Abstract
OBJECTIVE
To examine variations in timing of lower-limb amputation (LLA) across race/ethnicity and sex among older adults with a diabetic foot ulcer (DFU). It was hypothesized Black/African Americans were more likely to have LLA post-DFU earlier compared with non-Hispanic/Whites, and more men would receive LLA earlier post-DFU compared with women.
RESEARCH DESIGN AND METHODS
This was a retrospective cohort analysis of enrolled Medicare fee-for-service (FFS) beneficiaries with a diagnosis of DFU during the study period (2012–2017), allowing up to 5 years post-DFU. Final analytic sample contained 643,287 individuals; the subsample consisted of 68,633 individuals with LLA only. The primary outcome was mutually exclusive groups based on timing of LLA. Multinomial logistic regression was applied to assess likelihood of membership into a group post-DFU based on characteristics such as sex and race/ethnicity.
RESULTS
Black/African American beneficiaries had 1.98 (95% CI 1.93–2.03) times the odds of receiving an LLA within 1 year of DFU diagnosis compared with non-Hispanic/White beneficiaries relative to no amputation. Female beneficiaries had increased odds (odds ratio [OR] 1.07, 95% CI 1.02–1.11] between 1 and 3 years and OR 1.08 [95% CI 1.03–1.12] in ≥3 years) of a delayed LLA compared with men among those that underwent LLA.
CONCLUSIONS
Notably, these results present novel evidence on timing of LLA between racial groups and sex for Medicare FFS beneficiaries post-DFU. Results may be generalizable to individuals with Medicare FFS and DFU. Clinically more targeted, evidence-based decision making informs care decisions with opportunities to address inequities related to the social determinants of health that may lead to LLA.
Introduction
Diabetes is a chronic health condition that affects >24 million people in the U.S. and is expected to continue to grow (1,2). The risk of those with diabetes to experience a diabetic foot ulcer (DFU) during their lifetime is up to 25% (3), which is a known risk factor for future lower limb amputation (LLA), with rates between 11 and 30% up to 5 years after onset of the initial ulcer (4,5). Approximately 60% of all LLAs are nontraumatic and often related to diabetes or secondary to complications of diabetes in the U.S. (1). Notably, DFUs and LLAs do not seem to affect everyone equally, and little is known about the timing of when LLA occurs based on different patient factors. In the U.S., the prevalence and incidence of DFUs varies by race and sex, meaning that certain populations have a higher risk of complications associated with DFUs (1), while multiple studies have found that all-cause LLA rates vary by race and sex (6–8). Health disparities are systemic yet avoidable differences in the quality of health care not related to clinical needs, preferences, or appropriateness of interventions that adversely impact socially disadvantaged groups, including but not limited to factors such as race, ethnicity, sex, or sexual orientation (9).
The incidence of DFUs and amputation are higher in Black Americans or African Americans (Black/AA) than in the non-Hispanic White American population (1). Black/AA patients are more likely to undergo amputation and less likely to undergo revascularization procedures compared with White patients (10). Furthermore, Black patients are more likely to have complications following LLAs and a lower survival rate (11).
The literature on amputation secondary to DFU and disparities relative to race/ethnicity or sex is mixed and often unclear (12–15). This may in part stem from lack of consistent data collection to be used in analyses. The reason for race/ethnicity disparities in the U.S. are likely multifaceted. Studies that have adjusted for other potential factors that contribute to LLA (e.g., comorbid conditions, economic status, severity of disease, and insurance coverage) continue to find a persistent difference in LLA rates due to race/ethnicity. A study by Holman et al. (14) assessed limb salvage attempts among the elderly Medicare population with peripheral arterial disease (PAD) to determine whether differences in LLA existed based on race. They observed that Black patients were less likely than White patients to undergo limb salvage attempts prior to LLA among enrolled Medicare beneficiaries, yet the clinical reasons were unclear (14). PAD and associated amputation rates have been examined more specifically than in a population with an incident DFU.
