Abstract
Non-clear cell renal cell carcinoma (nccRCC) represents a heterogeneous histological group which is 20–25% of those with renal cell carcinoma (RCC). Patients with nccRCC have limited therapeutic options due to their exclusion from phase III randomized trials. The aim of the present study was to investigate the effectiveness and tolerability of pembrolizumabaxitinib combination in chromophobe and papillary metastatic RCC (mRCC) patients enrolled in the I-RARE (Italian Registry on rAre genitor-uRinary nEoplasms) observational ongoing study (Meet-URO 23). Baseline characteristics, objective response rate (ORR), disease control rate (DCR) and progression-free survival (PFS) and toxicities were retrospectively and prospectively collected from nccRCC patients treated in 14 Italian referral centers adhering to the Meet-Uro group, from December 2020 to April 2022. Only patients with chromophobe and papillary histology were considered eligible for the present pre-specified analysis. There were 32 eligible patients who received pembrolizumab-axitinib as first-line treatment, of whom 13 (40%) had chromophobe histology and 19 (60%) were classified as papillary RCC. The DCR was 78.1% whereas ORR was 43.7% (11 patients achieved stable disease and 14 patients obtained partial response: 9/19 papillary, 5/13 chromophobe). Six patients (18.7%) were primary refractory. Median PFS was 10.8 months (95%CI 1.7–11.5). Eleven patients (34.3%) interrupted the full treatment due to immune-related adverse events (irAEs): G3 hepatitis (n = 5), G3 hypophisitis (n = 1), G3 diarrhea (n = 1), G3 pancreatitis (n = 1), G3 asthenia (n = 1). Twelve patients (37.5%) temporarily interrupted axitinib only due to persistent G2 hand-foot syndrome or G2 hypertension. Pembrolizumab-axitinib combination could be an active and feasible first-line treatment option for patients with papillary or chromophobe mRCC.
Keywords: non-clear renal cell carcinoma, cromophobe renal cell carcinoma, papillary renal cell carcinoma, pembrolizumab, axitinib, renal cell carcinoma, combination
1. Introduction
According to the World Health Organization (WHO) 2016 histological classification of renal tumors, non-clear cell renal cell carcinoma (nccRCC) represents a heterogeneous histological group, which is 20–25% of those with RCC [1]. Papillary and chromophobe RCC account for 80% of non-clear RCC and harbor histological, chromosomal alterations and molecular pathway different from clear cell RCC (ccRCC) and other nccRCC [2].
Papillary RCCs can be classified into two clinically and biologically distinct subtypes, type 1 RCCs associated to MET or EGFR alterations and type 2 RCCs with aggressive behavior and associated to CDKN2A, SETD2 mutations [3]. Chromophobe RCCs are histologically similar to oncocytoma and frequently harbor mutations in TP53, mTOR and PTEN [4].
Patients with nccRCC have limited therapeutic options due to their exclusion from phase III randomized trials. Small prospective phase II trials investigated the role of everolimus and sunitinib in papillary and chromophobe RCC [5,6]. Crizotinib was shown to achieve an objective response and long-lasting disease control in patients with type 1 papillary RCC with MET mutations or amplification [7], whereas recently, cabozantinib demonstrated better activity in terms of the objective response rate (ORR) and progression-free survival (PFS) in papillary metastatic RCC (mRCC) compared to sunitinib [8].
The aim of the present study was to investigate the effectiveness and tolerability of the pembrolizumab-axitinib combination as a first-line treatment in chromophobe and papillary-metastatic RCC patients enrolled in the I-RARE study (Meet-URO 23).
2. Results
2.1. Overall Population
Thirty-two eligible patients received pembrolizumab-axitinib combination as first-line treatment in 14 centers adhering to the Meet-Uro network. Data collection was retrospective for 6/32 patients and prospective for 26/32 patients. Thirteen patients had chromophobe histology whereas 19 were classified as papillary RCC. The characteristics of the patients are reported in Table 1.
