Abstract
OBJECTIVE
To evaluate whether indexes of glycemic variability may overcome residual β-cell secretion estimates in the longitudinal evaluation of partial remission in a cohort of pediatric patients with new-onset type 1 diabetes.
RESEARCH DESIGN AND METHODS
Values of residual β-cell secretion estimates, clinical parameters (e.g., HbA1c or insulin daily dose), and continuous glucose monitoring (CGM) from 78 pediatric patients with new-onset type 1 diabetes were longitudinally collected during 1 year and cross-sectionally compared. Circadian patterns of CGM metrics were characterized and correlated to remission status using an adjusted mixed-effects model. Patients were clustered based on 46 CGM metrics and clinical parameters and compared using nonparametric ANOVA.
RESULTS
Study participants had a mean (± SD) age of 10.4 (± 3.6) years at diabetes onset, and 65% underwent partial remission at 3 months. β-Cell residual secretion estimates demonstrated weak-to-moderate correlations with clinical parameters and CGM metrics (r2 = 0.05–0.25; P < 0.05). However, CGM metrics strongly correlated with clinical parameters (r2 >0.52; P < 0.05) and were sufficient to distinguish remitters from nonremitters. Also, CGM metrics from remitters displayed specific early morning circadian patterns characterized by increased glycemic stability across days (within 63–140 mg/dL range) and decreased rate of grade II hypoglycemia (P < 0.0001) compared with nonremitters. Thorough CGM analysis allowed the identification of four novel glucotypes (P < 0.001) that segregate patients into subgroups and mirror the evolution of remission after diabetes onset.
CONCLUSIONS
In our pediatric cohort, combination of CGM metrics and clinical parameters unraveled key clinical milestones of glucose homeostasis and remission status during the first year of type 1 diabetes.
Introduction
A major focus in modern medicine is recognizing disease heterogeneity and identifying measurable parameters for individualized health outcomes. Type 1 diabetes generally is characterized by ill-defined progressive immune-mediated β-cell destruction (1).
After a diagnosis of type 1 diabetes and initiation of insulin therapy, the evolution of the metabolic status of patients is marked by a dichotomy in their propensity to enter or not enter partial remission (PR), resulting from the preservation of residual β-cell function. Although variable in intensity and duration, PR is characterized by low levels of glycemic fluctuations and daily insulin needs that eventually result in the demise of β-cells and a concomitant worsening of glycemic variability and glycated hemoglobin (HbA1c) levels (2). For these reasons, the currently accepted definition of PR is provided by the calculation of an insulin dose-adjusted HbA1c (IDAA1c) score (3). Early and accurate identification of patients who will experience a significant PR period is key in developing secondary type 1 diabetes prevention strategies.
Most phase 3 interventional trials aimed at preserving β-cell mass after the diagnosis of type 1 diabetes have failed to meet the study’s primary objectives, commonly defined as the persistence of C-peptide secretion above a certain threshold (e.g., peak C-peptide >200 pmol/L) (4). Recent data from type 1 diabetes prevention trials demonstrated that only specific subgroups of patients might respond to the defined interventions based on this primary objective (5). Globally, the mitigated response of patients with new-onset type 1 diabetes to a rather diverse portfolio of pharmacological protocols supports the heterogeneity of type 1 diabetes and the need for patient stratification (6). It also challenges whether peak C-peptide estimation is the best metric for clinically significant (i.e., positively witnessing glucose homeostasis) residual β-cell function.
In patients with symptomatic type 1 diabetes, the evaluation of insulin secretion through standard oral tolerance testing poorly represents glucose homeostasis since it does not integrate key aspects of insulin sensitivity and since glucose responsiveness of β-cells might only be observed in patients with high levels (i.e., >400 pmol/L) of peak C-peptide (7,8). Since C-peptide assays lack the power to discriminate residual β-cell mass from β-cell function (9), new tools inferred from routine clinical parameters are needed that may both reflect the presence and predict the evolution of significant residual β-cell function, which qualifies PR.
HbA1c variability is associated with a long-term risk of diabetes-related microvascular complications (10). With the generalized use of continuous glucose monitoring (CGM) systems, the intuitive clinical approach suggests that glucose variability parameters (also called CGM metrics) may strongly correlate with features of diabetes control related to β-cell function. This presumption is fueled by studies showing that CGM metrics (e.g., coefficient of variation [CV] and percentage of the time in hypoglycemia) refine the estimation of glucose control provided by HbA1c measurement and may help to better stratify existing phenotypes among patients with type 1 diabetes (11,12).
The objectives of our subsidiary analysis of the DIATAG (DIAbetes TAGging) study are to evaluate whether CGM metrics correlate with clinical parameters representing PR (e.g., HbA1c, total insulin daily dose [TDD], and IDAA1c) in pediatric patients with new-onset type 1 diabetes and how these CGM metrics may overcome residual β-cell secretion estimates from a longitudinal perspective, immediately after diagnosis. We thus also investigated how CGM metrics might help stratify patients according to the evolution of their level of glucose homeostasis during the first year of diabetes.
Research Design and Methods
Study Design and Participants
The DIATAG study was designed as a multicentric, prospective, and nonpharmacological trial to identify biomarkers of PR in children and adolescents with new-onset type 1 diabetes. The study protocol was approved by the principal ethical committee (Comité d’Ethique Hospitalo-Facultaire of Cliniques universitaires Saint-Luc [CUSL], 2018/04DEC/462) and local ethical committees of every participating institution. The parents and participants (>6 years old) gave their written informed consent prior to enrollment in the study. Patient enrollment is currently open. The trial is registered at ClinicalTrials.gov (NCT04007809). Patients eligible for the study were aged 6 months to 18 years and were diagnosed with type 1 diabetes within the last 21 days. Type 1 diabetes was diagnosed according to the International Society for Pediatric and Adolescent Diabetes guidelines (13), which included the presence of at least one positive serum anti-islet autoantibody (anti-GAD, anti-ZnT8 transporter, anti-insulin, or anti–insulinoma-associated antigen-2). Exclusion criteria are detailed elsewhere (NCT04007809).
