Abstract
The mucosal humoral immune response elicited following Shigella flexneri infection in patients living in Antananarivo districts (Madagascar Island) was evaluated by measuring the gut-derived, circulating immunoglobulin A (IgA) antibody-secreting cells (ASC) specific for the major bacterial antigen lipopolysaccharide (LPS). Fifty, 34, 11, and 5% of the S. flexneri-positive patients were infected with serotypes 2a, 1a, 4a, and 3a, respectively. The total number of IgA ASC in infected patients increased significantly, compared to the number in healthy controls, early after the onset of disease. The number of anti-homologous LPS IgA ASC varied among individuals and peaked between days 5 and 10 after the onset of the disease. In the S. flexneri 1a- and 2a-infected patients, the level of IgA ASC cross-reactivity to heterologous S. flexneri serotypes was weak. These data indicate that S. flexneri 2a and 1a are the predominant strains responsible for shigellosis in this area of endemicity and that the anti-LPS antibody response following natural infection is mainly directed against serotype-specific determinants.
The annual number of episodes of infection with Shigella spp. in developing countries throughout the world has been recently estimated to be 163.2 million, with 1.1 million deaths. Children younger than 5 years account for 70% of all episodes and 60% of all deaths (7). This dysenteric syndrome is caused by Shigella, an invasive enterobacterium whose virulence factors and mechanisms of colonic mucosal damage have been extensively studied (for a review, see reference 14). The disease is endemic in most of the developing countries, with Shigella flexneri and Shigella sonnei being the predominant species. However, Shigella dysenteriae 1 is responsible for devastating epidemics, possibly due to production of Shiga toxin (7).
Acquired immunity to Shigella infection is mainly characterized by the production of local secretory immunoglobulin A (IgA) and serum IgG, which are specific for some bacterial virulence proteins and for lipopolysaccharide (LPS), the major bacterial surface component (for a review, see reference 14). In an animal model, monoclonal IgAs directed against the serotype-specific determinants of the polysaccharidic O antigen (O-Ag) of the LPS are sufficient to confer protection (12). This is consistent with most of the studies reporting that the mucosal anti-LPS IgA-mediated antibody response is the major protective response against homologous reinfection (14), although recent data from a vaccine trial using a parenteral detoxified LPS conjugate suggest that serum anti-LPS IgG antibodies may also be protective (2).
Our knowledge of mucosal immune responses, especially those in the gut, in humans remains limited due to obvious limitations in investigation. In a large number of reports, the priming of local antibody responses following mucosal infection or oral vaccination has been studied by numbering specific circulating antibody-secreting cells (ASC) using an enzyme-linked immunospot assay (15). Concerning Shigella, circulating ASC have essentially been evaluated after oral immunization with live attenuated vaccines (3, 6, 8) to assess their ability to induce a mucosal immune response. Only two reports have studied, by measurement of circulating ASC directed against LPS homologous to that of the infective strain, the mucosal response induced by natural infection in areas of endemicity (10, 13). In this study, we showed that the IgA response is mainly directed against serotype-specific determinants. We therefore provide new insights into the circulating anti-LPS IgA ASC in S. flexneri-infected patients living in Madagascar, where, as in many developing countries, Shigella spp. are endemic and represent a significant cause of diarrhea (11).
MATERIALS AND METHODS
Recruitment of patients.
Patients were recruited in Antananarivo during the rainy season (between October and February) either at the Anatihazo dispensary or at the pediatric service of the Soavinandriana Hospital. All subjects suffering from diarrhea, with emission of 5 to 10 stools per day containing mucus and/or blood, and with no known previous history of shigellosis were included in the study. Stools and blood samples were collected at the time of examination (first sample). Patients positive for Shigella were invited to return to the dispensary or the hospital for follow-up and further blood collection 1 week (second sample) and 3 weeks (third sample) later. Oral rehydration serum and antimicrobial treatment (nalidixic acid, trimethoprim-sulfamethoxazole, or chloramphenicol) were given to the patients at the time of examination. Some individuals consulting at the Anatihazo dispensary for any reason other than symptoms of diarrheal disease and without any previous episode of Shigella infection were included in the control group. They were chosen to match the patient group in age and sex.
