Abstract
Plant debris are habitats favoring survival and multiplication of various microbial species. During continuing mycological surveys of saprobic microfungi from plant debris in Yunnan Province, China, several Corynespora-like and Dendryphiopsis-like isolates were collected from dead branches of unidentified perennial dicotyledonous plants. Four barcodes, i.e., ITS, LSU, SSU and tef1-α, were amplified and sequenced. Morphological studies and multigene phylogenetic analyses by maximum likelihood and Bayesian inference revealed three new Corynespora species (C. mengsongensis sp. nov., C. nabanheensis sp. nov. and C. yunnanensis sp. nov.) and a new Kirschsteiniothelia species (K. nabanheensis sp. nov.) within Dothideomycetes, Ascomycota. A list of identified and accepted species of Corynespora with major morphological features, host information and locality was compiled. This work improves the knowledge of species diversity of Corynespora and Kirschsteiniothelia in Yunnan Province, China.
Keywords: anamorphic Ascomycota, Kirschsteiniotheliales, morphology, phylogeny, Pleosporales, taxonomy
1. Introduction
Hyphomycetes are highly diverse and distributed in terrestrial and freshwater habitats. More than 1500 Hyphomycetes genera and 30,000 species have been recorded worldwide [1,2]. These fungi show distinct morphological features, which often allow for species identification, as DNA sequences have been hitherto unavailable for most genera and species. Given the large amount of hyphomycetes, it is challenging to classify their taxonomic placement based on morphology alone because some of them may belong to the same species or even to different genera. The introduction of molecular phylogenetic analyses led to a better understanding of the heterogenous genera and species and further clarified their taxonomic status. Investigating fungal diversity is an important task in assembling the fungal tree of life (AFToL) [3], which contributes to the knowledge of biological diversity and the exploration and utilization of fungal resources.
Corynespora was established by Güssow [4] with C. mazei as the type species. Wei [5] provided a historical review and considered C. mazei a synonym of the previously described Helminthosporium cassiicola Berk. & M.A. Curtis and transferred the latter species, resulting in the new combination Corynespora cassiicola (Berk. & M.A. Curtis) C.T. Wei. This genus is mainly characterized by distinct, determinate or percurrently extending conidiophores and monotretic, integrated, terminal conidiogenous cells that produce solitary or sometimes catenate, distoseptate conidia [6]. To date, 198 epithets for Corynespora have been listed in Species Fungorum [7], but many species associated with leaf spots were defined at least partially on the basis of host identity. Siboe et al. [8] provided a synopsis of basic characteristics of 50 accepted Corynespora species, but C. kenyensis was not discussed. An additional 93 additional species have since been added to the genus [9,10,11,12,13,14], 87 of which are present in two tables in a format similar to that used by Siboe et al. [8,9,13]. However, C. alternarioides [15], C. camagueyensis [16], C. garciniae [17], C. inornata [18], C. mulanjeensis [19] and C. obclavata [20] were not congeneric with the generic characters in producing euseptate or muriform conidia or synnematous conidiophores with polytretic conidiogenous cells and were excluded from Corynespora [21,22,23,24,25]. Corynespora cespitosa [26], C. endiandrae [11], C. leucadendri [10] and C. olivacea [27] show the main characters of Corynespora but were transferred to Helminthosporium by Voglmayr and Jaklitsch [28] based on morphological and phylogenetic analyses. “Corynespora aeria” [29], “C. ipomoeae” [30] (Art. F.5.1: no identifier number cited), “C. masseeanum” [31] (Art. 41.1: lacking a full and direct basionym reference) and C. ruelliae [32] (Art. 40.1: without assigning a type) were not validly published based on the rules of the International Code of Nomenclature for Algae, Fungi, and Plants [33]. Thus, Corynespora currently contains 129 valid species. Most Corynespora species were introduced primarily based on morphology, and only 10 species with DNA sequences have been used for multigene phylogenetic analyses [12].
Sivanesan [34] introduced the family Corynesporascaceae Sivan. with Corynesporasca carotae Sivan. (= Corynespora calicioidea (Berk. & Broome) M.B. Ellis) [2] as the type species, and first connected the teleomorph (Corynesporasca caryotae) and anamorph (Corynespora) state through cultural studies. Rossman et al. [35] recommended using Corynespora over Corynesporasca, considering its widespread use, priority and number of species. Subsequently, phylogenetic analyses of five gene regions, i.e., SSU, ITS, LSU, rpb2 and tef1-α, revealed that Corynespora smithii forms a separate, distant clade, together with the generic type, C. cassiicola, and is treated in the monotypic Corynesporascaceae in Pleosporales [28].
The genus Kirschsteiniothelia was erected by Hawksworth [36] with K. aethiops as the type species and is mainly characterized by superficial to semi-immersed, subglobose to globose, dark brown to black ascomata; cylindrical clavate, bitunicate, spored asci; and brown to dark brown, ellipsoidal, 1(–2)-septate ascospores with or without a mucilaginous sheath [36,37]. The genus has been linked with two anamorph types, viz., Dendryphiopsis-like and Sporidesmium-like, based on phylogenetic analyses [38]. The Dendryphiopsis-like asexual morph is characteristically macronematous, simple or branched at the apex, forming a stipe and head, brown to dark brown conidiophores with monotretic, integrated, terminal or discrete, determinate or percurrently extending conidiogenous cells that produce solitary, acrogenous, euseptate conidia [38]. The Sporidesmium-like asexual morph has macronematous, unbranched conidiophores with monoblastic, integrated, terminal, determinate or irregular extending conidiogenous cells that produce solitary, acrogenous, euseptate conidia with or without a mucilaginous sheath [38]. Based on morphology and molecular data, previous studies have confirmed that Dendryphiopsis is the anamorph of Kirschsteiniothelia [37,39], and Wijayawardene et al. [40] further demonstrated that Dendryphiopsis atra (generic type) is synonymous with Kirschsteiniothelia atra and suggested using Kirschsteiniothelia rather than Dendryphiopsis, considering the requirement for fewer name changes. Subsequently, seven Sporidesmium-like asexual morphs were reported in Kirschsteiniothelia [38,41,42,43,44].
The early taxonomic placements of Kirschsteiniothelia are uncertain. The genus was originally placed in Pleosporaceae by Hawksworth [36] and Barr [45] and subsequently assigned to Pleomassariaceae by Barr [46] based on asexual morph connection and morphology. Schoch et al. [47] revealed that K. aethiops (generic type) does not cluster with Pleosporaceae in phylogenetic analyses and suggested that Kirschsteiniothelia should be transferred to a new family. Schoch et al. [39] further showed that K. elaterascus and K. maritma cluster within Mytilinidion (Mytilinidiaceae) and Morosphaeria (Morosphaeriaceae), respectively, according to phylogenetic analyses [48], and both species were excluded from Kirschsteiniothelia by Boonmee et al. [37]. In addition, Boonmee et al. [37] introduced a new family, Kirschsteiniotheliaceae, to accommodate taxa grouping with K. aethiops based on morphology and phylogenetic analyses. Hernandez-Restrepo et al. [49] proposed the monotypic order Kirschsteiniotheliales for Kirschsteiniotheliaceae due to its distant relation to other orders in Dothideomycetes.
Yunnan Province is located in southwestern China. It lies at 21°09′–29°15′ N and 97°32′–106°12′ E and includes vast territory with distinct climatic characteristics and abundant natural resources. Its average annual temperatures is 12–22 °C, and the total annual precipitation is approximately 1500 mm. Such favorable conditions support more than 18,000 higher plant species (51.6% of China’s total) in this province, resulting in a very wide range of habitats favoring the growth of various microbial species. However, its mycobiota, especially microfungi, is poorly understood. During our survey of the taxonomy and diversity of saprobic microfungi in Yunnan Province, a Dendryphiopsis-like fungus and three Corynespora-like fungi were collected on dead branches from terrestrial habitats. Based on multilocus phylogenetic analyses and morphological characteristics, we introduced four novel species of Corynespora and Kirschsteiniothelia in Dothideomycetes. This study broadens our understanding of the diversity of Corynespora and Kirschsteiniothelia taxa.
2. Materials and Methods
2.1. Sample Collection, Isolation and Morphology
Samples of dead branches were collected randomly from humid environments and river banks, where there is a deep litter layer comprising rotten softwood, dead branches and decayed leaves of various plants in the forest ecosystems of Yunnan Province. Dead branches are a rich habitat for saprobic hyphomycetes. Samples were placed in ZiplocTM bags for transport to the laboratory, where they were processed and examined as described by Ma et al. [50]. Colonies on decaying wood surface were examined and visually observed with a stereomicroscope (Motic SMZ-168, Xiamen, China) from low (0.75 times) to high (5 times) magnification. Fresh colonies were picked with sterile needles at a stereomicroscope magnification of 5 times, placed on a slide with a drop of lactic acid–phenol solution (lactic acid, phenol, glycerin, sterile water; 1:1:2:1, respectively), then placed under an Olympus BX 53 light microscope fitted with an Olympus DP 27 digital camera (Olympus Optical Co., Tokyo, Japan) for microscopic morphological characterization. The tip of a sterile toothpick dipped in sterile water was used to capture the conidia of the target colony directly from the specimen; the conidia were then streaked on the surface of potato dextrose agar (PDA; 20% potato + 2% dextrose + 2% agar, w/v) and incubated in an incubator at 25 °C overnight. The single germinated conidia were transferred to fresh PDA plates [51]. Cultures were grown on PDA and incubated in an incubator at 25 °C for 2 weeks; then, morphological characters, including color, shape and size, were recorded. All fungal strains were stored in 10% sterilized glycerin at 4 °C for further studies. The studied specimens and cultures were deposited in the Herbarium of Jiangxi Agricultural University, Plant Pathology, Nanchang, China (HJAUP). The names of the new taxa were registered in Index Fungorum [2].
2.2. DNA Extraction, PCR Amplification and Sequencing
Fungal hyphae were scraped from the surface of colonies growing on PDA plates, transferred to 2 mL safe-lock tubes and ground with liquid nitrogen; then, DNA was extracted using a Solarbio fungal genomic DNA extraction kit (Solarbio, Beijing, China) according to the manufacturer’s instructions. DNA amplification was performed by polymerase chain reaction (PCR) using the respective loci (ITS, SSU, LSU and tef1-α). The following primer sets were used for these genes: ITS: ITS5/ITS4; SSU: NS1/NS4 [52]; LSU: 28S1-F/28S3-R [53]; and tef1-α: EF1-983F/EF1-2218R [54].
The final volume of the PCR reaction was 25 μL, comprising 1 μL of DNA template, 1 μL of each forward and reward primer, 12.5 μL of 2 × Power Taq PCR MasterMix and 9.5 μL of double-distilled water (ddH2O). The PCR thermal cycling conditions of ITS, SSU and LSU were initialized at 94 °C for 3 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 50 s, elongation at 72 °C for 1 min and a final extension at 72 °C for 10 min before being maintained at 4 °C; the tef1-α were initialized at 95 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s, elongation at 72 °C for 1 min and a final extension at 72 °C for 10 min before being maintained at 4 °C. The PCR products were checked by 1% agarose gel electrophoresis staining with ethidium bromide. Purification and DNA sequencing were carried out at Beijing Tsingke Biotechnology Co., Ltd. China. New sequences generated in this study were deposited in the NCBI GenBank (www.ncbi.nlm.nih.gov, accessed on 10 December 2022; Table 1 and Table 2).
Table 1.
List of Corynespora species and GenBank accessions used in the phylogenetic analyses. New sequences are indicated in bold.
| Taxon | Strain Number | GenBank Accession Numbers | |||
|---|---|---|---|---|---|
| SSU | LSU | ITS | tef1- α | ||
| Corynespora cassiicola | CBS 100822 | GU296144 | GU301808 | – | GU349052 |
| C. citricola | CBS 169.77 | – | – | FJ852594 | – |
| C. doipuiensis | MFLUCC 14–0022 | MN648318 | MN648326 | MN648322 | – |
| C. encephalarti | CBS 145555 | – | MK876424 | MK876383 | – |
| C. lignicola | MFLUCC 16–1301 | – | MN860554 | MN860549 | – |
| C. mengsongensis | HJAUP C2000T | OQ060575 | OQ060578 | OQ060574 | – |
| C. nabanheensis | HJAUP C2048T | OQ060576 | OQ060580 | OQ060577 | OQ067526 |
| C. pseudocassiicola | CPC 31708 | – | MH327830 | MH327794 | MH327877 |
| C. smithii | L120 | – | KY984297 | KY984297 | KY984435 |
| C. smithii | L130 | KY984419 | KY984298 | KY984298 | KY984436 |
| C. smithii | CABI 5649b | – | GU323201 | FJ852597 | GU349018 |
| C. smithii | CBS 139925 | – | KY984299 | KY984299 | – |
| C. submersa | MFLUCC 16–1101 | – | MN860553 | MN860548 | – |
| C. torulosa | CBS 136419 | – | MH877634 | MH866095 | – |
| C. thailandica | CBS 145089 | – | MK047505 | MK047455 | MK047567 |
| C. yunnanensis | HJAUP C2132T | OQ060584 | OQ060583 | OQ060579 | – |
| Periconia byssoides | H 4600 | AB797280 | AB807570 | LC014581 | AB808546 |
| P. digitata | CBS 510.77 | AB797271 | AB807561 | LC014584 | AB808537 |
| P. igniaria | CBS 845.96 | AB797277 | AB807567 | LC014586 | AB808543 |
| P. macrospinosa | CBS 135663 | KP184080 | KP184038 | KP183999 | – |
| P. pseudodigitata | KT 1395 | NG_064850 | NG_059396 | NR_153490 | AB808540 |
| Cyclothyriella rubronotata | TR, CBS 121892 | – | KX650541 | KX650541 | KX650516 |
| C. rubronotata | TR9, CBS 141486 | KX650507 | KX650544 | KX650544 | KX650519 |
“–”, sequence is unavailable. Strain with T (ex-type). Abbreviations: CABI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, U.K.; CBS: Central Bureau voor Schimmel cultures, Utrecht, The Netherlands; CPC: Collection of Pedro Crous housed at CBS; HJAUP: Herbarium of Jiangxi Agricultural University, Plant Pathology; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand; SSU: Small Subunit Ribosomal; LSU: Large Subunit Ribosomal; ITS: Internal Transcribed Spacer; tef1-α: Transcriptional Enhancer Factor 1-alpha; others are not registered abbreviations.
