Abstract
Staphylococcus aureus invades osteoblasts and can persist in the intracellular environment. The present study examined the role of osteoblast mitogen-activated protein kinase (MAPK) pathways in bacterial invasion. S. aureus infection of normal human and mouse osteoblasts resulted in an increase in the phosphorylation of the extracellular signal-regulated protein kinases (ERK 1 and 2). This stimulation of ERK 1 and 2 correlated with the time course of S. aureus invasion, and bacterial adherence induced the MAPK pathway. ERK 1 and 2 phosphorylation was time and dose dependent and required active S. aureus gene expression for maximal induction. The nonpathogenic Staphylococcus carnosus was also able to induce ERK 1 and 2 phosphorylation, albeit at lower levels than S. aureus. Phosphorylation of the stress-activated protein kinases was increased in both infected human and mouse osteoblasts; however, the p38 MAPK pathway was not activated in response to S. aureus. Finally, the transcription factor c-Jun, but not Elk-1 or ATF-2, was phosphorylated in response to S. aureus infection.
Osteomyelitis (OM) is an infection of bone that results from hematogenous seeding, spread of infection from a contiguous area such as the skin adjacent to a wound, surgical inoculation of bacteria into bone, or trauma coincident with staphylococcal infection (57). The presence of an inert, prosthetic orthopedic device increases the likelihood of disease, and removal of the implant may be required (16). Chronic or recurring OM can be a persistent clinical problem that is difficult to treat effectively and results in abnormal bone remodeling, leading to a vascular compromise in the infected area. Long periods of antibiotic treatment are utilized in an attempt to control OM recurrences; however, methicillin-resistant staphylococci are now commonplace, and therapeutic levels of antibiotics in necrotic bone are difficult to achieve unless antibiotic-impregnated beads or implants are used (9).
Staphylococcus aureus is a capable bone pathogen because it possesses several cell surface adhesion molecules that facilitate its binding to the bone matrix. These include fibronectin-binding proteins (18, 30), fibrinogen-binding proteins (6, 10, 36), elastin-binding adhesin (42), collagen-binding adhesin (43), and a broad-specificity adhesin (MAP) which facilitates low-affinity binding of S. aureus to several proteins, including osteopontin, collagen, bone sialoprotein, fibronectin, fibrinogen, and vitronectin (37). In addition, S. aureus contains surface proteins that are able to stimulate bone resorption (39) via increasing osteoclast activity (4). The resultant bone destruction facilitates bacterial invasiveness. S. aureus not only colonizes bone matrix but is internalized in vitro (17, 28, 29) and in vivo (49) by osteoblasts (bone-forming cells). With the notable exception of Listeria monocytogenes, very little work has been done to examine mechanisms of invasion and intracellular survival by gram-positive bacteria. The ability of S. aureus to invade osteoblasts as well as several other cell types (3, 5, 38, 55) may be critical to the pathogenesis of the organism.
Upon binding and stimulation of many eukaryotic surface receptors, the mitogen-activated protein kinase (MAPK) pathway can be activated (45). The MAPKs extracellular signal-regulated protein kinases (ERK 1 [p44 MAPK] and ERK 2 [p42 MAPK]) have been found to be activated during the invasion of Henle-407 cells by L. monocytogenes (52) and Salmonella enterica serovar Typhimurium (41). MAPKs are important mediators in many cellular functions, including cytokine, mitogenic, and stress responses and cytoskeletal rearrangement (12, 58). As a result of the involvement of ERK 1 and 2 in other bacterial invasion systems, the role of ERK 1 and 2 in the invasion of both normal mouse and human osteoblasts by S. aureus strain UAMS-1 was examined. As a potential negative control, the ERK 1 and 2 response was examined following infection with Staphylococcus carnosus, since this bacterium was reported to be unable to invade the human osteoblastic cell line MG-63 (29). In addition to ERK 1 and 2, other isoforms of MAPK exist, such as p38 MAPK (hyperosmolarity [HOG] kinase) (25) and p54-p46 MAPK (c-Jun N-terminal kinase [JNK], also known as stress-activated protein kinase [SAPK]) (15, 32). SAPK and p38 MAPK are phosphorylated in response to extracellular signals, including proinflammatory cytokines and cellular stresses (11, 23, 26, 47). These isoforms have also been implicated in the invasion of Henle-407 cells by L. monocytogenes (53) and S. enterica serovar Typhimurium (27). It is also clear that the transcription factors ATF-2 (a target of p38 MAPK and SAPK) (24, 56) and c-Jun (a target of SAPK and ERK 1 and 2) (15, 32, 46) are stimulated by proinflammatory cytokines. These transcription factors have been shown to become activated during S. enterica serovar Typhimurium invasion (27). Since it is known that S. aureus induces an inflammatory response, it is possible that the severe inflammation observed with OM is mediated by members of the MAPK family and the above-mentioned transcription factors. It has recently been demonstrated that p38 MAPK activation leads to induction of expression of the proinflammatory cytokines interleukin 12 (IL-12) p40 (2) and IL-6 (13) and that IL-6 production is induced via ATF-2 activation in osteoblasts (19). It is also known that IL-6 induces bone remodeling via osteoclastogenesis (14). Additionally, tumor necrosis factor alpha (TNF-α) mediates bone resorption (51), and SAPK is subsequently activated via phosphorylation when cells are exposed to TNF-α (26). The activation of SAPK and c-Jun may be the mechanism responsible for the increased TNF-α activity and mRNA transcript levels observed in an experimental model of S. aureus-induced OM (35).
In the present study, the role of intracellular signaling mechanisms in the invasion of normal mouse and human osteoblasts by S. aureus strain UAMS-1, an OM clinical isolate, was examined. S. aureus induced a time-dependent and dose-dependent activation of several members of the MAPK family, including ERK 1 and 2 and SAPK, but not p38 MAPK, upon association with normal mouse and human osteoblasts. In addition, S. carnosus infection of normal mouse osteoblasts induced this same ERK 1 and 2 response, albeit at a lower level than with S. aureus. Active bacterial gene expression is required for full stimulation of ERK 1 and 2. Attachment of the bacteria to the osteoblast surface results in ERK 1 and 2 phosphorylation, and the kinetics of ERK 1 and 2 activation correlates with the time course of invasion. Finally, invasion of normal mouse osteoblasts by S. aureus resulted in activation of c-Jun but not ATF-2 or ELK-1. These studies are the first to examine intracellular signaling in normal osteoblasts in response to S. aureus infection.
