Abstract
The objectives of this study were to verify, first, if arterial stiffness indices can discriminate between obese and healthy children. Second, to evaluate arterial stiffness index predictors and hemodynamic parameters in obese children. Arterial stiffness indices evaluated were pulse wave velocity (PWV), central systolic blood pressure (SBPc), and central pulse pressure (PPc). A cross-sectional, descriptive, comparative study design was used. The sample consisted of 78 normal-weight children (8.1 ± 1.96 years) and 58 obese children (9.0 ± 1.87 years). PWV, PPc, and SBPc were significantly higher in the group of obese children than in the control group. The ROC curve analysis showed that maximum PWV and SBPc sensitivity and specificity in differentiating obese from non-obese children occurred at 4.09 m/s and 86.17 mmHg, respectively. PPc did not exhibit a discriminatory capacity between the two groups. Peripheral systolic blood pressure (SBPp), peripheral pulse pressure (PPp), and PPc (R2 = 0.98) were predictors of increased PWV. Augmentation pressure, PPp, and reflection coefficient (R2 = 0.873) were predictors of PPc. Age, augmentation index, total vascular resistance, cardiac index, and mean fat percentage (R2 = 0.801) were predictors of SBPc.
Conclusion: This study shows for the first time that PWV > 4.09 m/s and SBPc > 86.17 mmHg are cut-off points associated with a higher risk of obesity. These results indicate that the simple, rapid, and noninvasive measurement of arterial stiffness adds prognostic information regarding cardiovascular risk, in addition to increased body mass index.
|
What is Known: • Overweight and obesity are strongly associated with comorbidities que contribute to the development of cardiovascular diseases. | |
|
What is New: • This is the first study to show that PWV and SBPc can discriminate obese from non-obese children. These results show that, in addition to an increased BMI, a simple, rapid, and noninvasive measurement of arterial stiffness adds prognostic information on cardiovascular risk. |
Keywords: Vascular stiffness, Pulse wave velocity, Systemic arterial hypertension, Pediatric obesity, Overweight
Introduction
Obesity is a chronic multifactorial disease associated with genetic, nutritional, hormonal, socioeconomic, and environmental factors [1]. It can affect all age groups and has a significant impact on physical and mental health [2]. The prevalence of childhood obesity has increased exponentially worldwide [3]. In Brazil, a recent meta-analysis by Ferreira et al. (2021) showed that the prevalence of obesity increased over 3 decades (1990: 6.5%; 2000: 7.9%; and 2010: 12.0%). It is higher in boys than in girls, increases with age, and is more prevalent in developed Brazilian regions [4].
Obesity is associated with eating disorders, depression, and anxiety, including body image distortions [2]. Some studies have exhibited worse aspects of quality of life in obese children and adolescents, compared to those with normal weight [5, 6]. As for physical health, physical inactivity stands out as an important contributor to increased cardiovascular risk, in addition to being independently associated with the development of chronic diseases [7, 8].
Among the complications of obesity, we highlight accelerated vascular aging, called arterial stiffness, which is an independent predictor for cardiovascular diseases (CVD), cognitive functional decline, and chronic kidney disease [9]. Arterial stiffness is part of a natural vascular aging process, but it can be accelerated by obesity, insulin resistance, and diabetes [10]. Pulse wave velocity (PWV), measured between the carotid and femoral arteries, is the gold standard for arterial stiffness analysis. Despite several studies demonstrating an increased PWV in this population, there are conflicting reports in the literature regarding this statement [11]. Hudson et al. demonstrated that increased adiposity is associated with higher PWV in obese adolescents [12]. Castro et al. observed that central systolic blood pressure (SBPc) and central pulse pressure (PPc), also considered to be indices of arterial stiffness, are higher in obese than in non-obese children and adolescents, regardless of dyslipidemia, arterial hypertension, or a sedentary lifestyle [13]. Increased body mass index (BMI) alone has been associated with increased peripheral SBP (SBPp) and SBPc and reduced myocardial perfusion.
To our knowledge, no study has verified whether arterial stiffness indices were able to discriminate between obese and non-obese children, nor to identify the associated predictors of arterial stiffness indices in a single measure. In addition to increased BMI, the results of this study may provide prognostic information on cardiovascular risk. The identification of arterial stiffness index predictors may assist with the implementation of preventive actions and early treatment in this population, averting irreversible cardiovascular system damage.
Materials and methods
This is a cross-sectional, prospective, and comparative study with children aged 5–12 years from a Basic Health Unit evaluated between November 2020 and October 2021. The exclusion criteria were children using continuous medication or diagnosed with diabetes, kidney diseases, cardiorespiratory diseases, or obstructive sleep apnea (OSA). The International Study of Asthma and Allergies in Childhood [14] and the Obstructive Apnea Syndrome in Child-18 [15] questionnaires were respectively used to identify asthma and OSA, which are common in this age group.
A total of 136 patients were evaluated. In a subsequent statistical analysis, the data presented 90% of normal approximation for all tests comparing arterial stiffness indices at a 5% significance level. Sociodemographic variables and socioeconomic class were assessed by means of the questionnaire of the Brazilian Association of Research Companies [16].
Participants were classified as normal-weight or obese in terms of age and sex by means of the BMI z-score distribution curves as recommended by the World Health Organization [17]. Waist circumference was measured, and the waist-to-height ratio (WHtR) was calculated. The percentage of body fat (%BF) was determined by triceps and subscapular skinfold measurements by two examiners using a skinfold caliper (Cescorf traditional clinical model), and calculation was carried out in terms of the equation proposed by Slaughter et al. [18]. Patients were also evaluated regarding their pubertal development and classified according to Tanner [19].
Cardiovascular parameters were performed according to previous studies by our group [20–23]. They were measured using the Mobil-O-Graph pulse wave analysis monitor (Mobil-O-Graph, IEM, Stolberg, Germany), which was clinically validated for peripheral and central blood pressure measurements [24]. The central or aortic pulse wave is generated from the peripheral oscillatory pulse wave using an algorithm that integrates arterial impedance and aortic hemodynamics in a mathematical model (ARC Solver algorithm). The ARC Solver method calculates PWV after analyzing the aortic pulse wave and its decomposition into ejection and reflection waves. AIx@75 is the percentage between augmentation pressure and PPc (AIx@75 = AP/PPc × 100).