Disparities with LLA are not limited to race/ethnicity but also exist based on sex. Among those with diabetes, despite equal hospitalization rates for uncontrolled diabetes, men are more likely to have an LLA (15). In contrast, women have higher mortality rates associated with LLAs attributed to diabetes (13). Some studies have proposed that the severity of associated comorbid health conditions is higher in women prior to LLA and that women are more likely to undergo more proximal LLAs (e.g., above the knee) compared with men (8).
There are several interventions available to patients that promote wound healing and possibly delay or prevent LLA (16–18). Previous studies have demonstrated effectiveness of providing nonsurgical measures (e.g., wound debridement, custom orthoses, accommodative footwear, and patient education) for those with DFU, yet there appear to be barriers that contribute to differences in who receives these interventions that may alter the trajectory of a patient’s health following onset of a DFU (16,17).
While there is evidence that these disparities in the pathway to LLA exist across race/ethnicity and sex, there is limited evidence on when in the time course differences begin to occur along the care pathway. A better understanding of how these care pathways are different can serve as a foundational element to improving patient outcomes by reducing disparities. Therefore, the purpose of this study was to examine variations in timing of LLA across race and sex among Medicare fee-for-service (FFS) beneficiaries with DFU. We hypothesized that a higher proportion of Black/AA individuals would receive an amputation in the first year following the index DFU compared with non-Hispanic/White individuals and that a higher proportion of men would receive amputation in the first year following the index DFU compared with women. These results will be foundational for future research to address inequities or health disparities among individuals who require prosthetic care.
Research Design and Methods
Data Source
Medicare 100% FFS beneficiary claims data were sourced through CareJourney (Arlington, VA). CareJourney is contracted with the Centers for Medicare and Medicaid Services (CMS) as a data broker able to provide analysis support on individual patient claims data for CMS beneficiaries. Medicare is the U.S. government–based health insurance program administered by CMS. Individuals are eligible for Medicare coverage who are ≥65 years old or those <65 years old who have certain disabilities or end-stage renal disease. The data are deidentified prior to release and therefore complies with the Health Insurance Portability and Accountability Act. Accordingly, the subsequent analysis is not considered human subject research and does not require approval from an Institutional Review Board.
Design and Cohort
This retrospective cohort study used eligible Medicare beneficiaries enrolled in the FFS program from 2012 to 2020. A sample data set requested through CareJourney was based on the restrictions set a priori and applied for extraction. The analytic sample was restricted to beneficiaries who had 1 year of eligibility before the DFU, with 5 years of eligibility after diagnosis and at least one claim with a diagnosis of DFU of the lower limb and no LLA claims prior to the DFU. For identification of DFU the International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes were used (Supplementary Table 1). The index date was based upon the date of the first DFU during the study period. Amputation surgery was defined by Current Procedural Terminology (CPT) codes (American Medical Association) or ICD-9 and ICD-10 codes subsequent to the DFU diagnosis (Supplementary Table 2).
Incident DFU occurred in 2012 through 2017, and then, individuals were followed for up to 5 years or until outcome occurred. Each beneficiary was followed until one of the following events occurred: death, enrollment change, or the end of the study period. Individuals were divided into their appropriate cohort based on when/whether a major LLA occurred following incident DFU. Across all cohorts, data extracted included risk adjustment score, mortality, area deprivation score index (ADI), and demographic variables (e.g., race/ethnicity, sex, age, comorbidities). Selected comorbidities retained include acute myocardial infarction, stroke or transient ischemic attack (TIA), chronic kidney disease, peripheral vascular disease, and heart condition (e.g., congestive heart failure or myocardial infarction). Race/ethnicity is collected by Medicare and self-reported. Individuals are asked to mark the race/ethnicity they identify with or to mark other. For example, the Medicare White category may include someone who identifies as Caucasian or of northern European descent but not considered Hispanic. The categories as collected by Medicare were maintained for this study.
Primary Outcome
Individuals were placed into mutually exclusive groups based on timing of LLA as follows: DFU with no subsequent amputation, LLA within 1 year post-DFU, LLA between 1 and 3 years post-DFU, and LLA >3 years post-DFU. LLA was determined based on the presence of the amputation procedure code after the index DFU diagnosis (Supplementary Table 2). Time was calculated between the index diagnosis of DFU and the date of amputation procedure.
