The mortality and morbidity associated with bacterial meningitis have remained significant despite advances in antimicrobial chemotherapy and supportive care. A major contributing factor is the incomplete understanding of the pathogenesis of this disease. For example, most cases of bacterial meningitis develop as a result of hematogenous spread, but it is unclear how circulating bacteria cross the blood-brain barrier (12, 25, 33, 46). Recent studies have shown that Escherichia coli K1, group B Streptococcus, Streptococcus pneumoniae, and Citrobacter spp., the important pathogens that cause meningitis, translocate from blood to the central nervous system (CNS) without altering the integrity of the blood-brain barrier (2, 23, 27, 36, 42). These studies of bacterial translocation from blood to the CNS have become possible because of the availability of both in vitro and in vivo models of the blood-brain barrier (1, 3, 4, 7, 11, 13, 17–20, 22, 26, 28, 32, 41, 43, 47). The in vitro model of the blood-brain barrier is composed of brain microvascular endothelial cells (BMEC), which exhibit transendothelial resistance of 200 to 600 Ω/cm2, a unique property of the brain microvascular endothelium monolayer compared to systemic vascular endothelium. The in vivo model of the blood-brain barrier utilizes experimental hematogenous meningitis in animals. In these experimental meningitis models, bacteria are injected via intravenous, intraperitoneal, subcutaneous, or intracardiac administration, resulting in bacteremia and subsequent entry into the CNS. At present, E. coli-BMEC interactions represent the most characterized system concerning how circulating bacteria cross the blood-brain barrier. This review summarizes our current understanding of the pathogenetic mechanisms involved in bacterial translocation of the blood-brain barrier, using E. coli as a paradigm.
A THRESHOLD LEVEL OF BACTEREMIA
Several studies of humans and experimental animals suggest a relationship between the magnitude of bacteremia and the development of meningitis due to E. coli, Haemophilus influenzae type b, and S. pneumoniae (Table 1), (5, 10, 13, 20, 22, 26, 44, 45). For example, Dietzman et al. (10) reported a significantly higher incidence of E. coli meningitis in neonates who had bacterial counts in blood >103 CFU/ml (6/11 [55%]) compared to those with bacterial counts in blood <103 CFU/ml (1/19 [5%]). Similarly, meningitis due to S. pneumoniae was observed more frequently in patients whose bacterial counts in blood were greater than 1 × 102 to 5 × 102 CFU/ml (6/7 [86%]) than those with bacterial counts in blood less than 1 × 102 to 5 × 102 CFU/ml (2/50 [4%]) (5, 45). For H. influenzae type b meningitis, the level of bacteremia required for the development of meningitis is around 102 CFU/ml of blood (5, 45). Consistent with these clinical findings, a high degree of bacteremia was shown to be a primary determinant for meningeal invasion by E. coli K1, group B Streptococcus, H. influenzae type b, and S. pneumoniae in an experimental hematogenous meningitis model (13, 20, 22, 26, 28). Thus, one of the reasons for the close association of E. coli K1, group B Streptococcus, H. influenzae type b, and S. pneumoniae with meningitis is their ability to escape from host defenses and then to achieve the threshold level of bacteremia necessary for invasion of the meninge. In contrast, it is unclear whether the development of meningococcal meningitis is associated with a threshold level of bacteremia as shown by inconsistent reports from clinical studies (Table 1) (44, 45). This issue is further hampered by the lack of a suitable animal model for studying the pathogenesis of meningococcal meningitis. E. coli is commonly associated with neonatal meningitis, and E. coli strains possessing the K1 capsular polysaccharide are predominant (approximately 80% among isolates from E. coli meningitis) (16, 24, 37). Of interest, rates of E. coli meningitis (defined as positive cerebrospinal fluid (CSF) cultures) were found to be similar between neonatal and adult animals developing a high degree of bacteremia (e.g., >105 CFU/ml of blood); however, an approximately 106-fold-greater inoculum of E. coli K1 was required to induce a similar high-level bacteremia in adult animals compared to neonatal animals (22). These findings indicate that the age dependency of E. coli meningitis is due to the relative resistance of adults to high-level bacteremia, which precedes the development of meningitis, and less likely due to greater invasion of neonatal BMEC compared to adults BMEC (43).
TABLE 1.
