Abstract
Chemokines, a subfamily of the cell cytokines, are low molecular weight proteins known to induce chemotaxis in leukocytes in response to inflammatory and pathogenic signals. A plethora of literature demonstrates that chemokines and their receptors regulate tumor progression and metastasis. With these diverse functionalities, chemokines act as a fundamental link between the tumor cells and their microenvironment. Recent studies demonstrate that the biology of chemokines and their receptor in metastasis is complex as numerous chemokines are involved in regulating site-specific tumor growth and metastasis. Successful treatment of disseminated cancer is a significant challenge. The most crucial problem for treating metastatic cancer is developing therapy regimes capable of overcoming heterogeneity problems within primary tumors and among metastases and within metastases (intralesional). This heterogeneity of malignant tumor cells can be related to metastatic potential, response to chemotherapy or specific immunotherapy, and many other factors. In this review, we have emphasized the role of chemokines in the process of metastasis and metastatic heterogeneity. Individual chemokines may not express the full potential to address metastatic heterogeneity, but chemokine networks need exploration. Understanding the interplay between chemokine-chemokine receptor networks between the tumor cells and their microenvironment is a novel approach to overcome the problem of metastatic heterogeneity. Recent advances in the understanding of chemokine networks pave the way for developing a potential targeted therapeutic strategy to treat metastatic cancer.
Keywords: Chemokines/receptors, Heterogeneity, Angiogenesis, Epithelial-mesenchymal plasticity, Cancer stem cells, Therapy resistance
1. Introduction
Metastasis, defined as secondary cancer that spreads from its site of origin to another part of the body, is the primary cause of cancer-related deaths. Although the successful eradication of primary tumors is possible with surgery and the continuous improvements in adjuvant chemotherapy and radiotherapy, treating secondary cancer or metastases still presents itself as a significant challenge.
Improvements in cancer therapy require a rational understanding of every step of metastasis. One of the essential aspects of treating metastasis is to answer whether metastases result from random survivors of tumor cells or a representative of a selective subpopulation of tumor cells existing within the primary tumor population [1]. Only with the condition that metastasis is a selective process, the metastasized cells represent a group of tumor cells with specialized properties. The uniquely acquired properties by tumor cells during metastasis allow us to design therapies directed against it. The primary tumors and metastases are heterogeneous regarding response to different therapy regimes such as chemotherapy, specific immunotherapy, or radiotherapy. Hence, to develop novel anticancer agents, the response of both the primary tumor and secondary tumors should determine the efficacy of the anticancer agent.
In light of the recent metastasis studies, both tumor cell properties and host-tumor cell interactions can influence the metastatic process. Chemokines are among several factors that facilitate the interplay between tumor cells, the host cells in their proximity, and at metastatic sites [2–7]. Interactions between chemokine receptors and their respective chemokines can regulate different processes affecting the metastatic cascade, such as invasion and migration of malignant cells to distinct organs, proliferation, survival, and angiogenesis, and control of leukocyte infiltration [2, 7–14]. An elaborative understanding of chemokines-chemokine receptor biology and the mechanisms of their actions in the metastatic processes will open additional avenues for therapeutic interventions. This review highlights the role of chemokines and their receptors on distant metastasis and metastatic heterogeneity.
2. Metastasis and metastatic heterogeneity
Metastasis is a process in which tumor cells disseminate from their primary site to distant organs and establish themselves as secondary tumors or metastases in that distant organ. The metastatic process is a cascade of rate-limiting interrelated steps [15]. The development of metastatic tumor foci is the most feared and catastrophic aspect of cancer. Metastasis accounts for most cancer deaths despite advances in primary tumors’ surgical resection and vigorous adjuvant therapies. There are many reasons for the failure in the treatment of metastases. Like, even before the diagnosis of primary cancer, metastases may already be present in the patient’s organs. In such cases, surgical resection, radiotherapy, or chemotherapy treatment is highly unlikely due to difficulties in treating metastases because of their location and undue toxic effects of the therapeutic agent in the metastatic site. Tumor heterogeneity and therapy-resistant variants within the primary tumor and metastases are essential factors responsible for tumor therapy’s refractory response.
Furthermore, interactions between tumor subpopulations and surrounding normal cells, such as metabolic cooperation, alter the sensitivity of whole tumor and metastases [16–19], compounding the problem of effective therapy for heterogeneous primary tumors and metastatic lesions. A single anticancer drug or treatment alone offers less probability of killing a malignant tumor and its metastases. Taken together, the successful treatment of cancer patients requires the development of new approaches capable of overcoming the problem of the heterogeneous response of a primary tumor and metastases to drug treatment [20]. In the next section, we will elaborate on the factors affecting metastatic heterogeneity.
2.1. Metastatic heterogeneity
The primary tumor consists of different subpopulations of tumor cells that can differ in expression of cell surface receptors [21], such as receptors for lectins [22], hormone, synthesis of cell products [23], specialized biosynthetic enzymes [24], and metabolic characteristics [25]. These subpopulations also differ with regard to their in vitro and in vivo growth rate, based on DNA content, karyotype, and marker chromosomes [1]. Tsuruo and colleagues, in their extensive study [26], reported that this heterogeneity extends in regard to drug sensitivity among cells populating parent tumors (in vitro clones) and their metastatic subpopulations. As discussed earlier, the primary and secondary tumors’ heterogeneous nature regarding cytotoxic drug sensitivity has profound implications on metastases’ treatment.
2.2. Clonal cooperation
In 1939, Koch [27] isolated a metastatic subline from the Ehrlich carcinoma tumor cells, suggesting that tumors may consist of cells with differing metastatic capabilities. However, in 1977, Fidler and Kripke [28] used B16 melanoma cells to demonstrate metastatic heterogeneity within a primary tumor. Their experimental results suggested that metastatic heterogeneity is not entirely dependent on the longevity of neoplasms. Later, many studies reported that the invasive and metastatic properties of clones from the B16 melanoma tumor are highly unstable during serial passage both in vitro and in vivo [29–31]. Later, Fidler’s group demonstrated that mixing and cocultivation of B16 clones dramatically reduce this metastatic instability [32]. Overall, the experimental results suggested that there is some form of “interaction” between tumor subpopulations that stabilize the subpopulations’ invasive-metastatic properties and maintain their relative proportions within the tumor, preventing dominance by a few or one subpopulation. However, this “stabi1izing interaction” between subpopulations is specific for cells from the same tumor [32]. The heterogeneity in metastatic properties and metastatic instability of clones were confirmed in diverse tumors [1].
The “stabi1izing interaction” or clonal cooperation between subpopulations can also define tumors as ecosystems of interactive subpopulations [33–37]. In 1983, Miller reported that the coinjection of nonmetastatic cells with metastatic cells could increase the former’s metastasis [38]. Similar cooperative heterotypic interactions were reported among EMT and non-EMT cells in prostate cancer metastasis [39] and studies proposing a leading invasive cell followed by “opportunistic” cells [40, 41].
2.3. Clonal/polyclonal origin of metastases
Metastases are the selected growth of specialized malignant cells that pre-exist as subpopulations within the parent tumor and are not a result of random survival of cells. However, this does not answer many fundamental questions, such as whether embolus released from the primary tumor originated from a single cell or a cellular aggregate comprising of tumor cells and host cells? Whether such cellular aggregate are homotypic or heterotypic? Do metastases originate from a single progenitor or multiple progenitor tumor cells? Fidler and Hart reported that metastases result from the proliferation of a single viable cell or a single cell within a homogenous/heterogeneous aggregate. Their study further demonstrated that the circulating embolus of tumor cells is likely to be homogeneous because of a clonal zone of a primary neoplasm [42]. Also, collective migration of tumor cells into the lymphatics or vasculature plays a significant role [40, 41, 43]. Circulating cellular aggregates, whether homotypic or heterotypic, are arrested more frequently in the encountered capillary beds and demonstrate a better survival rate [44]. Circulating cell emboli consisting of tumor cells, leukocytes, and platelets offer protection against host effector cells, turbulence within the circulation. They enable to complete the metastatic cascade to the tumor progenitor cells.
In 1982, using a metastatic variant of the K-1735 melanoma cells, Talmadge et al. demonstrated that different metastases could originate from different progenitor cells. Still, most metastases appear to be clonal in origin [45]. The multiple progenitors could explain the existence of biological heterogeneity among various metastases [42]. Although not definitive, literature dominates with studies suggesting the clonal nature of metastases using different approaches to address this question. [1]. However, in 1981, Poste et al. demonstrated that metastasis cells demonstrate a high spontaneously mutation rate [32] compared to non-metastatic tumorigenic cells; thus, clonal metastases may rapidly become heterogeneous. Recent studies utilizing next-generation sequencing analysis of primary and metastatic lesions show considerable diverse results in the mutational profile sustained in metastasis, showing both high and low complexity of mutational profile. Yet, analysis of genetic mutations through large-scale genomic sequencing efforts cannot explain the basic of metastatic growths [46, 47]. In the current view, the accumulation of somatic mutation in metastasis does not drive the development of metastasis beyond the driver mutations selected for primary tumor formation [43].
Recent studies examining circulating tumor cells (CTCs) report heterogeneous cell population [48–50], suggesting polyclonal seeding of metastases. In addition, CTC clusters have a higher metastatic potential comparison with single CTCs [51]. Moreover, recent studies using lineage tracing using fluorescence markers, barcode sequencing, and whole-genome sequencing have demonstrated a mostly polyclonal nature of metastasis [52–54]. In summary, CTCs are heterogeneous with the interaction between subclones, resulting in metastatic outgrowth (polyclonal or monoclonal). However, polyclonal metastasis suggests that different heterotypic interactions among clonal subpopulations initiate metastasis.
3. Chemokines and their receptors
The word chemokine originates from the Greek word “kinos,” meaning movement. As their name suggests, they can induce directed movement in the responsive cells. Chemokines, a family of low molecular weight cytokines, were discovered in the late 1980s and early 1990s based on leukocyte chemoattractant activity upon stimulation with proinflammatory agents [55]. Yoshimura discovered chemokine CCL2, one of the initially characterized chemokines, potent in the accumulation and activation of monocytes/macrophages during inflammation and cancer, which follows the identification of CXCL8 chemokine endowed with a potent chemotactic activity for neutrophils towards acute inflammatory responses.
Generally, chemokines are 8–15 kDa in size and structurally classified into four subfamilies (CXC, CC, C, and CX3C) [56, 57]. This structural classification’s foundation is the number and location of four conserved cysteine amino acid residues linked by disulfide bonds at the N-terminus of the chemokine ligands. Biologically, chemokines function by binding to G protein–coupled receptors (GPCR) [56, 58, 59], with their N-terminus outside the cell and C-terminus with serine and threonine phosphorylation sites in the cytoplasm. GPCRs have seven-transmembrane structural loops coupled to G protein for signal transduction. Upon specific ligand binding, chemokine receptors trigger a flux in intracellular calcium ions (calcium signaling), leading to chemotaxis and the onset of cell trafficking to the desired location. Each chemokine receptor binds to one of the four chemokine subfamilies. Thus, there is a similar classification of four subfamilies of the chemokine receptors as of their respective ligands. The classical family of chemokine receptors currently has four members [60]. Apart from the above-described conventional chemokine receptors, there are atypical chemokine receptors (ACKRs), the new and emerging class of regulators of the chemokine system. Although structurally related to conventional chemokine receptors, ACKRs fail to trigger classical chemokine receptor signaling upon chemokine binding. They can also regulate the activity of canonical chemokine receptors by sharing the ligands and forming heterodimers. ACKRs can also control the bioavailability of chemokines by scavenging, transportation, or storage. ACKRs have an anti-inflammatory role and regulate growth, survival, and metastatic processes in tumor cells [61, 62].
Functionally, chemokines and their receptors can be homeostatic and inflammatory. However, some chemokines and their receptors have both homeostatic and inflammatory functionalities. One of the remarkable features of chemokines or GPCRs is their overlapping activities or one chemokine’s ability to bind and activate more than one GPCR. Similarly, one GPCR may recognize more than one chemokine. This feature of “promiscuity of chemokine and their receptors” [63] endows them with an ability to compensate for another ligand during complex responses. Thus, chemokines and their GPCRs are redundant in activity, and the regulation of chemokine activities is complex. However, recent studies indicate that each chemokine or receptor has unique functionality under different physiological conditions [55]. The expression of chemokine receptors is not limited to leukocytes, but many non-leukocytic cell types express them. Similarly, chemokines/chemokine receptors can trigger diverse cellular migratory responses such as directed and undirected motility, such as haptokinesis, haptotaxis, and chemokinesis, including inducing cell adhesion and cell arrest [64].
Another essential feature of chemokines biology is their ability to undergo post-translational modification by interaction with the extracellular matrix (ECM) or tethering to “ACKR” [64]. Before we discuss chemokine’ functional role in cancer biology in detail, let us discuss some salient features of different chemokine families. The CXC subfamily or α-chemokine comprises members containing one non-conserved amino acid (denoted as X) between the first and second cysteine residues. Some CXC family chemokines have Glu-Leu-Arg (ELR) motif located at the N-terminus before the first cysteine amino acid residue [65]. This ELR motif is associated with whether the chemokine is angiogenic or angiostatic [66, 67] in nature. Thus, this family is further subdivided into two groups based on the presence or absence of an ELR motif [57, 58, 68]. The ELR+ chemokines are potent promoters of angiogenesis, display chemotaxis for endothelial cells, and recruit neutrophils, known for their synthesis and storage of angiogenic molecules [67, 69–72]. However, ELR− members potent inhibitors of angiogenesis [66, 72] and are known to recruit T and B cells. The CXC chemokines bind to the CXC receptor family comprising of six members.
The CC chemokine subfamily or β-chemokines comprise members with adjacent cysteine residues. The CC subfamily represents the largest sub-family of chemokines. Their family members display a diverse range of target cell specificities such as T cells, B cells, basophils, eosinophils, dendritic cells, mast cells, natural killer cells, monocytes, and macrophages [73–81]. The CC chemokines bind to the CC receptor family comprising of ten members. The majority of discovered chemokines and their respective receptors belong to the CC and CXC chemokine subfamilies.
The third group of chemokine family is the C chemokines or γ chemokines comprising of only two members with one cysteine residues on the N-terminus. These chemokines were initially described as lymphocyte-specific with XCL1 (lymphotactin-α) [82] and XCL2 (lymphotactin-β) as members. Their only chemokine receptor XCR1 was recently expressed on subsets of dendritic cells with the function of antigen cross-presentation [83].
The CX3C chemokine (δ-chemokines) subfamily contains a member CX3CL1 (Fractalkine) with three non-conserved amino acids between the first two cysteines [84]. CX3CL1 is a membrane-bound chemokine [85] shown to induce both the migration and the adhesion of leukocytes [64, 86, 87]. Table 1 summarizes the list of chemokine receptors along with their interacting ligands in humans and mice. The table also contains their expression summary in different tumor types, stromal, and immune cells.
Table 1.
List of chemokine receptors and their interacting ligands in humans and mice, expression in tumor type, and different stromal/immune cells
| S.no. | Chemokine receptor | Alternate name | Interacting ligand in humans | Interacting ligand in mice | Type of cancer cell | Type of stomal/immune cell |
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | CXCR1 | IL-8RA, CD181 | CXCL1, CXCL6, CXCL7, CXCL8 | CXCL1, CXCL7 | Breast [88], prostrate [89], lung [89], colorectal [89], melanoma [90] | Neutrophils [91], MDSCs [92], endothelial cells [93] |
| 2 | CXCR2 | IL-8RB | CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, CXCL8 | CXCL1, CXCL2, CXCL3, CXCL5, CXCL7 | Breast [94], prostrate [95], lung [89], colorectal [89], melanoma [90], pancreatic [96], renal [97] | Neutrophils [91], MDSCs [98], platelets, [99], endothelial cells [93], pancreatic fibroblasts [100] |
| 3 | CXCR3 | GPR9, CD183 | CXCL4, CXCL9, CXCL10, CXCL11, CXCL13 [101] | CXCL4, CXCL9, CXCL10, CXCL11 | Breast [102], colorectal [103], melanoma [104], leukemia [105], renal [97] | T cells [106], NKT cells [6], platelets [107] |
| 4 | CXCR4 | CD184 | CXCL12 | CXCL12 | Breast [108], prostrate [109], gastric [110], ovarian, [111], esophageal [112] | TAMs [113], endothelial [114], precursors of endothelial cells 12414810 neutrophils [115], MDSCs [116], platelets [117], |
| 5 | CXCR5 | BLR1, CD185 | CXCL13 | CXCL13 | Lymphomas [118], pancreatic [119], colon [119], head and neck [6] | B cells [120] T cells [121] |
| 6 | CXCR6 | BONZO, CD186 | CXCL16 | CXCL16 | Breast [122], prostrate [123], hepatocarcinoma [124] | Natural killer [125], natural killer T cells [124] |
| 7 | CXCR7 | GPR159, ACKR3 | CXCL11 [126–128], CXCL12 | CXCL11, CXCL12 | Breast 29257351 [127], prostrate 30952632 [129] | Endothelial 29257351 [127] |
| 8 | ? | CXCL14 | CXCL14 | Dendritic cells 28928016 [130] | ||
| 9 | ? | CXCL15 | ||||
| 10 | ? | CXCL17 | CXCL17 | |||
| 11 | CCR1 | CD191 | CCL3, CCL4,CC-L5,CCL7,-CCL8, CCL13,CC-L14,CC-L15,CC-L16,CCL23 | CCL3, CCL4,CC-L5,CCL6,-CCL7,CC-L9 | (Breast, prostrate, lung, colorectal, melanoma, pancreatic, renal, cervical, hepatocellular, multiple myeloma, T cell leukemia, osteosarcoma) [131] | Neutrophils [132], platelets [133] |
| 12 | CCR2 | CD192 | CCL2,CC-L7,CCL8,-CCL13,CC-L16 | CCL2,CC-L7,CCL12 | (Breast, glioma, lung, prostrate, melanoma, multiple myeloma) [131] | TAMs, [134], MDSCs, [135], monocytes [136], platelets [133] |
| 13 | CCR3 | CD193 | (Breast, cervical, renal) [131] | Platelets [133] | ||
| 14 | CCR4 | CD194, CNOT6 | CCL3, CCL5, CCL17, CCL22 | CCL3, CCL5, CCL17, CCL22 | (T cell leukemia, Hodgkin lymphoma, breast, melanoma, hepatocellular) [131] | T cells [137], TAMs [138], platelets [133] |
| 15 | CCR5 | CD195 | CCL2, CCL3, CCL4, CCL5, CCL8, CCL11, CCL13, CCL14, CCl16 | CCL2, CCL3, CCL4, CCL5 | Breast, cervical, lung, multiple myeloma, osteosarcoma, pancreatic, prostrate [139] | TAMs [140] |
| 16 | CCR6 | CD196 | CCL20 | CCL20 | (Colorectal, breast, hepatocellular, thyroid, ovarian, cutaneous T cell, laringeal) [141] | Thl7 [142], dendritic [143] |
| 17 | CCR7 | CD197 | CCL19, CCL21 | CCL19, CCL21 | (Breast, gastric, colorectal, lung, esophageal, leukemia) [144] | Th22, Treg, T cells, [145], dendritic [146], B cells [147] |
| 18 | CCR8 | CD198 | CCL1, CCL4, CCL16, CCL17, CCL18 | CCL1, CCL8 | Colon [148] | Treg [148] |
| 19 | CCR9 | CD199 | CCL25 | CCL25 | Melanoma [149], prostrate [150] | |
| 20 | CCR 10 | GPR2 | CCL27, CCL28 | CCL27, CCL28 | Melanoma [151] | |
| 21 | XCR1 | GPR5 | XCL1, XCL2 | XCL1 | Dendritic cells 28190711 [152] | |
| 22 | CX3CR1 | GPR13 | CX3CL1 | CX3CL1 | Pancreatic [153], prostrate [154], breast 27001765 [155] | TAMs 32060841 [156] |
Apart from the chemokine family mentioned above, leukotrienes, the biologically active eicosanoid lipid mediators, can act similarly to chemokines by critically modulating leukocyte migration. Leukotrienes are primarily synthesized by myeloid cells and have recently been shown to contribute to the inflammatory tumor microenvironment, resistance to immunotherapy, and metastasis [157, 158].
The chemokine system plays a pivotal role in cancer biology. Chemokines and their receptors can affect both the tumor cells and tumor microenvironment to enhance the selection of metastatic cells and eventually metastasis from the primary tumor. Various studies delineate chemokines’ role in enhancing cancer cell properties, such as chemokines supporting tumor growth and proliferation, epithelial to mesenchymal transition, cancer stem cell properties, and chemotherapy resistance. Similarly, chemokines modify the tumor progression and metastasis through leukocyte recruitment, stromal interactions, angiogenesis, and creating metastatic niches. The following section will delineate the general mechanisms (Fig. 1) by which these multifaceted chemokines intricately function and regulate metastatic progression.
Fig. 1.
