Abstract
Glucagon and insulin are the main regulators of blood glucose. While the actions of insulin are extensively mapped, less is known about glucagon. Besides glucagon’s role in glucose homeostasis, there are additional links between the pancreatic α-cells and the hepatocytes, often collectively referred to as the liver–α-cell axis, that may be of importance for health and disease. Thus, glucagon receptor antagonism (pharmacological or genetic), which disrupts the liver–α-cell axis, results not only in lower fasting glucose but also in reduced amino acid turnover and dyslipidemia. Here, we review the actions of glucagon on glucose homeostasis, amino acid catabolism, and lipid metabolism in the context of the liver–α-cell axis. The concept of glucagon resistance is also discussed, and we argue that the various elements of the liver–α-cell axis may be differentially affected in metabolic diseases such as diabetes, obesity, and nonalcoholic fatty liver disease (NAFLD). This conceptual rethinking of glucagon biology may explain why patients with type 2 diabetes have hyperglucagonemia and how NAFLD disrupts the liver–α-cell axis, compromising the normal glucagon-mediated enhancement of substrate-induced amino acid turnover and possibly fatty acid β-oxidation. In contrast to amino acid catabolism, glucagon-induced glucose production may not be affected by NAFLD, explaining the diabetogenic effect of NAFLD-associated hyperglucagonemia. Consideration of the liver–α-cell axis is essential to understanding the complex pathophysiology underlying diabetes and other metabolic diseases.
100 Years of Glucagon Research: Is It All About Glucose?
Glucagon was discovered in the early 1920s by Kimball and Murlin (1) and is thought to be one of the main regulators of glucose homeostasis together with its hormonal counterpart, insulin. Glucagon increases glucose levels, whereas insulin decreases glucose levels. It is probably the ratio of these two hormones that best determines hepatic glucose production, although the relative role of the two hormones in diabetic hyperglycemia is still debated (2). Importantly, the interplay between insulin and glucagon signaling depends on the nutritional state (i.e., postprandial vs. fasting conditions). Many patients with type 2 diabetes present with increased plasma levels of glucagon (hyperglucagonemia) and relative insulin deficiency, and restoring glucagon and insulin signaling may therefore be fundamental in diabetes therapy. However, focusing exclusively on the glucoregulatory effects of glucagon may be misleading, as glucagon receptor (GCGR) agonism and antagonism result in a range of metabolic disruptions, including changes in plasma levels of amino acids and lipids and hepatic fat content (3). Thus, focusing only on the glucoregulatory effects of glucagon offers an incomplete picture of its physiology and pathophysiology (4).
GCGR agonists and antagonists are currently being developed for treatment of obesity, nonalcoholic fatty liver disease (NAFLD), hypoglycemia, and hyperglycemia. However, it is not always appreciated that pharmacological levels of glucagon (i.e., treatment with a glucagon agonist) will not only activate its cognate receptor system (GCGR) but will also activate the glucagon-like peptide 1 (GLP-1) receptor (GLP-1R) system (although with lower potency) with combined effects on body weight and insulin secretion, whereby the diabetogenic effects of glucagon, mediated by hepatic glucose production, to some extent may be mitigated (1). It is understandable that GCGR antagonists are being investigated as potential glucose-lowering drugs, but data from phase 1 and 2 trials have been discouraging due to unfavorable side effects (5). As discussed in detail later, these side effects, which probably are inevitable consequences of blocking the physiological actions of glucagon, include both dyslipidemia and accumulation of triglycerides in hepatocytes (i.e., NAFLD) and hyperaminoacidemia. Conversely, the main findings in patients with extreme glucagon excess (glucagon-producing tumors) and glucagon deficiency (inactivating GCGR mutations) are hypoaminoacidemia (6) and hyperaminoacidemia (7), respectively, but not necessarily diabetes. A practical result of this is that plasma levels of amino acids are now used as a readout for GCGR activity in clinical trials of dual (glucagon/GLP-1) or triple (glucagon/GLP-1/glucose-dependent insulinotropic polypeptide [GIP]) receptor agonists (8). However, it must be noted that reduced plasma levels of amino acids during treatment with GCGR agonists may also reflect other mechanisms, such as weight loss, dietary changes, etc. Dual GLP-1/GCGR agonist treatment reduces plasma levels of amino acids independently of insulin and glucose levels and importantly also of GLP-1R activity (9). Dual GLP-1/GCGR agonism, but not isolated GLP-1R agonism (dulaglutide), additionally increases hepatic expression of amino acid transporters and carbamoyl phosphate synthase 1 (CPS-1) (9), a key enzyme in ureagenesis. These results indicate that the actions of glucagon on amino acid metabolism may outweigh its effect on glucose homeostasis under fed and fasted conditions.
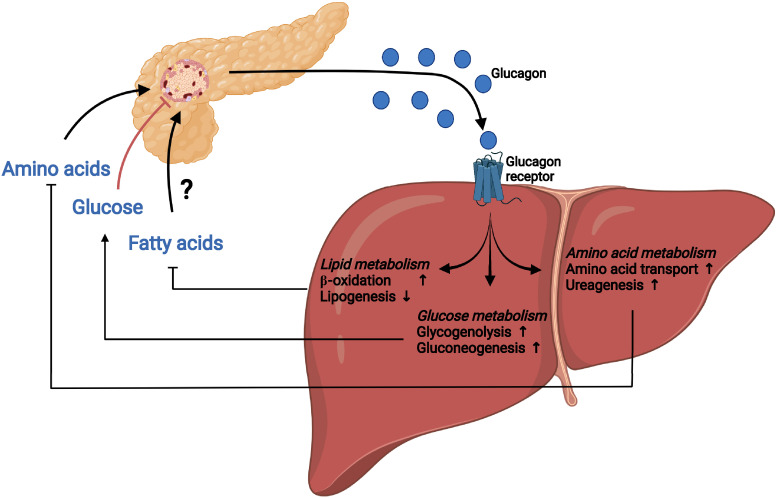
We (3) and others (10–12) have, in recent years, described and defined the liver–α-cell axis as a physiological feedback loop in which several amino acids stimulate the secretion of glucagon (glucagonotropic amino acids, some of which may also stimulate α-cell growth and proliferation, as shown in animal models), which, in turn, enhances transport into the hepatocytes and metabolism of amino acids via ureagenesis and gluconeogenesis. In this review, we describe how the liver–α-cell axis comprises more than a single feedback system merely controlling hepatic glucose turnover (Fig. 1); the axis also regulates amino acid and lipid homeostasis. Below, we will examine this more closely in the context of health as well as metabolic disease.
Figure 1.
