Abstract
Endotoxin (lipopolysaccharide [LPS]) tolerance is a state of altered immunity characterized, in part, by suppression of LPS-induced gamma interferon (IFN-γ) expression. However, the cellular mediators regulating LPS-induced production of IFN-γ in normal mice and the effect of LPS tolerance on these mediators has not been well characterized. Our studies show that macrophage dysfunction is the primary factor causing suppressed IFN-γ expression in LPS-tolerant mice. Specifically, LPS-tolerant macrophages have a markedly impaired ability to induce IFN-γ secretion by T cells and NK cells obtained from either control or LPS-tolerant mice. However, T cells and NK cells isolated from LPS-tolerant mice produce normal levels of IFN-γ when cocultured with control macrophages or exogenous IFN-γ-inducing factors. Assessment of important IFN-γ-regulating factors showed that interleukin-12 (IL-12) and costimulatory signals provided by IL-15, IL-18, and CD86 are largely responsible for LPS-induced IFN-γ expression in control mice. IL-10 is an inhibitor of IFN-γ production in both the control and LPS-tolerant groups. Expression of IL-12 and the IL-12 receptor β1 (IL-12Rβ1) and IL-12Rβ2 subunits are suppressed in the spleens of LPS-tolerant mice. LPS-tolerant splenocytes also exhibit decreased production of IL-15 and IL-15Rα. However, expression of IL-18 and the B7 proteins CD80 and CD86 are unchanged or increased compared to controls after induction of LPS tolerance. CD28, a major receptor for B7 proteins, is also increased in the spleens of LPS-tolerant mice. Expression of the inhibitory cytokine IL-10 and the IL-10R are sustained after induction of LPS tolerance. These data show that suppression of IFN-γ production in LPS-tolerant mice is largely due to macrophage dysfunction and provide insight into the cellular alterations that occur in LPS tolerance. This study also better defines the factors that mediate LPS-induced IFN-γ production in normal mice.
Endotoxin (lipopolysaccharide [LPS]) is an intrinsic component of the cell walls of gram-negative bacteria. Serious infections with gram-negative bacteria can lead to the development of the sepsis syndrome, a hyperinflammatory condition that is largely precipitated by LPS-induced secretion of proinflammatory cytokines (27, 30). Numerous investigators have reported transient endotoxemia in trauma and high-risk surgical patients due to the translocation of enteric bacteria and endotoxin across the gut (3, 4). Exposure of seriously injured patients to LPS may exacerbate the systemic inflammatory response syndrome, a major source of morbidity and mortality in this patient population. The LPS-induced inflammatory response can lead to systemic organ dysfunction and death. Conversely, prior sublethal exposure to LPS results in a state of tolerance to further LPS challenge. LPS tolerance is characterized by decreased production of macrophage-derived cytokines, such as tumor necrosis factor alpha, interleukin-1β (IL-1β), and IL-6, as well as lymphocyte-derived gamma interferon (IFN-γ) (11, 21). LPS tolerance is thought to be an adaptive mechanism designed to protect the host from further inflammatory injury. Whether or not this state of altered immunity leaves the host more susceptible to subsequent infections remains controversial. Some investigators have postulated that the suppressed cytokine response observed in the tolerant host will result in impaired antimicrobial immunity. However, recent reports have shown that LPS-tolerant mice are more resistant to systemic infection with Cryptococcus neoformans or Salmonella enterica serovar Typhimurium (19, 28). LPS tolerance has clinical relevance because the changes in immune function observed in this model parallel those seen following sepsis, major trauma, thermal injury, and high-risk surgery (1, 2, 8). A common feature of all of these conditions is suppression of IFN-γ production (12, 16).
IFN-γ appears to play an important role in the progression of sepsis and systemic inflammatory response syndrome through its ability to amplify the proinflammatory response (10, 24). The expression of IFN-γ is regulated by a complex interaction of macrophage- and lymphocyte-derived cytokines and cell surface proteins. The macrophage-derived cytokines IL-12, IL-15, and IL-18 are known positive regulators of IFN-γ expression (6, 18, 29). These cytokines act synergistically to induce IFN-γ production by T lymphocytes and natural killer (NK) cells. Induction of IFN-γ expression is also mediated through the T-cell receptor complex by interaction with the major histocompatability complex class II (MHC-II) on antigen-presenting cells (34). The accessory B7 proteins, of which CD80 and CD86 are the best defined, have also been shown to provide costimulatory signals for the induction of IFN-γ expression through interactions with surface CD28 on T cells and NK cells (5). However, the role of these factors in LPS-induced secretion of IFN-γ are not well characterized nor is the effect of LPS tolerance on the expression of these factors well understood. We characterized the role of known IFN-γ-regulating factors in LPS-induced secretion of IFN-γ and determined whether the expression and function of these mediators were altered in LPS tolerance. We report that IL-12 and costimulatory signals from IL-15 and IL-18 as well as the B7 protein CD86 are important mediators of LPS-induced IFN-γ expression by NK cells and T cells. IFN-γ production in response to LPS is independent of MHC-II. LPS tolerance is characterized by macrophage dysfunction and suppressed expression of the cytokines IL-12 and IL-15 but not IL-18. Expression of the B7 proteins CD80 and CD86 are increased in LPS tolerance. LPS-induced IL-10 production is unchanged or increased in LPS-tolerant mice and functions as an inhibitor of LPS-induced IFN-γ expression. These findings extend our current knowledge of the factors that regulate IFN-γ production after LPS challenge and characterize the alterations that occur in LPS tolerance.
MATERIALS AND METHODS
Reagents.
LPS (Escherichia coli serotype 0111:B4) and normal goat immunoglobulin G (IgG) were purchased from Sigma Chemical (St. Louis, Mo.). Recombinant IL-12, IL-15, and IL-18, monoclonal anti-CD3ɛ antibody, and polyclonal antibodies against IL-12, IL-15, IL-18, CD80, and CD86, as well as the CTLA-Ig fusion protein, were purchased from R&D Systems (Minneapolis, Minn.). Anti-CD28 antibody was purchased from Caltag Laboratories (Burlingame, Calif.). Polyclonal anti-IL-1 converting enzyme (ICE) p20 was purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Anti-MHC-II antibody was purchased from Leinco Technologies (St. Louis, Mo.). Clinical isolates of Pseudomonas aeruginosa and Staphylococcus aureus were obtained from the clinical microbiology laboratory at the Shriners Hospital for Children, Galveston Burns Unit, and were heat-killed at 56°C for 1 h.
Animal model.
All studies were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and met National Institutes of Health guidelines for the use of experimental animals in research. Female, 6- to 8-week-old BALB/c mice (Harlan Sprague Dawley, Indianapolis, Ind.) were used in all studies. Mice were housed in a monitored, light-dark cycled environment and provided standard lab chow and water ad libitum. LPS tolerance was induced by injecting mice intraperitoneally (i.p.) daily for 2 days with LPS (0.4 mg/kg of body weight/mouse in 0.2 ml of normal saline). Control mice received normal saline (0.2 ml) in the same regimen. On day 4, all mice received a challenge dose of LPS (4 mg/kg/mouse; i.p.). Sera and spleens were harvested after LPS challenge for assessment of cytokine expression. All LPS injections were given between 8 a.m. and noon.
Isolation of splenocytes and peritoneal macrophages.
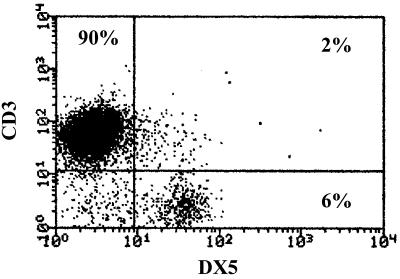
Spleens were aseptically harvested from mice and transferred to six-well culture plates containing RPMI 1640 medium supplemented with 10% fetal bovine serum and penicillin (10 U/ml)–streptomycin (10 μg/ml). This medium preparation was used in all experiments. Spleens were minced and passed over a sterile mesh, and erythrocytes were lysed. The remaining cells were resuspended in media and represent the whole spleen mononuclear cell population. Macrophage-depleted splenocytes were prepared by incubating the whole spleen mononuclear cell preparation (107 cells/ml) in 75-cm2 culture flasks for 16 to 18 h. The nonadherent, macrophage-depleted cell population was harvested, washed, and resuspended in media. For T-cell and NK-cell isolation, splenocytes were passed through T-cell enrichment columns (R&D Systems). The columns were packed with glass beads coated with Ig and anti-Ig antibody that selectively bind phagocytes and B lymphocytes, respectively. Flow cytometric analysis showed that approximately 90% of the cells in our isolates were T lymphocytes (CD3+/DX5−), 6% were NK cells (CD3−/DX5+), and 2% were NK/T cells (CD3+/DX5+) (Fig. 1). Therefore, this technique is highly selective for enrichment of the splenic T-lymphocyte and NK-cell populations. The viability of isolated cells was greater than 95% as determined by trypan blue exclusion. Resident peritoneal macrophages were harvested by peritoneal lavage with 10 ml of phosphate-buffered saline (PBS). The cells were washed (three times), resuspended in media, and used in isolated culture or coculture experiments with splenic T cells and NK cells.
FIG. 1.
Characterization of splenocytes after passage over T-cell enrichment columns. Isolated splenocytes were passed through T-cell enrichment columns and analyzed by flow cytometry after staining with FITC-conjugated anti-DX5 antibody and PE-conjugated anti-CD3 antibody. The percentage of cells staining with each antibody is indicated.
Peritoneal macrophages and splenic T-NK cells were also cocultured using Transwells, which are polycarbonate membranes with a 0.4-μm pore size designed to allow passage of soluble factors but prevent direct cell contact (Corning Costar, Cambridge, Mass.). T-NK cells (107/well) were cultured with peritoneal macrophages (5 × 105/well) either in direct contact or separated by Transwells. Cells were cultured for 24 h, conditioned media were harvested, and IFN-γ levels were determined.
ELISA for murine cytokines.
