Abstract
Choroidal melanoma is a rare malignant tumour, yet it is the most common primary intra-ocular neoplasm and second on the list of top ten most malignant melanoma sites in the body. Clinical presentation can be non-specific and includes photopsia, floaters, progressive visual field loss, and blurry vision. The tumour is quite often diagnosed clinically during fundus examination; however, the most valued diagnostic tests are A- and B-scan ultrasonography (US). Several factors affect prognosis, including the patient’s age, tumour size, histological features, and presence of metastases. Still, with primary treatment and tight surveillance, around 50% of choroidal melanoma patients metastasise.
Keywords: choroidal melanoma, eye cancer, management, prognosis
1. Introduction
The choroid is the layer between the sclera and the retina [1]. The choroid is part of the uveal tract of the eye, which consists of the iris, ciliary body, and choroid [2]. The tumour originates from the melanocytes situated in the uveal tract [2]. Uveal melanoma (UM) is the most common cancer of the eye and is the most common primary intraocular neoplasm in adults [3]. It represents 3 to 5% of all melanomas [4]. Out of these, choroidal is the largest population (90%), while 5 to 8% originate in the ciliary body and 3 to 5% originate in the iris [4].
2. Epidemiology
UM incidence differs by sex, race, and country [4]. The incidence of choroidal melanoma is 6 to 7.5 cases per million annually in populations of European descent [5]. The highest incidence is among people with blue eyes and lighter skin [6]. UM is slightly more common in males [7]. Melanomas can arise at any age; however, the majority of cases occur at about 55 years [5]. It has been also stated that the peak for diagnosing UM ranges from 70 to 79 years, with 63 years as an approximate median age of diagnosis [4]. Approximately 50% of patients die from metastatic disease [4].
3. Pathophysiology
The mechanism of how UM develops are still unclear; however, it may relate to oxidative damage to pigmented tissues, which are controlled by the type and degree of iris pigmentation. Another theory that has been proposed for the development of UM is the essential genetic sequencing of the pairing bases from adenine-to-cytosine alterations, while ciliochoroidal melanoma has been linked to adenine-to-thymine alterations. UM can also originate from new lesions already present as a pre-nevus lesion [4].
4. Genetics
UM is regarded as a sporadic event, though it is related to ocular melanocytosis and dysplastic nevus syndrome [7]. Various genetic mutations have been related to UM [8]. This involves abnormalities in chromosomes 1, 3, 6, and 8 [3]. Monosomy of chromosome 3 is associated with aggressive metastatic disease (5). It is the most common karyotypic abnormality present, accounting for 50 to 60% of cases [3].
Monosomy of chromosome 3 predicates a mortality rate higher than 50%, while disomy of chromosome 3 has a 100% survival rate [9]. Specific alterations in 3p and 1p losses and 8q gain loci are related to high-risk melanomas [5]. Damato and colleagues [10] reported that the 10-year mortality rate for UM is 71% in malignancies with loss of chromosome 3 and 8q gain, 55% in malignancies with monosomy 3, and 0% in malignancies with disomy 3. Another study by Cassoux and colleagues [11] reported that the two-year metastasis-free interval (MEI) is 100% in patients with disomy 3 and normal chromosome 8 but 85%, 82.1%, and 37.1% in disomy 3 and 8q gain, monosomy 3 and normal chromosome 8, as well as monosomy 3 and 8 or 8q gain, respectively.
Genetic studies have emphasised that almost all uveal melanomas contain oncogenic alterations in GNAQ and GNA11 [7]. The development of choroidal melanomas is also associated with breast cancer type 2 susceptibility protein (BRCA2) and BRCA-associated protein 1 (BAP1) genes [5]. Harbour and colleagues (12) reported that alterations in BAP1, which is encoded by a gene on chromosome 3p21 and is essential for neoplasm inhibition, were related to higher distant metastases risk. Tumours with BAP1 mutations are detected in up to half of all UM and often lead to metastasis within 5 years. These tumours are, therefore, considered to have a high risk of metastasis [12]. Splicing factor 3b subunit 1 (SF3B1) mutation often metastasises; however, it can take up to 15 years. These tumours are, therefore, considered to have an intermediate risk of metastasis [13]. UM with an alteration in the Eukaryotic Translation Initiation Factor 1A X-Linked (EIF1AX) gene rarely metastasises. These tumours, therefore, have a low risk of metastasising [14].
From a clinical point of view, RNA-based genetic analysis for choroidal melanoma prognosis, which tests the expression profile for 15 genes seeking to produce prognostic subgroups for metastatic risk, may be performed prior to commencing treatment [15].
5. Risk Factors
There is no known cause resulting in UM. It mostly affects individuals with light skin [7]. Other risk factors include iris nevi, freckles, light eye colour, outdoor activities, tanning, and abnormal cutaneous nevi [16]. According to the current literature, choroidal melanoma risk in patients with uveal tract nevus is low [17]. Other conditions that increases the incidence of choroidal melanomas are Nevus of Ota, dysplastic nevi [18], and oculodermal melanocytosis [19]. Ultraviolet exposure is considered to be a risk factor for choroidal melanomas [20]. However, more recent studies contradict this owing to the very low mutation burden observed in the tumour cells [21]. Additionally, a mutational inactivation of the BAP 1 tumour-suppressor gene increases the risk of metastasis in uveal melanoma [22].
6. History
The spectrum of symptoms for patients with choroidal melanoma is broad or non-specific and may depend on the tumour location [5]. The most common symptoms are blurry vision (37.8%), flashing lights (8.6%), visual field defect (6.1%), pain (2.4%), and metamorphopsia (2.2%), and patients can be asymptomatic during diagnosing time (30.2%) [23]. Choroidal melanomas can lead to exudative retinal detachment based on the tumour shape [24].
