11 (−8.28, 0.05) | 0.16 | 0.053 |
| T2 | 36.26 (17.58) | 28.17 (15.45) | 0.17 | 0.287 | 0.09 | 0.547 | 1.35 (−4.86, 7.57) | 0.49 | 0.669 |
The control group and the baseline measurement were chosen as the reference categories in the GEE model and its corresponding dummy variables were group = 1 and time = 0.
Mean (SD) = change of mean (SD) from T1 and T2 in relation to T0, respectively.
Exp experimental group, Control control group, MDS-UPDRS-III Movement Disorders Society United Parkinson’s Disease Rating Scale Part III, MM mindfulness meditation, HADS Hospital Anxiety and Depression Scale, PDQ-8 Parkinson’s Disease Questionnaire-8, SRTE stretching and resistance training exercise, T0 baseline, T1 8 weeks (immediate post-intervention), T2 20 weeks (three months post-intervention), CI confidence interval.
*p value ≤ 0.025; **p value ≤ 0.01; ***p value ≤ 0.001.
aGroup*time effect: T1 and T2 = additional change of scores of intervention group compared with control group at T1& T2, respectively.
For anxiety, no significant difference between groups at either time points (T1: β, −0.15 [95% CI, −1.19, 0.88; p = 0.77]; T2: β, −0.35 [95% CI, −1.56, 0.87, p = 0.58]). No significant differences within both groups in the anxiety scores across time points.
Secondary outcomes
The mindfulness group showed significant improvement compared with SRTE group in non-reacting aspect of mindfulness at T2 (β, 1.91 [95% CI, 0.54, 3.27; p = 0.006]) and marginally significant improvement in QoL at T1 (β, −4.11 [95% CI, −8.28, 0.05; p = 0.053]). For the mindfulness group, significant improvement was noted in the overall mindfulness (T1: d = 1.53, p = 0.010) and overall cognitive performance at T1 (T1: d = 0.37, p = 0.006), as well as increased non-judging aspect of mindfulness at T2 (T2: d = 0.48, p = 0.022). For the SRTE group, significant improvement was noted in MDS-UPDRS-III motor scores (T1: d = −0.19, p = 0.01), delayed recall (T1: d = 0.46, p = 0.008), and overall cognitive function (T1: d = 0.36, p = 0.011) at T1; whereas significant deterioration was noted in non-reacting aspect of mindfulness at T2 (d = −0.55, p = 0.001).
Adverse events
A participant (3%) from the mindfulness group reported temporary back pain after the 7th session. It was resolved by using of prop (placing a support cushion on the chair) and occasionally doing intermittent changes in body postures during the meditation practice; no medical attention was needed. Another participant (2.9%) from the SRTE group reported temporary mild knee pain during and after squatting exercises, however, no medical attention was required. No further serious adverse events were noted. No adverse events/accidents have been reported when participants accessed to community centers from their home.
Feedback from participants
Supplementary Table 2 summarizes the findings of the satisfaction evaluation. All participants were satisfied with the study. Regarding the open-ended question, surprisingly, 67.7% and 51.6% of participants from the mindfulness group reported improvement in their constipation and sleep outcomes, which were not quantitatively assessed in this study. Regardless of the satisfactory attendance (overall attendance = 82.5%) and acceptable attrition (overall attrition = 7.3% at T1 face-to-face follow-up, 10.3% at T2 telephone follow-up), 60% of the participants reported safety concerns on their way to join the center-based activities caused by the uncertainty and threats of social unrests.
Discussion
We observed that the mindfulness meditation group had greater improvement in psychospiritual outcomes than the conventional physical exercise group, which includes improved depressive symptoms at T1 and maintained non-reactivity at T2. In addition, the mindfulness group showed improved mindfulness and overall cognitive performance at T1 and increased non-judging awareness at T2, whereas the physical exercise group showed improved motor symptoms, delayed recall and overall cognitive performance at T1, with no statistically significant superiority noted. The effects of mindfulness meditation in improving immediate depressive symptoms were statistically significant with a small-to-moderate in size, and reaching marginal clinical significance in PD patients. Significant emotional distress or mental disorder among PD populations during and after large-scale social movements and COVID-19 pandemic was evident14,15, which is associated with higher subsequent psychiatric symptoms16. It shall be noting that the mindfulness program was not specifically designed to address the stress triggers owing to social unrest and/or COVID-19 pandemic, the treatment effects and between-group differences on psychospiritual outcomes would likely to be reduced. Given the unfavorable and unexpected stress triggers, the observed improvements were confirmative because interventions and assessments were conducted during the strikes of social unrest and/or the COVID-19 pandemic.
It is worth noting that the emotional reactivity of exercise group has significantly worsen at T2 (during the start of COVID pandemic) and such difference remained statistically significant when compared to the mindfulness meditation group. Previous neuroimaging studies have suggested that individuals who showed less openness and acceptance to experiences, exhibited stronger activation in limbic systems when labeling negative thoughts, stressful events and experiences17. Hence, anti-pandemic measures should consider providing mindfulness tips/techniques to ease the public emotional reactivity and mitigate pandemic-related distress, which in turn might help to inhibit habitual responses contributing to sharing of false information and ineffective coping.