IFN-γ, IL-12 p40, IL-12 p70, and IL-18 levels in serum and conditioned media were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocol (R&D Systems). Briefly, standards or experimental samples were added to microtiter plates coated with monoclonal antibody to the cytokine of interest and incubated for 2 h. After washing, horseradish peroxidase-conjugated, cytokine-specific antibody was added to each well, incubated for 2 h, and washed. Substrate solution was added and incubated for 30 min, and the reaction was terminated by the addition of stop solution. Cytokine levels were determined by measuring the optical density at 450 nm by using a microtiter plate reader (Dynatech Laboratories, Chantilly, Va.).
Flow cytometry.
Fluorescein isothiocyanate (FITC)-conjugated anti-CD14 antibody was purchased from Research Diagnostics (Flanders, N.J.). FITC-conjugated anti-CD3 and anti-CD19 antibodies as well as phycoerythrin (PE)-conjugated anti-CD80, -CD86 and -CD28 antibodies were purchased from Caltag Laboratories (Burlingame, Calif.). FITC-conjugated anti-DX5 antibody and PE-conjugated anti-IFN-γ antibodies were purchased from B-D Pharmingen (San Diego, Calif.). Isotype controls included FITC-conjugated rat IgG2a, rat IgM, and hamster IgG as well as PE-conjugated rat IgG1. For surface staining, splenocytes (106 in 0.1 ml of PBS) were incubated (4°C) with marker-specific antibodies or isotype controls (0.5 μg of antibody/106 cells) in polystyrene tubes for 30 min. Cells were washed with 2 ml of PBS and fixed in 1% paraformaldehyde. For intracellular cytokine staining, surface markers were stained as just described. Cells were then permeabilized using Cytofix/Cytoperm (B-D Pharmingen) and stained with anti-IFN-γ antibody or isotype control for 30 min. Cells were washed with 2 ml of PBS and fixed with 1% paraformaldehyde. All analyses were performed on a FACSort flow cytometer (Becton Dickinson, Mountain View, Calif.).
RT-PCR.
Total RNA were isolated using the TRI Reagent System (Molecular Research Center, Cincinnati, Ohio) according to the manufacturer's protocol. For reverse transcription-PCR (RT-PCR), 5 μg of total RNA was reverse transcribed (42°C for 50 min) using Superscript II reverse transcriptase (Gibco/BRL, Gaithersburg, Md.), oligo(dT) primers, and deoxynecleoside triphosphates. The RT product (3 to 6 μl) was amplified using Taq DNA polymerase (1 U/reaction; Sigma Chemical), deoxynecleoside triphosphates, and product-specific primers. The PCR was conducted for 35 to 40 cycles in a DNA thermal cycler (Barnstead/Thermolyne, Dubuque, Iowa). β-Actin served as an internal control in all experiments. The following 5′-to-3′ primers were used: for β-actin, forward primer 5′-CTACAATGAGCTGCGTGTGG-3′ and reverse primer 5′-AAGGAAGGCTGGAAGAGTGC-3′; for IFN-γ, forward primer 5′-AGCTCTGAGACAATGAACGC-3′ and reverse primer 5′-GGACAATCTCTTCCCCACCC-3′; for IL-12 p40, forward primer 5′-TCTGCAGAGAAGGTCACACTG-3′ and reverse primer 5′-GACTTCGGTAGATGTTTCCTC-3′; for IL-18, forward primer 5′-GCTTGAATCTAAATTATCAGTC-3′ and reverse primer 5′-GAAGATTCAAATTGCATCTTAT-3′. The PCR products were separated on 2% agarose gels containing 1× Tris-acetic acid-EDTA and ethidium bromide. Gels were analyzed by densitometry using a Fluor-S MultiImager (Bio-Rad, Hercules, Calif.). The predicted sizes of the PCR products were 528, 320, 374, and 420 bp for β-actin, IFN-γ, IL-12 p40, and IL-18, respectively.
RPA.
RNase protection assay (RPA) was performed using the Riboquant system (Pharmingen, San Diego, Calif.) per the manufacturer's instructions. Briefly, radiolabeled RNA probes were synthesized from DNA template sets using T7 RNA polymerase, 32P-UTP, and pooled nonradiolabeled nucleotides. Isolated mRNAs (10 μg of total RNA/sample) were hybridized with purified riboprobes and subjected to RNase digestion. DNA template sets included probes for the L32 and GADPH (glyceraldehyde-3-phosphate dehydrogenase) housekeeping genes that serve as internal controls. Protected RNA species were separated on 5% polyacrylamide denaturing gels by using 0.5× Tris-borate-EDTA running buffer. Gels were run at 50 W of constant power, and dried and protected fragments were visualized using autoradiography.
Immunoprecipitation and Western blotting.
Immunoprecipitation and Western blotting were utilized to determine IL-18 expression by splenic pan-leukocyte preparations. For immunoprecipitation, splenic pan-leukocytes (5 × 106/well) were cultured (37°C, 5% CO2) in six-well plates supplemented with or without LPS (1 μg/ml) for 24 h. Conditioned media were harvested and centrifuged (2,000 × g for 10 min) to remove residual cells. Cells were harvested, washed (three times) with PBS, and disrupted with lysis buffer (62 mM Tris base, 10 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 1 mM aprotinin, 14 mM leupeptin, 1 mM pepstatin, 80 μg of benzamidine/ml, 1% Triton X-100). Protein concentration was determined by the Bradford assay (Bio-Rad). Cell lysates (500 μg of protein in 0.5 ml) and conditioned media (2 ml) were incubated (4°C) overnight with anti-IL-18 antibody (1 to 2 μg/tube) followed by the addition of 30 μl of protein A-Sepharose beads and an additional 1-h incubation at room temperature. The beads were sequentially washed with lysis buffer (three times) and PBS (two times), followed by addition of 30 μl of Laemmli buffer and boiling for 5 min. The beads were pelleted by centrifugation (5,000 × g for 10 min), and the entire supernatant was loaded onto a 4 to 20% gradient denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel. Separated proteins were then transferred (100 V for 1 h at 4°C) to a nitrocellulose membrane (0.2-μm pore size; Bio-Rad) (25 mM Tris, 192 mM glycine, 20% methanol transfer buffer), and processed as described below.
For Western blotting, splenocytes were disrupted in lysis buffer and protein content was determined using the Bradford assay (Bio-Rad). In some studies, protein was harvested directly from whole spleen by homogenizing the tissue in lysis buffer by using a small tissue grinder. Proteins (100 μg/lane) were loaded onto a 4 to 20% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, electrophoresed under denaturing conditions, and transferred to a 0.2-μm-pore-size nitrocellulose membrane. The membrane was blocked overnight at 4°C with blocking buffer (5% nonfat dry milk in 0.1% Triton X-100–Tris-buffered saline [TTBS]) and incubated with primary antibody for 1 h at room temperature. After washing (three times) with blocking buffer, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody for 1 h and washed (five times) with TTBS. Immunoreactive proteins were visualized using the ECL system (Amersham).
Data analysis.
For comparisons of data from multiple groups, two-way analysis of variance was performed followed by Student's t test. A P value of <0.05 was considered significant.
RESULTS
Suppression of IFN-γ production in LPS-tolerant mice is macrophage dependent.
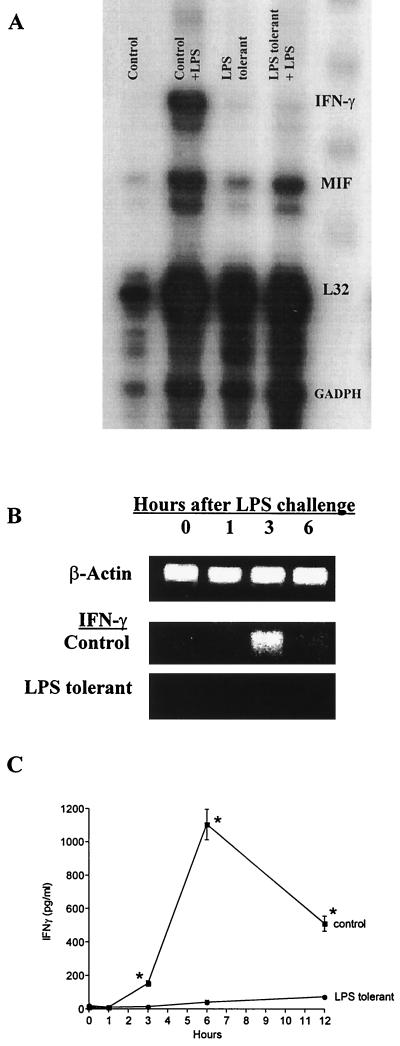
The expression of IFN-γ mRNA in mouse spleen and the secretion of IFN-γ protein into mouse sera after LPS challenge were markedly suppressed in LPS-tolerant mice (Fig. 2). Intraperitoneal challenge of control mice with LPS resulted in a marked increase in IFN-γ mRNA expression at 4 h postchallenge as determined by RPA (Fig. 2A). However, IFN-γ mRNA was not significantly induced in the spleens of LPS-tolerant mice challenged with LPS. Assessment of splenic IFN-γ mRNA expression over time using RT-PCR showed that IFN-γ expression peaked at 3 h after LPS challenge in control mice and was not detectable within the limits of our assay in LPS-tolerant mice (Fig. 2B). Serum IFN-γ levels peaked at 6 h after LPS challenge in control mice and were markedly decreased in LPS-tolerant mice (Fig. 2C).
FIG. 2.
IFN-γ mRNA expression and serum IFN-γ levels are suppressed in LPS-tolerant mice. (A) Spleens were harvested from control and LPS-tolerant mice 4 h following challenge with saline (0.2 ml; i.p.) or LPS (100 μg; i.p.), total RNA was isolated, and IFN-γ mRNA expression was determined by RPA. (B) Spleens were harvested from control and LPS-tolerant mice following LPS challenge (100 μg; i.p.) at the time points indicated. Total RNA was isolated, and IFN-γ mRNA expression was determined by semiquantitative RT-PCR. (C) Sera were harvested from control and LPS-tolerant mice after LPS challenge (100 μg; i.p.) at the time points indicated, and IFN-γ levels were determined using ELISA. n = 6 to 10 mice/group; ∗, significantly (P < 0.05) greater than LPS-tolerant group. Data shown are means ± standard error of the mean.
