Abstract
In 2022, Greece was the second most seriously affected European country in terms of the West Nile virus (WNV), after Italy. Specifically, Central Macedonia was the region with the most reported human cases (81.5%). In the present study, 30,816 female Culex pipiens sensu lato mosquitoes were collected from May to September 2022 in the seven regional units of Central Macedonia; they were then grouped into 690 pools and tested for WNV, while next-generation sequencing was applied to the samples, which showed a cycle threshold of Ct < 30 in a real-time RT-PCR test. WNV was detected in 5.9% of pools, with significant differences in the detection rate among regional units and months. It is of interest that in the Thessaloniki regional unit, where most of the human cases were observed, the virus circulation started earlier, peaked earlier, and lasted longer than in the other regional units. All sequences clustered into the Central European subclade of WNV lineage 2, and the virus strain differed from the initial Greek strain of 2010 by 0.52% and 0.27% at the nucleotide and amino acid levels, respectively. Signature substitutions were present, such as S73P and T157A in the prM and E structural proteins, respectively. The screening of mosquitoes provides useful information for virus circulation in a region with a potential for early warning, while the availability of whole-genome sequences is essential for further studies, including virus evolution.
Keywords: West Nile virus, Culex pipiens, Greece, lineage 2, whole-genome sequencing
1. Introduction
West Nile virus (WNV) was initially isolated in 1937 from the serum of a febrile case in Uganda [1]. The virus belongs to the genus Flavivirus in the Flaviviridae family and possesses a single-stranded positive-sense RNA genome. WNV is maintained in nature, in an enzootic cycle between mosquitoes (mainly of the Culex genus) and birds [2]. Humans and equines are dead-end hosts due to the low-level viremia that they develop, which is not enough for further virus transmission [3]. Most cases (approximately 80%) of WNV infections are asymptomatic, and 20% present as mild cases of illness, while approximately 1% of the patients develop a neuroinvasive disease (mainly encephalitis, meningitis, or acute flaccid paralysis) [4,5,6].
Although up to nine of the WNV genetic lineages have been identified [7], the sequences in human cases cluster only into lineages 1 and 2 [8]. In Europe, the first human outbreak occurred in 1962 in southern France [9], while WNV lineage 2 was first detected in 2004 in Hungary and Russia (as independent virus introductions), with the sequences forming the Central European/Hungarian and the Eastern European/Russian clades of WNV lineage 2, respectively [10,11].
The first outbreak in Greece occurred in 2010; it started near a river delta in the Central Macedonia Region, in the northern part of the country [12]. A total of 197 neuroinvasive cases were reported, and 33 of them had fatal outcomes [13]. The strain responsible (Nea Santa-Greece-2010) was first detected in Culex spp. mosquitoes; phylogenetic analysis showed that the strain belongs to the Central European subclade of WNV lineage 2 [14,15]. Since then, WNV human cases have been reported every year, except in 2015 and 2016, while 2018 was a record year, with 243 cases showing neuroinvasive disease [16]. Furthermore, there are several reports on virus detection in mosquitoes, equines, and birds [17,18,19,20,21,22,23,24,25,26].
In 2022, Greece was the second most seriously affected European country, with 284 reported human cases of WNV, following Italy (586 cases) [27]. The aim of the present study was to screen for WNV Culex pipiens sensu lato mosquitoes, collected in 2022 in the Central Macedonia Region, and to perform phylogenetic analysis based on whole WNV-genome sequences.
2. Materials and Methods
2.1. Mosquito Collection
A total of 30,816 female C. pipiens s.l. mosquitoes were collected from May to September 2022, in 139 sampling sites in the seven regional units of Central Macedonia, Greece (Chalkidiki, Imathia, Kilkis, Pella, Pieria, Serres, and Thessaloniki). Trapping was performed using CO2-baited light traps, with a constant CO2 outflow of 0.5 l/min, constructed by the Ecodevelopment mosquito control company (Thessaloniki, Greece). The selection of sites was based on environmental characteristics (e.g., terrain morphology—proximity to water bodies), and WNV detection in previous years. Additional traps were placed at 71 sites where new human cases were observed.
2.2. Mosquito Identification and Transportation
The mosquito identification was performed using morphological identification keys [28,29]. The mosquitoes were transferred to the laboratory on dry ice and kept at −80 °C until processing.
