Abstract
Murine Lyme borreliosis, caused by infection with the spirochete Borrelia burgdorferi, results in acute arthritis and carditis that regress as a result of B. burgdorferi-specific immune responses. B. burgdorferi-specific antibodies can attenuate arthritis in mice deficient in both B cells and T cells but have no effect on carditis. Because macrophages comprise the principal immune cell in carditis, T-cell responses that augment cell-mediated immunity may be important for carditis regression. To investigate this hypothesis, we examined the course of Lyme carditis in mice selectively deficient in B cells or αβ T cells. Our results show that carditis regresses in B-cell-deficient B10.Ak mice but not in αβ T-cell-deficient mice, independently of the mouse strain background. Despite prominent macrophage infiltrates, hearts from B. burgdorferi-infected αβ T-cell-deficient mice had less mRNA for tumor necrosis factor alpha as measured by reverse transcription-PCR compared to infected control mice. Anti-inflammatory cytokine mRNA levels were equivalent. Adoptive transfer of gamma interferon-secreting CD4+ T cells into infected αβ T-cell-deficient mice promoted carditis resolution. These results show that αβ T cells can promote resolution of murine Lyme carditis and are the first demonstration of a beneficial role for CD4+ T helper 1 cells in this disease.
Lyme borreliosis, due to infection with the spirochete Borrelia burgdorferi, has a variety of clinical manifestations, including arthritis and carditis (12). In murine Lyme borreliosis, these disease manifestations follow a predictable time course, with the most severe inflammatory responses detectable within the first 2 to 4 weeks of infection (9). Thereafter, acute inflammation in joints and hearts regresses even though animals remain persistently infected. Acquired immunity is necessary for disease regression, as shown by the persistence of acute inflammation in both joints and the hearts of infected severe combined immunodeficiency (SCID) mice, which lack T and B cells (10, 32).
B. burgdorferi-specific antibodies are critical for protective immunity and for arthritis modulation (8, 23). Passive immunization of mice with serum from B. burgdorferi-infected mice (immune serum) can prevent experimental infection (5), and administration of immune serum to infected SCID mice causes regression of arthritis (8). However, immune serum does not ameliorate carditis, which remains active in SCID mice. Carditis differs from arthritis in that >90% of the inflammatory infiltrate is comprised of macrophages (4, 30). Adaptive immune responses that promote macrophage activation rather than B-cell effector functions alone may be particularly important for resolving Lyme carditis in mice.
The dominance of B. burgdorferi-specific T helper 1 (Th1) cell responses in disease-susceptible mouse strains (19, 22) and in humans with chronic Lyme arthritis (21, 36) has led to the prevailing notion that T-cell responses that promote cell-mediated immunity are detrimental to the host. Several studies evaluating murine Lyme arthritis challenge this supposition. Pretreatment of mice with monoclonal antibody (MAb) to interleukin-12 (IL-12), a cytokine favoring Th1 cell development, increases the pathogen burden (1), and the absence of the Th1 cell cytokine gamma interferon (IFN-γ) enhances joint swelling (13). Some disease-resistant mouse strains also exhibit a Th1 cytokine profile after B. burgdorferi infection (17). We have shown that reduction in Th2 responses in a resistant mouse strain does not necessarily enhance arthritis severity (33). Moreover, fewer spirochetes can be detected in joints of mice deficient in the anti-inflammatory Th2 cell cytokine IL-10 (14), suggesting that in normal hosts, inflammation may be suppressed at the expense of the spirochete burden. Taken together, these findings suggest that Th1 cell effector functions could have a positive impact on the outcome of B. burgdorferi infection, but no study to date has directly shown this.
Lyme carditis, a macrophage-mediated pathology not directly influenced by B. burgdorferi-specific antibodies, is an especially suitable model for assessing the contribution of Th1 cells to disease regression. In this study, we examined the course of Lyme carditis in mice genetically deficient in B cells and those selectively deficient in αβ T cells. This experimental design allowed us to assess the impact of T-cell immune responses other than those affecting B-cell function, as well as the requirement for αβ T cells, the dominant T-cell population, in carditis regression. Our results confirm that immune serum does not reduce carditis and show that Th1 cells can promote resolution rather than exacerbation of this disease manifestation.
MATERIALS AND METHODS
Spirochetes.
An infectious pathogenic clone of B. burgdorferi strain N40 (cN40) was used in all experiments (6). Frozen aliquots of low-passage cN40 were thawed and grown to log phase in modified Barbour-Stoenner-Kelly (BSK II) medium at 33°C prior to each experiment (3). Spirochetes were visualized to assess viability and counted by dark-field microscopy using a Petroff-Hausser chamber before inoculation into mice.
