Abstract
Mice treated with viable Mycobacterium tuberculosis with no glycolipid trehalose dimycolate (TDM) on the outer cell wall (delipidated M. tuberculosis) by intraperitoneal or intratracheal inoculation presented an intense recruitment of polymorphonuclear cells into the peritoneal cavity and an acute inflammatory reaction in the lungs, respectively. In addition, lung lesions were resolved around the 32nd day after intratracheal inoculation. TDM-loaded biodegradable poly-dl-lactide-coglycolide microspheres as well as TDM-coated charcoal particles induced an intense inflammatory reaction. In addition, high levels of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), IL-12, IL-10, gamma interferon (IFN-γ), and IL-4 production were detected in lung cells, and nitric oxide (NO) production was high in culture supernatants of bronchoalveolar lavage cells. These in vivo data were confirmed by in vitro experiments using peritoneal macrophages cultured in the presence of TDM adsorbed onto coverslips. High levels of IFN-γ, IL-6, TNF-α, IL-12, IL-10, and NO were detected in the culture supernatants. Our results suggest that TDM contributes to persistence of infection through production of cytokines, which are important for the recruitment of inflammatory cells and maintenance of a granulomatous reaction. In addition, our findings are important for a better understanding of the immunostimulatory activity of TDM and its possible use as an adjuvant in experiments using DNA vaccine or gene therapy against tuberculosis.
Tuberculosis is a classical example of an infectious illness in which the disease process is caused by the immune response directed at the infectious agent. Innate and cell-mediated immune responses directed against bacteria and their products which are essentially nontoxic may lead to extensive tissue damage, wasting, and death (24). On the other hand, innate and cell-mediated responses also protect the host against the disease by arresting, killing, and removing multiplying bacteria. This effect determines disease progression by regulating the supply of mycobacterial components that drive the immunopathologic abnormalities (24). An important question that arises from this balance between protective and harmful effects of the immune response is whether distinct mycobacterial components could selectively induce protective responses and be suitable for use as new vaccines or immunotherapy for the disease.
Several factors which may have a role in the pathogenesis of disease have been isolated from mycobacteria, including cord factor, a component located at the external layer of the cell wall of mycobacteria. The structure of cord factor has been fully elucidated by Noll et al. (17), who showed that it was a glycolipid consisting of two mycolic acid molecules (an α-branched and β-hydroxylated fatty acid with 90 carbon atoms) linked to trehalose by the hydroxyl groups of carbons 6 and 6′, i.e., 6,6′-trehalose dimycolate (TDM). Four decades of research on TDM have uncovered a number of biological activities involved in the pathogenesis of mycobacterial diseases, including high toxicity to mice (3) and immunomodulation (12). Several other effects of TDM in addition to its toxicity (28), such as the development of a granulomatous reaction in mouse lungs after intravenous administration (21), adjuvant and immunostimulatory properties (10), and capacity to enhance nonspecific resistance to tumors (12, 16) and to bacterial infections (20, 22) in mice, suggest the participation of TDM in the host-parasite relationship at different levels. Indirect evidence has also been provided that TDM might be responsible for inhibiting fusion between adjacent membranes in vivo (29), suggesting a role in inhibition of phagosome-lysosomal fusion. More recently, recognition of a glycolipid antigen by CD1-restricted αβ+ T cells has also been reported (2).
Protective immunity to mycobacteria is dependent on several effector mechanisms working together, particularly at the infection's local microenvironment. These factors include the local cell population and its cytokine production and expression of adhesion (1) and costimulatory molecules. Local cytokines produced by inflammatory cells are thought to direct macrophage activation and T-cell development to a Th1 or Th2 pattern on the following days of infection, leading to control of antibacterial activity (18). Evasion of innate defense mechanisms and cell-mediated immunity are the keys to the success of Mycobacterium tuberculosis, and this microorganism must adapt to the intracellular environment in the macrophage (24). How is this immune response altered or manipulated by M. tuberculosis or its products so that it can up- or down-regulate the expression of cytokine molecules in the host? To help answer this question, we have used in the present study a model of pulmonary and intraperitoneal (i.p.) M. tuberculosis infection with no glycolipid TDM on the outer cell wall (delipidated M. tuberculosis) or TDM-loaded biodegradable poly-dl-lactide-coglycolide (PLGA) microspheres to study the local inflammatory events and establish the relationship of the nature and extent of the inflammatory reaction to cytokine production.
MATERIALS AND METHODS
Live TDM-depleted M. tuberculosis.
The depletion of TDM from live M. tuberculosis H37Rv was carried out by treating the bacteria with petroleum ether (boiling point, 35 to 60°C) as previously described (4). The viability of M. tuberculosis and solvent-treated bacteria (delipidated M. tuberculosis) was determined by plating serial fivefold dilutions of the bacterial suspension on Lowestein Jensen agar at 37°C for 21 days. The residual material extracted with petroleum ether contained several lipid components, including glycolipids, free mycolic acid, glycerides, menaquinones, and hydrocarbons. TDM, accounting for 90% of the petroleum ether extract, had physical and chemical characteristics of 6,6′-dimycoloyl trehalose, as previously described (27).
TDM preparation.
One hundred grams of heat-killed, dried M. tuberculosis H37Rv was repeatedly soaked in a mixture of chloroform-methanol (1:1 [vol/vol]), and the extracted residual material (18 g) was fractionated as previously reported for TDM purification (27). The purified glycolipid (0.530 g) migrated as a single band on thin-layer chromatography, similarly to commercially available TDM (Sigma, St. Louis, Mo.).
TDM-loaded microspheres and TDM-coated charcoal particles.