The liver–α-cell axis. The liver–α-cell axis constitutes a feedback loop in which circulating amino acids stimulate glucagon secretion from pancreatic α-cells and glucagon in turn controls hepatic amino acid uptake and metabolism, including increased amino acid transport and ureagenesis. The liver–α-cell axis also constitutes a feedback loop in which high levels of circulating glucose inhibit α-cell secretion of glucagon, resulting in decreased hepatic glucose metabolism and subsequent glucose production. In contrast, lower levels of circulating glucose result in increased α-cell secretion of glucagon and increased glucagon-mediated hepatic glucose metabolism, including increased glycogenolysis and gluconeogenesis, and glucose production. A final component of the liver–α-cell axis may be the regulation of hepatic lipid metabolism by increasing β-oxidation and decreasing lipogenesis; however, it is currently not established exactly how, and if, lipids regulate α-cell secretion.
Discovery and Definition of the Liver–α-Cell Axis
The cross talk between the liver and the pancreas is an established physiological concept. The inverse relationship between β-cell secretion and hepatic glucose production is an example and represents an essential regulatory mechanism of blood glucose levels. In type 2 diabetes, the liver–pancreas circuit is disrupted, in part due to reduced hepatic insulin sensitivity and clearance, eventually resulting in hyperglycemia and hyperinsulinemia. A similar interplay exists between the pancreatic α-cells and hepatic glucose production. The normal feedback in the axis is disrupted in patients with type 2 diabetes, resulting in hyperinsulinemia and, as alluded to above, hyperglucagonemia, which may compensate for the glucagon insensitivity. With increased insulin and glucagon levels, normal rates of glucose entry into the cells and substrate-induced ureagenesis may be maintained but at the cost of concomitant hyperglycemia. The importance of the cross talk between the liver and the β-cells has been recognized for many years; however, the α-cell part was discovered more recently. The key observation was the extreme α-cell hyperplasia upon GCGR antagonism by deletions of or destructive mutations in the GCGR gene. From experiments with deletion of the hepatic expression of GCGR, it was concluded that α-cell hyperplasia was probably due to a circulating factor (13). A trophic protein was suspected, but in 2015, Solloway et al. (10) showed that amino acids might be responsible and proposed the existence of “a nutrient-sensing circuit between liver and pancreas in which glucagon-dependent control of hepatic amino acid metabolism regulates α-cell mass.” Their study did not address whether and how acute regulation of glucagon secretion may also be controlled by the liver–α-cell axis. Soon after, we (3) proposed that both proliferation and α-cell secretion were regulated acutely in a similar manner. Further studies showed how upregulation of amino acid transporters (e.g., Slc38a5) was essential for the proliferative effects of glutamine in murine islets (11,12). In humans, the α-cell population is thought to be more or less constant throughout the life span, but extreme α-cell hyperplasia has been reported in patients with inactivating GCGR mutations (7). It was later demonstrated that other amino acid transporters (particularly Slc38a4) may be more important for the trophic effects in humans than Slc38a5, which was essential in mice but not expressed in human islets. This highlights that the molecular mediators driving the liver–α-cell axis may be species dependent, at least when it comes to the factors linking the liver and the α-cells. A key question has been whether glucagon controls the metabolism of all amino acids or only a selected group, perhaps the glucagonotropic ones. In glucagonoma patients, plasma levels of all amino acids are low (often extremely low), and conversely plasma levels of amino acids are high in pancreatectomized subjects; in particular, plasma levels of arginine, alanine, and serine are consistently increased (14). Regarding secretion, although studied for years, it is not clear which amino acids stimulate secretion of glucagon, as differences between species, experimental conditions, and administered doses of amino acids vary widely in the published studies. In studies involving perfused mouse pancreata, glutamine did not stimulate secretion of glucagon, whereas amino acids like alanine and arginine did (15); other studies involving higher doses showed stimulation, so the differences may be dose related (16). It is well known that both alanine and arginine stimulate glucagon secretion in humans. Intraduodenal infusion of glutamine also resulted in increased glucagon secretion in humans (17), but whether this is a direct effect on the pancreatic α-cells or an indirect effect by, e.g., increased secretion of GIP or transamination to glucagonotropic amino acids is, to our knowledge, unknown.
α-Cells as Amino Acid Sensors and Glucagon as an Essential Hepatic Regulator of Amino Acid Catabolism
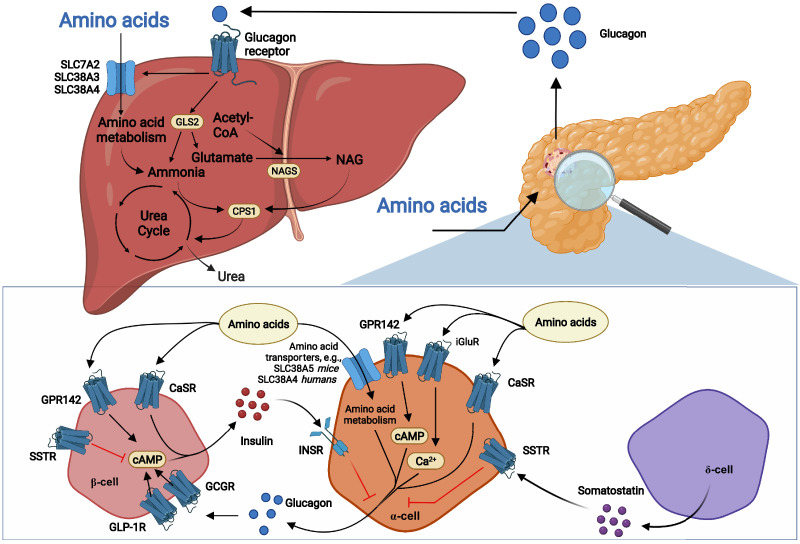
In perfused mouse pancreas, alanine, arginine, glycine, lysine, and proline stimulated glucagon secretion (15,18). The branch-chained amino acids (isoleucine, leucine, and valine) do not seem to acutely induce glucagon secretion (15). The mechanisms by which amino acids stimulate the α-cell are generally unknown (Fig. 2). Alanine increases the intracellular calcium concentration by unknown pathways (16), and arginine, a positively charged amino acid, may depolarize the α-cell upon uptake, as described for glucose-stimulated insulin secretion. Electrogenic amino acid transport (provided by some of the amino acid transporters expressed by the α-cell [11]) or metabolism of the amino acids in the α-cells may also depolarize the α-cell and activate pathways resulting in glucagon secretion. Additionally, α-cells express ionotropic glutamate receptors, which stimulate glucagon secretion upon glutamate activation (19). G protein–coupled receptors (GPRs), including the aromatic amino acid sensor GPR142 (20) and the l-amino acid–activated calcium-sensing receptor (CaSR), may also mediate amino acid-induced glucagon (and insulin) secretion.
