Abstract
The interaction of the innate immune system with the microbial world involves primarily two sets of molecules generally known as microbial pattern recognition receptors and microbial pattern recognition molecules, respectively. Examples of the former are the Toll receptors present particularly in macrophages and dendritic cells. Conversely, the microbial pattern recognition molecules are conserved protist homopolymers, such as bacterial lipopolysaccharides, lipoteichoic acids, peptidoglycans, glucans, mannans, unmethylated bacterial DNA, and double-strand viral RNA. However, for protists that lack most of these molecules, such as protozoans, the innate immune system must have evolved receptors that recognize other groups of microbial molecules. Here we present evidence that a highly purified protein encoded by a Leishmania brasiliensis gene may be one such molecule. This recombinant leishmanial molecule, a homologue of eukaryotic ribosomal elongation and initiation factor 4a (LeIF), strongly stimulates spleen cells from severe combined immunodeficient (SCID) mice to produce interleukin-12 (IL-12), IL-18, and high levels of gamma interferon. In addition, LeIF potentiates the cytotoxic activity of the NK cells of these animals. Because LeIF is a conserved molecule and because SCID mice lack T and B lymphocytes but have a normal innate immune system (normal reticuloendothelial system and NK cells), these results suggest that proteins may also be included as microbial pattern recognition molecules. The nature of the receptor involved in this innate recognition is unknown. However, it is possible to exclude the Toll receptor Tlr4 as a putative LeIF receptor because the gene encoding this receptor is defective in C3H/HeJ mice, the mouse strain used in the present studies.
Stimulation of the immune system by most microorganisms is usually carried out by molecules that interact with two distinct sets of host recognition molecules, namely, the microbial pattern recognition receptors and the antigen receptors of T and B lymphocytes (6, 7). These receptors are responsible for the initiation and expression of the innate and adaptive immune responses, respectively. Microbial pattern recognition receptors are germ line encoded, and it is estimated that their repertoire is restricted to a few hundred different receptors (12). In contrast, T and B lymphocyte antigen receptors are generated by somatic genetic mechanisms and their repertoires are on the order of approximately 1015 different specificities (2).
In general, the immune response is initiated by the recognition of the microbial pattern recognition molecules by host cells. This results in activation of the host cells to both produce several different cytokines involved in the inflammatory reaction and present antigenic epitopes to the highly specialized cognitive elements of the immune system. While the latter elements can recognize practically all sorts of foreign organic molecules, in particular, carbohydrates and proteins, the elements involved in the pattern recognition molecules are not as diversified. It is believed that the recognition repertoire of this system is limited to bacterial lipopolysaccharides (LPS), lipoteichoic acids, peptidoglycans, glucans, mannans, bacterial DNA, and double-stranded viral RNA (12). Although these molecules are chemically distinct from one another, they have some properties in common; e.g., they all are homopolymers, and with the exception of the nucleic acids, they are integral components of the bacterial and fungal cell walls and are therefore limited to these organisms of the microbial world. In addition, the immune-regulatory properties of nucleic acids are primarily associated with bacterial and viral DNA and RNA, respectively.
However, for microbes, such as protozoans, that lack cell walls and have nucleic acids that are weaker stimulatory molecules than the bacterial and viral counterparts (21), the vertebrate hosts must have evolved and developed receptor molecules that are capable of recognizing different microbial molecules to alert the innate immune system to subsequently initiate the specific immune response.
We have recently shown that a protozoan (Leishmania brasiliensis) gene homologous to eukaryotic ribosomal elongation and initiation factor 4A encodes a protein designated LeIF that stimulates peripheral blood mononuclear cells (PBMC) from both leishmaniasis patients and uninfected individuals (22, 23). Stimulation of these cells, as well as normal human monocyte-derived macrophages and dendritic cells, with LeIF resulted in the production of large quantities of gamma interferon (IFN-γ) and interleukin-12 (IL-12) p40 and p70 (18). Therefore, LeIF could be a microbial protein recognized by the elements of the innate immune system. However, because in these experiments we could not rule out the possibility that LeIF was recognized by contaminating T cells, i.e., by cognitive receptors, it became important to test if LeIF would stimulate the innate components of immunity in the absence of the cognitive elements of the immune system.
To investigate this possibility, we used severe combined immunodeficient (SCID) mouse spleen cells because these mice lack mature T and B lymphocytes but have functional macrophages and NK cells, essential components of the innate immune system. The results confirm and expand our former observation, in that LeIF activates macrophages to produce IL-12 and IL-18 and NK cells to produce IFN-γ.
MATERIALS AND METHODS
Animals.
SCID mice in the LPS low-responder genetic background (C3H/HeJ) aged 6 to 8 weeks were obtained from The Jackson Laboratory and maintained at our facilities in specific-pathogen-free condition.
Reagents.
