Abstract
Cognitive impairment is a prevalent neurological disorder that burdens families and the healthcare system. Current conventional therapies for cognitive impairment, such as cholinesterase inhibitors and N-methyl-d-aspartate receptor antagonists, are unable to completely stop or reverse the progression of the disease. Also, these medicines may cause serious problems with the digestive system, cardiovascular system, and sleep. Clinically, stimulation of acupoints has the potential to ameliorate the common symptoms of a variety of cognitive disorders, such as memory deficit, language dysfunction, executive dysfunction, reduced ability to live independently, etc. There are common acupoint stimulation mechanisms for treating various types of cognitive impairment, but few systematic analyses of the underlying mechanisms in this domain have been performed. This study comprehensively reviewed the basic research from the last 20 years and found that acupoint stimulation can effectively improve the spatial learning and memory of animals. The common mechanism may be that acupoint stimulation protects hippocampal neurons by preventing apoptosis and scavenging toxic proteins. Additionally, acupoint stimulation has antioxidant and anti-inflammatory effects, promoting neural regeneration, regulating synaptic plasticity, and normalizing neural circuits by restoring brain functional activity and connectivity. Acupoint stimulation also inhibits the production of amyloid β-peptide and the phosphorylation of Tau protein, suggesting that it may protect neurons by promoting correct protein folding and regulating the degradation of toxic proteins via the autophagy-lysosomal pathway. However, the benefits of acupoint stimulation still need to be further explored in more high-quality studies in the future.
Keywords: Acupoint stimulation, Acupuncture, Cognitive impairment, Dementia, Neuroprotection, Neural circuit
Introduction
Cognitive impairment is a common neurodegenerative disease. In the past two decades, dementia’s implication as a syndrome of global cognitive impairment has gained widespread recognition. More than 50 million individuals worldwide are affected by dementia, which has become the leading cause of disability and dependence among the elderly [1]. Alzheimer’s disease (AD) and vascular dementia (VD) are the two main causes of dementia [2, 3]. Mild cognitive impairment (MCI) is gaining attention as an intermediate stage between normal cognition and dementia, with a prevalence of 10–20% among those over the age of 65 [4]. Loss of neurons and synapses are the main pathological features of cognitive impairment, which leads to impairment of basic functions such as cognition and gradual loss of autonomous living ability [5]. This places a heavy burden on the patients’ families and the healthcare system. It is estimated that the global economic cost of dementia could rise to US$2 trillion by 2030 [6]. Drug therapy, including cholinesterase inhibitors (donepezil, galantamine, and rivastigmine) and N-methyl-d-aspartate receptor (NMDAR) antagonists (i.e., amantadine), is the currently available standard treatment for dementia. However, these drugs cannot prevent or reverse the progression of dementia and are associated with gastrointestinal, cardiovascular, and sleep disorders, as well as other side effects [7, 8]. Exploring new, safe, and effective therapies is a crucial aspect of neuroscience.
Traditional Chinese medicine (TCM), which includes acupuncture, electroacupuncture (EA), moxibustion, and other therapies, places a high priority on acupoint stimulation. These therapies have been demonstrated to be effective in treating multiple neurological disorders [9–11]. Acupuncture has been recommended by WHO as an effective complementary and alternative therapy in the treatment of VD [12], a recently published overview of systematic reviews of acupuncture for 77 diseases found that acupuncture significantly reduced the severity of VD symptoms [13], and it also shows potential in the treatment of other types of cognitive impairment. A systematic review and meta-analysis of 13 randomized controlled trials (RCTs) with 750 cases showed that acupuncture could help AD patients with cognitive impairment and that it was more effective than traditional Western medicine on the Mini-Mental State Examination (MMSE), the Activity of Daily Living (ADL), and the AD Assessment Scale-Cognitive scales, with no serious side effects [14]. Another study analyzed and compared 15 RCTs concerning acupuncture treatment for MCI, involving 1051 subjects, and found that compared with the control group, acupuncture treatment resulted in better clinical efficacy rates, as well as improved MMSE, Montreal Cognitive Assessment, clock-drawing task, and ADL scores, indicating that acupuncture is beneficial for improving cognitive function in elderly people with MCI [15]. Acupoint stimulation is also effective in improving cognitive deficits in memory, language, and executive function caused by surgery [16], chemotherapy [17], depression [18], and schizophrenia [19]. In addition, acupoint stimulation alone has better efficacy than Western medicine in improving patients' cognitive function and living ability, and synergistic therapy with Western medicine or Chinese herbal medicine may be superior to Western medicine alone [20–25].
Clinically, acupoint stimulation has the potential to treat symptoms of common cognitive disorders such as memory impairment, language impairment, executive dysfunction, and decreased functional capacity, among others. Taken together, this therapeutic strategy pays attention to symptoms when selecting acupoints. Similar acupoints have been selected, such as Baihui (GV20), Sishencong (EX-HN1), Fengchi (GB20), Shuigou (GV26), Shenting (GV24), Neiguan (PC6), Zusanli (ST36), Sanyinjiao (SP6), Shenmen (HT7), Taixi (KI3), Shenshu (BL23), Fenglong (ST40), Taichong (LR3), Dazhui (GV14), Xuanzhong (GB39), etc. It is suggested that there are common mechanisms of acupoint stimulation in the treatment of various forms of cognitive impairment. According to preliminary research, in models of cognitive impairment, acupoint stimulation can improve spatial learning and memory. In recent years, however, few systematic analyses of the underlying mechanisms in this domain have been conducted. Therefore, we reviewed the basic research from the past two decades to evaluate the common mechanisms of acupoint stimulation for the treatment of various forms of cognitive impairment and to provide new evidence for its clinical application.
Methods
Search strategy
For this study, the PubMed, Web of Science, and Embase databases were searched for the articles that were published between August 2001 and December 2022. The keywords included “acupuncture”, “electroacupuncture”, “moxibustion”, “transcutaneous acupoint electrical stimulation”, “neurocognitive disorders”, “cognitive defect”, “cognitive impairment”, and their related terms. A total of 1702 articles in English were identified.
Study selection
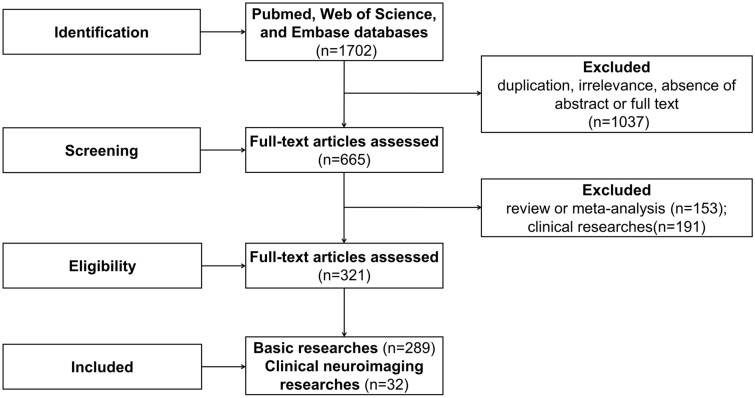
The following inclusion criteria were used for screening of the selected articles: stimulation methods included manual acupuncture (MA), EA, moxibustion, and transcutaneous acupoint electrical stimulation, and the main diseases studied included AD, VD, MCI, and cognitive impairment caused by various pathological factors. The 1702 articles identified by the search engine were then manually screened for those that met our inclusion criteria based on title and abstract. This led to the exclusion of 1037 articles due to duplication, irrelevance, lack of abstract, or unavailability of full text while the remaining 665 articles, including 289 basic studies, 223 clinical studies, and 153 reviews or meta-analyses were included. There were a total of 289 basic studies that constituted the underlying research. The 32 neuroimaging studies were selected from the 223 clinical studies because of their potential relevance to neural circuits. There were a total of 191 excluded clinical studies and 153 reviews or meta-analyses. Finally, 321 studies were analyzed. The flow chart of the search process is shown in Fig. 1.
Fig. 1.
Flow chart of the search strategy and process
Data extraction
Two authors have independently studied the titles, abstracts, and full texts of the retrieved articles. As a result, 321 articles were finally included, followed by data collection based on the predetermined criteria. Information from 56 recent representative studies is summarized in Table 1. Any disagreements were resolved through discussion among the authors.
Table 1.
Neuroprotective and neural circuit mechanisms of acupoint stimulation treating cognitive impairment
| References | Model/Subjects | Intervention methods | Acupoints | Acupoint stimulation parameters | Effect measurements | Biochemical measurements |
|---|---|---|---|---|---|---|
| [31] | d-galactose and Aβ1-40 injection rats | MA | GV24, GB13 | Twisting at 80 ± 5 times/min, 180° ± 5°, 2 min, with 1 min rest, 15 min, 6 days a week, 4 weeks | The escape/avoidance training: the number and duration of electric shock↓ | Serum: ChAT↑, AChE↓, SOD↑, MDA↓, ROS↓; Hippocampus: neurons number↑, cells intercellular space↓, TUNEL indexes↓, Bcl-2↑, Bax↓, CYC↓, caspase3↓, caspase 9↓, Bcl-2/Bax↑ |
| [32] | Aβ1-40 injection rats | EA | GV20, BL23 | 20 Hz, 30 min, 6 days a week, 4 weeks | MWM test: escape latency↓, platform cross number↑, Time spent in the target quadrant↓ | CA1: cell apoptosis↓; Hippocampus: Bcl-2 protein↑, Bax protein↓, synapsin-1↑, Notch1 mRNA↓, Hes1 mRNA↓ |
| [36] | MCAO/R rats | EA | GV20, GV24 | 1/20 Hz, 30 min, 10 days | Cerebral infarct volumes↓, MWM test: the latency and route length↓, platform cross number↑ | TUNEL indexes↓, NF-κB protein↓, p-IκB↓, Fas↓, Bax↓ |
| [37] | Aβ1–42 injection rats | Moxibustion | GV20, BL23 | Pre-moxibustion group: 15 min, 6 days a week, 8 weeks + 2 weeks; moxibustion group: 15 min, 6 days a week, 2 weeks | MWM test: escape latency↓, platform cross number↑, time spent in the target quadrant↑ | CA1: cell apoptosis (Pre-moxibustion group was superior to moxibustion group)↓, cell membrane ruptured↓ |
| [40] | BCCAO rats | EA | GV20, GV14, BL23 | 4 Hz, 2 mA, 20 min, 30 days | – | CA1: p53↓, Noxa↓ |
| [42] | APP/PS1 mice | EA | GV20, GV29, GV26 | 1 Hz, 1 mA, 20 min | MWM test: escape latency↓, platform cross number↑ | Hippocampus: APP↓, p-JNK↓, p-MKK7↓, c-Jun↓ |
| APP/PS1 mice | EA + SP600125 | GV20, GV29, GV26 | 1 Hz, 1 mA, 20 min | MWM test: escape latency↑, platform cross number↓ | Hippocampus: APP↓, P-JNK↓, p-MKK7↓, c-Jun↓ | |
| [44] | 2VO rats | MA | GV20, ST36 | 6 days per week, 2 weeks | MWM test: escape latency↑, swimming distance↑, time spent in the target quadrant↓ | Hippocampus: ROS↓, TUNEL indexes↓, Trx-1↑, TrxR-1↑, p-ASK1↓, p-JNK↓, p-p38↓, cleaved-caspase3↓ |
| 2VO rats | Trx-1 siRNA + MA | GV20, ST36 | 6 days per week, 2 weeks | MWM test: escape latency↑, swimming distance↑, time spent in the target quadrant↓ | Hippocampus: ROS↑, TUNEL indexes↑ | |
| [45] | APP/PS1 mice | EA + MA | EA: GV20, GV29; MA: GV26 | EA: 1 Hz, 1 mA, 20 min, MA: fast pricking, 4 weeks | MWM test: escape latency↓, Average swimming speed↑, Platform crossing frequency↑, time spent in the target quadrant↑ | Hippocampus: APP↓, BACE1↓, p-PKA/total-PKA↑ |
| [49] | D-galactose and Aβ1-40 injection rats | EA | GV24, GB13 | 30 Hz, 1 mA, 30 min, 6 days a week, 4 weeks | OFT: duration spent in the central zone↓, frequency of crossing↑; MWM test: escape latency↓, escape distance↓, latency to cross the platform for the first time↓, frequency of platform crossings↑, time spent in the target quadrant↑ | Hippocampus: Aβ↓, p-tau (s396)↓, p-tau (s404)↓ |
| [52] | APP/PS1 mice | EA + MA | EA: GV20, GV29; MA: GV26 | EA: 2 Hz, 1 mA, 20 min, MA: fast pricking, once every other day for 28 days | MWM test: escape latency↓, time spent in the target↑, platform cross frequency↑ | Hippocampus: glucose metabolism↑, p-Tau (Ser199, Ser202)↓, p-AKT (Ser473)↑, p-GSK3β (Ser9)↑ |
| [53] | SAMP8 mice | MA | CV17, CV12, CV6, SP10, ST36 | 30 s | Tightrope test: success rate↑; MWM test: the number of platform-site crossovers↑, the latency to first target-site crossover↓, percentage of time spent in the middle annulus↑ | Hippocampus:CA3↑, DG↑, SOD↑, GSH-Px↑, superoxide anion↓, protein carbonyl concentrations↓, HSP84↑, HSP86↑ |
| [54] | APP/PS1 mice | EA | GV20 | 1/20 Hz, 30 min, 5 days a week, 4 weeks | MWM test: escape latency↓, escape distance↓, frequency of platform crossings↑ time spent in the target quadrant↑, discrimination ratio↑ | Cortex and Hippocampus: glucose metabolism↑, GLUT1↑, GLUT3↑, Aβ↓, AMPK↑, AKT↑, p-mTOR/total-mTOR↓ |
| [55] | MCAO rats | EA | GV20, GV24 | 1–20 Hz, 2 mA, 30 min, 8 days | Neurological scores↓, cerebral infarction volume↓, MWM test: escape latency↓, platform crossings frequency↑ | Cell membrane ruptured↓, mitochondria damaged↓, PI3K↑, mTOR↑, Beclin-1↑, p53↓, p-Akt↑ |
| [56] | 5xFAD mice | EA | GV24, GB13 | 2 Hz, 0.3 mA, 15 min, 5 days a week, 4 weeks | MWM test: escape latency↓, platform crossings frequency↑, time spent in the target↑; Contextual and Cued test: freezing index↑ | Fl-APP↓, CTFs↓, Aβ positive area↓, plaque size↓, microglia activation↓, insoluble LC3B-II↓, insoluble SQSTM1↓, LC3B+ CTSD↑, LAMP1↑, total TFEB↑, nuclear TFEB↑, p-TFEB (S142)↑, LC3B +/Aβ1-42 +↑, APP-associated SQSTM1↓, 6E10 + plaques↓, plaque-associated LAMP1↓, p-MTOR↓, RPS6↓, p-AKT in the PFC↓, p-MAPK1 in the hippocampus↓ |
| 5xFAD mice | EA + CQ | GV24, GB13 | 2 Hz, 0.3 mA, 15 min, 5 days a week, 4 weeks | MWM test: platform crossings frequency↓, time spent in the target↓, Contextual and Cued test: freezing index↓ | Insoluble LC3B-II↑, insoluble SQSTM1↑, LAMP1↓, Fl-APP↑ | |
| 5xFAD mice | EA + AAV-sh-Tfeb | GV24, GB13 | 2 Hz, 0.3 mA, 15 min, 5 days a week, 4 weeks | MWM test: platform crossings frequency↓ | CTFs↑ | |
| [58] | MCAO rats | MA | GV20, GV26, GV14, GV16 | 20 min, 15 days | MWM test: escape latency↓, platform cross number↑ | TUNEL indexes↓, ATP↑, SOD↓, NO↓, iNOS↓, ROS↓, TOMM40↓, TIMM17A↓, Aβ↓, APP↑, COX↑ |
