Abstract
The phenotypic and functional plasticity of adipose tissue macrophages (ATMs) during obesity plays a crucial role in orchestration of adipose and systemic inflammation. Tonicity-responsive enhancer binding protein (TonEBP) (also called NFAT5) is a stress protein that mediates cellular responses to a range of metabolic insults. Here, we show that myeloid cell–specific TonEBP depletion reduced inflammation and insulin resistance in mice with high-fat diet–induced obesity but did not affect adiposity. This phenotype was associated with a reduced accumulation and a reduced proinflammatory phenotype of metabolically activated macrophages, decreased expression of inflammatory factors related to insulin resistance, and enhanced insulin sensitivity. TonEBP expression was elevated in the ATMs of obese mice, and Sp1 was identified as a central regulator of TonEBP induction. TonEBP depletion in macrophages decreased induction of insulin resistance–related genes and promoted induction of insulin sensitivity–related genes under obesity-mimicking conditions and thereby improved insulin signaling and glucose uptake in adipocytes. mRNA expression of TonEBP in peripheral blood mononuclear cells was positively correlated with blood glucose levels in mice and humans. These findings suggest that TonEBP in macrophages promotes obesity-associated systemic insulin resistance and inflammation, and downregulation of TonEBP may induce a healthy metabolic state during obesity.
Introduction
Obesity is associated with chronic low-grade inflammation characterized by progressive accumulation of immune cells in metabolic tissues, especially visceral adipose tissue (1). Adipose tissue inflammation is considered to be a main driving force for the development of insulin resistance and type 2 diabetes (T2D) in obese individuals (2). As a major cell type that contributes to inflammatory responses, macrophages accumulate in the adipose tissue of humans and rodents with increasing body weight (3) and adipose tissue macrophages (ATMs) are major effector cells that orchestrate adipose and systemic inflammation in obesity (4).
Macrophages are highly heterogeneous and possess broad plasticity, which means that they can change their phenotypes and distinct functional activation states according to local environmental factors and thereby play distinct roles in regulation of tissue homeostasis and inflammation (5). They are generally classified as proinflammatory M1 macrophages or anti-inflammatory M2 macrophages (6). Traditionally, ATMs are considered to accumulate during obesity due to recruitment of circulating monocytes and subsequent differentiation into macrophages (7,8). In addition to the increased number of ATMs, the phenotype of these cells dramatically changes during obesity. ATMs in the lean state typically have an anti-inflammatory–like phenotype, which helps to maintain tissue homeostasis. By contrast, ATMs during obesity respond to metabolic cues such as excess free fatty acids (FFAs), glucose, and gut-derived endotoxin and adopt a metabolically activated (MMe) phenotype that is distinct from M1 or M2 activation (9,10). MMe ATMs exert both detrimental and beneficial functions via independent proinflammatory and anti-inflammatory pathways during obesity (9,10). Thus, the balance between these two pathways in MMe ATMs could result in distinct metabolic states and influence the progression of obesity and its common comorbidities, including glucose intolerance and T2D.
Tonicity-responsive enhancer binding protein (TonEBP), also known as nuclear factor of activated T cells 5 (NFAT5), is a pleiotropic stress protein involved in distinct stress responses in various cell types (11). It can mediate physiological or pathological responses depending on the context. For example, TonEBP-mediated responses to osmotic stress, bacterial infection, and genotoxin-induced DNA damage are protective. By contrast, TonEBP-mediated responses to autoimmune and metabolic stresses are pathogenic in human diseases such as autoimmune diseases, acute kidney injury, hepatocellular carcinoma, atherosclerosis, and obesity (11). Many pathological conditions and agents stimulate TonEBP expression in T cells, macrophages, and dendritic cells, and elevated levels of TonEBP promote pathogenic activation of these cells in a cell type–specific manner (11). Numerous studies show that TonEBP promotes inflammatory and autoimmune diseases in humans and rodents. Conversely, downregulation of TonEBP reduces inflammation and thereby helps to prevent these diseases (11). Although the role of TonEBP in macrophages is well studied, relatively little is known about its intrinsic role in the identity and function of ATMs during obesity. Therefore, in this study we examined the impact of macrophage TonEBP in the context of obesity-associated inflammation and insulin resistance. We found that TonEBP expression was elevated in the ATMs of HFD-induced obese mice, and this promoted the development of obesity-associated inflammation and insulin resistance. Consequently, myeloid TonEBP–deficient mice showed reduced inflammation and insulin resistance and were protected against obesity-induced metabolic dysfunction. Thus, we identified TonEBP as a crucial factor in the activation states of ATMs and in the control of metabolic dysfunction during obesity.
Research Design and Methods
Mice
All animal procedures were approved by and performed according to guidelines of the Institutional Animal Care and Use Committee of the Ulsan National Institute of Science and Technology (UNISTACUC-20-27). Lysozyme 2-cre knock-in (LysM-cre) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). TonEBPfl/fl mice (12) were crossed with LysM-cre heterozygous mice to generate mice lacking TonEBP in myeloid cells. For the obese mice model, male mice aged 8 weeks were fed a control diet (CD) (10% fat as kcal; Research Diets, New Brunswick, NJ) or high-fat diet (HFD) (60% fat as kcal; Research Diets) for 12 weeks. Age-matched littermates without the Cre transgene were used as controls. Mouse bone marrow (BM)-derived macrophages (BMDMs) were obtained through culturing of BM cells with macrophage colony-stimulating factor (M-CSF) for 7 days (13). Mouse mononuclear cells were isolated from BM or blood with use of Histopaque-1077 (Sigma-Aldrich, St. Louis, MO). Monocytes were further purified by positive selection on CD11b microbeads followed by separation on MACS Columns (Miltenyi Biotec, Bergisch, Germany).
Human Samples
The study was approved by the Institutional Review Board of the Ulsan National Institute of Science and Technology (UNISTIRB-15-25-A). Human monocyte-derived macrophages were prepared by M-CSF (14). Human peripheral blood mononuclear cells (PBMCs) from healthy subjects and subjects with diabetes were provided by the Seoul National University Hospital Human Biobank, a member of the National Biobank of Korea, which is supported by the Ministry of Health and Welfare. All samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board–approved protocols.
Cell Culture, Transfection, and Adenovirus Infection
Human monocyte-like THP-1 cells (TIB-202; ATCC) were cultured in RPMI medium containing 10% FBS and then differentiated into macrophages through exposure to 5 ng/mL Phorbol 12-Myristate 13-Acetate (PMA) (Sigma-Aldrich) for 2 days. The murine macrophage cell line RAW264.7 (TIB-71; ATCC) was cultured in DMEM containing 10% FBS. All siRNA duplexes were purchased from Integrated DNA Technologies (Coralville, IA). PMA-differentiated THP-1 macrophages and RAW264.7 cells were transfected with scrambled (Scr) siRNA or siRNAs specific for target genes for 48 h with Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA). For overexpression, RAW264.7 cells were infected with an empty vector control virus or an adenovirus carrying the human TonEBP gene at a multiplicity of infection of 50 for 24 h.
Immunohistochemistry
Tissue sections of epididymal white adipose tissue (epi-WAT) and liver were stained with hematoxylin-eosin (H-E) for examination of morphological changes. We performed immunohistochemistry by incubating samples with primary antibodies as indicated. Images were recorded with an Olympus FV1000 confocal fluorescence microscope. Signal intensity was determined with ImageJ software (https://imagej.nih.gov/ij/).
Flow Cytometry
Cells were immunophenotyped by flow cytometry using multicolor fluorochrome-conjugated antibodies. Cell-surface markers were identified with use of primary antibodies purchased from BD Biosciences (Franklin Lakes, NJ) (Supplementary Table 1). Flow cytometry data were acquired on a BD LSRFortessa instrument (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR).
Real-time PCR
Total RNA was extracted from tissues and cells using TRIzol reagent (Invitrogen). After reverse transcription, real-time PCR was performed with CFX384 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). Measured cycle threshold values were normalized with the cyclophilin A or GAPDH reference gene and expressed as fold changes relative to control samples. The primers are described in Supplementary Table 2.
Immunoblotting, Cytokine Analysis, and Glucose Uptake Assay
Western blotting was performed with standard methods (15). The primary antibodies used are described in Supplementary Table 1. Reactive bands were detected through chemiluminescence with the ImageQuant LAS 4000 imaging system (GE Healthcare Life Sciences). Levels of cytokines in serum from mice and culture medium were measured with ELISA kits (R&D Systems, Minneapolis, MN). The glucose uptake rate was measured as previously described (16).
Luciferase Reporter Assay
Cells were transfected for 24 h with human TonEBP promoter–driven luciferase reporter vectors. The Renilla luciferase reporter plasmid was used as a control for transfection efficiency. Transfected cells were treated as indicated in the figure legends and lysed in passive lysis buffer. The luciferase assay was performed with the dual-luciferase reporter system (Promega, Madison, WI).
