Abstract
Antibodies against three long synthetic peptides (LSPs) derived from the glutamate-rich protein (GLURP) of Plasmodium falciparum were analyzed in three cohorts from Liberia, Ghana, and Senegal. Two overlapping LSPs, LR67 and LR68, are derived from the relatively conserved N-terminal nonrepeat region (R0), and the third, LR70, is derived from the R2 repeat region. A high prevalence of antibody responses to each LSP was observed in all three areas of endemic infection. Levels of cytophilic immunoglobulin G (IgG) antibodies against both GLURP regions were significantly correlated with protection from clinical P. falciparum malaria. Protected children from the Ghana cohort possessed predominantly IgG1 antibodies against the nonrepeat epitope and IgG3 antibodies against the repeat epitope. T-cell proliferation responses, studied in the cohort from Senegal, revealed that T-helper-cell epitopes were confined to the nonrepeat region. When used as immunogens, the LR67 and LR68 peptides elicited strong IgG responses in outbred mice and LR67 also induced antibodies in mice of different H-2 haplotypes, confirming the presence of T-helper-cell epitopes in these constructs. Mouse antipeptide antisera recognized parasite proteins as determined by immunofluorescence and immunoblotting. This indicates that synthetic peptides derived from relatively conserved epitopes of GLURP might serve as useful immunogens for vaccination against P. falciparum malaria.
Passive-transfer studies have unequivocally demonstrated that antibodies from West Africans can confer protection against East African (17) and Thai (25) Plasmodium falciparum parasites, showing that antibodies play a critical role in the development of immunity to clinical malaria. Human monocytes can act cooperatively with human antibodies to exert an antibody-dependent cellular inhibition of parasite growth (ADCI) both in vitro (5, 12, 19) and in the humanized SCID mouse model (2). This cooperative effect with monocytes depends on cytophilic antibodies and may explain why cytophilic subclasses (immunoglobulin G1 [IgG1] and IgG3) predominate in protected individuals while noncytophilic types (IgG2 and IgM) are more abundant in susceptible subjects (6). The importance of cytophilic antibodies has since been confirmed by several studies (1, 26, 28, 30).
In recent years, evidence has accumulated that cytophilic antibody responses to the glutamate-rich protein (GLURP) play a role in protection against P. falciparum malaria. Affinity-purified human IgG antibodies against nonrepeat as well as repeat epitopes of GLURP inhibit parasite growth in vitro in a monocyte-dependent manner (32, 33). In addition, the predominance of cytophilic subclasses against GLURP in protected individuals was demonstrated in two independent immunoepidemiological studies (9, 20). Four B-cell epitopes have been identified in the nonrepeat R0 region as targets of human antibodies which can promote monocyte-mediated growth inhibition of P. falciparum (32). Three of these epitopes, P1, P3, and P4, are structurally related, cross-reactive, and conserved among 30 field isolates and 14 laboratory lines of P. falciparum (29, 32). Of these, P3 may be potentially the most important epitope since affinity-purified human antibodies against P3 were found able to mediate the strongest ADCI effect in vitro (32).
Progress in the field of peptide synthesis now allows the production of relatively long synthetic polypeptide (LSP) chains. Such LSPs proved to be strongly immunogenic in mice (15, 16, 23). Clinical trials have confirmed this in humans and further demonstrated the safety and efficiency of this approach for vaccine development (22, 24).
In the present study we have synthesized two LSPs covering the conserved P3 epitope in the nonrepeat R0 region and one covering the variable R2 repeat region of GLURP and investigated humoral and cellular immune responses in exposed individuals in three different areas of endemic infection. We have also examined the immunogenicity of these LSPs in mice.
MATERIALS AND METHODS
Study area, study population, and clinical surveillance.
Field studies were conducted in three different areas of endemic infection, namely, Liberia, Ghana, and Senegal. Liberian plasma samples were from a previous study (32). Ghanaian samples were from a random subpopulation consisting of 104 sickle cell trait-negative children (age range, 3 to 15 years; 54% males) drawn from a larger cohort of 300 children described in detail previously (8, 9). Susceptible subjects were defined as children with at least one episode of clinical malaria during the morbidity survey (i.e., fever of >37.5°C and parasitaemia of >5,000 parasites per μl of blood). The parasite cutoff level of 5,000 parasites/μl was based on morbidity and gave a sensitivity and specificity of 90%. Protection was defined as absence of fever in the presence of parasitemia during the survey period. The Ghanaian Ministry of Health approved the study. Senegalese samples were from Dielmo, a village situated in a malaria-holoendemic area of Senegal where malaria transmission is perennial and intense, with 100 to 300 infective bites per person per year. Sixty subjects were studied: 30 adults (aged 47.5 ± 16.7 years [mean ± standard deviation]) and 30 children (mean age, 9.8 ± 2.3 years). Epidemiological follow-up carried out during the past 10 years in this village shows that the incidence of malaria attacks decreases dramatically after 10 years of age.
Forty-five samples from healthy Danish adults never exposed to malaria served as negative controls.
Recombinant GLURP and synthetic peptides.
The four P. falciparum GLURP antigens studied included a recombinant protein from the N-terminal nonrepeat region R0 (GLURP27–500) and three synthetic peptides LR67 (GLURP85–213), LR68 (GLURP191–287), and LR70 (GLURP801–920). The choice and sequences of the three LSPs were based on previous epitope-mapping studies (32). Thus, LR67 and LR68 contain the P3 epitope (amino acids 216 to 229) and LR70 contains 3 of the 11 repeat units, which constitute the C-terminal R2 repeat region (amino acids 816 to 1091). The peptides were chemically synthesized by solid-phase 5-fluorenylmethoxycarbonyl (Fmoc) chemistry as described previously (22) and purified to homogeneity by reverse-phase high-pressure liquid chromatography using C4 or C18 columns. Analytical high-pressure liquid chromatography and mass spectrometry were used to determine the purity of the final product (which was found to be >80% pure).