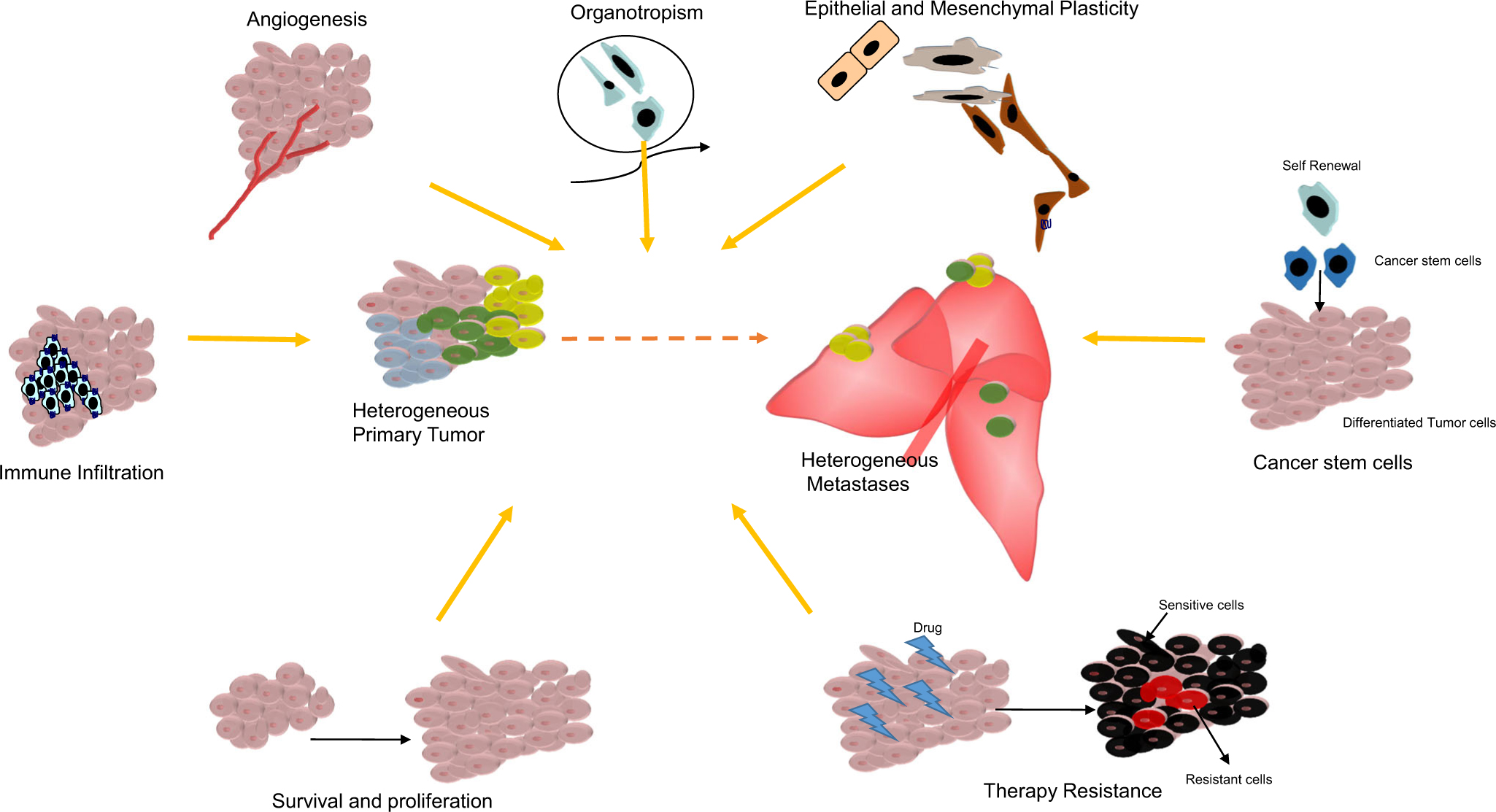
Different chemokine-regulated processes contributing to the tumor and metastatic heterogeneity. Summary of the general mechanisms by which multifaceted chemokines intricately function to regulate cancer cell properties and the tumor microenvironment to facilitate metastatic heterogeneity
3.1. Chemokines and organotropism
Organotropism or organ-specific metastasis, the non-random distribution of cancer cells among distant organs, is regulated by multiple factors, including the organ’s anatomical location, blood circulation pattern, and tumor-intrinsic factor organ-specific niches, and the interaction between tumor cells and the microenvironment of the metastatic sites. As discussed earlier, chemokines are equipped with chemo-attractive signaling that can regulate leukocyte trafficking to distant organ sites. Cancer cells and endothelial cells that express the chemokine receptor migrate towards their paired chemokine gradient at non-random organ-specific sites [87, 157–159]. Thus, it is logical to expect that chemokines would have been among the first genes shown to control metastasis’ molecular wheel [159]. Initial evidence showed that different cancer cells have aberrant expression of chemokine receptors, selective but not random. [160]. In 2001, Muller et al. were among the initial few groups to demonstrate chemokines’ role in organ-specific metastasis. The group revealed that CCR7 and CXCR4 expression on breast cancer cells influences the invasion and organ specificity of breast cancer metastasis [160] to preferred sites positive for CCL21 (ligand for CCR7) and CXCL12 (ligand for CXCR4) expression such as the lung, liver, and bone. However, breast cancer cells’ tendency to metastasize to the lung and brain is primarily determined by the vascular anatomy. Similarly, vascular anatomy dictates colorectal cancer’s tendency to metastasize to the liver. Thus, some organs are more susceptible to tumor metastasis in the body, such as the lung, brain, liver, lymph nodes, and bone marrow, while other organs such as the kidneys, pancreas, and skin are less prone [161].
Studies show that chemokine receptors in cancer cells enhance invasion and metastasis and define the cancer cell’s metastatic destination. A non-metastatic B16 melanoma cell line with no endogenous expression of Ccr7 metastasized to the lymph nodes on transfection with Ccr7 [162]. Similarly, CCR7 expression on a lung metastatic cell line showed metastasis to the lymph node [163]. Similarly, Cxcr4 expression on B16 melanoma cells induced metastasis to the lung [164]. In addition, microarray studies showed a very small number of differentially expressed genes on comparing primary tumors with corresponding metastases obtained from the same patient. Chemokine receptor genes are part of those differentially expressed gene pools and determine different tumors’ metastatic destinations [165–167].
The characteristics of neoplastic cells and the specific microenvironment of the secondary organ can influence the site of metastasis [15]. Since chemokines can guide cells with appropriate receptors to particular locations, metastatic cancer cells can hijack the chemokine receptor system to facilitate cellular migration to trigger metastasis at distant sites [161]. Such as breast and prostate cancers are the primary cancers that metastasize to bone [168]. Multiple cancer type/subtype-specific mechanistic axes exist for bone metastasis. However, CXCL12/CXCR4-mediated chemotaxis is shared among different cancer types [161]. Along the same lines, various resident cells in the lungs secrete CXCL12 and CCL21, directing breast cancer (CXCR4) and melanoma cells (CCR7) to the lung [160]. One specific axis is the CCR9 expression in a subset of patients with melanoma tumors that shows metastasis to a rare metastatic site—the intestine (positive for the paired CCL25 expression) [149, 169].
3.2. Chemokines and their receptors on leukocyte recruitment and activation in malignant tumors
The tumor cells’ intrinsic properties and different cells constituting the tumor milieu determine chemokines’ expression pattern and their receptor in tissue and dictate the frequency and type of leukocyte infiltrates within the specific microenvironment [7, 170–172]. These chemokine gradients and the recruited leukocytes infiltrate population changes under pathological and inflammatory stimuli and can critically modulate tumorigenesis and metastasis. In summary, firstly, the type of chemokine present in the microenvironment and secondly, the specific receptors expressed on the infiltrating cells are the deciding factor for the number and type of infiltrated leukocytes in primary tumors and secondary tumors.
A bilateral interaction occurs between tumor cells and infiltrating leukocytes at a different stage of tumor progression. The infiltrating leukocytes can synthesize cytokines, enzymes, and different growth inhibitory/stimulatory factors such as matrix metalloproteinases (MMPs), growth, and angiogenic factors [173–177] to initiate, maintain, or terminate tumor growth and metastasis [178, 179]. Apart from primary tumors, infiltrating leukocytes can also regulate metastatic secondary tumors by balancing stimulatory (immunosuppressive factors, tumor survival, or angiogenic factors) and inhibitory activities (potent cellular immune response) by interacting with different stromal cells in the metastatic microenvironment [180, 181].
To delineate one such chemokine network, CC chemokines is a well-known network for accumulating macrophages and lymphocytes at tumor sites [182, 183]. CC chemokines can preferentially recruit macrophages and T lymphocytes, NK cells, and dendritic cells into the tumors [7, 8]. Such stromal cells’ recruitment can promote tumor angiogenesis, cancer cell invasion, and/or disrupt immune surveillance and progression of the metastatic cascade [184–186]. Tumor-associated macrophages (TAMs) are one of the most abundant stromal cell types in solid tumors [187], and a high number of TAMs in the tumor correlates with poor overall survival in cancer patients [188–191]. Apart from TAM recruitment to primary tumors, such cells recruited to the metastatic sites are called metastasis-associated macrophages (MAMs) [138]. TAMs can protect cancer cells from antitumor immune reactions by directly suppressing T cell responses [192] and NK cell cytotoxicity [193, 194] through the expression of regulatory molecules such as arginase-1(ARG1), IL-10, and transforming growth factor-β (TGF-β). Therefore, the strategy to block the recruitment of TAMs and MAMs can improve the outcome of metastatic disease [195].
CCL2, initially considered a monocyte-specific chemokine, is essential in phytohemagglutinin (PHA)-stimulated leukocyte culture responsible for chemotaxis of T cell with a memory phenotype (CD45RA+, CD45RO+, CD29+, l-selectin+) [196]. Although monocytes respond to CCL2 within an hour, it requires at least 4 h to initiate a significant T cell response to CCL2. These data signify CCL2 as the link between monocytes and lymphocytes infiltration in the inflammatory sites. CCL2 involvement in T cell recruitment leads to further investigation of T cell analogous chemotactic response to other CC chemokines such as CCL7 [197–200]. Additionally, CCL2 receptor CCR2 can regulate the migration of IL-17-producing cells, promoting inflammation in autoimmune diseases [201] as well as cancer [202] that can suppress the adaptive immune response [203]. Recently, utilizing multiple murine tumors and metastasis models, Tu [204] et al. demonstrated that CCR2 inhibition combined with anti-PD-1 enhances tumor response to immune checkpoint therapy.
The chemokine ligands/receptor axes regulating spatiotemporal recruitment, retention, and MAM expression’s phenotype include CCL2-CCR2 [205] and CCL3- CCR1/CCR5 [138]. It is noteworthy to understand that upregulation of chemokines such as CCL2, CCL5, and CCL18 cannot only recruit monocytes/macrophages but also induce de novo synthesis of CCL3, CCL8, and CCL22 chemokines to reinforce the accumulation of metastasis-promoting immune cells such Treg cells as well as MAMs [138]. Monocyte-derived macrophages can secrete CCL18 to promote the secretion of Treg cells’ chemokines, including CCL2, CCL3, and CCL22 [206]. Similarly, MAMs in the metastatic lung predominantly express CCL8 to recruit Treg cells mediated through receptor CCR5 [207]. Also, CCL3 was identified as the principal mediator of the communication between the neoplastic epithelium and the peripheral tissues such as lung and brain in breast cancer–bearing mice. CCL3-induced monocyte chemoattractive protein chemokines cluster with CCL7, CCL8, CCL11, and CCL12 chemokines in the distant peripheral tissues [208].
Depletion of MAMs can reduce the metastatic tumor burden of breast cancer cells in mice [209, 210]. A few functional possibilities for these observations include a recent study demonstrating that similar to TAMs, MAMs can also protect cancer cells from tumoricidal immune reactions in the metastatic sites by suppressing cytotoxicity of CD8+ T cells [211]. CCL5 chemokine can prevent MAMs from becoming tumoricidal cells. Furthermore, a recent study suggests that monocytes’ recruitment and subsequent accumulation of MAMs are critical for circulating breast cancer cells to establish metastases [212].
Also, the CXC chemokine network deserves mention in the process of metastasis which is involved in the recruitment of neutrophils to primary and secondary tumors. Emerging evidence indicates that heterogeneous neutrophils with plastic sub-populations are actively involved in metastasis [91, 213, 214]. In brief, several studies now suggest that CXC chemokines/receptors mediate the accumulation of neutrophils in the pre-metastatic niche [98, 215–217].
Extensive studies on tumor-infiltrating leukocytes and lymphocytes suggest depressed functionalities of these immune cells against the tumor cells [173, 218, 219]. If these depressed functionalities of macrophages/lymphocytes or any other immune population are a cytokine defect, rather than an inherent defective immune population, then manipulating chemokines and their receptors may enhance antitumor responses [220, 221]. The extent of macrophage and lymphocyte infiltration into tumors of the same histological origin can vary widely. These cells are located predominantly at the tumor and host cellular interface and represent a potential target for therapy based on immune manipulation [222, 223].
The mechanism(s) of leukocytic recruitment and activation and the significance of this process in tumor growth, heterogeneity, and metastasis are intensely investigated [5, 11]. Recent reports rationalize that tumor-infiltrating immune cells provide selection pressure that can shape tumor heterogeneity, and high heterogeneity tumors are associated with less immune response and cell infiltration [224, 225]. However, a better understanding of “cross-talk” between the malignant tumor cell and different infiltrating leukocyte populations is essential before implementing therapeutic strategies. Similarly, the relationship between the type of therapy, patient prognosis, chemokine-receptor expression, and leukocyte infiltration remains poorly understood. Thus, the development of novel adjuvant therapies requires us to delineate the interrelations between the type of therapeutic approach resulting in leukocytic infiltration, their chemokine-receptor expression pattern, and the prognosis of cancer patients.
3.3. Chemokines and their receptor in tumor angiogenesis
Assembly of new vascular structures, neovascularization, is relatively quiescent under normal adult physiological conditions and is limited to wound healing and the female reproductive processes in adults [226–229]. However, a number of diseases, including cancer, can result in abnormal neovascularization. Angiogenesis is the primary process of adult pathological neovascularization [226–229], with vasculogenesis’ limited contribution [230]. Angiogenesis in tumors addresses sustenance from nutrients, oxygen supply, excretion of metabolic wastes, and carbon dioxide [231]. Angiogenesis is regulated by many angiogenic factors, metabolites such as carbohydrates, and lipids, enzymes, and members of the chemokine superfamily [67, 232].
Chemokine networks play essential roles in tumor angiogenesis [67] by the promotion or suppression of angiogenic factors such as VEGF and bFGF [67, 233, 234] in either a direct, parallel, or serial manner, proliferation [234–237], and migration [238, 239] of endothelial cells and through the recruitment of immune cells that support or inhibit angiogenesis to the tumor microenvironment [67, 238, 240, 241]. Specific chemokine members can act as pro-angiogenic molecules [67, 238], while others can be angiostatic [70, 232]. In addition, chemokines can also exert their angiogenic activity by upregulating metal metalloproteases such as MMP-2 and MMP-9 endothelial and tumor cells [242–244]. In turn, MMPs can degrade the extracellular matrix leading to endothelial cell migration, re-organization, and favoring angiogenesis [245].
The “angiogenic switch” or initiation of tumor angiogenesis is critical for tumor progression and metastasis [246]. Thus, tumor microvessel density is one of the most vital lines of evidence linking angiogenesis and metastasis. The correlation between tumor microvessel density and increased metastatic potential is present in all forms of cancers [247]. Notably, at the metastatic site, malignant tumor cells must proliferate and again undergo angiogenesis to result in a clinically relevant secondary tumor or macrometastases. Angiogenesis is essential for the growth of micrometastases. Thus, researchers propose that normal vessels’ cooption can be a mechanism for metastasis vascularization [248] or contribution of bone marrow–derived endothelial progenitor cells to early angiogenic stages metastatic growth [249].
Hypoxia can serve as a link between angiogenesis and tumor heterogeneity [250]. Two prime reasons result in a hypoxic tumor microenvironment. Firstly, with cancer cells’ proliferation, the inner core cells get away from the blood supply and turn hypoxic [247]. In turn, hypoxia upregulates the expression of many angiogenic growth factors in cancer cells [251–253]. Secondly, unlike normal blood vessels, tumor-associated capillaries are notoriously abnormal, tortuous, malformed, hyperplastic, and misguided. High expression of factors such as VEGF, TAMS, and angiogenic chemokines renders tumor vessels highly permeable and leaky in the tumor and metastatic environment [254]. Such irregularities in the tumor vascular network with leaky and compressed vessels make the network inefficient with poor blood flow and oxygen delivery. Low oxygenation or hypoxia can mediate cancer progression and metastasis and immunosuppression, therapy resistance, and particularly tumor heterogeneity [250].
Other than hypoxia, spatial and temporal heterogeneity of angiogenic molecules present within a single tumor and even between different metastases in a single organ [255] can result in the generation of multiple cancer cell subpopulations within the tumor and metastatic microenvironment. To exemplify, small tumors (3–4 mm in diameter) express more basic fibroblast growth factor (bFGF) and CXCL8, whereas large tumors (>10 mm in diameter) express more vascular endothelial growth factor (VEGF). On the same lines, immunostaining revealed high expression of bFGF and CXCL8 on the periphery of a large tumor and increased VEGF expression in the tumor center [256]. Similarly, matrix metalloproteinase-9 was overexpressed at the periphery of the tumor, characterized by rapidly dividing cells with VEGF expression which was localized in the center of the lesions [257]. Gradient expression of such angiogenic molecules can influence the nearby tumor cells resulting in subpopulations with differences in angiogenic potential, invasiveness, and metastatic capabilities [256, 258].
Among all the chemokines, CXCL8 is extensively studied as a potent mediator of angiogenesis. The pro-angiogenic activity of CXCL8 in vivo was confirmed by using the rat mesenteric window assay, the rat and rabbit corneal assay, and a subcutaneous sponge model [259–261]. Human recombinant CXCL8 was angiogenic when implanted in the rat cornea and induced proliferation and chemotaxis of human umbilical vein endothelial cells. [259] In addition, the angiogenic properties of conditioned media from activated monocytes and macrophages were attenuated by CXCL8 anti-sense oligonucleotides [259]. Furthermore, it was shown that CXCL8 could act directly on vascular endothelial cells by promoting their survival [262]. Studies from our lab and other groups suggest that CXCL8 stimulates both endothelial proliferation and capillary tube formation in vitro in a dose-dependent manner. These effects can be blocked by monoclonal CXCL8 antibodies [263]. In addition, CXCL8 was shown to inhibit apoptosis of endothelial cells [243]. Also, CXCL8 exerts its angiogenic activity by upregulating MMP-2 and MMP-9 in tumor and endothelial cells [242, 244]. Degradation of the extracellular matrix by MMPs is required for endothelial cell migration, organization, and, hence, angiogenesis [245]. Our group and others have demonstrated that CXCL8 directly enhances endothelial cell proliferation, survival, and MMP expression in CXCR1- and CXCR2-expressing endothelial cells, thus, may be an important player in the process of angiogenesis [74, 89, 264, 265].
CXC chemokines also include angiostatic members known to inhibit neovascularization [70, 232, 264, 266, 267]. The following examples briefly describe the angiostatic role of CXC chemokines: CXCL10 has been demonstrated to inhibit CXCL8- and FGF-2-induced angiogenesis [258]. Delivery, injection, or genetic manipulation of CXCL9 or CXCL10 expression into tumors has been shown to suppress tumor angiogenesis [268–270]. Also, intratumoral delivery of immunotherapeutic agents correlates with increased expression of CXCL9 and/or CXCL10 [271, 272]. Cell cycle–dependent expression of CXCR3 on endothelial cells mediates the angiostatic activity of CXCL9–11 [273]. Intratumoral expression of CXCL9 and CXCL10 results in decreased renal carcinoma tumor size in patients enrolled in clinical studies [274]. Interestingly, the presence of angiogenic and angiostatic CXC chemokine suggests that different chemokines’ relative expression/activities in the tumor microenvironment may affect tumor angiogenesis.
A plethora of recent studies now suggest that non ELR+ CXC chemokines and chemokine family other than CXC are also angiogenic. CC chemokines are now part of the growing list of angiogenic modulators and find implications in disease with inflammation-driven angiogenesis [237]. Initially, CC chemokines were shown to indirectly promote angiogenesis by first recruiting macrophages that release cytokines and growth factors necessary for forming a neovessel [275–277]. However, recent reports suggest CC chemokines’ direct action on endothelial cells leading to enhanced vascularity [278]. For example, CC chemokines can increase nitric oxide production and endothelial cell proliferation and migration, ultimately leading to increased angiogenesis [279, 280]. Stimulation of these can also increase VEGF production to further augment neovascularization [232, 266]. A wide variety of cells, including endothelial cells, smooth muscle cells, and inflammatory cells, can secrete CC chemokines under the inflammatory stimulus [281]. Additionally, CCL2 is associated with the increase of MMP14, essential for endothelial cell migration and neovessel formation [280]. CCL2 can also recruit endothelial progenitor cells to accelerate the endothelialization process [282]. Apart from CCL2 [279], CCL1 [283], CCL11 [237], CCL15 [284], and CCL16 [285] can initiate in vitro endothelial tubule formation.
Another interesting chemokine modulating angiogenesis is Fractalkine (FKN, CX3CL1), a CX3C chemokine family member. CX3CL1-CX3CR1 can regulate angiogenesis in primary tumors of the breast [286], liver [287], lung [288], melanoma [289], and multiple myeloma [290]. CX3CL1-CX3CR1 can also regulate angiogenesis in two ways, by recruitment of pro-angiogenic TAM [286, 291], and by directly acting on endothelial cells resulting in their proliferation, migration, and tube formation [292–294]. Apart from contributing to cancer angiogenesis, the CX3L1-CX3CR1 axis facilitates angiogenesis in inflammatory disease. CX3L1-CX3CR1 contributes to the pathogenesis of atherosclerosis [295, 296] by promoting leukocyte adhesion to endothelial cells [86, 87] and participates in rheumatoid arthritis through endothelial cell activation [297–299].
A biological imbalance in angiogenic and angiostatic chemokine production can contribute to several angiogenesis-dependent disorders such as cancer, rheumatoid arthritis, and psoriasis [89, 265, 300–304]. How a multitude of angiogenic and angiostatic chemokines function together, whether their functionality is gradient dependent and whether a synergistic effect exists of their action on different stromal players in primary tumor and metastases in regulating the cancer cell heterogeneous subpopulations is not clear. More studies are needed to define the contribution of tumor angiogenesis towards metastatic heterogeneity clearly. With an understanding of current literature, one can suggest a shift in the balance of expression of these angiogenic and angiostatic chemokines in favor of angiostasis by the pharmacological intervention of the specific expression chemokine check tumor and metastatic heterogeneity.
3.4. Chemokines and their receptors in epithelial to mesenchymal plasticity
Epithelial to mesenchymal transition (EMT) is a well-studied process for the process of embryonic development. Cancer cells are known to hijack such embryonic development processes like EMT [305] to enhance their dynamic state that offers numerous advantages to these cells for undergoing successful metastasis [306–309]. Cancer cells can exist in partial or intermediate plasticity with the acquired property of stemness [310]. Thus, EMT converges two hallmark properties of metastatic cells—invasiveness and stemness. Apart from the known role in cell invasiveness, EMT is emerging to contribute to stemness, immune escape, and resistance to therapy, and, most importantly, cancer cell phenotypic heterogeneity in primary tumors and metastasis [311, 312]. Chemokines and their receptors are emerging players of cancer cell EMT.
