Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder which is characterized by β-amyloid (Aβ) aggregation, τ-hyperphosphorylation, and loss of cholinergic neurons. The other important hallmarks of AD are oxidative stress, metal dyshomeostasis, inflammation, and cell cycle dysregulation. Multiple therapeutic targets may be proposed for the development of anti-AD drugs, and the “one drug–multiple targets” strategy is of current interest. Tacrine (THA) was the first clinically approved cholinesterase (ChE) inhibitor, which was withdrawn due to high hepatotoxicity. However, its high potency in ChE inhibition, low molecular weight, and simple structure make THA a promising scaffold for developing multi-target agents. In this review, we summarized THA-based hybrids published from 2006 to 2022, thus providing an overview of strategies that have been used in drug design and approaches that have resulted in significant cognitive improvements and reduced hepatotoxicity.
Keywords: Alzheimer, acetylcholinesterase, butyrylcholinesterase, amyloid-β, tacrine
1. Introduction
AD is a progressive multifarious neurodegenerative disorder which is described by a progressive loss of cognitive abilities, such as memory, language skills, and attention, as well as by spatial disorientation and depression. The pathological hallmarks of AD are extracellular accumulation of Aβ plaques composed of Aβ peptides, neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau protein, brain inflammation, and atrophy [1]. Aβ is formed from amyloid precursor protein (APP), which is cleaved by β-secretase (BACE-1) and γ-secretase. Thus, interfering with fibril formation, including metal cation chelation, the disruption of amyloid aggregation, and BACE-1 inhibition, are well-established approaches to the development of anti-AD drugs [2].
Oxidative stress and inflammation are also some of the hallmarks of AD [3]. An increase in reactive oxygen species (ROS) levels is caused by mitochondrial dysfunction, violation of the homeostasis of metal cations, the formation of Aβ fibrils, inflammatory processes, etc. [4].
Calcium regulation is important in learning and memory. The disruption of Ca2+ level homeostasis caused by the formation of Aβ leads to cell death [5,6]. Blocking Ca2+ channels is also one of the important strategies in the treatment of AD. Calcium channel blockers (CCBs), one of the more commonly used treatments for hypertension, are also considered as potential drug candidates for anti-AD therapy [7].
Glycogen synthase kinase-3 (GSK-3) is a serine-threonine kinase involved in neurodegeneration. GSK-3β isoform is found to be hyperactive in the brains of AD patients; GSK-3β inhibition is also one of the therapeutic strategies during anti-AD drug development [8,9].
Neurotransmitters such as noradrenaline, dopamine, serotonin (5-HT), and GABA are involved in the pathogenesis of AD; imbalances between neurotransmitters in the temporal cortex and hippocampus have been reported [10]. In addition, in the latter studies of AD, a deficiency of monoamines is found in the brainstem and hippocampus [11]. NMDA antagonists slow the decline in cognitive function in AD patients [3,12].
Different components of the cholinergic system are therapeutic targets in AD treatment. ACh is synthesized from acetyl coenzyme A and choline in the presence of choline acetyltransferase (ChAT). Then, ACh is released into the synapse and binds to either the G-protein coupled muscarinic receptors or the ionotropic nicotinic receptors to transmit signals from one neuron to the other [13]. ACh can be degraded by AChE or BuChE [14]. The activation of the muscarinic M1 receptor exerts a pro-cognitive effect, and an activation of the alpha7 nACh receptor might inhibit the formation of Aβ [15]. Thus, drugs capable of acting on muscarinic and nicotinic receptors are of interest [16,17]. Moreover, the mAChR antagonist scopolamine is used for inducing cognitive and behavioral deficits in animals [18].
Current clinical therapy for AD patients is based on the cholinergic hypothesis, which suggests that the decline of acetylcholine (ACh) levels causes cognitive and memory deficits [19]. An increase in the ACh concentration in a synaptic cleft by various ways, such as the inhibition of both acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE), is the key approach in the treatment of AD now. AChE inhibitors (AChEIs), including donepezil, galantamine, and rivastigmine, are FDA-approved drugs for AD treatment [20].
Tacrine (9-amine-1,2,3,4-tetrahydroacridine) (THA) was the first FDA-approved ChE inhibitor for the treatment of AD. THA was produced under the brand name Cognex® and the recommended dose was 40 mg per day. THA acts by inhibiting the metabolism of acetylcholine and thus prolonging its activity and raising levels in the cerebral cortex. Therapy with THA improves mental functioning in patients with mild-to-moderate dementia of Alzheimer disease [21,22]. THA undergoes first pass metabolism by the liver and is extensively metabolized by the cytochrome P450 system, which is supposedly the reason for its high hepatotoxicity. Thus, THA therapy is accompanied by increased serum alanine aminotransferase (ALT) and aspartate aminotransferase (ASAT) levels, which are indicative of liver damage and which were the reason for its withdrawal from use in 2013 [22].
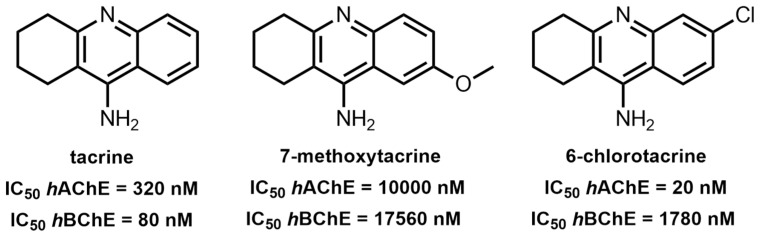
Intensive research resulted in the design of more potent 6-chlorotacrine (6-Cl-THA) and less toxic 7-methoxytacrine (7-MEOTA) drug candidates [23,24,25] (Figure 1).
Figure 1.
Tacrine (THA), 7-metoxytacrine (7-MEOTA), 6-chlorotacrine (6-Cl-THA), and their IC50 values.
Despite the hepatotoxicity of THA, its suitability for chemical modification makes it a widely used scaffold for drug development [26]. Chemical modification of amino groups in the THA molecule leads to a decrease in hepatotoxicity [27]. Thus, a conjugation of THA with a second pharmacophoric moiety resulting in THA-based hybrids, pioneered by Pang et al. [28], is still an area of active research and development.
Since the strategy of ChE inhibition was successful, much attention is paid to the development of drugs that effectively interact with this enzyme. The crystallographic structure of AChE reveals that it has a narrow 20A gorge with two binding sites, the catalytic active site (CAS) at the bottom and the peripheral anionic site (PAS) near the entrance [29,30]. AChE inhibitors can bind to either one or two sites. Importantly, AChE could also promote Aβ formation by interaction through the PAS of AChE, yielding the toxic AChE-Aβ complex [31]. Therefore, the dual binding inhibitors, which target both PAS and CAS, are of interest in AD treatment, and THA-linker-residue hybrids with appropriate linker length are being designed [32].
Based on the above-mentioned multiple cellular and pathological hallmarks of AD, several therapeutic strategies should be used in developing effective anti-AD therapy, and a potential drug candidate should affect several therapeutic targets at once to be effective. Thus, multi-target-directed THA-based hybrids have been of interest for years, and novel potential anti-AD THA-based drugs are still reported. Several reports have considered designing THA-like compounds by replacing or annulating the benzene ring in THA with different heterocyclic systems [33,34] and THA dimers [35]. Additionally, several reviews have reported THA-based hybrids. In 2017, Sameem et al. reported a short review of THA-based scaffolds as multi-target drugs (MTDLs) [36] and Wu et al. reported a review of several THA-based hybrids [27]. In 2019, Girek et al. summarized phyto-THA hybrids [37]. In addition, in 2020, Eckroat et al. summarized structural analogues of THA developed in 2015–2020 [38].
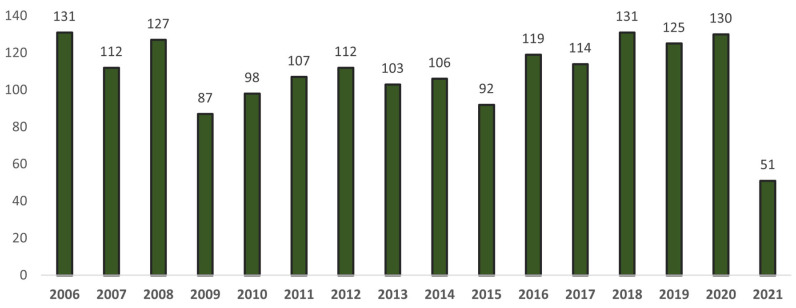
The development of new multi-target drugs based on THA and its analogues is a relevant task, and novel high-quality research works continue to be published. A quick search of the database of articles on THA and its analogues shows that the development of drugs against AD based on THA is of current interest (Figure 2).
Figure 2.
Result of a quick search of articles devoted to THA vs. year. Librarysearch.library.utoronto.ca. (accessed on 18 August 2022).
In this review, we sum up THA-linker-residue hybrids published from 2006 to 2022, with many of them being superior to currently clinically used drugs in terms of their multiplicity of biological action, low toxicity, and drug efficacy.
2. Summary of Tacrine-Based Hybrids Reported in 2006–2022
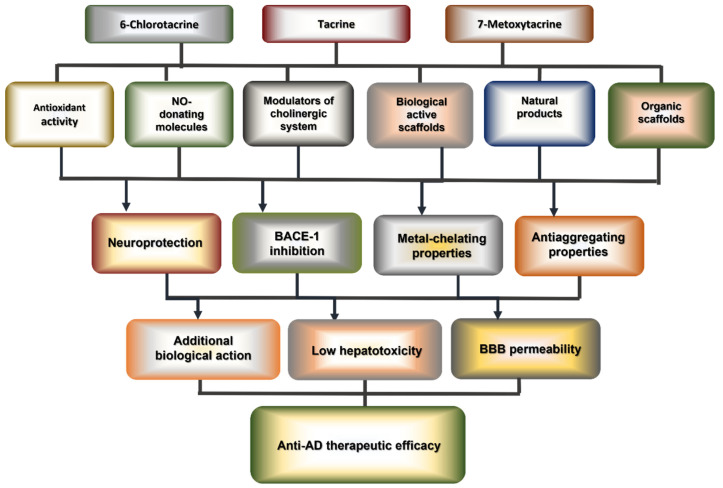
Because the number of articles devoted to the development of analogues of THA is enormous, we structured the articles published in 2006–2022 [30,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133] based on general patterns. This review is divided into several sections based on the biological action of the second ligand conjugated to the THA backbone (Figure 3, Table S1).
Figure 3.
THA-based hybrids with various biological activities, summarized in this review.
Tacrine hybrids with antioxidant activity (Section 3), NO-donors (Section 4), with biologically active molecules (Section 5), and with drugs that affect the cholinergic/serotonergic systems (Section 6) are summarized in various chapters. Additionally, two chapters are devoted to tacrine hybrids with natural products (Section 7) and organic ligands (Section 8). Below, the plan of this review is presented in the form of a diagram (Figure 4). In addition, in Table S1, we summarize all hybrids described in this review, present the best result of inhibitory activity among the series, and the spectrum of biological actions confirmed for hybrids.
Figure 4.
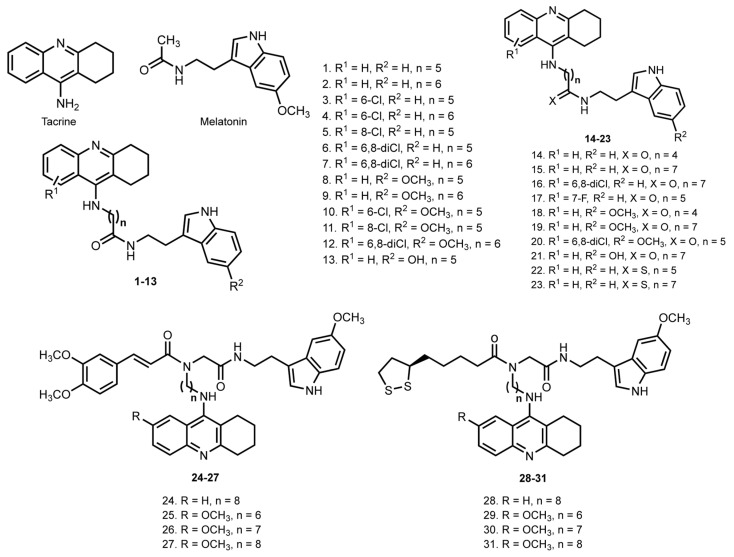
THA–melatonin hybrids 1–13 [39], an extended series of THA (6-Cl-THA)–melatonin hybrids 14–23 [40], and THA–melatonin hybrids with ferulic acid or lipoic acid 24–31 [41].
Additionally, we summarize the in vivo therapeutic efficacy of hybrids data presented in this review in Table S2.
3. Tacrine Hybrids with Antioxidant Activity
3.1. Tacrine–Melatonin Hybrids
In 2006, Rodríguez-Franco et al. [39] reported hybrids of THA with melatonin, a pineal neurohormone with strong antioxidant action [134] (Figure 4).
Hybrids were potent inhibitors of cholinesterases at the low nanomolar level. Additionally, hybrid 7 is still one of the most potent inhibitors of human AChE described with IC50 0.008 nM. An antioxidant activity of hybrids was determined by the oxygen radical absorbance capacity assay using fluorescein (ORAC-FL); hybrids showed potent peroxyl radical absorbance capacities ranging from 1.7- to 4-fold the value of trolox, a Vitamin E analogue which was used as a standard. Hybrids 1–12 proved the ability to cross the blood-brain barrier (BBB) in the PAMPA-BBB test.
In 2009, the same scientific group reported an extended series of THA–melatonin hybrids 14–23 [40]. All hybrids were potent inhibitors of mammalian ChEs at the low-nanomolar range. 6-Chloro- and 6,8-dichlorotacrine–melatonin hybrids 3 and 6 showed remarkable selectivity, being from 200- to 1000-fold more active toward hAChE than hBuChE and showing potent peroxyl radical absorbance capacities ranging from 1.5- to 4-fold the trolox value. Molecular modeling studies showed that hybrids target both the CAS and the PAS of AChE. A displacement of the binding of propidium iodide (PI) from the PAS at sub-micromolar concentrations was confirmed. In addition, an inhibition of Aβ self-aggregation and neuroprotective properties in a human neuroblastoma line were reported.
In 2005, racemic lipocrine, a lipoic acid with a derivative of THA, was reported [135]. This hybrid inhibited AChE effectively (IC50 0.253 nM) and reduced AChE-induced Aβ aggregation from ROS formation. Inspired by this, in 2016 Benchekroun et al. designed THA–melatonin hybrids 24–31 with ferulic acid (FA) or lipoic acid [41]. Hybrid 28 was the most effective inhibitor of BuChE with IC50 1.25 nM and AChE with IC50 3.62 nM. Additionally, 24–27 were potent antioxidants, showing values from 9.11 trolox equivalents. A neuroprotective effect for hybrids 25–27 was shown on SH-SY5Y neuroblastoma cells, with 26 being the best of the series. Additionally, 26 showed a neuroprotective effect against toxic insults mediated by hydrogen peroxide, Aβ1−40, and Aβ1−42. Hybrids 24–27 successfully induced the Nrf2 pathway in the AREc32 reporter cell line.
3.2. Other Hybrids with Antioxidant Activity
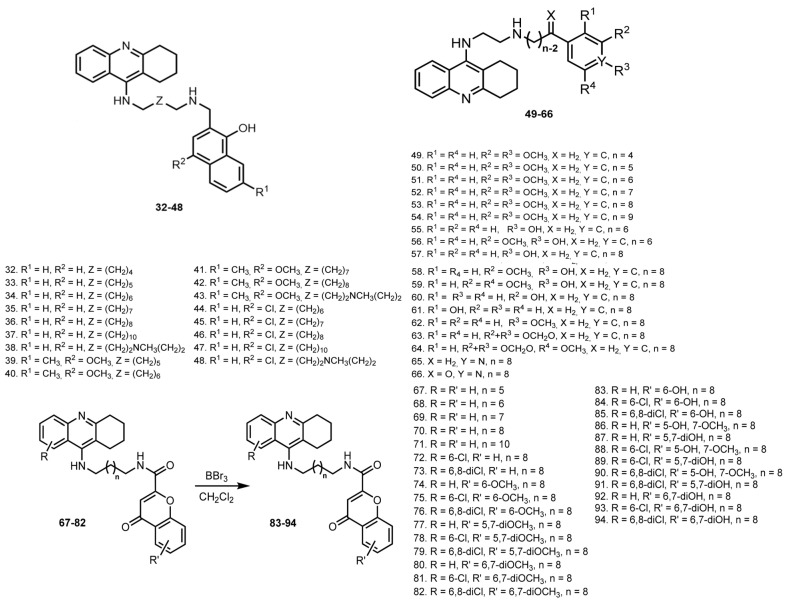
In 2010, Fernández-Bachiller et al. reported THA-based hybrids 32–48 with 8–hydroxyquinoline [42] (Figure 5). An inhibition of human cholinesterase showed IC50 values for all tested hybrids in the nano- and subnanomolar range (0.5–5.5 nM). Hybrid 40 was the best hBuChE inhibitor with IC50 2 nM. Hybrids conjugated with an unsubstituted 8-hydroxyquinoline fragment and a methylene tether of 7–10 carbons showed the best AChE inhibitory activities, with 35 showing IC50 20 nM. The antioxidant capacity of selected hybrids 35, 40, and 45 was confirmed by their competition with fluorescein in the radical capture. Hybrid 35 showed 3.3 trolox equivalents, hybrid 40 showed 2.6, and hybrid 45 showed 4.7.
Figure 5.
THA–hydroxyquinoline hybrids 32–48 [42], THA hybrids with benzene/pyridine moieties 49–66 [43,44], and THA–4-oxo-4H-chromene hybrids 67–94 [44].
The affinity of selected compounds for the PAS was confirmed by the displacement of PI. All hybrids showed permeability values over the above limit in the PAMPA-BBB test. The metal-chelating properties of 35 were confirmed by UV-Vis spectrometry in the presence of Cu2+. Finally, 35 showed negligible cell toxicity on human neuroblastoma cell line SHSY5Y.
In 2011, Luo et al. reported THA hybrids with substituted benzene or pyridine moieties 49–66 [43,44]. Most compounds showed selectivity for BuChE over AChE, and 58 was found to be the best inhibitor for both ChEs with its IC50 4.55 nM and 3.41 nM. Kinetic studies of the inhibition of AChE by 58 revealed a mixed-type inhibition, which was confirmed by a molecular modeling study. The antioxidant activity of 58–61 with hydroxyl group was proven via ORAC test; compounds showed potent peroxyl radical absorbance capacities ranging from 1.2- to 2.7-fold of the trolox value. Finally, 58 proved to inhibit self-mediated Aβ aggregation.
In 2012, Fernández-Bachiller et al. reported hybrids of THA with flavonoid scaffold 67–94 derived from 4-oxo-4H-chromene with possible antioxidant and BACE-1 inhibitory activities [45]. Hybrids 67–94 showed a selectivity for BuChE, with 70 as the most active inhibitor. Hybrid 88 was the best hAChE inhibitor with IC50 35 pM, and 74 was the most active with IC50 38 pM. Expectedly, hybrids with hydroxyl groups exhibited antioxidant capacities. Hybrid 83 was 1.3-fold more potent than the vitamin E analogue and was the best antioxidant. All hybrids were found to be potent inhibitors of human BACE-1 with IC50s from 2 to 22 μM, better than that of apigenin (IC50 38.5 μM), with the most active as 77 (IC50 2.1 μM). All hybrids (except for 92 and 93) showed the potential to cross the BBB in the PAMPA-BBB test. Finally, 83 showed potent combined inhibition of human BACE-1 and ChEs.
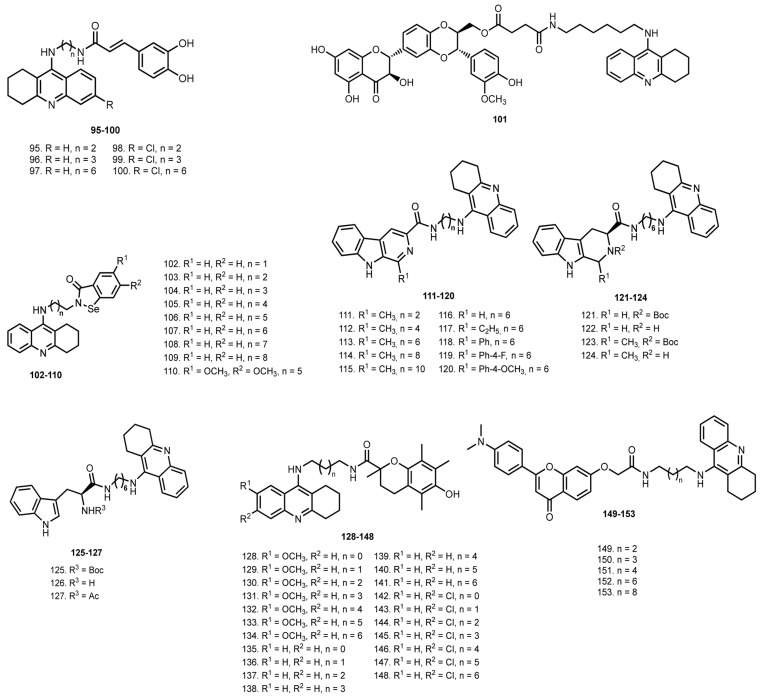
In 2012, Chao et al. reported hybrids of THA with caffeic acid 95–100 [46] (Figure 6). All hybrids inhibited ChEs, with the most potent AChE inhibitor being 99 (IC50 0.3 µM). Expectedly, 95–100 showed a radical scavenging activity in a DPPH test due to the presence of a hydroxyl group. Hybrid 99 showed the best antioxidant activity in 4.8 ± 0.9 µM. The inhibition activity of 99 besides Aβ self- or AChE-induced aggregation was proven, as well as its antioxidant properties. Finally, the Cu2+–chelating properties of 99 were proven by UV-Vis spectra.
Figure 6.
THA–caffeic acid hybrids 95–100 [46], THA–silibinin hybrid 101 [47], THA–Ebselen hybrids 102–110 [49], THA–(b-carbolines (pyrido [3,4-b]indoles) hybrids 111–127 [50], THA–trolox hybrids 128–148 [51], THA hybrids with N,N-dimethylated flavonoids 149–153 [52].
In 2012, Chen et al. reported a hybrid of THA 101 with a flavonolignan silibinin, a natural antioxidant [47,48,136]. Hybrid 101 loses quite a bit of inhibitory activity at BuChE (16-fold lower) and moderately at AChE (3.5-fold lower) when compared with THA. A lower hepatotoxicity of 101 in comparison with THA was revealed on hepatocellular carcinoma HePG2 cells. No histomorphological changes in liver tissue were observed after administration of 101 in vivo, in contrast to the THA administration. The antioxidant effect of 101 was confirmed by an evaluation of the lipid peroxidation products level in vivo after drug administration. In in vivo behavior tests on scopolamine-injected mice, 101 showed the same pro-cognitive effect as THA.
In 2013, Mao et al. reported THA hybrids with Ebselen 102–110, an organoselenium with antioxidant activity, anti-inflammatory, and neuroprotective activities [49,137,138]. Hybrids 102–110 inhibited both AChE and BuChE with nanomolar activity. Hybrid 106 was the best AChE inhibitor with IC50 6.32 nM among the derivatives with unsubstituted Ebselen moiety, whereas 110 with OMe-substituent showed a promising result in 2.55 nM.
Lineweaver–Burk plots of 110 against AChE revealed a mixed-type inhibition, which was also confirmed by molecular modelling. Expectedly, 106 and 110 proved to be antioxidants with eroxynitrite scavenging activity 1.17 and 1.26 times greater than that of ebselen, respectively. Unfortunately, 110 showed high toxicity on human hepatic stellate cells (HSC).
In 2014, Lan et al. reported THA–(b-carbolines (pyrido [3,4-b]indoles) hybrids 111–127 [50]. All hybrids inhibited both ChEs with IC50 values from sub-micromolar to nanomolar. Hybrid 122 was the best AChE inhibitor with IC50 21.6 nM and 125 was the best BuChE inhibitor with IC50 4.3 nM. In addition, 122 was the best hAChE inhibitor with IC50 63.2 nM. Kinetic study revealed 122 as a mixed-type inhibitor of AChE, which was also confirmed by molecular modeling studies. Additionally, 122 displayed the most potent antioxidant activity in 1.57 trolox equivalents, and 126 and 122 showed a neuroprotective effect on the rat pheochromocytoma cell line PC12 from H2O2-induced oxidative stress. Thioflavin T (ThT)-based fluorometric assay with curcumin as a control showed moderate to good antiaggregating potencies of hybrids (22.4–66.5% at 20 µM) with the most effective compounds being 126 and 122; 122 could also inhibit a Cu2+-induced Aβ aggregation. UV–Vis spectrometry revealed the ability of 122 to chelate Cu2+ ions. Finally, the PAMPA-BBB assay revealed the ability of hybrids to cross the BBB.
In 2015, Nepovimova et al. reported hybrids of THA with Trolox 128–148, a water-soluble analogue of vitamin E (±6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) and a “gold standard” antioxidant [51]. Hybrids 128–148 showed moderate inhibition activity toward hAChE with an IC50 from 13.29 to 0.08 μM. Hybrid 148 was the best hAChE inhibitor with IC50 80 nM, four-fold weaker than 6-Cl-THA. Overall, 128–148 displayed moderate to good antioxidant capacities, with 148 as the best antioxidant with IC50 44.09 μM. Mixed-type inhibition of AChE was established for 148 by kinetic assay. The low hepatotoxicity of 148 was shown on HepG2 cells, and a metabolic assay in human liver microsomes showed no potentially metabolic products emerging under experiment. Unfortunately, the limitations in the solubility of 148 did not allow the determining of LD50 in vivo.
In 2016, Luo et al. reported THA hybrids with N,N-dimethylated flavonoids 149–153 [52]. All hybrids inhibited ChEs in the nanomolar range. Hybrid 152 was the best AChE inhibitor with IC50 59.61 nM and 153 was the best BuChE inhibitor with IC50 24.67 nM. The antioxidant activity of hybrids was confirmed using oxygen radical absorbance capacity (ORAC) assay, in which hybrid 153 showed 3.2 trolox equivalents. The antiaggregating activity of hybrids was confirmed by ThT assay. A neuroprotective effect of 152 against H2O2-induced oxidative stress was shown on PC12 cells, as well as a registered reduction of intracellular ROS levels after treatment with 152.
In 2016, Chand et al. reported THA–(hydroxybenzoyl-pyridone) hybrids 154–156 [53] (Figure 7). All hybrids showed moderate inhibition activity with the most potent being 156 (IC50 0.57 µm against eeAChe). In addition, when antioxidant activity and metal-chelating properties of the hybrid were confirmed, hybrid 156 was the best antioxidant with EC50 = 204 μM.