2.3. Laboratory Handling, RNA Extraction, and WNV Detection
The mosquitoes were grouped into 690 pools (max. 50 mosquitoes per pool), based on the trapping sites and collection dates (Table 1). The specimens were then washed with distilled water and homogenized in phosphate-buffered saline, using glass beads (diameter 150–212 μm) in a FastPrep FP120 cell disrupter (Bio-101, Thermo Savant; Q-Biogene, Carlsbad, CA, USA). The total RNA was extracted from 200 μl of supernatant of the homogenized pools, using the QIAamp cador Pathogen Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. WNV was detected using a commercial real-time RT-PCR (RealStar WNV RT-PCR Kit, altona Diagnostics, Hamburg, Germany).
Table 1.
Detection of West Nile virus in Culex pipiens s.l. mosquitoes, shown per month and regional unit of Central Macedonia, Greece, 2022.
| Regional Unit | May | June | July | August | September | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Pools | No. Pos. Pools (%) | No. Pools | No. Pos. Pools (%) | No. Pools | No. Pos. Pools (%) | No. Pools | No. Pos. Pools (%) | No. Pools | No. Pos. Pools (%) | No. Pools | No. Pos. Pools (%) | |
| Chalkidiki | 10 | 0 (0) | 7 | 0 (0) | 6 | 0 (0) | 13 | 2 (15.4) | 9 | 0 (0) | 45 | 2 (4.4) |
| Imathia | 13 | 0 (0) | 15 | 0 (0) | 24 | 4 (16.7) | 28 | 6 (21.4) | 12 | 0 (0) | 92 | 10 (10.9) |
| Kilkis | 9 | 0 (0) | 6 | 0 (0) | 7 | 1 (14.3) | 8 | 3 (37.5) | 17 | 0 (0) | 47 | 4 (8.5) |
| Pella | 4 | 0 (0) | 6 | 0 (0) | 7 | 0 (0) | 14 | 1 (7.1) | 7 | 0 (0) | 38 | 1 (2.6) |
| Pieria | 7 | 0 (0) | 8 | 0 (0) | 11 | 1 (9.1) | 14 | 3 (21.4) | 9 | 0 (0) | 49 | 4 (8.2) |
| Serres | 13 | 0 (0) | 11 | 0 (0) | 16 | 0 (0) | 15 | 1 (6.7) | 17 | 0 (0) | 72 | 1 (1.4) |
| Thessaloniki | 69 | 0 (0) | 64 | 3 (4.7) | 72 | 10 (13.9) | 78 | 2 (2.6) | 64 | 4 (6.2) | 347 | 19 (5.5) |
| Total | 125 | 0 (0) | 117 | 3 (2.6) | 143 | 16 (11.2) | 170 | 18 (10.6) | 135 | 4 (3.0) | 690 | 41 (5.9) |
2.4. Next-Generation Sequencing and Phylogenetic Analysis
The samples that showed a cycle threshold (Ct) of less than 30 in the real-time RT-PCR test were chosen for further testing using a recently designed PCR-based next-generation sequencing (NGS) protocol [30]. The NGS libraries were prepared and quantified using the Ion Library TaqMan Quantitation kit; then, they were subjected to emulsion PCR on an Ion One Touch 2 system. After template enrichment, sequencing was performed on an Ion PGM sequencer, using a 316 Chip. Assembly and annotation were conducted in Geneious Prime, version 2021.2.1. The initial Greek sequence (HQ537483) was used as a reference. Whole-genome sequences were aligned with WNV lineage 2 sequences retrieved from the GenBank Database, and a maximum likelihood phylogenetic tree was constructed based on the best model, using MEGA version 11 software [31].
2.5. Maximum Likelihood Estimation, Minimum Infection Rate, and Statistical Analysis
The maximum likelihood estimation (MLE) value per 1000 mosquitoes for each regional unit was calculated using the PooledInfRate program, version 4.0 [32]. The minimum infection rate (MIR) was calculated as the ratio of positive pools to the total number of mosquitoes tested. A chi-square test was applied to determine whether the variation in the proportion of positive mosquito pools per regional unit and month was significant. The level of statistical significance was set to p < 0.001.
2.6. Data Visualization
The sites where mosquito trapping was performed were mapped using the geographic information system (GIS) application ArcGIS Pro, version 3.0 (Esri, Redlands CA, USA).