Habitual physical activity was assessed using the short version of the International Physical Activity Questionnaire (IPAQs) [25], and the Pediatric Quality of Life Inventory (PedsQL™) version 4.0 (pedsQL-4.0) questionnaire was used to assess quality of life [26].
Statistical analysis
The data were presented in frequency tables with absolute frequencies and their respective percentages and descriptive measures (mean, median, standard deviation, minimum, maximum, and 25th and 75th percentiles) for quantitative data. The normality of the variables was tested by the Kolmogorov–Smirnov test. Parametric tests were used for variables with a normal distribution (t-test for independent groups, and one-way ANOVA), and the Mann–Whitney test was used for those without a normal distribution.
The Chi-square test was used for the association of categorical variables. For numerical variables, Pearson’s correlation coefficient was used for parametric data, and Spearman’s correlation was used for non-parametric data. Multiple linear regression models were built by the stepwise method. The necessary assumptions (normality, independence, and homoscedasticity of residuals) were evaluated in all models, as well as multicollinearity by the variance inflation factor (VIF). Variables that presented a p-value lower than 0.20 in univariate analysis and correlation coefficients above 0.25 entered the multiple regression model. The ROC curve of the three main arterial stiffness indices—PWV, SBPc, and PPc—was used to discriminate obese from non-obese children. A 5% significance level was set in all tests; therefore, comparisons whose p-value was less than or equal to 5% were considered significant. The SPSS software version 25.0 was used for the analyses.
The present study was approved by the FCM-MG Research Ethics Committee (CAAE: 38,379,520.6.0000.5134). Parents or guardians provided written informed consent.
Results
The sample consisted of 136 patients, 58 obese (9.0 ± 1.87 years old) and 78 with normal weight (8.1 ± 1.96 years old) classified by BMI. The groups were similar in terms of sex (p = 0.400) and age (p = 0.060). Of the girls, 53.4% were obese and 46.2% had normal weight. The economic class level was lower in the normal-weight group (D/E class, 21.8%) than in the obese group (D/E class, 3.5%) (p < 0.001) and did not influence any arterial stiffness measurements. Quality of life was not associated with any significant difference between obese and normal-weight participants. As for development, the normal-weight group consisted of one postpubescent child (1.28%), 25 pubescent (32.05%), and 52 prepubescent (66.67%) children. The obese group had seven postpubescent (12.07%), 22 pubescent (37.93%), and 29 prepubescent (50%) children.
SBPp and DBPp were significantly higher in the obese group (106.67 mmHg and 62.23 mmHg) than in the normal-weight group (100.11 mmHg and 56.74 mmHg) (p < 0.001). Similar results were observed for SBPc and DBPc (p = 0.001), which were significantly higher in the obese group (95.51 mmHg and 64.19 mmHg) than in the normal-weight group (88.29 mmHg and 58.41 mmHg). PPp and pulse pressure amplification (PPA) did not differ between groups. On the other hand, PPc was significantly higher in the obese (31.41 mmHg) than in the normal-weight group (30.00 mmHg) (p < 0.031). Similarly, with regard to SBPc and PPc, obese children presented a significantly higher PWV (4.4 m/s) than normal-weight children (4.19 m/s) (p < 0.001). Augmentation pressure did not differ between groups (Table 1). As for hemodynamic parameters, stroke volume and cardiac output were significantly higher in the obese group (49.15 ml and 4.33 l/min) compared to the control group (46.29 ml and 3.95 l/min). In contrast, the cardiac index was significantly lower in the obese group (3.2 l/min/m2) than in the control group (4.13 l/min/m2) (p < 0.001). HR and total vascular resistance (TVR) did not differ between groups.
Table 1.
Comparison of arterial stiffness indices between obese and normal-weight children (control)
| Arterial stiffness indices | Obese (n = 58) | Control (n = 78) | p-value | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | Min–Max | Mean (SD) | Median (IQR) | Min–Max | ||
| PWV (m/s) | 4.40 (0.26) | 4.40 (4.23–4.87) | 3.6–5.0 | 4.19 (0.27) | 4.18 (3.97–4.67) | 3.6–5.0 | < 0.001 a |
| cPP (mmHg) | 31.41 (4.83) | 31.50 (27.67–39.33) | 20.3–41.3 | 30.00 (5.30) | 28.67 (26.0–42.33) | 20.3–41.3 | 0.031 b |
| cSBP (mmHg) | 95.51 (7.75) | 95.00 (89.33–108.33) | 74.0–13.0 | 88.29 (7.04) | 89.17 (82.6–98.67) | 72.3–100.0 | < 0.001 a |
PWV pulse wave velocity, cPP central pulse pressure, cSBP central systolic blood pressure, Min minimun, Max maximun, IQR interquartile range
aStudent T test
bMann–Whitney test
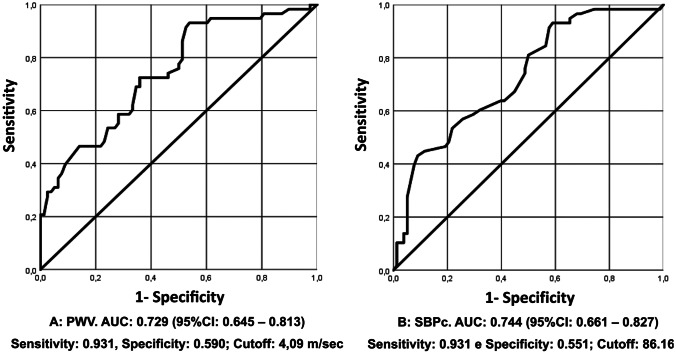
ROC curve analysis showed that the maximum PWV and SBPc sensitivity and specificity in differentiating obese and non-obese children occurred at 4.09 m/s and 86.17 mmHg, respectively (Fig. 1). The ROC curve for PPc showed no good discriminatory capacity in differentiating obese and non-obese children.