| [59] | LI/R rats | EA pretreatment | GV20, GB34, LR3, ST36, SP10 | 2/15 Hz, 1 mA, 30 min | Survival rate↑, MWM test: escape latency↓, distance to platform↓, time spent in the target quadrant↑, platform cross number↑ | CA1: number of normal cells↑, TUNEL + cells↓, microglia activation↓ |
| [60] | Aβ1-42 injection rats | EA | GV20, KI1 | 2/15 Hz, 1 mA, 30 min, 4 weeks | MWM test: escape latency↓, time spent in the target quadrant↑ | Hippocampus: ROS↓, 8-OH-dG↓, MDA↓, T-AOC↑, NOX2↓, swelling of neurons↓ |
| [61] | AF64A injection rats | LA | HT7 | Wavelength of 405 nm, 10 min, 2 weeks | MWM test: escape latency↓, retention time↑ | Hippocampus: ROS↓, MDA↓, CAT activity↑, SOD activity↑, AChE activity↓ |
| [69] | 2VO rats | MA | GV20, ST36 | Twisting < 90°, > 120 times per min, 30 s | MWM test: the escape latency↓, swimming distance↓, time spent in the target quadrant↑; infarct volume↓, neuronal cell loss↓ | O2−↓, NADPH oxidase activity↓, gp91phox protein↓, p47phox protein↓ |
| 2VO rats | MA + TBCA | GV20, ST36 | Twisting < 90°, > 120 times per min, 30 s | Infarct volume↑ | O2−↑ | |
| Gp91phox KO mice | MA | GV20, ST36 | Twisting < 90°, > 120 times per min, 30 s | MWM test: the escape latency↑, swimming distance↑, time spent in the target quadrant↓ | – | |
| [71] | 2VO rats | MA | GV20, ST36 | 2 weeks, suspend on day 7 | CBF↑, MWM test: the escape latency↓, time spent in the target quadrant↓ | CA1: injured neuron cell↓, ROS↓; Hippocampus: Nrf2 nuclear translocation↑, Nrf2↑, HO-1 protein↑, NQO1 protein↑, microglia activation↓, neuron death↓ |
| 2VO mice (Nrf2 KO) | MA | GV20, ST36 | 2 weeks, suspend on day 7 | MWM test: the escape latency↑, time spent in the target quadrant↑ | CA1: microglia activation↑, neuron death↑ | |
| [76] | APP/PS1 mice (mild AD) | EA | GV20, GV24 | 1/20 Hz, 1 mA, 30 min, 3 days a week, 16 weeks | MWM test: escape latency↓, platform cross number↑ | Parietal association cortex: number and area fraction of Aβ↓; colocalization of iNOS-Iba1↓, IL-1β-Iba1↓, CD206-Iba1↑, Arg1-Iba1↑; iNOS mRNA↓, IL-1β mRNA↓, CD206 mRNA↑, Arg1 mRNA↑; Entorhinal cortex: colocalization of Arg1-Iba1↑; iNOS mRNA↓, IL-1β mRNA↓, CD206 mRNA↑, Arg1 mRNA↑ |
| APP/PS1 mice (moderate AD) | EA | GV20, GV24 | 1/20 Hz, 1 mA, 30 min, 3 days a week, 16 weeks | MWM test: escape latency↓, platform cross number↑ | Parietal association cortex: number of Aβ↓; colocalization of iNOS-Iba1↓; Entorhinal cortex: number of Aβ↓; colocalization of iNOS-Iba1↓, Arg1-Iba1↑; IL- 1β mRNA↓, CD206 mRNA↑ | |
| [80] | BCCAO Mongolian gerbils | BVA | ST36 | 0.1 μg/g, 4 times every other day | Y-Maze task: spontaneous alternation↑ | CA1: neuronal cell death↓, microglia activation↓; Hippocampus: TLR4↓, CD14↓, TNF-α↓, iNOS↓, Bax↓, BDNF↑, p-ERK↑ |
| [81] | 2VO rats | MA | GV20, ST36 | 6 days per week, 2 weeks | MWM test: escape latency↓, time spent in the target↑ | Hippocampus and plasma: IL-6↓, TNF-α↓; Hippocampus: TLR4↓, TLR4 + microglia↓, MyD88↓, p-NF-κB↓, p65↓, miR-93↓ |
| [85] | SAMP8 mice | EA | GV20, ST36 | 2 or 10 Hz, 1 mA, 10 min, 2 weeks | MWM test: escape latency↓, travel distance↓, platform cross frequency↑ | CA1 and CA3: morphological and structural abnormalities of neurons↓, TUNEL indexes↓; Serum: IL-1β↓, IL-6↓, IL-18↓, TNF-α↓; Hippocampus: NLRP3↓, ASC↓, caspase-1↓, GSDM-D↓, IL-1β↓, IL-18↓ (10 Hz was superior to 2 Hz) |
| [86] | 2VO rats | MA | GV20, ST36 | Twisted 2 times per sec, 30 s, 2 weeks, suspend on day 7 | MWM test: escape latency↓, total distance↓, the time spent in the target quadrant↓ | CA1: cell apoptosis↓, ROS↓, 8-OHdG↓, SOD↑, TXNIP↓, NLRP3↓, caspase-1↓, IL-1β↓ |
| [90] | 2VO rats | MA | GV20, ST36 | 10 min, 6 days per week, 2 weeks | MWM test: escape latency↓, the time spent in the target quadrant↓ | Hippocampus: injured neurons↓, TNF-α↓, IL-6↓, microglia activation↓, α7nAChR + neurons↑, p-JAK2↑, p-STAT3↑ |
| 2VO rats | MA + α-BGT | GV20, ST36 | 10 min, 6 days per week, 2 weeks | MWM test: escape latency↑, the time spent in the target quadrant↓ | Hippocampus: injured neurons↑, TNF-α↑, IL-6↑ | |
| [93] | MCAO/R rats | EA | GV20, GV24 | 2/20 Hz, 0.2 mA, 30 min, 7 days | MWM test: escape latency↓, platform cross number↑, infarct volume↓ | Peri-infarct CA1 and sensorimotor cortex: ED1+ cells↓, GFAP+ cells↓, IL-1β↑, IL-10↓, P2X7R+/ED1+ cells↓, P2X7R +/GFAP+ cells↓, P2Y1R+/ED1+ cells↓, P2Y1R+/GFAP+ cells↓ |
| [94] | MID rats | MA | ST36 | Twisted 2 times/s for 30 s, 6 days a week, 2 weeks | MWM test: escape latency↓ | Pyramidal neurons number in the CA1↑ |
| [95] | SAMP8 rats | MA | CV17, CV12, CV6, SP10, ST36 | rotated twice a second, 30 s | MWM test: escape latency↓, retention time↓ | Neurons number in the CA3 and DG↑ |
| [96] | SAMP8 rats | MA + NSCs transplantation | CV17, CV12, CV6, SP10, ST36 | CV17, CV12, CV6, ST36: twisting reinforcing methods; SP10: twisting reducing method; 15 days, suspended on day 7 | MWM test: escape latency↓, retention time↓, platform cross frequency↑ | Hippocampus: bFGF↑, EGF↑, BDNF; DG and in vitro: NeuN +/BrdU+ NSCs↑, GFAP +/BrdU+ NSCs↑ |
| [97] | Brain irradiation rats | EA | GV20, ST36 |
2/15 Hz, 2–3 mA, from 3 days before irradiation to 2 weeks post-irradiation |
NORT: novel place exploration ratio↑, stool droppings↓, latency↓ | Hippocampus: activated microglia↓, BDNF↑; DCX + cells in the SGZ↑ |
| [98] | SAMP8 | MA | CV17, CV12, CV6, SP10, ST36 | CV17, CV12, CV6, ST36: twisting reinforcing methods; SP10: twisting reducing method, 15 days, suspended on day 8 | MWM test: escape latency↓, retention time↓ | BrdU+ cells in the DG and subfield of LV-CC↑ |
| [102] | 5xFAD mice | EA | GV20, GV24 | 2/20 Hz, 1 mA, 30 min, 5 days a week, 4 weeks | NLRT: location exploration↑ | MS/VDB and DG: NAA/Cr↑, Cho/Cr↑, the number of M1 mAChR+ cells↑; the neurons were arranged more neatly, with darker and more obvious Nissl bodies; MS/VDB: AChE↓, VAChT↑; DG: ChAT↑, Aβ fraction ratio↓; Hippocampus: DCX+ cells↑, Neuro-D1+ cells↑ |
| 5xFAD mice | EA + hM4Di | GV20, GV24 | 2/20 Hz, 1 mA, 30 min, 5 days a week, 4 weeks | – | MS/VDB and DG: Cho/Cr↓, the number of M1 mAChR+ cells↓; Hippocampus: DCX+ cells↓, Neuro-D1+ cells↓ | |
| [103] | BCAS mice | EA | GV20, GV14 | 2 Hz, 20 min, 7 days | MWM test: latency to locate platform↓, step through latency↓ | CC: fluoromyelin staining↓, MBP↓, BrdU + cells↑, BrdU +/NG2 + cells↓, BrdU +/CNPase + cells↑, NG2↓, PDGFRα↓, CNPase↑, Figf↑, Mdk↑, NT4/5↑, NT4/5 +/GFAP cells↑, p-TrkB + cells↑, p-TrkB +/PDGFRα + cells↑, p-TrkB +/ CNPase + cells↑, p-CREB + cells |
| BCAS mice | EA + ANA-12 | GV20, GV14 | 2 Hz, 20 min, 7 days | MWM test: latency to locate platform↑ | BrdU +/NG2 + cells↑, BrdU +/CNPase + cells↓ | |
| [111] | MCAO/R rats | EA | GV20, GV24 | 2/10 Hz, 1–3 mA, 30 min, 7 days | Cerebral infarction↓; MWM test: escape latency↓, retention time↓, platform cross number↑ | Hippocampus: vacuolization of mitochondria in the axons↓, BDNF↑, TrkB↑, PSD-95 |
| [114] | Aβ1-42 injection rats | EA | GV20, BL23 | 2 or 30 or 50 Hz, 1 mA, 15 days, suspended on day 7 | MWM test: escape latency↓, time spent in the target↑, platform cross number↑ | Synaptic curvatures↑, synaptic cleft width↓, postsynaptic density↑, GSK-3β↓, p-GSK-3β (Tyr216)↓, APP↓, p-GSK-3β (Ser9)↑, Aβ1–40↓ (50 Hz > 30 Hz > 2 Hz) |
| [116] | MCAO rats | EA | GV20, GV24 | 1/20 Hz, 30 min, 7 days | Infarct volume↓, MWM test: swimming duration and distances↓ | Dendritic spines density↑, Cdc42↑, Rac1↑, F-actin↑, RhoA↓ |
| [121] | MCAO rats | EA | EX-HN3, GV20 | 2 Hz, 1 mA, 10 min, 14 days | MWM test: latency↓, time spent in the target↓; NORT: recognition index↑ | Hippocampus and PFC: BDNF↑, TrkB↑, NMDAR1↑, AMPAR↑, GABAAR↑, CaMKII↑, NeuN↑, PSD-95↑ |
| [123] | Cerebral multi-infarction rats | MA | ST36 | Twisting two spins per second, 30 s, 6 days a week, 2 weeks | Radial arm maze test: working memory errors↓, reference memory errors↓ | fEPSP slope in the DG↑; p-ERK in the CA1 and DG; Hippocampus: PDE activity↓, cAMP↑, PKA activity↑, p-CREB↑ |
| Cerebral multi-infarction rats | MA + H89 | ST36 | Twisting two spins per second, 30 s, 6 days a week, 2 weeks | MWM test: escape latency↑, time spent in the target↓ | p-CREB↓ | |
| [127] | SAMP8 | EA | GV20, ST36 | 10 Hz, 1 mA, 30 min, 7 days | Y Maze Spontaneous Alternation Test: correct spontaneous alternation rate↑; MWM test: escape latency↓, time spent in the target↑, platform cross number↑ | CSF: Orexin A↓, Glutamate↓; Hippocampus and lateral hippocampus: Orexin A↓; Hippocampal CA1 and CA3: the pyramidal cells were orderly arranged, the metabolism of Nissl bodies↑; Hippocampus: cAMP↑, pPKA↑, PKA↑, pCREB↑, CREB↑, GluN1↑, GluN2A↑, GluA2↑, SYP↑, and PSD-95↑, Orenxin A↓, synapses↑; the mitochondrial bilayer membrane was clear, the internal cristae was orderly arranged, the myelin sheaths were intact, dense, and highly layered, synaptic structures were relatively complete and normal |
| [128] | 2VO rats | MA | MA1: GV20, ST36; MA2: GV20, GV24; MA3: ST36, SP10 | Twisting < 90°, 120 times/min, 30 s, 6 days a week, 2 weeks | MWM test: escape latency↓, swimming distance↓, time spent in the target↑ (MA1 was superior to MA2 and MA3) | – |
| 2VO rats | MA | GV20, ST36 | Twisting < 90°, 120 times/min, 30 s, 6 days a week, 2 weeks | – | fEPSP slope in the PP-DG↑, dopamine↑, DOPAC↑, HVA↑, epinephrine↑, D1R↑, D5R↑ | |
| 2VO rats | MA + SCH23390 | GV20, ST36 | Twisting < 90°, 120 times/min, 30 s, 6 days a week, 2 weeks | MWM test: escape latency↓, swimming distance↑, time spent in the target↓ | fEPSP slope↓ | |
| 2VO rats | MA + SKF38393 | GV20, ST36 | Twisting < 90°, 120 times/min, 30 s, 6 days a week, 2 weeks | – | fEPSP slope↑ | |
| [129] | 2VO rats | MA | GV20, ST36 | 10 min, 6 days a week, 2 weeks | – | fEPSP slope in the PP-DG↑, NE↑, β1-AR↑, β1-AR +/NeuN + cells↑ |
| 2VO rats | MA + propranolol | GV20, ST36 | 10 min, 6 days a week, 2 weeks | – | fEPSP slope in the PP-DG↓ | |
| 2VO rats | MA + atenolol | GV20, ST36 | 10 min, 6 days a week, 2 weeks | – | fEPSP slope in the PP-DG↓ | |
| [130] | 2VO rats | MA | GV20, ST36 | 10 min, 12 days, suspended on day 7 | NORT: recognition index↑; RAM test: working memory errors↓, new entries number↑ | LTP of population spike in the PP-DG↑, DBH↑, DBH activity↑ |
| [133] | MCAO rats | EA | GV20, GV24 | 20 Hz, 30 min, 7 days | Infarct volume↓, MWM test: latency↓, route length↓, platform cross number↑ | CA1: Glu↓, NMDAR2A↑, NMDAR2B↓, intracellular Ca2 +↓ |
| [134] | SCO rats | MA | MA1:GV20; MA2: TE4 | 5 min | PAT: latencies to enter the dark compartment↑ (MA1); MWM test: latency↓, percentages of time↑ (MA1) | CA1: ChAT↑, BDNF↑, CREB↑, CHT1↑, VAChT↑ (MA1) |
| [136] | POCD rats | EA | GV20, PC6, LI4 | 2/100 Hz, 4 mA, 30 min, 7 days | MWM test: escape latency↓, platform cross number↑ | Hippocampus: α7nAChR + neurons↑, TNF-α + neurons↓, IL-1β + neurons↓ |
| [143] | 3 × Tg-AD mice | EA | GV20, GV24 | 1/20 Hz, 1 mA, 30 min, 5 days a week, 4 weeks | NORT: recognition index↑ | ReHo in the amygdala, auditory cortex, DG, dorsal raphe nucleus, entorhinal cortex, hippocampus, somatosensory cortex, subiculum, substantia nigra, temporal cortex, and ventral tegmental area↑; postsynaptic sEPSC of hippocampus CA1↑; FC of the hippocampus with entorhinal cortex↑ |
| [144] | D-galactose injection rats | MA | ST36 | Twisting at 90–180°, 60–90 times/min; lifting and thrusting at 0.1–0.2 mm, 60–90 times/min; 30 min | —— | Glycol metabolism in the pyriform cortex of the right limbic system, the olfactory cortex of the right temporal lobe, the right amygdaloid body, the right hippocampus, the pyriform cortex of the left limbic system, and the olfactory cortex of the left temporal lobe↑ |
| [145] | MCAO rats | EA | GV20, GV24 | 1/20 Hz, 0.2 mA, 30 min, 14 days | MWM test: escape latency↓, platform cross number↑ | FC of the left RSC with the left hippocampus, left RSC, right RSC, left CG, right CG, right tegmentum of midbrain and right visual cortex↑ |
| [147] | AD patients (n = 14) | MA | LR3, LI4 | 3 min | – | ALFF in the left PoCG↑; FC of the right hippocampus with the left PrCG↑ |
| [148] | AD patients (n = 14) | MA | LR3, LI4 | 3 min | – | FC of the MFG with the left hippocampus↑ |
| [151] | AD patients (n = 14) | MA | LR3, LI4 | 3 min | – | FC of the bilateral CG with left PCu↓, FC of the right IPL, right MTG, with a cluster in left PCC↑ |
| [152] | D-gal injection rats | MA | HT7 | Twisted/rotated at 120–150 times per min, 3 min, 2 min break and repeated three times, 5 days a week, 6 weeks | Y-Maze test: total reaction time↓ | Glucose metabolism in the hippocampus, orbital cortex, cerebellum, piriform cortex, RSC, sensory cortex, and olfactory cortex↑ |
| [153] | MCI patients (n = 12) | MA | KI3 | Twisted at 60°, 120 times/min, 2 min | – | Activities in bilateral anterior cingulate gyrus, left medial frontal gyrus, left cuneus, left middle frontal gyrus, left lingual gyrus, right middle frontal gyrus, bilateral inferior frontal gyrus, left SFG, right cuneus, right STG, left subcallosal gyrus, bilateral PCu, right medial frontal gyrus, right SFG, left CG, left PrCG, and right FG↑ |
| [154] | MCI patients (n = 8), AD patients (n = 12) | MA | LR3, LI4 | 3 min | – | In the procession of acupuncture: activities in the bilateral CPL, bilateral MTG, bilateral FG, right PHG, left ITG, frontal lobe, bilateral IPL, right PoCG and occipital lobe↑, activities in the bilateral CPL, temporal lobe, bilateral SFG, right MFG, left PrCG, right PoCG, left PCL, left SPL, right lingual gyrus and limbic regions↓ of MCI patients; activities in the right CPL, bilateral frontal lobe, right IPL, right MOG↑, activities in the right STG, right MTG, bilateral MFG and left brain stem↓ of AD patients; In the second resting state after acupuncture: In MCI patients, activities in the bilateral CPL, bilateral FG, right MTG and right PHG, frontal lobe, right lentiform nucleus, left extra nuclear and right thalamus↑, activities in the bilateral CPL, bilateral MTG, left STG, right ITG and right FG, left SFG, left IFG, bilateral PrCG, right MFG, bilateral PoCG, left IPL, bilateral SPL, right angular and left SOG, left cuneus↓ of MCI patients; activities in the right CPL, left ITG, right MTG, bilateral SFG, left IFG, right MFG and bilateral PrCG, right MOG, bilateral SMG, right SPL↑, activities in the left CPL, bilateral PHG, right MFG, left lingual gyrus, right cingulate gyrus, left lentiform nucleus and right midbrain↓ of AD patients |