Animals.
These studies were performed with female mice of different strains: C57BL/10 (H-2b), CBA/J (H-2k), A/J (H-2a), DBA/2 (H-2d), and (CF1 × BALB/c)F1. All mice were 8 to 13 weeks old.
Antibody measurements.
The levels of plasma antibodies to the GLURP antigens were measured by enzyme-linked immunosorbent assay (ELISA) as described previously (33). Briefly, microtiter plates (Nunc, Roskilde, Denmark) were coated with the recombinant R0 at 0.5 μg/ml or the peptide LR67, LR68, or LR70 at 0.2 μg/ml. The plates were incubated overnight (4°C) and blocked with 2.5% skimmed milk for 1 h. Plasma samples diluted 1:200 (IgG) or 1:50 (IgG subclasses) were added in duplicate, and the mixtures were incubated at room temperature for 1 h. The plates were washed four times between steps. Color was developed with either peroxidase-conjugated rabbit anti-human IgG (Dako, Glostrup, Denmark) or horseradish peroxidase-conjugated mouse anti-IgG1, anti-IgG2 (Zymed Laboratories Inc, San Francisco, Calif.; [clones HP6069 and HP6002]), anti-IgG3, or anti-IgG4 (The Binding Site, Birmingham, United Kingdom [clones HP6050 and HP6023]), followed by H2O2 with o-phenylenediamine (Sigma, St. Louis, Mo.). Absorbance was read at 492 nm. The mean absorbance plus 3 standard deviations for samples from unexposed control donors was used as the cutoff value. To control interplate as well as day-to-day variation, all absorbance readings were transformed to arbitrary units by defining a hyperimmune plasma pool titrated on all plates as containing 1,000 U. An absorbance of more than 3,000 was assigned this value. Immunofluorescence assay (IFA) was performed as described previously (33).
For mouse IgG subclass detection, the Mouse MonoAb ID/SP kit (Zymed) was used as specified by the manufacturer. Sera were diluted 1:200, and the plates were washed extensively with phosphate-buffered saline (PBS) plus 0.05% Tween 20 (PBST) between each incubation step. Immunoblotting was performed as described previously (3) using goat anti-mouse immunoglobulins conjugated to alkaline phosphatase (Promega, Madison, Wis.) as the secondary antibody.
Competition ELISAs.
Synthetic peptide P3 or LR67 was added at various concentrations (0.017 μM to 9.0 × 10−7 μM) to each of 10 Liberian-adult plasma samples diluted in 1.25% (wt/vol) milk powder in PBST. The plasma dilutions used in these experiments were adjusted so as to give an absorbance of approximately 2,000. The peptide-antibody mixtures were incubated overnight at 4°C, and then the reactivity to LR67-coated ELISA plates was determined.
T-cell proliferation.
Peripheral blood mononuclear cells were isolated from heparinized whole blood by Histopaque-1077 density gradient centrifugation (Sigma). Cultures were performed in 96-well round-bottom plates (Nunclon) at a final concentration of 106 cells/ml in a total volume of 200 μl of culture medium (RPMI 1640 supplemented with 10% [vol/vol] heat-inactivated pooled human serum AB [CNTS], 1 mM glutamine, 35 mM HEPES, 1% sodium pyruvate, 2 g of sodium bicarbonate per liter, 10 mg of gentamicin per liter). The LSPs were tested at a concentration of 10 μg/ml. As a positive control, purified protein derivative was used at a final concentration of 2.5 μg/ml. The plates were incubated at 37°C in a water-saturated atmosphere containing 5% CO2. [3H]thymidine (1 μCi per well) was added after 6 days of culture, 16 h before harvesting, and the radioactivity incorporated was measured by liquid scintillation counting.
Statistical analysis and data presentation.
Statistical analysis was performed using the Sigma Stat software package (Jandel Scientific Corp., San Rafael, Calif.). The Mann-Whitney rank-sum test was used to compare antibody levels and age in protected and susceptible children. The age dependence of antibody responses was analyzed by Spearman's rank correlation test (rS). Multiple logistic regression (RSEPT statistical software [http://www.ci.tuwien.ac.at/R/src/base/]) was used in regression analysis to identify factors of importance in predicting clinical protection. Differences were considered statistically significant at P < 0.05.
RESULTS
Antipeptide reactivities of IgG antibodies from Liberian, Senegalese, and Ghanaian individuals exposed to P. falciparum malaria.