Mice.
B-cell-deficient, B10.Ak-Igh-6tm1Cgn (μMT) mice were kindly provided by Charles Janeway (Yale University School of Medicine); these mice lack mature B cells due to targeted disruption of the immunoglobulin (Ig) μ heavy-chain gene (20). Age- and sex-matched inbred control B10.A/SGSNJ (B10.Ak) mice were purchased from the Jackson Laboratories (Bar Harbor, Maine). Mice expressing the T-cell receptor (TCR) α−/− mutation on three genetic backgrounds, varying in their susceptibility to Lyme borreliosis, were used: (BALB/c × 129)F1 TCR α−/− intercrossed to homozygosity for the TCR α−/− mutation and heterozygote littermate controls (27), the N6 intercross of (BALB/c × 129)F1 TCR α−/− mice backcrossed six times with disease-susceptible C3H/HeN (C3H) mice, and B6.129S2-TCRαtm1Mom (B6 TCR α−/−) and B6 control mice, purchased from the Jackson Laboratories. Mice were housed in filter frame cages and screened by antibody and PCR to ensure absence of specific pathogens, including mouse hepatitis virus and parvovirus. Except where noted otherwise, all mice were infected at 4 to 5 weeks of age by intradermal inoculation with the indicated dose of cN40 in 100 μl of BSK II medium and sacrificed by carbon dioxide inhalation.
Passive immunization.
Immune mouse serum (IMS) was derived from B10.Ak mice inoculated 30 days previously with 104 cN40. Infection among serum donor mice was confirmed by culture prior to pooling of the sera. B10.Ak-Igh-6tm1Cgn and B10.Ak age-matched mice were passively immunized by subcutaneous injection of 500 μl of a 1:10 dilution of IMS or normal mouse serum (NMS) at days 12, 16, 20, and 23 of infection and then sacrificed for analysis on infection day 28.
Bb-specific IgG ELISA.
Immunoglobulin G (IgG) responses to cN40 lysates were analyzed in serial dilutions of serum specimens from infected mice by standard enzyme-linked immunosorbent assay (ELISA) techniques as previously described (33). Results are reported for a 1:20,000 dilution.
T-cell cytokine analysis.
T cells from infected mice were isolated from pooled lymph node (LN) cells by negative selection using rat anti-CD19 and anti-CD11b MAb, (Pharmingen, San Diego, Calif.) and Biomag goat anti-rat IgG and goat anti-mouse IgM magnetic beads (PerSeptive Biosystems, Framingham, Mass.), as specified by the manufacturer. Purified T cells were then separated into CD4+ and CD8+ populations by negative selection using rat anti-CD8 or rat anti-CD4 MAb (Pharmingen), respectively, and goat anti-rat IgG magnetic beads. The purity of each T-cell subpopulation was >95%, as assessed by flow cytometry. A total of 5 × 106 purified CD4+ and CD8+ T cells were stimulated in triplicate for 72 h with 50 μg of B. burgdorferi sonicate per ml and irradiated splenocytes from uninfected mice as described elsewhere (33). Harvested supernatants were assayed for IFN-γ and IL-4 by a sandwich ELISA as specified by the manufacturer (Pharmingen). Concentrations of cytokines were calculated based on standard curves obtained from serial dilutions of recombinant IFN-γ and IL-4 (Biosource, Camarillo, Calif.) (33).
T-cell adoptive transfer.
CD4+ and CD8+ T-cell subsets were purified by negative selection from the spleens and LNs of B6 mice 14 and 31 days after infection. Then 5 × 106 purified CD4+ or CD8+T cells were injected intravenously into the tail vein of TCR α−/− mice after the establishment of carditis at infection days 14 and 31. At the end of the experimental period, the presence of the transferred population was confirmed by flow cytometry of the splenocytes, and the cytokine production of T cells was assessed as described above.
Histopathology.
Hearts and hindlimb joints (knee and tibiotarsal joints) were immersion fixed in neutral buffered formalin (pH 7.2), demineralized (joints only), and then processed and stained with hematoxylin-eosin by routine histologic techniques (10). Tibiotarsal joints were scored for arthritis severity on a scale of 0 (negative) to 3 (severe), as described elsewhere (7). Carditis (active or chronic) was evaluated in sagittal sections through the heart, which included the aortic valve (2).
