Abstract
Pseudomonas aeruginosa binds to human respiratory mucins by mechanisms involving flagellar component-receptor interactions. The adhesion of P. aeruginosa strain PAK is mediated by the flagellar cap protein, FliD, without the involvement of flagellin. Two distinct types of FliD proteins have been identified in P. aeruginosa: A type, found in strain PAK, and B type, found in strain PAO1. In the present work, studies performed with the P. aeruginosa B-type strain PAO1 indicate that both the FliD protein and the flagellin of this strain are involved in the binding to respiratory mucins. Using polyacrylamide-based fluorescent glycoconjugates in a flow cytometry assay, it was previously demonstrated that P. aeruginosa recognizes Lex (or Lewis x) derivatives found at the periphery of human respiratory mucins. The aim of the present work was therefore to determine whether these carbohydrate epitopes (or glycotopes) are receptors for FliD proteins and flagellin. The results obtained by both flow cytometry and a microplate adhesion assay indicate that the FliD protein of strain PAO1 is involved in the binding of glycoconjugates bearing Lex or sialyl-Lex determinants, while the binding of flagellin is restricted to the glycoconjugate bearing Lex glycotope. In contrast, the type A cap protein of P. aeruginosa strain PAK is not involved in the binding to glycoconjugates bearing Lex, sialyl-Lex, or sulfosialyl-Lex glycotopes. This study demonstrates a clear association between a specific Pseudomonas adhesin and a specific mucin glycotope and demonstrates that fine specificities exist in mucin recognition by P. aeruginosa.
Pseudomonas aeuginosa is the major pathogen in the airways of patients suffering from cystic fibrosis (CF) and is currently responsible for most of the morbidity and mortality seen from this disease. This organism has been localized to the mucus of the airways of CF patients during colonization (3); thus, binding to mucin is of great importance in the pathogenesis of chronic airway colonization. Supporting the clinical observations, adhesion of this organism to human salivary and airway mucins has been demonstrated in vitro using liquid- and solid-phase adhesion assays (5, 7, 17, 19, 27, 28). Human airway mucins are a very broad family of polydisperse high-molecular-weight glycoproteins that are part of innate airway defenses. They are highly glycosylated and contain from one single to several hundred carbohydrate chains which form a combination of carbohydrate determinants of considerable diversity in CF and normal airway mucins (14, 20). Therefore the molecular interactions of P. aeruginosa and mucins is potentially a multiligand-adhesin phenomenon involving different carbohydrate epitopes, or glycotopes, of the mucin molecules as well as different peripheral structures on the organism.
By use of P. aeruginosa mutants, peripheral bacterial components, mostly flagellar proteins, have been identified as playing an important role in the binding of this bacterium to respiratory mucins (1, 2, 25). A role for pili which interact with the carbohydrate sequence GalNAcβ1-4Galβ (24), which is present in glycosphingolipids, such as asialo-GM1 and asialo-GM2 (11, 12), but not in human respiratory mucins, has also been excluded by use of nonpiliated mutants (18). However, the nature of the mucin determinants that are specifically recognized by the flagellar components is unknown.
Different approaches have been used in order to identify the mucin carbohydrate determinants responsible for the adhesion of P. aeruginosa to mucins. They are all based on the study of glycolipids or neoglycoconjugates bearing a single type of glycotope. Glycolipids or neoglycolipids have been used in solid-phase adhesion assays (16, 20). More recently, the synthesis of water-soluble polyacrylamide-based fluorescent glycoconjugates (4) has allowed the use of flow cytometry to analyze the interactions of glycotopes with various strains of P. aeruginosa (21). Under these conditions, a number of neutral and acidic Lewis blood group derivatives analogous to glycotopes found at the periphery of airway mucins are recognized by whole cells of P. aeruginosa (20–22). Some of these glycotopes such as the sialyl-Lewis x determinants are overexpressed in the airway mucins of patients chronically colonized with bacteria, especially in the mucins of patients suffering from CF (6).
The present study was therefore designed to determine if the P. aeruginosa flagellar protein FliD, which is a mucin-specific adhesin, recognizes any of the specific Lewis x determinants that bind to whole P. aeruginosa cells. A mutant of the flagellin gene of strain PAO1 was also used as a control. Flow cytometry and solid-phase binding assays were used to analyze the interactions of various mutants of P. aeruginosa defective in the expression of these flagellar proteins with polyacrylamide-based fluorescent neoglycoconjugates bearing neutral, sialylated, and/or sulfated Lewis x glycotopes.
MATERIALS AND METHODS
Neoglycoconjugates.