Recombinant mouse IL-12 and polyclonal sheep anti-murine IL-12 were kindly supplied by the Immunology Department of the Genetic Institute (Cambridge, Mass.). Recombinant human IL-15 and IL-2, murine granulocyte-macrophage colony-stimulating factor, and IL-4 were provided by Immunex Corp. (Seattle, Wash.). Recombinant mouse IL-5 and IFN-γ were from Pharmingen (San Diego, Calif.), and recombinant IL-18 was purchased from Pepro Tech, Inc. (Rocky Hill, N.J.). Purified anti-mouse IL-5 and IFN-γ monoclonal antibodies (MAbs) were from Pharmingen. Rabbit anti-asialo GM1 antiserum was purchased from Wako Chemicals (Dallas, Tex.). Purified anti-tumor necrosis factor alpha, anti-mouse CD11b (Mac-1), anti-CD80 (B7.1), anti-CD86 (B7.2), and anti-mouse CD32/16 were purchased from Pharmingen. LPS was purchased from Sigma Chemical Co. (St. Louis, Mo.). LeIF was prepared as previously described (22), followed by reverse-phase high-pressure liquid chromatography using a gradient of acetonitrile in water (both eluants contained 0.05% trifluoroacetic acid). Pure fractions were pooled and lyophilized before being redissolved in Tris buffer (10 mM, pH 8.0) prior to use. Figure 1 illustrates the high degree of purity of the protein used in these studies. Purified leishmanial recombinant proteins 8E and Ldp23 and mycobacterial recombinant proteins TbH9 and TbRa1 were provided by Corixa Corporation (Seattle, Wash.). All recombinant proteins contained less than 10 ng of endotoxin per mg of protein, as determined by the Limulus amoebocytelysate (LAL) assay.
FIG. 1.
High-pressure liquid chromatography profile of purified LeIF. Au, arbitrary units.
Cell preparation.
Spleens from C3H/HeJ SCID mice were removed aseptically and dissociated in RPMI medium containing 10% fetal bovine serum, 50 μM 2 β-mercaptoethanol, and 50 μg of gentamicin per ml. Single-cell suspensions were prepared after lyses of red blood cells with ammonium chloride. After two washes, the cells were counted and plated in 96-well flat-bottom plates at a density of 2 × 105/100 μl of RPMI 1640 medium. Bone marrow-derived macrophages were obtained from the bone marrow of C3H/HeJ SCID mice by Ficoll gradient, washed twice with RPMI 1640 medium, and plated in six-well plates at a density of 5 × 106 to 8 × 106 cells per well in 5 ml of RPMI 1640 medium. After 2 h at 37°C, the nonadherent cells were removed and the cultures were submitted to another overnight adherent cycle of purification. The adherent cells were cultured in RPMI 1640 medium plus 20 ng each of recombinant murine granulocyte-macrophage colony-stimulating factor and recombinant murine IL-4 per ml. Every 3 days, the cells were fed with fresh supplemented medium. After 8 to 12 days, the cells were harvested for assay. The cells were analyzed by FACScan cytoflurometer using the Cell Quest software (Becton Dickinson) and shown to be 98% MAC-1-positive cells.
IL-15-activated NK cells (LAK) were obtained from SCID mouse spleen cells after lysis of red blood cells and cultured at a density of 4 × 106 to 8 × 106 cells per well in six-well plates in a final volume of 5 ml of RPMI 1640 medium supplemented with 1% (vol/vol) nonessential amino acids (Gibco) plus 300 ng of human IL-15 per ml. After 5 days in culture, the cells were washed and purified in two cycles over Sephadex G-10 columns to remove adherent cells. LAK cells were then cultured for additional times varying from 7 to 40 days before they were assayed. A fluorescence-activated cell sorter (FACS) analysis showed that these populations were CD14−, B7.1/B7.2−, and asialo-GM+.
Cytotoxicity assays.
The specific cytotoxic activity of activated spleen cells against 51Cr-labeled YAC-1 target cells was measured in a standard 4-h chromium release assay at several effector-to-target ratios. Percent specific 51Cr release was calculated by using the following formula: (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100. Spontaneous release was obtained from target cells in the absence of effector cells, and maximum release was obtained by lysis of target cells with Triton X-100. The results are expressed as the mean of triplicate wells of one representative experiment from at least three identical experiments.
Cytofluorometric analysis.
The expression of cell surface markers was performed by standard procedures on a FACSCalibur (Becton Dickinson). The following fluorescein isothiocyanate-labeled antibodies, purchased from Pharmingen, were used for staining: anti-CD11b (MAC-1), anti-CD80 (B7-1), anti-CD86 (B7-2), and anti-CD14. Purified anti-mouse CD32/CD16 was used to block the nonspecific binding of MAbs to Fc receptors. For NK cell staining, spleen cells or activated NK cells were incubated with a 1/250 dilution of polyclonal rabbit anti-asialo-GM1 serum for 30 min at 4°C. Background fluorescence was assessed by using normal rabbit serum. Cells were washed and then incubated with biotinylated anti-rat immunoglobulin G, followed by incubation with phycoerythrin-conjugated streptavidin for 30 min at 4°C. Stained cells were analyzed by FACS.
ELISA.
The two-site enzyme-linked immunosorbent assay (ELISA) was employed to assay the levels of IFN-γ, IL-5, and IL-12. Briefly, 96-well plates were coated overnight at 4°C with 2 μg of either anti-murine IL-5 (TRFK5), IL-12 (C.17.15.10.12), or anti-IFN-γ (R4-6A2) MAbs per ml in bicarbonate buffer, washed with phosphate-buffered saline–0.05% Tween 20 (Sigma Chemical Co., St. Louis, Mo.), and blocked with phosphate-buffered saline–1% bovine serum albumin for 2 h. After washing, samples were added and incubated overnight at 4°C. Plates were washed, followed by the addition of biotinylated anti-mouse IL-5 (TRFK4), anti-mouse IL-12 (C.15.6.7.6), or anti-mouse IFN-γ (XMG1.2) MAbs at 0.5 mg/ml, and incubated for 2 h at room temperature. The plates were washed and incubated for an additional 1 h with streptavidin-peroxidase. After the last washing, the reactions were developed with tetramethylbenzidine (TMB) substrate and read at 450 nm.
RESULTS
IFN-γ production by SCID spleen cells stimulated with LeIF.
