Abstract
Despite a growing amount of data around the kinetics and durability of the antibody response induced by vaccination and previous infection, there is little understanding of whether or not a given quantitative level of antibodies correlates to protection against SARS-CoV-2 infection or reinfection. In this study, we examine SARS-CoV-2 anti-spike receptor binding domain (RBD) antibody titers and subsequent SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) tests in a large cohort of US-based patients. We analyzed antibody test results in a cohort of 22,204 individuals, 6.8% (n = 1,509) of whom eventually tested positive for SARS-CoV-2 RNA, suggesting infection or reinfection. Kaplan-Meier curves were plotted to understand the effect of various levels of anti-spike RBD antibody titers (classified into discrete ranges) on subsequent RT-PCR positivity rates. Statistical analyses included fitting a Cox proportional hazards model to estimate the age-, sex- and exposure-adjusted hazard ratios for S antibody titer, using zip-code positivity rates by week as a proxy for COVID-19 exposure. It was found that the best models of the temporally associated infection risk were those based on log antibody titer level (HR = 0.836 (p < 0.05)). When titers were binned, the hazard ratio associated with antibody titer >250 Binding Antibody Units (BAU) was 0.27 (p < 0.05, 95% CI [0.18, 0.41]), while the hazard ratio associated with previous infection was 0.20 (p < 0.05, 95% CI [0.10, 0.39]). Fisher exact odds ratio (OR) for Ab titers <250 BAU showed OR = 2.84 (p < 0.05; 95% CI: [2.30, 3.53]) for predicting the outcome of a subsequent PCR test. Antibody titer levels correlate with protection against subsequent SARS-CoV-2 infection or reinfection when examining a cohort of real-world patients who had the spike RBD antibody assay performed.
Keywords: COVID-19, SARS-CoV-2, Antibody, Spike, Nucleocapsid, Seroprevalence
Abbreviations: AUC, Area Under the Curve; BAU, Binding Antibody Units; HR, Hazard Ratio; IQR, Interquartile Range; OR, Odds Ratio; ROC, Receiver operating characteristic; RBD, Receptor Binding Domain; RT-PCR, Reverse Transcription Polymerase Chain Reaction
1. Introduction
As the COVID-19 pandemic persists, the number of vaccinated or previously infected individuals having antibodies to SARS-CoV-2 continues to grow. However, durability of the protection those antibodies provide against further SARS-CoV-2 infection is unclear, with some evidence suggesting waning protection over time [1,2], especially for immunocompromised individuals who are shown to produce lower amounts of antibodies following vaccination [3]. Previous studies have identified long-term presence of antibodies to the Spike protein of SARS-CoV-2 [4], but their quantitative levels and corresponding protection from the virus and other influencing factors are still being investigated [5]. Some evidence has shown that breakthrough infections are possible in those with low levels of antibodies [6], and although it is possible that no specific level of antibodies serves as a precise correlate of immunity, predictive modeling has shown that neutralization titers are highly predictive of immune protection [7]. Additionally, debate over the degree of protection conferred by immunity from previous infection compared to vaccination continues, with some suggesting that a combination of both could provide more robust protection [8]. However, previous research has shown the relationship between CD-4 T cell immunity after vaccine [9].
Here, we present results from an investigation using real-world data from a national diagnostic laboratory that provides evidence of antibody protection and persistence. Utilizing semi-quantitative antibody assays to the Spike (S) protein of SARS-CoV-2, we investigated the rate of infections and reinfections over time (determined by reverse transcription polymerase chain reaction (RT-PCR) testing) as a function of previous antibody levels, in hopes of identifying a threshold of protection. We hypothesize that any level of antibody presence will provide some protection from COVID-19 infection, and that this protection increases with higher titer and may be affected by previous infection.
2. Methods
2.1. Analysis plan
The study protocol included a retrospective, real-world data study using a population cohort of individuals tested by Labcorp®. Inclusion criteria required reported results for two Labcorp® tests: the SARS-CoV-2 Semi-quantitative spike antibody assay and followed by at least one SARS-CoV-2 RT-PCR test at least one month after the antibody test.
2.2. Data
The study used data from Labcorp®’s clinical testing operations, carried out at regional labs across the US. An IRB waiver was obtained to utilize the data in a deidentified manner for epidemiological research purposes. The data is maintained in a data warehouse and accessed by statisticians for analysis.