Figure 7.
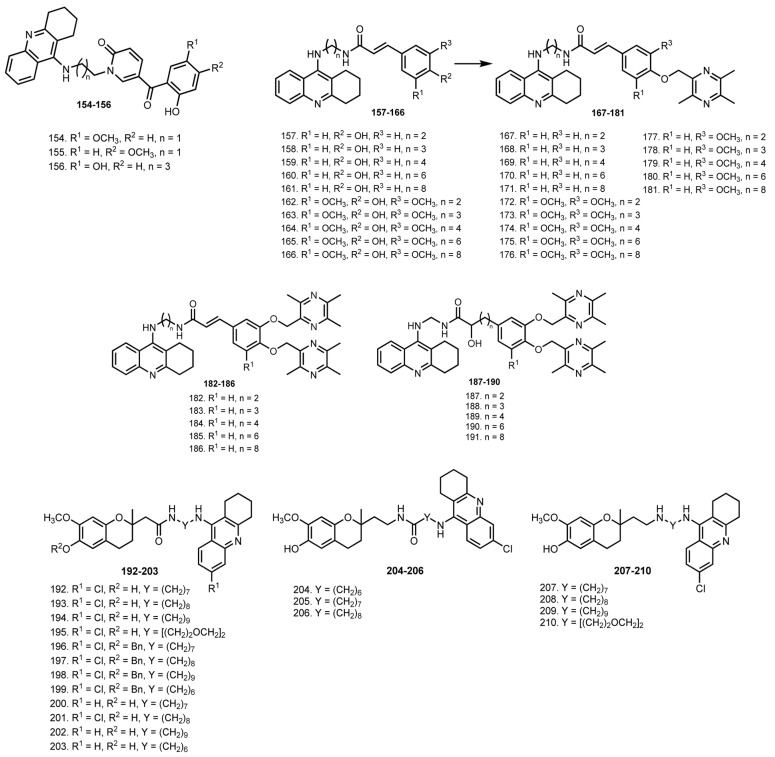
THA–(hydroxybenzoyl-pyridone) hybrids 154–156 [53], THA–phenolic acid dihybrids 157–166 and THA–phenolic acid–ligustrazine trihybrids 167–191 [54], and THA–antioxidant CR-6 hybrids 192–210 [56].
In 2018, Li et al. designed THA–phenolic acid dihybrids and THA–phenolic acid–ligustrazine trihybrids 157–191 [54]. All hybrids showed AChE and BuChE inhibitory activities. Hybrid 165 (IC50 3.9 nM) and trihybrid 175 (IC50 2.6 nM) exhibited the best AChE inhibitory activity. Aso, 165 was a potent inhibitor toward hAChE with IC50 65.2 nM, more effective that THA (IC50 116.8 nM). Kinetic and molecular modeling studies revealed 165 as a mixed-type inhibitor. Additionally, 165 could inhibit the self-mediated Aβ42 aggregation, which was confirmed via monitoring of the Aβ aggregation using an atomic force microscope (AFM). Expectedly, 165 showed potent peroxyl radical scavenging capacity with IC50 85.8 μM, and a neuroprotective effect on PC12 cells treated with CoCl2 was detected. MTT assay on HepG2 cells revealed a low hepatotoxicity of 165, and further in vivo tests with ALT and ASAT measurements revealed a lower hepatotoxicity of 165 in comparison with THA.
In 2020, the same scientific group reported significant improvements in cognitive function in APP/PS1 transgenic mice treated with 165 [55]. After 4 weeks of intragastric administration of 165 (1.27 mg/100 g), cognitive function and synaptic plasticity were improved. In addition, the level of Aβ plaques in the DG region in the APP/PS1 mice was reduced.
In 2020, Pérez-Areales et al. reported THA-based hybrids with antioxidant CR-6 192–210 [56]. The most potent hAChE inhibitors were amines 207 (IC50 442 pM), 208 (IC50 121 pM), and 209 (IC50 272 pM), which were 33-, 120-, and 53-fold more potent than the parent Cl-THA. The order of potencies was as follows: amines 207–210 > amides 192–195 > reverse amides 204–206 > O-benzylated amides 196–199. Expectedly, CR-6–chlorotacrine hybrids were found to be less potent inhibitors toward hBuChE than hAChE. However, amines 207 (IC50 17.4 nM), 208 (IC50 13.4 nM), and 209 (IC50 18.3 nM) were the most potent inhibitors of hBuChE, being 29-, 38-, and 28-fold more potent than the parent 6-Cl-THA. Because 6-Cl-THA is known to interact with CAS [139], the lead compound was expected to interact with AChE in the same way, which was confirmed using molecular dynamics simulations. Kinetic studies showed that the hybrid acts as a mixed-type inhibitor of hAChE. The in vitro antioxidant activity of all hybrids featuring a free hydroxyl group was revealed using DPPH assay, with IC50 values in the 6.9–22.9 μM range. Most of the hybrids were found to be inactive as BACE-1 inhibitors, with only the O-benzylated hybrids 196–199 showing weak inhibition. Favorable brain permeability of hybrids was confirmed using PAMPA-BBB assay. Chronic in vivo efficacy studies with 193 and 197 in double-transgenic APP/PS1 mice have shown positive tendencies in improving cognition and amyloid pathology.
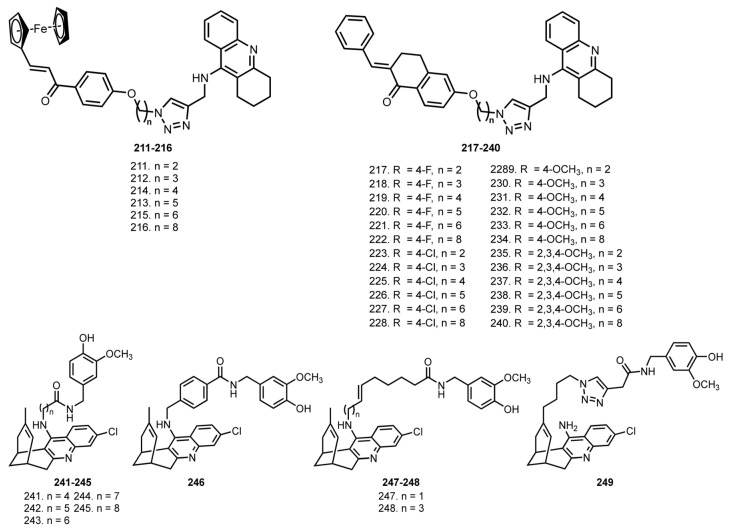
In 2021, Rani et al. reported hybrids of THA with chalcones 211–240, a scaffold with AChE-inhibitory, antioxidant, antiaggregating, anti-inflammatory, neuroprotective, and vasodilator activities [57,140] (Figure 8). Hybrids 216, 225, and 226 showed moderate activity against AChE, and only 225 showed above 50% inhibition at 10 µM against the BuChE. Molecular docking studies showed that 225 and 226 interact with residues of AchE. In vivo behavior studies showed that 216, 225, and 226 attenuated the effect of scopolamine treatment. Moreover, a recovery of scopolamine-induced glutathione depletion in the mice brain was confirmed in group treated with 216, 225 and 226. Additionally, a significant reduction in in-brain malondialdehyde level was detected.
Figure 8.
THA–triazole–chalkone conjugates 211–240 [57], huprine Y–capsaicin hybrids 241–249 [58].
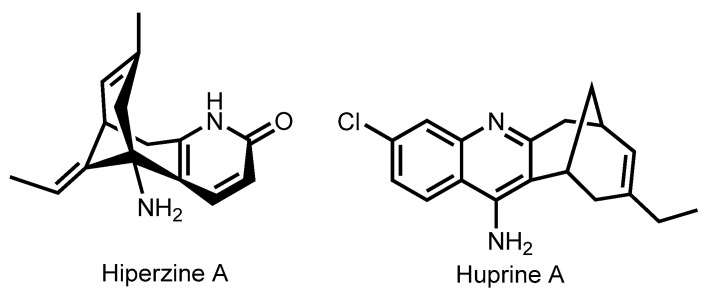
In 2021, Viayna et al. reported huprine Y-based hybrids 241–249 with an antioxidant capsaicin [58]. Huprines represent a family of potent and selective AChE inhibitors based on THA and (−)-huperzine A (HA) [141]. HA is a Chinese herb extract from Huperzia serrata, a reversible AChE inhibitor [142]. Despite the approval of HA by the FDA of China for AD therapy in 1994, the supply of this drug is still limited due to expensive synthesis [143,144]. Thus, design of potent HA analogs not requiring great synthetic effort is of interest (Figure 9).
Figure 9.
Huperzine A and THA–huperzine analogue Huprine A.
241–249 retained the high potency of the parent huprine Y against hAChE and hBuChE. The nine-atoms linker was found to be optimal for the inhibition of both hAChE and hBuChE, yielding 243, which surpassed the nanomolar potency of huprine Y. Kinetic studies of AChE inhibition showed a dual site binding, and the interaction of 243 with PAS was confirmed by a PI displacement. All compounds showed antioxidant activity in the DPPH assay, with the best antioxidant being 247 (IC50 = 31.7 µM), and proved to be BACE-1 inhibitors, with the most active being 241. The ability of hybrids to cross the BBB was proven in PAMPA-BBB assay. Additionally, biodistribution studies in C57BL6 mice revealed an ability of 243 and 249 to accumulate in the brain.
Finally, the therapeutic efficacy of 243 and 249 was investigated in young (5 month) and old (10 month) APP/PS1 mice. Mice treated with 249 enhanced learning and memory in old APP/PS1 mice in all the performed tests, while neither 249 nor 243 were effective in young transgenic mice. A decrease in the Aβ42/Aβ40 ratio in the brains of mice treated with 249 was revealed. In addition, 249 significantly increased the strength of synaptic transmission, and reduced hippocampal levels of the oxidative stress marker 4-HNE and the neuroinflammation (astrogliosis) marker GFAP.
3.3. Tacrine–Ferulic Acid Hybrids
FA is a well-known antioxidant with multiple biological actions [145]. Due to its multiple activity and ease of chemical modifications, the design of FA–THA hybrids is of interest (Figure 10) [146].
Figure 10.
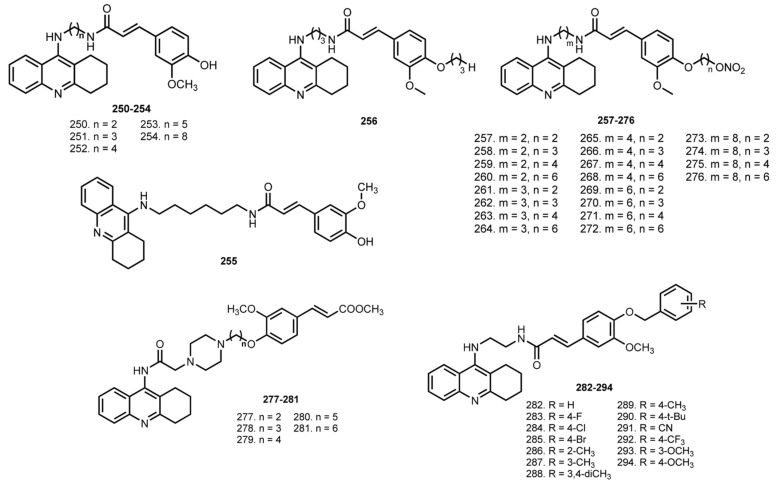
THA–ferulic acid hybrids 250–254 [59], 255 [61], THA–ferulic acid–NO-donor thihybrids 257–276 and model hybrid 256 [30], THA–ferulic acid hybrids with piperazine linker 277–281 [62], and hybrids 282–294 [63].
In 2008, Fang et al. reported THA–FA hybrids 250–254 [59]. All compounds inhibited ChEs, with the most potent hybrids being 252 and 253 toward both ChEs, suggesting the optimal length of linker in 6–7 atoms. Kinetic study of AChE inhibition by 253 showed reversible and noncompetitive inhibition. All hybrids showed moderate to good antioxidant activity confirmed by ORAC-fluorescein assay and hybrid 253 showed 1.5 trolox equivalents.
In 2010, an investigation of the in vivo anti-AD effectiveness of 254 was reported [60]. Unfortunately, no beneficial effect on scopolamine-induced cognition impairment was detected.
In 2012, Pi et al. reported an investigation of anti-AD properties of a similar THA–FA hybrid, 255, with n = 6 [61]. Hybrid 255 showed an ability to inhibit AChE-induced Aβ aggregation and reduce Aβ-induced oxidative stress in PC12 cells. Thus, 10 µM of 255 reduced the Aβ1–40-induced ROS production in C12 cells. In addition, 255 improved the cognitive impairment, increased ChAT and superoxide dismutase activity, and decreased AChE activity and malondialdehyde (MDA) levels in the Aβ i.c.v. AD model.
In 2012, Chen et al. designed THA–FA–NO donor trihybrids 257–276 [30]. NO-donating hybrids showed better or comparable inhibition activity compared to parent 250–254 and a decrease in antioxidant activity. The in vitro reactivity of 257–276 as NO-donators was confirmed using the Griess reaction [147], in which 262 and 273 showed the height levels of nitrite. Additionally, ex vivo organ bath tests (coronary arteries from rats) vascular relaxation assay for 251, 257, 262, 273, and 256 revealed a high activity for all hybrids. Hybrid 262 showed a comparable EC50 with positive control isosorbide dinitrate (ISDN). Hybrids 251, 262, and 256 were active in improving memory impairment in scopolamine-induced mice in a transfer latency time (TLT) test. Importantly, 262 possessed better performance than the non-nitrate hybrid 256. Finally, the levels of ASAT and ALT in serum of drug-treated mice were determined; 251 and 262 possessed higher safety than THA, and 262 showed the lowest hepatotoxicity.
Fu et al. designed THA–FA hybrids with piperazine linker [62]. All hybrids presented inhibitory activity for both ChEs and selectivity for AChE. The best AChE inhibitors were 279 with IC50 52.7 nM and 280 with IC50 61.7 nM. Low antiaggregating properties of hybrids were revealed, and the Cu2+-chelating properties of 280 were confirmed by UV-Vis spectroscopy. Finally, the protective effects of 280 against Aβ-induced neurotoxicity were shown on Neuro-2A cells.
In 2018, Zhu et al. reported THA–FA hybrids 282–294 with different substituents in the benzene ring [63]. Hybrid 288 was the most potent AChE inhibitor with IC50 37.02 nM. The presence of electron-withdrawing substituents contributed to the inhibition of BuChE; hybrid 292 with CF3 substituent showed IC50 52 nM. Molecular docking showed binding of 288 with both the CAS and PAS of AChE. Further, 285, 288, and 291 displayed inhibition on the aggregation of Aβ. When hybrids 285 and 288 were studied for in vivo behavioral analysis in scopolamine-induced cognition-impairment, treatment with 288 led to a remarkable improvement of memory in the scopolamine-induced cognitive impairment in the Morris water maze test. Finally, ALT and ASAT levels were measured after the treatment of animals with 285 and 288, and the hybrids proved to be safe, which was also confirmed by morphologic results.
4. Tacrine Hybrids with NO-Donating Molecules
Nitric oxide (NO) is a key signaling molecule involved in the regulation of many physiological processes [148]. NO plays roles in regulating synaptic plasticity, neurosecretion, and the sleep-wake cycle, and is considered as a molecule for the treatment of AD, which can cause therapeutic effects by increasing blood supply and regulating cerebral circulation [149,150,151,152,153,154]. In recent years, NO-donating and NO mimetic strategies in AD treatment proved to be effective, and several THA-based hybrids with NO-donating properties were reported (Figure 11).
Figure 11.
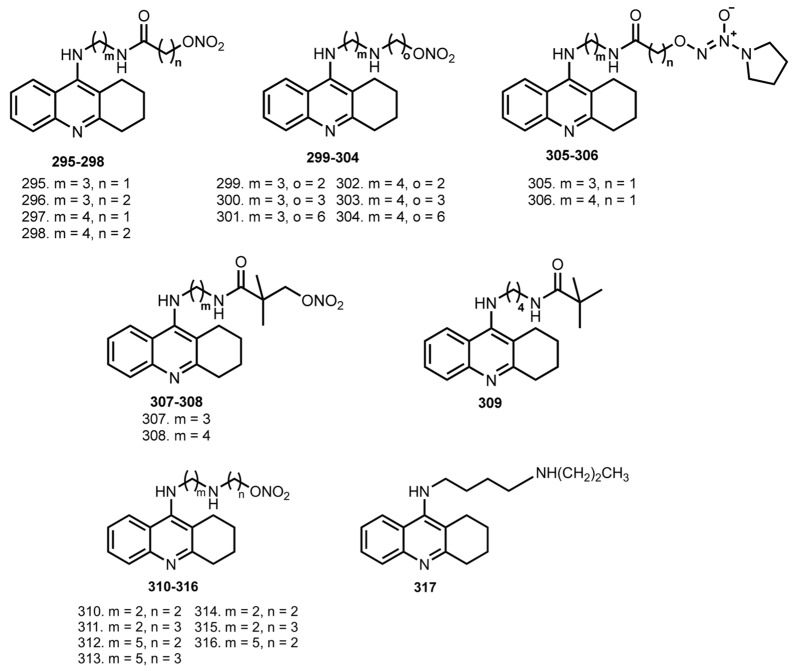
THA hybrids with NO-donating moieties 295–308 and model compound 309 [64], hybrids 310–316, and model 317 [65].
In 2008, Fang et al. designed and synthesized a series of THA hybrids with NO-donating nitrato- and diazeniumdiolate moieties [64]. With the exception of 308 (226.0 nM), all compounds inhibited AChE with IC50 from 5.2 to 93 nM. Inhibition of BuChE was also similar to THA, with IC50 values from 5.2 to 41.0 nM. Hybrid 308 showed selectivity for the inhibition of BuChE over AChE, with IC50 7 and 226 nM.
When the vasorelaxation effects of hybrids were accessed via a test with PGF2R-precontracted porcine pulmonary artery, 295, 303, and 308 showed moderate effect. When ASAT, lactate dehydogenase (LDH), albumin levels in serum, and concentration of protein in liver tissue after injection of 303 were measured, the hybrid did not show any hepatotoxicity.
In 2008, nitrate–THA hybrids 310–317 with shorter and longer diamine side chains were reported [65]. All hybrids retain the ChE inhibitory effect of THA, with the most interesting being 310 (IC50 9.1 nM) and 314 (IC50 7.7 nM). In in vivo tests on the scopolamine-induced cognition impairment animal model, hybrids 295, 303, and 310 showed improving recognition activity whereas the analogue 317 did not. This result indicates that the nitrate group of 303 may not only contribute to the vessel relaxant activity, but also is essential for the ChE inhibitory effect. Finally, 295 and 303 did not show obvious signs of hepatotoxicity.
5. Tacrine Hybrids with Biological Active Organic Scaffolds
5.1. Tacrine–Phenothiazine Hybrids
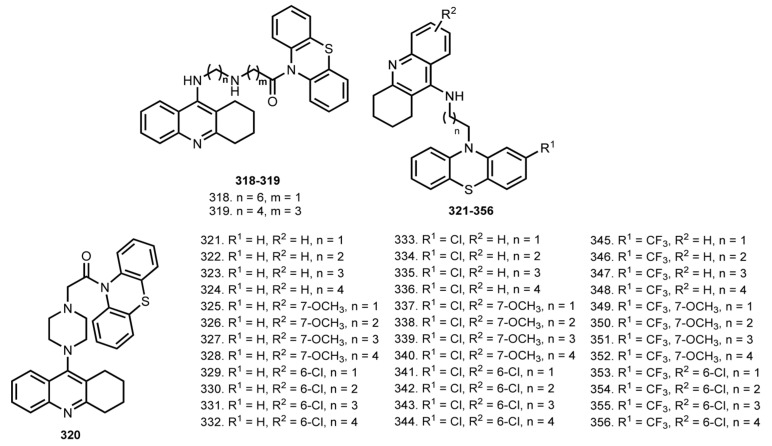
Phenothiazine is a first-generation heterocyclic anti-psychotic medication that can also prevent tau filament formation [155]. In 2014, Hui et al. reported THA–phenothiazine heterodimers 318–320, which were designed based on molecular docking simulation [66] (Figure 12). Hybrid 318 was the most potent AChE inhibitor with IC50 89 nM. Hybrid 318 proved the ability to reduce P-Tau accumulation in N2a cells, and the ability of 318 to bind Aβ fibrils was confirmed using surface plasmon resonance (SPR).
Figure 12.
In 2021, Gorecki et al. reported THA–phenothiazine heterodimers 321–356 [67]. All hybrids were potent hChEs inhibitors. 6-Cl-THA-based derivatives were more potent on hAChE (IC50 8–1500 nM) than THA analogues. The most selective compounds were 332 with IC50 8/190 nM and 321 with IC50 2040/15 nM. All compounds showed a toxicity on HePG2 cells in the micromolar range. Hybrids 330, 332, 336, and 344 proved their potential ability to cross the BBB. 6-Cl-THA-based hybrids showed the ability to inhibit τ (306–336) aggregation; the chain length was found to influence the inhibitory potency, with optimal 2–3 methylene units. Length of the linker also proved to be crucial in self-induced Aβ aggregation, with hybrids 332 and 335 showing the best inhibitory potency. Finally, in vivo safety studies revealed a good tolerance of hybrid 332.
5.2. Tacrine–Benzotiazole/Benzofuran Derivatives
Hybrids with Aβ-affinic benzofuran/benzotiazole moieties were repeatedly designed as dual action drugs capable of both Aβ binding and cholinesterase inhibition (Figure 13). Both benzotiazole and benzofuran are well-known scaffolds for Aβ binding; thus, 11C-Pittsburgh Compound-B (PiB) is a non-invasive tool for amyloid imaging in humans [156,157]
Figure 13.
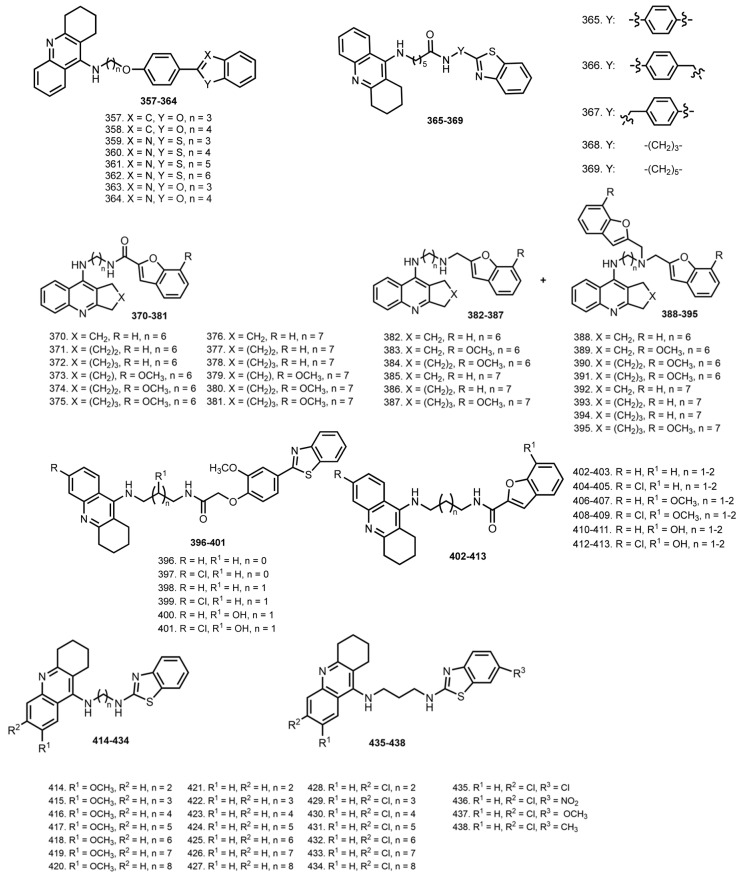
THA–benzofuran 357 and 358 and THA–benzotiazole hybrids 359–364 [68], THA–benzotiazole hybrids 365–369 [69], THA–benzofuran hybrids 370–395 [70], THA–benzotiazole hybrids 396–401 [71], THA–benzofurane hybrids 402–413 [72], THA–benzotiazole hybrids 414–434 and 435–438 [73].
Pioneer THA–benzofuran/benzotiazole conjugates were reported by Huang et al. [68]. Hybrids 357–364 inhibited ChEs with IC50 values in the micromolar range. Hybrid 359 exhibited the most potent inhibition of AChE with IC50 0.017 µM. Kinetics study of AChE inhibition showed 359 to bind both the CAS and PAS of AChE. The ability of hybrids to inhibit Aβ aggregation was assessed by the ThT assay, in which 359 demonstrated similar Aβ aggregation inhibitory activity with curcumin.
In 2013, Keri et al. also designed THA–benzotiazole hybrids 365–369 [69]. All hybrids displayed high inhibitory activities the against AChE enzyme, and with IC50 values in the micromolar range 365 was chosen as the lead compound with IC50 0.34 µM. All the compounds showed some ability to inhibit the Aβ42 self-aggregation, which was confirmed by ThT assay.
In 2016, Zha et al. reported hybrids 370–395 based on THA, as well as its analogues with different side cycle size, with benzofuran scaffolds [70]. The inhibitory activities against hAChE ranged from 7.49 µM to 0.86 nM. The most potent hybrid, 386, showed a subnanomolar inhibitory potency, 493 times more potent than THA. The most selective hBuChE inhibitors were hybrids with hexamethyl chain 388–391. Hybrid 386 was also the only derivative slightly selective for hAChE (2.5-fold). hBuChE inhibitory activity was associated with the presence of a 7-methoxy substituent on the benzofuran nucleus. The highest hBuChE inhibition was achieved with hybrids 384 and 390 (0.49 nM and 0.48 nM). Kinetic study of hAChE inhibition by 386 revealed mixed-type inhibition. Further, the ability of 386 to inhibit AChE-induced Aβ fibril formation was confirmed by a ThT assay. An inhibition of Aβ self-aggregation and an inhibitory potency against hBACE-1 were confirmed. Finally, treatment of scopolamine-induced ICR mice with 386 led to considerable amelioration in cognition impairment. In ASAT and ALT levels measurements in serum after treatment with similar doses of 386, THA and bis-THA were measured and revealed low hepatotoxicity of 386.
In 2019, THA–benzothiazole hybrids 396–401 were reported by Rajeshwari et al. [71]. The docking study revealed favorable interactions of hybrids with THA moiety binding CAS. All hybrids inhibited AChE (IC50 0.06–0.27 µM), but 397 exhibited the best inhibitory activity (IC50 0.06 µM). In addition, all hybrids inhibited Aβ self-aggregation. A neuroprotective effect of hybrids was confirmed on SH-SY5Y cells treated with Aβ peptide or ascorbate/iron. Hybrids 397, 398, and 401 prevented Aβ-induced cell toxicity; 396, 398, 399, and 401 also showed the ability to inhibit Aβ-self-aggregation. However, the log BB assessment showed that hybrids are not drug candidates for oral administration.