| [155] | MCI patients (n = 32) | MA | EX-HN1, EX-HN3, PC6, KI3, ST40, LR3 | twirled at ± 60°, 120 times per min, 5 days a week, 4 weeks | MMSE↑, MoCA↑, digit-symbol task↑, digit-span task↑, word recall task↑ | Insula, DLPFC, and hippocampus acted as central hubs. The insula received causal inflows from the thalamus, hippocampus, ACC, and primary somatosensory cortex. The hippocampus received causal inflows from the DLPFC, ACC, and mPFC. The DLPFC received causal inflows from the OFC, ACC, and primary motor cortex. The IPL received causal inflows from the DLPFC and culmen. The PCu received causal inflows from the FG, the thalamus received causal inflows from the caudate. The supplementary motor area received causal inflows from the insula. The somatosensory cortex received causal inflows from the MTG |
↑, upregulated by intervention; ↓, downregulated by intervention
Aβ, amyloid-beta; MA, manual acupuncture; GV24, Shenting; GB13, Benshen; ChAT, choline acetyltransferase; AChE, acetylcholinesterase; SOD, superoxide dismutase; MDA, malondialdehyde; ROS, reactive oxygen species; EA, electroacupuncture; GV20, Baihui; BL23, Shenshu; MWM, Morris Water Maze; MCAO/R, middle cerebral artery occlusion-reperfusion; NF-κB, nuclear factor-kappa B; phosphorylated IκB, phosphorylated NF-κB inhibitor; BCCAO, bilateral common carotid artery occlusion; GV14, Dazhui; GV29, Yintang; GV26, Shuigou; JNK, c-Jun N-terminal kinase; MKK7, mitogen-activated protein kinase kinase 7; SP600125, JNK inhibitor; 2VO, bilateral common carotid artery occlusion; ST36, Zusanli; Trx-1, thioredoxin-1; TrxR-1, thioredoxin reductase-1; ASK1, apoptosis signal-regulating kinase 1; APP, β-amyloid precursor protein; BACE1, Beta-site APP cleaving enzyme1; PKA, protein kinase; OFT, Open Field Test; Akt, protein kinase B; GSK3β, Glycogen synthase kinase-3; CV17, Danzhong; CV12, Zhongwan; CV6, Qihai; SP10, Xuehai; DG, dentate gyrus; GSH-Px, glutathione peroxidase; HSP84, heat shock protein 84; HSP86, heat shock protein 86; GLUT1, glucose transporter type 1; GLUT3, glucose transporter type 3; AMPK, AMP-activated protein kinase; mTOR, mammalian/mechanistic target of rapamycin; PI3K, phosphatidylinositol 3-kinase; FL-APP, full-length APP; LC3B, microtubule-associated protein light chain 3 beta; SQSTM1, sequestosome 1; CTSD, cathepsin D; LAMP1, lysosomal-associated membrane protein 1; TFEB, transcription factor EB; RPS6, ribosomal protein S6; PFC, prefrontal cortex; MAPK, mitogen-activated protein kinases; CQ, chloroquine; CTFs, beta C-terminal fragments; GV16, Fengfu; ATP, adenosine triphosphate; iNOS, inducible nitric oxide synthase; TOMM40, Translocase of Outer Mitochondrial Membrane-40; TOMM17A, Translocase of Outer Mitochondrial Membrane-17A; COX, cyclooxygenase; LI/R, Limb ischemia/reperfusion; GB34, Yanglingquan; LR3, Taichong; 8-OH-dG, 8-Hydroxy-2'-deoxyguanosine; T-AOC, total antioxidant capacity; NOX2, nicotinamide adenine dinucleotide phosphate oxidases 2; LA, laser acupuncture; HT7, Shenmen; CAT, catalase; TBCA, NADPH oxidase activator; CBF, cerebral blood flow; Nrf2, Nuclear factor erythroid 2-related factor2; HO-1, heme oxygenase-1; NQO1, NAD(P)H: Quinone Oxidoreductase 1; Arg1, arginase-1; BVA, Bee Venom Acupuncture; TLR4, Toll-like Receptor 4; TNF-α, Tumour necrosis factor alpha; BDNF, brain-derived neurotrophic factor; ERK, extracellular signal-regulated kinase; IL-6, interleukin-6; MyD88, myeloid differentiation primary response 88; IL-1β, interleukin-1beta; IL-18, interleukin-18; NLRP3, nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing-3; ASC, apoptosis-associated speck-like protein; GSDM-D, gasdermin-D; TXNIP, thioredoxin-interacting protein; α-BGT, α7nAChR antagonist α-bungarotoxin; α7nAChR, alpha7 nicotinic acetylcholine receptor; JAK2, Janus kinase 2; STAT3, signal transducer and activator of transcription 3; GFAP, Glial fibrillary acidic protein; P2X7R, P2X purinergic receptor 7, ligand-gated ion channel, 7; P2Y1R, P2Y purinergic receptor 1; MID, multi-infarction dementia; NSCs, neural stem cells; bFGF, basic fibroblast growth factor; EGF, epidermal growth factor; NeuN, neuronal nuclei; BrdU, bromodeoxyuridine; NORT, novel object recognition task; DCX, doublecortin; SGZ, subgranular zone; LV-CC, lateral ventricle-corpus callosum; NLRT, novel location recognition; MS/VDB, medial septum/vertical limb of the diagonal band of Broca; NAA, N-acetyl aspartate; Cr, creatine; Cho, choline; M1 mAChR, M1 muscarinic acetylcholine receptor; VAChT, vesicular acetylcholine transporter; BCAS, bilateral carotid artery stenosis; CC, corpus callosum; MBP, myelin basic protein; NG2, neural/glial antigen 2; CNPase, 2′,3′-cyclic nucleotide-3′-phosphodiesterase; PDGFRα, platelet-derived growth factor receptor alpha; Figf, C-Fos-Induced Growth Factor; Mdk, midkine; NT4/5, neurotrophin-4/5; TrkB, tropomysin related kinase B; CREB, cAMP Response Element-Binding Protein; ANA-12, TrkB antagonist; PSD-95, postsynaptic density protein-95; Cdc42, cell division cycle 42; Rac1, small GTPase Ras-related C3 botulinum toxin substrate 1; RhoA, Ras homolog gene family member A; EX-HN3, Yintang; NMDAR1, N-methyl-d-aspartate receptor 1; AMPAR, AMPA-type glutamate receptor; GABAAR, type A gamma-aminobutyric acid chloride channel; CaMKII, Calcium/calmodulin-dependent protein kinase II; fEPSP, field excitatory postsynaptic potential; cAMP, 3', 5' cyclic adenosine monophosphate; H89, N-(2-(4-bromocinnamylamino) ethyl)-5-isoquinolinesulfonamide; PP-DG, perforant pathway-dentate gyrus; CSF, cerebrospinal fluid; SYP, Synaptophysin; DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid; D1R, Dopamine D1 Receptor; D5R, Dopamine D5 Receptor; SCH23390, D1/D5 receptors antagonist; SKF38393, D1/D5 receptor agonist; NE, norepinephrine; β1-AR, beta 1-adrenergic receptor; RAM, Radial Arm Maze; DBH, Dopamine β-Hydroxylase; Glu, Glutamate; NMDAR2A, N-methyl-d-aspartate receptor 2A; NMDAR2B, N-methyl-d-aspartate receptor 2B; SCO, scopolamine; TE4, Yangchi; PAT, passive avoidance test; CHT1, choline transporter; POCD: Postoperative Cognitive Dysfunction; PC6: Neiguan; sEPSCs, spontaneous excitatory postsynaptic currents; FC: functional connectivity; RSC: retrosplenial cortex; CG: cingulate gyrus; LI4:Hegu; AD: Alzheimer’s disease; ALFF, amplitude of low-frequency fluctuation; PoCG, postcentral gyrus; PrCG, precentral gyrus; FG, fusiform gyrus; MFG, middle frontal lobe; PCu, precuneus; IPL, inferior parietal lobule; MTG, middle temporal gyrus; PCC, posterior cingulate cortex; MCI, mild cognitive impairment; KI3, Taixi; SFG, superior frontal gyrus; STG, superior temporal gyrus; CPL, cerebellum posterior lobe; PHG, parahippocampus; ITG, inferior temporal gyrus; PCL, paracentral lobule; SPL, superior parietal lobule; MOG, middle occipital lobe; SOG, superior occipital lobe; EX-HN1, Sishencong; ST40, Fenglong; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; DLPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; mPFC, medial prefrontal cortex; OFC, orbitofrontal cortex
Mechanisms of acupoint stimulation in the treatment of cognitive impairment
The pathological process of dementia has not been fully elucidated. However, a variety of pathophysiological processes may potentially contribute to dementia, such as age, genetic predisposition, cardiovascular disease, diabetes, psychiatric disorders, traumatic brain injury, and obesity [26, 27]. Most dementias are associated with a loss of hippocampal neurons. Numerous basic studies have shown that acupoint stimulation mainly in the form of MA and EA improves a variety of primary and secondary cognitive impairments, including those caused by AD, VD, Parkinson’s disease (PD), drugs, sepsis, and radiation brain injury, mainly using the measure of improved spatial learning memory in animal models. This study describes how acupoint stimulation helps protect, regenerate, and plasticize hippocampus neurons and restores proper function to neuronal circuits, as shown in Table1.
Neuroprotective mechanism of acupoint stimulation in the treatment of cognitive impairment
Anti-apoptosis
Apoptosis of neurons in the hippocampus or cortex is an important event in the progression of AD and VD diseases [28, 29]. Apoptotic pathways mainly include the intracellular mitochondrial and the cell surface death receptor pathway [30]. Damage signals by toxic proteins, oxidative stress, and inflammation generated during the pathology of cognitive impairment may indirectly induce these two apoptotic cascade pathways, causing neuronal apoptosis and ultimately cognitive impairment. Acupuncture promotes neuronal survival mainly by regulating factors involved in the intracellular apoptotic pathways. Acupuncture at Benshen (GB13) and GV24 increased Bcl-2 and decreased Bax, CYC, caspase-3, and caspase-9 in the hippocampi of AD rats [31]. Additionally, 20 Hz EA at GV20 and BL23 upregulated Bcl-2 and downregulated Bax [32], suggesting that both MA and EA could reduce hippocampal neuronal apoptosis in AD rats. Moreover, similar results were obtained in VD rats and POCD rats [33–35]. Furthermore, in rats with hypergravity-induced cognitive impairment, 2/15 Hz EA pretreatment at GV20 attenuated caspase-3 activity and neuronal apoptosis in the CA1 region [36]. Suspension moxibustion at GV20 and BL23 reduced apoptosis in rat hippocampal neurons exposed to amyloid β-peptide 1–42 (Aβ1–42). In particular, administering moxibustion before Aβ1-42 exposure improved the protection of neural structures and decreased apoptosis following Aβ1-42 exposure [37]. These results indicate that both electroacupuncture and moxibustion may prevent cognitive impairment by blocking neuronal apoptosis.
It has been shown that nuclear factor-κB (NF-κB) plays a considerable role in preventing neuronal death. It was found that 1–20 Hz EA at GV20 and GV24 inhibited the activation of NF-κB signaling induced by cerebral ischemia–reperfusion (I/R) and downregulated the expression of two key target genes related to apoptosis, Bax and Fas, downstream of the NF-κB pathway [38]. Moreover, it has been hypothesized that EA’s ability to suppress both endogenous and exogenous apoptotic pathways contributes to its beneficial effect on cognitive impairment in VD rats. Activation of p53, a downstream target gene of NF-κB, can inhibit the expression of anti-apoptotic genes and activate the expression of pro-apoptotic genes that are involved in mitochondria-dependent apoptotic pathways [39]. It was found that p53 expression in the hippocampus of VD rats was positively correlated with Noxa expression. Additionally, some studies demonstrated that EA may inhibit apoptosis in VD rats and may be correlated with suppressing the expression of p53 and Noxa in the CA1 region after exposure to 4 Hz EA at GV20, GV14, and BL23 [40]. It was reported that 1 Hz EA at GV20 and Yintang (GV29), and puncture at GV26 decreased the expression of mitogen-activated protein kinase kinase 7 (MKK7), p–c-Jun, p-MKK7, and c-Jun, thereby inhibiting the c-Jun N-terminal kinase (JNK) signaling pathway and regulating apoptotic signaling and reversing cognitive impairment in amyloid precursor protein (APP)/presenilin 1 (PS1) mice [41, 42]. In chronic cerebral hypoperfusion (CCH), there is an accumulation of cerebral reactive oxygen species (ROS) and oxidation of thioredoxin-1 (Trx-1). This leads to the activation of the signal-regulated kinase 1 (ASK1)-JNK/p38 pathway and apoptosis [43]. Acupuncture at GV20 and ST36 upregulated Trx-1 and thioredoxin reductase-1 (TrxR-1) expression and increased TrxR-1 activity while inhibiting ASK1-JNK/p38 pathway activation, while these effects were blocked by Trx-1 siRNA [44]. Thus, it is hypothesized that acupuncture can enhance Trx-1 and TrxR-1 while suppressing the ASK1-JNK/p38 pathway to alleviate VD-associated cognitive impairment. In this view, NF-κB/p53 and ASK1-JNK/P38 signaling cascades may have a significant role in the process through which acupuncture promotes neuronal survival by modulating apoptosis-related variables.
Scavenging toxic proteins
Amyloid β-peptides (Aβ) aggregation between neurons in regions including the hippocampus and cortex results in senile plaques, and increased levels of phosphorylated Tau protein (p-Tau) that further affect the stability of microtubules lead to neurofibrillary tangles, both of which cause neuronal death and cognitive impairment [3]. Acupuncture may improve cognitive impairment in AD rats by inhibiting the production of toxic proteins and promoting their clearance. First, acupuncture can inhibit the production of Aβ. Beta site amyloid precursor protein-cleaving enzyme 1 (BACE1) is a key protein involved in the production of the Aβ peptide, cleaving the peptide from the APP. It was found that 2 Hz EA at GV20 and GV29 and puncture at GV26 reduced the co-expression and deposition of BACE1 and APP in the hippocampus of APP/PS1 mice, thereby improving memory and learning ability [45]. Several studies have reported that acupuncture could also decrease the level of phosphorylation at specific Tau protein phosphorylation sites. Additionally, many serine phosphorylation sites on the Tau protein were found to be phosphorylated in AD mice, including Ser199, Ser202, Ser396, and Ser404 [46–48]. The mechanism underlying the improvement of cognitive function by EA at GV24 and GB13 may be related to the reduction of Aβ, p-Tau (Ser396), and p-Tau (Ser404) in the hippocampi of AD rats [49]. Additionally, 2 Hz EA at GV20 and BL23 caused a decrease in hippocampal Aβ and p-Tau (Ser404) [50]. EA with the same frequency at GV20, GV29, and GV26 enhanced glucose metabolism in APP/PS1 mice, activated protein kinase B (AKT), decreased glycogen synthase kinase-3beta (GSK-3β) activity, promoted GSK3β (Ser9) phosphorylation, and eventually inhibited hippocampal phosphorylation of Tau (Ser199 and Ser202) [51, 52].