Chromatin Immunoprecipitation Quantitative PCR
Chromatin immunoprecipitation (ChIP) was performed with a commercial kit (Millipore, Bedford, MA). In brief, cells were cross-linked with 1% formaldehyde followed by addition of 125 mmol/L glycine. After washing, cells were sonicated and immunoprecipitated with anti-IgG, anti-Sp1 IgG (ab231778; Abcam), and anti-C/EBPβ IgG (3082S; Cell Signaling Technology) antibodies at 4°C overnight. After elution and reverse cross-linking of the antibody/DNA complexes, DNA was purified with a DNA purification kit (QIAGEN, Redwood, CA) and subjected to real-time PCR with use of suitable primers. The primers are described in Supplementary Table 2. Immunoprecipitated DNA from each sample was normalized to its respective chromatin input and IgG controls.
Statistical Analysis
Data are expressed as the mean ± SEM or SD. The statistical significance of differences between two conditions was estimated with an unpaired t test. One-way ANOVA followed by Tukey post hoc test was used to compare multiple conditions. All statistical analyses were performed with GraphPad Prism 8.2 software (GraphPad, San Jose, CA).
Data and Resource Availability
The data sets generated during or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Myeloid TonEBP Deficiency Prevents HFD-Induced Glucose Intolerance and Insulin Resistance
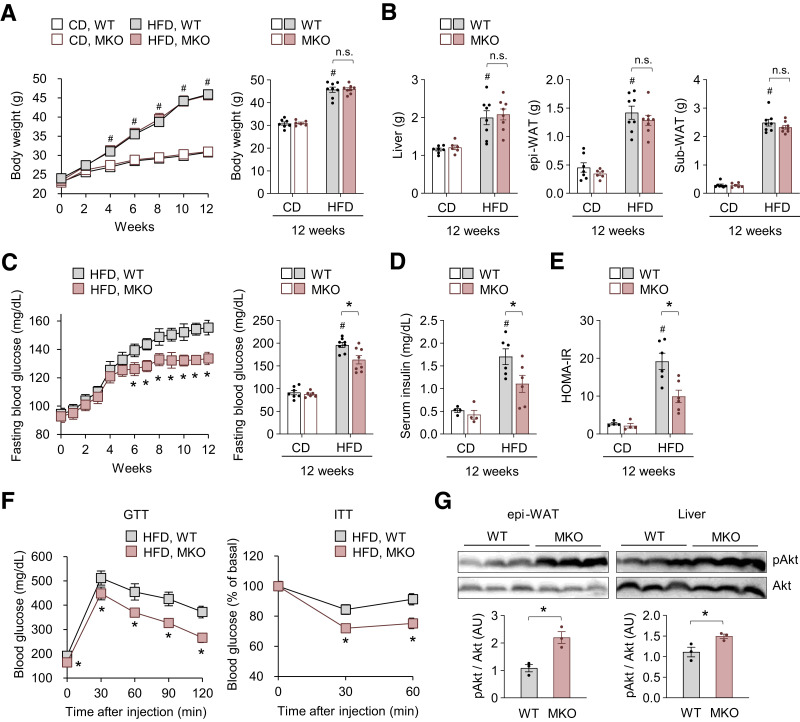
To investigate the intrinsic role of TonEBP in myeloid cells (i.e., macrophages) during the development of obesity and insulin resistance, we generated a mouse model with myeloid cell–specific genetic deletion of TonEBP (MKO) by crossing floxed TonEBP (TonEBPfl/fl) mice with TonEBPfl/fl; LysM-Cre+/− mice. TonEBPfl/fl cohoused littermates that did not express Cre recombinase (wild type [WT]) were used as controls. TonEBP levels were much lower in peritoneal macrophages and BMDMs from MKO mice than in those from their WT littermates, confirming the deletion of TonEBP in LysM-expressing macrophages (Supplementary Fig. 1A and B). MKO and WT mice showed similar weight gain (Fig. 1A) and food intake (Supplementary Fig. 1C) during feeding of CD or HFD for 12 weeks. Weights of the liver, epi-WAT, and subcutaneous white adipose tissue after feeding of HFD for 12 weeks did not differ between MKO and WT mice (Fig. 1B). In addition, MKO mice showed similar HFD-induced hypertriglyceridemia but reduced hypercholesterolemia in comparison with their WT counterparts (Supplementary Fig. 1D). HFD induced hyperglycemia and hyperinsulinemia and increased HOMA of insulin resistance index in mice (Fig. 1C–E). Notably, these effects were less severe in MKO mice than in WT mice (Fig. 1C–E). MKO mice showed improved glucose tolerance and insulin sensitivity at week 8 of HFD feeding (Fig. 1F). In addition, epi-WAT and livers isolated from MKO mice showed more potent insulin signaling than those isolated from WT mice (Fig. 1G). CD-fed MKO and WT mice had similar serum glucose and insulin levels (Fig. 1C and D). Collectively, these data demonstrate that TonEBP in myeloid cells is related to development of obesity-associated metabolic deterioration.
Figure 1.
Myeloid TonEBP deficiency prevents HFD-induced glucose intolerance and insulin resistance. Male MKO mice and their WT littermates aged 8 weeks were fed a normal CD (n = 14) or HFD (n = 15) for 12 weeks. A: Changes in body weight after switching to HFD. B: Liver, epi-WAT, and subcutaneous WAT (Sub-WAT) weights. C: Changes in fasting (18 h) blood glucose levels after switching to HFD. D: Circulating insulin concentrations after fasting for 18 h. E: HOMA of insulin resistance (HOMA-IR). F: Glucose tolerance test (GTT) (left) (n = 10) and insulin tolerance test (ITT) (right) (n = 11) after 8 weeks on HFD. For GTT, mice were fasted for 18 h and orally administered glucose (2 g/kg). Insulin tolerance test was performed with injection of human insulin (0.75 units/kg i.p.) to mice fasted for 6 h. Blood glucose concentrations were measured in blood taken from the tail vein at the indicated time points after glucose or insulin injection. The results of the insulin tolerance test are expressed as a percentage of basal blood glucose. G: Phosphorylation (p) of AKT in epi-WAT (left) and the liver (right) in response to insulin administration after 8 weeks on HFD (n = 3). Mice fasted overnight were injected with human insulin (0.75 units/kg i.p.) and scarified 30 min later. Tissues were rapidly removed and snap frozen in liquid nitrogen for immunoblotting. n represents the number of biologically independent animals (or samples). All data are presented as mean ± SEM. P values were determined with ANOVA with Tukey post hoc test. #P < 0.05 vs. CD; *P < 0.05. AU, arbitrary units; HOMA-IR, HOMA of insulin resistance; n.s., not significant.
Myeloid TonEBP Deficiency Reduces HFD-Induced Macrophage Accumulation and Inflammation in Adipose Tissue and the Liver
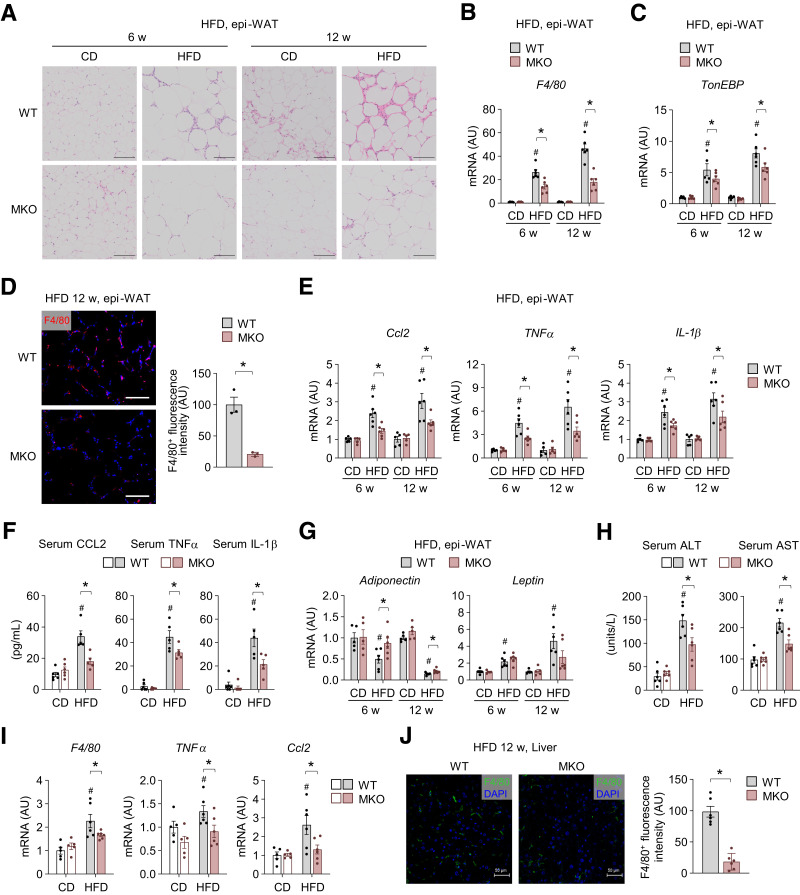
Given that increased macrophage infiltration and chronic inflammation in peripheral tissues, such as the liver and adipose tissue, are hallmarks of obesity-induced insulin resistance (17), we examined the epi-WAT and liver phenotypes. In comparison with their WT counterparts, HFD-fed MKO mice showed decreases of crown-like structures, which are hallmarks of adipose tissue inflammation (Fig. 2A). mRNA expression of F4/80 and TonEBP was increased in epi-WAT of mice fed HFD and lower in HFD-fed MKO mice (Fig. 2B and C). HFD-fed MKO mice showed decreases of F4/80+ ATMs (Fig. 2D), consistent with reduced F4/80 mRNA expression (Fig. 2B). Furthermore, expression of genes encoding CCL2, which is the important chemokine regulating infiltration of monocytes/macrophages, and proinflammatory cytokines related to insulin resistance, namely, TNFα and IL-1β, was lower in MKO mice than in their WT counterparts (Fig. 2E). Consistently, HFD-fed MKO mice had lower serum levels of CCL2, TNFα, and IL-1β (Fig. 2F). Notably, neither epi-WAT phenotype nor expression of these genes differed between WT and MKO mice fed CD for 12 weeks. MKO mice were also protected against HFD-induced downregulation of adiponectin, which is an adipokine positively associated with insulin sensitivity, compared with WT mice, but leptin level had not significantly change in either group (Fig. 2G). Collectively, these data suggest that TonEBP in myeloid cells promotes HFD-induced epi-WAT remodeling toward inflammation and insulin resistance.