3. Results
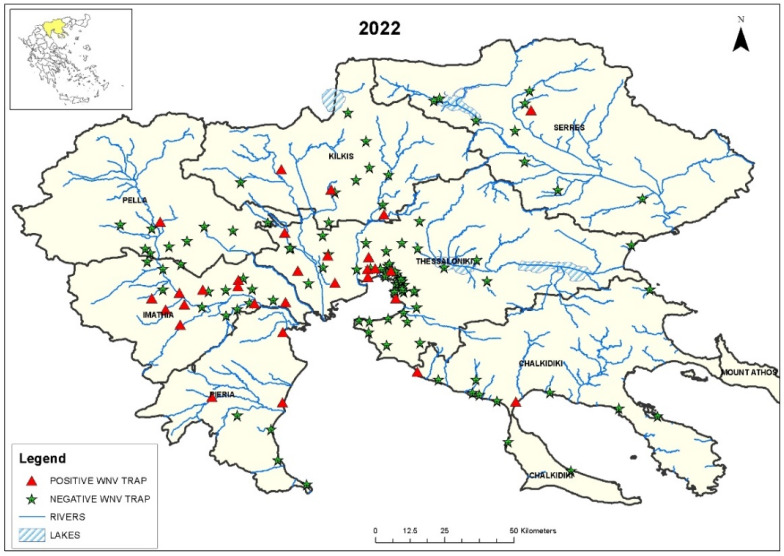
WNV RNA was detected in 41/690 (6.0%) C. pipiens s.l. mosquito pools (Table 1). The sites where the positive mosquito pools were detected in 2022 are shown in Figure 1. The rate of WNV-positive mosquito pools differed significantly per regional unit and month (p < 0.001). The virus was not detected in May; it started to be detectable in June (in the Thessaloniki regional unit), peaked in August, when positive mosquitoes were detected in all the regional units and continued to be detectable in September (in the Thessaloniki regional unit).
Figure 1.
Map of Central Macedonia, with the sites where WNV-positive and -negative C. pipiens s.l. mosquitoes were trapped from May to September 2022. The location of the region in Greece can be seen in the inset in the upper left corner.
The first WNV detection in the mosquitoes collected in the Central Macedonia Region was on 8 June 2022, in the regional unit of Thessaloniki (Table 2). Two weeks later, the first human case of the year was diagnosed in the same area [33]. In two additional regional units (Kilkis and Pieria), the virus detection in mosquitoes preceded the diagnosis of human cases (Table 2). The MLEs for the WNV infection rate per 1000 mosquitoes and the MIR per regional unit are shown in Table 3. In five regional units, the MLE and MIR values were >1.
Table 2.
Dates of WNV detection in mosquitoes and humans, per regional unit of the Central Macedonia region, Greece, 2022.
| Regional Unit | First Detection in Mosquitoes |
Diagnosis of First Clinical Cases |
|---|---|---|
| Chalkidiki | 8 August | 28 July |
| Imathia | 26 July | 12 July |
| Kilkis | 6 July | 18 July |
| Pieria | 18 July | 2 August |
| Serres | 18 August | 3 August |
| Pella | 24 August | 18 July |
| Thessaloniki | 8 June | 23 June |
Table 3.
MLE and MIR values per regional unit of Central Macedonia, Greece, 2022.
| Regional Unit | Mosquitoes | Pools | Positive Pools | MLE (95% CI) | MIR (95% CI) |
|---|---|---|---|---|---|
| Imathia | 5271 | 92 | 10 | 2.04 (1.03–3.61) | 1.90 (0.72–3.07) |
| Thessaloniki | 13507 | 347 | 19 | 1.48 (0.92–2.26) | 1.41 (0.77–2.04) |
| Kilkis | 1678 | 47 | 4 | 2.5 (0.81–5.89) | 2.38 (0.05–4.72) |
| Pieria | 2638 | 38 | 1 | 1.5 (0.48–3.55) | 1.44 (0.03–2.85) |
| Pella | 2779 | 49 | 4 | 0.39 (0.02–1.86) | 0.38 (0–1.12) |
| Serres | 3472 | 72 | 1 | 0.29 (0.02–1.4) | 0.29 (0–0.85) |
| Chalkidiki | 1553 | 45 | 2 | 1.34 (0.24–4.35) | 1.29 (0–3.07) |