Table 2.
List of Kirschsteiniothelia species and GenBank accessions used in the phylogenetic analyses. New sequences are in bold.
| Taxon | Strain | Genbank Accession Numbers | ||
|---|---|---|---|---|
| ITS | LSU | SSU | ||
| Acrospermum adeanum | M133 | EU940180 | EU940104 | EU940031 |
| A. compressum | M151 | EU940161 | EU940084 | EU940012 |
| A. gramineum | M152 | EU940162 | EU940085 | EU940013 |
| Anisomeridium ubianum | MPN94 | – | GU327709 | JN887379 |
| Flavobathelium epiphyllum | MPN67 | – | GU327717 | JN887382 |
| Kirschsteiniothelia aethiops | CBS 109.53 | – | AY016361 | AY016344 |
| K. aethiops | MFLUCC 16–1104 | MH182583 | MH182589 | MH182615 |
| K. aethiops | S–783 | MH182586 | MH182595 | MH182617 |
| K. aethiops | MFLUCC 15–0424 | KU500571 | KU500578 | KU500585 |
| K. aquatica T | MFLUCC 17–1685 | MH182587 | MH182594 | MH182618 |
| K. arasbaranica | IRAN 2509C | KX621986 | KX621987 | KX621988 |
| K. arasbaranica T | IRAN 2508C | KX621983 | KX621984 | KX621985 |
| K. cangshanensis T | MFLUCC 16–1350 | MH182584 | MH182592 | – |
| K. fluminicola T | MFLUCC 16–1263 | MH182582 | MH182588 | – |
| K. lignicola T | MFLUCC 10–0036 | HQ441567 | HQ441568 | HQ441569 |
| K. nabanheensis T | HJAUP C2004 | OQ023197 | OQ023273 | OQ023038 |
| K. nabanheensis | HJAUP C2006 | OQ023274 | OQ023275 | OQ023037 |
| K. phoenicis T | MFLUCC 18–0216 | MG859978 | MG860484 | MG859979 |
| K. rostrata T | MFLUCC 15–0619 | KY697280 | KY697276 | KY697278 |
| K. rostrata | MFLUCC 16–1124 | – | MH182590 | – |
| K. submersa T | MFLUCC 15–0427 | KU500570 | KU500577 | KU500584 |
| K. submersa | S–481 | – | MH182591 | MH182616 |
| K. submersa | S–601 | MH182585 | MH182593 | – |
| K. tectonae T | MFLUCC 12–0050 | KU144916 | KU764707 | – |
| K. thailandica T | MFLUCC 20–0116 | MT985633 | MT984443 | MT984280 |
| K. thujina | JF 13210 | KM982716 | KM982718 | KM982717 |
| Megalotremis verrucosa | MPN104 | – | GU327718 | JN887383 |
| Phyllobathelium anomalum | MPN 242 | – | GU327722 | JN887386 |
| P. firmum | ERP 3175 | – | GU327723 | – |
| Pseudorobillarda eucalypti | MFLUCC 12–0422 | KF827451 | KF827457 | KF827463 |
| P. phragmitis | CBS 398.61 | MH858101 | EU754203 | EU754104 |
| Strigula guangxiensis T | HMAS-L0138040 | NR_146255 | MK206256 | – |
| S. nemathora | MPN 72 | – | JN887405 | JN887389 |
| Tenuitholiascus porinoides T | HMAS-L0139638 | – | MK206259 | MK352441 |
| T. porinoides | HMAS-L0139639 | – | MK206258 | MK352442 |
| T. porinoides | HMAS-L0139640 | – | MK206260 | MK352443 |
“–”, sequence is unavailable; strain with T, ex type. Abbreviations: CBS: Central Bureau voor Schimmel cultures, Utrecht, the Netherlands; HJAUP: Herbarium of Jiangxi Agricultural University, Plant Pathology; HMAS: Fungarium-Lichenum of the Institute of Microbiology, Chinese Academy of Sciences; IRAN: Iranian Fungal Culture Collection, Iranian Research Institute of Plant Protection, Tehran, Iran; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand; ITS: internal transcribed spacer; LSU: large subunit ribosomal; SSU: small subunit ribosomal; others are not registered abbreviations.
2.3. Phylogenetic Analyses
The newly generated sequences, together with other sequences obtained from GenBank (four loci: ITS, LSU, SSU and tef1-α (Table 1); three loci: ITS, LSU and SSU (Table 2)), were separately aligned using the MAFFTv.7 [55] online server (http://maffTh.cbrc.jp/alignment/server/, accessed on 23 December 2022) and manually optimized when needed. Phylogenetic analyses were first conducted individually for each locus, then for a combined dataset of these loci. The four ITS, LSU, SSU and tef1-α alignment datasets and the three ITS, LSU and SSU alignment datasets were concatenated with Phylosuite software v1.2.2 [56], and absent sequence data in the alignments were treated with a question mark as missing data. Phylosuite software v1.2.2 [56] was used to construct separate phylogenetic trees based on ITS, LSU, SSU and tef1-α sequence data, as well as ITS, LSU and SSU sequence data. The concatenated and aligned datasets were analyzed separately using maximum likelihood (ML) and Bayesian inference (BI). The maximum-likelihood phylogenies were inferred using IQ-TREE [57] under an edge-linked partition model for 10000 ultrafast bootstraps [58]. For Corynespora, the final tree was selected among suboptimal trees from each run by comparing the likelihood scores using SYM+G4 for ITS, TNe+G4 for LSU+tef1-α and K2P+I for the SSU substitution model. Bayesian inference phylogenies were inferred using MrBayes 3.2.6 [59] under a partition model (2 parallel runs, 2000000 generations), in which the initial 25% of sampled data were discarded as burn-in. The best-fit model was SYM+G4 for ITS, GTR+F+G4 for LSU+tef1-α and K2P+I for SSU. For Kirschsteiniothelia, the final tree was selected among suboptimal trees from each run by comparing the likelihood scores using TIM2e+R3 for ITS+SSU and TN+F+G4 for the LSU substitution model. Bayesian inference phylogenies were inferred using MrBayes 3.2.6 [59] under a partition model (2 parallel runs, 2000000 generations), in which the initial 25% of sampled data were discarded as burn-in. The best-fit model was SYM+G4 for ITS+LSU+SSU. ModelFinder [60] was used to select the best-fit partition model (edge-linked) using the BIC criterion. The trees were viewed in FigTree v. 1.4.4 (http://tree.bio.ed.ac.uk/software/figtree, accessed on 10 December 2022) and further edited in Adobe Illustrator 2021.
3. Results
3.1. Molecular Phylogeny
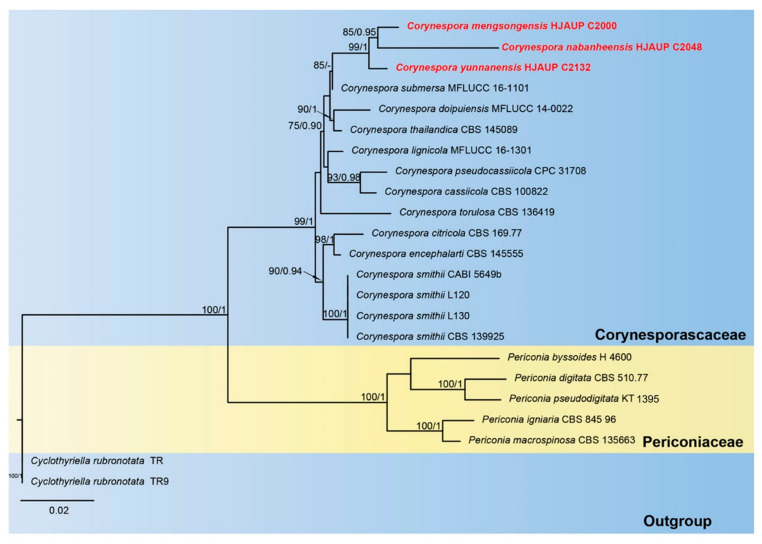
The phylogenetic tree (Figure 1) inferred from maximum-likelihood and Bayesian inference analyses based on combined ITS, LSU, SSU and tef1-α sequence data consisted of three families (Corynesporascaceae, Periconiaceae and Cyclothyriellaceae). The concatenated sequence matrix comprised 23 sequences with 3147 total characters (the combined dataset, ITS: 1–498, LSU: 499–1348, SSU: 1349–2374, tef1-α: 2375–3147), 537 distinct patterns, 375 parsimony-informative sites, 147 singleton sites and 2625 constant sites; Cyclothyriella rubronotata (TR) and C. rubronotata (TR9) were regarded as an outgroup. Maximum-likelihood and Bayesian inference analyses of the combined dataset resulted in phylogenetic reconstructions with largely similar topologies; the best-scoring ML tree is shown in Figure 1. Bootstrap support values for maximum likelihood higher than 75% and Bayesian posterior probabilities greater than 0.90 are shown above the nodes. The best-scoring ML consensus tree (lnL = –8859.832) with ultrafast bootstrap values from ML analyses and posterior probabilities from MrBayes analysis at the nodes is shown in Figure 1. The strains of Corynespora mengsongensis form a distinct clade sister to C. nabanheensis with good statistical support (ML/BI = 85/0.95); C. yunnanensis forms a high-support clade (ML/BI = 99/1.00) with the lineage consisting of C. mengsongensis and C. nabanheensis, and they form a sister clade to C. submersa (ML/BI =85/0.75).
Figure 1.
Phylogram of Pleosporales based on combined ITS, SSU, LSU and tef1-α sequences. ML and BI bootstrap support values above 75% and 0.90 are shown at the first and second position, respectively. The tree is rooted to Cyclothyriella rubronotata (TR) and C. rubronotata (TR9). Strains from the current study are indicated in red.
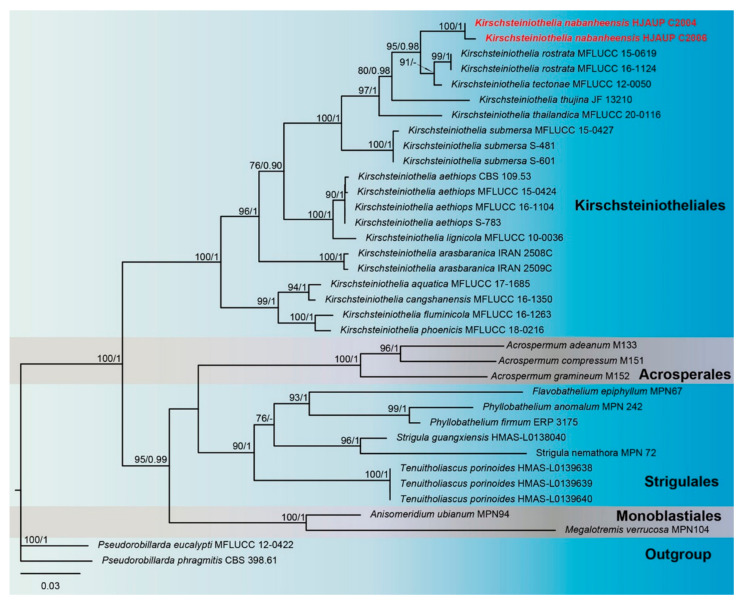
The phylogenetic tree (Figure 2) inferred from maximum-likelihood and Bayesian inference analyses based on combined ITS, LSU and SSU sequence data consisted of four orders (Acrospermales, Kirschsteiniotheliales, Monoblastiales and Strigulales). The concatenated sequence matrix comprised 36 sequences with 1260 total characters (combined dataset, ITS: 1–162, LSU: 163–471, SSU: 472–1260), 413 distinct patterns, 253 parsimony-informative sites, 183 singleton sites and 824 constant sites; Pseudorobillarda eucalypti (MFLUCC 12–0422) and P. phragmitis (CBS 398.61) were regarded as an outgroup. Maximum-likelihood and Bayesian inference analyses of the combined dataset resulted in phylogenetic reconstructions with largely similar topologies; the best-scoring ML tree is shown in Figure 2. Bootstrap support values for maximum likelihood higher than 75% and Bayesian posterior probabilities greater than 0.90 are shown above the nodes. The best-scoring ML consensus tree (lnL = –6307.741) with ultrafast bootstrap values from ML analyses and posterior probabilities from MrBayes analysis at the nodes is shown in Figure 2. The strains of K. nabanheensis form a separate clade closely related to K. thailandica, K. thujina, K. tectonae and K. rostrata, with strong statistical support (ML/BI = 95/0.98).