In 2020, Fancellu et al. reported THA–benzofurane hybrids 402–413 [72]. The best AChE inhibitors were 404, 408, and 412 with IC50 0.12, 0.13, and 0.13 µm. Additionally, all hybrids exhibited inhibitory activity in self-induced Aβ aggregation, and hybrids with the OH-group also showed high activity in Cu2+- induced Aβ aggregation. For 410, an anti-aggregating activity was also confirmed using transmission electron microscopic (TEM) images. Hybrids 408 and 412 prevented Aβ-induced cell toxicity, and 408 also showed cell protection from Asc/Fe-induced oxidative stress.
In 2021, Nepovimova et al. reported THA–benzotiazole hybrids 414–438 [73]. All hybrids were potent hAChE inhibitors with IC50 values in the micromolar to nanomolar range. Hybrids based on 7-MEOTA (414–420) displayed the poorest inhibition of hAChE; THA-based hybrids (421–427) showed moderate results, whereas hybrids based on 6-Cl-THA (428–434) were the best hAChE inhibitors. Based on a set of test results, hybrid 436 was chosen as lead, and its interactions with AChE were simulated by molecular docking. 6-Cl-THA moiety was found to occupy the PAS of hAChES, in contrast to its previously reported CAS binding [139]. The antiaggregating potential of 414–434 was confirmed using ThT assay. Additionally, the inhibition effects of 416 and 429 were confirmed using steady-state fluorescence and microscopy techniques. In addition, 436 showed the lowest hepatotoxicity, which was confirmed by the MTT test. The BBB penetration ability of hybrids was confirmed by a PAMPA-BBB test. Finally, an insignificant therapeutic effect of 436 was observed in scopolamine-treated mice.
5.3. Tacrine Hybrids with NSAIDS
Inflammation is an important therapeutic target, and is one of the important factors in clinical symptoms of AD [158]. Thus, nonsteroidal anti-inflammatory drugs (NSAIDs) are of interest in AD therapy [159]. NSAIDs were reported to reduce inflammatory markers and reverse spatial memory deficits in APPsw transgenic mice or improve memory and learning, and decrease stress-related behaviors in FAD5X/Ppara-null mice [160,161,162].
In 2013, Chen et al. reported THA–flurbiprofen hybrids 439–443 [74] (Figure 14). Hybrids 442 and 443 showed high activity toward both ChEs. Additionally, hybrid 442 showed a reduction in Aβ40 formation.
Figure 14.
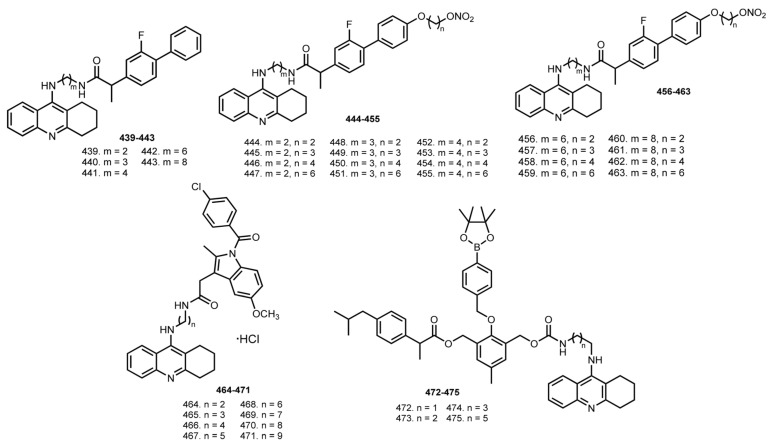
THA–flurbiprofen hybrids 439–443 [74], THA–flurbiprofen–NO–donating hybrids 444–455 [75], 456–463 [76], THA–indometacine hybrids 464–471 [77], ROS–responsive ibuprofen–THA hybrids 472–475 [78].
Similar hybrids 444–455 fortified with NO-donating ability were designed [75]. All hybrids showed comparable or better BuChE inhibitory activity (IC50s 3.9–13.9 nM) than parent hybrids 439–441. The best results were obtained for 447 and 455, with IC50 4309.5 and 1456.4 nM against AChE. Kinetic study revealed a mixed-type inhibition of 447. All hybrids showed promising levels of nitrite generated in Griess reactions. Vasorelaxation activity of 447 and 455 was confirmed in an ex vivo organ bath (coronary arteries from rats).
The same scientific group reported hybrids 456–463 [76]. All hybrids (ex. 463) exhibited similar or higher inhibitory activities compared with THA. The most potent hybrids were 456 and 460 with IC50 9.1 and 12.5 nM on AChE and IC50 2.5 and 1.0 nM against BuChE. Kinetic study of ChE inhibition for 456 revealed mixed-type inhibition. Griess reaction revealed the NO-releasing ability of all hybrids 456ȓ463. A vascular relaxation effect of 456 and 460 was confirmed on the coronary arteries of rats.
Behavior studies in vivo were performed using a scopolamine-induced impairment in passive avoidance test. An improving memory impairment in the group treated parent with 442 (hybrid without NO-donating group), and the group treated with 456 was observed compared to the scopolamine group. Hybrid 456 showed no difference in comparison with THA and hybrid 442 (p > 0.05). ASAT and ALT levels were determined after mice were treated with THA, hybrids 442, and 456 at equimolar doses. Hybrids 442 and 456 displayed higher safety than THA, and NO-donating hybrid 456 showed the lowest hepatotoxicity.
In 2021, Zawada et al. reported THA–indometacine hybrids 464–471 [77]. The IC50 values for hybrids range from 10 to 260 nM. The most active compound against AChE was 471 (IC50 10 nM). Kinetic study revealed a mixed-type inhibition for 471. A low toxicity on the HepG2 and EA.hy926 cells was revealed. Moreover, 471 showed antioxidant effects in DDPH and ABTS studies.
In 2021, Liu et al. designed ROS-responsive ibuprofen–THA hybrids 472–475 [78]. Low neurotoxicity of hybrids was proven on SH-SY5Y cells. Neuroprotective activities of 475 against H2O2, and H2O2-scavenging capability were shown. An ability of 475 to degrade into counterparts in the presence of H2O2 was confirmed by HPLC.
Hybrids 472–475 showed moderate or no obvious AChE inhibitory activity in the absence of H2O2. An ability of 475 to inhibit proinflammatory cytokines TNF-α and IL-1β in endotoxin lipopolysaccharide (LPS)-treated microglial cells (BV-2) was revealed, as well as the regulating of apoptosis-related proteins. In addition, 475 showed neglected hepatotoxicity in HepG2 cells. Finally, the therapeutic effect of 475 and the improving spatial memory of Aβ-induced AD model rats were confirmed.
5.4. Tacrine–Hupyridone Hybrids
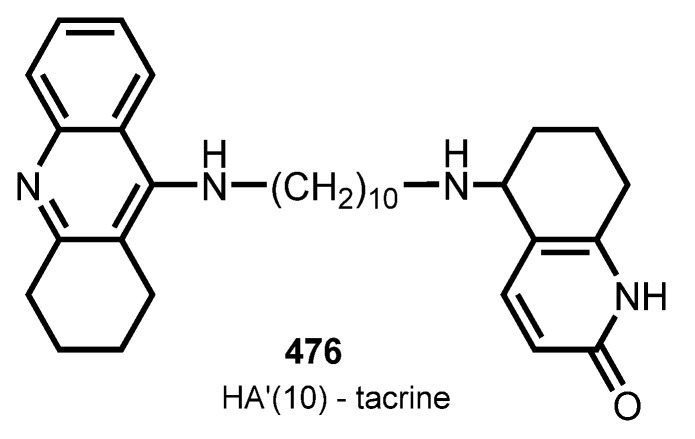
Pyridones have been utilized as privileged scaffolds in drug discovery [163]. THA–hupyridone hybrids were first described in 1999 [164,165]. In 2007, Li et al. summarized a study of therapeutic efficacy of several THA dimers, including THA–hupyridone hybrids [166]. In addition, in 2021 Mak et al. summarized multifunctional dimers, including THA–hupyridone [167]. Herein, we will provide the most potent hybrid HA’(10)–THA 476, first described by Carlier et al. [164] (Figure 15).
Figure 15.
Hybrid HA’(10)–THA 476 [164].
476 possessed a nanomolar AChE inhibition (IC50 8.8 nM). Since both THA and huperzine A can increase the expression of brain-derived neurotrophic factor (BDNF) in the brain, 476 was also suggested to elevate BDNF expression concurrently [168,169].
In 2018, Chen et al. reported 476 to prevent the surgery-induced decrease in BDNF in the hippocampus of aged mice [79]. Hybrid 476 might act on BDNF to enhance cognitive performance. Additionally, 476 proved to increase the expression of pAkt and pERK, and ChAT-positive area in the hippocampal regions of surgery-treated mice proved to effectively attenuate scopolamine-induced cognitive impairments in vivo and be less toxic than THA [80].
Recently, Xuan et al. reported that 476 produces cognitive-enhancing effects in APP/PS1 and Aβ oligomers-treated mice [81]. Neuroprotective effects of 476 were proved, including the inhibition of Aβ aggregation, the activation of the BDNF/TrkB pathway, the alleviation of neuroinflammation, and the decrease in AChE activity.
5.5. Tacrine–Donepezil Hybrids
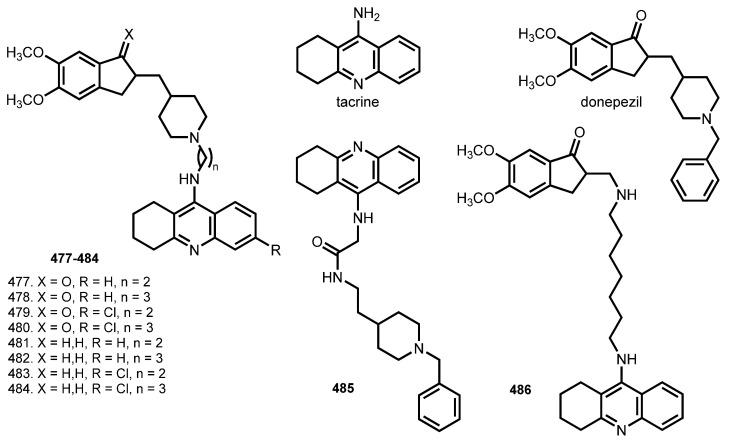
Donepezil is a specific and reversible inhibitor of AChE, and is an FDA-approved drug for the symptomatic treatment of AD [170]. THA–donepezil hybrids were designed by different scientific groups. The first donepezil-THA hybrid was reported in 2004 by Shao et al. [82].
Camps et al. reported [83] donepezil-THA hybrids in order to obtain more effective AChE inhibitors than previously reported by Shao et al. and Alonso et al. in 2004 [82,171] (Figure 16).
Figure 16.
Donepezil, THA, donepezil–THA hybrids 477–484 [83], and previously reported donepezil–THA hybrids 485, 486 [82].
All hybrids were hAChE inhibitors, exhibiting IC50 values in the subnanomolar range. Hybrids 477–484 were more potent AChE inhibitors than were 485 (IC50 6.0 nM) and 486 (IC50 25 nM). Hybrid 480 was the most effective inhibitor (IC50 90 pM). Hybrids 477, 478, 481, and 482 were more potent BuChE inhibitors than was 485 (IC50 76 nM), though none of them was as potent as 486 (IC50 0.6 nM). Hybrids 479 and 480 proved their ability to bind with PAS via displacement of ThT. In addition, six out of the eight hybrids exhibited an Aβ antiaggregating activity.
5.6. Tacrine–TPPU Hybrids
1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea (TPPU) is a potent soluble epoxide hydrolase (sEH) inhibitor [172]. sEH are able to metabolize epoxyeicosatrienoic acids (EETs), which reduce inflammation and oxidative stress, by epoxide ring opening to the corresponding diols by the soluble epoxide hydrolase [84]. sEH inhibition is a promising strategy for the treatment of pain, inflammation, cardiovascular diseases, and other conditions [173].
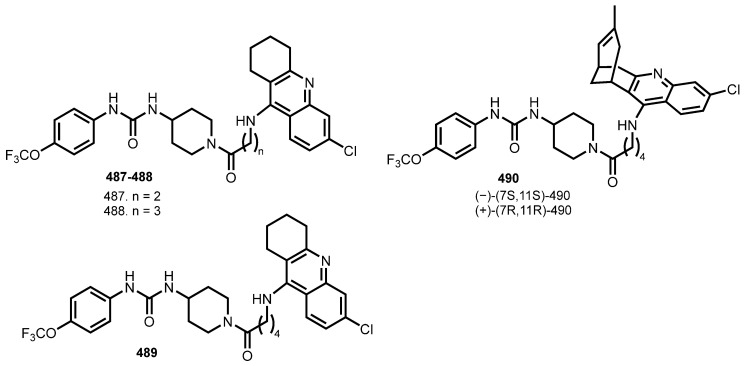
In 2022, Codony et al. reported hybrids 487–489 with dual targeting of sEH and AChE [84] (Figure 17). Dual inhibitors were designed by linking the scaffolds of TPPU, 6-Cl-THA, and huprine.
Figure 17.
6-Cl-THA–TPPU 487–489 and huprine–TPPU hybrids (−)-(7S,11S)-490, (+)-(7R,11R)-490 [84].
Most of the hybrids displayed well-balanced potencies in the low nanomolar range when tested in vitro on the two recombinant human enzymes, hsEH and hAChE. All hybrids retained the hsEH inhibitory activity of TPPU (IC50 3.7 nM) with IC50s in the subnanomolar to low nanomolar range, with hybrids 487 and (−)-490 displaying an even higher potency. Regarding hAChE inhibition, 487 and 489 retained the potency of 6-Cl-THA, and 488 was five-fold more potent. (−)-(7S,11S)-490 proved to be 850-fold more potent than its enantiomer, in line with the eudismic ratio of huprine Y. Hybrid 489 was chosen as the lead, with IC50 12.9 nM against hAChE and IC50 179 nM against hBuChE. Molecular dynamics simulations revealed 489 to interact with both sites of AChE. Hybrid 489 was the most stable compound in human microsomes. In vivo investigation in senescence-accelerated mouse-prone 8 (SAMP8) revealed a significant amelioration in short-term and long-term working memory after oral administration of 489 (2 mg kg/day).
5.7. Tacrine–Huprine Hybrids
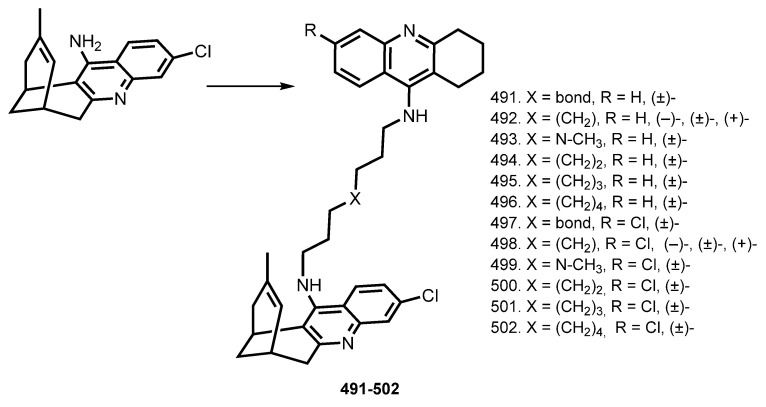
Huprine is a potent AChE inhibitor based on THA scaffold [174]. In 2012, Galdeano et al. reported enantiopure huprine—THA heterodimers 491–502 [85] (Figure 18). Hybrids in racemic form (±)-491, (±)-493–(±)-497, and (±)-499–(±)-502, as well as the enantiopure (−)-(7S,11S)- and (+)-(7R,11R)-heptamethylene-linked heterodimers (−)-492, (+)-492, (−)-498, and (+)-498 were synthesized and their biological activities were investigated.
Figure 18.
Huprine−tacrine heterodimers 491–502 [85].
Expectedly, ChEs inhibitory activity was governed by spacer length. The levorotatory (7S,11S)-huprine-based heterodimers were the eutomers with regard to hAChE inhibition, with (−)-492 and (−)-498 being five- to six-fold more potent than the dextrorotatory enantiomers. The most potent hybrids were racemic (±)-493 and (±)-499, and (±)-491 and (±)-497. Additionally, heterodimers inhibited hAChE-induced Aβ aggregation and blocked the chaperoning effect of AChE on PrP106−126 aggregation. An activity of heterodimers (−)-492, (+)-492, (±)-492, (±)-494, and (±)-495 toward self-induced Aβ aggregation and BACE-1 inhibition of hybrids (±)-496, (−)-498, (+)-498, and (±)-500–(±)-502 were revealed. Finally, ex vivo experiments proved the ability of (±)-494 and (±)-500 to cross the BBB and inhibit in-brain AChE activity.
5.8. Tacrine–Bifendate Hybrids
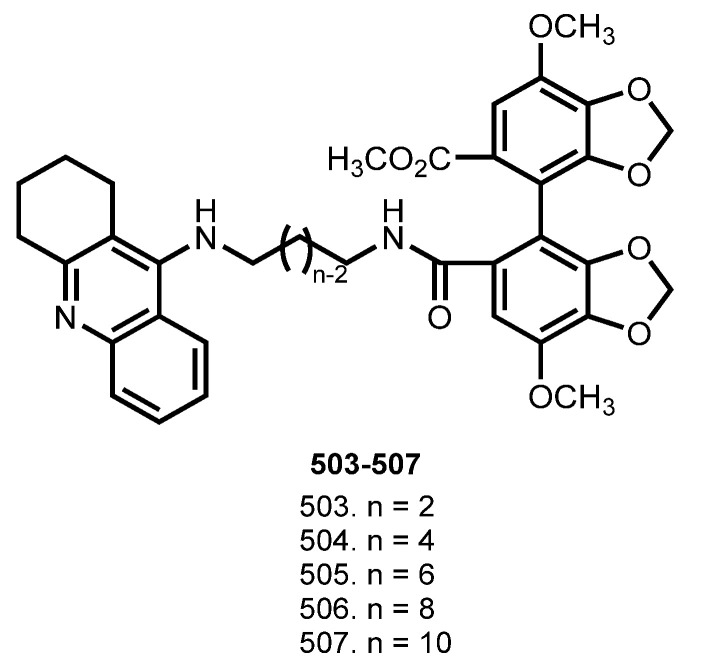
In 2018, Cen et al. reported hybrids of THA with Bifendate (503–507), which is used for the treatment of chronic viral hepatitis B in China, and which was also reported to protect the liver mitochondria in mice from THA-induced injury [86,175,176] (Figure 19).
Figure 19.
THA -Bifendate hybrids 503–507 [86].
Hybrids 503–507 showed potent inhibitory activities at the nanomolar concentrations and good selectivity for BuChE. Hybrid 506 was the most potent AChE inhibitor (IC50 27.32 nM). Hybrid 504 was the most potent inhibitor of BuChE (IC50 4.02 nM). In addition, 506 showed a high inhibition of hAChE. Hybrids 503–507 prevented the self-mediated Aβ aggregation, and the antiaggregating potential of 506 was confirmed by TEM study. Low hepatotoxicity of 506 was confirmed on HepG2 and HL-7702 cells. No increases in ALT and ASAT levels were observed after the administration of 506 in mice, but amelioration of the cognition functions in the scopolamine treated ICR mice was proven.
5.9. Tacrine hybrids with HDAC Inhibitors
Histone deacetylases (HDACs) are generally considered as therapeutic targets in the treatment of AD. The roles of histone deacetylases HDACs on cognitive impairments have been demonstrated in studies of AD animal models [177]. Furthermore, different types of HDACs may have distinct roles in the cognitive changes of AD.
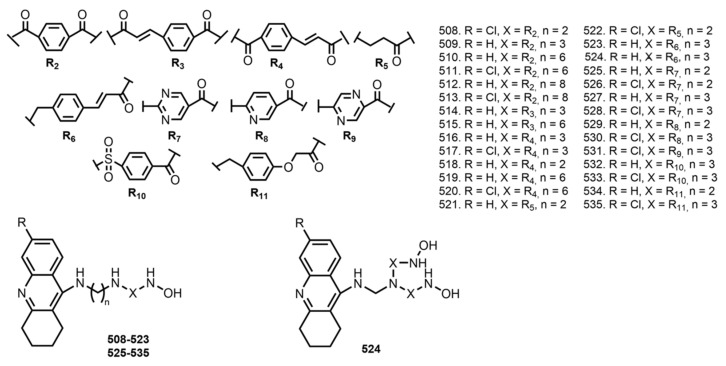
In 2020, Xu et al. reported THA-based hybrids 508–535 with HDAC inhibitors [87] (Figure 20). Well-established pharmacophore models such as SAHA, LBH589, and PXD101 [178] were used.
Figure 20.
THA (6-Cl-THA)–HDAC inhibitors hybrids 508–535 [87].
All hybrids inhibited ChEs, with improved inhibition on AChE compared to THA. Hybrids 517 and 535 were the most potent inhibitors of AChE (IC50 0.12 and 0.26 nM). The inhibitory potency on HDACs of hybrids 508–535 was determined against HeLa nuclear extract. Hybrids 511, 517–520, 523–524, 528, and 535 showed superior or comparable inhibitory potency when compared with reference compounds SAHA or PXD101; hybrids 517, 520, and 524 were the most potent, with IC50 0.23, 0.32, and 0.28 nM. An antioxidant activity of all hybrids (except 520) was confirmed using ABTS assay. Hybrids 508–535 also exhibited an inhibition of Aβ self-aggregation. An ability of hybrids to chelate copper ions was confirmed. Kinetic study of AChE inhibition by 517 revealed mixed–type inhibition. Additionally, hybrids 512, 517, 519, and 524 were predicted as a BBB penetrant.
5.10. Tacrine Hybrids with Thio Derivatives
Modulation of synaptic plasticity, especially the long-term potentiation (LTP), has been proposed as a potential therapeutic strategy for improving cognitive function of AD patients [179]. Compounds with mercapto group, such as dithiothreitol (DTT), glutathione (GSH), and N-acetyl cysteine (NAC), can facilitate the induction of LTP in normal rats and even reverse the LTP impairment in aged rats [180]. Inspired by this, several THA hybrids with thio derivatives were reported (Figure 21).
Figure 21.
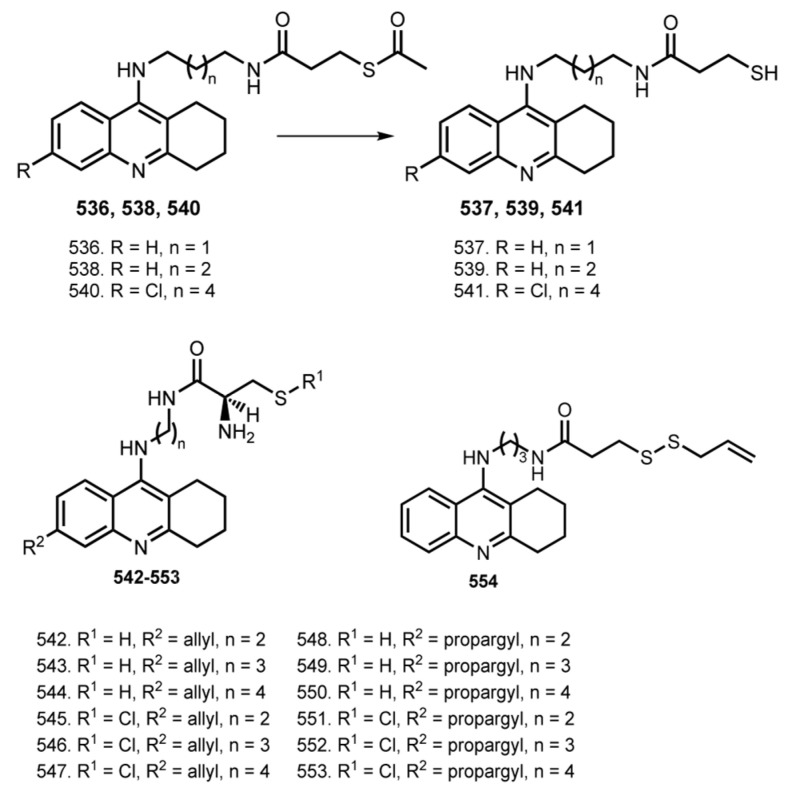
THA derivates conjugated with mercapto group 536–541, designed by Wang et al. [88], THA and 6-Cl-THA–based hybrids 542–553 with allyl and propargyl derivatives of cysteine [89], hybrid with H2S-releasing moiety 554 [90].
In 2012, Wang et al. designed THA derivates 536–541 conjugated with mercapto group [88]. Hybrids generally retained the ChE inhibitory effect, and 540 displayed the most potent inhibitory activity against AchE with pIC50 7.37 ± 0.02. Mercaptotacrine derivatives were more potent inhibitors of BuchE than AchE, similar to that of THA, except for 540. Effects of hybrids on high-frequency stimulation (HF)-induced LTP in the CA1 region of Sprague−Dawley rat hippocampal slices were evaluated and an increase in the magnitude of LTP during the incubation of hippocampal slices with 537 and 541 was detected. Additionally, an enhanced hippocampal LTP after intracerebroventricular (icv) injection of 541 was detected in vivo. A neuroprotective action of 536, 538, and 540 against H2O2 -induced oxidative stress was proven on human neuroblastoma cell line SH-SY5Y. All hybrids showed neuroprotective effects in a concentration-dependent manner, whereas hybrids 537, 539, and 541 presented a U-shaped dose-protection dependency. Finally, AST and ALT activities in serum samples were measured after administration of the drugs in vivo; hybrids 536 and 537 showed little hepatotoxicity.
In 2016, Keri et al. reported THA and 6-Cl-THA-based hybrids 542–553 with allyl and propargyl derivatives of cysteine [89]. Among the compounds investigated, the 6-Cl-THA-based hybrids presented high inhibitory activity in the submicromolar range, with the most active being 545 (IC50 0.30 µM against AChE). Hybrids 542, 547, 552, and 553 showed neuroprotection from H2O2-mediated oxidation on SH-SY5Y cells.
Levels of H2S and activity of its synthesized enzyme cystathione β-synthase (CBS) are severely reduced in the brains of AD patients [181,182]. Treatment with H2S or a H2S donor improves cognitive function in AD patients and rat models [183].