Table 1.
Characteristics of patients. IMDC (International Metastatic RCC Database Consortium); ECOG PS (Eastern Cooperative Oncology Group Performance Status); yrs (years); met (metastasis).
| N (%) | |
|---|---|
| Median Age | 68 yrs |
| M | 23/32 (72) |
| F | 9/32 (28) |
| Chromophobe RCC | 13/32 (40) |
| Papillary RCC | 19/32 (60) |
| Sarcomatoid features | 3/32 (9) |
| IMDC score | |
| good | 4/32 (12) |
| intermediate | 20/32 (62) |
| Poor | 8/32 (25) |
| Previous Nephrectomy | 23/32 (72) |
| Cytoreductive Nephrectomy | 17/32 (53) |
| ECOG PS | |
| 0 | 21/32 (65) |
| 1 | 7/32 (22) |
| 2 | 5/32 (13) |
| Synchronous metastatic disease | 13/32 (40) |
| Bone met | 4/32 (12) |
| Liver met | 13/32 (40) |
| Lung met | 8/32 (25) |
| Nodes met | 24/32 (75) |
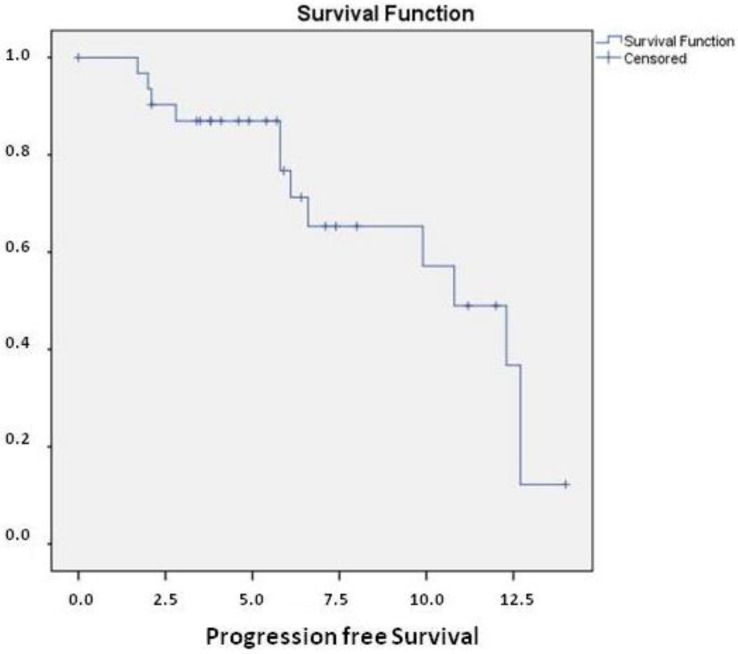
At a median follow-up of 7.1 months (95%CI 5.0–9.1), median PFS (mPFS) was 10.8 months (95%CI 7.8–13.7) (Figure 1).
Figure 1.
median Progression Free Survival (mPFS) in the overall population was 10.8 months (95%CI 7.8–13.7).
ORR was 43.7% whereas the DCR was 78.1% (11 patients achieved SD and 14 patients obtained PR as best response). Six patients (18.7%) were primary refractory (Table 2).
Table 2.
Radiological Response in overall population and according to different histologies.
| Overall Population (n = 32) |
Chromophobe Histology (n = 13) |
Papillary Histology (n = 19) |
|
|---|---|---|---|
| CR | 0 | 0 | 0 |
| PR | 14 (43.8) | 5 (16.1) | 9 (19.3) |
| SD | 11 (34.3) | 5 (19.3) | 6 (16.1) |
| PD | 6 (18.8) | 2 (12.9) | 4 (6.4) |
| NE | 1 (3.1) | 1 (3.1) | |
| DCR | 78.1% | 78.9% | 76.9% |
| ORR | 43.7% | 41.6% | 47.3% |
CR (Complete Response); PR (Partial Response); SD (Stable Disease); PD (Progressive Disease); NE (Not Evaluable); DCR (Disease Control Rate); ORR (Objective Response Rate).
