Abstract
Cymbopogon species essential oil (EO) carries significant importance in pharmaceuticals, aromatherapy, food, etc. The chemical compositions of Cymbopogon spp. Viz. Cymbopogon winterianus (citronella) Cymbopogon citratus (lemongrass), and Cymbopogon martini (palmarosa) were analyzed by gas chromatography–mass spectrometry (GC-MS), enantiomeric distribution by chiral GC-MS, and antimicrobial activities of some selected pure major compound and root and leaves EOs of citronella. The EO of leaves of Cymbopogon spp. showed comparatively higher yield than roots or other parts. Contrary to citral (neral and geranial) being a predominant compound of Cymbopogon spp., α-elemol (53.1%), α-elemol (29.5%), geraniol (37.1%), and citral (90.4%) were detected as major compounds of the root, root hair with stalk, leaf, and root stalk with shoot of citronella EO, respectively. Palmarosa leaves’ EO contains neral (36.1%) and geranial (53.1) as the major compounds. In the roots of palmarosa EO, the prime components were α-elemol (31.5%), geranial (25.0%), and neral (16.6%). Similarly, lemongrass leaves’ EO contains geraniol (76.6%) and geranyl acetate (15.2%) as major compounds, while the root EO contains a higher amount of geraniol (87.9%) and lower amount of geranyl acetate (4.4%). This study reports for the first time chiral terpenoids from Cymbopogon spp. EOs. Chiral GC-MS gave specific enantiomeric distributions of nine, six, and five chiral terpenoids in the root, root stalk with a shoot, and leaves of citronella EOs, respectively. Likewise, four and three chiral terpenoids in the root and leaves of lemongrass oil followed by two chiral terpenoids in the leaves and root of palmarosa EOs each. Additionally, the root and leaves’ EOs of citronella exhibit noticeable activity on bacteria such as Staphylococcus aureus, Staphylococcus epidermidis, and Streptococcus pyogenes and fungus such as Candida albicans, Microsporum canis, and Trichophyton mentagrophytes. So, geranial-, neral-, geraniol-, and citronellal-rich EOs can be used as an alternative antimicrobial agent.
Keywords: citronella, palmarosa, lemongrass, citral, enantiomeric distribution, antibacterial, antifungal
1. Introduction
The genus Cymbopogon (Poaceae) comprises up to 144 species distributed in Asia, America, and Africa [1,2], among which Cymbopogon winterianus Jowitt ex Bor, Cymbopogon citratus Stapf, and Cymbopogon martini (Roxb.) Will. Watson are the economically important species of the genus [3]. The species C. winterianus is popularly known as citronella grass, citronella, or Java citronella [4]. Citronella is one of the industrially important essential oils (EO), which is produced by steam or hydro-distillation of the whole plant [5] and has a characteristic lemon odor [6]. The world consumption of citronella EO has amounted to several thousand tons annually due to being the most important source of geraniol and citronellal [7]. It is also known for its natural insect-repellent property and is of great interest to the pharmaceutical and fragrance industry [8]. It has various therapeutic uses as an analgesic, anticonvulsant, anxiolytic, etc., and is a favorable agent for antifungal, antibacterial, antiparasitic, herbicidal [9], and nematicidal activities. In traditional practice, citronella EO has been used as an antipyretic, aromatic tea, vermifuge, diuretic, and in treating mental illness [10,11,12,13,14].
Cymbopogon citratus, also known as lemongrass [15], is widely cultivated worldwide due to its aromatic and medicinal properties [16]. It is rich in minerals, vitamins, and macronutrients (carbohydrates, protein, and small amounts of fat). Its leaves are also a good source of various bioactive compounds, including alkaloids, terpenoids, flavonoids, phenols, saponins, and tannins [17], which has led to numerous herbal therapies currently widely used in medicine [18]. Lemongrass has been traditionally used as a remedy for a variety of health conditions. Recent scientific studies have provided evidence supporting its antiviral, antimicrobial [19], antioxidant [20], antifungal [21], anticancer [22], sedative, and anti-inflammatory properties in several disease models [23,24]. It is used as a food flavoring and as a perfume and contains 1 to 2% EO on a dry weight basis [25]. The quality of lemongrass is generally determined by the amount of citral content, which is composed of isomers (neral and geranial), giving it the characteristic odor [26].
Cymbopogon martini (palmarosa) is an important, multiharvest, perennial, ad aromatic grass native to India. India is the principal producer and exporter of palmarosa EO [27,28,29]. EO of palmarosa is generally distilled (more frequently by steam or hydro-distillation) from the leaves, stems, flowers, bark or roots, or other elements of the plant [30]. The EO of palmarosa mainly consists of monoterpenoid components [31] such as citral, citronellol, and geranyl acetate [32], which is why these oils are mostly used as a culinary flavoring in Asia [33,34,35] and have demonstrated remarkable bioactivities, including herbicidal activity [36]. Moreover, the most important use of palmarosa is in the treatment of skin infections such as acne and also to stimulate cell regeneration while regulating the production of sebum [37].
EOs are expensive because of their low oil yields. In order to increase profit margins, EOs are being adulterated in different degrees with vegetable oils, synthetic compounds, or isolated natural compounds from less valued EOs [38]. So, there is a dire need to understand the composition of Cymbopogon winterianus, Cymbopogon citratus, and Cymbopogon martini. Thus, in the present study, we isolated the EOs from different tissues of these three Cymbopogon species and studied their composition using GC-MS and Chiral GC-MS, which can be used as a chemical fingerprint for the authentication of these Cymbopogon species. In addition to these, we studied the antibacterial and antifungal activity of some pure major components (geraniol, (±) citronellol, citral, and (−) citronellal) and the root and leaves of Cymbopogon winterianus Eos.
2. Results and Discussion
2.1. Essential Oil Yields
The EO yields of three Cymbopogon species, namely C. winterianus, C. citratus, and C. martini are presented in Table 1. The hydro-distillation of leaves of all three Cymbopogon species yielded 1.3–2.2% essential oil, which is remarkably high compared with other parts of the plant. The previously reported hydro-distillation yield of C. winterianus was 1.50 ± 0.15% (w/w) and with better extraction yield in comparison with steam distillation [39]. Similarly, the hydro-distillation extraction yield of C. citratus was 0.98%, which was quite lower as compared with microwave-assisted hydro-distillation. Our extraction yield was closely in agreement with previously reported microwave-assisted hydro-distillation yields [40]. In the case of C. martini leaves, the yield of extraction was (1.4756 w/w %), which is in close agreement with our hydro-distillation yield [41].
Table 1.