Figure 2.
Amino acids stimulate glucagon secretion, and glucagon in turn stimulates hepatic amino acid uptake and metabolism, including ureagenesis. Circulating amino acids stimulate glucagon secretion from pancreatic α-cells by mechanisms that may involve stimulation of ionotropic glutamate receptors (iGluR) and G protein–coupled receptors (GPRs), including CaSR and GPR142. Increased expression of amino acid transporters such as SLC38A5 (in mice) and SLC38A4 (in humans) and subsequent amino acid metabolism may also be involved. Amino acids may also stimulate insulin secretion from β-cells through GPR142 and CaSR, and insulin may inhibit glucagon release via the insulin receptor (INSR) on α-cells. Paracrine somatostatin inhibits cAMP generation and inhibits secretion via activation of somatostatin receptor (SSTR). Glucagon activates both the GLP-1R and the GCGR on β-cells and stimulates insulin secretion. Once secreted to the peripheral circulation, glucagon binds the GCGR and stimulates hepatic amino acid uptake (through amino acid transporters such as SLC7A2, SLC38A3, and SLC38A4) and metabolism. Glucagon receptor signaling activates glutaminase, yielding glutamate, a NAGS substrate, whereby NAG levels increase, activating CPS-1 and ureagenesis. Glutaminase activity also yields ammonia, a CPS-1 substrate, and thus activates CPS-1. GCGR activity also increases the expression and activity of urea cycle enzymes.
After secretion, glucagon binds to its hepatic receptors and increases amino acid uptake through increased expression of both system A and N amino acid transporters (21), as illustrated by downregulation of amino acid transporters in livers of GCGR knockout (KO) mice (22) and in mice treated with a GCGR antibody (11). Of interest in the context of obesity-induced glucagon resistance, decreased expression of amino acid transporters was also observed in livers of subjects with obesity (23), in individuals with simple steatosis, and in patients with nonalcoholic steatohepatitis (NASH) (24). In addition, glucagon increases hepatic amino acid metabolism by increasing the capacity and rate of the urea cycle (25). The urea cycle takes place in periportal hepatocytes and serves to scavenge toxic ammonia from all sources, including amino acid catabolism, by forming urea, a nontoxic molecule that is excreted in the urine (the latter process may also be stimulated by glucagon). Glucagon increases the transcription of several enzymes required for ureagenesis (26), and conversely, certain urea cycle enzymes are downregulated in GCGR KO (22) and GCGR antibody-treated mice (11), in subjects with obesity (23), and in individuals with simple steatosis and NASH (24). CPS-1 is found among these downregulated urea cycle enzymes (11,23,24), and in line with this, glucagon administration increases hepatic CPS-1 expression (26). CPS-1 is the first enzyme in the urea cycle and is activated by N-acetylglutamate (NAG), which is produced by NAG synthase (NAGS), the transcription of which is decreased in GCGR KO mice (22). Glucagon stimulates ureagenesis within 20 min (22), possibly by increasing NAG levels via increased hepatic glutaminase (GLS2) activity (27). Increased GLS2 activity may induce NAG formation and ureagenesis by increasing the concentration of its products, glutamate and ammonia, which are substrates for NAGS and CPS-1, respectively. Further supporting glucagon-induced GLS2 activation, hepatic Gls2 expression was downregulated in GCGR KO mice (22) and in GCGR antibody-treated mice (12) as well as in individuals with simple steatosis and NASH (24).
The decreased Gls2 expression would be expected to result in increased plasma levels of glutamine, but this is not observed in patients with NASH. A possible explanation is the overexpression of Gls1 in NASH (28). In accordance with this, glutaminolytic activity was found to be increased in NASH (29) due to upregulation of Gls1 in fibrogenic stellate cells, suggesting that there is a switch from relatively low glutamine catabolism in the healthy liver to higher glutamine consumption in NAFLD/NASH (30).
Ureagenesis is a highly substrate-driven process, and the glucagon-stimulated uptake of amino acids probably plays an important role. Glucagon appears to have a predominant role in the (acute) control of circulating amino acids compared with that of insulin. This implies that a pull-and-push regulation by glucagon and insulin, as is known to exist for hepatic glucose production, may not be evident for amino acid metabolism, where glucagon may be the master regulator.
As a result of glucagon-stimulated hepatic amino acid uptake and metabolism (ureagenesis), plasma amino acid levels decrease under conditions of glucagon excess and increase during glucagon deficiency. Alanine, arginine, glycine, lysine, and proline seem to be particularly affected by glucagon signaling (31), which is also the case in individuals who are overweight or have obesity and are subjected to a glucagon infusion (32). These amino acids may thus be the main signaling links in the liver–α-cell axis, as these amino acids stimulate glucagon secretion and their metabolism is affected by GCGR signaling.
α-Cells are amino acid sensors and relay important amino acid signals to β-cells via proglucagon-derived peptides. This α-cell–to–β-cell communication is required for intact insulin responses to protein stimulation and for maintenance of euglycemia (33), and it becomes especially important in the context of mixed-meal ingestion, upon which both plasma glucose and amino acid levels rise, stimulating both insulin and glucagon secretion. Upon mixed-meal ingestion, amino acids stimulate secretion of glucagon as well as insulin. The cosecretion of insulin with glucagon prevents inappropriate increases in plasma glucose (33). At the same time, toxic hyperammonemia resulting from increased amino acid catabolism is prevented by the amino acid–induced glucagon secretion (postprandial hyperglucagonemia), which enhances ureagenesis. The combined islet response therefore acts to regulate postprandial glycemia as well as plasma amino acid levels.
It would thus make sense that glucose does not inhibit amino acid–stimulated glucagon secretion or glucagon-mediated ureagenesis, as observed after protein-rich meals. Thus, alanine-stimulated ureagenesis is maintained by glucagon despite high glucose levels (34), and in perfused mouse pancreas, basal glucagon secretion was strongly suppressed by 12 mmol/L compared with 3.5 mmol/L glucose, but arginine still stimulated glucagon secretion at 12 mmol/L despite the high glucose and the concomitant, maximally stimulated insulin secretion (35). In perifused islets, amino acids still stimulated glucagon secretion at 10 mmol/L glucose (18,33).
A Third Component of the Liver–α-Cell Axis: Lipids?