Association of the magnitude of bacteremia with meningitis in infants and children
| Bacterium | No. of patients | CFU/ml of blood | No of patients with meningitis/total no. (percent) | Reference |
|---|---|---|---|---|
| E. coli | 30 | <1,000 | 1/19 (5) | 10 |
| >1,000 | 6/11 (55) | |||
| S. pneumoniae | 24 | ≤100 | 1/20 (5) | 5 |
| >100 | 3/4 (75) | |||
| 33 | <500 | 1/30 (3) | 45 | |
| ≥500 | 3/3 (100) | |||
| H. influenzae type b | 34 | <100 | 2/12 (17) | 45 |
| ≥100 | 11/22 (50) | |||
| 26 | ≤100 | 7/11 (63) | 5 | |
| >100 | 11/15 (73) | |||
| N. meningitidis | 35 | <500 | 7/22 (32) | 44 |
| ≥500 | 8/13 (62) | |||
| 12 | <1,000 | 5/8 (63) | 45 | |
| ≥1,000 | 1/4 (25) |
BACTERIAL STRUCTURES CONTRIBUTING TO INVASION OF BMEC
Recent studies indicate that a high degree of bacteremia is necessary but not sufficient for the development of meningitis from E. coli K1 and that invasion of BMEC is a prerequisite for E. coli K1 penetration of the blood-brain barrier in vivo (21). This was shown by the demonstration in infant rats with experimental hematogenous meningitis that several isogenic mutants of E. coli K1 strain RS 218 (018:K1:H7) were significantly less able to induce meningitis (defined as positive CSF cultures) than the parent strain despite similar levels of bacteremia, indicating that certain E. coli K1 structures are necessary for crossing the blood-brain barrier in vivo (Table 2). Similarly, many E. coli K1 structures which contribute to BMEC invasion in vitro have been identified (Table 3). The major findings are summarized below.
TABLE 2.
Development of bacteremia and meningitis (defined as positive CSF cultures) in newborn rats receiving E. coli K1 strain E44 (RS 218 Rifr, 018:K1:H7) or its isogenic mutants
| E. coli strain | No. of animals | Bacteremia (log CFU/ml of blood) | No. (%) of animals with positive CSF | Reference(s) |
|---|---|---|---|---|
| E44 | 19 | 7.18 ± 0.63 | 12 (63) | 48 |
| ΔompA | 22 | 7.05 ± 0.49 | 6 (27)a | |
| E44 | 24 | 7.51 ± 1.25 | 16 (67) | 20 |
| ΔibeA | 25 | 6.97 ± 1.21 | 4 (16)a | |
| E44 | 27 | 7.01 ± 1.17 | 15 (56) | 19 |
| ΔibeB | 25 | 7.06 ± 1.29 | 4 (16)a | |
| E44 | 24 | 7.53 ± 0.40 | 18 (75) | 47 |
| ΔibeC | 24 | 7.80 ± 0.67 | 10 (42)a | |
| E44 | 17 | 7.50 ± 0.32 | 14 (82) | 18 |
| ΔaslA | 22 | 7.60 ± 0.49 | 7 (32)a | |
| E44 | 51 | 7.22 ± 0.59 | 34 (6) | 3, 4 |
| ΔtraJ | 50 | 7.10 ± 0.44 | 23 (4)a | |
| E44 | 26 | 6.06 ± 1.49 | 10 (38) | 4, Wang et al.b |
| Δcnf1 | 28 | 6.07 ± 1.21 | 3 (11)a |
Significantly less than result for E44.
Wang et al., Abstr. 100th Gen. Meet. Am. Soc. Microbiol.
TABLE 3.
E. coli K1 structures contributing to invasion of BMEC
| K1 structurea | Orginb | K12 homologuec | Mechanism | Reference(s) |
|---|---|---|---|---|
| ompA | Rs218/E44 | ompA (98) | Ligand-receptor | 31, 32; Wang and Kim, unpublished |
| ibeA | 10A-23 (E44ibeB::TnphoA) | None | Ligand-receptor | 20 |
| ibeB | 7A-33 (E44ibeB::TnphoA) | p77211 (97) | Ligand-receptor interaction (?) | 19 |
| ibeC | 23A-20 (E44ibeC::TnphoA) | yijP (98) | Ligand-receptor interaction (?) | 47 |
| aslA | 27A-6 (E44aslA::TnphoA) | aslA (94) | ND | 18 |
| nilA | E44, DFI | None | ND | 3 |
| nilB | E44, DFI | None | ND | 3 |
| nilC | E44, DFI | None | ND | 3 |
| ygdP | E44, DFI | ygdP (98) | ND | 3 |
| traJ | E44, DFI, STM | Noned | ND | 3, 4 |
| cnf1 | E44, STM | None | RhoA GTPase | 4 |
| cigA | E44, STM | None | ND | 4 |
| b2146 (o412) | E44, STM | o412 (42–100) | ND | 4 |
| vibO (pmgl) | E44, STM | pmgI (60–74) | ND | 4 |
| b1983 (o347) | E44, STM | o347 (49–100) | ND | 4 |
| yaiU | E44, STM | yaiU (44–100) | ND | 4 |
ibe, invasion brain endothelial cells; nil, newborn bovine serum-inducing loci; cig, CNS invasion gene.
DFI, differential fluorescence induction assay; STM, signature-tagged mutagenesis.
Numbers in parentheses indicate percentages of nucleotide identity of DNA sequenced.
Homologous to F-like plasmid R1-19.