The study cohort was defined as all individuals who had a SARS-CoV-2 antibody result and a subsequent RT-PCR test. Patients of all ages and sexes were included who had a semi-quantitative antibody result between December 1, 2020, and August 30, 2021, with any RT-PCR test through August 30, 2021. No special consideration was made for the variant type of the follow-up RT-PCR test. Anti-S antibodies were detected, and antibody levels determined using a commercially available quantitative electrochemiluminescence immunoassay (Roche, Elecsys® Anti-SARS-CoV-2 S) that detects antibodies specific to the spike RBD (including, but not specific for, IgM, IgG, and IgA class) using recombinant spike proteins in a double-antigen sandwich assay format. The anti-S assay received Federal Drug Administration (FDA) Emergency Use Authorization (EUA) as a semi-quantitative assay prior to the development of an international standard for anti-SARS-CoV-2 immunoglobulin and as such, results reported as U/mL cannot be directly correlated to results generated by other assays. The manufacturer has since correlated results generated by this assay to the “binding antibody units” (BAU) of the first WHO International Standard for anti-SARS-CoV-2 immunoglobulin and found that manufacturer specific U/mL of the Elecsys(R) Anti-SARS-CoV-2 S assay can be considered to be equivalent to the BAU/mL of the International Standard.
2.3. Covariates
Covariates included semi-quantitative antibody titer levels (BAU), follow-up RT-PCR results, geographic region, “pandemic exposure” (the weekly COVID-19 positivity rate for a patient’s zip code), age, sex and previously confirmed COVID-19 infection status as determined either by RT-PCR testing or qualitative nucleocapsid antibody testing performed at least 30 days before the semi-quantitative assay. Multiple derived covariates were computed, including binning antibody titers into ranges of 0–250 BAU and 250+ BAU (“low” and “high” antibody levels, respectively, although in some parts of the analysis a 0 BAU bin was included as well), and age groups into 0–65 and 65+ years, log-transformed antibody titers, and exposure. The 250 BAU binning was selected because the EUA for this test only permitted reporting results up to 250 BAU until April 2021, after which the limit was raised to 2500 BAU.
2.4. Outcomes
The outcomes measured were the qualitative result of the RT-PCR SARS-CoV-2 test, either detected (positive) or not detected (negative).
2.5. Statistical analyses
Cox proportional-hazards models were used to estimate the influence of the covariates on the time-to-event between the antibody and PCR tests. All models used only right-censoring with no stratification of covariates, and baseline hazards were calculated using Breslow’s method. No additional penalty or regularization was added. Models were ranked by partial AIC and the top model was chosen through those criteria. In addition, Kaplan-Meier curves were generated for various stratifications of antibody titer.
Secondary analyses were performed with careful consideration to omit time dependence (e.g. Fisher Exact test as opposed to a Cox Proportional Hazard model). If it is presumed that antibody levels are relatively stable over a period of several months, we are able to examine the effect of antibody titers on subsequent infection. Receiver operating characteristic curve (ROC) and corresponding Area Under the Curve (AUC) was calculated using the antibody titer as a threshold and the outcome being a negative RT-PCR test (i.e. higher titer resulting in lower likelihood of becoming infected). To assess the effect of binning the titers, Fisher Exact odds ratios (OR), p-values and corresponding confidence intervals were calculated using a two-by-two contingency table of low vs. high and antibody by PCR positive or negative.
Statistical analysis was performed using Python 3.7 in Amazon Web Services® Sagemaker® using the Data Science kernel. P-values were reported to p < 0.05.
3. Results
A total of 22,204 patients were studied in this cohort, where 6.8% (n = 1509) had a subsequent positive RT-PCR test (Table 1). The cohort included patients who had up to 320 days between their semi-quantitative antibody test and a subsequent RT-PCR test (median (IQR): 52 (86) days). The geographic distribution included patients from 48 states, the median (IQR) age was 46 (25) years and 63.7% of the cohort were female (n = 14,149). Of patients with a semi-quantitative test and subsequent RT-PCR testing, 9.2% (n = 2,048) had evidence of previous infection through either RT-PCR or evidence of positive nucleocapsid qualitative results.
Table 1.