Consent was obtained from patients or their parents for the children. The human experimentation guidelines of the authors' institutions were followed in the conduct of clinical research.
Identification of the strains.
Stool samples were inoculated onto Hektoen and Salmonella-Shigella agar and incubated at 37°C for 48 h. Isolation and identification of Shigella spp. were performed by routine morphological, biochemical, and serological testing, and samples were sent for confirmation to the National Center of Salmonella and Shigella (Institut Pasteur, Paris, France).
LPS preparation.
For each Shigella species and serotype, purified LPS was prepared by the hot phenol-water method of Westphal and Jann (17).
Isolation of lymphocytes.
To study the kinetics of the appearance of IgA ASC, venous blood was collected in sterile EDTA-treated tubes at different time points after the onset of the disease for each of the recruited patients. Mononuclear cells were recovered by a Ficoll-Paque density gradient centrifugation (Pharmacia, Uppsala, Sweden). Interface cells were collected and washed three times in phosphate-buffered saline (PBS), resuspended in RPMI 1640 medium supplemented with 20% fetal calf serum (FCS) (Gibco-BRL, Cergy-Pontoise, France), and then frozen at −80°C in the presence of 20% dimethyl sulfoxide.
Detection of ASC.
Total IgA ASC and anti-LPS IgA ASC in peripheral blood mononuclear cells were enumerated using an enzyme-linked immunospot assay as previously described (15). Assays were performed only at the end of the study. Prior to use, cells were rapidly thawed to 37°C and mixed with 4 volumes of Plasmagel (Laboratoire Bellon, Neuilly Seine, France) previously diluted twice in RPMI 1640–10% FCS. Cell viability as assessed by trypan blue staining was about 70%. Briefly, 96-well plates (High-binding; Costar, Corning, N.Y.) were coated overnight with either 1 μg of purified LPS/well in carbonate buffer (pH 9.6) to detect the anti-LPS IgA ASC or 0.5 μg of goat anti-human IgA antibodies (Biosys, Compiègne, France)/well to quantify the total number of IgA ASC. Purified LPS corresponding to the infecting strain was used to enumerate IgA ASC specific for homologous LPS. IgA ASC cross-reacting with other S. flexneri serotypes were counted using LPS purified from the different serotypes tested. Wells were then treated with 1% bovine serum albumin (BSA)–PBS for 1 h at 37°C and washed three times with 0.05% Tween 20–PBS. Freshly thawed mononuclear cells (106 or 105) suspended in 100 μl of RPMI 1640–10% FCS were added to each well in duplicate or triplicate (depending on the total number of recovered cells) for 4 h at 37°C in 5% CO2. After the cells were washed three times with distilled water and then three times with 0.05% Tween 20–PBS, 100 μl of goat anti-human IgA alkaline phosphatase-conjugated antibodies (Biosys) was added at a dilution of 1:3,000 in 1% BSA–PBS for 2 h at 37°C. After three additional washes, the substrate 5-bromo-4-chloro-3-indolylphosphate (BCIP; Sigma-Aldrich Chimie, L'Isle d'Abeau Chesnes, France) was added at a concentration of 1 mg/ml in buffer consisting of 0.1 M 2-amino-2-methyl-1-propanol, 0.5 mM MgCl2, and 0.001% Triton X-405 and adjusted to pH 10.2. Spots were counted with no agar addition after 18 h of incubation in the dark at room temperature.
Statistical analysis.
The mean number of total IgA ASC/106 PBMC and the standard error were calculated for each group of patients. Mean numbers of anti-homologous LPS IgA ASC/106 PBMC and standard errors were calculated for each patient and correspond to duplicate or triplicate samples from two independent experiments. The significance of differences between groups was analyzed with Student's t test or the Kendall rank test. Differences were considered significant when P values were <0.05.
RESULTS AND DISCUSSION
Bacterial strain prevalence in the recruited patients.