Outer membrane protein A (OmpA) was identified as a potential contributor to E. coli K1 invasion of BMEC on the basis of its homology with Neisseria Opa proteins, which have been shown to be involved in invasion of eukaryotic cells (32). OmpA is a major outer membrane protein in E. coli, and its N-terminal domain crosses the membrane eight times in antiparallel β-strands with four relatively large and hydrophilic surface-exposed loops. The N-terminal portion of OmpA, not the C-terminal portion, and surface-exposed loops have been shown to contribute to E. coli K1 invasion of BMEC (32). For example, the synthetic peptides representing a part of the first loop and the tip of the second loop of OmpA have been shown to inhibit E. coli K1 invasion of BMEC (32). In addition, anti-OmpA antibody inhibited E. coli K1 invasion of BMEC, indicating that OmpA is exposed to the surface of the K1 encapsulated E. coli. OmpA interacts with the GlcNAcβ1-4GlcNAc epitope of the BMEC receptor glycoprotein, and its first, and second loops are shown to be the sites for the interaction with the carbohydrate epitope of the BMEC glycoprotein (31). The receptor glycoprotein is found to be present on BMEC but is not detectable on systemic vascular endothelial cells (31). In addition, purified OmpA and chitooligomers prepared from the polymer of 1,4-linked GlcNAc inhibited E. coli K1 invasion of BMEC (31, 32). These findings indicate that OmpA contributes to BMEC invasion via a ligand-receptor interaction. E. coli K1 OmpA is highly homologous to E. coli K-12 OmpA. The nucleotide sequence of the K1 ompA gene (GenBank accession no. AF234269) differs from that of the E. coli K-12 ompA in 20 of 1,038 nucleotides, and only 3 of the 325 deduced amino acid residues differ between the K1 and K-12 OmpA proteins. The function of OmpA in E. coli invasion of BMEC was also found to be similar for E. coli K1 and K12 OmpA, as shown by successful complementation of the noninvasive ompA deletion mutant of E. coli K1 to invade BMEC with the E. coli K-12 ompA gene (32; Y. Wang and K. S. Kim, unpublished data).
Ibe (invasion of brain endothelial cells) proteins A, B, and C were identified by cloning and characterization the TnphoA insertion sites from strains 10A-23, 7A-33, and 23A-20, respectively, which are the noninvasive mutants of E. coli K1 strain RS218 (018:K1:H7) (19, 20, 47). Sequence analysis indicates that the E. coli K1 ibeA, ibeB, and ibeC encode 50-, 50-, and 66-kDa membrane proteins, respectively. Both ibeB and ibeC were found to have K-12 homologues, p77211 and yijP, respectively, while ibeA was unique to E. coli K1. The roles of IbeA, IbeB, and IbeC in E. coli K1 invasion of BMEC were verified by deletion and complementation experiments; i.e., isogenic deletion mutants were less invasive in BMEC in vitro and less able to cross the blood-brain barrier in vivo (Table 2), and their invasion abilities were restored by complementation in trans with individual genes. Of interest, recombinant Ibe proteins inhibited E. coli K1 invasion of BMEC, suggesting that Ibe proteins contribute to BMEC invasion by ligand-receptor interactions. This concept was supported by the demonstration of a 45-kDa BMEC surface protein interactive with IbeA and a polyclonal antibody raised against this receptor protein inhibited E. coli K1 invasion of BMEC (29). Partial characterization by N-terminal and internal amino acid sequencing of this receptor protein reveals that it represents a novel albumin-like protein present on BMEC (29). Studies are in progress to identify whether BMEC possess receptor proteins for IbeB and IbeC.
E. coli K1 aslA was identified by cloning and sequencing of the TnphoA insertion site of the noninvasive mutant of E. coli K1 strain RS218, 27A-6 (18). This mutant exhibited significantly decreased invasion of BMEC in vitro and an attenuated ability to cause meningitis compared to the parent strain in the newborn rat model of experimental hematogensis meningitis (18). The role of AslA in E. coli K1 invasion of BMEC was also verified by deletion and complementation experiments (18). The E. coli K1 aslA sequence is highly homologous to E. coli K-12 aslA, a putative arylsulfatase-like gene. This E. coli K-12 gene was named because the deduced protein sequence contains sulfatase consensus motifs I and II, which are homologous (55 and 70% identity, respectively) to those of Klebsiella pneumoniae AtsA, an arylsulfatase involved in sulfate metabolism. The E. coli K1 aslA encodes a 52-kDa protein with two transmembrane domains and an amino-terminal signal sequence (18). Its deduced protein sequence indicates that E. coli K1 AslA is also a member of the arylsulfatase family of enzymes that contain highly conserved sulfatase motifs. In bacteria, these genes are expressed under conditions of sulfur starvation. Of interest, unlike the Klebsiella protein, both E. coli K1 and K-12 AslA proteins failed to exhibit in vitro arylsulfatase activity (18). It remains unclear how AslA contributes to E. coli K1 invasion of BMEC in vitro and traversal of the blood-brain barrier in vivo.