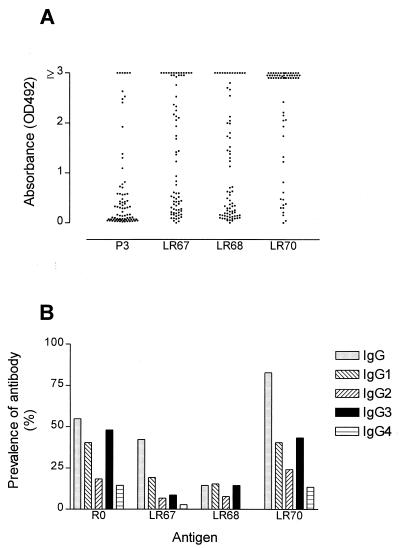
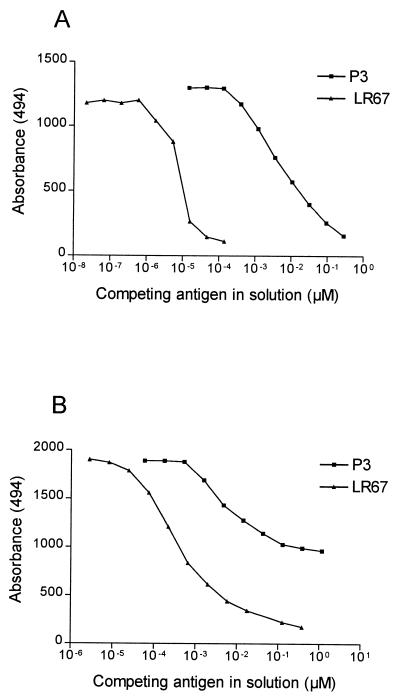
To study immune responses to both nonrepeat and repeat epitopes of GLURP, we synthesized two overlapping LSPs (LR67 and LR68) containing the conserved nonrepeat P3 B-cell epitope and one peptide (LR70) containing the R2 repeat unit. Epitopes defined by these LSPs all react with ADCI-effective human IgG antibodies (32). Antibody reactivities in plasma collected from three geographically different locations in Africa were studied. Each of the LSPs bound IgG antibodies in plasma from clinically immune Liberian adults (Fig. 1A). Of the 75 plasma samples tested, 58 (77%), 48 (64%), and 72 (96%) yielded positive reactions with the LR67, LR68, and LR70 peptides, respectively. For comparison, the anti-P3 IgG reactivity is shown for the same plasma samples. All 35 P3-reactive samples gave a positive ELISA reaction with at least one of the LR67 or LR68 peptides, and 28 samples reacted with all three constructs. Competition assays showed that the binding of antibodies to LR67 in 10 of these samples was completely (n = 7) or partially (n = 3) inhibited by prior incubation with peptide P3, suggesting that LR67 provides a good presentation of this protective epitope (Fig. 2). In agreement with this finding, antibody responses to P3 and LR67 were closely correlated (Spearman rS = 0.69 and P < 0.0001) whereas responses to P3 and LR70 were, as expected, not correlated (rS = −0.032, not statistically significant). Of the 40 P3-negative samples, 15 reacted with both LSPs, 9 reacted with LR67 only, 2 reacted with LR68 only, and 14 were negative on both peptides. Only three samples were negative in the LR70 ELISA. Two of these were also negative in the R2 ELISA, suggesting that the LR70 peptide provides an adequate presentation of the R2-repeat region.
FIG. 1.
ELISA titers against LSPs in two endemic populations. (A) Antibody levels in 75 Liberian adults clinically immune to malaria. The corresponding anti-P3 IgG levels are shown for comparison (32). The P3 epitope present on both LR67 and LR68 is a known target of ADCI-effective antibodies. (B) Prevalence of positive antibody titers to R0, LR67, LR68, and LR70 in Ghanaian children. The synthetic peptides used for the ELISAs are LR67 (GLURP85–213), LR68 (GLURP191–287), LR70 (GLURP800–919). OD492, optical density at 492 nm.
FIG. 2.
P3 is the major epitope in peptide LR67. Liberian plasma samples (n = 10) were preincubated with peptides P3 and LR67 at the indicated concentrations before being added to ELISA wells coated with LR67. Prior incubation with P3 completely (A) or partially (B) inhibited IgG antibody binding to LR67 in seven and three plasma samples, respectively.
The prevalence of antibody responses in a clinically followed-up cohort of Ghanaian children is summarized in Fig. 1B. The IgG responses to R0, LR67, and LR70 were associated with age (rS = 0.571 and P = 0.0001, rS = 0.242 and P = 0.019, and rS = 0.438, and P = 0.0001, respectively) and were associated with each other (P = 0.0001). The IgG reactivity to LR68, on the other hand, was correlated neither with age (rS = −0.058 and P = 0.580) nor with the R0-specific IgG response (rS = 0.188 and P = 0.071), probably reflecting the low prevalence of antibody to this peptide (Fig. 1B).
To determine whether these children develop IgG antibodies faster to one of the epitopes, we examined the association between age and levels of total IgG to LR67 and LR70 in a linear-regression model. The regressions equations were log (LR67-IgG) = 3.8841 + 0.0828 × age and log(LR70-IgG) = 4.1405 + 0.1256 × age. Thus, it seems that children develop antibodies to the repeat epitope 1.52 times faster than to the nonrepeat epitope (from comparison of the slopes). Regarding subclass responses, only IgG1 and IgG3 were significantly associated with age.
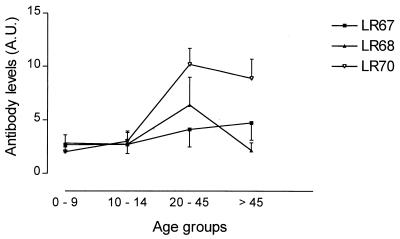
In agreement with the above data, antibody levels against the three LSPs in the Senegalese individuals were directed predominantly to LR70 (Fig. 3). The LR70 IgG titers only were significantly correlated with age (rS = 0.576 and P = 0.0001). Peptide-specific IgG responses were predominantly of the IgG1 and IgG3 subclasses (data not shown), reinforcing the previous observation obtained with recombinant GLURP antigens in the same village (20).
FIG. 3.
Mean antibody levels per age group in Senegalese individuals. A total of 46 villagers from Dielmo were divided into four age groups: 0 to 9 years (n = 10), 10 to 14 years (n = 13), 20 to 45 years (n = 12), and >45 years (n = 11). Arbitrary units (A.U.) are optical density values of the test sample divided by the mean optical density of a serum pool from donors never exposed to malaria.
GLURP antibody responses in relation to clinical protection against malaria.