Study Procedures
The baseline screening (i.e., blood draws and urine) was performed after an overnight fast between 5 and 21 days after diagnosis (Δ) to allow metabolic stabilization. After the initial hospitalization, the outpatient clinical follow-up in diabetes care centers was organized throughout routine visits at Δ+3, Δ+6, Δ+9, and Δ+12 months, during which an array of data was collected (i.e., raw CGM, demographic and clinical parameters [i.e., TDD, HbA1c, and IDAA1c], and insulin administration regimen [i.e., pump or multiple daily injections (MDI)]). All patients >4 years old were recommended to wear CGM devices (FreeStyle Libre, Abbott Laboratories; DexCom, DexCom, Inc.; and Enlite, Medtronic MiniMed). Data from the medical records of participants were gathered and registered inside the Research Electronic Data Capture (REDCap) system (14) provided by Vanderbilt University (Nashville, TN) and hosted at CUSL.
Glucagon Stimulation and β-Cell Function Tests
A subset of participants (i.e., patients who completed the full study protocol) underwent a glucagon stimulation test (GST) at Δ+3 months and Δ+12 months to evaluate the insulin secretion capacities of β-cells, as described elsewhere (15). In brief, after an overnight fast, patients were tested for capillary blood glucose. If pretest capillary glucose was between 70 and 250 mg/dL, 1 mg of glucagon (Glucagen; Novo Nordisk) was injected intravenously. C-peptide and plasma glucose were measured at 1 min preinjection and 2, 4, and 6 min postinjection. The C-peptide level was measured at the central laboratory of CUSL (Brussels, Belgium) using a two-site chemiluminescence immunoassay (LIAISON XL; DiaSorin, Antony, France).
The response to GST was evaluated by calculating the area under the curve over a 6-min interval as previously described (15), corresponding to stimulated C-peptide (CPEPSTIM) in this article. Peak C-peptide was determined as the maximal value of the latter during the GST test. Estimated C-peptide (CPEPEST) was calculated as described elsewhere (16) using values of basal C-peptide (CPEPBASAL; i.e., fasting) and plasma glucose obtained before stimulation.
Remission Status
Remission status was determined at each visit using the IDAA1c score as follows: HbA1c (%) + (4 × insulin dose [U/kg body weight/24 h]), with a score <9 defining the remission status (3). TDD was either reported by patients (i.e., MDI users) or calculated using the software for pump users.
Analysis of CGM Data
CGM data were extracted at each outpatient clinical visit from a 30-day interval before measurement of HbA1c (reference range 4–6% [20–42 mmol/mol]) (Tosoh G8; Tosoh, Tokyo, Japan). All CGM data were analyzed using R (R Core Team; R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, 2021; https://www.R-project.org/). Raw glycemic data were preprocessed using the R statistical package cgmanalysis (17). Data quality check (i.e., the exclusion of data sets if the time elapsed between CGM values was >45 min) and the calculation of a panel of 46 CGM metrics (Supplementary Table 1) were performed using the Iglu package that provides functions for outputting relevant metrics for data collected from CGM (18,19). These were further classified into eight clusters (i.e., global control measures, hypoglycemia, time in range, hyperglycemia, within-day and between-day and total glucose variability, and combination scores). These were further classified into eight clusters (i.e., global control measures, hypoglycemia, time in range, hyperglycemia, glucose global, within- and between-day variability, and combination scores). Hypoglycemia and hyperglycemia were classified in grade 1 (i.e., 54–70 mg/dL [GIH54–70], 180–250 mg/dL) or grade 2 (i.e., <54 mg/dL [GIIH<54], >250 mg/dL) according to the recommendations of the International Consensus on Use of Continuous Glucose Monitoring (20). The distribution of indices across global and within- and between-day variability was performed according to Rodbard (21).
Statistical Analysis
All statistical analyses were performed in R (R Core Team). The statistical significance level used for all analyses was 0.05. When appropriate and unless specified otherwise, data were log-transformed using y = loge(value + 0.0001) if 0 values were included. Demographic and clinical data are reported as the mean ± SD for continuous variables and as numbers and proportions for categorical variables. Comparisons between groups were performed using Student t test, χ2 test, and linear regression or their nonparametric equivalent (Mann-Whitney U test, Fisher exact test, and Kruskal-Wallis test, respectively) as appropriate. Multivariate regression was used to assess the impact of cofactors on peak C-peptide secretion. Cross-sectional comparisons between secretion, CGM, and clinical parameters were performed using linear mixed models with R packages lmer (22) and lmerTest (23) to take into account multiple measurements from the same patient. Models included the methods as fixed effects and patient identity as a random intercept.
Four-week CGM data were analyzed either on a daily or hourly basis. Data densities were inspected in both data sets (i.e., daily or circadian analyses). Differences in circadian patterns of CGM metrics were investigated between remitters and nonremitters using a linear mixed model (22,23) that includes remission status (remitter/nonremitter) as a fixed effect and patient identity as a random intercept. Residuals were inspected for normality on Q-Q plots in which the distribution of the residuals’ quantiles is compared with its theoretical normal one. This model was used to generate plots representing the amplitude of differences between both groups expressed in percentage of variation compared with the remitter group. P values were adjusted for multiple testing with the Benjamini and Hochberg false discovery rate procedure (24).
Principal component analysis was conducted in R based on CGM data across all outpatient clinical visits (n = 172). Prior to principal component analysis, glucose metrics were standardized and imputed by a regularized expectation–minimization principal component analysis algorithm with the missMDA package (25). Next, unsupervised hierarchical clustering was performed with the stats package (R) on standardized CGM data along the patients based on the Euclidean distance (i.e., CGM metrics). The number of clusters was determined based on a scree plot of the dendrogram height of the hierarchical clustering. As appropriate, comparisons between the groups were assessed by linear regression or its nonparametric equivalent (Kruskal-Wallis test).