| Total | 30,898 | 690 | 41 | 1.39 (1.01–1.86) | 1.33 (0.92–1.73) |
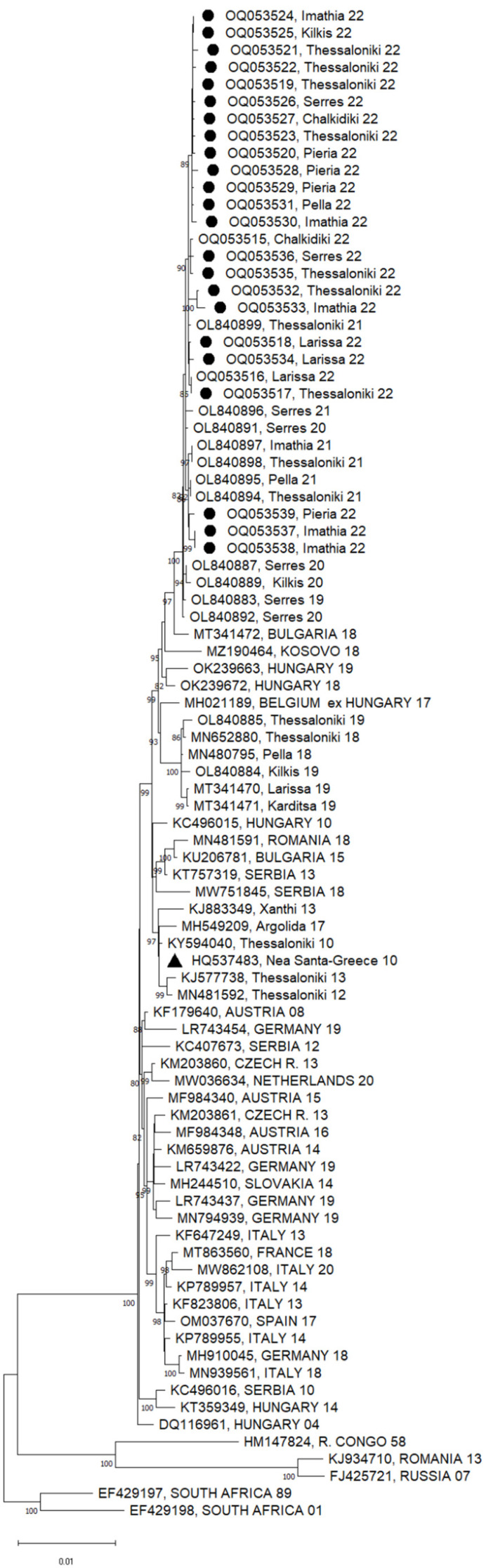
Twenty-three of the 41 WNV-positive pools presented a Ct value of less than 30 in the real-time RT-PCR test and were subjected to NGS. A total of 3,270,641 reads were taken, and WNV sequences were recovered from all samples, with > 99.5% genome coverage. All sequences clustered within the Central European/Hungarian subclade of WNV lineage 2 (Figure 2). Sequences from all seven of the regional units are included. The nucleotide (nt) and amino acid (aa) differences among the sequences of 2022 were < 0.01%. It was seen that the sequences in 2022 form a distinct subclade, together with the Greek sequences from 2020 and 2021, along with one sequence from 2019 (OL840883). The nt and aa differences from the initial Greek WNV strain (HQ537483) were 0.52% and 0.27%, respectively. The closest published sequences from other European countries were those from Bulgaria (MT341472) and Hungary (OK239672), which were both detected in 2018 (differing from the Greek sequences of 2022 by 0.37% and 0.48 at the nt level, and 0.22% and 0.22% at the aa level, respectively).
Figure 2.
A phylogenetic tree based on the complete nucleotide sequences of the WNV lineage 2 polyprotein (10,302 nt) using the maximum likelihood method and Tamura–Nei model. A discrete gamma distribution was used to model the evolutionary rate differences among sites (5 categories (+ G, parameter = 0.2474)). The tree is drawn to scale, with branch lengths measured according to the number of substitutions per site. The percentage of trees in which the associated taxa are clustered together is shown next to the branches; only values >80% are shown. The tree is drawn to scale. The sequences of the present study are marked with a circle; the first Greek sequence (HQ537483) is marked with a triangle. The names of countries are shown in capital letters; all other sequences are from Greece.
All the Greek sequences of 2020–2022, as well as one sequence from 2019 (OL840883), had an S73P substitution in the membrane glycoprotein precursor, prM, and a T157A substitution in the envelope glycoprotein. It is of interest that among the sequences included in the phylogenetic tree, the S73P substitution was present only in the sequences of the Eastern European subclade of WNV lineage 2 (KJ934710 and FJ425721). Furthermore, all sequences of 2021–2022, as well as one sequence from 2020 (OL840891), had a V106A substitution in the nonstructural protein, NS3.
