Abstract
The continuously expanding distribution of sand flies, proven vectors of Leishmania and of several phleboviruses, is a growing public health issue in Europe. Especially in Italy, visceral leishmaniasis (VL) is occurring with increasing incidence northward, in previously non-endemic provinces. Around the globe, disease elimination efforts largely focus on sand fly vector insecticidal control, often leading to the development of resistance. In Emilia-Romagna (ER), northern Italy, insecticides are heavily applied for agricultural and mosquito control, but not specifically against sand flies. Here, we investigated the sand fly species composition in certain environmental settings in ER provinces and monitored the presence of pyrethroid resistance mutations and pathogen circulation. Phlebotomus perfiliewi, a dominant vector of Leishmania infantum, was detected almost exclusively in the region. No mutations in the voltage-gated sodium channel gene, e.g., knock-down resistance mutations I1011M, L1014F/S, V1016G, or F1020S, were recorded. Pathogen monitoring revealed that almost 40% of the tested sand fly pools were positive for Leishmania, while the presence of Toscana and Fermo phleboviruses was also observed in much lower frequencies (≤3% positive pools). Regular epidemiological and entomological monitoring, alongside resistance surveillance, is highly recommended to ensure the sustainability and efficiency of vector control interventions.
Keywords: Phlebotomus, Leishmania, phleboviruses, pyrethroid resistance, kdr mutations, molecular diagnostics
1. Introduction
Leishmaniasis is second only to malaria among the deadliest protozoan diseases globally. The prevalence of leishmaniasis in Europe, largely under-reported, counts for less than 2% of the global prevalence [1]. However, in the last decades, the re-emergence of vector-borne diseases has been witnessed across the continent, making leishmaniasis a growing public health concern, especially for Mediterranean countries [2,3].
In Italy, as of 1990 in particular, canine leishmaniasis, caused by Leishmania infantum (Kinetoplastida: Trypanosomatidae), has been expanding northward into previously non-endemic regions, with an approximately 10-fold increase in seroprevalence rates (2.1% to 21.6%) since 2009 [4,5]. Phlebotomus perniciosus and P. perfiliewi are considered the dominant sand fly (Diptera: Psychodidae) vector species in the country [6]. Sand flies’ broadening distribution patterns, mainly attributed to global warming, environmental modifications, and their remarkable ecological plasticity, facilitate pathogen circulation and the establishment of new endemic disease foci [4].
Interestingly, the impact of sand fly distribution on public health is not restricted to leishmaniasis transmission. Phlebotomines are additionally incriminated as vectors of a variety of phleboviruses (family: Phenuiviridae, genus: Phlebovirus), such as Toscana virus (TOSV; a causative agent of neuroinvasive infections in humans), sand fly Naples and Sicilian virus, Fermo virus (FERMV), etc. [7,8,9,10]. Even though the circulation of the aforementioned viruses in the Mediterranean basin is well-evident, their public health significance remains greatly disregarded, as scarce information exists on their epidemiology and transmission cycles [11].
In the absence of preventive human vaccines and safe therapeutic drugs against leishmaniasis, apart from case management (early detection and treatment) [12], sand fly control, either by utilizing synthetic insecticides or by managing environmental habitats, stands as the cornerstone of disease elimination efforts in many endemic countries [13]. Emilia-Romagna (ER; north-eastern Italy), a leishmaniasis endemic region, is extensively cultivated, with the annual usage of pesticides for agricultural purposes reaching approximately 1400 tons [14]. Besides this, given the touristic exploitation of the region, during the last decades, pyrethroid sprayings (principally deltamethrin and permethrin, according to the European Union directive 528/2012 [15]) have been implemented against mosquito species, such as Culex pipiens and Aedes albopictus, as part of regional vector control programs and/or household-level interventions [16,17]. Although, so far, no targeted sand fly insecticidal control program has been applied in the country, it is highly anticipated that sand fly populations in ER provinces have been focally exposed to insecticides in areas where leishmaniasis is co-endemic with mosquito-borne diseases and in those proximal to agricultural areas.