Fig. 1.
ROC curve of pulse wave velocity (PWV) and central systolic blood pressure (cSBP)
The association of sociodemographic and anthropometric variables, as well as of cardiovascular parameters and arterial stiffness indices (PWV, PPc, and SBPc) in obese children are shown in Tables 2, 3, and 4, respectively. Figures 2, 3, and 4 represent the associations of different variables with PWV, SBPc, and PPc, respectively.
Table 2.
Association between sociodemographic and anthropometric characteristics, physical activity level, cardiovascular parameters, and other arterial stiffness indices with PWV in obese children
| PWV | BMI | p-value | |
|---|---|---|---|
| Obese (n = 58) | |||
| Sex | Male | 4.40 (0.22) | 0.930 a |
| Female | 4.40 (0.28) | ||
| Age (years) | 0.443* c | ||
| Body mass index (kg/m2) | 0.408* c | ||
| Waist-to-height ratio | 0.208 c | ||
| Body fat (%) | 0.301* c | ||
| IPAQs | High activity (n = 15) | 4.29 (0.33) | 0.126 b |
| Moderate activity (n = 22) | 4.46 (0.21) | ||
| Low activity (n = 21) | 4.42 (0.22) | ||
| Peripheral vascular parameters | |||
| SBPp (mmHg) | 0.977* c | ||
| PPp (mmHg) | 0.524* c | ||
| Central vascular parameters | |||
| SBPc (mmHg) | 0.927* c | ||
| PPc (mmHg) | 0.476* d | ||
| Stiffness parameters | |||
| AIx@75 (%) | 0.348* c | ||
| Pulse pressure amplification | 0.082 d | ||
| Reflection coefficient | 0.200 c | ||
| Augmentation pressure (mmHg) | 0.307* c | ||
| Hemodynamic parameters | |||
| Total vascular resistance (s*mmHg/ml) | 0.243 c | ||
| Cardiac index (l/min/m2) | −0.226 c | ||
| Heart rate (bpm) | 0.240 c |
BMI body mass index, IPAQs International Physical Activity Questionnaire Short form, SBPp peripheral systolic blood pressure, PPp peripheral pulse pressure, SBPc central systolic blood pressure, PPc central pulse pressure, AIx@75 augumentation index normalize to heart rate of 75 bpm
*Significant comparison (p < 0.05)
aStudent T test
bOne way ANOVA
cPearson’s correlation
dSpearman’s correlation
Table 3.
Association between sociodemographic and anthropometric characteristics, physical activity level, cardiovascular parameters, and other stiffness indices with PPc in obese children
| PPc | BMI | ||
|---|---|---|---|
| Obese (n = 58) | p-value | ||
| Sex | Male | 31 (27.6–34) [22.6–39.33] | 0.697 b |
| Female | 32.2 (28–35) [20.22–41.33] | ||
| Age | 0.148 c | ||
| Body mass index (kg/m2) | 0.246 c | ||
| Waist-to-height ratio | 0.117 c | ||
| Body fat (%) | 0.210 c | ||
| IPAQs | High activity (n = 15) | 29.93 (3.75) | 0.177 a |
| Moderate activity (n = 22) | 32.83 (5.43) | ||
| Low activity (n = 21) | 30.97 (4.66) | ||
| Peripheral vascular parameters | |||
| PPp (mmHg) | 0.754 * c | ||
| Stiffness parameters | |||
| AIx@75 (%) | 0.317* c | ||
| Augmentation pressure (mmHg) | 0.844* c | ||
| PWV (m/s) | 0.476* c | ||
| Reflection coefficient | 0.585* c | ||
| Pulse pressure amplification | −0.342* c | ||
| Hemodynamic parameters | |||
| Total vascular resistance (s*mmHg/ml) | 0.023 c | ||
| Heart rate (bpm) | −0.213 c |
BMI body mass index, IPAQs International Physical Activity Questionnaire Short form, PPp peripheral pulse pressure, AIx@75 augumentation index normalized to heart rate of 75 bpm
*Significant comparison (p < 0.05)
aOne way ANOVA
bMann–Whitney
cSpearman’s correlation
Table 4.
Association between sociodemographic and anthropometric characteristics, physical activity level, cardiovascular parameters, and other arterial stiffness indices with SBPc in obese children
| SBPc | BMI | ||
|---|---|---|---|
| Obese (n = 58) | p-value | ||
| Sex | Male | 95.4 (7.7) | 0.943 a |
| Female | 95.6 (7.9) | ||
| Age (years) | 0.500* d | ||
| Body mass index (kg/m2) | 0.481* d | ||
| Waist-to-height ratio | 0.263* d | ||
| Body fat (%) | 0.402* d | ||
| IPAQc | High activity (n = 15) | 29.93 (3.75) | 0.177 b |
| Moderate activity (n = 22) | 32.83 (5.43) | ||
| Low activity (n = 21) | 30.97 (4.66) | ||
| Peripheral vascular parameters | |||
| SBPp (mmHg) | 0.923* d | ||
| Stiffness parameters | |||
| AIx@75 (%) | 0.391* d | ||
| PWV (m/s) | 0.927* d | ||
| Augmentation pressure (mmHg) | 0.269* c | ||
| Reflection coefficient | 0.369* d | ||
| Pulse pressure amplification | −0.158* c | ||
| Parâmteros hemodinâmicos | |||
| Total vascular resistance (s*mmHg/ml) | 0.427* d | ||
| Cardiac index (l/min/m2) | −0.328* d |
BMI body mass index, IPAQs International Physical Activity Questionnaire Short form, pPP peripheral pulse pressure, pSBP peripheral systolic blood pressure, AIx@75 augumentation index normalized to heart rate of 75 bpm, PWV pulse wave velocity
*Significant comparison (p < 0.05)
aStudent T test
bOne way ANOVA
cSpearman’s correlation
dPearson’s correlation
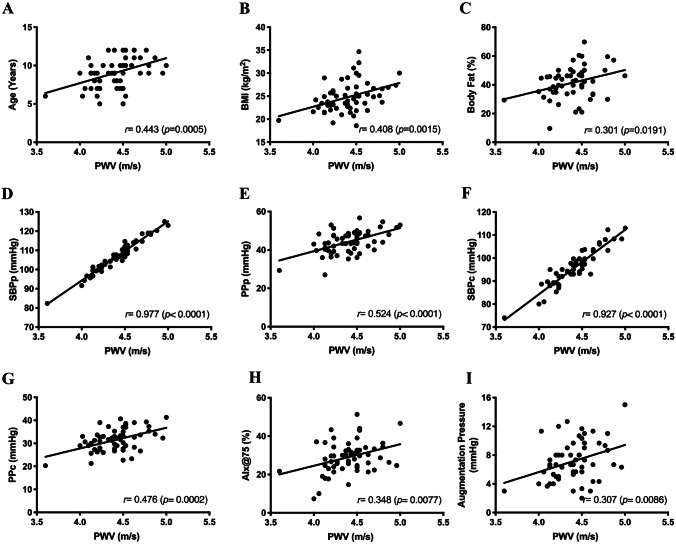
Fig. 2.