Microspheres were prepared using the emulsion-solvent evaporation technique (13, 14). Briefly, 5 mg of TDM (Sigma) and 125 mg of the biodegradable polymer PLGA (50:50) (Resomer RG 505; molecular weight, 78,000; Boehringer Ingelheim, Mannheim, Germany) were diluted in 30 ml of methylene chloride. This organic phase was mixed with 100 ml of an aqueous phase containing 3% polyvinyl alcohol (Mowiol 40–88; Sigma-Aldrich Chemicals) as surfactant to form a stable oil-in-water emulsion. A 6-h stirring at room temperature with a Eurostar homogenizer (600 rpm) was carried out to allow for organic solvent evaporation. Microspheres were collected by centrifugation at 10,000 × g and washed three times with sterile water, freeze-dried, and stored at 4°C. The presence of TDM was determined by thin-layer chromatography after particle dissolution in methylene chloride. Particle characterization was carried out by scanning electron microscopy. Briefly, an aqueous suspension of microspheres was dropped onto slides covered with poly-l-lysine (Sigma), allowed to dry in air under ambient conditions, and stuck onto metal stubs. Samples were coated with gold prior to examination by scanning electron microscopy in a JSM-5200 scanning microscope (JEOL). Unloaded PLGA microspheres, trehalose dibehenate (TDB) (Sigma), or unrelated lipid control (a mixture of triglycerides; Sigma)-loaded PLGA microspheres were prepared using the same procedure and used as controls. TDM-coated charcoal particles (50 to 100 μm in diameter) were prepared as previously described (26).
Animals, infection, and TDM-loaded microsphere and TDM-coated charcoal particle administration.
Young adult BALB/c mice were obtained from the vivarium of the School of Medicine of Ribeirão Preto, University of São Paulo, and were maintained under standard laboratory conditions. Mice were infected with 106 viable M. tuberculosis or delipidated M. tuberculosis cells intratracheal administration or with 3 × 106 organisms by i.p. administration under anesthesia using 200 μl of tribromoethanol (Sigma) in 2.5% phosphate-buffered saline (PBS). Infected animals were kept in biohazard facilities and were housed in cages within a laminar flow safety enclosure. Infected animals were sacrificed at 2, 8, or 16 days for recruitment-of-cell determinations and at 4, 16, or 32 days after infection for lung inflammatory reaction characterization. Microspheres were administered using the same route (200 mg/kg of body weight per mouse). Mice were sacrificed 60 days after microsphere treatment to allow for particle degradation and release of entrapped TDM. TDM-coated charcoal particles were administered by an intravenous route (100 μl) and sacrificed at different time points after injection. TDM or a lipid control of TDB or a mixture of triglycerides was also injected by the intravenous route (50 μg per animal).
Evaluation of lung granulomatous reaction and recruitment of cells to the peritoneal cavity.
The ability of M. tuberculosis, delipidated M. tuberculosis, TDM-PLGA microspheres, and TDM-coated charcoal particles to cause granulomatous reactions was assessed by histological analysis of the lungs. Lung tissues from four mice per experimental group were infused with fresh 10% formaldehyde in PBS at different times after each treatment. Sections made from paraffin blocks were stained with hematoxylin and eosin. Groups of five mice were injected i.p. with 3 × 106 viable M. tuberculosis or delipidated M. tuberculosis cells or with 50 μg of TDM. Control groups received PBS or lipid controls (TDB or a mixture of triglycerides). At 2, 8, and 16 days after the injection of stimulants the animals were killed with anesthetic and the cells from the peritoneal cavities were harvested by injection of 3 ml of PBS containing 5 μg of heparin/ml. The abdomens were gently massaged, and a blood-free cell suspension was carefully withdrawn with a syringe. Abdominal washings were placed in plastic tubes and total cell counts were performed immediately in a Newbauer chamber. Differential counting was obtained using Rosenfeld-stained cytospin preparations.
BALF.
Microsphere-treated mice were killed by an overdose of sodium pentobarbitone (i.p.), and 1.0 ml of RPMI-1640 (Sigma) at room temperature was instilled through a polyethylene cannula introduced into the trachea. Cells present in the bronchoalveolar lavage fluid (BALF) were recovered immediately. The procedure was repeated once.
Measurement of cytokines.
Levels of cytokines produced by lung cells were measured by enzyme-linked immunosorbent assay (ELISA). Total lung tissue was homogenized with an Ultraturrax T50 IKA (Labortechnik, Staufen, Germany) apparatus for 5 min at 4°C. Homogenized tissue was centrifuged at 10,000 × g for 15 min and the supernatant was filtered through a 0.22-μm-pore-size Millipore filter. Capture and biotinylated monoclonal antibodies for tumor necrosis factor alpha (TNF-α) (MP6-XT22, MP6-XT3), interleukin-10 (IL-10) (JES5-2A5, JES5-16E3), IL-6 (MP5-20F3, MP5-32C11), IL-4 (BVD4-1D11, BVD6-24G2), gamma interferon (IFN-γ) (R4-6A2, XMG1.2), and IL-12 (C15.6, C17.8) and recombinant cytokines were purchased from Pharmingen (San Diego, Calif.). Levels of TNF-α, IL-10, IL-6, IL-4, IFN-γ, and IL-12 were determined in the supernatants by ELISA as previously described (9) following the manufacturer's instructions (Pharmingen). A standard curve using preparations with known concentrations of mouse recombinant TNF-α (rTNF-α), rIL-10, rIL-6, rIL-4, rIFN-γ, or rIL-12, respectively, was performed for each assay. The detection limit was 15 pg per ml for all cytokines evaluated. Lipopolysaccharide (LPS) (50 μg per mouse [100 μl])- and mock-injected mice were used as positive and negative controls, respectively. TDB or a mixture of triglyceride (Sigma)-loaded microspheres was used as a control in microsphere experiments.