Essential oil yields for different Cymbopogon species from hydro-distillation.
| Cymbopogon Species | Different Parts | Essential Oil Yield (v/w %) | Color of Essential Oil |
|---|---|---|---|
| C. winterianus | Root | 0.3 | Pale yellow |
| Root hair and stalk (1:1) | 0.3 | Pale yellow | |
| Leaves | 2.2 | Light pale yellow | |
| Root stalk and shoot (1:1) | 0.8 | Pale yellow | |
| C. citratus | Root | 0.5 | Pale yellow |
| Leaves | 1.4 | Yellow | |
| C. martini | Root | 0.2 | Pale yellow |
| Leaves | 1.3 | Light pale yellow |
2.2. Chemical Composition of Cymbopogon Species
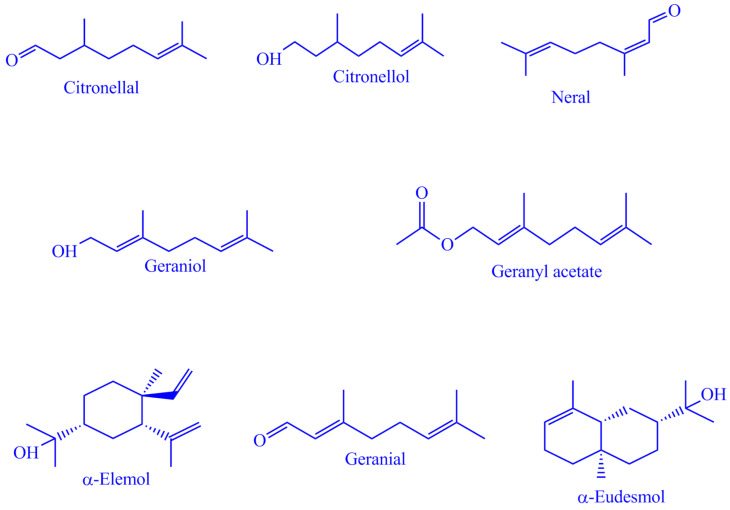
The major compounds present in the different tissues of cymbopogon species are presented in Figure 1. Several studies have been reported on the EO composition of C. winterianus, which reveals high variability in its chemical fingerprint. So, to reveal the mystery behind this variability for the first time, we studied the composition of EOs extracted from different parts of C. winterianus. The results of GC-MS analysis of C. winterianus EOs from different parts are presented in Supplementary File (Table S1), and Table 2 shows only the major selected constituents.
Figure 1.
Major compounds present in different Cymbopogon species.
Table 2.
Selected constituents of C. winterianus essential oils from different parts.
| RIdb | RIcalc | Compounds | Root | Root Hair and Stalk | Leaves | Root Stalk and Shoot |
|---|---|---|---|---|---|---|
| 981 | 981 | 6-Methyl-5-hepten-2-one | t | 0.1 | 0.2 | 0.8 |
| 1098 | 1095 | Linalool | 0.1 | 0.5 | 0.5 | 0.8 |
| 1151 | 1148 | Citronellal | 1.1 | 8.6 | 13.7 | 0.3 |
| 1226 | 1223 | Citronellol | 4.3 | 7.5 | 5.1 | 0.2 |
| 1238 | 1235 | Neral | 1.2 | 6.5 | 8.3 | 31.5 |
| 1250 | 1249 | Geraniol | 0.1 | 8.6 | 37.1 | 0.4 |
| 1268 | 1264 | Geranial | 1.9 | 10.7 | 11.0 | 59.0 |
| 1347 | 1350 | Citronellyl acetate | t | 1.9 | 1.9 | - |
| 1350 | 1356 | Eugenol | 0.7 | 1.2 | 1.0 | - |
| 1376 | 1379 | Geranyl acetate | 0.1 | 5.3 | 11.3 | 0.3 |
| 1387 | 1389 | trans-β-Elemene | 2.1 | 0.9 | 0.3 | t |
| 1417 | 1417 | (E)-β-Caryophyllene | 0.3 | 0.6 | 2.0 | 0.2 |
| 1444 | 1448 | (E)-Isoeugenol | 0.1 | 0.3 | 0.5 | 0.9 |
| 1509 | 1513 | γ-Cadinene | 0.7 | 0.7 | 0.7 | 0.1 |
| 1548 | 1548 | α-Elemol | 53.1 | 29.5 | 1.9 | 1.2 |
| 1630 | 1630 | γ-Eudesmol | 7.5 | 2.0 | 0.1 | t |
| 1659 | 1652 | α-Eudesmol | 18.9 | 5.3 | - | - |
In the root oil of C. winterianus, sesquiterpenoids were predominant, representing 84.5% of the total oil in which monoterpenoids were 9.8% and sesquiterpenes 4.7%. The result shows that the roots were rich in α-elemol (53.1%), α-eudesmol (18.9%), γ-eudesmol (7.5%), whereas the root hair and stalk oils are dominated by more than half by the monoterpenoids (52.1%), sesquiterpenoids (41.8%), and sesquiterpenes (3.1%) of the total oil. The principal compound of root hair and stalk was α-elemol (29.5%), geranial (10.7%), citronellal (8.6%), geraniol (8.6%), citronellol (7.5%), neral (6.5%), and geranyl acetate (5.3%). The composition of root and root hair and stalk oils are almost similar, presenting α-elemol as the major compound. The percentage of some compounds such as citronellol, citronellal, neral, geraniol, geranial, and geranyl acetate are higher in root hair and stalk EO, while, other compounds such as α-elemol, α-Eudesmol, and γ-Eudesmol are higher in the root oil of C. winterianus.
The leaves and root stalk and shoot oils largely contained monoterpenoids as their major components. The leaves’ EO contains (90.4%) monoterpenoids, (3.9%) sesquiterpenes, and (2.6%) sesquiterpenoids. The major compounds of leaves were geraniol (37.1%), followed by citronellal (13.7%), geranyl acetate (11.3%), geranial (11.0%), neral (8.3%), and citronellol (5.1%); this was in a close agreement with studies conducted by Rodrigues et al. [4], except the presence of α-elemol in high amounts, which might be due to the addition of roots in their studies during EO extraction. In another study, it is unclear whether the part used in their extracted EO is either the leaves, stems or both of C. winterianus; anyways, the principle components are citronellal, geraniol, and citronellol [44]. Similarly, contrasting the type of C. winterianus leaves EO composition and major component was geraniol (18.88%), citronellal (16.95%), elemenol (14.08%), and citronellol (12.57%), and we confirm that was not pure single-tissues EO, and there might be addition of other tissues [45]. Meanwhile, 94.2% monoterpenoids, 1.8% sesquiterpenoids, and 0.5% sesquiterpenes were found in root stalk and shoot EO, where citral was the predominant constituent, consisting of geranial (59.0%) and neral (31.5%).
The results of GC-MS analysis of Cymbopogon citratus EOs extracted from roots and leaves are tabulated in Supplementary File (Table S2), and Table 3 shows only the selected major constituents.
Table 3.
