Abstract
Levels of the serum opsonin mannan-binding lectin (MBL) were directly correlated with the probability of developing visceral leishmaniasis. Monocytes infected with MBL-opsonized Leishmania chagasi promastigotes secreted higher levels of tumor necrosis factor alpha and interleukin-6 than cells infected with nonopsonized parasites. Our findings indicate that MBL can modulate the clinical outcome of infection with L. chagasi and the function of infected macrophages.
Visceral leishmaniasis (VL) in Brazil is caused by the intracellular pathogen Leishmania chagasi and is almost always lethal if not treated (4). A persisting question has been why only a small proportion of infected individuals develop disease (4). Most infections will remain cryptic unless immunological suppression occurs (2). Young age, malnutrition, and human immunodeficiency virus infection are risk factors for VL (2, 4), but other host susceptibility factors remain unknown. Mannan-binding lectin (MBL), a multichain serum lectin and a component of innate immunity (10), is a candidate molecule for modifying disease progression because of its possible enhancing effect upon infections with intracellular pathogens (13, 14). It binds to carbohydrates present on many pathogens, including Leishmania (14), acting as an opsonin and “ante-antibody” by conferring protection before the establishment of adaptive immune responses (11). Three independent mutations at exon 1 result in amino acid substitutions in a collagenous region of the polypeptide chain (20, 28), which hinder assembly of subunits into the functional trimeric structure, render them vulnerable to degradation (18), and affect levels of MBL in serum.
Garred and colleagues proposed a dual role for MBL that explains the selective advantage for the wide range in levels of this collectin seen in a population (13): whereas low concentrations or exon 1 mutations have been associated with recurrent or severe infections in children and adults caused by extracellular pathogens (16, 29) and Plasmodium falciparum (19), high concentrations may enhance targeting of intracellular organisms to host phagocytes, the milieu preferred by these pathogens. We addressed this hypothesis by evaluating levels of MBL in VL in a case-control study of a population from Teresina, Piauí State, Brazil, where urban epidemics have occurred since 1980 (8) and where there is no transmission of Chagas' disease or cutaneous leishmaniasis. Individuals presenting with active VL (aVL) or a documented history of this disease and cured for at least 5 months (median, 316 days) (cVL) before sample collection were compared with healthy, Montenegro (leishmanin) skin test (MST)-positive or MST-negative individuals recruited from the same background and with no history of VL. Informed consent and institutional approval were obtained. Individuals were between 1 and 80 years of age (mean, 22.5 ± 16.2 years) and represent an admixture of African, Caucasian, and native American populations estimated, respectively, at 31, 21, and 48% (3). Diagnosis of VL was confirmed by isolation of parasites from bone marrow, and MST-positive individuals were considered to be infected. Serum MBL concentrations were measured in a double-antibody immune assay (30).
We took every step to ensure that, with the exception of aVL, the levels of MBL in serum measured were baseline. Levels vary during infection, reflecting acute phase reactions (12) or consumption by pathogens (1). The following data suggest that in cured and MST-positive individuals the load of L. chagasi is insufficient to trigger acute phase responses: (i) DNA-based detection of L. chagasi (9, 26) was positive in 76% of individuals with aVL versus 8% of patients with cVL or asymptomatic infection, and (ii) xenodiagnosis was negative in all MST-positive or cured individuals and positive in 40% of patients with aVL (9).
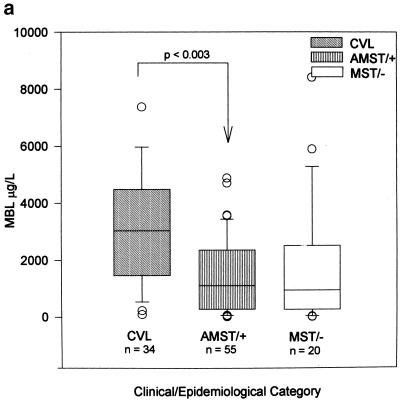
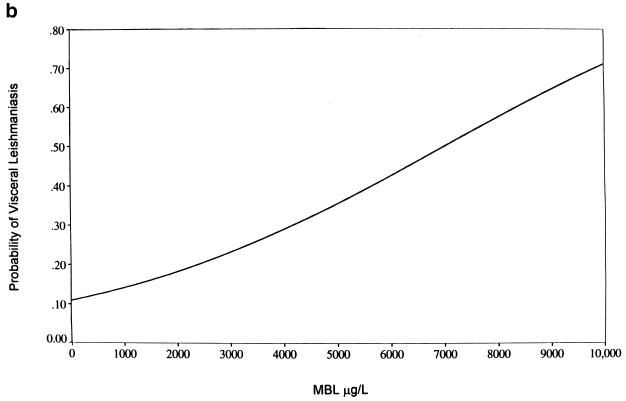
We thus examined the association between clinical outcome of infection with L. chagasi and MBL as a quantitative trait. MBL phenotypes (baseline levels) are associated with the outcome of infection with L. chagasi: levels were significantly higher (P < 0.003) in individuals with a history of VL than in infected, asymptomatic individuals (Fig. 1a) (median MBL concentrations in cVL patients [n = 34], 2,888 μg/liter; in MST-positive, asymptomatic individuals [n = 55], 1,113 μg/liter; and in exposed, MST-negative, healthy individuals [n = 20], 944 μg/liter). There was no association between levels of MBL and the sex or age of individuals. MBL phenotypes were directly correlated with the probability of developing VL (Fig. 1b), indicating a threshold effect of levels on the clinical outcome upon infection with L. chagasi.