MATERIALS AND METHODS
Bacterial strains.
S. aureus strain UAMS-1 (ATCC 49230) is a human OM clinical isolate (21). S. carnosus (ATCC 51365) is a nonpathogenic species reported to be incapable of invasion of osteoblasts (29).
Isolation and culture of normal mouse osteoblasts.
Osteoblasts were isolated from the calvariae of 1-day old BALB/c mice according to a method described elsewhere for chicken embryos (48). Bone-forming cells were isolated from mouse neonate calvariae by sequential collagenase-protease digestion. The periostea were removed, and the frontal bones were harvested free of the suture regions and incubated for 10 min at 37°C in 10 ml of digestion medium containing collagenase (375 U/ml; type VII; Sigma, St. Louis, Mo.) and protease (7.5 U/ml; Sigma). The digestion medium and released cells were removed and discarded. Ten milliliters of fresh digestion medium was then added, and the incubation was continued for 20 min. The cells were harvested by centrifugation and rinsed three times in 25 mM HEPES-buffered Hanks' balanced salt solution (HBSS) (pH 7.4). The digestion step was repeated twice, and the three cell isolates were pooled in mouse osteoblast growth medium (MOBGM) consisting of Dulbecco's modified Eagle medium containing 25 mM HEPES, 2 g of sodium bicarbonate/liter, 75 μg of glycine/ml, 10% fetal bovine serum (Sigma), 100 μg of ascorbic acid/ml, 40 ng of vitamin B12/ml, 2 μg of p-aminobenzoic acid/ml, 200 ng of biotin/ml, and penicillin-streptomycin-Fungizone (Abx-Amx) (100 U/ml–100 μg/ml–0.25 μg/ml; Sigma) (pH 7.4). The cells were seeded at 106/well in six-well plates and incubated in a humidified incubator at 37°C in a 5% CO2 atmosphere until they reached confluence (6 to 7 days). The medium was changed every 48 h after being seeded.
Normal human osteoblast cultures.
Normal human osteoblasts (Clonetics, San Diego, Calif.) were purchased and propagated according to the guidelines provided by the vendor. The cells were seeded in 25-cm2 flasks and incubated in a humidified incubator at 37°C in a 5% CO2 atmosphere in growth medium supplied by the manufacturer that contained 10% fetal calf serum, ascorbic acid, and gentamicin. After the cells reached ≈80% confluence (5 to 9 days), they were removed from the flasks by use of 0.025% trypsin–0.01% EDTA, washed in growth medium, and seeded in six-well plates. The osteoblasts were then maintained in growth medium and grown as described above until they reached confluence (6 to 7 days). The growth medium was changed every 48 h after being seeded. These commercially available cells have been extensively characterized as osteoblasts (20, 22).
Invasion assay to enumerate intracellular bacteria.
S. aureus and S. carnosus were grown overnight (16 h) in 5 ml of tryptic soy broth in a shaking water bath at 37°C. The bacteria were harvested by centrifugation for 10 min at 4,300 × g at 4°C and washed twice in 5 ml of HBSS. The pellets were then resuspended in 5 ml of MOBGM lacking Abx-Amx. Confluent cell layers of osteoblasts were washed three times with 4 ml of HBSS to remove the growth medium. The cultures were then infected at a multiplicity of infection (MOI) of 75 or 250:1 with either S. aureus or S. carnosus in 4 ml of MOBGM lacking Abx-Amx. Following an appropriate infection period, the cell cultures were washed three times with 4 ml pf HBSS and incubated for 3 h in 4 ml of MOBGM containing 25 μg of gentamicin/ml to kill the remaining extracellular S. aureus or S. carnosus cells. The osteoblast cultures were washed as described above and subsequently lysed by the addition of 1.2 ml of 0.1% Triton X-100 (Fisher Biotech, Fair Lawn, N.J.) with incubation for 5 min at 37°C. To quantify the number of bacteria internalized, suspension dilutions of the lysates were plated in triplicate on tryptic soy agar plates followed by incubation at 37°C overnight.
Preparation of osteoblast lysates.
Mouse or human osteoblasts were infected with live or UV-killed S. aureus strain UAMS-1 or S. carnosus at an MOI of 25, 75, or 250:1 for the live bacteria and 75 or 250:1 for the UV-killed organisms. Following an infection period, the osteoblasts were washed three times with 4 ml of ice-cold phosphate-buffered saline, pH 7.4, containing 0.4 mM Na3VO4 (Sigma), 1 mM NaF, and 0.1 mg of phenylmethylsulfonyl fluoride (Sigma)/ml and removed from the culture plates with a cell scraper (Costar). The cells were then concentrated by centrifugation at 10,000 × g for 1 min. The cell pellet was resuspended in 100 μl of lysis buffer (50 mM Tris-HCl [pH 7.6] containing 0.4 mM Na3VO4, 1 mM NaF, 0.1 mg of PMSF/ml, 0.01 mg of leupeptin [Sigma]/ml, and 1.0% Triton X-100). The resulting lysate was then subjected to centrifugation at 13,600 × g for 30 min. Equivalent amounts of protein from the Triton X-100-soluble fraction (10 to 20 μg) were mixed with concentrated 5× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (0.313 M Tris-HCl [pH 6.8], 10% [vol/vol] SDS, 25% [vol/vol] 2-mercaptoethanol, 50% [vol/vol] glycerol, 0.01% [wt/vol] bromophenol blue), boiled for 5 min, cleared by centrifugation at 13,600 × g for 5 min, and subjected to SDS-PAGE analysis.
SDS-PAGE and immunoblot analyses.
Osteoblast proteins were separated by SDS-PAGE as described by Laemmli (33). The proteins were then electrophoretically transferred to polyvinylidene difluoride membranes (Fisher Biotech) using the Mini Trans-Blot apparatus (Bio-Rad, Hercules, Calif.) at 300 mA for 1 h at 4°C according to the manufacturer's recommendations. The membranes were blocked with buffer A (Tris-buffered saline [Bio-Rad] containing 5.0% skim milk and 0.5% Tween 20 [Sigma]) for 1 h with gentle shaking and then washed with buffer B (Tris-buffered saline containing 0.1% Tween 20) three times for 5 min each time. A two-step detection method was used to identify phosphorylated proteins of interest. Blots were first incubated overnight with gentle shaking at 4°C with mouse anti-phospho-ERK 1 and 2 (p44-p42 MAPK) (New England Biolabs [NEB], Beverly, Mass.) diluted 1:5,000 in buffer A or with either rabbit anti-phospho-p38 MAPK (NEB), rabbit anti-phospho-MKK3-MKK6 (NEB), rabbit anti-phospho-SAPK (NEB), rabbit anti-phospho-c-Jun (NEB), rabbit anti-phospho-Elk-1, or rabbit anti-phospho-ATF-2 (NEB) diluted 1:1,000 in buffer A. The membranes were then washed three times the following day for 5 min each time in buffer B with gentle shaking, followed by incubation for 1 h with gentle shaking at room temperature in buffer A with either anti-mouse or anti-rabbit immunoglobulin G (1:5,000) conjugated to horseradish peroxidase (Jackson ImmunoResearch, West Grove, Pa.). Reactive proteins were then visualized by enhanced chemiluminescence (Amersham, Arlington Heights, Ill.) following exposure to X-ray film and subsequent film development.