RT-PCR of mRNA and densitometry.
mRNA was extracted from heart tissue homogenized in Tri-Reagent (Molecular Research Center, Inc., Cincinnati, Ohio) using the Tri-Reagent RNA isolation kit according to the manufacturer's recommendations. cDNA was synthesized by reverse transcription-PCR (RT-PCR) using random primers (Stratagene, La Jolla, Calif.) in a 50-μl total volume as previously described (25). Tenfold dilutions of cDNA (corresponding to 1, 0.1, and 0.01 μl of the 50-μl reaction volume) were amplified by PCR using oligonucleotide primers for tumor necrosis factor alpha (TNF-α), IFN-γ, transforming growth factor β (TGF-β), IL-4, IL-10, or β2 tubulin at a final concentration of 20 μM each in the presence of 10 mM deoxynucleoside triphosphates. PCR was carried out for 35 cycles, with initial template denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 1.5 min. Amplification was completed by a final incubation at 72°C for 10 min. Primer pairs for IFN-γ (405 bp) and IL-10 (177 bp) were purchased from Stratagene and Biosource (Camarillo, Calif.), respectively. The primer sequences and predicted amplified product sizes for the remaining cytokines were as follows: 5′-TNF-α, 5′-ATGAGCACAGAAAGCATGATC; 3′-TNF-α, 3′-TACAGGCTTGTCACTCGAATT (276 bp); 5′-TGF-β, 5′-CGGGGCGACCTGGGCACCATCCATGAC; 3′-TGF-β, 3′-CTGCTCCACCTTGGGCTTGCGACCCAC (404 bp); 5′-IL-4, 5′-ACGGAGATGGATGTGCCAAACGTC; 3′-IL-4, 3′-GCATCATGCAAATGGATTACTCGT (279 bp); 5′-β2 tubulin, 5′-GGCGCCCTCTGTGTAGTGGCCTTTGGCCCA; and 3′-β2 tubulin, 3′-CAGGCTGGTCAATGTGGCAACCAGATCGGT (300 bp). We analyzed 25 μl of a 100-μl reaction volume by 2% agarose gel electrophoresis for visualization of the expected product (Gibco Laboratories, Grand Island, N.Y.). Images were scanned and quantified by densitometry using Bio-Rad Multi-Analyst software. Bands were outlined by boxes of constant size; a background of one pixel depth outside the perimeter of the sample box was subtracted from the quantified scan (data not shown).
Quantitative B. burgdorferi detection by competitive PCR.
DNA was extracted from preweighed mouse ear samples using a modified Qiamp tissue kit protocol (Qiagen, Chatsworth, Calif.), and spirochete DNA was amplified using a 256-bp region of the B. burgdorferi flagellin gene as described earlier (18). Reaction mixtures contained 100 copies of a 356-bp competitive internal control consisting of a 303-bp segment of Staphylococcus aureus plasmid, pub112, flanked by the B. burgdorferi primer sequences found in the target. In addition, digoxigenin-11–dUTP was used in all reaction mixtures so that amplified products could be quantitated using the PCR ELISA detection kit (Boehringer Mannheim Biochemicals, Indianapolis, Ind.) as described previously (18).
RESULTS
B cells are not required for resolution of Lyme carditis in B10.Ak mice.
B. burgdorferi-infected SCID mice develop arthritis and carditis that persist through at least 60 days of infection. Passive transfer of IMS into infected SCID mice resolves arthritis but not carditis, suggesting that antibody alone is insufficient for carditis resolution. In fact, carditis regresses in infected B10.Ak-Igh-6tm1Cgn mice, which possess immature B cells but are unable to make antibodies (Table 1, panel A). The enhanced arthritis observed in infected B10.Ak-Igh-6tm1Cgn mice was attenuated with passive transfer of IMS (Table 1, panel B, P < 0.05 [Fisher exact test]). However, IMS had no apparent effect on the course of carditis, which remained active at 28 days of infection, even when the infecting spirochete dose was reduced 10-fold from 104 (panel A) to 103 (panel B) and the spirochete burden in ear tissues had been reduced to a level comparable to that of the controls.
TABLE 1.
Attenuation of arthritis, but not carditis, by passive transfer of immune mouse serum into B. burgdorferi- infected B10.Ak-Igh-6tm1Cgn micea
| Panel and mouse group | Mean arthritis severity ± SDb | Carditis prevalence (no. of animals affected/total no. of animals) | Mean no. of spirochetes/μg of tissue ± SDc |
|---|---|---|---|
| Panel A | |||
| B10.Ak-Igh-6tm1Cgn | 1.9 ± 0.6 | 6/6 | ND |
| B10.Ak-Igh-6tm1Cgn | 2.6 ± 0.4 | 0/7 | ND |
| Panel B | |||
| B10.Ak + NMS | 0 | 9/9 | 3 ± 1 |
| B10.Ak-Igh-6tm1Cgn + NMS | 1.2 ± 0.6 | 9/9 | >25 |
| B10.Ak-Igh-6tm1Cgn + IMS | 0.5 ± 0.1d | 11/11e | 4 ± 0 |
Results are representative of two separate passive immunization experiments.