Selected constituents of C. citratus essential oils extracted from root and leaves.
| RIdb | RIcalc | Compounds | C. citratus (Lemongrass) | |
|---|---|---|---|---|
| Root | Leaves | |||
| 1098 | 1095 | Linalool | 0.5 | 0.6 |
| 1178 | 1177 | trans-Isocitral | 0.4 | 0.4 |
| 1230 | 1232 | Epoxy geranial | 0.1 | 0.1 |
| 1238 | 1235 | Neral | 16.6 | 36.1 |
| 1250 | 1249 | Geraniol | 0.4 | 0.3 |
| 1268 | 1264 | Geranial | 25.0 | 53.1 |
| 1353 | 1358 | Neric acid | 0.3 | 0.6 |
| 1387 | 1389 | trans-β-Elemene | 1.4 | 0.1 |
| 1417 | 1417 | (E)-β-Caryophyllene | 0.2 | 1.0 |
| 1548 | 1548 | α-Elemol | 31.5 | 0.3 |
| 1577 | 1582 | Caryophyllene oxide | t | 1.3 |
| 1630 | 1630 | γ-Eudesmol | 5.2 | t |
| 1659 | 1652 | α-Eudesmol | 11.3 | - |
| 1663 | 1666 | 14-Hydroxy-epi-(Z)-caryophyllene | 1.0 | 0.1 |
C. citratus leaves’ EO contains neral (36.1%) and geranial (53.1%) monoterpene aldehydes as the major compounds, whereas (E)-β-caryophyllene (1.0%) and caryophyllene oxide (1.3%) contain γ-cadinene (0.81%) in lower concentrations, which is similar to the study previously conducted by Boukhatem et al. [18], except for the presence of β-myrcene in a significant amount. C. citratus leaves’ EO from Vietnam also showed a comparable content of citral (neral and geranial), and the content of β-myrcene can be used to distinguish the origin of the EO [46]. In the roots of C. citratus EO, a sesquiterpene alcohol and monoterpene aldehydes were predominant compounds. The prime components were α-elemol (31.5%), geranial (25.0%), neral (16.6%), α-eudesmol (11.3%), and γ-eudesmol (5.2%); trans-β-elemene (1.4%) was present in a lesser quantity. Thus, compounds such as α-elemol, α-eudesmol, and γ-eudesmol can be used to identify the source of C. citratus EO.
The results of GC-MS analysis of Cymbopogon martini EOs extracted from leaves and roots are presented in Supplementary File (Table S3), and Table 4 shows only the selected constituents. The C. martini leaves’ EO contains geraniol (76.6%), and geranyl acetate (15.2%) as major compounds, which is comparable to the previous study conducted by Jnanesha et al. [27]. Geraniol content in the C. martini leaves’ EO up to 80 days was noticeably increased, whereas geranyl acetate decreased significantly at that time and positively correlated [3]. Interestingly, one of the C. martini cultivars from India showed limonene as a major compound [47], while, the C. martini root EO contains a higher amount of geraniol (87.9%) compared with the leaves’ oil, which ultimately leads to a decrease in the content of geranyl acetate (4.4%). The underlying reasons for the differences in EOs composition could be attributed to genotype, edaphic variables, geographical location, pedo-climatic conditions, harvest time, extraction procedure, maturity of plant, different part of plant material, and analytical procedures.
Table 4.
Selected constituents of C. martini essential oils extracted from roots and leaves.
| RIdb | RIcalc | Compounds | C. martini (Palmarosa) | |
|---|---|---|---|---|
| Root | Leaves | |||
| 987 | 988 | Myrcene | 0.1 | 0.2 |
| 1044 | 1044 | trans-β-Ocimene | 0.2 | 0.9 |
| 1098 | 1095 | Linalool | 3.2 | 2.0 |
| 1238 | 1235 | Neral | t | 0.2 |
| 1250 | 1249 | Geraniol | 87.9 | 76.6 |
| 1268 | 1264 | Geranial | 0.2 | 0.6 |
| 1376 | 1379 | Geranyl acetate | 4.4 | 15.2 |
| 1417 | 1418 | (E)-β-Caryophyllene | 0.4 | 0.5 |
| 1713 | 1714 | (2E,6Z)-Farnesol | 3.0 | 1.3 |
2.3. Enantiomeric Distributions Analysis of Cymbopogon Species Essential Oils
The enantiomeric distributions of chiral terpenoids present in Cymbopogon species EOs are presented in Table 5. In previous studies, the enantiomeric distributions of chiral terpenoids have been successfully used for species identification and adulteration detection of different EOs [38,48]. To the best of our knowledge, this is the first report on enantiomeric distributions of chiral terpenoids from Cymbopogon species Viz C. winterianus, C. citratus, and C. martini. There were, altogether, nine chiral terpenoids detected in various parts of citronella Eos, among which linalool, terpinen-4-ol, bornyl acetate, borneol, α-terpineol, and (E)-β-caryophyllene were levorotatory. However, citronellal and citronellol were detected as dextrorotatory compounds. On the contrary, germacrene D was levorotatory in the root and dextrorotatory in leaves, as well as root stalk and shoots.
Table 5.
Enantiomeric distributions of chiral terpenoids present in Cymbopogon species.
| Chiral Compounds | C. winterianus (Citronella) | C. citratus (Lemongrass) | C. martini (Palmarosa) | ||||
|---|---|---|---|---|---|---|---|
| Root | Root Stalk and Shoot | Leaves | Root | Leaves | Root | Leaves | |
| Linalool | (+) 13.0: (−) 87.0 | (+) 32.4: (−) 67.6 | (+) 28.7: (−)71.3 | (+) 25.0: (−)75.0 | (+) 33.2: (−) 66.8 | (+) 95.9: (−) 4.1 | (+) 86.2: (−) 13.8 |
| Citronellal | (+) 100.0: (−) 0.0 | (+) 100.0: (−) 0.0 | (+) 100.0: (−)0.0 | (+) 100.0: (−)0.0 | - | - | - |
| Terpinen-4-ol | (+) 35.7: (−) 64.3 | - | - | - | - | - | - |
| Bornyl acetate | (+) 0.0: (−) 100.0 | - | - | - | - | - | - |
| Borneol | (+) 0.0: (−) 100.0 | (+) 0.0: (−) 100 | - | - | - | - | - |
| α-Terpineol | (+) 10.3: (−) 89.7 | - | - | - | - | - | - |
| Citronellol | (+) 83.7: (−)16.3 | (+) 54.2: (−) 45.8 | (+) 83.6: (−) 16.4 | (+) 60.2: (−) 39.8 | - | - | - |
| (E)-β-Caryophyllene | (+) 0.0: (−) 100.0 | (+) 0.0: (−) 100.0 | (+) 0.0: (−) 100.0 | (+) 0.0: (−) 100.0 | (+) 0.0: (−) 100.0 | (+) 0.0: (−) 100.0 | (+) 0.0: (−) 100.0 |
| Germacrene D | (+) 17.4: (−) 82.6 | (+) 100.0: (−) 0.0 | (+) 75.0: (−) 25.0 | - | (+) 100.0: (−) 0.0 | - | - |
“-” indicates not detected.