RESULTS
S. aureus infection results in phosphorylation of the mouse osteoblast proteins ERK 1 and 2.
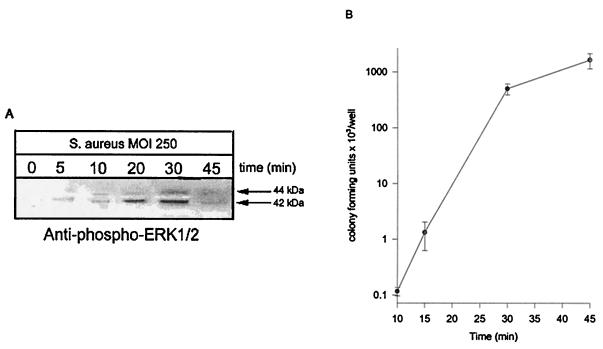
Osteoblast cultures infected with S. aureus at an MOI of 250:1 demonstrated a marked increase in ERK 1 and 2 phosphorylation over time (Fig. 1A). Immunoblots incubated with anti-phospho-ERK 1 and 2 antibodies demonstrated that ERK 1 and 2 are phosphorylated as early as 5 min following infection and are maximal at 30 min (Fig. 1A). Studies examining the kinetics of S. aureus invasion of normal mouse osteoblasts indicate that bacterial invasion correlates with observed ERK 1 and 2 phosphorylation (Fig. 1B). The numbers of intracellular S. aureus cells increased logarithmically up to approximately 30 min following infection.
FIG. 1.
ERK 1 and 2 phosphorylation correlates with the kinetics of S. aureus invasion. (A) ERK 1 and 2 phosphorylation is induced by S. aureus infection. Normal mouse osteoblasts were either uninfected (0 min) or infected with S. aureus strain UAMS-1 at an MOI of 250:1 for various times. Equivalent volumes of Triton X-100-soluble protein samples were resolved on an SDS-PAGE gel (12%), transferred to a polyvinylidene difluoride membrane, and analyzed for ERK 1 and 2 phosphorylation by immunoblotting and reaction with anti-phospho-ERK 1 and 2 antibody. The arrows on the right show the positions of the 44- and 42-kDa phosphorylated ERK isoforms induced by S. aureus infection. (B) Rate of osteoblast invasion by S. aureus. Normal mouse osteoblasts were infected with S. aureus strain UAMS-1 at an MOI of 250:1 for various times. The wells were then washed three times, followed by the addition of medium supplemented with gentamicin for 3 h to kill extracellular bacteria. Following a 3-h incubation, the wells were washed three times and the osteoblasts were lysed to enumerate intracellular S. aureus cells. The standard deviations are indicated. Both the exposed film and enumeration data are representative of results from three independent experiments.
Cytochalasin D does not affect ERK 1 and 2 phosphorylation; PD98059 reduces ERK 1 and 2 phosphorylation.
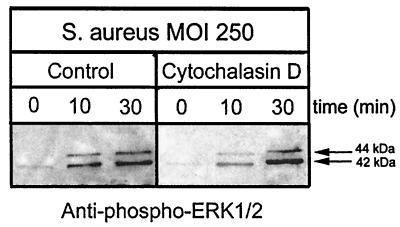
Experiments have demonstrated that inhibition of osteoblast actin polymerization with 5 μg of cytochalasin D/ml decreases intracellular S. aureus numbers by as much as 99.8% compared to control cultures (17). To examine whether invasion or attachment of S. aureus is the stimulus for ERK 1 and 2 phosphorylation, normal mouse osteoblast cultures were treated with cytochalasin D (5 μg/ml), and the ERK 1 and 2 phosphorylation levels were determined. The increase in ERK 1 and 2 phosphorylation observed in response to osteoblast infection by S. aureus was unaffected by treatment of cultures with cytochalasin D (Fig. 2). The data therefore suggest that ERK 1 and 2 phosphorylation is induced by S. aureus attachment to the osteoblast surface.
FIG. 2.
S. aureus attachment is sufficient to induce phosphorylation of ERK 1 and 2. Normal mouse osteoblasts were treated with cytochalasin D (5 μg/ml) or with the solvent dimethyl sulfoxide (Control) for 30 min. The osteoblasts were then either left uninfected (0 min) or infected with S. aureus strain UAMS-1 at an MOI of 250:1 for 10 or 30 min. Equivalent volumes of Triton X-100-soluble protein samples were resolved on an SDS-PAGE gel (12%), transferred to a polyvinylidene difluoride membrane, and analyzed for ERK 1 and 2 phosphorylation by immunoblotting and reaction with anti-phospho-ERK 1 and 2 antibody. The arrows on the right show the positions of the 44- and 42-kDa phosphorylated ERK isoforms induced by S. aureus infection. The exposed film is representative of results from three independent experiments.
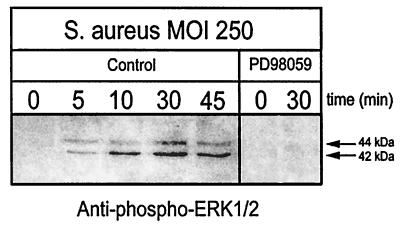
Treatment of osteoblast cultures with the MAPK kinase (MEK 1 and 2) inhibitor PD98059 reduces the numbers of intracellular S. aureus cells by as much as 80.5% compared to control cultures (J. K. Ellington and M. C. Hudson, unpublished results). Treatment of osteoblast cultures with PD98059 (100 μM) resulted in undetectable ERK 1 and 2 phosphorylation in response to S. aureus infection (Fig. 3). Therefore, inhibition of ERK 1 and 2 phosphorylation decreases invasion of osteoblasts by S. aureus. Taken together, these results indicate that the ERK 1 and 2 pathway is important in the invasion of normal mouse osteoblasts by S. aureus and that ERK 1 and 2 phosphorylation is induced by S. aureus attachment.