NO measurements.
NO production was assessed by measuring the amount of nitrite in supernatants of BALF cell cultures, using the Greiss reagent (30). The titer was determined using a standard curve with serial dilutions of NaNO2 (Sigma) and the detection limit was 3 μM per 105 cells.
Cell cultures.
BALF cells recovered from mice treated with TDM-loaded microspheres were cultured in RPMI medium containing 10% fetal calf serum (GIBCO-BRL, Grand Island, N.Y.), 10 mM HEPES, 20 mM sodium bicarbonate, and penicillin and streptomycin (GIBCO-BRL) (100 μg/ml). In addition, resident peritoneal macrophages were collected from naive mice by washing the peritoneal cavity with PBS and were cultured under the same conditions. Alternatively, resident peritoneal macrophages were layered onto a glass coverslip onto which 5 μl of purified TDM (Sigma) diluted in chloroform (1 mg/ml) was dropped and were allowed to dry. After incubation for 1 h in RPMI-1640 (Sigma) the coverslips were vigorously washed with the same medium and cultured in the presence of RPMI-1640 and 10% fetal calf serum (Gibco-BRL). Coverslips were removed after 2, 4, or 8 days, and the supernatants were collected and kept at −70°C until assayed by ELISA. Cells were also analyzed by immunocytochemistry.
Immunocytochemistry.
TDM, LPS, and control lipid in vitro-treated cells were fixed in 4% paraformaldehyde (Sigma). Slides were washed with PBS, blocked, and incubated overnight at 4°C with diluted monoclonal rat immunoglobulin G directed against IL-6, TNF-α, and IL-10 or control isotype (Pharmingen) at a final concentration of 7 μg/ml. A biotinylated polyclonal rabbit anti-rat immunoglobulin G diluted 1/100 was used as a secondary antibody. After washing, the slides were stained by avidin-biotin complex peroxidase (Dako A/S, Glostrup, Denmark). The chromogenic substrate was developed by incubation in diaminobenzidine for 10 min. Finally, the slides were stained with hematoxylin. For negative-control slides, all these steps were repeated, but with an irrelevant, isotype-matched or nonimmune serum substituted for the primary antibody.
Statistical analysis.
The results represent means ± standard deviations of the means. The significance of the difference between groups was calculated by Dunnett's test. Computer-assisted evaluation of the results was used to calculate the probability value of the data. A P of 0.05 was used as the limit of statistical significance.
RESULTS
Comparison of the inflammatory cell recruitment activity induced in mice by M. tuberculosis and delipidated M. tuberculosis.
The number of total cells as well as polymorphonuclear (PMN) and mononuclear (MN) cells in peritoneal fluid measured 2, 8, and 16 days after injection of 3 × 106 viable M. tuberculosis or delipidated M. tuberculosis organisms into the peritoneal cavity of mice is shown in Table 1. Inoculation of delipidated M. tuberculosis significantly increased the number of PMN cells in the peritoneal cavity up to 16 days after injection when compared with the inoculation of intact M. tuberculosis or with control groups receiving PBS, TDB, or a mixture of triglycerides. In contrast, intense infiltrates of MN cells were observed in the peritoneal cavity of mice inoculated with M. tuberculosis organisms or with the glycolipid DMT (Table 1). A similar pattern of cell recruitment was also observed in lungs of animals inoculated with delipidated M. tuberculosis or with M. tuberculosis (data not shown).
TABLE 1.
Effect of TDM on the leukocyte cell influx into the peritoneal cavity of mice several times after i.p. injection of viable M. tuberculosis or delipidated M. tuberculosis
| Injected preparation | Recruitment of indicated cells (per mm3) into the peritoneal cavity on indicated day after injectiona
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Totalb
|
PMN
|
MN
|
|||||||
| 2 | 8 | 16 | 2 | 8 | 16 | 2 | 8 | 16 | |
| M. tuberculosis | 7,520 ± 367c | 9,850 ± 415c | 9,740 ± 358c | 2,620 ± 140c | 3,090 ± 195c | 2,350 ± 145c | 5,488 ± 327c | 7,124 ± 386c | 7,640 ± 421c |
| Delipidated M. tuberculosis | 6,320 ± 326c | 9,740 ± 358c | 8,714 ± 406c | 5,366 ± 270c | 7,133 ± 264c | 6,450 ± 255c | 2,190 ± 146c | 3,412 ± 185c | 3,630 ± 204c |
| TDM | 7,850 ± 384c | 6,520 ± 329c | 3,160 ± 242c | 754 ± 88 | 815 ± 77 | 690 ± 58 | 6,490 ± 309c | 5,170 ± 261c | 2,754 ± 218c |
| TDB | 1,655 ± 216 | 1,590 ± 190 | 1,466 ± 128 | 1,040 ± 115 | 1,120 ± 132 | 984 ± 138 | 975 ± 129 | 1,218 ± 140 | 1,064 ± 121 |
| Triglycerides | 1,430 ± 158 | 1,513 ± 162 | 1,346 ± 109 | 830 ± 108 | 592 ± 72 | 628 ± 87 | 850 ± 90 | 914 ± 104 | 760 ± 88 |
| PBS | 1,025 ± 143 | 1,180 ± 212 | 1,236 ± 198 | 670 ± 95 | 894 ± 115 | 718 ± 104 | 614 ± 65 | 659 ± 86 | 741 ± 92 |
The number of total cells, PMN cells, and MN cells in peritoneal fluid was measured 2, 8, and 16 days after injection of 3 × 106 viable organisms (M. tuberculosis or delipidated M. tuberculosis) into the peritoneal cavity of mice or after inoculation of TDM, TDB, or a mixture of triglycerides (50 μg per animal).