Association between anthropometric characteristics, cardiovascular parameters, and other arterial stiffness indices with pulse wave velocity (PWV) in obese children. BMI, body mass index; SBPp and SBPc, peripheral and central systolic blood pressure; PPp and PPc, peripheral and central pulse pressure; AIx@75, augmentation index normalized to heart rate of 75 of bpm
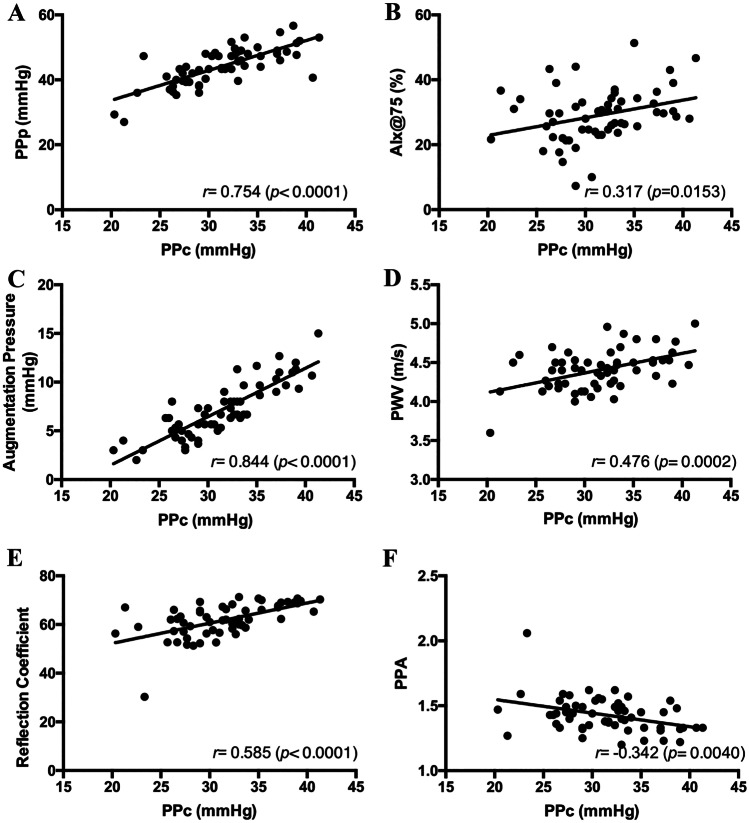
Fig. 3.
Association between cardiovascular parameters and other arterial stiffness indices with central pulse pressure (PPc) in obese children. PPp, peripheral pulse pressure; AIx@75, augumentation index normalized to heart rate of 75 bpm; PWV, pulse wave velocity; PPA, pulse pressure amplification
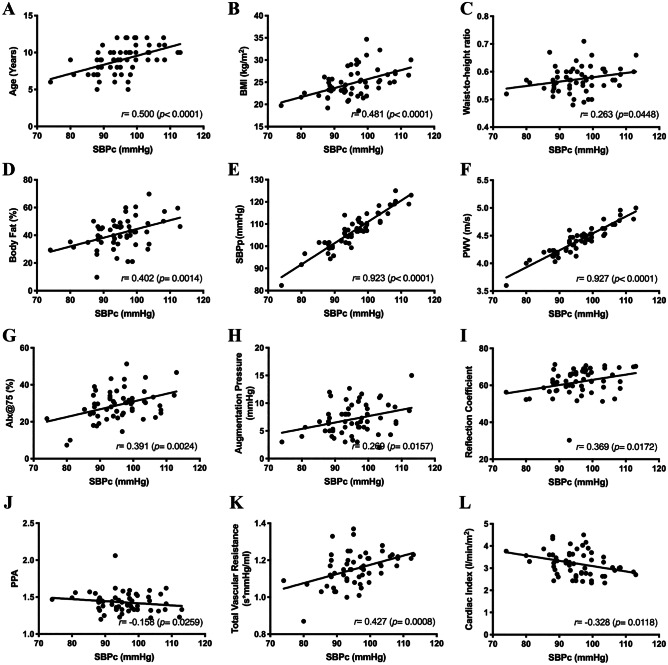
Fig. 4.
Association between anthropometric characteristics, cardiovascular parameters, and other arterial stiffness indices with central systolic blood pressure (SBPc) in obese children. BMI, body mass index; SBPp, peripheral systolic blood pressure; PWV, pulse wave velocity; AIx@75, augumentation index normalized to heart rate of 75 bpm; PPA, pulse pressure amplification
Table 2 shows that age and BMI had a direct and moderate correlation with PWV. Waist-to-height ratio (WHtR) and body fat percentage were weakly correlated with PWV. AIx@75 and augmentation pressure also were weakly correlated with PWV. SBPc and SBPp presented direct and high-intensity correlations with PWV. PPc and PPp correlated positively and at medium intensity with PWV.