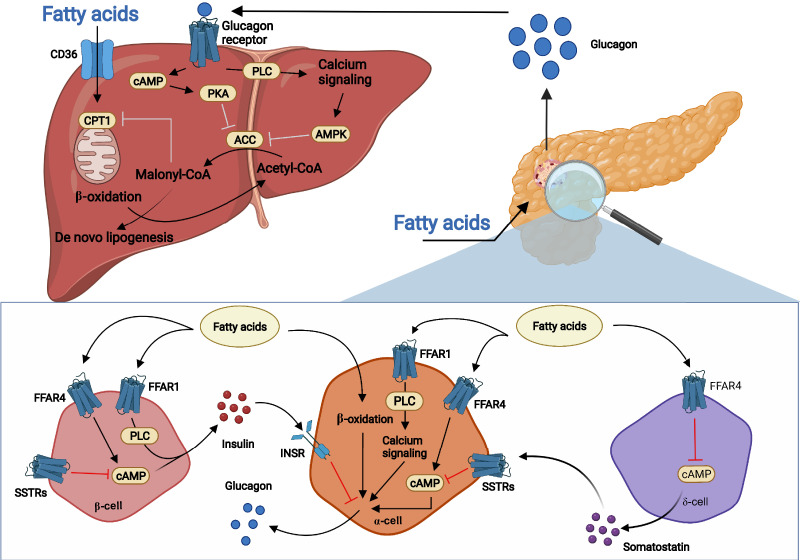
Glucagon stimulates β-oxidation of fatty acids (FAs) and inhibits triglyceride synthesis, reducing hepatic triglyceride content (1) (Fig. 3). The mechanism may involve protein kinase A and AMP-activated kinase–mediated inhibition of acetyl-CoA carboxylase, resulting in decreased malonyl-CoA levels and increased mitochondrial entry of FAs and β-oxidation. This may indirectly inhibit triglyceride synthesis by decreasing the availability of FAs for triglyceride synthesis and subsequent secretion (36). Altered transcription of genes related to lipid metabolism may also mediate glucagon-regulated lipid metabolism (1). These effects become evident in experiments involving impaired and increased glucagon action, which increases (37) and decreases (38) plasma and liver levels of cholesterol and triglycerides, respectively. GLP-1/GCGR coagonist treatment likewise decreases plasma triglyceride and hepatic fat levels more than GLP-1 analog (liraglutide) treatment alone (39). Besides the effects of glucagon on hepatic lipid metabolism, glucagon may also stimulate white adipose lipolysis. This has been clearly shown in rats; however, the literature regarding direct actions of glucagon on white adipose tissue in humans and mice is conflicting, with the majority of studies unable to show a lipolytic effect of glucagon (and mature human adipocytes do not seem to express a functional GCGR), and clear evidence that physiological concentrations of glucagon directly stimulate white adipose lipolysis in humans and mice has not been presented (40).
Figure 3.
Model for stimulation of glucagon secretion by FAs and effects of glucagon signaling on hepatic lipid metabolism. Circulating FAs, primarily LCFAs, may stimulate glucagon secretion from pancreatic α-cells through various known and unknown mechanisms. This may involve FFAR1 and FFAR4 with subsequent increase in intracellular Ca2+ and cAMP. Likewise, β-oxidation in α-cells may result in the release of glucagon. Fatty acids may also inhibit somatostatin release from δ-cells through decreased intracellular cAMP via FFAR4 activation. The reduced somatostatin release results in decreased activation of several somatostatin receptors (SSTRs) on α-cells, which results in less inhibition of cAMP generation. FAs can also stimulate insulin secretion from β-cells through FFAR1 and FFAR4, and insulin may inhibit glucagon release through the insulin receptor (INSR) on α-cells. Once secreted, glucagon binds the hepatic GCGRs, stimulates β-oxidation, and inhibits de novo lipogenesis. GCGR signaling inhibits acetyl-CoA carboxylase (ACC) via protein kinase A (PKA) and AMP-activated protein kinase (AMPK), resulting in decreased malonyl-CoA formation. As a result, mitochondrial entry of FAs and β-oxidation are increased, while the availability of FAs for triglyceride synthesis and subsequent export is reduced. PLC, phospholipase C.
While glucagon clearly influences lipid metabolism, the regulation of glucagon secretion by lipids is far from understood. The topic is complex because lipids and FAs are found in many different forms, which can have various effects. So far, studies have primarily focused on the effects of FAs and less on the effects of triglycerides and cholesterols.
Clinical studies in humans are conflicting about the stimulatory effect of FAs on circulating glucagon levels. No changes in glucagon levels were found following intravenous or oral administration of an intralipid emulsion in young males (41). On the other hand, 90-min intraduodenal infusions of primarily long-chain FAs (LCFAs) in men resulted in an increased glucagon response, although the increase was small compared with responses to protein infusion (42). Similarly, a 2-h intraduodenal fat emulsion infusion markedly increased plasma levels of glucagon in patients with type 2 diabetes (43). One study of healthy males showed that postprandial lipemia following a fat-enriched meal was associated with increased circulating glucagon regardless of FA levels (44). Other studies have shown that ingestion of medium-chain FAs and LCFAs acutely increased plasma levels of glucagon with no increase following ingestion of short-chain FAs (45).
More recent in vitro studies of isolated rodent and human islets showed increased glucagon secretion in response to short-term or long-term incubation with palmitate or oleate under both euglycemic (5.5 mmol/L) and hyperglycemic (15 mmol/L) conditions (1). The levels of LCFAs used for stimulation ranged from 20 μmol/L to 0.5 mmol/L, well within the physiological range of circulating FA levels.
Several G protein–coupled receptors with FA ligands have been identified, including free FA receptor 1 (FFAR1/GPR40) and FFAR4 (GPR120). FFAR1, which responds to medium and LCFAs, is expressed in pancreatic β-cells and may be involved in regulation of insulin secretion but is also expressed in α- and δ-cells (40). FFAR4 responds to LCFAs and is expressed in several tissues, including pancreatic islets. A recent study showed that the stimulatory effect of LCFAs on glucagon secretion was reduced in isolated islets from FFAR4 and FFAR1 KO mice, suggesting that both receptors contribute to FA-stimulated glucagon secretion. Overexpression of either FFAR4 or FFAR1 resulted in increased calcium mobilization in human islets following LCFA stimulation, suggesting calcium is required for palmitate-stimulated glucagon secretion (46).
FFAR1 signaling induced by medium and LCFAs is thought to involve the Gαq11 protein, which activates phospholipase C with subsequent diacylglycerol and inositol-trisphosphate formation. In β-cells, activation of FFAR1 leads to intracellular Ca2+ release from the endoplasmic reticulum and activation of voltage-dependent L-type Ca2+ channels, resulting in insulin secretion (47,48). For α-cells, LCFAs may also stimulate glucagon secretion by increasing intracellular Ca2+ concentration (49) and possibly through FFAR1 activation and phospholipase C signaling (50). A stimulated secretion of insulin and glucagon by activation of Gq signaling was recently shown in human islet cells (51).