In 2019, Cheng et al. reported THA-based hybrid 554 with H2S-releasing moieties (ACS81) [90]. Hybrid 554 improved cognitive and locomotor activity in AD mice, while also reducing inflammation and increasing synaptic plasticity in the hippocampus. Furthermore, hepatotoxicity studies confirmed that 554 was much safer than THA. Treatment with 554 was able to inhibit the AChE levels in the serum and hippocampus of AlCl3-treated AD mice with comparable effects to THA. Additionally, 554 inhibited hippocampal inflammation, as evidenced by the decreased mRNA expression of proinflammatory cytokines (TNF-α, IL-6, and IL-1β). Hybrid 554 also increased hippocampal H2S levels, decreased inflammation, and improved synaptic plasticity in the hippocampus. Importantly, 554 did not show evidence of hepatotoxicity or liver inflammation as measured by hepatic transaminases and proinflammatory cytokines.
5.11. Tacrine Hybrids with Fluorescent Probes
PI is well-known to bind the AChE [184]. Inspired by PI structure, in 2009 Camps et al. designed 6-Cl-THA-based hybrids 555–564 with 5-phenylpyrano [3,2-c]quinoline [91] (Figure 22). All hybrids showed nanomolar activity toward ChEs. The most potent hAChE inhibitor was 557. Hybrids 560–564 turned out to be two- to three-fold more potent toward the human enzyme, with no significant dependency on the length of the linker. Hybrids 560–564 expectedly proved to be more potent BuChE inhibitors that chloro-substituted 555–559, with IC50 values in the nanomolar range, up to three- to four-fold more potent than 6-Cl-THA. Molecular modeling and kinetic studies confirmed the dual site binding to hAChE. Hybrids 560, 563, and 564 can be considered as moderate inhibitors of Aβ self-aggregation. In addition, 564 showed as a potent BACE-1 inhibitor. Finally, these hybrids are able to cross BBB according to PAMPA-BBB assay.
Figure 22.
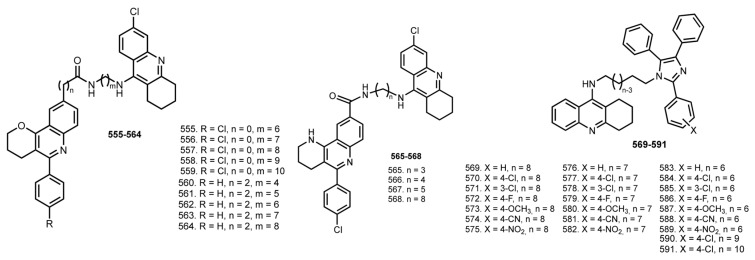
6-Cl-THA-5-phenylpyrano [3,2-c]quinoline hybrids 555–564 [91], 6-Cl-THA–tetrahydrobenzo[h][1,6]naphthyridine 565–568 [92], THA–lophine hybrids 569–591 [93].
In 2014, Pietro et al. reported similar THA hybrids with tetrahydrobenzo[h][1,6]naphthyridine [92].
All the 6-Cl-THA-based hybrids turned out to be potent inhibitors of hAChE, with 565 being the most potent (IC50 6.27 pM). Additionally, 565–568 exhibited an inhibition of Aβ42 and tau aggregation. All hybrids were predicted to cross the BBB.
In 2013, Costa et al. reported THA-based hybrids 569–591 with 2,4,5-triphenyl-1H-imidazole (lophine), which can be used as a fluorescent-labeling reagent and was reported as a ChE inhibitor [93,185]. Hybrids 569–591 were found to be potent inhibitors, with IC50 in the nanomolar range. The most active AChE inhibitor was 570 (IC50 5.87 nM) and the most active BuChE inhibitor was 581 (IC50 7.10 nM), which was inactive toward AChE.
5.12. Tacrine Hybrids with Ca2+ Channel Blocker
In 2006, Marco-Contelles designed hybrids 592–600 in which the aromatic moieties of THA are surrogated to nimodipine-like moiety [94] (Figure 23). Nimodipine is an FDA-approved selective blocker of L-type voltage-dependent Ca2+ channels [186].
Figure 23.
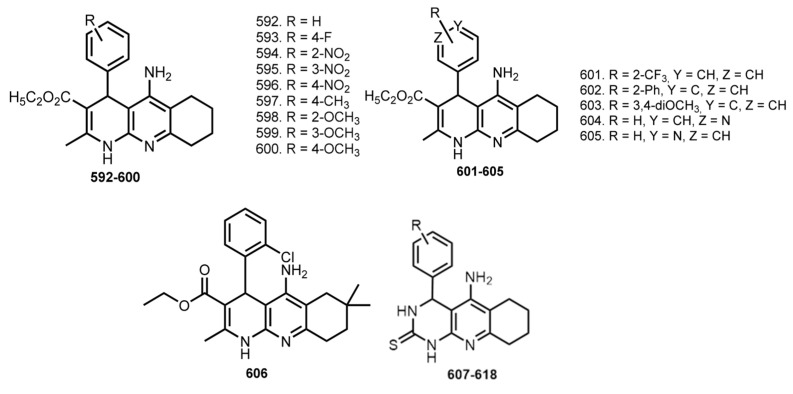
THA–nimodipine hybrids 592–600, 601–605 [94,95], THA–dihydropyridine hybrid 606 [97], THA-dihydropyirimidine-thione hybrids 607–618 [98].
The most potent inhibitor of AChE was 600 (IC50 45 nM). Hybrids 593 and 599 were also of high potency and excellent selectivity for AChE. A Ca2+ influx induced by K+ depolarization in SH-SY5Y cells was evaluated. Most of the hybrids demonstrated a significant Ca2+ blockade, with the most potent being 598 with a blockade similar to that obtained for nimodipine. Finally, 593, 599, and 600 showed neuroprotective properties against Ca2+ overload and H2O2-induced oxidative stress on SH-SY5Y cells.
In 2009, an expanded series of THA–nimodipine hybrids (601–605) was reported [95]. Most of the tacripyrines were more potent inhibitors of AChE than THA. Hybrid 600 was again chosen as the lead compound (IC50 45 nM). Molecular modeling results showed binding of (R)-600 with PAS. Hybrid 600 proved to be an inhibitor of AChE-induced Aβ40 aggregation and Aβ42 self-aggregation. Most hybrids promoted significant Ca2+ blockade, with the most potent being 604, whose activity was similar with nimodipine. PAMPA-BBB assay showed that almost all tacripyrines could cross the BBB and reach their biological targets.
In 2011, the same scientific group provided the pharmacological analysis of both enantiomers of 600 [96]. Both enantiomers showed similar results in inhibiting cholinesterase activity, AChE-induced Aβ aggregation, and Aβ self-aggregation in vitro. (S)-600 afforded significant protection against Aβ25–35-induced toxicity when tested on SH-SY5Y cells.
In 2015, Xiu-Lian et al. reported a similar THA-based hybrid 606 [97]. Hybrid 606 in low concentrations proved its ability to reduce tau phosphorylation levels, which was confirmed on HEK293/tau cells. In addition, its ability to inhibit the generation and release of Aβ was confirmed on mouse neuroblastoma N2a/APP cells.
In 2018, hybrids of THA with dihydropyirimidine-thiones 607–618 were reported [98]. Most tacripyrimidines showed selectivity for hBuChE with IC50 from 0.372 mM (616) to 154 mM (614). Additionally, most tacripyrimidines 607–618 inhibited hAChE with IC50 from 3.05 mM (611) to 31.0 mM (615). The most selective and potent hAChEI was 617 (hAChE: IC50 0.0373 mM). All tacripyrimidines except 616 significantly inhibited Ca2+ influx induced by K-depolarization in SH-SY5Y cells. A hepatotoxicity study revealed most tacripyrimidines to be similarly or slightly less toxic than THA. Finally, 611 was chosen as a well-balanced inhibitor of ChEs and a calcium channel blocker, with no toxicity toward HepG2 cells up to 300 mM and excellent predicted oral absorption and BBB permeability.
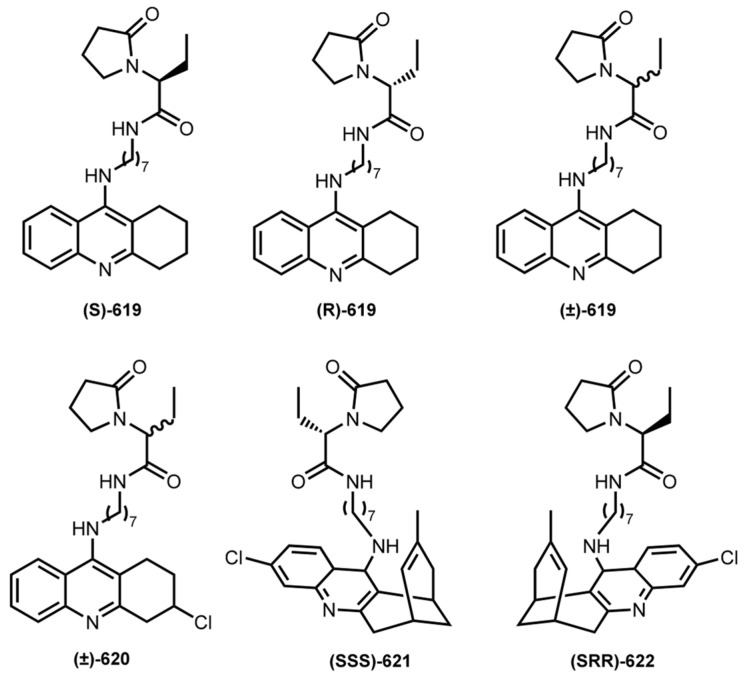
In 2015, Sola et al. reported hybrids 619–622 based on a THA, 6-Cl-THA, or huprine Y with 2-(2-oxopyrrolidin-1-yl)butyramide moiety of levetiracetam, an antiepileptic drug [99] that was reported to improve memory performance in mouse models of AD [187] (Figure 24).
Figure 24.
THA (S)-619, (R)-619, (±)-619, 6-Cl-THA (±)-620, huprine Y (SSS)-621, and (SRR)-622–based hybrids with 2-(2-oxopyrrolidin-1-yl)butyramide moiety of levetiracetam [99].
All hybrids were potent inhibitors of hAChE, with IC50 in the low nanomolar range. Huprine is a stereoactive drug, with (7R,11R)-huprine derivatives being more potent hBuChE inhibitors than (7S,11S)-counterparts [188]. Expectedly, 621 and 622 showed different inhibition activity. However, no differences in inhibitory activity of (7S,11S)-huprine Y hybrids were detected. Hybrids 621 and 622 exhibited a moderately potent Aβ42 and tau antiaggregating activity. The inhibition of mouse brain AChE after i.p. administration of the levetiracetam-based hybrids was also confirmed. A significant reduction in the frequency of spontaneous convulsions in APP/PS1 mice treated with the levetiracetam-huprine hybrid 621 was revealed. APP/PS1 mice treated with hybrids 619 and 621 exhibited significantly increased recognition indices when compared to vehicle-treated animals. In addition, immunohistochemical determination revealed a reduction of the Aβ burden in the cortex of APP/PS1 mice after chronic treatment with 621. Finally, chronic treatment with 621 led to a significant reduction of GFAP positive astrocytes around Aβ plaques and Iba1 positive microglial cells in APP/PS1 mice.
6. Tacrine Hybrids with Modulators of Cholinergic/Serotonergic System
6.1. Tacrine Hybrids with Modulators of Serotonin Receptors
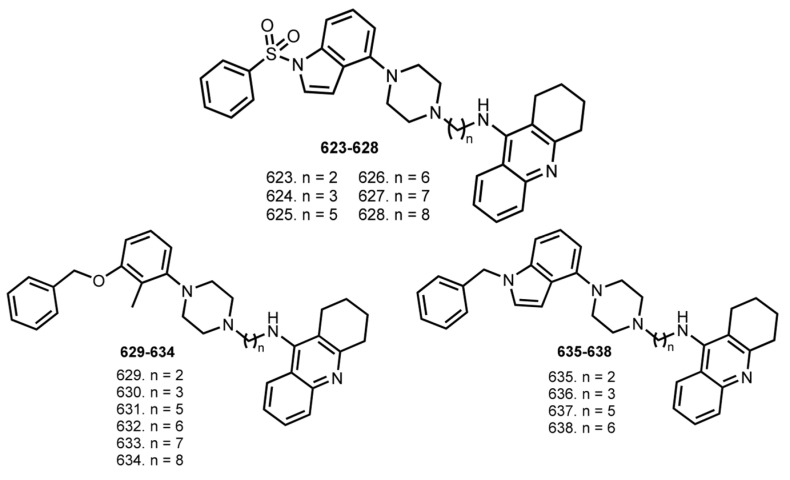
In 2016, Wigockowska et al. designed hybrids 623–628 as potential ChE inhibitors and 5-HT6 antagonists. As a 5-HT6 antagonist, 1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole was chosen (Figure 25) [100].
Figure 25.
THA-1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole hybrids 623–628 [100], THA–5HT6-agonist hybrids 629–638 [101].
All hybrids displayed high affinities for 5-HT6 receptor in the low nanomolar range. IC50 values for hybrids were in the range from 7.1 to 57.0 nM for AChE and from 8.2 to 21.3 nM for BuChE. Hybrid 626 was chosen for its balanced activity as lead compound based on a set of test results. Hybrid 626 significantly diminished serotonin-induced calcium mobilization, confirming its 5-HT6 antagonistic properties (Kb = 27.0 nM). A kinetic study revealed a non-competitive type of inhibition of AChE/BuChE. Hybrid 626 decreased the rats’ locomotion activity and reduced scopolamine-induced hyperlocomotion in rats.
In 2018, an expanded library of similar hybrids 629–638 was synthesized [101]. The affinity of hybrids for recombinant human 5-HT6 receptor was found to increase with the linker length and reached a Ki value of 18 nM for 632, which was confirmed by a radioligand binding assay. All hybrids were potent ChEs inhibitors, with the most active being 634 (IC50 50 nM against AChE) and 632 (IC50 14 nM against hAChE). A kinetic study revealed a non-competitive mode of action for 632. Further, the inhibitory effect on Aβ aggregation was determined by ThT assay, and the most active hybrids were 632, 633, 635–638. A PAMPA-BBB test showed a possible effective CNS permeability of hybrids. An in vitro metabolic stability study on human liver microsomes did not detect any hepatotoxic metabolites.
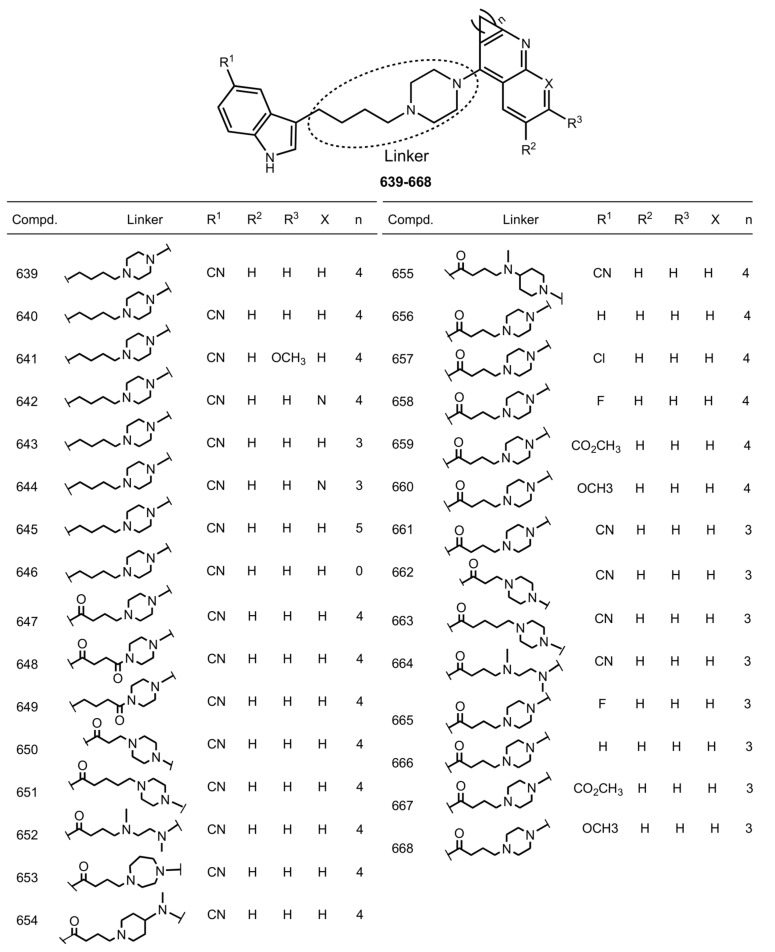
In 2017, Li et al. reported hybrids of THA with Vilazodone 639–668, an inhibitor of serotonin reuptake and partial agonist of 5-HT1A receptor [102,189] (Figure 26).
Figure 26.
THA–Vilazodone hybrids 639–668 [102].
Activities of hybrids such as 5-HT1A agonists and 5-HT reuptake inhibitors were evaluated; hybrid 643 showed relatively balanced activities against the three targets. Low hepatotoxicity of 643 was confirmed on HepG2 cells. Low cardiotoxicity of 643 was confirmed by hERG activity inhibition. The antidepressive effect of 643 was confirmed by the tail suspension test in vivo, and cognitive improvements were detected in scopolamine-treated mice.
6.2. Tacrine Hybrids with Modulator of Muscarinic Receptors
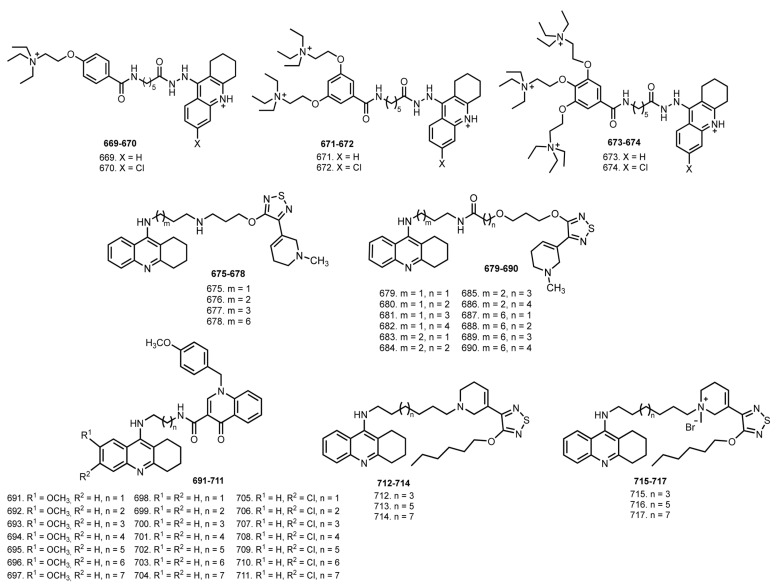
In 2007, Elsinghorst et al. reported THA-based hybrids 669–674 with gallamine, an archetypal muscarinic allosteric agent [103] (Figure 27). An increasing substitution of the gallamine-derived moiety tends to reduce the inhibitory potency. Hybrid 669 was found to be a potent inhibitor of AChE (IC50 500 pM). The interaction of the building blocks and hybrids 669–674 with M2 receptors was measured in receptors whose acetylcholine binding site was blocked by the radioligand [3H]NMS. Muscarinic allosteric ligands typically retard the dissociation of [3H]NMS by allosteric binding to [3H]NMS-occupied receptors, thereby prolonging the incubation time needed for reaching [3H]NMS equilibrium binding. All hybrids restrict [3H]NMS dissociation. Finally, hybrids showed an increase in the allosteric potency by factors of 100 relative to gallamine and 4800 relative to THA.
Figure 27.
Gallamine–THA hybrids 669–674 [103], THA–xanomeline hybrids with amine linker 675–678 and amide linker 679–690 [104], 7-MEOTA–BQCA hybrids 691–697, THA–BQCA hybrids 698–704, 6-Cl-THA–BQCA hybrids 705–711 [105], and THA–xanomeline hybrids 712–717 [106].
In 2010, Fang et al. reported THA-xanomeline hybrids 675–790 [104]. Xanomeline is an M1 activator, an M1/M4-preferring orthosteric agonist with antidementive properties in vivo [190]. All compounds were potent inhibitors of both cholinesterases. The most potent compound was 690 with pIC50 8.21 against eeAChE. The affinity of hybrids for unliganded receptors was determined using the orthosteric radioligand [3H]N-methylscopolamine ([3H]NMS). All hybrids induced an allosteric inhibition of [3H]NMS dissociation. The most potent log KXdiss of 680 was more than three log units higher compared to xanomeline. In vivo studies in rats revealed the ability of 687 to significantly enhance scopolamine action.
In 2018, Hepnarova et al. reported hybrids of THA 691–711 with benzylquinolone carboxylic acid (BQCA; 1-(4-methoxybenzyl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid), a selective positive allosteric modulator of M1 mAChRs which does not interact with the Ach site with pro-cognitive action [105,191,192]. 7-MEOTA-based hybrids 691–697, THA-based hybrids 698–704 and 6-Cl-THA-based hybrids 705–711 were potent inhibitors of cholinesterases. The most active hAChE inhibitors in each family were found as follows; 696, 699, and 706 with IC50 1.5 µM, 0.13 µM, and 42 nM from each subset. Unfortunately, all hybrids exerted an antagonistic profile of M1 mAChR, instead of the expected agonostic profile.
In 2020, Maspero et al. reported THA hybrids 712–717 with xanomeline, a selective muscarinic acetylcholine receptor agonist and M1/M4 preferring muscarinic acetylcholine receptor activator [106,193]. Hybrids 712–717 were able to inhibit AChE; eight methylene units were optimal for the highest AChE inhibition. The most active inhibitor was 715 with pIC50 9.55. However, hybrids 712–717 were unable to activate the M1 receptor subtype.
6.3. Tacrine Hybrids with Cannabinoid CB1 Receptor Antagonists
Cannabinoid signaling systems are involved in a variety of physiological processes. The selective CB1 antagonist/inverse agonist drug Rimonabant is an FDA-approved drug to treat obesity and metabolic-related disorders [194]. In 2007, Wise et al. reported [195] a combination of rimonabant and donezepil, a CB1 antagonist and an AChE inhibitor, as an effective memory-enhanced therapy.
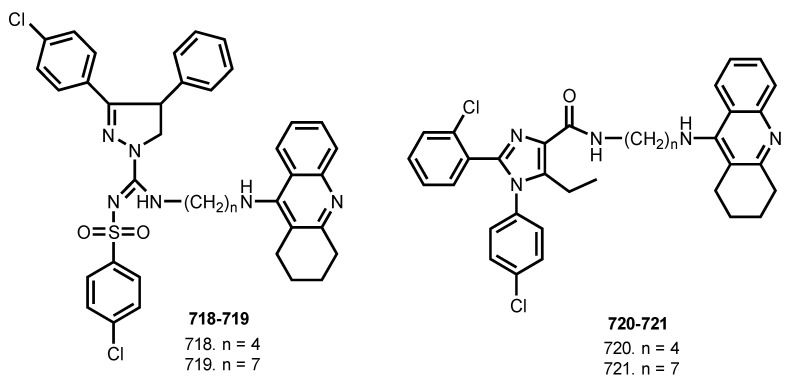
In 2010, Lange et al. reported hybrids of THA with cannabinoid CB1 receptor antagonists 718–721 [107] (Figure 28).
Figure 28.
AChEIs/CB1 receptor antagonists 718–721 [107].
Hybrids exhibited AChE inhibiting activities and significant cannabinoid CB1 receptor antagonistic properties. The most effective AchE inhibitor was 720 (pIC50 6.5). Hybrids 718–721 showed significant CB1 receptor affinities and, in general, acted as CB1 receptor antagonists, while 720 showed significant CB1 receptor affinity with Ki = 48 nM.
6.4. Tacrine Hybrids with Modulator of NMDA Receptors
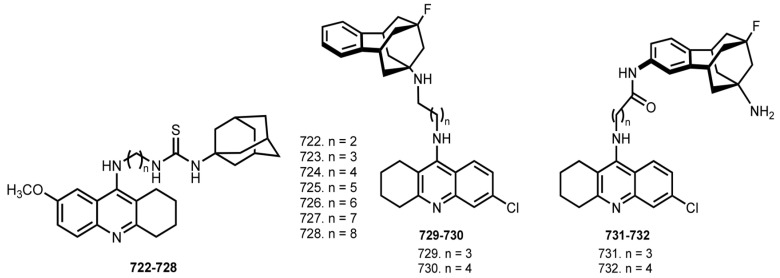
In 2013, Spilovska et al. designed 7-MEOTA-adamantylamine thioureas 722–728 [108] (Figure 29).
Figure 29.
7-MEOTA–adamantylamine hybrids 722–728 [108], benzohomoadamantane–6-Cl-THA hybrids 729–732 [109].
All hybrids exhibited good inhibitory activity toward ChEs. The most potent cholinesterase inhibitor was 725, with an IC50 0.47 µM for hAChE and 0.11 µM for hBuChE.
In 2019, Perez-Areales et al. designed benzohomoadamantane -6-Cl-THA hybrids 729–732 with unsubstituted amino groups [109]. All hybrids were potent hAChE inhibitors, 6- to 44-fold more potent than 6-Cl-THA. Hybrid 731 was the most potent hAChE inhibitor. The most potent hBuChE inhibitors were hybrids 730 and 732 (IC50 210 and 21 nM).
When the effects of hybrids on the increase in intracellular calcium evoked by NMDA in neurons loaded with Fura-2 was evaluated [196], the most potent NMDA antagonists were 730 and 731. Unfortunately, low BBB permeation for 731 and 732 was predicted, whereas when substituted at the bridgehead amino group 729 and 730 were predicted to be able to cross the BBB.
6.5. Tacrine Hybrids with Modulators of Opioid Receptors
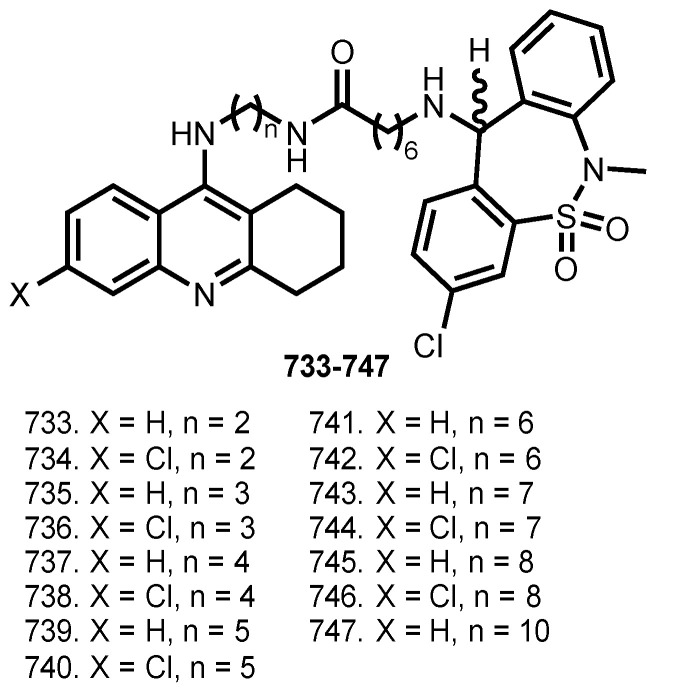
In 2016, Ceschi et al. reported hybrids of THA with antidepressant Tianeptine 733–747 [110] (Figure 30).