Factors associated with PPc are shown in Table 3. PPp and augmentation pressure showed a high and positive correlation with PPc. The associations of PPc with PWV, AIx@75, and reflection coefficient were positive and moderate. As expected, PPA was negatively correlated with PPc.
Age, BMI, and body fat percentage showed a direct and moderate correlation with SBPc. Waist-to-height ratio was weakly correlated with SBPc. SBPp and PWV showed direct and high-intensity correlations with SBPc. AIx@75, augmentation pressure, and reflection coefficient were weakly correlated with PWV. PPA and cardiac index were negatively correlated with PPc (Table 4).
The multiple linear regression analysis to predict the behavior of PWV, PPc, and SBPc is presented in Table 5. PWV was positively associated with SBPp and PPc and negatively associated with PPp. SBPp had the greatest effect (highest standardized coefficient) on PWV. The three variables together explain a 98% increase in PWV.
Table 5.
Coefficients of the reference equation for PWV. PPc and SBPc in obese children
| Variables | Coefficient | Standardized coefficient | CI 95% | p-value |
|---|---|---|---|---|
| PWV (R2 = 0.980) | ||||
| Constant | 1.017 | - | 0.887–1.148 | < 0.001 |
| SBPp (mmHg) | 0.030 | 0.944 | 0.029–0.031 | 0.006 |
| PPp (mmHg) | −0.004 | −0.088 | 0.887–1.148 | < 0.001 |
| PPc (mmHg) | 0.012 | 0.218 | 0.008–0.015 | < 0.001 |
| PPc (R2 = 0.873) | ||||
| Constant | −3.551 | −11.85–4.75 | 0.395 | |
| Augmentation pressure (mmHg) | 0.651 | 0.380 | 0.325–0.976 | 0.000 |
| PPp (mmHg) | 0.427 | 0.519 | 0.314–0.540 | 0.000 |
| Reflection coefficient | 0.185 | 0.268 | 0.076–0.294 | 0.001 |
| SBPc (R2 = 0.801) | ||||
| Constant | 4.389 | −28.590–37.368 | 0.790 | |
| Age (years) | 2.829 | 0.724 | 1.888–3.769 | 0.000 |
| AIx@75 (%) | 0.363 | 0.408 | 0.182–0.543 | 0.000 |
| Total vascular resistance (s*mmHg/ml) | 21.897 | 0.267 | 3.878–39.915 | 0.018 |
| Cardiac index (l/min/m2) | 6.243 | 0.485 | 2.485–10.001 | 0.002 |
| Body fat (%) | 0.224 | 0.333 | 0.077–0.370 | 0.004 |
PWV pulse wave velocity, SBPc central systolic blood pressure, PPp peripheral pulse pressure, PPc central pulse pressure, AIx@75 augmentation index normalized to heart rate of 75 bpm
The predictors of PPc were augmentation pressure, PPp, and reflection coefficient. PPp had the greatest effect (highest standardized coefficient) on PPc. The three variables together explain an 87.3% increase in PPc.
Age, AIx@75, TVR, cardiac index, and body fat percentage were directly associated and increased SBPc values. Increasing age had the greatest effect (higher standardized coefficient) on SBPc. The five variables together explain an 80.1% increase in SBPc.
Discussion
The present study shows for the first time that PWV and SBPc can differentiate between obese and non-obese children. In addition, we identified the predictors of increased PWV, SBPc, and PPc in this population.
PWV has been suggested as a noninvasive measurement to assess vascular health, especially in pediatric patients at risk for cardiovascular disease [27]. Corroborating the studies by Koopman et al. and Urbina et al. [28, 29], the present study demonstrated a significantly higher PWV in the obese group. Contrary to these findings, Charakida et al., Lurb et al. and Dangardt et al. [30–32] found significantly lower PWV in obese children compared to the control group. According to Dangardt et al. a lower PWV may reflect general vasodilation.
ROC curve analysis demonstrated the discriminatory strength of PWV in distinguishing obese from non-obese children, with maximum sensitivity and specificity at 4.09 m/s. Several PWV reference equations have been created for children and adolescents considered healthy [10, 27, 33]. Reusz et al. [33] evaluated PWV in 1008 children and adolescents (6–20 years old) and observed that PWV was positively correlated with age, height, weight, and blood pressure and negatively correlated with heart rate, but only age, height, and blood pressure remained as the main PWV predictors in multiple regression analysis. Fischer et al. [27] also evaluated children and adolescents (5–19.6 years old) and observed that PWV was positively correlated with age, height, weight, SBP, mean arterial pressure, and sex. Multiple regression analysis identified age, sex, and mean arterial pressure as independent predictors of PWV, explaining 42% of the overall variability. In addition to predictors such as age, height, and weight, Thurn et al. [10] also reported an association of PWV with BMI and body surface area, paternal hypertension, maternal obesity, and passive smoking. The present study analyzed associated factors and predictors of increased PWV in obese children. PWV correlated directly with age, BMI, WHtR, body fat percentage, and vascular pressures (SBPc, SBPp, PPc, and PPp). AIx@75 and augmentation pressure, indirect indices of arterial stiffness, and direct of reflection wave are also positively associated with PWV. Amplification pressure represents an increased SBPc due to increased wave reflection reaching the heart. Unlike previous studies, in the present study, only vascular variables (SBPp, PPp, and PPc) remained in the multiple regression model and explained 98% of the increased PWV. SBPp had the greatest effect (highest standardized coefficient) on PWV. In a systematic review and meta-analysis, Hudson et al. [34] reported that PWV varied in obese children according to the evaluated site, with increased stiffness in the carotid arteries and aorta. These findings may have important clinical implications as target organs are more exposed to central rather than peripheral blood pressure [35].