Figure 2.
Phylogram of Kirschsteiniotheliales, Acrosperales, Strigulales and Monoblastiales based on combined ITS, SSU and LSU sequences. The ML and BI bootstrap support values above 75% and 0.90 are shown at the first and second position, respectively. The tree is rooted to Pseudorobillarda eucalypti (MFLUCC 12–0422) and P. phragmitis (CBS 398.61). Strains from the current study are indicated in red.
3.2. Taxonomy
Corynespora mengsongensis Jing W. Liu & Jian Ma, sp. nov., Figure 3.
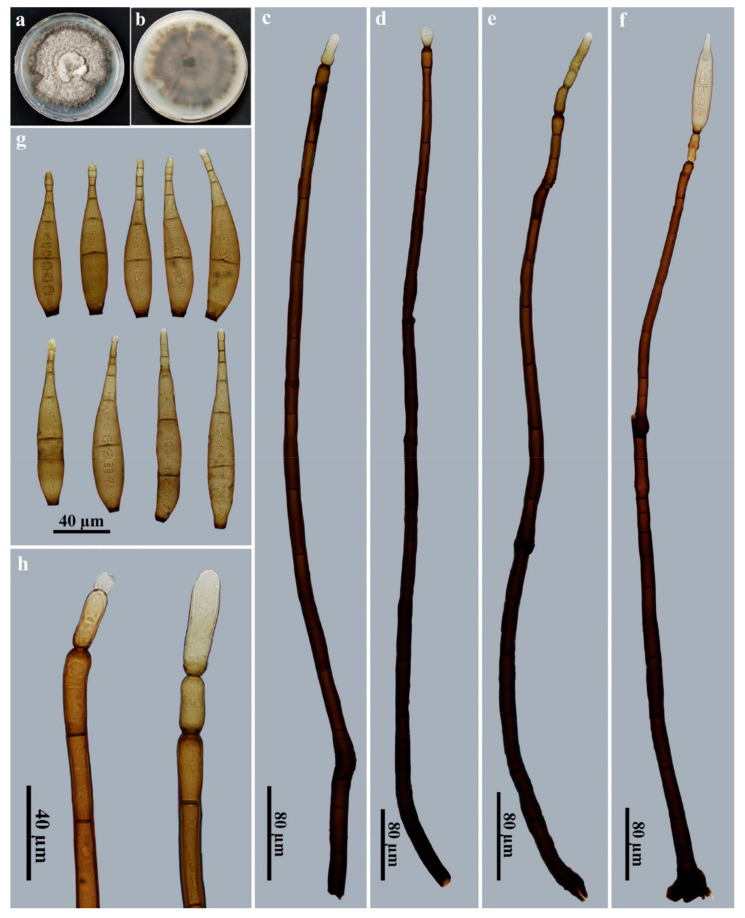
Figure 3.
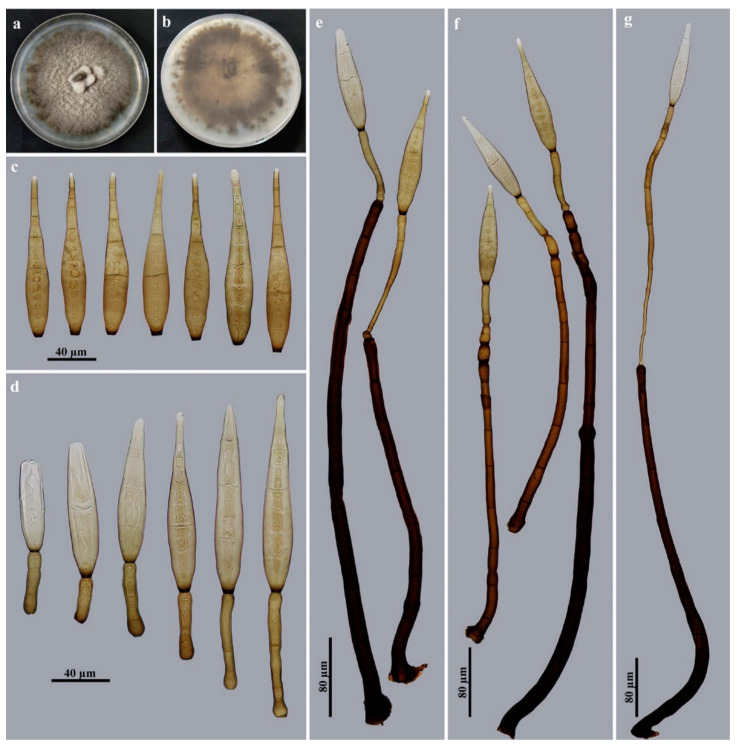
Corynespora mengsongensis (HJAUP M2000, holotype). (a) Surface of colony after 2 weeks on PDA; (b) reverse of colony after 2 weeks on PDA; (c–f) conidiophores, conidiogenous cells and conidia; (g) conidia; (h) conidiogenous cells with developing conidia.
Indexfungorum number: IF900076
Etymology: The name refers to Mengsong, the township where the fungus was collected.
Holotype: HJAUP M2000.
Description: Saprobic on decaying wood in terrestrial habitats. Teleomorph: undetermined. Anamorph (Figure 3): Hyphomycetes. Colonies on natural substratum are effuse, brown to dark brown and hairy. Mycelium is superficial and immersed, composed of branched, septate, pale brown to brown, smooth-walled hyphae. Conidiophores are macronematous, mononematous, unbranched, erect, straight or flexuous, cylindrical, smooth, brown to dark brown and septate, with up to 2 successive cylindrical extensions (267–)746–938 × 12.5–17 μm ( = 829 × 14.5 μm, n = 15). Conidiogenous cells are integrated, terminal, monotretic, cylindrical, pale brown to brown and smooth, with dimensions of 25–29 × 8.5–10.5 µm ( = 27 × 9.4 μm, n = 15). Conidia are acrogenous, solitary, obclavate, rostrate, rounded at the apex, straight or slightly curved, 13–18-distoseptate, brown to golden brown and smooth, with dimensions of 96–146 × 16.5–20.5 μm ( = 118.5 × 18.5 μm, n = 20), tapering to 3–4 μm near the apex and truncate at the base, with a protuberant dark-brown hilum that is 6–8 μm wide at the base.
Culture characteristics: Colony on PDA reaching 80–88 mm diam. after 2 weeks in an incubator under dark conditions at 25 °C; irregular circular, velvety surface with dense, gray–white mycelia along the entire margin; reverse brown to dark brown.
Material examined: China, Yunnan Province, Xishuangbanna Dai Autonomous Prefecture, Menghai County, Mengsong Township, on dead branches of an unidentified broadleaf tree, 12 July 2021, J.W. Liu (HJAUP M2000, holotype; ex-type culture permanently preserved in a metabolically inactive state, HJAUP C2000).
Notes: Phylogenetic analyses showed that C. mengsongensis cluster with C. nabanheensis (Figure 1). BLASTn analysis of C. mengsongensis (HJAUP C2000T) and C. nabanheensis (HJAUP C2048T) shows 90% identity (540/598, 22 gaps) using ITS, 97% identity (559/578, 3 gaps) using LSU and 99% identity (1021/1026, no gaps) using SSU. Corynespora mengsongensis are morphologically similar to C. merrilliopanacis [61], but the latter differ in terms of their longer conidiophores (260–1200 μm), with up to 5 successive cylindrical extensions and longer conidia (130–260 μm) with 12–25 distosepta. Furthermore, C. mengsongensis differ from C. nabanheensis, which have smaller conidiophores (282–528 × 6–8 μm) with 3–4 successive cylindrical extensions and smaller conidia (56–84 × 12–14 μm) with 9–13 distosepta.
Corynespora nabanheensis Jing W. Liu & Jian Ma, sp. nov., Figure 4.
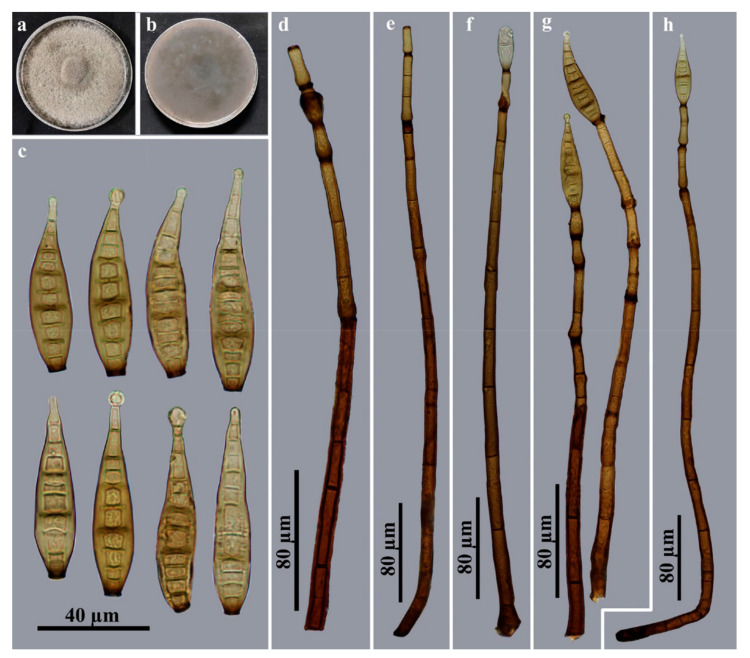
Figure 4.
Corynespora nabanheensis (HJAUP M2048, holotype). (a) Surface of colony after 2 weeks on PDA; (b) reverse of colony after 2 weeks on PDA; (c) conidia; (d,e) conidiophores and conidiogenous cells; (f–h) conidiophores, conidiogenous cells and conidia.
Index Fungorum number: IF900077
Etymology: The name refers to Nabanhe Nature Reserve, the locality where the fungus was collected.
Holotype: HJAUP M2048.
Description: Saprobic on decaying wood in terrestrial habitats. Teleomorph: undetermined. Anamorph (Figure 4): hHyphomycetes. Colonies on natural substratum are effuse, brown to dark brown and hairy. Mycelia are superficial and immersed, composed of branched, septate, pale brown to brown, smooth-walled hyphae. Conidiophores are macronematous, mononematous, unbranched, erect, straight or flexuous, cylindrical, smooth and brown to dark brown, with 10–17-septate with 3–4 successive cylindrical extensions and dimensions of 282–528 × 6–8 μm ( = 388 × 7 μm, n = 15). Conidiogenous cells are integrated, terminal, monotretic, cylindrical, pale brown to brown and smooth, with dimensions of 20–32 × 5–8 µm (= 25.5 × 6.5 μm, n = 15). Conidia are acrogenous, solitary, obclavate, rostrate and straight or slightly curved with 9–13-distoseptate, brown to golden brown, smooth and usually expanded to a rounded shape at the apex, with dimensions of 56–84 × 12–14 μm ( = 66.5 × 13 μm, n = 20) and 4–6 μm near the apex and truncated at the base, with a protuberant dark brown hilum that is 4–5 μm wide at the base.
Culture characteristics: Colonies on PDA reach 85–90 mm diam. after 2 weeks in an incubator under dark conditions at 25 °C, with an irregular, circular, velvety surface and dense, gray mycelia along the entire margin; reverse brown to black.
Material examined: China, Yunnan Province, Xishuangbanna Dai Autonomous Prefecture, the Nabanhe National Nature Reserve, on dead branches of an unidentified broadleaf tree, 12 July 2021, J.W. Liu (HJAUP M2048, holotype; ex-type culture permanently preserved in a metabolically inactive state HJAUP C2048).
Notes: Phylogenetic analyses showed that C. nabanheensis cluster with C. mengsongensis (Figure 1). BLASTn analysis of C. nabanheensis (HJAUP C2048T) and C. mengsongensis (HJAUP C2000T) shows 90% identity (540/598, 22 gaps) using ITS, 97% identity (559/578, 3 gaps) using LSU and 99% identity (1021/1026, no gaps) using SSU. Corynespora nabanheensis are morphologically similar to C. doipuiensis [12], but the latter differ in terms of their shorter and wider conidiophores (212–426 × 10–15 μm), with fewer successive cylindrical extensions and larger, obconical, guttulate, subhyaline to moderately brown conidia (136–165 × 5–25.5 μm). Furthermore, C. nabanheensis differ from C. mengsongensis, which have larger conidiophores (746–938 × 12.5–17 μm), with up to 2 successive cylindrical extensions and larger conidia (96–146 × 16.5–20.5 μm) with 13–18 distosepta.
Corynespora yunnanensis Jing W. Liu & Jian Ma, sp. nov., Figure 5.
Figure 5.
Corynespora yunnanensis (HJAUP M2132, holotype). (a) Surface of colony after 2 weeks on PDA; (b) reverse of colony after 2 weeks on PDA; (c) conidia; (d) conidiogenous cells and conidia; (e–g) conidiophores, conidiogenous cells and conidia.
Index Fungorum number: IF900078.
Etymology: The name refers to Yunnan, the province where the fungus was collected.
Holotype: HJAUP M2132.