FIG. 3.
ERK 1 and 2 is not phosphorylated when osteoblasts are treated with the MEK 1 and 2 inhibitor PD98059 followed by infection with S. aureus. Normal mouse osteoblasts were treated with PD98059 (100 μM) or with the solvent dimethyl sulfoxide (Control) for 60 min. The osteoblasts were then either left uninfected (0 min) or infected with S. aureus strain UAMS-1 at an MOI of 250:1 for various times. Equivalent volumes of Triton X-100-soluble protein samples were resolved on an SDS-PAGE gel (12%), transferred to a polyvinylidene difluoride membrane, and analyzed for ERK 1 and 2 phosphorylation by immunoblotting and reaction with anti-phospho-ERK 1 and 2 antibody. The arrows on the right show the positions of the 44- and 42-kDa phosphorylated ERK isoforms induced by S. aureus infection. The exposed film is representative of results from three independent experiments.
ERK 1 and 2 phosphorylation is dependent on S. aureus MOI.
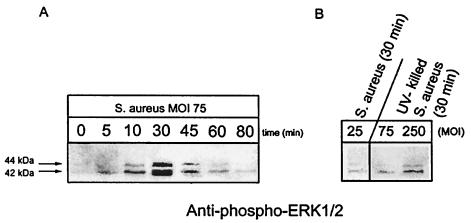
Maximal ERK 1 and 2 phosphorylation was observed at 30 min following infection in response to an MOI of 75:1 (Fig. 4A). There was 25-fold less ERK 1 and 2 phosphorylation at the same time point when osteoblasts were infected at an MOI of 25:1 (Fig. 4B). Therefore, all remaining studies examined phosphorylation responses at an MOI of 75:1 at 30 min following infection.
FIG. 4.
Dose-dependent S. aureus induction of ERK 1 and 2 phosphorylation. (A) Normal mouse osteoblasts were either left uninfected (0 min) or infected with S. aureus strain UAMS-1 at an MOI of 75:1 for various times. (B) Normal mouse osteoblasts were infected with live S. aureus strain UAMS-1 at an MOI of 25:1 or with UV-killed S. aureus strain UAMS-1 at an MOI of 250 or 75:1 for 30 min. Equivalent volumes of Triton X-100-soluble protein samples were resolved on a single SDS-PAGE gel (12%), transferred to a polyvinylidene difluoride membrane, and analyzed for ERK 1 and 2 phosphorylation by immunoblotting and reaction with anti-phospho-ERK 1 and 2 antibody. The arrows on the left show the positions of the 44- and 42-kDa phosphorylated ERK isoforms induced by S. aureus infection. The exposed film is representative of results from three independent experiments.
Interaction of mouse osteoblasts with UV-killed S. aureus results in reduced ERK 1 and 2 phosphorylation compared to infection with viable bacteria.
UV treatment of S. aureus was used to kill the bacteria while maintaining the integrity of surface structures, presumably including those that bind and interact with the osteoblast surface. When UV-killed S. aureus cells were incubated with normal mouse osteoblast cultures for 30 min (MOI, 75 and 250:1), ERK 1 and 2 phosphorylation was observed, albeit at lower levels than for live S. aureus cells (Fig. 4B). Viable S. aureus cells at an MOI of 75:1 induced eightfold-greater ERK 1 and 2 phosphorylation than UV-killed S. aureus at the same MOI (Fig. 4).
Infection of mouse osteoblasts by S. carnosus results in lower levels of ERK 1 and 2 phosphorylation than S. aureus infection.
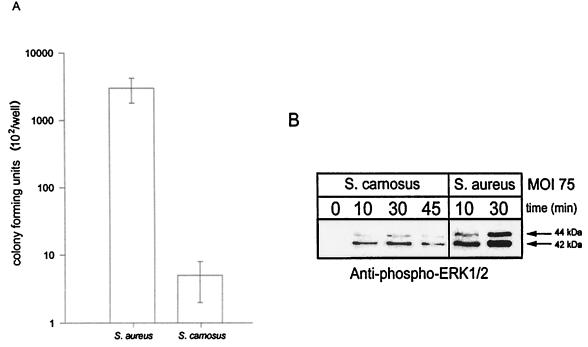
It has been reported that S. carnosus is incapable of invasion of the human osteoblast cell line MG-63 (29); however, we report that S. carnosus is capable of invasion of normal osteoblasts, albeit at a significantly lower level than S. aureus (Fig. 5A). The differential ability of S. carnosus and S. aureus to invade osteoblasts is also manifested in the ability to induce ERK 1 and 2 phosphorylation. Osteoblasts infected with S. carnosus demonstrated an increase in ERK 1 and 2 phosphorylation over time at the same MOI of 75:1 used in the S. aureus studies; however, the amount of phosphorylated ERK 1 and 2 induced by S. carnosus was threefold less than that induced by S. aureus (Fig. 5B).
FIG. 5.
Differences in S. aureus and S. carnosus invasion of osteoblasts. (A) S. carnosus is impaired in invasion of osteoblasts compared to S. aureus. Normal mouse osteoblasts were infected at an MOI of 75:1 with either S. aureus or S. carnosus for 45 min. The wells were then washed three times followed by the addition of growth medium containing gentamicin for 3 h. The wells were then washed, and the osteoblasts were lysed to enumerate intracellular bacteria. The standard deviations are indicated. (B) ERK 1 and 2 phosphorylation in response to S. aureus or S. carnosus infection. Normal mouse osteoblasts were either left uninfected (0 min) or infected with S. aureus strain UAMS-1 or S. carnosus, each at an MOI of 75:1, for various times. Equivalent volumes of Triton X-100-soluble protein samples were resolved on an SDS-PAGE gel (12%), transferred to a polyvinylidene difluoride membrane, and analyzed for ERK 1 and 2 phosphorylation by immunoblotting and reaction with anti-phospho-ERK 1 and 2 antibody. The arrows on the right show the positions of the 44- and 42-kDa phosphorylated ERK isoforms induced by S. aureus and S. carnosus. The exposed film is representative of results from three independent experiments.