The FAs may also be substrates for ATP generation, as etomoxir, inhibiting entry of LCFA-derived acetyl-CoA into the mitochondrial matrix and thereby β-oxidation, reduced palmitate-induced glucagon secretion, supporting that LCFAs may act via stimulation of receptor signaling as well as metabolism (52).
For FFAR4, the primary effect of LCFAs on glucagon secretion may be mediated via δ-cell signaling, as activation of FFAR4 with endogenous or synthetic FFAR4 agonists resulted in decreased somatostatin secretion in isolated mouse islets (53); this effect was lost in islets from both FFAR4 KO mice and δ-cell–specific FFAR4 KO mice.
A study by Croze et al. (53) showed that activation of FFAR4 signaling increased cAMP and calcium levels in both α- and β-cells in intact mouse islets in the presence of 5.5 mmol/L glucose, whereas an FFAR4 agonist reduced forskolin-induced cAMP levels and calcium levels in δ-cells in the presence of 16.8 mmol/L glucose, suggesting opposite effects of FFAR4 signaling in α- and β-cells versus δ-cells. FFAR4 activation in δ-cells will likely cause a suppression of cAMP generation and reduction of somatostatin release, thus alleviating the inhibitory effects of somatostatin on cAMP generation in α-cells.
Collectively, a potential model for the effects of LCFAs on glucagon secretion could include FFAR1 and FFAR4 signaling on α-cells by increasing levels of intracellular calcium, β-oxidation, and increased cAMP generation and indirectly via δ-cells by decreasing somatostatin release (Fig. 3).
The proposed model is largely based on in vitro experiments and is not directly translatable to in vivo settings; however, it points to a potential mechanism for LCFAs to increase glucagon secretion in health and disease. During fasting, circulating levels of FAs increase and may thus stimulate glucagon secretion to maintain fasting glucose levels. In metabolic disease, during which plasma FA levels are often increased, the increased flux of FAs could stimulate glucagon secretion, resulting in an inappropriate increase in hepatic glucose production. However, additional studies in humans are needed to further evaluate the role of LCFAs on glucagon release.
The Liver–α-Cell Axis in Disease
Ever since the first observations made with Roger Unger’s “Rabbit 30K” radioimmunoassay, increased plasma levels of glucagon (hyperglucagonemia) have been reported in individuals with obesity, type 2 diabetes, and different liver diseases (3). It is important to distinguish between fasting and postprandial (often mimicked by an oral glucose tolerance test [OGTT]) conditions. Postprandial hyperglucagonemia reported in the literature during OGTTs may not only reflect altered α-cell sensing (disruption of the liver–α-cell axis in glucose homeostasis) but also, perhaps equally importantly, cross-reactivity in the immunoassays with glucagon-like peptides secreted from the gut during an OGTT (i.e., glicentin and oxyntomodulin), whereas postprandial hyperglucagonemia after mixed meals may better reflect mainly pancreatic secretion of glucagon (54). GCGR antagonist studies in patients with type 2 diabetes consistently show an effect on fasting blood glucose, but postprandial hyperglycemia was not reduced with, e.g., the antagonist LY2409021 (55). This may have been due to unspecific effects of the antagonist on the incretin system (GIP and GLP-1). In monkeys, a monoclonal GCGR antibody reduced glucose excursions after OGTT (56), but it is possible that glucagon’s effect on glucose homeostasis is primarily exerted on fasting/baseline glycemia or, of course, in the case of insulin-driven hypoglycemia. In GCGR antagonist studies, impairments in lipid metabolism have also been found. These include increased low-density lipoprotein cholesterol and altered triglyceride metabolism. GCGR agonism appears to have opposite effects on the parameters reported above, with the exception of glucose (8).
As mentioned previously, hyperglucagonemia is characteristically observed in type 2 diabetes, but studies have suggested that hyperglucagonemia is found mainly in individuals who also have fatty liver disease, a condition occurring in up to 80% of patients with type 2 diabetes. Hyperglucagonemia was reported in patients with end-stage fatty liver disease (cirrhosis) in 1973 by Marco et al. (57), but it took almost 40 years before it was realized that this was likely due to the disruption of the liver–α-cell axis. Several studies have independently reported an association between liver disease and increased glucagon and amino acid levels. Importantly, even small increases in liver fat lead to elevations of both glucagon and amino acids (58). As discussed, not all amino acids are believed to be part of the liver–α-cell axis, or at least not all control α-cell function (15). We proposed a glucagon–alanine index (the product of fasting levels of glucagon and alanine in plasma) as a biomarker for the liver–α-cell axis (59), and this has been validated in several cohorts. An increase in the glucagon–alanine index associates with increases in liver transaminase levels (59), and this is also observed upon glucagon receptor antagonist treatment and in patients with NAFLD or NASH (22). Together, these findings support the glucagon–alanine index as a pathophysiological marker for disruption of the liver–α-cell axis. A simple procedure for evaluating glucagon resistance is, however, lacking. One group used a pancreatic clamp (somatostatin) to evaluate the acute effects of glucagon on glucose and amino acid levels (23). One challenge with such an approach is that the actions of endogenous glucagon are not evaluated. Another possibility is the development of a glucagon sensitivity index similar to the Matsuda index, as recently suggested by our group (S. Kjelden and N.J. Wewer Albrechtsen, unpublished observations).