Description: Saprobic on decaying wood in terrestrial habitats. Teleomorph: undetermined. Anamorph (Figure 5): Hyphomycete. Colonies on natural substratum are effuse, brown to dark brown and hairy. Mycelia are superficial and immersed, composed of branched, septate, pale brown to brown, smooth-walled hyphae. Conidiophores are macronematous, mononematous, unbranched, erect, straight or flexuous, cylindrical, smooth, septate and brown to dark brown, with 1–4 successive cylindrical extensions and dimensions of 380–844 × 8–16 μm ( = 547 × 12.5 μm, n = 15). Conidiogenous cells are integrated, terminal, monotretic, cylindrical, pale brown to brown and smooth, with dimensions of 44–120(–332) × 6–8 µm ( = 55.5 × 6.5 μm, n = 15). Conidia are acrogenous, solitary, obclavate, rostrate, rounded at the apex, straight or slightly curved, 3–16-distoseptate, brown to golden brown and smooth, with dimensions of 80–128 × 16–19 μm ( = 117 × 18 μm, n = 25), tapering to 4–8 μm near the apex, and truncated at the base with a protuberant dark brown hilum that is 6–8 μm wide at the base.
Culture characteristics: Colonies on PDA reach 78–85 mm diam. after 2 weeks in an incubator under dark conditions at 25 °C, with an irregular, circular, velvety surface with dense, gray mycelia along the entire margin; reverse brown to black.
Material examined: China, Yunnan Province, Xishuangbanna Dai Autonomous Prefecture, Jinghong City, Gasa Township, on dead branches of an unidentified broadleaf tree, 12 July 2021, J.W. Liu (HJAUP M2132, holotype; ex-type culture permanently preserved in a metabolically inactive state HJAUP C2132).
Notes: Phylogenetic analyses showed that C. yunnanensis cluster with C. mengsongensis and C. nabanheensis, and they form a sister clade to C. submersa (Figure 1). BLASTn analysis of C. yunnanensis (HJAUP C2132T) and C. mengsongensis (HJAUP C2000T) shows 99% identity (567/569, 2 gaps) using ITS, 98% identity (577/587, 7 gaps) using LSU and 99% identity (1028/1029, no gaps) using SSU. BLASTn analysis of C. yunnanensis (HJAUP C2132T) and C. nabanheensis (HJAUP C2048T) shows 91% identity (518/569, 20 gaps) using ITS, 97% identity (543/558, 1 gap) using LSU and 99% identity (1024/1028, no gaps) using SSU. BLASTn analysis of C. yunnanensis (HJAUP C2132T) and C. submersa (MFLUCC 16-1101) shows 100% identity (487/487, no gaps) using ITS and 99% identity (544/547, 1 gap) using LSU. Corynespora yunnanensis are morphologically similar to C. submersa [12], but the latter differ by in terms of their shorter and narrower conidiophores (150–370 × 10–12 μm) and larger, catenate conidia (100–150 × 16–24 μm), with 9–13 distosepta. Furthermore, C. yunnanensis differ from C. mengsongensis, which have larger conidiophores (746–938 × 12.5–17 μm), with up to 2 successive cylindrical extensions, and larger conidia (96–146 × 16.5–20.5 μm) with 13–18 distosepta, as well as from C. nabanheensis, which have smaller conidiophores (282–528 × 6–8 μm) and smaller conidia (56–84 × 12–14 μm) with 9–13 distosepta.
Kirschsteiniothelia nabanheensis Jing W. Liu & Jian Ma, sp. nov., Figure 6.
Figure 6.
Kirschsteiniothelia nabanheensis (HJAUP M2004, holotype). (a) Surface of colony after 2 weeks on PDA; (b) reverse of colony after 2 weeks on PDA; (c) conidia; (d) conidiogenous cells and conidia; (e–g) conidiophores, conidiogenous cells and conidia; (h) conidiophores and conidiogenous cells.
Index Fungorum number: IF900082.
Etymology: The name refers to Nabanhe Nature Reserve, the locality where the fungus was collected.
Holotype: HJAUP M2004.
Description: Saprobic on decaying wood in terrestrial habitats. Teleomorph: undetermined. Anamorph (Figure 6): Hyphomycetes. Colonies on natural substratum are effuse, brown to black and hairy. Mycelia are superficial and immersed, composed of branched, septate, pale brown to brown, smooth-walled hyphae. Conidiophores are macronematous, mononematous, erect, irregular or subscorpioid branched near the apex, solitary, smooth, cylindrical, straight to flexuous, septate and black–brown to brown, with dimensions of (200–)320–588 × 8–12 μm ( = 405 × 9.5 μm, n = 15). Conidiogenous cells are monotretic, integrated, terminal or intercalary, cylindrical or doliiform, determinate, smooth and brown to dark brown, with dimensions of 20–24 × 4–6 µm ( = 22 × 5 μm, n = 15). Conidia are acrogenous, solitary, obclavate or fusiform, sometimes rostrate, straight or slightly curved, 3–7-euseptate, dark brown to brown and smooth, with dimensions of 32–112 × 8–12 μm ( = 55.5 × 10 μm, n = 25), tapering to 3–4 μm near the apex, with a width of 4–5 μm at the base.
Culture characteristics: Colonies on PDA reach 85–90 mm diam. after 2 weeks in an incubator under dark conditions at 25 °C, with an irregular, circular, velvety surface with dense, gray–brown mycelia along the entire margin; reverse brown to dark brown.
Material examined: China, Yunnan Province, Xishuangbanna Dai Autonomous Prefecture, the Nabanhe National Nature Reserve, on dead branches of an unidentified broadleaf tree, 12 July 2021, J.W. Liu (HJAUP M2004, holotype; ex-type culture permanently preserved in a metabolically inactive state HJAUP C2004 = HJAUP C2006).
Notes: Phylogenetic analyses showed that K. nabanheensis cluster with K. thailandica, K. thujina, K. tectonae and K. rostrata, and they form a sister clade to K. submersa (Figure 2). BLASTn analysis of K. nabanheensis (HJAUP C2004T) and K. rostrata (MFLUCC 15–0619T) shows 85% identity (473/558, 27 gaps) using ITS, 95% identity (519/548, 4 gaps) using LSU and 99% identity (875/886, no gaps) using SSU. BLASTn analysis of K. nabanheensis (HJAUP C2004T) and K. tectonae (MFLUCC 12–0050T) shows 84% identity (458/548, 35 gaps) using ITS and 95% identity (523/548, 4 gaps) using LSU. Kirschsteiniothelia nabanheensis are morphologically similar to K. shimlaensis, but the latter differ in terms of their shorter and wider conidiophores (110–268 × 12–19 μm), and shorter and wider, obovoid, oblong, clavate or cylindrical conidia (41–81 × 13–17.5 μm) with 2–5(–6) eusepta [62]. Furthermore, K. nabanheensis differ from K. submersa which have smaller conidiophores (220–280 × 6–7 μm) and holoblastic conidiogenous cells producing smaller conidia (37.5–51.5 × 8.5–9.5 μm) with 4–6 eusepta [42].
4. Discussion
In this study, we collected saprophytic hyphomycetes on dead branches from terrestrial habitats in Yunnan Province, China. Based on the morphomolecular approach, four novel taxa are introduced: Corynespora mengsongensis sp. nov., C. nabanheensis sp. nov., C. yunnanensis sp. nov. and Kirschsteiniothelia nabanheensis sp. nov.
Corynespora show high morphological similarity to Corynesporina, Corynesporopsis, Hemicorynespora and Solicorynespora in terms of their terminal, monotretic, conidiogenous cells and differ only on the basis of single conidial characteristics (e.g., single or catenate, euseptate or distoseptate, basipetal chain or acropetal chain) [63]. The weak differentiation of these similar genera should only be maintained until sufficient molecular analysis allows for a more phylogenetic classification of genera. In addition, it is challenging to separate Corynespora from Helminthosporium based on morphology alone, as four Corynespora species, C. caespitosa, C. endiandrae, C. leucadendri and C. olivacea, were transferred to Helminthosporium based on molecular phylogenetic analyses, which led to the genus Helminthosporium also meeting the criteria of Corynespora [28].
The genus Corynespora produces conspicuous morphological features, and its generic type, C. cassiicola, is a ubiquitous species, mainly in tropical and subtropical areas, and has been recorded from a wide range of plants [64]. Most Corynespora species are known as saprobes and plant pathogens from woody and herbaceous hosts [8,9,13], but occasionally, C. cassiicola is also found in nematodes, sponges and human skin [64]. To date, 132 species of Corynespora (Table 3) have been be accepted worldwide, whereas four invalid names enclosed in quotation marks are also listed in Table 3. Many species have been identified only based on morphological studies, and only 13 species, including our three new species, have been subjected to molecular phylogenetic analyses. Morphological comparison is important for species identification, but the lack of a large amount of molecular data made it difficult to evaluate previously described Corynespora species by molecular methods. Thus, we recommend supplementary sequence data for previously described Corynespora species by re-examining their type materials or collecting fresh new specimens and using molecular phylogenetic analyses to evaluate their taxonomic placement as necessary.
Hawksworth [36] established the genus Kirschsteiniothelia and regarded K. aethiops as the type species. Boonmee et al. [37] treated the genus in a new family, Kirschsteiniotheliaceae, based on evidence from morphological and phylogenetic analyses. Hernandez-Restrepo et al. [49] raised Kirschsteiniotheliaceae to the new order Kirschsteiniotheliales in Dothideomycetes, although this order does not form a well-supported clade within Dothideomycetidae as a sister clade to Asterinales; the two orders diverged approximately 221 MYA according to divergence time estimates [65].
Sun et al. [38] accepted five former Dendryphiopsis species, D. arbuscula, D. binsarensis, D. biseptata, D. fascicularis and D. goaensis, in Kirschsteiniothelia following the latest treatment of Dendryphiopsis by Wijayawardene et al. [40]. However, these five species were invalidly introduced as new combinations in Kirschsteiniothelia on the basis of Art. F.5.1 (no identifier number cited) and Art. 41.1 (lacking a full and direct basionym reference) of the International Code of Nomenclature for Algae, Fungi, and Plants [33]. In addition, Sun et al. [38] provided a checklist for 35 Kirschsteiniothelia species including the distribution, habitat, host and morphology type of each species, but K. ebriosa [66] and K. vinigena [66] are not included. Subsequently, Verma et al. [62] described a new species, K. shimlaensis, from decaying stump in India.
Table 3.
Synopsis of conidial characteristics, host information and locality compared across Corynespora species.