Furthermore, emerging evidence indicated that the prolonged public health and virus-control measures implemented to combat the COVID-19 pandemic severely disrupted various aspects of daily life among the PD population worldwide, particularly in overcrowded cities like Hong Kong18. Virus-control measures implemented were as follows: social lockdown, closures of community-based leisure and recreational facilities, and suspension of rehabilitation, and home-based care services. The threat to health and the economic downturn associated with the COVID-19 pandemic incur a tremendous burden on the population’s mental health19. All these suggested that PD patients were at high risk of psychological distress during this period. Considering that stress was identified as a risk factor for PD progression and cognitive impairment, our study suggests integrating mindfulness training into evidence-based rehabilitation protocols. Psychologically informed physical therapy could be considered a favorable complementary behavioral approach to strengthening physical functions, psychospiritual resilience and cognitive reserve of PD patients, thereby, reducing their risk of psychiatric or cognitive comorbidities. As for future research directions, further efforts should be expended to test and translate center-based behavioral rehabilitation practices into home-based settings. A hybrid delivery mode is preferred to address the challenges imposed by the common barriers in participating in center-based rehabilitation and any future social unrests/infectious disease outbreaks.
Compared to our previous clinical trial of mindfulness yoga, there was inconsistency in the evidence of its effects on anxiety, motor symptoms, and HRQOL12,20,21. Such discrepancies might be due to the different teaching/training approaches of mindfulness practices, as well as confounding factors related to the external stressors. In the current study, the program aimed to cultivate mindfulness via meditation and breathing techniques. The training was relatively static and started with focused attention on an object to develop attentional stability and non-judgmental awareness of the current mental state22. This mentally-oriented approach might be particularly challenging for some participants as dopamine depletion in PD might affect frontal functions, leading to executive dysfunction, impaired attention and increased feelings of distractions23. In comparison, the previous study cultivated mindfulness mainly via yoga, which is a relatively dynamic and physically exerting form of mind-body training24. On top of emotional and cognitive regulating pathway, we suspect the physically exerting yoga training might provide additional symptomatic relief (i.e. to improve stiffness, muscle weakness, and postural instability) and address additional stress triggers owing to physical symptoms. These findings suggest mindfulness training via physically exerting mind-body approach might multiply the avenue of physical and psychospiritual benefits in PD patients. However, the mechanistic effects of these different use of individual mindfulness practices are still unknown. Future studies should extend the investigation into the use of individual mindfulness techniques (i.e. cultivating mindfulness via meditation vs yoga), and to further examine their therapeutic value and underlying mediating mechanisms among PD patients, preferably using both subjective (i.e. validated patient-reported outcomes such as HADS) and objective outcome measures related to stress and inflammatory responses (i.e. cortisol, IL-6 and TNF-α)25. Such evidence is important to guide the development of personalized mindfulness therapy to optimize its therapeutic benefits.
Both groups showed significant improvement in delayed recall and overall cognitive performance among PD patients. These findings are consistent with a 2018 systematic review of exercise interventions26, which concluded moderate-to-strong evidence on moderate-to-vigorous intensity physical exercise. It improved not only motor symptoms, but also cognitive function in PD patients, including global cognitive function, processing speed, sustained attention, and mental flexibility. Pickut and colleagues27 investigated the effects of an 8-week mindfulness-based intervention (MBI) on anatomical brain MRI scans using a voxel-based morphometry approach, and found that PD patients who received MBI (n = 14) showed increased gray matter density in the amygdala, the caudate nucleus, and the left occipital lobe compared to usual care control (n = 13)27. In addition, MBI studies consistently showed increased activity and structural changes in the insula, anterior and posterior cingulate cortex, striatum, and the medial and dorsolateral prefrontal cortex28. All brain regions have important roles to play in executive functioning, memory, information processing, attentional control, emotional regulation, and self-awareness. Emerging empirical evidence showed that mindfulness may improve emotional and cognitive functioning in PD patients29. Indeed, cognitive impairment is a common non-motor complication of PD, and 50% of the patients demonstrated a significant cognitive decline in the first 3–5 years of illness26. A prospective 3-year longitudinal study with a total of 182 PD patients shows that mild cognitive impairment (MCI) at PD diagnosis predicts a highly increased risk for early dementia30. Therefore, early screening for MCI and evidence-based behavioral interventions for cognitive rehabilitation for at-risk PD patients, such as physical exercise and mindfulness training, should be prescribed to improve their cognitive functioning and prevent subsequent development of dementia31.
Based on our knowledge, this is the first clinical trial of MBI for PD conducted during social unrest and the COVID-19 pandemic. This trial provides invaluable insights as a guide for further development and implements mindfulness as a complementary therapeutic option for PD patients in coping with unplanned external stressors and major adversities. Study strengths include an assessor-masked, randomized clinical design with multiple follow-up time points to elucidate the residual effects of the interventions, involvement of an active control group, comprehensive measurement of physio-psycho-cognitive, and spiritual outcomes. The data analysis followed an intention-to-treat principle.