Insecticide resistance, often fostered by the prolonged and excessive use of insecticides, critically impedes chemical control interventions against pests of agricultural and medical importance [18]. Regarding sand flies, information in the literature on the response profile of wild populations to insecticides around the globe remains seriously limited. However, recent phenotypic data (bioassays) and molecular analyses (e.g., detection of knock-down resistance (kdr) mutations L1014F/S in the voltage-gated sodium channel (VGSC) gene related to loss of sensitivity to pyrethroids) of dominant sand fly vector species, wild populations originating from countries with the highest leishmaniasis burden in north-eastern Asia and the Middle East, revealed that insecticide resistance in sand flies poses an up-coming and alarming issue [19].
Hence, regular monitoring of local sand fly populations constitutes a required precondition of integrated control campaigns, especially when incipient resistance, which could be missed by bioassays, needs to be detected as early as possible. The objective of the present study was to compile surveillance data from sand fly field collections in three ER provinces regarding (1) the sand fly species composition; (2) the presence of known pyrethroid resistance molecular markers (e.g., target-site mutations I1011M, L1014F/S, V1016G and F1020S in the VGSC); and (3) the Leishmania load, as well as the possible sand fly-borne phleboviruses, in an epidemiologically relevant macroarea.
2. Materials and Methods
2.1. Sampling Areas, Sand Fly Collection, and Sample Handling
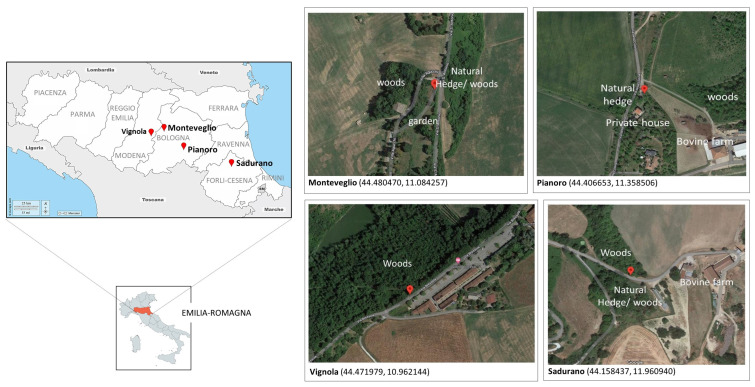
Multiple sand fly samplings were performed at four georeferenced sites in Vignola (Modena province; MO—1 sampling), Monteveglio and Pianoro (Bologna province; BO—8 samplings), and Sadurano (Forlì Cesena province; FC—1 sampling) in the Emilia-Romagna (ER) region between July and September 2021. Locations were selected in the interface between semi-natural environments (woods or hedges), agricultural areas/cultivated fields, and urbanized territories (streets, villas) (Figure 1; Table 1).
Table 1.
Sand fly sampling locations in ER, species composition, and monitoring of kdr mutations and pathogen presence.
| Location— Province (X, Y) |
Date | Environmental Settings | N | Species Composition (%) | kdr L1014F/S | Pathogen Detection | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n 1 | perfi | perni | n2 (p) | L (%) | P (n3) | Leish | TOSV | FERMV | ||||||
| Monteveglio—BO (44.480470, 11.084257) |
July 2021 | Abandoned villa in farmland, with sparse trees/shrubs, vineyards | 18,579 | 512 | 99.2 | 0.8 | 60 (12) | 100 | 59 (5462) |
24 | 0 | 1 | ||
| Pianoro—BO (44.406653, 11.358506) |
July 2021 | Inhabited villa in cattle pasture, sparse trees, grassland | 12,349 | 852 | 100 | 0 | 60 (12) | 100 | 49 (4562) |
21 | 1 | 2 | ||
| Sadurano—FC (44.158437, 11.960940) |
August 2021 | Hilly area proximal to farm | 1323 | 122 | 100 | 0 | 60 (12) | 100 | 9 (441) |
2 | 2 | 1 | ||
| Vignola—MO (44.471979, 10.962144) |
September 2021 | Area between villas, vineyards | 3455 | 158 | 100 | 0 | 60 (12) | 100 | 15 (1500) |
4 | 0 | 0 | ||
ER, Emilia-Romagna; BO, Bologna; FC, Forli-Cesena; MO, Modena; (X,Y), coordinates of sampling location; N, total number of collected sand flies per location; n1, total number of specimens identified molecularly by species; perfi, Phlebotomus perfiliewi; perni, Phlebotomus perniciosus; n2, total number of specimens genotyped for kdr mutations in pools; p, number of pools into which n2 specimens were divided; kdr, knock-down resistance; L, susceptible wild-type allele (leucine); F, resistant mutant allele (phenylalanine); S, resistant mutant allele (serine); P, number of pools into which n3 specimens were divided; n3, total number of specimens analyzed for the presence of pathogens in pools; Leish, Leishmania; TOSV, Toscana virus; FERMV, Fermo virus.
