Abstract
The incidence of oropharyngeal squamous cell carcinoma (OPSCC) has increased in the past decades due to carcinogenic HPV infection. As this patient group suffers from considerable mortality and treatment morbidity it is important to improve prognostic strategies in OPSCC. Inflammation plays a key role in cancer and the neutrophil-to-lymphocyte ratio (NLR) in blood has been suggested as a prognostic factor for OPSCC. This study aimed to investigate the prognostic impact of NLR on overall survival (OS) and recurrence-free survival (RFS) in a retrospective cohort of 1370 patients. Included patients had pretreatment neutrophil and lymphocyte counts available, as well as a known HPV status. Patients were treated with curative intent according to Danish national guidelines. We stratified patients in groups by NLR < 2, NLR 2–4, or NLR > 4 and analyzed the influence of the NLR tertile on OS and RFS. Kaplan–Meier curves illustrated survival probability in OS and RFS in the general cohort and were stratified by HPV status. We found that an increasing NLR was associated with inferior OS (HR = 1.5 for NLR > 4) and RFS (HR = 1.6 for NLR 2–4; HR = 1.8 for NLR > 4) in multivariable analysis. The Kaplan–Meier curves displayed inferior OS and RFS with an increasing NLR for both HPV+ and HPV− patients. In conclusion, we showed that an increasing NLR is prognostic for a worse outcome of OPSCC independently of HPV status. There are possible uses of NLR in prognostication and treatment de-escalation although further studies are warranted to determine the clinical utility.
Keywords: oropharyngeal squamous cell carcinoma, OPSCC, human papillomavirus, HPV, neutrophil-to-lymphocyte ratio, NLR
1. Introduction
Through the past decades, there has been an increase in the incidence of oropharyngeal squamous cell carcinomas (OPSCCs), notably in the Western World [1]. This increase has been driven by an increase in human papillomavirus (HPV)-positive OPSCC [2]. The incidence of oropharyngeal cancer worldwide in 2020 was 98,412 and oropharyngeal cancer resulted in 48,143 new deaths that year [3]. More than 90% of oropharyngeal cancers are classified as squamous cell carcinomas [4]. The 5-year relative survival rate of oropharyngeal cancer has increased from 33.1% in the period 1980–1984 to 58.5% in the period 2010–2014. Despite this improvement in survival, the mortality rate remains more than 40% after five years and it is thus of continued interest to further improve OPSCC treatment strategies and survival [1].
HPV-negative (HPV−) OPSCCs are phenotypically similar to non-oropharyngeal squamous cell carcinomas of the upper aerodigestive tract, with alcohol and tobacco being the main risk factors for the development of malignancy [5]. HPV-positive (HPV+) OPSCCs are most often found in the palatine tonsils or base of tongue, and the carcinogenic relationship between HPV infection and OPSCC has been well established in these anatomical sites [5,6]. HPV status also influences the growth pattern and histological appearance of OPSCC; HPV+ OPSCC tend to develop inside crypts in lingual and palatine tonsils, whereas HPV− OPSCC typically arise from surface epithelium [7].
The HPV status of a tumor has important prognostic value as HPV+ patients generally have a better prognosis than the HPV− OPSCC patients [8]. More than 200 different subtypes of HPV have been identified, and currently 14 subtypes are classified as high-risk for their oncogenic potential [9]. Besides HPV and p16-status, known prognostic factors regarding OPSCC are T stage, N stage, performance status, and smoking history [8].
Inflammation associated with cancer has been suggested to impact the prognosis of malignant disease both regarding overall survival (OS) and disease progression [10]. Increasing neutrophil levels in blood have been shown to correlate with lesser OS in head and neck squamous cell carcinomas (HNSCC) and conversely, increasing lymphocyte levels have been shown to correlate with improved OS [11]. Neutrophil-to-lymphocyte ratio (NLR) is an easily accessible marker for systemic inflammation and has been suggested as a prognostic factor for the outcome of many solid tumor malignancies, including gastrointestinal, hepatocellular, and non–small cell lung carcinomas [10,12]. NLR is a simple ratio of neutrophils and lymphocytes and contains information on the balance between acute and chronic inflammation of innate immunity, represented by neutrophils, and adaptive immunity, represented by lymphocytes [13]. It is of interest to gain more knowledge about easily accessible hematological parameters to further understand the underlying mechanisms in OPSCC as well as to improve the diagnosis and treatment of OPSCC patients.
The aim of this study was to investigate the prognostic impact of NLR on OS and recurrence-free survival (RFS) in a retrospective cohort of 1370 patients diagnosed with OPSCC in Eastern Denmark from 2000 to 2020. Of importance, HPV status was available for all patients and our study was more than double the size of previous studies to include HPV status. The recent increase in the incidence of OPSCC testifies to the significance of investigating factors that are useful in the prognostication of the disease. The large cohort size and general HPV availability in our study makes it ideally suited to contribute with valuable knowledge to the field.
2. Materials and Methods
2.1. Setting
The study was a retrospective cohort study based on an OPSCC database actively maintained at the Department of Otorhinolaryngology, Head and Neck Surgery and Audiology at Rigshospitalet, Copenhagen University Hospital [14]. Study data in the database were collected and managed using RedCap [15,16].
This cohort included all patients consecutively diagnosed with OPSCC in Eastern Denmark from 2000 to 2020. Currently, the database comprises 2918 patients with OPSCC and is continually updated with new cases. In Denmark, all patients regardless of socioeconomic status are diagnosed and treated in public hospitals and according to The Danish Head and Neck Cancer Study Group’s (DAHANCA) national treatment guidelines, resulting in a non-selected patient cohort [17].