Both HSP86 and HSP84 protect neuronal function by degrading misfolded proteins and preventing the formation and aggregation of Aβ and Tau [53]. It has been reported that acupuncture might promote correct folding in potentially toxic proteins; for example, acupuncture at Danzhong (CV17), Zhongwan (CV12), Qihai (CV6), Xuhai (SP10), and ST36 improved cognitive function and increased neuronal numbers in senescence-accelerated mouse prone 8 (SAMP8) mice, accompanied by increased expression of HSP84 and HSP86 [53].
By triggering autophagy, acupuncture can help remove misfolded and aggregated proteins. It was discovered that 1/20 Hz EA at GV20 caused AMP-activated protein kinase and AKT to become phosphorylated while inhibiting the phosphorylation of the mammalian rapamycin target (mTOR) [54]. Inactivating mTOR activated the autophagic pathways to manage the Aβ accumulation in the cortex and hippocampus regions. EA at the same frequency reversed the decrease in the microtubule-associated protein, 1A/1B-light chain 3 II (LC3II)/microtubule-associated protein, 1A/1B-light chain 3 I ratio, and Beclin-1 levels in the hippocampus after Aβ1-40 injection. It also induced the expression of autophagic precursors and larger autophagosomes, decreasing the Aβ levels. The co-localization of Aβ and LC3II suggests that EA-induced autophagy in Aβ1-40 injected rats eliminates the Aβ aggregations [55]. In contrast, Zheng et al. found that in the prefrontal cortices and hippocampi of 5xFAD mice, LC3B, sequestosome 1 (SQSTM1), and lysosome-associated membrane protein 1 (LAMP1) aggregated around or co-localized with APP/Aβ plaques, which may indicate defects in autophagic cargo recognition, trafficking to lysosomes, and lysosomal activity. The insoluble LC3B-II and SQSTM1 were decreased at GV24 and GB13 by 2 Hz EA, and the reduced LC3B+ cathepsin D (CTSD) area was restored in the prefrontal cortex and hippocampus. Increased CTSD and LAMP1 levels were found in both the precursor and mature forms, indicating that EA may not have an impact on the induction of autophagy but primarily promotes lysosomal biogenesis and destroys insoluble SQSTM1 and APP/Aβ. As a result of inhibiting AKT, mTOR complex 1, and mitogen-activated protein kinases 1, EA may also activate transcription factor EB (TFEB). Activated TFEB transcription increases autophagy-lysosomal degradation of APP, the production of APP C-terminal fragments, and Aβ [56]. Additionally, it was discovered that acupuncture at the GV20, GV14, GV26, and Fengfu (GV16) points inhibited the buildup of Aβ in the mitochondria as well as the expression of outer mitochondrial membrane 40, which is involved in encouraging Aβ influx into the mitochondria, thereby reducing mitochondrial dysfunction [57, 58].
In view, acupuncture prevents the synthesis of Aβ and the phosphorylation of Tau protein. Additionally, it encourages proper protein folding and controls the autophagic degradation of toxic proteins, which may help to prevent neuronal damage.
Anti-oxidation
The imbalance between oxidative and antioxidant systems leads to damage to hippocampal neurons. Acupuncture at GV20, GV14, GV26, and GV16 inhibited the production of two pro-oxidative stress factors (i) nitric oxide and (ii) inducible nitric oxide synthase (iNOS) in VD rats [58], while the levels of ROS and malondialdehyde (MDA) in the hippocampus were decreased by pretreatment with 2/15 Hz EA at GV20, Yanglingquan (GB34), LR3, ST36, and SP10 for 14 days before limb ischemia–reperfusion (LI/R) [59]. In AD rats, EA at the same frequency at GV20 and Yongquan (KI1) had comparable effects [60]. On the other hand, acupuncture at CV17, CV12, CV6, SP10, and ST36 was related to reductions in superoxide anions and carbon bases and increased levels of plasma glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) in the hippocampus. This also improved cognitive function in SAMP8 mice [53]. 2/15 Hz EA pretreatment at GV20, GB34, LR3, ST36, and SP10 also increased SOD activity in the CA1 region of VD rats, while LA at HT7 enhanced both SOD and catalase (CAT) activities in the hippocampi of AD rats [59, 61]. Acupuncture, meanwhile, demonstrated comparable effects in models of cognitive impairment brought on by various pathological conditions, including acute myocardial infarction/reperfusion, Parkinson's disease, surgery, and lipopolysaccharide administration [62–65].
As a result, increased SOD brought on by stimulation of acupoints may turn superoxide free radicals into hydrogen peroxide. GSH-Px and CAT can stop hydrogen peroxide from entering cells to produce hydroxyl free radicals, thereby reducing oxidative stress [66].
Additionally, it has been demonstrated that acupuncture inhibits nicotinamide adenine dinucleotide phosphate oxidases(NOXs), preventing the production of ROS. In the ischemic brain, NOX2 (gp91phox) and NOX4 are significant sources of ROS [67, 68]. Treatment with 2/15 Hz EA at GV20 and KI1 reduced abnormally elevated hippocampal NOX2 levels and Aβ1-42-induced hippocampal neuronal damage in AD rats [60]. Activation of NOX2 is dependent on the phosphorylation of the cytoplasmic protein p47phox [69]. Acupuncture at GV20 and ST36 suppressed bilateral common carotid artery occlusion (2VO)-induced elevation of gp91phox and p47phox in cognition-impaired rats [70]. Meanwhile, acupuncture increased the expression of nuclear factor erythroid2-related factor2 (Nrf2) and heme oxygenase 1 (HO-1) in VD rats [71]. On the one hand, Nrf2 is activated by binding to the antioxidant response element, which stimulates the transcription of antioxidant proteins such as HO-1 and participates in the synthesis of glutathione [72], while, on the other hand, there is a negative feedback regulatory loop between NOX4 and Nrf2. Under steady-state conditions, NOX4 generates superoxide and hydrogen peroxide to activate Nrf2 [73], and the activated Nrf2 inhibits NOX4 transcription to reduce ROS production [72]. Acupuncture may initiate this complex signaling pathway.
By decreasing ROS, MDA, and other oxides and raising SOD, GSH-Px, CAT, and other antioxidants in the hippocampal neurons, acupuncture may be able to restore the oxidation-antioxidant balance of the cognition-impaired brain. This regulation process may involve NOXs and Nrf2-related signaling pathways.
Anti-neuroinflammation
Cognitive functions have been found to suffer from chronic neuroinflammation [74]. A key component of neuroinflammation in the central nervous system is microglial activation. Excessive activation of M1-type microglia causes the release of large quantities of inflammatory mediators and the induction of neuronal apoptosis [75]. In the parietal association cortex and entorhinal cortex of mice with mild AD, EA (1/20 Hz) at GV20 and GV24 decreased the colocalization of iNOS/interleukin-1beta (IL-1β), and Iba1 (M1 microglial markers), and increased the colocalization of CD206/arginase-1 and Iba1 (M2 microglial markers), indicating that EA regulates microglial polarization [76]. Acupuncture at GV29 and Yingxiang (LI20) and 2 Hz EA at GV24 and GB13 prevented AD mice's hippocampi from becoming activated by microglia [56, 71]. According to another study, pretreatment with 2/15 Hz EA before LI/R decreased microglial activation in the CA1 region [36]. Additionally, in AD and VD rats, MA and EA reduced the production of pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and IL-1β [77, 78].
Through their cell surface receptor complexes, microglia interact with Aβ protofibrils, which in turn causes phagocytosis of Aβ and neuroinflammation. Toll-like receptor 2, Toll-like receptor 4, and their co-receptor CD14 are the microglial receptor complexes that recognize Aβ protofibrils [79]. Bee venom acupuncture at the ST36 acupoint enhanced cognitive performance in VD Mongolian gerbils, attenuated microglial activation in the hippocampus, and suppressed the expression of TLR4, CD14, and TNF-α [80]. Acupuncture at GV20 and ST36 suppressed TLR4 expression in the hippocampal microglia of VD rats, accompanied by reduced activation of miR-93 and myeloid differentiation primary response 88 (MyD88)/NF-κB signaling, suggesting that acupuncture may attenuate inflammation-related cognitive impairment by inhibiting miR-93-mediated TLR4/MyD88/NF-κB signaling in VD rats [81]. Other studies using EA reported similar results, implying that TLR4/MyD88/NF-κB signaling may have mediated EA’s effect in rats with VD and hepatic encephalopathy [82, 83].
Microglia promote the cleavage of pro-caspase-1 to active caspase-1 by activating NLR family pyrin domain-containing 3 (NLRP3), causing the release of pro-inflammatory cytokines such as IL-1β and TNF-α [84]. In SAMP8 mice, 10 Hz EA at GV20 and ST36 was more effective than 2 Hz EA in reducing the number of TUNEL+ cells and serum IL-1β and IL-6 levels in the CA1 region, which may be associated with the downregulation of hippocampal NLRP3/caspase-1 pathway-related proteins [85]. Acupuncture at the same acupoints restored the 2VO-induced elevation of hippocampus thioredoxin-interacting protein (TXNIP), NLRP3, caspase-1, and IL-1β, indicating that acupuncture may perform neuroprotective effects in VD rats by decreasing TXNIP-related oxidative stress and inflammation [86]. Another research obtained similar results and the NLRP3 activator abolished the anti-inflammation effect on the cognitive function of EA treatment [87]. Moreover, acupuncture repressed microglial activation and TNF-α, IL-6, and IL-1β levels, which may be related to the up-regulation of the α7-nicotinic acetylcholine receptor (α7nAChR) and its downstream pathway including high mobility group box 1 (HMGB1)/NF-κB or Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) pathway by acupuncture [88–92].
Some studies have revealed that 2/20 Hz EA at GV20 and GV24 reduced astrocyte proliferation and microglial/macrophage activation, decreased IL-1β secretion, and promoted IL-10 release, accompanied by a decrease in purinergic P2X receptor 7 (P2X7R)+ED1+, P2X7R+GFAP+, purinergic P2Y receptor 1 (P2Y1R)+ED1+, and P2Y1R+GFAP+ cells in the CA1 region of the peri-infarct hippocampus and sensorimotor cortex. These findings suggest that EA exerts anti-inflammatory effects by inhibiting astrocyte and microglial/macrophage P2X7R and P2Y1R-mediated neuroinflammation after middle cerebral artery occlusion/reperfusion injury and improves motor and memory functions [93].
The above evidence suggests that acupuncture may protect hippocampal neurons from neuroinflammatory damage by regulating TLR4/MyD88/NF-κB, HMGB1/NF-κB, JAK2-STAT3, and P2 purinergic receptor signaling pathways.
Acupoint stimulation modulates neural regeneration and neuroplasticity in the treatment of cognitive impairment
Promoting neural regeneration
Multiple studies have reported that acupuncture may stimulate neuronal regeneration. Acupuncture at ST36 has been demonstrated to enhance the level of pyramidal neurons in the CA1 region of VD rats [94]. Additional studies suggested that acupuncture at CV17, CV12, CV6, SP10, and ST36 elevated the number of CA3 and dentate gyrus (DG) neurons in the hippocampus [95]. It was reported that acupuncture at CV17, CV12, CV6, ST36, and SP10 increased the number of NeuN+/BrdU+ cells in the DG after neural stem cell (NSC) transplantation in SAMP8 mice, suggesting that acupuncture promotes NSC proliferation [96]. In rats with cognitive dysfunction after brain X-ray irradiation, 2/15 Hz EA at GV20, ST36 upregulated DCX+ neurons in the subgranular zone of the hippocampus [97]. Another study reported that acupuncture reversed declining cell proliferation in the DG and showed a stream-like distribution along the dorsum of the sulcus that extended from the left ventricle to the corpus callosum (CC) [98].
By regulating neurotrophic and nerve growth factors, acupuncture may improve neural regeneration. Treatment of AD mice with 2/15 Hz EA at GV20 enhanced the expression of brain-derived neurotrophic factor (BDNF) in the hippocampus and cortex [99]. Additionally, the basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) are two key mitogens involved in the proliferation of NSCs [100, 101]. Acupuncture upregulated the expression of BDNF, bFGF, and EGF in the hippocampus of SAMP8 mice after NSC transplantation [96]. An interesting finding was that the number of M1-type muscarinic acetylcholine receptors (M1 mAChR) in the medial septum (MS)/vertical limb of the diagonal band of Broca (VDB)-DG region increased after 2/20 Hz EA treatment at GV20 and GV24, with an increase in DCX+ cells and Neuro-D1+ cells, however, the above effects of EA vanished when hM4Di Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) were used to block cholinergic circuits in this area. This evidence revealed that M1 mAChR may also facilitate EA-induced neuronal regeneration in the MS/VDB-DG region, although further research is required to confirm this hypothesis [102].
Additionally, acupuncture may alleviate damage to the neuronal myelin sheath and stimulate the regeneration of oligodendrocytes, which will lead to neural regeneration. For instance, treatment with 2 Hz EA at GV20 and GV14 reduced the number of new oligodendrocyte precursor cells (OPCs) in the CC and increased the number of newly differentiated oligodendrocytes (OLs). In addition, EA also increased the levels of neurotrophin 4/5(NT4/5)-tropomysin related kinase B (TrkB) /cAMP Response Element-Binding Protein (CREB) in the OLs and OPCs of the CC, suggesting that the effects of EA on oligodendrocyte regeneration were related to NT4/5-TrkB signal transduction [103].
The aforementioned research suggests that acupuncture promotes neural regeneration by activating factors including BDNF, bFGF, and EGF, and by repairing injuries to neuronal myelin sheaths, hence reducing cognitive impairment.
Modulating synaptic plasticity
Synaptic plasticity plays a key role in learning and memory and includes both structural and functional plasticity [104]. Reduced hippocampal synaptic plasticity in AD and VD patients results in impaired spatial learning and memory [105, 106]. Structural plasticity depends on the synaptic ultrastructure, such as the synaptic curvature, synaptic cleft width, and postsynaptic density. Acupuncture at CV17, CV12, CV6, SP10, and ST36 increased the expression of synaptophysin (SYN, a presynaptic vesicle marker) in SAMP8 mice after receiving NSC transplantation [107]. In AD mice, treatment with 2 Hz EA increased SYN and the postsynaptic marker postsynaptic density protein 95 (PSD-95) and decreased synaptic ultrastructural deterioration [108–110].
Various studies have shown that BDNF/TrkB and its downstream signaling pathways may mediate the regulation of synaptic plasticity by acupuncture. It was reported that EA increased the expression of SYP, PSD-95, BDNF, and TrkB in the hippocampus [111, 112]. Furthermore, the binding of BDNF to TrkB activates the downstream phosphatidylinositol-3-kinase (PI3K) /AKT pathway. Acupuncture at GV29 and LI20 increased the expression of PSD-95, SYN, and growth-associated protein 43 in SAMP8 mice, and activated the PI3K/AKT signaling pathway and the phosphorylation of GSK-3β [55]. GSK-3β, in turn, downregulates BDNF and destroys synaptic plasticity by impairing CREB protein transcriptional activity. Potentially, 2/30/50 Hz EA can downregulate GSK-3β, leading to an increase in synaptic bending, a decrease in synaptic cleft width, and an increase in postsynaptic density. In resting settings, GSK-3β is highly active and can be triggered by phosphorylation at Tyr216 and inhibited by phosphorylation at Ser9 [113]. After EA treatment, pSer9-GSK-3β expression was found to be significantly reduced, while pTyr216-GSK-3β expression was found to be significantly increased; furthermore, EA with high frequency (50 Hz) was more effective than EA with low frequency (2 Hz) or medium frequency (30 Hz) [114]. PI3K/AKT, as molecules upstream of GSK-3β, can phosphorylate and inhibit GSK-3β. Consequently, it is hypothesized that the BDNF/TrkB-PI3K/AKT/GSK-3β signaling pathway may facilitate acupuncture’s modulation of synaptic structural plasticity in the hippocampus [115].
Rho GTPases and the Notch signaling pathway may also play roles in the regulation of synaptic structural plasticity by acupuncture. According to Lin et al., 20 Hz EA at GV20 and GV24 increased hippocampal dendritic spine densities in I/R-injured rats. This effect may have been caused by upregulating cell division cycle 42 and Ras-related C3 botulinum toxin substrate 1 and downregulating Ras homolog gene family member A, which would have regulated the F-actin cytoskeleton and stimulated the growth of local dendritic spines [116]. Guo et al. found that the levels of Notch1 and Hes1 were abnormally elevated in the hippocampus after Aβ injection, whereas 20 Hz EA at GV20 and BL23 downregulated hippocampal Notch1 and Hes1 expression and promoted synapsin-1 and SYN expression, suggesting that EA partially improves learning memory by downregulating the abnormally elevated Notch signaling pathway in AD rats [32].