Results
Study Participant Characteristics
The present subsidiary investigation of the DIATAG consortium study included 207 visits and 80 GSTs from 78 patients. All data were longitudinally collected during the first year after type 1 diabetes onset. Patients were mostly males (52%) and had a mean ± SD age of 10.4 ± 3.6 years at type 1 diabetes onset. The great majority of the cohort was under MDI (83.5%), and the incidence of PR (i.e., IDAA1c <9) at Δ+3 months was 65%. The baseline characteristics of the cohort are provided in Table 1. CPEPBASAL was measured for 78 patients at Δ+3 and Δ+12 months (n = 119), from which C-peptide was detectable (i.e., >0.01 pmol/mL) in all samples at Δ+3 months and all but two samples at Δ+12 months. On average, 1 GST was analyzed per patient (0 GST in 27 patients, 1 GST in 22 patients, and 2 GST in 29 patients). None of the patients with undetectable fasting C-peptide had detectable C-peptide levels after stimulation.
Table 1.
Characteristics of participants included in analysis
| Characteristic | Global (N = 78) | Remitters (N = 51) | Nonremitters (N = 27) | P value* |
|---|---|---|---|---|
| Distribution | ||||
| Age, years | 10.4 ± 3.6 | 10.9 ± 3.4 | 9.3 ± 4 | 0.09† |
| Sex, male, n (%) | 41 (52) | 30 (59) | 10 (37) | 0.1‡ |
| Pubertal, n (%) | 40 (51) | 29 (57) | 11 (40.7) | 0.2‡ |
| BMI (z score) | −0.7 ± 1.5 | −0.6 ± 1.5 | −1 ± 1.3 | 0.3† |
| Baseline diabetes characteristics | ||||
| HbA1c, % (mmol/mol) | 12.3 (111) ± 2.1 (23) | 12.1 (109) ± 2.2 (24) | 12.8 (116) ± 1.8 (19) | 0.13† |
| Presence of ketoacidosis, n (%) | 26 (33) | 13 (26) | 13 (48) | 0.04‡ |
| Glycemia (mg/dL) | 475 ± 190 | 462 ± 197 | 508 ± 193 | 0.3† |
| Weight loss (%) | 9.9 ± 15.47 | 6.9 ± 17.8 | 15.7 ± 6.8 | 0.003† |
| Insulin administration | ||||
| MDI, n (%) | 66 (83.5) | 45 (88) | 20 (77) | 0.3‡ |
| Insulin pump, n (%) | 9 (11.4) | 4 (8) | 5 (19) | 0.3‡ |
| Unknown, n (%) | 3 (5) | 2 (4) | 1 (4) | NA |
| Glycemic control£ (n = 78) | ||||
| HbA1c, % (mmol/mol) | 6.2 (44) ± 0.7 (8) | 6 (42) ± 0.5 (6) | 6.7 (50) ± 0.8 (8) | 2.5e-20† |
| Insulin doses (IU/kg/day) | 0.6 ± 0.3 | 0.5 ± 0.2 | 0.8 ± 0.3 | 6.1e-20† |
| IDAA1c | 8.5 ± 1.3 | 7.8 ± 0.8 | 9.9 ± 1.1 | 3.4e-38† |
| Fasting and stimulated C-peptide£ | ||||
| CPEPBASAL (pmol/mL) (n = 73) | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.2 ± 0.2 | 0.01¶ |
| Peak stimulated (pmol/mL) (n = 52) | 0.7 ± 0.4 | 0.6 ± 0.4 | 0.4 ± 0.3 | 0.01¶ |
| CPEPSTIM (pmol/mL/min) (n = 52) | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.4 ± 0.3 | 0.02¶ |
| CPEPEST (pmol/mL)§ (n = 73) | 0.6 ± 0.3 | 0.6 ± 0.2 | 0.4 ± 0.2 | 2.6e-06† |
Data are means ± SD unless otherwise indicated. Percentages may not total to 100 because of rounding.
NA, not applicable.
P value calculated between remitters and nonremitters. Results were considered significant when P < 0.05.
Student t test.
χ2.
Parameters evaluated at 3 months after diagnosis.
Wilcoxon test.
Calculated as described by Wentworth et al. (16).
Residual C-Peptide Secretion Estimates Strongly Correlate With Each Other but Only Weakly With Clinical Parameters
In our DIATAG cohort, we wanted to determine whether C-peptide measured on a single fasted blood test (i.e., CPEPBASAL or model-based stimulated C-peptide [CPEPEST]) was as efficient as the globally advocated gold-standard CPEPSTIM for evaluating residual endogenous secretion in children during the first year of type 1 diabetes. The influences of various cofactors on C-peptide measures were investigated using multivariate regression and revealed that peak C-peptide depended on sex and age (P < 0.05) but not on basal glycemia, BMI, or time of measurement (P > 0.05). However, in our DIATAG study, we decided not to adjust secretion data for sex and age for the following reasons: first, C-peptide versus sex and age correlations may be influenced by the disease pathogenesis itself more than by the specifically chosen parameters (26), and second, the inclusion of sex and age parameters in a recent model was shown to worsen its power to predict stimulated C-peptide (16).
All three methods (CPEPBASAL, CPEPEST, and CPEPSTIM) were very strongly correlated with each other (i.e., R > 0.84; P < 0.001), underlining the concordance of stimulated versus fasted C-peptide measures. CPEPEST was available for most patients, so we used the latter as our standard measure of β-cell secretion for cross-sectional analysis of the whole study cohort.