2.2. Study Population
HPV+/p16-positive(p16+) tumors were defined as HPV+, whereas HPV+/p16-negative (p16−), HPV−/p16+, or HPV−/p16− tumors were defined as HPV−. Patients with unknown HPV status (n = 40) or unknown p16 status (n = 43), were excluded from this study. Patients who had distant metastases (n = 47) at the time of diagnosis, patients who received palliative therapy (n = 133), patients who did not receive any treatment (n = 86), or patients who had unknown treatment (n = 15) were also excluded. Blood samples were collected prior to treatment initiation and patients without data on neutrophil and lymphocyte counts were excluded (n = 1184) (See Figure S1, Supplementary Materials).
The NLR was obtained by dividing the total pretreatment neutrophil count with the total pretreatment lymphocyte count per patient. Ferrandino et al. grouped patients in three groups with NLR-cutoffs for investigating OS of 2.1 and 3.4 while the cutoffs were 2.3 and 3.7 for cancer-specific survival (CSS) [18]. Rachidi et al. examined the effect of NLR on OS in HNSCC and divided patients in three groups with NLR-cutoffs 2.36 and 4.39 [11]. Other studies divided patients in two groups using NLR-cutoffs of 2.42 and 3 [19,20,21]. Based on these previous studies, we chose to separate our patient cohort in three groups of NLR < 2, NLR 2–4, and NLR > 4.
OPSCCs were classified in our database according to UICC 8th. In the novel UICC 8th staging system for OPSCC, different staging criteria are used for p16+ versus p16− carcinomas [22]. The differences in staging between p16+ and p16− carcinomas mainly concern N-staging, although there also is a small difference in T stage, as only p16− carcinomas have a subdivision of T4 in T4a and T4b [23]. In our database, the p16+ carcinomas were classified as either N0, N1, N2, or N3. The p16− carcinomas were classified as N0, N1, N2a, N2b, N2C, N3a, or N3b. We merged the T and N stages of both p16-statuses to be able to treat the study population as a whole entity. p16− N1 and p16+ N1 were grouped as N1. p16− N2a, N2b, and N2c and p16+ N2 were grouped as N2. N3a and N3b of p16− were grouped with N3 of p16+ as N3. T4a and T4b of p16− were grouped with T4 of p16+ as T4. UICC 8th group stages of both p16 statuses were grouped together.
2.3. Statistical Methods
R version 4.1.0 (18 May 2021) was used for statistical analyses [24]. Statistical significance was defined by a p-value of ≤0.05. A demographic Table 1 stratified by the NLR group was created in R using the package tableone [25]. Packages survminer and survival were used to perform survival analyses and create Kaplan–Meier estimators [26,27].
Table 1.
Characteristics of the 1370 patients in the study population.
| Variable | Overall | NLR < 2 | NLR 2–4 | NLR > 4 | p-Value |
|---|---|---|---|---|---|
| n = 1370 | n = 280 | n = 695 | n = 395 | ||
| Age, median (IQR) | 61 [55;68] | 58 [53;65] | 61 [54;69] | 63 [57;69] | <0.001 |
| Sex, n (%) | 0.029 | ||||
| Female | 380 (27.7) | 95 (33.9) | 186 (26.8) | 99 (25.1) | |
| Male | 990 (72.3) | 185 (66.1) | 509 (73.2) | 296 (74.9) | |
| HPV status, n (%) | 0.001 | ||||
| Negative | 493 (36.0) | 79 (28.2) | 246 (35.4) | 168 (42.5) | |
| Positive | 877 (64.0) | 201 (71.8) | 449 (64.6) | 227 (57.5) | |
| Smoking category, n (%) | 0.08 | ||||
| Never a smoker | 326 (23.8) | 87 (31.1) | 162 (23.3) | 77 (19.5) | |
| 0–10 pack years | 101 (7.4) | 26 (9.3) | 47 (6.8) | 28 (7.1) | |
| 10–20 pack year | 114 (8.3) | 16 (5.7) | 68 (9.8) | 30 (7.6) | |
| >20 pack years | 690 (50.4) | 122 (43.6) | 347 (49.9) | 221 (55.9) | |
| Unknown | 139 (10.1) | 29 (10.4) | 71 (10.2) | 39 (9.9) | |
| Performance status, n (%) | <0.001 | ||||
| PS0 | 953 (69.6) | 223 (79.6) | 504 (72.5) | 226 (57.2) | |
| PS1 | 228 (16.6) | 31 (11.1) | 115 (16.5) | 82 (20.8) | |
| PS2 | 40 (2.9) | 6 (2.1) | 13 (1.9) | 21 (5.3) | |
| PS3 | 6 (0.4) | 0 (0.0) | 2 (0.3) | 4 (1.0) | |
| Unknown | 143 (10.4) | 20 (7.1) | 61 (8.8) | 62 (15.7) | |
| Tumor location, n (%) | 0.480 | ||||
| Pharyngeal wall or unspecified location | 132 (9.6) | 23 (8.2) | 70 (10.1) | 39 (9.9) | |
| Palatine tonsils | 711 (51.9) | 156 (55.7) | 362 (52.1) | 193 (48.9) | |
| Lingual tonsil/Base of tongue or vallecula epiglottica | 429 (31.3) | 84 (30.0) | 218 (31.4) | 127 (32.2) | |
| Soft palate, Uvula, or palatal arches | 98 (7.2) | 17 (6.1) | 45 (6.5) | 36 (9.1) | |