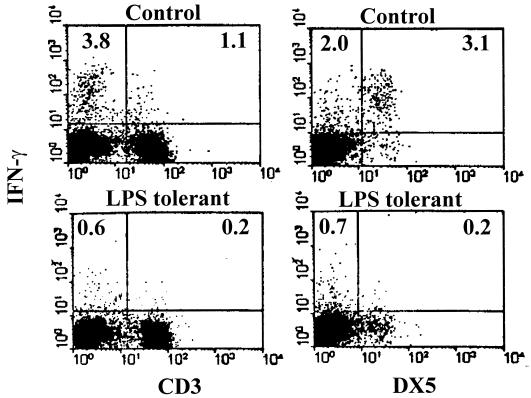
Further studies were undertaken to identify the cellular source of IFN-γ in the spleen and determine the effect of LPS tolerance on splenic IFN-γ production by specific lymphocyte populations. In these studies, splenocytes were isolated from control or LPS-tolerant mice 8 h after LPS challenge and IFN-γ production was determined by intracellular staining and flow cytometry (Fig. 3). These studies showed that the majority of IFN-γ producing cells were CD3 negative. In control mice, IFN-γ+CD3+ cells accounted for 1.1% of total splenocytes and 22.3% of IFN-γ-producing cells, whereas IFN-γ+CD3− cells comprised 3.8% of total splenocytes and 77.7% of IFN-γ+ cells. Most of the IFN-γ+CD3− cells were NK cells (DX5+). Specifically, IFN-γ+DX5+ cells comprised 3.1% of all splenocytes and 61.5% of IFN-γ+ cells. Therefore, CD3+ and DX5+ cells comprised more than 83% of LPS-induced, IFN-γ+ cells. In addition, approximately 2% of CD3+ cells produced IFN-γ in response to LPS challenge, whereas 74% of DX5+ cells were IFN-γ+. Induction of LPS tolerance resulted in marked reductions in IFN-γ production by both CD3+ and DX5+ populations. As a percentage of cells in each population, IFN-γ production was decreased by 83% in the CD3+ population and 91% for DX5+ cells in LPS-tolerant mice compared to control mice.
FIG. 3.
Characterization of IFN-γ-producing cells in the spleens of control and LPS-tolerant mice. Splenocytes were isolated from control and LPS-tolerant mice 8 h after LPS challenge. Intracellular staining for IFN-γ was performed after surface staining with anti-CD3 or anti-DX5 antibody. Cells were analyzed by flow cytometry. Numbers indicate the percentage of IFN-γ-producing cells as a percentage of total splenocytes.
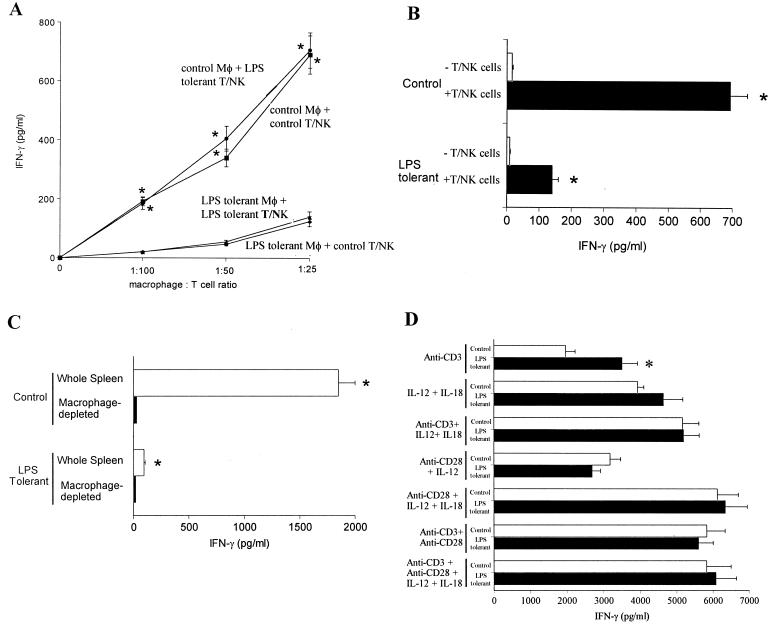
In order to identify the cellular source of impaired LPS responsiveness in the tolerant state, isolated splenic T cells and NK cells were cultured with control or LPS-tolerant peritoneal macrophages and their ability to secrete IFN-γ in response to LPS was determined (Fig. 4). As outlined previously, we utilized a column binding procedure to enrich splenic T and NK cells (Fig. 1). T and NK cells did not secrete IFN-γ in response to LPS when cultured in the absence of macrophages (Fig. 4A). Coculture of T and NK cells isolated from control or LPS-tolerant mice with control peritoneal macrophages resulted in similar levels of IFN-γ secretion that increased in proportion to the number of macrophages added. LPS-induced IFN-γ production by T cells and NK cells isolated from LPS-tolerant mice after incubation with control macrophages did not significantly differ from control T-NK cells incubated with control macrophages (Fig. 4A). However, IFN-γ production by T cells and NK cells obtained from either control or LPS-tolerant mice was decreased by 85 to 90% after coculture with LPS-tolerant macrophages (Fig. 4A). Additional studies were undertaken to determine whether IFN-γ was arising directly from the added peritoneal macrophages. In both control and LPS-tolerant mice, isolated peritoneal macrophages secreted only 2 and 9%, respectively, of the IFN-γ produced by macrophage-T-NK-cell cocultures (Fig. 4B). This does not exclude the possibility that macrophages require the presence of T-NK cells in order to secrete IFN-γ. However, our flow cytometry studies using whole spleen showed that macrophages (CD14+) comprised less than 2% of IFN-γ-producing cells after LPS stimulation (data not shown). Macrophage-T-NK cell cocultures obtained from LPS-tolerant mice secreted significantly (P < 0.05) less IFN-γ than control cocultures.
FIG. 4.
LPS-induced IFN-γ production is macrophage dependent. (A) Isolated splenic T cells and NK cells (106 cells/well in 96-well plates) and peritoneal macrophages (macrophage [Mφ]-to-T-NK cell ratios) from control and LPS-tolerant mice were cocultured in the presence of LPS (100 ng/ml) for 24 h. IFN-γ levels in conditioned media were determined by ELISA. ∗, significantly (P < 0.05) greater than T cells cultured with LPS-tolerant macrophages. (B) Isolated peritoneal macrophages (5 × 104/well in 96-well plates) were cultured with or without isolated splenic T-NK cells (106 cells/well, macrophage-to-T-NK cell ratio of 1:20) for 24 h in the presence of LPS (100 ng/ml). ∗, significantly (P < 0.05) greater than macrophages cultured in the absence of T-NK cells. (C) Whole or macrophage-depleted splenocytes (106 cells/well in 96-well plates) from control and LPS-tolerant mice were cultured with LPS (100 ng/ml) for 24 h. IFN-γ levels were determined by ELISA. ∗, significantly (P < 0.05) greater than macrophage-depleted splenocytes. (D) Splenic T cells and NK cells (106/well) obtained from control and LPS-tolerant mice were cultured in 96-well plates with the indicated factors for 24 h. IL-12 and IL-18 were added at concentrations of 1 and 10 ng/ml, respectively. Immobilized anti-CD3 and anti-CD28 antibodies were added at 10 μg/ml. IFN-γ levels in conditioned media were determined by ELISA. ∗, significantly (P < 0.05) greater than control. For all studies, n was 6 to 12 wells/group, with cells taken from at least three mice per group. Data shown are means ± standard error of the mean.
The role of macrophages in LPS-induced IFN-γ secretion was further elucidated using whole-splenocyte cultures as well as macrophage-depleted splenocytes (Fig. 4C). Whole-splenocyte cultures isolated from LPS-tolerant mice secreted significantly (P < 0.05) less IFN-γ into conditioned media than control splenocytes in response to LPS challenge (Fig. 4C). The macrophage dependence of LPS-induced IFN-γ production in the spleen was demonstrated by comparing the ability of whole splenic mononuclear cells and macrophage-depleted splenocytes to secrete IFN-γ in response to LPS. In both control and LPS-tolerant spleens, macrophage-depleted splenocytes secreted markedly less IFN-γ than the whole spleen mononuclear cell population (Fig. 4C).
Additional studies were performed to further assess T-cell and NK-cell function in the LPS-tolerant state by determining the ability of T cells and NK cells isolated from control and LPS-tolerant mice to respond to known IFN-γ-inducing factors (Fig. 4D). T cells and NK cells obtained from LPS-tolerant mice produced significantly more IFN-γ in response to the polyclonal T-cell activator anti-CD3 antibody than control T-NK cells. In response to other IFN-γ-inducing factors, such as IL-12, IL-18, and anti-CD28 antibody, LPS-tolerant T cells and NK cells were equally as responsive as T-NK cells obtained from control mice (Fig. 4D).
Induction of IFN-γ by LPS is regulated by multiple cytokines and B7-CD28 interactions.