We have previously shown that PBMC from leishmaniasis patients and normal donors produce IFN-γ in response to LeIF (22). However, the mechanism by which LeIF elicits this IFN-γ production was not systematically investigated. Three types of cells produce IFN-γ, CD4+ Th1 lymphocytes, CD8+ T lymphocytes, and NK cells. To investigate the requirement, or lack thereof, of T-cell recognition for the production of IFN-γ by normal cells stimulated with LeIF, spleen cells from T-cell deficient SCID mice were used. SCID mice lack mature T and B cells, and their spleens are highly enriched with NK cells (3, 4, 8, 11). Therefore, this is an ideal system with which to test the action of LeIF in the absence of specific immune recognition. Cells were activated with different concentrations of LeIF for 48 to 72 h, and the cytokine concentration was analyzed in the culture supernatants. The results shown in Fig. 2A indicate that LeIF stimulates resting NK cells in a dose-dependent manner for the production of high levels of IFN-γ. This effect was not due to LPS contamination because the LeIF preparations used in these studies contained less than 10 ng of endotoxin per mg of protein (LAL assay). In addition, the LPS inhibitor polymyxin B had little or no effect on LeIF-induced IFN-γ production by SCID spleen cells. In contrast, polymyxin B inhibited LPS-induced IFN-γ production by these cells by >90% (Fig. 2B). Moreover, treatment of LeIF with proteinase K totally abolished its activity (data not shown).
FIG. 2.
IFN-γ production by SCID C3H/HeJ mouse spleen cells induced by LeIF. (A) Spleen cells (2 × 105/100 μl) were stimulated with different concentrations of LeIF and incubated for 48 h. Supernatants were harvested and tested by ELISA for the presence of IFN-γ. (B) Spleen cells were incubated with LeIF (10 μg/ml) or LPS (1 μg/ml) with or without polymyxin B (10 μg/ml), and supernatants were harvested after 48 h and assayed for the presence of IFN-γ. Results represent the arithmetic mean of duplicate culture supernatants in one of five experiments with essentially the same results.
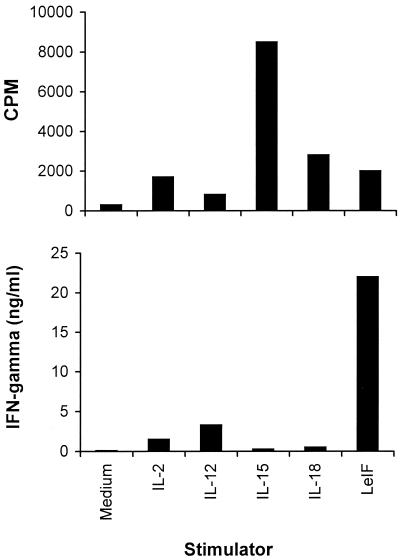
Compared with cytokines that typically induce IFN-γ production by NK cells, such as IL-12 and IL-18, LeIF induced greater quantities of IFN-γ than either IL-12 or IL-18, even when these inflammatory cytokines were used at high concentrations (10 U/ml and 100 ng/ml, respectively). In addition, IL-15, which is a cytokine that stimulates NK cells to proliferate via components of IL-2R, did not stimulate SCID mouse spleen cells to produce IFN-γ. Interestingly, LeIF induced high levels of IFN-γ production in the absence of a significant proliferative response of the SCID spleen cells (Fig. 3).
FIG. 3.
IFN-γ production by spleen cells of SCID C3H/HeJ mice stimulated with LeIF or cytokines is independent of cell proliferation. Spleen cells (2 × 105/100 μl) were incubated for 48 h in the presence or absence of 10 μg of LeIF per ml or with the optimal concentration of the cytokine IL-12 (10 U/ml), IL-15 (100 ng/ml), or IL-18 (100 ng/ml). Supernatants were harvested and tested by ELISA for the presence of IFN-γ (bottom). Cell proliferation was measured by incorporation of [3H]thymidine (top). Results represent the arithmetic mean of duplicate culture supernatants in one of three experiments with essentially the same results.
Because the recombinant LeIF used in these studies has a His tag (six histidines) in its N terminus, it became important to determine whether the stimulation of NK cells for the production of IFN-γ was due to LeIF or if the His tag portion of the recombinant protein induced this activity. To test this possibility, several recombinant proteins (with a His tag in the N terminus) from different origins, including two proteins from other species of Leishmania and two from Mycobacterium tuberculosis, were compared with LeIF. The results indicate that LeIF was the only microbial recombinant protein to induce IFN-γ production in SCID spleen cells. None of the other His tag recombinant protein tested stimulated these cells (data not shown).
Characterization of SCID spleen cells that interact with LeIF for production of IFN-γ.