S. aureus infection results in phosphorylation of the mouse osteoblast protein SAPK but not p38 MAPK.
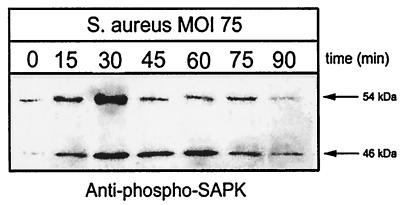
Two major stress-activated pathways in eukaryotic cells are SAPK and p38 MAPK (15, 25). Thus, it was hypothesized that one or both of these pathways would be activated during S. aureus infection of osteoblasts. Normal mouse osteoblast cultures infected with S. aureus at an MOI of 75:1 exhibited a marked increase in phosphorylation of the SAPK isoforms p54 and p46 (Fig. 6). Like ERK 1 and 2 phosphorylation observed in response to S. aureus infection, SAPK phosphorylation was maximal at 30 min following infection.
FIG. 6.
SAPK phosphorylation in response to S. aureus infection. Normal mouse osteoblasts were either left uninfected (0 min) or infected with S. aureus strain UAMS-1 at an MOI of 75:1 for various times. Equivalent volumes of Triton X-100-soluble protein samples were resolved on an SDS-PAGE gel (12%), transferred to a polyvinylidene difluoride membrane, and analyzed for SAPK phosphorylation by immunoblotting and reaction with anti-phospho-SAPK antibody. The arrows on the right show the positions of the 54- and 46-kDa phosphorylated SAPK isoforms induced by S. aureus infection. The exposed film is representative of results from three independent experiments.
In contrast to SAPK, the phosphorylation of p38 MAPK was not induced by S. aureus infection (data not shown). MKK3 and MKK6 are upstream effector proteins for p38 MAPK and were also examined to further confirm that the p38 MAPK stress pathway is not activated during infection of osteoblasts by S. aureus. The phosphorylation of MKK3-MKK6 was also unaffected by S. aureus infection (data not shown).
S. aureus infection results in phosphorylation of the mouse osteoblast protein c-Jun.
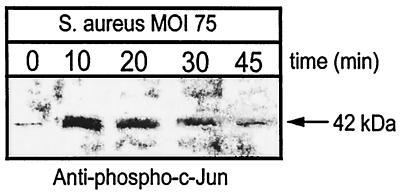
The transcription factor c-Jun is a known target of the SAPK pathway (15, 32). As shown in Fig. 7, there is a rapid and marked increase in c-Jun phosphorylation in normal osteoblasts in response to S. aureus infection. As early as 10 min postinfection, c-Jun phosphorylation is maximal, and it returns to near control levels by 45 min following infection. In contrast to c-Jun, the transcription factors ATF-2 and Elk-1 are not phosphorylated in response to S. aureus (data not shown). The c-Jun data suggest a potential mechanism whereby infection of osteoblasts with S. aureus leads to specific activation of host cell transcription.
FIG. 7.
c-Jun phosphorylation in response to S. aureus infection. Normal mouse osteoblasts were either left uninfected (0 min) or infected with S. aureus strain UAMS-1 at an MOI of 75:1 for various times. Equivalent volumes of Triton X-100-soluble protein samples were resolved on an SDS-PAGE gel (12%), transferred to a polyvinylidene difluoride membrane, and analyzed for c-Jun phosphorylation by immunoblotting and reaction with anti-phospho-c-Jun antibody. The arrow on the right shows the position of the 42-kDa phosphorylated protein induced by S. aureus infection. The exposed film is representative of results from three independent experiments.
S. aureus infection results in phosphorylation of the human osteoblast proteins ERK 1 and 2 and SAPK.
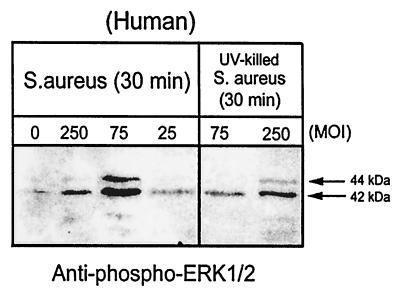
To extend studies using normal mouse osteoblasts to human cells, immunoblot analysis was performed on cell lysates of normal human osteoblasts. S. aureus-induced phosphorylation responses in normal mouse osteoblasts were also evident in normal human osteoblasts. Normal human osteoblasts infected with S. aureus for 30 min demonstrated dose-dependent ERK 1 and 2 phosphorylation, with maximal activation at an MOI of 75:1 (Fig. 8). ERK 1 and 2 phosphorylation increased threefold at an MOI of 75:1 compared to that at 25:1. Interestingly, the amount of phosphorylated ERK 1 and 2 then actually decreased twofold at an MOI of 250:1 compared to levels observed at 75:1. As for mouse osteoblasts, UV-killed S. aureus induced significantly lower levels of ERK 1 and 2 phosphorylation (Fig. 8). The amount of phosphorylated ERK 1 and 2 was threefold higher with live bacteria than with UV-killed organisms at an MOI of 75:1. SAPK phosphorylation in human osteoblast cultures infected with S. aureus also mirrored the results observed with the mouse osteoblasts (Fig. 9). The SAPK response in human cells is also maximal at 30 min following bacterial infection.
FIG. 8.
Dose-dependent S. aureus induction of ERK 1 and 2 phosphorylation in normal human osteoblasts. Normal human osteoblasts were either left uninfected (MOI, 0) or infected with live S. aureus at an MOI of 250, 75, or 25:1 or with UV-killed S. aureus at an MOI of 250 or 75:1 for 30 min. Equivalent volumes of Triton X-100-soluble protein samples were resolved on an SDS-PAGE gel (12%), transferred to a polyvinylidene difluoride membrane, and analyzed for ERK 1 and 2 phosphorylation by immunoblotting and reaction with anti-phospho-ERK 1 and 2 antibody. The arrows on the right show the positions of the 44- and 42-kDa phosphorylated ERK isoforms induced by live and UV-killed S. aureus. The exposed film is representative of results from three independent experiments.
FIG. 9.
SAPK phosphorylation in response to S. aureus infection of normal human osteoblasts. Normal human osteoblasts were either left uninfected (0 min) or infected with S. aureus strain UAMS-1 at an MOI of 75:1 for various times. Equivalent volumes of Triton X-100-soluble protein samples were resolved on an SDS-PAGE gel (12%), transferred to a polyvinylidene difluoride membrane, and analyzed for SAPK phosphorylation by immunoblotting and reaction with anti-phospho-SAPK antibody. The arrows on the right show the positions of the 54- and 46-kDa phosphorylated SAPK isoforms induced by S. aureus infection. The exposed film is representative of results from three independent experiments.