Demographic summary about the cohort.
| All | No Breakthrough Infection | With Breakthrough Infection | p | |
|---|---|---|---|---|
| n | 22,204 | 20,695 | 1509 | |
| Female (%) | 14,149 (63.7%) | 13,263 (64.1%) | 886 (58.7%) | <0.0001 |
| Age, median (IQR) | 46 (25) | 47 (25) | 45 (22) | 0.0008 |
| Region | <0.0001 | |||
| Northeast | 9132 (41.1%) | 8677 (41.9%) | 455 (30.1%) | |
| South | 8034 (36.2%) | 7433 (35.9%) | 601 (39.8%) | |
| Midwest | 1205 (5.4%) | 1119 (5.4%) | 86 (5.7%) | |
| West | 3794 (17.1%) | 3430 (16.6%) | 364 (24.1%) | |
| Unknown | 39 (0.2%) | 36 (0.2%) | 3 (0.2%) | |
| Previous infection (%) | 2048 (9.2%) | 1973 (9.5%) | 75 (5.0%) | <0.001 |
| Ab Semi-quant Result (U/mL- BAU), median (IQR) | ||||
| With previous infection | 229 (556) | 232 (566) | 53 (145) | <0.0001 |
| Without previous infection | 149 (626) | 171 (668) | 0 (67) | <0.0001 |
| Days between Ab Semi-quant and follow-up PCR, median (IQR) | 52 (86) | 52 (85) | 42 (6) | <0.0001 |
| Number of follow-up PCR tests after Semi-quant, median (IQR) | 1 (1) | 1 (1) | 1 (1) | 0.0033 |
| Number of Semi-quant per patient, median (IQR) | 1 (0) | 1 (0) | 1 (0) | <0.0001 |
| COVID-19 Positivity Rate in Patient's Zip at Time of PCR Test, median (IQR) | 6.5% (7%) | 6.4% (7%) | 8.9% (9%) | <0.0001 |
3.1. Survival analysis – Kaplan Meier and Cox proportional hazard models
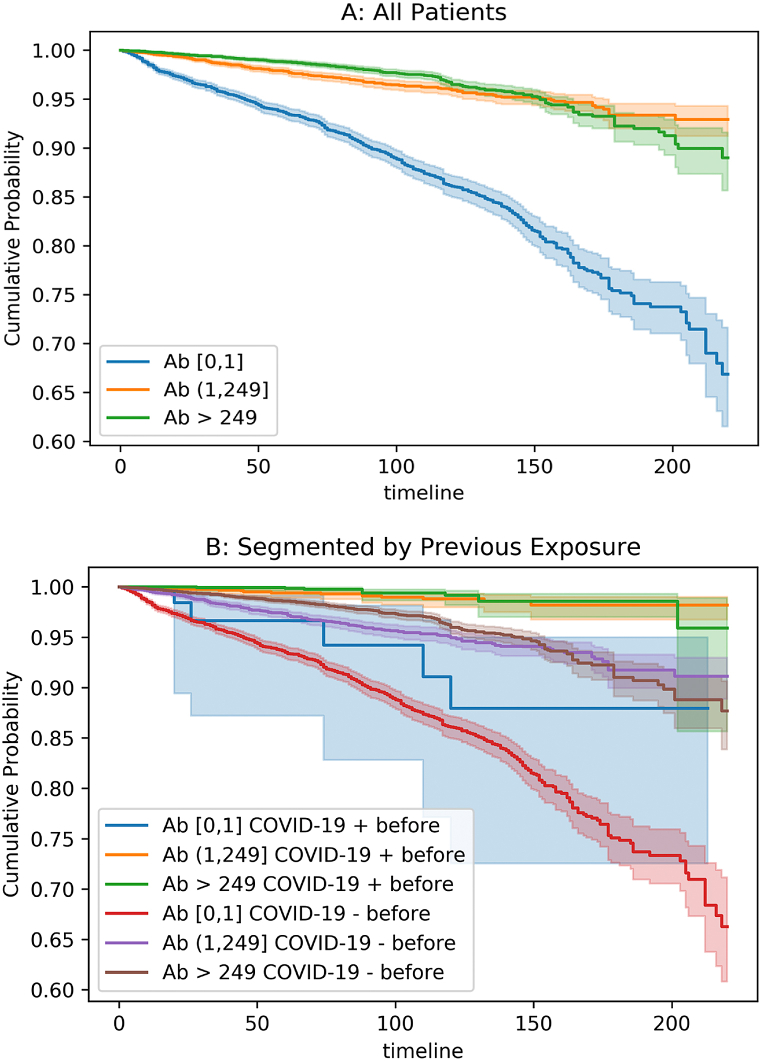
Kaplan Meier curves comparing threshold levels of semi-quantitative assay results identified censored cumulative probability for all patients, with highest cumulative probability of “survival,” or no infection over time, for anyone with some presence of antibodies (Fig. 1A). When stratified by previous infection status, those with confirmed previous COVID-19 infection and some level of antibody presence proved less likely to be re-infected than those without previous infection over time (Fig. 1B).