There were, altogether, five chiral terpenoids detected in lemongrass root and leaves EOs. Among these, linalool and (E)-β-caryophyllene were levorotatory and detected in both root and leaf oil. However, citronellol and citronellal were dextrorotatory and detected only in root EO. Germacrene D, on the other hand, was dextrorotatory but detected only in the lemongrass leaves’ oil. Lastly, only two chiral terpenoids were detected in palmarosa root and leaves’ EOs, namely linalool and (E)-β-caryophyllene; linalool was dextrorotatory and (E)-β-caryophyllene was 100% levorotatory.
Thus, the enantiomeric distributions of chiral terpenoids present in Cymbopogon species Viz C. winterianus, C. citratus, and C. martini will be helpful to establish the chemical fingerprint of these species and also in the adulteration detection of EOs of these species.
2.4. Antimicrobial Activity of Cymbopogon Winterianus Essential Oil and Some Major Compounds
The MIC values of Cymbopogon winterianus EOs and some pure compounds such as geraniol, (±) citronellol, citral, and (−) citronellal, and that of the positive control gentamicin against a panel of bacterial and fungal strains, were determined through a two-fold broth microdilution method. This study showed that the assayed root and leaves of C. winterianus EOs have variable microbial inhibitory activities, as presented in Table 6. Plants having secondary metabolites and the EOs of C. winterianus have demonstrated a broad range of antimicrobial activities against different pathogens [11,12,49,50]. The leaf part of C. winterianus EO showed effectiveness against Pseudomonas aeruginosa, with an MIC of 78.1 μg/mL and noticeable activity against Staphylococcus aureus, Staphylococcus epidermidis, and Streptococcus pyogenes, with an MIC of 156.3 μg/mL, while other panels of bacterial strains had no surprising results. The Eos of C. winterianus demonstrated weaker antibacterial activities than those of the positive control, gentamicin (MIC < 19.5 μg/mL). It is difficult to speculate as to which components in the root and leaves of C. winterianus Eos may be responsible for the antibacterial activity. In the case of Staphylococcus aureus, pure component citral (MIC = 78.1 μg/mL) is more active than EO. It might be due to the antagonistic effect of individual components present in EO. In the case of Pseudomonas aeruginosa, the leaf EO is more potent than either of the tested pure components, which might be due to the synergetic mechanism among components of EO.
Table 6.
Antimicrobial activity of C. winterianus essential oil and some major compounds.
| Name of Bacteria | C. winterianus | MICs (μg/mL) | |||||
|---|---|---|---|---|---|---|---|
| Leaves | Root | (±)-Citronellol | Citral (Neral:Geranial in 1:1 Ratio) | (−) Citronellal | Geraniol | Gentamicin | |
| Bacillus cereus | 312.5 | 312.5 | 312.5 | 156.3 | 312.5 | 312.5 | <19.5 |
| Propionibacterium acnes | 312.5 | 312.5 | 625 | 312.5 | 312.5 | 625 | <19.5 |
| Pseudomonas aeruginosa | 78.1 | 2500 | 312.5 | 312.5 | 312.5 | 312.5 | <19.5 |
| Serratia marcescens | 625 | 625 | 312.5 | 312.5 | 312.5 | 312.5 | <19.5 |
| Staphylococcus aureus | 312.5 | 156.3 | 312.5 | 78.1 | 312.5 | 312.5 | <19.5 |
| Staphylococcus epidermidis | 312.5 | 156.3 | 312.5 | 312.5 | 312.5 | 312.5 | <19.5 |
| Streptococcus pyogenes | 625 | 156.3 | 625 | 156.3 | 312.5 | 312.5 | <19.5 |
| Name of Fungus | Amphotericin B | ||||||
| Aspergillus niger | 78.1 | 2500 | 156.3 | 156.3 | 78.1 | 156.3 | <19.5 |
| Aspergillus fumigatus | 78.1 | 2500 | 156.3 | 156.3 | 156.3 | 312.5 | <19.5 |
| Candida albicans | 156.3 | 156.3 | 156.3 | 156.3 | 156.3 | 156.3 | <19.5 |
| Microsporum canis | 156.3 | 156.3 | 312.5 | 156.3 | 312.5 | 312.5 | <19.5 |
| Microsporum gypseum | 312.5 | 625 | 156.3 | 156.3 | 312.5 | 156.3 | <19.5 |
| Trichophyton mentagrophytes | 78.1 | 156.3 | 156.3 | 312.5 | 312.5 | 625 | <19.5 |
| Trichophyton rubrum | 312.5 | 312.5 | 312.5 | 312.5 | 312.5 | 312.5 | <19.5 |
The EO from the leaves of C. winterianus displayed potent antifungal activity against Aspergillus niger, Aspergillus fumigatus, and Trichophyton mentagrophytes (MIC = 78.1 μg/mL). Both the leaves and root parts of C. winterianus EOs showed good activity against Candida albicans, Microsporum canis, and Trichophyton mentagrophytes, with MIC values of 156.3μg/mL. The EOs of C. winterianus demonstrated weaker antifungal activities than those of the positive control, amphotericin B (MIC < 19.5 μg/mL). In the cases of Trichophyton mentagrophytes, Aspergillus fumigates, and Aspergillus niger, the synergetic effect is more pronounced in the leaves as compared with root EO. In the case of, Aspergillus niger, pure component (−) citronellal (MIC = 78.1 μg/mL) is more active than root EO. On the other hand, despite the absence of (−) citronellal, as indicated by chiral GC-MS analysis in leaves’ oil, it shows effectiveness against Aspergillus niger, which might be due to the synergistic effect of individual components of leaves EO. However, in the cases of Candida albicans and Trichophyton rubrum, there was no antagonistic and synergetic effect pronounced.
C. winterianus leaves EO showed promising antimicrobial properties and can be used in lieu of synthetic chemicals to counter microbial attacks. Additionally, the leaves of C. winterianus EO were more potent than the root oil. This may be due to differences in chemical compositions of EOs in roots and leaves. Alternatively, the antimicrobial properties of C. winterianus EO may be the presence of secondary metabolites such as citronellal, citronellol, geraniol, neral, geranial, and other components by synergistic and antagonistic mechanisms. The antifungal and antibacterial mechanisms of action of EOs are not clearly understood yet. However, it has been postulated that the hydrophobic constituents either disrupt cytoplasmic membranes via a cascade of different reactions leading to cytoplasmic leakage, cell lysis, and ultimate death, or via the inhibition of sporulation [51,52].