Figure 30.
THA–Tianeptine hybrids 733–747 [110].
THA-tianeptine hybrids were potent inhibitors of cholinesterases, the most active AChE inhibitor was 736 (IC50 6.79 nM), and 737 was the most active and selective in inhibiting BuChE (IC50 3.59 nM). Molecular modeling studies showed that THA moiety targets CAS, while tianeptine binds to PAS. Additionally, 737 and 739 were able to reduce the in vitro basal secretion of S100B, a calcium-binding protein which is known to regulate several processes associated with AD [197].
6.6. Tacrine Hybrids with MAO Inhibitors
MAO inhibitors (namely, two isoforms MAO-A and MAO-B) are considered as promising therapeutic agents for AD [198,199,200]. Ladostigil is a drug with cholinesterase and brain-selective monoamine oxidase inhibitory activities approved for phase IIb clinical trial [189].
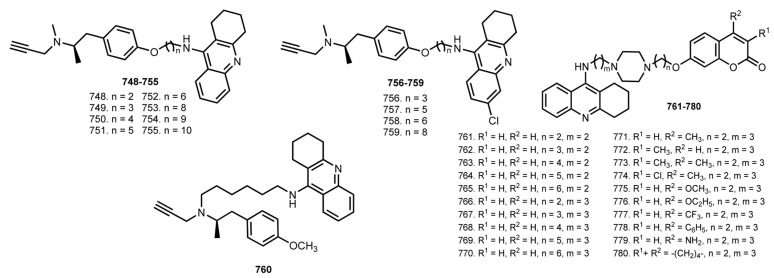
In 2013, Lu et al. reported a number of THA-based hybrids with drug Selegiline, a selective inhibitor of MAO-B [111,201]. (Figure 31)
Figure 31.
THA–selegiline hybrids 748–760 [111], THA–coumarin hybrids 761–780 [112].
Hybrids 748–751 were inhibitors of AChE, with the most active being 749 (IC50 36.1 nM). In addition, 755 exhibited the best IC50 toward BuChE in 2.03 nM. Hybrid 754 was chosen as lead due to balanced activity based on a set of test results. A kinetic study revealed a mixed-type inhibitory behavior for 754. Most of the hybrids were effective in inhibiting MAO-A and MAO-B in the sub-micromolar range. Hybrid 759 showed the highest inhibitory activity for both MAO-A (IC50 0.1926 mM) and MAO-B (IC50 0.1290 mM), and 754 exhibited the best balance of inhibition for both ChE and MAO. Finally, 754 proved to be an irreversible MAO-B inhibitor.
In 2015, Xie et al. designed THA-coumarin hybrids 761–780 [112]. Coumarin moiety was chosen due to its MAO inhibitory activity [202] and AChE inhibitory activity [203]. Hybrid 766 gave the highest AChE inhibitory activity with IC50 17.70 nM. The substituents in coumarin moiety were found to worsen the inhibitory activity. Hybrids 774 (IC50 31.88 nM for AChE) and 771 (IC50 50.76 nM for BuChE) were the most potent inhibitors, with their inhibitory activity 1.8- and 1.3-fold less than those of their no substituted analog 766. All hybrids showed inhibition activity against hMAO-A and hMAO-B, with the most selective toward MAO-B being 773 (IC50 0.24 mM). An inhibitory activity of 773 as a mixed-type competitive inhibitor was confirmed. Finally, 773 showed negligible toxicity on SH-SY5Y cells.
7. Tacrine Hybrids with Natural Products
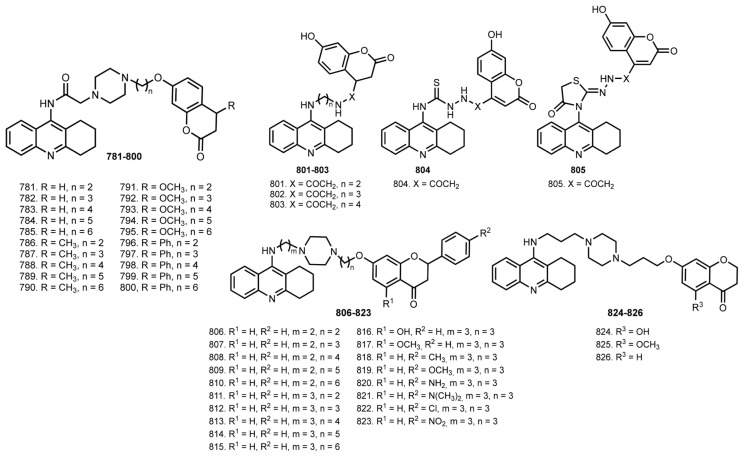
In 2013, Xie et al. reported THA–coumarin hybrids 781–800, structural analogues of hybrids 761–780 (Figure 32) [112,113]. Hybrids showed moderate activity toward both ChEs, with the best AChE inhibitor being 786 (IC50 0.092 µM), and the most effective BuChE inhibitor being 790 (IC50 0.099 µM). Kinetic study revealed a mixed-type inhibition for 786. An ability of hybrids to inhibit self-induced Aβ aggregation was confirmed using a ThT-test, with 786 as the most potent AChE inhibitor that also showed the highest inhibitory potency. The metal-chelating ability of hybrids was confirmed using UV-Vis spectrometry in the presence of Cu2+ and Fe2+. Finally, a low toxicity of 786 was confirmed on SH-SY5Y cells.
Figure 32.
THA–coumarin hybrids 781–800 [113], 801–805 [114], and THA–flavonoid hybrids 806–826 [115].
In 2014, Hamulakova et al. reported THA–coumarin hybrids 801–805 [114]. The most potent inhibitor of hAChE was 803 (IC50 0.0154 μM). A selectivity for hAChE was demonstrated by 803 (SI 21.30) and for hBuChE by 804 (SI 0.174).
In 2013, Li et al. reported THA–flavonoid hybrids 806–826 [115]. All hybrids inhibited both ChE, with the most potent being 825 (IC50 8.4 nM toward AChE) and 826 (IC50 25.8 nM toward BuChE). Further, most hybrids inhibited Aβ self-induced aggregation, with the most potent being 824. Hybrids 816 and 824 showed moderate metal-chelating ability. Additionally, 816 was non-toxic to SH-SY5Y cells.
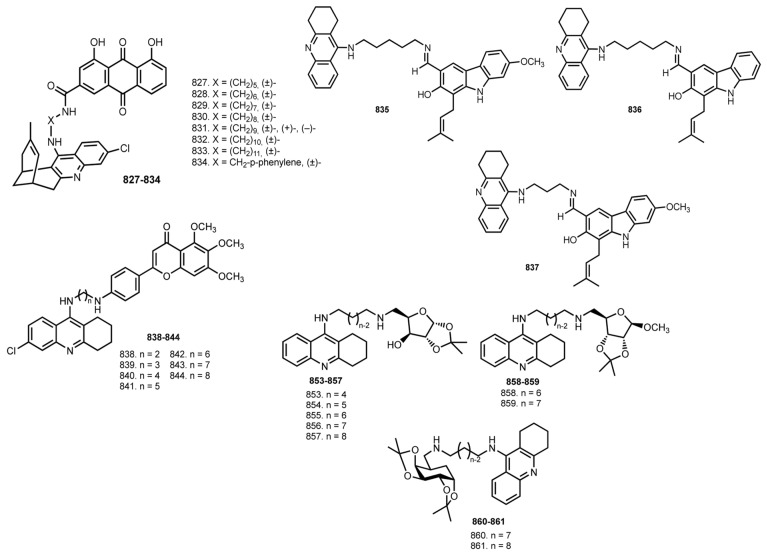
In 2014, Viayna et al. reported huprine Y-rhein hybrids 827–834 [116] (Figure 33). All racemic hybrids were potent inhibitors of hAChE, with IC50 values in the low nanomolar range. The most potent hAChE inhibitor was (±)-827 (IC50 1.07 nM). In addition, all hybrids were selective for hAChE. The binding mode to AChE was explored for 834 via docking studies, in which (−)-834 bound to AChE more favorably than did (+)-834. For all hybrids, a significant Aβ42 antiaggregating activity was confirmed. Additionally, (±)-830, (±)-832, and (±)-833, as well as (±)-834, exhibited a moderately potent BACE-1 inhibitory activity. The levorotatory (−)-831 was a far more potent hAChE inhibitor than its enantiomer (+)-831, with IC50 2930 and 2.39 nM. A kinetic study demonstrated that (−)-831 acts as a mixed-type inhibitor of hAChE. (+)-831 was two-fold more potent hBuChE inhibitor than (−)-831. In addition, both (−)-831 and (+)-831 proved to prevent the loss of synaptic proteins in hippocampal slices of 2-month-old C57bl6 mice. In vivo experiments with transgenic APP-PS1 mice showed that (+)- and (−)-831 can lower the levels of hippocampal total soluble Aβ and increase the levels of APP.
Figure 33.
Huprine Y-rhein hybrids 827-834 [116], THA-carbazoles hybrids 835–837 [117], 6-Cl-THA–Scutellarin hybrids 838–844 [118], THA–resveratrol hybrids 845–852 [119], THA hybrids with natural-based D-xylose, D-ribose, and and D-galactose 853–861 [120].
In 2014, Thiratmatrakul et al. reported hybrids of THA with phytochemicals carbazoles 835–837 [117]. All hybrids showed potent ABTS radical scavenging capacities with IC50 in the range of 8.34–11.24 µM, and selectivity against AChE over BuChE. Hybrid 835 displayed the most potent inhibitory activity and inhibition selectivity toward AChE, (IC50 0.48 µM). A neuroprotective effect of hybrids against H2O2 -induced oxidative stress was shown on NG108–15cells and 835 proved to be most potent in protecting cell damage. Additionally, neuroprotective effect of hybrids against Aβ peptide induced toxicity was shown on C6 astroglioma cells. Hybrid 835 was also the most potent in increasing cell viability. Behavioral studies indicated that 835 could improve scopolamine-induced cognitive deficits in mice.
In 2017, Spilovska et al. reported THA–scutellarin hybrids 838–844 [118]. The most active was 838 (IC50 1.63 nM against AChE) and the most potent inhibitor of hBuChE was 839 (IC50 174 nM). Only 843 and 844 showed lower cytotoxicity compared to the 6-Cl-THA.
In 2017, Jeřábek et al. reported THA-resveratrol hybrids 845–852 [119]. The most potent AChE inhibitor was 845 (IC50 0.8 µM). Some antiaggregating properties of hybrids were revealed by ThT assay. Only 852 showed no neurotoxicity on primary rat cerebellar granule neurons (CGNs). Nitrite production in LPS-treated glial cells was evaluated, which was significantly reduced by treatment of cells with 852. Finally, an ability of 852 to modulate the switch from the M1 to M2 phenotype on glial cells was investigated; a decrease in iNOS and slightly attenuating MRC1 expression was detected. Unfortunately, hepatotoxicity of hybrids on HePG2 cells was shown.
In 2018, Lopes et al. reported THA hybrids with natural-based D-xylose, D-ribose and D-galactose 853–861 [120]. Hybrid 857 showed an IC50 2.2 nM against AChE and of 4.93 nM against BuChE. Docking studies revealed that sugar moieties are stabilized in the PAS region through cation-π and CH/π interactions with Trp279.
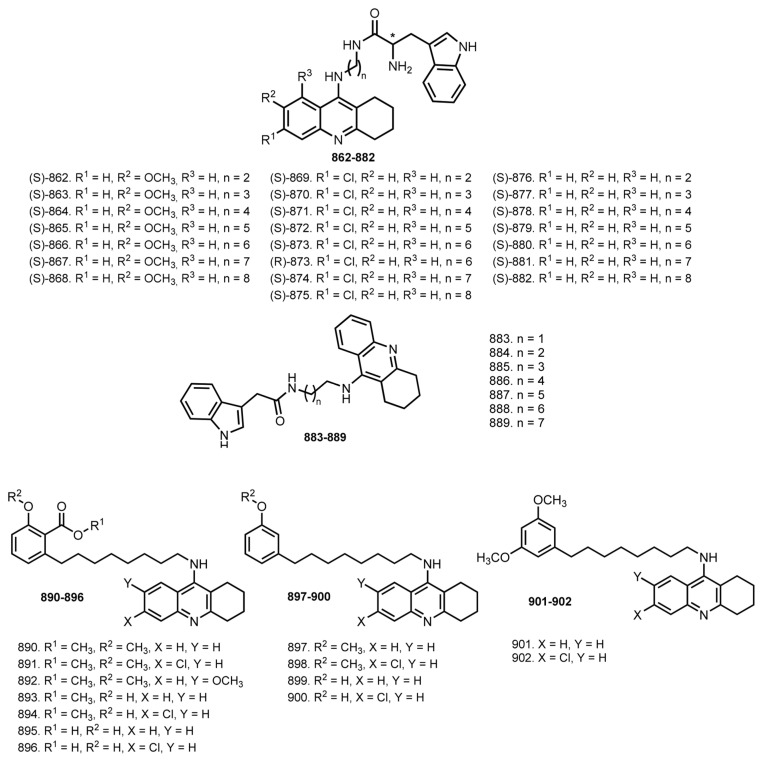
In 2019, Chalupova et al. designed THA–tryptophan heterodimers 862–882 [121] (Figure 34). All hybrids (except (S)-864) were potent inhibitors of hAChE; (S)-873 (IC50 6.3 nM) was chosen as lead compound based on a set of test results. S-enantiomer was found to be 15-fold more potent than the R- (9.1 nM vs. 140 nM). Moreover, the crystal structure confirmed the ability of (S)-873 to target both the CAS and PAS of AChE. PI displacement studies showed that the interaction of (S)-873 with PAS was about 6.9-fold weaker than that of PI. All hybrids were able to significantly inhibit Aβ42 -self-aggregation.
Figure 34.
THA–tryptophan hybrids 862–882 [121], THA–indole hybrids 883–889 [122], THA-based hybrids with anacardic acid 890–896, cardanol 897–900, and cardols 901 and 902 [123].
Cell toxicity studies revealed the order of toxicity is as follows: THA derivatives < 7-MEOTA analogues < 6-Cl-THA derivatives. The maximum tolerated dose of (S)-873 was found to be 70 mg/kg, meaning (S)-873 is safer than THA. The therapeutic effect of (S)-873 in a scopolamine-induced cognitive deficit rat model confirmed the pro-cognitive potential of the hybrid.
In 2019, Cheng et al. reported THA–indole hybrids 883–889 [122]. A moderate inhibition activity was shown by all hybrids. Hybrid 887 with IC50 0.173 μM against AChE was chosen as lead based on a set of test results. Kinetic study that revealed 887 is a mixed type ChE inhibitor. In addition, 887 exhibits a much stronger effect in modulating neural network activity compared to THA, indicating better antidementia and nootropic potentials.
In 2021, Rossi et al. reported THA-based hybrids conjugated with anacardic acid, cardanol, and cardols 890–902 [123]. All hybrids were effective AChE inhibitors, with the most active being 891 (IC50 2.54 nM). As BuChE inhibitors, 890, 891, and 893 were the top-ranked with IC50 0.0352 nM, 0.265 nM, and 0.177 nM. A crystal structure of 890 with hBuChE revealed that 890 accommodates the active site gorge of hBuChE. Toxicity studies revealed low hepato- and neurotoxicity in hybrids. Anti-inflammatory activity of 890 and 891 was shown on LPS-treated microglial BV-2 cells; a protective activity against neurotoxic insults was detected, as was the suppression of LPS-induced IL-1β, COX-2, and iNOS expression (TNF-α only for 891). The PAMPA-BBB test predicted that both hybrids have the potential to cross the BBB.
8. Tacrine Hybrids with Other Organic Scaffolds
In 2006, Elsinghorst et al. reported THA hybrids with trimethoxybenzene 903–917 [124] (Figure 35). The most active inhibitor of hAChE was 912 (IC50 5 nM). In addition, several hybrids showed selectivity toward hBuChE, with the most active inhibitor being 916 (IC50 0.139 nM).
Figure 35.
THA-trimethoxybenzene hybrids 903–917 [124], photoswitchable hybrids 918–921 [125], quinone–THA hybrids 922–939 [126], and THA–propargylamine hybrids 940–944 [127].
In 2014, Chen et al. reported a first example of photoswitchable THA-based hybrids [125]. Hybrids 918–921 showed reversible light-controlled behavior. The absorbances around 270 nm decreased upon UV-irradiation, whereas the absorbance band at 340 nm increased, and a new absorbance band maximum at 525 nm evolved. However, in all cases, the inhibitory activity against AChE was practically the same before and after irradiation of hybrids, except for the 921 (IC50 4.3 nM for “open” molecule and 1.8 nM for “closed’). Computational docking studies suggest that 921 might bind to both CAS and the PAS of AChE in both opened and closed forms. Additionally, when AChE-induced Aβ aggregation under the action of both photoforms of 921 were investigated, ring-closed 921 showed lower inhibition than a ring-open form.
In 2014, Nepovimova et al. designed quinone-THA hybrids 922–939 [126]. All hybrids were effective inhibitors of hAChE, with 930 being 752-fold more active in inhibiting hAChE than hBuChE. Hybrids 922–939 significantly prevented self-induced Aβ aggregation. Hybrids 926 and 930 were least neurotoxic on N2A cells. A neuroprotective effect of 926 and 930 was shown on N2A cells incubated with Aβ. The antioxidant properties of 926 and 930 were confirmed on TBH-stressed T67 cells pre-treated with sulforaphane. In addition, AChE inhibition was also confirmed ex vivo. A percentage of brain AChE inhibition versus untreated controls was evaluated after the drug’s injection; both 926 and 930 provide dose-dependent inhibition of cholinesterase activity in telencephalon at 1 h postdosing. Finally, 926 showed a superior hepatotoxicity profile to THA.
In 2015, Mao et al. reported THA–propargylamine hybrids 940–944 [127]. Hybrid 941 showed good inhibition activity for both ChEs (IC50 11.2 and 83.5 nM). Additionally, a mixed-type inhibitory behavior for 940 and 941 was revealed. Hybrid 940 showed the absence of neurotoxicity on SH-SY5Y cells. Finally, 940 and 941 showed low hepatotoxicity on HSC.
In 2015, Korabecny et al. designed 7-MEOTA hybrids 945–958 with p-anisidine [128] (Figure 36). All hybrids turned out to be potent inhibitors, but 955 showed the best inhibiting activity (IC50 1.35 µm against hAChE,) and 951 showed IC50 1.36 µm against hAChE. Kinetic analysis revealed a non-competitive type of inhibition of AChE by 951 and 955. In silico studies confirmed the dual binding site character of the selected ligands, with prevailing interactions with the PAS region of hAChE.
Figure 36.
7-MEOTA-p-anisidine hybrids 945–958 [128], THA-1,2,3-triazole hybrids 959–973 [129], Schiff base hybrids 974–989 [130].
In 2017, Najaf et al. reported THA–1,2,3-triazole hybrids 959–973 [129]. All hybrids showed moderate inhibition activity against ChEs, with the best inhibitor being 970 (IC50 0.521 mM); 968 was the best BuChE inhibitor (IC50 0.055 mM). Kinetic study confirmed mixed types of inhibition for both AChE and BuChE. In vivo evaluation of 970 confirmed memory improvement in scopolamine-induced impairment.
In 2019, Riazimontazer et al. reported THA–isatin schiff base hybrids 974–989 [130]. Most of the hybrids were potent ChE inhibitors with IC50 values from 0.42 nM to 79.66 nM. The most active, 984, showed IC50 against AChE 0.42 nM. Hybrid 977 exhibited the strongest inhibition of BuChE with IC50 0.11 nM. Kinetic study of AchE inhibition revealed a mixed-type inhibition for 984. In addition, 984 and 986 exhibit good inhibitory activity on AchE-induced Aβ aggregation. Metal-chelating properties for 986, 984, and 989 were shown.
In 2021, Yao et al. reported THA−pyrimidone hybrids 990–1039 [131] (Figure 37).
Figure 37.
THA—pyrimidone hybrids 990–1039 [131].
Br- and Cl- as substituents in THA unit were found to enhance AChE inhibition, fluorine-substituted pyridine groups were found to intensify to GSK-3β target, and alkylamine linkers with a linear chain of seven carbons were chosen as the most beneficial moiety. Hybrid 1035 was chosen as the compound with excellent dual AChE/GSK-3 inhibition (AChE: IC50 51.1 nM; GSK/3β: IC50 89.3 nM).
The docking studies for 1035 proved both CAS and PAS binding. Hybrid 1035 could fit the binding pockets of AChE and GSK-3β and exhibited good affinity with the interactions of several secondary bonds through the cooperation of the THA unit, alkylamine linker, and pyrimidone moiety, making it an excellent dual AChE/GSK-3β inhibitor. Additionally, 1035 was proven to regulate the tau protein pathway in SH-SY5Y-derived neurons, and to alleviate glyceraldehyde-induced cytotoxicity in DSH-SY5Y cells. A kinase selectivity profiling study showed that 1035 is a pan-GSK-3 inhibitor and possessed good kinase selectivity profiles. The capacity of 1035 to successfully permeate the BBB was confirmed by UPLC-MS/MS. Finally, treatment of scopolamine-induced ICR mice with 1035 led to significant amelioration of memory and spatial behavior.
In 2021, Ozten et al. reported carbamate hybrids of THA 1040–1052 [132] (Figure 38).
Figure 38.
THA–carbamate hybrids 1040–1052 [132].
All hybrids inhibited both ChEs, but 1050 was chosen as the best inhibitor of AChE and BuChE (IC50 22.15 nM and 16.96 nM).
In 2022, Przybyłowska et al. reported THA hybrids 1053–1066 with phosphorus moiety [133] (Figure 39).
Figure 39.
THA hybrids with phosphorus moieties 1053–1066 [133].
Hybrids were mostly more neurocytoxic than THA. Only 1064 showed a significant reduction of hepatotoxicity against HepG2 cells when compared with THA. In ChEs test inhibition, 1053 and 1058 showed similar activity with THA. The most active hybrid was 1055 with IC50 6.11 nM against AChE and 12.86 nM against BuChE. In addition, 1060 and 1053 were potent against BuChE, with IC50 1.969 nM and 6.753 nM.
9. Discussion
In summary, the most effective inhibitors of hAChE among those presented in this review are 7 and 565, which are hybrids of 6,8-dichlorotacrin-melatonin (7) and 6-chlorotacrin-tetrahydrobenzo[h][1,6]naphthyridine (565) with IC50 8 and 6 pm [39,93]. Additionally, hybrids with picomolar efficacy are 6-chlorotacrine-4-oxo-4H-chromene 88 with IC50 35 pm against hAChE and 74 with IC50 38 pm against hBuChE [45]. THA hybrid with anacardic acid 890 also showed picomolar activity against hBuChE with IC50 35 pm [123].
THA–melatonin hybrid 7, first described in 2006, is still one of the most potent hybrids described to date. At the same time, THA–melatonin hybrids 3 and 6 showed selectivity for one esterase, which also makes them promising scaffolds for the development of anti-AD drugs. The conjugation of additional lipoic or ferulic acid moieties reduces inhibition activity (24–31), apparently due to steric hindrances arising in the resulting hybrid when binding to the target ChE [41].
THA–silibinin hybrid 101 demonstrated low hepatotoxicity in vivo, but was not superior to THA in improving cognitive abilities, presumably due to high steric hindrance [47]. Hybrid 165 showed both cognitive improvements and a decrease in hepatotoxicity in vivo, which clearly indicates the effectiveness of antioxidant strategy [54]. In addition, a reduction in Aβ plaque levels in the APP/PS1 mice certainly proves the effectiveness of simultaneous anti-amyloid and anti-AChE approaches in the treatment of AD.
An extremely interesting result was observed for hybrids of THA and FA. Thus, 254 with linker length n = 8 did not show cognitive improvement in vivo, while hydride 255 with linker length n = 6 showed significant cognitive improvements [51,52]. This once again emphasizes the importance of choosing the linker length both for the effectiveness of cholinesterase inhibition and for anti-AD therapy in vivo. It is also important to note that THA-FA hybrids 277–281 were linked through a conformationally rigid piperazine linker [62]. Comparison of the inhibition data of these hybrids with their analogues clearly indicates that the restriction of the conformational mobility of the linker negatively affects the inhibitory effect.
Also, the effectiveness of introducing NO-donors into THA hybrids for anti-AD therapeutic efficacy should be noted. Thus, a comparison of the in vivo efficacy of hybrids 262 (with the NO-donating group) and 256 (without the NO-donating group) clearly indicates that the vasorelaxant effect of NO-donors not only reduces the hepatotoxicity of hybrids, but also improves cognitive functions in vivo better than hybrids that do not possess a vasorelaxant effect [53]. Convincing evidence for the effectiveness of NO-group donors was also concluded from the results of in vivo studies of hybrids 295, 310, and 317. Hybrid 317 (without the NO-donating group) did not show improvement in cognitive functions in tests in vivo, while 295 and 310 showed improvements in cognitive functions, apparently due to the vasorelaxant effect [64,65]. It is also worth noting the THA–flurbiprofen hybrids 439–443 which were then optimized by conjugation with NO-donor molecules to give 444–463 [74,75,76]. At the same time, 456 did not outperform THA in improving its cognitive functions; however, the reduced hepatoxicity of 456 is attributed by the authors to its ability to donate NO.
Despite the seeming simplicity of THA-linker-second residue hybrids synthesis, careful design is critical. Thus, among the THA–benzothiazole hybrids 359–364, 370–395, and 402–413 developed, only 386 showed high inhibitory activity along with low hepatotoxicity in vivo and therapeutic efficacy in vivo [68,69,70,72].
Not only the length of the linker is critical in drug design, but also its nature and donor properties. Thus, the amide linker between THA and benzofuran in 370–381 renders a negative effect on inhibitory activity compared to the amine linker in 382–387. Additionally, both aromatic moieties in a hybrid should not be sterically expanded to be able to pack into the enzyme binding site. Thus, 388–395 show selectivity for BuChE, apparently due to the steric impossibility of binding to AChE. Regarding the negative effect of an amide linker on inhibition activity, 781–800 exhibited moderate anti-AD activity, while in subsequent work, similar hybrids 761–780 were designed not with an amide, but with an amine linker [112,113]. Optimization of the molecular structure resulted in an increase in anticholinesterase activity, and in the ability of 761–780 to inhibit MAO.