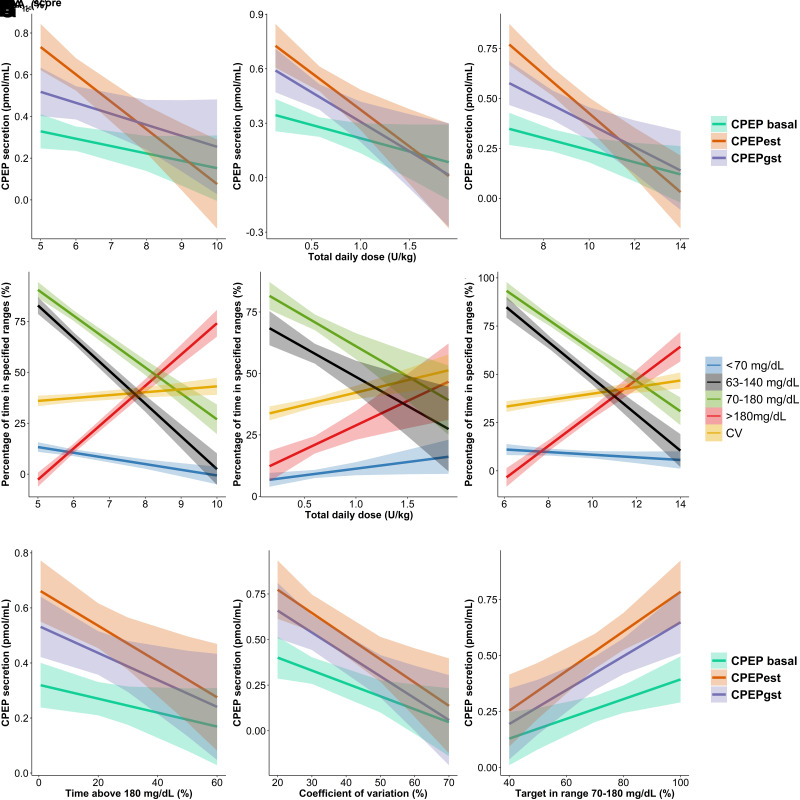
We next investigated whether these residual β-cell secretion estimates correlated with clinical parameters (i.e., HbA1c, TDD, and IDAA1c) and found only weak-to-moderate correlations between the latter (r2 between 0.05 and 0.25) (Fig. 1A–C). Notably, only CPEPEST correlated with all clinical parameters while exhibiting the strongest correlation with IDAA1c (r2 = 0.25). These results did not differ when considering the remission status (data not shown).
Figure 1.
Relations among β-cell residual secretion, routine clinical parameters of glycemic control, and CGM metrics during the first year of type 1 diabetes. Residual β-cell secretion was evaluated at 3 and 12 months after diagnosis. Routine clinical parameters and CGM metrics were obtained at 3, 6, 9, and 12 months after diagnosis. Correlation analyses were performed on all data. A–C and G–I represent linear regression with 95% CI bands (shaded zone) between endogenous residual insulin secretion (i.e., CPEPEST, CPEPBASAL, and CPEPSTIM) and HbA1c (A), daily insulin dose (B), IDAA1c score (C), time >180 mg/dL (G), CV (H), and time between 70 and 180 mg/dL (I). D–F represent linear regression with 95% CI bands (shaded zone) between CGM metrics (i.e., glycemia <70 mg/dL, between 63 and 140 mg/dL, between 70 and 180 mg/dL, and >180 mg/dL and CV) and HbA1c (D), insulin daily dose (E), and IDAA1c score (F). Regression coefficients (r2) are shown according to the secretion method (A–C, G–I) and CGM metrics (F–H). The level of significance of the correlations is represented after the r2 value as follows: *P < 0.05, **P < 0.01, ***P < 0.001. Significant regression coefficients are indicated in boldface.
CGM-Derived Metrics Strongly Correlate With Clinical Parameters but Only Weakly With Endogenous Insulin Secretion
Our next approach was to evaluate whether the time spent within different glycemic ranges and the CV might better reflect clinical parameters than β-cell secretion estimates (Fig. 1D–F). We analyzed 500,000 interstitial glucose values corresponding to a mean of 3,450 measures/patient. The percentage of time spent between 70 and 180 mg/dL (time in range [TIR70–180]), between 63 and 140 mg/dL (time in target [TIT63–140]), and >180 mg/dL (time above range [TAR>180]) demonstrated the strongest correlations with HbA1c levels (r2 = 0.52, r2 = 0.6, and r2 = 0.67, respectively; P < 0.0001) and IDAA1c (r2 = 0.53, r2 = 0.53, and r2 = 0.54, respectively; P < 0.0001) while showing the weakest correlations with TDD (r2 = 0.16, r2 = 0.1, and r2 = 0.09, respectively; P < 0.0001). In contrast, CV and time spent <70 mg/dL (time below range [TBR<70]) showed a weaker or no correlation with clinical parameters (Fig. 1D–F).
Finally, we investigated how the CGM metrics reflected residual β-cell secretion estimates. As shown in Fig. 1G–I, TAR>180 and TIR70–180 demonstrated the highest correlations with CPEPEST (r2 = 0.13 and r2 = 0.22, respectively; P < 0.01), while CV was equivalent in its correlations with CPEPEST and CPEPSTIM (r2 = 0.17; P < 0.01). Interestingly, TBR<70 did not correlate with β-cell residual secretion estimates (P = 0.77) while nearly reaching significance for GIIH<54 (P = 0.06, data not shown). Altogether, these data suggest that residual β-cell secretion only moderately reflects glucose homeostasis levels when evaluated using either clinical parameters (HbA1c and IDAA1c) or CGM metrics, especially hypoglycemia.