A recent study has shown that certain environmental conditions positively and negatively affect E. coli K1 invasion of BMEC in vitro and traversal of the blood-brain barrier in vivo (1). For example, the following growth condition enhanced E. coli invasion of BMEC: media supplemented with 50% newborn bovine serum or iron. Growth conditions that significantly repressed invasion included iron chelation and high osmolarity (1). Using differential fluorescence induction and screening of gfp fusion library, TraJ was identified as a contributor to E. coli K1 invasion of BMEC (3). As expected, TraJ was found to be differentially expressed at the transcriptional level; e.g., transcript levels of traJ increased when E. coli K1 was grown in the presence of serum compared to that in medium alone. A traJ mutant was less invasive in BMEC in vitro and less able to cross the blood-brain barrier in vivo (4). traJ belongs to a cluster of genes within the F-like plasmid R1-19 transfer region called the tra operon. It is speculative whether E. coli K1 TraJ is an invasive factor itself or is needed for the expression of a gene(s) required for efficient penetration of BMEC and/or whether the F-like plasmid tra operon is required for E. coli K1 invasion of BMEC. Studies with signature tagged mutagenesis also identified TraJ as well as cytotoxic necrotizing factor 1 (CNF1) as contributors to E. coli K1 invasion of BMEC (4). An isogenic cnf1-deletion mutant of E. coli K1 strain RS 218 is less invasive in BMEC in vitro and less able to penetrate the blood-brain barrier in vivo (Y. Wang, C. A. Wass, and K. S. Kim, Abstr. 100th Gen. Meet. Am. Soc. Microbiol. 2000, abstr. B-108, p. 65). CNF1 has been shown to activate Rho GTPases, resulting in polymerization of F-actin and increased formation of stress fibers (14, 39). Actin cytoskeletal rearrangements are required for E. coli K1 invasion of BMEC, as shown by invasive E. coli K1-associated F-actin condensation and blockade of invasion by the microfilament disrupting agents, cytochalasin D and latrunculin A (30). Taken together, these findings indicate that CNF1 contributes to E. coli K1 invasion of BMEC, most likely via Rho activation.
Recent studies have also indicated that other meningeal pathogens invade BMEC via ligand-receptor interations. For example, S. pneumoniae invades BMEC in part via interaction between cell wall phosphorylcholine and the BMEC platelet-activating factor receptor as shown by partial inhibition of pneumococcal invasion of BMEC by the platelet-activating factor receptor antagonists (36). Listeria monocytogenes invasion of BMEC has been shown to be mediated by internalin B (15). Two receptors for internalin B have been identified, gC1q-R (the receptor for the globular head of the complement component C1q) and Met tyrosine kinase (8, 40). However, it is unclear whether these receptors for internalin B are present on human BMEC. Group B Streptococcus and Citrobacter have also shown to invade BMEC (2, 27), but bacterial structures contributing to their invasion of BMEC have not been determined.
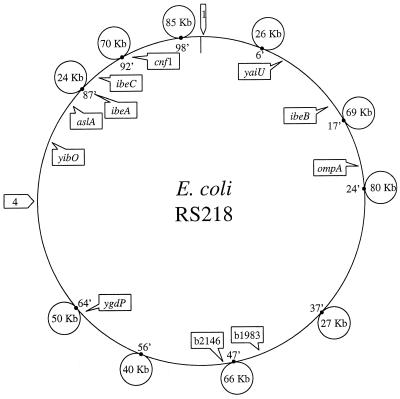
Comparative macrorestriction mapping and subtractive hybridization of the chromosomes of meningitis-causing E. coli K1 (e.g., strains RS 218 and C5) compared to nonpathogenic E. coli have identified 500 kb spread over at least 12 chromosomal loci specific to E. coli K1 (6, 38) (Fig. 1). As shown in Table 3 and Fig. 1, several structures contributing to E. coli K1 invasion of BMEC are unique to E. coli K1, such as ibeA, traJ, and cnf1, whereas other structures critical to E. coli K1 crossing of the blood-brain barrier are shown to have K-12 homologues such as ompA, ibeB, ibeC, and aslA. Thus, E. coli K1 determinants contributing to invasion of BMEC include K1 specific genes as well as K12 homologues. Mapping studies reveal that those E. coli loci involved in BMEC invasion are located at different regions of E. coli K1 chromosome (Fig. 1).
FIG. 1.
Sizes and chromosomal locations of E. coli K1 strain RS 218 (018:K1:H7)-specific segments identified by comparative macrorestriction mapping (38) are shown by small circles. Numbers in the circles represent sizes of K1-specific DNA segments. The sizes of two additional segments shown by arrows labeled 1 and 4, identified by subtractive hybridization (6) are currently unknown. Also shown are 11 E. coli K1 genes contributing to invasion of BMEC, indicated by squares which include K1-specific genes (e.g., ibeA, and cnf1) as well as genes which have K-12 homologues (e.g., ompA, ibeB, ibeC, and aslA).