4. Discussion
WNV infections constitute a serious threat to public health, especially to the elderly and immunocompromised persons. Since no WNV vaccine for humans is available, the only means of disease prevention is the limitation of mosquito bites. Prompt knowledge about the abundance of mosquitoes and their WNV infection rate in a particular region can direct decisions regarding the strategies needed for public and animal health prevention measures, including awareness among citizens, clinicians, and veterinarians, and mosquito control strategies. Therefore, mosquito surveillance and screening are useful tools by which to identify the areas with WNV circulation.
Previous studies in Central Macedonia showed that the detection rate in mosquitoes ranged from 1.3% to 9.46% [17,23,24,34,35]. A plethora of biotic and abiotic factors affect the WNV circulation level, with temperature and precipitation having a major role since they influence the life cycle of the vectors [36]. The area where WNV emerged in Greece in 2010 was close to a major wetland area (the Axios Delta) which is on the migration route of millions of birds, while the plain of the wider area is one of the largest rice-producing areas in Europe [37], suggesting that there are many flooded fields of arable land in the area. Global phenomena, such as the North Atlantic oscillation (NAO) and El Niño southern oscillation events, might also influence the disease’s emergence and incidence; it was found that 2010 was a record year for the NAO, as the NAO index had previously had a continuously negative value (from October 2009 to January 2011) [38]. In the following years, the distribution and the incidence of WNV human cases in Greece varied, with a peak in 2018 (a record WNV year for Europe), when cases were spread all over the mainland of the country [16]. Another exceptional year was 2020, when Greece was the most affected country in Europe. Nearly half of the cases (56/144) occurred in the regional unit of Serres, where the WNV detection rate in mosquitoes was 14.3%; it was suggested that the upsurge in the virus circulation was probably related to anthropogenic factors that had an influence on the ecosystem of a major wetland in the area [39]. The situation was totally different in 2022; although again, the most affected region was Central Macedonia, the outbreak started in the regional unit of Thessaloniki, which has the highest human population in Central Macedonia (1,091,424 in the census of 2022). There, the WNV detection in mosquitoes started earlier, peaked earlier, and lasted longer than in other units of Central Macedonia (Table 1). The longest virus circulation in Thessaloniki resulted in 115 human cases (out of 233 in the whole region) [33]. Specifically, the first cases were reported in the city of Thessaloniki (the largest city in the region, with a population of 814,000 in the census of 2022), instead of in the rural areas, and cases were continuously reported in the city until October. A factor that might have played a role could be the repeated heavy rainfalls during June, which led to an increased number of C. pipiens s.l. breeding sites in the urban system and, at the same time, made the mosquito control efforts less effective, due to the flushing out of the insecticides applied in the rainwater catchment basins.
Mosquitoes, horses, birds, and other animals can be used as sentinel species for WNV surveillance. Previous studies in Greece showed that in some cases, the virus was detected earlier in mosquitoes (by approx. two weeks) than in human cases [23,24]. This was also seen in 2022, as the first virus detection in mosquitoes in the country preceded the diagnosis of the first human case by two weeks, serving as an early warning system. The MLE and MIR values were > 1 in five of the regional units, and the early warning worked in three of them (Thessaloniki, Kilkis, and Pieria) (Table 2 and Table 3).
Since 2010, when the virus emerged in Greece, all sequences cluster into the Central European subclade of WNV lineage 2, except for one case in 2018 that was caused by a strain belonging to the Eastern European subclade [40]. The sequences of the present study constituted a distinct subclade, being evolutionary variants of a strain introduced into the country in 2019 (OL840883). Specific substitutions characterized the sequences of 2020–2022; it is not known whether they play any role in the biological properties of the strains. The availability of more sequences, covering a broad spatial and temporal distribution of WNV strains in Europe, will lead to accurate phylogenetic and phylogeography studies.
5. Conclusions
An intense WNV circulation occurred in 2022 in Greece, especially in the Central Macedonia Region, where 5.9% of the tested C. pipiens s.l. pools were positive. The screening of mosquitoes provides information about virus circulation in an area, which is useful for both veterinary health and human public health, while the availability of whole-genome sequences is the basis for further studies, including those on virus evolution.
Acknowledgments
We thank Victoria Basba and Vassileios Antalis at Ecodevelopment for performing the mosquito identification.
Author Contributions
Conceptualization, supervision, reviewing, and editing A.P.; writing—original draft K.T.; methodology K.T., S.P., K.S., S.G., S.M. and S.K.; investigation S.G., S.M. and S.K.; provision of biological material S.G., S.M. and S.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Nucleotide sequences identified in the study were submitted to the GenBank DataBase under the accession numbers OQ053517–OQ053539.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could appear to influence the work reported in this paper.