The median duration of treatment was 7.5 months (95%CI 6.3–8.7).
The median OS was not reached and 94.6% of patients were alive at 1 year.
Eleven patients (34.3%) interrupted the full treatment due to immune-related adverse events (irAEs): G3 hepatitis (n = 5), G3 hypophisitis (n = 1), G3 pancreatitis (n = 1), G3 diarrhea (n = 3), 1 asthenia G3; 12 (37.5%) patients interrupted axitinib due to persistent G2 hand-foot syndrome or G2 hypertension.
2.2. Chromophobe Renal Cell Carcinoma
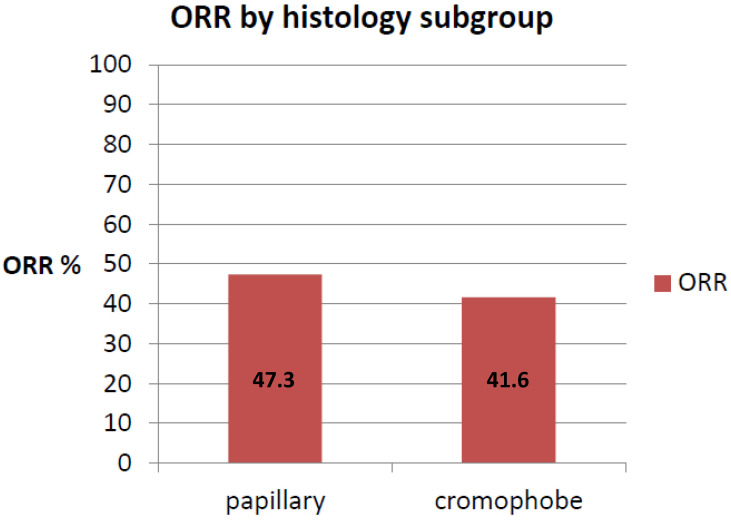
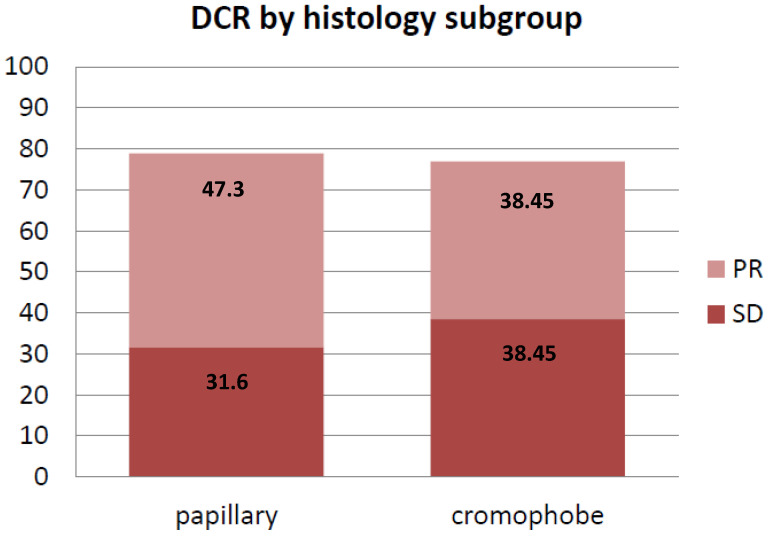
In chromophobe RCC patients, mPFS was not reached. Those experiencing disease progression at one year were 4/13 (30.7%). ORR was 41.6% whereas the DCR was 76.9% (five patients achieved PR and five patients achieved SD) (Figure 2 and Figure 3). Two patients were primary refractory (Table 2). One patient was not evaluable due to rapid clinical deterioration.
Figure 2.