DISCUSSION
When normal mouse osteoblasts were infected with S. aureus strain UAMS-1, phosphorylation of ERK 1 and 2 was increased, with the greatest activation at 30 min following infection. The maximal numbers of intracellular S. aureus cells correlated well with this maximal ERK 1 and 2 phosphorylation. These results clearly indicate that activation of ERK 1 and 2 occurs during invasion of osteoblasts by S. aureus. Activated ERK 1 and 2 probably then effects cytoskeletal rearrangement by inducing actin reorganization. Invasion of epithelial cells by L. monocytogenes correlates with greater phosphorylation of the p44 isoform (ERK-1) than the p42 isoform (ERK-2) (52); however, the situation is different with S. aureus invasion of osteoblasts. Infection of both normal mouse and human osteoblasts by S. aureus resulted in greater ERK-2 than ERK-1 phosphorylation. MAPKs are a group of threonine and serine kinases, and they have many diverse functions within a cell. ERK 1 and 2 are regulated by phosphorylation of a tyrosine and a threonine residue, and both sites must be phosphorylated for maximal activity (44). The ERK 1 and 2 signaling pathway is utilized by a variety of growth and differentiation factors (8, 31, 40), and activation of ERK 1 and 2 can result in phosphorylation of many different substrates. These include the transcription factors ATF-2, Elk-1 (1), and c-Jun (46). ERK 1 and 2 phosphorylation can also activate phospholipase A2 (34). Phospholipase A2 activation results in the production of leukotrienes after arachidonic acid release (50). Leukotrienes, which are hypothesized to open calcium channels on the host cell membrane, are important in the invasion of Henle-407 cells by S. enterica serovar Typhimurium (41). We have recently demonstrated that calcium channels are also important in the invasion of normal mouse osteoblasts by S. aureus (Ellington and Hudson, unpublished).
ERK 1 and 2 phosphorylation in response to different MOIs was examined. When the MOI increased from 25:1 to 75:1, ERK 1 and 2 phosphorylation increased 25- and 3-fold in mouse and human osteoblasts, respectively; however, at an MOI of 250:1, ERK 1 and 2 phosphorylation actually decreased twofold in human cells compared to that at an MOI of 75:1. This reduced ERK 1 and 2 phosphorylation observed at the highest MOI used may be attributed to cell death via necrosis and/or apoptosis. We have recently demonstrated that S. aureus does induce osteoblast apoptosis (54). When osteoblasts were pretreated with the MEK 1 and 2 inhibitor PD98059, ERK 1 and 2 phosphorylation was undetectable in infected osteoblast cultures. This correlates with data from our laboratory demonstrating that following inhibition of MEK 1 and 2 with PD98059, the numbers of intracellular S. aureus cells decreased by as much as 80.5% (Ellington and Hudson, unpublished). These findings indicate that the activation of ERK 1 and 2 is a significant event in S. aureus invasion of osteoblasts.
To examine whether bacterial attachment was responsible for activation of ERK 1 and 2, osteoblast cultures were pretreated with cytochalasin D, which inhibits S. aureus invasion of osteoblasts (17). The data demonstrate that ERK 1 and 2 phosphorylation is still induced when osteoblasts are treated with cytochalasin D, indicating that ERK 1 and 2 activation occurs prior to bacterial invasion. In addition, UV-killed S. aureus cells were able to induce ERK 1 and 2 phosphorylation in both normal mouse and human osteoblasts, albeit at lower levels than with live S. aureus. This indicates that active bacterial gene expression may be required for maximal ERK 1 and 2 activation and further suggests that interaction of a bacterial ligand with an osteoblast receptor results in an increase in ERK 1 and 2 phosphorylation. Alternatively, data from experiments with killed bacteria could suggest that active secretion of soluble factors by bacteria plays a role in osteoblast responses. Experiments are currently in progress to examine whether secretion of soluble factors by S. aureus results in ERK 1 and 2 activation.
S. carnosus was utilized to examine how osteoblasts respond to a presumably noninvasive species of staphylococcus (29). Live and UV-killed S. carnosus did induce ERK 1 and 2 phosphorylation in normal mouse osteoblasts, albeit at significantly lower levels than S. aureus. Viable intracellular S. carnosus cells were also recovered from osteoblasts, but the numbers of intracellular bacteria were also significantly lower than the numbers of intracellular S. aureus cells. Differences in numbers of viable intracellular bacteria between species appear to be much greater than the differences in the levels of ERK 1 and 2 phosphorylation observed. These data may indicate that S. carnosus is debilitated in the ability to survive in the osteoblast intracellular environment compared to S. aureus.
In addition to the phosphorylation of ERK 1 and 2 observed during challenge with S. aureus, the phosphorylation of SAPK was also increased in both normal mouse and human osteoblasts; however, another stress pathway including p38 MAPK was not activated in response to infection by S. aureus. These data indicate that osteoblasts respond to S. aureus infection by activation of the SAPK pathway but not the p38 MAPK pathway.
To examine downstream activity in normal mouse osteoblasts challenged with S. aureus, possible transcription factors that are targets of ERK 1 and 2 and SAPK were examined. During osteoblast infection by S. aureus, a rapid increase in c-Jun phosphorylation was observed. The kinetics of c-Jun activation could indicate that the SAPK pathway (15, 32) and the ERK 1 and 2 pathway (46) both converge on c-Jun, resulting in its rapid phosphorylation in response to S. aureus infection; however, it is possible that another factor is responsible for c-Jun activation. In any case, phosphorylation of c-Jun probably then affects osteoblast gene expression, possibly inducing proinflammatory cytokine expression. Such cytokine expression has been observed in response to S. aureus invasion in both normal mouse and human osteoblasts (7).
The overall mechanism of S. aureus invasion of osteoblasts clearly involves a variety of converging signal transduction pathways. S. aureus invasion of osteoblasts is a complex process, in which the bacterium exploits the host cell machinery and escapes the extracellular environment and humoral immune response. Further investigation of eukaryotic cellular processes that become activated in response to S. aureus may allow modified treatment strategies for bone infection and possibly other S. aureus-induced diseases.
ACKNOWLEDGMENTS
This work was supported by funding to M. C. Hudson from the Foundation for the Carolinas and the UNC Charlotte Foundation.