FIG. 1.
MBL levels correlate to clinical outcome upon exposure to L. chagasi. (a) Box-whisker plots of serum MBL concentrations in different clinical-epidemiological categories: levels of MBL in serum are significantly (Kruskal-Wallis: H = 14.2, 3 df, P < 0.003) associated with clinical outcome upon infection with L. chagasi. (b) Logistic regression analysis of the probability of developing VL according to serum MBL concentrations: levels of MBL are directly and significantly correlated (likelihood ratio test = 11.55; 1 df, P < 0.001) with the probability of developing VL.
We next examined the association between exon 1 genotypes and outcome upon infection with L. chagasi. DNA was extracted with a Puregene kit (Gentra Systems), and PCR-restriction fragment length polymorphism typing of exon 1 was done as described previously (20). We determined that 50% of individuals had at least one exon 1 mutation (allele frequencies were 0.71, 0.16, 0.09, and 0.04 for A, B, C, and D, respectively; genotypes were AA, 50%; AO, 42%; OO, 8%; n = 108), the second highest frequency described to date (17, 21). Mutations were more frequent among MST-positive, healthy individuals and MST-negative individuals than among individuals who developed VL. Conversely, wild-type genotypes were more frequent among VL patients (Table 1). However, neither stepwise nor multiple regression analysis revealed a significant association between exon 1 genotypes and outcome upon exposure to or infection with L. chagasi. Exon 1 genotypes did not have a major effect on outcome. MBL phenotypes depend, however, on the set of alleles not only at exon 1 but also at the promoter. The LX promoter haplotype has the same effect on levels of MBL as do exon 1 structural mutants (20). We plan to examine whether promoter haplotypes associated with lower levels of protein transcription are more frequent in asymptomatic individuals.
TABLE 1.
MBL genotypes associated with clinical outcome of exposure to L. chagasi
| Genotypea | No. (%) of unrelated individuals with indicated genotype by clinical status:
|
|||
|---|---|---|---|---|
| aVL/cVL | MST+b | MST−b | Total | |
| AA | 28 (60) | 20 (46) | 6 (33) | 54 |
| AO | 17 (36) | 18 (42) | 10 (56) | 45 |
| OO | 2 (4) | 5 (12) | 2 (11) | 9 |
| AO/OO | 19 (40) | 23 (54) | 12 (67) | 54 |
| Total | 47 | 43 | 18 | 108 |
Nomenclature for the alleles (21): A for the functional, wild-type allele; B, C, and D for the individual mutant alleles, which are collectively called O since they all result in lower levels of protein.
MST+, MST positive; MST−, MST negative.
We next investigated the physiological basis for the associations observed in this population. Innate immunity can determine the outcome of adaptive immune responses to pathogens (22). MBL may regulate the availability of pathogen-derived polymannose structures for CD1b molecules (24) and of oligosaccharide for induction of T cells (32) and may affect the function of macrophages (6). We therefore examined whether MBL modulates the pattern of cytokines secreted by monocytes infected with MBL-opsonized L. chagasi promastigotes. First passage, stationary-growth-phase promastigotes were grown as described previously (14), washed three times, and resuspended in Hanks balanced salt solution containing 10 mM CaCl2 (Gibco) at 20 × 106 parasites/ml. Aliquots were mixed with an equal volume of recombinant human MBL (30) at the final concentrations indicated in Fig. 2. Parasites were incubated on ice for 1 h and then washed three times in Hanks balanced salt solution-Ca2+ buffer, resuspended in the same volume of the original aliquot, and used to infect peripheral blood mononuclear cells (PBMCs) or the myelomonocytic cell line THP-1. PBMCs (3.5 × 106/well in a 24-well plate) and THP-1 cells (1 × 106/well in a 24-well plate) were washed and cultured in complete RPMI medium containing 10% fetal calf serum without antibiotics. Gamma interferon was added at a final concentration of 100 ng/ml. After 18 h, cultures were infected with opsonized or nonopsonized promastigotes at a parasite-to-cell ratio of 1:2 for THP-1 cells and 1:3.5 for PBMCs. After 24 h, cytokines were measured in supernatants by capture assay (PharMingen). Levels of LPS in supernatants were below the detection limit of the limulus amebocyte assay (BioWhittaker).