Figure 2.
Myeloid TonEBP deficiency reduces HFD-induced macrophage accumulation and inflammation in adipose tissue and the liver. A–J: WT and MKO mice were fed CD or HFD for 6 and 12 weeks. A: Representative images of H-E–stained sections of epi-WAT from mice fed CD (n = 5) or HFD (n = 6). B and C: mRNA levels of F4/80 (B) and TonEBP (C) in epi-WAT from mice fed CD (n = 5) or HFD (n = 6). D: Representative images (left) and quantification (right) of F4/80 immunostaining in epi-WAT of mice fed HFD for 12 weeks (n = 3). E: mRNA levels of Ccl2, TNF-α, and IL-1β in epi-WAT from mice fed CD (n = 5) or HFD (n = 6). F: Serum levels of CCL2, TNF-α, and IL-1β in mice fed CD (n = 6) or HFD (n = 5) for 12 weeks. G: mRNA levels of adiponectin and leptin in epi-WAT from mice fed CD (n = 5) or HFD (n = 6). H–J: WT and MKO mice were analyzed after 12 weeks on CD or HFD (n = 6). H: Serum ALT and AST concentrations. I: mRNA levels of F4/80, TNF-α, and Ccl2 in the liver. J: Representative images (left) and quantification (right) of F4/80 immunostaining in livers of HFD-fed mice (n = 6). n represents the number of biologically independent animals (or samples). All data are presented as mean ± SEM. Scale bars, 100 μm (A) and 50 μm (D and J). P values were determined with ANOVA with Tukey post hoc test. #P < 0.05 vs. CD; *P < 0.05. AU, arbitrary units; w, weeks.
Chronic HFD feeding causes hepatic steatosis and inflammation (1,18). HFD-fed WT mice had elevated circulating levels of ALT and AST (Fig. 2H), but these levels were attenuated in HFD-fed MKO mice (Fig. 2H). mRNA expression of F4/80, TNFα, and Ccl2 was also lower in livers of HFD-fed MKO mice (Fig. 2I). Consistent with the reduced mRNA level of F4/80, livers of HFD-fed MKO mice contained fewer F4/80+ macrophages (Fig. 2J).
Taken together, these data demonstrate that TonEBP mediates the detrimental effects of obesity, such as macrophage accumulation, inflammation, and hepatic steatosis in adipose tissue and the liver.
TonEBP Deficiency Promotes Macrophage Polarization Toward Improvement of Insulin Sensitivity
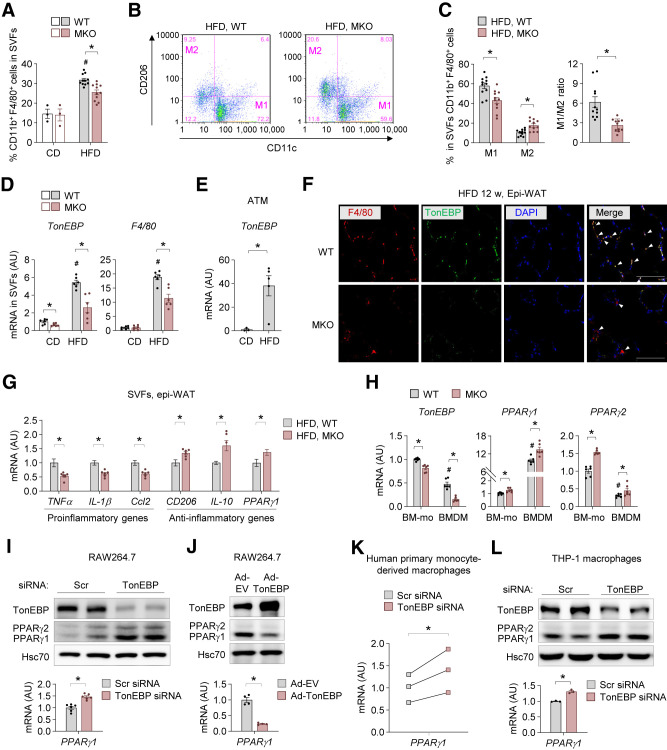
In both mice and human subjects, the activation state of ATMs is causally linked to obesity-induced insulin resistance (17) and steatohepatitis (19). Therefore, we examined macrophage populations in stromal vascular fractions (SVFs) of epi-WAT. Consistent with results of a previous study (20), HFD feeding increased the population of ATMs (CD11b+F4/80+) in WT mice, and this was suppressed by myeloid TonEBP deficiency (Fig. 3A and Supplementary Fig. 2A). More importantly, TonEBP deficiency in myeloid cells reduced the population of proinflammatory M1-like ATMs (CD11b+F4/80+CD11c+CD206−) in HFD-fed mice (Fig. 3B and C and Supplementary Fig. 2B). By contrast, the population of anti-inflammatory M2-like ATMs (CD11b+F4/80+CD11c−CD206+) was larger in HFD-fed MKO mice than in HFD-fed WT mice (Fig. 3B and C and Supplementary Fig. 2B). Hence, the ratio of M1-like to M2-like ATMs was lower in MKO mice (Fig. 3C), indicating that inflammatory responses are reduced in epi-WAT of HFD-fed MKO mice. Notably, mRNA expression of TonEBP was elevated in SVFs of epi-WAT from HFD-fed mice (Fig. 3D). As expected given the decrease in epi-WAT (Fig. 2B and C), mRNA expression of F4/80 was lower in SVFs of epi-WAT from HFD-fed MKO mice (Fig. 3D). We further confirmed that TonEBP expression was markedly higher in ATMs from epi-WAT from HFD-fed mice than in those from epi-WAT from CD-fed mice (Fig. 3E). Furthermore, immunofluorescence staining of epi-WAT from HFD-fed WT mice showed colocalization of TonEBP with F4/80, whereas TonEBP was not detected in macrophages of epi-WAT from HFD-fed MKO mice (Fig. 3F), suggesting that TonEBP expression increases in ATMs during obesity.
Figure 3.
TonEBP deficiency promotes macrophage polarization toward improvement of insulin sensitivity. A–C: Flow cytometry analysis of cells isolated from SVFs of epi-WAT from MKO and WT mice fed CD (n = 3) or HFD (n = 11) for 12 weeks. A: Percentage of total macrophages (CD11b+F4/80+) in SVFs. B: Representative flow cytometry plots showing the frequencies of M1 (CD11c+CD206−) and M2 (CD11c−CD206+) macrophages within the macrophage population in mice fed HFD. C: Quantification of M1 and M2 macrophages (left) and the M1-to-M2 ratio (right). D: mRNA levels of TonEBP and F4/80 in SVFs of epi-WAT from mice fed HFD (n = 6). E: ATMs were isolated from SVFs of epi-WAT from WT mice fed CD (n = 3) or HFD (n = 5) for 12 weeks with anti-CD11b antibody coupled to magnetic beads. mRNA levels of TonEBP. F: Representative images of immunofluorescence staining of F4/80 (red), TonEBP (green), and DAPI (blue) in epi-WAT from WT and MKO mice fed HFD for 12 weeks (n = 3). Colocalization of F4/80 and TonEBP is shown in yellow in the merged image (arrows). Scale bar, 50 μm. G: mRNA levels of metabolically activated pro- and anti-inflammatory genes in SVFs of epi-WAT from mice fed HFD (n = 6). H: mRNA levels of TonEBP, PPARγ1, and PPARγ2 in BM-Mo and BMDMs from WT and MKO mice (n = 6). I: Representative protein levels of TonEBP and PPARγ (top) and mRNA levels of PPARγ1 (bottom) in RAW264.7 cells transfected with Scr or TonEBP-targeted siRNA (n = 3). J: Representative protein levels of TonEBP and PPARγ (top) and mRNA levels of PPARγ1 (bottom) in RAW264.7 cells infected with adenovirus expressing TonEBP (Ad-TonEBP) or empty vector (Ad-EV) (n = 4). K: mRNA levels of PPARγ1 in primary human monocyte–derived macrophages transfected with Scr or TonEBP-targeted siRNA. Primary human mononuclear cells were isolated from blood, and monocytes were further purified using CD14 microbead positive selection. The macrophages were obtained from a 7-day culture with human M-CSF (20 ng/mL), and transfected with the indicated siRNA (n = 3). L: Representative protein levels of TonEBP and PPARγ (top) and mRNA levels of PPARγ1 (bottom) in THP-1–derived macrophages transfected with Scr or TonEBP-targeted siRNA (n = 6). n represents the number of biologically independent animals (or samples) (A–H and K) or independent experiments with at least two replicates (I, J, and L). All data are presented as mean ± SEM. (or SD). P values were determined with ANOVA with Tukey post hoc test. #P < 0.05 vs. CD or BM-Mo (H). *P < 0.05. mRNA levels were normalized to those of cyclophilin A (internal control). mRNA levels in SVFs were further normalized to those of F4/80 to correct for the amount of macrophages (G). AU, arbitrary units.