7. Physical Examination and Evaluation
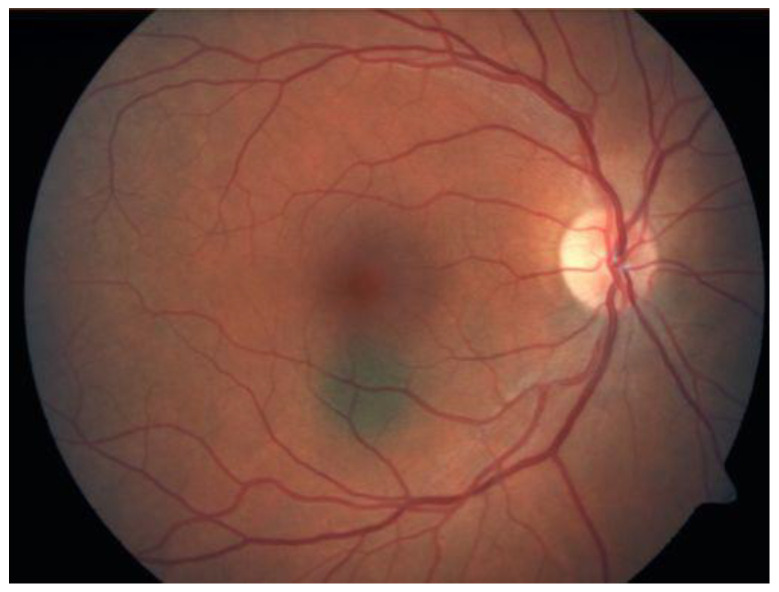
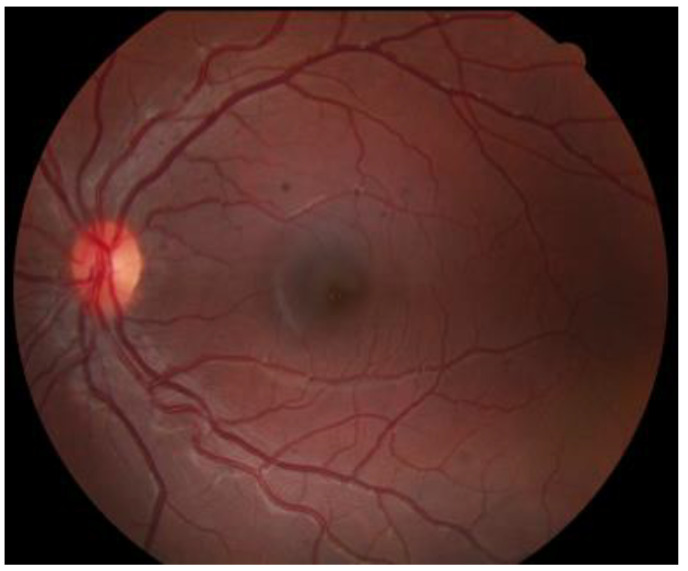
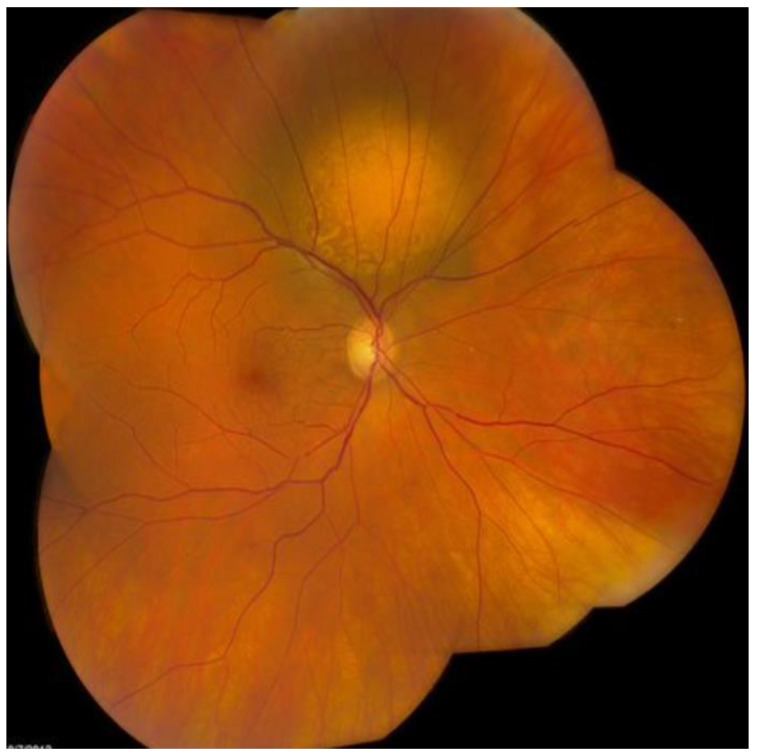
It can be challenging to distinguish choroidal melanoma from benign pigmented nevus (Figure 1); moreover, management may be complex as few nevi convert into choroidal melanomas (1 in 8000) (4). Fundus examination, fundus photography, and ocular ultrasound are the main evaluation aspects for ocular tumours [25].
Figure 1.
Choroidal nevus.
Uveal nevi are generally flat, slate-gray lesions without sharply demarcated margins; their size is limited to about 6 mm in diameter [25,26]. However, there is considerable overlap in size distributions of nevi and indeterminate lesions compared with small melanomas [26,27]. Choroidal melanomas should be suspected if there is orange pigmentation upon fundus examination, tumour thickness > 2 mm, tumour largest basal diameter > 5 mm and hollow acoustic density on the B-scan, subretinal fluid on the optical coherence tomography (OCT), and symptoms of visual acuity loss to 20/50 or worse [28,29].
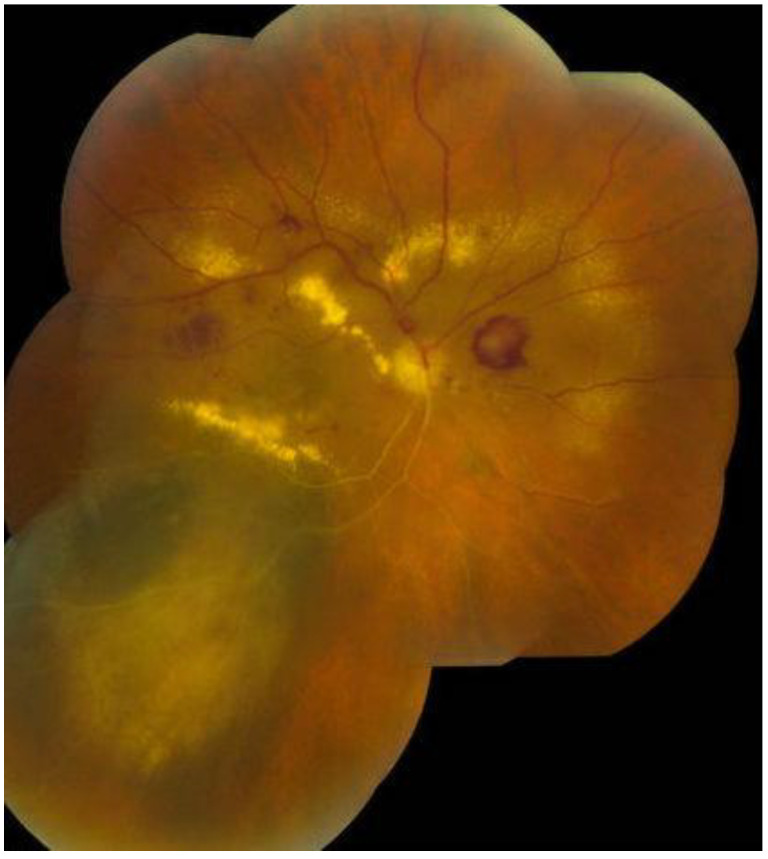
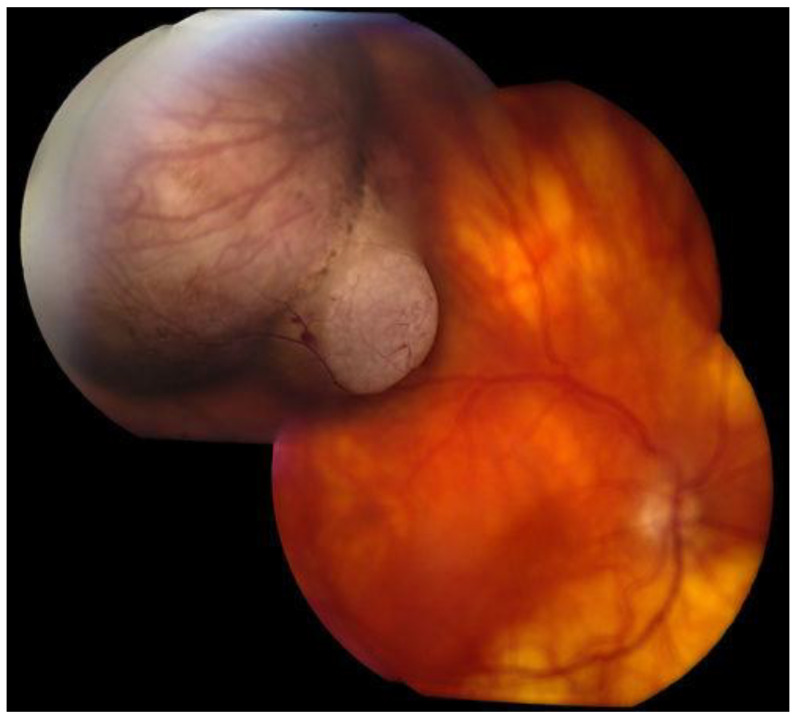
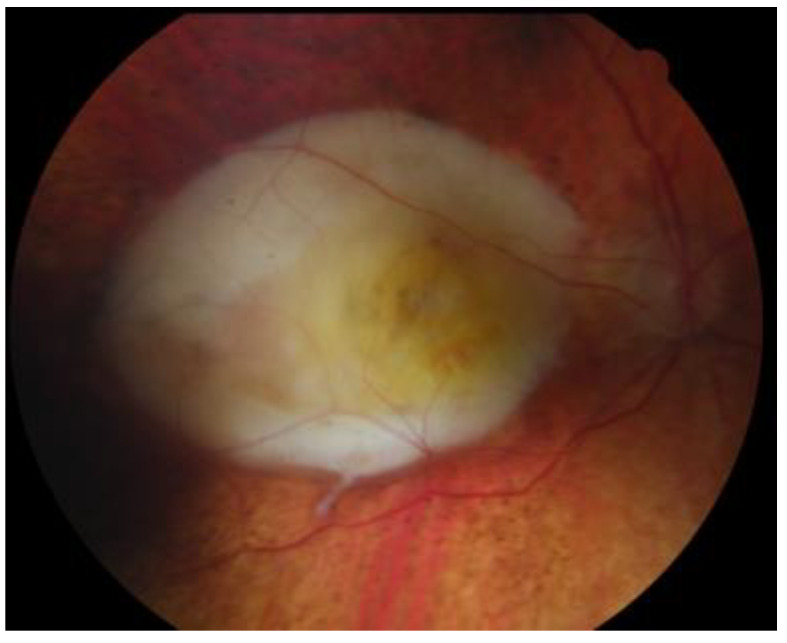
Upon dilated fundus examination, choroidal melanoma resembles a mushroom collar-button, or dome-shaped tumour with orange lipofuscin pigmentation as well as surface vasculature. Moreover, in melanomas > 4 mm in thickness, it can lead to exudative retinal detachment. Choroidal melanomas are notably pigmented; however, they may vary from darkly pigmented (Figure 2) to amelanotic (Figure 3) [5].
Figure 2.
Darkly pigmented melanoma.
Figure 3.