The neoglycoconjugates (Gly-PAA) used in this study were made commercially and were obtained from Syntesome (Munich, Germany). In order to synthesize the neoglycoconjugates (Table 1), oligosaccharides (Gly) are linked via a 3-carbon spacer arm [-(CH2)3-] to a polyacrylamide type matrix (PAA) (4). In these compounds, approximately every fifth amide group of the polymer chains is N substituted by the carbohydrate on the spacer arm. Their molecular weights are about 40,000, and the carbohydrate content is about 20% (4). Neoglycoconjugates labeled with a fluorescent probe (Gly-PAA-Flu) were used for flow cytometry analysis.
TABLE 1.
Synthetic neoglycoconjugates used in this study
| Carbohydrate | Neoglycoconjugate |
|---|---|
| Lex | Galβ1,4[Fucα1,3]GlcNAcβ-PAAa |
| Sialyl-Lex | NeuAcα2,3Galβ1,4[Fucα1-3]GlcNAcβ-PAA |
| Sulfosialyl-Lex | NeuAcα2,3Galβ1,4[HSO3-6][Fucα1,3]GlcNAcβ-PAA |
PAA, polyacrylamide carrier. The carbohydrate content is 20%.
Bacterial strains and culture conditions.
The bacterial strains used in the study are shown in Table 2. They were grown in tryptic soy broth (TSB medium; Difco, Detroit, Mich.) for 18 h at 37°C. The following antibiotics were used to maintain plasmids and chromosomal insertions in P. aeruginosa strains PAO1 and PAK: gentamicin at 100 μg/ml and carbenicillin at 300 μg/ml for complementation experiments. After centrifugation of the cultures at 4,000 × g for 30 min, the cell pellet was washed twice with phosphate-buffered saline (PBS) containing 5% (vol/vol) TSB and then suspended in the same solution. The optical density was adjusted to obtain a bacterial suspension of approximately 107 CFU/ml. The exact number of bacteria was determined by dilution and plating of the suspension.
TABLE 2.
Bacterial strains used in this study
| Bacterial strain | Relevant characteristic(s) | Source or reference |
|---|---|---|
| PAO1 | Wild-type P. aeruginosa | M. Vasil |
| PAO1-D | PAO1 fliD:Gmr | 2 |
| PAO1-D(375) | PAO1-D− carrying the empty vector pPZ375 | This study |
| PAO1-D(375Db) | PAO1-D− carrying the fliD complementing plasmid | This study |
| PAO1-C | PAO1 fliC:Gmr | 8 |
| PAK | Wild-type P. aeruginosa | D. Bradley |
| PAK-D | PAK fliD::Gmr | 1 |
Flow cytometry binding analysis.
Before each experiment, fluorescent neoglyconjugates were dissolved in filtered, double-distilled water to obtain a 13.5 μM solution. Bacteria were suspended at a final concentration of 2 × 106 CFU/ml in PBS containing 1% (wt/vol) bovine serum albumin (BSA), and 0.5-ml aliquots were incubated with increasing concentrations (6.25 to 125 nM) of fluorescent neoglycoconjugates. Fluorescent polyacrylamide devoid of carbohydrate glycotopes could not be used as a control because it is not commercially available. Therefore, controls were obtained by suspending bacteria in 0.5 ml of PBS containing 1% BSA but omitting neoglycoconjugates from the incubation mixture.
The mixtures were analyzed by flow cytometry as described previously (21, 22), but using a FACScalibur cytometer (Becton Dickinson) and Q cell software for acquisition and analysis, respectively. The green fluorescence was set on a logarithmic scale, and the mean fluorescence was converted into equivalent bound particles using fluorescent calibrated beads (Immuno-Britt; Coulter Counter), after deduction of the control fluorescence. The concentration of fluorescent neoglycoconjugates and the equivalent bound particles obtained for each concentration were used to construct Scatchard plots. Binding capacities and apparent dissociation constants (Kd) for the interaction of different fluorescent neoglycoconjugates with P. aeruginosa strains were calculated by Scatchard plots using the nonlinear progression data analysis program Enzfitter (Cambridge). The dissociation constants were compared using Student's t test (14).
Adhesion assay.