Consistent with the increased macrophage content, metabolically activated proinflammatory and anti-inflammatory gene expression was increased in SVFs of epi-WAT from HFD-fed WT mice (Supplementary Fig. 2C). Importantly, expression of mRNAs encoding proinflammatory-related TNFα, IL-1β, and CCL2 was lower in MKO mice when normalized to F4/80 mRNA levels, while expression of mRNAs encoding anti-inflammatory–related CD206, IL-10, and PPARγ1 was higher (Fig. 3G). These data indicate that TonEBP expression in myeloid cells contributes to the increase of proinflammatory-like ATMs and to the decrease in anti-inflammatory–like ATMs in HFD-fed mice. Based on these findings, we investigated whether TonEBP intrinsically affects the polarization state of macrophages. To this end, BM cells were differentiated into BMDMs with culture in the presence of M-CSF, which drives differentiation of monocytes into unactivated M0 macrophages (21). Interestingly, TonEBP deficiency increased basal expression of anti-inflammatory–related genes (CD206 and PPARγ1) without affecting the expression of the proinflammatory-related genes TNFα, IL-1β, and Ccl2 (Supplementary Fig. 2D) in BMDMs (M0). Additionally, the percentage of BMDMs (Supplementary Fig. 2E), and mRNA expression of F4/80 (Supplementary Fig. 2F), did not differ between these two groups of mice, demonstrating that TonEBP does not modulate the capacity of monocytes for macrophages differentiation. Taken together, these data demonstrate that TonEBP suppresses polarization toward anti-inflammatory macrophages during differentiation of macrophages. Similar effects were observed in macrophages differentiated from the human monocyte cell line THP-1. Two siRNAs (nos. 1 and 2, Supplementary Fig. 3A and B) targeting different regions of TonEBP mRNA reduced expression of proinflammatory-associated genes and increased expression of anti-inflammatory–associated genes in THP-1–derived macrophages, suggesting that TonEBP suppresses polarization of differentiated human macrophages toward an anti-inflammatory phenotype.
Given the importance of PPARγ in priming monocytes toward anti-inflammatory polarization and in suppression of the inflammatory response in macrophages (22), we further explored the role of TonEBP in PPARγ expression in macrophages. mRNA expression of TonEBP decreased on M-CSF–induced differentiation of BM-derived monocytes (BM-Mo) into macrophages (Fig. 3H). Notably, mRNA expression of both PPARγ1 and PPARγ2 was higher in BM-Mo and BMDM from MKO mice than in the corresponding cells from WT mice (Fig. 3H). mRNA levels of PPARγ1 were also higher in SVFs from 8-week-old MKO mice than in those from WT mice (Supplementary Fig. 3C). Similarly, siRNA-mediated TonEBP knockdown increased PPARγ1 expression in murine RAW264.7 macrophages (Fig. 3I). Conversely, overexpression of TonEBP with an adenoviral vector reduced mRNA expression of PPARγ1 (Fig. 3J). Importantly, TonEBP depletion increased PPARγ1 expression in primary human monocyte–derived macrophages obtained from three donors as previously described (23) (Fig. 3K) and in THP-1–derived macrophages (Fig. 3L). Furthermore, TonEBP knockdown stimulated induction of PPARγ1 by the M2 inducer IL-4 (20 ng/mL) (24) (Supplementary Fig. 3D). However, TonEBP knockdown did not affect STAT6 activation or C/EBPβ expression, which are involved in M2 polarization (25) (Supplementary Fig. 3D). These data suggest that TonEBP modulates the proinflammatory/anti-inflammatory phenotype at least partly by suppressing the PPARγ1 expression in monocytes/macrophages.
We next asked how TonEBP suppressed the PPARγ1 expression. Since TonEBP suppresses the PPARγ2 promoter via blocking the recruitment of C/EBPβ (26) and there is C/EBPβ binding site in the PPARγ1 promoter (27), we examined C/EBPβ binding to the PPARγ1 promoter. ChIP analysis using specific primers for the binding site (Supplementary Table 2) showed that C/EBPβ binding to the PPARγ1 promoter was elevated in response to TonEBP knockdown (Supplementary Fig. 3E). These data demonstrate that TonEBP suppresses the PPARγ1 promoter via blocking the recruitment of C/EBPβ.
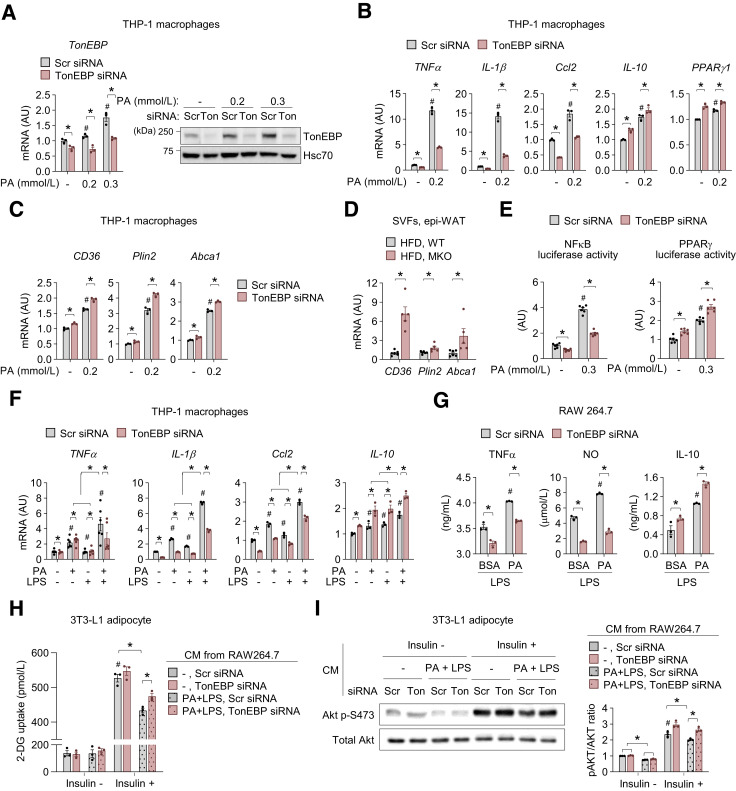
TonEBP Deficiency in Macrophages Improves Insulin Sensitivity of Adipocytes Under Obesity-Mimicking Conditions In Vitro
To address how TonEBP regulates proinflammatory responses in MMe ATMs, we examined the effects of TonEBP on macrophage responses to saturated FFA palmitate (PA), the main driver of the MMe phenotype (9,10). PA increased TonEBP expression in THP-1 macrophages (Fig. 4A). Consistent with previous findings (9,10), it also increased mRNA expression of the proinflammatory-genes TNFα, IL-β, and Ccl2, which are responsible for the development of obesity-induced insulin resistance (28), and of IL-10 (8) and PPARγ1 (22), which encode anti-inflammatory proteins that play important roles as modulators of insulin sensitivity (Fig. 4B). Notably, TonEBP knockdown markedly reduced PA-mediated induction of TNFα, IL-1β, and Ccl2 expression but enhanced expression of IL-10 and PPARγ1 (Fig. 4B). Similar effects were observed in mouse BMDM and RAW264.7 cells (Supplementary Fig. 4A–C). TonEBP depletion also promoted PA-mediated induction of genes involved in lipid metabolism (Cd36, Plin2, and Abca1) that are regulated by PPARγ (9,10) in THP-1 macrophages (Fig. 4C). Furthermore, mRNA levels of these genes were higher in SVFs of epi-WAT from HFD-fed MKO mice (Fig. 4D). Given the importance of the opposing actions of nuclear factor-κB (NF-κB) and PPARγ on MMe phenotype in ATMs (9,10), we examined whether TonEBP affects PA-stimulated transcriptional activity of NF-κB and PPARγ in macrophages. PA-stimulated NF-κB activation was decreased by TonEBP depletion consistent with the transcriptional activator function of TonEBP (29,30). Consistent with the suppressor function of TonEBP for the PPARγ1 promoter (see above), PA-stimulated PPARγ activation was increased by TonEBP knockdown (Fig. 4E). Collectively, these data demonstrate that TonEBP promotes the proinflammatory phenotype of MMe macrophages via at least two independent mechanisms: the activation of the proinflammatory NF-κB pathway and the suppression of anti-inflammatory PPARγ pathway.