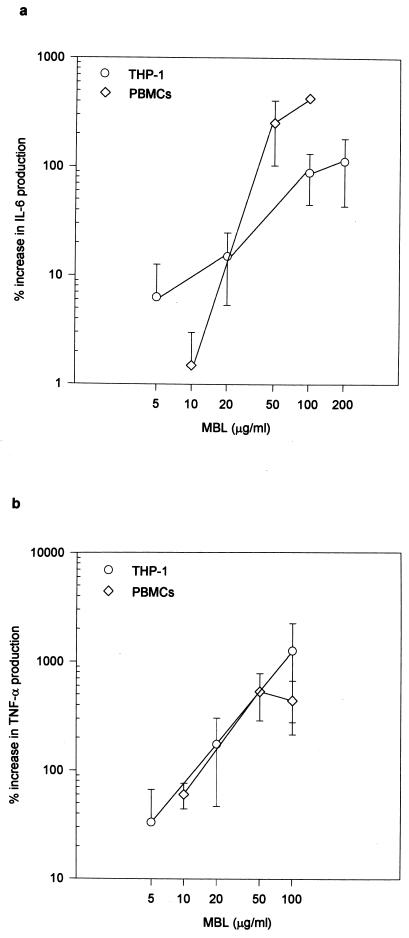
FIG. 2.
MBL modulates production of cytokines by human mononuclear cells. Cells were infected with stationary-phase promastigotes preincubated with the indicated dose of MBL. MBL modulates production of IL-6 (panel a: P < 0.001 for THP-1 and PBMCs by analysis of variance [ANOVA]) and of TNF-α (panel b: P < 0.04 for THP-1 and PBMCS by ANOVA and Kruskal-Wallis) by gamma interferon-primed THP-1 cells and PBMCs infected with L. chagasi. Results are expressed as percentage of increase of cytokine production over that obtained with cells infected with nonopsonized parasites. Data shown are means ± standard errors of the means of three experiments each for both cell types at the indicated concentrations. Mean cytokine production for cells infected with nonopsonized parasites was 5,160 ± 2,230 (a) and 49 ± 20 (b) pg/ml and 1,840 ± 1,580 (a) and 319 ± 179 (b) pg/ml for THP-1 cells and PBMCs, respectively.
Secretion of interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) was enhanced in a dose-dependent manner (respectively, P < 0.001 and P < 0.04) in both THP-1 cells and normal human PBMCs infected with promastigotes opsonized with MBL (Fig. 2a and b). We examined and did not see differences in the levels of IL-1α, IL-10, or IL-12. IL-6 and TNF-α are inflammatory cytokines, and higher levels of MBL could enhance microbicidal mechanisms in the macrophage by increasing production of these cytokines. IL-6 and TNF-α are, however, clearly elevated in VL (5, 31), and in spite of their microbicide-enhancing capacity, they do not promote control of the parasite. Increased IL-6 production is related to polyclonal B-cell activation and hypergammaglobulinemia, hallmarks of VL (31). This cytokine also inhibits the leishmanicidal capacities of macrophages infected with Leishmania mexicana (15). Mice deficient in IL-6 can control infection with Leishmania major (23, 27). Although TNF-α mediates leishmanicidal functions of macrophages, the parasite load is significant in VL in spite of high circulating levels of this cytokine (5). Il-6 and TNF-α affect T-cell development (7, 25) and could be involved in the suppression of parasite-specific cellular responses seen in VL (4). MBL may be involved in this suppression by modulating production of these inflammatory cytokines by L. chagasi-infected macrophages.
We show that, in vitro, MBL affects the function of L. chagasi-infected cells and that, in vivo, levels of MBL are directly correlated with the probability of developing VL upon infection. These results support the concept that MBL may indeed be a “double-edged sword” and that intermediary levels of MBL may be the most desirable phenotype for innate protection against a broad range of pathogens (13).