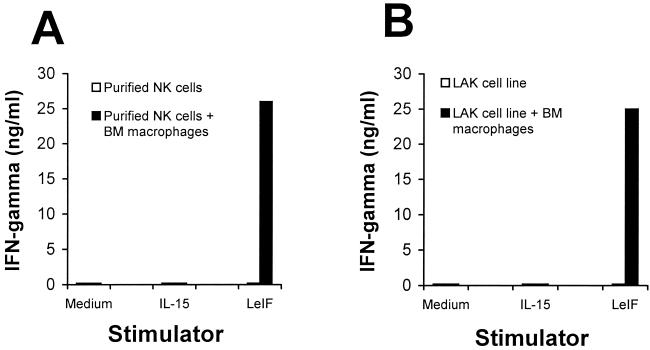
Because the only cell population capable of producing IFN-γ in the SCID mouse spleen is NK cells, it became interesting to determine if LeIF stimulates these cells directly or indirectly, for example, via cytokines produced by activated macrophages. To investigate this possibility, a direct approach was initially used. NK cells were purified from SCID spleen cells after removal of macrophages and other adherent cells by two passages over Sephadex G-10 columns. The purified (enriched) NK cells were stimulated with either LeIF or IL-15. No IFN-γ could be detected in the supernatant of these cultures (Fig 4A). However, excellent proliferation was obtained when these cells were stimulated with IL-15, thus confirming their viability after the two passages over Sephadex G-10 columns (data not shown). To dissect further the cells involved in the induction by LeIF of IFN-γ production by SCID spleen cells, a lymphokine-activated NK cell line (LAK) devoid of antigen-presenting cells was generated. This LAK cell line was obtained after several cycles of stimulation of macrophage-depleted (with Sephadex G-10 column) SCID spleen cells with IL-15. FACS analysis revealed the following cell surface pattern: CD14−, B7.1/B7.2−, and asialo-GM1+ (data not shown). Stimulation of these highly purified NK cells with LeIF again resulted in no production of IFN-γ. However, when bone marrow macrophages were added to the purified NK or to the LAK cells, great quantities of this cytokine were produced by both cell populations (Fig. 4). No IFN-γ was detected in the culture supernatants of bone marrow macrophages alone either incubated with medium or in the presence of LeIF (data not shown). These results indicate first that LeIF does not act directly on the NK cells and second that its ability to stimulate IFN-γ production by these cells is dependent on antigen-presenting cells.
FIG. 4.
LeIF induction of IFN-γ production by spleen cells of SCID C3H/HeJ mice is macrophage dependent. (A) Macrophage-depleted (Sephadex G-10 adherence) SCID mouse spleen cells (2 × 105/100 μl) were cultured in the presence or absence of LeIF (10 μg/ml) for 72 h and assayed for IFN-γ production. (B) SCID mouse bone marrow (BM)-derived macrophages restore the ability of purified NK or LAK cells to produce IFN-γ. In panels A and B, the viability of NK and LAK cells was confirmed by proliferation upon stimulation with IL-15 (data not shown). Results represent the arithmetic mean of duplicate culture supernatants in one of two experiments with essentially the same results.
Characterization of the cytokines involved in the LeIF-stimulated macrophages necessary for IFN-γ production by NK cells.
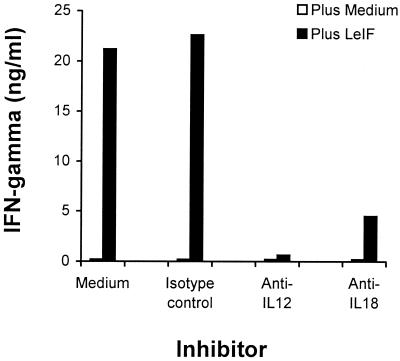
One of the most intriguing characteristics of LeIF is its strong ability to induce both human PBMC and PBMC-derived macrophages to produce IL-12 (18, 22). Because this cytokine and IL-18, another macrophage-derived cytokine, are actively involved in the activation of both T cells and NK cells for the production of IFN-γ (13, 15, 25), we investigated the participation of these cytokines in IFN-γ production by SCID mouse spleen cells stimulated with LeIF. Cells were stimulated with LeIF in the presence or in the absence of rabbit anti-IL-12 or rabbit anti-IL-18 antibody for 72 h. Supernatants were harvested and analyzed for the presence of IFN-γ. Figure 5 clearly shows that both antisera inhibited IFN-γ production by LeIF-stimulated SCID mouse spleen cells. Because IL-12 acts synergistically with IL-18 to induce IFN-γ production by T cells (10, 13, 24), these results strongly suggest that the potent activation of SCID mouse spleen cells by LeIF for the production of IFN-γ is caused by the activation of macrophages and/or dendritic cells to secrete both IL-1 and IL-18.
FIG. 5.
LeIF induction of IFN-γ production by spleen cells of SCID C3H/HeJ mice is IL-12 and IL-18 dependent. Spleen cells (2 × 105/100 μl) were incubated for 48 h with LeIF (10 μg/ml) in the presence or absence of a 1/500 dilution of either anti-IL-12 or anti IL-18 antibody or with a 1/500 dilution of an isotype-matched control normal immunoglobulin. Supernatants were collected and tested by ELISA for IFN-γ production. Results represent the arithmetic mean of duplicate culture supernatants in one of two experiments with essentially the same results.
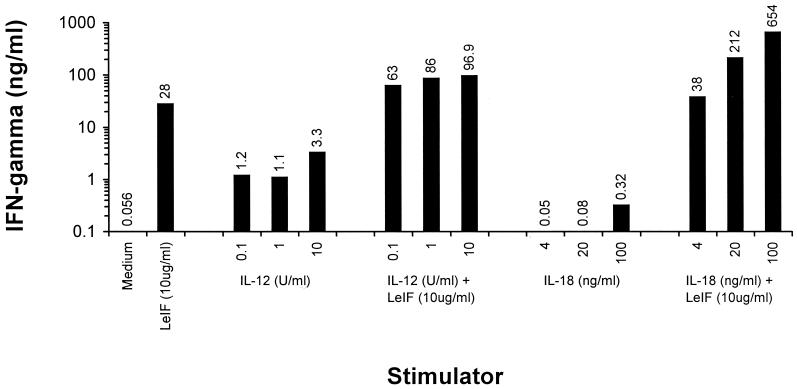
This synergistic effect was further confirmed by experiments in which recombinant IL-12 or IL-18 was added to LeIF-stimulated SCID spleen cells. Stimulation of these cells with 0.1, 1, and 10 U of IL-12 per ml resulted in the production of 1.2, 1.1, and 3.3 ng of IFN-γ per ml, respectively. In contrast, the addition of 10 μg of LeIF per ml to these cultures resulted in the production of 63, 86, and 96.9 ng of IFN-γ per ml, respectively (Fig. 6). The same pattern of synergistic response became even more evident when exogenous IL-18 was used instead of IL-12. When the intrinsic ability of IL-18 alone to induce the production of IFN-γ was compared with that of its combination with LeIF, the results were striking. Undetectable or little IFN-γ production (less than 1 ng/ml) was obtained when SCID spleen cell cultures are stimulated with 4 to 100 ng of IL-18 per ml alone. In contrast, up 654 ng of IFN-γ per ml was obtained when 10 μg of LeIF per ml was added to these cultures (Fig. 6).