Figure 4.
TonEBP deficiency in macrophages improves insulin sensitivity of adipocytes under obesity-mimicking conditions in vitro. A–C: THP-1–derived macrophages were transfected with Scr or TonEBP-targeted siRNA. siRNA-transfected cells were exposed to BSA (-) or PA (0.2 or 0.3 mmol/L) for 6 or 18 h. A: TonEBP mRNA levels at 6 h (left) and representative immunoblots of TonEBP and Hsc70 at 18 h (right) (n = 3). B and C: mRNA expression of the indicated genes at 18 h. D and E: RAW264.7 cells transfected with the indicated siRNA transfected a second time with κB- (D) or PPRE (E)-luciferase reporter vector. Cells were exposed to BSA (-) or PA (0.3 mmol/L) for 12 h. Luciferase activity was measured (n = 4). F: THP-1–derived macrophages transfected with Scr or TonEBP-targeted siRNA were exposed to BSA or PA (0.2 mmol/L) in the absence or presence of LPS (0.01 ng/mL) for 18 h. mRNA levels of TNFα, IL-1β, Ccl2, and IL-10, which are related to the insulin response (n = 3). G: RAW264.7 cells transfected with Scr or TonEBP-targeted siRNA were exposed to BSA or PA (0.2 mmol/L) in the presence of LPS (1 ng/mL) for 18 h. Concentrations of TNFα, NO, and IL-10 in CM from cells (n = 3). H and I: RAW264.7 cells transfected with the indicated siRNA were incubated in the absence or presence of PA (0.2 mmol/L) plus LPS (1 ng/mL) for 24 h, and CM was collected. Differentiated 3T3-L1 adipocytes were exposed to CM for 24 h. Cells were serum starved for 2 h and then incubated for 30 min in low-glucose media containing 20 μmol/L 6-NBDG without or with 100 nmol/L insulin. H: Stimulation of glucose (2-DG) uptake in response to insulin in 3T3-L1 adipocytes exposed to CM from RAW264.7 cells treated as indicated (n = 3). I: Representative immunoblots of phosphorylated (p-)Akt in response to insulin (left), and the ratio of phosphorylated to total Akt (right) in 3T3-L1 adipocytes exposed to CM from RAW264.7 cells treated as indicated (n = 3). n represents the number of independent experiments with more than three replicates (A–C and E–I) or biologically independent samples (D). All data are presented as mean ± SD (or SEM). P values were determined with ANOVA with Tukey post hoc test. #P < 0.05 vs. Scr siRNA; PA (-) (A–C and E), Scr siRNA; PA (-); LPS (-) (F), Scr siRNA; BSA; LPS (G), Scr siRNA; PA (-); insulin (-) (H and I). *P < 0.05. AU, arbitrary units; Ton, TonEBP.
Circulating levels of lipopolysaccharide (LPS) and PA are increased in obese subjects (31,32) and animal models of obesity (33,34). The synergistic interaction between them is known to promote proinflammatory responses via TLR4/NF-kB signaling and stimulate the development of obesity-related diseases including T2D (35). Thus, to mimic the conditions of obesity in vitro, we activated macrophages with 0.1 or 1 ng/mL LPS and/or 0.2 mmol/L PA, which are similar to the concentrations found in the serum of obese subjects (31,32). mRNA expression of TNFα, IL-1β, Ccl2, and IL-10 was higher in cells treated with PA plus LPS than in cells treated with PA or LPS alone in THP-1 macrophages (Fig. 4F). Notably, mRNA expression of TNFα, IL-1β, and Ccl2 was lower in TonEBP-deficient cells, while mRNA expression of IL-10 was higher (Fig. 4F). Obesity-induced activation of ATMs impairs insulin sensitivity of nearby adipocytes (36). For investigation of whether TonEBP depletion in macrophages changes the insulin response of adipocytes, an indirect coculture experiment was performed with mature 3T3-L1 adipocytes and conditioned medium (CM) from RAW264.7 cells. Similar to findings in THP-1 macrophages, the expression of insulin response–associated genes was higher in RAW264.7 cells treated with PA plus LPS than in those treated with LPS alone, whereas the levels of TNFα and iNOS in TonEBP-deficient RAW264.7 cells were lower than in control cells after treatment with both LPS alone and the combination of PA and LPS, and the level of IL-10 was higher (Fig. 4G and Supplementary Fig. 4D). Exposure to CM from PA plus LPS–stimulated cells reduced insulin-stimulated glucose uptake and Akt phosphorylation compared with exposure to CM from vehicle-treated cells (Fig. 4H and I). Notably, exposure to CM from TonEBP-deficient RAW264.7 cells improved insulin-stimulated glucose uptake and Akt phosphorylation compared with exposure to CM from control cells stimulated with PA plus LPS (Fig. 4H and I). Collectively, these data demonstrate that TonEBP in macrophages impairs insulin sensitivity of adipocytes and that macrophage TonEBP plays an important role in obesity-induced inflammation and subsequent insulin resistance.
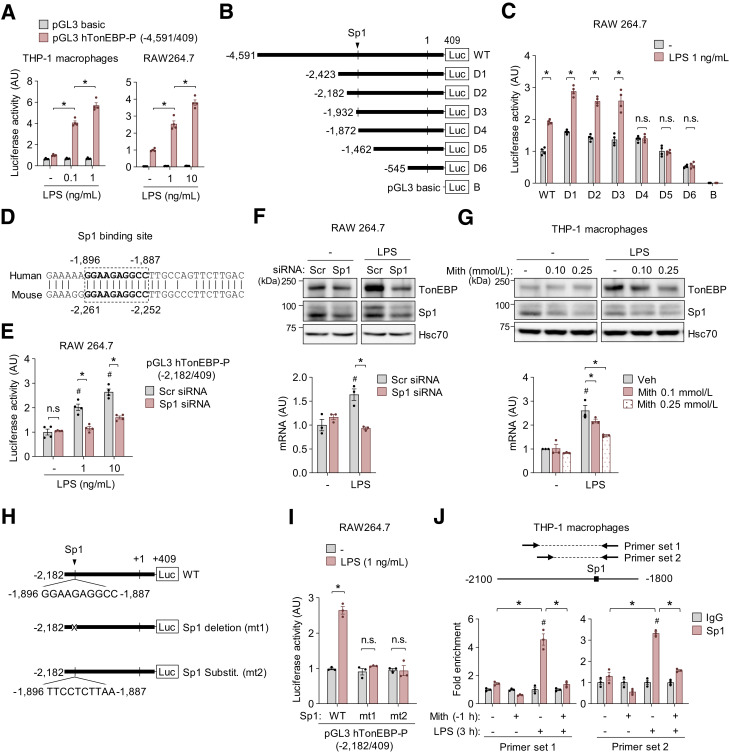
Sp1 Is a Central Mediator of TonEBP Induction in Response to TLR4 Stimulation in Macrophages
TonEBP expression in macrophages is upregulated through TLR4 stimulation by LPS and various pathologic stimuli (11), and here, we showed that TonEBP expression was increased by PA treatment (Fig. 4A and Supplementary Fig. 4B). Therefore, we investigated transcriptional regulation of TonEBP in response to LPS and PA, a potent agonist of TLR4. We constructed a pGL3 luciferase reporter vector by inserting a 5 kb promoter sequence (−4,591/409) of the human TonEBP gene. Murine RAW264.7 and human THP-1 macrophages transfected with this reporter construct exhibited luciferase activity, which was stimulated by LPS and PA (Fig. 5A and Supplementary Fig. 5A). To define DNA regions responsible for stimulation of TonEBP, we generated serial deletion constructs (Fig. 5B). RAW264.7 cells transfected with a construct in which the sequence between −1,931 and −1,873 (D4) was deleted did not exhibit LPS-stimulated luciferase activity (Fig. 5C). Database analysis of the sequences in this region revealed similarity to a nonconsensus recognition sequence (−1,896 to −1,887) of the transcription factor Sp1 that is remarkably conserved in human and mouse (Fig. 5D). Therefore, we investigated whether Sp1 is required for induction of TonEBP in response to LPS and PA. The mRNA expression of Sp1 was increased by treatment with LPS (1 ng/mL) for 18 h but not by PA (0.2 and 0.3 mmol/L) (Supplementary Fig. 5B). Notably, Sp1 knockdown reduced LPS-induced TonEBP promoter–driven luciferase expression without influencing luciferase activity under unstimulated conditions (Fig. 5E). Consistently, Sp1 knockdown decreased TonEBP expression induced by LPS (Fig. 5F). Similarly, LPS-induced TonEBP expression was reduced by the selective Sp1-DNA-binding inhibitor mithramycin A in THP-1–derived macrophages (Fig. 5G). Mithramycin A also reduced PA-induced mRNA expression of TonEBP in RAW264.7 cells (Supplementary Fig. 5C).