The signals activated by ligand CXCL8 through CXCR1/2 receptors result in a few well-investigated downstream signaling pathways that are linked to phenotypic plasticity [313, 314]. With a direct mechanism of action, enhanced secretion of CXCL8 in cancer cells that underwent EMT plays a role in acquiring and maintaining this plasticity, acting in an autocrine manner [315]. CXCL8 can also act in a paracrine manner on adjacent cancer cells to induce a mesenchymal phenotype. While serving indirectly, CXCL8 can activate endothelial cells or create neutrophil infiltration into the tumor site. Activation of endothelial cells results in angiogenesis [316], while neutrophils in TME can secrete additional factors, furthermore promoting EMT in the cancer cells [317, 318]. Apart from independent mechanistic actions of CXCL8, Cheng et al. in 2014 demonstrated that chemokine CCL20 could synergize with CXCL8 to bring collaborative induction of the epithelial-mesenchymal transition in colorectal cancer cells [319].
Another upcoming axis of chemokine/receptor player in EMT is CCR7/CCL21 that has implications of inducing EMT in different cancer cells such as breast [320], lung [321], oral squamous cell carcinoma [322], and pancreas [323]. This upregulation of EMT associated with the CCR7/CCL21 axis can also enhance stemness in cancers such as oral squamous cell carcinoma [322] and pancreatic carcinoma [323]. Lastly, chemokines such as CCL20 [319, 324] and CXCL5 [325, 326], and receptors such as CXCR2 [325–327] and CXCR4 [328], have been associated with bringing EMT in different cancer cells.
3.5. Chemokines and their receptors in cancer stem cell concept
As described earlier, genetic and phenotypic heterogeneity is a significant challenge in cancer management. EMP and cancer stemness are two interlinked axes that can account for cancer cells’ non-genetic phenotypic plasticity [312]. Max Askanazy, a pathologist by profession, came forward with the cancer stem cell concept stating that differentiated ovarian teratomas are derived from a single multipotent cell type [329]. With decades of research, scientists elucidated cancer cells’ ability to initiate heterogeneous tumors and a relevant explanation for metastasis mirroring heterogeneity of primary tumor with cancer stem cell concept (CSC) [310]. The current CSC models state that CSC needs not to be a rare minority of tumor cells with a fixed population but dynamic. Normal stem cells need not originate CSC; the reprogrammed somatic cell can give rise to a malignant CSC, and finally, these cells can be proliferative, not quiescent. With this redefinition, tumor-initiating cells (TICs) and metastasis initiating cells (MICs), cancer cells capable of giving rise to overt secondary growth in a distant organ, are CSCs by nature [306]. The origin of MICs is elusive, with the question of whether these cells arise in the primary tumor or during the metastatic journey or at a secondary site on interaction with stromal components. Still, importantly MICs must possess the ability to survive metastatic cascade.
As stated earlier, the role of chemokines and their receptors expands beyond cellular motility and also finds relevance in maintaining cancer stem cells [5]. The chemokine/receptor axis of CXCR4-CXCL12, one of the most well-defined chemokine/receptor players, is emerging as an important player in maintaining CSC. The evidence came from high levels of CXCR4 expression in CD44+/CD133+ prostate cancer stem cells (CSCs) [330]. In this study, Dubrovska et al. demonstrated that increased CXCR4 expression on CD44+/CD133+ prostate cancer CSC promotes adhesion to the extracellular protein fibronectin and their proliferation with activation of the PI3K pathway in a CXCL12-dependent manner. The combined facilitated adhesion and proliferation by CXCR4/CXCL12 are essential for initiating secondary tumors in distant organs.
Moreover, both a CXCR4 receptor antagonist (AMD3100) and antibody could decrease tumor size and these prostate cancer progenitor cells’ population. Additional evidence supporting cancer stemness linked with CXCR4/CXCL12 axis in prostate cancer came from the reports of Jung et al. showing CXCL12 expression results in the development of aggressive metastatic castration-resistant prostate cancer through induction of cancer stemness and neuroendocrine phenotypes [331]. Similarly, in breast cancer, the co-culture of the cancer cells with breast cancer–associated fibroblasts enhanced CXCL12 secretion, resulting in high spheroid formation with an enriched population of CSCs [332]. Another supporting evidence came from a study showing that CXCR4 expression enhances breast cancer cells’ ability to form tumor mammospheres [122]. Lastly, elaborative research conducted in the luminal-A subtype of breast carcinoma showed that overexpression of CXCL12 elevated the proportion of CD44+/CD24− ALDH-expressing cells along with stemness markers such as sox2, Oct4, and Nanog [333].
Another chemokine receptor axis playing a significant role in promoting CSC enrichment is CXCR1/CXCR2 receptors in conjunction with CXCL1 and CXCL8 chemokines. One of the pioneer studies reported the role of CXCL8/CXCR1 in breast cancer CSCs by isolating and characterizing CSC populations in 33 cell lines using expression analysis of aldehyde dehydrogenase [334]. Gene expression profiling of these isolated aldehyde dehydrogenase positive CSCs identified a 413-gene signature that included CXCL8/CXCR1. Functionally, recombinant CXCL8 increased mammosphere formation and the ALDEFLUOR-positive population in breast cancer cell lines. Later, an elegant study from Ginestier et al. demonstrated that blockade of CXCR1 using either a CXCR1-specific blocking antibody or repertaxin, a small-molecule CXCR1 inhibitor, selectively depleted the aldehyde dehydrogenase positive breast cancer CSC population. In their in vivo studies, CXCR1 blockade induced massive apoptosis in the bulk tumor population via FASL/FAS signaling and decreased metastasis at distant organs [335]. In 2013, Singh et al. delineated that the mammosphere-promoting effect of CXCL8 is partly mediated through Src and EGFR/HER2-dependent pathways. Thus, a combination of CXCR1/2 inhibitors and HER2-targeted therapies has the potential to reduce breast CSC activity [336]. In addition to the role of CXCL8 chemokine in CSCs, CXCL1-chemokine partner of CXCR2, secreted from TAMs, is reported to enhance tumor spheroids and CSC subpopulation in human TNBC cells [337]. Chen et al. observed similar results in pancreatic carcinoma that overexpression of CXCL8 self-renews pancreatic CSC through CXCR1 [338]. Apart from the CXCR4/CXCL12 and CXCR1/2/-CXCL1/8 axes mentioned above, other chemokines are also shown to generate CSCs in breast cancer such as CCL2, [339], CCL5, [340], CCR5 [341], and CXCR7/ACKR3 [342].
3.6. Chemokines and their receptors and therapy resistance
Cancer being a dynamic disease, tumors become more heterogeneous over time—heterogeneity results in spatial or temporal distinct tumor-cell subpopulations within a tumor [343]. With high levels of heterogeneity in a tumor comes differential drug sensitivity levels to treatment and inferior therapeutic outcomes. Also, selective pressure of a drug treatment can expand pre-existing subclonal drug-tolerant populations, leading to drug treatment resistance. In summary, heterogeneity is the powerhouse for drug resistance; and an evaluation of tumor heterogeneity is required for effective drug treatment in primary and secondary tumors.
On the other hand, chemotherapy resistance is often intertwined with the metastasis process. Various clinical observations such as higher frequency of metastatic tumors observed in chemoresistant primary tumors, low chemotherapy response rate in metastatic settings, and a correlation between poor chemotherapy sensitivity and metastatic occurrence [344] supports this interlink. These observations also suggest that gain of chemoresistance in tumors may select MIC cells [306]. One possible mechanism for this interlink is that chemotherapeutic treatment’s toxicity results in the secretion of proinflammatory cytokines/chemokines and bioactive lipids by tumor and cells of tumor microenvironment termed as cytokine storm [345]. This pool of secreted chemokines such as CXCL12, CCL2, CCL4, and others is related to the process of metastasis through inflammation [345]. Other important mechanisms benefiting both the gain of chemoresistance and generation of MICs are CSC-like features like enhanced DNA damage response [346], detoxifying enzymes such as ALDH [347], and effective drug efflux pumps [348] as well as EMP [349, 350].
Various in vitro and in vivo studies [351–355] provide evidence that CXCR1/CXCR2 and their CXCL1/CXCL8 ligand axes can directly promote chemoresistance in breast cancer. This study also demonstrated that chemotherapy treatment, along with CXCR1/CXCR2 inhibition, reduces primary tumor burden, metastasis, angiogenesis, and therapy resistance [351–356]. CXCL8/CXCR2 also connects chemoresistance to metastasis through CSCs, EMP, or immune infiltration in breast tumor settings [357–359]. To delineate this axis, Samantha et al. showed that reactive oxygen species generated by chemotherapy treatment induce the production of CXCL8 through activation of the hypoxia-inducible factor. Induced CXCL8, in turn, elevated tumor spheroids and ALDH-expressing cells under the chemotherapy settings [360]. Later in support of these observations, a study showed that treatment of mice with CXCR1/2 inhibitor reparixin decreased the number of tumor-initiating cells elevated under chemotherapy administration [361]. We also demonstrated that doxorubicin- and paclitaxel-resistant breast cancer cells had upregulated CXCR2 ligands, stem cell, and mesenchymal markers with higher metastatic capability in comparison with parent cells [357]. Furthermore, tumors derived from these resistant cells had higher infiltration of neutrophils and T helper 17 cells with increased IL-17 receptor, CXCR2, and CXCR2 ligands within the metastatic lungs [358]. We also demonstrated that chemotherapy resistance induced IL-17 increased CXCR2 ligands cells’ expression and enhanced neutrophil chemotaxis in CXCR2-dependent manner. Lastly, the therapy-resistant breast cancer cells enhanced the secretion of pro-tumorigenic MMP9 in neutrophils [359].
In 2012, Massagués and colleagues uncovered a mechanistically defined molecular interlink between metastasis and chemoresistance in breast cancer [362]. The group demonstrated that paracrine signaling of CXCL1/2 interconnects cancer cells with stromal cells like endothelial and myeloid cells to drive both metastasis and chemoresistance processes. In brief, chemotherapeutic agents trigger TNF-α production by endothelial and other stromal cells to upregulate the CXCL1/2 expression in cancer cells. Secreted CXCL1/2 attracts CD11b+Gr1+ myeloid cells into the tumor and creates a proinflammatory environment in the lungs. Infiltrated myeloid cells produce S100A8/9 to enhance cancer cell survival in primary breast tumors and secondary lung tumors. Inhibition of CXCR2 can break the CXCL1/2-S100A8/9 loop to improve chemotherapy response and decreases metastatic burden.
Another chemokine receptor family member shown to play a role in breast cancer’s chemoresistance is CXCR4 [363, 364] and CCR5 [341]. Importantly, CXCL12/CXCR4 axis also offers resistance to endocrine therapy by activating both ERα [365] and ERβ estrogen receptors in the presence of tamoxifen treatment [364, 366]. Similarly, CXCR7/ACKR3 can stabilize ERα estrogen receptor and render tamoxifen treatment in luminal-A breast cancer cells insensitive [367]. Apart from the delineated role of CXC-chemokine/receptor in chemoresistance, recent reports suggest that the CC-chemokines subfamily offers chemoresistance to the platinum drugs cisplatin, carboplatin, and oxaliplatin treatments in different cancer [368].
3.7. Chemokines and their receptors in cell survival, proliferation, and senescence
For the successful establishment of metastases, cellular survival and proliferation signals are needed at various metastatic cascade stages. For example, during dissemination, detachment of cancer cells from ECM may induce anoikis. Also, cancer cells may encounter apoptotic death signals on entering the new environment present at the metastatic sites, and lastly, cancer cells need to proliferate to establish distant metastases. Another important phenomenon is exiting from metastatic dormancy characterized by growth arrest and survival [369]. Furthermore, dormancy reactivation requires intrinsic and extrinsic signals, a specialized microenvironment, and an immune escape [186, 370, 371].
Evidence for chemokines and chemokine receptors regulating survival and proliferation comes from various reports demonstrating the regulation of tumor growth or inhibition by chemokines through activation of different signaling pathways. One of the first indications came from chemokines CXCL1 and CXCL8, enhancing the proliferation of different melanoma cells [372–375]. Other CXC families of chemokines are involved in many cancers, including CXCL12 [376–380], CXCL2, and CXCL3 [381]. Similarly, overexpression of CXC receptors such as CXCR4 [379, 382], CXCR2 [96, 383–385], and CXCR6 [386, 387] can enhance tumor growth and progression of many cancers. Altogether, numerous reports demonstrate that CXC chemokines derived from different cellular sources [388, 389], whether acting in an autocrine or paracrine manner or alone or in synchrony with other growth regulators such as IL-6 [390], can enhance cancer cell proliferation, colonization at metastatic sites, and anchorage-independent cell growth [391] and lowers cancer cell apoptosis [392] and dormancy [393] and cell cycle arrest [351]. Various reports demonstrate increased breast cancer cell proliferation under hormonal stimulation mediated through chemokines such as CXCL12 acting through either CXCR4 or CXCR7/ACKR3 [366, 394–396].
Similarly, the CC family of chemokines and receptors can promote proliferation and provide growth-stimulating regulatory modes of tumor cells in different tumors [388, 397–402]. However, chemokine receptors in this family inhibit tumor cells’ proliferation, such as CCR1 expression in human hepatocellular carcinoma cells [403]. In contrast to cellular survival, chemokines can also regulate cellular senescence. Senescence can be defined as the process in which cells undergo permanent proliferation arrest and cannot enter the cell cycle [404, 405]. However, such senescent cells are not metabolically arrested and can secrete many pro-inflammatory factors, including CXCL8 [405, 406], termed as senescence-associated secretory phenotype (SASP). Thus, senescent cells have two contradictory properties, growth arrest and proinflammatory SASP, leading to their dual role in tumor biology [407]. Senescence mediated through chemokines has been shown to promote metastasis by governing leukocytes entering the organ site [408, 409], creation of metastasis-promoting TME [410], induction of EMT [411], promoting tumor cell invasiveness [412, 413], and inducing collective invasion of the cancer cells enhancing the survival of non-senescent cancer cells [414].
3.8. Chemokine network: the link between metastatic heterogeneity and metastatic niches
Distant organs are characterized by hostile environments for the CTCs that will eventually undergo anoikis, apoptosis, or cell death due to many factors such as the absence of survival signals, energy metabolites, or incompatible stromal interactions in the host tissue [186, 415]. So, does primary tumor reeducate, corrupt, or influence this distant hostile environment to initiate metastases? A growing body of literature suggested that cancer cells and the distant organ’s stroma evolve together to initiate metastasis [184, 416, 417]. Cancer cells in the primary tumor can systemically recruit stromal cells to a distant site to prepare the metastasis milieu even before the occurrence of metastatic colonization. The conditioning continues even after the establishment of metastases [418]. This preparation leads to pre-metastatic niche formation that undergoes continuous cellular and molecular changes to prepare fertile soil for metastatic seeding.
In 2005, the group led by Dr. Lyden pioneered the research on a pre-metastatic niche [419]. By definition, a pre-metastatic niche is a supportive or receptive microenvironment for metastatic overgrowth of specialized cancer cells in a distant secondary organ, regulated by primary tumor factors such as secreted cytokines, exosomes, and mobilization of bone marrow–derived cells (BMDCs) [418–422]. Even after 15 years, this field still attracts more focus and attention [423–427]. Summarizing the understanding of current literature, we can assign specific characteristics to a pre-metastatic niche such as inflammation, organotropism, immune escape, angiogenesis, and the cascade of anchorage, survival, and proliferation [425, 426]. Chemokines are the well-established molecular hallmark of inflammation.
Additionally, we have already discussed organotropism, immune infiltration, angiogenesis, and interlink of anchorage, survival, and proliferation under the light of chemokines/receptors. Thus, chemokines and their network can orchestrate each characteristic of the metastatic niche. Moreover, we have also discussed the role of chemokines in EMP, CSCs, and therapy resistance, significant processes contributing to metastatic heterogeneity. Thus, there is an overlap between metastatic niches’ characteristics and the chemokine-regulated processes contributing to metastatic heterogeneity (Fig. 2). Summarizing the overall idea, chemokine/receptor biology is the link between metastatic heterogeneity and metastatic niches. Thereby, the creation of metastatic niches at the distant organ site to support secondary tumors may also indirectly facilitate the seeding of different metastatically capable clones to survive in the new microenvironment resulting in the heterogeneous nature of metastases.
Fig. 2.
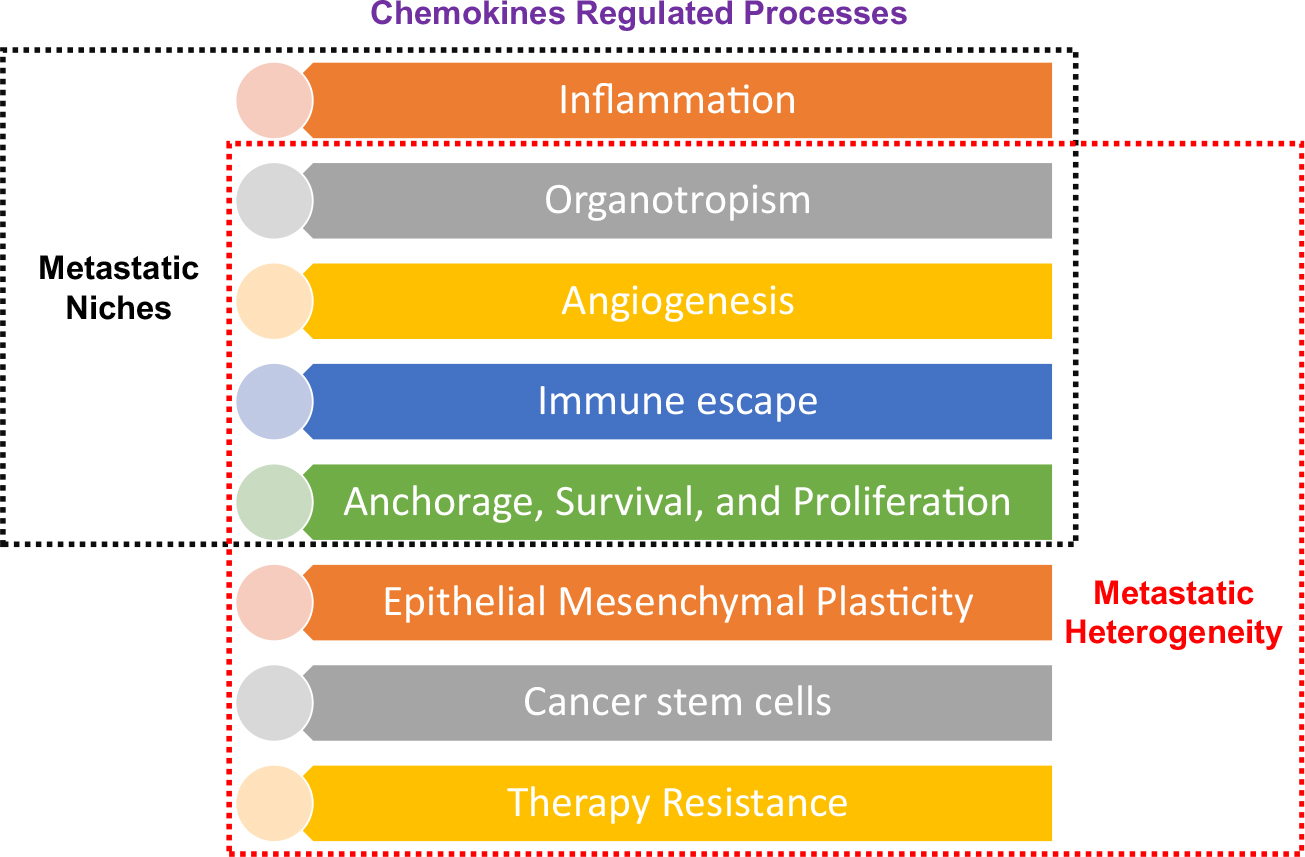
Chemokine network-the link between metastatic heterogeneity and metastatic niches. List of different chemokine regulated properties contributing to metastatic heterogeneity and creation of metastatic niches. There is an overlap between the different properties shared by these two processes, indicating metastatic niches to metastatic heterogeneity
3.9. Challenges for clinical implications
Yet, there is no clinically available anti-metastatic therapy; thus, the community of cancer researchers is engaged on a current mission to find effective ways of treating and preventing metastatic tumor spread. Recent studies are unveiling the layers of a complex interaction between tumor cells and the host cell, the understanding required for effective inhibition of metastasis.
Several chemokine receptor inhibitors are under evaluation in preclinical studies and clinical trials to treat different primary tumors and metastasis (Table 2). In preclinical settings, chemokine receptor inhibitors showed promising results in reducing metastatic burden when used in combination with chemotherapy or immune checkpoint therapy. The following are brief details of clinical trials blocking chemokine/chemokine receptors in patients with a metastatic burden. Based on the preclinical evidence of a reduction in metastasis [469–471], blocking of both CCL2 and CCR2 was evaluated in clinical trials of metastatic castration–resistant prostate cancer patients (NCT00992186) and treatment of patients with bone metastasis (NCT01015560). With the concept of blocking MDSC recruitment to tumors and the pre-metastatic niche, CXCR2 antagonists are in clinical trials for metastatic castration–resistant prostate cancer (NCT03177187) and the combination of CXCR2 antagonist with immune checkpoint inhibitor pembrolizumab for metastatic melanoma patients (NCT03161431). Additionally, CXCR4 antagonist BL-040 is in phase II clinical trial for metastatic pancreatic cancer (NCT02907099). The CXCR4 antagonist-balixafortide, combined with eribulin chemotherapy, has completed phase I trials in HER2-negative patients with heavily pretreated and relapsed metastatic breast cancer [437].
Table 2.