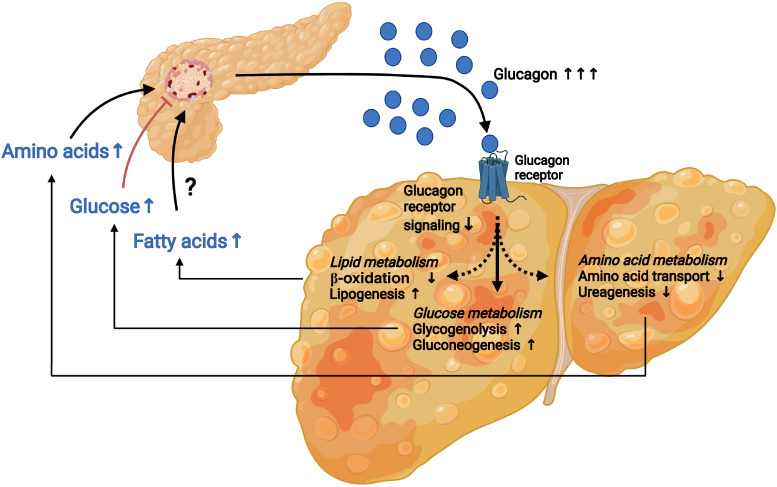
In mechanistic studies of how metabolic diseases affect the liver–α-cell axis, we and others have found that the glucagon-induced amino acid catabolism is impaired in isolated hepatocytes as well as in mice and patients with NAFLD compared with non-NAFLD conditions, whereas the effect on glucose production is not (23,60). The effect is probably due to impairment of amino acid transportation into the hepatocytes and metabolism of nitrogen atoms from the amino acids (ureagenesis) in the mitochondria (e.g., via reduced CPS-1 and ornithine aminotransferase capacity). Deficient transcriptional and nontranscriptional activity of glucagon signaling can be termed glucagon resistance (61). A key question is whether glucagon resistance develops as an epiphenomenon to insulin resistance or if these are separate pathways. In humans undergoing bariatric surgery, we found that markers of glucagon resistance coexisted with insulin resistance before surgery, but whereas insulin resistance disappeared after the operation, glucagon resistance did not in those with a remaining hepatic fibrosis score of one to two 12 months after surgery (although hepatic steatosis resolved) (62). Decades of research have shown increased glucagon levels in obesity, but very few human studies have stratified hyperglucagonemia based on NAFLD activity score (NAS) and fibrosis scores used for classifying NAFLD and alcoholic liver disease. The NAS includes markers of inflammation, but earlier studies mainly focused on hepatic steatosis, which may be inappropriate. An important confounder is obesity itself, and therefore studying individuals with obesity with and without NAFLD is essential to isolate impairments of the liver–α-cell axis in disease. Interestingly, based on the current studies, the liver–α-cell axis may be extremely sensitive to minor alterations in hepatic fat content, to other components in the NAS, and to fibrosis (62). What is surprising is the lack of further deterioration in markers of the liver–α-cell axis in individuals with increasing severity of NAFLD. This suggests that the liver–α-cell axis is a sensitive feedback system that is affected by minor changes in the liver (Fig. 4).
Figure 4.
The liver–α-cell axis in disease. Under conditions of type 2 diabetes and fatty liver disease, the liver–α-cell feedback loop is affected. Impaired hepatic glucagon signaling (glucagon resistance) decreases amino acid uptake and metabolism, resulting in hyperaminoacidemia and increased stimulation of glucagon secretion from pancreatic α-cells. Likewise, reduced glucagon signaling decreases hepatic β-oxidation, increases lipogenesis, and elevates circulating free FA concentrations, which may contribute to increased α-cell secretion of glucagon. Lastly, the hepatic glucose production is not affected by the reduced glucagon signaling. This results in increased hepatic glucose metabolism and hyperglycemia by the increased circulating levels of glucagon secreted from pancreatic α-cells in response to increased levels of amino acids and possibly FAs.
As for insulin resistance, in which insulin production is increased concomitantly with decreasing insulin sensitivity, whereby normal plasma glucose levels are maintained, the liver–α-cell axis may constitute a vital regulatory response to increasing glucagon resistance. Thus, decreased amino acid uptake and catabolism results in increased circulating amino acid levels, which in turn is balanced by increased α-cell secretion of glucagon. However, as a result of enhanced glucagon secretion, the hepatic glucose production is also increased. Biased GCGR signaling (such as tipping the balance from Gs to Gq downstream pathways [61]) in disease may be crucial for directing glucagon therapeutics toward improvements in amino acid and lipid metabolism without a diabetogenic effect.
From a pathophysiological and evolutionary perspective, it can be argued that such a feedback system should be rapidly adaptive in the context of fasting and postprandial conditions to ensure that toxic amounts of nitrogen do not accumulate, glucose levels are balanced, and lipids are catabolized as fuel or stored appropriately.
Prolonged fasting represents a special condition with hyperglucagonemia. An accelerated ureagenesis would not be expedient in this situation, but since the entire glucose production is maintained by gluconeogenesis, there is also an increasing need for removal of urea from amino acid–derived glucose production. The increased glucagon secretion in this situation may be supported by decreasing plasma glucose and insulin levels (which will immediately result in increased glucagon secretion) and perhaps increasing concentrations of free FAs, as discussed above.
Concluding Remarks
For a long time, glucagon has been considered an enigmatic hormone, and although it has been known to exist and to influence glucose metabolism for almost 100 years, there are still numerous unsolved puzzles regarding its physiological actions, particularly its role in diabetes development. The liver–α-cell axis offers an explanation for the effects and complications observed in GCGR agonist and antagonist studies and, importantly, probably explains the development of hyperglucagonemia in metabolic diseases such as diabetes, obesity, and NAFLD.
Article Information
Acknowledgments. The authors acknowledge their collaborators (in particular Professor Lise Lotte Gluud, University of Copenhagen, Professor Hendrik Vilstrup, University of Aarhus, and Katherina Maruszczak, Paracelsus Medical University) and the scientific community for their efforts in delineating the liver–α-cell axis.
Funding. Nicolai J. Wewer Albrechtsen is supported by the Danish Diabetes Association, Novo Nordisk Foundation Excellence Emerging Investigator Grant—Endocrinology and Metabolism (application no. NNF19OC0055001), European Foundation for the Study of Diabetes Future Leader Award (NNF21SA0072746), and Independent Research Fund Denmark, Sapere Aude (1052-00003B). The Novo Nordisk Foundation Center for Protein Research is supported financially by the Novo Nordisk Foundation (grant agreement NNF14CC0001). The salary of Michael M. Richter was partly covered by the Danish Diabetes Association via the grant mentioned above.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.M.R., K.D.G., and N.J.W.A. wrote the manuscript and generated the figures. E.E., F.K.K., M.P.S., J.J.H., M.W.-S., and S.A.S.K. revised the manuscript. All authors approved the final version of the manuscript.
Prior Presentation. Parts of this work were presented at the 82nd Scientific Sessions of the American Diabetes Association, New Orleans, LA, 3–7 June 2022.