Figure 5.
Sp1 is a central mediator of TonEBP induction in response to TLR4 stimulation in macrophages. A: RAW264.7 cells were transfected with the human (h)TonEBP promoter–luciferase construct containing the −4,591 to 409 region or the pGL3 basic vector. Luciferase activity was measured after LPS (8 h) or PA (10 h) treatment as indicated (n = 4). B: The TonEBP promoter-luciferase construct containing the −4,591 to 409 region of the human TonEBP gene and a series of 5′ promoter deletion mutants. “Sp1” denotes the predicted Sp1 binding sequence. C: RAW264.7 cells were transfected with each of the 5′ deletion mutants of the TonEBP promoter construct. Luciferase activity was measured 8 h after treatment with vehicle (-) or 1 ng/mL LPS (n = 3). D: Nucleotide sequence alignment of the promoters of the human and mouse TonEBP genes (National Center for Biotechnology Information [NCBI], Basic Local Alignment Search Tool [BLAST], and alignment tools). The conserved predicted Sp1 binding sites are shown in boldface type. E and F: RAW264.7 cells were transfected with Scr or Sp1-targeted siRNA. E: siRNA-transfected cells were transfected with the −2,182/409 promoter construct including the Sp1 binding site. Luciferase activity was measured 8 h after treatment with 0, 1, or 10 ng/mL LPS (n = 3). F: siRNA-transfected RAW264.7 cells were treated with vehicle (-) or LPS (1 ng/mL) for 18 h (for protein) or 6 h (for RNA). Representative immunoblots of TonEBP, Sp1, and Hsc70 (top), and TonEBP mRNA levels (bottom) (n = 3). G: THP-1–derived macrophages were treated with vehicle (-) or mithramycin A (Mith) (0.1 and 0.25 mmol/L) for 1 h followed by vehicle (-) or LPS (0.1 ng/mL) for 18 h (for protein) or 6 h (for RNA). Representative immunoblots of TonEBP, Sp1, and Hsc70 (top) and TonEBP mRNA levels (bottom) (n = 3). H: The TonEBP promoter–luciferase construct containing the −2,182 to 409 region of the human TonEBP gene (Sp1 WT) and the mutant −2,182/409 construct in which the Sp1 site was deleted (mt1) or mutated (mt2). I: RAW264.7 cells were transfected with the −2,182/409 promoter construct and Sp1-WT, -mt1, or -mt2. Luciferase activity was measured after treatment with vehicle (-) or LPS (1 ng/mL) for 8 h (n = 3). J: THP-1–derived macrophages were pretreated for 1 h (−1 h) with vehicle (-) or mithramycin A (200 μmol/L), and then treated with vehicle (-) or LPS (1 ng/mL) for 3 h. ChIP analysis of Sp1 and normal rabbit IgG on the TonEBP promoter was performed as described in Research Design and Methods. Two primer sets were designed for ChIP quantitative PCR at the predicted Sp1 binding site region of the TonEBP promoter. We calculated relative occupancy by performing quantitative PCR analysis and normalizing the CT values with input controls. All data are presented as mean ± SD (n = 3). n represents the number of independent experiments with more than three replicates. P values were determined with ANOVA with Tukey post hoc test. #P < 0.05 vs. Scr siRNA; LPS (-) (E–G) or IgG (J). *P < 0.05. AU, arbitrary units; n.s., not significant; Veh, vehicle.
To investigate whether reduction of TonEBP expression on Sp1 knockdown was dependent on the Sp1 binding sequence in the promoter, we constructed Sp1-deleted (mt1) and -substituted (mt2) mutants (Fig. 5H). Neither mutant exhibited LPS-induced transcriptional activity (Fig. 5I), confirming that the Sp1 binding site functions in LPS-induced TonEBP expression. Next, to investigate whether Sp1 binds to the Sp1 binding site in the TonEBP promoter, we performed ChIP quantitative PCR in THP-1 macrophages (Fig. 5J). When DNA was precipitated from THP-1 macrophages with use of an anti-Sp1 antibody, fragments of the TonEBP promoter containing the Sp1 binding sequence detected with two primer sets (A and B) were enriched in LPS-treated cells, and this enrichment was reduced by mithramycin A (Fig. 5J). Taken together, these data demonstrate that Sp1 binds to the Sp1 binding site in the TonEBP promoter and thereby induces TLR4-mediated TonEBP expression.
TonEBP mRNA Expression Level in PBMCs Is Positively Correlated With Blood Glucose Levels in Mice and Humans
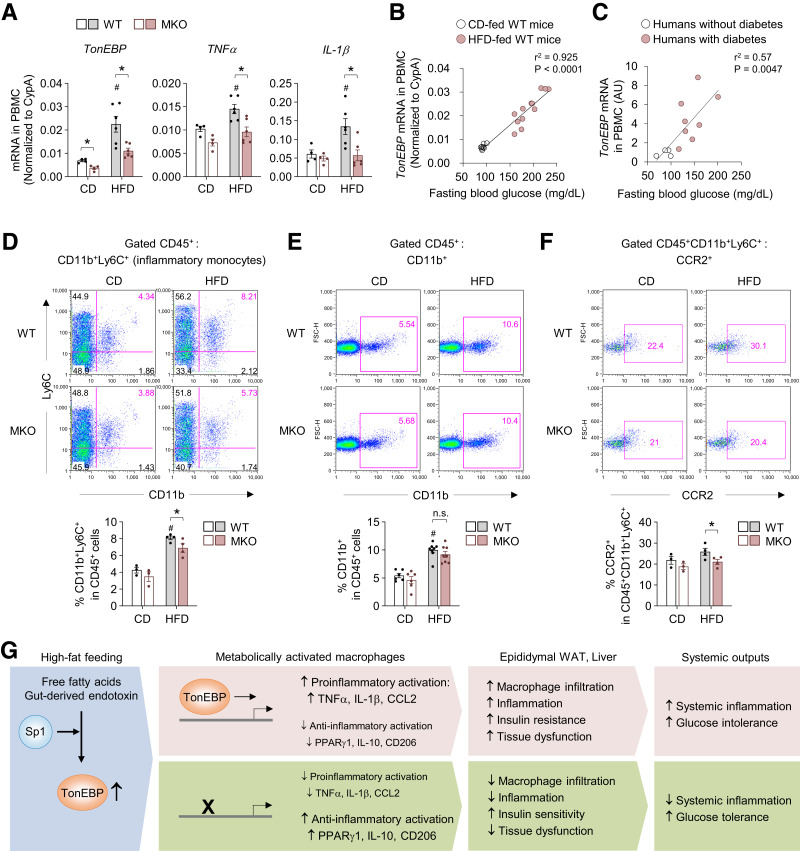
PBMCs from subjects with chronic inflammatory diseases are proinflammatory and release inflammatory cytokines and chemoattractant mediators into the systemic circulation (37,38). Therefore, we investigated mRNA expression of TonEBP in PBMCs. Feeding an HFD increased mRNA expression of TonEBP, TNFα, and IL-1β in PBMCs from WT mice, but this effect was significantly attenuated in PBMCs from MKO mice (Fig. 6A). Notably, fasting blood glucose levels were positively correlated with mRNA levels of TonEBP, TNFα, and IL-1β in PMBCs (Fig. 6B and Supplementary Fig. 6A). In addition, there was a significant association between the mRNA levels of TonEBP and TNFα (Supplementary Fig. 6B). More importantly, mRNA expression of TonEBP was higher in PBMCs from patients with diabetes than from subjects without diabetes and was positively correlated with fasting blood glucose levels (Fig. 6C and Supplementary Table 3). These data suggest that TonEBP plays a critical role in the proinflammatory state of PBMCs and that variability of TonEBP expression in PBMCs can affect this state in patients with diabetes and diabetic mice.
Figure 6.
The TonEBP mRNA expression level in PBMCs is positively correlated with blood glucose levels in mice and humans. A: mRNA levels of TonEBP, TNFα, and IL-1β in PBMCs from WT and MKO mice fed CD (n = 4) or HFD (n = 5) for 12 weeks. B: Correlation between fasting blood glucose levels and mRNA expression of TonEBP in PBMCs from WT mice fed CD (n = 8) and HFD (n = 12) for 12 weeks. C: Correlation betwseen fasting blood glucose levels and mRNA expression of TonEBP in PBMCs from individuals without diabetes (n = 4) and with diabetes (n = 8). D–F: Representative flow cytometry plots (top) and quantification (bottom) of myeloid cells (CD45+CD11b+) (CD, n = 6; HFD, n = 8) (D), inflammatory monocytes (CD45+CD11b+Ly6C+) (CD, n = 3; HFD, n = 4) (E), and CCR2 expression on inflammatory monocytes (CD, n = 3; HFD, n = 4) (F) in PBMCs from WT and MKO mice fed CD or HFD for 12 weeks. G: Proposed model for the role of macrophage TonEBP in development of systemic insulin resistance and inflammation during obesity. n represents the number of biologically independent samples. Correlations were assessed with nonparametric Spearman test. All data are presented as mean ± SEM. P values were determined with ANOVA with Tukey post hoc test. #P < 0.05 vs. CD; *P < 0.05. AU, arbitrary units; FSC-H, forward scatter-height; n.s., not significant.