3. Materials and Methods
3.1. Plant Material and Isolation of Essential Oils
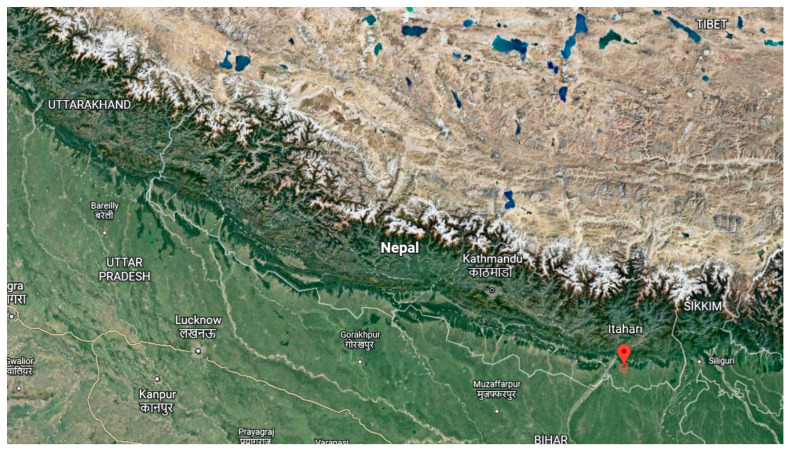
Three cultivated species of Cymbopogon, namely C. winterianus, C. citratus, and C. martini were collected in March, 2021 from Sunsari (26°42′19.6″ N 87°15′29.7″ E), Nepal, presented in Figure 2. Different parts of plants were separated, washed, and then hydrodistilled for 6 h using a Clevenger apparatus, as previously described [48]. The obtained EOs were dried with anhydrous sodium sulfate and stored in bottles at 4 °C until further research was conducted. The Cymbopogon species EOs yields are summarized in Table 1.
Figure 2.
The geographical location of Cymbopogon species collection site from Google Earth.
3.2. Chemical Composition Analysis by Gas Chromatography/Mass Spectrometry (GC-MS)
Analysis of the chemical constituents in the Cymbopogen species (C. winterianus, C. citratus, and C. martini) EOs was carried out using Shimadzu GCMS-QP2010(Shimadzu Corp, Columbia, MD, USA) Ultra under the following condition: mass selective detector (MSD), operated in the EI mode (electron energy = 70 eV), with scan range = 40–400 m/z and scan rate of 3.0 scans/s, as previously described [53,54]. Identification of the individual components of the EOs was determined by comparison of the retention indices determined by reference to a homologous series of n-alkanes and comparison of the mass spectral fragmentation patterns (over 80% similarity match) with those reported in the literature [43] and our own in-house library [42] using the LabSolutions GC-MS solution software version 4.45 (Shimadzu Scientific Instruments, Columbia, MD, USA).
3.3. Enantiomeric Analysis by Chiral Gas Chromatography-Mass Spectrometry (CGC-MS)
A Shimadzu GC-MS-QP2010S with EI mode (70 eV) and B-Dex 325 chiral capillary GC column was used to perform the enantiomeric analysis of Cymbopogen species (C. winterianus, C. citratus, and C. martini) Eos, as previously described [55]. A comparison of retention times and mass spectral fragmentation patterns with authentic samples obtained from Sigma-Aldrich (Milwaukee, WI, USA) was used to identify the enantiomers. Table 5 shows the enantiomeric distribution of chiral terpenoids from Cymbopogen species (C. winterianus, C. citratus, and C. martini) EOs
3.4. Bacterial Strains Tested
Tryptic soy agar medium was used to culture all tested bacterial strain. A 5000 μg/mL solution of EOs was prepared in dimethyl sulfoxide (DMSO), and twofold dilution in 100 μL of cation-adjusted Mueller Hinton broth (CAMHB) (Sigma-Aldrich, St. Louis, MO, USA) was added to the top well of a 96-well microdilution plate. The prepared stock solution of EOs was then serially twofold diluted in fresh CAMHB to obtain final concentrations of 2500, 1250, 625, 312.5, 156.3, 78.1, 39.1, and 19.5 μg/mL. The freshly harvested bacteria with approximately 1.5 × 108 CFU/mL final concentration were added to each well of 96-well microdilution plates and were incubated at 37 °C for 24 h. Gentamicin (Sigma-Aldrich, St. Louis, MO, USA) and DMSO were used as positive and negative controls, respectively [56]. Seven microorganisms were used to evaluate the antibacterial activities of C. winterianus (leaves and root) EOs: Five Gram-positive bacteria, Bacillus cereus (ATCC-14579), Staphylococcus epidermidis (ATCC-12228), Propionibacterium acnes (ATCC-11827), Staphylococcus aureus (ATCC-29213), and Streptococcus pyogenes (ATCC-19615) and two Gram-negative bacteria, Serratia marcescens (ATCC-14756) and Pseudomonas aeruginosa (ATCC-27853), using the microbroth dilution technique.
3.5. Fungal Strains Tested
All tested fungi were cultured yeast-nitrogen base growth medium (Sigma-Aldrich, St. Louis, MO, USA). Stock solutions (5000 μg/mL) of C. winterianus (leaves and root) EOs were prepared in DMSO and diluted as above. The freshly harvested fungi with approximately 7.5 × 107 CFU/mL final concentrations were added to each well of 96-well microdilution plates and were incubated at 35 °C for 24 h. DMSO and amphotericin B (Sigma-Aldrich, St. Louis, MO, USA) were negative and positive antifungal controls, respectively, as previously described [56]. Seven fungal strains were used: Aspergillus niger (ATCC-16888), Candida albicans (ATCC-18804), Microsporum canis (ATCC-11621), Trichophyton mentagrophytes (ATCC-18748), Aspergillus fumigatus (ATCC-96918), Microsporum gypseum (ATCC-24102), and Trichophyton rubrum (ATCC-28188).
4. Conclusions
As far as we are aware, this is the first report on the EOs of three Cymbopogon species (C. winterianus, C. citratus, and C. martini) from Sunsari, eastern Nepal that includes not only chemical composition analysis by GC-MS but also enantiomeric composition by chiral GC-MS. The results show variations in volatiles’ compositions and enantiomeric distributions of chiral terpenoids. The yield of extraction varies depending upon the part used. The study can be used to create a benchmark for future Cymbopogon species’ EOs assessments, as well as authentication for adulteration or consumer safety. In addition, the antibacterial and antifungal activity of some selected pure compounds and leaves of Cymbopogon winterianus EO (rich in citral, citronellal, citronellol, and geraniol) suggests that it can be used in lieu of synthetic antimicrobial agents. It is unclear which of the individual components is responsible for the antimicrobial activity. However, it is likely that synergistic effects are more pronounced for the components’ activity.