CGM Variables Are Robust and Sufficient Parameters to Distinguish Remitters From Nonremitters
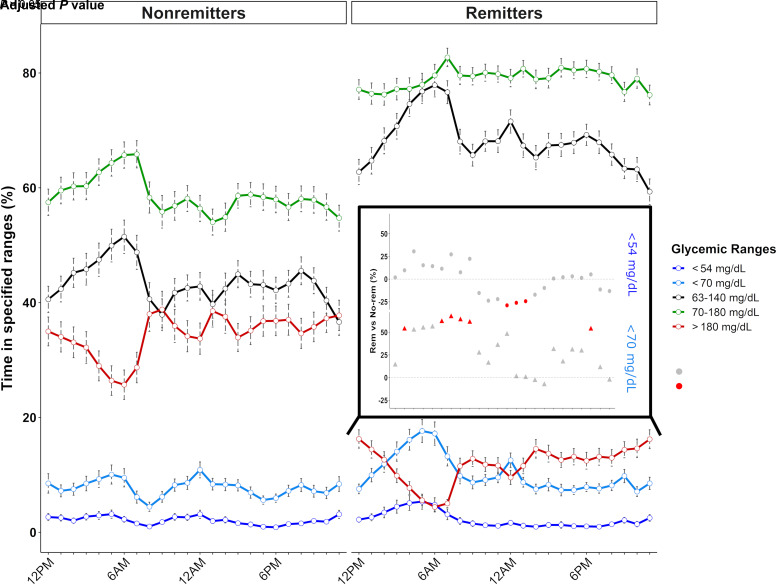
CGM metrics showed strong correlations with clinical parameters and allowed deeper characterization of glucose homeostasis (i.e., hypoglycemia episodes and glucose variability). We investigated whether a comprehensive analysis of CGM values might distinguish remitters from nonremitters. CGM metrics were analyzed on an hourly basis and compared with the patient remission status as a preanalytic evaluation of CGM demonstrated that specific periods of the day exhibited amplified differences between both groups (Fig. 2).
Figure 2.
Daily patterns of time spent in defined glycemic ranges during the first year of type 1 diabetes regarding the remission status. Routine clinical parameters and CGM metrics were obtained at 3, 6, 9, and 12 months after diagnosis. Lines represent the mean percentage of time spent <54 mg/dL (dark blue), <70 mg/dL (light blue), between 63 and 140 mg/dL (black), between 70 and 180 mg/dL (green), and >180 mg/dL (red). Error bars represent the SEs. The inset panel represents the daily variation in the amplitude of differences for values <70 mg/dL (triangles) and <54 mg/dL (circles) between remission groups based on a linear mixed model (i.e., the percentage >0% [dashed black line] defining higher values in remitters, percentage <0% defining lower values in remitters). The significance level of the differences is represented by the color of the points as follows: red dots correspond to P < 0.05, and gray dots represent P > 0.5. No-rem, nonremitters; Rem, remitters.
We first analyzed the time spent within different glycemic ranges, including hypoglycemia (i.e., TBR<70 and GIIH<54), time in range (i.e., TIT63–140 and TIR70–180), and hyperglycemia (TAR>180). As expected, remitters spent more time in time in range (TIR70–180 [34%] and TIT63–140 [57%]) and less time in hyperglycemia (TAR>180 [−61%]) during the whole day (Supplementary Table 2). Moreover, while differences between both remission groups regarding time in range (TIR70–180) were the highest during the day, differences in TIT63–140 and TAR>180 peaked in the early morning (i.e., 4–7 a.m.). During this specific period, we observed in remitters a peak in low-to-normal glucose values (TBR<70 and TIT63–140) but not in frank hypoglycemia (GIIH<54), suggesting that most TBR<70 episodes were in the 63–70 mg/dL range (Fig. 2). The score assessing the risk of hypo- and hyperglycemia (average daily risk range score) remained low and stable over the 24 h in remitters while peaking during the day in nonremitters (Supplementary Fig. 1A).
Corroborating the early-morning glycemic pattern mentioned above, remitters experienced decreased total, between-day (interquartile range), and within-day glycemic variability throughout the whole day [i.e., CV <36% at all time points (27)], with differences between both groups reaching −22% and −49% for total and between-day variability, respectively, at 6 a.m. (Supplementary Fig. 1B and C).
Deep Characterization of CGM Metrics Defines Different Remission Clusters
To investigate the evolution of glucotypes during the first year of type 1 diabetes, we performed a principal component analysis based on a panel of 46 daily CGM metrics (Supplementary Table 1). The horizontal axis (PC1) was principally driven by hyperglycemia, time in range, within-day variability, and global diabetes control indices, and the vertical axis (PC2) was driven by hypoglycemia and total variability indices (e.g., CV) (Supplementary Fig. 2A and B). While PC1 segregated the HbA1c and allowed the distinction between extreme values of IDAA1c (<7.5 and >10) (Supplementary Fig. 2C and D), there remained an overlap between both remission groups for intermediate values (Supplementary Fig. 2D).