REFERENCES
- 1.Abdel-Hafiz H A, Heasley L E, Kyriakis J M, Avruch J, Kroll D J, Johnson G L, Hoeffler J P. Activating transcription factor-2 DNA-binding activity is stimulated by phosphorylation catalyzed by p42 and p54 microtubule-associated protein kinases. Mol Endocrinol. 1992;6:2079–2089. doi: 10.1210/mend.6.12.1337144. [DOI] [PubMed] [Google Scholar]
- 2.Aicher A, Shu G L, Magaletti D, Mulvania T, Pezzutto A, Craxton A, Clark E A. Differential role for p38 mitogen-activated protein kinase in regulating CD40-induced gene expression in dendritic cells and B cells. J Immunol. 1999;163:5786–5795. [PubMed] [Google Scholar]
- 3.Almeida R A, Matthews K R, Cifrian E, Guidry A J, Oliver S P. Staphylococcus aureus invasion of bovine mammary epithelial cells. J Dairy Sci. 1996;79:1021–1026. doi: 10.3168/jds.S0022-0302(96)76454-8. [DOI] [PubMed] [Google Scholar]
- 4.Arora M, Shah N, Meghji S, Henderson B, Harris M, Nair S, Wilson M, Gray C M, Jones S J, Boyde A. Effect of Staphylococcus aureus extracellular proteinaceous fraction in an isolated osteoclastic resorption assay. J Bone Miner Res. 1998;16:158–161. [Google Scholar]
- 5.Bayles K W, Wesson C A, Liou L E, Fox L K, Bohach G A, Trumble W R. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect Immun. 1998;66:336–342. doi: 10.1128/iai.66.1.336-342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boden M K, Flock J I. Cloning and characterization of a gene for a 19 kDa fibrinogen-binding protein from Staphylococcus aureus. Mol Microbiol. 1994;12:599–606. doi: 10.1111/j.1365-2958.1994.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 7.Bost K L, Ramp W K, Nicholson N C, Bento J L, Marriott I, Hudson M C. Staphylococcus aureus infection of mouse or human osteoblasts induces high levels of interleukin-6 and interleukin-12 production. J Infect Dis. 1999;180:1912–1920. doi: 10.1086/315138. [DOI] [PubMed] [Google Scholar]
- 8.Boulton T G, Nye S H, Robbins D J, Ip N Y, Radziejewska E, Morgenbesser S D, DePinho R A, Panayotatos N, Cobb M H, Yancopoulos G D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- 9.Calhoun M D, Mader J T. Treatment of osteomyelitis with a biodegradable antibiotic implant. Clin Orthopaed Rel Res. 1997;341:206–214. [PubMed] [Google Scholar]
- 10.Cheung A I, Projan S J, Edelstein R E, Fischetti V A. Cloning, expression and nucleotide sequence of a Staphylococcus aureus gene (fbpA) encoding a fibrinogen-binding protein. Infect Immun. 1995;63:1914–1920. doi: 10.1128/iai.63.5.1914-1920.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 12.Davis R J. The mitogenic-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–14556. [PubMed] [Google Scholar]
- 13.De Cesaris P, Starace D, Riccioli A, Padula F, Filippini A, Ziparo E. Tumor necrosis factor-alpha induces interleukin-6 production and integrin ligand expression by distinct transduction pathways. J Biol Chem. 1998;273:7566–7571. doi: 10.1074/jbc.273.13.7566. [DOI] [PubMed] [Google Scholar]
- 14.de la Mata J, Uy H L, Guise T A, Story B, Boyce B F, Mundy G R, Roodman G D. Interleukin-6 enhances hypercalcemia and bone resorption mediated by parathyroid hormone-related protein in vivo. J Clin Investig. 1995;95:2846–2852. doi: 10.1172/JCI117990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 16.Dougherty S H. Pathobiology of infection in prosthetic devices. Rev Infect Dis. 1988;10:1102–1117. doi: 10.1093/clinids/10.6.1102. [DOI] [PubMed] [Google Scholar]
- 17.Ellington J K, Reilly S S, Ramp W K, Smeltzer M S, Kellam J F, Hudson M C. Mechanisms of Staphylococcus aureus invasion of cultured osteoblasts. Microb Pathog. 1999;26:317–323. doi: 10.1006/mpat.1999.0272. [DOI] [PubMed] [Google Scholar]
- 18.Flock J I, Froman G, Jonsson K, Guss B, Signas C, Nilsson B, Raucci G, Hook M, Wadstrom T, Lindberg M. Cloning and expression of the gene for fibronectin-binding protein from Staphylococcus aureus. EMBO J. 1987;6:2351–2357. doi: 10.1002/j.1460-2075.1987.tb02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franchimont N, Durant D, Rydziel S, Canalis E. Platelet-derived growth factor induces interleukin-6 transcription in osteoblasts through the activator protein-1 complex and activating transcription factor-2. J Biol Chem. 1999;274:6783–6789. doi: 10.1074/jbc.274.10.6783. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher J A, Gundle R, Bereford J N. Isolation and culture of bone forming cells (osteoblasts) from human bone. In: Jones G E, editor. Methods in molecular medicine: human cell culture protocols. Totowa, N.J: Humana Press; 1996. pp. 233–241. [DOI] [PubMed] [Google Scholar]
- 21.Gillaspy A F, Hickmon S B, Skinner R A, Thomas J R, Nelson C L, Smeltzer M S. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect Immun. 1995;63:3373–3380. doi: 10.1128/iai.63.9.3373-3380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gundle R, Beresford J N. The isolation and culture of cells from explants of human trabecular bone. Calcif Tissue Int. 1995;56:S8–S10. [Google Scholar]
- 23.Gupta S, Barrett T, Whitmarsh A J, Cavanagh J, Sluss H K, Derijard B, Davis R J. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S, Campbell D, Derijard B, Davis R J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 25.Han J, Lee J D, Bibbs L, Ulevitch R J. An ERK1/2 targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 26.Han J, Lee J D, Jiang Y, Li Z, Feng L, Ulevitch R J. Characterization of the structure and function of a novel ERK1/2 kinase (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- 27.Hobbie S, Chen L M, Davis R, Galan J E. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol. 1997;159:5550–5559. [PubMed] [Google Scholar]
- 28.Hudson M C, Ramp W K, Nicholson N C, Williams A S, Nousiainen M T. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog. 1995;19:409–419. doi: 10.1006/mpat.1995.0075. [DOI] [PubMed] [Google Scholar]