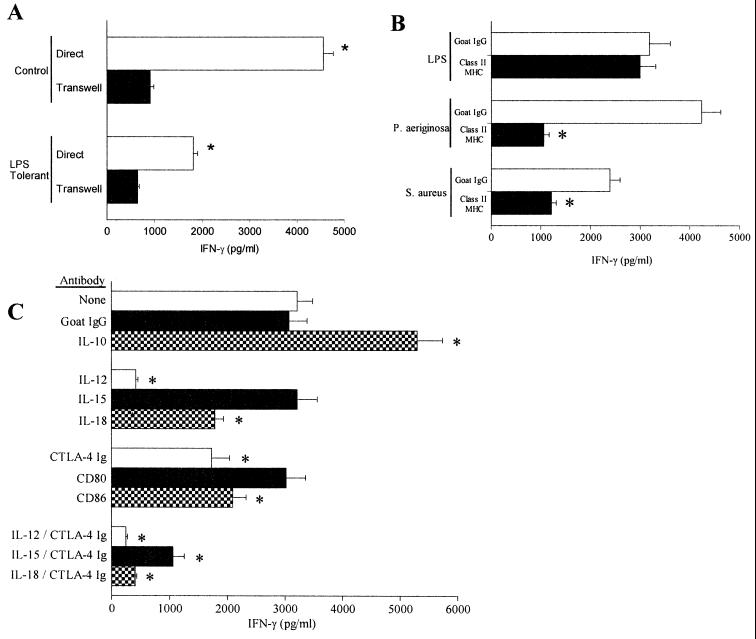
In order to define factors that mediate LPS-induced IFN-γ production, macrophages and T-NK cells were cocultured either in direct contact or separated from direct contact using Transwells. Transwells are porous membranes that allow the passage of soluble factors such as cytokines but prevent direct cell interaction. The goal of these studies was to determine the importance of direct cell contact in LPS-induced IFN-γ production. Control cells cocultured using Transwells exhibited an 80% decrease in LPS-induced IFN-γ production compared to cells cultured in direct contact (Fig. 5A). Cocultures obtained from LPS-tolerant mice also showed a 64% decrease in IFN-γ production when separated by Transwells compared to cells cultured in direct contact.
FIG. 5.
Cellular mediators of LPS-induced production of IFN-γ. (A) Peritoneal macrophages (5 × 105/well) and splenic T-NK cells (107 cells/well in 24-well plates) were cultured with LPS (100 ng/ml) either in direct contact or separated by Transwells for 24 h. IFN-γ levels in conditioned media were determined by ELISA. ∗, significantly (P < 0.05) greater than cells cultured in Transwells; n = 6 to 10 wells/group. (B) Determination of MHC-II dependence for LPS-induced production of IFN-γ. Splenocytes (106/well in 96-well plates) were stimulated with LPS (100 ng/ml), P. aeruginosa (107 CFU/well), or S. aureus (107 CFU/well) during coculture with goat IgG (10 μg/ml) or antibody against mouse MHC-II (10 μg/ml). Conditioned media were harvested to assess IFN-γ levels by ELISA after 24 h of culture. ∗, significantly (P < 0.05) decreased IFN-γ levels compared to goat IgG. (C) Mouse splenocytes were stimulated with LPS (100 ng/ml) during coculture with the indicated antibodies. IFN-γ levels in conditioned media were determined by ELISA after 24 h of culture. All antibodies were added at a concentration of 10 μg/ml, which is least fivefold greater than the 50% effective dose for each antibody. CTLA-4 Ig fusion protein was added at 1 μg/ml. ∗, significantly (P < 0.05) altered IFN-γ production compared to cells cultured with goat IgG. Data shown are means ± standard error of the mean.
Additional studies were undertaken to elucidate the roles of specific mediators in LPS-induced IFN-γ production. The role of MHC-II in LPS-induced production of IFN-γ was determined by adding antibodies specific for mouse MHC-II to splenocyte cultures that were stimulated with LPS, heat-killed P. aeruginosa cells, or heat-killed S. aureus cells (Fig. 5B). Culture of LPS-stimulated mouse splenocytes with anti-MHC-II antibody did not decrease IFN-γ secretion compared to splenocytes cultured with nonspecific goat IgG. However, addition of anti-MHC-II antibody decreased IFN-γ secretion in response to P. aeruginosa or S. aureus by 72 and 53%, respectively.
We also investigated the relative roles of cytokines and B7 proteins in LPS-induced secretion of IFN-γ. Compared to cells cultured without antibodies or with nonspecific goat IgG, addition of anti-IL-10 antibody increased LPS-induced IFN-γ production by 63% (Fig. 5C). However, anti-IL-12 antibody reduced LPS-induced IFN-γ secretion by 84%. Anti-IL-15 antibody alone did not significantly change LPS-induced IFN-γ secretion compared to that of the control, but addition of anti-IL-18 antibody reduced LPS-induced secretion of IFN-γ by approximately 30%. The combination of anti-IL-12 antibody with either anti-IL-15 or anti-IL-18 antibody reduced IFN-γ to the same level as anti-IL-12 antibody alone (data not shown). The CTLA-4 Ig fusion protein, an inhibitor of B7-CD28 interactions, significantly decreased LPS-induced IFN-γ secretion by 32%. Blocking antibodies against CD80 did not significantly reduce IFN-γ production compared to controls, but antibodies against CD86 significantly inhibited IFN-γ secretion by 25% (Fig. 5C). Studies were also performed using the combination of CTLA-4 Ig and antibodies to specific cytokines. The combination of CTLA-4 Ig and anti-IL-12 antibody decreased LPS-induced IFN-γ secretion to levels that were not significantly different from those observed with anti-IL-12 antibody alone. However, the combination of anti-IL-15 or anti-IL-18 antibody with CTLA-4 Ig fusion protein significantly (P < 0.05) inhibited LPS-induced IFN-γ secretion compared to either cytokine or CTLA-4 fusion protein alone.
LPS tolerance causes suppressed production of IL-12 and IL-15, but not IL-18 or B7 proteins.
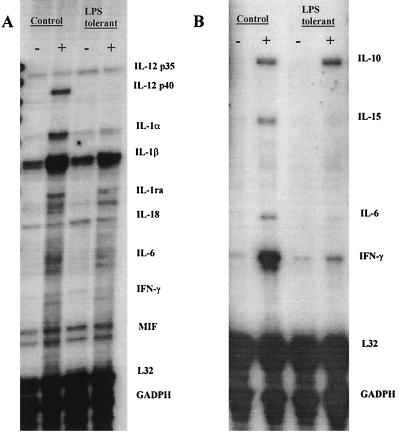
In order to identify mechanisms of suppressed IFN-γ production in LPS-tolerant mice, we evaluated levels of IFN-γ-regulating factors in control and LPS-tolerant mice at baseline and after LPS challenge. IL-12 p35 mRNA was constitutively expressed in the spleens of control and LPS-tolerant mice (Fig. 6A). LPS challenge did not significantly change IL-12 p35 expression in either group. In contrast, IL-12 p40 mRNA did not exhibit constitutive expression in either control or LPS-tolerant mice but was induced 1 h after LPS challenge in control mice but not in LPS-tolerant mice. Like IL-12 p35, IL-18 mRNA was constitutively expressed in the spleens of control and LPS-tolerant mice and expression was not significantly changed after LPS challenge in either control or LPS-tolerant mice (Fig. 6A). IL-10 mRNA was not constitutively expressed but was induced in the spleens of both control and LPS-tolerant mice 4 h after LPS challenge (Fig. 6B). LPS stimulated expression of IL-15 and IFN-γ mRNAs in the spleens of control mice but not in LPS-tolerant mice 4 h after LPS challenge.
FIG. 6.
Cytokine mRNA expression in control and LPS-tolerant mice after LPS challenge. (A) Control and LPS-tolerant mice were challenged with either saline (0.2 ml IP) or LPS (100 μg/mouse; i.p.), and spleens were harvested after 1 h. Total RNA was isolated, and cytokine expression was determined by RPA (DNA template, mCK-2b). (B) Control and LPS-tolerant mice were challenged with LPS as described above. Spleens were harvested 4 h after LPS challenge, RNA was isolated, and cytokine mRNA expression was determined by RPA (DNA template mCK-1). −, saline challenged; +, LPS challenged.
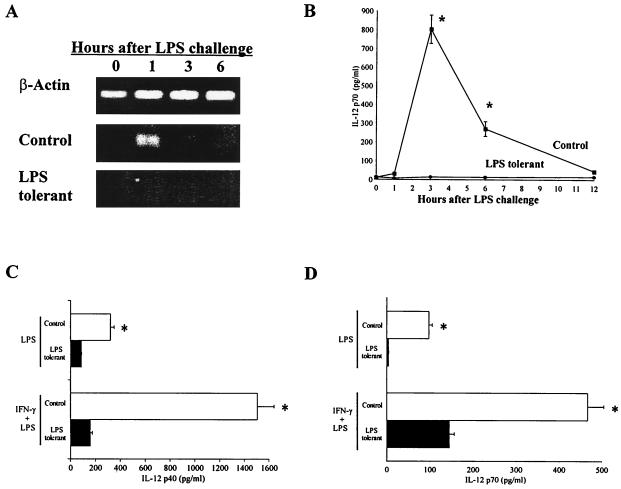
Because of its key role in the induction of IFN-γ production, studies were undertaken to better define the effects of LPS tolerance on IL-12 expression (Fig. 7). These studies showed that LPS-induced splenic IL-12 p40 mRNA and serum IL-12 p70 levels were suppressed in LPS-tolerant mice. IL-12 p40 mRNA peaked in the spleens of control mice at 1 h after LPS challenge and was not detectable in the spleens of LPS-tolerant mice within the limits of our RT-PCR assay (Fig. 7A). IL-12 p70 protein peaked in serum at 3 h after LPS challenge and was markedly decreased in LPS-tolerant mice (Fig. 7B). We also performed ex vivo studies to determine the effects of LPS tolerance on IL-12 secretion by isolated peritoneal macrophages. Peritoneal macrophages showed suppressed secretion of IL-12 p40 and IL-12 p70 after the induction of LPS tolerance (Fig. 7C and D). Priming of macrophages with IFN-γ augmented LPS-induced secretion of IL-12 p40 and IL-12 p70 in both control and LPS-tolerant macrophages. However, IFN-γ production remained significantly (P < 0.05) lower in LPS-tolerant macrophages than in the control.
FIG. 7.