Acknowledgments
We thank Peter Garred for sharing data before publication and John David, Phillip Stahl, Antonio Campos Neto, Richard C. Lewontin, and Siamon Gordon for helpful suggestions.
We acknowledge the financial support of the Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (grant 20.1145/95-2 to I.K.F.D.M.S.), World Health Organization (grant M8/181/4/C.256 to C.H.N.C.), Pediatric Scientist Development Program (NICHD grant K12-HD00850 to J.E.E.), and the National Institutes of Health.
REFERENCES
- 1.Aittoniemi J, Rintala E, Miettinen A, Soppi E. Serum mannan-binding lectin (MBL) in patients with infection: clinical and laboratory correlates. APMIS. 1997;105:617–622. doi: 10.1111/j.1699-0463.1997.tb05062.x. [DOI] [PubMed] [Google Scholar]
- 2.Alvar J. Leishmaniasis and AIDS co-infection: the Spanish example. Parasitol Today. 1994;10:160–163. doi: 10.1016/0169-4758(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 3.Arpini-Sampaio Z, Costa M C B, Melo A A, Carvalho M F V A, Deus M S M, Simões A A. Genetic polymorphisms and ethnic admixture in African-derived black communities of northeastern Brazil. Human Biol. 1999;71:67–77. [PubMed] [Google Scholar]
- 4.Badaró R, Jones T C, Lorenço R, Cerf B J, Sampaio D, Carvalho E M, Rocha H, Teixeira R, Johnson W D., Jr A prospective study of visceral leishmaniasis in an endemic area of Brazil. J Infect Dis. 1986;154:639–649. doi: 10.1093/infdis/154.4.639. [DOI] [PubMed] [Google Scholar]
- 5.Barral-Netto M, Badaró R, Barral A, Almeida R P, Santos S B, Badaró F, Pedral-Sampaio D, Carvalho E M, Falcoff E, Falcoff R. Tumor necrosis factor (cachectin) in visceral leishmaniasis. J Infect Dis. 1991;163:853–857. doi: 10.1093/infdis/163.4.853. [DOI] [PubMed] [Google Scholar]
- 6.Chaka W, Verheul A F M, Vaishnav V V, Cherniak R, Scharringa J, Verhoef J, Snippe H, Hoepelman A I M. Induction of TNF-α in human peripheral blood mononuclear cells by the mannoprotein of Cryptococcus neoformans involves human mannose binding protein. J Immunol. 1997;159:2979–2985. [PubMed] [Google Scholar]
- 7.Cope A P, Liblau R S, Yang X-D, Congia M, Laudanna C, Schreiber R D, Probert L, Kollias G, McDevitt H O. Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J Exp Med. 1997;185:1573–1584. doi: 10.1084/jem.185.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa C H, Pereira H F, Araújo M V. Epidemia de leishmaniose visceral no Estado de Piauí, Brasil, 1980–1986. Rev Saude Publica Sao Paulo. 1990;24:361–372. doi: 10.1590/s0034-89101990000500003. [DOI] [PubMed] [Google Scholar]
- 9.Costa C H, Gomes R B, Silva M R, Garcez L M, Ramos P K, Santos R S, Shaw J J, David J R, Maguire J H. Competence of the human host as a reservoir for Leishmania chagasi. J Infect Dis. 2000;182:997–1000. doi: 10.1086/315795. [DOI] [PubMed] [Google Scholar]
- 10.Epstein J L, Eichbaum Q, Sheriff S, Ezekowitz R A B. The collectins in innate immunity. Curr Opin Immunol. 1996;8:29–35. doi: 10.1016/s0952-7915(96)80101-4. [DOI] [PubMed] [Google Scholar]
- 11.Ezekowitz R A B. Ante-antibody immunity. Curr Biol. 1991;1:60–62. doi: 10.1016/0960-9822(91)90132-g. [DOI] [PubMed] [Google Scholar]
- 12.Ezekowitz R A B, Day L, Herman G A. Human mannose-binding protein is an acute phase reactant that shares sequence homology with other vertebrate lectins. J Exp Med. 1988;167:1034–1046. doi: 10.1084/jem.167.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garred P, Harboe M, Oettinger T, Koch C, Svejgaard A. Dual role of mannan-binding protein in infections: another case of heterosis? Eur J Immunogenet. 1994;21:125–131. doi: 10.1111/j.1744-313x.1994.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 14.Green P J, Feizi T, Stoll M S, Thiel S, Prescott A, McConville M J. Recognition of the major cell surface glycoconjugates of Leishmania parasites by the human serum mannan-binding protein. Mol Biochem Parasitol. 1994;66:319–328. doi: 10.1016/0166-6851(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 15.Hatzigeorgiou D E, He S, Sobel J, Grabstein K H, Hafner A, Ho J L. IL-6 down-modulates the cytokine-enhanced antileishmanial activity in human macrophages. J Immunol. 1993;151:3682–3692. [PubMed] [Google Scholar]