| Treatment type, n (%) | 0.028 | ||||
| Radiotherapy | 517 (37.7) | 96 (34.3) | 248 (35.7) | 173 (43.8) | |
| Chemo- and radiotherapy | 728 (53.1) | 159 (56.8) | 391 (56.3) | 178 (45.1) | |
| Primary surgery | 104 (7.6) | 20 (7.1) | 47 (6.8) | 37 (9.4) | |
| Primary surgery with adjuvant radiotherapy | 13 (0.9) | 3 (1.1) | 7 (1.0) | 3 (0.8) | |
| Primary surgery with adjuvant chemo-and radiotherapy | 8 (0.6) | 2 (0.7) | 2 (0.3) | 4 (1.0) | |
| T stage, n (%) | 0.074 | ||||
| T1 | 352 (25.7) | 80 (28.6) | 174 (25.0) | 98 (24.8) | |
| T2 | 542 (39.6) | 117 (41.8) | 286 (41.2) | 139 (35.2) | |
| T3 | 272 (19.9) | 53 (18.9) | 136 (19.6) | 83 (21.0) | |
| T4a or T4b | 201 (14.7) | 29 (10.4) | 97 (14.0) | 75 (19.0) | |
| Unknown | 3 (0.2) | 1 (0.4) | 2 (0.3) | 0 (0.0) | |
| N stage, n (%) | <0.001 | ||||
| N0 | 300 (21.9) | 62 (22.1) | 131 (18.8) | 107 (27.1) | |
| N1 | 703 (51.3) | 166 (59.3) | 372 (53.5) | 165 (41.8) | |
| N2 (including N2a, N2b, N2c) | 306 (22.3) | 47 (16.8) | 164 (23.6) | 95 (24.1) | |
| N3 (including N3a, N3b) | 60 (4.4) | 5 (1.8) | 28 (4.0) | 27 (6.8) | |
| Unknown | 1 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.3) | |
| UICC 8th group stage, n (%) | <0.001 | ||||
| Stage I | 606 (44.2) | 148 (52.9) | 304 (43.7) | 154 (39.0) | |
| Stage II | 283 (20.7) | 69 (24.6) | 137 (19.7) | 77 (19.5) | |
| Stage III | 221 (16.1) | 33 (11.8) | 124 (17.8) | 64 (16.2) | |
| Stage IV | 246 (18.0) | 28 (10.0) | 123 (17.7) | 95 (24.1) | |
| Unknown | 14 (1.0) | 2 (0.7) | 7 (1.0) | 5 (1.3) |
We performed univariable and multivariable COX proportional hazards regression analysis to investigate the significance of NLR on OS and RFS. The multivariable analysis was adjusted for the variables of age, sex, HPV status, smoking category, performance status, tumor location, T stage, N stage, and NLR tertile. To avoid confounding between treatment type and tumor staging, treatment type was not included in the COX regression analysis, as the clinically appropriate treatment type is chosen depending on stage. Alcohol status was not included in the analyses as a large proportion of patient cases in the study population had unknown alcohol status (n = 416, 30.4%) and an inclusion would have hindered our multivariable analysis.
We created Kaplan–Meier survival curves to visualize the survival probability of OS and RFS for the study population. Patients were censored at the last day of follow-up if they had not presented with the outcomes of death or recurrence.
A proportional hazard test with Schoenfeld residuals was used for testing the assumptions for the COX regression analysis of categorical variables (See Figure S2, Supplementary Materials). We found non-proportionality for all variables except the UICC 8th group stage, which was identified as a time-dependent variable. To account for this, the UICC 8th group stage was included as a stratification factor rather than a predictor in our multivariable analysis, thus allowing the baseline hazard function to differ. We tested for the linearity of the continuous covariate patient age by plotting the Martingale residuals against continuous covariates (See Figure S3, Supplementary Materials).
3. Results
We included 1370 OPSCC patients with available NLR and HPV status in this study. Two hundred and eighty patients were categorized as NLR < 2, 695 as NLR 2–4, and 395 had an NLR > 4. Median age at diagnosis increased with the increasing NLR group (p < 0.001); in the NLR < 2 group median age was 58 years, in the NLR 2–4 group median age was 61 years, and in the NLR > 4 group median age was 63 years. We observed an inverse relationship between the increasing NLR group and distribution of HPV positivity (p = 0.001), with 71.8% of tumors being HPV positive in NLR < 2, 64.6% HPV positive in NLR 2–4, and 57.5% HPV positive in NLR > 4. Higher NLR-group entailed a worse performance score (PS) (p < 0.001); 79.6% of patients had PS0 in the NLR < 2 group, 72.5% had PS0 in the NLR 2–4 group, whereas 57.2% of patients had PS0 in the NLR > 4 group. The percentage of patients with a high UICC 8th group stage also increased with an increasing NLR (p < 0.001); 24.1% of patients in the NLR > 4 group had a UICC 8th group stage IV tumor at diagnosis compared with 17.7% in group NLR 2–4 and 10.0% in group NLR < 2 (Table 1).