BACTERIAL TRAFFICKING OF BMEC
Transcytosis of BMEC by E. coli K1, group B Streptococcus, S. pneumoniae, and Citrobacter spp. occur without any change in the integrity of monolayers (2, 23, 27, 36, 42). Transmission electron microscopy revealed that Citrobacter has been shown to invade and replicate in BMEC (2). In contrast, E. coli K1 invades BMEC via a zipper-like mechanism and transmigrates through BMEC in an enclosed vacuole without intracellular multiplication (30). E. coli K1 invasion of BMEC requires actin cytoskeletal rearrangements and induces tyrosine phophorylation of focal adhesion kinase and paxillin, a cytoskeletal protein known to associate with focal adhesion kinase. Furthermore, using focal adhesion kinase-dominant-negative mutants, focal adhesion kinase activity and its autophosphorylation site tyrosine 397 are shown to be critical for E. coli K1 invasion of BMEC (35). The autophosphorylation site (Tyr 397) of focal adhesion kinase has been shown to bind Src kinases and phosphatidylinositol 3-kinase, and binding of one or both is required for focal adhesion kinase-mediated functions. Src kinases, however, were not critical in E. coli K1 invasion of BMEC. This was shown by the demonstration that pretreatment of BMEC with the Src kinase-specific inhibitor, PP1, did not affect E. coli K1 invasion of BMEC and also overexpression of a Src kinase-dominant-negative mutant did not block E. coli K1 invasion of BMEC (35).
In contrast, phosphatidylinositol 3-kinase activation and its association with focal adhesion kinase are required for E. coli K1 invasion of BMEC (34). This was shown by blockade of both phosphatidylinositol 3-kinase activation and E. coli K1 invasion of BMEC with specific phosphatidylinositol 3-kinase inhibitor (LY294002) as well as using dominant negative mutants of phosphatidylinositol 3-kinase and focal adhesion kinase. Phosphatidylinositol 3-kinase activation was abolished by focal adhesion kinase-dominant-negative mutants (34), indicating that focal adhesion kinase is upstream of phosphatidylinositol 3-kinase in E. coli K1 invasion of BMEC. Phosphatidylinositol 3-kinase has been shown to participate in actin reorganization, recruitment of early endosome proteins, and movement of the endosomes along the microtubules. It remains to be determined how focal adhesion kinase and phosphatidylinositol 3-kinase activation contribute to E. coli K1 invasion of BMEC.
Phospholipase A2, particularly cytosolic phospholipase A2, has been shown to contribute to E. coli K1 invasion of BMEC. This was shown by the demonstration that AACOCF3, a selective cytosolic phospholipase A2 inhibitor, blocked E. coli K1 invasion of BMEC and E. coli K1 invasion was significantly decreased in BMEC derived from cytosolic phospholipase A2 knockout mice compared to that in BMEC from control mice (9). Phospholipase A2 hydrolyzes phospholipids at their sn-2 position, resulting in the release of fatty acids, e.g., arachidonic acid. Actin cytoskeletal rearrangements in mammalian cells have been linked to intracellular signaling via metabolites of arachidonic acid. These findings indicate that focal adhesion kinase, phosphatidylinositol 3-kinase, and cytostolic phospholipase A2 activation contribute to E. coli K1 invasion of BMEC, presumably via affecting the signaling mechanisms associated with BMEC actin cytoskeletal arrangements.
It should be noted that bacterial trafficking mechanisms in BMEC are shown to differ between E. coli K1 and other meningitis-causing bacteria such as L. monocytogenes and group B Streptococcus (Table 4). For example, BMEC actin cytoskeletal rearrangements are shown to be a prerequisite for BMEC invasion by E. coli K1, L. monocytogenes, and group B Streptococcus (15, 27, 30). However, L. monocytogenes invasion of BMEC depends on Src kinases, not on focal adhesion kinase and cytosolic phospholipase A2 (9, 35). In contrast, group B Streptococcus invasion of BMEC was independent of phosphatidylinositol 3-kinase and cytosolic phospholipase A2 activation (9, 34). BMEC vacuoles containing E. coli K1 were found to have markers for early and late endosomes but devoid of lysosomal markers (K. J. Kim and K. S. Kim, unpublished data), suggesting that there is an escape from transport to lysosome and/or a blockade of fusion to lysosome. Additional studies are needed to understand the trafficking mechanisms involved in bacterial transcytosis of BMEC.
TABLE 4.
Comparison of host cell cytoskeleton and signaling mechanisms in bacterial invasion of BMEC
| Bacterium | Mechanism
|
References | ||||
|---|---|---|---|---|---|---|
| Actin cytoskeletal rearrangements | Activation ofa:
|
|||||
| FAK | Src | PI3K | cPLA2 | |||
| E. coli K1 | + | + | − | + | + | 9, 34, 35 |
| Group B Streptococcus | + | ND | ND | − | − | 9, 27, 34 |
| L. monocytogenes | + | − | + | + | − | 9, 15, 34, 35 |
+, active participation in BMEC invasion; −, no role in BMEC invasion; ND, not examined.
