Abstract
Type III-mediated translocation of exoenzyme S (ExoS) into HT-29 epithelial cells by Pseudomonas aeruginosa causes complex alterations in cell function, including inhibition of DNA synthesis, altered cytoskeletal structure, loss of readherence, microvillus effacement, and interruption of signal transduction. ExoS is a bifunctional protein having both GTPase-activating (GAP) and ADP-ribosyltransferase (ADPRT) functional domains. Comparisons of alterations in HT-29 cell function caused by P. aeruginosa strains that translocate ExoS having GAP or ADPRT mutations allowed the independent and coordinate functions of the two activities to be assessed. An E381A ADPRT mutation revealed that ExoS ADPRT activity was required for effects of ExoS on DNA synthesis and long-term cell rounding. Conversely, the R146A GAP mutation appeared to have little impact on the cellular effects of ExoS. While transient cell rounding was detected following exposure to the E381A mutant, this rounding was eliminated by an E379A-E381A ADPRT double mutation, implying that residual ADPRT activity, rather than GAP activity, was effecting transient cell rounding by the E381A mutant. To explore this possibility, E381A and R146A-E381A mutants were examined for their ability to ADP-ribosylate Ras in vitro or in vivo. While no ADP-ribosylation of Ras was detected by either mutant in vitro, both mutants were able to modify Ras when translocated by the bacteria, with the R146A-E381A mutant causing more efficient modification than the E381A mutant, in association with increased inhibition of DNA synthesis. Comparisons of Ras ADP-ribosylation by wild-type and E381A mutant ExoS by two-dimensional electrophoresis found the former to ADP-ribosylate Ras at two sites, while the latter modified Ras only once. These studies draw attention to the key role of ExoS ADPRT activity in causing the effects of bacterially translocated ExoS on DNA synthesis and cell rounding. In addition, the studies provide insight into the enhancement of ExoS ADPRT activity within the eukaryotic cell microenvironment and into possible modulatory roles that the GAP and ADPRT domains might have on the function of each other.
The opportunistic pathogen, Pseudomonas aeruginosa, causes serious infections in compromised individuals through the production of multiple virulence factors. ExoS has been implicated in bacterial virulence (21), but an understanding of its cellular mechanism of action has been complicated by its type III-mediated secretion, which requires contact between P. aeruginosa and target cells for ExoS translocation (45). Consistent with ExoS contributing to P. aeruginosa virulence, bacterial-eukaryotic coculture studies comparing the effects of P. aeruginosa strain 388 and its ExoS isogenic mutant on HT-29 epithelial cell function showed ExoS production to be associated with a decrease in DNA synthesis, long-term alterations in cell morphology, microvillus effacement, and a loss of the ability to readhere (34). Epithelial cell sensitivity to ExoS parallels the opportunistic nature of P. aeruginosa infection, with normal confluent epithelial monolayers being resistant to the effects of bacterially translocated ExoS, while compromised epithelial monolayers are sensitive to ExoS (11, 33).
ExoS is a bifunctional molecule containing an amino-terminal YopE-like domain that disrupts cytoskeletal structure (38) and a carboxy-terminal domain containing ADP-ribosyltransferase (ADPRT) activity (26). Transfection and transient expression of the region encoding N-terminal residues 1 to 234 of ExoS in Chinese hamster ovary (CHO) cells caused a disruption of actin filaments by affecting Rho family proteins that regulate cytoskeletal structure (38). Subsequent in vitro studies related this actin disruption to GAP activity located within the amino terminus of ExoS that is specific for the low-molecular-mass G (LMMG) proteins, Rho, Rac, and Cdc42 (18). In vitro analyses found the ADPRT activity of ExoS to target another family of LMMG proteins, the Ras family, with Ras, Rap, Rab, and Ral identified as preferred substrates (4, 5). Of these LMMG proteins, Ras (32) and, more recently, Ral (J. E. Fraylick, T. S. Vincent, J. C. Olson, Abstr. 100th Gen. Meet. Am. Soc. Microbiol. 2000, abstr. B113, p. 67, 2000) have been confirmed as in vivo substrates of ExoS ADPRT activity following bacterial translocation. The ADP-ribosylation of Ras by ExoS was shown to inhibit guanine nucleotide exchange factor (GEF)-mediated Ras GDP-to-GTP exchange in vitro, which in turn affects Ras–Raf-1 interaction in vivo (15, 20, 43). These data are consistent with inhibitory effects of ExoS on DNA synthesis relating, at least in part, to the ADP-ribosylation of cellular Ras, which leads to an interference of the Ras–Raf-1 proliferative pathway.