Adherence of P. aeruginosa to human respiratory mucins and to neoglycoconjugates was quantified using a microtiter plate assay (27, 28) Respiratory mucins were prepared from sputum of a patient with chronic bronchitis by ultracentrifugation as described previously (6). Respiratory mucins (100 μg/ml) and neoglycoconjugates (5 μg/ml) were dissolved in 0.1 M sodium carbonate-sodium hydrogenocarbonate, pH 9 (26). One hundred microliters of these solutions was used to coat the wells of a microtiter plate overnight at 37°C. The wells were then rinsed with PBS to remove excess unbound mucins, and the bacterial suspension was added at a concentration between 5 × 106 and 5 × 107 CFU/ml. After incubation at 37°C for 30 min, the unbound bacteria were removed by washing the wells with PBS. The bacteria adhering to mucins or to neoglycoconjugates were desorbed by adding a 0.5% solution of Triton X-100 for 15 min. Desorbed bacteria were quantified by dilution and plating of the contents of the wells on MacConkey agar. A set of uncoated wells was used as negative controls. Only the experiments with little or no background binding to uncoated wells were considered valid. All experiments were performed at least four times with three wells per experiment. Results were expressed in hundreds of CFU per well. They corresponded to the average number of bacteria of each well.
RESULTS
Adherence of a fliD mutant of P. aeruginosa strain PAO1 to human respiratory mucins.
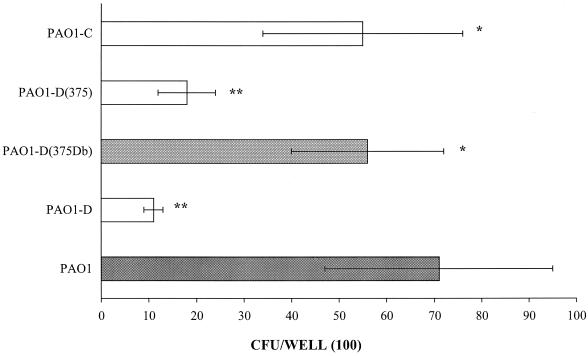
The adhesion of strain PAO1 and a fliD mutant of this strain was examined. Using the microtiter plate adhesion assay, the number of bacteria adhering to mucins was (439 ± 86) × 102 CFU/well for the parental strain (PAO1) (Fig. 1). This number was significantly decreased (P < 0.05) with the fliD mutant (PAO1-D). Adhesion assays were also performed with strain PAO1-D complemented with the homologous fliD gene on the multicopy plasmid vector pPZ375 [strain PAO1-D(375Db)] or containing vector alone [strain PAO1-D(375)]. Complementation restored adhesion to a level that was not significantly different from that of the wild-type parent strain [(349 ± 70) × 102 CFU/well], while it was significantly higher (P < 0.005) than that of the vector control. These results indicate that, as previously described for strains PAK and PAK-NP (2), the binding of P. aeruginosa strain PAO1 to respiratory mucins is partly mediated by the flagellar cap protein, FliD.
FIG. 1.
Comparative binding of P. aeruginosa PAO1 and its fliD and fliC mutants to human airway mucins. PAO1-D, fliD mutant of PAO1; PAO1-D(375Db), PAO1-D complemented with the complete fliD gene on a multicopy plasmid vector, pPZ375Db; PAO1-D(375), PAO1-D with the vector pPZ375; PAO1-C, fliC mutant of PAO1. Differences in binding between strain PAO1 and its mutants marked by one asterisk were not significant, whereas those marked by two asterisks were considered significant (P < 0.05) by Student's t test. The binding of PAO1-D(375Db) and that of PAO1-D(375) were also significantly different.
Binding of glycoconjugates bearing Lex, sialyl-Lex, and sulfosialyl-Lex glycotopes to strain PAO1 and its fliD mutant. (i)Flow cytometry analysis.
The Kd values obtained for the binding of the fluorescent glycoconjugates to strain PAO1 were calculated to be 39 ± 7 nM for Lex-PAA-Flu, 31 ± 3 nM for sialyl-Lex-PAA-Flu, and 59 ± 7 nM for sulfosialyl-Lex-PAA-Flu (Table 3). The Kd values obtained for the binding to the fliD mutant PAO1-D were calculated to be 70 ± 8 nM for Lex-PAA-Flu, 61 ± 6 nM for sialyl-Lex-PAA-Flu, and 72 ± 9 nM for 6-sulfosialyl-Lex-PAA-Flu (Table 3). The differences between the binding of Lex-PAA-Flu and sialyl Lex-PAA-Flu to strains PAO1 and PAO1-D were significant (P < 0.005), while no differences were found between the binding of sulfosialyl-Lex-PAA-Flu to the parental strain and to the mutated strain.
TABLE 3.