Amelanotic choroidal melanoma.
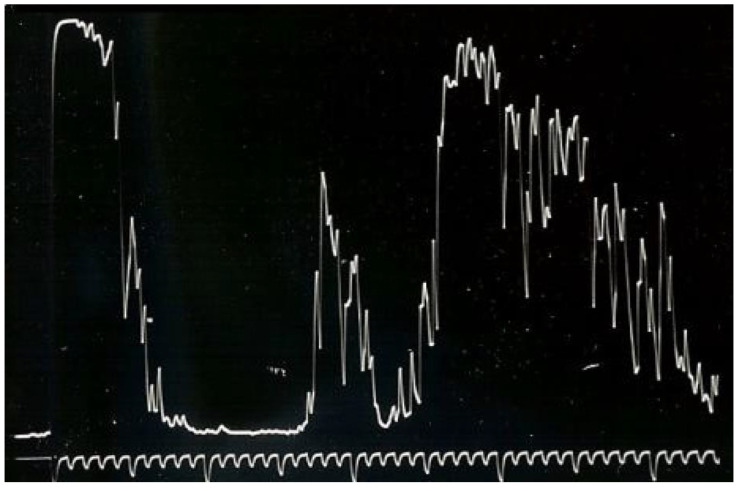
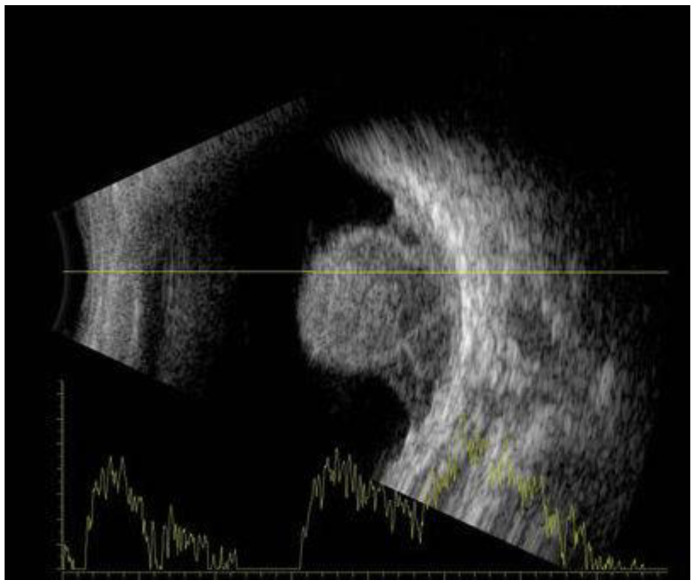
The most valued diagnostic tests are A- and B-scan US. Choroidal melanomas on A-scan US reveal medium to low internal reflectivity with vascular pulsations (Figure 4). Upon B-scan US, choroidal melanoma shows as a mushroom or dome-shaped tumour, indicating extension through Bruch’s membrane. The mass is acoustically silent, indicating a dark appearance inside the tumour (Figure 5). The combination of A and B-scan ultrasonography for tumours > 3 mm in thickness has a 95% accuracy in choroidal melanomas diagnosis [1,5].
Figure 4.
A-scan US reveals medium to low internal reflectivity with vascular pulsations.
Figure 5.
B-scan US shows a mushroom or dome-shaped tumour. The mass is acoustically silent, indicating a dark appearance inside the tumour.
Fluorescein angiography (FA) and indocyanine green angiography (ICGA) may be carried out to help distinguish melanomas from underlying pathologies [30]. FA can be performed to assess for intrinsic tumour circulation (double circulation) and extensive dye leakage [5]. ICGA can be used to image the micro-circulation of choroidal melanomas [1]. Melanomas also appear as clumps of hyperautofluorescence on autofluorescence [1].
Furthermore, optical coherence tomography (OCT) and optical coherence tomography angiography(OCTA) have been used recently to capture superficial and deep retinal and choroidal structures non-invasively, providing more accurate details of the lesion [31]. Melanomas on OCT reveal serous retinal detachment, normal retinal thickness, debris on back of retina, and intact photoreceptors [1].