Values reported are the mean of tibiotarsal joint scores from individual mice for each group, as assessed on a scale of 0 to 3.
Values were rounded to the nearest integer; values equivalent to >25 spirochetes were above the cutoff for enumeration. ND, not determined.
Significantly different (P < 0.05, Fisher exact test) from value for B10.Ak-Igh-6tm1Cgn mice treated with NMS.
Carditis in this group was equivalent in severity to that observed in B10.Ak mice and B10.Ak-Igh-6tm1Cgn + NMS.
αβ T cells are required for resolution of carditis in B. burgdorferi-infected mice.
Carditis regression in B-cell-deficient but not SCID mice, which lack both B and T cells, strongly suggests a role for T cells in this process. We therefore examined the evolution of carditis in B. burgdorferi-infected TCR α−/− mice, which lack αβ T cells due to targeted disruption of the TCR α chain. These mice were chosen over mice deficient in all T cells (TCR βδ−/− mice) because they permit assessment of the effects of αβ T cells when potentially regulatory γδ T cells are still present (35). All B. burgdorferi-infected mice developed carditis within 14 days of infection (Table 2), but this disease manifestation remained active only in αβ T-cell-deficient mice, independent of genetic background (Table 2 and Fig. 1A). In contrast, all control mice resolved inflammation by day 45 (Table 2 and Fig. 1B). Although B. burgdorferi-specific IgG titers were lower in TCR α−/− mice compared to control mice, there was no difference in arthritis severity between the TCR α−/− and control mice at 14 days of infection and equal regression of arthritis by 45 days (data not shown).
TABLE 2.
Persistence of carditis in mice deficient in αβ T cells
| Mouse strain | Carditisa at:
|
|
|---|---|---|
| Day 14 | Day 45 | |
| BALB/c × 129 | ||
| TCR α+/− | 5/5 | 0/5 |
| TCR α−/− | 4/4 | 5/5b |
| C3H | ||
| TCR α+/− | 4/4 | 0/3 |
| TCR α−/− | 4/4 | 3/3b |
| B6 | ||
| TCR α+/+ | 4/4 | 0/4 |
| TCR α−/− | 4/4 | 4/4b |
Carditis is reported as the number of mice with active carditis/total number of hearts examined in each group. In some cases, hearts from control and experimental groups were used for other types of analyses and were therefore not available for histopathology. These results are reflective of a total of seven separate experiments.
Differences in carditis prevalence at the 45-day time point were significant (P = 0.0025, Fisher exact test).
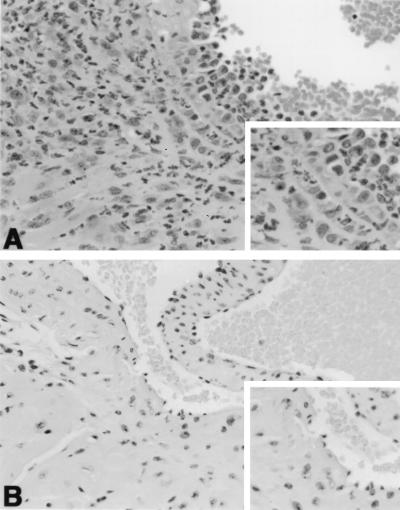
FIG. 1.
Hematoxylin-eosin stain of representative heart tissues. (A) Heart base of a B6 TCR α−/− mouse at 45 days of infection. Note margination of leukocytes along the endothelial surface above the aortic valve, and infiltration of leukocytes in the wall and adventitia of the root of the aorta (upper left and insert) and ventricular myocardium (lower left). (A) Heart base of a B6 mouse at 45 days of infection. There is no inflammation remaining near the aortic valve (valve leaflet in center and insert) or root of the aorta (upper left). Magnification, ×175.
Cytokine production by B. burgdorferi-infected TCR α−/− mice.
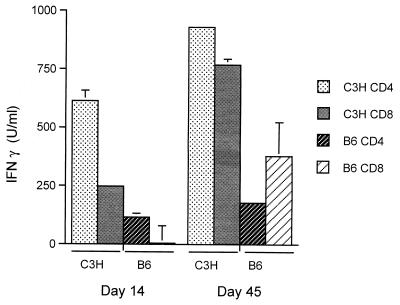
Previous studies have shown that T cells from either C3H or B6 mice produce IFN-γ but not IL-4 after B. burgdorferi infection (17, 22). Purified CD4+ and CD8+ LN T-cell subsets isolated from control C3H mice infected for 14 or 45 days produced IFN-γ (Fig. 2), a result consistent with the known Th1 dominance of this strain (19, 22). While T cells from B6 mice produced less IFN-γ, the pattern of cytokine expression at 14 and 45 days of infection was identical to that of C3H mice (Fig. 2), with no IL-4 detected in culture supernatants of T cells from either mouse strain.