List of chemokine/chemokine receptor inhibitors tested in different tumor types in preclinical models and clinical trials. GEM gemcitabine, PTX paclitaxel, FX FOLFIRINOX; inhibitors in red ink are used for metastatic or relapse therapy
| Tumor Type | Receptor Target | Inhibitors | Clinical Trials |
|---|---|---|---|
| Brain tumor | CXCR4 | PRX177561 + Bevacizumab + Suntininib (438) POL5551 + aVGEF (439, 440) AMD3465 (441) |
USL311 + Lomustine (NCT02765165) AMD3100 (NCI2012–00149; NCI2013–02012) |
| ACKR3 | X7Ab + Temozolomide (442) | ||
| Breast Cancer | CCR1 | CCX9588 + Anti-PDL1 (443) | |
| CCL2 | CNTO888 + Radiotherapy (444) | ||
| CXCR2 | Reparixin + PTX NCT0237038 (445) | ||
| CXCR4 |
LY2510924 (NCT02737072) (446) Balixafortide + Erbulin NCT01837095(374) USL311 + Lomustine (NCT02765165) |
||
| Colon & Gastric Cancer | CCR1 | BL5923 (447) | |
| CCR4 | AF399/420/1802 (448) | ||
| CCR5 | Meraviroc + Chemotherapy(NCT0136813) (449) | ||
| CCR7 | si RNA (450) | ||
| CXCR2 | Reparixin + 5-fluorouracil (451) | ||
| CXCR4 | LY2510924 NCT02737072 (446) | ||
| Hematologic Malignancies | CCR1 | CCX721 (452, 453) | |
| CCR4 | Anti-CCR4 CAR T-cells (454) | Mogamulizumab (NCT01728805) (455) | |
| CCR7 | MSM R707 (456) | ||
| CXCR4 | AMD3100 + Ara-C (457) BKT140 + Rituximab (458) LY2510924 (459),(458) |
PF-06747143 (NCT02954653) AMD3100 NCT00512252 (460) BMS936564 NCT01120457 (461) | |
| Hepatocellular carcinoma | CCR2 | 747 + Sorafenib (462) RDC018 (417) |
|
| Lung Cancer | CCR4 | AF399/420/1802 (448) | |
| CXCR4 | AMD3100 + VIC-008 (463) | LY2510924 NCT02737072 (446) | |
| Ovarian & Prostate cancer | CCR2 | iCCR2 (464) | |
| CCL2 | CNTO888 NCT00992186 (465, 466) | ||
| CCR7 | siRNA (450) | ||
| CXCR2 | SB225002 + Sorafenib (467) | NCT03177187 | |
| SB265610 + Docetaxel (468) | |||
| CXCR4 | AMD3100 (469) | LY2510924 NCT02737072 (446) | |
| Pancreatic cancer | CCR2 | PF-04136309 + GEM (470) CCX872 + Anti-PD1 (471) |
PF-04136309 + nab-PTX + GEM
NCT02732938 (472) PF-04136309 + FX NCT01413022 (473) CCX872 + FX NCT02345408 (474) |
| CXCR2 | CXCR2−/− (475) CXCR2−/− + Anti-PD1 (149) SB225002 + RS504393 + FX (475) |
AZD5069 NCT02583477 | |
| CXCR4 | AMD3100 + Anti-PDL1 (476) | BL-040 (NCT02907099) | |
| Renal Carcinoma | CCR4 | Affi5 (477) | |
| Skin tumor | CCR4 | AF399/420/1802 (448) | |
| CXCR2 | Navarixin + Anti-MEK (476) | NCT03161431 |
As discussed above, several clinical trials are utilizing chemokine antagonists and inhibitors; thus, targeting chemokines and their receptors for treating metastasis is not new [14, 472] but challenging for various reasons [14]. Firstly, both tumor cells and a wide range of host cells can express chemokines/receptors. Thus, blocking a chemokines/receptors pair can lead to potential side effects, such as normal immune cells expressing the same receptor will be affected. Immune cells are required to clear residual tumor cells and to prevent the residual disease-preventing relapse. Also, administration of homeostatic dosage is required to avoid unwanted immune reactions and allergies. Secondly, chemokines/receptors’ promiscuous nature increases their interaction’s complexity, and inhibition or inactivation of a chemokine/receptor pair may lead to compensatory effects. Thirdly, blocking specific chemokine–chemokine receptors may not serve as effective targets in the entire metastasis process and may have a restricted therapeutic window. Similarly, chemokines’ profile changes with different cancer stages, drug treatment, and chemotherapy resistance, again limiting the therapeutic window. Lastly, chemokines as therapeutic agents must target cancer cell dissemination and already-established metastases and overcome metastasis heterogeneity.
Apart from challenges associated with targeting chemokines/receptors, targeting metastatic heterogeneity is also demanding. Ideally, we should approach targeting genomic instability as a source of metastasis heterogeneity. With the multitude of genes involved and other potential heterogeneity problems involved during a clinical course, it is a more daunting task than targeting cellular heterogeneity, though targeting cellular heterogeneity has important implications for the treatment of metastases. Cells present in the primary tumor do not need to represent the tumor cells populating different metastases. The extraordinary level of cellular diversity limits a single anticancer drug’s success, or a single treatment, to eliminate all cancer cells present in a malignant tumor and metastases.
Thus, new therapeutic targets or modalities should focus on the characteristics that permit malignant cells to metastasize or somehow limit the number of different cancer cell subpopulations within a tumor or slow tumor cells’ potential to generate new variants. Notably, the primary tumor response towards a drug and the response of the metastatic subpopulations readout should determine the efficacy of a treatment. Using a combination of anticancer therapies, the type of combination used, the administration sequence, and the time interval between successive administrations may eliminate tumor cells’ different subpopulations.
4. Conclusion and future directions
Dysregulation of chemokines/chemokine receptors’ biology in various tumor progression stages and metastasis is a cancer hallmark. The gain of the expression of chemokine receptors by cancer cells promotes their “specific” metastasis to organs that are positive for the expression of the respective ligand. In their literature review of homeostatic chemokine receptors [159], Zlotnik et al. suggested a model that chemokines/receptors present on normal or cancer cells constitute a cellular highway guiding these cells to specific organs and account for a non-random metastatic destination. Moreover, primary tumors and metastases should be viewed as multi-chemokine organs, with chemokine expression dependent on the factors such as temporal/spatial localization of the chemokine source, the amount of chemokine production, whether the source of chemokine is at the tumor site or metastatic organ, and the type of cell expressing the corresponding receptors such as cancer cell, leukocytes, endothelial cells, and stromal cells. All these factors will govern which chemokine/receptor will dominate the malignancy. Identifying the specific networks of chemokines/receptors present on tumor cells and their interaction with tumor milieu opens a broad avenue for the treatment of metastasis. Furthermore, deciphering molecular mechanisms of chemokines regulating tumor phenotypes affecting metastasis will identify the cellular and molecular targets helpful in designing effective molecular targeted therapeutics.
In the last three decades, chemokine/receptor biology has made extensive progress. New chemokines/chemokine receptors were identified, characterized, and delineated their role in different biological processes like angiogenesis, tumorigenesis, host defense, immune surveillance, and the creation of metastatic niches. Many antagonists of chemokine receptors are under investigation in various clinical trials for different cancer. However, it is crucial to understand that clinical trials on chronic inflammatory diseases such as rheumatoid arthritis, AIDS, and others have not yielded significant results by targeting a single chemokine/receptor [13]. Hence, the current understanding of chemokine biology suggests exploration of chemokine/receptor antagonists in combination with currently used chemotherapeutic drugs or targeting multiple pairs of chemokine/receptors to treat metastasis. Besides, the expression of various chemokines and their receptors is associated with survival analysis of different cancer patients; thus, in the future, expression of chemokines/receptors can become a prognostic biomarker. Chemokine/receptor expression can also be included in “molecular signatures” that can determine tumor aggressiveness, select appropriate treatments for cancer patients, and respond to chemotherapy drugs.
Another future approach for treating cancer and metastasis is to take advantage of the “immune infiltration” property of chemokines. Induction of chemokines that mount an antitumor immune response in the tumor microenvironment through viral delivery of chemokines, nanoparticle delivery, or reactivating epigenetic blocks that lowers antitumor chemokines in the tumor milieu can be used as therapy [11]. However, to utilize chemokines/receptors as targets in cancer therapy, extensive research unraveling the interplay between metastatic heterogeneity and chemokine/receptor heterogeneity at the tumor and metastases is needed.
Acknowledgements
We thank Dipakkumar R. Prajapati for reviewing the manuscript.
Funding
This work was supported in part by grants R01CA228524 and Cancer Center Support Grant (P30CA036727) from the National Cancer Institute, National Institutes of Health.
Footnotes
Declarations
Competing interests The authors declare no competing interests.
References
- 1.Singh RK, & Talmadge JE (2008). The evolution of diversity within tumors and metastases. Selected Aspects of Cancer Progression: Metastasis, Apoptosis and Immune Response Cancer Growth and Progression (pp. 59–90). Springer Life Sciences. [Google Scholar]
- 2.Singh S, Sadanandam A, & Singh RK (2007). Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis Reviews, 26, 453–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, & Lesniak MS (2013). Chemokines in tumor progression and metastasis. Oncotarget, 4(12), 2171–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Do HTT, Lee CH, & Cho J (2020). Chemokines and their receptors: multifaceted roles in cancer progression and potential value as cancer prognostic markers. Cancers, 12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morein D, Erlichman N, & Ben-Baruch A (2020). Beyond cell motility: the expanding roles of chemokines and their receptors in malignancy. Frontiers in Immunology, 11, 952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcuzzi E, Angioni R, Molon B, & Calì B (2018). Chemokines and chemokine receptors: orchestrating tumor metastasization. International journal of molecular sciences, 20(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balkwill F (2004). Cancer and the chemokine network. Nature Reviews Cancer, 4(7), 540–550. [DOI] [PubMed] [Google Scholar]
- 8.Ruffini PA, Morandi P, Cabioglu N, Altundag K, & Cristofanilli M (2007). Manipulating the chemokine-chemokine receptor network to treat cancer. Cancer, 109(12), 2392–2404. [DOI] [PubMed] [Google Scholar]
- 9.Ransohoff RM (2009). Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity, 31(5), 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandercappellen J, Van Damme J, & Struyf S (2008). The role of CXC chemokines and their receptors in cancer. Cancer Letters, 267(2), 226–244. [DOI] [PubMed] [Google Scholar]
- 11.Vilgelm AE, & Richmond A (2019). Chemokines modulate immune surveillance in tumorigenesis, metastasis, and response to immunotherapy. Frontiers in Immunology, 10, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mollica Poeta V, Massara M, Capucetti A, & Bonecchi R (2019). Chemokines and chemokine receptors: new targets for cancer immunotherapy. Frontiers in Immunology, 10, 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagarsheth N, Wicha MS, & Zou W (2017). Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nature Reviews Immunology, 17(9), 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borsig L, Wolf MJ, Roblek M, Lorentzen A, & Heikenwalder M (2014). Inflammatory chemokines and metastasis–tracing the accessory. Oncogene, 33(25), 3217–3224. [DOI] [PubMed] [Google Scholar]
- 15.Fidler IJ (2003). The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature Reviews. Cancer, 3(6), 453–458. [DOI] [PubMed] [Google Scholar]
- 16.Heppner GH, Dexter DL, DeNucci T, Miller FR, & Calabresi P (1978). Heterogeneity in drug sensitivity among tumor cell subpopulations of a single mammary tumor. Cancer Research, 38(11 Pt 1), 3758–3763. [PubMed] [Google Scholar]
- 17.Heppner GH, & Miller BE (1983). Tumor heterogeneity: biological implications and therapeutic consequences. Cancer Metastasis Reviews, 2(1), 5–23. [DOI] [PubMed] [Google Scholar]
- 18.Miller BE, Miller FR, & Heppner GH (1981). Interactions between tumor subpopulations affecting their sensitivity to the antineoplastic agents cyclophosphamide and methotrexate. Cancer Research, 41(11 Pt 1), 4378–4381. [PubMed] [Google Scholar]
- 19.Loewenstein WR (1979). Junctional intercellular communication and the control of growth. Biochimica et Biophysica Acta, 560(1), 1–65. [DOI] [PubMed] [Google Scholar]
- 20.Hunter KW, Amin R, Deasy S, Ha NH, & Wakefield L (2018). Genetic insights into the morass of metastatic heterogeneity. Nature Reviews. Cancer, 18(4), 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raz A, McLellan WL, Hart IR, Bucana CD, Hoyer LC, Sela BA, et al. (1980). Cell surface properties of B16 melanoma variants with differing metastatic potential. Cancer Research, 40(5), 1645–1651. [PubMed] [Google Scholar]
- 22.Reading CL, Belloni PN, & Nicolson GL (1980). Selection and in vivo properties of lectin-attachment variants of malignant murine lymphosarcoma cell lines. Journal of the National Cancer Institute, 64(5), 1241–1249. [PubMed] [Google Scholar]
- 23.Mathieson BJ, Zatz MM, Sharrow SO, Asofsky R, Logan W, & Kanellopoulos-Langevin C (1982). Separation and characterization of two component tumor lines within the AKR lymphoma, AKTB-1, by fluorescence-activated cell sorting and flow microfluorometry analysis. II. Differential histopathology of sIg+ and sIg− sublines. Journal of Immunology, 128(4), 1832–1838. [PubMed] [Google Scholar]
- 24.Domińguez OV, & Huseby RA (1968). Heterogeneity of induced testicular interstitial cell tumors of mice as evidenced by steroid biosynthetic enzyme activities. Cancer Research, 28(2), 348–353. [PubMed] [Google Scholar]
- 25.Kim J, & DeBerardinis RJ (2019). Mechanisms and implications of metabolic heterogeneity in cancer. Cell Metabolism, 30(3), 434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuruo T, & Fidler IJ (1981). Differences in drug sensitivity among tumor cells from parental tumors, selected variants, and spontaneous metastases. Cancer Research, 41(8), 3058–3064. [PubMed] [Google Scholar]
- 27.Kosh F (1939). Zur frage der metastazenbildung bei Impftumoren. ZKrebsforsch., 48, 495–505. [Google Scholar]
- 28.Fidler IJ, & Hart IR (1981). The origin of metastatic heterogeneity in tumors. European Journal of Cancer, 17(5), 487–494. [DOI] [PubMed] [Google Scholar]
- 29.Bosslet K, & Schirrmacher V (1982). High-frequency generation of new immunoresistant tumor variants during metastasis of a cloned murine tumor line (ESb). International Journal of Cancer Journal International du Cancer, 29(2), 195–202. [DOI] [PubMed] [Google Scholar]
- 30.Isaacs JT, Wake N, Coffey DS, & Sandberg AA (1982). Genetic instability coupled to clonal selection as a mechanism for tumor progression in the Dunning R-3327 rat prostatic adenocarcinoma system. Cancer Research, 42(6), 2353–2371. [PubMed] [Google Scholar]
- 31.Raz A (1982). Regional emergence of metastatic heterogeneity in a growing tumor. Cancer Letters, 17(2), 153–160. [DOI] [PubMed] [Google Scholar]
- 32.Poste G, Doll J, & Fidler IJ (1981). Interactions among clonal subpopulations affect stability of the metastatic phenotype in polyclonal populations of B16 melanoma cells. Proceedings of the National Academy of Sciences of the United States of America, 78(10), 6226–6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merlo LM, Pepper JW, Reid BJ, & Maley CC (2006). Cancer as an evolutionary and ecological process. Nature Reviews. Cancer, 6(12), 924–935. [DOI] [PubMed] [Google Scholar]
- 34.Moreno E (2008). Is cell competition relevant to cancer? Nature Reviews. Cancer, 8(2), 141–147. [DOI] [PubMed] [Google Scholar]
- 35.Neelakantan D, Drasin DJ, & Ford HL (2015). Intratumoral heterogeneity: clonal cooperation in epithelial-to-mesenchymal transition and metastasis. Cell Adhesion & Migration, 9(4), 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabassum DP, & Polyak K (2015). Tumorigenesis: it takes a village. Nature Reviews. Cancer, 15(8), 473–483. [DOI] [PubMed] [Google Scholar]
- 37.Cleary AS, Leonard TL, Gestl SA, & Gunther EJ (2014). Tumour cell heterogeneity maintained by cooperating subclones in Wnt-driven mammary cancers. Nature, 508(7494), 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller FR (1983). Tumor subpopulation interactions in metastasis. Invasion & Metastasis, 3(4), 234–242. [PubMed] [Google Scholar]
- 39.Celià-Terrassa T, Meca-Cortés O, & Mateo F (2012). Martínez de Paz A, Rubio N, Arnal-Estapé A, et al. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. The Journal of Clinical Investigation, 122(5), 1849–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chapman A (2014). Fernandez del Ama L, Ferguson J, Kamarashev J, Wellbrock C, Hurlstone A. Heterogeneous tumor subpopulations cooperate to drive invasion. Cell Reports, 8(3), 688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westcott JM, Prechtl AM, Maine EA, Dang TT, Esparza MA, Sun H, et al. (2015). An epigenetically distinct breast cancer cell subpopulation promotes collective invasion. The Journal of Clinical Investigation, 125(5), 1927–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fidler IJ, & Hart IR (1981). Biological and experimental consequences of the zonal composition of solid tumors. Cancer Research, 41(8), 3266–3267. [PubMed] [Google Scholar]
- 43.Lambert AW, Pattabiraman DR, & Weinberg RA (2017). Emerging biological principles of metastasis. Cell., 168(4), 670–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicolson GL, Brunson KW, & Fidler IJ (1978). Specificity of arrest, survival, and growth of selected metastatic variant cell lines. Cancer Research, 38(11 Pt 2), 4105–4111. [PubMed] [Google Scholar]
- 45.Talmadge JE, Wolman SR, & Fidler IJ (1982). Evidence for the clonal origin of spontaneous metastases. Science, 217(4557), 361–363. [DOI] [PubMed] [Google Scholar]
- 46.Garraway LA, & Lander ES (2013). Lessons from the cancer genome. Cell, 153(1), 17–37. [DOI] [PubMed] [Google Scholar]
- 47.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., & Kinzler KW (2013). Cancer genome landscapes. Science, 339(6127), 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoecklein NH, Hosch SB, Bezler M, Stern F, Hartmann CH, Vay C, et al. (2008). Direct genetic analysis of single disseminated cancer cells for prediction of outcome and therapy selection in esophageal cancer. Cancer Cell, 13(5), 441–453. [DOI] [PubMed] [Google Scholar]
- 49.Kang Y, & Pantel K (2013). Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell, 23(5), 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. (2013). Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science, 339(6119), 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. (2014). Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell., 158(5), 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maddipati R, & Stanger BZ (2015). Pancreatic cancer metastases harbor evidence of polyclonality. Cancer Discovery, 5(10), 1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McFadden DG, Papagiannakopoulos T, Taylor-Weiner A, Stewart C, Carter SL, Cibulskis K, et al. (2014). Genetic and clonal dissection of murine small cell lung carcinoma progression by genome sequencing. Cell., 156(6), 1298–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagenblast E, Soto M, Gutiérrez-Ángel S, Hartl CA, Gable AL, Maceli AR, et al. (2015). A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature., 520(7547), 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang P, & Wang JM (2018). Chemokines: the past, the present and the future. Cellular & Molecular Immunology, 15(4), 295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murphy PM (2001). Chemokines and the molecular basis of cancer metastasis. The New England Journal of Medicine, 345(11), 833–835. [DOI] [PubMed] [Google Scholar]
- 57.Zlotnik A, & Yoshie O (2000). Chemokines: a new classification system and their role in immunity. Immunity, 12(2), 121–127. [DOI] [PubMed] [Google Scholar]
- 58.Murphy PM (2002). International Union of Pharmacology. XXX. Update on chemokine receptor nomenclature. Pharmacological Reviews, 54(2), 227–229. [DOI] [PubMed] [Google Scholar]
- 59.Thelen M (2001). Dancing to the tune of chemokines. Nature Immunology, 2(2), 129–134. [DOI] [PubMed] [Google Scholar]
- 60.Nibbs RJ, & Graham GJ (2013). Immune regulation by atypical chemokine receptors. Nature Reviews. Immunology, 13(11), 815–829. [DOI] [PubMed] [Google Scholar]
- 61.Massara M, Bonavita O, Mantovani A, Locati M, & Bonecchi R (2016). Atypical chemokine receptors in cancer: friends or foes? Journal of Leukocyte Biology, 99(6), 927–933. [DOI] [PubMed] [Google Scholar]
- 62.Lokeshwar BL, Kallifatidis G, & Hoy JJ (2020). Atypical chemokine receptors in tumor cell growth and metastasis. Advances in Cancer Research, 145, 1–27. [DOI] [PubMed] [Google Scholar]
- 63.Mantovani A (1999). The chemokine system: redundancy for robust outputs. Immunology Today, 20(6), 254–257. [DOI] [PubMed] [Google Scholar]
- 64.Hughes CE, & Nibbs RJB (2018). A guide to chemokines and their receptors. The FEBS Journal, 285(16), 2944–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baggiolini M, Dewald B, & Moser B (1997). Human chemokines: an update. Annual Review of Immunology, 15, 675–705. [DOI] [PubMed] [Google Scholar]
- 66.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, et al. (1995). The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. The Journal of Biological Chemistry, 270(45), 27348–27357. [DOI] [PubMed] [Google Scholar]
- 67.Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, & Belperio JA (2006). Cancer CXC chemokine networks and tumour angiogenesis. European Journal of Cancer (Oxford, England: 1990), 42(6), 768–778. [DOI] [PubMed] [Google Scholar]
- 68.Murphy PM, Baggiolini M, Charo IF, Hébert CA, Horuk R, Matsushima K, et al. (2000). International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacological Reviews, 52(1), 145–176. [PubMed] [Google Scholar]
- 69.Scapini P, Morini M, Tecchio C, Minghelli S, Di Carlo E, Tanghetti E, et al. (2004). CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-A. Journal of Immunology, 172(8), 5034–5040. [DOI] [PubMed] [Google Scholar]
- 70.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, et al. (2000). CXC chemokines in angiogenesis. Journal of Leukocyte Biology, 68(1), 1–8. [PubMed] [Google Scholar]