There are two main forms of synaptic plasticity, namely, long-term potentiation (LTP) and long-term depression. LTP is considered to be the molecular basis of memory formation [117]. Early-LTP induction requires activation of the postsynaptic NMDAR and Ca2+ flux into the activated NMDAR channel, which results in the activation of calcium/calmodulin-dependent protein kinase II (CaMKII) and a rapid increase in the number of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR) at the synapse [118]. Late-LTP involves the activation of 3′, 5′ cyclic adenosine monophosphate (cAMP) by activated CaMKII, regulating the protein kinase A (PKA)/CREB pathway and increasing the number of AMPAR [119, 120]. EA increased the levels of CaMKII in the hippocampus [121, 122] while acupuncture at ST36 alleviated disordered LTP in the perforant pathway-dentate gyrus (PP-DG), and counteracted decreases in cAMP, PKA, and p-CREB in cerebral multi-infarction rats. Increased CREB phosphorylation and the improvement in cognitive function were both blocked by the PKA inhibitor N- [2-(p-bromocinnamyl amino) ethyl] -5-isoquinoline-sulfonamide [123]. Zheng et al. obtained similar results, observing enhanced LTP in Schaffer collaterals [124] and suggesting that the cAMP/PKA/CREB pathway may be involved in LTP induction by acupoint stimulation.
In AD patients, elevated levels of Orexin A in the cerebrospinal fluid were related to elevated levels of phosphorylated Tau and Aβ in the cerebrospinal fluid, as well as diminished cognitive scores [125, 126]. The latest study found that 10 Hz EA alleviated learning and memory impairment in SAMP8 mice, reduced the level of Orexin A in the cerebrospinal fluid, improved the synaptic structure and synaptic transmission of the hippocampus, increased the level of glutamate, SYP, PSD-95, cAMP, pPKA/PKA, and pCREB/CREB protein levels. Orexin A-RNAi adenovirus was utilized to silence the orexin gene, simulating the abovementioned consequences of EA. It is hypothesized that 10 Hz EA therapy alters glutamatergic synaptic plasticity mediated by cAMP/PKA/CREB by decreasing cerebrospinal fluid Orexin A levels [127]. Dopamine (DA) and norepinephrine (NE) and their receptors may also play important roles in synaptic plasticity. Acupuncture at ST36 and GV20 alleviated LTP injury in the PP-DG of VD rats and promoted the release of DA in the hippocampus while reversing the decreases in the DA receptors D1R and D5R in the DG region. It was also reported that the level of NE in the hippocampus and the numbers of β1-adrenergic receptors (β1-AR) in the DG region were increased by acupuncture. The protective effect of acupuncture on LTP was eliminated by blocking the D1/D5 receptor and use of a β1-AR antagonist, suggesting that the mechanism by which acupuncture maintains the LTP in VD rats may be related to increased levels of DA and NE and the activation of the D1/D5 receptor and β1-AR [128, 129]. They also observed that acupuncture promoted the expression and activity of dopamine β-hydroxylase in the cerebrospinal fluid of CCH rats [130], suggesting that acupuncture may also induce and maintain LTP in the hippocampus by regulating DA to NE conversion. Studies have demonstrated that DA acting on D1/D5 receptors increases cAMP production and improves NMDA channel function [131]. Hippocampal β-AR activation activates PKA by increasing intracellular cAMP production, thereby reducing the threshold for LTP induction [132]. However, it has to be determined whether these variations influence synaptic plasticity by modulating the classical LTP induction pathway. It has been observed that electrical stimulation at EX-HN3 and GV20 at 2 Hz increased the expression of NMDAR1, AMPAR, and -aminobutyric acid type A receptor [121]. Further research is required to determine whether the underlined result is related to EA’s role in the induction of LTP. Other studies demonstrated that middle cerebral artery occlusion (MCAO) elevates the concentrations of Ca2+, Glu, and NMDA2B in hippocampus cells and that excessive Glu continually activates postsynaptic NMDAR, leading to excessive Ca2+ influx and poor cognitive function. These abnormalities were eradicated with the use of 1/20 Hz EA at GV20 and GV24. Meanwhile, EA reversed the decline in NMDAR2A caused by MCAO. NMDAR2A contributes to neuronal regeneration, while NMDAR2B mediates oxidative stress-induced neuronal apoptosis. These results suggest that EA may reduce Ca2+ influx by inhibiting glutamate neurotoxicity and downregulating NMDAR2B expression [133].
The degeneration of cholinergic innervation is one of the causes of decreased neuroplasticity and memory decline. Acupuncture at GV24 and GB13 can enhance the learning and memory of AD model rats, and the mechanism may be related to the modulation of the activities of choline acetyltransferase (ChAT) and acetylcholinesterase (AChE) [31]. Similar effects were observed in rats treated with acupuncture for lipopolysaccharide administration [64], long-term corticosterone administration [134], and PD-induced cognitive impairment [62]. ChAT and AChE are the enzymes responsible for synthesizing and hydrolyzing acetylcholine (ACh), and their levels regulate ACh metabolism. Lee et al. reported that acupuncture at GV20 improved cognitive dysfunction induced by scopolamine, alleviated the decline in hippocampal ChAT levels, and restored the expression of the hippocampal choline transporter 1 and vesicular acetylcholine transporter [135], suggesting that acupuncture may regulate ACh metabolism, and may contribute to the circulation of choline from the synaptic cleft back to the presynaptic terminal, and improve the efficiency of vesicle filling. Li et al. reported that 2/20 Hz EA at GV20 and GV24 improved pattern separation disorders in 5xFAD mice, up-regulated ChAT and vesicular acetylcholine transporter, and down-regulated AChE in the MS/VDB-DG region, while the use of hM4Di DREADDs reversed the above effects of EA, suggesting that EA may improve early pattern separation by activating cholinergic system in MS/VDB-DG [102]. According to the reported study of Liu et al., 2/100 Hz EA at GV20, PC6, and LI4 could enhance learning and memory in rats with postoperative cognitive impairment, as well as upregulate α7nAChR+ neurons in the hippocampus regions [136]. Treatment with 2 Hz EA at GV20 inhibited the lipopolysaccharide-induced decrease in α7nAChR activity [64]. Cholinergic signaling can enhance hippocampal function, and the α7nAChR contributes to LTP induction in the hippocampus due to its high Ca2+ permeability [137, 138]. Therefore, it is speculated that acupuncture may activate the α7nAChR to protect synaptic plasticity by regulating the hippocampal cholinergic pathway.
Neural circuit mechanism of acupoint stimulation in the treatment of cognitive impairment
The Papez circuit in the limbic system is involved in spatial learning and episodic memory [139]. The Papez circuit is composed of the medial temporal lobe (MTL, consisting of the entorhinal cortex, hippocampus, parahippocampal gyrus, and amygdala), mammillary bodies, anterior thalamic nuclei, and the cingulate cortex, which are interconnected by white matter tracts consisting of the fornix, papillary thalamic tract, and cingulate tract [140]. Both AD and VD patients may experience disruptions in the Papez circuit [141, 142]. Several lines of evidence demonstrate that acupuncture recovers the activity and functional connectivity (FC) of brain areas connected with this circuit. Lin et al. found that 1/20 Hz EA at GV20 and GV24 significantly increased FC between the hippocampus and entorhinal cortex, and this was also demonstrated by diffusion tensor imaging [143]. A PET study showed that acupuncture at ST36 mainly activated regions in the bilateral limbic system (piriform cortex), bilateral temporal lobe (olfactory cortex), right amygdala, and right hippocampus [144]. In MCAO rats, 1/20 Hz EA at GV20 and GV24 enhanced the FC between the retrosplenial cortex (RSC) and the hippocampus, cingulate gyrus (CG), and midbrain [145]. The RSC plays a significant role in learning and memory because it is more closely connected to the anterior thalamic nuclei within the cingulate cortex and receives projections from all of the anterior thalamic nuclei [146]. Zheng et al. found that acupuncture at LR3 and LI4 upregulated the amplitude of low-frequency fluctuations (ALFF) in the right superior frontal gyrus (SFG) and downregulated ALFF in the left posterior central gyrus, inferior cingulate cortex, right middle cingulate cortex, right inferior frontal gyrus (IFG), right hippocampus, and right inferior temporal gyrus in AD patients. Acupuncture also improved the FC between the hippocampus and the anterior central gyrus [147] as well as between the frontal and temporal lobes and the hippocampus [148].
The default mode network (DMN) is primarily involved in the formation of autobiographical memory and spans different cortical regions, including the precuneus (PCu), posterior cingulate cortex (PCC), inferior parietal lobe (IPL), and temporal and medial prefrontal cortex [149], which are heavily projected to the MTL [150]. These regions play different roles in resting-state brain activity, with the left middle temporal gyrus (MTG) providing information, the IPL spatial attention, and the PCC information integration [151]. Acupuncture can also regulate DMN activity and FC, especially with the frontal and temporal lobes, and can regulate FC within the DMN as well as FC between DMN and other cognition-related brain regions. Using PET imaging, Lai et al. revealed that acupuncture at HT7 enhanced glucose metabolism in the hippocampus, thalamus, hypothalamus, frontal lobe, and temporal lobe of AD rats [152]. Using fMRI technology, Chen et al. found that acupuncture at KI3 could activate the CG, frontal lobe, precuneus, and other brain regions in elderly MCI patients and healthy elderly volunteers [153]. Acupuncture at LR3 and LI4 reduced the elevated left SFG and right IFG activity in MCI patients and activated the previously inhibited CG and fusiform gyrus, suggesting that acupuncture can bidirectionally regulate brain activity in MCI patients [154]. Meanwhile, acupuncture may increase the FC among the left PCC, right MTG, and right IPL while decreasing the FC between the bilateral CG and left PCu in the DMN of AD patients. Tan et al. found that after acupuncture, the FC between the hippocampus and the insula, the dorsolateral prefrontal cortex, and PCu, and the insula and dorsolateral prefrontal cortex was increased [155].
Acupoint stimulation may improve cognitive impairment in MCI and AD patients and animal models by modulating the activity and FC of related brain regions within the Papez circuit and DMN. Furthermore, deep acupuncture at KI3 causes more significant alterations in brain function than superficial acupuncture, though additional research is required to evaluate whether this remains true for other acupuncture locations. The choice of acupoints and their associated parameters varies throughout studies, as does their standardization. All of these factors must be taken into consideration to produce results that can be used universally.
Discussion and conclusion
Treatment of cognitive impairment using acupoint stimulation, especially MA and EA, has promising results. The acupoints chosen for the preliminary research were mostly found in the upper and lower limbs and the head and neck, such as GV20, ST36, GV24, GV14, and HT7. Among these, GV20 was the most commonly used. Data mining showed that the use of GV20 or the combination of other acupoints centered on GV20 might have a better therapeutic effect on AD and VD in clinical studies [156, 157], and basic studies showed a high degree of similarity. The combination of GV20 and ST36 has been the subject of the most research and was found to be effective in every pathological condition targeted by acupoint stimulation for cognitive impairment, particularly in the treatment of neuroinflammation. The parameters of EA are also crucial in determining its efficacy, and low and medium frequency EA, primarily 2 Hz and 2/15 Hz, has been the subject of the most research and yielded great results. However, some studies showed that while 2 Hz EA improved learning and memory in rats, 50 Hz was more effective [158], and another study reported that 30/100 Hz EA also improved cognitive function [77]. Therefore, to standardize treatment settings, additional research is required to examine the efficacy of using various EA frequencies and the efficacy of variable frequency EA versus single frequency EA.
In conclusion, it has been demonstrated that acupoint stimulation can enhance spatial learning and memory function in cognitive impairment models. Specifically, MA and EA have been shown to directly inhibit cognition-related neuronal apoptotic pathways or to indirectly inhibit apoptosis by scavenging toxic proteins, battling oxidative stress, and reducing neuroinflammation, as shown in Fig. 2. In particular, acupoint stimulation can inhibit the production of Aβ and the phosphorylation of Tau protein and mitigate neuronal damage by promoting correct protein folding and regulating the autophagic degradation of toxic proteins. Acupoint stimulation prevents oxidative damage by regulating NOXs and Nrf2-related signaling pathways. Acupoint stimulation may also protect hippocampal neurons from neuroinflammatory damage by modulating the TLR4/MyD88/NF-κB, HMGB1/NF-κB, JAK2/STAT3, and P2 purinergic receptor signaling pathways. Additionally, acupoint stimulation can stimulate neural regeneration by increasing BDNF, bFGF, and EGF. It can also alter the synaptic structure and functional plasticity by controlling the BDNF/TrkB-PI3K/AKT/GSK-3β, cAMP/PKA/CREB signaling pathways, as well as glutamatergic, dopaminergic, noradrenergic, and cholinergic innervation (shown in Fig. 3). All of these effects of acupoint stimulation may contribute to the functional recovery of neurons in the hippocampal CA1, CA3, and DG regions, as well as the synaptic transmission in the PP-DG and Schaffer collaterals in the hippocampal trisynaptic circuit, to restore hippocampal activity, and FC with several brain regions in the Papez circuit and DMN. Moreover, acupoint stimulation altered functional activity and connectivity in the cingulate cortex, temporal lobe, and frontal lobe, among other locations (shown in Fig. 4). These pathways, as shown by molecular, cellular, and neuroimaging data, facilitate the amelioration of cognitive impairment by acupoint stimulation. This study illustrates the effectiveness of acupoint stimulation in treating cognitive impairment. However, additional high-quality research is necessary.
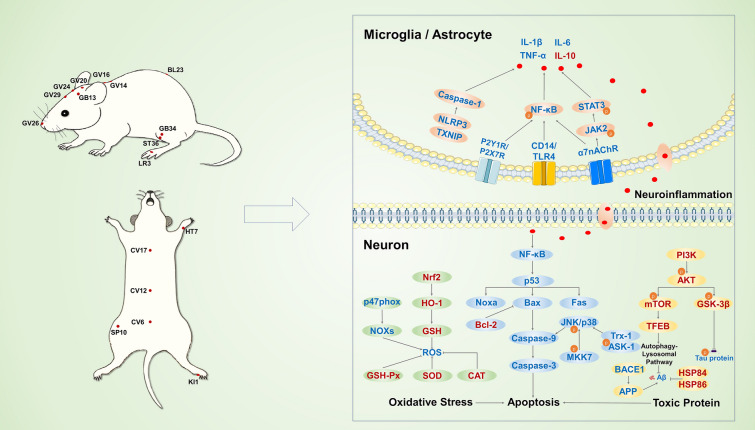
Fig. 2.
The neuroprotective mechanisms involved in acupoint stimulation for the treatment of cognitive impairment. The acupoints with neuroprotective effects for the treatment of cognitive impairment are marked in the image on the left. The neuroprotective mechanisms of acupoint stimulation are shown in the image on the right. Red characters, upregulated by acupoint stimulation; blue characters, downregulated by acupoint stimulation
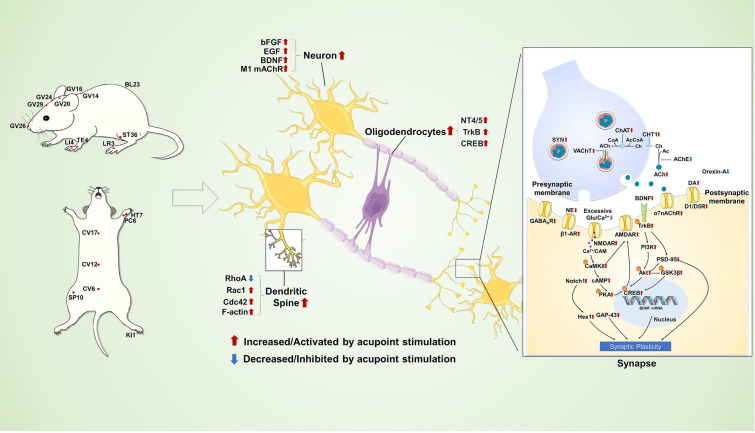
Fig. 3.
The neural regeneration and neuroplasticity mechanisms involved in acupoint stimulation for the treatment of cognitive impairment. The acupoints with neural regenerative and neuroplastic effects for the treatment of cognitive impairment are marked in the image on the left. Neural regeneration and neuroplasticity mechanisms of acupoint stimulation are shown in the image on the right
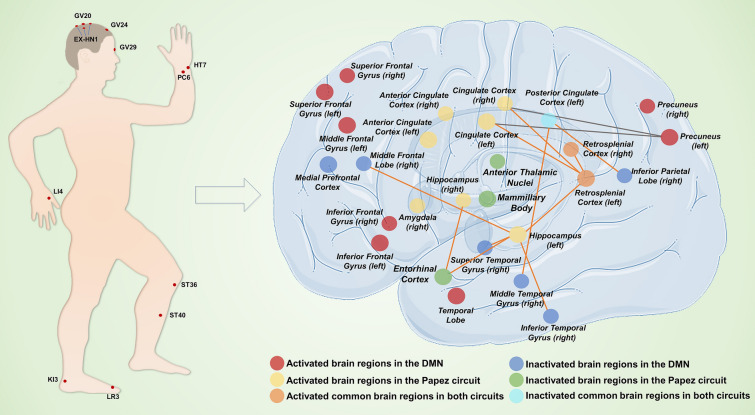
Fig. 4.