ORR according to histology subgroup.
Figure 3.
DCR according to histology subgroup.
2.3. Papillary Renal Cell Carcinoma
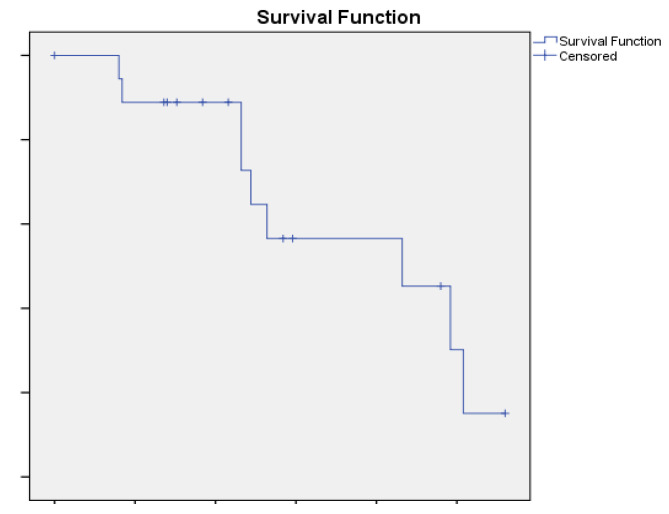
In papillary RCC patients, mPFS was 10.8 months (95% CI4.48–17.12, Figure 4). ORR was 47.3% whereas the DCR was 78.9% (9 patients had PR whereas 6 patients had SD) (Figure 2 and Figure 3). Four patients had progressive disease (PD) as best response (Table 2).
Figure 4.
median Progression Free Survival (mPFS) in the papillary RCC patients was 10.8 months (95%CI 4.48–17.12).
3. Discussion
Chromophobe and papillary renal cell carcinoma are a rare subtype of RCC, classified as nccRCC, that account for 5% and 10–15% of RCC cases, respectively [9,10]. Favorable prognosis is attributed to papillary type 1 and chromophobe-localized RCC whose cancer specific survivals at 5 years are 87.4% and 86.7% compared to 68.9% for ccRCC [11]. Otherwise, papillary type 2 is more aggressive and tend to rapid metastatic spread [12].
The choice of therapy for nccRCC is often a challenge and limited evidence is available to guide clinicians in treatment, due to its relative rarity. Although immune-checkpoint inhibitors (ICI) based combinations can be considered as standard of care for metastatic RCC [13], pivotal trials included only patents with ccRCC, therefore nccRCC have limited personalized treatments [14,15].
Recently, Procopio et al. demonstrated the efficacy of cabozantinib in metastatic collecting duct carcinoma (CDC) [16]. The study prospectively enrolled patients for central pathology review and was a breakthrough for the treatment of nccRCC considering the rarity of disease and that the only prospective trial for CDC was dated 2007 and included patients treated with platinum-based chemotherapy [16].
Pale et al., in a randomized phase II trial including only patients with papillary mRCC, reported a significant improvement in both PFS and response rate with the dual VEGF/MET inhibitor cabozantinib compared to sunitinib (9.0 months (95% CI6–12) vs. 5.6 months (95%CI 3–7), respectively) [8]. For papillary and chromophobe RCC, ESMO, and NCCN, guidelines still suggest tyrosine-kinase-inhibitors (TKI) monotherapy or everolimus, but the results of these approaches demonstrate that more effective treatment options for nccRCC are needed. Indeed, enrollment in clinical trials is recommended [17,18].
To date, pembrolizumab-axitinib combination has been demonstrated to prolong PFS, OS, and ORR in metastatic ccRCC compared to sunitinib. Nevertheless, papillary and chromophobe RCC represent different renal malignancy and treatments should not be habitually extrapolated from RCC. There are biological differences between clear and non-clear RCC that raise some doubts about transposing our knowledge for ccRCC to nccRCC. Nevertheless, pembrolizumab monotherapy showed an attractive ORR in papillary and chromophobe RCC (28.8% and 9.5%, respectively) and 4.2 months (95% CI 2.9–5.6) as PFS in a phase II prospective trial [19].