Binding of fluorescent glycoconjugates bearing Lex, sialyl-Lex, and 6-sulfosialyl-Lex to P. aeruginosa strain PAO1 and to its fliD mutants
| Glycoconjugate |
Kd (nM ± SD)a for strain:
|
Comparison of Kd valuesb
|
||||
|---|---|---|---|---|---|---|
| PAO1 | PAO1-D | PAO1-D(375Db) | PAO1-D(375) | PAO1 vs PAO1-D | PAO1 vs PAO1-D(375Db) | |
| LPF | 39 ± 7 | 70 ± 8 | 46 ± 8 | 64 ± 5 | P < 0.005 | NS |
| Sialyl-LPF | 31 ± 3 | 61 ± 6 | 40 ± 10 | 67 ± 7 | P < 0.005 | NS |
| 6-Sulfosialyl-LPF | 59 ± 7 | 72 ± 9 | 77 ± 8 | 78 ± 10 | NS | NS |
The number of data points for Scatchard analysis ranged between 20 and 30.
NS, not significant.
LPF, Lex-PAA-Flu.
The Kd values obtained for the binding of Lex (46 ± 8 nM)- and sialyl-Lex-PAA-Flu (40 ± 10 nM) to strain PAO1-D complemented with the fliD gene (Table 3) were practically identical to the Kd values obtained for the binding of these two glycoconjugates to the parental strain. Moreover, significant differences were observed between the binding of Lex- and sialyl-Lex-PAA-Flu to the strain complemented with the fliD gene and that to the strain complemented with the vector alone (Table 3). These studies demonstrate significantly reduced affinity of the fliD mutant of strain PAO1 for glycoconjugates bearing Lex and sialyl-Lex oligosaccharides.
(ii) Microtiter plate adherence assay.
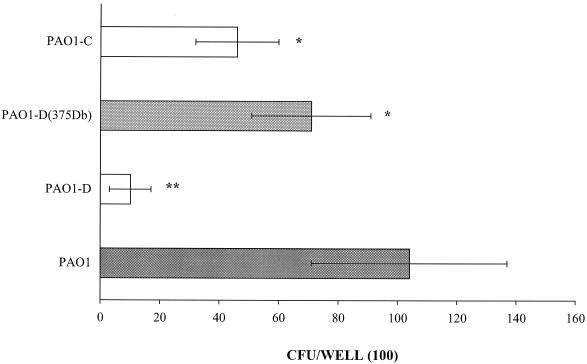
To confirm the role of FliD in the recognition of Lex and sialyl-Lex, which are oligosaccharides that are found in mucins, adhesion assays were performed using microtiter plates coated with the unlabeled glycoconjugates. The number of bacteria adhering to sialyl-Lex-PAA was found to be significantly higher for strain PAO1 [(71 ± 3) × 102 CFU/well] than for strain PAO1-D [(11 ± 2) × 102 CFU/well] (Fig. 2). After complementation with the fliD gene, the adherence of PAO1-D to sialyl-Lex was restored, but this was not the case after complementation with the vector alone. Similar results were obtained for the adherence to Lex-PAA (Fig. 3).
FIG. 2.
Comparative binding of P. aeruginosa PAO1 and its fliD and fliC mutants to the polyacrylamide derivative bearing the sialyl-Lex glycotope. PAO1-D, fliD mutant of PAO1; PAO1-D(375 Db), PAO1-D complemented with the complete fliD gene on a multicopy plasmid vector, pPZ375Db; PAO1-D(375), PAO1-D with the vector pPZ375; PAO1-C, fliC mutant of PAO1. Differences in binding between strain PAO1 and its mutants marked by one asterisk were not significant, whereas those marked by two asterisks were considered significant (P < 0.05) by Student's t test. The binding of PAO1-D(375Db) and that of PAO1-D(375) were also significantly different.
FIG. 3.
Comparative binding of P. aeruginosa PAO1 and its fliD and fliC mutants to the polyacrylate derivative bearing the Lex glycotope. PAO1-D, fliD mutant of PAO1; PAO1-D(375 Db), PAO1-D complemented with the complete fliD gene on a multicopy plasmid vector, pPZ375Db; PAO1-C, fliC mutant of PAO1. Differences in binding between strain PAO1 and its mutants marked by one asterisk were not significant, whereas those marked by two asterisks were considered significant.
Adherence of fliC mutant of P. aeruginosa strain PAO1 to respiratory mucins.
Using the microtiter adhesion assay for mucins, adhesion of a fliC mutant of the b type flagellin-bearing strain PAO1 was measured at (111 ± 19) × 102 CFU/well; this was significantly different (P < 0.005) from the number obtained for the parental strain [(439 ± 86) × 102 CFU/well], indicating that flagellin of this strain is also involved in the binding to human respiratory mucins (Fig. 1). This is in contrast to the lack of a role for the a-type flagellin of strain PAK in mucin binding (25).