Acknowledgments
The authors are thankful to the APRC and ARC for GC-MS and Chiral GC-MS analysis. We acknowledge Sunita Satyal for their constructive suggestions and support.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28020543/s1, Table S1: Chemical composition of Cymbopogon winterianus essential oils extracted from different parts, Table S2: Chemical composition of Cymbopogon citratus essential oil extracted from roots and leaves, and Table S3: Chemical composition of Cymbopogon martini essential oils extracted from roots and leaves.
Author Contributions
Conceptualization D.K.P., P.K.O. and P.S.; methodology, P.S. and N.S.D.; validation, P.S. and W.N.S.; formal analysis, S.D., D.K.P., P.K.O., A.P., R.S., S.M. and A.R.; investigation, S.D., P.K.O., D.K.P., S.M., N.S.D., S.T. and A.R.; data curation, P.S. and W.N.S.; writing—original draft preparation, S.M., P.K.O., S.D. and D.K.P.; writing—review and editing, D.K.P. and P.S.; supervision, P.S.; project administration, P.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Data Availability Statement
Data included in the article/Supplementary Material are referenced in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Huang X., Feng Y., Huang Y., Li H. Chemical Composition, Antioxidant and the Possible Use as Skin-Care Ingredient of Clove Oil (Syzygium aromaticum (L.) Merr. & Perry) and Citronella Oil (Cymbopogon goeringii) from China. J. Essent. Oil Res. 2013;25:315–323. doi: 10.1080/10412905.2013.775082. [DOI] [Google Scholar]
- 2.Avoseh O., Oyedeji O., Rungqu P., Nkeh-Chungag B., Oyedeji A. Cymbopogon Species; Ethnopharmacology, Phytochemistry and the Pharmacological Importance. Molecules. 2015;20:7438–7453. doi: 10.3390/molecules20057438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kakaraparthi P.S., Srinivas K.V.N.S., Kumar J.K., Kumar A.N., Rajput D.K., Anubala S. Changes in the Essential Oil Content and Composition of Palmarosa (Cymbopogon martini) Harvested at Different Stages and Short Intervals in Two Different Seasons. Ind. Crops Prod. 2015;69:348–354. doi: 10.1016/j.indcrop.2015.02.020. [DOI] [Google Scholar]
- 4.Rodrigues K.A.D.F., Dias C.N., do Amaral F.M.M., Moraes D.F.C., Mouchrek Filho V.E., Andrade E.H.A., Maia J.G.S. Molluscicidal and Larvicidal Activities and Essential Oil Composition of Cymbopogon winterianus. Pharm. Biol. 2013;51:1293–1297. doi: 10.3109/13880209.2013.789536. [DOI] [PubMed] [Google Scholar]
- 5.Kandimalla R., Kalita S., Choudhury B., Dash S., Kalita K., Kotoky J. Chemical Composition and Anti-Candidiasis Mediated Wound Healing Property of Cymbopogon nardus Essential Oil on Chronic Diabetic Wounds. Front. Pharmacol. 2016;7:198. doi: 10.3389/fphar.2016.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beneti S.C., Rosset E., Corazza M.L., Frizzo C.D., Di Luccio M., Oliveira J.V. Fractionation of Citronella (Cymbopogon winterianus) Essential Oil and Concentrated Orange Oil Phase by Batch Vacuum Distillation. J. Food Eng. 2011;102:348–354. doi: 10.1016/j.jfoodeng.2010.09.011. [DOI] [Google Scholar]
- 7.Akbar N., Saxena B.K. Isolation of Geraniol Content from Various Essential Oils. Asian J. Exp. Chem. 2009;4:14–17. [Google Scholar]
- 8.Sajo M.E.J., Song S.-B., Bajgai J., Kim Y.-J., Kim P.-S., Ahn D.-W., Khanal N., Lee K.-J. Applicability of Citronella Oil (Cymbopogon winteratus) for the Prevention of Mosquito-Borne Diseases in the Rural Area of Tikapur, Far-Western Nepal. Rural Remote Health. 2015;15:3532. doi: 10.22605/RRH3532. [DOI] [PubMed] [Google Scholar]
- 9.Lins L., Dal Maso S., Foncoux B., Kamili A., Laurin Y., Genva M., Jijakli M.H., De Clerck C., Fauconnier M.L., Deleu M. Insights into the Relationships between Herbicide Activities, Molecular Structure and Membrane Interaction of Cinnamon and Citronella Essential Oils Components. Int. J. Mol. Sci. 2019;20:4007. doi: 10.3390/ijms20164007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker B.P., Grant J.A., Malakar-Kuenen R. Citronella & Citronella Oil Profile. New York State IPM Program; New York, NY, USA: 2018. [Google Scholar]
- 11.De Toledo L.G., Ramos M.A.D.S., Spósito L., Castilho E.M., Pavan F.R., Lopes É.D.O., Zocolo G.J., Silva F.A.N., Soares T.H., Dos Santos A.G., et al. Essential Oil of Cymbopogon nardus (L.) Rendle: A Strategy to Combat Fungal Infections Caused by Candida Species. Int. J. Mol. Sci. 2016;17:1252. doi: 10.3390/ijms17081252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pontes E.K.U., Melo H.M., Nogueira J.W.A., Firmino N.C.S., de Carvalho M.G., Catunda Júnior F.E.A., Cavalcante T.T.A. Antibiofilm Activity of the Essential Oil of Citronella (Cymbopogon nardus) and Its Major Component, Geraniol, on the Bacterial Biofilms of Staphylococcus aureus. Food Sci. Biotechnol. 2019;28:633–639. doi: 10.1007/s10068-018-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sellamuthu P.S., Sivakumar D., Soundy P. Antifungal Activity and Chemical Composition of Thyme, Peppermint and Citronella Oils in Vapor Phase against Avocado and Peach Postharvest Pathogens: Essential Oils Control Postharvest Diseases. J. Food Saf. 2013;33:86–93. doi: 10.1111/jfs.12026. [DOI] [Google Scholar]
- 14.Timung R., Barik C.R., Purohit S., Goud V.V. Composition and Anti-Bacterial Activity Analysis of Citronella Oil Obtained by Hydrodistillation: Process Optimization Study. Ind. Crops Prod. 2016;94:178–188. doi: 10.1016/j.indcrop.2016.08.021. [DOI] [Google Scholar]