This study has limitations and must be acknowledged. Although the number of included patients was significantly smaller than originally planned because of the COVID-19 pandemic and associated nationwide regulations, several significant changes were still detected. Nevertheless, the sample size of the current study is above the average of existing MBI clinical trials for PD patients (number of studies = 7; range = 13–138; mean sample size = 50)12,32–37. We believe that these findings will provide valuable evidence to guide the future MBI research for PD. In addition, local government and university guidelines prohibited face-to-face non-clinical services and research activities at T2. Thus, we were unable to perform a face-to-face examination at T2, the sustained intervention effects on motor symptoms, functional mobility, and cognition remained unknown. Thirdly, we did not measure participants’ expectation regarding the effects of each intervention nor measuring their physical activity levels during the study period. Future clinical trial should assess participants’ expectations and physical activity levels as co-variables, which allows a better interpretation of the therapeutic outcomes between groups38. The incorporation of singing bowls might add a potential layer of complexity of the meditation effects. For instance, participants’ feedback collected from the satisfaction evaluation revealed improved constipation outcomes owing to the resonant vibrations generated by the singing bowls being felt by the body and intestines. Future study could consider adopting a qualitative approach to explore participants’ experience of meditation with and without sound catalyst. Furthermore, we observed a higher drop-out and lower attendance for the mindfulness meditation group compared to the physical exercise group. One possibility is that PD patients might be more interested to attend rehabilitation activities with physical training components. However, owing to the on-going protests that occurred during the intervention period, safety concerns while traveling to community center might have an impact on the intervention compliance. Lastly, selection bias may arise because the study participants were enrolled through convenience sampling. There were more females (57%) than males that participated in this study, while PD is 1.5 times more common in males than in females. Such pattern may indicate that women with PD might be more active in reaching out to community resources and more willing to join group-based rehabilitation services. We excluded people who had severe motor limitations, uncontrolled/active psychiatric disorders, or significant cognitive impairment. Thus, the PD population is only partially represented. All these factors may limit the generalizability of the study findings to the entire PD population.
As compared with physical exercises, mindfulness meditation appeared to be a feasible strategy for managing depressive symptoms and maintaining emotional stability, with comparable benefits in cognitive performance, among patients with mild-to-moderate PD. These findings suggest that mindfulness meditation might be a promising complementary lifestyle practice for cultivating non-reactivity and managing depressive mood among patients with PD, which exerts similar benefits on cognition as compared to conventional physical exercise. Given the additional stress triggers related to the unfavorable external conditions (i.e. social unrest and COVID-19 pandemic) which were likely to reduce the treatment effects, such improvements on psychospiritual outcomes are confirmative. Considering that PD is not just a psychologically distressing life event but is also associated with physical and cognitive impairment, health care professionals should integrate mindfulness into evidence-based exercise prescription for PD rehabilitation. Further investigation is warranted to establish the mechanistic effect and compliance of various forms of mindfulness practices. Alternative delivery modes including telehealth should also be included in the further investigation given social unrest and/or infectious disease outbreaks39–41.
Methods
Study design
This assessor-masked, multicentered, randomized clinical trial (RCT) was conducted among PD patients between August 1, 2019 and February 28, 2020 comparing mindfulness meditation with an active exercise control (stretching and resistance training exercise). The trial has been prospectively registered in the HKU Clinical Trials Registry identifier: HKUCTR-2681. Recruitment was conducted via convenience sampling at two out-patient neurology clinics of regional public hospitals and via the Community Rehabilitation Network. Meanwhile, the study implementation was conducted at three community wellness centers. Ethical approval was obtained from the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (HKU/HA HKW IRB) (Reference Number: UW 19-446). All participants provided written informed consent and all obtained data were anonymous. This report followed the Consolidated Standards of Reporting Trials (CONSORT 2010) guidelines and its extension to non-pharmacological interventions42. Ethics approval for this study was obtained from the Institutional Review Board of the University of Hong Kong/ Hospital Authority Hong Kong West Cluster (HKU/HA HKW IRB) (Reference Number: UW 19-446).
Study participants
Individuals were eligible to participate and included in the trial if they were: (i) diagnosed with idiopathic PD (Hoehn and Yahr stage II-IV); (ii) aged ≥18 years; and (iii) able to give written consent. Individuals were excluded if they were: (i) regularly practicing meditation once a week or more during the past 6 months; (ii) currently receiving pharmacological treatments (such as antidepressants) or surgical treatments (such as deep brain stimulation) for uncontrolled/active psychiatric disorders (such as including schizophrenia, psychosis, major depressive disorder, etc.); (iii) were currently participating in any other behavioral or pharmacological trial; (iv) screened with significant cognitive impairment as indicated by the abbreviated mental test lower than 6; or (v) had serious medical conditions except for PD or other contraindication that might hinder their full participation to the intervention (e.g., severe hearing or vision impairment, etc.).
Sampling and recruitment strategies
Subjects were recruited via referrals from two major sources: (i) neurological outpatient clinics from two local regional hospitals, which are the Queen Mary Hospital and Pamela Youde Nethersole Eastern Hospital; and (ii) three PD support groups, which the Community Rehabilitation Network (CRN), Hong Kong Parkinson’s Disease Association (HKPDA), and Hong Kong Parkinson’s Disease Foundation (HKPDF). The intervention was conducted in three community wellness centers to enhance the sample representativeness and participants’ accessibility, recruitment, and adherence. Community wellness is located in three major districts in Hong Kong, namely, Hong Kong-West, Hong Kong-East, and Kowloon. Combinations of recruitment strategies were used, including electronic mailing, printed flyers within the patient support centers, and neurological clinics, and newsletters disseminated among patient support groups. All promotional materials and registration were made accessible online.