| Species | Conida | Host/Locality | References | ||||
|---|---|---|---|---|---|---|---|
| Production | Morphology | Color | Size (µm) | Septation | |||
| Corynespora acaciae | Solitary | Obclavate | Dark brown | 16–30 × 6–8 | 1–5 | On phyllodes of Acacia pycnantha, Australia | [67] |
| C. acalyphae | Solitary | Obclavate, rostrate | Pale brown to brown | 85–120 × 9–11 | 8–16 | On dead branches of Acalypha hamiltoniana, Indonesia | [68] |
| C. achradis | Solitary or catenate | Obclavate, rostrate | Pale olivaceous brown | 60–95 × 6–7 | 5–10 | On leaves of Achras sapota, Brunei | [69] |
| “C. aeria” | Solitary | Obclavate | Subhyaline to olivaceous | Up to 350 × 2–5 | 1–5 | Isolated from air, India | [29] |
| C. albiziicola | Solitary | Obclavate, ellipsoid or clavate | Pale olivaceous yellow | 20–70.1 × 10–18.5 | 1–6 | On leaves of Albizia lebbek, India | [70] |
| C. alstoniae | Solitary or catenate | Cylindrical to obclavate | Subhyaline to light olivaceous | 48–154 × 8–21.5 | 2–15 | On leaves of Alstonia scholaris, Nepal | [71] |
| C. annonacea | Solitary or catenate | Obclavate to obclavate–cylindrical | Subhyaline to olivaceous brown | 25–135 × 10–18 | 1–10 | On living leaves of Annona squamosa, India | [72] |
| C. aquatica | Solitary | Obclavate to cylindrical | Pale brown | 34–46 × 3–4.5 | (1–)2(–3) | On decaying leaves submerged in stream, Mexico | [24] |
| C. arctespora | Solitary or catenate | Cylindrical to obclavate | Brown to pale brown | 13–63 × 4–7 | 2–20 | On twigs of Vaccinium, USA | [73] |
| C. asclepiadacearum | Mostly solitary | Obclavato-cylindric to cylindrical | Pale olivaceous brown | 44–192 × 10–25 | Up to 26 | On leaves of Cryptolepis buchananii, India | [74] |
| C. azadirachtiana | Solitary or catenate | Obclavate | Pale yellow | 32–303.5 × 7–21.5 | 1–20 | On leaves of Azadirachta indica, India | [75] |
| C. barleriicola | Solitary | Obclavate to cylindrical | Olivaceous yellow | 41–246 × 10–18.5 | 3–14 | On leaves of Barleria cristata, India | [75] |
| C. bdellomorpha | Solitary | Obclavate | Mid to dark-reddish brown | 90–138 × 12–17 | 12–19 | On dead stems of Chusquea valdiviensis, Chile | [26] |
| C. beilschmiediae | Solitary | Obclavate | Pale brown to brown | 52–144.5 × 8.5–11 | 7–19 | On dead branches of Beilschmiedia intermedia, China | [76] |
| C. bombacearum | Solitary or catenate | Obclavato-cylindrical to cylindrical | Pale to mid-olivaceous | 26–206 × 8.5–17 | Up to 15 | On leaves of Bombax malabaricum, India | [77] |
| C. bombacina | Solitary or catenate | Obclavate to cylindrical | Light olivaceous | 45–180 × 10–16 | 5–15 | On living leaves of Bombax ceiba, India | [78] |
| C. calicioidea | Solitary | Obclavate | Subhyaline to pale golden brown | 50–170 × 10–15 | 6–21 | On wood, Sri Lanka | [79] |
| C. carrisae | Solitary | Obclavate to cylindrical | Olivaceous to very light brown | 75–242 × 6–14 | 4–17 | On leaves of Carissa spinarum, India | [80] |
| C. caryotae | Solitary | Obclavate to elongate | Pinkish brown | 45–120 × 6–10 | Up to 18 | On dead rachis of Caryota mitis, Singapore | [81] |
| C. cassiae | Solitary | Obclavate | Pale brown to olivaceous brown | 107.5–214 × 11–14 | 10–21 | On dead branches of Cassia surattensis, China | [76] |
| C. cassiicola | Solitary or catenate | Obclavate to cylindrical | Subhyaline to pale olivaceous brown | 40–220 × 9–22 | 4–20 | On leaves of Cassia, Cuba | [5] |
| C. catenulata | Solitary or catenate | Obclavate to obclavato-cylindrical | Dark olivaceous yellow to pale olivaceous brown | 27.5–225.5 × 11–19 | 1–24 | On leaves of Clerodendrum indicum, India | [75] |
| C. catharanthicola | Solitary or catenate | Cylindrical | Brown | 140–310 × 5.5–11 | 4–25 | On leaves of Catharanthus roseus, China | [82] |
| C. celastri | Solitary | Obclavate to obclavato-cylindrical | Olivaceous to very light brown | 55–120 × 8–15 | 7–17 | On living leaves of Celastrus paniculatus, India | [83] |
| C. chinensis | Catenate | Obclavate | Pale brown | 31–61 × 5–7.5 | 1–5 | On dead branches of Angiospermae, China | [14] |
| C. citricola | Solitary or catenate | Cylindrical to obclavate | Subhyaline | 48–150 × 4.5–8 | 4–18 | On leaves of Citrus aurantiifolia, Australia | [79] |
| C. clerodendrigena | Solitary or catenate | Obclavate to cylindrical | Light olivaceous | 60–220 × 16–22 | 3–13 | On leaves of Clerodendrum viscosum, India | [84] |
| C. colebrookiana | Solitary or catenate | Obclavate, rarely cylindrical | Pale yellow | 45–330 × 6–22 | 4–16 | On leaves of Colebrookea oppositifolia, India | [75] |
| C. combreli | Solitary | Obclavate, rostrate | Pale olivaceous brown to olivaceous brown | 40–122 × 8–11 | 4–10 | On dead branches of Combretum zeyheri, Zambia | [85] |
| C. cubensis | Solitary or catenate | Cylindrical to obclavate | Pale brown to dark rusty brown | 40–80 × 8–12 | 6–15 | On dead petiole of Coccothrinax, Cuba | [86] |
| C. cucurbiticola | Solitary or catenate | Obclavato-cylindrical | Subhyaline to pale olivaceous | 38.5–230 × 6.5–20 | 6–23 | On leaves of Coccinia grandis, Nepal | [87] |
| C. curvispora | Solitary or catenate | Narrow obclavate | Straw-colored to mid-brown | 40–250 × 10–12 | 5–10 | On fallen herbaceous stems, USA | [88] |
| C. doipuiensis | Solitary | Obclavate to cylindrical | Subhyaline to moderately brown | 136–165 × 5–25.5 | – | On dead herbaceous branches, Thailand | [12] |
| C. donacis | Solitary | Obclavate | Olivaceous brown | 45–70 × 8–12 | 10–14 | On dead branches of Donax, China | [89] |
| C. elaeidicola | Solitary or catenate | Cylindrical to obclavate | Subhyaline or pale olivaceous brown | 43–65 × 4–7 | 3–7 | On dead leaves of Elaeis guineensis, Malaysia | [27] |
| C. encephalarti | Solitary | Obclavate | Medium olivaceous brown | 100–150 × 11–15 | 1–12 | On leaves of Encephalartos, South Africa | [90] |
| C. eranthemi | Solitary | Obclavate | Brown to pale olivaceous brown | 65–176 × 11–14 | 5–25 | On leaves of Eranthemum wattii, Singapore | [32] |
| C. erythropsidis | Solitary | Ellipsoid, doliiform to broad clavate | Pale brown to olivaceous brown | 25–31 × 9–12 | 4 | On dead branches of Erythropsis colorata, China | [91] |
| C. euphorbiacearum | Solitary or catenate | Obclavate | Subhyaline to light olivaceous brown | 59–235 × 11–22.5 | 5–18 | On leaves of Manihot esculenta, India | [71] |
| C. euryae | Solitary | Obclavate | Pale brown to brown | 36–67 × 6–9 | 5–9 | On dead branches of Eurya inaequalis, China | [92] |
| C. fici-altissimae | Solitary | Obclavate, rostrate | Dark brown | 55–85 × 9–12 | 11–18 | On dead branches of Ficus altissima, China | [89] |
| C. fici-benjaminae | Solitary | Obclavate | Pale olivaceous brown | 51.5–71 × 8–11 | 5–10 | On dead branches of Ficus benjamina, China | [76] |
| C. ficigena | Solitary | Obclavate to cylindrical | Light olivaceous brown | 90–165 × 9–20 | 7–13 | On leaves of Ficus religiosa, India | [93] |
| C. flagellata | Solitary | Obclavate, rostrate, smooth or verrucose | Dark brown | 50–100 × 9–11 | 5–10 | On wood of Citrus, Ghana | [94] |
| C. fujianensis | Solitary | Obclavate | Brown | 31–90 × 6.5–10 | 4–10 | On dead branches of Myrioneuron faberi, China | [95] |
| C. gigaspora | Solitary | Obclavate, rostrate | Pale to dark golden brown | 100–270 × 19–28 | 9–52 | On dead wood, Sri Lanka | [79] |
| C. gorakhpurensis | Solitary | Obclavate to ellipsoid | Pale olivaceous yellow | 21–157 × 13–20 | 3–13 | On leaves of Erythrina indica, India | [70] |
| C. gracilis | Solitary | Cylindric to obclavate | Olivaceous | 92–138 × 5–7 | 10–22 | On dead branches of Piper betle, Indonesia | [68] |
| C. gymnocladi | Solitary | Obclavate | Brown to dark brown | 15–40 × 7–10.5 | 2–6 | On dead branches of Gymnocladus chinensis, China | [92] |
| C. hamata | Solitary | Obclavate, hamatate at apex | Pale olivaceous brown | 158–198 × 9–11 | 14–19 | On dead wood, Indonesia | [68] |
| C. hansfordii | Solitary | Obclavate, rostrate | Straw-colored to brown | 70–100 × 9–13 | 7–10 | On dead wood, Uganda | [27] |
| C. hemigraphidis | Solitary | Obclavate | Pale olivaceous brown | 72–218 × 12–15 | 5–25 | On leaves of Hemigraphis alternat, Singapore | [32] |
| C. heterospora | Solitary | Cylindrical to obclavate | Pale olivaceous brown to olivaceous brown | 75–170 × 6–20 | 6–12 | On leaves of Manihot utilissima, Malaysia | [96] |
| C. holopoteleae | Solitary or catenate | Obclavato-cylindrical to cylindrical | Mid olivaceous | 23–234 × 3.6–19.5 | 0–17 | On leaves of Holoptelea integrifolia, India | [77] |
| C. holopteleicola | Solitary | Obclavate to obclavato-cylindrical | Olivaceous brown | 33–148 × 5–20 | 0–11 | On living leaves of Holoptelea integrifolia, India | [72] |
| C. homaliicola | Solitary | Obclavate, cylindrical | Subhyaline to straw-colored | 110–220 × 11–22 | 13–28 | On dead branches of Homalium aylmeri, Sierra Leone | [79] |
| “C. ipomoeae” | Solitary or catenate | Obclavate to cylindrical | Subhyaline to pale olivaceous | 40–380 × 5–15 | 2–35 | On leaves of Ipomoea obscura, India | [30] |
| C. jasminicola | Solitary | Obclavate | Pale olivaceous | 39.5–176 × 10–21 | 2–18 | On leaves of Jasminum arborescens, Nepal | [87] |
| C. kamatii | Solitary | Obclavate | Straw-colored | 60–70 × 10–13 | 7–12 | On dead twigs of Vitis, India | [69] |
| C. kenyensis | Solitary | Obclavate to obpyriform, rostrate | Subhyaline to pale brown | 60–125 × 16–25 | 8–15 | On dead stems of Sericostachys scandens, Kenya | [8] |
| C. keskaliicola | Solitary or catenate | Obclavato-cylindrical to cylindrical | Mid olivaceous | 64–164 × 16–28 | Up to 17 | On leaves of Hemidesmus indicus, India | [74] |
| C. laevistipitata | Solitary | Broadly ellipsoid | Red–brown | 17.5–24 × 7–8 | (0–)1–2 (–3) | On Pertusaria ophthalmiza (lichen), USA | [97] |
| C. lanneicola | Solitary | Obclavate | Straw-colored to brown | 40–58 × 10–15 | 4–5 | On dead branches of Lannea afzelii, Sierra Leone | [79] |
| C. lasianthi | Solitary | Obclavate, sometimes rostrate | Pale brown to dark brown | 50–103.5 × 8.5–10 | 4–8 | On dead branches of Lasianthus chinensis, China | [76] |
| C. leptoderridicola | Solitary | Obclavate, rostrate | Subhyaline to straw-colored | 70–120 × 14–17 | 6–16 | On dead branches of Leptoderris fasciculata, Sierra Leone | [79] |
| C. leucaenae | Solitary | Obclavate, obovoid or ellipsoid | Pale yellow | 16–298 × 10–19 | 1–28 | On leaves of Leucaena leucocephala, India | [70] |
| C. lignicola | Solitary or catenate | Cylindrical | Subhyaline to pale brown | 110–156 × 7–9 | – | On submerged decaying wood, China | [12] |
| C. ligustri | Solitary or catenate | Obclavate to cylindrical | Straw-colored to brown | 25–225 × 7.5–30 | 4–20 | On leaves of Ligustrum lucidum, China | [98] |
| C. litseae | Solitary | Obclavate | Pale brown to olivaceous brown | 105–235 × 10–12 | 14–34 | On dead branches of Litsea elongata, China | [99] |
| C. longispora | Solitary | Cylindrical | Subhyaline to pale brown | 120–330 × 5.5–8 | 11–24 | On dead herbaceous stems, India | [100] |
| C. maculiformis | Solitary or catenate | Cylindrical to obclavate | Subhyaline to pale olivaceous brown | 20–86 × 5–10 | 2–8 | On rotten wood, Czech Republic | [101] |
| “C. masseeanum” | Solitary | Elongate to obclavate | Pale olivaceous | 80–120 × 18–20 | 7–11 | On branches of Helicteres Isora, India | [31] |
| C. matuszakii | Solitary or catenate | Cylindrical to obclavate | Pale brown to straw-colored or mid brown | 56–260 × 10–12.5 | 2–10 | On herbaceous stems of Compositae, USA | [102] |
| C. merremiae | Solitary or catenate | Cylindrical to obclavate | Pale olivaceous brown to pale brown | 37–150 × 6–12.5 | 4–22 | On leaves of Merremia hirta, China | [98] |
| C. merrilliopanacis | Solitary | Obclavate, rostrate | Straw-colored to brown | 130–260 × 17–21 | 12–25 | On dead branches of Merrilliopanax listeri, China | [61] |
| C. micheliae | Solitary | Obclavate, rostrate | Subyhaline to brown | 333–360 × 15–19 | 12–28 | On dead branches of Michelia champaca, China | [61] |
| C. millettiae | Solitary or catenate | Obclavate, smooth | Olivaceous brown to mid brown | 30–182 × 7.5–14 | 2–15 | On leaves of Millettia, China | [98] |
| C. moracearum | Solitary | Obclavate to cylindrical | Light olivaceous brown | 27–163 × 12–20 | 5–16 | On living leaves of Ficus hispida, India | [103] |
| C. morindae-tinctoriae | Solitary | Obclavate | Pale olivaceous | 44–127 × 15–26.5 | 6–15 | On leaves of Morinda tinctoria, India | [104] |
| C. myrioneuronis | Solitary | Obclavate | Pale brown to brown | 30–46 × 6.5–8 | 3–4 | On dead branch of Myrioneuron faberi, China | [92] |