Cell numbers are expressed as the means of five mice per group ± standard errors of the means.
P < 0.001 when compared to the PBS group.
Inflammation, cytokines, and NO production induced by TDM-PLGA microspheres.
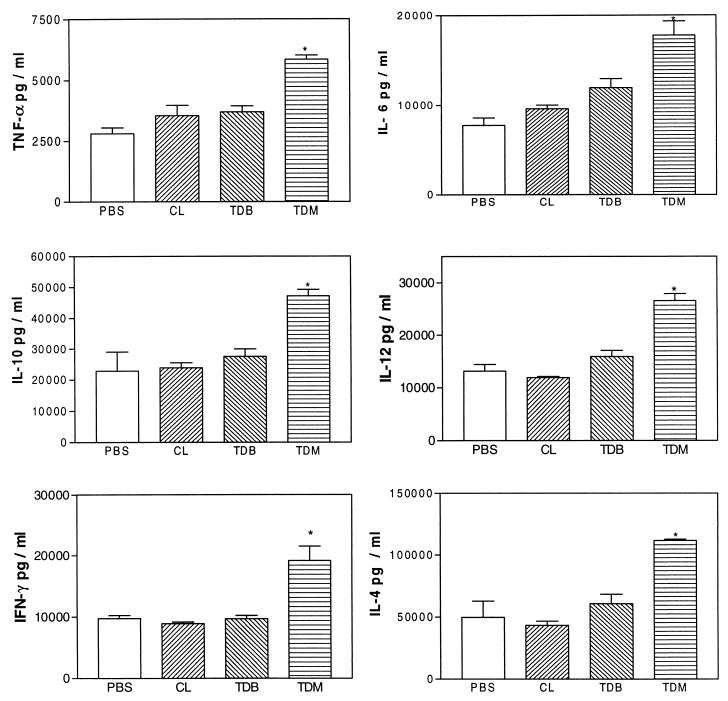
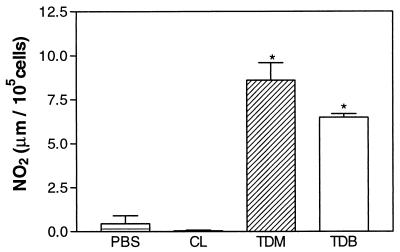
To determine whether TDM induces inflammation, cytokines, and NO production a drug delivery system based on biodegradable microspheres was used. This system consists of microparticles formed by a polymeric matrix where the lipid is entrapped. The microparticles of PLGA (50:50) have a slow degradation time, and thus the treated mice were sacrificed 60 days after treatment to ensure that most of the lipid was released (14). Mice treated with TDM-loaded PLGA microspheres showed an intense infiltrate of MN cells in the lungs; however, control mice treated with TDB-loaded or unloaded PLGA microspheres were unable to induce an inflammatory reaction (data not shown). Levels of IL-6, TNF-α, IL-10, IFN-γ, IL-12, and IL-4 found in homogenates of bulk lung cells from mice treated with TDM-PLGA microspheres presenting intense inflammatory reactions in the lungs were significantly higher than levels in PBS-treated mice (Fig. 1). In addition, supernatants from cultures of BALF cells collected from animals treated 60 days before with TDM-PLGA microspheres showed higher levels of NO production than with PBS (negative control) (Fig. 2).
FIG. 1.
Levels of IL-6, TNF-α, IL-10, IL-12, IL-4, and IFN-γ determined by ELISA in the homogenate of bulk lung cells from mice treated with TDM-PLGA microspheres or with encapsulated microspheres containing lipids (CL) or TDB. Mice were killed 60 days after treatment and cytokines were determined by ELISA. Data representing a typical experiment repeated three times are expressed as means ± standard deviations for five mice in each group. Asterisks indicate a significant difference between the TDM-PLGA-injected and PBS control groups (P < 0.01).
FIG. 2.
NO levels in the supernatant culture of BALF cells recovered from mice inoculated with TDM-PLGA microspheres or controls as described for Fig. 1. The nitrite level was assayed as described in Materials and Methods. Data representing a typical experiment repeated three times are expressed as means ± standard deviations for five mice in each group. Asterisks indicate a significant difference between the experimental and PBS control groups (P < 0.01).
Inflammation induced by TDM-coated charcoal particles.
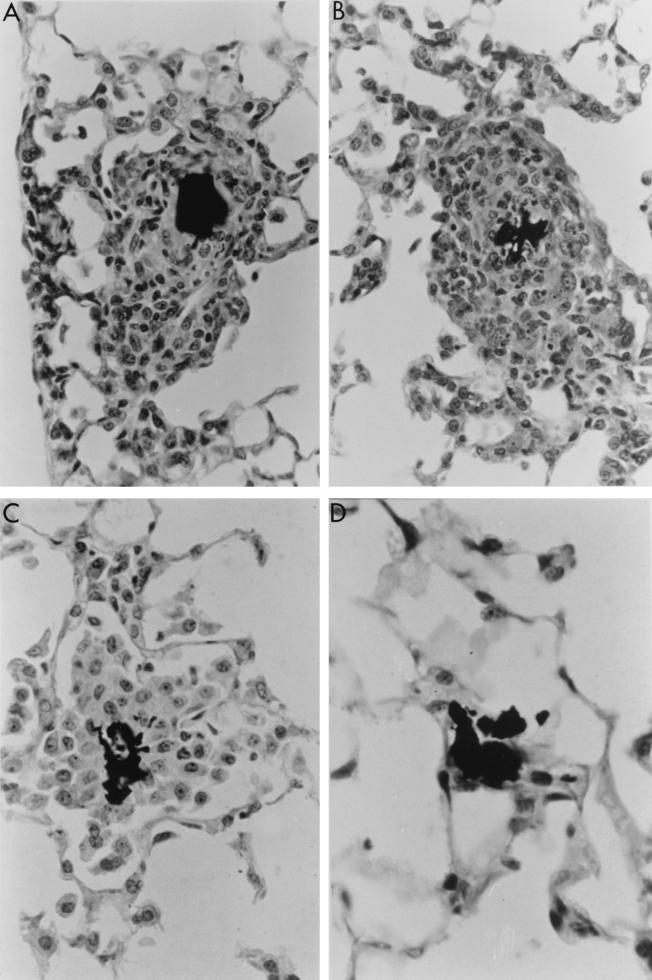
Intravenous administration of TDM coated to charcoal particles induced an intense inflammatory reaction around the particles that were trapped in the lungs, as shown in Fig. 3. This reaction was more intense after 4 to 8 days, when it was detected around approximately 80% of the particles. The histological picture of the lung inflammatory reaction around TDM-coated charcoal particles was characterized by several layers of large MN cells. Ninety-nine percent of uncoated particles or particles coated with lipid control were scored as negative at 2, 4, and 8 days postinoculation.