IL-12 expression is suppressed in LPS-tolerant mice. (A) LPS-induced splenic IL-12 mRNA expression in control and LPS-tolerant mice. Spleens were harvested from mice following LPS challenge at the indicated time points, total RNA was isolated, and IL-12 mRNA expression was determined using RT-PCR. (B) Sera were harvested from LPS-challenged mice at the time points indicated, and IL-12 levels were determined by ELISA. n = 6 to 10 mice/group; ∗, significantly (P < 0.05) greater than LPS-tolerant group. (C) Thioglycolate-elicited peritoneal macrophages (2 × 105/well in 96-well plates) were rendered LPS tolerant by incubation with LPS (10 ng/ml) for 24 h. The macrophages were washed (three times) with media and then primed with IFN-γ (100 ng/ml) for 16 h. Cells were then challenged with LPS (100 ng/ml) for 24 h, conditioned media were harvested, and IL-12 p40 levels were determined by ELISA. (D) Peritoneal macrophages were isolated and treated as above. IL-12 p70 levels were determined by ELISA. n = 6 per group; ∗, significantly (P < 0.05) greater than LPS-tolerant group. Data shown are means ± standard error of the mean.
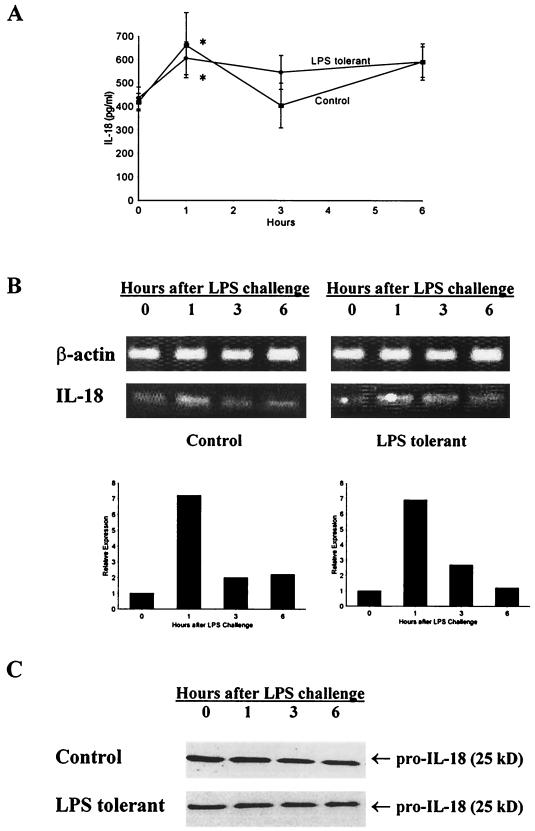
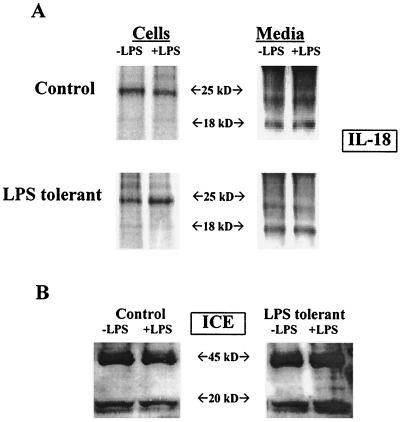
Because IL-18 secretion is mediated through complex mechanisms that include intracellular cleavage of pro-IL-18 by ICE, studies were undertaken to further characterize the effect of LPS tolerance on IL-18 synthesis and secretion. IL-18 was constitutively expressed in both control and LPS-tolerant mice and was not suppressed by LPS tolerance (Fig. 8). IL-18 was present in mouse serum prior to challenge with LPS and showed a small, but significant, increase that peaked 1 h after LPS challenge (Fig. 8A). When serum IL-18 levels of control and LPS-tolerant mice were compared, no significant difference was observed at any of the time points studied. IL-18 mRNA was also constitutively expressed in the spleens of control and LPS-tolerant mice (Fig. 8B). LPS treatment increased splenic IL-18 mRNA expression at 1 h after challenge in both control and LPS-tolerant mice, and levels of IL-18 mRNA expression were not different between groups (Fig. 8B). Pro-IL-18 (p25) protein was also present in the spleens of mice prior to and following LPS challenge in both control and LPS-tolerant mice (Fig. 8C). Levels of pro-IL-18 were similar among mice from both groups, and stimulation with LPS did not increase pro-IL-18 levels in the spleens of mice from either group. We did not observe mature IL-18 (p18) in our Western blots of whole mouse spleen. In an ex vivo model, we showed a predominance of p25 in spleen cell lysates (Fig. 9A). Analysis of conditioned media showed a predominance of p18 that was constitutively released by both control and LPS-tolerant splenocytes. Comparison of control and LPS-tolerant splenocytes did not reveal a significant difference in levels of p18 or p25 between the groups. Pro-IL-18 is cleaved by ICE to yield the mature, secreted form of IL-18. We measured ICE levels in splenocytes isolated from control and LPS-tolerant mice (Fig. 9B). Splenocytes obtained from both control and LPS-tolerant mice exhibited constitutive expression of the ICE precursor (p45) as well as the mature component p20. The induction of LPS tolerance did not change expression of either p20 or p45 (Fig. 9B).
FIG. 8.
LPS-induced IL-18 production by control and LPS-tolerant mice. (A) Serum IL-18 levels at different time points after LPS challenge in control and LPS-tolerant mice. Sera were harvested from mice at the time points indicated after challenge with LPS (100 μg; i.p.), and IL-18 levels were determined by ELISA. ∗, significantly (P < 0.05) greater than IL-18 levels prior to LPS challenge; n = 6 to 8 mice/group. Data shown are means ± standard error of the mean. (B) Splenic IL-18 mRNA expression in control and LPS-tolerant mice. Spleens were harvested from control and LPS-tolerant mice after LPS challenge, total RNA was isolated, and IL-18 mRNA expression was determined by semiquantitative RT-PCR. Densitometry was performed to quantitate relative IL-18 mRNA expression in mouse spleens. (C) Splenic IL-18 protein levels in control and LPS-tolerant mice after LPS challenge. Spleens were harvested from mice after LPS challenge, total protein was harvested, and IL-18 levels were determined by Western blot analysis. (B and C) Data are representative of results from three different mice.
FIG. 9.
IL-18 and ICE production by isolated splenocytes from control and LPS-tolerant mice. (A) Splenic mononuclear cells were harvested from mice and cultured (107 cells/well in six-well plates) with or without LPS (100 ng/ml) for 24 h. Cells and conditioned media were harvested, and IL-18 levels were measured by immunoprecipitation and Western blotting. (B) Splenic mononuclear cells were harvested and cultured (107 cells/well in six-well plates) with LPS (100 ng/ml) for 24 h. ICE content was determined by Western blotting.
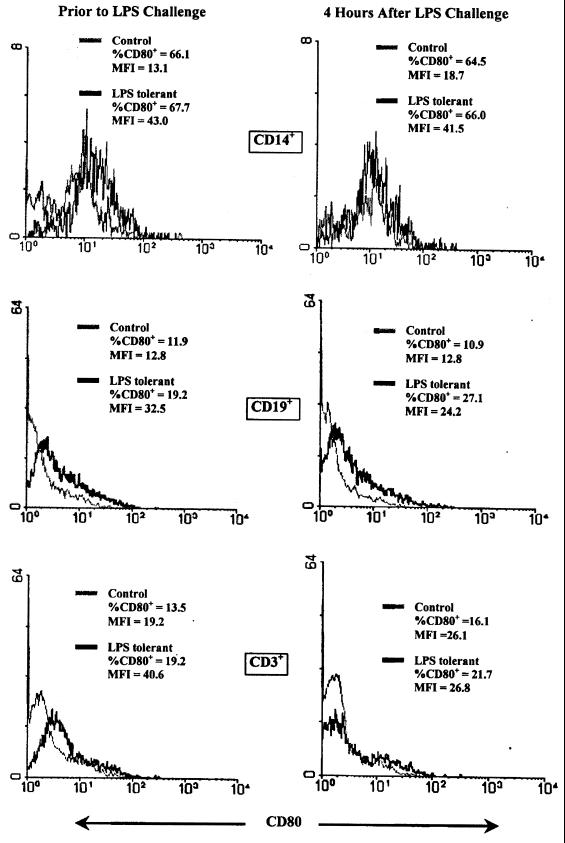
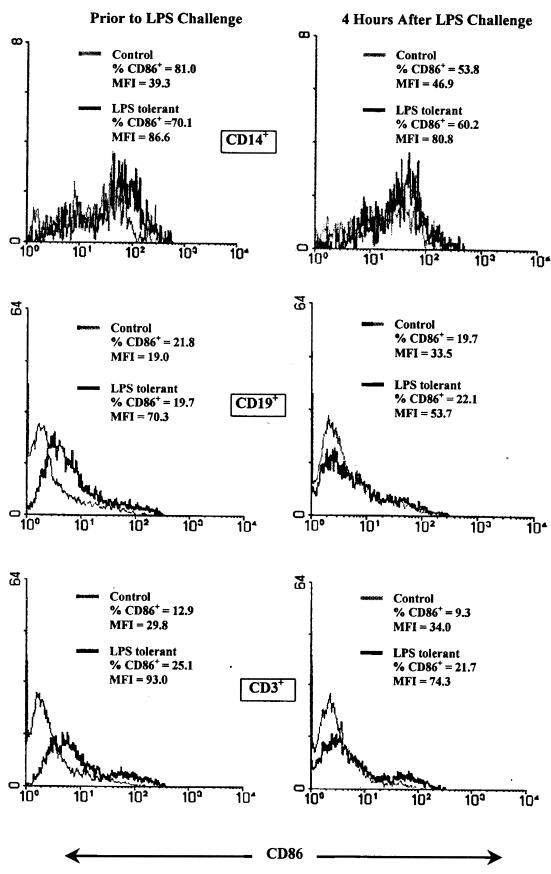
The effect of LPS tolerance on expression of the B7 proteins CD80 and CD86 was determined using flow cytometry either prior to or 4 h after LPS challenge. B7 protein expression by splenic macrophages (CD14+), B lymphocytes (CD19+), and T lymphocytes (CD3+) was ascertained. The data presented are representative of three separate analyses. Among the macrophage population, the percentage of CD14+CD80+ cells was not different when comparing the control and LPS-tolerant groups either prior to or after LPS challenge (Fig. 10). In both groups, approximately 65% of CD14+ cells expressed cell surface CD80. However, the level of CD80 expression per cell as indicated by mean fluorescence intensity (MFI) was increased in CD14+ cells from LPS-tolerant mice at baseline and following LPS stimulation by more than onefold compared to that of controls. Among B cells, the percentage of cells expressing CD80 in the LPS-tolerant group was approximately 70% greater and MFI was increased by more than twofold in LPS-tolerant mice. Following LPS challenge, the percentage of B cells expressing CD80 and the MFI remained increased by at least onefold compared to that of the controls. The percentage of T cells expressing CD80 in LPS-tolerant mice was increased by approximately 50%, and the MFI was elevated by more than onefold prior to LPS challenge. However, after LPS stimulation, the percentage of T cells expressing CD80 and MFI in the LPS-tolerant group was not different from the control. The percentage of CD14+ cells expressing CD86 decreased in both groups 4 h after LPS challenge and was not significantly different between groups either before or after LPS challenge. However, the MFI for CD86 was increased by approximately onefold in LPS-tolerant mice both before and after LPS challenge (Fig. 11). The percentage of B cells from LPS-tolerant mice expressing CD86 was not different from the controls. However, the MFI was increased by more than twofold at baseline and by approximately 60% after LPS challenge in the LPS-tolerant group compared to the control. T cells from LPS-tolerant mice exhibited an increase in both the percentage of cells expressing CD86 and in MFI both at baseline and after LPS challenge.