Obesity-induced inflammation and metabolic dysfunction in humans and mice are associated with increased numbers of circulating inflammatory monocytes (39,40). Therefore, we examined blood inflammatory CD11b+Ly6C+ monocytes in mice, which are considered the counterpart of human inflammatory monocytes (5). Whereas MKO mice had a similar percentage of total myeloid cells (CD11b+) compared with WT mice (Fig. 6D), TonEBP deficiency in myeloid cells attenuated the increase of circulating inflammatory monocytes induced by HFD (Fig. 6E). More importantly, TonEBP deficiency reduced surface expression of CCR2, a chemokine receptor that mediates migration of monocytes and is highly expressed by inflammatory monocytes (Fig. 6F and Supplementary Fig. 6C and D). Additionally, deficiency of TonEBP in myeloid cells did not affect the populations of monocyte subsets (Supplementary Fig. 7A–C) among cells isolated from BM. Collectively, these data raise the possibility that TonEBP contribute to the differentiation of circulating inflammatory monocytes and their migration capacity via CCR2-CCL2 signaling during obesity.
Discussion
In adipocytes, TonEBP suppresses white adipocyte beiging by repressing thermogenesis. Accordingly, mice lacking TonEBP specifically in adipocytes exhibit reduced weight gain and insulin resistance (41). Results of the current study demonstrated that mice lacking TonEBP in myeloid cells show reduced inflammation and insulin resistance, even in the absence of changes in weight gain and adiposity (Fig. 6G). These findings suggest that TonEBP affects metabolic physiology in at least two cell types, namely, adipocytes and macrophages. Adipose tissue is a bona fide endocrine organ that regulates systemic metabolic homeostasis and plays a crucial role in metabolic homeostasis and dysfunction through cross talk between cell types and organs. TonEBP depletion in myeloid cells also has beneficial effects on obesity-induced hepatic dysfunction. Given the important role of islet-associated macrophages in T2D (42), it is possible that TonEBP influences the obesity-associated islet inflammation and β-cell dysfunction. Thus, we suggest that TonEBP represents a key node in this cross talk by regulating multiple aspects of the physiological response to metabolic stimuli and obesity in metabolic tissues.
Increasing evidence highlights the importance of cross talk between ATMs and other immune cells in shaping the immunometabolic profile of the adipose tissue contributing to inflammation and insulin resistance during obesity. While ATMs of obese mice trigger the reduction of Tregs (43,44), which is responsible for improvement of insulin sensitivity and reduced inflammation in obese humans and mice (45), they promote the accumulation of mast cells and CD8+ effector T cells, which leads to macrophage recruitment into adipose tissue (46,47) and inflammation and insulin resistance in obesity (48,49). Thus, the complex interplay between ATMs and other immune cells might also have contributed to the beneficial effect in HFD-feeding MKO mice.
PBMCs are implicated in the mechanisms linking immune-inflammation to the modulation of chronic disease development (50). Hence, these cells may be a new source of noninvasive diagnostic and prognostic biomarkers. Hyperglycemia functions as a crucial driving force of these processes by modulating the response of PBMCs (51). Increased TonEBP activity in monocytes is associated with early diabetic nephropathy in humans (52). Data from various human cohorts also indicate that single nucleotide polymorphisms of TonEBP are associated with inflammation, diabetic nephropathy, and the risk of T2D (11). Importantly, our finding that mRNA levels of TonEBP are elevated in PMBCs of patients with diabetes and mice with HFD-induced diabetes supports these previous findings. TonEBP mRNA expression in PMBCs was positively correlated with blood glucose levels in human and mice. In addition, there was a strong association between TonEBP and TNFα mRNA levels in PMBCs of obese mice, suggesting that TonEBP plays a critical role in the proinflammatory state of PBMCs and that variability of TonEBP expression can affect this state. Therefore, targeting TonEBP levels in PBMCs is potentially a new therapeutic strategy to counteract metabolic diseases such as T2D, and these levels are also potentially an additional biomarker to predict outcomes.
Obese patients have increased FFA levels in blood (32,53). Elevated saturated fatty acids, particularly PA, lead to inflammatory responses, which are an important risk factor for the onset of obesity-associated metabolic disorders (35,54). In adipose tissue, macrophages are surrounded by adipocytes, which constantly release FFAs. PA and its metabolites promote potent metabolic inflammation in macrophages via various signaling pathways (54). Here, we show that TonEBP depletion in human and mouse macrophages decreases induction of proinflammatory gene expression and cytokine secretion but promotes expression of genes related to anti-inflammatory macrophages in response to PA and thereby improves insulin signaling and glucose uptake in adipocytes. In addition, PA can induce and enhance inflammatory reactions via distinct mechanisms, including endoplasmic reticulum stress. Future studies should investigate the impact of TonEBP on various PA-mediated cellular events, including endoplasmic reticulum stress. Furthermore, the molecular mechanism by which TonEBP regulates PA-mediated cellular events involved in MMe macrophages remains to be determined.
Many pathological conditions and agents induce TonEBP gene expression via TLR4 stimulation, and increased expression of TonEBP promotes its homeostatic and pathologic functions (11). In the current study, we provide evidence that the transcription factor Sp1 is a crucial mediator of transcriptional regulation of the TonEBP gene induced by TLR4 activation (Fig. 5). This is interesting in view of the previous finding that Sp1 and TonEBP play distinct roles in expression of pro- and anti-inflammatory genes. First, TonEBP and Sp1 have opposite roles in transcriptional regulation of IL-10, a potent anti-inflammatory and immunosuppressive molecule in macrophages. Sp1 is a major transcription factor involved in LPS-mediated induction of IL-10 gene expression and is recruited to a putative binding site in the promoter region in mouse and human macrophages (55). By contrast, TonEBP suppresses LPS-mediated transactivation of the IL-10 gene. Interestingly, TonEBP suppresses transcription of the IL-10 gene by reducing chromatin accessibility and thus recruitment of Sp1 to its promoter (23). The current study and these previous findings suggest there is a previously unrecognized bidirectional regulatory loop between Sp1 and TonEBP in the context of LPS-mediated IL-10 expression. Specifically, Sp1 activates TonEBP transcription in response to LPS. TonEBP, in turn, negatively regulates recruitment of Sp1 to the IL-10 promoter and thereby abrogates the capacity of Sp1 to stimulate IL-10 transcription. Thus, the reciprocal actions of Sp1 and TonEBP are an important aspect of regulation of IL-10 expression. Second, TonEBP and Sp1 are important positive regulators of NF-kB p65, which is a central signaling hub in inflammatory responses. Sp1 facilitates p65 binding to promoters and promotes expression of its proinflammatory target genes, such as Ccl2 (56). Similarly, TonEBP promotes transcriptional activity of NF-κB via interaction with p65 and expression of its proinflammatory target genes (29). This regulatory relationship between Sp1 and TonEBP has functional implications for counteracting the anti-inflammatory role of Sp1 and stimulating the proinflammatory role of TonEBP and Sp1, thereby promoting proinflammatory activation of macrophages. These results demonstrate a mechanism that may help to explain the differential regulation of pro- and anti-inflammatory genes. Further investigations of cross talk between TonEBP, Sp1, and NF-κB in macrophages will likely provide important insights into the mechanisms underlying pro- and anti-inflammatory polarization, providing leads for therapeutic targeting of this cross talk in inflammation biology.
In summary, our findings reveal that macrophage TonEBP is a crucial driver of obesity-associated inflammation and insulin resistance. Depletion of TonEBP profoundly alters the ratio of proinflammatory to anti-inflammatory MMe macrophages and reduces macrophage accumulation in adipose tissue and the liver. Considering the key role of macrophages in adipose tissue function, targeted modulation of TonEBP activity in ATMs might open up new avenues for inducing healthy adipose tissue remodeling to prevent progression of obesity-associated morbidities.
Article Information
Acknowledgments. The authors thank Dr. Wolfgang Neuhofer (Department of Nephrology, Helios Klinikum Erfurt, Erfurt, Germany) for generously providing TonEBPfl/fl mice.