Lately, Graham et al. reported the outcome of nccRCC patients treated with IO, reporting improvement in OS compared to TKI monotherapy. Despite 49 papillary and 28 chromophobe patients being treated with the ICI-based combination, only 14 patients were treated with the IO-TKI combination [15] with limited data supporting this association.
Despite the rarity of these subtypes of RCC, we collected 32 patients treated with pembrolizumab-axitinib combination with a median follow-up of 7.1 months, which is consistent to previous reports in literature. In our cohort of previously untreated patients with chromophobe or papillary mRCC, pembrolizumab-axitinib combination showed therapeutic potential with an overall ORR of 43.7%, DCR of 78.1% and mPFS of 10.8 months. The subgroup analysis for different histology showed promising ORR and DCR in both groups, 47.3% and 78.9% in papillary patients and 41.6% and 76.9% for chromophobe patients, respectively.
The safety profile of pembrolizumab-axitinib combination in the present study seems consistent with what has been observed in the KEYNOTE-426 trial [20] that showed in the experimental arm a discontinuation rate of either drugs in 30.5% of patients and reported arterial hypertension as the most common AE.
The limits of our trial include the short follow-up, a lack of central radiological and histological revision, and the partially retrospective collection of data. Indeed, an ambispective trial is not ideal to study the effectiveness and tolerability of IO-combination because some data have been collected retrospectively, within the limit of a retrospective collection.
Based on our results, pembrolizumab-axitinib combination could be considered a potential option for chromophobe and papillary RCC patients.
4. Materials and Methods
Baseline characteristics, ORR, disease control rate (DCR), PFS, and toxicities were retrospectively and prospectively collected from nccRCC patients treated in 14 Italian referral centers adhering to the Meet-Uro group, from December 2020 to April 2022. Only patients with chromophobe or papillary histology treated with the pembrolizumab-axitinib combination as first line were considered eligible for the present analysis. Data were extracted from patients enrolled in the I-RARE study (Meet-URO 23).
Meet-URO23 (I-RARE study) is a multicentre trial aimed to collect data about rare genitourinary cancers. The registry is an ambispective observational real-world collection of patient characteristics, treatment and outcome. The study was approved by the ethics committee of Istituto Oncologico Veneto, Padua, Italy (No. 2021/19/PU).
Written informed consent was provided by all the patients or a legally authorized representative. All participating centers received local ethics approval for data collections. The study was conducted in accordance with good clinical practice and the Declaration of Helsinki.
Considering the explorative intent of this observational study a sample size calculation was not provided.
Primary endpoint was the PFS, defined as the time from the start of pembrolizumab-axitinib combination to radiological or clinical progression or death, whichever occurred first. Real-world physician-assessed progression and response were based on radiographic criteria using Response Evaluation Criteria in Solid Tumors (RECIST) guidelines [21], with imaging assessments occurring about every three months. In case of missing radiological assessment due to rapid clinical deterioration, clinical criteria were used to define progression. PFS is considered as an important measure of treatment benefit and can be evaluated earlier with fewer patients and no confounding data due to subsequent treatment.
Secondary endpoints were ORR, DCR, overall survival (OS) and toxicity. ORR included partial and complete responses (CR+PR), whereas DCR includes ORR plus stable diseases (SD) as best responses. OS was calculated from the pembrolizumab combination start to death for any cause. Toxicities were measured according to the Common Terminology Criteria for Adverse Events, version 5.0 (CTCAE v 5.0)
Patients without progression or death were considered censored at the date of last follow-up.