Binding of glycoconjugates bearing Lex, sialyl-Lex, and 6-sulfosialyl-Lex glycotopes to the fliC mutant of P. aeruginosa strain PAO1.
Using flow cytometry analysis, the Kd obtained for the binding of the fluorescent glycoconjugates to the fliC mutant of strain PAO1 (PAO1-C) was calculated at 62 ± 10 nM for Lex-PAA-Flu and at 40 ± 9 and 58 ± 8 nM for the binding of sialyl-Lex and 6-sulfosialyl-Lex, respectively (Table 4). Comparison of these values with those obtained for the parental strain showed that there was no difference in the binding of sialyl-Lex- and 6-sulfosialyl-Lex-PAA-Flu. Conversely, the Kd values indicated that the binding of Lex-PAA-Flu to strain PAO1-C was significantly decreased (Table 4).
TABLE 4.
Binding of fluorescent glycoconjugates bearing Lex, sialyl-Lex, and 6-sulfosialyl-Lex epitopes to P. aeruginosa strain PAO1 and to its fliC mutant
| Glycoconjugate |
Kd (nM ± SD)a for strain:
|
Comparison of Kd valuesb | |
|---|---|---|---|
| PAO1 | PAO1C− | ||
| LPFc | 39 ± 7 | 62 ± 10 | P < 0.05 |
| Sialyl-LPF | 31 ± 3 | 40 ± 9 | NS |
| 6-Sulfosialyl-LPF | 59 ± 7 | 58 ± 8 | NS |
The number of data points for Scatchard analysis ranged between 41 and 49.
NS, not significant.
LPF, Lex-PAA-Flu.
Binding of Lex-, sialyl-Lex-, and 6-sulfosialyl-Lex-PAA-Flu to fliD mutants of P. aeruginosa PAK.
Adhesion of strain PAK to mucins is mediated by FliD (2); therefore, the role of the Lewis antigens in PAK FliD-mediated adhesion was also investigated. As shown in Table 5, a mutation in the fliD gene of P. aeruginosa PAK did not produce a significant change in the binding to the fluorescent neoglycoconjugates in the liquid-phase assay. Using a microtiter plate adhesion assay, there was also no difference in the binding of PAK and PAK-D strains to sialyl-Lex-PAA [(15 ± 6) and (16 ± 12) × 102 CFU/well, respectively). These numbers are in the range of those seen with the PAO1 fliD mutant, supporting the conclusion that PAK FliD is not involved in binding to the Lewis glycotopes.
TABLE 5.
Binding of fluorescent glycoconjugates bearing Lex, sialyl-Lex, and 6-sulfosialyl-Lex to P. aeruginosa strain PAK and to its fliD mutant
| Glycoconjugate |
Kd (nM ± SD)a for strain:
|
Comparison of Kd values | |
|---|---|---|---|
| PAK | PAK-D | ||
| LPFb | 48 ± 7 | 46 ± 5 | NSc |
| Sialyl-LPF | 40 ± 6 | 43 ± 10 | NS |
| 6-Sulfosialyl-LPF | 65 ± 8 | 58 ± 9 | NS |
The number of data points for Scatchard analysis ranged between 36 and 44.
LPF, Lex-PAA-Flu.
NS, not significant.
DISCUSSION
P. aeruginosa binding to human respiratory mucins is mediated by the interaction of a bacterial component(s) with carbohydrate moieties of mucins (5, 17, 27, 28). However, there are differences in binding from one strain to another for the same mucins. For instance, this binding is greater for strains 1244 and PAO1 than for strain PAK (17, 23). While it is possible that one or the other strain may possess multiple adhesins to mediate these differences, another possible explanation may be differences in receptor recognition mediated by the known adhesins. Concerning the mucin adhesins, only the flagellar system of P. aeruginosa strain PAK has been shown to date to play an important role in mucin binding (25), and the flagellar cap protein, FliD, was shown to be a mucin-specific adhesin for this strain (2). However, P. aeruginosa contains two distinct types of flagellar cap proteins, designated A and B types, which are inherited with their corresponding a- and b-type flagellins (1). The flagellar caps show only 43% identity at the amino acid level, even though they belong to the same species and no immunological cross-reactivity between them is detectable by use of polyclonal antibodies (1). This suggests that they do not share recognizable linear or conformational epitopes that may be involved in adhesion. The role of the B-type cap in adhesion had not been previously studied, since it was assumed that caps were structurally similar, but this has now been shown not to be the case (2). Strains carrying the B-type cap, however, make up only 20% of mucoid isolates from CF individuals (R. Ramphal, personal communication). Thus, the increased mucin binding of strains carrying B-type caps alluded to above may be aided by other adhesins. This investigation thus further demonstrates a role for the flagellar cap protein in mucin adhesion and points out important fine specificities in the recognition of carbohydrates by the FliD protein.