Allocation concealment, assignment of interventions and blinding
Prescreening was done via telephone calls and in neurology clinics, and participants who met the criteria underwent baseline assessments. Afterward, participants were randomly assigned to experimental or control groups at a ratio of 1:1 through a computerized permuted block randomization generator, in which randomized sizes of 4, 6, and 8 were used. The randomization sequence was generated by an independent research coordinator and the group assignments were concealed on cards placed inside sequentially numbered, sealed opaque envelopes until the assignment occurred. Outcome assessors were blinded to subject allocation and all assessments were conducted at CRN centers.
Interventions
Mindfulness meditation
The mindfulness meditation program was adapted from the structured eight-week mindfulness-based stress reduction (MBSR) program conceptualized by Jon Kabat-Zin43 and our previous experiences of teaching mindfulness among local PD patients12,20, and was delivered in neutral terminology free from any particular religious affiliation. Previous PD literature reported that frequent and lengthy mind-body interventions (more than 3 times per week, with a total time >180 min per week) were associated with reduced effectiveness owing to the decreased adherence and increased physical intolerance experienced by PD patients, and suggested a moderate dose of about 120 min per week as a feasible and practical mind-body practice for PD patients10. Hence, compared to the standard MBSR program (eight weekly 2.5-hour sessions, one all-day session between sessions 6 and 7, and daily 45-min homework), the tested mindfulness meditation program was modified to eight 1.5-hour sessions, and 20-min home-based practice twice a week. The weekly session consisted of standardized core elements of mindfulness practices: (1) body scan, (2) guided meditation focusing one’s attention on the breath, (3) guided meditation facilitated by singing bowls with focus on being aware of bodily sensations and non-judgmental awareness, and (4) practicing being fully aware during everyday activities by using the breath as an anchor for the attention (Supplementary Table 3. Overview of the modified mindfulness meditation program). Each session started with 15-min of introduction and sharing, followed by 60-min of guided mindfulness meditation (30-min was facilitated with the use of singing bowls) and mindful breathing techniques, and 15-min of closing and sharing. Owing to the physical symptoms of PD patients including tremor, rigidity and bradykinesia, prolonged holding of the same pose is not recommended44. Hence, verbal cues of gentle movements were incorporated every 10-min during the guided meditation practice, so as to avoid exacerbation of physical symptoms. To compensate for the impaired attention and cognition in PD patients, 30-min of the guided meditation practice was facilitated with the use of singing bowls. Previous research has highlighted the combinations of high and low frequency sounds generated by singing bowls could produce resonant vibrations as a catalyst and sensational stimuli for mental activation to induce a deep relaxation response and immersive experiences of meditation45,46. During the guided meditation facilitated by singing bowls, participants were instructed to lie down on yoga mats, in a half-circle around the room with their heads pointed towards the singing bowls, which were placed on the floor near their heads. Participants were instructed to focus their awareness on sound and vibrations created and sensations felt by the body, and merely observe non-judgmentally for any sensations, emotions, or thoughts that may arise during the meditation practice.
Conventional stretching and resistance training exercise
This group of participants received weekly 90-min sessions of full-body physical exercise for 8 weeks. In addition, all participants were encouraged to perform 20-min home-based practice twice a week. The physical exercise protocol consisted of a progressive set of warm-ups, stretching, and resistance training exercises (SRTE) with moderate intensity, and cool-down exercises. The protocol was reviewed by two physiotherapists to confirm the validity for patients with PD, its safety has been demonstrated in our previous published trial12.
The purpose of adopting an active exercise control group was two-fold: (i) to counteract the confounding effects of regular social interaction among peers and instructors during weekly gatherings; and (ii) physical exercise is a standard treatment to provide PD patients additional symptomatic relief on top of pharmacotherapy. The format of interventions was comparable (group), contact time (8 weekly 90-minute sessions), number of participants per group (15–20 participants per session), and venue (activity rooms in community wellness centers). Each intervention was delivered to the participants according to a manualized protocol in which all instructors were trained. Mindfulness meditation was delivered by an instructor with MBSR teacher qualifications, whereas physical exercise was delivered by a qualified fitness instructor. All instructors were experienced in teaching people with chronic illnesses. All participants in each group of interventions were given information booklets covering instructions for home practice.
Data collection and outcome measures
Outcome assessors were trained and masked to group allocation. Each participant was invited to the nearby community rehabilitation center to conduct a face-to-face clinical assessment and interview. If indicated, all assessments were conducted during the “on” state of medications to minimize motor fluctuations among participants. There were three assessment time points, including baseline (T0), immediate post-intervention (T1, 8 weeks), and three-month post-intervention (T2, 20 weeks). Telephone follow-up was offered as an alternative option for those who declined face-to-face clinical assessment to collect self-reported outcomes.