Baseline demographic and clinical characteristics have been described using frequencies and percentages for categorical variables. Descriptive analysis was made using median values and ranges. Survival curves were built by the Kaplan–Meier method. All statistical analyses were performed using SPSS software (version 19.00, SPSS, Chicago, IL, USA). The median follow-up was calculated using the reverse Kaplan–Meier method [22].
5. Conclusions
Pembrolizumab-axitinib combination is an effective option for chromophobe and papillary RCC but further studies, including only these subtypes of RCC, are needed to prospectively confirm the efficacy of novel treatments with combinations for non-clear RCC.
Author Contributions
Conceptualization, M.S. (Marco Stellato), M.M., G.P., E.V. and D.S.; Formal analysis, M.S. (Marco Stellato) and E.V.; Investigation, M.S. (Marco Stellato), S.B., M.M., A.M. (Andrea Malgeri) and A.M. (Alessia Mennitto); Resources, M.M., F.P., U.D.G., R.I., P.E., V.P., A.M. (Alessia Mennitto), A.C., M.S. (Matteo Santoni), C.C. and L.F.; Data curation, M.B., U.D.G., M.D.N., R.I., M.G.V. and P.E.; Writing—original draft, M.S. (Marco Stellato) and S.B.; Writing—review & editing, S.B., M.B., U.D.G., B.A.M., G.P. and E.V.; Visualization, L.F.; Supervision, G.P. and E.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Istituto Oncologico Veneto IOV–IRCCS of Padua (protocol code 2019/19/PU—22 February 2021 date of approval).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Moch H., Cubilla A.L., Humphrey P.A., Reuter V.E., Ulbright T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Hsieh J.J., Purdue M.P., Signoretti S., Swanton C., Albiges L., Schmidinger M., Heng D.Y., Larkin J., Ficarra V. Renal cell carcinoma. Nat. Rev. Dis. Prim. 2017;3:17009. doi: 10.1038/nrdp.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linehan W.M., Spellman P.T., Ricketts C.J., Creighton C.J., Fei S.S., Davis C., Wheeler D.A., Murray B.A., Schmidt L., Vocke C.D., et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N. Engl. J. Med. 2016;374:135–145. doi: 10.1056/NEJMoa1505917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis C.F., Ricketts C.J., Wang M., Yang L., Cherniack A.D., Shen H., Buhay C., Kang H., Kim S.C., Fahey C.C., et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell. 2014;26:319–330. doi: 10.1016/j.ccr.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong A.J., Halabi S., Eisen T., Broderick S., Stadler W.M., Jones R.J., Garcia J.A., Vaishampayan U.N., Picus J., Hawkins R.E., et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): A multicentre, open-label, randomised phase 2 trial. Lancet. Oncol. 2016;17:378–388. doi: 10.1016/S1470-2045(15)00515-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tannir N.M., Jonasch E., Albiges L., Altinmakas E., Ng C.S., Matin S.F., Wang X., Qiao W., Dubauskas Lim Z., Tamboli P., et al. Everolimus Versus Sunitinib Prospective Evaluation in Metastatic Non-Clear Cell Renal Cell Carcinoma (ESPN): A Randomized Multicenter Phase 2 Trial. Eur. Urol. 2016;69:866–874. doi: 10.1016/j.eururo.2015.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schöffski P., Wozniak A., Escudier B., Rutkowski P., Anthoney A., Bauer S., Sufliarsky J., van Herpen C., Lindner L.H., Grünwald V., et al. Crizotinib achieves long-lasting disease control in advanced papillary renal-cell carcinoma type 1 patients with MET mutations or amplification. EORTC 90101 CREATE trial. Eur. J. Cancer. 2017;87:147–163. doi: 10.1016/j.ejca.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Pal S.K., Tangen C., Thompson I.M.J., Balzer-Haas N., George D.J., Heng D.Y.C., Shuch B., Stein M., Tretiakova M., Humphrey P., et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: A randomised, open-label, phase 2 trial. Lancet. 2021;397:695–703. doi: 10.1016/S0140-6736(21)00152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe H., Okada M., Kaji Y., Satouchi M., Sato Y., Yamabe Y., Onaya H., Endo M., Sone M., Arai Y. New response evaluation criteria in solid tumours-Revised RECIST Guideline (version 1.1) Jpn. J. Cancer Chemother. 2009;36:2495–2501. [PubMed] [Google Scholar]