The exceptionally successful design of hybrids with analogues of PI deserves special attention. The known possibility of the second moiety to bind to the AChE provides a successful design of the anti-AChE drug, in which the optimal length of the linker between the CAS and PAS binding fragment should be selected. Thus, 565 is one of the most potent inhibitors of hAChE reported to date, with an IC50 6.27 pM [92]. It is interesting to note that the design of 565 is also a result of careful drug development, as originally synthesized 555–564 were not effective inhibitors [91]. However, the successful design made it possible to obtain one of the strongest inhibitors described to date. In vivo studies on the efficacy of 565 would be extremely interesting.
Of obvious interest are hybrids acting on the cholinergic system. Thus, 592–605, which are both cholinesterase inhibitors and calcium channel blockers, were developed and the leader compound (S)-600 was chosen, of which studies of the in vivo therapeutic efficacy would be very interesting [95,96]. In addition, similar hybrids 607–618, representing a weak structural analogue based on dihydropyirimidine-thiones, are also able to block calcium channels [98]. For 611, the study of anti-AD activity for (R) and (S) enantiomers would certainly be interesting.
Hybrids 669–674 and 675–690 are all cholinesterase inhibitors and muscarinic receptor agonists; 687 shows therapeutic efficacy in vivo [103,104]. Both the hybrid series 675–690 and 712–717 are THA–xanomeline hybrids [104,106]. At the same time, the first series are successful activators of M1 receptors, while the second series are not able to affect receptors, which once again confirms the importance of not only the choice of the second moiety, but also the design of the linker. Additionally, THA–BOCA hybrids 691–711 turned out to be antagonists of muscarinic receptors, which confirms the complexity of receptor-targeted drug design [105]. The design of hybrids of THA with a memantine residue is a classic “dual drug” idea, while 722–728 do not show significant anticholinesterase activity [108]. At the same time, the change in the linker and the conjugation of the free amino group to the adamantane core significantly increased the activity of the resulting hybrids, and 729–732 turned out to be promising cholinesterase inhibitors and NMDA receptor antagonists [109]. Unfortunately, these hybrids are presumably unable to cross the BBB, possibly due to steric hindrance. Hybrids 759 and 773 are promising anticholinergic agents and MAO-B inhibitors, and their in vivo efficacies as anti-AD agents are also of interest [111,112].
Of particular note is the therapeutic efficacy of hybrids based on Huprine Y. Thus, Huprine Y–capsaicin hybrid 249 significantly enhanced learning and memory in old APP/PS1 mice [58]. Huprine-THA heterodimers (±)-494 and (±)-500 inhibited mouse brain AChE activity [85]. In addition, Huprine Y-based hybrid (SSS)-621 with 2-(2-oxopyrrolidin-1-yl)butyramide moiety of levetiracetam significantly increased recognition indices in vivo, reduced Aβ burden in the cortex, and reduced GFAP positive astrocytes around Aβ plaques [99]. Additionally, both enantiomers of hybrid (+)- and (−)-831 are able to lower the levels of hippocampal Aβ and increase the levels of APP both in initial and advanced stages of AD in vivo [116]. It should also be noted that the right and left hybrids (+) and (−)-831 showed a tremendous difference in the inhibition of hAChE, and both showed anti-AD efficacy in vivo.
THA-carbazoles hydride 835 is not an effective cholinesterase inhibitor compared to the other hybrids described above, but demonstrated an ability to cause cognitive improvements in vivo [117]. As hybrids of THA with natural products, resveratrol 845–852 showed high hepatotoxicity [119]. THA hybrids with sugar moieties 853–861 and THA–anacardic acid hybrids 890–891 showed high efficacy along with low toxicity, and their further studies are promising [120,123]. An interesting result is the activity of the (S)-873 enantiomer toward AChE [121].
The design of photoactive THA hybrids 918–921 is an interesting concept, but its practical applicability is questionable [125]. The spectacular work on the development of quinone–THA hybrids by Nepovimova et al., in which 926 is a selective AChE inhibitor, exhibits multiple activities and inhibits AChE in vivo [126]. Hybrids 940–944 with propargylamine as the second residue are striking in their simplicity and efficiency; further research on hybrids would be interesting [132]. 7-MEOTA-based hybrids with p-anisidine 945–958 are not as active, presumably due to the low activity of the parental 7-MEOTA [128]. Despite the fact that simple THA–triazole hybrids 959–973 are not active compared to other previously described hybrids, 970 showed therapeutic efficacy in vivo [129]. Hybrids of THA with isatin, especially 984, also showed good activity [130]. An extremely interesting THA hybrid is pyrimidone 1035, which exhibits AChE/GSK-3 inhibition activity and also exhibits therapeutic efficacy in vivo [131].
Comparison of structurally similar hybrids 67–94, 149–153, and 806–826 based on THA and flavonoids is interesting [45,52,115]. Thus, 67–94 are able to inhibit BACE-1, while the inhibitory ability of the hybrids of the other two series has not been evaluated [45]. Hybrids 67–94 and 149–153 exhibit antioxidant activity, while 806–826 are not antioxidants. The most successful choliesterase inhibitors are hybrids 67–94; the lead compounds in this series are 88 with IC50 0.035 nm against AChE and 74 with 0.038 nM against BuChE, while hybrids 149–153 and 806–826 demonstrated weaker inhibitory activity. Thus, the most successful design of THA–flavonoid hybrids is reported by Fernández-Bachiller et al. [45].
Comparison of structurally similar hybrids 128–148 and 192–210 with antioxidants Trolox and Cp-6 is also of interest [51,56]. Hybrids 128–148 with an amide linker showed moderate inhibitory activity, while hybrids 192–201 showed picomolar activity. The importance of the linker choice is confirmed again, which is confirmed by the order of activities in the second series: amines 207–210 > amides 192–195 > reverse amides 204–206 > O-benzylated amides 196–199. That is, the amine linker between THA and the second residue without an acceptor amide bond plays one of the key roles in the activity of the hybrid.
10. Conclusions
The design of THA hybrids is a promising alternative to anti-AD drugs used in clinical practice due to their high efficiency and multiple biological actions. In addition, synthesis of THA hybrids results in reducing hepatotoxicity. A wide possibility opens up with the conjugation of THA with a second biological active moiety, which provides an additional biological activity and several mechanisms of anti-AD therapy in one drug. Thus, in this review, many hybrids are able not only to inhibit cholinesterase, but also to affect amyloid aggregation and reduce the number of Aβ plaques in the brain. Additionally, many of the described hybrids showed a high selectivity for one of the cholinesterase, are able to donate NO and cause a vasorelaxant effect, inhibit BACE-1, cause an antidepressant and a neuroprotective effect, and chelate metal cations, thereby counteracting the formation of toxic amyloid aggregates. The impressive results of in vivo studies also confirm that THA derivatives are able to improve cognitive functions by not only cholinesterase inhibition, but also by reducing the number of amyloid plaques in AD brains, while not causing noticeable hepatotoxicity.
Abbreviations
AD—Alzheimer’s disease, Aβ—β-amyloid, ACh—acetylcholine, AChE—acetylcholinesterase, BuChE—butyrylcholinesterase, 6-Cl-THA—6-chlorotacrine, 7-MEOTA—7-metoxytacrine, HDAC—Histone deacetylase, THA—tacrine, i.c.v.—intracerebroventricular injection, i.p.—intraperitoneal injection, i.g.—intragastric administration, i.d.—intradermal injection, ORAC-FL—radical absorbance capacity assay using fluorescein, ALT—alanine aminotransferase, TPPU—N-[1-(1-Oxopropyl)-4-piperidinyl]-N′-[4-(trifluoromethoxy)phenyl]-urea, ASAT—aspartate aminotransferase, LDH—lactate dehydogenase, NSAIDs—nonsteroidal anti-inflammatory drugs, isosorbide mononitrate (ISMNI), MAOs—monoamine oxidases, FAD—flavin adenine dinucleotide, ROS—reactive oxygen species, TEM—transmission electron microscopic, LTP—long-term potentiation, CAS—catalytic active site, PAS—peripheral anionic site, GSK-3—glycogen synthase kinase-3, APP—amyloid precursor protein, LPS—bacterial endotoxin lipopolysaccharide, AFM—atomic force microscope, SPR—surface plasmon resonance, HSC—human hepatic stellate cells, PI—propidium iodide.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24021717/s1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was financially supported by the Ministry of Education and Science of the Russian Federation, Agreement No. 075-15-2022-264. (unique scientific facility “Scanning ion-conductance microscope with a confocal module” (registration number 2512530).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Takahashi R.H., Nagao T., Gouras G.K. Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer’s disease. Pathol. Int. 2017;67:185–193. doi: 10.1111/pin.12520. [DOI] [PubMed] [Google Scholar]
- 2.Das B., Yan R. Role of BACE1 in Alzheimer’s synaptic function. Transl. Neurodegener. 2017;6:23. doi: 10.1186/s40035-017-0093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali M.M., Ghouri R.G., Ans A.H., Akbar A., Toheed A. Recommendations for Anti-inflammatory Treatments in Alzheimer’s Disease: A Comprehensive Review of the Literature. Cureus. 2019;11:e462. doi: 10.7759/cureus.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang W.-J., Zhang X., Chen W.-W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016;4:519–522. doi: 10.3892/br.2016.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbel-Ornath M., Hudry E., Boivin J.R., Hashimoto T., Takeda S., Kuchibhotla K.V., Hou S., Lattarulo C.R., Belcher A.M., Shakerdge N., et al. Soluble oligomeric amyloid-β induces calcium dyshomeostasis that precedes synapse loss in the living mouse brain. Mol. Neurodegener. 2017;12:27. doi: 10.1186/s13024-017-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drews A., De S., Flagmeier P., Wirthensohn D.C., Chen W.-H., Whiten D.R., Rodrigues M., Vincke C., Muyldermans S., Paterson R.W., et al. Inhibiting the Ca2+ Influx Induced by Human CSF. Cell Rep. 2017;21:3310–3316. doi: 10.1016/j.celrep.2017.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodison W.V., Frisardi V., Kehoe P.G. Calcium Channel Blockers and Alzheimer’s Disease: Potential Relevance in Treatment Strategies of Metabolic Syndrome. J. Alzheimer’s Dis. 2012;30:S269–S282. doi: 10.3233/JAD-2012-111664. [DOI] [PubMed] [Google Scholar]
- 8.Lauretti E., Dincer O., Praticò D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta—Mol. Cell Res. 2020;1867:118664. doi: 10.1016/j.bbamcr.2020.118664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper C., Killick R., Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J. Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gsell W., Jungkunz G., Riederer P. Functional Neurochemistry of Alzheimer’s Disease. Cur. Pharm. Dis. 2004;10:265–293. doi: 10.2174/1381612043386473. [DOI] [PubMed] [Google Scholar]
- 11.Werner F., Coveñas R. Classical Neurotransmitters and Neuropeptides Involved in Major Depression in a Multi-neurotransmitter System: A Focus on Antidepressant Drugs. Curr. Med. Chem. 2013;20:4853–4858. doi: 10.2174/09298673113206660280. [DOI] [PubMed] [Google Scholar]
- 12.Ishibashi M., Yamazaki Y., Miledi R., Sumikawa K. Nicotinic and muscarinic agonists and acetylcholinesterase inhibitors stimulate a common pathway to enhance GluN2B-NMDAR responses. Proc. Natl. Acad. Sci. USA. 2014;111:12538–12543. doi: 10.1073/pnas.1408805111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwomoh L., Tejeda G.S., Tobin A.B. Targeting the M1 muscarinic acetylcholine receptor in Alzheimer’s disease. Neuronal Signal. 2022;6:NS20210004. doi: 10.1042/NS20210004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colovic M.B., Krstic D.Z., Lazarevic-Pasti T., Bondzic A.M., Vasic V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013;11:315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terry A.V., Jr., Callahan P., Hernandez C.M. Nicotinic ligands as multifunctional agents for the treatment of neuropsychiatric disorders. Biochem. Pharmacol. 2015;97:388–398. doi: 10.1016/j.bcp.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoskin J.L., Al-Hasan Y., Sabbagh M.N. Nicotinic Acetylcholine Receptor Agonists for the Treatment of Alzheimer’s Dementia: An Update. Nicotine Tob. Res. 2019;21:370–376. doi: 10.1093/ntr/nty116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenlee W., Clader J., Asberom T., McCombie S., Ford J., Guzik H., Kozlowski J., Li S., Liu C., Lowe D., et al. Muscarinic agonists and antagonists in the treatment of Alzheimer’s disease. Il Farm. 2001;56:247–250. doi: 10.1016/S0014-827X(01)01102-8. [DOI] [PubMed] [Google Scholar]
- 18.Furuie H., Yamada K., Ichitani Y. MK-801-induced and scopolamine-induced hyperactivity in rats neonatally treated chronically with MK-801. Behav. Pharmacol. 2013;24:678–683. doi: 10.1097/FBP.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 19.Francis P.T., Palmer A.M., Snape M., Wilcock G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marucci G., Buccioni M., Ben D.D., Lambertucci C., Volpini R., Amenta F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology. 2021;190:108352. doi: 10.1016/j.neuropharm.2020.108352. [DOI] [PubMed] [Google Scholar]
- 21.Tayeb H.O., Yang H.D., Price B.H., Tarazi F.I. Pharmacotherapies for Alzheimer’s disease: Beyond cholinesterase inhibitors. Pharmacol. Ther. 2012;134:8–25. doi: 10.1016/j.pharmthera.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 22.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet] National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD, USA: 2012. [PubMed] [Google Scholar]
- 23.Recanatini M., Cavalli A., Belluti F., Piazzi L., Rampa A., Bisi A., Gobbi S., Valenti P., Andrisano V., Bartolini M., et al. SAR of 9-Amino-1,2,3,4-tetrahydroacridine-Based Acetylcholinesterase Inhibitors: Synthesis, Enzyme Inhibitory Activity, QSAR, and Structure-Based CoMFA of Tacrine Analogues. J. Med. Chem. 2000;43:2007–2018. doi: 10.1021/jm990971t. [DOI] [PubMed] [Google Scholar]
- 24.Soukup O., Jun D., Zdarova-Karasova J., Patocka J., Musilek K., Korabecny J., Krusek J., Kaniakova M., Sepsova V., Mandikova J., et al. A Resurrection of 7-MEOTA: A Comparison with Tacrine. Curr. Alzheimer Res. 2013;10:893–906. doi: 10.2174/1567205011310080011. [DOI] [PubMed] [Google Scholar]
- 25.Korabecny J., Musilek K., Holas O., Binder J., Zemek F., Marek J., Pohanka M., Opletalova V., Dohnal V., Kuca K. Synthesis and in vitro evaluation of N-alkyl-7-methoxytacrine hydrochlorides as potential cholinesterase inhibitors in Alzheimer disease. Bioorg. Med. Chem. Lett. 2010;20:6093–6095. doi: 10.1016/j.bmcl.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 26.Quintanova C., Keri R.S., Marques S.M., Fernandes G.M., Cardoso S.M., Luísa Serralheiro M., Amélia Santos M. Design, synthesis and bioevaluation of tacrine hybrids with cinnamate and cinnamylidene acetate derivatives as potential anti-Alzheimer drugs. MedChemComm. 2015;6:1969–1977. doi: 10.1039/C5MD00236B. [DOI] [Google Scholar]
- 27.Wu W.-Y., Dai Y.-C., Li N.-G., Dong Z.-X., Gu T., Shi Z.-H., Xue X., Tang Y.-P., Duan J.-A. Novel multitarget-directed tacrine derivatives as potential candidates for the treatment of Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2017;32:572–587. doi: 10.1080/14756366.2016.1210139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlier P.R., Chow E.S., Han Y., Liu J., El Yazal J., Pang Y.-P. Heterodimeric Tacrine-Based Acetylcholinesterase Inhibitors: Investigating Ligand−Peripheral Site Interactions. J. Med. Chem. 1999;42:4225–4231. doi: 10.1021/jm990224w. [DOI] [PubMed] [Google Scholar]
- 29.Harel M., Sonoda L.K., Silman I., Sussman J.L., Rosenberry T.L. Crystal Structure of Thioflavin T Bound to the Peripheral Site of Torpedo californica Acetylcholinesterase Reveals How Thioflavin T Acts as a Sensitive Fluorescent Reporter of Ligand Binding to the Acylation Site. J. Am. Chem. Soc. 2008;130:7856–7861. doi: 10.1021/ja7109822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y., Sun J., Fang L., Liu M., Peng S., Liao H., Lehmann J., Zhang Y. Tacrine–Ferulic Acid–Nitric Oxide (NO) Donor Trihybrids as Potent, Multifunctional Acetyl- and Butyrylcholinesterase Inhibitors. J. Med. Chem. 2012;55:4309–4321. doi: 10.1021/jm300106z. [DOI] [PubMed] [Google Scholar]
- 31.Carvajal F.J., Inestrosa N.C. Interactions of AChE with Aβ Aggregates in Alzheimer’s Brain: Therapeutic Relevance of IDN 5706. Front. Mol. Neurosci. 2011;4:19. doi: 10.3389/fnmol.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pourshojaei Y., Abiri A., Eskandari K., Haghighijoo Z., Edraki N., Asadipour A. Phenoxyethyl Piperidine/Morpholine Derivatives as PAS and CAS Inhibitors of Cholinesterases: Insights for Future Drug Design. Sci. Rep. 2019;9:19855. doi: 10.1038/s41598-019-56463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khoobi M., Ghanoni F., Nadri H., Moradi A., Hamedani M.P., Homayouni Moghadam F., Emami S., Vosooghi M., Zadmard R., Foroumadi A., et al. New tetracyclic tacrine analogs containing pyrano[2,3-c]pyrazole: Efficient synthesis, biological assessment and docking simulation study. Eur. J. Med. Chem. 2015;89:296–303. doi: 10.1016/j.ejmech.2014.10.049. [DOI] [PubMed] [Google Scholar]
- 34.Pourabdi L., Khoobi M., Nadri H., Moradi A., Moghadam F.H., Emami S., Mojtahedi M.M., Haririan I., Forootanfar H., Ameri A., et al. Synthesis and structure-activity relationship study of tacrine-based pyrano[2,3-c]pyrazoles targeting AChE/BuChE and 15-LOX. Eur. J. Med. Chem. 2016;123:298–308. doi: 10.1016/j.ejmech.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 35.Roldán-Peña J.M., Alejandre-Ramos D., López Ó., Maya I., Lagunes I., Padrón J.M., Peña-Altamira L.E., Bartolini M., Monti B., Bolognesi M.L., et al. New tacrine dimers with antioxidant linkers as dual drugs: Anti-Alzheimer’s and antiproliferative agents. Eur. J. Med. Chem. 2017;138:761–773. doi: 10.1016/j.ejmech.2017.06.048. [DOI] [PubMed] [Google Scholar]
- 36.Sameem B., Saeedi M., Mahdavi M., Shafiee A. A review on tacrine-based scaffolds as multi-target drugs (MTDLs) for Alzheimer’s disease. Eur. J. Med. Chem. 2017;128:332–345. doi: 10.1016/j.ejmech.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 37.Girek M., Szymański P. Phyto-Tacrine Hybrids as Promising Drugs to Treat Alzheimer’s Disease. ChemistrySelect. 2019;4:5776–5790. doi: 10.1002/slct.201803672. [DOI] [Google Scholar]
- 38.Eckroat T.J., Manross D.L., Cowan S.C. Merged Tacrine-Based, Multitarget-Directed Acetylcholinesterase Inhibitors 2015–Present: Synthesis and Biological Activity. Int. J. Mol. Sci. 2020;21:5965. doi: 10.3390/ijms21175965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodríguez-Franco M.I., Fernández-Bachiller M.I., Pérez C., Hernández-Ledesma B., Bartolomé B. Novel Tacrine−Melatonin Hybrids as Dual-Acting Drugs for Alzheimer Disease, with Improved Acetylcholinesterase Inhibitory and Antioxidant Properties. J. Med. Chem. 2006;49:459–462. doi: 10.1021/jm050746d. [DOI] [PubMed] [Google Scholar]
- 40.Fernández-Bachiller M.I., Pérez C., Campillo N.E., Páez J.A., González-Muñoz G.C., Usán P., García-Palomero E., López M.G., Villarroya M., García A.G., et al. Tacrine-Melatonin Hybrids as Multifunctional Agents for Alzheimer’s Disease, with Cholinergic, Antioxidant, and Neuroprotective Properties. ChemMedChem. 2009;4:828–841. doi: 10.1002/cmdc.200800414. [DOI] [PubMed] [Google Scholar]
- 41.Benchekroun M., Romero A., Egea J., León R., Michalska P., Buendía I., Jimeno M.L., Jun D., Janockova J., Sepsova V., et al. The Antioxidant Additive Approach for Alzheimer’s Disease Therapy: New Ferulic (Lipoic) Acid Plus Melatonin Modified Tacrines as Cholinesterases Inhibitors, Direct Antioxidants, and Nuclear Factor (Erythroid-Derived 2)-Like 2 Activators. J. Med. Chem. 2016;59:9967–9973. doi: 10.1021/acs.jmedchem.6b01178. [DOI] [PubMed] [Google Scholar]
- 42.Fernández-Bachiller M.I., Pérez C., González-Muñoz G.C., Conde S., López M.G., Villarroya M., García A.G., Rodríguez-Franco M.I. Novel Tacrine−8-Hydroxyquinoline Hybrids as Multifunctional Agents for the Treatment of Alzheimer’s Disease, with Neuroprotective, Cholinergic, Antioxidant, and Copper-Complexing Properties. J. Med. Chem. 2010;53:4927–4937. doi: 10.1021/jm100329q. [DOI] [PubMed] [Google Scholar]
- 43.Luo W., Li Y.-P., He Y., Huang S.-L., Tan J.-H., Ou T.-M., Li D., Gu L.-Q., Huang Z.-S. Design, synthesis and evaluation of novel tacrine-multialkoxybenzene hybrids as dual inhibitors for cholinesterases and amyloid beta aggregation. Bioorg. Med. Chem. 2011;19:763–770. doi: 10.1016/j.bmc.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 44.Luo W., Li Y.-P., He Y., Huang S.-L., Li D., Gu L.-Q., Huang Z.-S. Synthesis and evaluation of heterobivalent tacrine derivatives as potential multi-functional anti-Alzheimer agents. Eur. J. Med. Chem. 2011;46:2609–2616. doi: 10.1016/j.ejmech.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 45.Fernández-Bachiller M.I., Pérez C., Monjas L., Rademann J., Rodríguez-Franco M.I. New Tacrine–4-Oxo-4 H -chromene Hybrids as Multifunctional Agents for the Treatment of Alzheimer’s Disease, with Cholinergic, Antioxidant, and β-Amyloid-Reducing Properties. J. Med. Chem. 2012;55:1303–1317. doi: 10.1021/jm201460y. [DOI] [PubMed] [Google Scholar]
- 46.Chao X., He X., Yang Y., Zhou X., Jin M., Liu S., Cheng Z., Liu P., Wang Y., Yu J., et al. Design, synthesis and pharmacological evaluation of novel tacrine–caffeic acid hybrids as multi-targeted compounds against Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2012;22:6498–6502. doi: 10.1016/j.bmcl.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 47.Chen X., Zenger K., Lupp A., Kling B., Heilmann J., Fleck C., Kraus B., Decker M. Tacrine-Silibinin Codrug Shows Neuro- and Hepatoprotective Effects in Vitro and Pro-Cognitive and Hepatoprotective Effects in Vivo. J. Med. Chem. 2012;55:5231–5242. doi: 10.1021/jm300246n. [DOI] [PubMed] [Google Scholar]
- 48.Zenger K., Chen X., Decker M., Kraus B. In-vitro stability and metabolism of a tacrine–silibinin codrug. J. Pharm. Pharmacol. 2013;65:1765–1772. doi: 10.1111/jphp.12070. [DOI] [PubMed] [Google Scholar]
- 49.Mao F., Chen J., Zhou Q., Luo Z., Huang L., Li X. Novel tacrine–ebselen hybrids with improved cholinesterase inhibitory, hydrogen peroxide and peroxynitrite scavenging activity. Bioorg. Med. Chem. Lett. 2013;23:6737–6742. doi: 10.1016/j.bmcl.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 50.Lan J.-S., Xie S.-S., Li S.-Y., Pan L.-F., Wang X.-B., Kong L.-Y. Design, synthesis and evaluation of novel tacrine-(β-carboline) hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2014;22:6089–6104. doi: 10.1016/j.bmc.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 51.Nepovimova E., Korabecny J., Dolezal R., Babkova K., Ondrejicek A., Jun D., Sepsova V., Horova A., Hrabinova M., Soukup O., et al. Tacrine–Trolox Hybrids: A Novel Class of Centrally Active, Nonhepatotoxic Multi-Target-Directed Ligands Exerting Anticholinesterase and Antioxidant Activities with Low In Vivo Toxicity. J. Med. Chem. 2015;58:8985–9003. doi: 10.1021/acs.jmedchem.5b01325. [DOI] [PubMed] [Google Scholar]