Human respiratory mucins, the other partner in the bacteria-mucin interaction, have significant numbers of potential receptors, since there are a large number of glycotopes at their periphery, especially neutral or acidic Lewis derivatives (13, 20). Using purified whole mucins, it has been difficult to define specific receptors. However, some progress has been made in defining mucin receptors, with the recognition of the involvement of certain sugars in binding of whole bacteria. One approach that has been successful at identifying potential glycotopes that are recognized by P. aeruginosa has been the synthesis of neoglycolipids containing one specific putative receptor (15, 19). Such an approach has now been applied to the study of mucin receptors with the ability to synthesize neoglycoproteins that carry glycotopes that can be obtained in significant quantities (4). In the context of CF, the Lewis derivatives such as Lex, sialyl-Lex, or 6-sulfosialyl-Lex appear to be increased in airway mucins and have been shown to bind to whole P. aeruginosa cells (21, 22). Recognizing that the FliD protein was a mucin-specific adhesin provided an opportunity to test the interaction between a specific adhesin and single putative receptors.
Polyacrylamide-based glycoconjugates that have predetermined properties, such as molecular mass, solubility, matrix flexibility, distance between the glycotopes, and density of substitution by the different glycotopes, are a suitable tool for measuring interactions between lectins and their carbohydrate receptors (4, 9, 10). The adhesion of bacteria to these neoglycoconjugates can be studied by two different methods: an adhesion assay with unlabeled glycoconjugates applied to a microtiter plate or a flow cytometry assay with polyacrylamide-based glycoconjugates labeled with fluorescein. Fluorescent neoglycoconjugates bearing Lex determinant and its sialylated or sulfated derivatives bound specifically to several strains of P. aeruginosa (21, 22). Scatchard analysis of the data obtained by flow cytometry showed that the affinity of the fluorescent glycoconjugate bearing the sialyl-Lex glycotope was higher than that observed for the other glycoconjugates. The binding of fluorescent glyconjugates bearing blood group A or sialy-N-acetyllactosamine to these strains was not saturable (no Kd could be calculated) and was therefore considered nonspecific (21). In the present work, the binding of polyacrylamide-based glycoconjugates bearing Lex, sialyl-Lex, and 6-sulfosialyl-Lex to strains PAO1 and PAK and to their fliD or fliC mutants was compared. The results obtained for the binding of the neoglycoconjugates to P. aeruginosa strain PAO1 indicated that FliD protein was involved in the binding of this strain to glycoconjugates bearing Lex and sialyl-Lex glycotopes. This was confirmed by using a microtiter plate adhesion assay with unlabeled glycoconjugates bearing Lex and sialyl-Lex glycotopes. In contrast, mutation in the fliD gene of strain PAK did not change the binding of the fluorescent conjugates compared to that with the parental strain, indicating that the specific ligand of PAK FliD is not one of the Lex derivatives that is recognized by the PAO1 FliD.
The studies performed with the strain PAO1 fliC mutant indicated that PAO1 flagellin may be involved in the specific binding of glycoconjugates bearing the Lex glycotope, but not in the binding of the glycoconjugates bearing the sialyl-Lex and 6-sulfosialyl-Lex glycotopes, which recognize the FliD protein. This was confirmed for FliD by using a microtiter plate adhesion assay with unlabeled fluorescent glycoconjugates bearing Lex glycotopes. This suggests a specificity of the interactions between protein and the glycotopes and not a nonspecific interaction with fluorescein, as could have occurred. Both strains bound to 6-sulfosialyl-Lex glycotopes. However, their binding was not modified after mutations either in the fliD or in the fliC gene. This is assumed to be some form of nonspecific binding.
These data suggest that the interactions between P. aeruginosa and carbohydrates are more complex than has been thought and that different components of the adhesin-flagellar system, flagellin and FliD, which differ from one strain to another, do not necessarily recognize the same glycotopes of human respiratory mucins. Since CF mucins are characterized by a high content of sialyl-Lex glycotopes (6), the FliD protein and flagellin may be involved in specific binding to these glycotopes in vivo. Recent results have also demonstrated that glycoconjugates bearing the 6-sulfosialyl-Lex glycotope, also found in abundance in CF respiratory mucins (13), was recognized by whole P. aeruginosa (22). In the future, it will be important to find out the bacterial component involved in the binding to this ligand. In conclusion, the recognition of human respiratory mucins by the adhesin-flagellar system appears to be a mutifactorial phenomenon, involving different flagellar components and different carbohydrate receptors. Further studies will still be necessary to link these in some quantitative way to airway colonization of CF patients. However, these studies define for the first time the association between a specific P. aeruginosa adhesin and a mucin glycotope.