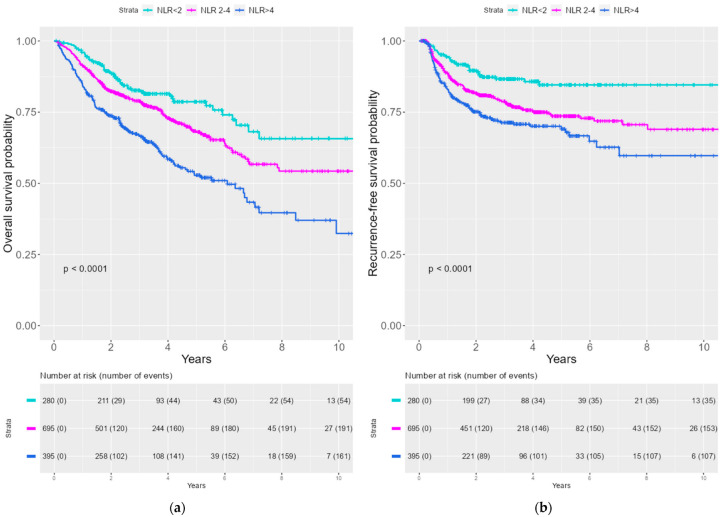
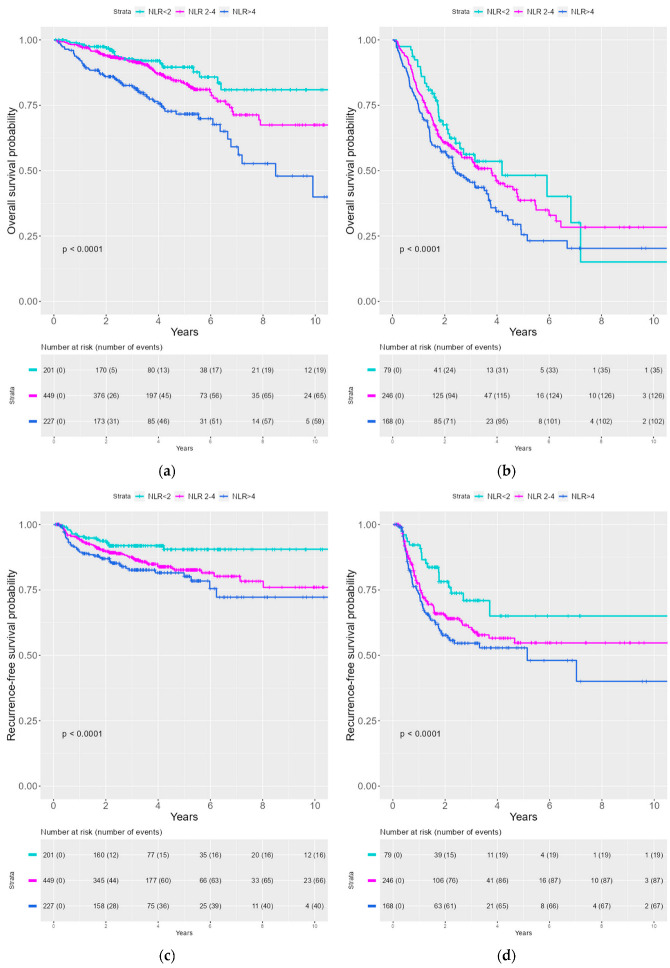
Kaplan–Meier curves were created to illustrate the survival probability for OS and RFS stratified by NLR. A significantly lower survival probability was seen with an increasing NLR for both OS and RFS (See Figure 1). We divided the patient cohort into HPV− and HPV+ patients. The same tendency for correlation between the increasing NLR group and inferior survival probability for OS and RFS was observed both the HPV− and the HPV+ cohort (See Figure 2).
Figure 1.
Kaplan–Meier curves of OS and RFS stratified by NLR tertile (a) Kaplan–Meier curves of OS by NLR tertile (b) Kaplan–Meier curve of RFS by NLR tertile.
Figure 2.
Kaplan–Meier curves of OS and RFS stratified for NLR in HPV+ and HPV− patients. (a) OS by NLR for HPV+ patients (b) OS by NLR for HPV− patients (c) RFS by NLR for HPV+ patients (d) RFS by NLR for HPV− patients.
In the multivariable analysis, we observed that high NLR influenced OS with HR = 1.5 (p = 0.02) in the highest tertile (NLR < 4) with the lowest tertile (NLR < 2) as a reference (See Table 2). When investigating the effect of NLR on RFS we found a HR of 1.6 (p = 0.02) for the middle tertile (NLR 2–4) and 1.8 (p = 0.003) for the highest tertile (NLR > 4) of NLR with the lowest tertile (NLR < 2) as a reference (See Table 3).
Table 2.
COX Proportional Hazards Regression Analysis, OS.