Fig. 1.
Kaplan-Meier curves showing time to positive RT-PCR tests, where negative RT-PCR tests indicate negativity, for all patients (A) and for patients categorized by previous positive (+) or negative (−) confirmed-COVID-19 before semi-quantitative anti-spike assay by RT-PCR or nucleocapsid assay (B). X-axis is days, y-axis is proportion of cohort who remains negative RT-PCR. Buckets are 0, 1–249, and 250+ representing spike antibody titer. A: All patients segmented by antibody titer level (Ab). B: All patients segmented by both antibody titer level (Ab) and evidence of previous infection through positive PCR test and/or Nucleocapsid (N) antibody test positivity.
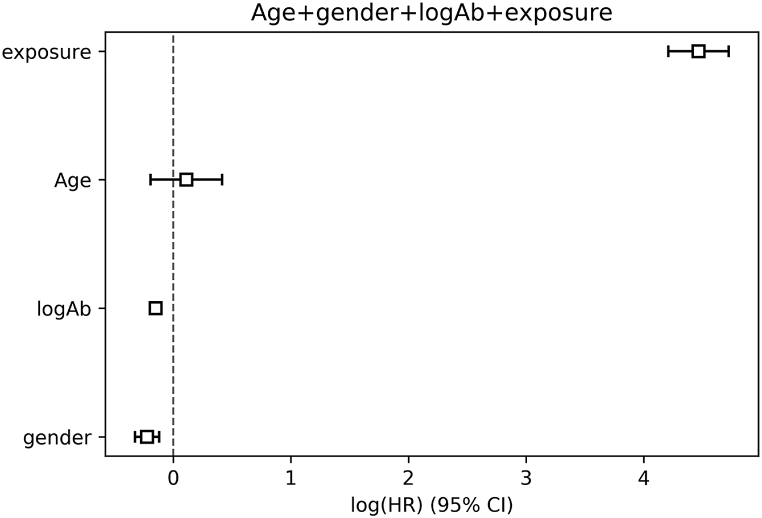
Survival analysis was conducted by using Cox proportional hazard models and analyzed for statistical significance at p < 0.05. Multiple candidate models were compared (Fig. 2, Figure S4 for alternative model), and the top model was one that included age, sex, threshold classification levels of antibodies, and exposure (positivity by week per patient geography). The coefficients for the hazard ratio analysis are shown in Fig. 2. The hazard ratio for log-transformed antibody titer is HR = 0.836 (p < 0.05); the HR for exposure is HR = 57.4 (p < 0.05).
Fig. 2.
Hazard Ratio Forest plot showing log-scaled hazards for top model candidate.
Examination of stratified antibody threshold levels identifies trends in infection or reinfection in one month, three-month and six-month intervals (Table 2). For those with no S antibodies present in the sera, 6.8% (n = 212) had an infection within a month, versus only 1.0% (n = 14) of those with at least 10–100 BAU titers and 1.1% (n = 29) of those with over 250 BAU titers. By six months, those same groups had 12.8% (n = 397), 1.9% (n = 27) and 1.9% (n = 51) infected, respectively, showing that even small presence of antibodies provides better protection through one to six months than none at all.
Table 2.
Infection rates over 1, 3 and 6 month intervals per antibody threshold levels.
| COVID-19 Semi-Quant. Ab (BAU) | Positive in 1 month | Positive in 3 months | Positive in 6 months |
|---|---|---|---|
| 0 | 212 (6.8%) | 310 (10.0%) | 397 (12.8%) |
| 0–10 | 11 (2.7%) | 19 (4.6%) | 26 (6.3%) |
| 11–100 | 14 (1.0%) | 23 (1.6%) | 27 (1.9%) |
| 101–249 | 12 (1.1%) | 19 (1.7%) | 25 (2.3%) |
| 250 | 5 (0.3%) | 9 (0.5%) | 32 (1.7%) |
| 251+ | 29 (1.1%) | 47 (1.7%) | 51 (1.9%) |
3.2. Receiver operating characteristic analysis & fisher exact odds ratios
Receiver operating curve for untransformed antibody titer levels result in an AUC of 0.7 (Figure S3). Antibody level is significantly associated with COVID-19 contraction, as determined by Fisher exact testing. Patients who had low antibody level showed higher risk of COVID-19 contraction than those of high antibody level (Odds Ratio (OR): 2.77; 95% Confidence Interval (CI) [2.23, 3.43]; p-value: <0.0001).