Funding Statement
This work was supported by the European Union’s Horizon 2020 research and innovation program VEO (grant number 874735) (A.P., S.P. and K.T.) and by the Operational Program for Competitiveness, Entrepreneurship, and Innovation project EMPROS (code: Τ2ΕΔΚ-02070) (A.P., K.T., S.G., S.K. and S.M.). The National Reference Centre for Arboviruses is supported by the National Public Health Organization in Greece.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Smithburn K.C., Hughes T.P., Burke A.W., Paul J.H. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. Hyg. 1970;20:471–492. doi: 10.4269/ajtmh.1940.s1-20.471. [DOI] [Google Scholar]
- 2.Lim S.M., Koraka P., Osterhaus A.D., Martina B.E. West Nile virus: Immunity and pathogenesis. Viruses. 2011;3:811–828. doi: 10.3390/v3060811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.David S., Abraham A.M. Epidemiological and clinical aspects on West Nile virus, a globally emerging pathogen. Infect. Dis. 2016;48:571–586. doi: 10.3109/23744235.2016.1164890. [DOI] [PubMed] [Google Scholar]
- 4.Rossi S.L., Ross T.M., Evans J.D. West Nile virus. Clin. Lab. Med. 2010;30:47–65. doi: 10.1016/j.cll.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer L.D., Li J., Shi P.Y. West Nile virus. Lancet Neurol. 2007;6:171–181. doi: 10.1016/S1474-4422(07)70030-3. [DOI] [PubMed] [Google Scholar]
- 6.Sejvar J.J. West Nile Virus Infection. Microbiol. Spectr. 2016;4:175–199. doi: 10.1128/microbiolspec.EI10-0021-2016. [DOI] [PubMed] [Google Scholar]
- 7.Fall G., Di Paola N., Faye M., Dia M., Freire C.C.M., Loucoubar C., Zanotto P.M.A., Faye O., Sall A.A. Biological and phylogenetic characteristics of West African lineages of West Nile virus. PLoS Negl. Trop. Dis. 2017;11:e0006078. doi: 10.1371/journal.pntd.0006078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casimiro-Soriguer C.S., Perez-Florido J., Fernandez-Rueda J.L., Pedrosa-Corral I., Guillot-Sulay V., Lorusso N., Martinez-Gonzalez L.J., Navarro-Mari J.M., Dopazo J., Sanbonmatsu-Gamez S. Phylogenetic Analysis of the 2020 West Nile Virus (WNV) Outbreak in Andalusia (Spain) Viruses. 2021;13:836. doi: 10.3390/v13050836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joubert L., Oudar J., Hannoun C., Beytout D., Corniou B., Guillon J.C., Panthier R. [Epidemiology of the West Nile virus: Study of a focus in Camargue. IV. Meningo-encephalomyelitis of the horse] Ann. Inst. Pasteur. 1970;118:239–247. [PubMed] [Google Scholar]
- 10.Bakonyi T., Ivanics E., Erdelyi K., Ursu K., Ferenczi E., Weissenbock H., Nowotny N. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg. Infect. Dis. 2006;12:618–623. doi: 10.3201/eid1204.051379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Platonov A.E., Karan L.S., Shopenskaia T.A., Fedorova M.V., Koliasnikova N.M., Rusakova N.M., Shishkina L.V., Arshba T.E., Zhuravlev V.I., Govorukhina M.V., et al. [Genotyping of West Nile fever virus strains circulating in southern Russia as an epidemiological investigation method: Principles and results] Zh. Mikrobiol. Epidemiol. Immunobiol. 2011;2:29–37. (In Russian) [PubMed] [Google Scholar]
- 12.Papa A., Danis K., Baka A., Bakas A., Dougas G., Lytras T., Theocharopoulos G., Chrysagis D., Vassiliadou E., Kamaria F., et al. Ongoing outbreak of West Nile virus infections in humans in Greece, July-August 2010. Eurosurveillance. 2010;15:19644. doi: 10.2807/ese.15.34.19644-en. [DOI] [PubMed] [Google Scholar]
- 13.Danis K., Papa A., Theocharopoulos G., Dougas G., Athanasiou M., Detsis M., Baka A., Lytras T., Mellou K., Bonovas S., et al. Outbreak of West Nile virus infection in Greece, 2010. Emerg. Infect. Dis. 2011;17:1868–1872. doi: 10.3201/eid1710.110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papa A., Bakonyi T., Xanthopoulou K., Vazquez A., Tenorio A., Nowotny N. Genetic characterization of West Nile virus lineage 2, Greece, 2010. Emerg. Infect. Dis. 2011;17:920–922. doi: 10.3201/eid1705.101759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papa A., Xanthopoulou K., Gewehr S., Mourelatos S. Detection of West Nile virus lineage 2 in mosquitoes during a human outbreak in Greece. Clin. Microbiol. Infect. 2011;17:1176–1180. doi: 10.1111/j.1469-0691.2010.03438.x. [DOI] [PubMed] [Google Scholar]