| Variable | Univariable | p-Value | Multivariable | p-Value |
|---|---|---|---|---|
| Age | 1.0 | <0.001 | 1.0 | <0.001 |
| Sex | ||||
| Female | ref | ref | ||
| Male | 1.2 | 0.09 | 1.3 | 0.02 |
| HPV status | ||||
| Negative | ref | ref | ||
| Positive | 0.2 | <0.001 | 0.4 | <0.001 |
| Smoking category | ||||
| Never a smoker | ref | ref | ||
| 0–10 pack years | 1.3 | 0.3 | 1.2 | 0.6 |
| 10–20 pack year | 1.6 | 0.06 | 0.9 | 0.8 |
| >20 pack years | 4.5 | <0.001 | 2.1 | <0.001 |
| Unknown | 3.0 | <0.001 | 1.7 | 0.02 |
| Performance status | ||||
| PS0 | ref | ref | ||
| PS1 | 4.1 | <0.001 | 2.0 | <0.001 |
| PS2 | 8.2 | <0.001 | 3.1 | <0.001 |
| PS3 | 43.8 | <0.001 | 12.3 | <0.001 |
| Unknown | 3.4 | <0.001 | 2.2 | <0.001 |
| Tumor location | ||||
| Pharyngeal wall or unspecified location | ref | ref | ||
| Palatine tonsils | 0.4 | <0.001 | 0.9 | 0.3 |
| Lingual tonsil/Base of tongue or vallecula epiglottica | 0.4 | <0.001 | 0.7 | 0.05 |
| Soft palate, Uvula, or palatal arches | 0.8 | 0.3 | 0.7 | 0.1 |
| T stage | ||||
| T1 | ref | ref | ||
| T2 | 1.7 | 0.001 | 1.6 | 0.007 |
| T3 | 3.5 | <0.001 | 2.3 | <0.001 |
| T4a or T4b | 4.8 | <0.001 | 2.4 | <0.001 |
| Unknown | 2.3 | 0.4 | 1.1 | 0.9 |
| N stage | ||||
| N0 | ref | ref | ||
| N1 | 0.6 | <0.001 | 1.1 | 0.7 |
| N2 (including N2a, N2b, N2c) | 2.2 | <0.001 | 2.2 | <0.001 |
| N3 (including N3a, N3b) | 2.0 | 0.001 | 2.7 | 0.001 |
| Unknown | 212.5 * | <0.001 | 5.4 | 0.2 |
| UICC 8th group stage | ||||
| Stage I | ref | |||
| Stage II | 2.2 | <0.001 | - | - |
| Stage III | 4.1 | <0.001 | - | - |
| Stage IV | 9.3 | <0.001 | - | - |
| Unknown | 11.3 | <0.001 | - | - |
| NLR tertile | ||||
| NLR < 2 | ref | ref | ||
| NLR 2–4 | 1.5 | 0.01 | 1.2 | 0.3 |
| NLR > 4 | 2.5 | <0.001 | 1.5 | 0.02 |
* Only one patient was included in this variable analysis and the HR could not be counted as reliable despite statistical significance.
Table 3.
COX Proportional Hazards Regression Analysis, RFS.
| Variable | Univariable | p-Value | Multivariable | p-Value |
|---|---|---|---|---|
| Age | 1.0 | 0.7 | 1.0 | 0.05 |
| Sex | ||||
| Female | ref | ref | ||
| Male | 1.3 | 0.03 | 1.4 | 0.3 |
| HPV status | ||||
| Negative | ref | ref | ||
| Positive | 0.3 | <0.001 | 0.4 | <0.001 |
| Smoking category | ||||
| Never a smoker | ref | ref | ||
| 0–10 pack years | 1.5 | 0.1 | 1.4 | 0.2 |
| 10–20 pack year | 1.7 | 0.06 | 1.1 | 0.8 |
| >20 pack years | 3.1 | <0.001 | 1.7 | 0.004 |
| Unknown | 1.6 | 0.1 | 1.3 | 0.4 |
| Performance status | ||||
| PS0 | ref | ref | ||
| PS1 | 2.5 | <0.001 | 1.5 | 0.01 |
| PS2 | 4.1 | <0.001 | 2.0 | 0.01 |
| PS3 | 2.6 | 0.99 | 0.0 | 0.99 |
| Unknown | 2.2 | <0.001 | 1.6 | 0.007 |
| Tumor location | ||||
| Pharyngeal wall or unspecified location | ref | ref | ||
| Palatine tonsils | 0.7 | 0.03 | 1.3 | 0.3 |
| Lingual tonsil/Base of tongue or vallecula epiglottica | 0.8 | 0.2 | 1.3 | 0.2 |
| Soft palate, Uvula, or palatal arches | 1.1 | 0.8 | 1.1 | 0.7 |
| T stage | ||||
| T1 | ref | ref | ||
| T2 | 1.7 | 0.002 | 1.8 | 0.003 |
| T3 | 2.9 | <0.001 | 2.4 | <0.001 |
| T4a or T4b | 3.7 | <0.001 | 2.3 | <0.001 |
| Unknown | 3.6 | 0.2 | 6.9 | 0.1 |
| N stage | ||||
| N0 | ref | ref | ||
| N1 | 0.9 | 0.3 | 1.3 | 0.2 |
| N2 (including N2a, N2b, N2c) | 2.7 | <0.001 | 2.1 | 0.001 |
| N3 (including N3a, N3b) | 2.4 | 0.001 | 2.3 | 0.008 |
| Unknown | NA | NA | NA | NA |
| UICC 8th group stage | ||||
| Stage I | ref | |||
| Stage II | 1.6 | 0.01 | - | - |
| Stage III | 2.6 | <0.001 | - | - |
| Stage IV | 6.1 | <0.001 | - | - |
| Unknown | 4.0 | 0.02 | - | - |
| NLR tertile | ||||
| NLR < 2 | ref | ref | ||
| NLR 2–4 | 1.9 | 0.001 | 1.6 | 0.02 |
| NLR > 4 | 2.6 | <0.001 | 1.8 | 0.003 |
4. Discussion
This population-based cohort study, including 1370 patients with OPSCC from the world’s largest consecutive database of HPV-tested OPSCCs, aimed to investigate the prognostic impact of NLR for OS and RFS. In our multivariable analysis for OS, we found a significant HR = 1.5 (p = 0.02) for NLR > 4. When investigating the effect on RFS, we found a HR = 1.6 (p = 0.02) for NLR 2–4, and HR = 1.8 (p = 0.003) for NLR > 4. The Kaplan–Meier survival probability curves illustrated a statistically significant (p < 0.0001) difference between NLR groups and OS and RFS with inferior survival rates in the increasing NLR group. This difference was observed both in general and when stratified by HPV status.