FIG. 2.
CD4+ and CD8+ T cells from B. burgdorferi-infected mice produce IFN-γ after in vitro restimulation. CD4+ and CD8+ T cells were purified by negative selection from regional lymph nodes from infected C3H or B6 mice at the indicated day of infection. Bars represent the mean units of IFN-γ present per milliliter of supernatant of pooled CD4+ or CD8+ T cells stimulated with B. burgdorferi sonicate as determined by ELISA ± the standard error of the mean. Results are representative of three experiments.
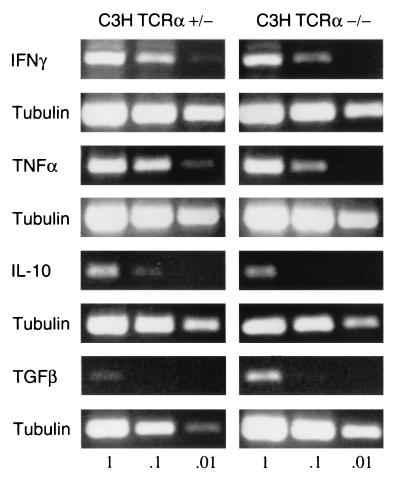
We also analyzed mRNA from 14-day-infected heart tissues to determine whether there were substantial differences in the levels of proinflammatory and anti-inflammatory cytokines in the absence of αβ T cells (Fig. 3). mRNAs for TNF-α, IFN-γ, TGF-β, and IL-10 were present in both infected TCR α−/− and infected control TCR α+/− hearts (Fig. 3). When analyzed by densitometry and adjusted for tubulin amplification, less TNF-α was present in TCR α−/− hearts compared to controls (most notable at the 1:10 dilution of cDNA template). The increase in TGF-β in TCR α−/− hearts suggested visually in Fig. 3 was not substantiated by densitometry. IL-4 mRNA could not be detected in any of the experimental groups.
FIG. 3.
Cytokine production in B. burgdorferi-infected C3H hearts in the absence of αβ T cells. mRNA was extracted from 14-day-infected C3H TCR α−/− and TCR α+/− hearts and cDNAs generated by RT as described in Materials and Methods. Serial tenfold dilutions of the cDNA products were amplified by PCR and sized by agarose gel electrophoresis. Tubulin amplification was performed in parallel for each cytokine examined. These results are representative of three separate analyses of C3H or B6 hearts and show a relative reduction in TNF-α in TCR α−/− hearts compared to controls (confirmed by band densitometry; data not shown).
IFN-γ-producing CD4+ T cells facilitate carditis resolution in B. burgdorferi-infected TCR α−/− mice.
Adoptive transfer experiments were performed to determine the effect of T cells on established carditis in B. burgdorferi-infected B6 TCR α−/− mice (Table 3). Infected TCR α−/− mice received either CD4+ or CD8+ T-cell subsets isolated from wild-type mice infected for a similar duration, commencing on day 14 of infection. These T-cell subsets produced IFN-γ but not IL-4 after in vitro restimulation prior to transfer and when reanalyzed from LN cells retrieved from recipient mice at infection day 41 (Table 3). No exacerbation of arthritis or carditis was noted when T-cell-deficient mice were reconstituted with T-cell subsets. Moreover, recipients of IFN-γ secreting CD4+ T cells had a statistically significant reduction in the prevalence of carditis, with the majority of mice resolving inflammation by the end of the experimental period (Table 3, P = 0.009, Fisher exact test).
TABLE 3.
CD4+ Th1 cells facilitate carditis resolution in TCR α−/− mice
| Mouse group | Carditisa at:
|
Mean B. burgdorferi-specific antibodyb level (OD405) ± SD at day 41 | Mean cytokine concn (U/ml)c ± SD
|
||
|---|---|---|---|---|---|
| Day 14 | Day 41 | IFN-γ | IL-4 | ||
| B6 TCR α+/+ | 10/10 | 0/10 | 0.519 ± 0.077 | 53 ± 15 | ≤0.02 |
| B6 TCR α−/− | 10/10 | 8/8 | 0.239 ± 0.098 | 42 ± 12 | ≤0.02 |
| B6 TCR α−/− + CD4+ T cells | ND | 3/9d | 0.512 ± 0.075 | 222 ± 21 | ≤0.02 |
| B6 TCR α−/− + CD8+ T cells | ND | 8/10 | 0.295 ± 0.050 | 133 ± 13 | ≤0.02 |
Carditis is reported as the number of mice with acute carditis/total number of mice examined. ND, not determined. The results are representative of three experiments.