Sandflies were collected overnight using CDC miniature light traps baited with dry ice, set before 5 p.m. and removed at 7 a.m. the day after. The collection bags were refrigerated and transferred to the laboratory, where, prior to any further handling/analysis, they were anesthetized at 4 °C. Males were separated from females, and engorged females were removed. Specimens that were not immediately processed were conserved at −80 °C. A sub-sample of the collected sand flies was identified morphologically, using a light microscope and specific morphological keys [20]. Females were either (i) group in pools comprised of approximately 50–100 specimens from the same sampling location and collection date and submitted to molecular analysis for pathogen detection (manipulation of the females was kept to a minimum to optimize pathogen detection); or (ii) individually stored (60 sand fly individuals from each sampling location) for molecular verification of species and genotyping of kdr mutations.
Figure 1.
Sand fly sampling locations in Emilia-Romagna provinces, northern Italy. Red pins correspond to the study sites, whose coordinates are given in parentheses (X, Y). The base layers of the left panel’s maps were obtained from d-maps.com [21], and sampling locations’ screenshots were obtained from Google maps (accessed on 22 December 2022).
2.2. Genetic Material Extraction from Sand Flies
Genomic DNA was extracted from 240 individual female sand flies (60 specimens from each sampling location) using the DNazol reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). For pathogen detection, pools of sand flies were homogenized using a pellet pestle, and DNA and RNA were automatically extracted using BioSprint 96 (Qiagen, Germany); RNA was retro-transcribed (Super Script II, Invitrogen, Carlsbad, CA, USA). The quantity and purity of DNA/RNA were assessed using a NanoDrop 2000c spectrophotometer (Thermo Scientific, Waltham, MA, USA).
2.3. Molecular Identification of Species and Genotyping of Mutations in the Voltage-Gated Sodium Channel (VGSC)
Discrimination of the sand fly species relied on the PCR amplification of a 700 bp mitochondrial cytochrome oxidase subunit I (COI) genomic fragment, using primers LCO1490 and HCO2198 [22,23] and Taq DNA polymerase (EnzyQuest, Heraklion, Greece). The applied thermal protocol was as follows: 94 °C for 2 min, 35 cycles × (94 °C for 45 s, 50 °C for 30 s, 72 °C for 45 s), 72 °C for 10 min. After agarose gel visualization of a small PCR product quantity (5 μL), the rest was purified using the Nucleospin PCR & Gel Clean-Up Kit (Macherey Nagel, Dueren, Germany) and then subjected to Sanger sequencing (GENEWIZ, Azenta Life Sciences, Griesheim, Germany) using the LCO1490 primer and BLASTn analysis.
The presence of kdr mutations associated with resistance to pyrethroid insecticides and previously detected in sand flies (L1014F/S) and/or other insect species (I1011M, V1016G, and F1020S) populations was monitored by genotyping the VGSC domain IIS6. The genomic sequence was amplified by Taq DNA polymerase (EnzyQuest, Heraklion, Greece) using primers Vssc8F and Vssc1bR, as described in Gomes et al., 2017 [24]. The DNA template (2 μL) used in this diagnostic assay consisted of a mixture of genomic DNAs extracted individually from up to 5 sand flies of the same species and sampling location. The pooled DNA included 1.5 μL of each individual gDNA and ddH2O up to 10 μL. The reaction’s thermal conditions were as follows: 94 °C for 2 min, 35 cycles × (94 °C for 45 s, 56 °C for 30 s, 72 °C for 30 s), 72 °C for 10 min. The approximately 400 bp generated PCR fragments, after visualization in agarose gel, were purified using the Nucleospin PCR & Gel Clean-Up Kit (Macherey Nagel, Dueren, Germany) and then subjected to Sanger sequencing (GENEWIZ, Azenta Life Sciences, Germany) using the Vssc8F primer. Sequences were analyzed using the sequence alignment editor BioEdit 7.2 (https://bioedit.software.informer.com/7.2/). Reference VGSC partial genomic sequences were obtained from GenBank for Phlebotomus papatasi (MH401419.1) and P. perfiliewi (MG779187.1)
2.4. Pathogen Detection
Detection of the Leishmania parasite in female sand fly pools relied on a TaqMan quantitative PCR (qPCR) assay targeting a 122 bp fragment of the kinetoplast DNA, as described by Galletti et al., 2011 [25].