- 15.Sagradas J., Costa G., Figueirinha A., Castel-Branco M.M., Silvério Cabrita A.M., Figueiredo I.V., Batista M.T. Gastroprotective Effect of Cymbopogon citratus Infusion on Acute Ethanol-Induced Gastric Lesions in Rats. J. Ethnopharmacol. 2015;173:134–138. doi: 10.1016/j.jep.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Gautam M., Agrawal M. Influence of Metals on Essential Oil Content and Composition of Lemongrass (Cymbopogon citratus (D.C.) Stapf.) Grown under Different Levels of Red Mud in Sewage Sludge Amended Soil. Chemosphere. 2017;175:315–322. doi: 10.1016/j.chemosphere.2017.02.065. [DOI] [PubMed] [Google Scholar]
- 17.Muala W.C.B., Desobgo Z.S.C., Jong N.E. Optimization of Extraction Conditions of Phenolic Compounds from Cymbopogon citratus and Evaluation of Phenolics and Aroma Profiles of Extract. Heliyon. 2021;7:e06744. doi: 10.1016/j.heliyon.2021.e06744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boukhatem M.N., Ferhat M.A., Kameli A., Saidi F., Kebir H.T. Lemon Grass (Cymbopogon citratus) Essential Oil as a Potent Anti-Inflammatory and Antifungal Drugs. Libyan J. Med. 2014;9:25431. doi: 10.3402/ljm.v9.25431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassolé I.H.N., Lamien-Meda A., Bayala B., Obame L.C., Ilboudo A.J., Franz C., Novak J., Nebié R.C., Dicko M.H. Chemical Composition and Antimicrobial Activity of Cymbopogon citratus and Cymbopogon giganteus Essential Oils Alone and in Combination. Phytomedicine. 2011;18:1070–1074. doi: 10.1016/j.phymed.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence R., Lawrence K., Srivastava R., Gupta D. Antioxidant Activity of Lemon Grass Essential Oil (Cympopogon citratus) Grown in North Indian Plains. Sci. Temper. 2015;4:23–29. [Google Scholar]
- 21.Nguefack J., Dongmo J.B.L., Dakole C.D., Leth V., Vismer H.F., Torp J., Guemdjom E.F.N., Mbeffo M., Tamgue O., Fotio D., et al. Food Preservative Potential of Essential Oils and Fractions from Cymbopogon citratus, Ocimum gratissimum and Thymus vulgaris against Mycotoxigenic Fungi. Int. J. Food Microbiol. 2009;131:151–156. doi: 10.1016/j.ijfoodmicro.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Bayala B., Bassole I.H.N., Maqdasy S., Baron S., Simpore J., Lobaccaro J.-M.A. Cymbopogon citratus and Cymbopogon giganteus Essential Oils Have Cytotoxic Effects on Tumor Cell Cultures. Identification of Citral as a New Putative Anti-Proliferative Molecule. Biochimie. 2018;153:162–170. doi: 10.1016/j.biochi.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Han X., Parker T.L. Lemongrass (Cymbopogon flexuosus) Essential Oil Demonstrated Anti-Inflammatory Effect in Pre-Inflamed Human Dermal Fibroblasts. Biochim. Open. 2017;4:107–111. doi: 10.1016/j.biopen.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartatie E.S., Prihartini I., Widodo W., Wahyudi A. Bioactive Compounds of Lemongrass (Cymbopogon citratus) Essential Oil from Different Parts of the Plant and Distillation Methods as Natural Antioxidant in Broiler Meat. Mater. Sci. Eng. 2019;532:012018. doi: 10.1088/1757-899X/532/1/012018. [DOI] [Google Scholar]
- 25.Piaru S.P., Perumal S., Cai L.W., Mahmud R., Majid A.M.S.A., Ismail S., Man C.N. Chemical Composition, Anti-Angiogenic and Cytotoxicity Activities of the Essential Oils of Cymbopogan citratus (Lemon Grass) against Colorectal and Breast Carcinoma Cell Lines. J. Essent. Oil Res. 2012;24:453–459. doi: 10.1080/10412905.2012.703496. [DOI] [Google Scholar]
- 26.Pesimo A.R. Harnessing the Solar Energy in Extracting Essential Oil for Community Based Perfumery and Aromatherapy. Open Access Libr. J. 2017;04:1–11. doi: 10.4236/oalib.1103905. [DOI] [Google Scholar]
- 27.Jnanesha A.C. Variation in the Essential Oil Yield and Chemical Composition of Palmarosa Biomass Cymbopogon martini (Roxb.) Wats. Var. Motia Burk) Under Different Location in Semi Arid Tropic Regions of India. Ind. J. Pure Appl. Biosci. 2019;7:107–113. doi: 10.18782/2582-2845.7867. [DOI] [Google Scholar]
- 28.Rajeswara Rao B.R., Rajput D.K. Effect of Planting Technique and Number of Irrigations on Biomass and Essential Oil Yields of Palmarosa (Cymbopogon martinii (Roxb.) Wats. Var. Motia Burk.) J. Med. Aromat. Plants Sci. 2007;29:177–181. [Google Scholar]
- 29.Randriamiharisoa R.P., Gaydou E.M. Composition of Palmarosa (Cymbopogon martinii) Essential Oil from Madagascar. J. Agric. Food Chem. 1987;35:62–66. doi: 10.1021/jf00073a015. [DOI] [Google Scholar]
- 30.Lawrence K., Lawrence R., Parihar D., Srivastava R., Charan A. Antioxidant Activity of Palmarosa Essential Oil (Cymbopogon martini) Grown in North Indian Plains. Asian Pac. J. Trop. Biomed. 2012;2:S888–S891. doi: 10.1016/S2221-1691(12)60330-X. [DOI] [Google Scholar]
- 31.Dubey V.S., Bhalla R., Luthra R. An Esterase Is Involved in Geraniol Production during Palmarosa Inflorescence Development. Phytochemistry. 2003;63:257–264. doi: 10.1016/S0031-9422(03)00114-6. [DOI] [PubMed] [Google Scholar]
- 32.Smitha G.R., Rana V.S. Variations in Essential Oil Yield, Geraniol and Geranyl Acetate Contents in Palmarosa (Cymbopogon martinii, Roxb. Wats. Var. Motia) Influenced by Inflorescence Development. Ind. Crops Prod. 2015;66:150–160. doi: 10.1016/j.indcrop.2014.12.062. [DOI] [Google Scholar]
- 33.Ganjewala D. Cymbopogon Essential Oils Chemical Compositions and Bioactivities. Int. J. Essent. Oil Ther. 2009;3:56–65. [Google Scholar]
- 34.Prashar A., Hili P., Veness R.G., Evans C.S. Antimicrobial Action of Palmarosa Oil (Cymbopogon martinii) on Saccharomyces cerevisiae. Phytochemistry. 2003;63:569–575. doi: 10.1016/S0031-9422(03)00226-7. [DOI] [PubMed] [Google Scholar]
- 35.Zheljazkov V.D., Astatkie T., Norton U., Jeliazkova E.A. Gypsum and Coal-Bed Methane Water Modify Growth Media Properties, Nutrient Uptake, and Essential Oil Profile of Lemongrass and Palmarosa. Agronomy. 2019;9:282. doi: 10.3390/agronomy9060282. [DOI] [Google Scholar]
- 36.Hong S.-Y., Choi J.-S., Kim S.-M. Herbicidal Activity of Essential Oil from Palmarosa (Cymbopogon martini) Korean J. Weed Sci. 2011;31:96–102. doi: 10.5660/KJWS.2011.31.1.096. [DOI] [Google Scholar]