TRAVERSAL OF THE BLOOD-BRAIN BARRIER AS LIVE BACTERIA
Previous studies of E. coli K1 meningitis have shown that the K1 capsule is a critical determinant in the development of meningitis (22). This was shown by the demonstration of sterile CSF cultures from animals infected with K1− strains, which was interpreted to indicate that the K1 capsule was necessary for the bacterial crossing of the blood-brain barrier. A recent study, however, has shown that both E. coli K1+ and K1− strains are able to traverse BMEC in vitro and enter the CNS in vivo, but infections caused by K1+ strains resulted in positive CSF cultures (17). Thus, the K1 capsule has, in addition to its well-recognized serum resistance and antiphagocytic properties, a role in the traversal of E. coli K1 across the blood-brain barrier as live bacteria. The nature of this novel BMEC activity that is bactericidal to E. coli strains without a capsule is currently unknown. This has been shown not to be related to NO, peroxynitrites, superoxides, and other oxygen radicals (17). Similarly, most opaque variants of S. pneumoniae are shown to be killed in BMEC (36), but the basis of this BMEC killing activity is unclear.
CONCLUSION
A major limitation to advances in prevention and therapy of bacterial meningitis is our incomplete understanding of the pathogenesis of this disease, such as how circulating bacteria cross the blood-brain barrier. Successful isolation and cultivation of BMEC, which constitute the blood-brain barrier, and the development of an experimental hematogenous animal model that closely mimics the pathogenesis of human meningitis enabled dissection of the mechanisms of bacterial translocation across the blood-brain barrier. The studies, so far, have identified that crossing of the blood-brain barrier by E. coli K1, group B Streptococcus, H. influenzae type b, and S. pneumoniae require a high degree of bacteremia. However, a high degree of bacteremia alone is not sufficient for the development of meningitis. The microbial basis for successful traversal of the blood-brain barrier by circulating bacteria is incompletely understood. Recent studies with E. coli K1 have shown that several microbial determinants such as the K1 capsule, OmpA, Ibe proteins, AslA, TraJ, and CNF1 contribute to invasion of BMEC, which is required for successful penetration into the CNS in experimental hematogenous meningitis. In addition, bacterial trafficking of BMEC by E. coli K1 requires BMEC actin cytoskeletal reorganizations and activations of focal adhesion kinase, phosphatidylinositol 3-kinase, and cytosolic phospholipase A2. Of interest, these E. coli trafficking mechanisms are shown to differ from those of other meningitis-causing bacteria, such as L. monocytogenes and group B Streptococcus. Structural genomic studies have identified DNA segments specific to the prototypes of meningitis-causing E. coli K1 (e.g., strains RS 218 and C5), and their sequencing is in progress. It is, however, unclear whether sequence information specific to E. coli K1 will identify all the microbial determinants relevant to the pathogensis of E. coli K1 meningitis. This was exemplified by the identification of E. coli K1 structures that contribute to crossing of the blood-brain barrier in vivo but having highly homologous structures in the E. coli K-12 genome (e.g., ompA, ibeB, ibeC, and aslA). Thus, it is likely that E. coli K1 determinants which contribute to crossing of the blood-brain barrier are not clustered within K1-specific segments and include K1-specific genes as well as K-12 homologues.
ACKNOWLEDGMENTS
The information contained in the review for E. coli K1 was derived from studies carried out by the former and current members of K.S.K.'s laboratory.
This work was supported by NIH grants RO1 NS 26310, AI 47225, and HL 61951.