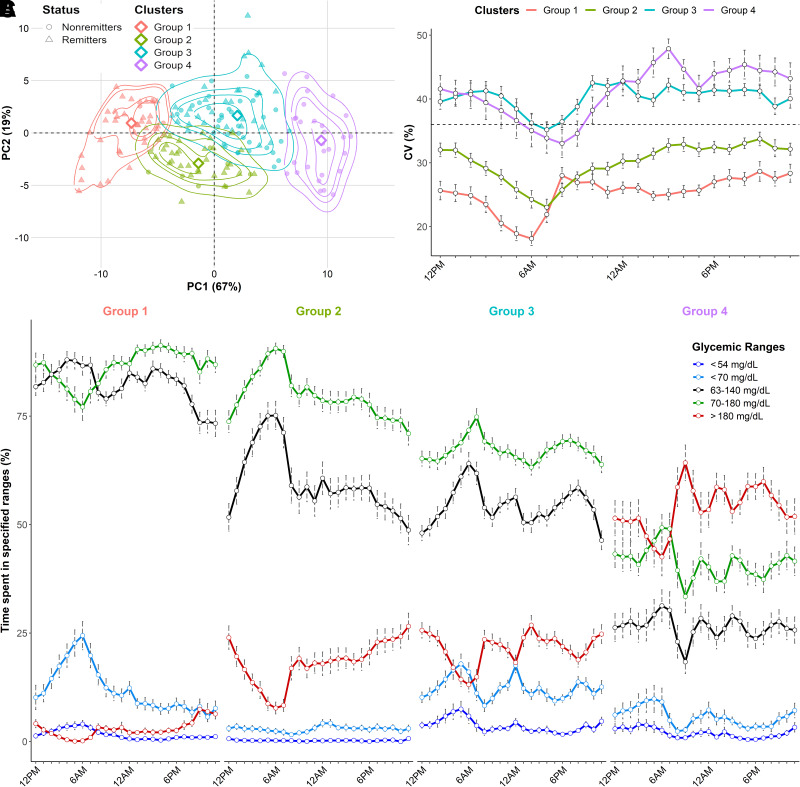
To better understand the overlap between remission groups and identify subgroups of patients with similar glucose profiles, we performed unsupervised hierarchical clustering on CGM metrics and clinical parameters (Supplementary Fig. 3). We identified four clusters of patients who were distinctly segregated across the principal component analysis (Fig. 3A) and showed distinctive glycemic patterns (Fig. 3B and C). Groups significantly differed from each other for all clinical parameters and CGM metrics (P < 0.05) (Supplementary Table 3). Metrics of time in range (TIR70–180 and TIT63–140) were the highest in group 1 but were progressively dissociated (i.e., decrease in TIT63–140) during the daytime in group 2 and the whole day in group 3; a net drop in these values occurred in group 4. Episodes of hyperglycemia (TAR>180 and hyperglycemia >250 mg/dL) first appeared during the day in group 2, extended to nighttime in group 3, and peaked across the entire day in group 4 (Fig. 3C). Regarding hypoglycemia, we found that the mean incidence of TBR<70 was equivalently high in groups 1 and 3 (Supplementary Table 3). However, as shown in remitters, TBR<70 specifically increased in the early morning in group 1 while remaining stable during the whole day in group 3 with a concomitant increase of GIIH<54.
Figure 3.
Illustration and characterization of glycemic clusters identified by unsupervised hierarchical clustering on CGM metrics and clinical data during the first year of diabetes. Routine clinical parameters and CGM metrics were obtained at 3, 6, 9, and 12 months after diagnosis. A: Repartition of the clustering groups across the principal component analysis data. The empirical distributions of the patients across each group are represented by isoprobability contours of kernel densities at 25th, 50th, 75th, and 95th percentiles. The medoid of each group is represented by a diamond. B: Circadian evolution of CV (%) according to the clustering groups. The dashed line represents the threshold of 36%. C: Daily patterns of time spent in defined glycemic ranges <54 mg/dL (dark blue), <70 mg/dL (light blue), between 63 and 140 mg/dL (black), between 70 and 180 mg/dL (green), and >180 mg/dL (red). Error bars represent the SEs. Gray horizontal bars represent nighttime, and orange horizontal bars represent daytime.
Completing these observations, glycemic variability (CV and interquartile range) was the lowest in group 1, increased in group 2, and peaked in group 3 during nighttime and in group 4 during daytime. Notably, CV remained below the threshold of 36% in the first two groups (Fig. 3B and Supplementary Table 3).
Conclusions
Current methods for screening residual C-peptide secretion are rather invasive and poorly performed in reflecting the evolution of β-cell function and routine glucose parameters (28–31). A comprehensive composite evaluation of clinical parameters (i.e., TDD, HbA1c, and IDAA1c) and CGM data are required to decipher glucose homeostasis evolution during the first year after type 1 diabetes onset and provide clues to achieve outcome-focused patient stratification (11).
Using cross-sectional measures of residual β-cell secretion, clinical parameters of glucose control, and 4-week CGM, our study showed that clinical parameters (including IDAA1c) showed a strong correlation with various CGM metrics and yet a moderate correlation with β-cell secretion estimates. Using CGM data, we identified specific circadian patterns among remission groups for most CGM metrics (including TIT63–140, TBR<70, and between-day glucose variability), which peaked in their discriminative features in the early morning period. Finally, integrating CGM metrics and clinical parameters, we identified four clinically meaningful clusters that exhibit specific glucotypes and reflect the progressive loss of glucose homeostasis during the first year after type 1 diabetes onset.
There is controversy in using C-peptide as a forefront marker of residual β-cell function, as it fails to correlate with individual patient phenotypes (29,30). Accordingly, secretion estimates yielded a maximal correlation coefficient of 0.5 with routine parameters of glycemic control (i.e., HbA1c, TDD, and IDAA1c), concurring with results from previous studies (28–31). Our results support the study of Buckingham et al. (29), who demonstrated that most patients with new-onset type 1 diabetes and IDAA1c ≥9 have detectable C-peptide secretion (>0.2 pmol/mL) in ranges not strictly parallel to HbA1c levels. These observations illustrate the “C-peptide secretion versus glucose homeostasis” discrepancy that can be attributed, at least partially, to individual insulin sensitivity and β-cell glucose responsiveness during the first postdiagnosis year (7–9,28,32).
When analyzing glycemic values, we hypothesized that CGM metrics (TIR70-180 and TAR>180) represent glucose homeostasis better than β-cell secretion testing (29,31). Using a proof-of-concept method, we found that routinely assessed CGM metrics allowed for a distinction between remitters and nonremitters. Daily glucose profile analysis identified the early morning as the most sensitive time to evaluate this distinction (Fig. 2 and Supplementary Fig. 1). Indeed, several metrics (e.g., TBR<70, TIT63–140, and interquartile range) were the most powerful for patient stratification, highlighting that a special focus on the early morning period might provide the highest yields in the search for analytic tools to segregate remitters from nonremitters.
Our analysis also fueled the characterization of hypoglycemia in remitters and the importance of the severity of hypoglycemic episodes as indirect markers of residual β-cell secretion. We demonstrated for the first time the predominance of early morning low-grade hypoglycemia in remitters compared with nonremitters (Fig. 2), suggesting that TBR<70 in the 63–70 mg/dL range might be a clinically significant meaningful marker of β-cell function. This was partially suggested by previous studies showing frequent TBR<70 in secretors (29), minimal increase in TBR<70 in remitters (33), and a high proportion (>50%) of TBR<70 in the 65–70 mg/dL range during PR (33). However, we found for the first time that high-grade hypoglycemia (GIIH<54) tended to negatively correlate with β-cell residual secretion, confirming that increased dependence on exogenous insulin fosters the occurrence of severe hypoglycemia in patients with new-onset type 1 diabetes.