Analysis
Descriptive statistics were calculated for the total sample. Mean, SD, and range were assessed for continuous data, and count and percentage were calculated for categorical data. Multinomial logistic regression was applied to assess likelihood of membership into a group post-DFU based on individual characteristics such as sex, race/ethnicity (non-Hispanic White, Black/AA, Hispanic, Asian, Native American, other, or unknown). Two models were developed. The first model examined the full sample to assess potential differences in which individuals experienced amputation as well as timing of amputation (i.e., no amputation, <1 year, 1–3 years, and ≥3 years) while controlling for age, sex, race/ethnicity, comorbidity, ADI, and hierarchical condition category (HCC). HCC is a risk-adjustment designed by CMS to estimate risk scores and performs similarly to the Charlson Comorbidity Index (19). A second model was a subanalysis, which included only those who lived for the entire study period and experienced an amputation, to assess the likelihood of when LLA occurred while controlling for age, sex, race/ethnicity, comorbidity, ADI, and HCC.
Results
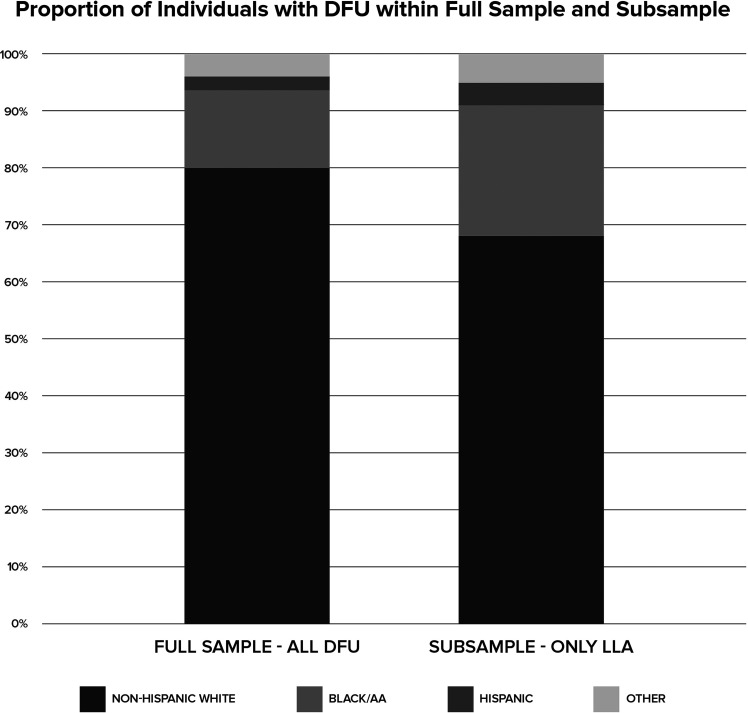
The final analytic sample contained 643,287 enrolled Medicare FFS beneficiaries, of whom 49.7% survived >5 years after the index DFU (Table 1). The subanalysis included 68,633 beneficiaries who had experienced an LLA within the 5 year study period, of whom 42% died during the study period but after the LLA. The proportion of male to female beneficiaries in the full sample was fairly balanced (53.6% men), while the subsample of LLA was less balanced (66.9% men). The age of the LLA subsample was younger (67.7 ± 12.4 years) than the full sample (74.0 ± 12.3 years), while the HCC among those with LLA appeared to be similar to the full sample (2.4 ± 1.8 vs. 2.5 ± 1.9, respectively). The proportion of the combined minority or non-Hispanic White race/ethnicities increased from the full sample (Black/AA 13.6%, Hispanic 2.6%) to the subsample containing only those with LLA (Black/AA 22.3%, Hispanic 3.8%) (Fig. 1). Most of the full sample presented with chronic kidney disease (78.8%), peripheral vascular disease (71.2%), and heart disease (84.2%). However, very few presented with stroke/TIA (21.2%) or with acute myocardial infarction (10.5%). Among the subsample of those who received an amputation, there was an increased percentage of those with chronic kidney disease (89.7%), peripheral vascular disease (92.3%), and heart disease (89%).
Table 1.