Because Liberian and Senegalese samples were obtained predominantly from clinically immune individuals, associations between antibody levels and protection could be addressed only in the Ghanaian samples. Based on the clinical data, the Ghanaian cohort was divided into two groups: one group included children who experienced one or more clinical attacks during the follow-up, and the other group included the children who did not experience any attacks. We found that the protected group had higher levels of IgG to R0 and to each LSP construct than did the susceptible group (Table 1). The difference was most pronounced for the LR67- and LR70-specific IgG responses. The levels of specific cytophilic IgG subclasses (IgG1 and IgG3) were significantly higher in protected than in susceptible children (Table 1). This association was not observed for the levels of noncytophilic (IgG2 and IgG4) subclasses. In addition, we found that the levels of GLURP-specific antibodies belonging to the cytophilic subclasses were associated with protection against high levels of parasitemia. This correlation was significant for IgG1 antibodies against all GLURP regions (Spearman rS = −0.49, −0.38, −0.33, and −0.43, and P = 0.002, 0.02, 0.04, and 0.006 for GLURP-R0, LR67, LR68, and LR70, respectively) and for IgG3 antibodies against LR70 (rS = −0.33 and P = 0.04).
TABLE 1.
Levels of IgG subclass antibodies directed against different regions of GLURP in Ghanaian children protected from or susceptible to P. falciparum malaria
| Antigen | Subclass | Antibody level (median and interquartile range) in:
|
Pa | Pb | |
|---|---|---|---|---|---|
| Protected children (n = 67) | Susceptible children (n = 26) | ||||
| R0 | IgG | 535.9 (133.1–535.9) | 159.4 (60.6–535.9) | 0.016c | 0.020c |
| IgG1 | 59.2 (25.0–213.9) | 29.4 (23.2–50.6) | 0.072 | 0.120 | |
| IgG2 | 22.0 (14.8–48.6) | 17.5 (14.9–29.2) | 0.179 | 0.244 | |
| IgG3 | 64.7 (13.6–349.9) | 14.2 (11.0–73.5) | 0.015c | 0.034c | |
| IgG4 | 13.0 (10.8–19.7) | 12.7 (11.3–15.4) | 0.546 | 0.231 | |
| LR67 | IgG | 144.0 (52.6–535.9) | 52.8 (41.4–94.8) | 0.006d | 0.013c |
| IgG1 | 16.7 (11.9–62.1) | 11.3 (9.4–16.7) | 0.001d | 0.010d | |
| IgG2 | 17.5 (10.0–30.0) | 10.6 (8.1–24.2) | 0.105 | 0.245 | |
| IgG3 | 10.4 (7.8–13.2) | 7.5 (6.8–10.9) | 0.019c | 0.119 | |
| IgG4 | 10.7 (7.3–15.0) | 8.3 (7.0–15.1) | 0.656 | 0.294 | |
| LR68 | IgG | 83.7 (48.5–152.2) | 50.5 (37.0–66.5) | 0.015c | 0.021c |
| IgG1 | 19.3 (12.6–42.5) | 12.2 (9.0–24.2) | 0.007d | 0.023c | |
| IgG2 | 14.9 (10.0–28.7) | 11.0 (9.4–24.7) | 0.179 | 0.318 | |
| IgG3 | 11.0 (8.4–18.5) | 9.0 (7.8–18.6) | 0.239 | 0.475 | |
| IgG4 | 11.0 (9.1–12.9) | 11.6 (8.5–12.5) | 0.342 | 0.155 | |
| LR70 | IgG | 366.2 (127.7–535.9) | 109.9 (48.5–433.7) | 0.008d | 0.015c |
| IgG1 | 35.0 (17.6–84.0) | 20.5 (14.0–55.7) | 0.133 | 0.568 | |
| IgG2 | 25.1 (14.0–58.4) | 15.9 (9.3–77.5) | 0.164 | 0.321 | |
| IgG3 | 17.9 (10.3–70.4) | 8.5 (7.8–17.8) | 0.002d | 0.010d | |
| IgG4 | 9.7 (8.4–12.3) | 9.9 (7.8–12.0) | 0.605 | 0.232 | |
P determined from the Mann-Whitney rank sum test.
Multiple logistic-regression analysis was performed on levels of antibody after normalization by log transformation to compensate for the antibody reactivity which is due to age-related exposure to malaria.
0.01 < P < 0.05.
0.001 < P < 0.01.
Because the levels of GLURP-specific antibodies increase with age, the association between the levels of total IgG and IgG subclasses and the clinical status of the children was further analyzed in a logistic regression model with the immune responses as independent variables. This analysis showed that for all antigens, total IgG was a strong indicator of protection and, regarding IgG subclass responses, IgG1 against LR67 and LR68 and IgG3 against R0 and LR70 were the strongest predictors of protection (Table 1).
T-cell responses to LSPs in Senegalese individuals.
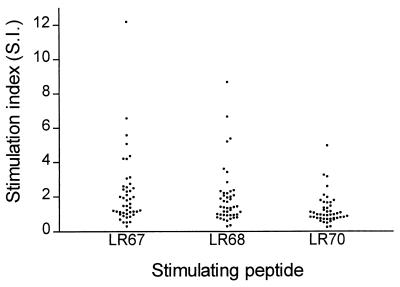
To ascertain their putative potential to elicit T-cell reactivity in humans, LSPs were tested in a lymphocyte proliferation assay using peripheral blood mononuclear cells obtained from the Senegalese individuals. A total of 49 samples from the 60 subjects gave analyzable results in the proliferation assay. Of these, 40, 34, and 10% gave a positive stimulation index of more than 2 with the LR67, LR68, and LR70 peptides, respectively (Fig. 4).
FIG. 4.