The primary outcome, psychological distress in terms of anxiety, and depressive symptoms were measured by the validated Hospital Anxiety and Depression Scale (HADS) (Chinese-Cantonese)47,48. HADS is a self-report questionnaire consisting of anxiety and depression subscales. Each subscale consists of seven items and each item is rated on a four-point scale. A high score represents a high level of psychological distress. HADS has been suggested to be utilized in the PD population because somatic symptoms that may potentially overlap Parkinsonian manifestations are not assessed on this scale49. In addition, HADS focuses on measuring the negative emotions of anxiety and depression, which were reported as the most prominent psychological factors among PD patients. The Cronbach alphas for the HADS-anxiety and HADS-depression subscales were 0.86 and 0.78. The test-retest reliability for subscales, as assessed by intraclass correlation coefficient (ICC), were 0.86 and 0.84 for the HADS-anxiety and HADS-depression50. Although no published data regarding minimal clinically important difference of HADS score was reported in PD, previous literature reported the MCID for the HADS, triangulated from the distribution-based, anchor-based, and Delphi-based findings, was 1.7 points in cardiovascular diseases51, meanwhile, the MCID of subscale scores for anxiety and depression were 1.32 and 1.40 in pulmonary diseases, respectively52.
Secondary outcomes included were: (i) PD-related motor symptoms as measured using the validated Movement Disorder Society-Unified Parkinson’s Disease Rating Scale, part III motor examination (MDS-UPDRS-III) (Chinese version)53, which covers domains related to tremor, rigidity, bradykinesia, gait, and postural instability; (ii) mobility was measured based on the Timed-up-and-go (TUG) test by the United States Food and Drug Administration approved EncephaLog application using a smartphone device54; (iii) cognition as measured by the Montreal Cognitive Assessment (MoCA) protocol (Hong Kong version)55, which consists of seven subtests that examine varying cognitive domains, including visuospatial/executive, naming, memory, attention, language, abstraction, and orientation; (iv) mindfulness as measured using the 20-item Five-Facet Mindfulness Questionnaire (Short-form) (FFMQ-SF) (Chinese version), which assesses five mindfulness domains including observing, describing, acting with awareness, nonjudgment of inner experience, and non-reaction to inner experience. FFMQ-SF has been validated in community adults recruiting from out-patient clinics (n = 156) and community settings (n = 230) which shared similar socio-demographic characteristic as compared to our study participants (Cronbach’s alpha = 0.80–0.83)56; and (v) HRQOL as assessed using the validated Parkinson’s Disease Questionnaire-8 (PDQ-8) (Chinese version)57,58, which yields a summary index score that captures disease-specific HRQOL in terms mobility, activities of daily living, emotional wellbeing, social support, cognitions, communication, bodily discomfort and stigma.
Satisfactory, safety and adverse event
A satisfactory evaluation questionnaire (10-item) was administered to both groups at the last intervention session to collect feedback and perceived effects of the study. Adverse events were identified during the intervention sessions and by follow-up interview questions about significant intervention-induced discomfort, pain, or harm. Participants were instructed to inform the research team if they encountered any adverse event related to the study.
Sample size
A moderate effect size of 0.59 was reported for people who presented with depressive symptoms according to a meta-analysis of mindfulness interventions compared with exercises59. Assuming an attrition rate of 15%, a total sample size of 112 participants was required in order to have 80% power to determine an effect of at least 0.59 at a 5% level of significance. We have originally planned three batches of intervention sessions during the project period (August 2019–2020) (1st batch: Sept-Oct 2019; 2nd batch: Jan-Feb 2020; 3rd batch: May-Jun 2020). However, given the local government’s enforcement of stringent public health measures toward COVID-19, which include mandatory closing of all community-based leisure and recreational facilities and suspending research and non-clinical services since Jan 2020, all recruitment and community group-based rehabilitation sessions were suspended since Jan 2020 and till the project end date (August 2020). Therefore, only a total of 68 participants (61% of the proposed sample size) were included in this study.
Statistical analysis
The socio-demographic and clinical outcomes of the participants at each time point were summarized using descriptive statistics. We applied the intention-to-treat (ITT) principle under which all participants were included in the analysis as randomized. The changes of the primary and secondary outcomes at immediate post-intervention (T1, 8 weeks) and three-month post-intervention (T1, 20 weeks) from baseline (T0) between the mindfulness meditation and SRTE groups were compared by the generalized estimating equations (GEE). The extra-covariance among the repeated measurements at T1 and T2 were accounted by a first-order auto-regressive working covariance matrix. The covariates included baseline value, group, time and group by time interaction. The GEE possessed the virtue of including participants in the analysis provided they had at least one follow-up measurements. Nevertheless, drop- and non-drop-out cases were compared to check for any differences in demographic characteristics and health conditions among PD participants. IBM SPSS 25 software was used for statistical analysis. The primary objectives of this study are to examine the immediate post-intervention effects of mindfulness meditation on anxiety and depression compared to stretching and resistance training exercise. To account for multiplicity, we consider 2.5% as the nominal level of significance by Bonferroni adjustment.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Supplementary table 1; Supplementary table 2; Supplementary table 3
Acknowledgements
This research project is funded by the Seed Fund for Basic Research for New Staff, the University of Hong Kong. The authors would like to thank the Hong Kong Society for Rehabilitation, Hong Kong Parkinson’s Disease Foundation and Hong Kong Parkinson’s Disease Association for their support, and all the participants who contributed for the study. We would also like to thank the Pure International (HK) Limited for venue sponsorships. We were also grateful to the research assistants, Mr. Leo Chun Chung Lam, Mr. Geoff Ho, and Ms. Charis Ann Lai for their dedicated assistance and contribution to this research paper.