Footnotes
References
- 1. Müller TD, Finan B, Clemmensen C, DiMarchi RD, Tschöp MH. The new biology and pharmacology of glucagon. Physiol Rev 2017;97:721–766 [DOI] [PubMed] [Google Scholar]
- 2. Holst JJ, Holland W, Gromada J, et al. Insulin and glucagon: partners for life. Endocrinology 2017;158:696–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wewer Albrechtsen NJ, Kuhre RE, Pedersen J, Knop FK, Holst JJ. The biology of glucagon and the consequences of hyperglucagonemia. Biomarkers Med 2016;10:1141–1151 [DOI] [PubMed] [Google Scholar]
- 4. Finan B, Capozzi ME, Campbell JE. Repositioning glucagon action in the physiology and pharmacology of diabetes. Diabetes 2020;69:532–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheen AJ, Paquot N, Lefèbvre PJ. Investigational glucagon receptor antagonists in phase I and II clinical trials for diabetes. Expert Opin Investig Drugs 2017;26:1373–1389 [DOI] [PubMed] [Google Scholar]
- 6. Almdal TP, Heindorff H, Bardram L, Vilstrup H. Increased amino acid clearance and urea synthesis in a patient with glucagonoma. Gut 1990;31:946–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Larger E, Wewer Albrechtsen NJ, Hansen LH, et al. Pancreatic α-cell hyperplasia and hyperglucagonemia due to a glucagon receptor splice mutation. Endocrinol Diabetes Metab Case Rep 2016;2016:16–0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bossart M, Wagner M, Elvert R, et al. Effects on weight loss and glycemic control with SAR441255, a potent unimolecular peptide GLP-1/GIP/GCG receptor triagonist. Cell Metab 2022;34:59–74.e10 [DOI] [PubMed] [Google Scholar]
- 9. Li W, Kirchner T, Ho G, et al. Amino acids are sensitive glucagon receptor-specific biomarkers for glucagon-like peptide-1 receptor/glucagon receptor dual agonists. Diabetes Obes Metab 2020;22:2437–2450 [DOI] [PubMed] [Google Scholar]
- 10. Solloway MJ, Madjidi A, Gu C, et al. Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of α-cell mass. Cell Rep 2015;12:495–510 [DOI] [PubMed] [Google Scholar]
- 11. Kim J, Okamoto H, Huang Z, et al. Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic α cell hyperplasia in mice. Cell Metab 2017;25:1348–1361.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dean ED, Li M, Prasad N, et al. Interrupted glucagon signaling reveals hepatic α cell axis and role for l-glutamine in α cell proliferation. Cell Metab 2017;25:1362–1373.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Longuet C, Robledo AM, Dean ED, et al. Liver-specific disruption of the murine glucagon receptor produces α-cell hyperplasia: evidence for a circulating α-cell growth factor. Diabetes 2013;62:1196–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boden G, Master RW, Rezvani I, Palmer JP, Lobe TE, Owen OE. Glucagon deficiency and hyperaminoacidemia after total pancreatectomy. J Clin Invest 1980;65:706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galsgaard KD, Jepsen SL, Kjeldsen SAS, Pedersen J, Wewer Albrechtsen NJ, Holst JJ. Alanine, arginine, cysteine, and proline, but not glutamine, are substrates for, and acute mediators of, the liver-α-cell axis in female mice. Am J Physiol Endocrinol Metab 2020;318:E920–E929 [DOI] [PubMed] [Google Scholar]
- 16. El K, Gray SM, Capozzi ME, et al. GIP mediates the incretin effect and glucose tolerance by dual actions on alpha cells and beta cells. Sci Adv 2021;7:eabf1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang J, Wu T, Greenfield JR, Samocha-Bonet D, Horowitz M, Rayner CK. Effects of intraduodenal glutamine on incretin hormone and insulin release, the glycemic response to an intraduodenal glucose infusion, and antropyloroduodenal motility in health and type 2 diabetes. Diabetes Care 2013;36:2262–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Capozzi ME, Svendsen B, Encisco SE, et al. β Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 2019;4:e126742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cabrera O, Jacques-Silva MC, Speier S, et al. Glutamate is a positive autocrine signal for glucagon release. Cell Metab 2008;7:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rudenko O, Shang J, Munk A, et al. The aromatic amino acid sensor GPR142 controls metabolism through balanced regulation of pancreatic and gut hormones. Mol Metab 2019;19:49–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Le Cam A, Freychet P. Glucagon stimulates the A system for neutral amino acid transport in isolated hepatocytes of adult rat. Biochem Biophys Res Commun 1976;72:893–901 [DOI] [PubMed] [Google Scholar]
- 22. Winther-Sørensen M, Galsgaard KD, Santos A, et al. Glucagon acutely regulates hepatic amino acid catabolism and the effect may be disturbed by steatosis. Mol Metab 2020;42:101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suppli MP, Bagger JI, Lund A, et al. Glucagon resistance at the level of amino acid turnover in obese subjects with hepatic steatosis. Diabetes 2020;69:1090–1099 [DOI] [PubMed] [Google Scholar]
- 24. Eriksen PL, Vilstrup H, Rigbolt K, et al. Non-alcoholic fatty liver disease alters expression of genes governing hepatic nitrogen conversion. Liver Int 2019;39:2094–2101 [DOI] [PubMed] [Google Scholar]
- 25. Petersen KF, Hansen BA, Vilstrup H. Time dependent stimulating effect of glucagon on the capacity of urea-N synthesis in rats. Horm Metab Res 1987;19:53–56 [DOI] [PubMed] [Google Scholar]
- 26. Snodgrass PJ, Lin RC, Müller WA, Aoki TT. Induction of urea cycle enzymes of rat liver by glucagon. J Biol Chem 1978;253:2748–2753 [PubMed] [Google Scholar]
- 27. Lacey JH, Bradford NM, Joseph SK, McGivan JD. Increased activity of phosphate-dependent glutaminase in liver mitochondria as a result of glucagon treatment of rats. Biochem J 1981;194:29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simon J, Nuñez-García M, Fernández-Tussy P, et al. Targeting hepatic glutaminase 1 ameliorates non-alcoholic steatohepatitis by restoring very-low-density lipoprotein triglyceride assembly. Cell Metab 2020;31:605–622.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Du K, Chitneni SK, Suzuki A, et al. Increased glutaminolysis marks active scarring in nonalcoholic steatohepatitis progression. Cell Mol Gastroenterol Hepatol 2020;10:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 2015;62:1148–1155 [DOI] [PubMed] [Google Scholar]