The neural circuit mechanisms involved in acupoint stimulation for the treatment of cognitive impairment. The acupoints used in neural circuit study for the treatment of cognitive impairment are marked in the image on the left. Neural circuit mechanisms of acupoint stimulation are shown in the image on the right
Acknowledgements
The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for their linguistic assistance during the preparation of this manuscript.
Abbreviations
- 2VO
Bilateral common carotid artery occlusion
- Ac
Acetic acid
- AcCoA
Acetyl-coenzyme A
- ACh
Acetylcholine
- AChE
Acetylcholinesterase
- AD
Alzheimer’s disease
- ADL
Activity of daily living
- AKT
Protein kinase B
- ALFF
Amplitude of low-frequency fluctuations
- AMPAR
α-Amino-3-hydroxy-5-methyl-4-isoazolpropionic acid receptors
- APP
Amyloid precursor protein
- ASK-1
Apoptosis signal-regulating kinase
- Aβ
Amyloid β-peptide
- Aβ1-42
Amyloid β-peptide 1–42
- BACE1
Beta site amyloid precursor protein-cleaving enzyme 1
- BDNF
Brain-derived neurotrophic factor
- bFGF
Basic fibroblast growth factor
- CaM
Calmodulin
- CaMKII
Calcium/calmodulin-dependent protein kinase II
- cAMP
3′, 5′ Cyclic adenosine monophosphate
- CAT
Catalase
- CC
Corpus callosum
- CCH
Chronic cerebral hypoperfusion
- Cdc42
Cell division cycle 42
- CG
Cingulate gyrus
- Ch
Choline
- ChAT
Choline acetyltransferase
- CHT1
Choline transporter
- CoA
Coenzyme A
- CREB
CAMP response element-binding protein
- CTSD
Cathepsin D
- D1/D5R
Dopamine D1/D5 receptor
- DA
Dopamine
- DG
Dentate gyrus
- DMN
Default mode network
- DREADDs
Designer Receptors Exclusively Activated by Designer Drugs
- EA
Electroacupuncture
- EGF
Epidermal growth factor
- FC
Functional connectivity
- GABAAR
Type A gamma-aminobutyric acid chloride channel
- GAP-43
Growth associated protein 43
- Glu
Glutamate
- GSH
Glutathione
- GSH-Px
Glutathione peroxidase
- GSK-3β
Glycogen synthase kinase-3beta
- HMGB1
High mobility group box 1
- HO-1
Heme oxygenase-1
- HSP84
Heat shock protein 84
- HSP86
Heat shock protein 86
- I/R
Ischemia–reperfusion
- IFG
Inferior frontal gyrus
- IL-10
Interleukin-10
- IL-1β
Interleukin-1beta
- IL-6
Interleukin-6
- iNOS
Inducible nitric oxide synthase
- IPL
Inferior parietal lobe
- JAK2
Janus kinase 2
- JNK
C-Jun N-terminal kinase
- LA
Laser acupuncture
- LAMP1
Lysosome-associated membrane protein 1
- LC3II
Microtubule-associated protein 1A/1B-light chain 3 II
- LI/R
Limb ischemia–reperfusion
- LTP
Long-term potentiation
- M1 mAChR
M1-type muscarinic acetylcholine receptors
- MA
Manual acupuncture
- MCAO
Middle cerebral artery occlusion
- MCI
Mild cognitive impairment
- MDA
Malondialdehyde
- MKK7
Mitogen-activated protein kinase kinase 7
- MMSE
Mini-Mental State Examination
- MTG
Middle temporal gyrus
- MS
Medial septum
- MTL
Medial temporal lobe
- mTOR
Mammalian rapamycin target
- MyD88
Myeloid differentiation primary response 88
- NE
Norepinephrine
- NF-κB
Nuclear factor-κB
- NLRP3
NLR family pyrin domain-containing 3
- NMDAR
N-methyl-d-aspartate receptor
- NOXs
Nicotinamide adenine dinucleotide phosphate oxidases
- Nrf2
Nuclear factor erythroid 2-related factor2
- NSC
Neural stem cell
- NT4/5
Neurotrophin 4/5
- OLs
Oligodendrocytes
- OPCs
Oligodendrocyte precursor cells
- p
Phosphorylated
- P2X7R
Purinergic P2X receptor 7
- P2Y1R
Purinergic P2Y receptor 1
- PCC
Posterior cingulate cortex
- PCu
Precuneus
- PD
Parkinson’s disease
- PI3K
Phosphatidylinositol-3-kinase
- PKA
Protein kinase A
- PP-DG
Perforant pathway-dentate gyrus
- PS1
Presenilin 1
- PSD-95
Postsynaptic density protein 95
- p-Tau
Phosphorylated Tau protein
- Rac1
Small GTPase Ras-related C3 botulinum toxin substrate 1
- RCTs
Randomized controlled trials
- RhoA
Ras homolog gene family member A
- ROS
Reactive oxygen species
- RSC
Retrosplenial cortex
- SAMP8
Senescence-accelerated mouse prone 8
- SFG
Superior frontal gyrus
- SOD
Superoxide dismutase
- SQSTM1
Sequestosome 1
- STAT3
Signal transducer and activator of transcription 3
- SYN
Synaptophysin
- TFEB
Transcription factor EB
- TLR4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor alpha
- TrkB
Tropomysin related kinase B
- Trx-1
Thioredoxin-1
- TrxR-1
Thioredoxin reductase-1
- TXNIP
Thioredoxin interacting protein
- VAChT
Vesicular acetylcholine transporter
- VD
Vascular dementia
- VDB
Vertical limb of the diagonal band of Broca
- α7nAChR
α7-Nicotinic acetylcholine receptor
- β1-AR
β1-Adrenergic receptors
Author contributions
ZFX and ZLC: conceptualization, ZCZ, LYC, YG, and DL: methodology, data collection, and manuscript writing. JYZ, TG, and LL: data collection and analysis. WF: preparation of the figures. ZFX, SRQ, and YDZ: review and editing. All authors contributed to the article and approved the submitted version. All authors read and approved the final manuscript.
Funding
This study was financially supported by the National Key R&D Program of China No. 2019YFC1712200-2019YFC1712204, the National Natural Science Foundation of China No. 82074534, Scientific Research Program of Tianjin Municipal Education Commission No. 2018KJ026, College Students' Innovative Entrepreneurial Training Plan No. 202210063027, The Natural Science Foundation of Tianjin No. 20JCQNJC00920.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree to the publishing of this article in Chinese Medicine.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zichen Zhang and Liuyi Chen have contributed equally to this work and share first authorship
Contributor Information
Zhifang Xu, Email: xuzhifangmsn@hotmail.com.
Zelin Chen, Email: chenzelin328@163.com.
References
- 1.Collaborators, G. 2. N. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18: 88–106. 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed]
- 2.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 3.Jagust W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat Rev Neurosci. 2018;19:687–700. doi: 10.1038/s41583-018-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312:2551–2561. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elahi FM, Miller BL. A clinicopathological approach to the diagnosis of dementia. Nat Rev Neurol. 2017;13:457–476. doi: 10.1038/nrneurol.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wimo A, Guerchet M, Ali GC, Wu YT, Prina AM, Winblad B, et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13:1–7. doi: 10.1016/j.jalz.2016.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsunaga S, Kishi T, Nomura I, Sakuma K, Okuya M, Ikuta T, et al. The efficacy and safety of memantine for the treatment of Alzheimer’s disease. Expert Opin Drug Saf. 2018;17:1053–1061. doi: 10.1080/14740338.2018.1524870. [DOI] [PubMed] [Google Scholar]
- 8.Haake A, Nguyen K, Friedman L, Chakkamparambil B, Grossberg GT. An update on the utility and safety of cholinesterase inhibitors for the treatment of Alzheimer’s disease. Expert Opin Drug Saf. 2020;19:147–157. doi: 10.1080/14740338.2020.1721456. [DOI] [PubMed] [Google Scholar]
- 9.Ma R, Liu X, Clark J, Williams GM, Doi SA. The impact of acupuncture on neurological recovery in spinal cord injury: a systematic review and meta-analysis. J Neurotrauma. 2015;32:1943–1957. doi: 10.1089/neu.2014.3866. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Zhang Z, Qin S, Fan W, Li W, Liu J, et al. Acupuncture for Parkinson’s disease: efficacy evaluation and mechanisms in the dopaminergic neural circuit. Neural Plast. 2021;2021:9926445. doi: 10.1155/2021/9926445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, Yuxin F, Zhang Z, Liu J, Qin S. Arousal effect and potential mechanism of dopamine-mediated acupuncture on traumatic brain injury. Acupuncture Herbal Med. 2021;1:22–30. doi: 10.1097/HM9.0000000000000005. [DOI] [Google Scholar]
- 12.Organization WH (2002) Acupuncture: Review and Analysis of Reports on Controlled Clinical Trials.
- 13.Lu L, Zhang Y, Tang X, Ge S, Wen H, Zeng J, Wang L, Zeng Z, Rada G, Ávila C, Vergara C, Tang Y, Zhang P, Chen R, Dong Y, Wei X, Luo W, Wang L, Guyatt G, Tang C, Xu N. Evidence on acupuncture therapies is underused in clinical practice and health policy. BMJ. 2022;376:e067475. doi: 10.1136/bmj-2021-067475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Q, Luo D, Chen L, Liang FX, Chen R. Effectiveness of acupuncture for Alzheimer’s disease: an updated systematic review and meta-analysis. Curr Med Sci. 2019;39(3):500–511. doi: 10.1007/s11596-019-2065-8. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Wang Q, Du S, Pu Y, Xu G. Acupuncture for mild cognitive impairment in elderly people: Systematic review and meta-analyses. Medicine (Baltimore) 2020;99(39):e22365. doi: 10.1097/MD.0000000000022365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Y, Wang T, Yang L, Zou X, Zhou J, Wu J, et al. Acupuncture for post-operative cognitive dysfunction: a systematic review and meta-analysis of randomized controlled trials. Acupunct Med. 2021;39:423–431. doi: 10.1177/0964528420961393. [DOI] [PubMed] [Google Scholar]
- 17.Tong T, Pei C, Chen J, Lv Q, Zhang F, Cheng Z. Efficacy of acupuncture therapy for chemotherapy-related cognitive impairment in breast cancer patients. Med Sci Monit. 2018;24:2919–2927. doi: 10.12659/MSM.909712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, Gong W, Ma X, Wang S, Wang X, Guo T, et al. Factor analysis of electroacupuncture and selective serotonin reuptake inhibitors for major depressive disorder: an 8-week controlled clinical trial. Acupunct Med. 2020;38:45–52. doi: 10.1136/acupmed-2017-011412. [DOI] [PubMed] [Google Scholar]
- 19.Sun ZL, Liu J, Guo W, Jiang T, Ma C, Li WB, et al. Serum brain-derived neurotrophic factor levels associate with cognitive improvement in patients with schizophrenia treated with electroacupuncture. Psychiatry Res. 2016;244:370–375. doi: 10.1016/j.psychres.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 20.Zhou J, Peng W, Xu M, Li W, Liu Z. The effectiveness and safety of acupuncture for patients with Alzheimer disease: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94(22):e933. doi: 10.1097/MD.0000000000000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou S, Dong L, He Y, Xiao H. Acupuncture plus herbal medicine for Alzheimer’s disease: a systematic review and meta-analysis. Am J Chin Med. 2017;45(7):1327–1344. doi: 10.1142/S0192415X17500732. [DOI] [PubMed] [Google Scholar]
- 22.Zhao L, Zhang H, Zheng Z, Huang J. Electroacupuncture on the head points for improving gnosia in patients with vascular dementia. J Tradit Chin Med. 2009;29(1):29–34. doi: 10.1016/s0254-6272(09)60027-3. [DOI] [PubMed] [Google Scholar]
- 23.Yang JW, Shi GX, Zhang S, Tu JF, Wang LQ, Yan CQ, Lin LL, Liu BZ, Wang J, Sun SF, Yang BF, Wu LY, Tan C, Chen S, Zhang ZJ, Fisher M, Liu CZ. Effectiveness of acupuncture for vascular cognitive impairment no dementia: a randomized controlled trial. Clin Rehabil. 2019;33(4):642–652. doi: 10.1177/0269215518819050. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Zhao L, Yang S, Chen Z, Li Y, Peng X, Yang Y, Zhu M. Clinical observation on effect of scalp electroacupuncture for mild cognitive impairment. J Tradit Chin Med. 2013;33(1):46–50. doi: 10.1016/s0254-6272(13)60099-0. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Yang H, Zhang J, Zhang B, Liu T, Gan L, Zheng J. Efficacy and safety assessment of acupuncture and nimodipine to treat mild cognitive impairment after cerebral infarction: a randomized controlled trial. BMC Complement Altern Med. 2016;16(1):361. doi: 10.1186/s12906-016-1337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med. 2014;30:421–442. doi: 10.1016/j.cger.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deckers K, van Boxtel MP, Schiepers OJ, de Vugt M, Muñoz Sánchez JL, Anstey KJ, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 2015;30:234–246. doi: 10.1002/gps.4245. [DOI] [PubMed] [Google Scholar]
- 28.Obulesu M, Lakshmi MJ. Apoptosis in Alzheimer’s disease: an understanding of the physiology, pathology and therapeutic avenues. Neurochem Res. 2014;39(12):2301–2312. doi: 10.1007/s11064-014-1454-4. [DOI] [PubMed] [Google Scholar]
- 29.Sairanen T, Karjalainen-Lindsberg ML, Paetau A, Ijäs P, Lindsberg PJ. Apoptosis dominant in the periinfarct area of human ischaemic stroke–a possible target of antiapoptotic treatments. Brain. 2006;129(Pt 1):189–199. doi: 10.1093/brain/awh645. [DOI] [PubMed] [Google Scholar]
- 30.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15(6):725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Tang C, Liao W, Zhu M, Liu M, Sun N. The antiapoptotic and antioxidative stress effects of Zhisanzhen in the Alzheimer’s disease model rat. NeuroReport. 2019;30:628–636. doi: 10.1097/WNR.0000000000001243. [DOI] [PubMed] [Google Scholar]
- 32.Guo HD, Tian JX, Zhu J, Li L, Sun K, Shao SJ, et al. Electroacupuncture suppressed neuronal apoptosis and improved cognitive impairment in the AD model rats possibly via downregulation of notch signaling pathway. Evid Based Complement Alternat Med. 2015;2015:393569. doi: 10.1155/2015/393569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu F, Jiang YJ, Zhao HJ, Yao LQ, Chen LD. Electroacupuncture ameliorates cognitive impairment and regulates the expression of apoptosis-related genes Bcl-2 and Bax in rats with cerebral ischaemia-reperfusion injury. Acupunct Med. 2015;33:478–484. doi: 10.1136/acupmed-2014-010728. [DOI] [PubMed] [Google Scholar]
- 34.Yun YC, Jang D, Yoon SB, Kim D, Choi DH, Kwon OS, et al. Laser acupuncture exerts neuroprotective effects via regulation of Creb, Bdnf, Bcl-2, and bax gene expressions in the hippocampus. Evid Based Complement Alternat Med. 2017;2017:7181637. doi: 10.1155/2017/7181637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Q, Li Y, Yin C, Yu J, Zhao J, Yan L, Wang Q. Electro-acupuncture pretreatment ameliorates anesthesia and surgery-induced cognitive dysfunction via inhibiting mitochondrial injury and neuroapoptosis in aged rats. Neurochem Res. 2022;47:1751–1764. doi: 10.1007/s11064-022-03567-3. [DOI] [PubMed] [Google Scholar]
- 36.Feng S, Wang Q, Wang H, Peng Y, Wang L, Lu Y, et al. Electroacupuncture pretreatment ameliorates hypergravity-induced impairment of learning and memory and apoptosis of hippocampal neurons in rats. Neurosci Lett. 2010;478:150–155. doi: 10.1016/j.neulet.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Du Y, Liu R, Sun G, Meng P, Song J. Pre-moxibustion and moxibustion prevent Alzheimer’s disease. Neural Regen Res. 2013;8:2811–2819. doi: 10.3969/j.issn.1673-5374.2013.30.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng X, Yang S, Liu J, Huang J, Peng J, Lin J, et al. Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med Rep. 2013;7:1516–1522. doi: 10.3892/mmr.2013.1392. [DOI] [PubMed] [Google Scholar]
- 39.Wawryk-Gawda E, Chylińska-Wrzos P, Lis-Sochocka M, Chłapek K, Bulak K, Jędrych M, et al. P53 protein in proliferation, repair and apoptosis of cells. Protoplasma. 2014;251:525–533. doi: 10.1007/s00709-013-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Y, Zeng Y. Electroacupuncture protected pyramidal cells in hippocampal CA1 region of vascular dementia rats by inhibiting the expression of p53 and Noxa. CNS Neurosci Ther. 2011;17:599–604. doi: 10.1111/j.1755-5949.2010.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishina H, Wada T, Katada T. Physiological roles of SAPK/JNK signaling pathway. J Biochem. 2004;136:123–126. doi: 10.1093/jb/mvh117. [DOI] [PubMed] [Google Scholar]