- 16.Hibberd M L, Sumiya M, Summerfield J A, Booy R, Levin M. Association of variants of the gene for mannose-binding lectin with susceptibility to meningococcal disease. Lancet. 1999;353:1049–1053. doi: 10.1016/s0140-6736(98)08350-0. [DOI] [PubMed] [Google Scholar]
- 17.Hoal-van Helden E, Epstein J, Victor T C, Hon D, Lewis L-A, Zurakowski D, Ezekowitz R A B, van Helden P D. Mannose-binding protein B allele confers protection against tuberculous meningitis. Pediatr Res. 1999;45:459–464. doi: 10.1203/00006450-199904010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Lipscombe R J, Sumiya M, Summerfield J A, Turner M W. Distinct physicochemical characteristics of human mannose binding protein expressed by individuals of differing genotype. Immunology. 1995;85:660–667. [PMC free article] [PubMed] [Google Scholar]
- 19.Luty A J F, Kun J F J, Kremsner P G. Mannose-binding lectin plasma levels and gene polymorphisms in Plasmodium falciparum malaria. J Infect Dis. 1998;178:1221–1224. doi: 10.1086/515690. [DOI] [PubMed] [Google Scholar]
- 20.Madsen H O, Garred P, Thiel S, Kurtzhals J A, Lamm L U, Ryder L P, Svejgaard A. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155:3013–3020. [PubMed] [Google Scholar]
- 21.Madsen H O, Satz L, Hogh B, Svejgaard A, Garred P. Different molecular events result in low protein levels of mannan-binding lectin in populations from southeast Africa and South America. J Immunol. 1998;161:3169–3175. [PubMed] [Google Scholar]
- 22.Medzhitov R, Janeway C A., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 23.Moskowitz N H, Brown D R, Reiner S L. Efficient immunity against Leishmania major in the absence of IL-6. Infect Immun. 1997;65:2448–2450. doi: 10.1128/iai.65.6.2448-2450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prigozy T I, Sieling P A, Clemens D, Stewart P L, Behar S M, Porcelli S A, Brenner M B, Modlin R L, Kronenberg M. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity. 1997;6:187–197. doi: 10.1016/s1074-7613(00)80425-2. [DOI] [PubMed] [Google Scholar]
- 25.Rincón M, Anguita J, Nakamura T, Fikrig E, Flavell R A. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodgers M R, Popper S, Wirth D. Amplification of kinetoplast DNA as a tool in the detection and diagnosis of Leishmania. Exp Parasitol. 1990;71:267–275. doi: 10.1016/0014-4894(90)90031-7. [DOI] [PubMed] [Google Scholar]
- 27.Saha B, Saini A, Germond R, Perrin P J, Harlan D M, Davis T A. Susceptibility or resistance to Leishmania infection is dictated by the macrophages evolved under the influence of IL-3 or GM-CSF. Eur J Immunol. 1999;29:2319–2329. doi: 10.1002/(SICI)1521-4141(199907)29:07<2319::AID-IMMU2319>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Sumiya M, Super M, Tabona P, Levinsky R J, Arai T, Turner M W, Summerfield J A. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337:1569–1570. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- 29.Super M, Thiel S, Lu J, Levinsky R J, Turner M W. Association of low levels of mannan-binding protein with a common defect in opsonisation. Lancet. 1989;ii:1236–1238. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- 30.Super M, Gillies S D, Foley S, Sastry K, Schweinle J-E, Silverman V J, Ezekowitz R A B. Distinct and ovelapping functions of allelic forms of human mannose binding protein. Nat Genet. 1992;2:50–55. doi: 10.1038/ng0992-50. [DOI] [PubMed] [Google Scholar]
- 31.van der Poll T, Zijlstra E E, Mevissen M. Interleukin 6 during active visceral leishmaniasis and after treatment. Clin Immunol Immunopathol. 1995;77:111–114. doi: 10.1016/0090-1229(95)90144-2. [DOI] [PubMed] [Google Scholar]
- 32.Velupillai P, Harn D A. Oligosaccharide-specific induction of interleukin 10 production by B220+ cells from schistosome-infected mice: a mechanism for regulation of CD4+ T cell subsets. Proc Natl Acad Sci USA. 1994;91:18–22. doi: 10.1073/pnas.91.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]