- 71.Luster AD (1998). Chemokines–chemotactic cytokines that mediate inflammation. The New England Journal of Medicine, 338(7), 436–445. [DOI] [PubMed] [Google Scholar]
- 72.Strieter RM, Kunkel SL, Arenberg DA, Burdick MD, & Polverini PJ (1995). Interferon gamma-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochemical and Biophysical Research Communications, 210(1), 51–57. [DOI] [PubMed] [Google Scholar]
- 73.Baggiolini M, Dewald B, & Moser B (1994). Interleukin-8 and related chemotactic cytokines–CXC and CC chemokines. Advances in Immunology, 55, 97–179. [PubMed] [Google Scholar]
- 74.Bischoff SC, Krieger M, Brunner T, Rot A, von Tscharner V, Baggiolini M, et al. (1993). RANTES and related chemokines activate human basophil granulocytes through different G protein-coupled receptors. European Journal of Immunology, 23(3), 761–767. [DOI] [PubMed] [Google Scholar]
- 75.Dahinden CA, Geiser T, Brunner T, von Tscharner V, Caput D, Ferrara P, et al. (1994). Monocyte chemotactic protein 3 is a most effective basophil- and eosinophil-activating chemokine. The Journal of Experimental Medicine, 179(2), 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, & Luster AD (1996). Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nature Medicine, 2(4), 449–456. [DOI] [PubMed] [Google Scholar]
- 77.Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, & Yoshie O (1997). The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. The Journal of Biological Chemistry, 272(23), 15036–15042. [DOI] [PubMed] [Google Scholar]
- 78.Jose PJ, Griffiths-Johnson DA, Collins PD, Walsh DT, Moqbel R, Totty NF, et al. (1994). Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. The Journal of Experimental Medicine, 179(3), 881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kameyoshi Y, Dörschner A, Mallet AI, Christophers E, & Schröder JM (1992). Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. The Journal of Experimental Medicine, 176(2), 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam N, et al. (1996). Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. The Journal of Clinical Investigation, 97(3), 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, & Dahinden CA (1992). RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. The Journal of Experimental Medicine, 176(6), 1489–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelner GS, Kennedy J, Bacon KB, Kleyensteuber S, Largaespada DA, Jenkins NA, et al. (1994). Lymphotactin: a cytokine that represents a new class of chemokine. Science, 266(5189), 1395–1399. [DOI] [PubMed] [Google Scholar]
- 83.Kroczek RA, & Henn V (2012). The role of XCR1 and its ligand XCL1 in antigen cross-presentation by murine and human dendritic cells. Frontiers in Immunology, 3, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, et al. (1997). A new class of membrane-bound chemokine with a CX3C motif. Nature., 385(6617), 640–644. [DOI] [PubMed] [Google Scholar]
- 85.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, et al. (1997). Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature., 387(6633), 611–617. [DOI] [PubMed] [Google Scholar]
- 86.Segerer S, Hughes E, Hudkins KL, Mack M, Goodpaster T, & Alpers CE (2002). Expression of the fractalkine receptor (CX3CR1) in human kidney diseases. Kidney International, 62(2), 488–495. [DOI] [PubMed] [Google Scholar]
- 87.Umehara H, & Imai T (2001). Role of fractalkine in leukocyte adhesion and migration and in vascular injury. Drug News & Perspectives, 14(8), 460–464. [DOI] [PubMed] [Google Scholar]
- 88.Ruffini PA (2019). The CXCL8-CXCR1/2 axis as a therapeutic target in breast cancer stem-like cells. Frontiers in Oncology, 9, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, et al. (2016). The CXCL8-CXCR1/2 pathways in cancer. Cytokine & Growth Factor Reviews, 31, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu S, Saxena S, Varney ML, & Singh RK (2017). CXCR1/2 chemokine network regulates melanoma resistance to chemotherapies mediated by NF-κB. Current Molecular Medicine, 17(6), 436–449. [DOI] [PubMed] [Google Scholar]
- 91.Wu L, Saxena S, Awaji M, Singh RK. Tumor-associated neutrophils in cancer: going pro. Cancers. 2019;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Greene S, Robbins Y, Mydlarz WK, Huynh AP, Schmitt NC, Friedman J, et al. (2020). Inhibition of MDSC trafficking with SX-682, a CXCR1/2 inhibitor, enhances NK-Cell immunotherapy in head and neck cancer models. Clinical Cancer Research, 26(6), 1420–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh S, Wu S, Varney M, Singh AP, & Singh RK (2011). CXCR1 and CXCR2 silencing modulates CXCL8-dependent endothelial cell proliferation, migration and capillary-like structure formation. Microvascular Research, 82(3), 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharma B, Nannuru KC, Saxena S, Varney ML, & Singh RK (2019). CXCR2: a novel mediator of mammary tumor bone metastasis. International journal of molecular sciences, 20(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Y, He Y, Butler W, Xu L, Chang Y, Lei K, et al. (2019). Targeting cellular heterogeneity with CXCR2 blockade for the treatment of therapy-resistant prostate cancer. Science translational medicine, 11(521). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Purohit A, Varney M, Rachagani S, Ouellette MM, Batra SK, & Singh RK (2016). CXCR2 signaling regulates KRAS(G12D)-induced autocrine growth of pancreatic cancer. Oncotarget., 7(6), 7280–7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun KH, Sun GH, Wu YC, Ko BJ, Hsu HT, & Wu ST (2016). TNF-α augments CXCR2 and CXCR3 to promote progression of renal cell carcinoma. Journal of Cellular and Molecular Medicine, 20(11), 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, et al. (2016). CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell, 29(6), 832–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kral JB, Schrottmaier WC, Salzmann M, & Assinger A (2016). Platelet interaction with innate immune cells. Transfusion Medicine and Hemotherapy: Offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie, 43(2), 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Awaji M, Saxena S, Wu L, Prajapati DR, Purohit A, Varney ML, et al. (2020). CXCR2 signaling promotes secretory cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. The FASEB Journal, 34(7), 9405–9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jenh CH, Cox MA, Hipkin W, Lu T, Pugliese-Sivo C, Gonsiorek W, et al. (2001). Human B cell-attracting chemokine 1 (BCA-1; CXCL13) is an agonist for the human CXCR3 receptor. Cytokine., 15(3), 113–121. [DOI] [PubMed] [Google Scholar]
- 102.Kundu N, Ma X, Brox R, Fan X, Kochel T, Reader J, et al. (2019). The chemokine receptor CXCR3 isoform B drives breast cancer stem cells. Breast Cancer: Basic and Clinical Research, 13, 1178223419873628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kawada K, Hosogi H, Sonoshita M, Sakashita H, Manabe T, Shimahara Y, et al. (2007). Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene, 26(32), 4679–4688. [DOI] [PubMed] [Google Scholar]
- 104.Doron H, Amer M, Ershaid N, Blazquez R, Shani O, Lahav TG, et al. (2019). Inflammatory activation of astrocytes facilitates melanoma brain tropism via the CXCL10-CXCR3 signaling axis. Cell Reports, 28(7), 1785–98.e6. [DOI] [PubMed] [Google Scholar]
- 105.Manukyan G, Papajik T, Mikulkova Z, Urbanova R, Kraiczova VS, Savara J, et al. (2020). High CXCR3 on leukemic cells distinguishes IgHV (mut) from IgHV (unmut) in chronic lymphocytic leukemia: evidence from CD5(high) and CD5(low) clones. Journal of Immunology Research, 2020, 7084268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De Simone G, Mazza EMC, Cassotta A, Davydov AN, Kuka M, Zanon V, et al. (2019). CXCR3 identifies human naive CD8(+) T cells with enhanced effector differentiation potential. Journal of Immunology, 203(12), 3179–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pinedo HM, Verheul HM, D’Amato RJ, & Folkman J (1998). Involvement of platelets in tumour angiogenesis? Lancet (London, England), 352(9142), 1775–1777. [DOI] [PubMed] [Google Scholar]
- 108.Xu C, Zhao H, Chen H, & Yao Q (2015). CXCR4 in breast cancer: oncogenic role and therapeutic targeting. Drug Design, Development and Therapy, 9, 4953–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arya M, Patel HR, McGurk C, Tatoud R, Klocker H, Masters J, et al. (2004). The importance of the CXCL12-CXCR4 chemokine ligand-receptor interaction in prostate cancer metastasis. Journal of Experimental Therapeutics & Oncology, 4(4), 291–303. [PubMed] [Google Scholar]
- 110.Zhao BC, Wang ZJ, Mao WZ, Ma HC, Han JG, Zhao B, et al. (2011). CXCR4/SDF-1 axis is involved in lymph node metastasis of gastric carcinoma. World Journal of Gastroenterology, 17(19), 2389–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y, Ren CC, Yang L, Xu YM, & Chen YN (2019). Role of CXCL12-CXCR4 axis in ovarian cancer metastasis and CXCL12-CXCR4 blockade with AMD3100 suppresses tumor cell migration and invasion in vitro. Journal of Cellular Physiology, 234(4), 3897–3909. [DOI] [PubMed] [Google Scholar]
- 112.Kaifi JT, Yekebas EF, Schurr P, Obonyo D, Wachowiak R, Busch P, et al. (2005). Tumor-cell homing to lymph nodes and bone marrow and CXCR4 expression in esophageal cancer. Journal of the National Cancer Institute, 97(24), 1840–1847. [DOI] [PubMed] [Google Scholar]
- 113.Hughes R, Qian BZ, Rowan C, Muthana M, Keklikoglou I, Olson OC, et al. (2015). Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Research, 75(17), 3479–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liekens S, Schols D, & Hatse S (2010). CXCL12-CXCR4 axis in angiogenesis, metastasis and stem cell mobilization. Current Pharmaceutical Design, 16(35), 3903–3920. [DOI] [PubMed] [Google Scholar]
- 115.De Filippo K, & Rankin SM (2018). CXCR4, the master regulator of neutrophil trafficking in homeostasis and disease. European Journal of Clinical Investigation, 48(Suppl 2), e12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu Y, Fang F, Jiao H, Zheng X, Huang L, Yi X, et al. (2019). Activated hepatic stellate cells regulate MDSC migration through the SDF-1/CXCR4 axis in an orthotopic mouse model of hepatocellular carcinoma. Cancer Immunology, Immunotherapy: CII, 68(12), 1959–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vandercappellen J, Van Damme J, & Struyf S (2011). The role of the CXC chemokines platelet factor-4 (CXCL4/PF-4) and its variant (CXCL4L1/PF-4var) in inflammation, angiogenesis and cancer. Cytokine & Growth Factor Reviews, 22(1), 1–18. [DOI] [PubMed] [Google Scholar]
- 118.Charbonneau B, Wang AH, Maurer MJ, Asmann YW, Zent CS, Link BK, et al. (2013). CXCR5 polymorphisms in non-Hodgkin lymphoma risk and prognosis. Cancer Immunology, Immunotherapy: CII, 62(9), 1475–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meijer J, Zeelenberg IS, Sipos B, & Roos E (2006). The CXCR5 chemokine receptor is expressed by carcinoma cells and promotes growth of colon carcinoma in the liver. Cancer Research, 66(19), 9576–9582. [DOI] [PubMed] [Google Scholar]
- 120.Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, & Moser B (1998). B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. The Journal of Experimental Medicine, 187(4), 655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bai M, Zheng Y, Liu H, Su B, Zhan Y, & He H (2017). CXCR5(+) CD8(+) T cells potently infiltrate pancreatic tumors and present high functionality. Experimental Cell Research, 361(1), 39–45. [DOI] [PubMed] [Google Scholar]
- 122.Ablett MP, O’Brien CS, Sims AH, Farnie G, & Clarke RB (2014). A differential role for CXCR4 in the regulation of normal versus malignant breast stem cell activity. Oncotarget., 5(3), 599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kapur N, Mir H, Sonpavde GP, Jain S, Bae S, Lillard JW Jr., et al. (2019). Prostate cancer cells hyper-activate CXCR6 signaling by cleaving CXCL16 to overcome effect of docetaxel. Cancer Letters, 454, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tao H, & Chen X (2019). Role of CXCR6-deficient natural killer T cells and CD4 T cells in hepatocarcinogenesis. Gastroenterology, 157(4), 1169–1170. [DOI] [PubMed] [Google Scholar]
- 125.Choreño-Parra JA, Jiménez-Álvarez LA, Muñoz-Torrico M, Ramírez-Martínez G, Jiménez-Zamudio LA, Salinas-Lara C, et al. (2020). Antigens of Mycobacterium tuberculosis stimulate CXCR6+ natural killer cells. Frontiers in Immunology, 11, 582414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang C, Chen W, & Shen J (2018). CXCR7 targeting and its major disease relevance. Frontiers in Pharmacology, 9, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qian T, Liu Y, Dong Y, Zhang L, Dong Y, Sun Y, et al. (2018). CXCR7 regulates breast tumor metastasis and angiogenesis in vivo and in vitro. Molecular Medicine Reports, 17(3), 3633–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, et al. (2003). An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. The Journal of Experimental Medicine, 197(11), 1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li S, Fong KW, Gritsina G, Zhang A, Zhao JC, Kim J, et al. (2019). Activation of MAPK signaling by CXCR7 leads to enzalutamide resistance in prostate cancer. Cancer Research, 79(10), 2580–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tanegashima K, Takahashi R, Nuriya H, Iwase R, Naruse N, Tsuji K, et al. (2017). CXCL14 acts as a specific carrier of CpG DNA into dendritic cells and activates Toll-like receptor 9-mediated adaptive immunity. EBioMedicine., 24, 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Korbecki J, Kojder K, Simińska D, Bohatyrewicz R, Gutowska I, Chlubek D, et al. (2020). CC chemokines in a tumor: a review of pro-cancer and anti-cancer properties of the ligands of receptors CCR1, CCR2, CCR3, and CCR4. International Journal of Molecular Sciences, 21(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yamamoto T, Kawada K, Itatani Y, Inamoto S, Okamura R, Iwamoto M, et al. (2017). Loss of SMAD4 promotes lung metastasis of colorectal cancer by accumulation of CCR1+ tumor-associated neutrophils through CCL15-CCR1 Axis. Clinical Cancer Research, 23(3), 833–844. [DOI] [PubMed] [Google Scholar]
- 133.Clemetson KJ, Clemetson JM, Proudfoot AE, Power CA, Baggiolini M, & Wells TN (2000). Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood., 96(13), 4046–4054. [PubMed] [Google Scholar]
- 134.Li X, Yao W, Yuan Y, Chen P, Li B, Li J, et al. (2017). Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut., 66(1), 157–167. [DOI] [PubMed] [Google Scholar]
- 135.Flores-Toro JA, Luo D, Gopinath A, Sarkisian MR, Campbell JJ, Charo IF, et al. (2020). CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proceedings of the National Academy of Sciences of the United States of America, 117(2), 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, & Coughlin SR (1994). Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proceedings of the National Academy of Sciences of the United States of America, 91(7), 2752–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fujii K, Sakamoto Y, Masaki A, Murase T, Tashiro Y, Yonekura K, et al. (2021). Immunohistochemistry for CCR4 C-terminus predicts CCR4 mutations and mogamulizumab efficacy in adult T-cell leukemia/lymphoma. The Journal of Pathology Clinical Research, 7(1), 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Argyle D, & Kitamura T (2018). Targeting macrophage-recruiting chemokines as a novel therapeutic strategy to prevent the progression of solid tumors. Frontiers in Immunology, 9, 2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aldinucci D, Borghese C, & Casagrande N (2020). The CCL5/CCR5 axis in cancer progression. Cancers, 12(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nie Y, Huang H, Guo M, Chen J, Wu W, Li W, et al. (2019). Breast phyllodes tumors recruit and repolarize tumor-associated macrophages via secreting CCL5 to promote malignant progression, which can be inhibited by CCR5 inhibition therapy. Clinical Cancer Research, 25(13), 3873–3886. [DOI] [PubMed] [Google Scholar]
- 141.Kadomoto S, Izumi K, & Mizokami A (2020). The CCL20-CCR6 axis in cancer progression. International Journal of Molecular Sciences, 21(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Paulissen SM, van Hamburg JP, Dankers W, & Lubberts E (2015). The role and modulation of CCR6+ Th17 cell populations in rheumatoid arthritis. Cytokine, 74(1), 43–53. [DOI] [PubMed] [Google Scholar]
- 143.Singh TP, Zhang HH, Borek I, Wolf P, Hedrick MN, Singh SP, et al. (2016). Monocyte-derived inflammatory Langerhans cells and dermal dendritic cells mediate psoriasis-like inflammation. Nature Communications, 7, 13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mishan MA, Ahmadiankia N, & Bahrami AR (2016). CXCR4 and CCR7: two eligible targets in targeted cancer therapy. Cell Biology International, 40(9), 955–967. [DOI] [PubMed] [Google Scholar]
- 145.Bromley SK, Thomas SY, & Luster AD (2005). Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nature Immunology, 6(9), 895–901. [DOI] [PubMed] [Google Scholar]
- 146.Riol-Blanco L, Sánchez-Sánchez N, Torres A, Tejedor A, Narumiya S, Corbí AL, et al. (2005). The chemokine receptor CCR7 activates in dendritic cells two signaling modules that independently regulate chemotaxis and migratory speed. Journal of Immunology, 174(7), 4070–4080. [DOI] [PubMed] [Google Scholar]
- 147.Payne D, Drinkwater S, Baretto R, Duddridge M, & Browning MJ (2009). Expression of chemokine receptors CXCR4, CXCR5 and CCR7 on B and T lymphocytes from patients with primary antibody deficiency. Clinical and Experimental Immunology, 156(2), 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Villarreal DO, L’Huillier A, Armington S, Mottershead C, Filippova EV, Coder BD, et al. (2018). Targeting CCR8 induces protective antitumor immunity and enhances vaccine-induced responses in colon cancer. Cancer Research, 78(18), 5340–5348. [DOI] [PubMed] [Google Scholar]
- 149.Amersi FF, Terando AM, Goto Y, Scolyer RA, Thompson JF, Tran AN, et al. (2008). Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clinical Cancer Research, 14(3), 638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Singh S, Singh UP, Stiles JK, Grizzle WE, & Lillard JW Jr. (2004). Expression and functional role of CCR9 in prostate cancer cell migration and invasion. Clinical Cancer Research, 10(24), 8743–8750. [DOI] [PubMed] [Google Scholar]
- 151.Murakami T, Cardones AR, Finkelstein SE, Restifo NP, Klaunberg BA, Nestle FO, et al. (2003). Immune evasion by murine melanoma mediated through CC chemokine receptor-10. The Journal of Experimental Medicine, 198(9), 1337–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brewitz A, Eickhoff S, Dähling S, Quast T, Bedoui S, Kroczek RA, et al. (2017). CD8(+) T cells orchestrate pDC-XCR1(+) dendritic cell spatial and functional cooperativity to optimize priming. Immunity., 46(2), 205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang H, Cai J, Du S, Guo Z, Xin B, Wang J, et al. (2017). Fractalkine/CX3CR1 induces apoptosis resistance and proliferation through the activation of the AKT/NF-κB cascade in pancreatic cancer cells. Cell Biochemistry and Function, 35(6), 315–326. [DOI] [PubMed] [Google Scholar]
- 154.Liu P, Liang Y, Jiang L, Wang H, Wang S, & Dong J (2018). CX3CL1/fractalkine enhances prostate cancer spinal metastasis by activating the Src/FAK pathway. International Journal of Oncology, 53(4), 1544–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shen F, Zhang Y, Jernigan DL, Feng X, Yan J, Garcia FU, et al. (2016). Novel small-molecule CX3CR1 antagonist impairs metastatic seeding and colonization of breast cancer cells. Molecular Cancer Research, 14(6), 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Conroy MJ, & Lysaght J (2020). CX3CL1 signaling in the tumor microenvironment. Adv. Exp. Med. Biol, 1231, 1–12. [DOI] [PubMed] [Google Scholar]
- 157.Tian W, Jiang X, Kim D, Guan T, Nicolls MR, & Rockson SG (2020). Leukotrienes in tumor-associated inflammation. Frontiers in Pharmacology, 11, 1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jala VR, Bodduluri SR, Satpathy SR, Chheda Z, Sharma RK, & Haribabu B (2017). The yin and yang of leukotriene B(4) mediated inflammation in cancer. Seminars in Immunology, 33, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zlotnik A, Burkhardt AM, & Homey B (2011). Homeostatic chemokine receptors and organ-specific metastasis. Nature Reviews. Immunology, 11(9), 597–606. [DOI] [PubMed] [Google Scholar]
- 160.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. (2001). Involvement of chemokine receptors in breast cancer metastasis. Nature, 410(6824), 50–56. [DOI] [PubMed] [Google Scholar]
- 161.Gao Y, Bado I, Wang H, Zhang W, Rosen JM, & Zhang XH (2019). Metastasis organotropism: redefining the congenial soil. Developmental Cell, 49(3), 375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wiley HE, Gonzalez EB, Maki W, Wu MT, & Hwang ST (2001). Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. Journal of the National Cancer Institute, 93(21), 1638–1643. [DOI] [PubMed] [Google Scholar]
- 163.Cunningham HD, Shannon LA, Calloway PA, Fassold BC, Dunwiddie I, Vielhauer G, et al. (2010). Expression of the C-C chemokine receptor 7 mediates metastasis of breast cancer to the lymph nodes in mice. Translational Oncology, 3(6), 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Murakami T, Maki W, Cardones AR, Fang H, Tun Kyi A, Nestle FO, et al. (2002). Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Research, 62(24), 7328–7334. [PubMed] [Google Scholar]
- 165.Ye QH, Qin LX, Forgues M, He P, Kim JW, Peng AC, et al. (2003). Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nature Medicine, 9(4), 416–423. [DOI] [PubMed] [Google Scholar]
- 166.Bohn OL, Nasir I, Brufsky A, Tseng GC, Bhargava R, MacManus K, et al. (2009). Biomarker profile in breast carcinomas presenting with bone metastasis. International Journal of Clinical and Experimental Pathology, 3(2), 139–146. [PMC free article] [PubMed] [Google Scholar]
- 167.Fingleton B (2007). Molecular targets in metastasis: lessons from genomic approaches. Cancer Genomics & Proteomics, 4(3), 211–221. [PubMed] [Google Scholar]
- 168.Budczies J, von Winterfeld M, Klauschen F, Bockmayr M, Lennerz JK, Denkert C, et al. (2015). The landscape of metastatic progression patterns across major human cancers. Oncotarget, 6(1), 570–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Letsch A, Keilholz U, Schadendorf D, Assfalg G, Asemissen AM, Thiel E, et al. (2004). Functional CCR9 expression is associated with small intestinal metastasis. The Journal of Investigative Dermatology, 122(3), 685–690. [DOI] [PubMed] [Google Scholar]