B. burgdorferi-specific antibodies are reported as the means of values for individual mice in each group.
Cytokine ELISA data reflect the mean of triplicate measurements. The lower detection limits for IFN-γ and IL-4 were 30 and 0.02 U/ml, respectively.
Significantly different (P = 0.009, Fisher exact test) from carditis prevalence in unreconstituted TCR α−/− mice.
DISCUSSION
The dominance of CD4+ Th1 cells in mouse strains susceptible to B. burgdorferi-induced disease and in humans with Lyme arthritis has led to the notion that Th1 cell effector functions impair host control of the pathogen and promote disease (22, 34). Our present study evaluated the role of T cells in resolution of Lyme carditis and provides evidence that CD4+ Th1 cells may have a beneficial effect on this disease manifestation. B. burgdorferi-infected mice deficient in αβ T cells and unable to resolve carditis in comparison to similarly infected immunocompetent mice do resolve this disease manifestation when reconstituted with infection-primed IFN-γ-secreting CD4+ T cells. Although B. burgdorferi-specific antibody is also enhanced in CD4+ T-cell-reconstituted TCR α−/− mice, T cell effects on antibody production alone are unlikely to account for these findings. Immune serum does not resolve carditis in SCID, B-cell-deficient, or αβ T-cell-deficient mice (7, 8, 23). Our results in B10.Ak B-cell-deficient mice confirm these observations and show also that although immune serum can reduce arthritis severity and the number of spirochetes in ear tissues within 2 weeks of administration, carditis regression is not accelerated.
Previous studies in SCID mice (10, 31) and major histocompatibility complex MHC class II-deficient mice lacking CD4+ T cells show that T cells are not required for the development of Lyme carditis, which is composed largely of macrophages (29). However, cardiac infiltrates in immunocompetent mice contain small numbers of CD4+ and CD8+ T cells (29), which likely modulate the activity of macrophages responding to spirochetes. mRNAs for proinflammatory cytokines (IL-1β, TNF-α, and IFN-γ) derived from both macrophages and T cells have been demonstrated in heart tissues within the first week of infection and persist for at least 42 days (15). We found that mRNA for T-cell-associated cytokines was also present in TCR α−/− mice, which possess other cell populations, including natural killer cells and γδ cells that may produce cytokines in this setting. We were unable to detect the Th2-cell cytokine IL-4 by either cytokine ELISA of restimulated draining LN cells or by RT-PCR of heart tissue mRNA. Moreover, equivalent levels of IL-10 mRNA were noted in TCR α−/− and control infected mouse hearts. This observation is consistent with a recent study showing that IL-10 and TGF-β mRNA levels in C3H mouse hearts during peak or resolving carditis are no different than those of uninfected controls (26). Taken together, these findings suggest that other non-Th2 associated T-cell effector functions facilitate carditis resolution. In this regard, the lower level of TNF-α mRNA in infected TCR α−/− mice was particularly noteworthy and unexpected given the degree of carditis observed. Because TNF-α production is subject to feedback regulation, one cannot directly correlate mRNA levels with the observed pathology. However, diminished expression of mRNA for this cytokine could indicate that macrophages are not fully activated in the absence of αβ T cells. Other cell populations that produce IFN-γ, a cytokine promoting macrophage activation, appear unable to compensate for αβ T-cell deficiency. Inefficient macrophage clearance of spirochetes or their antigens within hearts may be one reason carditis remains active through 45 days in TCR α−/− mice. While the experimental conditions of our study delineate a beneficial role for Th1 cells on carditis, these conditions do preclude a role for local production of antibody, which through opsonization of antigens may have an impact on carditis evolution in immunologically intact hosts. Indeed, any immune parameter that assists macrophage function may help eliminate spirochete products and resolve carditis.