Lymphocyte proliferative responses in 46 Senegalese subjects with LSPs as stimulating antigens. A stimulation index (geometric mean cpm of stimulated cultures divided by geometric mean cpm of control cultures) of more than 2 was considered positive.
Immunogenicity of synthetic peptide constructs.
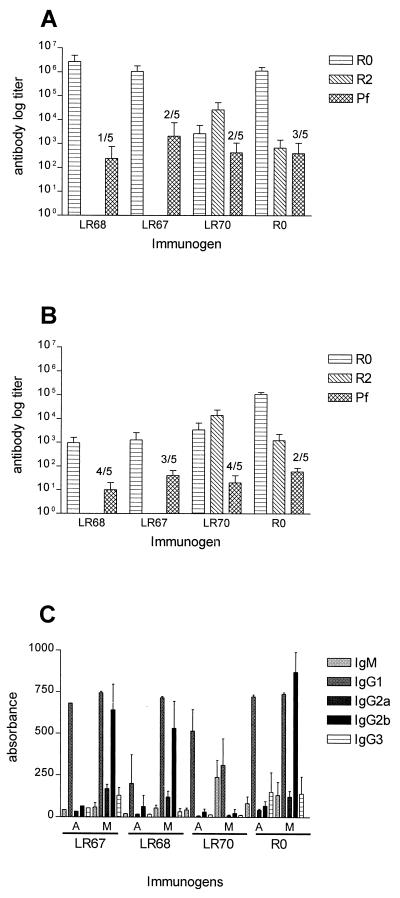
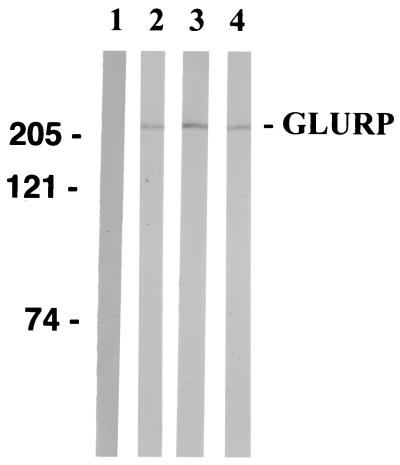
(CF1 × BALB/c)F1 mice (13) were immunized subcutaneously with each of the LSPs formulated in either alum or Montanide. Plasma samples were taken on days 0, 14, 28, and 35 and tested by ELISA. When formulated in alum, all the peptide constructs induced low antibody responses; however, when formulated in Montanide, all the peptides elicited boostable high-titer IgG responses (Fig. 5A and B). The nonrepeat sequences (LR67 and LR68) induced markedly higher antibody levels than did the repeat construct (LR70). Therefore, the experimental animals produced specific antibodies against all immunogens. LR67 and LR68 elicited predominantly IgG1 and IgG2b responses, and LR70 elicited a mixed IgG1 and IgM response (Fig. 5B). The mouse antibodies generated by the LSPs recognized a 220-kDa protein band in Western blotting using a schizont extract (Fig. 6) and bound to native parasite protein as determined by IFA on mature NF54 schizonts. Mean day 35 IFA titers for LR67-, LR68-, and LR70-immunized mice were 2,060, 440, and 250, respectively. As expected, the anti-LR70 antisera displayed reactivity with recombinant R2 in ELISA and the anti-LR67 and anti-LR68 antisera displayed strong reactivity with the recombinant R0 and with each of the two immunizing LSPs, probably due to the P3 epitope they share (four of five mice in each group recognize peptide P3), whereas they were nonreactive with LR70. In agreement with this finding, anti-LR70 antibodies did not bind to the LR67 and LR68 peptides. Nevertheless anti-LR70 antibodies also reacted with the R0 recombinant, confirming the presence of a previously observed cross-reactive epitope between R0 and the C-terminal R2 repeat region (33) and hence located outside the region defined by the two LSPs.
FIG. 5.
Antipeptide responses in mice. Groups of mice were immunized with LR67, LR68, LR70, or R0 in Montanide (A) or alum (B). Day 35 plasma samples were tested for antibody reactivity on ELISA plates coated with R0 or R2 or by IFA (pf). ELISA titer was the inverse plasma dilution giving an absorbance of 0.1. The number of animals with a negative IFA titer is indicated above the bars. (C) Isotype response of mice for which results are presented in panels A and B. The measurements represent the mean of duplicate assays from five individual mouse plasma samples. A, alum; M, Montanide.
FIG. 6.
Western blot reactivity of mouse antipeptide antisera with P. falciparum NF54 blood-stage schizont extract. Sera from mice immunized with LSPs were diluted 200-fold and used as the primary antibody. Lanes; 1, prebleed; 2, anti-LR67; 3, anti-LR68; 4, anti-LR70. The positions of molecular markers are indicated on the left in kilodaltons.
IgG responses in mice of different H-2 haplotypes immunized with LR67.
Since the prevalences of human antibody and T-cell responses were highest against LR67, we decided to focus on this antigen. Antigen recognition is influenced by the major histocompatibility complex composition of the host, and the immunogenicity of LR67 in four strains of mice representing different H-2 haplotypes was therefore investigated. Plasma samples were collected after two recall injections and tested for ELISA reactivity against the immunogen. LR67 elicited specific antibodies in all strains with inverse ELISA titers of 4.5 × 105, 6.8 × 104, 4.3 × 105, and 5.3 × 104 in A/J, C57BL/10, CBA, and DBA/2 mice, respectively. As in the first experiment, the specific IgG responses were predominantly of the cytophilic subclasses IgG1 and IgG2b (Fig. 5C).