REFERENCES
- 1.Badger J L, Kim K S. Environmental growth conditions influence the ability of Escherichia coli K1 to invade brain microvascular endothelial cells and confer serum resistance. Infect Immun. 1998;66:5692–5697. doi: 10.1128/iai.66.12.5692-5697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badger J L, Stins M F, Kim K S. Citrobacter freundii invades and replicates in human brain microvascular endothelial cells. Infect Immun. 1999;67:4208–4215. doi: 10.1128/iai.67.8.4208-4215.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badger J L, Wass C A, Kim K S. Identification of E. coli K1 genes contributing to human brain microvascular endothelia cell invasion by differential fluorescence induction. Mol Microbiol. 2000;36:174–182. doi: 10.1046/j.1365-2958.2000.01840.x. [DOI] [PubMed] [Google Scholar]
- 4.Badger J L, Wass C A, Weissman S J, Kim K S. Application of signature-tagged mutagenesis for the identification of Escherichia coli K1 genes that contribute to invasion of human brain microvascular endothelial cells Infect. Immun. 2000;68:5056–5061. doi: 10.1128/iai.68.9.5056-5061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell L M, Alpert G, Campos J M, Plotkin S A. Routine quantitative blood cultures in children with Haemophilus influenzae or Streptococcus pneumoniae bacteremia. Pediatrics. 1985;76:901–904. [PubMed] [Google Scholar]
- 6.Bonacorsi S P P, Clermont O, Tinsley C, Le Gall I, Beaudoin J-C, Elion J, Nassif X, Bingen E. Identification of regions of the Escherichia coli chromosome specific for neonatal meningitis-associated strains. Infect Immun. 2000;68:2096–2101. doi: 10.1128/iai.68.4.2096-2101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowman P D, Betz A L, Ar D, Wolinsky J S, Penney J B, Shivers R R, Goldstein G W. Primary culture of capillary endothelium from rat brain. In Vitro. 1981;17:353–362. doi: 10.1007/BF02618147. [DOI] [PubMed] [Google Scholar]
- 8.Braun L, Ghebrehiwet B, Cossart P. gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes. EMBO J. 2000;19:1458–1466. doi: 10.1093/emboj/19.7.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das, A., L. Asatryan, M. A. Reddy, C. A. Wass, M. Stins, S. Joshi, J. V. Boventre, and K. S. Kim. Differential role of cytosolic phospholipase A2 in the invasion of brain microvascular endothelial cells by Escherichia coli and Listeria monocytogenes. J. Infect. Dis., in press. [DOI] [PubMed]
- 10.Dietzman D E, Fischer G W, Schoenknecht F D. Neonatal Escherichia coli septicemia bacterial counts in blood. J Pediatr. 1974;85:128–130. doi: 10.1016/s0022-3476(74)80308-2. [DOI] [PubMed] [Google Scholar]
- 11.Dorovini-Zis K, Prameya R, Bowman P D. Culture and characterization of microvascular endothelial cells derived from human brain. Lab Investig. 1991;64:425–436. [PubMed] [Google Scholar]
- 12.Feigin R D, McCracken G H, Jr, Klein J O. Diagnosis and management of meningitis. Pediatr Infect Dis J. 1992;11:785–814. doi: 10.1097/00006454-199209000-00039. [DOI] [PubMed] [Google Scholar]
- 13.Ferrieri P, Burke B, Nelson J. Production of bacteremia and meningitis in infant rats with group B streptococcal serotypes. Infect Immun. 1980;27:1023–1032. doi: 10.1128/iai.27.3.1023-1032.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flatau G, Lemichez E, Gauthier M, Chardin P, Fiorentini C, Boquet P. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- 15.Greiffenberg L, Goebel W, Kim K S, Weiglein I, Bubert A, Engelbrecht F, Stins M, Kuhn M. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect Immun. 1998;66:5260–5267. doi: 10.1128/iai.66.11.5260-5267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross R J, Ward L R, Threlfall E J, Cheasty T, Rowe B. Drug resistance among Escherichia coli strains isolated from cerebrospinal fluid. J Hyg. 1982;90:195–198. doi: 10.1017/s0022172400028850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman J A, Wass C, Stins M F, Kim K S. The capsule supports survival but not traversal of Escherichia coli K1 across the blood-brain barrier. Infect Immun. 1999;67:3566–3570. doi: 10.1128/iai.67.7.3566-3570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman J A, Badger J L, Zhang Y, Huang S-H, Kim K S. Escherichia coli K1 aslA contributes to invasion of brain microvascular endothelial cells in vitro and in vivo. Infect Immun. 2000;68:5062–5067. doi: 10.1128/iai.68.9.5062-5067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang S-H, Chen Y-H, Fu Q, Stins M, Wang Y, Wass C, Kim K S. Identification and characterization of an Escherichia coli invasion gene locus, ibeB, required for penetration of brain microvascular endothelial cells. Infect Immun. 1999;67:2103–2109. doi: 10.1128/iai.67.5.2103-2109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang S-H, Wass C A, Fu Q, Prasadarao P N V, Stins M, Kim K S. Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect Immun. 1995;63:4470–4475. doi: 10.1128/iai.63.11.4470-4475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim K S. Escherichia coli invasion of brain microvascular endothelial cells as a pathogenetic basis of meningitis. Subcell Biochem. 2000;33:47–59. doi: 10.1007/978-1-4757-4580-1_3. [DOI] [PubMed] [Google Scholar]
- 22.Kim K S, Itabashi H, Gemski P, Sadoff J, Warren R L, Cross A S. The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat. J Clin Investig. 1992;90:897–905. doi: 10.1172/JCI115965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim K S, Wass C A, Cross A S. Blood brain barrier permeability during the development of experimental bacterial meningitis in the rat. Exp Neurol. 1997;45:253–257. doi: 10.1006/exnr.1997.6458. [DOI] [PubMed] [Google Scholar]