Several previous studies on smaller or selected cohorts have been published on NLR as a prognostic marker for the outcome of OPSCC, as well as HNSCC located at other anatomical subsites. In the largest study, Ferrandino et al. included 5840 patients with HNSCC; of these, 1952 patients had OPSCC [18]. In their multivariable subgroup analysis of OS in OPSCC they found HR = 1.25 in the middle tertile (2.1–3.4) and HR = 1.95 for the top tertile (>3.4) of NLR. For CSS they found a HR = 1.48 in the top tertile of NLR. This study was based on US Veteran Affairs patients, 99% of who were male, and did not include HPV status. Rachidi et al. included 543 patients with HNSCC of whom 89 patients had HPV-status available [11]. In their multivariable analysis for OS, they found a HR = 1.13 for the middle tertile (2.36–4.39) and HR = 2.39 in the highest tertile (>4.39) of NLR. Ng et al. included 848 patients of whom 674 had HPV status available [20]. They found a HR = 1.64 in the multivariable analysis for OS in patients with NLR > 3 compared to NLR < 3. Other smaller studies found NLR to be prognostic for OS, disease-free survival, as well as local and regional tumor control [19,21,28].
In the present study we enrolled 1370 patients, all with available HPV status. This non-selected cohort included patients from a database comprising all patients diagnosed with OPSCC in Eastern Denmark from 2000 to 2020. It is not clear if patients in earlier studies were treated according to a defined treatment guideline, although all were treated with curative intent [11,18,19,20,21,28]. Our cohort solely comprised patients diagnosed and treated according to the Danish national guidelines created by DAHANCA, with treatment being either by radiotherapy, chemoradiotherapy, surgery, or surgery with adjuvant therapy [17].
Previous attempts to determine a normal level of NLR in healthy subjects have yielded reference intervals of 0.78–3.53 and 0.88–4.0 [29,30]. The large inter-individual variation in NLR complicates the decision related to an optimal cutoff value and has resulted in many different cutoff values being used in previous studies investigating NLR and OPSCC [11,18,19,20,21,28,31]. Due to dissimilarity in cutoff values, comparisons of results from different studies should be made with caution. With this reservation in mind, we deemed it acceptable to compare our findings with those of previous studies as the cutoffs used were very near the cutoffs chosen in our study.
Infection can naturally affect the NLR, and it is a possible limitation of our study that we did not adjust for concurrent infection at time of diagnosis [13]. It was not possible to adjust for infection in our analysis, as our OPSCC database does not contain this information. We have no reason to suspect that there would be an uneven distribution of infections at time of diagnosis, skewing towards patients with a certain outcome. In consequence, infections are not expected to have impacted the association between NLR and outcomes OS and RFS. An obvious limitation of the study is that NLR is not a specific biomarker for cancer and can be influenced by comorbidities [18]. Additionally, it has been proposed that an elevated NLR may be caused by an advanced malignant disease concerning tumor mass, nodal stage, and number of metastases [10]. Both comorbidity and advanced disease can naturally decrease OS. The effect of these factors on RFS is more speculative, but one possible explanation could be that clinicians choose a less aggressive, though still curatively intended, treatment plan for patients with comorbidity or advanced disease. A predictable consideration that could be taken about this study is whether there was an actual correlation between NLR and OS and RFS or if the observed correlation was due to comorbidity, advanced tumor stage and HPV status. We excluded patients with distant metastasis at diagnosis as well as patients who did not receive curative treatment. Additionally, we adjusted for interacting covariates in our multivariable analysis, performance status being the most indicative of patient comorbidity. These measures significantly reduced the possibility of confounding by comorbidity and advanced disease on the correlation found between NLR and RFS or OS.
The tumor microenvironment (TME) plays an important role in the progression of OPSCC [32]. In several solid cancers, thoroughly investigated in melanoma and colorectal cancer, a high density of tumor-infiltrating lymphocytes (TILs) correlates with a better prognosis [33]. This relationship has also been investigated in OPSCC, with a higher infiltration of the tumor with CD4+ and CD8+ T cells associated with a better OS, DSS as well as a lower T stage [32]. This clinical advantage was seen regardless of HPV status, although there was a stronger infiltration in HPV+ tumors. A study by Nordfors et al. found a positive correlation between CD8+ TIL counts and improved OS and DFS in HPV+ OPSCC as well as insignificant trends for effect on OS in HPV− OPSCC. No correlation was found between the infiltration of CD4+ and OS in this study [34]. TIL counts have been suggested as a possible routine prognostic tool in OPSCC [35]. The role of TILs in OPSCC could be, at least in part, an explanation for the improved OS and RFS in patients with lower NLR, although further investigation is needed to expand on whether there is a link between lymphocyte counts, and by extension NLR, and lymphocyte infiltration in tumor tissue.
Current treatment of OPSCC in all treatment modalities is associated with significant morbidity. It is of interest to determine novel strategies for risk stratification and prognostication of OPSCCs for the purpose of treatment de-escalation in patients, where current treatment strategies are unnecessarily aggressive and result in morbidity that could possibly be avoided. Perhaps NLR in combination with HPV/p16 status and TIL density could be included in a such prognostication strategy. Further trials are warranted, especially with focus on confounding comorbidity, for NLR to be included in prognostication for treatment de-escalation.