Sample characteristics
| Full sample (N = 643,287) | Only amputation subsample (n = 68,633) | |||
|---|---|---|---|---|
| Variable | Mean (SD) | Range | Mean (SD) | Range |
| HCC score | 2.4 (1.8) | 0–17.9 | 2.5 (1.9) | 0–17.9 |
| Age (years) | 74.0 (12.3) | 18–114 | 67.7 (12.4) | 21–106 |
| National ADI (weighted) | 50.0 (20.2) | 0–98.7 | 52.3 (19.9) | 3.0–98.1 |
| No. (%) | No. (%) | |||
| Sex | ||||
| Male | 344,555 (53.6) | 45,930 (66.9) | ||
| Female | 298,732 (46.4) | 22,703 (33.1) | ||
| Race/ethnicity | ||||
| Non-Hispanic/White | 515,412 (80.1) | 47,460 (69.2) | ||
| Black/AA | 87,215 (13.6) | 15,272 (22.3) | ||
| Asian | 7,139 (1.1) | 898 (1.3) | ||
| Hispanic | 16,744 (2.6) | 2,589 (3.8) | ||
| Native American | 6,735 (1.0) | 608 (0.9) | ||
| Other | 6,517 (1.0) | 1,318 (1.9) | ||
| Unknown | 3,525 (0.6) | 488 (0.7) | ||
| Death | ||||
| Yes | 323,670 (50.3) | 28,838 (42.0) | ||
| No | 319,617 (49.7) | 39,795 (58.0) | ||
| Timing of LLA | ||||
| LLA <1 year | 37,442 (5.8) | 42,616 (63.1) | ||
| LLA 1–3 years | 17,172 (2.7) | 15,065 (22.3) | ||
| LLA ≥3 years | 14,019 (2.2) | 9,866 (14.6) | ||
| No LLA | 574,654 (89.3) | — | ||
| Comorbidities | ||||
| Chronic kidney disease | 136,107 (21.2) | 7,053 (10.3) | ||
| Peripheral vascular disease | 185,216 (28.8) | 5,285 (7.7) | ||
| Stroke/TIA | 506,576 (78.8) | 50,803 (74.0) | ||
| Heart disease | 101,921 (15.8) | 7,547 (11.0) | ||
| Acute myocardial infarction | 575,946 (89.5) | 57,257 (83.4) | ||
Figure 1.
Comparison by proportion of the full sample that includes all individuals with DFU (left) to the subsample of only those with LLA (right).
The regression results of the full sample demonstrated that the odds of LLA within 1 year of DFU was significantly higher across all race categories compared with non-Hispanic White beneficiaries (Table 2). Specifically, the analysis revealed that Black/AA beneficiaries had 2.18 (95% CI 2.13–2.23) times the odds of receiving an LLA within 1 year of the DFU diagnosis compared with non-Hispanic White beneficiaries. The increased odds of LLA was sustained across race/ethnicity category for Black/AA beneficiaries to receive an LLA between 1 and 3 years post-DFU diagnosis, with an odds ratio [OR] of 1.38 (95% CI 1.33–1.44). This dropped slightly to an OR of 1.22 (95% CI 1.17–1.28) for ≥3 years post-DFU diagnosis compared with non-Hispanic White beneficiaries. There was also a significant difference between men and women with respect to timing of LLA, where women had reduced odds of an earlier LLA compared with men while controlling for covariates across all groups (Table 2). Notably, those with peripheral vascular disease had >10 times the odds of experiencing an LLA within 1 year of DFU (OR 10.75, 95% CI 10.18–11.36), and odds of LLA decreased over time.
Table 2.