Imaging methods used in the staging of uveal melanomas at baseline and follow-up include liver ultrasound (US); computed tomography (CT) of the head, chest, abdomen, and pelvis; whole body positron emission tomography (PET); or magnetic resonance imaging (MRI) [32]. The function of FNAB is undefined as choroidal melanoma is currently diagnosed clinically with no invasive approaches needed, which may lead to subsequent seeding [1]. However, currently, FNAB is taken more regularly at diagnosis, which aids in developments in the use of gene-expression profiling and cytogenetic analysis [4].
8. Differential Diagnoses
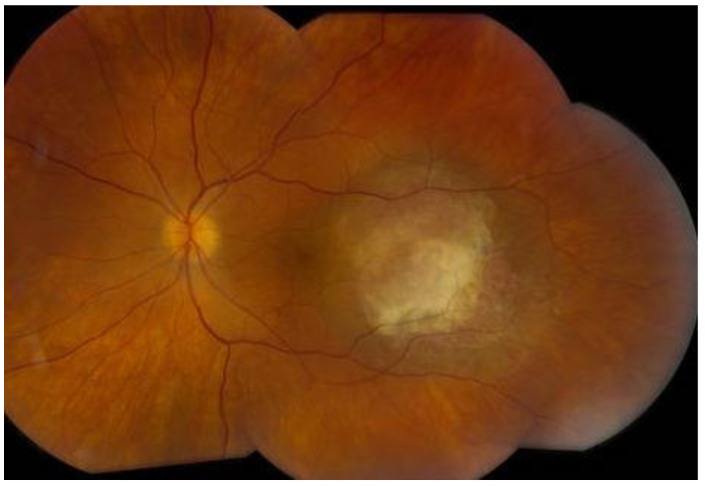
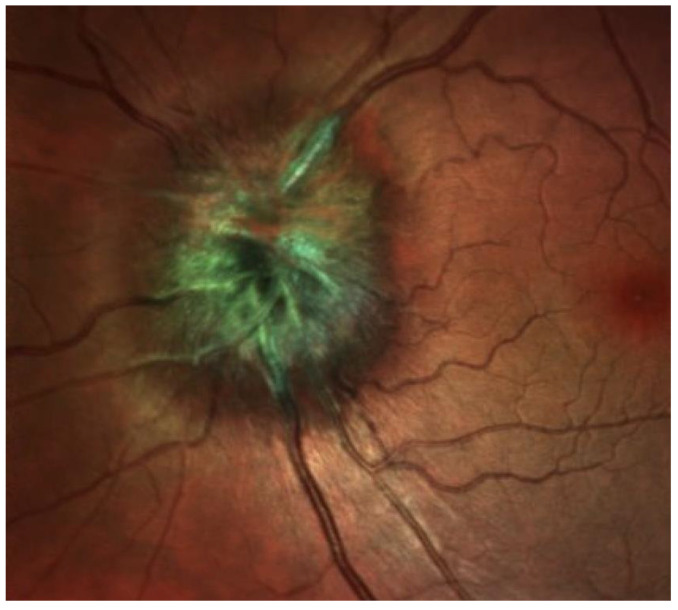
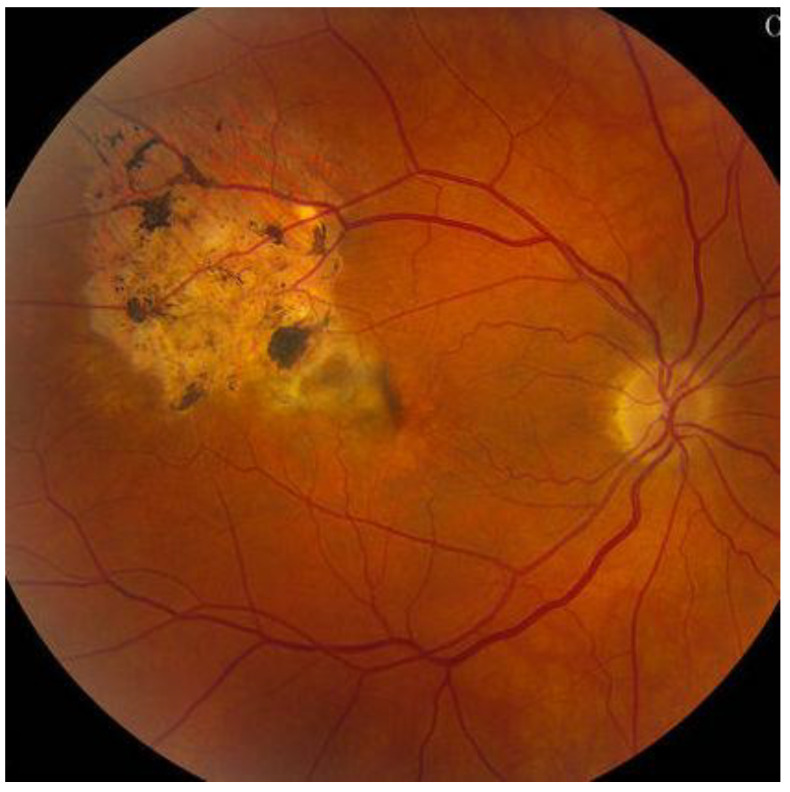
The Collaborative Ocular Melanoma Study (COMS) reported a 0.48% clinical misdiagnosis rate, indicating that most intraocular malignancies may only be diagnosed clinically [4]. Shields and colleagues [33] reported that among 400 referrals for evaluation for posterior UM, 8% were diagnosed with haemangiomas (Figure 6), 9.5% were diagnosed with retinal pigment-epithelium hypertrophy (Figure 7), 23.5% were diagnosed with disciform degeneration (Figure 8), and 26.5% were diagnosed with choroidal nevi (Figure 1). It can be also misdiagnosed as melanocytoma (Figure 9), choroidal osteoma (Figure 10), or choroidal metastasis (Figure 11) [34].
Figure 6.
Choroidal haemangiomas.
Figure 7.
Retinal pigment epithelium hypertrophy.
Figure 8.
Disciform scar.
Figure 9.
Melanocytoma.
Figure 10.
Choroidal osteoma.
Figure 11.
Choroidal metastasis.
9. Classifications
- Size according to COMS:
-
-Small: 4.0–8.0 mm diameter and/or 1.0–2.4 mm height;
-
-Medium: 6–16 mm diameter and/or 2.5–10.0 mm height;
-
-Large: >16 mm diameter and/or >10.0 mm height [35].
-
-
Based on the cell type: epithelioid, spindle, mixed [34].
The tumour, Node, Metastasis (TNM) staging system has several limitations for the classification and prognostication of uveal melanomas, such as the lack of lymph node dissemination, unlike other tumours including conjunctival melanoma [36]. In fact, Cai and colleagues [36] reported that gene-expression profiling is superior in choroidal melanoma classification compared with the TNM classification system.
10. Management
UM was historically treated by enucleation [7]. However, treatment of localised UM can currently be classified into eye salvage treatment or enucleation [4]. Eye salvage treatments are generally classified into radiation therapy such as plaque radiation therapy or particle beam radiotherapy, surgical approaches such as local surgical resection, and laser therapy such as transpupillary thermotherapy (TTT) or laser photocoagulation [37].