- 37.da Rocha Neto A.C., Navarro B.B., Canton L., Maraschin M., Di Piero R.M. Antifungal Activity of Palmarosa (Cymbopogon martinii), Tea Tree (Melaleuca alternifolia) and Star Anise (Illicium verum) Essential Oils against Penicillium expansum and Their Mechanisms of Action. LWT. 2019;105:385–392. doi: 10.1016/j.lwt.2019.02.060. [DOI] [Google Scholar]
- 38.Ojha P.K., Poudel D.K., Rokaya A., Satyal R., Setzer W.N., Satyal P. Comparison of Volatile Constituents Present in Commercial and Lab-Distilled Frankincense (Boswellia carteri) Essential Oils for Authentication. Plants. 2022;11:2134. doi: 10.3390/plants11162134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verma R.S., Verma S.K., Tandon S., Padalia R.C., Darokar M.P. Chemical Composition and Antimicrobial Activity of Java Citronella (Cymbopogon winterianus Jowitt Ex Bor) Essential Oil Extracted by Different Methods. J. Essent. Oil Res. 2020;32:449–455. doi: 10.1080/10412905.2020.1787885. [DOI] [Google Scholar]
- 40.Ranitha M., Abdurahman H.N., Ziad A.S., Azhari H.N., Thana Raj S. A Comparative Study of Lemongrass (Cymbopogon citratus) Essential Oil Extracted by Microwave-Assisted Hydrodistillation (MAHD) and Conventional Hydrodistillation (HD) Method. IJCEA. 2014;5:104–108. doi: 10.7763/IJCEA.2014.V5.360. [DOI] [Google Scholar]
- 41.Thakker M.R., Parikh J.K., Desai M.A. Isolation of Essential Oil from the Leaves of Cymbopogon martinii Using Hydrodistillation: Effect on Yield of Essential Oil, Yield of Geraniol and Antimicrobial Activity. J. Essent. Oil Bear. Plants. 2016;19:1943–1956. doi: 10.1080/0972060X.2016.1231087. [DOI] [Google Scholar]
- 42.Satyal P. Ph.D. Thesis. The University of Alabama in Huntsville; Huntsville, AL, USA: 2015. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. [Google Scholar]
- 43.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing; Carol Stream, IL, USA: 2007. [Google Scholar]
- 44.Simic A., Rančic A., Sokovic M.D., Ristic M., Grujic-Jovanovic S., Vukojevic J., Marin P.D. Essential Oil Composition of Cymbopogon winterianus and Carum carvi and Their Antimicrobial Activities. Pharm. Biol. 2008;46:437–441. doi: 10.1080/13880200802055917. [DOI] [Google Scholar]
- 45.Hu H.-L., Zhou D., Wang J.-W., Wu C., Li H.-J., Zhong J., Xiang Z., Sun C. Chemical Composition of Citronella (Cymbopogon winterianus) Leaves Essential Oil and Gastric Toxicity of Its Major Components to Drosophila melanogaster Larvae. J. Essent. Oil Bear. Plants. 2022;25:315–325. doi: 10.1080/0972060X.2022.2077142. [DOI] [Google Scholar]
- 46.Trang D.T., Hoang T.K.V., Nguyen T.T.M., Van Cuong P., Dang N.H., Dang H.D., Nguyen Quang T., Dat N.T. Essential Oils of Lemongrass (Cymbopogon citratus Stapf) Induces Apoptosis and Cell Cycle Arrest in A549 Lung Cancer Cells. BioMed Res. Int. 2020;2020:e5924856. doi: 10.1155/2020/5924856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verma R.S., Singh S., Padalia R.C., Tandon S., Kt V., Chauhan A. Essential Oil Composition of the Sub-Aerial Parts of Eight Species of Cymbopogon (Poaceae) Ind. Crops Prod. 2019;142:111839. doi: 10.1016/j.indcrop.2019.111839. [DOI] [Google Scholar]
- 48.Ojha P.K., Poudel D.K., Dangol S., Rokaya A., Timsina S., Satyal P., Setzer W.N. Volatile Constituent Analysis of Wintergreen Essential Oil and Comparison with Synthetic Methyl Salicylate for Authentication. Plants. 2022;11:1090. doi: 10.3390/plants11081090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cofelice M., Cinelli G., Lopez F., Di Renzo T., Coppola R., Reale A. Alginate-Assisted Lemongrass (Cymbopogon nardus) Essential Oil Dispersions for Antifungal Activity. Foods. 2021;10:1528. doi: 10.3390/foods10071528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phuyal A., Ojha P.K., Guragain B., Chaudhary N.K. Phytochemical Screening, Metal Concentration Determination, Antioxidant Activity, and Antibacterial Evaluation of Drymaria diandra Plant. Beni-Suef Univ. J. Basic Appl. Sci. 2019;8:16. doi: 10.1186/s43088-019-0020-1. [DOI] [Google Scholar]
- 51.Nazzaro F., Fratianni F., Coppola R., De Feo V. Essential Oils and Antifungal Activity. Pharmaceuticals. 2017;10:86. doi: 10.3390/ph10040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nazzaro F., Fratianni F., De Martino L., Coppola R., De Feo V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals. 2013;6:1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar Poudel D., Dangol S., Rokaya A., Maharjan S., Kumar Ojha P., Rana J., Dahal S., Timsina S., Dosoky N.S., Satyal P., et al. Quality Assessment of Zingiber officinale Roscoe Essential Oil from Nepal. Nat. Prod. Commun. 2022;17:1934578X221080322. doi: 10.1177/1934578X221080322. [DOI] [Google Scholar]
- 54.Bhandari D.P., Poudel D.K., Satyal P., Khadayat K., Dhami S., Aryal D., Chaudhary P., Ghimire A., Parajuli N. Volatile Compounds and Antioxidant and Antimicrobial Activities of Selected Citrus Essential Oils Originated from Nepal. Molecules. 2021;26:6683. doi: 10.3390/molecules26216683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poudel D.K., Ojha P.K., Rokaya A., Satyal R., Satyal P., Setzer W.N. Analysis of Volatile Constituents in Curcuma Species, Viz. C. aeruginosa, C. zedoaria, and C. longa, from Nepal. Plants. 2022;11:1932. doi: 10.3390/plants11151932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poudel D.K., Rokaya A., Ojha P.K., Timsina S., Satyal R., Dosoky N.S., Satyal P., Setzer W.N. The Chemical Profiling of Essential Oils from Different Tissues of Cinnamomum camphora L. and Their Antimicrobial Activities. Molecules. 2021;26:5132. doi: 10.3390/molecules26175132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in the article/Supplementary Material are referenced in the article.