FIG. 6.
LeIF synergizes with IL-12 and IL-18 to induce IFN-γ production by spleen cells of SCID C3H/HeJ mice. Spleen cells (2 × 105/100 μl) were incubated for 48 h with LeIF (10 μg/ml) in the presence or absence of various concentrations of either IL-12 or IL-18. Supernatants were harvested and tested by ELISA for IFN-γ production. Numbers above the bars represent the actual concentrations of INF-γ for the different conditions. Results represent the arithmetic mean of duplicate culture supernatants in one of two experiments with essentially the same results.
Stimulation of NK cell cytotoxicity by LeIF.
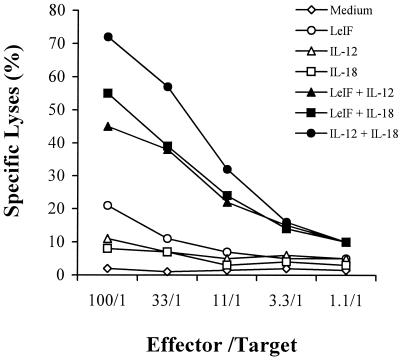
Because LeIF-stimulated SCID spleen cells result in IL-12- and IL-18-dependent production of high levels of IFN-γ by NK cells, we next investigate if this stimulation would also result in the activation of the cytolytic pathway of these cells. In these experiments, SCID spleen cells were stimulated for 24 h with LeIF alone or in combination with either IL-12 or IL-18. NK cell cytolytic activity was measured by a standard 51Cr release assay using YAC-1 cells as targets. Figure 7 illustrates the results. As can be seen, LeIF alone caused an increase in the cytolytic activity of the NK cells. In contrast, little or no killing activity was observed when NK cells were stimulated with either IL-12 or IL-18 alone. However, an excellent synergistic effect was observed when these two cytokines were used to stimulate NK cells. Moreover, LeIF could substitute for either one of these cytokines to achieve strong activation of killing activity by NK cells. These results confirm that, like the activation of NK cells for IFN-γ production, LeIF induces the lytic activity of these cells indirectly, i.e., via the stimulation of both IL-12 and IL-18 by SCID mouse spleen cells.
FIG. 7.
LeIF synergizes with IL-12 and IL-18 to induce NK cell killing activity by spleen cells of SCID C3H/HeJ mice. Spleen cells (2 × 105/100 μl) were incubated for 24 h with LeIF (10 μg/ml) in the presence or absence of various concentrations of either IL-12 or IL-18. In addition, cells were incubated with LeIF alone, IL-12 alone, IL-18 alone, or only medium. Cells were washed and incubated at various effector-to-target ratios with YAC-1 cells (5 × 103/well) previously labeled with 51Cr for 30 min at 37°C. NK cell killing activity was measured after 4 h by 51Cr release from YAC-1 target cells. Percent specific lysis was calculated as described in Materials and Methods. The results are those of one representative experiment of three experiments with essentially the same results.
Stimulation of SCID mice by LeIF leads to selective activation of a distinct subset of NK cells.
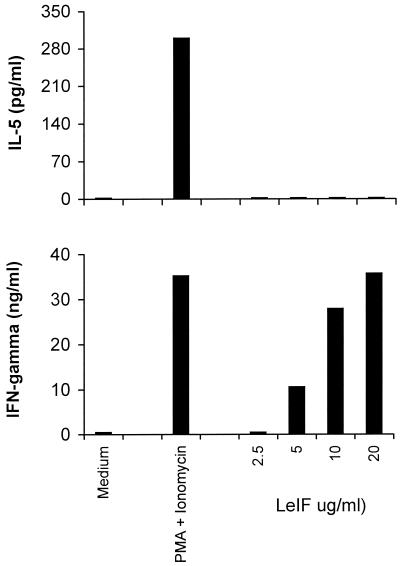
Stimulation of lymphoid cells from both leishmania-infected humans and mice with LeIF results in the preferential production of Th1 cytokines (18, 22, 23). In addition, LeIF stimulates normal human monocyte-derived cells to produce IL-12. In order to investigate the pattern of cytokine stimulation by LeIF in the absence of T-cell recognition, SCID mouse spleen cells were stimulated with LeIF and the production of both IFN-γ and IL-5 in the culture supernatants was determined by ELISA. IFN-γ and IL-5 have been shown to be produced by distinct subsets of NK cells, namely, NK1 and NK2, respectively (16). Because IL-5 is a Th2 cytokine, it is believed that activation of NK2 cells could be associated with the Th2 pattern of immune response during the innate phase of activation of the immune system. The results shown in Fig. 8 clearly point to the production of only IFN-γ by SCID spleen cells stimulated with various concentrations of LeIF. No IL-5 could be detected in the supernatants of these cells. In contrast, when the spleen cells were stimulated with phorbol myristate acetate (PMA) and ionomycin, both IFN-γ and IL-5 were produced, indicating that both the NK1 and NK2 cell subsets were activated after polyclonal stimulation.