A one-step Reverse Transcriptase PCR (RT-PCR) assay was performed to investigate the possible presence of phleboviruses in pools of sand fly samples, targeting a 370 bp nucleotide region of the S genomic segment, according to Lambert et al., 2009 [26]. The amplicons obtained with the pan-phlebo-PCR were sequenced and subjected to BLAST analysis for virus identification, based on GenBank. TOSV presence was monitored via specific real-time PCR, as described by Perez-Ruiz et al., 2007 [27].
3. Results
3.1. Sand Fly Collections and Species Identification
A total of N = 35,706 sand flies were collected from the four sampling locations, with 86.6% of them originating from Monteveglio and Pianoro (BO, eight samplings). A sub-group of n1 = 1644 samples was identified molecularly by species, relying on the COI marker. Phlebotomus perfiliewi was recorded almost exclusively (overall percentage: 99.2%) in all sampling locations, apart from four P. perniciosus individuals from Monteveglio (BO). In n2 = 240 Phlebotomus samples (out of the aforementioned n1), 60 from each location grouped into pools (p) of 5 individuals, the VGSC IIS6 region was genotyped for the presence of any pyrethroid resistance mutations. The investigation of Leishmania and phlebovirus load in the collected populations was conducted in n3 = 11,965 female sand flies (out of the N Phlebotomus samples) grouped into P = 132 pools of 50–100 individuals from the same sampling location and collection date (Table 1).
3.2. Monitoring of Knock-Down Resistance (kdr) Mutations
Sequencing of the VGSC domain IIS6 to monitor the occurrence of target-site mutations conferring resistance to pyrethroid insecticides revealed the presence of the wild-type allele TTA (leucine) at position 1014 in all the analyzed samples, n2 = 240 (Table 1). Similarly, wild-type codons were detected in all the other three VGSC loci investigated, i.e., ATT (isoleucine) at 1011, GTG (valine) at 1016, and TTC (phenylalanine) at 1020.
3.3. Detection of Leishmania and Phleboviruses
A total of 132 pools (P) of Phlebotomus sp. sand flies (n3 = 11,965 specimens, in groups of 50–100) were screened for the presence of Leishmania parasites and for TOSV and FERMV. Monitoring resulted in a total of 51 pools (38.6%) positive for Leishmania, out of which 45 were gathered from Monteveglio and Pianoro (BO). A lower percentage of pools was found to harbor TOSV or FERMV infection: 2.3% and 3%, respectively. Notably, co-infection with Leishmania and TOSV was recorded in a pool from Monteveglio (Table 1).
4. Discussion
The ongoing northward spread of visceral leishmaniasis (VL) in Italy highlights the necessity of seasonal surveillance programs, encompassing epidemiological and entomological parameters to alleviate disease transmission risk spatiotemporally. In support of this requirement, here we surveyed accordingly (semi-)rural areas close to small animal farms and/or cultivated fields and human residences in the Bologna, Modena, and Forli-Cesena provinces in Emilia-Romagna (ER), an important VL focus in the country.
Phlebotomus perfiliewi, consistently ranking as the most widespread sand fly species in ER, especially in the hilly central parts, was the dominant species documented in our collected samples. P. perniciosus, the main leishmaniasis vector in Italy, followed with a low percentage (<1%), in line with past literature data [28]. This finding could be possibly attributed to microhabitat preferences and/or the slightly different seasonal dynamics the two species exhibit. Indications of these species, along with P. neglectus, colonizing northern-latitude European regions beyond the confined endemic ones [5,29] raise concerns regarding the aggravation of the leishmaniasis epidemiological scenario on the continent.