| C. mengsongensis | Solitary | Obclavate to cylindrical, rostrate | Brown to golden brown | 96–146 × 16.5–20.5 | 13–18 | On dead branches, China | This study |
| C. nana | Solitary | Obclavate | Subhyaline to pale olivaceous brown | 49.5–110 × 9–18.5 | 4–14 | On leaves of Lantana indica, India | [104] |
| C. nabanheensis | Solitary | Obclavate to cylindrical, expanded to a rounded shape at the apex | Pale brown to brown | 56–84 × 12–14 | 9–13 | On dead branches, China | This study |
| C. occidentalis | Solitary | Ovoid to ellipsoidal | Subyhaline to pale brown | 30–54 × 15–19 | 3–6 | On leaves of Cordia collococca, Cuba | [105] |
| C. palmicola | Solitary | Obclavate to subcylindrical | Pale brown | 40–70 × 6–9 | 5–7 | On leaves of Cocos australis, Paraguay | [106] |
| C. parapyrenariae | Solitary | Obclavate | Pale brown to brown | 70–100 × 11–14 | 5–9 | On dead branches of Parapyrenaria multisepala, China | [99] |
| C. parvispora | Solitary | Ovoid | Brown | 13–15 × 4.5–7.5 | 1–2 | On dead twigs of Gynotroches axillaris, Singapore | [81] |
| C. pedaliacearum | Solitary or catenate | Obclavato-cylindrical to slightly acicular | Pale olivaceous | 16–163 × 3.2–6 | 3–28 | On leaves of Sesamum indicum, India | [107] |
| C. peristrophicola | Solitary | Obclavate to obclavato-cylindrical | Olivaceous to very light brown | 60–135 × 5–16 | 5–12 | On leaves of Peristrophe bicalyculata, India | [80] |
| C. phylloshureae | Solitary | Obclavate | Brown | 30–50 × 8–10 | 6–10 | On dead branches of Phyllostachys sulphurea, China | [89] |
| C. pogostemonicola | Solitary | Obclavate to obclavato-cylindrical | Olivaceous to olivaceous brown | 77–288 × 8–14 | 5–24 | On leaves of Pogostemon plectrantoides, India | [108] |
| C. polyphragmia | Solitary or catenate | Obclavate | Pale to mid golden brown | 110–280 × 14–17 | 10–25 | On decorticated branches of Camellia japonica, Japan | [109] |
| C. pongamiicola | Solitary | Obclavate, ellipsoidal, clavate or club-shaped | Light olivaceous yellow | 18–65.2 × 8–16.5 | 1–6 | On living leaves of Pongamia pinnata, India | [110] |
| C. premnigena | Solitary or catenate | Obclavate to obclavato-cylindrical | Subhyaline to pale yellow | 52–265 × 10–15 | 1–19 | On leaves of Premna mucronata, India | [75] |
| C. proliferata | Solitary or catenate | Obclavate, rostrate | Pale brown to brown | 30–300 × 9–12 | 3–17 | On wood of Fagus sylvatica, the Netherlands | [111] |
| C. pruni | Solitary or catenate | Obclavate | Olivaceous brown or brown | 50–130 × 10–16 | 4–9 | On bark of Prunus serotina, USA | [27] |
| C. pseudocassiicola | Solitary | Subcylindrical to obclavate | Medium brown | 95–160 × 9–10 | (4–)8–12(–17) | On leaves of Byrsonima, Colombia | [112] |
| C. queenslandica | Solitary | Obclavate | Pale brown | 72–114 × 8–10 | 6–9 | On phyllodes of Acacia leiocalyx, Australia | [15] |
| C. rhapidis-humilis | Solitary | Obclavate, rostrate | Pale brown to olivaceous brown | 90–130 × 6–8 | 12–16 | On dead branches of Rhapis humilis, China | [94] |
| C. rhododendri | Solitary | Obclavate to long rostrate | Pale brown to olivaceous brown | 180–400 × 7.5–11 | 19–36 | On dead branches of Rhododendron hainanense, China | [113] |
| C. ripogoni | Solitary | Obclavate | Brown | 60–160 × 10–13.5 | 7–15 | On dead stems of Ripogonum scandens, New Zealand | [9] |
| C. rosacearum | Solitary or catenate | Obclavate to obclavato-cylindrical | Subhyaline to pale olivaceous brown | 26.5–269 × 9–18.5 | 1–18 | On leaves of Eriobotrya japonica, India | [104] |
| “C. ruelliae” | Solitary | Obclavate | Brown to pale olivaceous brown | 60–150 × 12–15 | 5–16 | On leaves of Ruellia macrophylla and Ruellia dipteracanthus, Singapore | [32] |
| C. sacchari | Solitary | Obclavate, rostrate, verrucose or smooth | Pale brown to olivaceous brown | 80–120 × 8–9 | 10–14 | On dead branches of Saccharum sinense, China | [114] |
| C. salasiae | Solitary | Ellipsoidal, doliiform | Brown | 17–20 × 8–12 | 0–2 | On dead stems of grass, Cuba | [115] |
| C. schleichericola | Solitary or catenate | Obclavate | Pale olivaceous brown | 22.5–66 × 3.8–8.5 | 1–12 | On leaves of Schleichera trijuga, India | [107] |
| C. scolopiae | Solitary | Obclavate | Pale brown to brown | 90–150 × 10–13 | 8–11 | On dead branches of Scolopia chinensis, China | [116] |
| C. sed-acaciae | Solitary | Obclavate | Pale brown to olivaceous brown | 40–70 × 11–13.5 | 8–12 | On dead branches of Acacia confusa, China | [113] |
| C. sidae | Solitary | Obclavate to obclavato-cylindrical | Olivaceous brown to very light brown | 25–220 × 7–17 | 2–24 | On leaves of Sida acuta, India | [117] |
| C. sinensis | Catenate | Obclavate or fusiform, ellipsoid | Brown | 21–42 × 8–9.5 | 3(–4) | On dead branches, China | [13] |
| C. siwalika | Solitary | Obclavate, rostrate | Pale straw-colored to golden brown | 88–140 × 15–20 | 9–19 | On branches of Helicteres isora, India | [109] |
| C. smithii | Solitary or catenate | Cylindrical | Subhyaline to golden brown | 70–410 × 12–19 | 7–45 | On bark of Ilex, UK | [79] |
| C. solani | Solitary or catenate | Obclavate to cylindrical | Olivaceous yellow | 80.6–276 × 8–10 | 1–17 | On leaves of Solanum indicum, India | [118] |
| C. subcylindrica | Catenate | Broadly ellipsoid, subcylindrical | Pale brown | 18–60(–90) × 5–13 | 0–3(–6) | On leaves of Lippia sidoides, Brazil | [63] |
| C. submersa | Solitary or catenate | Obclavate, rostrate | Subhyaline to golden brown | 100–150 × 16–24 | 9–13 | On submerged decaying wood, China | [12] |
| C. supkharii | Solitary | Obclavate | Pale olivaceous brown | 22.5–142.5 × 10–17.5 | 2–11 | On leaves of Phyllanthus parvifolius, India | [119] |
| C. tanaceti | Solitary | Obclavate, smooth or verruculose | Pale brown to olivaceous brown | 60–104 × 12–16 | 7–12 | On dead branches of Tanacetum vulgare, China | [116] |
| C. tectonae | Solitary | Obclavate, rostrate, verrucose or smooth | Pale brown to olivaceous brown | 110–160 × 10–12 | 12–18 | On dead branches of Tectona grandis, China | [114] |
| C. thailandica | Mostly solitary | Obclavate | Brown | 80–110 × 10–12 | 4–8 | On wood, Thailand | [120] |
| C. thorii | Catenate | Subcylindrical, broadly ellipsoid to almost obovoid | Pale brown to medium olivaceous brown | 20–30 × 5–7 | (0–)1(–3) | On thallus, apothecia of Lecanora, Japan | [121] |
| C. titarpaniensis | Solitary | Obclavate to cylindrical | Olivaceous brown to light brown | 50–340 × 5–20 | 5–35 | On living leaves of Lepidagathis, India | [122] |
| C. tomenticola | Solitary | Cylindrical | Olivaceous brown to brown | 50–230 × 10.5–20.5 | 3–6 | On living leaves of Terminalia tomentosa, India | [110] |
| C. toonae | Solitary | Obclavate, rostrate | Pale brown to dark brown | 65–144 × 7–9 | 4–14 | On dead branches of Toona sinensis, China | [114] |
| C. torulosa | Solitary | Clavate | Dark olivaceous brown | 35–60 × 13–20 | 3–5 | On dead leaves of Musa sapientum Brazil | [123] |
| C. tremae | Solitary | Obclavate to obclavato-cylindrical | Light brown to brown | 50–160 × 4–12 | 5–20 | On dead petiole of Trema orientalis, India | [124] |
| C. trematicola | Solitary | Obclavate to ellipsoid | Pale olivaceous brown | 104–296 × 11–16 | 1–12 | On leaves of Trema orientalis, India | [118] |
| C. trichiliae | Solitary | Obclavate, rostrate | Subhyaline to straw-colored | 53–74 × 9–11 | 4–6 | On branches of Trichilia heudelotii, Sierra Leone | [27] |
| C. trichoides | Solitary | Obclavato-cylindrical or obclavate | Pale olivaceous brown | 29–170 × 10–15 | 3–14 | On leaves of Triumfetta rhomboidea, Nepal | [87] |
| C. ulmacearum | Solitary | Obclavate | Subhyaline to pale olivaceous brown | 15–106 × 3.5–10 | 2–16 | On leaves of Trema orientalis, India | [107] |
| C. vismiae | Solitary or catenate | Obclavate, rostrate | Pale olivaceous brown or straw-colored | 55–107 × 6–9 | 3–5 | On leaves of Vismia guineensis, Sierra Leone | [85] |
| C. viticis | Solitary or catenate | Cylindrical | Pale brown | 80–383 × 6–9 | – | On leaves of Vitex rotundifolia, China | [98] |
| C. viticola | Solitary or catenate | Obclavate, cylindrical to obovoid | Pale olivaceous brown | 34–170 × 7–17.5 | 1–14 | On leaves of Cayratia carnosa, India | [118] |
| C. woodfordiana | Solitary or catenate | Obclavate, rostrate | Light olivaceous brown | 40–170 × 9.5–17 | 4–14 | On leaves of Woodfordia fruticosa, India | [71] |
| C. yerbae | Solitary or catenate | Obclavate | Subhyaline to pale golden brown | 72–170 × 16–18 | 8–19 | On dead branches of Ilex paraguayensis, Argentina | [26] |
| C. yunnanensis | Solitary | Obclavato-cylindrical, rostrate | Brown to golden brown | 80–128 × 16–19 | 3–16 | On dead branches, China | This study |
| C. ziziphae | Solitary | Obclavato-cylindrical, cylindrical or clavate | Mid olivaceous brown to straw-colored | 33–215 × 10–27 | Up to 15 | On leaves of Ziziphus giraldii, India | [77] |
All conidia are smooth, except where indicated; 2“–”, the number of septation is not given.
Kirschsteiniothelia is one genus of many lignicolous fungi encountered in aquatic and terrestrial habitats. Following the treatment of Sun et al. [38], the genus currently consists of 39 species including K. nabanheensis [38,62,66], but most species have been identified based on morphological studies, and to date, only 17 species are represented by DNA sequences in GenBank. Kirschsteiniothelia has mainly been reported in the USA (nine species), China (eight species) and Thailand (six species), and little published information is has been recorded in other regions [38,62,66]. Thus, it is unclear whether is closely related with geographic regions.
Studies conducted to date on Corynespora and Kirschsteiniothelia have mainly focused on their alpha taxonomy, and most knowledge of both genera is related to woody and herbaceous hosts, whereas we have a less developed understanding of many natural substrates, such as dung, insects and other fungi, including lichens. Because most species of both genera lack cultures, some of them may have received scant consideration in single-spore isolation before the advent of Sanger sequencing and even have particular substrate requirements. Similarly, little attention has been accorded to the roles of these genera in decomposition and nutrient recycling, their geographical distribution, substrate specificities and teleomorph relationships. Therefore, it is not yet possible to quantify their roles in ecosystem function. Although this study broadens our understanding of the diversity of Corynespora and Kirschsteiniothelia taxa, additional large-scale surveys of fungal resources in aquatic and terrestrial habitats within different geographic regions and with different ecological environments, host information and climatic conditions are needed, which will contribute to a comprehensive knowledge of the fungal diversity of these genera. Further collaboration will also be necessary to quantify their functional roles and strengthen our ability to conserve fungal resources.
Author Contributions
J.L., Y.H., X.L., J.X., Z.X. and J.M. designed the study and were involved in the writing of the paper. J.L. and X.S. were responsible for sample collections. J.L. and L.Z. were involved in phylogenetic analyses. R.F.C.-R., R.C. and J.M. contributed to planning and editing of the paper. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All sequences generated in this study were submitted to GenBank.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This project was supported by the National Natural Science Foundation of China (Nos. 32160006, 31970018, 31360011).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Seifert K., Morgan-Jones G., Gams W., Kendrick B. The genera of hyphomycetes. CBS Biodivers. Ser. 2011;9:1–997. [Google Scholar]
- 2.Index Fungorum. [(accessed on 6 January 2023)]. Available online: http://www.indexfungorum.org/Names/Names.asp.
- 3.Celio G.J., Padamsee M., Dentinger B.T.M., Bauer R., McLaughlin D.J. Assembling the Fungal Tree of Life: Constructing the Structural and Biochemical Database. Mycologia. 2006;98:850–859. doi: 10.1080/15572536.2006.11832615. [DOI] [PubMed] [Google Scholar]
- 4.Güssow H.T. Notes on a disease of cucumbers. II. J. Royal Agric. Soc. Engl. 1905;65:271–272. [Google Scholar]
- 5.Wei C.T. Notes on Corynespora. Mycol. Pap. 1950;34:1–10. [Google Scholar]
- 6.Ellis M.B. Dematiaceous Hyphomycetes. Kew, Commonwealth Mycological Institute; Surrey, UK: 1971. [Google Scholar]
- 7.Species Fungorum. 2023. [(accessed on 6 January 2023)]. Available online: http://www.speciesfungorum.org/Names/Names.asp.