- 16.Pervanidou D., Vakali A., Georgakopoulou T., Panagiotopoulos T., Patsoula E., Koliopoulos G., Politis C., Stamoulis K., Gavana E., Pappa S., et al. West Nile virus in humans, Greece, 2018: The largest seasonal number of cases, 9 years after its emergence in the country. Eurosurveillance. 2020;25:1900543. doi: 10.2807/1560-7917.ES.2020.25.32.1900543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papa A., Papadopoulou E., Kalaitzopoulou S., Tsioka K., Mourelatos S. Detection of West Nile virus and insect-specific flavivirus RNA in Culex mosquitoes, central Macedonia, Greece. Trans. R. Soc. Trop. Med. Hyg. 2014;108:555–559. doi: 10.1093/trstmh/tru100. [DOI] [PubMed] [Google Scholar]
- 18.Valiakos G., Touloudi A., Iacovakis C., Athanasiou L., Birtsas P., Spyrou V., Billinis C. Molecular detection and phylogenetic analysis of West Nile virus lineage 2 in sedentary wild birds (Eurasian magpie), Greece, 2010. Eurosurveillance. 2011;16:19862. doi: 10.2807/ese.16.18.19862-en. [DOI] [PubMed] [Google Scholar]
- 19.Marka A., Diamantidis A., Papa A., Valiakos G., Chaintoutis S.C., Doukas D., Tserkezou P., Giannakopoulos A., Papaspyropoulos K., Patsoula E., et al. West Nile virus state of the art report of MALWEST Project. Int. J. Environ. Res. Public Health. 2013;10:6534–6610. doi: 10.3390/ijerph10126534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouzalas I.G., Diakakis N., Chaintoutis S.C., Brellou G.D., Papanastassopoulou M., Danis K., Vlemmas I., Seuberlich T., Dovas C.I. Emergence of Equine West Nile Encephalitis in Central Macedonia, Greece, 2010. Transbound. Emerg. Dis. 2016;63:e219–e227. doi: 10.1111/tbed.12334. [DOI] [PubMed] [Google Scholar]
- 21.Sofia M., Giannakopoulos A., Giantsis I.A., Touloudi A., Birtsas P., Papageorgiou K., Athanasakopoulou Z., Chatzopoulos D.C., Vrioni G., Galamatis D., et al. West Nile Virus Occurrence and Ecological Niche Modeling in Wild Bird Species and Mosquito Vectors: An Active Surveillance Program in the Peloponnese Region of Greece. Microorganisms. 2022;10:1328. doi: 10.3390/microorganisms10071328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valiakos G., Plavos K., Vontas A., Sofia M., Giannakopoulos A., Giannoulis T., Spyrou V., Tsokana C.N., Chatzopoulos D., Kantere M., et al. Phylogenetic Analysis of Bird-Virulent West Nile Virus Strain, Greece. Emerg. Infect. Dis. 2019;25:2323–2325. doi: 10.3201/eid2512.181225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papa A., Gewehr S., Tsioka K., Kalaitzopoulou S., Pappa S., Mourelatos S. Detection of flaviviruses and alphaviruses in mosquitoes in Central Macedonia, Greece, 2018. Acta Trop. 2020;202:105278. doi: 10.1016/j.actatropica.2019.105278. [DOI] [PubMed] [Google Scholar]
- 24.Tsioka K., Gewehr S., Kalaitzopoulou S., Pappa S., Stoikou K., Mourelatos S., Papa A. Detection and molecular characterization of West Nile virus in Culex pipiens mosquitoes in Central Macedonia, Greece, 2019–2021. Acta Trop. 2022;230:106391. doi: 10.1016/j.actatropica.2022.106391. [DOI] [PubMed] [Google Scholar]
- 25.Chaintoutis S.C., Dovas C.I., Danis K., Gewehr S., Mourelatos S., Hadjichristodoulou C., Papanastassopoulou M. Surveillance and Early Warning of West Nile Virus Lineage 2 Using Backyard Chickens and Correlation to Human Neuroinvasive Cases. Zoonoses Public Health. 2015;62:344–355. doi: 10.1111/zph.12152. [DOI] [PubMed] [Google Scholar]
- 26.Chaintoutis S.C., Dovas C.I., Papanastassopoulou M., Gewehr S., Danis K., Beck C., Lecollinet S., Antalis V., Kalaitzopoulou S., Panagiotopoulos T., et al. Evaluation of a West Nile virus surveillance and early warning system in Greece, based on domestic pigeons. Comp. Immunol. Microbiol. Infect. Dis. 2014;37:131–141. doi: 10.1016/j.cimid.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 27.European Centre for Disease Prevention and Control Weekly Updates: 2022 West Nile Virus Transmission Season. [(accessed on 11 December 2022)]. Available online: https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/disease-data-ecdc.