- 170.Brigati C, Noonan DM, Albini A, & Benelli R (2002). Tumors and inflammatory infiltrates: friends or foes? ClinExpMetastasis, 19(3), 247–258. [DOI] [PubMed] [Google Scholar]
- 171.Coussens LM, & Werb Z (2002). Inflammation and cancer. Nature, 420(6917), 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ojalvo LS, King W, Cox D, & Pollard JW (2009). High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. American Journal of Pathology, 174(3), 1048–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Varney ML, Johansson SL, & Singh RK (2005). Tumour-associated macrophage infiltration, neovascularization and aggressiveness in malignant melanoma: role of monocyte chemotactic protein-1 and vascular endothelial growth factor-A. Melanoma Research, 15(5), 417–425. [DOI] [PubMed] [Google Scholar]
- 174.Lu Y, Cai Z, Xiao G, Liu Y, Keller ET, Yao Z, et al. (2007). CCR2 expression correlates with prostate cancer progression. Journal of Cellular Biochemistry, 101(3), 676–685. [DOI] [PubMed] [Google Scholar]
- 175.Lu Y, Cai Z, Xiao G, Keller ET, Mizokami A, Yao Z, et al. (2007). Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Research, 67(8), 3646–3653. [DOI] [PubMed] [Google Scholar]
- 176.Kuroda T, Kitadai Y, Tanaka S, Yang X, Mukaida N, Yoshihara M, et al. (2005). Monocyte chemoattractant protein-1 transfection induces angiogenesis and tumorigenesis of gastric carcinoma in nude mice via macrophage recruitment. Clinical Cancer Research, 11(21), 7629–7636. [DOI] [PubMed] [Google Scholar]
- 177.Nakashima E, Mukaida N, Kubota Y, Kuno K, Yasumoto K, Ichimura F, et al. (1995). Human MCAF gene transfer enhances the metastatic capacity of a mouse cachectic adenocarcinoma cell line in vivo. PharmRes, 12(11), 1598–1604. [DOI] [PubMed] [Google Scholar]
- 178.Perussia B (1992). Tumor infiltrating cells. Laboratory Investigation, 67(2), 155–157. [PubMed] [Google Scholar]
- 179.Mantovani A, Schioppa T, Porta C, Allavena P, & Sica A (2006). Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Reviews, 25(3), 315–322. [DOI] [PubMed] [Google Scholar]
- 180.Locati M, Otero K, Schioppa T, Signorelli P, Perrier P, Baviera S, et al. (2002). The chemokine system: tuning and shaping by regulation of receptor expression and coupling in polarized responses. Allergy, 57(11), 972–982. [DOI] [PubMed] [Google Scholar]
- 181.Sica A, Saccani A, & Mantovani A (2002). Tumor-associated macrophages: a molecular perspective. IntImmunopharmacol, 2(8), 1045–1054. [DOI] [PubMed] [Google Scholar]
- 182.Balkwill F, & Mantovani A (2001). Inflammation and cancer: back to Virchow? Lancet (London, England), 357(9255), 539–545. [DOI] [PubMed] [Google Scholar]
- 183.Zlotnik A (2006). Chemokines and cancer. International Journal of Cancer, 119(9), 2026–2029. [DOI] [PubMed] [Google Scholar]
- 184.Joyce JA, & Pollard JW (2009). Microenvironmental regulation of metastasis. Nature Reviews. Cancer, 9(4), 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kitamura T, Qian BZ, & Pollard JW (2015). Immune cell promotion of metastasis. Nature Reviews. Immunology, 15(2), 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Quail DF, & Joyce JA (2013). Microenvironmental regulation of tumor progression and metastasis. Nature Medicine, 19(11), 1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Qian BZ, & Pollard JW (2010). Macrophage diversity enhances tumor progression and metastasis. Cell., 141(1), 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, et al. (2012). Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One, 7(12), e50946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yin S, Huang J, Li Z, Zhang J, Luo J, Lu C, et al. (2017). The prognostic and clinicopathological significance of tumor-associated macrophages in patients with gastric cancer: a meta-analysis. PLoS One, 12(1), e0170042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Guo B, Cen H, Tan X, & Ke Q (2016). Meta-analysis of the prognostic and clinical value of tumor-associated macrophages in adult classical Hodgkin lymphoma. BMC Medicine, 14(1), 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhao X, Qu J, Sun Y, Wang J, Liu X, Wang F, et al. (2017). Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget, 8(18), 30576–30586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ruffell B, & Coussens LM (2015). Macrophages and therapeutic resistance in cancer. Cancer Cell, 27(4), 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Krneta T, Gillgrass A, Chew M, & Ashkar AA (2016). The breast tumor microenvironment alters the phenotype and function of natural killer cells. Cellular & Molecular Immunology, 13(5), 628–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wu Y, Kuang DM, Pan WD, Wan YL, Lao XM, Wang D, et al. (2013). Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology (Baltimore, Md), 57(3), 1107–1116. [DOI] [PubMed] [Google Scholar]
- 195.Mantovani A, Marchesi F, Malesci A, Laghi L, & Allavena P (2017). Tumour-associated macrophages as treatment targets in oncology. Nature Reviews. Clinical Oncology, 14(7), 399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Carr MW, Roth SJ, Luther E, Rose SS, & Springer TA (1994). Monocyte chemoattractant protein 1 acts as a T lymphocyte chemoattractant. ProcNatlAcadSciUSA., 91(9), 3652–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, et al. (1998). CCR5 is characteristic of Th1 lymphocytes. Nature., 391(6665), 344–345. [DOI] [PubMed] [Google Scholar]
- 198.Loetscher P, & Clark-Lewis I (2001). Agonistic and antagonistic activities of chemokines. Journal of Leukocyte Biology, 69(6), 881–884. [PubMed] [Google Scholar]
- 199.Loetscher P, Moser B, & Baggiolini M (2000). Chemokines and their receptors in lymphocyte traffic and HIV infection. Advances in Immunology, 74, 127–180. [DOI] [PubMed] [Google Scholar]
- 200.Youngs SJ, Ali SA, Taub DD, & Rees RC (1997). Chemokines induce migrational responses in human breast carcinoma cell lines. International Journal of Cancer, 71(2), 257–266. [DOI] [PubMed] [Google Scholar]
- 201.Kara EE, McKenzie DR, Bastow CR, Gregor CE, Fenix KA, Ogunniyi AD, et al. (2015). CCR2 defines in vivo development and homing of IL-23-driven GM-CSF-producing Th17 cells. Nature Communications, 6, 8644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jala VR, Bodduluri SR, Ghosh S, Chheda Z, Singh R, Smith ME, et al. (2021). Absence of CCR2 reduces spontaneous intestinal tumorigenesis in the Apc(Min) (/+) mouse model. International Journal of Cancer. [DOI] [PubMed] [Google Scholar]
- 203.Fein MR, He XY, Almeida AS, Bružas E, Pommier A, Yan R, et al. (2020). Cancer cell CCR2 orchestrates suppression of the adaptive immune response. J Exp Med, 217(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tu MM, Abdel-Hafiz HA, Jones RT, Jean A, Hoff KJ, Duex JE, et al. (2020). Inhibition of the CCL2 receptor, CCR2, enhances tumor response to immune checkpoint therapy. Communications Biology, 3(1), 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lim SY, Yuzhalin AE, Gordon-Weeks AN, & Muschel RJ (2016). Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget, 7(19), 28697–28710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schraufstatter IU, Zhao M, Khaldoyanidi SK, & Discipio RG (2012). The chemokine CCL18 causes maturation of cultured monocytes to macrophages in the M2 spectrum. Immunology, 135(4), 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Halvorsen EC, Hamilton MJ, Young A, Wadsworth BJ, LePard NE, Lee HN, et al. (2016). Maraviroc decreases CCL8-mediated migration of CCR5(+) regulatory T cells and reduces metastatic tumor growth in the lungs. Oncoimmunology, 5(6), e1150398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Farmaki E, Kaza V, Papavassiliou AG, Chatzistamou I, & Kiaris H (2017). Induction of the MCP chemokine cluster cascade in the periphery by cancer cell-derived Ccl3. Cancer Letters, 389, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, et al. (2009). A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One, 4(8), e6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, et al. (2015). CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. The Journal of Experimental Medicine, 212(7), 1043–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kitamura T, Doughty-Shenton D, Cassetta L, Fragkogianni S, Brownlie D, Kato Y, et al. (2017). Monocytes differentiate to immune suppressive precursors of metastasis-associated macrophages in mouse models of metastatic breast cancer. Frontiers in Immunology, 8, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen Q, Zhang XH, & Massagué J (2011). Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell, 20(4), 538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wu M, Ma M, Tan Z, Zheng H, & Liu X (2020). Neutrophil: a new player in metastatic cancers. Frontiers in Immunology, 11, 565165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Masucci MT, Minopoli M, & Carriero MV (2019). Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Frontiers in Oncology, 9, 1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, & Naora H (2019). Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. The Journal of Experimental Medicine, 216(1), 176–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang D, Sun H, Wei J, Cen B, & DuBois RN (2017). CXCL1 is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Research, 77(13), 3655–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X, et al. (2016). Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell, 30(2), 243–256. [DOI] [PubMed] [Google Scholar]
- 218.Dinapoli MR, Calderon CL, & Lopez DM (1996). The altered tumoricidal capacity of macrophages isolated from tumor-bearing mice is related to reduce expression of the inducible nitric oxide synthase gene. Journal of Experimental Medicine, 183(4), 1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Young MRI, & Wright MA (1992). Myelopoiesis-associated immune suppressor cells in mice bearing metastatic Lewis lung carcinoma tumors: gamma-interferon plus tumor necrosis factor-alpha synergistically reduces immune suppressor and tumor growth-promoting activities of bone marrow cells and diminishes tumor recurrence and metastasis. Cancer Research, 52, 6335–6340. [PubMed] [Google Scholar]
- 220.Huang S, Singh RK, Xie K, Gutman M, Berry KK, Bucana CD, et al. (1994). Expression of the JE/MCP-1 gene suppresses metastatic potential in murine colon carcinoma cells. Cancer Immunology, Immunotherapy, 39(4), 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Huang S, Xie K, Singh RK, Gutman M, & Bar-Eli M (1995). Suppression of tumor growth and metastasis of murine renal adenocarcinoma by syngeneic fibroblasts genetically engineered to secrete the JE/MCP-1 cytokine. Journal of Interferon & Cytokine Research, 15(7), 655–665. [DOI] [PubMed] [Google Scholar]
- 222.Talmadge JE, Key M, & Fidler IJ (1981). Macrophage content of metastatic and nonmetastatic rodent neoplasms. Journal of Immunology, 126(6), 2245–2248. [PubMed] [Google Scholar]
- 223.Whiteside TL, Miescher S, Hurlimann J, & Moretta L (1986). von F, V. Clonal analysis and in situ characterization of lymphocytes infiltrating human breast carcinomas. Cancer Immunology, Immunotherapy, 23(3), 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.McDonald KA, Kawaguchi T, Qi Q, Peng X, Asaoka M, Young J, et al. (2019). Tumor heterogeneity correlates with less immune response and worse survival in breast cancer patients. Annals of Surgical Oncology, 26(7), 2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Miranda A, Hamilton PT, Zhang AW, Pattnaik S, Becht E, Mezheyeuski A, et al. (2019). Cancer stemness, intratumoral heterogeneity, and immune response across cancers. Proceedings of the National Academy of Sciences of the United States of America, 116(18), 9020–9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Folkman J, & Cotran R (1976). Relation of vascular proliferation to tumor growth. International Review of Experimental Pathology, 16, 207–248. [PubMed] [Google Scholar]
- 227.Folkman J (1985). Tumor angiogenesis. Advances in Cancer Research, 43, 175–203. [DOI] [PubMed] [Google Scholar]
- 228.Folkman J, & Klagsbrun M (1987). Angiogenic factors. Science, 235(4787), 442–447. [DOI] [PubMed] [Google Scholar]
- 229.Leibovich SJ, & Wiseman DM (1988). Macrophages, wound repair and angiogenesis. Progress in Clinical and Biological Research, 266, 131–145. [PubMed] [Google Scholar]
- 230.Wulf GG, Jackson KA, & Goodell MA (2001). Somatic stem cell plasticity: current evidence and emerging concepts. Experimental Hematology, 29(12), 1361–1370. [DOI] [PubMed] [Google Scholar]
- 231.Hanahan D, & Weinberg RA (2011). Hallmarks of cancer: the next generation. Cell., 144(5), 646–674. [DOI] [PubMed] [Google Scholar]
- 232.Strieter RM, Belperio JA, Phillips RJ, & Keane MP (2004). CXC chemokines in angiogenesis of cancer. Seminars in Cancer Biology, 14(3), 195–200. [DOI] [PubMed] [Google Scholar]
- 233.Martin D, Galisteo R, & Gutkind JS (2009). CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. The Journal of Biological Chemistry, 284(10), 6038–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Salcedo R, & Oppenheim JJ (2003). Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation (New York, NY: 1994), 10(3–4), 359–370. [DOI] [PubMed] [Google Scholar]
- 235.Carmeliet P (2005). VEGF as a key mediator of angiogenesis in cancer. Oncology, 69(Suppl 3), 4–10. [DOI] [PubMed] [Google Scholar]
- 236.Hwang J, Son KN, Kim CW, Ko J, Na DS, Kwon BS, et al. (2005). Human CC chemokine CCL23, a ligand for CCR1, induces endothelial cell migration and promotes angiogenesis. Cytokine, 30(5), 254–263. [DOI] [PubMed] [Google Scholar]
- 237.Salcedo R, Young HA, Ponce ML, Ward JM, Kleinman HK, Murphy WJ, et al. (2001). Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. Journal of Immunology, 166(12), 7571–7578. [DOI] [PubMed] [Google Scholar]
- 238.Chow MT, & Luster AD (2014). Chemokines in cancer. Cancer Immunology Research, 2(12), 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Perollet C, Han ZC, Savona C, Caen JP, & Bikfalvi A (1998). Platelet factor 4 modulates fibroblast growth factor 2 (FGF-2) activity and inhibits FGF-2 dimerization. Blood, 91(9), 3289–3299. [PubMed] [Google Scholar]
- 240.Schruefer R, Lutze N, Schymeinsky J, & Walzog B (2005). Human neutrophils promote angiogenesis by a paracrine feedforward mechanism involving endothelial interleukin-8. American Journal of Physiology Heart and Circulatory Physiology, 288(3), H1186–H1192. [DOI] [PubMed] [Google Scholar]
- 241.Metzemaekers M, Vanheule V, Janssens R, Struyf S, & Proost P (2017). Overview of the mechanisms that may contribute to the non-redundant activities of interferon-inducible CXC chemokine receptor 3 ligands. Frontiers in Immunology, 8, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD, et al. (2000). Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clinical Cancer Research, 6(5), 2104–2119. [PubMed] [Google Scholar]
- 243.Li A, Varney ML, Valasek J, Godfrey M, Dave BJ, & Singh RK (2005). Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis, 8(1), 63–71. [DOI] [PubMed] [Google Scholar]
- 244.Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, & Bar-Eli M (1997). Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. The American Journal of Pathology, 151(4), 1105–1113. [PMC free article] [PubMed] [Google Scholar]
- 245.McCawley LJ, & Matrisian LM (2000). Matrix metalloproteinases: multifunctional contributors to tumor progression. Molecular Medicine Today, 6(4), 149–156. [DOI] [PubMed] [Google Scholar]
- 246.Hanahan D, & Folkman J (1996). Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell, 86(3), 353–364. [DOI] [PubMed] [Google Scholar]
- 247.Bielenberg DR, & Zetter BR (2015). The contribution of angiogenesis to the process of metastasis. Cancer Journal, 21(4), 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jeong HS, Jones D, Liao S, Wattson DA, Cui CH, Duda DG, et al. (2015). Investigation of the lack of angiogenesis in the formation of lymph node metastases. Journal of the National Cancer Institute, 107(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Gao D, Nolan D, McDonnell K, Vahdat L, Benezra R, Altorki N, et al. (2009). Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochimica et Biophysica Acta, 1796(1), 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Martin JD, Fukumura D, Duda DG, Boucher Y, & Jain RK (2016). Reengineering the tumor microenvironment to alleviate hypoxia and overcome cancer heterogeneity. Cold Spring Harbor Perspectives in Medicine, 6(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Iruela-Arispe ML, & Dvorak HF (1997). Angiogenesis: a dynamic balance of stimulators and inhibitors. Thrombosis and Haemostasis, 78(1), 672–677. [PubMed] [Google Scholar]
- 252.Klagsbrun M, & D’Amore PA (1991). Regulators of angiogenesis. Annual Review of Physiology, 53, 217–239. [DOI] [PubMed] [Google Scholar]
- 253.Folkman J (1992). The role of angiogenesis in tumor growth. Seminars in Cancer Biology, 3(2), 65–71. [PubMed] [Google Scholar]
- 254.Owen JL, & Mohamadzadeh M (2013). Macrophages and chemokines as mediators of angiogenesis. Frontiers in Physiology, 4, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kumar R, Kuniyasu H, Bucana CD, Wilson MR, & Fidler IJ (1998). Spatial and temporal expression of angiogenic molecules during tumor growth and progression. Oncology Research, 10(6), 301–311. [PubMed] [Google Scholar]
- 256.Fidler IJ (1990). Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Research, 50(19), 6130–6138. [PubMed] [Google Scholar]
- 257.Kitadai Y, Bucana CD, Ellis LM, Anzai H, Tahara E, & Fidler IJ (1995). In situ mRNA hybridization technique for analysis of metastasis-related genes in human colon carcinoma cells. The American Journal of Pathology, 147(5), 1238–1247. [PMC free article] [PubMed] [Google Scholar]
- 258.Yu JL, Rak JW, Carmeliet P, Nagy A, Kerbel RS, & Coomber BL (2001). Heterogeneous vascular dependence of tumor cell populations. The American Journal of Pathology, 158(4), 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, et al. (1992). Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science., 258(5089), 1798–1801. [DOI] [PubMed] [Google Scholar]
- 260.Strieter RM, Kunkel SL, Elner VM, Martonyi CL, Koch AE, Polverini PJ, et al. (1992). Interleukin-8. A corneal factor that induces neovascularization. The American Journal of Pathology, 141(6), 1279–1284. [PMC free article] [PubMed] [Google Scholar]
- 261.Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, et al. (2000). The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. Journal of Immunology, 165(9), 5269–5277. [DOI] [PubMed] [Google Scholar]
- 262.Yoshida S, Ono M, Shono T, Izumi H, Ishibashi T, Suzuki H, et al. (1997). Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Molecular and Cellular Biology, 17(7), 4015–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Shono T, Ono M, Izumi H, Jimi SI, Matsushima K, Okamoto T, et al. (1996). Involvement of the transcription factor NF-kappaB in tubular morphogenesis of human microvascular endothelial cells by oxidative stress. Molecular and Cellular Biology, 16(8), 4231–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Waugh DJ, & Wilson C (2008). The interleukin-8 pathway in cancer. Clinical Cancer Research, 14(21), 6735–6741. [DOI] [PubMed] [Google Scholar]
- 265.Salazar N, & Zabel BA (2019). Support of tumor endothelial cells by chemokine receptors. Frontiers in Immunology, 10, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Salcedo R, & Oppenheim JJ (2003). Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation, 10(3–4), 359–370. [DOI] [PubMed] [Google Scholar]
- 267.Nannuru KC, Singh S, & Singh RK (2010). Chemokines and metastasis. In Theicher B & Bagley RG (Eds.), Tumor Microenvironment: Cancer Drug Discovery and Development (pp. 601–632). Springer Sciences. [Google Scholar]
- 268.Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, & Strieter RM (1996). Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. The Journal of Clinical Investigation, 97(12), 2792–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sgadari C, Angiolillo AL, Cherney BW, Pike SE, Farber JM, Koniaris LG, et al. (1996). Interferon-inducible protein-10 identified as a mediator of tumor necrosis in vivo. ProcNatlAcadSciUSA, 93(24), 13791–13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Sgadari C, Farber JM, Angiolillo AL, Liao F, Teruya-Feldstein J, Burd PR, et al. (1997). Mig, the monokine induced by interferon-gamma, promotes tumor necrosis in vivo. Blood, 89(8), 2635–2643. [PubMed] [Google Scholar]
- 271.Dorsey R, Kundu N, Yang Q, Tannenbaum CS, Sun H, Hamilton TA, et al. (2002). Immunotherapy with interleukin-10 depends on the CXC chemokines inducible protein-10 and monokine induced by IFN-gamma. Cancer Research, 62(9), 2606–2610. [PubMed] [Google Scholar]
- 272.Ruehlmann JM, Xiang R, Niethammer AG, Ba Y, Pertl U, Dolman CS, et al. (2001). MIG (CXCL9) chemokine gene therapy combines with antibody-cytokine fusion protein to suppress growth and dissemination of murine colon carcinoma. Cancer Research, 61(23), 8498–8503. [PubMed] [Google Scholar]
- 273.Romagnani P, Annunziato F, Lazzeri E, Cosmi L, Beltrame C, Lasagni L, et al. (2001). Interferon-inducible protein 10, monokine induced by interferon gamma, and interferon-inducible T-cell alpha chemoattractant are produced by thymic epithelial cells and attract T-cell receptor (TCR) alphabeta+ CD8+ single-positive T cells, TCRgammadelta+ T cells, and natural killer-type cells in human thymus. Blood, 97(3), 601–607. [DOI] [PubMed] [Google Scholar]
- 274.Kondo T, Ito F, Nakazawa H, Horita S, Osaka Y, & Toma H (2004). High expression of chemokine gene as a favorable prognostic factor in renal cell carcinoma. The Journal of Urology, 171(6 Pt 1), 2171–2175. [DOI] [PubMed] [Google Scholar]
- 275.Ehling J, Bartneck M, Wei X, Gremse F, Fech V, Möckel D, et al. (2014). CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut., 63(12), 1960–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Goede V, Brogelli L, Ziche M, & Augustin HG (1999). Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. International Journal of Cancer, 82(5), 765–770. [DOI] [PubMed] [Google Scholar]
- 277.Leung SY, Wong MP, Chung LP, Chan AS, & Yuen ST (1997). Monocyte chemoattractant protein-1 expression and macrophage infiltration in gliomas. Acta Neuropathologica (Berlin), 93(5), 518–527. [DOI] [PubMed] [Google Scholar]
- 278.Hong KH, Ryu J, & Han KH (2005). Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood., 105(4), 1405–1407. [DOI] [PubMed] [Google Scholar]