Of the 56 recruited patients with clinical symptoms of diarrhea and no known prior episode of clinical dysentery, 32 were infected with Shigella species, 2 were infected with Salmonella enterica serovar Typhi, and 22 were negative for bacteria. Among the Shigella-infected patients, 21 were infected with S. flexneri, 10 were infected with S. dysenteriae 1, and 1 was infected with S. sonnei. The most representative serotypes in the S. flexneri-infected patients were serotypes 2a (9 of 18) and 1a (6 of 18), with serotypes 4a and 3a being less frequently encountered (5 and 1 of 18, respectively). These results are in accordance with data recently published on the global burden of Shigella infection and point to S. flexneri 2a as being the major strain encountered in the developing countries (7). In contrast, the median percentage of S. dysenteriae 1-infected subjects was higher in our study than that reported for other areas of endemicity (7). Increasing numbers of S. dysenteriae 1 cases were noted in Madagascar during the last 10 years (Unit of Diarrheal Diseases, Institut Pasteur of Antananarivo, unpublished data), with a concomitant dramatic increase of multiantibiotic resistance (1). S. sonnei was less frequently encountered in our study than usually reported (7).
Clinical data for the S. flexneri-infected patients.
The main problem in such a study is to get a complete set of samples. Usually, samples are easily recovered at the onset of the disease after informed written consent of the patients who are consulting. But it is much more difficult to have them come back for recovery of further samples during the time course of infection, particularly if the antibiotic treatment has been efficient. Consequently, complete sets of samples were recovered from only 10 out of the 21 S. flexneri-infected patients and none were recovered from the S. dysenteriae 1- and S. sonnei-infected patients. Clinical features of the 10 S. flexneri-infected patients included in the study are as follows. S. flexneri 1a-infected patients 1 to 3 were 50 (female), 26 (male), and 16 (female) years old, respectively. Patients 4 to 8 were infected with S. flexneri 2a and were 16 (female), 20 (female), 75 (male), 41 (female), and 48 (female) years old, respectively. Patients 9 (male, 4 years old) and 10 (female, 47 years old) were infected with S. flexneri 4a. All of them received oral rehydration serum and antibiotic treatment (metronidazole, chloramphenicol, or trimethoprim-sulfamethoxazole) except patients 1 and 9, who were not given antibiotics at the onset of the disease. Patient 4 was given a second antibiotic treatment (nalidixic acid) since symptoms persisted 1 week after the first treatment.
Total IgA ASC in S. flexneri-infected patients.
Induction of the mucosal response in the S. flexneri-infected patients was monitored during the course of infection. Blood samples were first collected on admission (i.e., between 2 and 4 days after onset [first sample]) and then in the course of infection (1 week later [second sample]) and at the convalescence stage (3 weeks later [third sample]). As a complete set of samples was obtained for 10 S. flexneri-infected patients, 10 subjects were enrolled as a control group (see Materials and Methods). At any given time point during the course of infection, the total IgA ASC number in the infected patients varied considerably between individuals. In contrast, control subjects showed a more constant level of total IgA ASC. Moreover, at the onset of the disease (first sample), the mean number of total IgA ASC was significantly higher in the S. flexneri-infected patients (373 ± 387 IgA ASC/106 PBMC) than in the control subjects (87 ± 53 IgA-ASC/106 PBMC; P = 0.016). During the infection (second sample) and in the convalescence stage (third sample), the mean number of total IgA ASC slightly decreased and the difference between infected patients and healthy controls became nonsignificant (second sample, 197 ± 223 IgA ASC/106 PBMC; third sample, 184 ± 284 IgA ASC/106 PBMC; P = 0.07 and 0.14, respectively). To summarize, the total IgA ASC number increases at the onset of the disease but less than 10% of total IgA ASC are specific for Shigella LPS. This has been observed by Kantele et al. (5), who suggested that a polyclonal activation might occur at the onset of diarrheal diseases, leading to the proliferation of ASC with diverse specificities.
IgA ASC specific for homologous LPS in S. flexneri-infected patients.