The presence of the Leishmania parasite was recorded in approximately 40% of the tested sand fly pools. Previous surveillance studies, during 2018–2020, showed infection rates of <17% (examining pools of 50 females, in contrast to pools of 50–100 here) [10]. L. infantum has been exclusively documented in the surveyed regions in the past, based on specific molecular typing, implying that the isolates herein also belong to this species. In 2012, after almost 30 years, human leishmaniasis re-emerged in northern Italy, with P. perfiliewi “driving” several outbreaks (of canine leishmaniasis, as well) so far [29,30,31,32]. Interestingly, Calzolari et al., 2019 [31] described that L. infantum strains circulating in ER differ genetically based on their reservoir host (humans or dogs), suggesting that two distinct but overlapping Leishmania transmission cycles may occur in the region. The high infection prevalence marked here conjugated with P. perfiliewi abundance depicts the active risk of leishmaniasis transmission, potentially among both humans and dogs. Besides this, the co-circulation of sand-fly-borne phleboviruses, i.e., the Toscana (TOSV) and Fermo (FERMV) viruses, in the region is again attested to here. Apart from the importance of such viruses in human health per se, their potential to enhance Leishmania infection when both are present inside the host has been proposed [33], yet possible interactions of the two pathogens have been insufficiently investigated.
Regarding insecticide resistance, the application of molecular diagnostic tools revealed the absence of known knock-down resistance (kdr) mutations in the analyzed populations from ER. To the best of our knowledge, this is the first attempt to monitor resistance by the application of molecular diagnostics in sand flies originating from Italy. In the past, phenotypic profiling of insecticide resistance in important vector species populations (i.e., P. perfiliewi, P. perniciosus, and P. papatasi) from central and southern Italy indicated susceptibility to pyrethroid and/or acetyl-cholinesterase inhibitor insecticides [34,35]. Globally, investigations into the insecticide resistance status of sand fly populations and the background molecular mechanisms remain narrow. Nevertheless, resistance against widely used insecticides, such as DDT and pyrethroids, has arisen in countries with high disease endemicity and a history of immense insecticidal pressure for medical or agricultural purposes, such as India, Turkey, Iran, etc. Interestingly, mutations at the voltage-gated sodium channel (VGSC), L1014F and L1014S, conferring resistance to pyrethroids have been recorded focally in populations from India, Sri Lanka, and Turkey [19].
Worldwide sand fly chemical control is usually integrated into mosquito control programs, rather than being directly targeted [13]. Presumably, in the environmental settings we selected for samplings (semi-natural environments with woods, hedges, farms, cultivated fields, villas, and streets), the selection pressure from insecticide applications has been rare or absent. No regional spraying programs have operated there, and the use of insecticides at the household/farm level might be limited, possibly accounting for the absence of kdr mutations.
Field studies have revealed the restricted dispersal patterns (spatial movements) of some sand fly species in their distribution areas [36,37], indicating that any resistance trait could be focally present and, thus, difficult to detect in occasional field samplings in wider peridomicile settings. The possibility that pyrethroid resistance, either target-site or detoxification-based, may occur in the examined populations cannot be excluded. It is noteworthy that, especially in ER, but also in neighboring regions of northern Italy, pyrethroid resistance has been frequently recorded, phenotypically and molecularly, in Ae. albopictus and Cx. pipiens mosquito populations, probably due to urban chemical interventions conducted locally at the regional (by Municipalities) and/or household level [38,39].
The development of additional molecular diagnostic markers, to more reliably capture incipient insecticide resistance in sand flies, requires further molecular studies and, therefore, the availability of genomic resources (e.g., annotation of P450 monooxygenases and other detoxification genes, phylogenomic analyses, etc.). Genome sequencing of important sand fly vector species would greatly open up the possibility for efficient insecticide resistance management tools [39].
5. Conclusions
The Emilia-Romagna region, an epidemiologically important visceral leishmaniasis epicenter of Italy, receives heavy synthetic insecticide loads on an annual basis as part of both agricultural and public health interventions. Thus, monitoring for sandfly species, pathogen circulation, and insecticide resistance traits in this area is of major importance. In our study, co-circulation of Leishmania and sand-fly-borne phleboviruses was demonstrated in local populations, in line with previous findings of regional surveillance studies. Molecular analysis of pyrethroid resistance traits in P. perfiliewi populations, the dominant sand fly species of the region and a major L. infantum vector, revealed no occurrence of knock-down resistance mutations. However, given the selection of insecticide resistance in several sand fly populations from leishmaniasis endemic countries, systematic monitoring via the deployment of molecular markers and/or phenotypical assessment is highly recommended to facilitate early diagnosis of incipient resistance and assist with the application of evidence-based local control interventions.