Results of adjusted regression for the full sample (N = 643,287), which consisted of all Medicare FFS beneficiaries with DFU during the study period
| Patient characteristic | LLA <1 year post-DFU | P value | LLA 1–3 years post-DFU | P value | LLA ≥3 years post-DFU | P value |
|---|---|---|---|---|---|---|
| HCC score | 1.01 (1.00–1.01) | 0.0026 | 0.98 (0.97–0.99) | <0.0001 | 0.90 (0.89–0.91) | <0.0001 |
| Age | 0.97 (0.96–0.97) | <0.0001 | 0.95 (0.95–0.95) | <0.0001 | 0.94 (0.94–0.94) | <0.0001 |
| National ADI (weighted) | 1.01 (1.01–1.01) | <0.0001 | 1.00 (1.00–1.01) | <0.0001 | 1.00 (1.00–1.00) | <0.0001 |
| Sex (ref = male) | ||||||
| Female | 0.61 (0.59–0.62) | <0.0001 | 0.64 (0.62–0.66) | <0.0001 | 0.63 (0.61–0.66) | <0.0001 |
| Race/ethnicity (ref = Non-Hispanic White) | ||||||
| Black/AA | 1.98 (1.93–2.03) | <0.0001 | 1.38 (1.33–1.44) | <0.0001 | 1.22 (1.17–1.28) | <0.0001 |
| Asian | 1.44 (1.31–1.58) | 0.37 | 1.08 (0.94–1.25) | 0.17 | 0.95 (0.81–1.11) | 0.15 |
| Hispanic | 1.65 (1.56–1.74) | 0.0005 | 1.21 (1.11–1.31) | 0.66 | 1.08 (0.98–1.19) | 0.63 |
| Native American | 1.29 (1.16–1.43) | 0.0025 | 0.89 (0.74–1.05) | 0.0002 | 0.66 (0.54–0.82) | <0.0001 |
| Other | 2.07 (1.90–2.25) | <0.0001 | 1.80 (1.60–2.01) | <0.0001 | 1.64 (1.45–1.86) | <0.0001 |
| Unknown | 1.32 (1.16–1.51) | 0.038 | 1.13 (0.94–1.36) | 0.58 | 1.06 (0.87–1.28) | 0.98 |
| Comorbidities (ref = none) | ||||||
| Chronic kidney disease | 2.00 (1.92–2.09) | <0.0001 | 1.80 (1.71–1.91) | <0.0001 | 1.07 (1.02–1.12) | 0.0056 |
| Peripheral vascular disease | 10.75 (10.18–11.36) | <0.0001 | 5.35 (5.06–5.67) | <0.0001 | 2.81 (2.68–2.95) | <0.0001 |
| Stroke/TIA | 1.08 (1.05–1.11) | <0.0001 | 1.21 (1.17–1.25) | <0.0001 | 1.19 (1.14–1.24) | <0.0001 |
| Heart disease | 1.19 (1.14–1.24) | <0.0001 | 1.03 (0.97–1.08) | 0.35 | 0.89 (0.84–0.93) | <0.0001 |
| Acute myocardial infarction | 1.33 (1.29–1.37) | <0.0001 | 1.49 (1.43–1.56) | <0.0001 | 1.44 (1.38–1.52) | <0.0001 |
The results are presented as the OR (95% CI) of when an amputation occurs after the index DFU relative to those who did not receive an amputation. Model adjusted for comorbidity, age, sex, HCC, and ADI.
The results of the second regression model only examining those with LLA revealed that female beneficiaries had increased odds (OR 1.07 [95% CI 1.02–1.11] between 1 and 3 years group and OR 1.08 [95% CI 1.03–1.12] in ≥3 years group) of receiving an LLA later than men while controlling for covariates (reference category was those with amputation <1 year post-DFU). With respect to experiencing an LLA early (<1 year post-DFU), race was significantly associated with reduced odds of a delayed LLA occurring (Table 3). Based on comorbidity, a similar pattern continued in that peripheral vascular disease was associated with increased odds of an earlier LLA.
Table 3.