11. Radiation Therapy
Brachytherapy secures a radioactive plaque to the episclera, allowing for transmission of local radiation to the malignancy at a fixed dose [4]. Iodine-125 (I-125) and Ruthenium-106 (Ru-106) are the most common radioisotopes used. I-125 is a γ-emitting radioisotope that penetrates the tissues more deeply than β-radiation Ru-106 [4]. The recurrence rates for 125 I and 106 Ru are 7–10% and 14.7%, respectively [4]. It is associated with good local control; however, it is related to complications such as macular oedema (24.5%), neovascular glaucoma (28.3%), cataracts (44%), and radiation-induced retinopathy (45–67%). Additionally, 58% of UM patients who experience these complications may suffer from moderate loss of vision and poor VA (BCVA < 5/200) [38,39].
Intravitreal anti-vascular endothelial growth factor (IVEGF) following brachytherapy was reported to delay or reduce the loss of vision and macular oedema rates [2]. Brachytherapy is not recommended in UM patients with large basal diameters, extraocular metastases, no light perception vision, and blind painful eyes [40].
Proton beam therapy studies have also reported positive outcomes. A study of all stages of UM revealed that, with a medium follow-up of 5 years, proton-beam-targeted therapy has a 96.4% control rate locally and preserves the eyes in 95% of cases [41].
12. Enucleation
The most common surgery for patients with UM is enucleation. It is indicated in the cases of extraocular spread, large tumour diameter, circumferential tumour invasion, and patients suffering from vision loss [39]. Transscleral resection and transretinal endoresection are alternative surgical approaches [4]. Transscleral resection can be used in patients with wide malignancies that are not suitable for radiation therapy and aim for eye salvage treatment [4]. This option has proven to preserve vision; however, it is associated with various complications such as failed vitreoretinal surgery (44–70%), ocular hypertension (21%), retinal detachment (21%), and submacular haemorrhage (16%) [38,39,42]. Transscleral resection has higher recurrence rates in contrast to brachytherapy [43]. Puusaari et al. [44] found that the actuarial recurrence rate was 41% in the resection group vs. 7% in the brachytherapy group five years following intervention. Similarly, Augsburger et al. [45] reported that actuarial 5-year survival rates of 85.2% in the resection group and 81.8% in the brachytherapy group with a rate of severe early vision loss were higher in the resection group. Similar visual outcomes were reported by Kivela et al. and Bechrakis et al. [39]. Bechrakis et al. [39] also reported that the risk of developing neovascular glaucoma was significantly higher in the brachytherapy group in comparison with transscleral resection (33.3% vs. 5.6%). However, there were no differences between the groups in terms of eye retention and mortality rates [39]. In other studies by Puusaari et al. [44] and Caminal et al. [38], the 5-year actuarial enucleation rates were 28% and 29.1%, respectively. Foulds et al. [46] reported that the 5-year overall survival rates in patients with choroidal melanoma who underwent local resection were 79% vs. 54% in patients who underwent enucleation.
13. Laser Therapy
Laser therapy, such as transpupillary thermotherapy (TTT) or laser photocoagulation, injects light sensitive mixtures and free radicals to destruct the vascular supplies of the tumour and decreases the recurrences locally [4]. The efficacy of TTT has been reported when used for treating residual choroidal melanomas as well as adjuvant treatment following brachytherapy [47,48,49]. Tarmann and colleagues [50] reported that brachytherapy with TTT resulted in worse VA results and did not improve tumour control. For small and unclassified lesions with few risk factors, TTT has been reported to be effective as an initial treatment in approximately 80% of cases [51,52]. However, Shields and colleagues [52] advised that, when possible, small choroidal melanomas with multiple risk factors should be managed with treatment modalities other than TTT.
14. Choroidal Melanoma Treatment
Management modalities for choroidal melanoma depend on the VA of the involved eye, VA of the other eye, tumour location and size, and existence of extraocular spreading [1]. Factors that were identified to predict choroidal nevus transformation to choroidal melanoma include thickness > 2 mm, SRF, symptoms, orange pigmentation, tumour margin < 3 mm to disk, lack of surrounding, and US hollowness [53]. If the choroidal nevi do not show any features, the patients should be followed up every six months for the first year and then once each year thereafter. If there are one or two features, the patient must be followed up every 4 to 6 months [1].
Small and medium-sized choroidal melanomas may be managed by radiation therapy, laser treatment, and location tumour resection, whereas treatment options for larger choroidal melanomas include enucleation or orbital exenteration, if there is metastasis into the orbit [54].
Patients usually tend to fear death due to cancer and worry about losing vision in the affected eye. They tend to worry about disfigurement that may result after enucleation of the affected eye. Furthermore, they often worry that they may experience loss of vision or blindness in the other eye. Many factors should, therefore, be taken into consideration before determining which treatment approach should be employed as it may affect the patient’s overall quality of life as well as emotional and psychological function [1].
15. Management for Metastatic Disease
Even with primary treatment and tight surveillance, approximately 50% of UM patients will have metastases [4]. UM spreads through haematogenous spread, mainly to the liver [7]. Shields and colleagues [55] analysed > 8000 patients and found that 25% of choroidal melanoma patients had developed metastatic disease at 10 years.
The probabilities for secondary spread are related to the size of the initial malignancy at detection time; at 10 years, metastasis was diagnosed in 49% of large melanoma, 26% of medium melanoma, and 12% of small melanoma [55]. The five-year relative survival rate is 70 to 80%, notwithstanding developments in the management of the initial malignancy, with no documentation of reduction in metastatic death [56,57].
Management modalities for metastatic UM are limited. For resectable disease, surgical resection is the main approach [7]. In cases of distant metastatic disease, the main treatment option is systemic chemotherapy [1]. Options include chemo-embolisation, radio-embolisation, and chemotherapeutics agents, which have been shown to have limited efficacy [7]. Studies have reported that there can be 0–15% response rates when using chemotherapeutics agents such as treosulfan, cisplatin, temozolomide, dacarbazine, and fotemustine [2]. A selective kinase inhibitor known as selumetinib was shown to enhance response rates and progression-free survival in metastatic UM patients in contrast to chemotherapy [58]. Nevertheless, there was no improvement in overall survival [58].
Metastatic UM treatment is still a major challenge. Clinical trials are needed for ongoing research including treatments aimed at the PI3K and/or MAPK pathway, and epigenetic alteration with HDAC and DNA methyltransferase inhibitors [59]. Other clinical trials based on guiding the immune system in targeting malignant cells are still under development. IMCgp100 has demonstrated a favourable safety form and tumour shrinkage in metastatic UM [60,61].
16. Surveillance
Patients with UM are at lifetime risk of having metastasis even with treatment of primary malignancy [7]. Usual sites of metastases comprise the bones (16%), lungs (24%), and liver (90%) [62,63]. There are no consensus guidelines concerning optimal surveillance tests after initial management. Multiple imaging options such as chest X-ray (CXR), abdominal ultrasound (US), CT, MRI, and PET have been used [4].
Of these, MRI has demonstrated the highest sensitivity in identifying small liver lesions [2]. The National Guidelines for the Management of Uveal Melanoma in the UK advise that non-ionising imaging of the liver (US or MRI) should be conducted for all patients to avoid excessive radiation during metastatic surveillance [64].
Based on gene-expression profiling or cytogenetics, patients with low-risk disease should be considered for routine imaging, including chest CT, and abdomen and pelvis MRI every 6 to 12 months. In contrast, patients with a higher metastatic recurrence risk warrant a tighter follow-up, with imaging taken every 3 to 6 months [2].