- 279.Stamatovic SM, Keep RF, Mostarica-Stojkovic M, & Andjelkovic AV (2006). CCL2 regulates angiogenesis via activation of Ets-1 transcription factor. Journal of Immunology, 177(4), 2651–2661. [DOI] [PubMed] [Google Scholar]
- 280.Gálvez BG, Genís L, Matías-Román S, Oblander SA, Tryggvason K, Apte SS, et al. (2005). Membrane type 1-matrix metalloproteinase is regulated by chemokines monocyte-chemoattractant protein-1/ccl2 and interleukin-8/CXCL8 in endothelial cells during angiogenesis. The Journal of Biological Chemistry, 280(2), 1292–1298. [DOI] [PubMed] [Google Scholar]
- 281.Ridiandries A, Tan JT, & Bursill CA (2016). The role of CC-chemokines in the regulation of angiogenesis. International Journal of Molecular Sciences, 17(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Fujiyama S, Amano K, Uehira K, Yoshida M, Nishiwaki Y, Nozawa Y, et al. (2003). Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circulation Research, 93(10), 980–989. [DOI] [PubMed] [Google Scholar]
- 283.Bernardini G, Spinetti G, Ribatti D, Camarda G, Morbidelli L, Ziche M, et al. (2000). I-309 binds to and activates endothelial cell functions and acts as an angiogenic molecule in vivo. Blood., 96(13), 4039–4045. [PubMed] [Google Scholar]
- 284.Hwang J, Kim CW, Son KN, Han KY, Lee KH, Kleinman HK, et al. (2004). Angiogenic activity of human CC chemokine CCL15 in vitro and in vivo. FEBS Letters, 570(1–3), 47–51. [DOI] [PubMed] [Google Scholar]
- 285.Strasly M, Doronzo G, Cappello P, Valdembri D, Arese M, Mitola S, et al. (2004). CCL16 activates an angiogenic program in vascular endothelial cells. Blood., 103(1), 40–49. [DOI] [PubMed] [Google Scholar]
- 286.Reed JR, Stone MD, Beadnell TC, Ryu Y, Griffin TJ, & Schwertfeger KL (2012). Fibroblast growth factor receptor 1 activation in mammary tumor cells promotes macrophage recruitment in a CX3CL1-dependent manner. PLoS One, 7(9), e45877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Li F, Wang Z, Liu Y, & Li J (2010). Down-regulation of fractalkine inhibits the in vitro and in vivo angiogenesis of the hepatocellular carcinoma HepG2 cells. Oncology Reports, 24(3), 669–675. [PubMed] [Google Scholar]
- 288.Schmall A, Al-Tamari HM, Herold S, Kampschulte M, Weigert A, Wietelmann A, et al. (2015). Macrophage and cancer cell cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving lung cancer. American Journal of Respiratory and Critical Care Medicine, 191(4), 437–447. [DOI] [PubMed] [Google Scholar]
- 289.Ren T, Chen Q, Tian Z, & Wei H (2007). Down-regulation of surface fractalkine by RNA interference in B16 melanoma reduced tumor growth in mice. Biochemical and Biophysical Research Communications, 364(4), 978–984. [DOI] [PubMed] [Google Scholar]
- 290.Marchica V, Toscani D, Corcione A, Bolzoni M, Storti P, Vescovini R, et al. (2019). Bone marrow CX3CL1/Fractalkine is a new player of the pro-angiogenic microenvironment in multiple myeloma patients. Cancers, 11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zheng J, Yang M, Shao J, Miao Y, Han J, & Du J (2013). Chemokine receptor CX3CR1 contributes to macrophage survival in tumor metastasis. Molecular Cancer, 12(1), 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ryu J, Lee CW, Hong KH, Shin JA, Lim SH, Park CS, et al. (2008). Activation of fractalkine/CX3CR1 by vascular endothelial cells induces angiogenesis through VEGF-A/KDR and reverses hindlimb ischaemia. Cardiovascular Research, 78(2), 333–340. [DOI] [PubMed] [Google Scholar]
- 293.Lee SJ, Namkoong S, Kim YM, Kim CK, Lee H, Ha KS, et al. (2006). Fractalkine stimulates angiogenesis by activating the Raf-1/MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways. American Journal of Physiology Heart and Circulatory Physiology, 291(6), H2836–H2846. [DOI] [PubMed] [Google Scholar]
- 294.Volin MV, Huynh N, Klosowska K, Reyes RD, & Woods JM (2010). Fractalkine-induced endothelial cell migration requires MAP kinase signaling. Pathobiology: Journal of Immunopathology, Molecular and Cellular Biology, 77(1), 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Combadiere C, Potteaux S, Gao JL, Esposito B, Casanova S, Lee EJ, et al. (2003). Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation, 107(7), 1009–1016. [DOI] [PubMed] [Google Scholar]
- 296.Eriksson EE (2004). Mechanisms of leukocyte recruitment to atherosclerotic lesions: future prospects. CurrOpinLipidol, 15(5), 553–558. [DOI] [PubMed] [Google Scholar]
- 297.Blaschke S, Koziolek M, Schwarz A, Benohr P, Middel P, Schwarz G, et al. (2003). Proinflammatory role of fractalkine (CX3CL1) in rheumatoid arthritis. The Journal of Rheumatology, 30(9), 1918–1927. [PubMed] [Google Scholar]
- 298.Nanki T, Urasaki Y, Imai T, Nishimura M, Muramoto K, Kubota T, et al. (2004). Inhibition of fractalkine ameliorates murine collagen-induced arthritis. Journal of Immunology, 173(11), 7010–7016. [DOI] [PubMed] [Google Scholar]
- 299.Volin MV, Woods JM, Amin MA, Connors MA, Harlow LA, & Koch AE (2001). Fractalkine: a novel angiogenic chemokine in rheumatoid arthritis. The American Journal of Pathology, 159(4), 1521–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Singh RK, & Fidler IJ (1996). Regulation of tumor angiogenesis by organ-specific cytokines. In Gunthert U & Birchmeier W (Eds.), Attempts to Understand Metastasis Formation II (pp. 1–11). Springer-Verlag. [Google Scholar]
- 301.Folkman J (1995). Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Medicine, 1(1), 27–31. [DOI] [PubMed] [Google Scholar]
- 302.Keane MP, Arenberg DA, Lynch III JP, Whyte RI, Iannettoni MD, Burdick MD, et al. (1997). The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. Journal of Immunology, 159(3), 1437–1443. [PubMed] [Google Scholar]
- 303.Arenberg DA, Polverini PJ, Kunkel SL, Shanafelt A, Hesselgesser J, Horuk R, et al. (1997). The role of CXC chemokines in the regulation of angiogenesis in non- small cell lung cancer. Journal of Leukocyte Biology, 62(5), 554–562. [DOI] [PubMed] [Google Scholar]
- 304.Strieter RM, Polverini PJ, Arenberg DA, Walz A, Opdenakker G, Van Damme J, et al. (1995). Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. JLeukocBiol, 57(5), 752–762. [DOI] [PubMed] [Google Scholar]
- 305.Kalluri R, & Weinberg RA (2009). The basics of epithelial-mesenchymal transition. The Journal of Clinical Investigation, 119(6), 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Celià-Terrassa T, & Kang Y (2016). Distinctive properties of metastasis-initiating cells. Genes & Development, 30(8), 892–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Nieto MA (2013). Epithelial plasticity: a common theme in embryonic and cancer cells. Science., 342(6159), 1234850. [DOI] [PubMed] [Google Scholar]
- 308.Varga J, & Greten FR (2017). Cell plasticity in epithelial homeostasis and tumorigenesis. Nature Cell Biology, 19(10), 1133–1141. [DOI] [PubMed] [Google Scholar]
- 309.Gupta PB, Pastushenko I, Skibinski A, Blanpain C, & Kuperwasser C (2019). Phenotypic plasticity: driver of cancer initiation, progression, and therapy resistance. Cell Stem Cell, 24(1), 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Celià-Terrassa T, & Jolly MK (2020). Cancer stem cells and epithelial-to-mesenchymal transition in cancer metastasis. Cold Spring Harbor Perspectives in Medicine, 10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Sistigu A, Di Modugno F, Manic G, & Nisticò P (2017). Deciphering the loop of epithelial-mesenchymal transition, inflammatory cytokines and cancer immunoediting. Cytokine & Growth Factor Reviews, 36, 67–77. [DOI] [PubMed] [Google Scholar]
- 312.Jolly MK, & Celià-Terrassa T (2019). Dynamics of phenotypic heterogeneity associated with EMT and stemness during cancer progression. Journal of Clinical Medicine, 8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Zhao Z, Wang S, Lin Y, Miao Y, Zeng Y, Nie Y, et al. (2017). Epithelial-mesenchymal transition in cancer: Role of the IL-8/IL-8R axis. Oncology Letters, 13(6), 4577–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Long X, Ye Y, Zhang L, Liu P, Yu W, Wei F, et al. (2016). IL-8, a novel messenger to cross-link inflammation and tumor EMT via autocrine and paracrine pathways (Review). International Journal of Oncology, 48(1), 5–12. [DOI] [PubMed] [Google Scholar]
- 315.Fernando RI, Castillo MD, Litzinger M, Hamilton DH, & Palena C (2011). IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Research, 71(15), 5296–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Sparmann A, & Bar-Sagi D (2004). Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell, 6(5), 447–458. [DOI] [PubMed] [Google Scholar]
- 317.Freisinger CM, & Huttenlocher A (2014). Live imaging and gene expression analysis in zebrafish identifies a link between neutrophils and epithelial to mesenchymal transition. PLoS One, 9(11), e112183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Fousek K, Horn LA, & Palena C (2021). Interleukin-8: a chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacology & Therapeutics, 219, 107692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Cheng XS, Li YF, Tan J, Sun B, Xiao YC, Fang XB, et al. (2014). CCL20 and CXCL8 synergize to promote progression and poor survival outcome in patients with colorectal cancer by collaborative induction of the epithelial-mesenchymal transition. Cancer Letters, 348(1–2), 77–87. [DOI] [PubMed] [Google Scholar]
- 320.Li F, Zou Z, Suo N, Zhang Z, Wan F, Zhong G, et al. (2014). CCL21/CCR7 axis activating chemotaxis accompanied with epithelial-mesenchymal transition in human breast carcinoma. Medical Oncology (Northwood, London, England), 31(9), 180. [DOI] [PubMed] [Google Scholar]
- 321.Zhong G, Chen L, Yin R, Qu Y, Bao Y, Xiao Q, et al. (2017). Chemokine (C-C motif) ligand 21/C-C chemokine receptor type 7 triggers migration and invasion of human lung cancer cells by epithelial-mesenchymal transition via the extracellular signal-regulated kinase signaling pathway. Molecular Medicine Reports, 15(6), 4100–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Chen Y, Shao Z, Jiang E, Zhou X, Wang L, Wang H, et al. (2020). CCL21/CCR7 interaction promotes EMT and enhances the stemness of OSCC via a JAK2/STAT3 signaling pathway. Journal of Cellular Physiology, 235(9), 5995–6009. [DOI] [PubMed] [Google Scholar]
- 323.Zhang L, Wang D, Li Y, Liu Y, Xie X, Wu Y, et al. (2016). CCL21/CCR7 axis contributed to CD133+ pancreatic cancer stem-like cell metastasis via EMT and Erk/NF-κB pathway. PLoS One, 11(8), e0158529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Hou KZ, Fu ZQ, & Gong H (2015). Chemokine ligand 20 enhances progression of hepatocellular carcinoma via epithelial-mesenchymal transition. World Journal of Gastroenterology, 21(2), 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Zhou SL, Zhou ZJ, Hu ZQ, Li X, Huang XW, Wang Z, et al. (2015). CXCR2/CXCL5 axis contributes to epithelial-mesenchymal transition of HCC cells through activating PI3K/Akt/GSK-3β/Snail signaling. Cancer Letters, 358(2), 124–135. [DOI] [PubMed] [Google Scholar]
- 326.Qiu WZ, Zhang HB, Xia WX, Ke LR, Yang J, Yu YH, et al. (2018). The CXCL5/CXCR2 axis contributes to the epithelial-mesenchymal transition of nasopharyngeal carcinoma cells by activating ERK/GSK-3β/snail signalling. Journal of Experimental & Clinical Cancer Research: CR, 37(1), 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Liu G, An L, Zhang H, Du P, & Sheng Y (2019). Activation of CXCL6/CXCR1/2 axis promotes the growth and metastasis of osteosarcoma cells in vitro and in vivo. Frontiers in Pharmacology, 10, 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Cheng Y, Song Y, Qu J, Che X, Song N, Fan Y, et al. (2018). The chemokine receptor CXCR4 and c-MET cooperatively promote epithelial-mesenchymal transition in gastric cancer cells. Translational Oncology, 11(2), 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Maehle AH (2011). Ambiguous cells: the emergence of the stem cell concept in the nineteenth and twentieth centuries. Notes and Records of the Royal Society of London, 65(4), 359–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Dubrovska A, Elliott J, Salamone RJ, Telegeev GD, Stakhovsky AE, Schepotin IB, et al. (2012). CXCR4 expression in prostate cancer progenitor cells. PLoS One, 7(2), e31226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Jung Y, Cackowski FC, Yumoto K, Decker AM, Wang J, Kim JK, et al. (2018). CXCL12γ promotes metastatic castration-resistant prostate cancer by inducing cancer stem cell and neuroendocrine phenotypes. Cancer Research, 78(8), 2026–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Huang M, Li Y, Zhang H, & Nan F (2010). Breast cancer stromal fibroblasts promote the generation of CD44+CD24− cells through SDF-1/CXCR4 interaction. Journal of Experimental & Clinical Cancer Research: CR, 29(1), 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Kong L, Guo S, Liu C, Zhao Y, Feng C, Liu Y, et al. (2016). Overexpression of SDF-1 activates the NF-κB pathway to induce epithelial to mesenchymal transition and cancer stem cell-like phenotypes of breast cancer cells. International Journal of Oncology, 48(3), 1085–1094. [DOI] [PubMed] [Google Scholar]
- 334.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. (2009). Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Research, 69(4), 1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, et al. (2010). CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. The Journal of Clinical Investigation, 120(2), 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Singh JK, Farnie G, Bundred NJ, Simões BM, Shergill A, Landberg G, et al. (2013). Targeting CXCR1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2 via HER2-dependent and -independent mechanisms. Clinical Cancer Research, 19(3), 643–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Wang N, Zheng Y, Gu J, Cai Y, Wang S, Zhang F, et al. (2017). Network-pharmacology-based validation of TAMS/CXCL-1 as key mediator of XIAOPI formula preventing breast cancer development and metastasis. Scientific Reports, 7(1), 14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Chen L, Fan J, Chen H, Meng Z, Chen Z, Wang P, et al. (2014). The IL-8/CXCR1 axis is associated with cancer stem cell-like properties and correlates with clinical prognosis in human pancreatic cancer cases. Scientific Reports, 4, 5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Tsuyada A, Chow A, Wu J, Somlo G, Chu P, Loera S, et al. (2012). CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Research, 72(11), 2768–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Zhang Y, Yao F, Yao X, Yi C, Tan C, Wei L, et al. (2009). Role of CCL5 in invasion, proliferation and proportion of CD44+/CD24− phenotype of MCF-7 cells and correlation of CCL5 and CCR5 expression with breast cancer progression. Oncology Reports, 21(4), 1113–1121. [PubMed] [Google Scholar]
- 341.Jiao X, Velasco-Velázquez MA, Wang M, Li Z, Rui H, Peck AR, et al. (2018). CCR5 governs DNA damage repair and breast cancer stem cell expansion. Cancer Research, 78(7), 1657–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Tang X, Li X, Li Z, Liu Y, Yao L, Song S, et al. (2016). Downregulation of CXCR7 inhibits proliferative capacity and stem cell-like properties in breast cancer stem cells. Tumour Biology: The journal of the International Society for Oncodevelopmental Biology and Medicine, 37(10), 13425–13433. [DOI] [PubMed] [Google Scholar]
- 343.Dagogo-Jack I, & Shaw AT (2018). Tumour heterogeneity and resistance to cancer therapies. Nature Reviews. Clinical Oncology, 15(2), 81–94. [DOI] [PubMed] [Google Scholar]
- 344.Gonzalez-Angulo AM, Morales-Vasquez F, & Hortobagyi GN (2007). Overview of resistance to systemic therapy in patients with breast cancer. Advances in Experimental Medicine and Biology, 608, 1–22. [DOI] [PubMed] [Google Scholar]
- 345.Karagiannis GS, Condeelis JS, & Oktay MH (2019). Chemotherapy-induced metastasis: molecular mechanisms, clinical manifestations, therapeutic interventions. Cancer Research, 79(18), 4567–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Wang QE (2015). DNA damage responses in cancer stem cells: implications for cancer therapeutic strategies. World Journal of Biological Chemistry, 6(3), 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Honoki K, Fujii H, Kubo A, Kido A, Mori T, Tanaka Y, et al. (2010). Possible involvement of stem-like populations with elevated ALDH1 in sarcomas for chemotherapeutic drug resistance. Oncology Reports, 24(2), 501–505. [DOI] [PubMed] [Google Scholar]
- 348.Dean M, Fojo T, & Bates S (2005). Tumour stem cells and drug resistance. Nature Reviews Cancer, 5(4), 275–284. [DOI] [PubMed] [Google Scholar]
- 349.Thiery JP, Acloque H, Huang RY, & Nieto MA (2009). Epithelial-mesenchymal transitions in development and disease. Cell, 139(5), 871–890. [DOI] [PubMed] [Google Scholar]
- 350.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, et al. (2015). Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature, 527(7579), 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Shao N, Chen LH, Ye RY, Lin Y, & Wang SM (2013). The depletion of interleukin-8 causes cell cycle arrest and increases the efficacy of docetaxel in breast cancer cells. Biochemical and Biophysical Research Communications, 431(3), 535–541. [DOI] [PubMed] [Google Scholar]
- 352.Brandolini L, Cristiano L, Fidoamore A, De Pizzol M, Di Giacomo E, Florio TM, et al. (2015). Targeting CXCR1 on breast cancer stem cells: signaling pathways and clinical application modelling. Oncotarget, 6(41), 43375–43394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Xu H, Lin F, Wang Z, Yang L, Meng J, Ou Z, et al. (2018). CXCR2 promotes breast cancer metastasis and chemoresistance via suppression of AKT1 and activation of COX2. Cancer Letters, 412, 69–80. [DOI] [PubMed] [Google Scholar]
- 354.Chen DR, Lu DY, Lin HY, & Yeh WL (2014). Mesenchymal stem cell-induced doxorubicin resistance in triple negative breast cancer. BioMed Research International, 2014, 532161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Sharma B, Nawandar DM, Nannuru KC, Varney ML, & Singh RK (2013). Targeting CXCR2 enhances chemotherapeutic response, inhibits mammary tumor growth, angiogenesis, and lung metastasis. Molecular Cancer Therapeutics, 12(5), 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Shi Z, Yang WM, Chen LP, Yang DH, Zhou Q, Zhu J, et al. (2012). Enhanced chemosensitization in multidrug-resistant human breast cancer cells by inhibition of IL-6 and IL-8 production. Breast Cancer Research and Treatment, 135(3), 737–747. [DOI] [PubMed] [Google Scholar]
- 357.Sharma B, Varney ML, Saxena S, Wu L, & Singh RK (2016). Induction of CXCR2 ligands, stem cell-like phenotype, and metastasis in chemotherapy-resistant breast cancer cells. Cancer Letters, 372(2), 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Wu L, Awaji M, Saxena S, Varney ML, Sharma B, & Singh RK (2020). IL-17-CXC chemokine receptor 2 axis facilitates breast cancer progression by up-regulating neutrophil recruitment. The American Journal of Pathology, 190(1), 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Wu L, Saxena S, Goel P, Prajapati DR, Wang C, & Singh RK (2020). Breast cancer cell-neutrophil interactions enhance neutrophil survival and pro-tumorigenic activities. Cancers, 12(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Samanta D, Gilkes DM, Chaturvedi P, Xiang L, & Semenza GL (2014). Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proceedings of the National Academy of Sciences of the United States of America, 111(50), E5429–E5438. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 361.Jia D, Li L, Andrew S, Allan D, Li X, Lee J, et al. (2017). An autocrine inflammatory forward-feedback loop after chemotherapy withdrawal facilitates the repopulation of drug-resistant breast cancer cells. Cell Death & Disease, 8(7), e2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, et al. (2012). A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell, 150(1), 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Dubrovska A, Hartung A, Bouchez LC, Walker JR, Reddy VA, Cho CY, et al. (2012). CXCR4 activation maintains a stem cell population in tamoxifen-resistant breast cancer cells through AhR signalling. British Journal of Cancer, 107(1), 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Greco SJ, Patel SA, Bryan M, Pliner LF, Banerjee D, & Rameshwar P (2011). AMD3100-mediated production of interleukin-1 from mesenchymal stem cells is key to chemosensitivity of breast cancer cells. American Journal of Cancer Research, 1(6), 701–715. [PMC free article] [PubMed] [Google Scholar]
- 365.Rhodes LV, Bratton MR, Zhu Y, Tilghman SL, Muir SE, Salvo VA, et al. (2011). Effects of SDF-1-CXCR4 signaling on microRNA expression and tumorigenesis in estrogen receptor-alpha (ER-α)-positive breast cancer cells. Experimental Cell Research, 317(18), 2573–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Sauvé K, Lepage J, Sanchez M, Heveker N, & Tremblay A (2009). Positive feedback activation of estrogen receptors by the CXCL12-CXCR4 pathway. Cancer Research, 69(14), 5793–5800. [DOI] [PubMed] [Google Scholar]
- 367.Hao M, Weng X, Wang Y, Sun X, Yan T, Li Y, et al. (2018). Targeting CXCR7 improves the efficacy of breast cancer patients with tamoxifen therapy. Biochemical Pharmacology, 147, 128–140. [DOI] [PubMed] [Google Scholar]
- 368.Reyes ME, de La Fuente M, Hermoso M, Ili CG, & Brebi P (2020). Role of CC chemokines subfamily in the platinum drugs resistance promotion in cancer. Frontiers in Immunology, 11, 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Ghajar CM (2015). Metastasis prevention by targeting the dormant niche. Nature Reviews. Cancer, 15(4), 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Giancotti FG (2013). Mechanisms governing metastatic dormancy and reactivation. Cell, 155(4), 750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Sosa MS, Bragado P, & Aguirre-Ghiso JA (2014). Mechanisms of disseminated cancer cell dormancy: an awakening field. Nature Reviews. Cancer, 14(9), 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Hayashi S, Kurdowska A, Cohen AB, Stevens MD, Fujisawa N, & Miller EJ (1997). A synthetic peptide inhibitor for alpha-chemokines inhibits the growth of melanoma cell lines. The Journal of Clinical Investigation, 99(11), 2581–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Fujisawa N, Hayashi S, & Miller EJ (1999). A synthetic peptide inhibitor for alpha-chemokines inhibits the tumour growth and pulmonary metastasis of human melanoma cells in nude mice. Melanoma Research, 9(2), 105–114. [DOI] [PubMed] [Google Scholar]