FIG. 8.
Selective Th1 cytokine production by spleen cells of SCID C3H/HeJ mice stimulated with LeIF. Spleen cells (2 × 105/100 μl) were stimulated with different concentrations of LeIF and incubated for 48 h. As a control, cells were stimulated with 2 ng of PMA per ml plus 2 ng of ionomycin per ml. Supernatants were harvested and tested by ELISA for the presence of IFN-γ and IL-5. Results represent the arithmetic mean of duplicate culture supernatants in one of two experiments with essentially the same results.
DISCUSSION
The innate immune system is activated by several microbial homopolymers usually associated with microorganisms' cell walls. In addition, bacterial DNA and double-stranded viral RNA are also potent activators of this system. These microbial homopolymers have been generally referred to as pathogen-associated molecular patterns and are widely distributed in the protist kingdom. However, higher protists, such as protozoans, lack cell walls and their nucleic acids activate the immune system to a lesser extent than do bacterial DNA and viral RNA (21). Therefore, recognition of these organisms by the host innate immune system must be done by alternative stimulating molecules.
LeIF, a recombinant protein encoded by an L. brasiliensis gene, may be one such molecule. The gene that encodes it is present in all members of the Leishmania genus thus far analyzed, and the protein is expressed in both the promastigote and amastigote forms of these parasites. LeIF induces human PBMC from either leishmaniasis patients or normal individuals to produce IL-12 and IFN-γ production (22). In addition, LeIF induces the production of IL-12 by monocyte-derived macrophages and dendritic cells (18). Therefore, LeIF could be a microbial molecule capable of alerting the innate immune system to initiate an immune response.
In the present study, we investigated the ability of LeIF to activate the innate immune system under a stringent condition, i.e., in the total absence of the cognitive elements of the immune system. These studies were carried out with cells from SCID mice because these animals lack mature T and B cells and their spleens have a normal reticuloendothelial system and are highly enriched with NK cells (3, 4, 8, 11). They are therefore ideal for investigation of the activation of the noncognitive elements of the immune system. Our results demonstrated that naive SCID mouse spleen cells stimulated with LeIF produced IL-12, IL-18, and high levels of IFN-γ in a dose-dependent manner. This response was clearly not due to possible contamination of LeIF with LPS because this activity was resistant to polymyxin B and because the levels of LPS present in the preparation of LeIF used in these studies were below the sensitivity of the LAL assay.
Compared with other recombinant proteins from leishmania or M. tuberculosis, LeIF was revealed to be a unique microbial protein that is able to induce NK cells to produce IFN-γ because only LeIF, and none of the proteins tested, stimulated NK cells.
Studies designed to elucidate the mechanisms of action of LeIF indicated that this molecule stimulates NK cells indirectly. This conclusion was drawn based on the following experiments: First, removal of macrophages from SCID mouse spleen cells totally abrogated the ability of LeIF to stimulate the macrophage-depleted cells to produce IFN-γ. Likewise, macrophage-free NK cell lines generated from SCID mouse spleen cells by using IL-15 did not produce IFN-γ upon stimulation with LeIF or IL-15, despite extensive proliferation in response to the latter stimulus. However, reconstitution of these cells with bone marrow-derived macrophages completely restored the ability of these cells to produce large quantities of IFN-γ upon stimulation with LeIF.
These experiments also led to the characterization of the molecular events triggered by LeIF for the activation of NK cells. Because we have shown earlier (18, 22, 23) that LeIF stimulates human macrophages and dendritic cells to produce IL-12 and IL-18, these cytokines became prime candidates to explain the LeIF stimulation of murine NK cells to produce IFN-γ. Indeed, inhibition experiments using either anti-IL-12 or IL-18 antibodies clearly demonstrated that both cytokine are responsible for the LeIF activation of SCID mouse spleen cells for the production of IFN-γ. Moreover, it appears that LeIF stimulates the right balance and physiological levels of these cytokines by macrophages. This possibility is supported by the experiments designed to evaluate the synergism between these two cytokines. These experiments revealed that the levels of added exogenous IL-12 or IL-18 necessary for optimal production of IFN-γ by SCID spleen cells were much higher than the levels of both IL-12 and IL-18 produced by the SCID macrophages upon stimulation with LeIF. Curiously, the levels of IL-12 and IL-18 induced by LeIF in the culture supernatants were below the sensitivity of the ELISA used in these studies. Only mRNAs for both cytokines could be detected in LeIF-stimulated cells (data not shown).
One possible explanation for these findings is that LeIF, in addition to inducing the production of the cytokines themselves, also induces up-regulation of the IL-12 and IL-18 receptors on NK cells. This possibility is supported by the experiments that showed that addition of either exogenous IL-12 or exogenous IL-18 to SCID mouse spleen cells stimulated with LeIF resulted in an exceptional synergistic effect in the form of a level of IFN-γ production not achieved by the addition of the two cytokines together in the absence of LeIF. Experiments to address this possibility are in progress. Alternatively, it is possible that LeIF induces other factors that might act synergistically with IL-12 or IL-18.
The production of very high levels of IFN-γ by LeIF-stimulated SCID mouse spleen cells may be attributed to the absence of T cells in SCID mice and consequently to the lack of regulatory cytokines produced by these cells, such as, for example, IL-10. Indeed, LeIF, in addition to inducing IL-12, also induces the production of IL-10 by both human and conventional mouse cells (18). However, no IL-10 could be detected in cultures of LeIF-activated SCID mouse spleen cells (data not shown). Because IL-10 is a well-known inhibitor of IFN-γ production, this observation could explain the abundant production of IFN-γ by LeIF-stimulated SCID mouse spleen cells in this study.