- 24.Korhonen T K, Valtonen M V, Parkkinen J, Vaisanen-Rhen V, Finne J, Ørskov F, Ørskov I, Svenson S B, Mäkelä P H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985;48:486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leib S L, Tauber M G. Pathogenesis of bacterial meningitis. Infect Dis Clin N Am. 1999;13:527–548. doi: 10.1016/s0891-5520(05)70093-3. [DOI] [PubMed] [Google Scholar]
- 26.Moxon E R, Ostrow P T. Haemophilus influenzae meningitis in infant rats: role of bacteremia in pathogenesis of age-dependent inflammatory responses in cerebrospinal fluid. J Infect Dis. 1977;135:303–307. doi: 10.1093/infdis/135.2.303. [DOI] [PubMed] [Google Scholar]
- 27.Nizet V, Kim K S, Stins M, Jonas M, Chi E Y, Nguyen D, Rubens C E. Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun. 1997;65:5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersdorf R G, Swarner D R, Garcia M. Studies on the pathogenesis of meningitis. II. Development of meningitis during pneumococcal bacteremia. J Clin Investig. 1962;41:320–327. doi: 10.1172/JCI104485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasadarao N V, Wass C A, Huang S-H, Kim K S. Identification and characterization of a novel Ibe10 binding protein that contributes to Escherichia coli invasion of brain microvascular endothelial cells. Infect Immun. 1999;67:1131–1138. doi: 10.1128/iai.67.3.1131-1138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasadarao N V, Wass C A, Stins M F, Shimada H, Kim K S. Outer membrane protein A-promoted actin condensation of brain microvascular endothelial cells is required for Escherichia coli invasion. Infect Immun. 1999;67:5775–5783. doi: 10.1128/iai.67.11.5775-5783.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasadarao N V, Wass C A, Kim K S. Endothelial cell GlcNAcβ1-4GlcNAc epitopes for outer membrane protein A enhance traversal of Escherichia coli across the blood-brain barrier. Infect Immun. 1996;64:154–160. doi: 10.1128/iai.64.1.154-160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prasadarao N V, Wass C A, Weiser J N, Stins M F, Huang S-H, Kim K S. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64:146–153. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quagliarello V Q, Scheld W M. Bacterial meningitis: pathogenesis, pathophysiology and progress. N Engl J Med. 1992;327:864–871. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- 34.Reddy M A, Prasadarao N V, Wass C A, Kim K S. Phosphatidylinositol 3-kinase activation and interaction with focal adhesion kinase in Escherichia coli K1 invasion of human brain microvascular endothelial cells. J Biol Chem. 2000;275:36769–36774. doi: 10.1074/jbc.M007382200. [DOI] [PubMed] [Google Scholar]
- 35.Reddy M A, Wass C A, Kim K S, Schlaepfer D D, Prasadarao N V. Involvement of focal adhesion kinases in Escherichia coli invasion of human brain microvascular endothelial cells. Infect Immun. 2000;68:6425–6430. doi: 10.1128/iai.68.11.6423-6430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ring A, Weiser J N, Tuomanen E I. Pneumococcal trafficking across the blood brain barrier. Molecular analysis of a novel bi-directional pathway. J Clin Investig. 1998;102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robbins J B, McCracken G H, Jr, Gotschlich E C, Orskov F, Orskov I, Hanson L A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974;290:1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- 38.Rode C K, Melkerson-Watson L J, Johnson A T, Bloch C A. Type-specific contributions to chromosome size differences in Escherichia coli. Infect Immun. 1999;19:230–236. doi: 10.1128/iai.67.1.230-236.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor 1. Nature. 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 40.Shen Y, Naujokas M, Park M, Ireton K. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell. 2000;103:501–510. doi: 10.1016/s0092-8674(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 41.Smith A L, Smith D H, Averill D R, Marino J, Moxon E R. Production of Haemophilus influenzae b meningitis in infant rats by intraperitoneal inoculation. Infect Immun. 1973;8:278–290. doi: 10.1128/iai.8.2.278-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stins M F, Badger J L, Kim K S. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb Pathog. 2001;30:19–28. doi: 10.1006/mpat.2000.0406. [DOI] [PubMed] [Google Scholar]
- 43.Stins M F, Nemani P V, Wass C, Kim K S. Escherichia coli binding to and invasion of brain microvascular endothelial cells derived from humans and rats of different ages. Infect Immun. 1999;67:5522–5525. doi: 10.1128/iai.67.10.5522-5525.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan T D, LaScolea L J., Jr Neisseria meningitidis bacteremia in children: quantitation of bacteremia and spontaneous clinical recovery without antibiotic therapy. Pediatrics. 1987;80:63–67. [PubMed] [Google Scholar]
- 45.Sullivan T D, LaScolea L J, Neter E. Relationship between the magnitude of bacteremia in children and the clinical disease. Pediatrics. 1982;69:699–702. [PubMed] [Google Scholar]
- 46.Tuomanen E. Entry of pathogens into the central nervous system. FEMS Microbiol Rev. 1996;18:289–299. doi: 10.1111/j.1574-6976.1996.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Huang S-H, Wass C A, Stins M F, Kim K S. The gene locus yijP contributes to Escherichia coli K1 invasion of brain microvascular endothelial cells. Infect Immun. 1999;67:4751–4756. doi: 10.1128/iai.67.9.4751-4756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]