To better understand the heterogeneity of type 1 diabetes evolution the first year after onset, we identified four clusters of glucotypes supporting the clinical impression that the emergence of glycemic dysregulations follows a continuum, first appearing during the daytime before progressing to the nighttime with a concomitant increase in glycemic variability, hyperglycemia, and subsequent GIIH<54 (Fig. 3). Additionally, our data also show that patients with new-onset type 1 diabetes were distributed across all groups from Δ+3 months (i.e., 38.2% in group 1, 25.5% in group 2, 29.1% in group 3, and 7% of patients in group 4), highlighting that levels of glycemic dysregulation might be heterogeneous from the first months after diabetes onset. Altogether, these results provide new insights into understanding the patchiness of type 1 diabetes phenotypes (12) and challenge considerations of PR as a dichotomic event. In that regard, CGM metrics provide additional information to segregate patients, especially with intermediate IDAA1c values.
Our subsidiary analysis of the DIATAG cohort demonstrates several strengths. This is the first pediatric multicentric cross-sectional study that integrates CGM, clinical parameters, and residual β-cell secretion data to uncover the characteristics of PR and identify new glucotypes during the first year of type 1 diabetes. Furthermore, cohort characteristics (e.g., the ratio of MDI regimen and the same level of care) and user-friendly standardized methods to analyze CGM data support our classification’s external validity and translational in clinical routine (18).
Our study was limited by cross-sectional analysis of all three parameters (i.e., clinic, secretion, and CGM) that were only available for a subset of patients, as the study trial is still open. Nonetheless, the impact of these biases was limited. On the one hand, CPEPEST allowed reliable secretion measurement for the great majority of the patients (>95%). On the other hand, the first end point of CGM analysis was to depict an overview of type 1 diabetes glucotypes during the first year rather than evaluating the individual evolution of patients across the groups. The sensor manufacturer might also influence data. Notably, the impact is limited, as most of the data were obtained from FreeStyle Libre (i.e., >90%), and no sensor-specific pattern was observed within the principal component analysis.
Our study confirmed that β-cell secretion estimates, evaluated using either a single blood test or stimulation testing, were only weakly correlated with glucose homeostasis. CGM metrics (e.g., hyperglycemia and time in range) demonstrated a strong correlation with routine clinical parameters (i.e., HbA1c and IDAA1c score) and demonstrated, for most of them, a specific circadian pattern that distinguished both remission groups, specifically in the early morning period. Moreover, we identified TIT63–140, GIIH<54, and between-day glucose variability as key parameters to distinguish remitters from nonremitters. Finally, we showed that using a combination of CGM metrics and clinical parameters allowed for identifying four categories of patient groups that experienced varying degrees of glucose homeostasis during the first year of type 1 diabetes. We believe that integrating various CGM metrics as end points in residual β-cell function prevention trials might provide clinically relevant and precise clues to evaluate patient response to treatment protocols.
Article Information
Acknowledgments. The authors thank Brieuc Van Nieuwenhuyse, Pôle de PEDI, UCLouvain, Brussels, Belgium, for advice in the interpretation of the results and critical reviewing of the initial draft; Gaetan de Valensart and Thierry Barrea, Pediatric Diabetes Unit, CUSL, Brussels, Belgium, for the active collaboration in the study management and collection of CGM data; and the investigators of the DIATAG Working Group for the indefatigable support in the recruitment of patients and management of the samples.
Funding. This research was supported by funding from the Fonds de la Recherche Scientifique (Fonds pour la Formation à la Recherche dans l’Industrie et dans l’Agriculture grant, FRS starting grant), Belgian Society for Pediatric Endocrinology and Diabetology, and Société Francophone du Diabète. Publication fees were partially supported by funding from the Fondation Universitaire de Belgique.
The content is solely the responsibility of the authors and does not represent the official views of these organizations.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Authors Contributions. O.G.P. contributed to the design, implementation, and coordination of the study, collected and interpreted data, wrote the first draft of the manuscript, and revised the manuscript. A.D. contributed to the coordination of the study, collected and interpreted data, wrote the first draft of the manuscript, and revised the manuscript. M.M. participated in the design, analysis of the data, wrote the first draft of the manuscript and revised the manuscript. J.L., I.G., M.d.B., M.-C.L., N.S. and T.M. contributed to the implementation and coordination of the study, collected the data, reviewed the first draft, and revised the manuscript. L.G. participated in the design, supervised data analysis, reviewed the first draft, and revised the manuscript. P.A.L. conceived the research question and the study design, interpreted the data, wrote the first draft, and revised the manuscript. P.A.L. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in poster form at the 82nd Scientific Sessions of the American Diabetes Association, New Orleans, LA, 3–7 June 2022 and in oral form at the ENDO2022 Congress, Atlanta, GA, 11–14 June 2022.
Footnotes
Clinical trial reg. no. NCT04007809, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.20217125.
O.G.P., A.D., and M.M. contributed equally to this work.
A complete list of the members of the DIATAG Working Group can be found in the supplementary material online.