Recent studies have demonstrated that stimulation of NK cells can cause up-regulation of CD28 (14). In addition, it has been proposed that the interaction of CD28+ NK cells with B7+ accessory cells is an important asset in the control of infections like those caused by Toxoplasma gondii (5). In the present studies, the participation of these costimulatory molecules on the activation of NK cells by LeIF was investigated. FACS analysis revealed up-regulation of B7.1 but not of B7.2 after 36 h of stimulation of SCID mouse spleen cells with LeIF. However, addition of anti-B7.1 and anti-B7.2 during the activation of NK cells with LeIF did not interfere with the ability of NK cells to produce IFN-γ (data not shown). These results suggest that the interaction of CD28, B7.1, and B7.2 does not participate in the LeIF activation of NK cells for the production of IFN-γ.
Compared to the potent activation of NK cells for the production of IFN-γ, the killing activity of these cells was stimulated to a lesser extent by LeIF alone. However, after the exogenous addition of IL-12 or IL-18, LeIF appreciably augmented the killing activity of NK cells. These results suggest that the signals involved in signal transduction mechanisms for the production of cytokines, particularly IFN-γ, and cytotoxicity are not the same in NK cells.
Also interesting was the observation that LeIF stimulated the generation of only the NK1 response phenotype in SCID mouse spleen cells. In contrast, the polyclonal activators PMA and ionomycin stimulated both the NK1 and NK2 phenotypes. These results are apparently the first to expand to mice the former observation with human cells suggesting that NK cells can differentiate into cells of two phenotypes similar to those described for T cells. The production of large amounts of IFN-γ and undetectable levels of IL-5 in LeIF-stimulated cells could be a determining factor in the Th1-biased immune response to this molecule that is observed in both human and mouse cells. However, because NK cells do not produce IL-4 (9) and IL-5 is not implicated in the generation of Th2 responses, one cannot directly correlate the activation of the two subsets of NK cells as determinant factors in the generation of the two subsets of cytokine-producing T cells (Th1 and Th2).
We do not have any information on a possible unique physical or chemical characteristic(s) of the LeIF molecule that could explain its biological properties. LeIF is a polypeptide comprised of 403 amino acids with a predicted molecular mass of 45.3 kDa and an isoelectric point of 5.9. The molecule contains 46 strongly basic amino acids, 55 strongly acidic amino acids, 91 polar amino acids, and 148 hydrophobic amino acids. A Kyte-Doolittle hydrophilicity plot suggests that the molecule has no major clusters of hydrophobic amino acids. LeIF is a cytoplasmic (ribosomal) protein involved in the translation machinery. The molecule contains sequence elements characteristic of several demonstrated or putative ATP-dependent RNA helicases represented by eukaryotic initiation factor 4A, which is believed to be a relatively abundant protein. Moreover, LeIF is present in both the promastigote and amastigote parasite forms of all of the Leishmania species thus far tested, as revealed by both Northern and Western blot analyses (22). Indeed, we have cloned, expressed, and analyzed the L. major homologue of L. brasiliensis LeIF. At the protein level, these two molecules have 99.8% homology and 98.3% total homology. In addition, we have determined that the biological activity of LeIF resides in the N-terminal half of the molecule (23). Unfortunately, we have been unable to purify the native form of LeIF to homogeneity and to unquestionably attribute to the native molecule the biological properties of the recombinant protein.
In conclusion, we report here that a recombinant protein obtained from L. brasiliensis is a powerful stimulator of SCID mouse spleen cells for the production of IL-12, IL-18, and IFN-γ in the absence of the cognitive recognition elements of the immune system. Because of this property and because LeIF does not fit the classical pathogen-associated molecular patterns, this work points to an important finding, i.e., the description of alternative microbial components (proteins) that alert the host to the presence of an infecting or foreign organism. Incidentally, protein molecules extracted from other protozoans, such as T. gondii, and from Trypanosoma cruzi have been reported to stimulate mononuclear cells from noninfected mice to produce IL-12 and other inflammatory cytokines (1, 20). The nature of the possible cell receptor involved in the activation of the innate immune system by LeIF is not known and is currently under investigation. It is possible, however, to exclude the recently described Toll receptor Tlr4 as a putative LeIF receptor because the gene encoding this receptor is defective with a loss-of-function mutation in C3H/HeJ mice (17, 19), the mouse strain used in the present studies.
ACKNOWLEDGMENTS
We thank Thomas Vedvick for performing N-terminal sequencing and Pamela J. Ovendale for excellent technical assistance.
This work was supported by National Institutes of Health grant AI25038.