- 29.Jevon M, Guo C, Ma B, Mordan N, Nair S P, Harris M, Henderson B, Bentley G, Meghji S. Mechanisms of internalization of Staphylococcus aureus by cultured human osteoblasts. Infect Immun. 1999;67:2677–2681. doi: 10.1128/iai.67.5.2677-2681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonsson K, Signas C, Muller H P, Lindberg M. Two different genes encode fibronectin binding proteins in Staphylococcus aureus: the complete nucleotide sequence and characterization of the second gene. Eur J Biochem. 1991;202:1041–1048. doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- 31.Kohno M. Diverse mitogenic agents induce rapid phosphorylation of a common set of cellular proteins at tyrosine in quiescent mammalian cells. J Biol Chem. 1985;260:1771–1779. [PubMed] [Google Scholar]
- 32.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 33.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Lin L L, Lin A Y, Knopf J L. Cytosolic phospholipase A2 is coupled to hormonally regulated release of arachidonic acid. Proc Natl Acad Sci USA. 1992;89:6147–6151. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Littlewood-Evans A J, Hattenberger M R, Luscher C, Pataki A, Zak O, O'Reilly T. Local expression of tumor necrosis factor alpha in an experimental model of acute osteomyelitis in rats. Infect Immun. 1997;65:3438–3443. doi: 10.1128/iai.65.8.3438-3443.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDevitt D, Francois P, Vaudaux P, Foster T J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 37.McGavin M H, Krajewska-Pietrasik D, Ryden C, Hook M. Identification of a Staphylococcus aureus extracellular matrix-binding protein with broad specificity. Infect Immun. 1993;61:2479–2485. doi: 10.1128/iai.61.6.2479-2485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murai M, Usui A, Seki K, Sakurada J, Masuida S. Intracellular localization of Staphylococcus aureus within primary cultured mouse kidney cells. Microbiol Immunol. 1992;36:431–443. doi: 10.1111/j.1348-0421.1992.tb02042.x. [DOI] [PubMed] [Google Scholar]
- 39.Nair S, Song Y, Mehgji S, Reddi K, Harris M, Ross A, Poole S, Wilson M, Henderson B. Surface-associated proteins from Staphylococcus aureus demonstrate potent bone resorbing activity. J Bone Miner Res. 1995;10:726–734. doi: 10.1002/jbmr.5650100509. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura K D, Martinez R, Weber M J. Tyrosine phosphorylation of specific proteins after mitogen stimulation of chicken embryo fibroblasts. Mol Cell Biol. 1983;3:380–390. doi: 10.1128/mcb.3.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pace J, Hayman M J, Galan J E. Signal transduction and invasion of epithelial cells by Salmonella typhimurium. Cell. 1993;72:505–514. doi: 10.1016/0092-8674(93)90070-7. [DOI] [PubMed] [Google Scholar]
- 42.Park P W, Rosenbloom J, Abrams W R, Mecham R P. Molecular cloning and expression of the gene for elastin-binding protein (ebpS) in Staphylococcus aureus. J Biol Chem. 1996;271:15803–15809. doi: 10.1074/jbc.271.26.15803. [DOI] [PubMed] [Google Scholar]
- 43.Patti J M, Jonsson H, Guss B, Switalski L M, Wiberg K, Lindberg M, Hook M. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J Biol Chem. 1992;267:4766–4772. [PubMed] [Google Scholar]
- 44.Payne D M, Rossomando A J, Martino P, Erickson A K, Her J H, Shabanowitz J, Hunt D F, Weber M J, Sturgill T W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (ERK1/2) EMBO J. 1991;10:885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pelech S L, Sanghera J S. MAP kinases: charting the regulatory pathways. Science. 1992;257:1355–1356. doi: 10.1126/science.1382311. [DOI] [PubMed] [Google Scholar]
- 46.Pulverer B J, Kyriakis J M, Avruch J, Nikolakaki E, Woodgett J R. Phosphorylation of c-jun mediated by ERK1/2s. Nature. 1991;353:670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 47.Raingeaud J, Whitmarsh A J, Barrett T, Derijard B, Davis R J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramp W K, Lenz L G, Kaysinger K K. Medium pH modulates matrix, mineral, and energy metabolism in cultured chick osteoblast-like cells. Bone Miner. 1994;24:59–73. doi: 10.1016/s0169-6009(08)80131-6. [DOI] [PubMed] [Google Scholar]
- 49.Reilly S S, Hudson M C, Kellam J F, Ramp W K. Internalization of Staphylococcus aureus by embryonic chicken osteoblasts in vivo. Bone. 2000;26:63–70. doi: 10.1016/s8756-3282(99)00239-2. [DOI] [PubMed] [Google Scholar]
- 50.Rouzer C A, Matsumoto T, Samuelsson B. Single protein from human leukocytes possesses 5-lipoxygenase and leukotriene A4 synthase activities. Proc Natl Acad Sci USA. 1986;83:857–861. doi: 10.1073/pnas.83.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stashenko P, Obernesser M S, Dewhirst F E. Effects of immune cytokines on bone. Immunol Investig. 1989;18:239–249. doi: 10.3109/08820138909112240. [DOI] [PubMed] [Google Scholar]
- 52.Tang P, Rosenshine I, Finlay B B. Listeria monocytogenes, an invasive bacterium, stimulates MAP kinase upon attachment to epithelial cells. Mol Biol Cell. 1994;5:455–464. doi: 10.1091/mbc.5.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang P, Sutherland C L, Gold M R, Finlay B B. Listeria monocytogenes invasion of epithelial cells requires the MEK-1/ERK-2 mitogen-activated protein kinase pathway. Infect Immun. 1998;66:1106–1112. doi: 10.1128/iai.66.3.1106-1112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tucker K A, Reilly S S, Leslie C S, Hudson M C. Intracellular Staphylococcus aureus induces apoptosis in mouse osteoblasts. FEMS Microbiol Lett. 2000;186:151–156. doi: 10.1111/j.1574-6968.2000.tb09096.x. [DOI] [PubMed] [Google Scholar]
- 55.Usui A, Murai K, Seki J, Sakurada J, Masuida S. Conspicuous ingestion of Staphylococcus aureus organisms by mouse fibroblasts in vitro. Microbiol Immunol. 1992;36:545–550. doi: 10.1111/j.1348-0421.1992.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 56.van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J. 1995;14:1798–1810. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waldvogel F A, Medoff G, Swartz M N. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med. 1970;282:198–206. doi: 10.1056/NEJM197001222820406. [DOI] [PubMed] [Google Scholar]
- 58.Waskiewicz A J, Cooper J A. Mitogen and stress response pathways: ERK1/2 cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]