- 52.Luo W., Wang T., Hong C., Yang Y., Chen Y., Cen J., Xie S., Wang C. Design, synthesis and evaluation of 4-dimethylamine flavonoid derivatives as potential multifunctional anti-Alzheimer agents. Eur. J. Med. Chem. 2016;122:17–26. doi: 10.1016/j.ejmech.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 53.Chand K., Alsoghier H.M., Chaves S., Santos M.A. Tacrine-(hydroxybenzoyl-pyridone) hybrids as potential multifunctional anti-Alzheimer’s agents: AChE inhibition, antioxidant activity and metal chelating capacity. J. Inorg. Biochem. 2016;163:266–277. doi: 10.1016/j.jinorgbio.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Li G., Hong G., Li X., Zhang Y., Xu Z., Mao L., Feng X., Liu T. Synthesis and activity towards Alzheimer’s disease in vitro: Tacrine, phenolic acid and ligustrazine hybrids. Eur. J. Med. Chem. 2018;148:238–254. doi: 10.1016/j.ejmech.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 55.Li K., Jiang Y., Li G., Liu T., Yang Z. Novel Multitarget Directed Tacrine Hybrids as Anti-Alzheimer’s Compounds Improved Synaptic Plasticity and Cognitive Impairment in APP/PS1 Transgenic Mice. ACS Chem. Neurosci. 2020;11:4316–4328. doi: 10.1021/acschemneuro.0c00574. [DOI] [PubMed] [Google Scholar]
- 56.Pérez-Areales F., Garrido M., Aso E., Bartolini M., De Simone A., Espargaró A., Ginex T., Sabate R., Pérez B., Andrisano V., et al. Centrally Active Multitarget Anti-Alzheimer Agents Derived from the Antioxidant Lead CR-6. J. Med. Chem. 2020;63:9360–9390. doi: 10.1021/acs.jmedchem.0c00528. [DOI] [PubMed] [Google Scholar]
- 57.Rani A., Singh A., Kaur J., Singh G., Bhatti R., Gumede N., Kisten P., Singh P., Sumanjit, Kumar V. 1H-1,2,3-triazole grafted tacrine-chalcone conjugates as potential cholinesterase inhibitors with the evaluation of their behavioral tests and oxidative stress in mice brain cells. Bioorg. Chem. 2021;114:105053. doi: 10.1016/j.bioorg.2021.105053. [DOI] [PubMed] [Google Scholar]
- 58.Viayna E., Coquelle N., Cieslikiewicz-Bouet M., Cisternas P., Oliva C.A., Sánchez-López E., Ettcheto M., Bartolini M., De Simone A., Ricchini M., et al. Discovery of a Potent Dual Inhibitor of Acetylcholinesterase and Butyrylcholinesterase with Antioxidant Activity that Alleviates Alzheimer-like Pathology in Old APP/PS1 Mice. J. Med. Chem. 2021;64:812–839. doi: 10.1021/acs.jmedchem.0c01775. [DOI] [PubMed] [Google Scholar]
- 59.Fang L., Kraus B., Lehmann J., Heilmann J., Zhang Y., Decker M. Design and synthesis of tacrine–ferulic acid hybrids as multi-potent anti-Alzheimer drug candidates. Bioorg. Med. Chem. Lett. 2008;18:2905–2909. doi: 10.1016/j.bmcl.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 60.Fleck C., Appenroth D., Fang L., Schott Y., Lehmann J., Decker M. Investigation into the in vivo effects of five novel tacrine/ferulic acid and β-carboline derivatives on scopolamine-induced cognitive impairment in rats using radial maze paradigm. Arzneimittelforschung. 2011;60:299–306. doi: 10.1055/s-0031-1296291. [DOI] [PubMed] [Google Scholar]
- 61.Pi R., Mao X., Chao X., Cheng Z., Liu M., Duan X., Ye M., Chen X., Mei Z., Liu P., et al. Tacrine-6-Ferulic Acid, a Novel Multifunctional Dimer, Inhibits Amyloid-β-Mediated Alzheimer’s Disease-Associated Pathogenesis In Vitro and In Vivo. PLoS ONE. 2012;7:e31921. doi: 10.1371/journal.pone.0031921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fu Y., Mu Y., Lei H., Wang P., Li X., Leng Q., Han L., Qu X., Wang Z., Huang X. Design, Synthesis and Evaluation of Novel Tacrine-Ferulic Acid Hybrids as Multifunctional Drug Candidates against Alzheimer’s Disease. Molecules. 2016;21:1338. doi: 10.3390/molecules21101338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu J., Yang H., Chen Y., Lin H., Li Q., Mo J., Bian Y., Pei Y., Sun H. Synthesis, pharmacology and molecular docking on multifunctional tacrine-ferulic acid hybrids as cholinesterase inhibitors against Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2018;33:496–506. doi: 10.1080/14756366.2018.1430691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fang L., Appenroth D., Decker M., Kiehntopf M., Roegler C., Deufel T., Fleck C., Peng S., Zhang Y., Lehmann J. Synthesis and Biological Evaluation of NO-Donor-Tacrine Hybrids as Hepatoprotective Anti-Alzheimer Drug Candidates. J. Med. Chem. 2008;51:713–716. doi: 10.1021/jm701491k. [DOI] [PubMed] [Google Scholar]
- 65.Fang L., Appenroth D., Decker M., Kiehntopf M., Lupp A., Peng S., Fleck C., Zhang Y., Lehmann J. NO-Donating Tacrine Hybrid Compounds Improve Scopolamine-Induced Cognition Impairment and Show Less Hepatotoxicity. J. Med. Chem. 2008;51:7666–7669. doi: 10.1021/jm801131a. [DOI] [PubMed] [Google Scholar]
- 66.Hui A., Chen Y., Zhu S., Gan C., Pan J., Zhou A. Design and synthesis of tacrine-phenothiazine hybrids as multitarget drugs for Alzheimer’s disease. Med. Chem. Res. 2014;23:3546–3557. doi: 10.1007/s00044-014-0931-2. [DOI] [Google Scholar]
- 67.Gorecki L., Uliassi E., Bartolini M., Janockova J., Hrabinova M., Hepnarova V., Prchal L., Muckova L., Pejchal J., Karasova J.Z., et al. Phenothiazine-Tacrine Heterodimers: Pursuing Multitarget Directed Approach in Alzheimer’s Disease. ACS Chem. Neurosci. 2021;12:1698–1715. doi: 10.1021/acschemneuro.1c00184. [DOI] [PubMed] [Google Scholar]
- 68.Huang L., Su T., Shan W., Luo Z., Sun Y., He F., Li X. Inhibition of cholinesterase activity and amyloid aggregation by berberine-phenyl-benzoheterocyclic and tacrine-phenyl-benzoheterocyclic hybrids. Bioorg. Med. Chem. 2012;20:3038–3048. doi: 10.1016/j.bmc.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 69.Keri R.S., Quintanova C., Marques S.M., Esteves A.R., Cardoso S.M., Santos M.A. Design, synthesis and neuroprotective evaluation of novel tacrine–benzothiazole hybrids as multi-targeted compounds against Alzheimer’s disease. Bioorg. Med. Chem. 2013;21:4559–4569. doi: 10.1016/j.bmc.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 70.Zha X., Lamba D., Zhang L., Lou Y., Xu C., Kang D., Chen L., Xu Y., Zhang L., De Simone A., et al. Novel Tacrine–Benzofuran Hybrids as Potent Multitarget-Directed Ligands for the Treatment of Alzheimer’s Disease: Design, Synthesis, Biological Evaluation, and X-ray Crystallography. J. Med. Chem. 2016;59:114–131. doi: 10.1021/acs.jmedchem.5b01119. [DOI] [PubMed] [Google Scholar]
- 71.Rajeshwari R., Chand K., Candeias E., Cardoso S., Chaves S., Santos M. New Multitarget Hybrids Bearing Tacrine and Phenylbenzothiazole Motifs as Potential Drug Candidates for Alzheimer’s Disease. Molecules. 2019;24:587. doi: 10.3390/molecules24030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fancellu G., Chand K., Tomás D., Orlandini E., Piemontese L., Silva D.F., Cardoso S.M., Chaves S., Santos M.A. Novel tacrine–benzofuran hybrids as potential multi-target drug candidates for the treatment of Alzheimer’s Disease. J. Enzyme Inhib. Med. Chem. 2020;35:211–226. doi: 10.1080/14756366.2019.1689237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nepovimova E., Svobodova L., Dolezal R., Hepnarova V., Junova L., Jun D., Korabecny J., Kucera T., Gazova Z., Motykova K., et al. Tacrine-Benzothiazoles: Novel class of potential multitarget anti-Alzheimeŕs drugs dealing with cholinergic, amyloid and mitochondrial systems. Bioorg. Chem. 2021;107:104596. doi: 10.1016/j.bioorg.2020.104596. [DOI] [PubMed] [Google Scholar]
- 74.Chen Y., Sun J., Peng S., Liao H., Zhang Y., Lehmann J. Tacrine-Flurbiprofen Hybrids as Multifunctional Drug Candidates for the Treatment of Alzheimer’s Disease. Arch. Pharm. 2013;346:865–871. doi: 10.1002/ardp.201300074. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y., Sun J., Huang Z., Liao H., Peng S., Lehmann J., Zhang Y. NO-donating tacrine derivatives as potential butyrylcholinesterase inhibitors with vasorelaxation activity. Bioorg. Med. Chem. Lett. 2013;23:3162–3165. doi: 10.1016/j.bmcl.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 76.Chen Y., Sun J., Huang Z., Liao H., Peng S., Lehmann J., Zhang Y. Design, synthesis and evaluation of tacrine–flurbiprofen–nitrate trihybrids as novel anti-Alzheimer’s disease agents. Bioorg. Med. Chem. 2013;21:2462–2470. doi: 10.1016/j.bmc.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 77.Zawada K., Czarnecka K., Girek M., Kręcisz P., Trejtnar F., Mandíková J., Jończyk J., Bajda M., Staśkiewicz M., Wójtowicz P., et al. New hybrids of tacrine and indomethacin as multifunctional acetylcholinesterase inhibitors. Chem. Pap. 2021;75:249–264. doi: 10.1007/s11696-020-01295-y. [DOI] [Google Scholar]
- 78.Liu Z., Zhang B., Xia S., Fang L., Gou S. ROS-responsive and multifunctional anti-Alzheimer prodrugs: Tacrine-ibuprofen hybrids via a phenyl boronate linker. Eur. J. Med. Chem. 2021;212:112997. doi: 10.1016/j.ejmech.2020.112997. [DOI] [PubMed] [Google Scholar]
- 79.Chen H., Wu X., Gu X., Zhou Y., Ye L., Zhang K., Pan H., Wang J., Wei H., Zhu B., et al. Tacrine(10)-Hupyridone Prevents Post-operative Cognitive Dysfunction via the Activation of BDNF Pathway and the Inhibition of AChE in Aged Mice. Front. Cell. Neurosci. 2018;12:396. doi: 10.3389/fncel.2018.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen H., Xiang S., Huang L., Lin J., Hu S., Mak S.-H., Wang C., Wang Q., Cui W., Han Y. Tacrine(10)-hupyridone, a dual-binding acetylcholinesterase inhibitor, potently attenuates scopolamine-induced impairments of cognition in mice. Metab. Brain Dis. 2018;33:1131–1139. doi: 10.1007/s11011-018-0221-7. [DOI] [PubMed] [Google Scholar]
- 81.Xuan Z., Gu X., Yan S., Xie Y., Zhou Y., Zhang H., Jin H., Hu S., Mak M.S.H., Zhou D., et al. Dimeric Tacrine(10)-hupyridone as a Multitarget-Directed Ligand To Treat Alzheimer’s Disease. ACS Chem. Neurosci. 2021;12:2462–2477. doi: 10.1021/acschemneuro.1c00182. [DOI] [PubMed] [Google Scholar]
- 82.Shao D., Zou C., Luo C., Tang X., Li Y. Synthesis and evaluation of tacrine–E2020 hybrids as acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2004;14:4639–4642. doi: 10.1016/j.bmcl.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 83.Camps P., Formosa X., Galdeano C., Gómez T., Muñoz-Torrero D., Scarpellini M., Viayna E., Badia A., Clos M.V., Camins A., et al. Novel Donepezil-Based Inhibitors of Acetyl- and Butyrylcholinesterase and Acetylcholinesterase-Induced β-Amyloid Aggregation. J. Med. Chem. 2008;51:3588–3598. doi: 10.1021/jm8001313. [DOI] [PubMed] [Google Scholar]
- 84.Codony S., Pont C., Griñán-Ferré C., Di Pede-Mattatelli A., Calvó-Tusell C., Feixas F., Osuna S., Jarné-Ferrer J., Naldi M., Bartolini M., et al. Discovery and In Vivo Proof of Concept of a Highly Potent Dual Inhibitor of Soluble Epoxide Hydrolase and Acetylcholinesterase for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2022;65:4909–4925. doi: 10.1021/acs.jmedchem.1c02150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Galdeano C., Viayna E., Sola I., Formosa X., Camps P., Badia A., Clos M.V., Relat J., Ratia M., Bartolini M., et al. Huprine–Tacrine Heterodimers as Anti-Amyloidogenic Compounds of Potential Interest against Alzheimer’s and Prion Diseases. J. Med. Chem. 2012;55:661–669. doi: 10.1021/jm200840c. [DOI] [PubMed] [Google Scholar]
- 86.Cen J., Guo H., Hong C., Lv J., Yang Y., Wang T., Fang D., Luo W., Wang C. Development of tacrine-bifendate conjugates with improved cholinesterase inhibitory and pro-cognitive efficacy and reduced hepatotoxicity. Eur. J. Med. Chem. 2018;144:128–136. doi: 10.1016/j.ejmech.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 87.Xu A., He F., Zhang X., Li X., Ran Y., Wei C., James Chou C., Zhang R., Wu J. Tacrine-hydroxamate derivatives as multitarget-directed ligands for the treatment of Alzheimer’s disease: Design, synthesis, and biological evaluation. Bioorg. Chem. 2020;98:103721. doi: 10.1016/j.bioorg.2020.103721. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y., Guan X.-L., Wu P.-F., Wang C.-M., Cao H., Li L., Guo X.-J., Wang F., Xie N., Jiang F.-C., et al. Multifunctional Mercapto-tacrine Derivatives for Treatment of Age-Related Neurodegenerative Diseases. J. Med. Chem. 2012;55:3588–3592. doi: 10.1021/jm300124p. [DOI] [PubMed] [Google Scholar]
- 89.Keri R.S., Quintanova C., Chaves S., Silva D.F., Cardoso S.M., Santos M.A. New Tacrine Hybrids with Natural-Based Cysteine Derivatives as Multitargeted Drugs for Potential Treatment of Alzheimer’s Disease. Chem. Biol. Drug Des. 2016;87:101–111. doi: 10.1111/cbdd.12633. [DOI] [PubMed] [Google Scholar]
- 90.Cheng X., Gu J., Pang Y., Liu J., Xu T., Li X., Hua Y., Newell K.A., Huang X.-F., Yu Y., et al. Tacrine–Hydrogen Sulfide Donor Hybrid Ameliorates Cognitive Impairment in the Aluminum Chloride Mouse Model of Alzheimer’s Disease. ACS Chem. Neurosci. 2019;10:3500–3509. doi: 10.1021/acschemneuro.9b00120. [DOI] [PubMed] [Google Scholar]
- 91.Camps P., Formosa X., Galdeano C., Muñoz-Torrero D., Ramírez L., Gómez E., Isambert N., Lavilla R., Badia A., Clos M.V., et al. Pyrano[3,2-c]quinoline−6-Chlorotacrine Hybrids as a Novel Family of Acetylcholinesterase- and β-Amyloid-Directed Anti-Alzheimer Compounds. J. Med. Chem. 2009;52:5365–5379. doi: 10.1021/jm900859q. [DOI] [PubMed] [Google Scholar]
- 92.Di Pietro O., Pérez-Areales F., Juárez-Jiménez J., Espargaró A., Clos M.V., Pérez B., Lavilla R., Sabaté R., Luque F.J., Muñoz-Torrero D. Tetrahydrobenzo[h][1,6]naphthyridine-6-chlorotacrine hybrids as a new family of anti-Alzheimer agents targeting β-amyloid, tau, and cholinesterase pathologies. Eur. J. Med. Chem. 2014;84:107–117. doi: 10.1016/j.ejmech.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 93.da Costa J.S., Lopes J.P.B., Russowsky D., Petzhold C.L., de Borges A.C., Ceschi M.A., Konrath E., Batassini C., Lunardi P.S., Gonçalves C.A.S. Synthesis of tacrine-lophine hybrids via one-pot four component reaction and biological evaluation as acetyl- and butyrylcholinesterase inhibitors. Eur. J. Med. Chem. 2013;62:556–563. doi: 10.1016/j.ejmech.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 94.Marco-Contelles J., León R., de los Ríos C., Guglietta A., Terencio J., López M., García A., Villarroya M. Novel Multipotent Tacrine−Dihydropyridine Hybrids with Improved Acetylcholinesterase Inhibitory and Neuroprotective Activities as Potential Drugs for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2006;49:7607–7610. doi: 10.1021/jm061047j. [DOI] [PubMed] [Google Scholar]
- 95.Marco-Contelles J., León R., de los Ríos C., Samadi A., Bartolini M., Andrisano V., Huertas O., Barril X., Luque F.J., Rodríguez-Franco M., et al. Tacripyrines, the First Tacrine−Dihydropyridine Hybrids, as Multitarget-Directed Ligands for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2009;52:2724–2732. doi: 10.1021/jm801292b. [DOI] [PubMed] [Google Scholar]
- 96.Bartolini M., Pistolozzi M., Andrisano V., Egea J., López M.G., Iriepa I., Moraleda I., Gálvez E., Marco-Contelles J., Samadi A. Chemical and Pharmacological Studies on Enantiomerically Pure p-Methoxytacripyrines, Promising Multi-Target-Directed Ligands for the Treatment of Alzheimer’s Disease. ChemMedChem. 2011;6:1990–1997. doi: 10.1002/cmdc.201100239. [DOI] [PubMed] [Google Scholar]
- 97.Wang X.-L., Xiong Y., Yang Y., Tuo Q., Wang X., Chen R., Tian Q., Zhang Z., Yan X., Yang Z., et al. A novel tacrine-dihydropyridine hybrid (-)SCR1693 induces tau dephosphorylation and inhibits Aβ generation in cells. Eur. J. Pharmacol. 2015;754:134–139. doi: 10.1016/j.ejphar.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 98.Chioua M., Buzzi E., Moraleda I., Iriepa I., Maj M., Wnorowski A., Giovannini C., Tramarin A., Portali F., Ismaili L., et al. Tacripyrimidines, the first tacrine-dihydropyrimidine hybrids, as multi-target-directed ligands for Alzheimer’s disease. Eur. J. Med. Chem. 2018;155:839–846. doi: 10.1016/j.ejmech.2018.06.044. [DOI] [PubMed] [Google Scholar]
- 99.Sola I., Aso E., Frattini D., López-González I., Espargaró A., Sabaté R., Di Pietro O., Luque F.J., Clos M.V., Ferrer I., et al. Novel Levetiracetam Derivatives That Are Effective against the Alzheimer-like Phenotype in Mice: Synthesis, in Vitro, ex Vivo, and in Vivo Efficacy Studies. J. Med. Chem. 2015;58:6018–6032. doi: 10.1021/acs.jmedchem.5b00624. [DOI] [PubMed] [Google Scholar]
- 100.Więckowska A., Kołaczkowski M., Bucki A., Godyń J., Marcinkowska M., Więckowski K., Zaręba P., Siwek A., Kazek G., Głuch-Lutwin M., et al. Novel multi-target-directed ligands for Alzheimer’s disease: Combining cholinesterase inhibitors and 5-HT 6 receptor antagonists. Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2016;124:63–81. doi: 10.1016/j.ejmech.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 101.Więckowska A., Wichur T., Godyń J., Bucki A., Marcinkowska M., Siwek A., Więckowski K., Zaręba P., Knez D., Głuch-Lutwin M., et al. Novel Multitarget-Directed Ligands Aiming at Symptoms and Causes of Alzheimer’s Disease. ACS Chem. Neurosci. 2018;9:1195–1214. doi: 10.1021/acschemneuro.8b00024. [DOI] [PubMed] [Google Scholar]
- 102.Li X., Wang H., Xu Y., Liu W., Gong Q., Wang W., Qiu X., Zhu J., Mao F., Zhang H., et al. Novel Vilazodone–Tacrine Hybrids as Potential Multitarget-Directed Ligands for the Treatment of Alzheimer’s Disease Accompanied with Depression: Design, Synthesis, and Biological Evaluation. ACS Chem. Neurosci. 2017;8:2708–2721. doi: 10.1021/acschemneuro.7b00259. [DOI] [PubMed] [Google Scholar]
- 103.Elsinghorst P.W., Cieslik J.S., Mohr K., Tränkle C., Gütschow M. First Gallamine−Tacrine Hybrid: Design and Characterization at Cholinesterases and the M 2 Muscarinic Receptor. J. Med. Chem. 2007;50:5685–5695. doi: 10.1021/jm070859s. [DOI] [PubMed] [Google Scholar]
- 104.Fang L., Jumpertz S., Zhang Y., Appenroth D., Fleck C., Mohr K., Tränkle C., Decker M. Hybrid Molecules from Xanomeline and Tacrine: Enhanced Tacrine Actions on Cholinesterases and Muscarinic M 1 Receptors. J. Med. Chem. 2010;53:2094–2103. doi: 10.1021/jm901616h. [DOI] [PubMed] [Google Scholar]
- 105.Hepnarova V., Korabecny J., Matouskova L., Jost P., Muckova L., Hrabinova M., Vykoukalova N., Kerhartova M., Kucera T., Dolezal R., et al. The concept of hybrid molecules of tacrine and benzyl quinolone carboxylic acid (BQCA) as multifunctional agents for Alzheimer’s disease. Eur. J. Med. Chem. 2018;150:292–306. doi: 10.1016/j.ejmech.2018.02.083. [DOI] [PubMed] [Google Scholar]
- 106.Maspero M., Volpato D., Cirillo D., Chen N.Y., Messerer R., Sotriffer C., De Amici M., Holzgrabe U., Dallanoce C. Tacrine-xanomeline and tacrine-iperoxo hybrid ligands: Synthesis and biological evaluation at acetylcholinesterase and M1 muscarinic acetylcholine receptors. Bioorg. Chem. 2020;96:103633. doi: 10.1016/j.bioorg.2020.103633. [DOI] [PubMed] [Google Scholar]
- 107.Lange J.H.M., Coolen H.K.A.C., van der Neut M.A.W., Borst A.J.M., Stork B., Verveer P.C., Kruse C.G. Design, Synthesis, Biological Properties, and Molecular Modeling Investigations of Novel Tacrine Derivatives with a Combination of Acetylcholinesterase Inhibition and Cannabinoid CB 1 Receptor Antagonism. J. Med. Chem. 2010;53:1338–1346. doi: 10.1021/jm901614b. [DOI] [PubMed] [Google Scholar]
- 108.Spilovska K., Korabecny J., Kral J., Horova A., Musilek K., Soukup O., Drtinova L., Gazova Z., Siposova K., Kuca K. 7-Methoxytacrine-Adamantylamine Heterodimers as Cholinesterase Inhibitors in Alzheimer’s Disease Treatment—Synthesis, Biological Evaluation and Molecular Modeling Studies. Molecules. 2013;18:2397–2418. doi: 10.3390/molecules18022397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pérez-Areales F.J., Turcu A.L., Barniol-Xicota M., Pont C., Pivetta D., Espargaró A., Bartolini M., De Simone A., Andrisano V., Pérez B., et al. A novel class of multitarget anti-Alzheimer benzohomoadamantane chlorotacrine hybrids modulating cholinesterases and glutamate NMDA receptors. Eur. J. Med. Chem. 2019;180:613–626. doi: 10.1016/j.ejmech.2019.07.051. [DOI] [PubMed] [Google Scholar]
- 110.Ceschi M.A., da Costa J.S., Lopes J.P.B., Câmara V.S., Campo L.F., de Borges A.C., Gonçalves C., de Souza D., Konrath E.L., Karl A.L.M., et al. Novel series of tacrine-tianeptine hybrids: Synthesis, cholinesterase inhibitory activity, S100B secretion and a molecular modeling approach. Eur. J. Med. Chem. 2016;121:758–772. doi: 10.1016/j.ejmech.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 111.Lu C., Zhou Q., Yan J., Du Z., Huang L., Li X. A novel series of tacrine–selegiline hybrids with cholinesterase and monoamine oxidase inhibition activities for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2013;62:745–753. doi: 10.1016/j.ejmech.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 112.Xie S.-S., Wang X., Jiang N., Yu W., Wang K.D.G., Lan J.-S., Li Z.-R., Kong L.-Y. Multi-target tacrine-coumarin hybrids: Cholinesterase and monoamine oxidase B inhibition properties against Alzheimer’s disease. Eur. J. Med. Chem. 2015;95:153–165. doi: 10.1016/j.ejmech.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 113.Xie S.-S., Wang X.-B., Li J.-Y., Yang L., Kong L.-Y. Design, synthesis and evaluation of novel tacrine–coumarin hybrids as multifunctional cholinesterase inhibitors against Alzheimer’s disease. Eur. J. Med. Chem. 2013;64:540–553. doi: 10.1016/j.ejmech.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 114.Hamulakova S., Janovec L., Hrabinova M., Spilovska K., Korabecny J., Kristian P., Kuca K., Imrich J. Synthesis and Biological Evaluation of Novel Tacrine Derivatives and Tacrine−Coumarin Hybrids as Cholinesterase Inhibitors. J. Med. Chem. 2014;57:7073–7084. doi: 10.1021/jm5008648. [DOI] [PubMed] [Google Scholar]
- 115.Li S.-Y., Wang X.-B., Xie S.-S., Jiang N., Wang K.D.G., Yao H.-Q., Sun H.-B., Kong L.-Y. Multifunctional tacrine–flavonoid hybrids with cholinergic, β-amyloid-reducing, and metal chelating properties for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2013;69:632–646. doi: 10.1016/j.ejmech.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 116.Viayna E., Sola I., Bartolini M., De Simone A., Tapia-Rojas C., Serrano F.G., Sabaté R., Juárez-Jiménez J., Pérez B., Luque F.J., et al. Synthesis and Multitarget Biological Profiling of a Novel Family of Rhein Derivatives As Disease-Modifying Anti-Alzheimer Agents. J. Med. Chem. 2014;57:2549–2567. doi: 10.1021/jm401824w. [DOI] [PubMed] [Google Scholar]
- 117.Thiratmatrakul S., Yenjai C., Waiwut P., Vajragupta O., Reubroycharoen P., Tohda M., Boonyarat C. Synthesis, biological evaluation and molecular modeling study of novel tacrine–carbazole hybrids as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014;75:21–30. doi: 10.1016/j.ejmech.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 118.Spilovska K., Korabecny J., Sepsova V., Jun D., Hrabinova M., Jost P., Muckova L., Soukup O., Janockova J., Kucera T., et al. Novel Tacrine-Scutellarin Hybrids as Multipotent Anti-Alzheimer’s Agents: Design, Synthesis and Biological Evaluation. Molecules. 2017;22:1006. doi: 10.3390/molecules22061006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jeřábek J., Uliassi E., Guidotti L., Korábečný J., Soukup O., Sepsova V., Hrabinova M., Kuča K., Bartolini M., Peña-Altamira L.E., et al. Tacrine-resveratrol fused hybrids as multi-target-directed ligands against Alzheimer’s disease. Eur. J. Med. Chem. 2017;127:250–262. doi: 10.1016/j.ejmech.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 120.Lopes J.P.B., Silva L., da Costa Franarin G., Ceschi M.A., Lüdtke D.S., Dantas R.F., de Salles C., Silva F.P., Jr., Senger M.R., Guedes I.A., et al. Design, synthesis, cholinesterase inhibition and molecular modelling study of novel tacrine hybrids with carbohydrate derivatives. Bioorg. Med. Chem. 2018;26:5566–5577. doi: 10.1016/j.bmc.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 121.Chalupova K., Korabecny J., Bartolini M., Monti B., Lamba D., Caliandro R., Pesaresi A., Brazzolotto X., Gastellier A.-J., Nachon F., et al. Novel tacrine-tryptophan hybrids: Multi-target directed ligands as potential treatment for Alzheimer’s disease. Eur. J. Med. Chem. 2019;168:491–514. doi: 10.1016/j.ejmech.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 122.Cheng Z.-Q., Zhu K.-K., Zhang J., Song J.-L., Muehlmann L.A., Jiang C.-S., Liu C.-L., Zhang H. Molecular-docking-guided design and synthesis of new IAA-tacrine hybrids as multifunctional AChE/BuChE inhibitors. Bioorg. Chem. 2019;83:277–288. doi: 10.1016/j.bioorg.2018.10.057. [DOI] [PubMed] [Google Scholar]