REFERENCES
- 1.Almeida I C, Camargo M M, Procopio D O, Silva L S, Mehlert A, Travassos L R, Gazzinelli R T, Ferguson M A. Highly purified glycosylphosphatidylinositols from Trypanosoma cruzi are potent proinflammatory agents. EMBO J. 2000;19:1476–1485. doi: 10.1093/emboj/19.7.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis M M, Bjorkman P J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. . (Erratum, 335:744, 1988.) [DOI] [PubMed] [Google Scholar]
- 3.Dorshkind K, Keller G M, Phillips R A, Miller R G, Bosma G C, O'Toole M, Bosma M J. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J Immunol. 1984;132:1804–1808. [PubMed] [Google Scholar]
- 4.Garni-Wagner B A, Witte P L, Tutt M M, Kuziel W A, Tucker P W, Bennett M, Kumar V. Natural killer cells in the thymus. Studies in mice with severe combined immune deficiency. J Immunol. 1990;144:796–803. [PubMed] [Google Scholar]
- 5.Hunter C A, Ellis-Neyer L, Gabriel K E, Kennedy M K, Grabstein K H, Linsley P S, Remington J S. The role of the CD28/B7 interaction in the regulation of NK cell responses during infection with Toxoplasma gondii. J Immunol. 1997;158:2285–2293. [PubMed] [Google Scholar]
- 6.Janeway C A., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Janeway C A., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 8.Kumar V, Hackett J, Jr, Tutt M M, Garni-Wagner B A, Kuziel W A, Tucker P W, Bennett M. Natural killer cells and their precursors in mice with severe combined immunodeficiency. Curr Top Microbiol Immunol. 1989;152:47–52. doi: 10.1007/978-3-642-74974-2_7. [DOI] [PubMed] [Google Scholar]
- 9.Lauwerys B R, Garot N, Renauld J C, Houssiau F A. Cytokine production and killer activity of NK/T-NK cells derived with IL-2, IL-15, or the combination of IL-12 and IL-18. J Immunol. 2000;165:1847–1853. doi: 10.4049/jimmunol.165.4.1847. [DOI] [PubMed] [Google Scholar]
- 10.Lauwerys B R, Renauld J C, Houssiau F A. Synergistic proliferation and activation of natural killer cells by interleukin 12 and interleukin 18. Cytokine. 1999;11:822–830. doi: 10.1006/cyto.1999.0501. [DOI] [PubMed] [Google Scholar]
- 11.Lauzon R J, Siminovitch K A, Fulop G M, Phillips R A, Roder J C. An expanded population of natural killer cells in mice with severe combined immunodeficiency (SCID) lack rearrangement and expression of T cell receptor genes. J Exp Med. 1986;164:1797–1802. doi: 10.1084/jem.164.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medzhitov R, Janeway C. Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 13.Micallef M J, Tanimoto T, Kohno K, Ikeda M, Kurimoto M. Interleukin 18 induces the sequential activation of natural killer cells and cytotoxic T lymphocytes to protect syngeneic mice from transplantation with Meth A sarcoma. Cancer Res. 1997;57:4557–4563. [PubMed] [Google Scholar]
- 14.Nandi D, Gross J A, Allison J P. CD28-mediated costimulation is necessary for optimal proliferation of murine NK cells. J Immunol. 1994;152:3361–3369. [PubMed] [Google Scholar]
- 15.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–264. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 16.Peritt D, Robertson S, Gri G, Showe L, Aste-Amezaga M, Trinchieri G. Differentiation of human NK cells into NK1 and NK2 subsets. J Immunol. 1998;161:5821–5824. [PubMed] [Google Scholar]
- 17.Poltorak A, He X, Smirnova I, Liu M Y, Huffel C V, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 18.Probst P, Skeiky Y A, Steeves M, Gervassi A, Grabstein K H, Reed S G. A Leishmania protein that modulates interleukin (IL)-12, IL-10 and tumor necrosis factor-alpha production and expression of B7–1 in human monocyte-derived antigen-presenting cells. Eur J Immunol. 1997;27:2634–2642. doi: 10.1002/eji.1830271024. [DOI] [PubMed] [Google Scholar]
- 19.Qureshi S T, Lariviere L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. . (Erratum, 189:1518, 1999.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sher A, Oswald I P, Hieny S, Gazzinelli R T. Toxoplasma gondii induces a T-independent IFN-gamma response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-alpha. J Immunol. 1993;150:3982–3989. [PubMed] [Google Scholar]
- 21.Shoda L K, Kegerreis K A, Suarez C E, Roditi I, Corral R S, Bertot G M, Norimine J, Brown W C. DNA from protozoan parasites Babesia bovis, Trypanosoma cruzi, and T. brucei is mitogenic for B lymphocytes and stimulates macrophage expression of interleukin-12, tumor necrosis factor alpha, and nitric oxide. Infect Immun. 2001;69:2162–2171. doi: 10.1128/IAI.69.4.2162-2171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skeiky Y A, Guderian J A, Benson D R, Bacelar O, Carvalho E M, Kubin M, Badaro R, Trinchieri G, Reed S G. A recombinant leishmania antigen that stimulates human peripheral blood mononuclear cells to express a Th1-type cytokine profile and to produce interleukin 12. J Exp Med. 1995;181:1527–1537. doi: 10.1084/jem.181.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skeiky Y A, Kennedy M, Kaufman D, Borges M M, Guderian J A, Scholler J K, Ovendale P J, Picha K S, Morrissey P J, Grabstein K H, Campos-Neto A, Reed S G. LeIF: a recombinant Leishmania protein that induces an IL-12-mediated Th1 cytokine profile. J Immunol. 1998;161:6171–6179. [PubMed] [Google Scholar]
- 24.Tominaga K, Yoshimoto T, Torigoe K, Kurimoto M, Matsui K, Hada T, Okamura H, Nakanishi K. IL-12 synergizes with IL-18 or IL-1beta for IFN-gamma production from human T cells. Int Immunol. 2000;12:151–160. doi: 10.1093/intimm/12.2.151. [DOI] [PubMed] [Google Scholar]
- 25.Tomura M, Zhou X Y, Maruo S, Ahn H J, Hamaoka T, Okamura H, Nakanishi K, Tanimoto T, Kurimoto M, Fujiwara H. A critical role for IL-18 in the proliferation and activation of NK1.1+ CD3− cells. J Immunol. 1998;160:4738–4746. [PubMed] [Google Scholar]