In conclusion, our Kaplan–Meier survival probability estimators showed reduced OS and RFS in higher groups of NLR. This trend was evident for HPV+ and HPV− separately when stratifying for HPV status. We found inferior RFS with the increasing NLR group, as well as inferior OS in group NLR > 4. Our findings confirmed that a high NLR is a prognostic predictor for inferior OS and RFS, and we found that the significant effect of NLR on OS and RFS was independent of tumor HPV status in the largest non-selected OPSCC cohort including HPV status to date.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v15010198/s1, Figure S1: Flowchart of patient exclusion from the database population to the final included study population; Figure S2: Proportional Hazards test with Schoenfeld residuals for categorical covariates; Figure S3: Non-linearity test with Martingale Residual of continuous covariate patient age.
Author Contributions
Conceptualization, M.M.J., K.K.J., M.G.-Z., C.G. and C.v.B.; Formal analysis, M.M.J.; Funding acquisition, M.M.J., K.K.J. and C.v.B.; Investigation, M.M.J., S.K.B., M.G.-Z., C.M. and A.-L.F.C.; Methodology, M.M.J. and K.K.J.; Writing—original draft, M.M.J.; Writing—review and editing, M.M.J., K.K.J., S.K.B., M.G.-Z., A.-L.F.C., A.B.G. and C.v.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the REGIONAL ETHICS COMMITTEE OF THE CAPITAL REGION OF DENMARK (protocol code H-20072877, date of approval: 26 November 2022 and 22 April 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data generated at our central large-scale facility available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by KRÆFTENS BEKÆMPELSE, grant number R351-A20200.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jensen J.S., Jensen D.H., Grønhøj C., Karnov K.K.S., Nørregaard C., Agander T.K., Specht L., von Buchwald C. Incidence and survival of oropharyngeal cancer in Denmark: A nation-wide, population-based study from 1980 to 2014. Acta Oncol. 2018;57:269–275. doi: 10.1080/0284186X.2017.1390251. [DOI] [PubMed] [Google Scholar]
- 2.Carlander A.F., Jakobsen K.K., Bendtsen S.K., Garset-Zamani M., Lynggaard C.D., Jensen J.S., Grønhøj C., von Buchwald C. A Contemporary Systematic Review on Repartition of HPV-Positivity in Oropharyngeal Cancer Worldwide. Viruses. 2021;13:1326. doi: 10.3390/v13071326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Lambert R., Sauvaget C., de Camargo Cancela M., Sankaranarayanan R. Epidemiology of cancer from the oral cavity and oropharynx. Eur. J. Gastroenterol. Hepatol. 2011;23:633–641. doi: 10.1097/MEG.0b013e3283484795. [DOI] [PubMed] [Google Scholar]
- 5.Pytynia K.B., Dahlstrom K.R., Sturgis E.M. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50:380–386. doi: 10.1016/j.oraloncology.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlstrom K.R., Calzada G., Hanby J.D., Garden A.S., Glisson B.S., Li G., Roberts D.B., Weber R.S., Sturgis E.M. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: A Staging System in Need of Repair. Cancer. 2013;119:81–89. doi: 10.1002/cncr.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haeggblom L., Ramqvist T., Tommasino M., Dalianis T., Näsman A. Time to change perspectives on HPV in oropharyngeal cancer. A systematic review of HPV prevalence per oropharyngeal sub-site the last 3 years. Papillomavirus Res. 2017;4:1–11. doi: 10.1016/j.pvr.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen C.G., Jensen D.H., Carlander A.-L.F., Kiss K., Andersen L., Olsen C.H., Andersen E., Garnæs E., Cilius F., Specht L., et al. Novel nomograms for survival and progression in HPV+ and HPV− oropharyngeal cancer: A population-based study of 1542 consecutive patients. Oncotarget. 2016;7:71761–71772. doi: 10.18632/oncotarget.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lechner M., Liu J., Masterson L., Fenton T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022;19:306–327. doi: 10.1038/s41571-022-00603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guthrie G.J.K., Charles K.A., Roxburgh C.S.D., Horgan P.G., McMillan D.C., Clarke S.J. The systemic inflammation-based neutrophil–lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Rachidi S., Wallace K., Wrangle J.M., Day T.A., Alberg A.J., Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. 2016;38:E1068–E1074. doi: 10.1002/hed.24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Templeton A.J., Mcnamara M.G., Šeruga B., Vera-Badillo F.E., Aneja P., Ocaña A., Leibowitz-Amit R., Sonpavde G., Knox J.J., Tran B., et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 13.Song M., Graubard B.I., Rabkin C.S., Engels E.A. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci. Rep. 2021;11:464. doi: 10.1038/s41598-020-79431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamani M., Grønhøj C., Jensen D.H., Carlander A.F., Agander T., Kiss K., Olsen C., Baandrup L., Nielsen F.C., Andersen E., et al. The current epidemic of HPV-associated oropharyngeal cancer: An 18-year Danish population-based study with 2169 patients. Eur. J. Cancer. 2020;134:52–59. doi: 10.1016/j.ejca.2020.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L., McLeod L., Delacqua G., Delacqua F., Kirby J., et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DAHANCA The Danish Head and Neck Cancer Study Group (DAHANCA) [(accessed on 15 November 2022)]. Available online: https://www.dahanca.dk/CA_Adm_Web_Page?WebPageMenu=1&CA_Web_TabNummer=0.