Author contributions
J.Y.Y.K. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. (1) Research project: (a) study concept and design, (b) acquisition, analysis, or interpretation of data, (c) statistical analysis. (2) Manuscript preparation: (a) drafting of the manuscript, (b) critical revision of the manuscript for important intellectual content. (3) Obtained funding. (4) Administrative, technical, or material support. (5) Study supervision. J.Y.Y.K.: 1abc, 2ab, 3, 4, 5; E.P.H.C.: 1bc, 2b; J.Y.H.W.: 1b, 2b, 4; K.Y.W.L.: 1b, 2b, 4; M.-H.H.: 1b, 2b; D.Y.T.F.: 1c, 2b, 3; J.C.Y.K.: 1b, 2b, 4; S.Y.Y.P.: 1b, 2b, 4, 5; M.A.: 1ab, 2b, 4, 5
Data availability
Individual participant data that underlie the results reported in this article, after deidentification (text, tables, and figures), are available for researchers who provide a methodologically sound proposal with ethics approval. Proposal should be directed to the corresponding author (email: jojo.yykwok@gmail.com). To gain access, data requestors will need to sign a data access agreement. Data are available for 5 years.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-023-00452-w.
References
- 1.Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397:2284–2303. doi: 10.1016/S0140-6736(21)00218-X. [DOI] [PubMed] [Google Scholar]
- 2.Kwok JYY, et al. Symptom burden and unmet support needs of patients with Parkinson’s disease: a cross-sectional study in Asia-Pacific regions. J. Am. Med. Dir. Assoc. 2021;22:1255–1264. doi: 10.1016/j.jamda.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Kwok JYY, Lee JJ, Auyeung M, Chan ML, Chan HYL. Letting nature take its course: a qualitative exploration of the illness and adjustment experiences of Hong Kong Chinese people with Parkinson’s disease. Health Soc. Care Community. 2020;28:2343–2351. doi: 10.1111/hsc.13055. [DOI] [PubMed] [Google Scholar]
- 4.Choi, E. P. H., Hui, B. P. H. & Wan, E. Y. F. Depression and Anxiety in Hong Kong during COVID-19. Int. J. Environ. Res. Public Health17, 10.3390/ijerph17103740 (2020). [DOI] [PMC free article] [PubMed]
- 5.Dommershuijsen LJ, et al. Mental health in people with Parkinson’s disease during the COVID-19 pandemic: potential for targeted interventions? npj Parkinson’s Dis. 2021;7:95. doi: 10.1038/s41531-021-00238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou WK, et al. Civil unrest, COVID-19 stressors, anxiety, and depression in the acute phase of the pandemic: a population-based study in Hong Kong. Soc. Psychiatry Psychiatr. Epidemiol. 2021;56:1499–1508. doi: 10.1007/s00127-021-02037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helmich RC, Bloem BR. The impact of the COVID-19 pandemic on Parkinson’s disease: hidden sorrows and emerging opportunities. J. Parkinson’s Dis. 2020;10:351–354. doi: 10.3233/JPD-202038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson KI, et al. Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med. Sci. Sports Exerc. 2019;51:1242–1251. doi: 10.1249/MSS.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleshner F. Physical activity and stress resistance: sympathetic nervous system adaptations prevent stress-induced immunosuppression. Exerc. Sport Sci. Rev. 2005;33:120–126. doi: 10.1097/00003677-200507000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Kwok JYY, Choi KC, Chan HYL. Effects of mind–body exercises on the physiological and psychosocial well-being of individuals with Parkinson’s disease: a systematic review and meta-analysis. Complementary Therapies Med. 2016;29:121–131. doi: 10.1016/j.ctim.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 11.van der Heide A, et al. Stress and mindfulness in Parkinson’s disease – a survey in 5000 patients. npj Parkinsons Dis. 2021;7:7. doi: 10.1038/s41531-020-00152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwok, J. Y. Y. et al. The effects of mindfulness yoga versus stretching and resistance training exercises on anxiety and depression for people with Parkinson’s disease: a randomized clinical trial. JAMA Neurol. 10.1001/jamaneurol.2019.0534 (2019). [DOI] [PMC free article] [PubMed]
- 13.McLean G, Lawrence M, Simpson R, Mercer S. Mindfulness-based stress reduction in Parkinson’s disease: a systematic review. BMC Neurol. 2017;17:92. doi: 10.1186/s12883-017-0876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni MY, et al. Depression and post-traumatic stress during major social unrest in Hong Kong: a 10-year prospective cohort study. Lancet. 2020;395:273–284. doi: 10.1016/S0140-6736(19)33160-5. [DOI] [PubMed] [Google Scholar]
- 15.Hou WK, et al. Probable depression and suicidal ideation in Hong Kong amid massive civil unrest. Ann. Epidemiol. 2021;54:45–51. doi: 10.1016/j.annepidem.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Hou WK, Bonanno GA. Emotions in everyday life during social movements: prospective predictions of mental health. J. Counseling Psychol. 2018;65:120–131. doi: 10.1037/cou0000236. [DOI] [PubMed] [Google Scholar]
- 17.Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosom. Med. 2007;69:560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- 18.Zhao SZ, et al. Mental health crisis under COVID-19 pandemic in Hong Kong, China. Int. J. Infect. Dis. 2020;100:431–433. doi: 10.1016/j.ijid.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes EA, et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. lancet Psychiatry. 2020;7:547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwok JYY, Kwan JCY, Auyeung M, Mok VCT, Chan HYL. The effects of yoga versus stretching and resistance training exercises on psychological distress for people with mild-to-moderate Parkinson’s disease: Study protocol for a randomized controlled trial. Trials. 2017;18:1–13. doi: 10.1186/s13063-017-2223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwok JYY, et al. Effects of mindfulness yoga versus conventional physical exercises on symptom experiences and health-related quality of life in people with Parkinson’s disease: the potential mediating roles of anxiety and depression. Ann. Behav. Med. Publ. Soc. Behav. Med. 2022;56:1068–1081. doi: 10.1093/abm/kaac005. [DOI] [PubMed] [Google Scholar]