Adjusted logistic regression results of subsample: only those who receive an LLA (n = 68,633) and odds of timing of LLA
| Patient characteristic | LLA 1–3 years post-DFU | P value | LLA ≥3 years post-DFU | P value |
|---|---|---|---|---|
| HCC score | 0.97 (0.96–0.98) | <0.0001 | 0.89 (0.88–0.90) | <0.0001 |
| Age | 0.98 (0.98–0.98) | <0.0001 | 0.98 (0.97–0.98) | <0.0001 |
| National ADI (weighted) | 1.00 (1.00–1.00) | 0.012 | 1.00 (1.00–1.00) | <0.0001 |
| Sex (ref = male) | ||||
| Female | 1.07 (1.02–1.11) | 0.0017 | 1.08 (1.03–1.12) | 0.0009 |
| Race/ethnicity (ref = Non-Hispanic White) | ||||
| Black/AA | 0.74 (0.71–0.78) | 0.001 | 0.69 (0.65–0.72) | 0.01 |
| Asian | 0.77 (0.66–0.91) | 0.38 | 0.69 (0.58–0.84) | 0.34 |
| Hispanic | 0.78 (0.71–0.86) | 0.28 | 0.73 (0.65–0.81) | 0.50 |
| Native American | 0.72 (0.59–0.88) | 0.13 | 0.57 (0.45–0.72) | 0.0082 |
| Other | 0.90 (0.79–1.03) | 0.14 | 0.84 (0.73–0.98) | 0.11 |
| Unknown | 0.89 (0.71–1.10) | 0.45 | 0.83 (0.66–1.05) | 0.35 |
| Comorbidities (ref = none) | ||||
| Chronic kidney disease | 0.94 (0.87–1.00) | 0.06 | 0.58 (0.54–0.61) | <0.0001 |
| Peripheral vascular disease | 0.53 (0.49–0.58) | <0.0001 | 0.30 (0.28–0.33) | <0.0001 |
| Stroke/TIA | 1.13 (1.09–1.18) | <0.0001 | 1.14 (1.09–1.20) | <0.0001 |
| Heart disease | 0.92 (0.86–0.98) | 0.016 | 0.85 (0.79–0.91) | <0.0001 |
| Acute myocardial infarction | 1.11 (1.06–1.17) | <0.0001 | 1.08 (1.02–1.14) | 0.012 |
Data are presented as the OR (95% CI). Reference is to those who received an LLA within 1 year of the index DFU.
Conclusions
This retrospective cohort analysis was conducted to assess how timing of LLA was associated with key patient factors, race and sex, among Medicare FFS beneficiaries. While this study builds on a broad foundation of existing literature reporting racial disparities in LLA rates, these results present novel evidence related to timing of LLA between racial groups compared with non-Hispanic White as well as between sexes for Medicare FFS beneficiaries following an episode of DFU.
When comparing the proportion of individuals with LLA based on race/ethnicity, the hypothesis was supported as there was an increase in the proportion of Black/AA individuals receiving amputation following DFU (Fig. 1). Upon further examination, it is evident that Black/AA individuals are more likely to have an amputation within the first year following DFU as opposed to non-Hispanic White beneficiaries who have amputations delayed (>1 year). This would suggest the non-Black/AA population may have access to and increased use of conservative wound management techniques or there is less access to and adherence to such treatment options within the Black/AA population. The magnitude of ORs also indicates the decision to amputate may be influenced more by other unexplained underlying variables as race/ethnicity independently persists as a significant factor even while controlling for other sociodemographic variables (e.g., ADI). There is continued debate about the socioecological factors unique to the U.S. that contribute to this link between racial/ethnic background and poor health outcomes (20). For example, socioeconomic status is a complex construct that may impact an individual’s ability to access to wound and ulcer treatments and subsequently contribute to early LLA. While ADI in this particular study did not emerge as a measure that significantly influenced timing of LLA, unmeasured variables remain, such as health literacy that has been linked to negative outcomes among those with diabetes (21). This is relevant to the aging Black/AA Medicare population specifically due to historical sociopolitical roots associated with antiliteracy laws and poor access to education in the U.S. (21).
Historical policy and beliefs in the U.S. also may impact outcomes due to sex. Sex differences have been explored less in relation to LLA rates and potential disparities. The hypothesis that men would receive an amputation earlier was upheld. From the current results, women appear to have a greater likelihood of delayed amputation. The increased likelihood for delayed amputation may contribute to the overall increased prevalence of amputation for men as women may die before amputation is provided (8,15). Additionally, this finding is in alignment with recent evidence from a cohort of Swedish individuals that reported greater likelihood for women to have a more proximal level amputation at later ages compared with men (8). This suggests amputation in women is delayed until the patient dies or a distal amputation is no longer viable. The diminished health state at the time of amputation coincides with increased mortality after LLA for women (8). It is possible that women have LLAs later than men due to biological differences, such as hormones that may be protective against neuropathy, thus resulting in less severe clinical symptoms than men at the same age alerting individuals to an issue (15).