- 8.Siboe G.M., Kirk P.M., Cannon P.F. New dematiaceous hyphomycetes from Kenyan rare plants. Mycotaxon. 1999;73:283–302. [Google Scholar]
- 9.McKenzie E.H.C. Three new phragmosporous hyphomycetes on Ripogonum from an ‘ecological island’ in New Zealand. Mycotaxon. 2010;111:183–196. doi: 10.5248/111.183. [DOI] [Google Scholar]
- 10.Quaedvlieg W., Verkley G.J.M., Shin H.D., Barreto R.W., Alfenas A.C., Swart W.J., Groenewald J.Z., Crous P.W. Sizing up Septoria. Stud. Mycol. 2013;75:307–390. doi: 10.3114/sim0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crous P.W., Wingfield M.J., Schumacher R.K., Summerell B.A., Giraldo A., Gené J., Guarro J., Wanasinghe D.N., Hyde K.D., Camporesi E., et al. Fungal Planet description sheets: 281–319. Persoonia. 2014;33:212–289. doi: 10.3767/003158514X685680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyde K.D., de Silva N.I., Jeewon R., Bhat D.J., Phookamsak R., Doilom M., Boonmee S., Jayawardena R.S., Maharachchikumbura S.S.N., Senanayake I.C., et al. AJOM new records and collections of fungi: 1–100. Asian J. Mycol. 2020;3:22–294. doi: 10.5943/ajom/3/1/3. [DOI] [Google Scholar]
- 13.Xu Z.H., Kuang W.G., Qiu L., Zhang X.G., Castañeda-Ruíz R.F., Ma J. Corynespora sinensis sp. nov. from Jiangxi, China. Mycotaxon. 2020;135:803–809. doi: 10.5248/135.803. [DOI] [Google Scholar]
- 14.Liu J.W., Zhang X.G., Castañeda-Ruíz R.F., Ma J. Corynespora chinensis sp. nov. from Hainan, China. Mycotaxon. 2022;137:203–207. doi: 10.5248/137.203. [DOI] [Google Scholar]
- 15.Sutton B.C., Pascoe I.G. Some dematiaceous hyphomycetes from branches and phyllodes on Acacia in Australia. Aust. Syst. Bot. 1988;1:127–138. doi: 10.1071/SB9880127. [DOI] [Google Scholar]
- 16.Castañeda-Ruíz R.F. Deuteromycotina de Cuba. Hyphomycetes III. Instituto de Investigaciones Fundamentales en Agricultura Tropical “Alejandro de Humboldt”; Habana, Cuba: 1985. pp. 1–42. [Google Scholar]
- 17.Ellis M.B. Dematiaceous hyphomycetes. II. Mycol. Pap. 1961;79:1–23. [Google Scholar]
- 18.Deighton F.C. Observations on Phaeoisariopsis. Mycol. Res. 1990;94:1096–1102. doi: 10.1016/S0953-7562(09)81340-3. [DOI] [Google Scholar]
- 19.Sutton B.C. Mitosporic fungi from Malawi. Mycol. Pap. 1993;167:1–93. [Google Scholar]
- 20.Dyko B.J., Sutton B.C. New and interesting dematiaceous hyphomycetes from Florida. Mycotaxon. 1979;8:119–124. [Google Scholar]
- 21.Castañeda-Ruíz R.F., Zhang X.G., Li D.W., Gusmão L.F.P., Pérez-Martínez S., Sosa D. Notes on Vamsapriya and V. camagueyensis comb. nov. Mycotaxon. 2017;132:553–557. doi: 10.5248/132.553. [DOI] [Google Scholar]
- 22.Delgado-Rodríguez G., Mena-Portales J., Calduch M., Decock C. Hyphomycetes (Hongos Mitospóricos) del area protegida Mil Cumbres, Cuba Occidental. Cryptog. Mycol. 2002;23:277–293. [Google Scholar]
- 23.Heredia G., Li D.W., Wendt L., Reblová M., Arias R.M., Gamboa-Angulo M., Štěpánek V., Stadler M., Castañeda-Ruíz R.F. Natonodosa speciosa gen. et sp. nov. and rediscovery of Poroisariopsis inornata: Neotropical anamorphic fungi in Xylariales. Mycol. Prog. 2020;19:15–30. doi: 10.1007/s11557-019-01537-8. [DOI] [Google Scholar]
- 24.Castañeda-Ruíz R.F., Heredia G.P., Arias R.M., Saikawa M., Minter D.W., Stadler M., Guarro J., Decock C. Two new hyphomycetes from rainforests of Mexico, and Briansuttonia, a new genus to accommodate Corynespora alternarioides. Mycotaxon. 2004;89:297–305. [Google Scholar]
- 25.Castañeda-Ruíz R.F., Kendrick B. Conidial fungi from Cuba: II. Univ. Waterloo. Biol. Ser. 1990;33:1–61. [Google Scholar]
- 26.Ellis M.B. Dematiaceous hyphomycetes. IV. Mycol. Pap. 1963;87:1–42. [Google Scholar]
- 27.Ellis M.B. Dematiaceous hyphomycetes. I. Mycol. Pap. 1960;76:1–36. [Google Scholar]
- 28.Voglmayr H., Jaklitsch W.M. Corynespora, Exosporium and Helminthosporium revisited—New species and generic reclassification. Stud. Mycol. 2017;87:43–76. doi: 10.1016/j.simyco.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swapana S., Nair N.N. Corynespora aeria: A new species recorded amongst aero mycoflora of Kerala, India. World J. Pharm. Pharm. Sci. 2015;4:487–492. [Google Scholar]
- 30.Verma N.K., Surywanshi J.S., Rai A.N. Corynespora ipomoeae, a novel taxon of dematiaceous hyphomycetes. J. Mycol. Pl. Pathol. 2014;44:466–469. [Google Scholar]
- 31.Kirk P.M. Nomenclatural novelties. Index Fungorum. 2014;120:1. [Google Scholar]
- 32.Yen J.M., Lim G. Étude sur les champignons parasites du Sud-Est asiatique. 39. Les Corynespora de Malaisie. Cryptog. Mycol. 1980;1:83–90. [Google Scholar]
- 33.Turland N.J., Wiersema J.H., Barrie F.R., Greuter W., Hawksworth D.L., Herendeen P.S., Knapp S., Kusber W.H., Li D.Z., Marhold K., et al. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress, Shenzhen, China, July 2017. Koeltz Botanical Books; Glashütten, Germany: 2018. Regnum Vegetabile 159. [DOI] [Google Scholar]
- 34.Sivanesan A. Corynesporasca caryotae gen. et sp. nov. with a Corynespora anamorph, and the family Corynesporascaceae. Mycol. Res. 1996;100:783–788. doi: 10.1016/S0953-7562(96)80022-0. [DOI] [Google Scholar]
- 35.Rossman A.Y., Crous P.W., Hyde K.D., Hawksworth D.L., Aptroot A., Bezerra J.L., Bhat J.D., Boehm E., Braun U., Boonmee S., et al. Recommended names for pleomorphic genera in Dothideomycetes. IMA Fungus. 2015;6:507–523. doi: 10.5598/imafungus.2015.06.02.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawksworth D.L. Kirschsteiniothelia, a new genus for the Microthelia incrustans-group (Dothideales) Bot. J. Linn. Soc. 1985;91:181–202. doi: 10.1111/j.1095-8339.1985.tb01144.x. [DOI] [Google Scholar]
- 37.Boonmee S., Ko T.W.K., Chukeatirote E., Hyde K.D., Chen H., Cai L., McKenzie E.H.C., Jones E.B.G., Kodsueb R., Hassan B.A. Two new Kirschsteiniothelia species with Dendryphiopsis anamorphs cluster in Kirschsteiniotheliaceae fam. nov. Mycologia. 2012;104:698–714. doi: 10.3852/11-089. [DOI] [PubMed] [Google Scholar]
- 38.Sun Y.R., Jayawardena R.S., Hyde K.D., Wang Y. Kirschsteiniothelia thailandica sp. nov. (Kirschsteiniotheliaceae) from Thailand. Phytotaxa. 2021;490:172–182. doi: 10.11646/phytotaxa.490.2.3. [DOI] [Google Scholar]
- 39.Schoch C., Crous P.W., Groenewald J.Z., Boehm E., Burgess T.I., De Gruyter J., De Hoog G.S., Dixon L., Grube M., Gueidan C. A class-wide phylogenetic assessment of Dothideomycetes. Stud. Mycol. 2009;64:1–15. doi: 10.3114/sim.2009.64.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wijayawardene N.N., Crous P.W., Kirk P.M., Hawksworth D.L., Boonmee S., Braun U., Dai D.Q., D’souza M.J., Diederich P., Dissanayake A., et al. Naming and outline of Dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Divers. 2014;69:1–55. doi: 10.1007/s13225-014-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bao D.F., Luo Z.L., Liu J.K., Bhat D.J., Sarunya N., Li W.L., Su H.Y., Hyde K.D. Lignicolous freshwater fungi in China III: Three new species and a new record of Kirschsteiniothelia from northwestern Yunnan Province. Mycosphere. 2018;9:755–768. doi: 10.5943/mycosphere/9/4/4. [DOI] [Google Scholar]
- 42.Su H.Y., Hyde K.D., Maharachchikumbura S.S.N., Ariyawansa H.A., Luo Z.L., Promputtha I., Tian Q., Lin C.G., Shang Q.J., Zhao Y.C., et al. The families Distoseptisporaceae fam. nov.; Kirschsteiniotheliaceae, Sporormiaceae and Torulaceae, with new species from freshwater in Yunnan Province, China. Fungal Divers. 2016;80:375–409. doi: 10.1007/s13225-016-0362-0. [DOI] [Google Scholar]
- 43.Hyde K.D., Norphanphoun C., Abreu V.P., Bazzicalupo A., Chethana K.W.T., Clericuzio M., Dayarathne M.C., Dissanayake A.J., Ekanayaka A.H., He M.Q., et al. Fungal diversity notes 603–708: Taxonomic and phylogenetic notes on genera and species. Fungal Divers. 2017;87:1–235. doi: 10.1007/s13225-017-0391-3. [DOI] [Google Scholar]
- 44.Li G.J., Hyde K.D., Zhao R.L., Hongsanan S., Abdel-Aziz F.A., Abdel-Wahab M.A., Alvarado P., Alves-Silva G., Ammirati J.F., Ariyawansa H.A., et al. Fungal diversity notes 253–366: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016;78:1–237. doi: 10.1007/s13225-016-0366-9. [DOI] [Google Scholar]
- 45.Barr M.E. Prodromus to Class Loculoascomycetes. Lubrecht & Cramer, Limited; Amherst, MA, USA: 1987. pp. 1–168. [Google Scholar]
- 46.Barr M.E. Notes on the Pleomassariaceae. Mycotaxon. 1993;49:129–142. [Google Scholar]
- 47.Schoch C.L., Shoemaker R.A., Seifert K.A., Hambleton S., Spatafora J.W., Crous P.W. A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia. 2006;98:1041–1052. doi: 10.1080/15572536.2006.11832632. [DOI] [PubMed] [Google Scholar]
- 48.Suetrong S., Schoch C.L., Spatafora J.W., Kohlmeyer J., Volkmann-Kohlmeyer B., Sakayaroj J., Phongpaichit S., Tanaka K., Hirayama K., Jones E.B.G. Molecular systematics of the marine Dothideomycetes. Stud. Mycol. 2009;64:155–173. doi: 10.3114/sim.2009.64.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hernandez-Restrepo M., Gené J., Castañeda-Ruíz R.F., Mena-Portales J., Crous P.W., Guarro J. Phylogeny of saprobic microfungi from Southern Europe. Stud. Mycol. 2017;86:53–97. doi: 10.1016/j.simyco.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma J., Wang Y., Ma L.G., Zhang Y.D., Castañeda-Ruíz R.F., Zhang X.G. Three new species of Neosporidesmium from Hainan, China. Mycol. Prog. 2011;10:157–162. doi: 10.1007/s11557-010-0685-2. [DOI] [Google Scholar]
- 51.Goh T.K. Single-spore isolation using a hand-made glass needle. Fungal Divers. 1999;2:47–63. [Google Scholar]
- 52.White T.J., Bruns T.D., Lee S.B., Taylor J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols: A Guide to Methods and Applications. Academic Press; New York, NY, USA: 1990. pp. 315–322. [DOI] [Google Scholar]
- 53.Xia J.W., Ma Y.R., Li Z., Zhang X.G. Acrodictys-like wood decay fungi from southern China, with two new families Acrodictyaceae and Junewangiaceae. Sci. Rep. 2017;7:7888. doi: 10.1038/s41598-017-08318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rehner S.A., Buckley E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 2005;97:84–98. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- 55.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang D., Gao F.L., Jakovlić I., Zou H., Zhang J., Li W.X., Wang G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020;20:348–355. doi: 10.1111/1755-0998.13096. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen L.T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoang D.T., Chernomor O., von Haeseler A., Minh B.Q., Vinh L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2017;35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., von Haeseler A., Jermiin L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shang Z.Q., Zhang X.G. Two new Corynespora species from Jiangsu, China. Mycotaxon. 2007;100:155–158. [Google Scholar]