Funding. This research was funded by National Research Foundation grants (NRF-2019R1A2C1089260, NRF-2018R1A5A1024340, and NRF-2017R1E1A1A01074673) of Korea. This work was also supported by Ulsan National Institute of Science and Technology funds (1.200037.01).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. H.H.L., G.W.J., B.J.Y., H.M.K., and S.Y.C. made substantial contributions to study conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and revisions of the article critical for important intellectual content and provided the final approval of the version to be submitted. E.J.Y., K.S.S., D.K.K., H.-K.P., B.H.K., and W.L.-K. made substantial contributions to acquisition of data and the analysis and interpretation of data. All authors read and agreed to the published version of the manuscript. H.M.K. and S.Y.C. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.21170848.
H.H.L., G.W.J., and B.J.Y. contributed equally.
References
- 1. Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867 [DOI] [PubMed] [Google Scholar]
- 2. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011;121:2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des 2008;14:1225–1230 [DOI] [PubMed] [Google Scholar]
- 5. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005;5:953–964 [DOI] [PubMed] [Google Scholar]
- 6. Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014;41:14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes 2012;61:346–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kratz M, Coats BR, Hisert KB, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab 2014;20:614–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coats BR, Schoenfelt KQ, Barbosa-Lorenzi VC, et al. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep 2017;20:3149–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi SY, Lee-Kwon W, Kwon HM. The evolving role of TonEBP as an immunometabolic stress protein. Nat Rev Nephrol 2020;16:352–364 [DOI] [PubMed] [Google Scholar]
- 12. Küper C, Beck FX, Neuhofer W. Generation of a conditional knockout allele for the NFAT5 gene in mice. Front Physiol 2015;5:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weischenfeldt J, Porse B. Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protoc 2008;2008:pdb.prot5080 [DOI] [PubMed] [Google Scholar]
- 14. Troegeler A, Lastrucci C, Duval C, et al. An efficient siRNA-mediated gene silencing in primary human monocytes, dendritic cells and macrophages. Immunol Cell Biol 2014;92:699–708 [DOI] [PubMed] [Google Scholar]
- 15. Mahmood T, Yang PC. Western blot: technique, theory, and trouble shooting. N Am J Med Sci 2012;4:429–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jung DW, Ha HH, Zheng X, Chang YT, Williams DR. Novel use of fluorescent glucose analogues to identify a new class of triazine-based insulin mimetics possessing useful secondary effects. Mol Biosyst 2011;7:346–358 [DOI] [PubMed] [Google Scholar]
- 17. Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol 2011;11:738–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest 2008;118:829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alisi A, Carpino G, Oliveira FL, Panera N, Nobili V, Gaudio E. The role of tissue macrophage-mediated inflammation on NAFLD pathogenesis and its clinical implications. Mediators Inflamm 2017;2017:8162421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Strissel KJ, Stancheva Z, Miyoshi H, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007;56:2910–2918 [DOI] [PubMed] [Google Scholar]
- 21. Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 2006;177:7303–7311 [DOI] [PubMed] [Google Scholar]
- 22. Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007;447:1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi SY, Lee HH, Lee JH, et al. TonEBP suppresses IL-10-mediated immunomodulation. Sci Rep 2016;6:25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang JT, Welch JS, Ricote M, et al. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature 1999;400:378–382 [DOI] [PubMed] [Google Scholar]
- 25. Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 2011;11:750–761 [DOI] [PubMed] [Google Scholar]
- 26. Lee JH, Lee HH, Ye BJ, Lee-Kwon W, Choi SY, Kwon HM. TonEBP suppresses adipogenesis and insulin sensitivity by blocking epigenetic transition of PPARγ2. Sci Rep 2015;5:10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu Y, Qi C, Korenberg JR, et al. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc Natl Acad Sci U S A 1995;92:7921–7925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med 2008;14:222–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee HH, Sanada S, An SM, et al. LPS-induced NFκB enhanceosome requires TonEBP/NFAT5 without DNA binding. Sci Rep 2016;6:24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jeong GW, Lee HH, Lee-Kwon W, Kwon HM. Microglial TonEBP mediates LPS-induced inflammation and memory loss as transcriptional cofactor for NF-κB and AP-1. J Neuroinflammation 2020;17:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Al-Attas OS, Al-Daghri NM, Al-Rubeaan K, et al. Changes in endotoxin levels in T2DM subjects on anti-diabetic therapies. Cardiovasc Diabetol 2009;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mook S, Halkes Cj Cj, Bilecen S, Cabezas MC. In vivo regulation of plasma free fatty acids in insulin resistance. Metabolism 2004;53:1197–1201 [DOI] [PubMed] [Google Scholar]
- 33. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–1772 [DOI] [PubMed] [Google Scholar]
- 34. Lam TK, van de Werve G, Giacca A. Free fatty acids increase basal hepatic glucose production and induce hepatic insulin resistance at different sites. Am J Physiol Endocrinol Metab 2003;284:E281–E290 [DOI] [PubMed] [Google Scholar]
- 35. Khodabandehloo H, Gorgani-Firuzjaee S, Panahi G, Meshkani R. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl Res 2016;167:228–256 [DOI] [PubMed] [Google Scholar]
- 36. Lauterbach MA, Wunderlich FT. Macrophage function in obesity-induced inflammation and insulin resistance. Pflugers Arch 2017;469:385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sirota P, Hadi E, Djaldetti M, Bessler H. Difference in inflammatory cytokine production by mononuclear cells from obese and non-obese schizophrenic patients. Acta Psychiatr Scand 2015;132:301–305 [DOI] [PubMed] [Google Scholar]
- 38. Akhter N, Madhoun A, Arefanian H, et al. Oxidative stress induces expression of the Toll-like receptors (TLRs) 2 and 4 in the human peripheral blood mononuclear cells: implications for metabolic inflammation. Cell Physiol Biochem 2019;53:1–18 [DOI] [PubMed] [Google Scholar]
- 39. Friedrich K, Sommer M, Strobel S, et al. Perturbation of the monocyte compartment in human obesity. Front Immunol 2019;10:1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takahashi K, Mizuarai S, Araki H, et al. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J Biol Chem 2003;278:46654–46660 [DOI] [PubMed] [Google Scholar]
- 41. Lee HH, An SM, Ye BJ, et al. TonEBP/NFAT5 promotes obesity and insulin resistance by epigenetic suppression of white adipose tissue beiging. Nat Commun 2019;10:3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ying W, Fu W, Lee YS, Olefsky JM. The role of macrophages in obesity-associated islet inflammation and β-cell abnormalities. Nat Rev Endocrinol 2020;16:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cipolletta D, Feuerer M, Li A, et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012;486:549–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Onodera T, Fukuhara A, Jang MH, et al. Adipose tissue macrophages induce PPARγ-high FOXP3(+) regulatory T cells. Sci Rep 2015;5:16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eller K, Kirsch A, Wolf AM, et al. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes 2011;60:2954–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med 2009;15:940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009;15:914–920 [DOI] [PubMed] [Google Scholar]
- 48. Divoux A, Moutel S, Poitou C, et al. Mast cells in human adipose tissue: link with morbid obesity, inflammatory status, and diabetes. J Clin Endocrinol Metab 2012;97:E1677–E1685 [DOI] [PubMed] [Google Scholar]
- 49. Gurung P, Moussa K, Adams-Huet B, Devaraj S, Jialal I. Increased mast cell abundance in adipose tissue of metabolic syndrome: relevance to the proinflammatory state and increased adipose tissue fibrosis. Am J Physiol Endocrinol Metab 2019;316:E504–E509 [DOI] [PubMed] [Google Scholar]
- 50. van Dijk SJ, Feskens EJ, Bos MB, et al. Consumption of a high monounsaturated fat diet reduces oxidative phosphorylation gene expression in peripheral blood mononuclear cells of abdominally overweight men and women. J Nutr 2012;142:1219–1225 [DOI] [PubMed] [Google Scholar]
- 51. Schiekofer S, Andrassy M, Chen J, et al. Acute hyperglycemia causes intracellular formation of CML and activation of ras, p42/44 MAPK, and nuclear factor kappaB in PBMCs. Diabetes 2003;52:621–633 [DOI] [PubMed] [Google Scholar]
- 52. Yang B, Hodgkinson AD, Oates PJ, Kwon HM, Millward BA, Demaine AG. Elevated activity of transcription factor nuclear factor of activated T-cells 5 (NFAT5) and diabetic nephropathy. Diabetes 2006;55:1450–1455 [DOI] [PubMed] [Google Scholar]
- 53. Pankow JS, Duncan BB, Schmidt MI, et al.; Atherosclerosis Risk in Communities Study . Fasting plasma free fatty acids and risk of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes Care 2004;27:77–82 [DOI] [PubMed] [Google Scholar]
- 54. Korbecki J, Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm Res 2019;68:915–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ma W, Lim W, Gee K, et al. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J Biol Chem 2001;276:13664–13674 [DOI] [PubMed] [Google Scholar]
- 56. Boekhoudt GH, Guo Z, Beresford GW, Boss JM. Communication between NF-kappa B and Sp1 controls histone acetylation within the proximal promoter of the monocyte chemoattractant protein 1 gene. J Immunol 2003;170:4139–4147 [DOI] [PubMed] [Google Scholar]