- 22.Keng S-L, Smoski MJ, Robins CJ. Effects of mindfulness on psychological health: a review of empirical studies. Clin. Psychol. Rev. 2011;31:1041–1056. doi: 10.1016/j.cpr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang C, Lv L, Mao S, Dong H, Liu B. Cognition deficits in Parkinson’s disease: mechanisms and treatment. Parkinsons Dis. 2020;2020:2076942. doi: 10.1155/2020/2076942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Worle, L. & Pfeiff, E. Yoga as therapeutic exercise: a practical guide for manual therapists, 1st ed. (Churchill Livingstone, 2010).
- 25.Pascoe MC, Thompson DR, Jenkins ZM, Ski CF. Mindfulness mediates the physiological markers of stress: systematic review and meta-analysis. J. Psychiatr. Res. 2017;95:156–178. doi: 10.1016/j.jpsychires.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 26.da Silva FC, et al. Effects of physical exercise programs on cognitive function in Parkinson’s disease patients: a systematic review of randomized controlled trials of the last 10 years. PloS One. 2018;13:e0193113. doi: 10.1371/journal.pone.0193113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pickut BA, et al. Mindfulness based intervention in Parkinson’s disease leads to structural brain changes on MRI: a randomized controlled longitudinal trial. Clin. Neurol. Neurosurg. 2013;115:2419–2425. doi: 10.1016/j.clineuro.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 28.van der Heide A, et al. Stress and mindfulness in Parkinson’s disease: clinical effects and potential underlying mechanisms. Mov. Disord. Off. J. Mov. Disord. Soc. 2021;36:64–70. doi: 10.1002/mds.28345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cash TV, Ekouevi VS, Kilbourn C, Lageman SK. Pilot study of a mindfulness-based group intervention for individuals with Parkinson’s disease and their caregivers. Mindfulness. 2016;7:361–371. doi: 10.1007/s12671-015-0452-1. [DOI] [Google Scholar]
- 30.Pedersen KF, Larsen JP, Tysnes OB, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol. 2013;70:580–586. doi: 10.1001/jamaneurol.2013.2110. [DOI] [PubMed] [Google Scholar]
- 31.Chandler MJ, et al. Comparative effectiveness of behavioral interventions on quality of life for older adults with mild cognitive impairment: a randomized clinical trial. JAMA Netw. Open. 2019;2:e193016. doi: 10.1001/jamanetworkopen.2019.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickut B, et al. Mindfulness training among individuals with Parkinson’s disease: neurobehavioral effects. Parkinson’s Dis. 2015;2015:816404. doi: 10.1155/2015/816404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Son HG, Choi E-O. The effects of mindfulness meditation-based complex exercise program on motor and nonmotor symptoms and quality of life in patients with Parkinson’s disease. Asian Nurs. Res. 2018;12:145–153. doi: 10.1016/j.anr.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Vandenberg BE, et al. Mindfulness-based lifestyle programs for the self-management of Parkinson’s disease in Australia. Health Promotion Int. 2019;34:668–676. doi: 10.1093/heapro/day021. [DOI] [PubMed] [Google Scholar]
- 35.Birtwell K, Dubrow-Marshall L, Dubrow-Marshall R, Duerden T, Dunn A. A mixed methods evaluation of a Mindfulness-Based Stress Reduction course for people with Parkinson’s disease. Complementary Therapies Clin. Pract. 2017;29:220–228. doi: 10.1016/j.ctcp.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Shah-Zamora D, et al. Mindfulness based stress reduction in people with Parkinson’s disease and their care partners. Complementary Therapies Clin. Pract. 2021;43:101377. doi: 10.1016/j.ctcp.2021.101377. [DOI] [PubMed] [Google Scholar]
- 37.Advocat J, et al. The effects of a mindfulness-based lifestyle program for adults with Parkinson’s disease: a mixed methods, wait list controlled randomised control study. BMC Neurol. 2016;16:1–11. doi: 10.1186/s12883-016-0685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frisaldi E, Shaibani A, Benedetti F. Why we should assess patients’ expectations in clinical trials. Pain. Ther. 2017;6:107–110. doi: 10.1007/s40122-017-0071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chau PH, et al. Feasibility, acceptability, and efficacy of virtual reality training for older adults and people with disabilities: single-arm pre-post study. J. Med. Internet Res. 2021;23:e27640. doi: 10.2196/27640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwok JYY, Lee JJ, Choi EPH, Chau PH, Auyeung M. Stay mindfully active during the coronavirus pandemic: a feasibility study of mHealth-delivered mindfulness yoga program for people with Parkinson’s disease. BMC Complementary Med. Therapies. 2022;22:37. doi: 10.1186/s12906-022-03519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong CL, et al. Effects of immersive virtual reality for preventing and managing anxiety, nausea and vomiting among paediatric cancer patients receiving their first chemotherapy: a study protocol for an exploratory trial. PloS one. 2021;16:e0258514. doi: 10.1371/journal.pone.0258514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P. CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann. Intern. Med. 2017;167:40–47. doi: 10.7326/M17-0046. [DOI] [PubMed] [Google Scholar]
- 43.Kabat-Zinn, J. Full catastrophe living: using the wisdom of your body and mind to face stress, pain, and illness, 15th anniversary ed. (Delta Trade Paperback/Bantam Dell, 2005).