This study is not without limitations. First, the analysis is dependent on Medicare race/ethnicity data, which can be subject to a high rate of misreporting and small sample sizes. However, previous studies have demonstrated that misreporting of race/ethnicity does not limit the usefulness of Medicare data for comparing rates among racial and ethnic groups (22). Another consideration is that race/ethnicity is a self-reported variable collected by Medicare. This study is a secondary analysis that relied on the existing data.
There is also limitation with regards to side of amputation and exact cause for amputation. This information is not specified in the LLA procedure codes; thus, it is possible that some of the LLAs reported are not directly due to the DFU pathophysiology (e.g., trauma) or could have been patient choice versus attempting limb salvage or aggressive wound care. However, given the volume of the current sample size, it is unlikely patient choice alone could reach significant enough of volume to explain the differences in timing of LLA among the Medicare population (20,21).
Also, due to the window of the index period, individuals with incident DFU in 2016 and 2017 would have reached final outcome in 4 or 3 years, respectively, due to data ending in 2020. However, the influence of this difference in time window for a small percentage of beneficiaries was mitigated through the regression approach and subsequently considered to have low impact on the findings.
Regarding generalizability, this study represents Medicare FFS enrolled beneficiaries from 2012 through 2017 and may not represent those who are enrolled in other Medicare programs, those uninsured, or those insured through private plans.
The distribution of non-Hispanic White beneficiaries compared with Black/AA or other racial minorities in this study does not match the current total U.S. population racial makeup. It does however align with 2018 (cms.gov) estimates of Medicare FFS beneficiaries with ∼9% identifying as Black/AA and 79% as non-Hispanic White.
Finally, there may be variations in LLA due to clinical practice based on geography, but this current data sample did not include the specific variables on hospital or location to determine potential effects. A future study may include this as an aim related to timing of LLA.
Using national Medicare FFS data, this study found that Black/AA individuals are more likely to receive an earlier LLA within 1 year of DFU diagnosis compared with non-Hispanic White individuals. Furthermore, the pattern of disparities persisted when examining only those who received an amputation, noting a higher proportion of earlier amputations among individuals who identify as Black/AA, Hispanic, Asian, Native American, or other. A possible way to improve outcomes for minority beneficiaries is through increased access to early primary care and interdisciplinary groups that provide specialized care for diabetic foot infections, wounds, or ulcers.
The ability to drive equitable care starts with identification of clear disparities. This evidence allows for more targeted, evidence-based decision making to change potentially problematic care decisions made based on nonhealth-related factors. Health policies should address inequities related to the social determinants of health that may contribute to growing disparities that lead to different treatment of DFUs and ultimately the timing of LLA. Future research should explore whether these disparities continue into the patient’s post LLA care pathway, notably the opportunity for prosthetic rehabilitation.
Article Information
Acknowledgments. The authors acknowledge CareJourney analysts who accessed the data and created cohorts.
Funding. This study was partially funded by a grant provided by the American Orthotic and Prosthetic Association through the Center for Orthotic and Prosthetic Learning.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. T.A.M. contributed to study design and wrote the manuscript. J.H.C. contributed to study conception and reviewed and edited the manuscript. N.B. contributed to data analysis and to the research design and methods section. S.R.W. contributed to study conception and design and reviewed and edited the manuscript. All authors discussed and reviewed the results and approved the final version of the manuscript. N.B. and S.R.W. are the guarantors of this work and, as such, had full access to the data in the study and take responsibility for the integrity of the data and the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.20372667.