- 374.Singh S, Singh AP, Sharma B, Owen LB, & Singh RK (2010). CXCL8 and its cognate receptors in melanoma progression and metastasis. Future oncology (London, England), 6(1), 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Payne AS, & Cornelius LA (2002). The role of chemokines in melanoma tumor growth and metastasis. The Journal of Investigative Dermatology, 118(6), 915–922. [DOI] [PubMed] [Google Scholar]
- 376.Hall JM, & Korach KS (2003). Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Molecular Endocrinology (Baltimore, Md), 17(5), 792–803. [DOI] [PubMed] [Google Scholar]
- 377.Zhou Y, Larsen PH, Hao C, & Yong VW (2002). CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. The Journal of Biological Chemistry, 277(51), 49481–49487. [DOI] [PubMed] [Google Scholar]
- 378.Barbero S, Bonavia R, Bajetto A, Porcile C, Pirani P, Ravetti JL, et al. (2003). Stromal cell-derived factor 1alpha stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Research, 63(8), 1969–1974. [PubMed] [Google Scholar]
- 379.Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, et al. (2002). Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Research, 62(21), 6304–6311. [PubMed] [Google Scholar]
- 380.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, & Kipps TJ (2000). Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood, 96(8), 2655–2663. [PubMed] [Google Scholar]
- 381.Bendall L (2005). Chemokines and their receptors in disease. Histology and Histopathology, 20(3), 907–926. [DOI] [PubMed] [Google Scholar]
- 382.Messmer D, Fecteau JF, O’Hayre M, Bharati IS, Handel TM, & Kipps TJ (2011). Chronic lymphocytic leukemia cells receive RAF-dependent survival signals in response to CXCL12 that are sensitive to inhibition by sorafenib. Blood, 117(3), 882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Wang B, Hendricks DT, Wamunyokoli F, & Parker MI (2006). A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Research, 66(6), 3071–3077. [DOI] [PubMed] [Google Scholar]
- 384.Sharma B, Nannuru KC, Varney ML, & Singh RK (2015). Host Cxcr2-dependent regulation of mammary tumor growth and metastasis. Clinical & Experimental Metastasis, 32(1), 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Nannuru KC, Sharma B, Varney ML, & Singh RK (2011). Role of chemokine receptor CXCR2 expression in mammary tumor growth, angiogenesis and metastasis. Journal of Carcinogenesis, 10, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Ha HK, Lee W, Park HJ, Lee SD, Lee JZ, & Chung MK (2011). Clinical significance of CXCL16/CXCR6 expression in patients with prostate cancer. Molecular Medicine Reports, 4(3), 419–424. [DOI] [PubMed] [Google Scholar]
- 387.Darash-Yahana M, Gillespie JW, Hewitt SM, Chen YY, Maeda S, Stein I, et al. (2009). The chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation-associated cancers. PLoS One, 4(8), e6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Riverso M, Kortenkamp A, & Silva E (2014). Non-tumorigenic epithelial cells secrete MCP-1 and other cytokines that promote cell division in breast cancer cells by activating ERα via PI3K/Akt/mTOR signaling. The International Journal of Biochemistry & Cell Biology, 53, 281–294. [DOI] [PubMed] [Google Scholar]
- 389.Jin K, Pandey NB, & Popel AS (2017). Crosstalk between stromal components and tumor cells of TNBC via secreted factors enhances tumor growth and metastasis. Oncotarget, 8(36), 60210–60222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Hartman ZC, Poage GM, den Hollander P, Tsimelzon A, Hill J, Panupinthu N, et al. (2013). Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Research, 73(11), 3470–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Romero-Moreno R, Curtis KJ, Coughlin TR, Miranda-Vergara MC, Dutta S, Natarajan A, et al. (2019). The CXCL5/CXCR2 axis is sufficient to promote breast cancer colonization during bone metastasis. Nature Communications, 10(1), 4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Gao W, Mei X, Wang J, Zhang X, & Yuan Y (2015). ShRNA-mediated knock-down of CXCR7 increases TRAIL-sensitivity in MCF-7 breast cancer cells. Tumour Biology: The journal of the International Society for Oncodevelopmental Biology and Medicine, 36(9), 7243–7250. [DOI] [PubMed] [Google Scholar]
- 393.Khazali AS, Clark AM, & Wells A (2018). Inflammatory cytokine IL-8/CXCL8 promotes tumour escape from hepatocyte-induced dormancy. British Journal of Cancer, 118(4), 566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Boudot A, Kerdivel G, Habauzit D, Eeckhoute J, Le Dily F, Flouriot G, et al. (2011). Differential estrogen-regulation of CXCL12 chemokine receptors, CXCR4 and CXCR7, contributes to the growth effect of estrogens in breast cancer cells. PLoS One, 6(6), e20898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Luker KE, Lewin SA, Mihalko LA, Schmidt BT, Winkler JS, Coggins NL, et al. (2012). Scavenging of CXCL12 by CXCR7 promotes tumor growth and metastasis of CXCR4-positive breast cancer cells. Oncogene, 31(45), 4750–4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Salazar N, Munoz D, Kallifatidis G, Singh RK, Jorda M, & Lokeshwar BL (2014). The chemokine receptor CXCR7 interacts with EGFR to promote breast cancer cell proliferation. Molecular Cancer, 13, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Ghadjar P, Rubie C, Aebersold DM, & Keilholz U (2009). The chemokine CCL20 and its receptor CCR6 in human malignancy with focus on colorectal cancer. International Journal of Cancer Journal International du Cancer, 125(4), 741–745. [DOI] [PubMed] [Google Scholar]
- 398.Wang J, Seethala RR, Zhang Q, Gooding W, van Waes C, Hasegawa H, et al. (2008). Autocrine and paracrine chemokine receptor 7 activation in head and neck cancer: implications for therapy. Journal of the National Cancer Institute, 100(7), 502–512. [DOI] [PubMed] [Google Scholar]
- 399.Han R, Gu S, Zhang Y, Luo A, Jing X, Zhao L, et al. (2018). Estrogen promotes progression of hormone-dependent breast cancer through CCL2-CCR2 axis by upregulation of Twist via PI3K/AKT/NF-κB signaling. Scientific Reports, 8(1), 9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Fang WB, Jokar I, Zou A, Lambert D, Dendukuri P, & Cheng N (2012). CCL2/CCR2 chemokine signaling coordinates survival and motility of breast cancer cells through Smad3 protein- and p42/44 mitogen-activated protein kinase (MAPK)-dependent mechanisms. The Journal of Biological Chemistry, 287(43), 36593–36608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Yao M, Fang W, Smart C, Hu Q, Huang S, Alvarez N, et al. (2019). CCR2 chemokine receptors enhance growth and cell-cycle progression of breast cancer cells through SRC and PKC activation. Molecular Cancer Research, 17(2), 604–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Murooka TT, Rahbar R, & Fish EN (2009). CCL5 promotes proliferation of MCF-7 cells through mTOR-dependent mRNA translation. Biochemical and Biophysical Research Communications, 387(2), 381–386. [DOI] [PubMed] [Google Scholar]
- 403.Lu P, Nakamoto Y, Nemoto-Sasaki Y, Fujii C, Wang H, Hashii M, et al. (2003). Potential interaction between CCR1 and its ligand, CCL3, induced by endogenously produced interleukin-1 in human hepatomas. The American Journal of Pathology, 162(4), 1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Hernandez-Segura A, Nehme J, & Demaria M (2018). Hallmarks of cellular senescence. Trends in Cell Biology, 28(6), 436–453. [DOI] [PubMed] [Google Scholar]
- 405.Kim YH, & Park TJ (2019). Cellular senescence in cancer. BMB Reports, 52(1), 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, et al. (2008). Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell, 133(6), 1006–1018. [DOI] [PubMed] [Google Scholar]
- 407.Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, & Alimonti A (2019). Cellular Senescence: Aging, Cancer, and Injury. Physiological Reviews, 99(2), 1047–1078. [DOI] [PubMed] [Google Scholar]
- 408.Eggert T, Wolter K, Ji J, Ma C, Yevsa T, Klotz S, et al. (2016). Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell, 30(4), 533–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Iannello A, Thompson TW, Ardolino M, Lowe SW, & Raulet DH (2013). p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. The Journal of Experimental Medicine, 210(10), 2057–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Ruan JW, Liao YC, Lua I, Li MH, Hsu CY, & Chen JH (2012). Human pituitary tumor-transforming gene 1 overexpression reinforces oncogene-induced senescence through CXCR2/p21 signaling in breast cancer cells. Breast Cancer Research: BCR, 14(4), R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Smit MA, & Peeper DS (2010). Epithelial-mesenchymal transition and senescence: two cancer-related processes are crossing paths. Aging., 2(10), 735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, et al. (2008). Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biology, 6(12), 2853–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Ohanna M, Giuliano S, Bonet C, Imbert V, Hofman V, Zangari J, et al. (2011). Senescent cells develop a PARP-1 and nuclear factor-{kappa}B-associated secretome (PNAS). Genes & Development, 25(12), 1245–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Kim YH, Choi YW, Lee J, Soh EY, Kim JH, & Park TJ (2017). Senescent tumor cells lead the collective invasion in thyroid cancer. Nature Communications, 8, 15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Hanahan D, & Coussens LM (2012). Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell, 21(3), 309–322. [DOI] [PubMed] [Google Scholar]
- 416.Polyak K, Haviv I, & Campbell IG (2009). Co-evolution of tumor cells and their microenvironment. Trends in Genetics: TIG, 25(1), 30–38. [DOI] [PubMed] [Google Scholar]
- 417.Barcellos-Hoff MH, Lyden D, & Wang TC (2013). The evolution of the cancer niche during multistage carcinogenesis. Nature Reviews. Cancer, 13(7), 511–518. [DOI] [PubMed] [Google Scholar]
- 418.McAllister SS, & Weinberg RA (2014). The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nature Cell Biology, 16(8), 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the premetastatic niche. Nature., 438(7069), 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. (2012). Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature Medicine, 18(6), 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. (2014). Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell, 25(4), 501–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature., 527(7578), 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Sleeman JP (2012). The metastatic niche and stromal progression. Cancer Metastasis Reviews, 31(3–4), 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Chin AR, & Wang SE (2016). Cancer tills the premetastatic field: mechanistic basis and clinical implications. Clinical Cancer Research, 22(15), 3725–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Liu Y, & Cao X (2016). Characteristics and significance of the pre-metastatic niche. Cancer Cell, 30(5), 668–681. [DOI] [PubMed] [Google Scholar]
- 426.Celià-Terrassa T, & Kang Y (2018). Metastatic niche functions and therapeutic opportunities. Nature Cell Biology, 20(8), 868–877. [DOI] [PubMed] [Google Scholar]
- 427.Murgai M, Giles A, & Kaplan R (2015). Physiological, tumor, and metastatic niches: opportunities and challenges for targeting the tumor microenvironment. Critical Reviews in Oncogenesis, 20(3–4), 301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Gravina GL, Mancini A, Marampon F, Colapietro A, Delle Monache S, Sferra R, et al. (2017). The brain-penetrating CXCR4 antagonist, PRX177561, increases the antitumor effects of bevacizumab and sunitinib in preclinical models of human glioblastoma. Journal of Hematology & Oncology, 10(1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Gagner JP, Sarfraz Y, Ortenzi V, Alotaibi FM, Chiriboga LA, Tayyib AT, et al. (2017). Multifaceted C-X-C chemokine receptor 4 (CXCR4) inhibition interferes with anti-vascular endothelial growth factor therapy-induced glioma dissemination. The American Journal of Pathology, 187(9), 2080–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Pham K, Luo D, Siemann DW, Law BK, Reynolds BA, Hothi P, et al. (2015). VEGFR inhibitors upregulate CXCR4 in VEGF receptor-expressing glioblastoma in a TGFbetaR signaling-dependent manner. Cancer Letters, 360(1), 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Ling X, Spaeth E, Chen Y, Shi Y, Zhang W, Schober W, et al. (2013). The CXCR4 antagonist AMD3465 regulates oncogenic signaling and invasiveness in vitro and prevents breast cancer growth and metastasis in vivo. PLoS One, 8(3), e58426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Salazar N, Carlson JC, Huang K, Zheng Y, Oderup C, Gross J, et al. (2018). A chimeric antibody against ACKR3/CXCR7 in combination with TMZ activates immune responses and extends survival in mouse GBM models. Molecular Therapy, 26(5), 1354–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Jung H, Bischof A, Ebsworth K, Ertl L, Schall T, & Charo I (2015). Combination therapy of chemokine receptor inhibition plus PDL-1 blockade potentiates anti-tumor effects in a murine model of breast cancer. Journal for Immunotherapy of Cancer, 3(S2). [Google Scholar]
- 434.Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T, et al. (2014). Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature, 515(7525), 130–133. [DOI] [PubMed] [Google Scholar]
- 435.Schott AF, Goldstein LJ, Cristofanilli M, Ruffini PA, McCanna S, Reuben JM, et al. (2017). Phase Ib pilot study to evaluate reparixin in combination with weekly paclitaxel in patients with HER-2-negative metastatic breast cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 23(18), 5358–5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Galsky MD, Vogelzang NJ, Conkling P, Raddad E, Polzer J, Roberson S, et al. (2014). A phase I trial of LY2510924, a CXCR4 peptide antagonist, in patients with advanced cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 20(13), 3581–3588. [DOI] [PubMed] [Google Scholar]
- 437.Pernas S, Martin M, Kaufman PA, Gil-Martin M, Gomez Pardo P, Lopez-Tarruella S, et al. (2018). Balixafortide plus eribulin in HER2-negative metastatic breast cancer: a phase 1, single-arm, dose-escalation trial. The Lancet Oncology, 19(6), 812–824. [DOI] [PubMed] [Google Scholar]
- 438.Kitamura T, Fujishita T, Loetscher P, Revesz L, Hashida H, Kizaka-Kondoh S, et al. (2010). Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model. Proceedings of the National Academy of Sciences of the United States of America, 107(29), 13063–13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Beziaud L, Boullerot L, Tran T, Mansi L, Marie-Joseph EL, Ravel P, et al. (2018). Rapalog combined with CCR4 antagonist improves anticancer vaccines efficacy. International Journal of Cancer, 143(11), 3008–3018. [DOI] [PubMed] [Google Scholar]
- 440.Halama N, Zoernig I, Berthel A, Kahlert C, Klupp F, Suarez-Carmona M, et al. (2016). Tumoral Immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell, 29(4), 587–601. [DOI] [PubMed] [Google Scholar]
- 441.Shuyi Y, Juping D, Zhiqun Z, Qiong P, Wuyang J, Ting L, et al. (2014). A critical role of CCR7 in invasiveness and metastasis of SW620 colon cancer cell in vitro and in vivo. Cancer Biology & Therapy, 7(7), 1037–1043. [DOI] [PubMed] [Google Scholar]
- 442.Wang J, Hu W, Wang K, Yu JUN, Luo B, Luo G, et al. (2016). Repertaxin, an inhibitor of the chemokine receptors CXCR1 and CXCR2, inhibits malignant behavior of human gastric cancer MKN45 cells in vitro and in vivo and enhances efficacy of 5-fluorouracil. International Journal of Oncology, 48(4), 1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Vallet S, Raje N, Ishitsuka K, Hideshima T, Podar K, Chhetri S, et al. (2007). MLN3897, a novel CCR1 inhibitor, impairs osteoclastogenesis and inhibits the interaction of multiple myeloma cells and osteoclasts. Blood, 110(10), 3744–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Dairaghi DJ, Oyajobi BO, Gupta A, McCluskey B, Miao S, Powers JP, et al. (2012). CCR1 blockade reduces tumor burden and osteolysis in vivo in a mouse model of myeloma bone disease. Blood, 120(7), 1449–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Perera LP, Zhang M, Nakagawa M, Petrus MN, Maeda M, Kadin ME, et al. (2017). Chimeric antigen receptor modified T cells that target chemokine receptor CCR4 as a therapeutic modality for T-cell malignancies. American Journal of Hematology, 92(9), 892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.(2018). Mogamulizumab tops standard of care for CTCL. Cancer Discovery, 8(2), OF1–OOF. [DOI] [PubMed] [Google Scholar]
- 447.Micallef IN, Stiff PJ, Nademanee AP, Maziarz RT, Horwitz ME, Stadtmauer EA, et al. (2018). Plerixafor plus granulocyte colony-stimulating factor for patients with non-Hodgkin lymphoma and multiple myeloma: long-term follow-up report. Biology of Blood and Marrow Transplantation, 24(6), 1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, et al. (2009). Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood., 113(24), 6206–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Peng S-B, Zhang X, Paul D, Kays LM, Gough W, Stewart J, et al. (2015). Identification of LY2510924, a novel cyclic peptide CXCR4 antagonist that exhibits antitumor activities in solid tumor and breast cancer metastatic models. Molecular Cancer Therapeutics, 14(2), 480–490. [DOI] [PubMed] [Google Scholar]
- 450.Cho B-S, Zeng Z, Mu H, Wang Z, Konoplev S, McQueen T, et al. (2015). Antileukemia activity of the novel peptidic CXCR4 antagonist LY2510924 as monotherapy and in combination with chemotherapy. Blood., 126(2), 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Uy GL, Rettig MP, Motabi IH, McFarland K, Trinkaus KM, Hladnik LM, et al. (2012). A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood., 119(17), 3917–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Liu S-H, Gu Y, Pascual B, Yan Z, Hallin M, Zhang C, et al. (2017). A novel CXCR4 antagonist IgG1 antibody (PF-06747143) for the treatment of hematologic malignancies. Blood Advances, 1(15), 1088–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Yao W, Ba Q, Li X, Li H, Zhang S, Yuan Y, et al. (2017). A natural CCR2 antagonist relieves tumor-associated macrophage-mediated immunosuppression to produce a therapeutic effect for liver cancer. EBioMedicine., 22, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Li B, Zeng Y, Reeves PM, Ran C, Liu Q, Qu X, et al. (2018). AMD3100 augments the efficacy of mesothelin-targeted, immune-activating VIC-008 in mesothelioma by modulating intratumoral immunosuppression. Cancer Immunology Research, 6(5), 539–551. [DOI] [PubMed] [Google Scholar]
- 455.Binder PS, Cullinan D, Nywening T, Wilkinson-Ryan I, Belt B, Goedegebuure P, et al. (2017). CCR2 blockade alters the tumor microenvironment immune infiltrate and enhances antitumor activity in ovarian cancer. Gynecologic Oncology, 145, 36. [Google Scholar]
- 456.Sandhu SK, Papadopoulos K, Fong PC, Patnaik A, Messiou C, Olmos D, et al. (2013). A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemotherapy and Pharmacology, 71(4), 1041–1050. [DOI] [PubMed] [Google Scholar]
- 457.Pienta KJ, Machiels J-P, Schrijvers D, Alekseev B, Shkolnik M, Crabb SJ, et al. (2012). Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Investigational New Drugs, 31(3), 760–768. [DOI] [PubMed] [Google Scholar]
- 458.Ribatti D, Devapatla B, Sharma A, & Woo S (2015). CXCR2 inhibition combined with sorafenib improved antitumor and antiangiogenic response in preclinical models of ovarian cancer. PLoS One, 10(9), e0139237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Di Mitri D, Toso A, Chen JJ, Sarti M, Pinton S, Jost TR, et al. (2014). Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature., 515(7525), 134–137. [DOI] [PubMed] [Google Scholar]
- 460.Righi E, Kashiwagi S, Yuan J, Santosuosso M, Leblanc P, Ingraham R, et al. (2011). CXCL12/CXCR4 blockade induces multimodal antitumor effects that prolong survival in an immunocompetent mouse model of ovarian cancer. Cancer Research, 71(16), 5522–5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, et al. (2013). Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Research, 73(3), 1128–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Jung H, Ertl L, Janson C, Schall T, Charo I (2016). Abstract A107: Inhibition of CCR2 potentiates the checkpoint inhibitor immunotherapy in pancreatic cancer. A107–A. [Google Scholar]
- 463.Noel M, Lowery M, Ryan D, Wolpin B, Bullock A, Britten C, et al. (2017). Phase Ib study of PF-04136309 (an oral CCR2 inhibitor) in combination with nab-paclitaxel/gemcitabine in first-line treatment of metastatic pancreatic adenocarcinoma. Annals of Oncology, 28, v257. [Google Scholar]
- 464.Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, et al. (2016). Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. The Lancet Oncology, 17(5), 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Linehan D, Noel MS, Hezel AF, Wang-Gillam A, Eskens F, Sleijfer S, et al. (2018). Overall survival in a trial of orally administered CCR2 inhibitor CCX872 in locally advanced/metastatic pancreatic cancer: Correlation with blood monocyte counts. Journal of Clinical Oncology, 36(5_suppl), 92. [Google Scholar]
- 466.Chao T, Furth EE, & Vonderheide RH (2016). CXCR2-dependent accumulation of tumor-associated neutrophils regulates T-cell immunity in pancreatic ductal adenocarcinoma. Cancer Immunology Research, 4(11), 968–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Young HL, Rowling EJ, Bugatti M, Giurisato E, Luheshi N, Arozarena I, et al. (2017). An adaptive signaling network in melanoma inflammatory niches confers tolerance to MAPK signaling inhibition. The Journal of Experimental Medicine, 214(6), 1691–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Berlato C, Khan MN, Schioppa T, Thompson R, Maniati E, Montfort A, et al. (2017). A CCR4 antagonist reverses the tumor-promoting microenvironment of renal cancer. Journal of Clinical Investigation, 127(3), 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. (2011). CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature, 475(7355), 222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Wolf MJ, Hoos A, Bauer J, Boettcher S, Knust M, Weber A, et al. (2012). Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell, 22(1), 91–105. [DOI] [PubMed] [Google Scholar]
- 471.Zhao L, Lim SY, Gordon-Weeks AN, Tapmeier TT, Im JH, Cao Y, et al. (2013). Recruitment of a myeloid cell subset (CD11b/Gr1 mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology (Baltimore, Md), 57(2), 829–839. [DOI] [PubMed] [Google Scholar]
- 472.Garin A, & Proudfoot AE (2011). Chemokines as targets for therapy. Experimental Cell Research, 317(5), 602–612. [DOI] [PubMed] [Google Scholar]