- 28.Darsie R.F., Jr., Samanidou-Voyadjoglou A. Keys for the identification of the mosquitoes of Greece. J. Am. Mosq. Control Assoc. 1997;13:247–254. [PubMed] [Google Scholar]
- 29.Becker N., Petric D., Zgomba M., Boase C., Madon M., Dahl C., Kaiser A. Mosquitoes and Their Control. Springer; Berlin, Germany: 2010. [Google Scholar]
- 30.Pappa S., Chaintoutis S.C., Dovas C.I., Papa A. PCR-based next-generation West Nile virus sequencing protocols. Mol. Cell. Probes. 2021;60:101774. doi: 10.1016/j.mcp.2021.101774. [DOI] [PubMed] [Google Scholar]
- 31.Tamura K., Stecher G., Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biggerstaff B. PooledInfRate, Version 4.0: An Excel© Add-In to Compute Infection Rates from Pooled Data. Centers for Disease Control and Prevention. Centers for Disease Control and Prevention; Fort Collins, CO, USA: 2022. [Google Scholar]
- 33.National Public Health Organization . Weekly Epidemiological Report for West Nile Virus disease, Greece, 06.12.2022. National Public Health Organization; Athens, Greece: 2022. [Google Scholar]
- 34.Papa A., Xanthopoulou K., Tsioka A., Kalaitzopoulou S., Mourelatos S. West Nile virus in mosquitoes in Greece. Parasitol. Res. 2013;112:1551–1555. doi: 10.1007/s00436-013-3302-x. [DOI] [PubMed] [Google Scholar]
- 35.Patsoula E., Beleri S., Tegos N., Mkrtsian R., Vakali A., Pervanidou D. Entomological Data and Detection of West Nile Virus in Mosquitoes in Greece (2014-2016), Before Disease Re-Emergence in 2017. Vector Borne Zoonotic Dis. 2020;20:60–70. doi: 10.1089/vbz.2018.2422. [DOI] [PubMed] [Google Scholar]
- 36.Paz S., Malkinson D., Green M.S., Tsioni G., Papa A., Danis K., Sirbu A., Ceianu C., Katalin K., Ferenczi E., et al. Permissive summer temperatures of the 2010 European West Nile fever upsurge. PLoS ONE. 2013;8:e56398. doi: 10.1371/journal.pone.0056398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraehmer H., Thomas C., Vidotto F. Rice production in Europe. In: Chauhan B., Jabran K., Mahajan G., editors. Rice Production Worldwide. Springer; Cham, Switzerland: 2017. [DOI] [Google Scholar]
- 38.Papa A. West Nile virus infections in humans--focus on Greece. J. Clin. Virol. 2013;58:351–353. doi: 10.1016/j.jcv.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 39.Papa A., Tsioka K., Gewehr S., Kalaitzopouou S., Pervanidou D., Vakali A., Kefaloudi C., Pappa S., Louka X., Mourelatos S. West Nile fever upsurge in a Greek regional unit, 2020. Acta Trop. 2021;221:106010. doi: 10.1016/j.actatropica.2021.106010. [DOI] [PubMed] [Google Scholar]
- 40.Papa A., Papadopoulou E., Chatzixanthouliou C., Glouftsios P., Pappa S., Pervanidou D., Georgiou L. Emergence of West Nile virus lineage 2 belonging to the Eastern European subclade, Greece. Arch. Virol. 2019;164:1673–1675. doi: 10.1007/s00705-019-04243-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Nucleotide sequences identified in the study were submitted to the GenBank DataBase under the accession numbers OQ053517–OQ053539.