In the present study, peripheral and central vascular pressures were higher in obese children. Stabouli et al. [36] demonstrated that arterial stiffness is higher in overweight and obese children in the presence of arterial hypertension. These authors reported that peripheral and central blood pressures, BMI, and hemodynamic parameters including stroke volume, cardiac output, total peripheral vascular resistance, and cardiac index were all associated with increased 24-h PWV. However, in multiple regression analysis, only 24-h peripheral and central blood pressures and cardiac index were independent predictors of 24-h PWV. Li et al. [37] presented a possible explanation for the correlation between hypertension, arterial stiffness, and childhood obesity. According to the authors, sympathetic activation leads to increased left ventricular ejection, leading to increased PPc and PWV. Increased heart rate and stroke volume can lead to increased cardiac output, which leads to increased mean arterial pressure. In the present study, obese children had higher cardiac output and stroke volume values without heart rate changes.
We also evaluated the SBPs ROC curve, which showed that the maximum SBPc sensitivity and specificity to differentiate obese and non-obese children occurred at 86.17 mmHg and area under the curve (AUC) of 0.744. Measured at the aortic root, the SBPc is the result of the interaction between the stroke volume ejected by the left ventricle, the damping capacity of the great arteries, and the pressure waves propagated and reflected in the arterial tree [38]. The behavior of the aorta as a blood reservoir prevents an overly increased SBPc and a sharp flow drop during diastole, due to elastic recoil favoring coronary artery filling at this stage [38]. In the present study, SBPc was significantly higher in the group of obese children than in the control group. Age, BMI, and body fat percentage showed a direct and moderate correlation with SBPc. SBPp and PWV showed direct and high-intensity correlations with SBPc in the group of obese children. Peluso et al. [35] reported that children and adolescents with high SBPc presented a higher association with vascular changes (increased carotid intima-media thickness and arterial stiffness) compared with high SBPp. Increased SBPc in children and adolescents can be explained by an increased amplitude of incident and reflected wave components associated with an increased stroke volume and/or aortic arterial stiffness [13]. In line with these data, in the present study, the obese group had increased stroke volume.
Corroborating the study by Castro et al., in our study, the PPc was higher in obese than in normal-weight children. PPp, augmentation pressure, PWV, AIx@75, and reflection coefficient were factors associated with this increase. In multiple regression analysis, augmentation pressure, PPp, and reflection coefficient explained 87.3% of the increased PPc. As expected, PPA was negatively correlated with PPc. Garcia-Espinosa et al. [39] also reported increased PPc in obese children, in addition to increased PWV and SBPc associated with BMI.
In this study, we compared hemodynamic parameters between the obese and control group. Despite the stroke volume and cardiac output being significantly higher in the obese group, when these data were normalized by the body surface, the cardiac index was significantly lower in the obese group compared to the control group. Our results corroborate those found by Castro et al. [13]. These authors observed that stroke volume and cardiac output were significantly higher in obese children and adolescents aged 5–15 years. On the other hand, the cardiac index was significantly lower in this population. According to Castro et al., the higher stroke volume may be related to a state of hyperdynamic circulation. Cardiac index changes are associated with clinically critical alterations of the cardiac functioning of obese people as a result of body composition variations, and low cardiac index is related to poor tissue perfusion.
Obesity represents a chronic hypoxic state associated with decreased nitric oxide (NO) bioavailability. These decreased NO levels lead to the increased production of hypoxia-inducible factor-1α (HIF-1α), which is involved in the regulation of several metabolic pathway genes, including pro-inflammatory adipokines, endothelial NO synthase (eNOS), and insulin signaling components [40]. Our results suggest that the low cardiac index observed in obese patients can cause vascular tissue hypoxia, and this decreased NO bioavailability may contribute to inducing greater expression of pro-inflammatory cytokines. The metabolic and inflammatory pathogenesis caused by low cardiac index may be related to vascular and cardiac dysfunctions observed in obese patients.
Some limitations of our analyses must be taken into account. First, it is possible that some variable that has not been evaluated may be a predictor of arterial stiffness indices. Second, this study was limited to a single center, reducing the external applicability of the data. Third, the data were collected in the period of the pandemic COVID-19, leading to a sedentary lifestyle, eating habits, and psychological problems which may have influenced arterial stiffness measures.
In conclusion, this is the first study to show that PWV and SBPc can discriminate obese from non-obese children. In addition, we have highlighted the associated factors and predictors of arterial stiffness indices, PWV, PPc, and SBPc, in obese children. These results show that, in addition to an increased BMI, a simple, rapid, and noninvasive measurement of arterial stiffness adds prognostic information on cardiovascular risk.
Acknowledgements
This work was supported by the Fundação Educacional Lucas Machado – FELUMA, Faculdade Ciências Médicas-Minas Gerais (FCM-MG), Pós-Graduação em Ciências da Saúde. Scheinbein, GHA is undergraduate student from medical course from FCM-MG.
Abbreviations
- AIx@75
Augmentation index normalized to heart rate of 75 bpm
- AP
Augmentation pressure
- AUC
Area under the curve
- %BF
Percentage of body fat
- BMI
Body mass index
- DBPc
Central diastolic blood pressure
- CI
Cardiac index
- CO
Cardiac output
- PPc
Central pulse pressure
- SBPc
Central systolic blood pressure
- CVD
Cardiovascular diseases
- HIF-1α
Hypoxia-inducible factor-1α
- HR
Heart rate
- IPAQs
Internacional physical activity questionnaire short version
- NO
Nitric oxide
- OSA
Obstructive sleep apnea
- DBPp
Peripheral diastolic blood pressure
- PedsQL 4.0
Pediatric Quality of Life Inventory, Version 4.0
- PPA
Pulse pressure amplification (pPP/cPP ratio)
- PPp
Peripheral pulse pressure
- SBPp
Peripheral systolic blood pressure
- PWV
Pulse wave velocity
- RC
Reflection coefficient
- SV
Systolic volume
- TVR
Total vascular resistance
- WHtR
Waist-to-height ratio
Authors’ contributions
Bittencourt, JC: selected the patients; conceived and outlined the study; coordinated and supervised the data collection; analyzed and interpreted the data; drafted, edited, and critically reviewed the manuscript for important intellectual content. Scheinbein, GHA; Oliveira-Junior, WC; Bassi, RL; Moura, LB; Correa, ALD, Bernardes, RGL; Freitas, LS; Lemos, JC: contributed to the data collection and reviewed the manuscript for important intellectual content. Gonçalves GKN: analyzed and interpreted the data; drafted, edited, and critically reviewed the manuscript for important intellectual content. Rodrigues-Machado, MG: conceived and designed the study; analyzed and interpreted the data; drafted, edited, and critically revised the manuscript for important intellectual content. All the authors read and approved the final version of the manuscript.