17. Prognosis and Survival
Nearly 50% of UM patients will suffer from metastases, with the liver being the most common initially affected organ. Around 20–30% of UM patients die within 5 years and 45% die within 15 years [2]. Several prognostic factors are related to metastatic death, such as increasing tumour size, increasing patient age, lymphocytic infiltration, epithelioid cell type, ciliary body involvement, and multiple biomarkers such as human HLA molecules [7,65]. Damato and colleagues [66] stated that the most significant factors that predict metastatic death are chromosome 3 loss, epithelioid cell histopathology, and basal tumour diameter.
Unfortunately, the survival rates of patients suffering from UM have not improved in the past 30 years, even with the availability of various treatment modalities [1]. The percentage of patients with hepatic metastasis is approximately 80%, 92%, and only 1% after one year, two years, and over five years, respectively, regardless of the management option [67].
18. Conclusions
Choroidal melanomas are rare, yet it is crucial to be cautious when examining a choroidal mass. Early detection is vital; therefore, fundus examination must be considered as most choroidal melanoma cases are diagnosed clinically. The patient must be educated about the life expectancy, lifetime risk of potential metastases, management modalities, and expected vision outcome.
Author Contributions
N.S. conceptualisation, literature review, writing—original draft preparation, review, editing, and supervision; D.M. and A.E. visualisation, writing, and formal analysis. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. Figures: All figures illustrated in this review were retrieved from the Retina Gallery via https://www.retinagallery.com/index.php (accessed on 21 November 2022). Retina Gallery is a library of free and non-copyrighted Retinal images. The authors declare no conflict of interest with Retina Gallery.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Singh P., Singh A. Choroidal melanoma. Oman J. Ophthalmol. 2012;5:3. doi: 10.4103/0974-620X.94718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J., Manson D.K., Marr B.P., Carvajal R.D. Treatment of uveal melanoma: Where are we now? Ther. Adv. Med. Oncol. 2018;10:1758834018757175. doi: 10.1177/1758834018757175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Violanti S.S., Bononi I., Gallenga C.E., Martini F., Tognon M., Perri P. New insights into molecular oncogenesis and therapy of uveal melanoma. Cancers. 2019;11:694. doi: 10.3390/cancers11050694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krantz B.A., Dave N., Komatsubara K.M., Marr B.P., Carvajal R.D. Uveal melanoma: Epidemiology, etiology, and treatment of primary disease. Clin. Ophthalmol. 2017;11:279. doi: 10.2147/OPTH.S89591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terzidou C., Trivli A., Dalianis G., Apessou D., Spandidos D.A., Goulielmos G.N. Advanced choroidal melanoma with a desirable aesthetic outcome after enucleation: A case report. Oncol. Lett. 2018;16:511–514. doi: 10.3892/ol.2018.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadowsky D., Delijani K., Lim J., Cabrera M. Uveal melanoma. Georget. Med. Rev. 2022;6:24. doi: 10.52504/001c.36973. [DOI] [Google Scholar]
- 7.Vasalaki M., Fabian I.D., Reddy M.A., Cohen V.M., Sagoo M.S. Ocular oncology: Advances in retinoblastoma, uveal melanoma and conjunctival melanoma. Br. Med. Bull. 2017;121:107–119. doi: 10.1093/bmb/ldw053. [DOI] [PubMed] [Google Scholar]
- 8.Xia F., Yu Z., Deng A., Gao G. Identification of molecular subtyping system and four-gene prognostic signature with immune-related genes for uveal melanoma. Exp. Biol. Med. 2022;247:246–262. doi: 10.1177/15353702211053801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bornfeld N., Prescher G., Becher R., Hirche H., Jöckel K., Horsthemke B. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347:1222–1225. doi: 10.1016/S0140-6736(96)90736-9. [DOI] [PubMed] [Google Scholar]
- 10.Damato B., Dopierala J.A., Coupland S.E. Genotypic profiling of 452 choroidal melanomas with multiplex ligation-dependent probe amplification. Clin. Cancer Res. 2010;16:6083–6092. doi: 10.1158/1078-0432.CCR-10-2076. [DOI] [PubMed] [Google Scholar]
- 11.Cassoux N., Rodrigues M.J., Plancher C., Asselain B., Levy-Gabriel C., Lumbroso-Le Rouic L., Piperno-Neumann S., Dendale R., Sastre X., Desjardins L., et al. Genome-wide profiling is a clinically relevant and affordable prognostic test in posterior uveal melanoma. Br. J. Ophthalmol. 2014;98:769–774. doi: 10.1136/bjophthalmol-2013-303867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harbour J.W., Onken M.D., Roberson E.D., Duan S., Cao L., Worley L.A., Laurin Council M., Matatall K.A., Helms C., Bowcock A.M. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yavuzyigitoglu S., Koopmans A.E., Verdijk R.M., Vaarwater J., Eussen B., Van Bodegom A., Paridaens D., Kiliç E., de Klein A., Rotterdam Ocular Melanoma Study Group Uveal melanomas with SF3B1 mutations: A distinct subclass associated with late-onset metastases. Ophthalmology. 2016;123:1118–1128. doi: 10.1016/j.ophtha.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Martin M., Maßhöfer L., Temming P., Rahmann S., Metz C., Bornfeld N., van de Nes J., Klein-Hitpass L., Hinnebusch A.G., Horsthemke B., et al. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat. Genet. 2013;45:933. doi: 10.1038/ng.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harbour J.W. Molecular Diagnostics for Melanoma. Springer; Berlin/Heidelberg, Germany: 2014. A prognostic test to predict the risk of metastasis in uveal melanoma based on a 15-gene expression profile; pp. 427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald K.A., Krema H., Chan A.-W. Cutaneous signs and risk factors for ocular melanoma. J. Am. Acad. Dermatol. 2021;84:1732–1734. doi: 10.1016/j.jaad.2020.08.099. [DOI] [PubMed] [Google Scholar]
- 17.Smith J.H., Padnick-Silver L., Newlin A., Rhodes K., Rubinstein W.S. Genetic study of familial uveal melanoma: Association of uveal and cutaneous melanoma with cutaneous and ocular nevi. Ophthalmology. 2007;114:774–779. doi: 10.1016/j.ophtha.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 18.Singh A.D., Kalyani P., Topham A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology. 2005;112:1784–1789. doi: 10.1016/j.ophtha.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Shields C.L., Kaliki S., Livesey M., Walker B., Garoon R., Bucci M., Feinstein E., Pesch A., Gonzalez C., Lally S.E., et al. Association of ocular and oculodermal melanocytosis with the rate of uveal melanoma metastasis: Analysis of 7872 consecutive eyes. JAMA Ophthalmol. 2013;131:993–1003. doi: 10.1001/jamaophthalmol.2013.129. [DOI] [PubMed] [Google Scholar]