Author Contributions
Conceptualization, M.C., J.V.; Sample collection, M.D., A.G., M.S., M.C.; Methodologies, S.B., M.C., K.M.; Data analysis and interpretation, S.B., M.C.; Writing—original draft preparation, S.B., M.C., K.M.; Writing—review and editing, S.B., M.C., K.M., J.V., R.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Hellenic Foundation for Research and Innovation (HFRI) under the 3rd Call for HFRI PhD Fellowships awarded to S.B. (Fellowship Number: 11078) and also by Fondation Santé through a research grant awarded to K.M. Part of this research received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 731060 (INFRAVEC2).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization. [(accessed on 2 November 2022)]. Available online: https://www.euro.who.int/__data/assets/pdf_file/0007/246166/Fact-sheet-Leishmaniasis-Eng.pdf.
- 2.Alvar J., Vélez I.D., Bern C., Herrero M., Desjeux P., Cano J., Jannin J., den Boer M. Who Leishmaniasis Control the WHO Leishmaniasis Control Team Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza J.C. Global Health Impacts of Vector-Borne Diseases: Workshop Summary. Forum on Microbial Threats; Board on Global Health; Health and Medicine Division. National Academies Press; Washington, DC, USA: 2016. [(accessed on 28 October 2022)]. Vector-borne disease emergence and spread in the European Union; p. A11. National Academies of Sciences, Engineering, and Medicine. Available online: https://www.ncbi.nlm.nih.gov/books/NBK390443/ [PubMed] [Google Scholar]
- 4.Maroli M., Rossi L., Baldelli R., Capelli G., Ferroglio E., Genchi C., Gramiccia M., Mortarino M., Pietrobelli M., Gradoni L. The northward spread of leishmaniasis in Italy: Evidence from retrospective and ongoing studies on the canine reservoir and phlebotomine vectors. Trop. Med. Int. Health. 2008;13:256–264. doi: 10.1111/j.1365-3156.2007.01998.x. [DOI] [PubMed] [Google Scholar]
- 5.Mendoza-Roldan J., Benelli G., Panarese R., Iatta R., Furlanello T., Beugnet F., Zatelli A., Otranto D. Leishmania infantum and Dirofilaria immitis infections in Italy, 2009–2019: Changing distribution patterns. Parasites Vectors. 2020;13:193. doi: 10.1186/s13071-020-04063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maroli M., Feliciangeli M.D., Bichaud L., Charrel R.N., Gradoni L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2013;27:123–147. doi: 10.1111/j.1365-2915.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 7.Alkan C., Kasap O.E., Alten B., De Lamballerie X., Charrel R.N. Sandfly-Borne Phlebovirus Isolations from Turkey: New Insight into the Sandfly fever Sicilian and Sandfly fever Naples Species. PLoS Negl. Trop. Dis. 2016;10:e0004519. doi: 10.1371/journal.pntd.0004519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calzolari M., Chiapponi C., Bellini R., Bonilauri P., Lelli D., Moreno A., Barbieri I., Pongolini S., Lavazza A., Dottori M. Isolation of three novel reassortant phleboviruses, Ponticelli I, II, III, and of Toscana virus from field-collected sand flies in Italy. Parasites Vectors. 2018;11:84. doi: 10.1186/s13071-018-2668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calzolari M., Ferrarini G., Bonilauri P., Lelli D., Chiapponi C., Bellini R., Dottori M. Co-circulation of eight different phleboviruses in sand flies collected in the Northern Apennine Mountains (Italy) Infect. Genet. Evol. 2018;64:131–134. doi: 10.1016/j.meegid.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Calzolari M., Romeo G., Callegari E., Bonilauri P., Chiapponi C., Carra E., Rugna G., Taddei R., Lelli D., Dottori M. Co-Circulation of Phleboviruses and Leishmania Parasites in Sand Flies from a Single Site in Italy Monitored between 2017 and 2020. Viruses. 2021;13:1660. doi: 10.3390/v13081660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moriconi M., Rugna G., Calzolari M., Bellini R., Albieri A., Angelini P., Cagarelli R., Landini M.P., Charrel R.N., Varani S. Phlebotomine sand fly–borne pathogens in the Mediterranean Basin: Human leishmaniasis and phlebovirus infections. PLoS Negl. Trop. Dis. 2017;11:e0005660. doi: 10.1371/journal.pntd.0005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization Leishmaniasis. 2020. [(accessed on 28 September 2022)]. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1.