References
- 1. Tan T-W, Armstrong DG, Concha-Moore KC, et al. Association between race/ethnicity and the risk of amputation of lower extremities among Medicare beneficiaries with diabetic foot ulcers and diabetic foot infections. BMJ Open Diabetes Res Care 2020;8:e001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wittbrodt E, Kong AM, Moore-Schiltz L, Juneau P. All-cause and diabetes-related healthcare costs among US adults with type 2 diabetes initiating exenatide once weekly or insulin glargine. Diabetes Obes Metab 2018;20:672–680 [DOI] [PubMed] [Google Scholar]
- 3. Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–1724 [DOI] [PubMed] [Google Scholar]
- 4. Skrepnek GH, Mills JL Sr, Armstrong DG. A diabetic emergency one million feet long: disparities and burdens of illness among diabetic foot ulcer cases within emergency departments in the United States, 2006-2010. PLoS One 2015;10:e0134914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care 2003;26:491–494 [DOI] [PubMed] [Google Scholar]
- 6. Arya S, Binney Z, Khakharia A, et al. Race and socioeconomic status independently affect risk of major amputation in peripheral artery disease. J Am Heart Assoc 2018;7:e007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. George J, Navale SM, Schiltz NK, Siccha M, Klika AK, Higuera CA. Racial disparities in above-knee amputations after TKA: a national database study. Clin Orthop Relat Res 2017;475:1809–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamrad I, Söderberg B, Örneholm H, Hagberg K. SwedeAmp—the Swedish Amputation and Prosthetics Registry: 8-year data on 5762 patients with lower limb amputation show sex differences in amputation level and in patient-reported outcome. Acta Orthop 2020;91:464–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Braveman P. Health disparities and health equity: concepts and measurement. Annu Rev Public Health 2006;27:167–194 [DOI] [PubMed] [Google Scholar]
- 10. Barshes NR, Sharath S, Zamani N, Smith K, Serag H, Rogers SO. Racial and geographic variation in leg amputations among Texans. Tex Public Health J 2018;70:22–27 [PMC free article] [PubMed] [Google Scholar]
- 11. Curran T, Zhang JQ, Lo RC, et al. Risk factors and indications for readmission after lower extremity amputation in the American College of Surgeons National Surgical Quality Improvement Program. J Vasc Surg 2014;60:1315–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGrath RP, Snih SA, Markides KS, et al. The burden of health conditions across race and ethnicity for aging Americans: disability-adjusted life years. Medicine (Baltimore) 2019;98:e17964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lefebvre KM, Chevan J. The persistence of gender and racial disparities in vascular lower extremity amputation: an examination of HCUP-NIS data (2002-2011). Vasc Med 2015;20:51–59 [DOI] [PubMed] [Google Scholar]
- 14. Holman KH, Henke PK, Dimick JB, Birkmeyer JD. Racial disparities in the use of revascularization before leg amputation in Medicare patients. J Vasc Surg 2011;54:420–426, 426.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peek ME. Gender differences in diabetes-related lower extremity amputations. Clin Orthop Relat Res 2011;469:1951–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sutherland BL, Pecanac K, Bartels CM, Brennan MB. Expect delays: poor connections between rural and urban health systems challenge multidisciplinary care for rural Americans with diabetic foot ulcers. J Foot Ankle Res 2020;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crawford F, Chappell FM, Lewsey J, et al. Risk assessments and structured care interventions for prevention of foot ulceration in diabetes: development and validation of a prognostic model. Health Technol Assess 2020;24:1–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blumberg SN, Warren SM. Disparities in initial presentation and treatment outcomes of diabetic foot ulcers in a public, private, and Veterans Administration hospital. J Diabetes 2014;6:68–75 [DOI] [PubMed] [Google Scholar]
- 19. Li P, Kim MM, Doshi JA. Comparison of the performance of the CMS Hierarchical Condition Category (CMS-HCC) risk adjuster with the Charlson and Elixhauser comorbidity measures in predicting mortality. BMC Health Serv Res 2010;10:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hughes K, Sehgal N. Racial/ethnic disparities in lower extremity amputation vs revascularization: a brief review. J Natl Med Assoc 2018;110:560–563 [DOI] [PubMed] [Google Scholar]
- 21. Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care 2021;44:258–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Escarce J, Mcguire TG. Methods for using Medicare data to compare procedure rates among Asians, blacks, Hispanics, Native Americans, and whites. Health Serv Res 2003;38:1303–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]