FIG. 10.
Effect of LPS tolerance on CD80 expression by mouse splenocytes. Splenocytes were harvested prior to or 4 h after LPS (100 μg; i.p.) challenge, and CD80 expression was determined by flow cytometry. Unseparated total splenocytes were stained with PE-conjugated anti-CD80 antibody and FITC-conjugated anti-CD14, -CD19, or -CD3 antibody. Data are expressed as the percentage of each subpopulation staining positively for CD80 and as the mean fluorescence intensity (MFI) of CD80 staining.
FIG. 11.
Effect of LPS tolerance on CD86 expression by mouse splenocytes. Splenocytes were harvested from control or LPS-tolerant mice either prior to or 4 h after LPS (100 μg; i.p.) challenge, and CD86 expression was determined by flow cytometry. Unseparated, total splenocytes were stained with PE-conjugated anti-CD86 antibody and FITC-conjugated anti-CD14, -CD19, or -CD3 antibody. Data are expressed as the percentage of each subpopulation staining positively for CD86 and the mean fluorescence intensity (MFI) of CD86 staining.
LPS tolerance causes decreased expression of IL-12R and IL-15R.
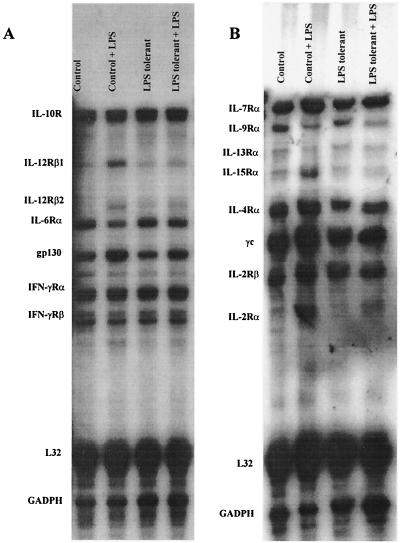
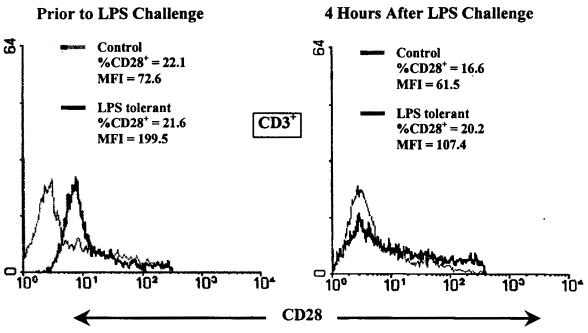
The effect of LPS tolerance on splenic cytokine receptor mRNA expression was determined by RPA 4 h after LPS challenge (Fig. 12). IL-10 receptor (IL-10R) and IL-12Rβ1 mRNAs were constitutively expressed in the spleens of both control and LPS-tolerant mice (Fig. 12A). LPS challenge did not change IL-10R mRNA expression in either group, but LPS stimulation increased expression of IL-12Rβ1 mRNA to a greater level in control mice than in LPS-tolerant mice. Unlike IL-12Rβ1, IL-12Rβ2 mRNA was not constitutively expressed in control spleen but was constitutively expressed in the spleens of LPS-tolerant mice. Challenge of control mice with LPS induced a greater level of IL-12Rβ2 expression than in LPS-tolerant mice. Both IFN-γRα and IFN-γRβ mRNA were constitutively expressed in the spleens of both groups; their levels were not increased by LPS stimulation nor were their significant differences between groups. IL-15Rα subunit mRNA was constitutively expressed by splenocytes from both groups, but induction of IL-15Rα mRNA by LPS was suppressed in the LPS-tolerant group compared to the control (Fig. 12B). The IL-2Rβ subunit, the second component of the functional IL-15R, was also constitutively expressed by splenocytes from both groups, but levels were not increased by LPS challenge nor were there significant differences between groups. Splenic IL-2Rα levels were significantly lower in LPS-tolerant mice compared to controls after LPS challenge, but IL-2Rβ and γc were strongly expressed in both groups. Expression of CD28 by splenocytes from control and LPS-tolerant mice was determined by flow cytometry (Fig. 13). The percentage of cells expressing CD28 was not different between groups either prior to or after LPS challenge. However, the MFI was increased by nearly twofold prior to LPS challenge and by approximately 70% after LPS challenge in the LPS-tolerant group.
FIG. 12.
Effect of LPS tolerance on cytokine receptor expression. Control and LPS-tolerant mice were challenged with saline (0.2 ml/mouse) or LPS (100 μg/mouse). Splenic RNA was harvested 4 h after challenge, and cytokine receptor mRNA levels were determined by RPA. Shown are results obtained with DNA template sets mCR-3 (A) and mCR-1 (B).
FIG. 13.
CD28 expression is increased in LPS-tolerant mice. Splenocytes were harvested from control and LPS-tolerant mice either prior to or 4 h after LPS (100 μg/mouse) challenge, and CD28 levels were determined by flow cytometry. Unseparated total splenocytes were stained with PE-conjugated anti-CD28 antibody and FITC-conjugated anti-CD3 antibody. Data are presented as the percentage of CD3+ cells expressing CD28 and the mean fluorescence intensity (MFI) of CD28 staining.
Splenocytes from LPS-tolerant mice remain responsive to exogenous IFN-γ-inducing factors.
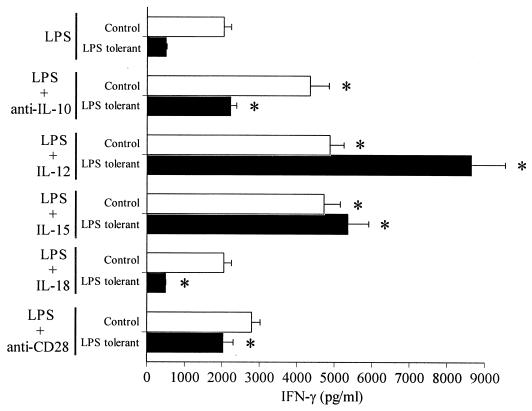
Because LPS tolerance is associated with down-regulation of some IFN-γ-inducing cytokines and their receptors, we examined the abilities of normal and LPS-tolerant splenocytes to respond to exogenously applied IFN-γ-regulating factors to determine whether signaling pathways associated with cytokine receptors were affected (Fig. 14). Comparisons were made between control and LPS-tolerant splenocytes cultured with LPS alone or with LPS plus IFN-γ-regulating factors. Addition of antibody to IL-10 significantly (P < 0.05) increased IFN-γ production after LPS challenge in both control and LPS-tolerant mice compared to cells cultured with LPS alone. Treatment of splenocytes from LPS-tolerant mice with anti-IL-10 antibody returned LPS-induced IFN-γ production to levels observed in control splenocytes challenged with LPS alone. Addition of IL-12 or IL-15 also significantly (P < 0.05) increased LPS-induced secretion of IFN-γ in both control and LPS-tolerant mice compared to splenocytes cultured with LPS alone. Interestingly, LPS-tolerant splenocytes were more responsive to stimulation with LPS plus exogenous IL-12 than control splenocytes. Stimulation with LPS and exogenous IL-18 did not significantly change IFN-γ secretion compared to cells challenged with LPS alone while treatment with anti-CD28 antibody increased LPS-induced secretion of IFN-γ in the LPS-tolerant group but not in the control group compared to cells challenged with LPS alone.
FIG. 14.
Response of control and LPS-tolerant splenocytes to exogenous IFN-γ-regulating factors. Splenocytes (106/well) were isolated from control or LPS-tolerant mice and cultured with LPS (100 ng/ml) and exogenous IFN-γ-regulating factors. IL-12 was added at 1 ng/ml. IL-15 and IL-18 were added at 10 ng/ml. Anti-IL-10 and anti-CD28 antibodies were added at 1 and 10 μg/ml, respectively. Conditioned media were harvested after 24 h, and IFN-γ levels were determined by ELISA. ∗, significantly (P < 0.05) different from splenocytes cultured with LPS alone; n = 4 per group. Data shown are means ± standard error of the mean.