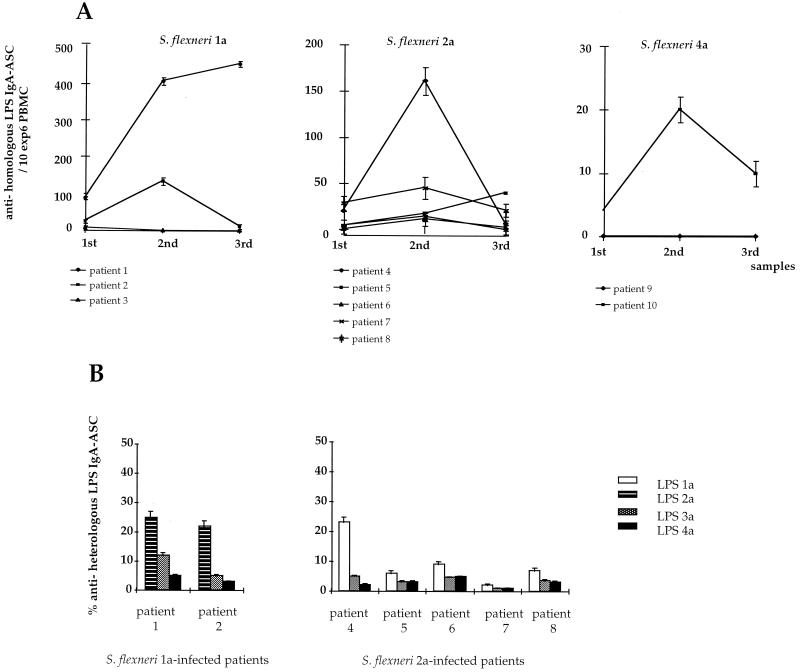
The LPS O-Ag molecule carries both species- and serotype-specific determinants that may be the targets of the humoral immune response (2). The number of anti-homologous LPS IgA ASC/106 PBMC corresponds to the number of IgA ASC recognizing both types of determinants. These cells were enumerated for each individual at each time point using purified LPS corresponding to each of the relevant S. flexneri strains. As shown in Fig. 1A, the anti-homologous LPS IgA ASC response significantly increased between days 9 and 11 (second sample) in comparison to the responses observed in the first and third samples (P = 0.015 for both of them). An exception was observed for patients 1 and 5, in whom the response peaked between days 19 and 22 (third sample). These data are consistent with those previously reported for subjects infected with Shigella in an area of endemicity (10, 13). In volunteers immunized with different Shigella vaccine candidates (3, 6, 8), the response peaks earlier, at day 7 after vaccination or even at day 5 if a large inoculum is used. The difference can be explained by the fact that, in controlled vaccine trials, the day of inoculation is known, which is not the case for naturally infected patients. It could also be due to the inoculum used in the vaccine trials, which is usually higher than the infecting dose in areas of endemicity. Nevertheless, this kinetics appeared to be a general scheme for the priming of mucosal responses following natural infection with intestinal pathogens or their vaccine counterparts (4). It is noteworthy that, for two patients, the response peaked later. For patient 1, this could be explained by the fact that this patient was not given an antibiotic. Therefore, we may hypothesize that a longer persistence of the bacteria within the host could be responsible for the persistence of the immune response. For patient 5, who was treated with antibiotics, it is possible that the second sample was recovered 1 or 2 days before the peak of the response, as the day when the ASC response peaks may vary from one individual to another.
FIG. 1.
(A) Anti-homologous LPS IgA ASC response in S. flexneri 1a-, 2a-, and 4a-infected patients. Mean values and standard errors are represented for each patient at each time point. (B) Anti-heterologous LPS IgA ASC response in S. flexneri 1a- and 2a-infected patients. Cross-reactivity for serotypes 2a, 3a, and 4a and 1a, 3a, and 4a was evaluated at the peak of the response for each of the S. flexneri 1a- and 2a-infected patients, respectively. It is expressed as the percentage of IgA ASC recognizing heterologous LPS among the anti-homologous LPS IgA ASC.