Data Availability
All data are available in eletronic PDF and XLS files upon request to Maria da Glória Rodrigues Machado via e-mail maria.machado@cienciasmedicasmg.edu.br
Declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of the Faculdade Ciências Médicas de Minas Gerais under the protocol number 08812019000005134.
Consent to participate
Informed assent was obtained from all minors’ participants included in the study. Written informed consent was obtained from the parents or legal guardians.
Consent for publication
All parents or legal guardians provided informed consent regarding the publishing of data about their children.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Juliana Cabral Bittencourt, Email: drajulianabittencourt@gmail.com.
Giovanna Hermont Abbes Scheinbein, Email: giovannascheinbein@gmail.com.
Walmer Cardoso de Oliveira Junior, Email: cardosowalmer@gmail.com.
Roberta Leão Bassi, Email: robertalbassi@gmail.com.
Luiza Bretas Moura, Email: luizapbg@gmail.com.
Ana Luisa Drumond Correa, Email: analuisadrumond@gmail.com.
Raquel Gil de Lima Bernardes, Email: raquelgbernardes@gmail.com.
Leticia Silveira Freitas, Email: leticiafreitas@icloud.com.
Julia Correa Lemos, Email: julia_correa1@hotmail.com.
Gleisy Kelly Neves Gonçalves, Email: goncalvesgk@gmail.com.
Maria da Glória Rodrigues-Machado, Email: maria.machado@cienciasmedicasmg.edu.br.
References
- 1.Meneguetti BT, Cardoso MH, Ribeiro CFA, et al. Neuropeptide receptors as potential pharmacological targets for obesity. Pharmacol Therap. 2019;196:59–78. doi: 10.1016/j.pharmthera.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Sanyaolu A, Okorie C, Qi X et al (2019). Childhood and adolescent obesity in the United States: a public health concern. Glob Pediatr Health 6:2333794X1989130. 10.1177/2333794X19891305. [DOI] [PMC free article] [PubMed]
- 3.Risk Factor Collaboration NCD, (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira CM, dos Reis ND, de Castro AO, et al. Prevalence of childhood obesity in Brazil: systematic review and meta-analysis. J Pediatria. 2021;97:490–499. doi: 10.1016/j.jped.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’avila HF, Poll FA, Reuter CP, et al. Health-related quality of life in adolescents with excess weight. J Pediatria. 2019;95:495–501. doi: 10.1016/j.jped.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Poeta LS, de Duarte M, de Giuliano I. Qualidade de vida relacionada à saúde de crianças obesas. Rev Assoc Med Bras. 2010;56:168–172. doi: 10.1590/S0104-42302010000200014. [DOI] [PubMed] [Google Scholar]
- 7.Janssen I, LeBlanc AG. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int J Behav Nutr Phys Act. 2010;7:40. doi: 10.1186/1479-5868-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salmon J, Dunstan D, Owen N. Should we be concerned about children spending extended periods of time in sedentary pursuits even among the highly active? Int J Pediatr Obes. 2008;3:66–68. doi: 10.1080/17477160802169672. [DOI] [PubMed] [Google Scholar]
- 9.Aroor AR, Jia G, Sowers JR. Cellular mechanisms underlying obesity-induced arterial stiffness. Am J Physiol-Regul, Integ Comparat Physiol. 2018;314:R387–R398. doi: 10.1152/ajpregu.00235.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thurn D, Doyon A, Sözeri B, et al. Aortic pulse wave velocity in healthy children and adolescents: reference values for the vicorder device and modifying factors. AJHYPE. 2015;28:1480–1488. doi: 10.1093/ajh/hpv048. [DOI] [PubMed] [Google Scholar]
- 11.Cote AT, Harris KC, Panagiotopoulos C, et al. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol. 2013;62:1309–1319. doi: 10.1016/j.jacc.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 12.Hudson L, Kinra S, Wong I et al (2017) Is arterial stiffening associated with adiposity, severity of obesity and other contemporary cardiometabolic markers in a community sample of adolescents with obesity in the UK? bmjpo 1:e000061. 10.1136/bmjpo-2017-000061 [DOI] [PMC free article] [PubMed]
- 13.Castro JM, García-Espinosa V, Curcio S, et al. Childhood obesity associates haemodynamic and vascular changes that result in increased central aortic pressure with augmented incident and reflected wave components, without changes in peripheral amplification. Int J Vasc Med. 2016;2016:1–8. doi: 10.1155/2016/3129304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Maçãira EF, Algranti E, Stelmach R, et al. Determinação de escore e nota de corte do módulo de asma do International Study of Asthma and Allergies in Childhood para discriminação de adultos asmáticos em estudos epidemiológicos. J Bras Pneumol. 2005;31:477–485. doi: 10.1590/S1806-37132005000600004. [DOI] [Google Scholar]
- 15.Santos Fernandes FMV, da Cruz Vieira Veloso Teles R. Application of the Portuguese version of the Obstructive Sleep Apnea-18 survey to children. Br J Otorhinol. 2013;79:720–726. doi: 10.5935/1808-8694.20130132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ABEP. Brazilian Criteria 2015 and social class distribution update for 2016. [cited October 19th, 2020]. Available from: http://www.abep.org/criterio-brasil
- 17.World Health Organization (2006) WHO Child growth standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age Methods and development. WHO (nonserial publication). Geneva, Switzerland WHO. Available in: https://www.who.int childgrowth/standards/en/ and https://www.who.int/childgrowth/en/. Accessed 15 Mar 2020