- 31. Boden G, Rezvani I, Owen OE. Effects of glucagon on plasma amino acids. J Clin Invest 1984;73:785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vega RB, Whytock KL, Gassenhuber J, et al. A metabolomic signature of glucagon action in healthy individuals with overweight/obesity. J Endocr Soc 2021;5:bvab118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Capozzi ME, Wait JB, Koech J, et al. Glucagon lowers glycemia when β-cells are active. JCI Insight 2019;5:e129954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hamberg O, Vilstrup H. Regulation of urea synthesis by glucose and glucagon in normal man. Clin Nutr (Edinburgh, Scotland) 1994;13:183–191 [DOI] [PubMed] [Google Scholar]
- 35. Svendsen B, Larsen O, Gabe MBN, et al. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep 2018;25:1127–1134.e2 [DOI] [PubMed] [Google Scholar]
- 36. Longuet C, Sinclair EM, Maida A, et al. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab 2008;8:359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guzman CB, Zhang XM, Liu R, et al. Treatment with LY2409021, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes Obes Metab 2017;19:1521–1528 [DOI] [PubMed] [Google Scholar]
- 38. Nason SR, Kim T, Antipenko JP, et al. Glucagon-receptor signaling reverses hepatic steatosis independent of leptin receptor expression. Endocrinology 2020;161:bqz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Day JW, Ottaway N, Patterson JT, et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol 2009;5:749–757 [DOI] [PubMed] [Google Scholar]
- 40. Galsgaard KD, Pedersen J, Knop FK, Holst JJ, Wewer Albrechtsen NJ. Glucagon receptor signaling and lipid metabolism. Front Physiol 2019;10:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lindgren O, Carr RD, Deacon CF, et al. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. J Clin Endocrinol Metab 2011;96:2519–2524 [DOI] [PubMed] [Google Scholar]
- 42. Ryan AT, Luscombe-Marsh ND, Saies AA, et al. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Am J Clin Nutr 2013;98:300–311 [DOI] [PubMed] [Google Scholar]
- 43. Xie C, Wang X, Jones KL, et al. Role of endogenous glucagon-like peptide-1 enhanced by vildagliptin in the glycaemic and energy expenditure responses to intraduodenal fat infusion in type 2 diabetes. Diabetes Obes Metab 2020;22:383–392 [DOI] [PubMed] [Google Scholar]
- 44. Niederwanger A, Ciardi C, Tatarczyk T, et al. Postprandial lipemia induces pancreatic α cell dysfunction characteristic of type 2 diabetes: studies in healthy subjects, mouse pancreatic islets, and cultured pancreatic α cells. Am J Clin Nutr 2014;100:1222–1231 [DOI] [PubMed] [Google Scholar]
- 45. Mandøe MJ, Hansen KB, Hartmann B, Rehfeld JF, Holst JJ, Hansen HS. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans. Am J Clin Nutr 2015;102:548–555 [DOI] [PubMed] [Google Scholar]
- 46. Suckow AT, Polidori D, Yan W, et al. Alteration of the glucagon axis in GPR120 (FFAR4) knockout mice: a role for GPR120 in glucagon secretion. J Biol Chem 2014;289:15751–15763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shapiro H, Shachar S, Sekler I, Hershfinkel M, Walker MD. Role of GPR40 in fatty acid action on the beta cell line INS-1E. Biochem Biophys Res Commun 2005;335:97–104 [DOI] [PubMed] [Google Scholar]
- 48. Ferdaoussi M, Bergeron V, Zarrouki B, et al. G protein-coupled receptor (GPR)40-dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia 2012;55:2682–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fujiwara K, Maekawa F, Dezaki K, Nakata M, Yashiro T, Yada T. Oleic acid glucose-independently stimulates glucagon secretion by increasing cytoplasmic Ca2+ via endoplasmic reticulum Ca2+ release and Ca2+ influx in the rat islet alpha-cells. Endocrinology 2007;148:2496–2504 [DOI] [PubMed] [Google Scholar]
- 50. Wang L, Zhao Y, Gui B, et al. Acute stimulation of glucagon secretion by linoleic acid results from GPR40 activation and [Ca2+]i increase in pancreatic islet alpha-cells. J Endocrinol 2011;210:173–179 [DOI] [PubMed] [Google Scholar]
- 51. Walker JT, Haliyur R, Nelson HA, et al. Integrated human pseudoislet system and microfluidic platform demonstrate differences in GPCR signaling in islet cells. JCI Insight 2020;5:e137017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Briant LJB, Dodd MS, Chibalina MV, et al. CPT1a-dependent long-chain fatty acid oxidation contributes to maintaining glucagon secretion from pancreatic islets. Cell Rep 2018;23:3300–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Croze ML, Flisher MF, Guillaume A, et al. Free fatty acid receptor 4 inhibitory signaling in delta cells regulates islet hormone secretion in mice. Mol Metab 2021;45:101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wewer Albrechtsen NJ, Kjeldsen SAS, Jensen NJ, et al. On measurements of glucagon secretion in healthy, obese, and Roux-en-Y gastric bypass operated individuals using sandwich ELISA. Scand J Clin Lab Invest 2022;82:75–83 [DOI] [PubMed] [Google Scholar]
- 55. Hædersdal S, Lund A, Maagensen H, et al. The glucagon receptor antagonist LY2409021 has no effect on postprandial glucose in type 2 diabetes. Eur J Endocrinol 2022;186:207–221 [DOI] [PubMed] [Google Scholar]
- 56. Yan H, Gu W, Yang J, et al. Fully human monoclonal antibodies antagonizing the glucagon receptor improve glucose homeostasis in mice and monkeys. J Pharmacol Exp Ther 2009;329:102–111 [DOI] [PubMed] [Google Scholar]
- 57. Marco J, Diego J, Villanueva ML, Diaz-Fierros M, Valverde I, Segovia JM. Elevated plasma glucagon levels in cirrhosis of the liver. N Engl J Med 1973;289:1107–1111 [DOI] [PubMed] [Google Scholar]
- 58. Gar C, Haschka SJ, Kern-Matschilles S, et al. The liver-alpha cell axis associates with liver fat and insulin resistance: a validation study in women with non-steatotic liver fat levels. Diabetologia 2021;64:512–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wewer Albrechtsen NJ, Færch K, Jensen TM, et al. Evidence of a liver-alpha cell axis in humans: hepatic insulin resistance attenuates relationship between fasting plasma glucagon and glucagonotropic amino acids. Diabetologia 2018;61:671–680 [DOI] [PubMed] [Google Scholar]
- 60. De Chiara F, Heebøll S, Marrone G, et al. Urea cycle dysregulation in non-alcoholic fatty liver disease. J Hepatol 2018;69:905–915 [DOI] [PubMed] [Google Scholar]
- 61. Wewer Albrechtsen NJ. The glucose-mobilizing effect of glucagon at fasting is mediated by cyclic AMP. Am J Physiol Endocrinol Metab 2021;321:E571–E574 [DOI] [PubMed] [Google Scholar]
- 62. Pedersen JS, Rygg MO, Kristiansen VB, et al. Nonalcoholic fatty liver disease impairs the liver-alpha cell axis independent of hepatic inflammation and fibrosis. Hepatol Commun 2020;4:1610–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]