DISCUSSION
In the present study we have examined the antigenicity and immunogenicity of three LSPs derived from two well-defined regions within P. falciparum GLURP, the relatively conserved N-terminal nonrepeat region (R0) and the immunodominant R2 repeat region. Since both regions are targeted by human IgG antibodies which are effective in ADCI (32, 33) it was proposed that cytophilic antibodies against these regions can connect merozoites to effector cells, such as monocytes, through FcγRII receptors. In the present study we have focused on GLURP peptides containing either the P3 epitope or the R2 repeat unit because both epitopes are known targets of ADCI-effective human antibodies (32, 33). Three lines of evidence suggest that R0-derived LSPs provide an efficient presentation of the P3 epitope. First, the LR67 and LR68 ELISA titers are strongly correlated with the P3 ELISA titers in Liberian plasma samples. Second, prior incubation with soluble P3 peptide can efficiently inhibit the binding of serum IgG antibodies to LR67. Full inhibition of antibody reactivity to the long peptide LR67 by the shorter P3 peptide could not be obtained in all samples, suggesting that, as expected, the LSP defines, in addition to the dominant epitope described previously for P3 (32), another minor B-cell epitope(s). Third, antibodies induced by LR67 in animals react with P3 peptide. These results support the choice of LR67 as a valuable synthetic molecule for further immunogenicity studies.
We found that levels of human IgG antibodies against all GLURP peptides are significantly associated with the absence of disease in children from Ghana. Moreover, we demonstrate that each of the three LSPs can be targeted by cytophilic antibody responses in individuals living in three different areas of endemic infection, thus validating the choice of the LSP sequences. Logistic regression models, which take into account the effect of age-related exposure, consistently identified cytophilic IgG classes (in particular IgG1 and IgG3) as strong predictors of protection in Ghanaian children. It appears that IgG1 preferentially recognizes the nonrepeat epitope in protected children whereas IgG3 recognizes the B-cell epitope in the repeat region. This confirms and extends earlier reports of isotype differences between these two regions of GLURP (9, 20). The reason for this difference is not clear; one possibility is that differences in antigen presentation could have consequences for the quality of the T-helper-cell responses to nonrepeat versus repeat sequences, which in turn might affect the Ig subclass production. The finding that children acquire IgG antibodies faster to the B-cell epitope in the R2 repeat region than to the epitope in the conserved nonrepeat region is in accord with several observations that dominant B-cell epitopes are located in the repeated GLURP regions (31, 34).
Previous studies have demonstrated that human antibodies against both repeat and nonrepeat regions of GLURP can inhibit parasite growth in vitro (32, 33). The present finding that high levels of anti-GLURP IgG1 and IgG3 antibodies correlate with low parasite densities in Ghanaian children supports the notion that anti-GLURP antibodies act in collaboration with monocytes to control parasite multiplication in vivo.
T-cell epitopes were detected most frequently in the sequences defined by LR67 and LR68 and less frequently in those defined by LR70. The precise location of T-cell epitopes within GLURP is not yet known; however, the present data demonstrate that the nonrepeat region contains one or more T-helper-cell epitopes, recognized by individuals naturally exposed to malaria. These results are reminiscent of those recorded with other malaria molecules, in which the repeat region was the main target for antibodies and in which T-cell epitopes were frequently located in the nonrepeat regions (4, 10).
Immunogenicity studies with mice confirmed the results from human T-cell studies since strong antibody responses were elicited by the two nonrepeat LSPs. Antibody titers to LR67 and LR68 were approximately 3 orders of magnitude higher than antibody titers to LR70. The relatively high prevalence of T-cell responses in immune individuals, as well as the strong immune responses consistently elicited by LR67 in five strains of mice with different H-2 haplotypes, further suggested that we might expect little genetic restriction against this immunogen.
Since the network of cross-reactivity among the many P. falciparum glutamate-rich proteins (GLURPs) has been repeatedly stressed (18), one could expect that antibodies to glutamate-rich regions may also react with other GLURPs. It is therefore remarkable that the epitopes defined by the LSPs were purely GLURP specific: they did not display cross-reactivity to any other blood-stage protein, as shown by Western blotting. Nevertheless, the previously described cross-reactive epitope that is shared between GLURP nonrepeat and repeat regions (33) was confirmed here through the reactivity of anti-LR70 antiserum with R0 recombinant.
Ideally, immunizations should elicit GLURP-specific cytophilic IgG subclasses in humans. Switching of the antibody response from IgM to one of the IgG subclasses requires cytokines secreted by different subsets of T-helper cells. It is still unknown which cytokines induce switching to the cytophilic subclasses in humans; therefore it remains to be seen whether immunization with LR67 in Montanide elicits a cytophilic antibody response in humans and one that will be ADCI effective. Montanide is among the few new adjuvants that can be used in humans (7, 11, 14, 21, 27). The combination of LR67, which is a target of IgG1 protective responses in areas of endemic infection, with Montanide was shown to induce in mice high-titer antibodies that recognized native parasite protein. Given the clinical expertise which has accumulated with Montanide and with LSP formulations (24), our results suggest that LR67 in Montanide deserves to be investigated for safety and immunogenicity in phase I clinical trials.
ACKNOWLEDGMENTS
We thank M. Paulli Andersen for technical assistance.
This work was supported by the Scientific and Technological Co-operation with the Developing Countries INCO program of the Commission of the European Communities (contract CT 950021) and The Enhancement of Research Capacity in developing countries (ENRECA) program of the Danish International Development Assistance (DANIDA) (grant 14.Dan.8.L.306). L.H. is supported by the Danish Medical Research Council (SSVF) (grant 9802405) and by the Danish Research Council for Development Research (RUF) (grant 90900).