- 44.Malling ASB, et al. The influence of posture duration on hand tremor during tasks with attention-distraction in persons with Parkinson’s disease. J. Neuroeng. Rehabil. 2019;16:61. doi: 10.1186/s12984-019-0534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldsby TL, Goldsby ME, McWalters M, Mills PJ. Effects of singing bowl sound meditation on mood, tension, and well-being: an observational study. J. Evid.-based complementary alternative Med. 2017;22:401–406. doi: 10.1177/2156587216668109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pacchetti C, et al. Active music therapy in Parkinson’s disease: an integrative method for motor and emotional rehabilitation. Psychosom. Med. 2000;62:386–393. doi: 10.1097/00006842-200005000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Leung CM, Ho S, Kan CS, Hung CH, Chen CN. Evaluation of the Chinese version of the Hospital Anxiety and Depression Scale. A cross-cultural perspective. Int J. Psychosom. 1993;40:29–34. [PubMed] [Google Scholar]
- 48.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Blazquez C, et al. Psychometric attributes of the Hospital Anxiety and Depression Scale in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2009;24:519–525. doi: 10.1002/mds.22321. [DOI] [PubMed] [Google Scholar]
- 50.Marinus J, Leentjens AF, Visser M, Stiggelbout AM, van Hilten JJ. Evaluation of the hospital anxiety and depression scale in patients with Parkinson’s disease. Clin. Neuropharmacol. 2002;25:318–324. doi: 10.1097/00002826-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Lemay KR, Tulloch HE, Pipe AL, Reed JL. Establishing the minimal clinically important difference for the hospital anxiety and depression scale in patients with cardiovascular disease. J. Cardiopulm. Rehabil. Prev. 2019;39:E6–E11. doi: 10.1097/HCR.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 52.Puhan MA, Frey M, Büchi S, Schünemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual. Life Outcomes. 2008;6:46. doi: 10.1186/1477-7525-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goetz CG, et al. Movement disorder society-sponsored revision of the unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 54.Tchelet, K., Stark-Inbar, A. & Yekutieli, Z. Pilot study of the encephalog smartphone application for gait analysis. Sensors19, 10.3390/s19235179 (2019). [DOI] [PMC free article] [PubMed]
- 55.Wong A, et al. Montreal Cognitive Assessment 5-minute protocol is a brief, valid, reliable, and feasible cognitive screen for telephone administration. Stroke. 2015;46:1059–1064. doi: 10.1161/STROKEAHA.114.007253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou J, Wong SY, Lo HH, Mak WW, Ma HS. Validation of a Chinese version of the Five Facet Mindfulness Questionnaire in Hong Kong and development of a short form. Assessment. 2014;21:363–371. doi: 10.1177/1073191113485121. [DOI] [PubMed] [Google Scholar]
- 57.Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing. 1997;26:353–357. doi: 10.1093/ageing/26.5.353. [DOI] [PubMed] [Google Scholar]
- 58.Tsang KL, et al. Translation and validation of the standard Chinese version of PDQ-39: a quality-of-life measure for patients with Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2002;17:1036–1040. doi: 10.1002/mds.10249. [DOI] [PubMed] [Google Scholar]
- 59.Cramer H, Lauche R, Langhorst J, Dobos G. Yoga for depression: a systematic review and meta-analysis. Depression Anxiety. 2013;30:1068–1083. doi: 10.1002/da.22166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1; Supplementary table 2; Supplementary table 3
Data Availability Statement
Individual participant data that underlie the results reported in this article, after deidentification (text, tables, and figures), are available for researchers who provide a methodologically sound proposal with ethics approval. Proposal should be directed to the corresponding author (email: jojo.yykwok@gmail.com). To gain access, data requestors will need to sign a data access agreement. Data are available for 5 years.