4. Discussion
4.1. Any antibody levels give protection, higher antibody levels give more protection
This study provides evidence that the presence of any detectable anti-S RBD antibodies provides some level of protection against infection or reinfection with SARS-CoV-2. The introduction of the Delta variant, and more recently the Omicron variant, brought the concept of reinfection and “breakthrough” infection (post-vaccine infection) to the forefront of public policy planning and made this research especially relevant to understanding the dynamics of semi-quantitative antibody titer levels on protective immunity.
We introduced multiple methods to study the effect of antibody levels on the subsequent risk of infection. All methods resulted in similar conclusions: having a titer level of 0 BAU, or no anti-S RBD antibodies, is associated with a dramatically higher risk of infection, while higher antibody levels afford a significant degree of protection from infection over time. According to the Cox Proportional Hazards model, for each unit increase of antibody level, there is reduction in risk by 0.86 (p < 0.05). Having an antibody titer over 250 BAU has a Boolean hazard ratio of 0.27 (p < 0.05, 95% CI [0.18, 0.41]), while the hazard ratio associated with previous infection was 0.20 (p < 0.05, 95% CI [0.10, 0.39]). This means a patient with an antibody titer under 250 BAU is about three times more likely to contract COVID-19 than somebody with a measurement over 250 BAU. A fixed-time analysis using a Fisher Exact test shows similar outcomes with an OR = 2.77 (95% CI: [2.23–3.43]; p < 0.05). The Kaplan-Meier curve shown in Fig. 1 supports these conclusions; age and sex were not found to be significant. Table 2 follows this analysis for further proof of trending infection over time, but also showing similar protection for any patient with titers >10 BAU and more limited protection for those with none.
We also note substantial differences in the demographics of patients found in Table 1, even comparing the breakthrough infection cohort to the no-previous infection cohort. For example, we observed more males and a slightly younger population in the cohort of patients with a previous infection. Further, the Northeast region of the US had a much higher frequency of patients in the no-previous infection cohort. These differences require further study, but the authors hypothesize that exposure risk likely is a major driver in the differences we observe (and thus why we included the exposure covariate in our Cox-PH models).
Further, the ROC curve (Figure S3) demonstrates the entire range of antibodies plotted against True Positive Rate (TPR) and False Positive Rate (FPR). Every point along the (antibody titer level) curve shows predictive power towards predicting persistent protection. This demonstrates that higher levels of antibody titer levels correspond to higher levels of protection from COVID-19. The AUC (area under the curve) of 0.70 is likely under-represented because of our study design choice (i.e. only including patients with a negative test as “uninfected” as opposed to untested).
4.2. Strengths and limitations
The dataset is incomplete in a number of ways: it does not include vaccination dates and types, if any, or clinical history of previous infection, if any. Our use of zip code positivity in a given week as a proxy for individual exposure may over- or underestimate an individual’s exposure.
A more serious limitation is the coarse-grained temporal sampling of both antibody titers and PCR-positivity. An ideal version of this study would sample both at high time resolution, whereas we required one of each type of test, in a particular order, and for the majority of patients that was all we had.
In addition, testing has at times been restricted by checklists that favored symptomatic patients, who are more likely to test positive. These restrictions can create an upward bias in both the exposure estimates and the estimated hazards. The exclusion of patients who never had a PCR test following an initial antibody test may similarly introduce an upward bias in estimated hazards, if those patients were less likely to be positive. However, we would not expect these biases to correlate with antibody titer, so the they may impact the magnitude, but not the direction or significance of the observed effects. It is also possible that a patient knowing their antibody titer level may change their overall exposure risk, in a way that we are not able to anticipate through this study.
We could not infer the temporal dynamics of the antibody titers from this dataset, and our Cox models implicitly assume no effect of passing time on titer. In addition, the start date for our time-to-event models, which is the time of the initial antibody test, can be considered arbitrary or left-censored, since it is not tied to a specific patient event, apart from the test itself. However, we argue that it cannot be completely arbitrary or the structure apparent in the Kaplan-Meier curves would not exist.
Furthermore, the anti-S RBD assay underwent changes in April, 2021 that increased its dynamic range: prior to that time, the maximum FDA approved quantitative titer value was 250 BAU, and it was subsequently moved to 2500 BAU. This means some of our titers are thresholded at different levels. The only antibody assay that had sufficient volume to conduct this study was IgG, even though IgM and IgA play a vital role in the dynamics of protection. Additionally, we could not look at other markers of protective immunity such as T-cell responses and other long-term immunity markers. The time period of this study limits our analysis to the original, Alpha, and Delta variants (the Omicron wave was not part of the study period).