- 42.Tang Y, Xu A, Shao S, Zhou Y, Xiong B, Li Z. Electroacupuncture ameliorates cognitive impairment by inhibiting the JNK signaling pathway in a mouse model of Alzheimer’s disease. Front Aging Neurosci. 2020;12:23. doi: 10.3389/fnagi.2020.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu W, Wang XR, Du SQ, Yan CQ, Yang NN, Lin LL, et al. Anti-oxidative and anti-apoptotic effects of acupuncture: role of thioredoxin-1 in the hippocampus of vascular dementia rats. Neuroscience. 2018;379:281–291. doi: 10.1016/j.neuroscience.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 45.Tang Y, Shao S, Guo Y, Zhou Y, Cao J, Xu A, et al. Electroacupuncture mitigates hippocampal cognitive impairments by reducing BACE1 deposition and activating PKA in APP/PS1 double transgenic mice. Neural Plast. 2019;2019:2823679. doi: 10.1155/2019/2823679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quintanilla RA, von Bernhardi R, Godoy JA, Inestrosa NC, Johnson GV. Phosphorylated tau potentiates Aβ-induced mitochondrial damage in mature neurons. Neurobiol Dis. 2014;71:260–269. doi: 10.1016/j.nbd.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 47.Foidl BM, Humpel C. Differential hyperphosphorylation of Tau-S199, -T231 and -S396 in organotypic brain slices of Alzheimer mice a model to study early tau hyperphosphorylation using okadaic acid. Front Aging Neurosci. 2018;10:113. doi: 10.3389/fnagi.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neddens J, Temmel M, Flunkert S, Kerschbaumer B, Hoeller C, Loeffler T, et al. Phosphorylation of different tau sites during progression of Alzheimer’s disease. Acta Neuropathol Commun. 2018;6:52. doi: 10.1186/s40478-018-0557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Hu S, Lin H, He J, Tang C. Electroacupuncture at GV24 and bilateral GB13 improves cognitive ability via influences the levels of Aβ, p-tau (s396) and p-tau (s404) in the hippocampus of Alzheimer’s disease model rats. NeuroReport. 2020;31:1072–1083. doi: 10.1097/WNR.0000000000001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang M, Xv GH, Wang WX, Meng DJ, Ji Y. Electroacupuncture improves cognitive deficits and activates PPAR-γ in a rat model of Alzheimer’s disease. Acupunct Med. 2017;35:44–51. doi: 10.1136/acupmed-2015-010972. [DOI] [PubMed] [Google Scholar]
- 51.Qi Y, Dou DQ, Jiang H, Zhang BB, Qin WY, Kang K, et al. Arctigenin attenuates learning and memory deficits through PI3k/Akt/GSK-3β pathway reducing tau hyperphosphorylation in Aβ-induced AD mice. Planta Med. 2017;83:51–56. doi: 10.1055/s-0042-107471. [DOI] [PubMed] [Google Scholar]
- 52.Xu A, Zeng Q, Tang Y, Wang X, Yuan X, Zhou Y, et al. Electroacupuncture protects cognition by regulating tau phosphorylation and glucose metabolism via the AKT/GSK3β signaling pathway in Alzheimer’s disease model mice. Front Neurosci. 2020;14:585476. doi: 10.3389/fnins.2020.585476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang S, Guo X, Li G, Zhang X, Li J, Jia Y, et al. Acupuncture promotes expression of Hsp84/86 and delays brain ageing in SAMP8 mice. Acupunct Med. 2019;37:340–347. doi: 10.1136/acupmed-2017-011577. [DOI] [PubMed] [Google Scholar]
- 54.Liu W, Zhuo P, Li L, Jin H, Lin B, Zhang Y, et al. Activation of brain glucose metabolism ameliorating cognitive impairment in APP/PS1 transgenic mice by electroacupuncture. Free Radic Biol Med. 2017;112:174–190. doi: 10.1016/j.freeradbiomed.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 55.Wang HL, Liu FL, Li RQ, Wan MY, Li JY, Shi J, et al. Electroacupuncture improves learning and memory functions in a rat cerebral ischemia/reperfusion injury model through PI3K/Akt signaling pathway activation. Neural Regen Res. 2021;16:1011–1016. doi: 10.4103/1673-5374.300454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng X, Lin W, Jiang Y, Lu K, Wei W, Huo Q, et al. Electroacupuncture ameliorates beta-amyloid pathology and cognitive impairment in Alzheimer disease via a novel mechanism involving activation of TFEB (transcription factor EB) Autophagy. 2021;17:3833–3847. doi: 10.1080/15548627.2021.1886720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu CE, Seltman H, Peskind ER, Galloway N, Zhou PX, Rosenthal E, et al. Comprehensive analysis of APOE and selected proximate markers for late-onset Alzheimer’s disease: patterns of linkage disequilibrium and disease/marker association. Genomics. 2007;89:655–665. doi: 10.1016/j.ygeno.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su X, Wu Z, Mai F, Fan Z, Du S, Qian H, et al. ‘Governor vessel-unblocking and mind-regulating’ acupuncture therapy ameliorates cognitive dysfunction in a rat model of middle cerebral artery occlusion. Int J Mol Med. 2019;43:221–232. doi: 10.3892/ijmm.2018.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y, Zhou J, Li J, Yang SB, Mo LQ, Hu JH, et al. Electroacupuncture pretreatment prevents cognitive impairment induced by limb ischemia-reperfusion via inhibition of microglial activation and attenuation of oxidative stress in rats. Brain Res. 2012;1432:36–45. doi: 10.1016/j.brainres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Wu G, Li L, Li HM, Zeng Y, Wu WC. Electroacupuncture ameliorates spatial learning and memory impairment via attenuating NOX2-related oxidative stress in a rat model of Alzheimer’s disease induced by Aβ1–42. Cell Mol Biol. 2017;63:38–45. doi: 10.14715/cmb/2017.63.4.7. [DOI] [PubMed] [Google Scholar]
- 61.Sutalangka C, Wattanathorn J, Muchimapura S, Thukham-Mee W, Wannanon P, Tong-un T. Laser acupuncture improves memory impairment in an animal model of Alzheimer’s disease. J Acupunct Meridian Stud. 2013;6:247–251. doi: 10.1016/j.jams.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Wattanathorn J, Sutalangka C. Laser Acupuncture at HT7 acupoint improves cognitive deficit, neuronal loss, oxidative stress, and functions of cholinergic and dopaminergic systems in animal model of Parkinson’s disease. Evid Based Complement Alternat Med. 2014;2014:937601. doi: 10.1155/2014/937601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan S, Zhang X, Bo Y, Li W, Zhang H, Jiang Q. The effects of electroacupuncture treatment on the postoperative cognitive function in aged rats with acute myocardial ischemia-reperfusion. Brain Res. 2014;1593:19–29. doi: 10.1016/j.brainres.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Han YG, Qin X, Zhang T, Lei M, Sun FY, Sun JJ, et al. Electroacupuncture prevents cognitive impairment induced by lipopolysaccharide via inhibition of oxidative stress and neuroinflammation. Neurosci Lett. 2018;683:190–195. doi: 10.1016/j.neulet.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Liu PR, Cao F, Zhang Y, Peng S. Electroacupuncture reduces astrocyte number and oxidative stress in aged rats with surgery-induced cognitive dysfunction. J Int Med Res. 2019;47:3860–3873. doi: 10.1177/0300060519860026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomas C, Mackey MM, Diaz AA, Cox DP. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: implications for diseases associated with iron accumulation. Redox Rep. 2009;14:102–108. doi: 10.1179/135100009X392566. [DOI] [PubMed] [Google Scholar]
- 67.Li H, Wang Y, Feng D, Liu Y, Xu M, Gao A, et al. Alterations in the time course of expression of the Nox family in the brain in a rat experimental cerebral ischemia and reperfusion model: effects of melatonin. J Pineal Res. 2014;57:110–119. doi: 10.1111/jpi.12148. [DOI] [PubMed] [Google Scholar]
- 68.Wang J, Liu Y, Shen H, Li H, Wang Z, Chen G. Nox2 and Nox4 participate in ROS-induced neuronal apoptosis and brain injury during ischemia-reperfusion in rats. Acta Neurochir Suppl. 2020;127:47–54. doi: 10.1007/978-3-030-04615-6_8. [DOI] [PubMed] [Google Scholar]
- 69.El-Benna J, Dang PM, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med. 2009;41:217–225. doi: 10.3858/emm.2009.41.4.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi GX, Wang XR, Yan CQ, He T, Yang JW, Zeng XH, et al. Acupuncture elicits neuroprotective effect by inhibiting NAPDH oxidase-mediated reactive oxygen species production in cerebral ischaemia. Sci Rep. 2015;5:17981. doi: 10.1038/srep17981. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Wang XR, Shi GX, Yang JW, Yan CQ, Lin LT, Du SQ, et al. Acupuncture ameliorates cognitive impairment and hippocampus neuronal loss in experimental vascular dementia through Nrf2-mediated antioxidant response. Free Radic Biol Med. 2015;89:1077–1084. doi: 10.1016/j.freeradbiomed.2015.10.426. [DOI] [PubMed] [Google Scholar]
- 72.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Brewer AC, Murray TV, Arno M, Zhang M, Anilkumar NP, Mann GE, et al. Nox4 regulates Nrf2 and glutathione redox in cardiomyocytes in vivo. Free Radic Biol Med. 2011;51:205–215. doi: 10.1016/j.freeradbiomed.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25(2):181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 75.Jazwa A, Cuadrado A. Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr Drug Targets. 2010;11:1517–1531. doi: 10.2174/1389450111009011517. [DOI] [PubMed] [Google Scholar]
- 76.Li L, Li L, Zhang J, Huang S, Liu W, Wang Z, et al. Disease stage-associated alterations in learning and memory through the electroacupuncture modulation of the cortical microglial M1/M2 polarization in mice with Alzheimer’s disease. Neural Plast. 2020;2020:8836173. doi: 10.1155/2020/8836173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fang Y, Sui R. Electroacupuncture at the wangu acupoint suppresses expression of inflammatory cytokines in the hippocampus of rats with vascular dementia. Afr J Tradit Complement Altern Med. 2016;13:17–24. doi: 10.21010/ajtcam.v13i5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Wang Q, Ren B, Guo T, Qiang J, Cao H, et al. “Olfactory three-needle” enhances spatial learning and memory ability in SAMP8 mice. Behav Neurol. 2020;2020:2893289. doi: 10.1155/2020/2893289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang J, Wise L, Fukuchi KI. TLR4 cross-talk with NLRP3 inflammasome and complement signaling pathways in Alzheimer’s disease. Front Immunol. 2020;11:724. doi: 10.3389/fimmu.2020.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cai M, Lee JH, Yang EJ. Bee venom ameliorates cognitive dysfunction caused by neuroinflammation in an animal model of vascular dementia. Mol Neurobiol. 2017;54:5952–5960. doi: 10.1007/s12035-016-0130-x. [DOI] [PubMed] [Google Scholar]
- 81.Wang L, Yang JW, Lin LT, Huang J, Wang XR, Su XT, et al. Acupuncture attenuates inflammation in microglia of vascular dementia rats by inhibiting miR-93-mediated TLR4/MyD88/NF-κB signaling pathway. Oxid Med Cell Longev. 2020;2020:8253904. doi: 10.1155/2020/8253904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bu Y, Li WS, Lin J, Wei YW, Sun QY, Zhu SJ, Tang ZS. Electroacupuncture attenuates immune-inflammatory response in hippocampus of rats with vascular dementia by inhibiting TLR4/MyD88 signaling pathway. Chin J Integr Med. 2022;28:153–161. doi: 10.1007/s11655-021-3350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang J, Gong Z, Kong Y, Huang Y, Wang H, Kang Y, Zhan S. Electroacupuncture synergistically inhibits proinflammatory cytokine production and improves cognitive function in rats with cognitive impairment due to hepatic encephalopathy through p38MAPK/STAT3 and TLR4/NF-κB signaling pathways. Evid Based Complement Alternat Med. 2021;2021:7992688. doi: 10.1155/2021/7992688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hemonnot AL, Hua J, Ulmann L, Hirbec H. Microglia in Alzheimer disease: well-known targets and new opportunities. Front Aging Neurosci. 2019;11:233. doi: 10.3389/fnagi.2019.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hou Z, Qiu R, Wei Q, Liu Y, Wang M, Mei T, et al. Electroacupuncture improves cognitive function in senescence-accelerated P8 (SAMP8) mice via the NLRP3/caspase-1 pathway. Neural Plast. 2020;2020:8853720. doi: 10.1155/2020/8853720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Du SQ, Wang XR, Zhu W, Ye Y, Yang JW, Ma SM, et al. Acupuncture inhibits TXNIP-associated oxidative stress and inflammation to attenuate cognitive impairment in vascular dementia rats. CNS Neurosci Ther. 2018;24:39–46. doi: 10.1111/cns.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun L, Yong Y, Wei P, Wang Y, Li H, Zhou Y, Ruan W, Li X, Song J. Electroacupuncture ameliorates postoperative cognitive dysfunction and associated neuroinflammation via NLRP3 signal inhibition in aged mice. CNS Neurosci Ther. 2022;28:390–400. doi: 10.1111/cns.13784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Z, Liu T, Yin C, Li Y, Gao F, Yu L, Wang Q. Electroacupuncture pretreatment ameliorates anesthesia and surgery-induced cognitive dysfunction via activation of an α7-nAChR signal in aged rats. Neuropsychiatr Dis Treat. 2021;17:2599–2611. doi: 10.2147/NDT.S322047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou Y, Hu C, Mao C, Li S, Cui Y, Qian Y. Electroacupuncture ameliorates tibial fracture-induced cognitive dysfunction by elevating α7nAChR expression and suppressing mast cell degranulation in the hippocampus of rats. Evid Based Complement Alternat Med. 2022;2022:3182220. doi: 10.1155/2022/3182220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cao Y, Wang L, Lin LT, Wang XR, Ma SM, Yang NN, et al. Acupuncture attenuates cognitive deficits through α7nAChR mediated anti-inflammatory pathway in chronic cerebral hypoperfusion rats. Life Sci. 2021;266:118732. doi: 10.1016/j.lfs.2020.118732. [DOI] [PubMed] [Google Scholar]
- 91.Han D, Liu Z, Wang G, Zhang Y, Wu Z. Electroacupuncture Improves cognitive deficits through increasing regional cerebral blood flow and alleviating inflammation in CCI rats. Evid Based Complement Alternat Med. 2017;2017:5173168. doi: 10.1155/2017/5173168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li CL, Lin YK, Chen HA, Huang CY, Huang MT, Chang YJ. Smoking as an independent risk factor for hepatocellular carcinoma due to the α7-nachr modulating the JAK2/STAT3 signaling axis. J Clin Med. 2019;8:1391. doi: 10.3390/jcm8091391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang J, You X, Liu W, et al. Electroacupuncture ameliorating post-stroke cognitive impairments via inhibition of peri-infarct astroglial and microglial/macrophage P2 purinoceptors-mediated neuroinflammation and hyperplasia. BMC Complement Altern Med. 2017;17(1):480. doi: 10.1186/s12906-017-1974-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li F, Yan CQ, Lin LT, Li H, Zeng XH, Liu Y, et al. Acupuncture attenuates cognitive deficits and increases pyramidal neuron number in hippocampal CA1 area of vascular dementia rats. BMC Complement Altern Med. 2015;15:133. doi: 10.1186/s12906-015-0656-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li G, Zhang X, Cheng H, Shang X, Xie H, Zhang X, et al. Acupuncture improves cognitive deficits and increases neuron density of the hippocampus in middle-aged SAMP8 mice. Acupunct Med. 2012;30:339–345. doi: 10.1136/acupmed-2012-010180. [DOI] [PubMed] [Google Scholar]
- 96.Zhao L, Zhou C, Li L, Liu J, Shi H, Kan B, et al. Acupuncture improves cerebral microenvironment in mice with Alzheimer’s disease treated with hippocampal neural stem cells. Mol Neurobiol. 2017;54:5120–5130. doi: 10.1007/s12035-016-0054-5. [DOI] [PubMed] [Google Scholar]
- 97.Fan XW, Liu HH, Wang HB, Chen F, Yang Y, Chen Y, et al. Electroacupuncture improves cognitive function and hippocampal neurogenesis after brain irradiation. Radiat Res. 2017;187:672–681. doi: 10.1667/RR14561.1. [DOI] [PubMed] [Google Scholar]