- 10.Mendhiratta N., Muraki P., Sisk A.E.J., Shuch B. Papillary renal cell carcinoma: Review. Urol. Oncol. 2021;39:327–337. doi: 10.1016/j.urolonc.2021.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Garje R., Elhag D., Yasin H.A., Acharya L., Vaena D., Dahmoush L. Comprehensive review of chromophobe renal cell carcinoma. Crit. Rev. Oncol. Hematol. 2021;160:103287. doi: 10.1016/j.critrevonc.2021.103287. [DOI] [PubMed] [Google Scholar]
- 12.Cheville J.C., Lohse C.M., Zincke H., Weaver A.L., Blute M.L. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am. J. Surg. Pathol. 2003;27:612–624. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Pignot G., Elie C., Conquy S., Vieillefond A., Flam T., Zerbib M., Debré B., Amsellem-Ouazana D. Survival analysis of 130 patients with papillary renal cell carcinoma: Prognostic utility of type 1 and type 2 subclassification. Urology. 2007;69:230–235. doi: 10.1016/j.urology.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 14.Stellato M., Santini D. ImmuneOncology-tyrosine kinase inhibitors combination, sometimes less is more. Int. Immunopharmacol. 2021;98:107673. doi: 10.1016/j.intimp.2021.107673. [DOI] [PubMed] [Google Scholar]
- 15.Sepe P., Ottini A., Pircher C.C., Franza A., Claps M., Guadalupi V., Verzoni E., Procopio G. Characteristics and Treatment Challenges of Non-Clear Cell Renal Cell Carcinoma. Cancers. 2021;13:3807. doi: 10.3390/cancers13153807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham J., Wells J.C., Dudani S., Gan C.L., Donskov F., Lee J.-L., Kollmannsberger C.K., Meza L., Beuselinck B., Hansen A., et al. Outcomes of patients with advanced non-clear cell renal cell carcinoma treated with first-line immune checkpoint inhibitor therapy. Eur. J. Cancer. 2022;171:124–132. doi: 10.1016/j.ejca.2022.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Procopio G., Sepe P., Claps M., Buti S., Colecchia M., Giannatempo P., Guadalupi V., Mariani L., Lalli L., Fucà G., et al. Cabozantinib as First-line Treatment in Patients With Metastatic Collecting Duct Renal Cell Carcinoma: Results of the BONSAI Trial for the Italian Network for Research in Urologic-Oncology (Meet-URO 2 Study) JAMA Oncol. 2022;8:910–913. doi: 10.1001/jamaoncol.2022.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escudier B., Porta C., Schmidinger M., Rioux-Leclercq N., Bex A., Khoo V., Grünwald V., Gillessen S., Horwich A. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019;30:706–720. doi: 10.1093/annonc/mdz056. [DOI] [PubMed] [Google Scholar]
- 19.Powles T., Albiges L., Bex A., Grünwald V., Porta C., Procopio G., Schmidinger M., Suárez C., de Velasco G. ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021;32:1511–1519. doi: 10.1016/j.annonc.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 20.McDermott D.F., Lee J.-L., Ziobro M., Suarez C., Langiewicz P., Matveev V.B., Wiechno P., Gafanov R.A., Tomczak P., Pouliot F., et al. Open-Label, Single-Arm, Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Non-Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021;39:1029–1039. doi: 10.1200/JCO.20.02365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rini B.I., Plimack E.R., Stus V., Gafanov R., Hawkins R., Nosov D., Pouliot F., Alekseev B., Soulières D., Melichar B., et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 22.Schemper M., Smith T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request.