FIG. 3.
Mouse lung inflammatory reaction 4 (A), 8 (B), and 16 (C) days after injection of charcoal particles coated with TDM. Note the strong inflammatory reaction around the particles. Charcoal particles without TDM (D) presented no inflammatory reaction 4 days after inoculation. Magnification, ×400.
Cytokines and NO production induced by TDM in macrophage cultures.
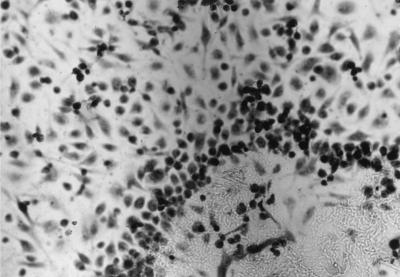
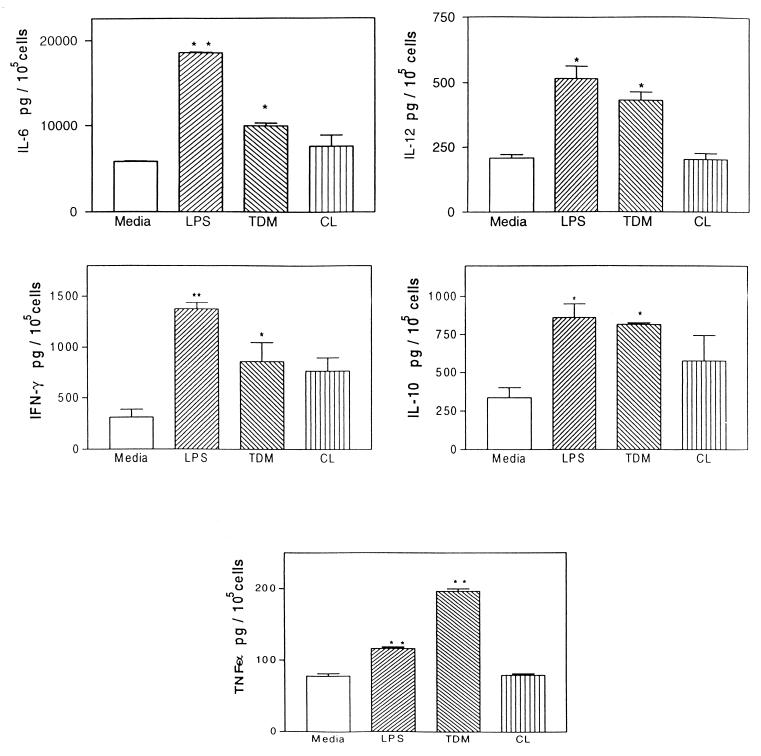
Peritoneal macrophages were layered onto a glass coverslip to which TDM was previously allowed to adhere. As shown in Fig. 4, there was a clear migration of cells toward the spot of TDM, a fact not observed for coverslips containing TDB or a mixture of triglycerides as the control lipid. Supernatants collected after 2 days of this culture presented higher levels of IL-6, TNF-α, IL-10, IL-12, and IFN-γ (Fig. 5) than supernatants obtained from coverslips containing a mixture of triglycerides as a control lipid. Similar data were also observed using immunocytochemistry analysis (data not shown). Nitrite production was detected in the supernatant of TDM-stimulated cultures 24 h after stimulation (data not shown).
FIG. 4.
Migration of peritoneal macrophages toward the center of a glass coverslip to which TDM was previously allowed to adhere. Magnification, ×200.
FIG. 5.
Levels of IL-6, TNF-α, IL-10, IL-12, and IFN-γ determined by ELISA in the culture supernatants of peritoneal macrophages in the presence of TDM. Data representing a typical experiment repeated three times are expressed as means ± standard deviations for five glass coverslips in each group which TDM was previously allowed to adhere. Asterisks indicate a significant difference between the experimental (cells stimulated with TDM or LPS) and medium control groups (∗, P < 0.05; ∗∗, P < 0.01).
DISCUSSION
TDM is a potent immunomodulator that increases nonspecific resistance to infectious agents and limits tumor growth (12, 16, 20). Direct or indirect attraction of macrophages by TDM to the site of inoculation may be one component of its mechanism of action. Other TDM action would be the priming of newly arrived macrophages at the site of inoculation (19). Our results using either delipidated mycobacteria or microspheres showed that TDM has an important role in recruitment of cells for granuloma formation in tuberculosis and in modulation of expression or production of important immunological mediators, including cytokines and NO.