- 98.Cheng H, Yu J, Jiang Z, Zhang X, Liu C, Peng Y, et al. Acupuncture improves cognitive deficits and regulates the brain cell proliferation of SAMP8 mice. Neurosci Lett. 2008;432:111–116. doi: 10.1016/j.neulet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 99.Li X, Guo F, Zhang Q, Huo T, Liu L, Wei H, et al. Electroacupuncture decreases cognitive impairment and promotes neurogenesis in the APP/PS1 transgenic mice. BMC Complement Altern Med. 2014;14:37. doi: 10.1186/1472-6882-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kumano G, Smith WC. FGF signaling restricts the primary blood islands to ventral mesoderm. Dev Biol. 2000;228:304–314. doi: 10.1006/dbio.2000.9937. [DOI] [PubMed] [Google Scholar]
- 101.Zhang S, Xie R, Zhao T, Yang X, Han L, Ye F, et al. Neural stem cells preferentially migrate to glioma stem cells and reduce their stemness phenotypes. Int J Oncol. 2014;45:1989–1996. doi: 10.3892/ijo.2014.2629. [DOI] [PubMed] [Google Scholar]
- 102.Li L, Li J, Dai Y, Yang M, Liang S, Wang Z, Liu W, Chen L, Tao J. Electro-acupuncture improve the early pattern separation in Alzheimer’s disease mice via basal forebrain-hippocampus cholinergic neural circuit. Front Aging Neurosci. 2021;13:770948. doi: 10.3389/fnagi.2021.770948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ahn SM, Kim YR, Kim HN, Shin YI, Shin HK, Choi BT. Electroacupuncture ameliorates memory impairments by enhancing oligodendrocyte regeneration in a mouse model of prolonged cerebral hypoperfusion. Sci Rep. 2016;6:28646. doi: 10.1038/srep28646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 105.Gutiérrez-Vargas JA, Múnera A, Cardona-Gómez GP. CDK5 knockdown prevents hippocampal degeneration and cognitive dysfunction produced by cerebral ischemia. J Cereb Blood Flow Metab. 2015;35:1937–1949. doi: 10.1038/jcbfm.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu B, Sun A, He Y, Qian F, Xi S, Long D, et al. Loss of thin spines and small synapses contributes to defective hippocampal function in aged mice. Neurobiol Aging. 2018;71:91–104. doi: 10.1016/j.neurobiolaging.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 107.Zhao L, Liu JW, Kan BH, Shi HY, Yang LP, Liu XY. Acupuncture accelerates neural regeneration and synaptophysin production after neural stem cells transplantation in mice. World J Stem Cells. 2020;12:1576–1590. doi: 10.4252/wjsc.v12.i12.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cai M, Lee JH, Yang EJ. Electroacupuncture attenuates cognition impairment via anti-neuroinflammation in an Alzheimer’s disease animal model. J Neuroinflammation. 2019;16:264. doi: 10.1186/s12974-019-1665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dong W, Yang W, Li F, Guo W, Qian C, Wang F, et al. Electroacupuncture improves synaptic function in SAMP8 mice probably via inhibition of the AMPK/eEF2K/eEF2 signaling pathway. Evid Based Complement Alternat Med. 2019;2019:8260815. doi: 10.1155/2019/8260815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma C, Zhou Y, Yi W, Zhou X, Guo W, Xu X, Luo J, Luo Z, Liu A, Chen D. Electroacupuncture of the Baihui and Shenting acupoints for vascular dementia in rats through the miR-81/IL-16/PSD-95 pathway. Ann Transl Med. 2022;10:540. doi: 10.21037/atm-22-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin R, Li X, Liu W, Chen W, Yu K, Zhao C, et al. Electro-acupuncture ameliorates cognitive impairment via improvement of brain-derived neurotropic factor-mediated hippocampal synaptic plasticity in cerebral ischemia-reperfusion injured rats. Exp Ther Med. 2017;14:2373–2379. doi: 10.3892/etm.2017.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pei W, Meng F, Deng Q, Zhang B, Gu Y, Jiao B, et al. Electroacupuncture promotes the survival and synaptic plasticity of hippocampal neurons and improvement of sleep deprivation-induced spatial memory impairment. CNS Neurosci Ther. 2021;27:1472–1482. doi: 10.1111/cns.13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maqbool M, Mobashir M, Hoda N. Pivotal role of glycogen synthase kinase-3: a therapeutic target for Alzheimer’s disease. Eur J Med Chem. 2016;107:63–81. doi: 10.1016/j.ejmech.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 114.Yu CC, Wang Y, Shen F, Kong LH, Wang YW, Zhou H, et al. High-frequency (50 Hz) electroacupuncture ameliorates cognitive impairment in rats with amyloid beta 1–42-induced Alzheimer’s disease. Neural Regen Res. 2018;13:1833–1841. doi: 10.4103/1673-5374.238620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y, Zheng A, Yang H, Wang Q, Ren B, Guo T, et al. “Olfactory three-needle” acupuncture enhances synaptic function in Aβ(1–42)-induced Alzheimer’s disease via activating PI3K/AKT/GSK-3β signaling pathway. J Integr Neurosci. 2021;20:55–65. doi: 10.31083/j.jin.2021.01.224. [DOI] [PubMed] [Google Scholar]
- 116.Lin R, Wu Y, Tao J, Chen B, Chen J, Zhao C, et al. Electroacupuncture improves cognitive function through Rho GTPases and enhances dendritic spine plasticity in rats with cerebral ischemia-reperfusion. Mol Med Rep. 2016;13:2655–2660. doi: 10.3892/mmr.2016.4870. [DOI] [PubMed] [Google Scholar]
- 117.Malenka RC, Nicoll RA. Long-term potentiation–a decade of progress. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 118.Herring BE, Nicoll RA. Long-term potentiation: from CaMKII to AMPA receptor trafficking. Annu Rev Physiol. 2016;78:351–365. doi: 10.1146/annurev-physiol-021014-071753. [DOI] [PubMed] [Google Scholar]
- 119.Duffy SN, Craddock KJ, Abel T, Nguyen PV. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn Memory (Cold Spring Harbor, N.Y.) 2001;8:26–34. doi: 10.1101/lm.36301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moulder KL, Jiang X, Chang C, Taylor AA, Benz AM, Conti AC, et al. A specific role for Ca2+-dependent adenylyl cyclases in recovery from adaptive presynaptic silencing. J Neurosci. 2008;28:5159–5168. doi: 10.1523/JNEUROSCI.5317-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zheng Y, Qin Z, Tsoi B, Shen J, Zhang ZJ. Electroacupuncture on trigeminal nerve-innervated acupoints ameliorates poststroke cognitive impairment in rats with middle cerebral artery occlusion: involvement of neuroprotection and synaptic plasticity. Neural Plast. 2020;2020:8818328. doi: 10.1155/2020/8818328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dai Y, Zhang Y, Yang M, Lin H, Liu Y, Xu W, Ding Y, Tao J, Liu W. Electroacupuncture increases the hippocampal synaptic transmission efficiency and long-term plasticity to improve vascular cognitive impairment. Mediators Inflamm. 2022;2022:5985143. doi: 10.1155/2022/5985143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li QQ, Shi GX, Yang JW, Li ZX, Zhang ZH, He T, et al. Hippocampal cAMP/PKA/CREB is required for neuroprotective effect of acupuncture. Physiol Behav. 2015;139:482–490. doi: 10.1016/j.physbeh.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 124.Zheng CX, Lu M, Guo YB, Zhang FX, Liu H, Guo F, et al. Electroacupuncture ameliorates learning and memory and improves synaptic plasticity via activation of the PKA/CREB signaling pathway in cerebral hypoperfusion. Evid Based Complement Alternat Med. 2016;2016:7893710. doi: 10.1155/2016/7893710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Treu SP, Plante DT. Cerebrospinal fluid orexin in Alzheimer’s disease: a systematic review and meta-analysis. Sleep Med. 2021;85:230–238. doi: 10.1016/j.sleep.2021.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shimizu S, Takenoshita N, Inagawa Y, Tsugawa A, Hirose D, Kaneko Y, et al. Positive association between cognitive function and cerebrospinal fluid orexin a levels in Alzheimer’s disease. J Alzheimers Dis. 2020;73:117–123. doi: 10.3233/JAD-190958. [DOI] [PubMed] [Google Scholar]
- 127.Hou Z, Yang X, Li Y, Chen J, Shang H. Electroacupuncture enhances neuroplasticity by regulating the orexin A-mediated cAMP/PKA/CREB signaling pathway in senescence-accelerated mouse prone 8 (SAMP8) mice. Oxid Med Cell Longev. 2022;2022:8694462. doi: 10.1155/2022/8694462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ye Y, Li H, Yang JW, Wang XR, Shi GX, Yan CQ, et al. Acupuncture attenuated vascular dementia-induced hippocampal long-term potentiation impairments via activation of D1/D5 receptors. Stroke. 2017;48:1044–1051. doi: 10.1161/STROKEAHA.116.014696. [DOI] [PubMed] [Google Scholar]
- 129.Xiao LY, Wang XR, Yang JW, Ye Y, Zhu W, Cao Y, et al. Acupuncture prevents the impairment of hippocampal LTP through β1-AR in vascular dementia rats. Mol Neurobiol. 2018;55:7677–7690. doi: 10.1007/s12035-018-0943-x. [DOI] [PubMed] [Google Scholar]
- 130.Xiao LY, Yang JW, Wang XR, Ye Y, Yang NN, Yan CQ, et al. Acupuncture rescues cognitive impairment and upregulates dopamine-β-hydroxylase expression in chronic cerebral hypoperfusion rats. Biomed Res Int. 2018;2018:5423961. doi: 10.1155/2018/5423961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kimura K, Sela S, Bouvier C, Grandy DK, Sidhu A. Differential coupling of D1 and D5 dopamine receptors to guanine nucleotide binding proteins in transfected GH4C1 rat somatomammotrophic cells. J Neurochem. 1995;64:2118–2124. doi: 10.1046/j.1471-4159.1995.64052118.x. [DOI] [PubMed] [Google Scholar]
- 132.Nguyen PV, Gelinas JN. Noradrenergic gating of long-lasting synaptic potentiation in the hippocampus: from neurobiology to translational biomedicine. J Neurogenet. 2018;32:171–182. doi: 10.1080/01677063.2018.1497630. [DOI] [PubMed] [Google Scholar]
- 133.Zhang Y, Mao X, Lin R, Li Z, Lin J. Electroacupuncture ameliorates cognitive impairment through inhibition of Ca(2+)-mediated neurotoxicity in a rat model of cerebral ischaemia-reperfusion injury. Acupunct Med. 2018;36:401–407. doi: 10.1136/acupmed-2016-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee B, Sur BJ, Kwon S, Jung E, Shim I, Lee H, et al. Acupuncture stimulation alleviates corticosterone-induced impairments of spatial memory and cholinergic neurons in rats. Evid Based Complement Alternat Med. 2012;2012:670536. doi: 10.1155/2012/670536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee B, Sur B, Shim J, Hahm DH, Lee H. Acupuncture stimulation improves scopolamine-induced cognitive impairment via activation of cholinergic system and regulation of BDNF and CREB expressions in rats. BMC Complement Altern Med. 2014;14:338. doi: 10.1186/1472-6882-14-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu PR, Zhou Y, Zhang Y, Diao S. Electroacupuncture alleviates surgery-induced cognitive dysfunction by increasing α7-nAChR expression and inhibiting inflammatory pathway in aged rats. Neurosci Lett. 2017;659:1–6. doi: 10.1016/j.neulet.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 137.Chen L, Yamada K, Nabeshima T, Sokabe M. alpha7 Nicotinic acetylcholine receptor as a target to rescue deficit in hippocampal LTP induction in beta-amyloid infused rats. Neuropharmacology. 2006;50:254–268. doi: 10.1016/j.neuropharm.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 138.Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Escobar I, Xu J, Jackson CW, Perez-Pinzon MA. Altered neural networks in the papez circuit: implications for cognitive dysfunction after cerebral ischemia. J Alzheimers Dis. 2019;67:425–446. doi: 10.3233/JAD-180875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vann SD, Nelson AJ. The mammillary bodies and memory: more than a hippocampal relay. Prog Brain Res. 2015;219:163–185. doi: 10.1016/bs.pbr.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zarei M, Patenaude B, Damoiseaux J, Morgese C, Smith S, Matthews PM, et al. Combining shape and connectivity analysis: an MRI study of thalamic degeneration in Alzheimer’s disease. Neuroimage. 2010;49:1–8. doi: 10.1016/j.neuroimage.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 142.Jang SH, Kwon HG. Neural injury of the Papez circuit following hypoxic-ischemic brain injury: A case report. Medicine (Abingdon) 2016;95:e5173. doi: 10.1097/MD.0000000000005173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lin B, Zhang L, Yin X, Chen X, Ruan C, Wu T, Liu Z, Huang J. Modulation of entorhinal cortex-hippocampus connectivity and recognition memory following electroacupuncture on 3×Tg-AD model: evidence from multimodal MRI and electrophysiological recordings. Front Neurosci. 2022;16:968767. doi: 10.3389/fnins.2022.968767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lu Y, Huang Y, Tang C, Shan B, Cui S, Yang J, et al. Brain areas involved in the acupuncture treatment of AD model rats: a PET study. BMC Complement Altern Med. 2014;14:178. doi: 10.1186/1472-6882-14-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang Q, Li J, Huang S, Yang M, Liang S, Liu W, et al. Functional connectivity of the retrosplenial cortex in rats with ischemic stroke is improved by electroacupuncture. Acupunct Med. 2021;39:200–207. doi: 10.1177/0964528420921190. [DOI] [PubMed] [Google Scholar]
- 146.Shibata H. Efferent projections from the anterior thalamic nuclei to the cingulate cortex in the rat. J Comp Neurol. 1993;330:533–542. doi: 10.1002/cne.903300409. [DOI] [PubMed] [Google Scholar]
- 147.Zheng W, Su Z, Liu X, Zhang H, Han Y, Song H, et al. Modulation of functional activity and connectivity by acupuncture in patients with Alzheimer disease as measured by resting-state fMRI. PLoS ONE. 2018;13:e0196933. doi: 10.1371/journal.pone.0196933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang Z, Liang P, Zhao Z, Han Y, Song H, Xu J, et al. Acupuncture modulates resting state hippocampal functional connectivity in Alzheimer disease. PLoS ONE. 2014;9:e91160. doi: 10.1371/journal.pone.0091160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 151.Liang P, Wang Z, Qian T, Li K. Acupuncture stimulation of Taichong (Liv3) and Hegu (LI4) modulates the default mode network activity in Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2014;29:739–748. doi: 10.1177/1533317514536600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lai X, Ren J, Lu Y, Cui S, Chen J, Huang Y, et al. Effects of acupuncture at HT7 on glucose metabolism in a rat model of Alzheimer’s disease: an 18F-FDG-PET study. Acupunct Med. 2016;34:215–222. doi: 10.1136/acupmed-2015-010865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen S, Xu M, Li H, Liang J, Yin L, Liu X, et al. Acupuncture at the Taixi (KI3) acupoint activates cerebral neurons in elderly patients with mild cognitive impairment. Neural Regen Res. 2014;9:1163–1168. doi: 10.4103/1673-5374.135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang Z, Nie B, Li D, Zhao Z, Han Y, Song H, et al. Effect of acupuncture in mild cognitive impairment and Alzheimer disease: a functional MRI study. PLoS ONE. 2012;7:e42730. doi: 10.1371/journal.pone.0042730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tan TT, Wang D, Huang JK, Zhou XM, Yuan X, Liang JP, et al. Modulatory effects of acupuncture on brain networks in mild cognitive impairment patients. Neural Regen Res. 2017;12:250–258. doi: 10.4103/1673-5374.200808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Feng S, Ren Y, Fan S, Wang M, Sun T, Zeng F, et al. Discovery of acupoints and combinations with potential to treat vascular dementia: a data mining analysis. Evid Based Complement Alternat Med. 2015;2015:310591. doi: 10.1155/2015/310591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yu C, Wang L, Kong L, Shen F, Du Y, Kong L, et al. Acupoint combinations used for treatment of Alzheimer’s disease: a data mining analysis. J Tradit Chin Med. 2018;38:943–952. doi: 10.1016/S0254-6272(18)30995-6. [DOI] [PubMed] [Google Scholar]
- 158.Li W, Kong LH, Wang H, Shen F, Wang YW, Zhou H, et al. High-frequency electroacupuncture evidently reinforces hippocampal synaptic transmission in Alzheimer’s disease rats. Neural Regen Res. 2016;11:801–806. doi: 10.4103/1673-5374.182708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.