DISCUSSION
LPS tolerance is characterized, in part, by suppressed expression of macrophage-, NK-cell-, and T-cell-derived cytokines. Decreased production of IFN-γ, a cytokine derived primarily from T cells and NK cells, has been previously described for LPS-tolerant mice and humans (2, 17) and is consistent with our observations. However, the cellular alterations that cause decreased IFN-γ production following induction of LPS tolerance are not well understood. In our studies, modified macrophage function was the primary factor resulting in suppressed IFN-γ secretion in LPS-tolerant mice. LPS-tolerant macrophages had a reduced ability to stimulate IFN-γ production by T cells and NK cells obtained from either control or LPS-tolerant mice, whereas T cells and NK cells isolated from LPS-tolerant mice responded normally to control macrophages (Fig. 2). In addition, T cells and NK cells obtained from LPS-tolerant mice secreted IFN-γ at levels that were comparable to those of control cells in response to exogenously added inducers of IFN-γ, such as IL-12 and IL-18, and at higher levels in response to the polyclonal T-cell activator anti-CD3 antibody. We also demonstrated that NK cells, followed by T cells, comprise the most abundant population of cells in the spleen that produce IFN-γ in response to LPS (Fig. 3). T cells and NK cells accounted for more than 83% of splenic IFN-γ-producing cells. The induction of LPS tolerance caused marked suppression of IFN-γ production by both cell types. Our results also showed that macrophages produce small amounts of IFN-γ in response to LPS (Fig. 4) and are likely to account for some of the remaining IFN-γ-producing cells in the spleen. However, our flow cytometry studies showed that macrophages comprise less than 2% of cells that produce IFN-γ in the mouse spleen.
IFN-γ expression is induced by a variety of factors and is regulated through a complex interaction between cytokines, accessory molecules, and the T-cell receptor complex (5, 6, 18, 29, 34). IL-12, IL-15, and IL-18 are macrophage-derived cytokines that act synergistically to induce IFN-γ secretion by T cells and NK cells (6, 18, 29). Activation of the T-cell receptor complex by antigen-laden MHC-II or of CD28 by B7 proteins has also been shown to be a stimulus for the synthesis and secretion of IFN-γ (5, 34). However, the roles of these factors in LPS-induced production of IFN-γ have not been well defined. Results from our studies show that IL-12, IL-15, and IL-18 play functional roles in LPS-induced production of IFN-γ, with IL-12 being the predominant factor. Specifically, treatment of normal mouse splenocytes with antibodies to IL-12 almost completely abrogated LPS-induced IFN-γ production (Fig. 5). Anti-IL-18 antibody also significantly lowered IFN-γ expression but to a lesser extent than anti-IL-12 antibody. Antibodies against IL-15 did not inhibit LPS-induced IFN-γ production when added alone but, like anti-IL-18 antibody, potentiated the inhibitory effect of CTLA-4 Ig. The results of our studies also demonstrated that IFN-γ expression in response to LPS is independent of MHC-II. However, B7-CD28 interactions appear to partly mediate LPS-induced production of IFN-γ. These findings extend previous investigations (20) showing that LPS-induced T-cell proliferation is MHC-II unrestricted but dependent on B7 interactions. Our study shows that CD86 plays a more functional role in LPS-induced IFN-γ production than CD80 (Fig. 5). Mattern et al. (20) reported that CD80 was a primary cofactor for LPS-induced T-cell proliferation. These findings suggest that the B7 proteins regulate different, but complementary, aspects of T-cell activation.
The primary goal of these studies was to define mechanisms by which LPS tolerance suppresses IFN-γ production. Therefore, we examined the effects of LPS tolerance on known IFN-γ-regulating factors. As described above, IFN-γ expression was markedly suppressed at the transcriptional level in splenocytes from LPS-tolerant mice (Fig. 6). However, expression of the IFN-γRα and IFN-γRβ subunits were not affected (Fig. 12). In agreement with previous studies (2, 15), we demonstrated markedly decreased production of IL-12 after induction of LPS tolerance. The decrease in IL-12 production was due primarily to suppression of IL-12 p40 transcription. IL-12 p35 was constitutively expressed in both the control and LPS-tolerant groups, and LPS-induced expression was not affected by LPS tolerance. Our studies also extend a prior report (2) by showing that LPS-induced expression of both the IL-12Rβ1 and IL-12Rβ2 subunits was decreased, but not ablated, in the LPS-tolerant state. However, T cells obtained from LPS-tolerant mice appear to be equally as responsive to exogenous IL-12 as control T cells. These data confirm normal T-cell function and further support the hypothesis of impaired macrophage function as the source of suppressed IFN-γ production in LPS tolerance. Because T cells from LPS-tolerant mice respond normally to control macrophages and to exogenously applied cytokines and costimulatory factors, signal transduction pathways leading to IFN-γ induction must remain intact. IL-12R mRNA levels were measured in total splenocytes, in which there are multiple IL-12R-expressing cell types, such as T cells (33), NK cells (32) and dendritic cells (22). Because we do not know the relative levels of IL-12R expression by each population, we cannot exclude the possibility that some IFN-γ-producing cells may have normal or increased levels of IL-12R expression. Likewise, IL-12 can induce its own receptor (32), so it is possible that suppressed IL-12 expression by LPS-tolerant macrophages may lead to decreased IL-12R expression on T cells and NK cells. Application of exogenous IL-12 may be able to restore IL-12R expression on splenocytes from LPS-tolerant mice. However, further studies will need to be performed to characterize IL-12R regulation and the effect of LPS tolerance.
Like IL-12, LPS-induced expression of IL-15 and the IL-15Rα subunit were transcriptionally suppressed in LPS-tolerant splenocytes (Fig. 6 and 12). Also, addition of exogenous IL-15 in combination with LPS resulted in the restoration of IFN-γ production to control levels (Fig. 14). In contrast, IL-18 expression was unchanged in LPS-tolerant mice compared to controls. IL-18 and ICE, an important factor in the processing of IL-18, were constitutively expressed at similar levels in both the control and LPS-tolerant groups. Addition of exogenous IL-18 in combination with LPS had no effect on IFN-γ production compared to splenocytes challenged with LPS alone in either group. Together, these findings suggest that suppression of IL-12 and IL-15, but not IL-18, contributes to the decreased IFN-γ production observed in LPS-tolerant mice.
We also show that IL-10 plays an inhibitory role in LPS-induced IFN-γ secretion. This finding is consistent with previous reports of IL-10-mediated inhibition of a variety of immune responses (9, 13, 31). LPS was a potent stimulus for IL-10 expression in both control and LPS-tolerant mice (Fig. 6) and that anti-IL-10 antibody enhanced LPS-induced IFN-γ production in both groups (Fig. 5 and 14). We also observed constitutive expression of IL-10R at similar levels in mice from both groups and that expression of IL-10R was not changed by LPS challenge (Fig. 12). Our studies extend prior findings by better defining the role of IL-10 in IFN-γ suppression and characterizing the effects of LPS tolerance on IL-10 and IL-10R expression. Specifically, expression and function of IL-10 remains intact after induction of LPS tolerance. In contrast, the production of IL-12 and IFN-γ is suppressed. This observation suggests that different pathways mediate LPS-induced expression of IL-10 and IL-12 and that the IL-10-regulating pathways remain intact in this model. Recent studies have demonstrated down-regulation of TLR4, an important early activator of LPS-induced signal transduction (25). In addition, decreased mitogen-activated protein kinase phosphorylation and impaired translocation of the transcription factor nuclear factor κB (NF-κB) in LPS tolerance have been demonstrated (21). Transcription of the IL-12 and IL-15 genes, both of which are suppressed in LPS tolerance and are key factors in LPS-induced expression of IFN-γ, are highly dependent on activation of the NF-κB pathway. The persistence of IL-10 expression in LPS-tolerant mice suggests that IL-10 gene expression is independent of TLR4 activation and NF-κB nuclear translocation. Further studies are needed to characterize factors that are important in LPS-induced expression of IL-10.
In the present study, expression of the B7 proteins CD80 and CD86 were unchanged or increased among splenic macrophages, B cells, and T cells from LPS-tolerant mice (Fig. 10 and 11). This finding confirms the observation of Wolk et al. (31), who showed increased CD80 expression by LPS-tolerant human monocytes. Our study extends this finding by showing that T cells and B cells from LPS-tolerant mice also exhibit increased CD80 expression. In contrast to their report, we demonstrate that CD86 expression is increased in macrophages as well as T cells and B cells from LPS-tolerant mice. We examined B7 expression on T cells and B cells as well as the CD14+ population because reports indicate that B7 proteins expressed by activated lymphocytes as well as antigen presenting cells provide costimulatory signals for activation of cellular immune responses (7, 23). Likewise, levels of CD28, a major receptor for B7 proteins, were increased on T cells isolated from the spleens of LPS-tolerant mice (Fig. 13). However, the functional significance of this finding is not known. A recent study showed that the inhibitory effect of IL-10 on T-cell function was mediated, in part, by inhibition of CD28 phosphorylation and activation (14). Our studies clearly show that IL-10 plays an inhibitory role in LPS tolerance. Whether or not the effect of IL-10 in this model is mediated through inhibition of CD28 remains to be determined. An additional factor that may be functional in LPS tolerance is CTLA-4. CTLA-4, which is expressed primarily on activated T cells, binds B7 proteins but, in contrast to CD28, serves as an inhibitor of many T-cell functions (26). The potential role of CTLA-4 in the immunological alterations observed in LPS tolerance remains to be determined.
In conclusion, our results indicate that suppression of IFN-γ production in LPS-tolerant mice is macrophage dependent and that T-cell and NK-cell function appears to be normal. Suppressed expression of the IFN-γ-inducing factors IL-12 and IL-15, but not IL-18 or B7 proteins, are key elements in the impaired IFN-γ production associated with LPS tolerance.
ACKNOWLEDGMENTS
This work was supported by NIH grant K08 GM61243 and research grant 8650 from the Shriners of North America to E.R.S. T.E.K. is the recipient of a NIH Postdoctoral Fellowship GM08256 for Trauma and Burn Research at the Shriners Burns Institute-Galveston.