REFERENCES
- 1.Aribot G, Rogier C, Sarthou J L, Trape J F, Balde A T, Druilhe P, Roussilhon C. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, west Africa) Am J Trop Med Hyg. 1996;54:449–457. doi: 10.4269/ajtmh.1996.54.449. [DOI] [PubMed] [Google Scholar]
- 2.Badell E, Oeuvray C, Moreno A, Soe Soe, van Rooijen N, Bouzidi A, Druilhe P. Human malaria in immunocompromised mice: an in vivo model to study defence mechanisms against Plasmodium falciparum. J Exp Med. 2000;192:1653–1659. doi: 10.1084/jem.192.11.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borre M B, Dziegiel M, Hogh B, Petersen E, Rieneck K, Riley E, Meis J F, Aikawa M, Nakamura K, Harada M, Wind A, Jakobsen P H, Cowland J, Jepsen S, Axelsen N, Vuust J. Primary structure and localization of a conserved immunogenic Plasmodium falciparum glutamate rich protein (GLURP) expressed in both the preerythrocytic and erythrocytic stages of the vertebrate life cycle. Mol Biochem Parasitol. 1991;49:119–131. doi: 10.1016/0166-6851(91)90135-s. [DOI] [PubMed] [Google Scholar]
- 4.Bottius E, BenMohamed L, Brahimi K, Gras H, Lepers J P, Raharimalala L, Aikawa M, Meis J, Slierendregt B, Tartar A, Thomas A, Druilhe P. A novel Plasmodium falciparum sporozoite and liver stage antigen (SALSA) defines major B, T helper, and CTL epitopes. J Immunol. 1996;156:2874–2884. [PubMed] [Google Scholar]
- 5.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouharoun-Tayoun H, Druilhe P. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect Immun. 1992;60:1473–1481. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cano C A. The multi-epitope polypeptide approach in HIV-1 vaccine development. Genet Anal. 1999;15:149–153. doi: 10.1016/s1050-3862(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 8.Dodoo D, Theander T G, Kurtzhals J, Koram K, Riley E, Akanmori D B, Nkrumah F K, Hviid L. Antibody levels to conserved parts of Plasmodium falciparim merozoite surface protein 1 (PfMSP1) Ghanaian children are not associated with protection from clinical malaria. Infect Immun. 1999;67:2131–2137. doi: 10.1128/iai.67.5.2131-2137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodoo D, Theisen M, Kurtzhals J A, Akanmori B D, Koram K A, Jepsen S, Nkrumah F K, Theander T G, Hviid L. Naturally acquired antibodies to the glutamate-rich protein are associated with protection against Plasmodium falciparum malaria. J Infect Dis. 2000;181:1202–1205. doi: 10.1086/315341. [DOI] [PubMed] [Google Scholar]
- 10.Fidock D A, Gras-Masse H, Lepers J P, Brahimi K, BenMohamed L, Mellouk S, Guerin-Marchand C, Londono A, Raharimalala L, Meis J F. Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J Immunol. 1994;153:190–204. . (Erratum, 153:5347.) [PubMed] [Google Scholar]
- 11.Genton B, Al Yaman F, Anders R, Saul A, Brown G, Pye D, Irving D O, Briggs W R, Mai A, Ginny M, Adiguma T, Rare L, Giddy A, Reber-Liske R, Stuerchler D, Alpers M P. Safety and immunogenicity of a three-component blood-stage malaria vaccine in adults living in an endemic area of Papua New Guinea. Vaccine. 2000;18:2504–2511. doi: 10.1016/s0264-410x(00)00036-0. [DOI] [PubMed] [Google Scholar]
- 12.Khusmith S, Druilhe P. Cooperation between antibodies and monocytes that inhibit in vitro proliferation of Plasmodium falciparum. Infect Immun. 1983;41:219–223. doi: 10.1128/iai.41.1.219-223.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klausen J, Magnusson M, Andersen A B, Koch C. Characterization of purified protein derivative of tuberculin by use of monoclonal antibodies: isolation of a delayed-type hypersensitivity reactive component from M. tuberculosis culture filtrate. Scand J Immunol. 1994;40:345–349. doi: 10.1111/j.1365-3083.1994.tb03471.x. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence G W, Saul A, Giddy A J, Kemp R, Pye D. Phase I trial in humans of an oil-based adjuvant SEPPIC MONTANIDE ISA 720. Vaccine. 1997;15:176–178. doi: 10.1016/s0264-410x(96)00150-8. [DOI] [PubMed] [Google Scholar]
- 15.Lopez J A, Gonzalez J M, Tolle R, Renggli J, Eberl G, Betschart B, Bujard H, Duombo O, Arevalo M, Herrera S, Corradin G, Roggero M A. Immunogenicity of synthetic peptides corresponding to the non-repeat regions of the Plasmodium falciparum circumsporozoite protein. Vaccines (Cold Spring Harbor) 1996;96:255–260. [Google Scholar]
- 16.Lopez J A, Gonzalez J M, Kettner A, Arevalo-Herrera M, Herrera S, Corradin G, Roggero M A. Synthetic polypeptides corresponding to the non-repeat regions from the circumsporozoite protein of Plasmodium falciparum: recognition by human T-cells and immunogenicity in owl monkeys. Ann Trop Med Parasitol. 1997;91:253–265. [PubMed] [Google Scholar]
- 17.McGregor I A, Carrington S P, Cohen S. Treatment of East African P. falciparum malaria with West African gammaglobulin. Trans R Soc Trop Med Hyg. 1963;57:170–175. [Google Scholar]
- 18.Moelans I I, Lal A A, Konings R N, Schoenmakers J G. Sequence of a 16-kilodalton sexual stage and sporozoite surface antigen of Plasmodium reichenowi and comparison with Pfs16 of Plasmodium falciparum. Mol Biochem Parasitol. 1992;50:349–350. doi: 10.1016/0166-6851(92)90232-9. [DOI] [PubMed] [Google Scholar]