Contributor Information
DIATAG Working Group::
Philippe A. Lysy, Olivier G. Pollé, Antoine Delfosse, Paola Gallo, Thierry Barrea, Gaetan De Valensart, Chloé Brunelle, Joachim Docquir, Jacques Louis, Nicolas Oberweis, Inge Gies, Willem Staels, Jesse Vanbesien, Christel Van den Brande, Marieke den Brinker, Mieke Van Eyde, Nicole Seret, Olimpia Chivu, Sophie Lambert, Marie-Christinne Lebrethon, Anne-Simone Parent, Catherine Sondag, Dominique Beckers, Thierry Mouraux, and Laure Boutsen
References
- 1. van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 2011;91:79–118 [DOI] [PubMed] [Google Scholar]
- 2. Nielens N, Pollé O, Robert A, Lysy PA. Integration of routine parameters of glycemic variability in a simple screening method for partial remission in children with type 1 diabetes. J Diabetes Res 2018;2018:5936360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mortensen HB, Hougaard P, Swift P, et al.; Hvidoere Study Group on Childhood Diabetes . New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care 2009;32:1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palmer JP, Fleming GA, Greenbaum CJ, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes 2004;53:250–264 [DOI] [PubMed] [Google Scholar]
- 5. Atkinson MA, Roep BO, Posgai A, Wheeler DCS, Peakman M. The challenge of modulating β-cell autoimmunity in type 1 diabetes. Lancet Diabetes Endocrinol 2019;7:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Linsley PS, Greenbaum CJ, Nepom GT. Uncovering pathways to personalized therapies in type 1 diabetes. Diabetes 2021;70:831–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rickels MR, Evans-Molina C, Bahnson HT, et al.; T1D Exchange β-Cell Function Study Group . High residual C-peptide likely contributes to glycemic control in type 1 diabetes. J Clin Invest 2020;130:1850–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu MG, Keenan HA, Shah HS, et al. Residual β cell function and monogenic variants in long-duration type 1 diabetes patients. J Clin Invest 2019;129:3252–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oram RA, Sims EK, Evans-Molina C. Beta cells in type 1 diabetes: mass and function; sleeping or dead? Diabetologia 2019;62:567–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gorst C, Kwok CS, Aslam S, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care 2015;38:2354–2369 [DOI] [PubMed] [Google Scholar]
- 11. Kahkoska AR, Adair LA, Aiello AE, et al. Identification of clinically relevant dysglycemia phenotypes based on continuous glucose monitoring data from youth with type 1 diabetes and elevated hemoglobin A1c. Pediatr Diabetes 2019;20:556–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Battaglia M, Ahmed S, Anderson MS, et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 2020;43:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mayer-Davis EJ, Kahkoska AR, Jefferies C, et al. ISPAD Clinical Practice Consensus Guidelines 2018: definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes 2018;19(Suppl. 27):7–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris PA, Taylor R, Minor BL, et al.; REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Philippe MF, Benabadji S, Barbot-Trystram L, Vadrot D, Boitard C, Larger E. Pancreatic volume and endocrine and exocrine functions in patients with diabetes. Pancreas 2011;40:359–363 [DOI] [PubMed] [Google Scholar]
- 16. Wentworth JM, Bediaga NG, Giles LC, et al.; Type 1 Diabetes TrialNet Study Group; Immune Tolerance Network Study Group . Beta cell function in type 1 diabetes determined from clinical and fasting biochemical variables. Diabetologia 2019;62:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vigers T, Chan CL, Snell-Bergeon J, et al. cgmanalysis: an R package for descriptive analysis of continuous glucose monitor data. PLoS One 2019;14:e0216851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Broll S, Urbanek J, Buchanan D, et al. Interpreting blood GLUcose data with R package iglu. PLoS One 2021;16:e0248560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fabris C, Patek SD, Breton MD. Are risk indices derived from CGM interchangeable with SMBG-based indices? J Diabetes Sci Technol 2015;10:50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodbard D. New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes Technol Ther 2009;11:551–565 [DOI] [PubMed] [Google Scholar]
- 22. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 2015;67:1–48 [Google Scholar]
- 23. Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Softw 2017;82:1–26 [Google Scholar]
- 24. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57:289–300 [Google Scholar]
- 25. Josse J, Husson F. missMDA: a package for handling missing values in multivariate data analysis. J Stat Softw 2016;70:1–31 [Google Scholar]
- 26. Pecheur A, Barrea T, Vandooren V, Beauloye V, Robert A, Lysy PA. Characteristics and determinants of partial remission in children with type 1 diabetes using the insulin-dose-adjusted A1c definition. J Diabetes Res. 2014;2014:851378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Monnier L, Colette C, Wojtusciszyn A, et al. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care 2017;40:832–838 [DOI] [PubMed] [Google Scholar]
- 28. Ruan Y, Willemsen RH, Wilinska ME, Tauschmann M, Dunger DB, Hovorka R. Mixed-meal tolerance test to assess residual beta-cell secretion: beyond the area-under-curve of plasma C-peptide concentration. Pediatr Diabetes 2019;20:282–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buckingham B, Cheng P, Beck RW, et al.; Diabetes Research in Children Network (DirecNet) and Type 1 Diabetes TrialNet Study Groups . CGM-measured glucose values have a strong correlation with C-peptide, HbA1c and IDAAC, but do poorly in predicting C-peptide levels in the two years following onset of diabetes. Diabetologia 2015;58:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hao W, Gitelman S, DiMeglio LA, Boulware D; Type 1 Diabetes TrialNet Study Group . Fall in C-peptide during first 4 years from diagnosis of type 1 diabetes: Variable relation to age, HbA1c, and insulin dose. Diabetes Care 2016;39:1664–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carr ALJ, Oram RA, Marren SM, McDonald TJ, Narendran P, Andrews RC. Measurement of peak C-peptide at diagnosis informs glycemic control but not hypoglycemia in adults with type 1 diabetes. J Endocr Soc 2021;5:bvab127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chmelova H, Cohrs CM, Chouinard JA, et al. Distinct roles of β-cell mass and function during type 1 diabetes onset and remission. Diabetes 2015;64:2148–2160 [DOI] [PubMed] [Google Scholar]
- 33. Addala A, Zaharieva DP, Gu AJ, et al. Clinically serious hypoglycemia is rare and not associated with time-in-range in youth with new-onset type 1 diabetes. J Clin Endocrinol Metab 2021;106:3239–3247 [DOI] [PMC free article] [PubMed] [Google Scholar]