REFERENCES
- 1.Adib-Conquy M, Adrie C, Moine P, Asehnoune K, Fitting C, Pinsky M R, Dhainaut J F, Cavaillon J M. NF-kappaB expression in mononuclear cells of patients with sepsis resembles that observed in lipopolysaccharide tolerance. Am J Respir Crit Care Med. 2000;162:1877–1883. doi: 10.1164/ajrccm.162.5.2003058. [DOI] [PubMed] [Google Scholar]
- 2.Balkhy H H, Heinzel F P. Endotoxin fails to induce IFN-γ in endotoxin-tolerant mice: deficiencies in both IL-12 heterodimer production and IL-12 responsiveness. J Immunol. 1999;162:3633–3638. [PubMed] [Google Scholar]
- 3.Buttenschoen K, Berger D, Strecker W, Buttenschoen D C, Stenzel K, Pieper T, Beger H G. Association of endotoxemia and production of antibodies against endotoxins after multiple injuries. J Trauma. 2000;48:918–923. doi: 10.1097/00005373-200005000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Buttenschoen K, Fleischmann W, Haupt U, Kinzl L, Buttenschoen D C. Translocation of endotoxin and acute-phase proteins in malleolar fractures. J Trauma. 2000;48:241–245. doi: 10.1097/00005373-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Chang J T, Segal B M, Shevach E M. Role of costimulation in the induction of the IL-12/IL-12 receptor pathway and the development of autoimmunity. J Immunol. 2000;164:100–106. doi: 10.4049/jimmunol.164.1.100. [DOI] [PubMed] [Google Scholar]
- 6.Fehniger T A, Yu H, Cooper M A, Suzuki K, Shah M H, Caligiuri M A. Cutting edge: IL-15 costimulates the generalized Shwartzman reaction and innate immune IFN-gamma production in vivo. J Immunol. 2000;164:1643–1647. doi: 10.4049/jimmunol.164.4.1643. [DOI] [PubMed] [Google Scholar]
- 7.Furukawa Y, Mandelbrot D A, Libby P, Sharpe A H, Mitchell R N. Association of B7-1 co-stimulation with the development of graft arterial disease. Studies using mice lacking B7-1, B7-2, or B7-1/B7-2. Am J Pathol. 2000;157:473–484. doi: 10.1016/S0002-9440(10)64559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goebel A, Kavanagh E, Lyons A, Saporoschetz I B, Soberg C, Lederer J, Mannick J A, Rodrick M L. Injury induces deficient interleukin-12 production, but interleukin-12 therapy after injury restores resistance to infection. Ann Surg. 2000;231:253–261. doi: 10.1097/00000658-200002000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodier M R, Londei M. Lipopolysaccharide stimulates the proliferation of human CD56+CD3- NK cells: a regulatory role of monocytes and IL-10. J Immunol. 2000;165:139–147. doi: 10.4049/jimmunol.165.1.139. [DOI] [PubMed] [Google Scholar]
- 10.Grohmann U, Van Snick J, Campanile F, Silla S, Giampietri A, Vacca C, Renauld J C, Fioretti M C, Puccetti P. IL-9 protects mice from Gram-negative bacterial shock: suppression of TNF-alpha, IL-12, and IFN-gamma, and induction of IL-10. J Immunol. 2000;164:4197–4203. doi: 10.4049/jimmunol.164.8.4197. [DOI] [PubMed] [Google Scholar]
- 11.Heagy W, Hansen C, Nieman K, Rodriguez J L, West M A. Impaired mitogen-activated protein kinase activation and altered cytokine secretion in endotoxin-tolerant human monocytes. J Trauma. 2000;49:806–814. doi: 10.1097/00005373-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Hershman M J, Appel S H, Wellhausen S R, Sonnenfeld G, Polk H C., Jr Interferon-gamma treatment increases HLA-DR expression on monocytes in severely injured patients. Clin Exp Immunol. 1989;77:67–70. [PMC free article] [PubMed] [Google Scholar]
- 13.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk A H. Induction of interleukin-10 producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joss A, Akdis M, Faith A, Blaser K, Akdis C A. IL-10 directly acts on Tcells by specifically altering the CD28 co-stimulation pathway. Eur J Immunol. 2000;30:1683–1690. doi: 10.1002/1521-4141(200006)30:6<1683::AID-IMMU1683>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 15.Karp C L, Wysocka M, Ma X, Marovich M, Factor R E, Nutman T, Armant M, Wahl L, Cuomo P, Trinchieri G. Potent suppression of IL-12 production from monocytes and dendritic cells during endotoxin tolerance. Eur J Immunol. 1998;28:3128–3136. doi: 10.1002/(SICI)1521-4141(199810)28:10<3128::AID-IMMU3128>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 16.Kox W J, Bone R C, Krausch D, Docke W D, Kox S N, Wauer H, Egerer K, Querner S, Asadullah K, von Baehr R, Volk H D. Interferon gamma-1 b in the treatment of compensatory anti-inflammatory response syndrome. A new approach: proof or principle. Arch Intern Med. 1997;157:389–393. [PubMed] [Google Scholar]
- 17.Lauw F N, ten Hove T, Dekkers P E, de Jonge E, van Deventer S J, van Der Poll T. Reduced Th1, but not Th2, cytokine production by lymphocytes after in vivo exposure of healthy subjects to endotoxin. Infect Immun. 2000;68:1014–1018. doi: 10.1128/iai.68.3.1014-1018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauwerys B R, Renauld J C, Houssiau F A. Synergistic proliferation and activation of natural killer cells by interleukin 12 and interleukin 18. Cytokine. 1999;11:822–830. doi: 10.1006/cyto.1999.0501. [DOI] [PubMed] [Google Scholar]
- 19.Lehner M D, Ittner J, Bundschuh D S, van Rooijen N, Wendel A, Hartung T. Improved innate immunity of endotoxin-tolerant mice increases resistance to Salmonella enterica serovar Typhimurium infection despite attenuated cytokine response. Infect Immun. 2001;69:463–471. doi: 10.1128/IAI.69.1.463-471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattern T, Flad H D, Brade L, Rietschel E T, Ulmer A J. Stimulation of human T lymphocytes by LPS is MHC unrestricted, but strongly dependent on B7 interactions. J Immunol. 1998;160:3412–3418. [PubMed] [Google Scholar]
- 21.Medvedev A E, Kopydlowski K M, Vogel S N. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–5574. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- 22.Nagayama H, Sato K, Kawasaki H, Enomoto M, Morimoto C, Tadokoro K, Juji T, Asano S, Takahashi T A. IL-12 responsiveness and expression of IL-12 receptor in human peripheral blood monocyte-derived dendritic cells. J Immunol. 2000;165:59–66. doi: 10.4049/jimmunol.165.1.59. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima A, Watanabe N, Yoshino S, Yagita H, Okumura K, Azuma M. Requirement of CD28-CD86 co-stimulation in the interaction between antigen-primed T helper type 2 and B cells. Int Immunol. 1997;9:637–644. doi: 10.1093/intimm/9.5.637. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura S, Otani T, Ijiri Y, Motoda R, Kurimoto M, Orita K. IFN-gamma-dependent and -independent mechanisms in adverse effects caused by concomitant administration of IL-18 and IL-12. J Immunol. 2000;164:3330–3336. doi: 10.4049/jimmunol.164.6.3330. [DOI] [PubMed] [Google Scholar]
- 25.Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K, Akira S. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164:3476–3479. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- 26.Ostrov D A, Shi W, Schwartz J C, Almo S C, Nathenson S G. Structure of murine CTLA-4 and its role in modulating T cell responsiveness. Science. 2000;290:816–819. doi: 10.1126/science.290.5492.816. [DOI] [PubMed] [Google Scholar]
- 27.Parrillo J E, Parker M M, Natanson C, Suffredini A F, Danner R L, Cunnion R E, Ognibene F P. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;13:227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- 28.Rayhane N, Fitting C, Lortholary O, Dromer F, Cavaillon J M. Administration of endotoxin associated with lipopolysaccharide tolerance protects mice against fungal infection. Infect Immun. 2000;68:3748–3753. doi: 10.1128/iai.68.6.3748-3753.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tominaga K, Yoshimoto T, Torigoe K, Kurimoto M, Matsui K, Hada T, Okamura H, Nakanishi K. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. Int Immunol. 2000;12:151–160. doi: 10.1093/intimm/12.2.151. [DOI] [PubMed] [Google Scholar]
- 30.van Deuren M, van der Ven-Jongekrijg A K, van Dalen R, Sauerwein R W, van der Meer J W. Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J Infect Dis. 1995;172:433–439. doi: 10.1093/infdis/172.2.433. [DOI] [PubMed] [Google Scholar]
- 31.Wolk K, Docke W D, von Baehr V, Volk H D, Sabat R. Impaired antigen presentation by human monocytes during endotoxin tolerance. Blood. 2000;96:218–223. [PubMed] [Google Scholar]
- 32.Wu C Y, Gadina M, Wang K, O'Shea J, Seder R A. Cytokine regulation of IL-12 receptor beta2 expression: differential effects on human T and NK cells. Eur J Immunol. 2000;30:1364–1374. doi: 10.1002/(SICI)1521-4141(200005)30:5<1364::AID-IMMU1364>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 33.Yamane H, Igarashi O, Kato T, Nariuchi H. Positive and negative regulation of IL-12 receptor expression of naive CD4(+) T cells by CD28/CD152 co-stimulation. Eur J Immunol. 2000;30:3171–3180. doi: 10.1002/1521-4141(200011)30:11<3171::AID-IMMU3171>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 34.Zhang F, Nakamura T, Aune T M. TCR and IL-12 receptor signals cooperate to activate an individual response element in the IFN-gamma promoter in effector Th cells. J Immunol. 1999;163:728–735. [PubMed] [Google Scholar]