- 123.Rossi M., Freschi M., de Camargo Nascente L., Salerno A., de Melo Viana Teixeira S., Nachon F., Chantegreil F., Soukup O., Prchal L., Malaguti M., et al. Sustainable Drug Discovery of Multi-Target-Directed Ligands for Alzheimer’s Disease. J. Med. Chem. 2021;64:4972–4990. doi: 10.1021/acs.jmedchem.1c00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Elsinghorst P.W., González Tanarro C.M., Gütschow M. Novel Heterobivalent Tacrine Derivatives as Cholinesterase Inhibitors with Notable Selectivity Toward Butyrylcholinesterase. J. Med. Chem. 2006;49:7540–7544. doi: 10.1021/jm060742o. [DOI] [PubMed] [Google Scholar]
- 125.Chen X., Wehle S., Kuzmanovic N., Merget B., Holzgrabe U., König B., Sotriffer C.A., Decker M. Acetylcholinesterase Inhibitors with Photoswitchable Inhibition of β-Amyloid Aggregation. ACS Chem. Neurosci. 2014;5:377–389. doi: 10.1021/cn500016p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nepovimova E., Uliassi E., Korabecny J., Peña-Altamira L.E., Samez S., Pesaresi A., Garcia G.E., Bartolini M., Andrisano V., Bergamini C., et al. Multitarget Drug Design Strategy: Quinone–Tacrine Hybrids Designed To Block Amyloid-β Aggregation and To Exert Anticholinesterase and Antioxidant Effects. J. Med. Chem. 2014;57:8576–8589. doi: 10.1021/jm5010804. [DOI] [PubMed] [Google Scholar]
- 127.Mao F., Li J., Wei H., Huang L., Li X. Tacrine-propargylamine derivatives with improved acetylcholinesterase inhibitory activity and lower hepatotoxicity as a potential lead compound for the treatment of Alzheimers disease. J. Enzyme Inhib. Med. Chem. 2015;30:995–1001. doi: 10.3109/14756366.2014.1003212. [DOI] [PubMed] [Google Scholar]
- 128.Korabecny J., Andrs M., Nepovimova E., Dolezal R., Babkova K., Horova A., Malinak D., Mezeiova E., Gorecki L., Sepsova V., et al. 7-Methoxytacrine-p-Anisidine Hybrids as Novel Dual Binding Site Acetylcholinesterase Inhibitors for Alzheimer’s Disease Treatment. Molecules. 2015;20:22084–22101. doi: 10.3390/molecules201219836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Najafi Z., Mahdavi M., Saeedi M., Karimpour-Razkenari E., Asatouri R., Vafadarnejad F., Moghadam F.H., Khanavi M., Sharifzadeh M., Akbarzadeh T. Novel tacrine-1,2,3-triazole hybrids: In vitro, in vivo biological evaluation and docking study of cholinesterase inhibitors. Eur. J. Med. Chem. 2017;125:1200–1212. doi: 10.1016/j.ejmech.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 130.Riazimontazer E., Sadeghpour H., Nadri H., Sakhteman A., Tüylü Küçükkılınç T., Miri R., Edraki N. Design, synthesis and biological activity of novel tacrine-isatin Schiff base hybrid derivatives. Bioorg. Chem. 2019;89:103006. doi: 10.1016/j.bioorg.2019.103006. [DOI] [PubMed] [Google Scholar]
- 131.Yao H., Uras G., Zhang P., Xu S., Yin Y., Liu J., Qin S., Li X., Allen S., Bai R., et al. Discovery of Novel Tacrine–Pyrimidone Hybrids as Potent Dual AChE/GSK-3 Inhibitors for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2021;64:7483–7506. doi: 10.1021/acs.jmedchem.1c00160. [DOI] [PubMed] [Google Scholar]
- 132.Ozten O., Zengin Kurt B., Sonmez F., Dogan B., Durdagi S. Synthesis, molecular docking and molecular dynamics studies of novel tacrine-carbamate derivatives as potent cholinesterase inhibitors. Bioorg. Chem. 2021;115:105225. doi: 10.1016/j.bioorg.2021.105225. [DOI] [PubMed] [Google Scholar]
- 133.Przybyłowska M., Dzierzbicka K., Kowalski S., Demkowicz S., Daśko M., Inkielewicz-Stepniak I. Design, synthesis and biological evaluation of novel N -phosphorylated and O -phosphorylated tacrine derivatives as potential drugs against Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2022;37:1012–1022. doi: 10.1080/14756366.2022.2045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun T.C., Liu X.C., Yang S.H., Song L.L., Zhou S.J., Deng S.L., Tian L., Cheng L.Y. Melatonin Inhibits Oxidative Stress and Apoptosis in Cryopreserved Ovarian Tissues via Nrf2/HO-1 Signaling Pathway. Front. Mol. Biosci. 2020;7:163. doi: 10.3389/fmolb.2020.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rosini M., Andrisano V., Bartolini M., Bolognesi M.L., Hrelia P., Minarini A., Tarozzi A., Melchiorre C. Rational Approach To Discover Multipotent Anti-Alzheimer Drugs. J. Med. Chem. 2005;48:360–363. doi: 10.1021/jm049112h. [DOI] [PubMed] [Google Scholar]
- 136.Yang N., Jia X., Wang D., Wei C., He Y., Chen L., Zhao Y. Silibinin as a natural antioxidant for modifying polysulfone membranes to suppress hemodialysis-induced oxidative stress. J. Memb. Sci. 2019;574:86–99. doi: 10.1016/j.memsci.2018.12.056. [DOI] [Google Scholar]
- 137.Schewe T. Molecular actions of Ebselen—An antiinflammatory antioxidant. Gen. Pharmacol. Vasc. Syst. 1995;26:1153–1169. doi: 10.1016/0306-3623(95)00003-J. [DOI] [PubMed] [Google Scholar]
- 138.Porciúncula L.O., Rocha J.B.T., Boeck C.R., Vendite D., Souza D.O. Ebselen prevents excitotoxicity provoked by glutamate in rat cerebellar granule neurons. Neurosci. Lett. 2001;299:217–220. doi: 10.1016/S0304-3940(01)01519-1. [DOI] [PubMed] [Google Scholar]
- 139.Wlodek S.T., Antosiewicz J., McCammon J.A., Straatsma T.P., Gilson M.K., Briggs J.M., Humblet C., Sussman J.L. Binding of tacrine and 6-chlorotacrine by acetylcholinesterase. Biopolymers. 1996;38:109–117. doi: 10.1002/(SICI)1097-0282(199601)38:1<109::AID-BIP9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 140.Tran T.-D., Nguyen T.-C.-V., Nguyen N.-S., Nguyen D.-M., Nguyen T.-T.-H., Le M.-T., Thai K.-M. Synthesis of Novel Chalcones as Acetylcholinesterase Inhibitors. Appl. Sci. 2016;6:198. doi: 10.3390/app6070198. [DOI] [Google Scholar]
- 141.Mezeiova E., Soukup O., Korabecny J. Huprines—An insight into the synthesis and biological properties. Russ. Chem. Rev. 2020;89:999–1039. doi: 10.1070/RCR4938. [DOI] [Google Scholar]
- 142.Yang G., Wang Y., Tian J., Liu J.-P. Huperzine A for Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. PLoS ONE. 2013;8:e74916. doi: 10.1371/journal.pone.0074916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang H.Y., Tang X.C. Neuroprotective effects of huperzine A: New therapeutic targets for neurodegenerative disease. Trends Pharmacol. Sci. 2006;27:619–625. doi: 10.1016/j.tips.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 144.Muñoz-Torrero D., Camps P. Huprines for Alzheimer’s disease drug development. Expert Opin. Drug Discov. 2008;3:65–81. doi: 10.1517/17460441.3.1.65. [DOI] [PubMed] [Google Scholar]
- 145.Kim J.K., Park S.U. A recent overview on the biological and pharmacological activities of ferulic acid. Excli J. 2019;18:132–138. doi: 10.17179/excli2019-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Meng G., Meng X., Ma X., Zhang G., Hu X., Jin A., Zhao Y., Liu X. Application of Ferulic Acid for Alzheimer’s Disease: Combination of Text Mining and Experimental Validation. Front. Neuroinform. 2018;12:31. doi: 10.3389/fninf.2018.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Simola N., Morelli M., Carta A.R. The 6-Hydroxydopamine model of parkinson’s disease. Neurotox. Res. 2007;11:151–167. doi: 10.1007/BF03033565. [DOI] [PubMed] [Google Scholar]
- 148.Tsikas D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the l-arginine/nitric oxide area of research. J. Chromatogr. B. 2007;851:51–70. doi: 10.1016/j.jchromb.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 149.Kerwin J.F., Heller M. The arginine-nitric oxide pathway: A target for new drugs. Med. Res. Rev. 1994;14:23–74. doi: 10.1002/med.2610140103. [DOI] [PubMed] [Google Scholar]
- 150.Esplugues J.V. NO as a signalling molecule in the nervous system. Br. J. Pharmacol. 2002;135:1079–1095. doi: 10.1038/sj.bjp.0704569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Balez R., Ooi L. Getting to NO Alzheimer’s Disease: Neuroprotection versus Neurotoxicity Mediated by Nitric Oxide. Oxid. Med. Cell. Longev. 2016;2016:3806157. doi: 10.1155/2016/3806157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Webb D.J., Megson I.L. Nitric oxide donor drugs: Current status and future trends. Expert Opin. Investig. Drugs. 2002;11:587–601. doi: 10.1517/13543784.11.5.587. [DOI] [PubMed] [Google Scholar]
- 153.Thatcher G., Bennett B., Reynolds J. Nitric Oxide Mimetic Molecules as Therapeutic Agents in Alzheimers Disease. Curr. Alzheimer Res. 2005;2:171–182. doi: 10.2174/1567205053585945. [DOI] [PubMed] [Google Scholar]
- 154.Chegaev K., Federico A., Marini E., Rolando B., Fruttero R., Morbin M., Rossi G., Fugnanesi V., Bastone A., Salmona M., et al. NO-donor thiacarbocyanines as multifunctional agents for Alzheimer’s disease. Bioorg. Med. Chem. 2015;23:4688–4698. doi: 10.1016/j.bmc.2015.05.050. [DOI] [PubMed] [Google Scholar]
- 155.Jafari S., Fernandez-Enright F., Huang X.-F. Structural contributions of antipsychotic drugs to their therapeutic profiles and metabolic side effects. J. Neurochem. 2012;120:371–384. doi: 10.1111/j.1471-4159.2011.07590.x. [DOI] [PubMed] [Google Scholar]
- 156.Krasnovskaya O., Spector D., Zlobin A., Pavlov K., Gorelkin P., Erofeev A., Beloglazkina E., Majouga A. Metals in Imaging of Alzheimer’s Disease. Int. J. Mol. Sci. 2020;21:9190. doi: 10.3390/ijms21239190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ono M., Kawashima H., Nonaka A., Kawai T., Haratake M., Mori H., Kung M.-P., Kung H.F., Saji H., Nakayama M. Novel Benzofuran Derivatives for PET Imaging of β-Amyloid Plaques in Alzheimer’s Disease Brains. J. Med. Chem. 2006;49:2725–2730. doi: 10.1021/jm051176k. [DOI] [PubMed] [Google Scholar]
- 158.Kinney J.W., Bemiller S.M., Murtishaw A.S., Leisgang A.M., Salazar A.M., Lamb B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dokmeci D. Ibuprofen and Alzheimer’s disease. Folia Med. 2004;46:5–10. [PubMed] [Google Scholar]
- 160.Lim G.P., Yang F., Chu T., Chen P., Beech W., Teter B., Tran T., Ubeda O., Ashe K.H., Frautschy S.A., et al. Ibuprofen Suppresses Plaque Pathology and Inflammation in a Mouse Model for Alzheimer’s Disease. J. Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Weggen S., Eriksen J.L., Das P., Sagi S.A., Wang R., Pietrzik C.U., Findlay K.A., Smith T.E., Murphy M.P., Bulter T., et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 162.Wilkinson B.L., Cramer P.E., Varvel N.H., Reed-Geaghan E., Jiang Q., Szabo A., Herrup K., Lamb B.T., Landreth G.E. Ibuprofen attenuates oxidative damage through NOX2 inhibition in Alzheimer’s disease. Neurobiol. Aging. 2012;33:e21–e197. doi: 10.1016/j.neurobiolaging.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang Y., Pike A. Pyridones in drug discovery: Recent advances. Bioorg. Med. Chem. Lett. 2021;38:127849. doi: 10.1016/j.bmcl.2021.127849. [DOI] [PubMed] [Google Scholar]
- 164.Carlier P.R., Du D.M., Han Y., Liu J., Pang Y.P. Potent, easily synthesized huperzine A-tacrine hybrid acetylcholinesterase inhibitors. Bioorg. Med. Chem. Lett. 1999;9:2335–2338. doi: 10.1016/S0960-894X(99)00396-0. [DOI] [PubMed] [Google Scholar]
- 165.Camps P., El Achab R., Görbig D.M., Morral J., Muñoz-Torrero D., Badia A., Baños J.E., Vivas N.M., Barril X., Orozco M., et al. Synthesis, in Vitro Pharmacology, and Molecular Modeling of Very Potent Tacrine−Huperzine A Hybrids as Acetylcholinesterase Inhibitors of Potential Interest for the Treatment of Alzheimer’s Disease. J. Med. Chem. 1999;42:3227–3242. doi: 10.1021/jm980620z. [DOI] [PubMed] [Google Scholar]
- 166.Li W., Kan K., Carlier P., Pang Y., Han Y. East Meets West in the Search for Alzheimers Therapeutics–Novel Dimeric Inhibitors from Tacrine and Huperzine A. Curr. Alzheimer Res. 2007;4:386–396. doi: 10.2174/156720507781788918. [DOI] [PubMed] [Google Scholar]
- 167.Mak S., Li W., Fu H., Luo J., Cui W., Hu S., Pang Y., Carlier P.R., Tsim K.W., Pi R., et al. Promising tacrine/huperzine A-based dimeric acetylcholinesterase inhibitors for neurodegenerative disorders: From relieving symptoms to modifying diseases through multitarget. J. Neurochem. 2021;158:1381–1393. doi: 10.1111/jnc.15379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nagahara A.H., Merrill D.A., Coppola G., Tsukada S., Schroeder B.E., Shaked G.M., Wang L., Blesch A., Kim A., Conner J.M., et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat. Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Giuffrida M.L., Copani A., Rizzarelli E. A promising connection between BDNF and Alzheimer’s disease. Aging. 2018;10:1791–1792. doi: 10.18632/aging.101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Knowles J. Donepezil in Alzheimer’s disease: An evidence-based review of its impact on clinical and economic outcomes. Core Evid. 2006;1:195–219. [PMC free article] [PubMed] [Google Scholar]
- 171.Alonso D., Dorronsoro I., Rubio L., Muñoz P., García-Palomero E., Del Monte M., Bidon-Chanal A., Orozco M., Luque F.J., Castro A., et al. Donepezil–tacrine hybrid related derivatives as new dual binding site inhibitors of AChE. Bioorg. Med. Chem. 2005;13:6588–6597. doi: 10.1016/j.bmc.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 172.Rose T.E., Morisseau C., Liu J.-Y., Inceoglu B., Jones P.D., Sanborn J.R., Hammock B.D. 1-Aryl-3-(1-acylpiperidin-4-yl)urea Inhibitors of Human and Murine Soluble Epoxide Hydrolase: Structure−Activity Relationships, Pharmacokinetics, and Reduction of Inflammatory Pain. J. Med. Chem. 2010;53:7067–7075. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jonnalagadda D., Wan D., Chun J., Hammock B.D., Kihara Y. A Soluble Epoxide Hydrolase Inhibitor, 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) Urea, Ameliorates Experimental Autoimmune Encephalomyelitis. Int. J. Mol. Sci. 2021;22:4650. doi: 10.3390/ijms22094650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Camps P., El Achab R., Morral J., Muñoz-Torrero D., Badia A., Baños J.E., Vivas N.M., Barril X., Orozco M., Luque F.J. New Tacrine−Huperzine A Hybrids (Huprines): Highly Potent Tight-Binding Acetylcholinesterase Inhibitors of Interest for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2000;43:4657–4666. doi: 10.1021/jm000980y. [DOI] [PubMed] [Google Scholar]
- 175.Chang J., Wang Q., Li Y. Synthesis and Biological Activity of Wuweizisu C and Analogs. Curr. Top. Med. Chem. 2009;9:1660–1675. doi: 10.2174/156802609789941933. [DOI] [PubMed] [Google Scholar]
- 176.Li Y., Li Y. Effect of dimethyl diphenyl bicarboxylate (DDB) on 9-amino-1,2,3,4-tetrahydroacridine-induced hepatotoxicity in mice. Yao Xue Xue Bao. 2001;36:493–497. [PubMed] [Google Scholar]
- 177.Xu K., Dai X.-L., Huang H.-C., Jiang Z.-F. Targeting HDACs: A Promising Therapy for Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2011;2011:143269. doi: 10.1155/2011/143269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xie R., Li Y., Tang P., Yuan Q. Design, synthesis and biological evaluation of novel 2-aminobenzamides containing dithiocarbamate moiety as histone deacetylase inhibitors and potent antitumor agents. Eur. J. Med. Chem. 2018;143:320–333. doi: 10.1016/j.ejmech.2017.08.041. [DOI] [PubMed] [Google Scholar]
- 179.Jeong W.-H., Kim W.-I., Lee J.-W., Park H.-K., Song M.-K., Choi I.-S., Han J.-Y. Modulation of Long-Term Potentiation by Gamma Frequency Transcranial Alternating Current Stimulation in Transgenic Mouse Models of Alzheimer’s Disease. Brain Sci. 2021;11:1532. doi: 10.3390/brainsci11111532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang Y.-J., Wu P.-F., Long L.-H., Yu D.-F., Wu W.-N., Hu Z.-L., Fu H., Xie N., Jin Y., Ni L., et al. Reversal of aging-associated hippocampal synaptic plasticity deficits by reductants via regulation of thiol redox and NMDA receptor function. Aging Cell. 2010;9:709–721. doi: 10.1111/j.1474-9726.2010.00595.x. [DOI] [PubMed] [Google Scholar]
- 181.Eto K., Asada T., Arima K., Makifuchi T., Kimura H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002;293:1485–1488. doi: 10.1016/S0006-291X(02)00422-9. [DOI] [PubMed] [Google Scholar]
- 182.Giuliani D., Ottani A., Zaffe D., Galantucci M., Strinati F., Lodi R., Guarini S. Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms. Neurobiol. Learn. Mem. 2013;104:82–91. doi: 10.1016/j.nlm.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 183.Chu Q.-J., He L., Zhang W., Liu C.-L., Ai Y.-Q., Zhang Q. Hydrogen sulfide attenuates surgical trauma-induced inflammatory response and cognitive deficits in mice. J. Surg. Res. 2013;183:330–336. doi: 10.1016/j.jss.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 184.Macdonald I.R., Martin E., Rosenberry T.L., Darvesh S. Probing the Peripheral Site of Human Butyrylcholinesterase. Biochemistry. 2012;51:7046–7053. doi: 10.1021/bi300955k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lopes J.P.B., Silva L., Ceschi M.A., Lüdtke D.S., Zimmer A.R., Ruaro T.C., Dantas R.F., de Salles C.M.C., Silva F.P., Jr., Senger M.R., et al. Synthesis of new lophine–carbohydrate hybrids as cholinesterase inhibitors: Cytotoxicity evaluation and molecular modeling. MedChemComm. 2019;10:2089–2101. doi: 10.1039/C9MD00358D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sobrado M., López M., Carceller F., García A., Roda J. Combined nimodipine and citicoline reduce infarct size, attenuate apoptosis and increase bcl-2 expression after focal cerebral ischemia. Neuroscience. 2003;118:107–113. doi: 10.1016/S0306-4522(02)00912-0. [DOI] [PubMed] [Google Scholar]
- 187.Shi J.-Q., Wang B.-R., Tian Y.-Y., Xu J., Gao L., Zhao S.-L., Jiang T., Xie H.-G., Zhang Y.-D. Antiepileptics Topiramate and Levetiracetam Alleviate Behavioral Deficits and Reduce Neuropathology in APPswe/PS1dE9 Transgenic Mice. CNS Neurosci. Ther. 2013;19:871–881. doi: 10.1111/cns.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Camps P., Cusack B., Mallender W.D., El Achab R.E., Morral J., Muñoz-Torrero D., Rosenberry T.L. Huprine X is a novel high-affinity inhibitor of acetylcholinesterase that is of interest for treatment of Alzheimer’s disease. Mol. Pharmacol. 2000;57:409–417. [PubMed] [Google Scholar]
- 189.Schneider L.S., Geffen Y., Rabinowitz J., Thomas R.G., Schmidt R., Ropele S., Weinstock M. Low-dose ladostigil for mild cognitive impairment. Neurology. 2019;93:e1474–e1484. doi: 10.1212/WNL.0000000000008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bender A.M., Jones C.K., Lindsley C.W. Classics in Chemical Neuroscience: Xanomeline. ACS Chem. Neurosci. 2017;8:435–443. doi: 10.1021/acschemneuro.7b00001. [DOI] [PubMed] [Google Scholar]
- 191.Yeatman H.R., Lane J.R., Choy K.H.C., Lambert N.A., Sexton P.M., Christopoulos A., Canals M. Allosteric Modulation of M1 Muscarinic Acetylcholine Receptor Internalization and Subcellular Trafficking. J. Biol. Chem. 2014;289:15856–15866. doi: 10.1074/jbc.M113.536672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shirey J.K., Brady A.E., Jones P.J., Davis A.A., Bridges T.M., Kennedy J.P., Jadhav S.B., Menon U.N., Xiang Z., Watson M.L., et al. A Selective Allosteric Potentiator of the M1 Muscarinic Acetylcholine Receptor Increases Activity of Medial Prefrontal Cortical Neurons and Restores Impairments in Reversal Learning. J. Neurosci. 2009;29:14271–14286. doi: 10.1523/JNEUROSCI.3930-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xin R., Chen Z., Fu J., Shen F., Zhu Q., Huang F. Xanomeline Protects Cortical Cells From Oxygen-Glucose Deprivation via Inhibiting Oxidative Stress and Apoptosis. Front. Physiol. 2020;11:656. doi: 10.3389/fphys.2020.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Micale V., Drago F., Noerregaard P.K., Elling C.E., Wotjak C.T. The Cannabinoid CB1 Antagonist TM38837 With Limited Penetrance to the Brain Shows Reduced Fear-Promoting Effects in Mice. Front. Pharmacol. 2019;10:207. doi: 10.3389/fphar.2019.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wise L.E., Iredale P.A., Stokes R.J., Lichtman A.H. Combination of Rimonabant and Donepezil Prolongs Spatial Memory Duration. Neuropsychopharmacology. 2007;32:1805–1812. doi: 10.1038/sj.npp.1301297. [DOI] [PubMed] [Google Scholar]
- 196.Torres E., Duque M.D., López-Querol M., Taylor M.C., Naesens L., Ma C., Pinto L.H., Sureda F.X., Kelly J.M., Vázquez S. Synthesis of benzopolycyclic cage amines: NMDA receptor antagonist, trypanocidal and antiviral activities. Bioorg. Med. Chem. 2012;20:942–948. doi: 10.1016/j.bmc.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cristóvão J.S., Gomes C.M. S100 Proteins in Alzheimer’s Disease. Front. Neurosci. 2019;13:463. doi: 10.3389/fnins.2019.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cai Z. Monoamine oxidase inhibitors: Promising therapeutic agents for Alzheimer’s disease (Review) Mol. Med. Rep. 2014;9:1533–1541. doi: 10.3892/mmr.2014.2040. [DOI] [PubMed] [Google Scholar]
- 199.Shahid Nadeem M., Azam Khan J., Kazmi I., Rashid U. Design, Synthesis, and Bioevaluation of Indole Core Containing 2-Arylidine Derivatives of Thiazolopyrimidine as Multitarget Inhibitors of Cholinesterases and Monoamine Oxidase A/B for the Treatment of Alzheimer Disease. ACS Omega. 2022;7:9369–9379. doi: 10.1021/acsomega.1c06344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sterling J., Herzig Y., Goren T., Finkelstein N., Lerner D., Goldenberg W., Miskolczi I., Molnar S., Rantal F., Tamas T., et al. Novel dual inhibitors of AChE and MAO derived from hydroxy aminoindan and phenethylamine as potential treatment for Alzheimer’s disease. J. Med. Chem. 2002;45:5260–5279. doi: 10.1021/jm020120c. [DOI] [PubMed] [Google Scholar]
- 201.Fowler J.S., Logan J., Volkow N.D., Shumay E., McCall-Perez F., Jayne M., Wang G.-J., Alexoff D.L., Apelskog-Torres K., Hubbard B., et al. Evidence that Formulations of the Selective MAO-B Inhibitor, Selegiline, which Bypass First-Pass Metabolism, also Inhibit MAO-A in the Human Brain. Neuropsychopharmacology. 2015;40:650–657. doi: 10.1038/npp.2014.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Piazzi L., Rampa A., Bisi A., Gobbi S., Belluti F., Cavalli A., Bartolini M., Andrisano V., Valenti P., Recanatini M. 3-(4-{[Benzyl(methyl)amino]methyl}phenyl)-6,7-dimethoxy-2 H -2-chromenone (AP2238) Inhibits Both Acetylcholinesterase and Acetylcholinesterase-Induced β-Amyloid Aggregation: A Dual Function Lead for Alzheimer’s Disease Therapy. J. Med. Chem. 2003;46:2279–2282. doi: 10.1021/jm0340602. [DOI] [PubMed] [Google Scholar]
- 203.Chimenti F., Secci D., Bolasco A., Chimenti P., Bizzarri B., Granese A., Carradori S., Yáñez, M., Orallo F., Ortuso F., et al. > Synthesis, Molecular Modeling, and Selective Inhibitory Activity against Human Monoamine Oxidases of 3-Carboxamido-7-Substituted Coumarins. J. Med. Chem. 2009;52:1935–1942. doi: 10.1021/jm801496u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.