Among the 10 patients, 4 mounted a high response, 4 mounted a weak response, and 2 did not respond at all. Patients 1 and 4 showed the highest anti-homologous LPS IgA ASC response among the subjects infected with S. flexneri 1a and 2a, respectively. Patient 4 was given a second antibiotic treatment, because clinical symptoms persisted after the first treatment. One explanation could be that the persistence of the infecting strain between the recovery of the first and second samples (either due to a high dose not totally eliminated by the first antibiotic treatment or to resistance of the strain to the antibiotic treatment) led to a stronger induction of the host immune response. This may also explain the result obtained for patient 1, who mounted the highest response of the S. flexneri 1a-infected subjects and who was not given antibiotic treatment. In patients 3 and 9, no ASC response was detectable. As the presence of ASC in the blood has been shown to be a transient phenomenon, we may hypothesize that, for these patients, recovery of samples during the time course of infection was unsuitable for ASC detection. However, for patient 9, a 4-year-old child, the clinical data indicated that his body weight was less than expected for children at this age. The negative response in this patient could therefore be explained by malnutrition, which has been shown to affect the gastrointestinal tract status and subsequently the induction of local immune responses (16). In conclusion, as reported in previous studies (10, 13), we observed a high variability in the magnitude of the response between patients, regardless of the infecting strain and the time course of infection.
IgA ASC cross-reactivity to heterologous S. flexneri serotypes in S. flexneri 1a- and 2a-infected patients.
We also evaluated the anti-LPS IgA ASC response directed against species-specific determinants for the different S. flexneri serotypes. IgA ASC cross-reactivity for heterologous S. flexneri serotypes, i.e., serotypes different from the serotype of the infecting strain, representing the most common serotypes in general, and in Madagascar in particular, was evaluated. Due to limitations in cells obtained, the number of IgA ASC specific for heterologous LPS was evaluated at the peak of the response in the S. flexneri 1a- and S. flexneri 2a-infected patients only. Therefore, samples from S. flexneri 1a-infected subjects were tested for IgA ASC cross-reactivity to serotypes 2a, 3a, and 4a and those from S. flexneri 2a-infected patients were tested for IgA ASC cross-reactivity to serotypes 1a, 3a, and 4a. As shown in Fig. 1B, in the S. flexneri 1a-infected subjects, about 20% of the anti-S. flexneri 1a LPS IgA ASC cross-reacted with S. flexneri 2a LPS. A lower level of cross-reactivity to S. flexneri 1a LPS was observed for anti-S. flexneri 2a LPS IgA ASC in the S. flexneri 2a-infected patients. IgA ASC cross-reactivity to S. flexneri 3a or 4a was weak (less than 10% in most of the patients) for both S. flexneri 1a- and 2a-infected patients. No IgA ASC specific for other Shigella species, i.e., S. dysenteriae 1 or S. sonnei, were detectable in any patient (data not shown). Control subjects were negative for all the serotypes tested (data not shown). Therefore, depending on the patient and the infecting strain, between 60 and 90% of anti-LPS IgA ASC recognized serotype-specific determinants. These data show that, for infection with S. flexneri 1a or 2a, the anti-LPS IgA ASC response is mainly directed against serotype-specific determinants on the O-Ag, indicating that species-specific epitopes common to the different S. flexneri serotypes tested are only weakly recognized. It is noteworthy that the structure of the repeated unit constituting the S. flexneri 1a O-Ag is closely related to the corresponding structure for serotype 2a, whereas it significantly differs from those for S. flexneri 3a and 4a (9). This may explain why cross-reactivity between 1a and 2a differs from that between 3a and 4a. In addition, this suggests that the differences in the structure of the repeated unit for the different serotypes in the S. flexneri species (9) are sufficient to induce major conformational changes in the LPS O-Ag, which result in the priming of specific subsets of B cells during the initiation of the humoral response. No cross-reactivity to S. sonnei was observed in the S. flexneri-infected patients. However, a weak cross-reactivity to S. flexneri 2a and 6 has been reported for patients infected with S. sonnei (10). This suggests that the response to heterologous strains may also depend on the infecting strain.
The data provided here emphasize the need for the development of serotype-specific Shigella vaccines. As monoclonal antibodies specific for serotype determinants are protective in an experimental model of shigellosis (12; A. Phalipon, unpublished data), we may imagine the design of multivalent chemically defined synthetic vaccines by combining characterized protective serotype-specific determinants corresponding to the most prevalent strains existing in a given geographical area.