Treatment of viable M. tuberculosis cells with petroleum ether solvent removed lipid substances from the outer cell wall, and 90 to 95% of all bacilli (delipidated M. tuberculosis) continued to be viable after the extraction procedure. The lipid extract was fractionated by column chromatography (17, 28) and the TDM component was obtained, as shown by physical and chemical analysis (31). The response of mice to i.p. administration of live M. tuberculosis or delipidated M. tuberculosis organisms was studied by evaluating the influx of leukocytes into the peritoneal cavity. The i.p. inflammatory picture induced by inoculation of delipidated M. tuberculosis was drastically altered when compared with that induced by intact M. tuberculosis, especially in terms of the nature of the reaction, the types of cells involved, and the onset and duration of the inflammatory reaction. Inoculation with M. tuberculosis as well as delipidated M. tuberculosis significantly increased the number of inflammatory cells in the peritoneal cavity up to 16 days after injection compared with the control group receiving only PBS, and the number declined afterwards for delipidated M. tuberculosis-injected mice. The kinetics of cell recruitment induced by delipidated M. tuberculosis revealed an intense increase in PMN from the beginning of infection that declined over 16 days. In contrast, the MN cell phase, beginning at 2 days and with a gradual increase over 16 days after injection, was observed for M. tuberculosis inoculation (Table 1). Treatment of animals with TDM significantly increased the total cell numbers at 2 and 8 days after injection. When differential cell counts were performed on these peritoneal washouts. MN cells showed the greatest increase when compared to the cells of the control groups injected with control lipids (TDB or a mixture of triglycerides). As previously demonstrated (27), the lung inflammation induced by inoculation of delipidated M. tuberculosis was also significantly altered when compared to that induced by intact M. tuberculosis. The lesion induced by delipidated M. tuberculosis was characterized as an acute inflammatory reaction, mainly because of the presence of PMN cells and a small number of MN cells. A gradual decrease of the cellular infiltrate occurred from the 4th day after inoculation onwards, with disappearance of the infiltrate between the 16th and 32nd days (27). The changes that occurred during the cell recruitment caused by delipidated M. tuberculosis in relation to that caused by intact M. tuberculosis could be related to the presence or absence of TDM at the reaction site. Although TDM accounted for 90% of the residual material extracted with petroleum ether from M. tuberculosis, the extraction procedure also isolated several other lipid components, including glycolipids, free mycolic acid, glycerides, menaquinones, and hydrocarbons. The importance of these components in inducing the inflammatory reaction will be investigated.
As far as multiplication of the organism is concerned, the tubercle bacillus can grow in culture without TDM on its surface. Thus, TDM is not essential for bacterial multiplication in vitro, although the bacillus constantly synthesizes it (27). On the other hand, TDM could be essential for bacillus multiplication in the host, because it may protect the microorganism against destruction by phagocytes (27, 29). Unprotected bacilli, i.e., bacilli without the outer glycolipid layer, may undergo phagocytosis by leukocytes and may be eliminated if there is not enough time to synthesize a new lipid layer for protection against the destroying mechanisms of leukocytes. If the bacilli escape from destruction, they can form a new protective lipid layer and behave as normal bacilli, triggering the entire process of chronic tubercular lesions (25). We already demonstrated (27) that the viability of microorganisms having no external lipid layer was drastically reduced during the first 2 weeks after inoculation, and no viable organisms were observed in any of the organs studied from the 20th day on. Microorganisms that had been previously delipidated and then recomposed with TDM were able to survive and multiply in the animals' organs in a manner similar to that observed for the intact bacilli (27).
The data reported in the present paper suggest that the inflammatory activity of TDM is important for the development of a specific immune response to the tubercle bacillus, since it is responsible for the recruitment and maintenance of a large number of activated macrophages around the lesion, thus permitting these cells to present antigens to T lymphocytes. As previously demonstrated (27), delayed-type hypersensitivity did not occur when delipidated Mycobacterium bovis BCG was inoculated. Delipidated bacilli induced an acute inflammatory reaction, and the cells present at the lesion site, comprising predominantly PMN cells, were unable to present antigens to T lymphocytes, and therefore no animal sensitization to mycobacterial antigens occurred (27).
In order to clarify the role of TDM in recruitment of inflammatory cells and its capacity for priming or activating newly arrived macrophages at the site of inoculation, we developed a model of inflammatory reaction by injecting TDM-coated charcoal particles or TDM encapsulated in PLGA microspheres. When TDM was adsorbed to charcoal particles (50 to 100 μm in diameter) and inoculated into mice, embolization of these particles occurred in the pulmonary circulation, and typical epithelioid granulomas developed around them. A decreased inflammatory reaction was observed around the charcoal particles in lungs collected 16 days after inoculation, and the absence of lesions was found on the 32nd day. These results suggest that progressive elimination of TDM from these particles took place. If the duration of the inflammatory process was related to TDM clearance, we could speculate that the chronic development of granuloma, which contains live bacilli, may derive from molecules of this glycolipid, which could be constantly synthesized and could maintain the inflammatory process by being transferred to the surface of the bacilli. The high intensity of inflammatory reactions induced in lungs of mice by administration of TDM-PLGA microspheres was associated with high amounts of IL-6, TNF-α, IL-10, IFN-γ, IL-12, and IL-4 observed in homogenates of bulk lung cells from these animals. Similar results were obtained in vitro by stimulation of peritoneal macrophages with TDM.