- 20.Shah C.P., Weis E., Lajous M., Shields J.A., Shields C.L. Intermittent and chronic ultraviolet light exposure and uveal melanoma: A meta-analysis. Ophthalmology. 2005;112:1599–1607. doi: 10.1016/j.ophtha.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Jager M.J., Shields C.L., Cebulla C.M., Abdel-Rahman M.H., Grossniklaus H.E., Stern M.-H., Carvajal R.D., Belfort R.N., Jia R., Shields J.A., et al. Uveal melanoma. Nat. Rev. Dis. Prim. 2020;6:24. doi: 10.1038/s41572-020-0158-0. [DOI] [PubMed] [Google Scholar]
- 22.Kaler C.J., Dollar J.J., Cruz A.M., Kuznetsoff J.N., Sanchez M.I., Decatur C.L., Licht J.D., Smalley K.S., Correa Z.M., Kurtenbach S., et al. BAP1 loss promotes suppressive tumor immune microenvironment via upregulation of PROS1 in class 2 uveal melanomas. Cancers. 2022;14:3678. doi: 10.3390/cancers14153678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damato E.M., Damato B.E. Detection and time to treatment of uveal melanoma in the United Kingdom: An evaluation of 2384 patients. Ophthalmology. 2012;119:1582–1589. doi: 10.1016/j.ophtha.2012.01.048. [DOI] [PubMed] [Google Scholar]
- 24.Ebert J.J., Di Nicola M., Williams B.K., Jr. Operative complications of posterior uveal melanoma surgery. Int. Ophthalmol. Clin. 2022;62:15–33. doi: 10.1097/IIO.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 25.Solnik M., Paduszyńska N., Czarnecka A.M., Synoradzki K.J., Yousef Y.A., Chorągiewicz T., Rejdak R., Toro M.D., Zweifel S., Dyndor K., et al. Imaging of uveal melanoma—Current standard and methods in development. Cancers. 2022;14:3147. doi: 10.3390/cancers14133147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane A.M., Egan K.M., Kim I.K., Gragoudas E.S. Mortality after diagnosis of small melanocytic lesions of the choroid. Arch. Ophthalmol. 2010;128:996–1000. doi: 10.1001/archophthalmol.2010.166. [DOI] [PubMed] [Google Scholar]
- 27.Harbour J.W., Paez-Escamilla M., Cai L., Walter S.D., Augsburger J.J., Correa Z.M. Are risk factors for growth of choroidal nevi associated with malignant transformation? Assessment with a validated genomic biomarker. Am. J. Ophthalmol. 2019;197:168–179. doi: 10.1016/j.ajo.2018.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalvin L.A., Shields C.L., Ancona-Lezama D.A., Michael D.Y., Di Nicola M., Williams B.K., Jr., Lucio-Alvarez J.A., Ang S.M., Maloney S.M. Combination of multimodal imaging features predictive of choroidal nevus transformation into melanoma. Br. J. Ophthalmol. 2019;103:1441–1447. doi: 10.1136/bjophthalmol-2018-312967. [DOI] [PubMed] [Google Scholar]
- 29.Shields C.L., Sioufi K., Srinivasan A., Di Nicola M., Masoomian B., Barna L.E., Bekerman V.P., Say E.A., Mashayekhi A., Emrich J., et al. Visual outcome and millimeter incremental risk of metastasis in 1780 patients with small choroidal melanoma managed by plaque radiotherapy. JAMA Ophthalmol. 2018;136:1325–1333. doi: 10.1001/jamaophthalmol.2018.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Negretti G.S., Kalafatis N.E., Shields J.A., Shields C.L. Choroidal melanoma masquerading as central serous chorioretinopathy. Ophthalmol. Retina. 2022 doi: 10.1016/j.oret.2022.08.013. in press . [DOI] [PubMed] [Google Scholar]
- 31.Obuchowska I., Konopińska J. Importance of optical coherence tomography and optical coherence tomography angiography in the imaging and differentiation of choroidal melanoma: A review. Cancers. 2022;14:3354. doi: 10.3390/cancers14143354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X., Wang L., Zhang L., Tang F., Wei X. Application of multimodal and molecular imaging techniques in the detection of choroidal melanomas. Front. Oncol. 2021;10:617868. doi: 10.3389/fonc.2020.617868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shields J.A., Augsburger J.J., Brown G.C., Stephens R.F. The differential diagnosis of posterior uveal melanoma. Ophthalmology. 1980;87:518–522. doi: 10.1016/S0161-6420(80)35201-9. [DOI] [PubMed] [Google Scholar]
- 34.Choroidal Melenoma. 2016. [(accessed on 15 May 2020)]. Available online: https://www.aao.org/topic-detail/choroidal-melanoma-europe.
- 35.Margo C.E. The collaborative ocular melanoma study: An overview. Cancer Control. 2004;11:304–309. doi: 10.1177/107327480401100504. [DOI] [PubMed] [Google Scholar]
- 36.Cai L., Paez-Escamilla M., Walter S.D., Tarlan B., Decatur C.L., Perez B.M., Harbour J.W. Gene expression profiling and PRAME status versus tumor-node-metastasis staging for prognostication in uveal melanoma. Am. J. Ophthalmol. 2018;195:154–160. doi: 10.1016/j.ajo.2018.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shields J.A., Shields C.L. Management of posterior uveal melanoma: Past, present, and future: The 2014 Charles L. Schepens lecture. Ophthalmology. 2015;122:414–428. doi: 10.1016/j.ophtha.2014.08.046. [DOI] [PubMed] [Google Scholar]
- 38.Caminal J., Padrón-Pérez N., Arias L., Masuet-Aumatell C., Gutiérrez C., Piulats J., Pera J., Català J., Rubio M.J., Arruga J. Transscleral resection without hypotensive anaesthesia vs iodine-125 plaque brachytherapy in the treatment of choroidal melanoma. Eye. 2016;30:833–842. doi: 10.1038/eye.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bechrakis N.E., Bornfeld N., Zöller I., Foerster M.H. Iodine 125 plaque brachytherapy versus transscleral tumor resection in the treatment of large uveal melanomas. Ophthalmology. 2002;109:1855–1861. doi: 10.1016/S0161-6420(02)01273-3. [DOI] [PubMed] [Google Scholar]
- 40.Force T., Simpson E.R., Gallie B., Laperrierre N., Beiki-Ardakani A., Kivelä T., Raivio V., Heikkonen J., Desjardins L., Dendale R., et al. The American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy. 2014;13:1–14. doi: 10.1016/j.brachy.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Seibel I., Cordini D., Rehak M., Hager A., Riechardt A.I., Böker A., Heufelder J., Weber A., Gollrad J., Besserer A., et al. Local recurrence after primary proton beam therapy in uveal melanoma: Risk factors, retreatment approaches, and outcome. Am. J. Ophthalmol. 2015;160:628–636. doi: 10.1016/j.ajo.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 42.Willerding G.D., Cordini D., Moser L., Krause L., Foerster M.H., Bechrakis N.E. Neoadjuvant proton beam irradiation followed by transscleral resection of uveal melanoma in 106 cases. Br. J. Ophthalmol. 2016;100:463–467. doi: 10.1136/bjophthalmol-2015-307095. [DOI] [PubMed] [Google Scholar]