Overall, the strengths of this research include the sheer size and geographic distribution of the testing patterns observed. Due to the large numbers of patients tested by Labcorp, thousands of results could be aggregated quickly to analyze how antibody titer correlates with positivity risk. Further, the findings of this research show that the semi-quantitative value can have utility in helping clinicians and patients understand their own level of protection. However, we do not believe that this paper supports any notion that antibodies from previous SARS-CoV-2 infection is a good substitute for immunity when other research has suggested that “hybrid immunity” is achieved by having both vaccination and previous infection [8] (and because higher antibody levels correlate to more protection, a previously infected patient’s best line of defense is to become vaccinated).
5. Conclusion
In this research, we examine the effects of antibody titer levels from a semi-quantitative assay performed by Labcorp and the effect on RT-PCR testing outcomes for SARS-CoV-2. The cohort consisted of 22,204 Labcorp patients with both a semi-quantitative antibody test and a subsequent RT-PCR test covering a wide-range of patient demographics from all across the United States, spanning all age ranges and sexes. A collection of statistical tests was performed, including Cox Proportional Hazard modeling, Fisher Exact test, Kaplan-Meier curve generation, and ROC analysis to examine how much a patient’s antibody titer level predicted protective status from infection (or reinfection/breakthrough infection). The results suggest that any increase in antibody levels provide an increasing level of protection, as shown in the Cox Proportional Hazard model to be HR = 0.27 (p < 0.05, 95% CI [0.18, 0.41]) while the hazard ratio associated with previous infection was HR = 0.20 (p < 0.05, 95% CI [0.10, 0.39]). The results can provide further evidence on the utility of semi-quantitative antibody results and provide more clinical interpretation of what a given level means. The results demonstrated here can shed light on the interpretation of exposure risk when measured with a spike protein antibody diagnostic assay, and perhaps can be a springboard for further research into clinicians and patients understanding when a patient needs to be vaccinated against SARS-CoV-2 in the future.
Author contribution statement
Adam Sullivan; David Alfego, Ph.D; Pingsha Hu, Ph.D: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Laura Gillim, Ph.D: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ajay Grover, Ph.D: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Chris Garcia, M.D: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Oren Cohen, M.D: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Stanley Letovsky, Ph.D: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
All authors are employees of Labcorp where this study was performed.
Data availability statement
The authors do not have permission to share data.
Declaration of interest’s statement
The authors declare no conflict of interest.
Data statement
The data presented here are deidentified but have requirements to protect patient privacy. Email alfegod@labcorp.com to request access to underlying data.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e13103.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Choe P.G., Kang C.K., Suh H.J., Jung J., Song K.H., Bang J.H., et al. Waning antibody responses in asymptomatic and symptomatic SARS-CoV-2 infection. Emerg. Infect. Dis. 2021;27(1):327. doi: 10.3201/eid2701.203515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altmann D.M., Boyton R.J. Waning immunity to SARS-CoV-2: implications for vaccine booster strategies. Lancet Respir. Med. 2021;9(12):1356–1358. doi: 10.1016/S2213-2600(21)00458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geisen U.M., Berner D.K., Tran F., Sümbül M., Vullriede L., Ciripoi M., et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann. Rheum. Dis. 2021;80(10):1306–1311. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfego D., Sullivan A., Poirier B., Williams J., Adcock D., Letovsky S. A population-based analysis of the longevity of SARS-CoV-2 antibody seropositivity in the United States. EClinicalMedicine. 2021:36. doi: 10.1016/j.eclinm.2021.100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacerda M.V.G., Bargieri D.Y. Protection against COVID-19: beyond antibodies. Lancet Infect. Dis. 2021;22(1):4–5. doi: 10.1016/S1473-3099(21)00561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., et al. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 8.Crotty S. Hybrid immunity. Science. 2021;372:1392–1393. [Google Scholar]
- 9.Esparcia-Pinedo L., Martínez-Fleta P., Ropero N., Vera-Tomé P., Reyburn H.T., Casasnovas J.M., et al. CD4+ T cell immune specificity changes after vaccination in healthy and COVID-19 convalescent subjects. Front. Immunol. 2022;12 doi: 10.3389/fimmu.2021.755891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.