- 13.Wilson A.L., Courtenay O., Kelly-Hope L.A., Scott T.W., Takken W., Torr S.J., Lindsay S.W. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 2020;14:e0007831. doi: 10.1371/journal.pntd.0007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malagoli C., Costanzini S., Heck J.E., Malavolti M., De Girolamo G., Oleari P., Palazzi G., Teggi S., Vinceti M. Passive exposure to agricultural pesticides and risk of childhood leukemia in an Italian community. Int. J. Hyg. Environ. Health. 2016;219:742–748. doi: 10.1016/j.ijheh.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.EU Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making availa-ble on the market and use of biocidal products. [(accessed on 28 October 2022)];Off. J. Eur. Union. 2012 L167:1–123. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:167:0001:0123:EN:PDF. [Google Scholar]
- 16.Pichler V., Giammarioli C., Bellini R., Veronesi R., Arnoldi D., Rizzoli A., Lia R.P., Otranto D., Ballardini M., Cobre P., et al. First evidence of pyrethroid resistance in Italian populations of West Nile virus vector Culex pipiens. Med. Veter. Ѐntomol. 2022;36:390–395. doi: 10.1111/mve.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canali M., Rivas-Morales S., Beutels P., Venturelli C. The Cost of Arbovirus Disease Prevention in Europe: Area-Wide Integrated Control of Tiger Mosquito, Aedes albopictus, in Emilia-Romagna, Northern Italy. Int. J. Environ. Res. Public Health. 2017;14:444. doi: 10.3390/ijerph14040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porretta D., Mastrantonio V., Lucchesi V., Bellini R., Vontas J., Urbanelli S. Historical samples reveal a combined role of agriculture and public-health applications in vector resistance to insecticides. Pest Manag. Sci. 2022;78:1567–1572. doi: 10.1002/ps.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balaska S., Fotakis E.A., Chaskopoulou A., Vontas J. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl. Trop. Dis. 2021;15:e0009586. doi: 10.1371/journal.pntd.0009586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dantas-Torres F., Tarallo V.D., Otranto D. Morphological keys for the identification of Italian phlebotomine sand flies (Diptera: Psychodidae: Phlebotominae) Parasites Vectors. 2014;7:479. doi: 10.1186/s13071-014-0479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.d-maps.com Free Maps. [(accessed on 22 December 2022)]. Available online: https://d-maps.com/carte.php?num_car=8072&lang=en.
- 22.Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c ox-idase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- 23.Gutiérrez M.A.C., Vivero R.J., Vélez I.D., Porter C.H., Uribe S. DNA Barcoding for the Identification of Sand Fly Species (Diptera, Psychodidae, Phlebotominae) in Colombia. PLoS ONE. 2014;9:e85496. doi: 10.1371/journal.pone.0085496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomes B., Purkait B., Deb R.M., Rama A., Singh R.P., Foster G.M., Coleman M., Kumar V., Paine M., Das P., et al. Knockdown resistance mutations predict DDT resistance and pyrethroid tolerance in the visceral leishmaniasis vector Phlebotomus argentipes. PLoS Negl. Trop. Dis. 2017;11:e0005504. doi: 10.1371/journal.pntd.0005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galletti E., Bonilauri P., Bardasi L., Fontana M.C., Ramini M., Renzi M., Dosa G., Merialdi G. Development of a minor groove binding probe based real-time PCR for the diagnosis and quantification of Leishmania infantum in dog specimens. Res. Veter Sci. 2011;91:243–245. doi: 10.1016/j.rvsc.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Lambert A.J., Lanciotti R.S. Consensus Amplification and Novel Multiplex Sequencing Method for S Segment Species Identification of 47 Viruses of the Orthobunyavirus, Phlebovirus, and Nairovirus Genera of the Family Bunyaviridae. J. Clin. Microbiol. 2009;47:2398–2404. doi: 10.1128/JCM.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-Ruiz M., Collao X., Navarro-Marí J.-M., Tenorio A. Reversetranscription, real-time PCR assay for detection of Toscana virus. J. Clin. Virol. 2007;39:276–281. doi: 10.1016/j.jcv.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Corradetti A. Phlebotomus and leishmaniasis in North-Central Italy (Apennine Region) Sci. Rep. Ist. Super. Sanità. 1962;2:103–109. [Google Scholar]