REFERENCES
- 1.Cassel-Béraud A M, Coulanges P, Richard C. Evolution des résistances aux antibiotiques des souches de Shigella dysenteriae type 1 (bacilles de Shiga) isolées à Tananarive et sur la côte Est de Madagascar. Arch Inst Pasteur Madagascar. 1989;56:71–76. [PubMed] [Google Scholar]
- 2.Cohen D, Ashkenazi S, Green M S, et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–159. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 3.Coster T S, Hoge C W, VanDeVerg L L, Hartman A B, Oaks E V, Venkatesan M M, Cohen D, Robin G, Fontaine-Thompson A, Sansonetti P J, Hale T L. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect Immun. 1999;67:3437–3443. doi: 10.1128/iai.67.7.3437-3443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantele A. Peripheral blood antibody-secreting cells in the evaluation of the immune response to an oral vaccine. J Biotechnol. 1996;44:217–224. doi: 10.1016/0168-1656(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 5.Kantele A M, Takanen R, Arvilommi H. Immune response to acute diarrhea seen as circulating antibody-secreting cells. J Infect Dis. 1988;158:1011–1015. doi: 10.1093/infdis/158.5.1011. [DOI] [PubMed] [Google Scholar]
- 6.Kärnell A, Li A, Zhao C R, Karlsson K, Minh N B, Lindberg A A. Safety and immunogenicity of the auxotrophic Shigella flexneri 2a vaccine SFL1070 with a deleted aroD gene in adult Swedish volunteers. Vaccine. 1995;13:88–99. doi: 10.1016/0264-410x(95)80017-8. [DOI] [PubMed] [Google Scholar]
- 7.Kotloff K L, Winickoff J P, Ivanoff B, et al. Global burden of Shigella infections: implications for vaccine development and implementation. Bull W H O. 1999;77:651–666. [PMC free article] [PubMed] [Google Scholar]
- 8.Li A, Cam P D, Islam D, et al. Immune response in Vietnamese children after a single dose of an auxotrophic, live Shigella flexneri Y vaccine strain SFL 124. J Infect Dis. 1994;28:11–23. doi: 10.1016/s0163-4453(94)94006-1. [DOI] [PubMed] [Google Scholar]
- 9.Lindberg A A, Kärnell A, Weintraub A. The lipopolysaccharide of Shigella bacteria as a virulence factor. Rev Infect Dis. 1991;13:S279–S284. doi: 10.1093/clinids/13.supplement_4.s279. [DOI] [PubMed] [Google Scholar]
- 10.Orr N, Robin G, Lowell G, Cohen D. Presence of specific immunoglobulin A-secreting cells in peripheral blood after natural infection with Shigella sonnei. J Clin Microbiol. 1992;30:2165–2168. doi: 10.1128/jcm.30.8.2165-2168.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peccarère J L, Boudon A, Collet M, et al. A propos de l'isolement de 367 souches de Shigellaà Madagascar. Arch Inst Pasteur Madagascar. 1981;48:45–53. [PubMed] [Google Scholar]
- 12.Phalipon A, Kaufmann M, Michetti P, Cavaillon J M, Huerre M, Sansonetti P J, Kraehenbuhl J P. Monoclonal IgA antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J Exp Med. 1995;182:769–778. doi: 10.1084/jem.182.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raqib R, Tzipori S, Islam M, Lindberg A A. Immune responses to Shigella dysenteriae 1 and Shigella flexneri lipopolysaccharide and polysaccharide antigens in Bangladeshi patients with shigellosis. Serodiagn Immunother Infect Dis. 1993;1:37–45. [Google Scholar]
- 14.Sansonetti P J, Phalipon A. Shigellosis: from molecular pathogenesis of infection to protective immunity and vaccine development. Res Immunol. 1996;147:595–602. doi: 10.1016/s0923-2494(97)85227-3. [DOI] [PubMed] [Google Scholar]
- 15.Sedgwick J D, Holt P G. A solid phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;57:301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- 16.Wapnir R A. Zinc deficiency, malnutrition and the gastrointestinal tract. J Nutr. 2000;130:1388.S–1392S. doi: 10.1093/jn/130.5.1388S. [DOI] [PubMed] [Google Scholar]
- 17.Westphal O, Jann J. Bacterial lipopolysaccharides: extraction with phenol-water and further application of the procedures. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]