- 18.Ferrandino R.M., Roof S., Garneau J., Haidar Y., Bates S.E., Park Y.H.A., Bauml J.M., Genden E.M., Miles B., Sigel K. Neutrophil-to-lymphocyte ratio as a prognostic indicator for overall and cancer-specific survival in squamous cell carcinoma of the head and neck. Head Neck. 2020;42:2830–2840. doi: 10.1002/hed.26329. [DOI] [PubMed] [Google Scholar]
- 19.Fanetti G., Alterio D., Marvaso G., Gandini S., Rojas D.P., Gobitti C., Minatel E., Revelant A., Caroli A., Francia C.M., et al. Prognostic significance of neutrophil-to-lymphocyte ratio in HPV status era for oropharyngeal cancer. Oral Dis. 2020;26:1384–1392. doi: 10.1111/odi.13366. [DOI] [PubMed] [Google Scholar]
- 20.Ng S.P., Bahig H., Jethanandani A., Sturgis E.M., Johnson F.M., Elgohari B., Gunn G.B., Ferrarotto R., Phan J., Rosenthal D.I., et al. Prognostic significance of pre-treatment neutrophil-to-lymphocyte ratio (NLR) in patients with oropharyngeal cancer treated with radiotherapy. Br. J. Cancer. 2021;124:628–633. doi: 10.1038/s41416-020-01106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.So Y.K., Lee G., Oh D., Byeon S., Park W., Chung M.K. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Patients with Human Papillomavirus–Positive Oropharyngeal Cancer. Otolaryngol. Neck Surg. 2018;159:303–309. doi: 10.1177/0194599818764651. [DOI] [PubMed] [Google Scholar]
- 22.Panwar A., Interval E., Lydiatt W.M. Emergence of a Novel Staging System for Oropharyngeal Squamous Cell Carcinoma Based on HPV Status. Oncology. 2017;31:e33–e40. [PubMed] [Google Scholar]
- 23.Würdemann N., Wagner S., Sharma S.J., Prigge E.-S., Reuschenbach M., Gattenlöhner S., Klussmann J.P., Wittekindt C. Prognostic Impact of AJCC/UICC 8th Edition New Staging Rules in Oropharyngeal Squamous Cell Carcinoma. Front. Oncol. 2017;7:129. doi: 10.3389/fonc.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2021. [Google Scholar]
- 25.Yoshida K. Tableone: Create “Table 1” to Describe Baseline Characteristics. 2020. [(accessed on 1 October 2022)]. R Package Version 0.11.1. CRAN R Proj. Available online: https://CRAN.R-project.org/package=tableone.
- 26.Kassambara A., Kosinski M., Biecek P. Survminer: Drawing Survival Curves Using “Ggplot2”. 2020. [(accessed on 1 October 2022)]. R Package Version 0.4.8. Available online: https://CRAN.R-project.org/package=survminer.
- 27.Therneau T.M. Survival: A Package for Survival Analysis in R. 2021. [(accessed on 1 October 2022)]. R Packag. Version 2.38. Available online: https://CRAN.R-project.org/package=survival.
- 28.Young C.A., Murray L.J., Karakaya E., Thygesen H.H., Sen M., Prestwich R.J.D. The Prognostic Role of the Neutrophil-to-Lymphocyte Ratio in Oropharyngeal Carcinoma Treated with Chemoradiotherapy. Clin. Med. Insights Oncol. 2014;8:81–86. doi: 10.4137/CMO.S15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forget P., Khalifa C., Defour J.-P., Latinne D., Van Pel M.-C., De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res. Notes. 2017;10:12. doi: 10.1186/s13104-016-2335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo H., He L., Zhang G., Yu J., Chen Y., Yin H., Goyal H., Zhang G.-M., Xiao Y., Gu C., et al. Normal Reference Intervals of Neutrophil-To-Lymphocyte Ratio, Platelet-To-Lymphocyte Ratio, Lymphocyte-To-Monocyte Ratio, and Systemic Immune Inflammation Index in Healthy Adults: A Large Multi-Center Study from Western China. Clin. Lab. 2019;65:255–265. doi: 10.7754/Clin.Lab.2018.180715. [DOI] [PubMed] [Google Scholar]
- 31.Mascarella M.A., Mannard E., Silva S.D., Zeitouni A. Neutrophil-to-lymphocyte ratio in head and neck cancer prognosis: A systematic review and meta-analysis. Head Neck. 2018;40:1091–1100. doi: 10.1002/hed.25075. [DOI] [PubMed] [Google Scholar]
- 32.Welters M.J.P., Santegoets S.J., Van Der Burg S.H. The Tumor Microenvironment and Immunotherapy of Oropharyngeal Squamous Cell Carcinoma. Front. Oncol. 2020;10:545385. doi: 10.3389/fonc.2020.545385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giraldo N.A., Sanchez-Salas R., Peske J.D., Vano Y., Becht E., Petitprez F., Validire P., Ingels A., Cathelineau X., Fridman W.H., et al. The clinical role of the TME in solid cancer. Br. J. Cancer. 2019;120:45–53. doi: 10.1038/s41416-018-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nordfors C., Grün N., Tertipis N., Ährlund-Richter A., Haeggblom L., Sivars L., Du J., Nyberg T., Marklund L., Munck-Wikland E., et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur. J. Cancer. 2013;49:2522–2530. doi: 10.1016/j.ejca.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Almangush A., Jouhi L., Atula T., Haglund C., Mäkitie A.A., Hagström J., Leivo I. Tumour-infiltrating lymphocytes in oropharyngeal cancer: A validation study according to the criteria of the International Immuno-Oncology Biomarker Working Group. Br. J. Cancer. 2022;126:1589–1594. doi: 10.1038/s41416-022-01708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated at our central large-scale facility available upon reasonable request.