Infection with M. tuberculosis leads to activation of macrophages and lymphocytes and to granuloma formation (18). Accumulation of inflammatory cells in the presence of TDM occurs at the sites of infection, following cell adhesion to endothelial cells and migration from blood vessels into tissues. Cell-cell adhesion is essential for interaction of leukocytes for granuloma formation, migration of cells through vessel walls, and activation of the specific immune response against the pathogen. Increased levels of expression of adhesion and costimulatory molecules have been associated with increased cell adhesion and migration and with activation of a specific immune response both in vivo and in vitro (11) and may be brought about by cytokines such as TNF-α, IL-12, IL-6, IL-1, IFN-γ, IL-2, and IL-4 (5, 6, 32). Cytokines are also involved in inflammation and antigen presentation. IFN-γ and IL-10 can induce up- and down-regulation of costimulatory molecules, respectively (6, 32). Preliminary results from our group have demonstrated that the level of CD11b, CD40, and CD80 molecules in alveolar macrophages from M. tuberculosis-infected mice was higher than that observed in delipidated M. tuberculosis-infected mice. The low level of cytokine production associated with a small number of cells expressing adhesion and costimulatory molecules in mice infected with delipidated M. tuberculosis could suggest that lipids in the mycobacterial cell wall are important in modulating the inflammatory process.
NO was detected in supernatant cultures of BALF from mice treated with TDM microspheres and in supernatant cultures of peritoneal macrophages treated in vitro with TDM. Our results are in agreement with another study indicating that TDM induces NO (8). On the other hand, several reports have previously described the induction of the NO pathway in murine, rat, and human macrophages after M. tuberculosis infection (7, 15, 23). Therefore, our data using TDM microspheres in vivo and peritoneal macrophages treated with TDM in vitro suggest that TDM could be one of the bacterial components responsible for this stimulation. The observation that the control glycolipid (TDB) elicited a highly significant level of NO production is being investigated.
The capacity of macrophages to simultaneously secrete cytokines and NO in vivo and in vitro after TDM stimulation suggests that TDM could be of particular relevance for the immune response contribution to inflammation. In addition, the cytokines induced by TDM have great potential as immunomodulators and adjuvants in both infectious diseases and cancer. We propose that cytokine inducers such as TDM may be attractive candidates for therapeutic and adjuvant use in experiments using DNA vaccine or gene therapy against tuberculosis.
ACKNOWLEDGMENTS
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
REFERENCES
- 1.Albeda S M, Bulk C A. Integrins and other cell adhesion molecules. FASEB J. 1990;4:2868–2880. [PubMed] [Google Scholar]
- 2.Beckman E M, Porcelli S A, Morita C T, Behar S M, Furlong S T, Brenner M B. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 3.Behling C A, Perez R L, Kidd M R, Staton G W, Jr, Hunter R L. Induction of pulmonary granulomas, macrophage procoagulant activity, and tumor necrosis factor-alpha by trehalose glycolipids. Ann Clin Lab Sci. 1993;23:256–266. [PubMed] [Google Scholar]
- 4.Bloch H. Studies on the virulence of tubercle bacilli. Isolation and biological properties of constituent of virulent organisms. J Exp Med. 1950;91:197–217. doi: 10.1084/jem.91.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creery W D, Diaz-Mitoma F, Filion L, Kumar A. Differential modulation of B7–1 and B7–2 isoform expression on human monocytes by cytokines which influence the development of T helper cell phenotype. Eur J Immunol. 1996;26:1273–1277. doi: 10.1002/eji.1830260614. [DOI] [PubMed] [Google Scholar]
- 6.Freedman A S, Freeman G J, Rhynhart K, Nadler L M. Selective induction of B7/BB-1 on interferon gamma stimulated monocytes: a potential mechanism for amplification of T cell activation through the CD28 pathway. Cell Immunol. 1991;137:429–437. doi: 10.1016/0008-8749(91)90091-o. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg S S, Xie J, Kolls J, Manson C, Didier P. Rapid induction of mRNA for nitric oxide synthase II in rat alveolar macrophage by intratracheal administration of Mycobacterium tuberculosis and Mycobacterium avium. Proc Soc Exp Biol Med. 1995;209:46–53. doi: 10.3181/00379727-209-43876. [DOI] [PubMed] [Google Scholar]
- 8.Guillemard E, Geniteau-Legendre M, Kergot R, Lemaire G, Gessani S, Labarre C, Quero A M. Simultaneous production of IFN-γ, IFN-α/β and nitric oxide in peritoneal macrophages from TDM-treated mice. J Biol Regul Homeost Agents. 1998;12:106–111. [PubMed] [Google Scholar]
- 9.Haagmans B L, van den Eertwegh A J, Claassen E, Horzinek M C, Schijns V E. Tumor necrosis factor-alpha production during cytomegalovirus infection in immunosuppressed rats. J Gen Virol. 1994;75:779–787. doi: 10.1099/0022-1317-75-4-779. [DOI] [PubMed] [Google Scholar]
- 10.Koike Y, Yoo Y C, Mitobe M, Oka T, Okuma K, Tono-oka S, Azuma I. Enhancing activity of mycobacterial cell derived adjuvants on immunogenicity of recombinant human hepatitis B virus vaccine. Vaccine. 1998;16:1982–1989. doi: 10.1016/s0264-410x(98)00084-x. [DOI] [PubMed] [Google Scholar]