- 18.Slaughter MH, Lohman TG, Boileau RA, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–723. [PubMed] [Google Scholar]
- 19.Tanner JM (1962) Growth at adolescence. 2nd ed. Oxford: Blackwell Scientific publications
- 20.Alves C, Pinho JF, dos Santos LM, et al. Augmentation index, a predictor of cardiovascular events, is increased in children and adolescents with primary nephrotic syndrome. Pediatr Nephrol. 2020;35:815–827. doi: 10.1007/s00467-019-04434-0. [DOI] [PubMed] [Google Scholar]
- 21.Duarte SV, Souza Rajão J, Pinho JF et al (2019) Changes in aortic pulse wave components, pulse pressure amplification, and hemodynamic parameters of children and adolescents with type 1 diabetes. Pediatr Diabetes 20:202–209. 10.1111/pedi.12782 [DOI] [PubMed]
- 22.Rossi-Monteiro EM, Sefair LR, Lima MC, et al. Pediatric obstructive sleep-disordered breathing is associated with arterial stiffness. Eur J Pediatr. 2022;181:725–734. doi: 10.1007/s00431-021-04238-1. [DOI] [PubMed] [Google Scholar]
- 23.Santos LM dos, Gomes IC, Pinho JF et al (2021) Predictors and reference equations for augmentation index, an arterial stiffness marker, in healthy children and adolescents. Clinics. 76:e2350. 10.6061/clinics/2021/e2350 [DOI] [PMC free article] [PubMed]
- 24.Wei W, Tölle M, Zidek W, van der Giet M. Validation of the mobil-O-Graph: 24 h-blood pressure measurement device. Blood Press Monitori. 2010;15:225–228. doi: 10.1097/MBP.0b013e328338892f. [DOI] [PubMed] [Google Scholar]
- 25.Kowalski KC, Crocker PR, Donen RM (2004) The physical activity questionnaire for older children (PAQ-C) and adolescents (PAQ-A) manual. [cited October 19th, 2020]. Available from: https://www.researchgate.net/publication/228441462
- 26.Varni JW, Seid M, Rode CA. The PedsQLTM: measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Fischer DC, Schreiver C, Heimhalt M, et al. Pediatric reference values of carotid-femoral pulse wave velocity determined with an oscillometric device. J Hypertens. 2012;30:2159–2167. doi: 10.1097/HJH.0b013e3283582217. [DOI] [PubMed] [Google Scholar]
- 28.Koopman LP, McCrindle BW, Slorach C, et al. Interaction between myocardial and vascular changes in obese children: a pilot study. J Am Soc Echocardiog. 2012;25:401–410.e1. doi: 10.1016/j.echo.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 29.Urbina EM, Kimball TR, Khoury PR, et al. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens. 2010;28:1692–1698. doi: 10.1097/HJH.0b013e32833a6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charakida M, Jones A, Falaschetti E, et al. Childhood obesity and vascular phenotypes. J Am Coll Cardiol. 2012;60:2643–2650. doi: 10.1016/j.jacc.2012.08.1017. [DOI] [PubMed] [Google Scholar]
- 31.Dangardt F, Osika W, Volkmann R, et al. Obese children show increased intimal wall thickness and decreased pulse wave velocity. Clin Physiol Funct Imag. 2008;28:287–293. doi: 10.1111/j.1475-097X.2008.00806.x. [DOI] [PubMed] [Google Scholar]
- 32.Lurbe E, Torro I, Garcia-Vicent C, et al. Blood Pressure and obesity exert independent influences on pulse wave velocity in youth. Hypertension. 2012;60:550–555. doi: 10.1161/HYPERTENSIONAHA.112.194746. [DOI] [PubMed] [Google Scholar]
- 33.Reusz GS, Cseprekal O, Temmar M, et al. Reference values of pulse wave velocity in healthy children and teenagers. Hypertension. 2010;56:217–224. doi: 10.1161/HYPERTENSIONAHA.110.152686. [DOI] [PubMed] [Google Scholar]
- 34.Hudson LD, Rapala A, Khan T, et al. Evidence for contemporary arterial stiffening in obese children and adolescents using pulse wave velocity: a systematic review and meta-analysis. Atherosclerosis. 2015;241:376–386. doi: 10.1016/j.atherosclerosis.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Peluso G, García-Espinosa V, Curcio S, et al. High central aortic rather than brachial blood pressure is associated with carotid wall remodeling and increased arterial stiffness in childhood. High Blood Press Cardiovasc Prev. 2017;24:49–60. doi: 10.1007/s40292-017-0179-2. [DOI] [PubMed] [Google Scholar]
- 36.Stabouli S, Kollios K, Nika T, et al. Ambulatory hemodynamic patterns, obesity, and pulse wave velocity in children and adolescents. Pediatr Nephrol. 2020;35:2335–2344. doi: 10.1007/s00467-020-04694-1. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Haseler E, Chowienczyk P, Sinha MD. Haemodynamics of hypertension in children. Curr Hypertens Rep. 2020;22:60. doi: 10.1007/s11906-020-01044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bulas J, Potocarova M, Kupcova V, et al. Central systolic blood pressure increases with aortic stiffness. Bratisl Lek Listy. 2019;120:894–898. doi: 10.4149/BLL_2019_150. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Espinosa V, Bia D, Castro J, et al. Peripheral and central aortic pressure, wave-derived reflection parameters, local and regional arterial stiffness and structural parameters in children and adolescents: impact of body mass index variations. High Blood Press Cardiovasc Prev. 2018;25:267–280. doi: 10.1007/s40292-018-0264-1. [DOI] [PubMed] [Google Scholar]
- 40.Jia G, Aroor AR, DeMarco VG, et al. Vascular stiffness in insulin resistance and obesity. Front Physiol. 2015;6:231. doi: 10.3389/fphys.2015.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in eletronic PDF and XLS files upon request to Maria da Glória Rodrigues Machado via e-mail maria.machado@cienciasmedicasmg.edu.br