ACKNOWLEDGMENTS
This investigation was supported by the Association Vaincre la Mucoviscidose, by the Réseau Régional d'Etude des Interactions Hôtes-Microorganismes (to P. R.), and by NHLBI grant HL-33622 (to R. R.).
REFERENCES
- 1.Arora S K, Dasgupta N, Lory S, Ramphal R. Identification of two distinct types of flagellar cap proteins, FliD, in Pseudomonas aeruginosa. Infect Immun. 2000;68:1474–1479. doi: 10.1128/iai.68.3.1474-1479.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora S K, Ritchings B W, Almira E C, Lory S, Ramphal R. The Pseudomonas aeruginosa flagellar cap protein, FliD, is responsible for mucin adhesion. Infect Immun. 1998;66:1000–1007. doi: 10.1128/iai.66.3.1000-1007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baltimore R S, Christie C D, Smith G J. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis. Implications for the pathogenesis of progressive lung deterioration. Am Rev Respir Dis. 1989;140:1650–1661. doi: 10.1164/ajrccm/140.6.1650. [DOI] [PubMed] [Google Scholar]
- 4.Bovin N V. Polyacrylamide-based glycoconjugates as tools in glycobiology. Glycoconj J. 1998;15:431–446. doi: 10.1023/a:1006963717646. [DOI] [PubMed] [Google Scholar]
- 5.Carnoy C, Ramphal R, Scharfman A, Lo-Guidice J-M, Houdret N, Klein A, Galabert C, Lamblin G, Roussel P. Altered carbohydrate composition of salivary mucins from patients with cystic fibrosis and the adhesion of Pseudomonas aeruginosa. Am J Respir Cell Mol Biol. 1993;9:323–334. doi: 10.1165/ajrcmb/9.3.323. [DOI] [PubMed] [Google Scholar]
- 6.Davril M, Degroote S, Humbert P, Galabert C, Dumur V, Lafitte J-J, Lamblin G, Roussel P. The sialylation of bronchial mucins secreted by patients suffering from cystic fibrosis or from chronic bronchitis is related to the severity of airway infection. Glycobiology. 1999;9:311–321. doi: 10.1093/glycob/9.3.311. [DOI] [PubMed] [Google Scholar]
- 7.Devaraj N, Sheykhnazari M, Warren W S, Bhavanandan V P. Differential binding of Pseudomonas aeruginosa to normal and cystic fibrosis tracheobronchial mucins. Glycobiology. 1994;4:307–316. doi: 10.1093/glycob/4.3.307. [DOI] [PubMed] [Google Scholar]
- 8.Fleiszig, S. M. J., S. K. Arora, R. Van, and R. Ramphal. FlhA, a component of the flagellum assembly apparatus of Pseudomonas aeruginosa, plays a role in internalization by corneal epithelial cells. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 9.Galanina O E, Tuizikov A B, Rapoport E, Le Pendu J, Bovin N V. Carbohydrate-based probes for detection of cellular lectins. Anal Biochem. 1998;265:258–269. doi: 10.1006/abio.1998.2859. [DOI] [PubMed] [Google Scholar]
- 10.Game S M, Rajapurohit P K, Clifford M, Bird M I, Priest R, Bovin N V, Nifant'ev N E, O'Beirne G, Cook N D. Scintillation proximity assay for E-, P- and L-selectin utilizing polyacrylamide-based neoglycoconjugates as ligands. Anal Biochem. 1998;258:127–135. doi: 10.1006/abio.1998.2576. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S K, Berk R S, Masinick S, Hazlett L D. Pili and lipopolysaccharide of Pseudomonas aeruginosa bind to the glycolipid asialo GM1. Infect Immun. 1994;62:4572–4579. doi: 10.1128/iai.62.10.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krivan H C, Ginsburg V, Roberts D D. Pseudomonas aeruginosa and Pseudomonas cepacia bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2) Arch Biochem Biophys. 1988;260:493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- 13.Lo-Guidice J-M, Wieruszeski J-M, Lemoine J, Verbert A, Roussel P, Lamblin G. Sialylation and sulfation of the carbohydrate chains in respiratory mucins from a patient with cystic fibrosis. J Biol Chem. 1994;269:18794–18813. [PubMed] [Google Scholar]