- 11.Larson S, Springer T A. Structure and function of leukocyte integrins. Immunol Rev. 1990;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 12.Lemaire G, Tenu J P, Petit J F, Lederer E. Natural and synthetic trehalose diesters as immunomodulators. Med Res Rev. 1986;6:243–247. doi: 10.1002/med.2610060302. [DOI] [PubMed] [Google Scholar]
- 13.Lewis D H. Controlled release of bioactive agents from lactide/glycolide polymers. In: Chasin M, Langer R, editors. Biodegradable polymers as drug delivery systems. New York, N.Y: Marcel Dekker; 1990. pp. 1–43. [Google Scholar]
- 14.Lima K M, Rodrigues Júnior J M. Poly-dl-lactide-co-glycolide microspheres as a controlled release antigen delivery system. Braz J Med Biol Res. 1999;32:171–180. doi: 10.1590/s0100-879x1999000200005. [DOI] [PubMed] [Google Scholar]
- 15.Nabeshima S, Nomoto M, Matsuzaki G, Kishihara K, Taniguchi H, Yoshida S, Nomoto K. T-cell hyporesponsiveness induced by activated macrophages through nitric oxide production in mice infected with Mycobacterium tuberculosis. Infect Immun. 1999;67:3221–3226. doi: 10.1128/iai.67.7.3221-3226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolibe D, Masse R, Tenu J P, Lepoivre M, Petit J F. Activation of rat alveolar macrophages and protection against i.v. injected tumor cells by intratracheal administration of trehalose dimycolate. Cancer Immunol Immunother. 1986;23:200–206. doi: 10.1007/BF00205650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noll H, Bloch H, Asselineau J, Lederer E. The chemical structure of the cord factor of Mycobacterium tuberculosis. Biochim Biophys Acta. 1956;20:299–309. doi: 10.1016/0006-3002(56)90289-x. [DOI] [PubMed] [Google Scholar]
- 18.Orme I M, Cooper A M. Cytokine/chemokine cascades in immunity to tuberculosis. Immunol Today. 1999;7:307–312. doi: 10.1016/s0167-5699(98)01438-8. [DOI] [PubMed] [Google Scholar]
- 19.Oswald I P, Afround S, Bray D, Petit J F, Lemaire G. Low response of BALB/c macrophages to priming and activating signals. J Leukoc Biol. 1992;52:315–322. doi: 10.1002/jlb.52.3.315. [DOI] [PubMed] [Google Scholar]
- 20.Parant M, Parant F, Chedid L, Drapier J C, Petit J F, Wietzerbin J, Lederer E. Enhancement of nonspecific immunity to bacterial infection by cord factor (6,6′-trehalose dimycolate) J Infect Dis. 1977;135:771–777. doi: 10.1093/infdis/135.5.771. [DOI] [PubMed] [Google Scholar]
- 21.Retzinger G S, Meredith S C, Hunter R L, Takayama K, Kézdy F J. Identification of the physiologically active state of the mycobacterial glycolipid trehalose 6,6′-dimycolate and the role of fibrinogen in the biologic activities of trehalose 6,6′-dimycolate monolayers. J Immunol. 1982;129:735–744. [PubMed] [Google Scholar]
- 22.Ribi E, Granger D L, Milner K C, Yamamoto K, Strain S M, Parker R, Smith R W, Brehmer W, Azuma I. Induction of resistance to tuberculosis in mice with defined components of mycobacteria and some unrelated materials. Immunology. 1982;46:297–305. [PMC free article] [PubMed] [Google Scholar]
- 23.Rich E A, Torres M, Sada E, Finegan C K, Hamilton B D, Toossi Z. Mycobacterium tuberculosis (Mtb)-stimulated production of nitric oxide by human alveolar macrophages and relationship of nitric oxide production to growth inhibition of Mtb. Tuber Lung Dis. 1997;78:247–255. doi: 10.1016/s0962-8479(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 24.Rook G A W, Hernandez-Pando R. The pathogenesis of tuberculosis. Annu Rev Microbiol. 1996;50:259–284. doi: 10.1146/annurev.micro.50.1.259. [DOI] [PubMed] [Google Scholar]
- 25.Silva C L. Inflammation induced by micolic acid-containing glycolipids of Mycobacterium bovis (BCG) Braz J Med Biol Res. 1985;18:327–335. [PubMed] [Google Scholar]
- 26.Silva C L, Ekizlerian S M. Granulomatous reactions induced by lipids extracted from Fonsecaea pedrosi, Fonsecaea compactum, Cladasporium carrionii and Phialophora verrucosum. J Gen Microbiol. 1985;131:187–194. doi: 10.1099/00221287-131-1-187. [DOI] [PubMed] [Google Scholar]
- 27.Silva C L, Ekizlerian S M, Fazioli R A. Role of cord factor in the modulation of infection caused by Mycobacteria. Am J Pathol. 1985;118:238–247. [PMC free article] [PubMed] [Google Scholar]
- 28.Silva C L, Faccioli L H. Tumor necrosis factor (cachectin) mediates induction of cachexia by cord factor from mycobacteria. Infect Immun. 1988;56:3067–3071. doi: 10.1128/iai.56.12.3067-3071.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spargo B J, Crowe L M, Ioneda T, Beaman B L, Crowle J H. Cord factor (α,α-trehalose 6-6′-dimycolate) inhibits fusion between phospholipid vesicles. Proc Natl Acad Sci USA. 1991;88:737–740. doi: 10.1073/pnas.88.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stuehr D J, Nathan C F. Nitric oxide. A macrophage product responsible for cystostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989;169:1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas D W, Matida A K, Silva C L, Ioneda T. Esters of trehalose from Corynebacterium diphtheriae: a modified purification procedure and studies on the structure of their constituent hydroxylated fatty acids. Chem Phys Lipids. 1979;23:267–282. [Google Scholar]
- 32.Willems F, Marchant A, Delville J P, Gerard C, Delvaux A, Velu T, de Boer M, Goldman M. Interleukin-10 inhibits B7 and intercellular adhesion molecule 1 expression on human monocytes. Eur J Immunol. 1994;24:1007–1009. doi: 10.1002/eji.1830240435. [DOI] [PubMed] [Google Scholar]