- 62.Verma R.K., Prasher I.B., Sushma , Gautam A.K., Rajeshkumar K.C., Castañeda-Ruíz R.F. Kirschsteiniothelia shimlaensis sp. nov. from Himachal Pradesh, India. Mycotaxon. 2021;136:401–407. doi: 10.5248/136.401. [DOI] [Google Scholar]
- 63.Siqueira M.V., Braun U., Souza-Motta C.M. Corynespora subcylindrica sp. nov., a new hyphomycete species from Brazil and a discussion on the taxonomy of corynespora-like genera. Sydowia. 2008;60:113–122. [Google Scholar]
- 64.Farr D.F., Rossman A.Y. Fungal Databases. U.S. National Fungus Collections, USDA; Washington, DC, USA: 2022. [Google Scholar]
- 65.Hongsanan S., Hyde K.D., Phookamsak R., Wanasinghe D.N., McKenzie E.H.C., Sarma V.V., Boonmee S., Lücking R., Bhat D.J., Liu N.G., et al. Refined families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere. 2020;11:1553–2107. doi: 10.5943/mycosphere/11/1/13. [DOI] [Google Scholar]
- 66.Rodríguez-Andrade E., Stchigel A.M., Guarro J., Cano-Lira J.F. Fungal diversity of deteriorated sparkling wine and cork stoppers in Catalonia, Spain. Microorganisms. 2020;8:12. doi: 10.3390/microorganisms8010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swart H.J. Australian leaf-inhabiting fungi. XVIII. Corynespora acaciae sp. nov. Trans. Br. Mycol. Soc. 1985;84:175–177. doi: 10.1016/S0007-1536(85)80239-4. [DOI] [Google Scholar]
- 68.Wulandari N.F. Three new species of Corynespora from Indonesia. Mycotaxon. 2006;97:21–27. [Google Scholar]
- 69.Ellis M.B. More Dematiaceous Hyphomycetes. Kew, Commonwealth Mycological Institute; Surrey, UK: 1976. [Google Scholar]
- 70.Sharma N., Singh P.N., Kamal Three new taxa of Corynespora causing foliar blight in forest plants of North Eastern Uttar Pradesh. J. Mycol. Pl. Pathol. 2003;33:26–32. [Google Scholar]
- 71.Meenu K., Singh A., Singh S.K. Some new species of Corynespora. Indian Phytopath. 1997;50:17–24. [Google Scholar]
- 72.Kumar S., Singh R., Gond D.K., Saini D.C. Two new species of Corynespora from Uttar Pradesh, India. Mycosphere. 2012;3:864–869. doi: 10.5943/mycosphere/3/5/11. [DOI] [Google Scholar]
- 73.Carris L.M. Vaccinium fungi: Corynespora arctespora, comb. nov. Mycotaxon. 1987;30:127–132. [Google Scholar]
- 74.Dubey R.K., Rai A.N. Two new hyphomycetous fungi from India. Indian Phytopath. 2003;56:486–490. [Google Scholar]
- 75.Sharma N., Chaudhary R.K., Kamal Five undescribed species of Corynespora. Indian Phytopath. 2002;55:458–463. [Google Scholar]
- 76.Zhang K., Fu H.B., Zhang X.G. Taxonomic studies of Corynespora from Hainan, China. Mycotaxon. 2009;109:85–93. doi: 10.5248/109.85. [DOI] [Google Scholar]
- 77.Jain S.L., Rai A.N., Mehta P. Additions to the genus Corynespora from India. Indian Phytopath. 2002;55:51–56. [Google Scholar]
- 78.Kumar S., Singh A., Singh R., Dubey N.K. Corynespora bombacina causing foliar disease on Bombax ceiba from Sonebhadra forest of Uttar Pradesh, India. Can. J. Plant Prot. 2013;1:76–77. [Google Scholar]
- 79.Ellis M.B. Some species of Corynespora. Mycol. Pap. 1957;65:1–15. [Google Scholar]
- 80.Singh R., Kamal Two new species of Corynespora from northeastern Uttar Pradesh, India. Mycotaxon. 2011;118:123–129. doi: 10.5248/118.123. [DOI] [Google Scholar]
- 81.Subramanian C.V. Hyphomycetes from South East Asia—Novelties from Singapore and Malaysia. Kavaka. 1994;22:52–76. [Google Scholar]
- 82.Chi P.K. Fungal Diseases of Cultivated Medicinal Plants in Guangdong Province. Guangdong Science and Technology Press; Guangzhou, China: 1994. pp. 1–275. [Google Scholar]
- 83.Kumar S., Singh R. Corynespora celastri sp. nov. on Celastraceae from India. Stud. Fung. 2016;1:125–129. doi: 10.5943/sif/1/1/12. [DOI] [Google Scholar]
- 84.Singh A., Kumar S., Singh R., Dubey N.K. Corynespora clerodendrigena sp. nov. causing foliar disease on Clerodendrum viscosum from Sonebhadra forest of Uttar Pradesh, India. Plant Pathol. Quar. 2013;3:15–17. doi: 10.5943/ppq/3/1/3. [DOI] [Google Scholar]
- 85.Ellis M.B. Dematiaceous hyphomycetes. V. Mycol. Pap. 1963;93:1–33. [Google Scholar]
- 86.Holubová-Jechová V., Mercado Sierra A. Studies on hyphomycetes from Cuba II. Hyphomycetes from the Isla de la Juventud. Ceská Mykol. 1984;38:96–120. [Google Scholar]
- 87.Meenu , Kharwar R.N., Bhartiya H.D. Some new forms of genus Corynespora from Kathmandu Valley of Nepal. Indian Phytopath. 1998;51:146–151. [Google Scholar]
- 88.Raja H.A., Stchigel A.M., Miller A.N., Crane J.L., Shearer C.A. Hyphomycetes from the great Smoky mountains national park, including three new species. Fungal Divers. 2007;26:271–286. [Google Scholar]
- 89.Zhang X.G., Xu J.J. Taxonomic studies of Corynespora from Guangxi, China. Mycotaxon. 2005;92:431–436. [Google Scholar]
- 90.Crous P.W., Carnegie A.J., Wingfield M.J., Sharma R., Mughini G., Noordeloos M.E., Santini A., Shouche Y.S., Bezerra J.D.P., Dima B., et al. Fungal Planet description sheets: 868–950. Persoonia. 2019;42:291–473. doi: 10.3767/persoonia.2019.42.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang X.M., Zhang X.G. A new species of Corynespora from Yunnan, China. Mycotaxon. 2007;101:77–79. [Google Scholar]
- 92.Ma J., Zhang X.G. Three new species of Corynespora from China. Mycotaxon. 2007;99:353–358. [Google Scholar]
- 93.Singh A., Kumar S., Singh R., Dubey N.K. A new species of Corynespora causing foliar disease on Ficus religiosa from forest of Sonebhadra, Uttar Pradesh, India. Mycosphere. 2012;3:890–892. doi: 10.5943/mycosphere/3/6/2. [DOI] [Google Scholar]
- 94.Zhang X.G., Ji M. Taxonomic studies of Corynespora from Yunnan, China. Mycotaxon. 2005;92:425–429. [Google Scholar]
- 95.Ma L.G., Ma J., Zhang Y.D., Zhang X.G. Craspedodidymum and Corynespora spp. nov. and a new anamorph recorded from southern China. Mycotaxon. 2011;117:351–358. doi: 10.5248/117.351. [DOI] [Google Scholar]
- 96.Yen J.M. Étude sur les champignons parasites du Sud-Est asiatique. 37. Champignons Parasites de Malaisie. 20. Bull. Soc. myc. Fr. 1980;96:18–25. [Google Scholar]
- 97.Heuchert B., Braun U. On some dematiaceous lichenicolous hyphomycetes. Herzogia. 2006;19:11–21. [Google Scholar]
- 98.Guo Y.L. Four new species of Corynespora. Acta Mycol. Sin. 1984;3:161–169. [Google Scholar]
- 99.Ma J., Zhang K., Zhang X.G. Taxonomic studies of Corynespora from Hainan, China. Mycotaxon. 2008;104:153–157. [Google Scholar]
- 100.Saikia U.N., Sarbhoy A.K. Hyphomycetes of North-East India II. Indian Phytopath. 1980;33:466–470. [Google Scholar]
- 101.Holubová-Jechová V. Revisiones generum obscurorum hyphomycetum: Four genera described by A.C.J. Corda. Sydowia. 1994;46:238–246. [Google Scholar]
- 102.Morgan-Jones G. Notes on hyphomycetes. LX. Corynespora matuszakii, an undescribed species with narrow, cylindrical, catenate conidia and highly-reduced conidial cell lumina. Mycotaxon. 1988;33:483–487. [Google Scholar]
- 103.Singh A., Kumar S., Singh R., Dubey N.K. A new species of Corynespora from Sonebhadra forest of Uttar Pradesh, India. Curr. Res. Envir. App. Myc. 2014;4:149–151. doi: 10.5943/cream/4/2/1. [DOI] [Google Scholar]
- 104.Meenu K., Kamal New species of Corynespora. Mycol. Res. 1998;102:344–346. doi: 10.1017/S0953756297005455. [DOI] [Google Scholar]
- 105.Castañeda-Ruíz R.F. Fungi Cubenses III. Instituto de Investigaciones Fundamentales en Agricultura Tropical “Alejandro de Humboldt”; Habana, Cuba: 1988. pp. 1–27. [Google Scholar]
- 106.Braun U., Crous P.W., Nakashima C. Cercosporoid fungi (Mycosphaerellaceae) 2. Species on monocots (Acoraceae to Xyridaceae, excluding Poaceae) IMA Fung. 2014;5:203–390. doi: 10.5598/imafungus.2014.05.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singh A., Singh S.K., Kamal Three new species of Corynespora from India. J. Mycol. Pl. Pathol. 2000;30:44–49. [Google Scholar]
- 108.Kumar S., Singh R., Saini D.C., Kamal A new species of Corynespora Güssow from terai forest of northeastern Uttar Pradesh, India. Mycosphere. 2012;3:410–412. doi: 10.5943/mycosphere/3/4/3. [DOI] [Google Scholar]
- 109.Ellis M.B. Dematiaceous hyphomycetes. III. Mycol. Pap. 1961;82:1–55. [Google Scholar]
- 110.Singh D.P., Mall T.P. Two novel additions to Corynespora Güssow from India. Int. J. Plant Sci. 2011;6:321–324. [Google Scholar]
- 111.Loerakker W.M. A new species of Corynespora. Persoonia. 1975;8:220–222. [Google Scholar]
- 112.Crous P.W., Wingfield M.J., Burgess T.I., Hardy G.E.S.t.J., Gené J., Guarro J., Baseia I.G., García D., Gusmão L.F.P., Souza-Motta C.M., et al. Fungal Planet description sheets: 716–784. Persoonia. 2018;40:240–393. doi: 10.3767/persoonia.2018.40.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang K., Ma J., Zhang X.G. Two new species of Corynespora from Hainan, China. Mycotaxon. 2008;104:159–163. [Google Scholar]
- 114.Zhang X.G., Shi C.H. Taxonomic studies of Corynespora from China. Mycotaxon. 2005;92:417–423. [Google Scholar]
- 115.Castañeda-Ruíz R.F., Guarro J., Cano J. Notes on conidial fungi. I. A new species of Corynespora. Mycologia. 1995;87:271–272. doi: 10.1080/00275514.1995.12026529. [DOI] [Google Scholar]
- 116.Zhang G.M., Zhang X.G. Two new species of Corynespora from Guangdong, China. Mycotaxon. 2007;99:347–352. [Google Scholar]
- 117.Kumar S., Singh R. Biodiversity, distribution and taxonomy of conidial fungus Corynespora (Corynesporascaceae) associated with Malvaceae. J. Biodivers. Endanger. Species. 2016;4:166. doi: 10.4172/2332-2543.1000166. [DOI] [Google Scholar]
- 118.Sharma N., Chaudhary S., Kamal Three new species of genus Corynespora. Indian Phytopathol. 2002;55:178–181. [Google Scholar]
- 119.Sharma N., Soni K.K., Jamaluddin , Verma R.K. A new species of Corynespora from central India. Indian Phytopathol. 2005;58:503–504. [Google Scholar]
- 120.Crous P.W., Luangsa-ard J.J., Wingfield M.J., Carnegie A.J., Hernández-Restrepo M., Lombard L., Roux J., Barreto R.W., Baseia I.G., Cano-Lira J.F., et al. Fungal Planet description sheets: 785–867. Persoonia. 2018;41:238–417. doi: 10.3767/persoonia.2018.41.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhurbenko M.P., Braun U., Heuchert B., Kobzeva A.A. New lichenicolous hyphomycetes from Eurasia. Herzogia. 2015;28:584–598. doi: 10.13158/heia.28.2.2015.584. [DOI] [Google Scholar]
- 122.Kushwaha P., Singh R., Kumar S. Corynespora titarpaniensis sp. nov., on Lepidagathis from Central India. Mycotaxon. 2017;132:271–279. doi: 10.5248/132.271. [DOI] [Google Scholar]
- 123.Crous P.W., Wingfield M.J., Guarro J., Cheewangkoon R., van der Bank M., Swart W.J., Stchigel A.M., Cano-Lira J.F., Roux J., Madrid H., et al. Fungal Planet description sheets: 154–213. Persoonia. 2013;31:188–296. doi: 10.3767/003158513X675925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kumar S., Singh R. Diversity, distribution and taxonomy of Corynespora associated with Cannabaceae and Ulmaceae. Plant Pathol. Quar. 2016;6:225–231. doi: 10.5943/ppq/6/2/11. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All sequences generated in this study were submitted to GenBank.