The results of our studies, which demonstrate a beneficial role for CD4+ T cells in the host immune response to B. burgdorferi infection, contrast with a recent report describing their potential for causing disease (24). In that study, B. burgdorferi-infected B6 Rag−/− mice reconstituted with naive CD4+ T cells developed severe lung inflammation and myocarditis, two disease manifestations atypical for murine Lyme borreliosis. Naive CD4+ T cells transferred into uninfected Rag−/− mice undergo homeostatic proliferation to fill the available lymphoid compartments, a process that increases the T-cell pool and allows for the expansion of self-reactive T cells (11, 16, 28). The differences between the outcome of T-cell reconstitution in our study using TCR α−/− mice and those using Rag−/− mice can be explained by both homeostatic proliferation of transferred T cells and by the presentation of foreign antigens to fuel the T-cell response. TCR α−/− mice possess B cells and produce low levels of antibodies that control pathogen burden and also modulate disease through the opsonization and clearance of proinflammatory lipoprotein antigens. This critical arm of host defense is absent from Rag−/− mice, which still possess dendritic cells and macrophages that phagocytose and present Borrelia antigens to naive T cells. Naive T cells transferred into infected Rag−/− mice can be readily activated by these professional antigen-presenting cells. In this study, we attempted to preserve the natural evolution of B. burgdorferi infection and host immune response by transferring infection-primed T cells into TCR α−/− mice with established carditis. In this setting, IFN-γ secreting CD4+ T cells did not exacerbate disease but instead promoted the complete resolution of carditis. These results provide the first example of a beneficial role for Th1 cells on the course of Lyme borreliosis.
ACKNOWLEDGMENTS
We thank Jialing Mao, Mary Campbell, Debby Beck, and Gordon Terwilliger for technical assistance and Ruth Montgomery and Joseph Craft for review of the manuscript.
This work was supported by National Institutes of Health grants AR42637, BAA-94-31, AI 45253, AI26815, AI27855, and AR38932; the G. Harold and Leila Y. Mathers Charitable Foundation; the American Heart Association; and the Arthritis Foundation.
REFERENCES
- 1.Anguita J, Persing D H, Rincon M, Barthold S W, Fikrig E. Effect of anti-interleukin 12 treatment on murine Lyme borreliosis. J Clin Investig. 1996;97:1028–1034. doi: 10.1172/JCI118494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong A L, Barthold S W, Persing D H, Beck D S. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am J Trop Med Hyg. 1992;47:249–258. doi: 10.4269/ajtmh.1992.47.249. [DOI] [PubMed] [Google Scholar]
- 3.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 4.Barthold S W, Beck D S, Hansen G M, Terwilliger G A, Moody K D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 5.Barthold S W, Bockenstedt L K. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect Immun. 1993;61:4696–4702. doi: 10.1128/iai.61.11.4696-4702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold S W, de Souza M S, Janotka J L, Smith A L, Persing D H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:419–420. [PMC free article] [PubMed] [Google Scholar]
- 7.Barthold S W, deSouza M, Feng S. Serum-mediated resolution of Lyme arthritis in mice. Lab Investig. 1996;74:57–67. [PubMed] [Google Scholar]
- 8.Barthold S W, Feng S, Bockenstedt L K, Fikrig E, Feen K. Protective and arthritis-resolving activity in serum of mice infected with Borrelia burgdorferi. Clin Infect Dis. 1997;25(Suppl. 1):S9–S17. doi: 10.1086/516166. [DOI] [PubMed] [Google Scholar]
- 9.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease following intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 10.Barthold S W, Sidman C L, Smith A L. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am J Trop Med Hyg. 1992;47:605–613. doi: 10.4269/ajtmh.1992.47.605. [DOI] [PubMed] [Google Scholar]
- 11.Bell E B, Sparshott S M, Drayson M T, Ford W L. The stable and permanent expansion of functional T lymphocytes in athymic nude rats after a single injection of mature T cells. J Immunol. 1987;139:1379–1384. [PubMed] [Google Scholar]
- 12.Bockenstedt L K, Malawista S E. Lyme disease. In: Rich R R, editor. Clinical immunology. St. Louis, Mo: Mosby-Year Book; 1995. pp. 1234–1249. [Google Scholar]
- 13.Brown C R, Reiner S L. Experimental Lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect Immun. 1999;67:3329–3333. doi: 10.1128/iai.67.7.3329-3333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown J P, Zachary J F, Teuscher C, Weis J J, Wooten R M. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect Immun. 1999;67:5142–5150. doi: 10.1128/iai.67.10.5142-5150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle M K, Telford III S R, Criscione L, Lin S R, Spielman A, Gravallese E M. Cytokines in murine Lyme carditis: Th1 cytokine expression follows expression of proinflammatory cytokines in a susceptible mouse strain. J Infect Dis. 1998;177:242–246. doi: 10.1086/517364. [DOI] [PubMed] [Google Scholar]