- 43.Caminal J.M., Lorenzo D., Gutierrez C., Slocker A., Piulats J.M., Cobos E., Garcia-Bru P., Morwani R., Santamaria J.F., Arias L. Local resection in choroidal melanoma: A review. J. Clin. Med. 2022;11:7156. doi: 10.3390/jcm11237156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puusaari I., Damato B., Kivelä T. Transscleral local resection versus iodine brachytherapy for uveal melanomas that are large because of tumour height. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007;245:522–533. doi: 10.1007/s00417-006-0461-9. [DOI] [PubMed] [Google Scholar]
- 45.Augsburger J.J., Lauritzen K., Gamel J.W., DeBrakeleer D.J., Lowry J.C., Eisenman R. Matched Group Study of Surgical Resection versus Cobalt-60 Plaque Radiotherapy for Primary Choroidal or Ciliary Body Melanoma. SLACK Incorporated; West Deptford, NJ, USA: 1990. pp. 682–688. [PubMed] [Google Scholar]
- 46.Foulds W.S., Damato B.E., Burton R.L. Local resection versus enucleation in the management of choroidal melanoma. Eye. 1987;1:676–679. doi: 10.1038/eye.1987.110. [DOI] [PubMed] [Google Scholar]
- 47.Badiyan S.N., Rao R.C., Apicelli A.J., Acharya S., Verma V., Garsa A.A., DeWees T., Speirs C.K., Garcia-Ramirez J., Esthappan J., et al. Outcomes of iodine-125 plaque brachytherapy for uveal melanoma with intraoperative ultrasonography and supplemental transpupillary thermotherapy. Int. J. Radiat. Oncol.*Biol.*Phys. 2014;88:801–805. doi: 10.1016/j.ijrobp.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 48.Saakian S., Val’skiĭ V., Semenova E., Amirian A. Transpupillary thermotherapy in the treatment of recurrent and residual choroidal melanomas: Preliminary results. Vestn. Oftalmol. 2009;125:11–15. [PubMed] [Google Scholar]
- 49.Harbour J.W., Meredith T.A., Thompson P.A., Gordon M.E. Transpupillary thermotherapy versus plaque radiotherapy for suspected choroidal melanomas. Ophthalmology. 2003;110:2207–2214. doi: 10.1016/S0161-6420(03)00858-3. [DOI] [PubMed] [Google Scholar]
- 50.Tarmann L., Wackernagel W., Avian A., Mayer C., Schneider M., Winkler P., Langmann G. Ruthenium-106 plaque brachytherapy for uveal melanoma. Br. J. Ophthalmol. 2015;99:1644–1649. doi: 10.1136/bjophthalmol-2015-306666. [DOI] [PubMed] [Google Scholar]
- 51.Turcotte S., Bergeron D., Rousseau A.P., Mouriaux F. Primary transpupillary thermotherapy for choroidal indeterminate melanocytic lesions. Can. J. Ophthalmol. 2014;49:464–467. doi: 10.1016/j.jcjo.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Mashayekhi A., Shields C.L., Rishi P., Atalay H.T., Pellegrini M., McLaughlin J.P., Patrick K.A., Morton S.J., Remmer M.H., Parendo A., et al. Primary transpupillary thermotherapy for choroidal melanoma in 391 cases: Importance of risk factors in tumor control. Ophthalmology. 2015;122:600–609. doi: 10.1016/j.ophtha.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 53.Shields C.L., Furuta M., Berman E.L., Zahler J.D., Hoberman D.M., Dinh D.H., Mashayekhi A., Shields J.A. Choroidal nevus transformation into melanoma: Analysis of 2514 consecutive cases. Arch. Ophthalmol. 2009;127:981–987. doi: 10.1001/archophthalmol.2009.151. [DOI] [PubMed] [Google Scholar]
- 54.Jovanovic P., Mihajlovic M., Djordjevic-Jocic J., Vlajkovic S., Cekic S., Stefanovic V. Ocular melanoma: An overview of the current status. Int. J. Clin. Exp. Pathol. 2013;6:1230. [PMC free article] [PubMed] [Google Scholar]
- 55.Shields C.L., Furuta M., Thangappan A., Nagori S., Mashayekhi A., Lally D.R., Kelly C.C., Rudich D.S., Nagori A.V., Wakade O.A., et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch. Ophthalmol. 2009;127:989–998. doi: 10.1001/archophthalmol.2009.208. [DOI] [PubMed] [Google Scholar]
- 56.Burr J.M., Mitry E., Rachet B., Coleman M. Survival from uveal melanoma in England and Wales 1986 to 2001. Ophthalmic Epidemiol. 2007;14:3–8. doi: 10.1080/09286580600977281. [DOI] [PubMed] [Google Scholar]
- 57.Singh A.D., Turell M.E., Topham A.K. Trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 58.Carvajal R.D., Sosman J.A., Quevedo J.F., Milhem M.M., Joshua A.M., Kudchadkar R.R., Linette G.P., Gajewski T.F., Lutzky J., Lawson D.H., et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: A randomized clinical trial. JAMA. 2014;311:2397–2405. doi: 10.1001/jama.2014.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guan X., Wang H., Ma F., Qian H., Yi Z., Xu B. The efficacy and safety of programmed cell death 1 and programmed cell death 1 ligand inhibitors for advanced melanoma: A meta-analysis of clinical trials following the PRISMA guidelines. Medicine. 2016;95:e3134. doi: 10.1097/MD.0000000000003134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez-Perez D., Viñal D., Solares I., Espinosa E., Feliu J. Gp-100 as a novel therapeutic target in uveal melanoma. Cancers. 2021;13:5968. doi: 10.3390/cancers13235968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen L.N., Carvajal R.D. Tebentafusp for the treatment of HLA-A* 02: 01–positive adult patients with unresectable or metastatic uveal melanoma. Expert Rev. Anticancer Ther. 2022;22:1017–1027. doi: 10.1080/14737140.2022.2124971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willson J.K., Albert D.M., Diener-West M., McCaffrey L., Moy C.S., Scully R.E. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the collaborative ocular melanoma study (COMS). COMS report no. 15. Arch. Ophthalmol. 2001;119:670–676. doi: 10.1001/archopht.119.5.670. [DOI] [PubMed] [Google Scholar]
- 63.Diener-West M., Reynolds S.M., Agugliaro D.J., Caldwell R., Cumming K., Earle J.D., Green D.L., Hawkins B.S., Hayman J., Jaiyesimi I., et al. Screening for metastasis from choroidal melanoma: The collaborative ocular melanoma study group report 23. J. Clin. Oncol. 2004;22:2438–2444. doi: 10.1200/JCO.2004.08.194. [DOI] [PubMed] [Google Scholar]
- 64.Nathan P., Cohen V., Coupland S., Curtis K., Damato B., Evans J., Fenwick S., Kirkpatrick L., Li O., Marshall E., et al. Uveal melanoma UK national guidelines. Eur. J. Cancer. 2015;51:2404–2412. doi: 10.1016/j.ejca.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 65.Singh A., Shields C., Shields J. Prognostic factors in uveal melanoma. Melanoma Res. 2001;11:255–263. doi: 10.1097/00008390-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 66.Damato B., Duke C., Coupland S.E., Hiscott P., Smith P.A., Campbell I., Douglas A., Howard P. Cytogenetics of uveal melanoma: A 7-year clinical experience. Ophthalmology. 2007;114:1925–1931.e1. doi: 10.1016/j.ophtha.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 67.Kaštelan S., Zimak D.M., Ivanković M., Marković I., Antunica A.G. Liver metastasis in uveal melanoma—Treatment options and clinical outcome. Front. Biosci.-Landmark. 2022;27:72. doi: 10.31083/j.fbl2702072. [DOI] [PubMed] [Google Scholar]