- 14.Partridge S R, Baker M S, Walker M R, Wilson M. Clusterin, a putative complement regulator, binds to the cell surface of Staphylococcus aureus clinical isolates. Infect Immun. 1996;64:4324–4329. doi: 10.1128/iai.64.10.4324-4329.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramphal R, Carnoy C, Fièvre S, Michalski J-C, Houdret N, Lamblin G, Strecker G, Roussel P. Pseudomonas aeruginosa recognizes carbohydrate chains containing type 1 (Galβ1-3GlcNAc) or type 2 (Galβ1-4GlcNAc) disaccharide units. Infect Immun. 1991;59:700–704. doi: 10.1128/iai.59.2.700-704.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramphal R, Houdret N, Koo L, Lamblin G, Roussel P. Differences in adhesion of Pseudomonas aeruginosa to mucin glycopeptides from sputa of patients with cystic fibrosis and chronic bronchitis. Infect Immun. 1989;57:3066–3071. doi: 10.1128/iai.57.10.3066-3071.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramphal R, Koo L, Ishimoto K S, Totten P A, Lara J-C, Lory S. Adhesion of Pseudomonas aeruginosa pilin-deficient mutants to mucin. Infect Immun. 1991;59:1307–1311. doi: 10.1128/iai.59.4.1307-1311.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy M S. Human tracheobronchial mucin: purification and binding to Pseudomonas aeruginosa. Infect Immun. 1992;60:1530–1535. doi: 10.1128/iai.60.4.1530-1535.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenstein I J, Yuen C-T, Stoll M S, Feizi T. Differences in the binding specificities of Pseudomonas aeruginosa M35 and Escherichia coli C600 for lipid-linked oligosaccharides with lactose-related core regions. Infect Immun. 1992;60:5078–5084. doi: 10.1128/iai.60.12.5078-5084.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roussel P, Lamblin G. Human mucosal mucins in diseases. In: Montreuil J, Vliegenthart J F G, Schachter H, editors. Glycoproteins and disease. Amsterdam, The Netherlands: Elsevier; 1996. pp. 351–393. [Google Scholar]
- 21.Scharfman A, Degroote S, Beau J, Lamblin G, Roussel P, Mazurier J. Pseudomonas aeruginosa binds to neoglycoconjugates bearing mucin carbohydrate determinants and predominantly to sialyl-Lewis x conjugates. Glycobiology. 1999;9:757–764. doi: 10.1093/glycob/9.8.757. [DOI] [PubMed] [Google Scholar]
- 22.Scharfman A, Delmotte P, Beau J, Lamblin G, Roussel P, Mazurier J. Sialyl-Lex and sulfo-sialyl-Lex determinants are receptors for P. aeruginosa. Glycoconjug J. 2000;17:729–734. doi: 10.1023/a:1011091112884. [DOI] [PubMed] [Google Scholar]
- 23.Scharfman A, Kroczynski H, Carnoy C, Van Brussel E, Lamblin G, Ramphal R, Roussel P. Adhesion of Pseudomonas aeruginosa to respiratory mucins and expression of mucin-binding proteins are increased by limiting iron during growth. Infect Immun. 1996;64:5417–5420. doi: 10.1128/iai.64.12.5417-5420.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheth H B, Lee K K, Wong W Y, Srivastava G, Hindsgaul O, Hodges R S, Paranchych W, Irvin R T. The pili of Pseudomonas aeruginosa strains PAK and PAO bind specifically to the carbohydrate sequence βGalNAc(1-4)βGal found in glycosphingolipids asialo-GM1 and asialo-GM2. Mol Microbiol. 1994;11:715–723. doi: 10.1111/j.1365-2958.1994.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 25.Simpson D A, Ramphal R, Lory R. Genetic analysis of Pseudomonas aeruginosa adherence: distinct genetic loci control attachment to epithelial cells and mucins. Infect Immun. 1992;60:3771–3779. doi: 10.1128/iai.60.9.3771-3779.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veerman E C, Bank V M, Namavar F, Appelmelk B J, Bolscher J G, Nieuw Amerongen A V. Sulfated glycans on oral mucin as receptors for Helicobacter pylori. Glycobiology. 1997;7:737–743. doi: 10.1093/glycob/7.6.737. [DOI] [PubMed] [Google Scholar]
- 27.Vishwanath S, Ramphal R. Adherence of Pseudomonas aeruginosa to human tracheobronchial mucin. Infect Immun. 1984;45:197–202. doi: 10.1128/iai.45.1.197-202.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vishwanath S, Ramphal R. Tracheobronchial mucin receptor for Pseudomonas aeruginosa: predominance of amino sugars in binding sites. Infect Immun. 1985;48:331–335. doi: 10.1128/iai.48.2.331-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]