- 16.Ernst B, Lee D-S, Chang J M, Sprent J, Surh C D. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 17.Ganapamo F, Dennis V A, Philipp M T. Early induction of gamma interferon and interleukin-10 production in draining lymph nodes from mice infected with Borrelia burgdorferi. Infect Immun. 2000;68:7162–7165. doi: 10.1128/iai.68.12.7162-7165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Germer J, Ryckmann B, Moro M, Hofmeister E, Barthold S W, Bockenstedt L, Persing D H. Quantitative detection of Borrelia burgdorferi with a microtiter-based competitive PCR assay. Mol Diagnosis. 1999;4:185–193. doi: 10.1016/s1084-8592(99)80022-8. [DOI] [PubMed] [Google Scholar]
- 19.Kang I, Barthold S W, Persing D H, Bockenstedt L K. T helper cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–3111. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 21.Lengl-Janssen B, Strauss A F, Steere A C, Kamradt T. The T helper cell response in Lyme arthritis: differential recognition of Borrelia burgdorferi outer surface protein A in patients with treatment-resistant or treatment-responsive Lyme arthritis. J Exp Med. 1994;180:2069–2078. doi: 10.1084/jem.180.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matyniak J E, Reiner S L. T helper phenotype and genetic susceptibility in experimental Lyme disease. J Exp Med. 1995;181:1251–1254. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKisic M D, Barthold S W. T-cell-independent responses to Borrelia burgdorferi are critical for protective immunity and resolution of Lyme disease. Infect Immun. 2000;68:5190–5197. doi: 10.1128/iai.68.9.5190-5197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKisic M D, Redmond W L, Barthold S W. T cell-mediated pathology in murine Lyme borreliosis. J Immunol. 2000;165:6096–6099. doi: 10.4049/jimmunol.164.12.6096. [DOI] [PubMed] [Google Scholar]
- 25.Montgomery R R, Malawista S E, Feen K J M, Bockenstedt L K. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: Exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–270. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montgomery, R. R., X.-M. Wang, and S. E. Malawista. 2001. Murine Lyme disease: no evidence for active immune downregulation in resolving or subclinical infection. J. Infect. Dis., in press. [DOI] [PubMed]
- 27.Philpott K L, Viney J L, Kay G, Rastan S, Gardiner E M, Chae S, Hayday A C, Owen M J. Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science. 1992;256:1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 28.Rocha B, Dautigny N, Pereira P. Peripheral T lymphocytes: expansion potential and homeostatic regulation of pool sizes and CD4/8 ratios in vivo. Eur J Immunol. 1989;19:905–911. doi: 10.1002/eji.1830190518. [DOI] [PubMed] [Google Scholar]
- 29.Ruderman E M, Kerr J S, Telford III S R, Spielman A, Glimcher L H, Gravallese E M. Early murine Lyme carditis has a macrophage predominance and is independent of major histocompatibility complex class II-CD4+ T cell interactions. J Infect Dis. 1995;171:362–370. doi: 10.1093/infdis/171.2.362. [DOI] [PubMed] [Google Scholar]
- 30.Ruderman E M, Kerr J S, Telford III S R, Spielman A, Glimcher L H, Gravallese E M. Early murine Lyme carditis is macrophage-mediated and does not require Class II MHC antigen. Arth Rheum. 1993;36:S42. [Google Scholar]
- 31.Schaible U E, Kramer M D, Museteanu C, Zimmer G, Mossman H, Simon M M. The severe combined immunodeficiency (scid) mouse: a laboratory model for the analysis of Lyme arthritis and carditis. J Exp Med. 1989;170:1427–1432. doi: 10.1084/jem.170.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaible U E, Gay S, Museteanu C, Kramer M D, Zimmer G, Eichmann K, Museteanu U, Simon M M. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manisfests predominantly in the joints, heart, and liver. Am J Pathol. 1990;137:811–820. [PMC free article] [PubMed] [Google Scholar]
- 33.Shanafelt M-C, Kang I, Barthold S W, Bockenstedt L K. Modulation of murine Lyme borreliosis by interruption of the B7/CD28 T cell costimulatory pathway. Infect Immun. 1998;66:266–271. doi: 10.1128/iai.66.1.266-271.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sigal L H. Lyme disease: a review of aspects of its immunology and immunopathogenesis. Annu Rev Immunol. 1997;15:63–92. doi: 10.1146/annurev.immunol.15.1.63. [DOI] [PubMed] [Google Scholar]
- 35.Vincent M S, Roessner K, Lynch D, Wilson D, Cooper S M, Tschopp J, Sigal L H, Budd R C. Apoptosis of Fashigh CD4+ synovial T cells by Borrelia-reactive Fas-ligandhigh γδ T cells in Lyme arthritis. J Exp Med. 1996;184:2109–17. doi: 10.1084/jem.184.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yssel H, Shanafelt M D, Sodenberg C, Schneider R, Peltz G. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J Exp Med. 1991;174:593–601. doi: 10.1084/jem.174.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]