- 19.Oeuvray C, Bouharoun-Tayoun H, Gras-Masse H, Bottius E, Kaidoh T, Aikawa M, Filgueira M C, Tartar A, Druilhe P. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood. 1994;84:1594–1602. [PubMed] [Google Scholar]
- 20.Oeuvray C, Theisen M, Rogier C, Trape J F, Jepsen S, Druilhe P. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect Immun. 2000;68:2617–2620. doi: 10.1128/iai.68.5.2617-2620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto L A, Berzofsky J A, Fowke K R, Little R F, Merced-Galindez F, Humphrey R, Ahlers J, Dunlop N, Cohen R B, Steinberg S M, Nara P, Shearer G M, Yarchoan R. HIV-specific immunity following immunization with HIV synthetic envelope peptides in asymptomatic HIV-infected patients. AIDS. 1999;13:2003–2012. doi: 10.1097/00002030-199910220-00002. [DOI] [PubMed] [Google Scholar]
- 22.Roggero M A, Filippi B, Church P, Hoffman S L, Blum-Tirouvanziam U, Lopez J A, Esposito F, Matile H, Reymond C D, Fasel N. Synthesis and immunological characterization of 104-mer and 102-mer peptides corresponding to the N- and C-terminal regions of the Plasmodium falciparum CS protein. Mol Immunol. 1995;32:1301–1309. doi: 10.1016/0161-5890(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 23.Roggero M A, Meraldi V, Lopez J A, Eberl G, Romero J C, Matile H, Betschart B, Corradin G, Renggli J. The synthetic, oxidized C-terminal fragment of the Plasmodium berghei circumsporozoite protein elicits a high protective response. Eur J Immunol. 2000;30:2679–2685. doi: 10.1002/1521-4141(200009)30:9<2679::AID-IMMU2679>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 24.Roggero M A, Weilenmann C, Bonelo A, Audran R, Renggli J, Spertini F, Corradin G, Lopez J A. Plasmodium falciparum CS C-terminal fragment: preclinical evaluation and phase I clinical studies. Parassitologia. 1999;41:421–424. [PubMed] [Google Scholar]
- 25.Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, Foucault C, Chongsuphajaisiddhi T, Druilhe P. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg. 1991;45:297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- 26.Sarthou J L, Angel G, Aribot G, Rogier C, Dieye A, Toure Balde A, Diatta B, Seignot P, Roussilhon C. Prognostic value of anti-Plasmodium falciparum-specific immunoglobulin G3, cytokines, and their soluble receptors in West African patients with severe malaria. Infect Immun. 1997;65:3271–3276. doi: 10.1128/iai.65.8.3271-3276.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saul A, Lawrence G, Smillie A, Rzepczyk C M, Reed C, Taylor D, Anderson K, Stowers A, Kemp R, Allworth A, Anders R F, Brown G V, Pye D, Schoofs P, Irving D O, Dyer S L, Woodrow G C, Briggs W R, Reber R, Sturchler D. Human phase I vaccine trials of 3 recombinant asexual stage malaria antigens with Montanide ISA720 adjuvant. Vaccine. 1999;17:3145–3159. doi: 10.1016/s0264-410x(99)00175-9. [DOI] [PubMed] [Google Scholar]
- 28.Shi Y P, Sayed U, Qari S H, Roberts J M, Udhayakumar V, Oloo A J, Hawley W A, Kaslow D C, Nahlen B L, Lal A A. Natural immune response to the C-terminal 19-kilodalton domain of Plasmodium falciparum merozoite surface protein 1. Infect Immun. 1996;64:2716–2723. doi: 10.1128/iai.64.7.2716-2723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stricker K, Vuust J, Jepsen S, Oeuvray C, Theisen M. Conservation and heterogeneity of the glutamate-rich protein (GLURP) among field isolates and laboratory lines of Plasmodium falciparum. Mol Biochem Parasitol. 2000;111:123–130. doi: 10.1016/s0166-6851(00)00304-2. [DOI] [PubMed] [Google Scholar]
- 30.Taylor R R, Allen S J, Greenwood B M, Riley E M. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am J Trop Med Hyg. 1998;58:406–413. doi: 10.4269/ajtmh.1998.58.406. [DOI] [PubMed] [Google Scholar]
- 31.Theisen M, Cox G, Hogh B, Jepsen S, Vuust J. Immunogenicity of the Plasmodium falciparum glutamate-rich protein expressed by vaccinia virus. Infect Immun. 1994;62:3270–3275. doi: 10.1128/iai.62.8.3270-3275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theisen M, Soe Soe, Jessing S, Okkels L, Danielsen S, Oeuvray C, Druilhe P, Jepsen S. Identification of a major linear B cell epitope of the Plasmodium falciparum Glutamate-rich protein (GLURP), targeted by human antibodies mediating parasite killing. Vaccine. 2000;19:204–212. doi: 10.1016/s0264-410x(00)00181-x. [DOI] [PubMed] [Google Scholar]
- 33.Theisen M, Soe Soe, Oeuvray C, Thomas A W, Vuust J, Danielsen S, Jepsen S, Druilhe P. The glutamate-rich protein (GLURP) of Plasmodium falciparum is a target for antibody-dependent monocyte-mediated inhibition of parasite growth in vitro. Infect Immun. 1998;66:11–17. doi: 10.1128/iai.66.1.11-17.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theisen M, Vuust J, Gottschau A, Jepsen S, Hogh B. Antigenicity and immunogenicity of recombinant glutamate-rich protein of Plasmodium falciparum expressed in Escherichia coli. Clin Diagn Lab Immunol. 1995;2:30–34. doi: 10.1128/cdli.2.1.30-34.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]