- 29.Defilippo F., Carrera M., Lelli D., Canziani S., Moreno A., Sozzi E., Manarolla G., Chiari M., Marco F., Cerioli M.P., et al. Distribution of Phlebotomine Sand Flies (Diptera: Psychodidae) in the Lombardy Region, Northern Italy. Insects. 2022;13:463. doi: 10.3390/insects13050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michelutti A., Toniolo F., Bertola M., Grillini M., Simonato G., Ravagnan S., Montarsi F. Occurrence of Phlebotomine sand flies (Diptera: Psychodidae) in the northeastern plain of Italy. Parasites Vectors. 2021;14:164. doi: 10.1186/s13071-021-04652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calzolari M., Carra E., Rugna G., Bonilauri P., Bergamini F., Bellini R., Varani S., Dottori M. Isolation and Molecular Typing of Leishmania infantum from Phlebotomus perfiliewi in a Re-Emerging Focus of Leishmaniasis, Northeastern Italy. Microorganisms. 2019;7:644. doi: 10.3390/microorganisms7120644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calzolari M., Romeo G., Bergamini F., Dottori M., Rugna G., Carra E. Host preference and Leishmania infantum natural infection of the sand fly Phlebotomus perfiliewi in northern Italy. Acta Trop. 2022;226:106246. doi: 10.1016/j.actatropica.2021.106246. [DOI] [PubMed] [Google Scholar]
- 33.Rath C.T., Schnellrath L.C., Damaso C.R., De Arruda L.B., Vasconcelos P.F.D.C., Gomes C., Laurenti M.D., Silva T.C.C., Vivarini D.C., Fasel N., et al. Amazonian Phlebovirus (Bunyaviridae) potentiates the infection of Leishmania (Leishmania) amazonensis: Role of the PKR/IFN1/IL-10 axis. PLoS Negl. Trop. Dis. 2019;13:e0007500. doi: 10.1371/journal.pntd.0007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavagino A., Ansaldi G. Susceptibility tests on Phlebotomus perniciosus and Phlebotomus perfiliewi wild populations in Sicily. Parassitologia. 1991;33:349–351. [PubMed] [Google Scholar]
- 35.Maroli M., Cianchi T., Bianchi R., Khoury C. Testing insecticide susceptibility of Phlebotomus perniciosus and P. papatasi (Diptera: Psychodidae) in Italy. Ann. Ist. Super. Sanita. 2002;38:419–423. [PubMed] [Google Scholar]
- 36.Casanova C., Costa A.I.P., Natal D. Dispersal pattern of the sand fly Lutzomyia neivai (Diptera: Psychodidae) in a cutaneous leishmaniasis endemic rural area in Southeastern Brazil. Mem. Inst. Oswaldo Cruz. 2005;100:719–724. doi: 10.1590/S0074-02762005000700006. [DOI] [PubMed] [Google Scholar]
- 37.Galvis-Ovallos F., Casanova C., Bergamaschi D.P., Galati E.A.B. A field study of the survival and dispersal pattern of Lutzomyia longipalpis in an endemic area of visceral leishmaniasis in Brazil. PLoS Negl. Trop. Dis. 2018;12:e0006333. doi: 10.1371/journal.pntd.0006333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pichler V., Bellini R., Veronesi R., Arnoldi D., Rizzoli A., Lia R.P., Otranto D., Montarsi F., Carlin S., Ballardini M., et al. First evidence of resistance to pyrethroid insecticides in Italian Aedes albopictus populations 26 years after invasion. Pest Manag. Sci. 2018;74:1319–1327. doi: 10.1002/ps.4840. [DOI] [PubMed] [Google Scholar]
- 39.Haas J., Hayward A., Buer B., Maiwald F., Nebelsiek B., Glaubitz J., Bass C., Nauen R. Phylogenomic and functional characterization of an evolutionary conserved cytochrome P450-based insecticide detoxification mechanism in bees. Proc. Natl. Acad. Sci. USA. 2022;119:e2205850119. doi: 10.1073/pnas.2205850119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.