The identification of ExoS as a bifunctional protein brings into question the independent and coordinate roles of ExoS GTPase-activating (GAP) and ADPRT activities in the effects of ExoS on eukaryotic cell function. To assess the functional interplay of these two activities following bacterial translocation, ExoS with mutations in either GAP and/or ADPRT functions was translocated into HT-29 epithelial cells using a P. aeruginosa strain, PA103ΔexoUexoT (PA103ΔUT), that lacks production of known type III effector proteins (42). The studies found that ExoS ADPRT activity was required for severe effects of ExoS on cell function, including the decrease in eukaryotic cell DNA synthesis and long-term alterations in cell morphology. GAP function, alternatively, was found to contribute minimally to the effects of ExoS on cell morphology, but loss of GAP activity appeared to modulate the effects of ExoS ADPRT activity on cell function.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
P. aeruginosa strains used in these studies include the parental strain 388 (22); strain 388ΔexoS (388ΔS), an isogenic mutant that lacks production of ExoS (28); and strain PA103ΔexoUexoT::Tc (PA103ΔUT), a derivative of the strain PA103 from which the exoU and exoT genes were deleted (42). The relevant phenotypes of these strains are compared in Table 1. PA103ΔUT served as the host strain for the production and type III-mediated translocation of ExoS constructs shown in Table 2. All bacterial strains were stored in 10% skim milk at −70°C and grown in preparation for coculture in ExoS induction medium (TSBD-N) (22), as previously described (35), for 16 h. For culture with eukaryotic cells, bacteria were diluted based on the culture optical density at 590 nm to approximately 107 CFU/ml in McCoy's 5A tissue culture medium (Gibco-BRL, Gaithersburg, Md.), containing 0.6% bovine serum albumin (Sigma, St. Louis, Mo.) (McCoys-BSA). Bacterial dilutions were subsequently plated and counted to calculate the multiplicity of infection (MOI) for each experiment. An average MOI of 50 was used in these studies, with an MOI ranging from 10 to 100 previously being found to be optimal for examining the effects of ExoS on eukaryotic cell function and Ras modification (32, 34, 35).
TABLE 1.
P. aeruginosa strain phenotypes
| Strain | Phenotype
|
Reference | |||||
|---|---|---|---|---|---|---|---|
| Type III | ExoS | ExoT | ExoU | ExoY | ETA | ||
| 388 | + | + | + | − | + | − | 22 |
| 388ΔS | + | − | + | − | + | − | 28 |
| PA103ΔUT | + | − | − | − | − | + | 42 |
TABLE 2.
Domain function of ExoS plasmid constructsa
| Plasmid | GAP activity | ADPRT activity | ADPRT sp actb | Source or reference |
|---|---|---|---|---|
| pUCP | − | − | <0.01 | 40 |
| ExoS | + | + | 6.3 ± 0.5 | 28 |
| E381A | + | − | <0.01 | 31, 42 |
| R146A | − | + | 5.4 ± 0.4 | This study |
| R146-E381A | − | − | <0.01 | This study |
| E379-E381A | + | −/− | <0.01 | This study |
| R146A-E379A-E381A | − | −/− | <0.01 | This study |
See also Fig. 1.
Expressed as femtomoles of ADP-ribose transferred to SBTI per nanogram of ExoS (± the standard deviation where applicable).
Construction of ExoS mutants.
The pUCP vector (40), the pUCP vector containing the exoS gene cloned from strain 388 (28), and the pUCP vector encoding an E381A mutant form of ExoS with <0.02% wild-type ADPRT activity (31, 42) have been previously described and were kindly provided by Dara Frank (Medical College of Wisconsin, Milwaukee, Wis.). The R146A GAP and E379A-E381A ADPRT mutations were introduced into the PstI-BamHI fragment of the exoS gene cloned in the pUCP vector (45) using the QuikChange PCR-based site-directed mutagenesis system (Stratgene, La Jolla, Calif.). The primers for the R146A mutation were as follows: forward, 5′-CGGAGATGGGGCGCTAGCTTCGCTGAGCACCG-3′, and reverse, 5′-CGGTGCTCAGCGAAGCTAGCGCCCCATCTCCG-3′. For the E379A-E381A ADPRT double mutation, the primers were as follows: forward, 5′-CGAACTACAAGAATGCAAAAGCGATTCTCTATAACAAAG-3′, and reverse, 5′-CTTTGTTATAGAGAATCGCTTTTGCATTCTTGTAGTTCG-3′. The R146A-E381A double mutant was constructed using the R146A primers and the pUCP E381A exoS mutant as a template. Alternatively, the R146A-E379A-E381A mutant was constructed using the E379A-E381A primers and the pUCP R146A exoS mutant as template. PCR reaction mixtures contained 50 ng of plasmid DNA, a 25 μM concentration of each primer, a 25 μM concentration of each deoxynucleotide triphosphate, 5 μl of 10× Pfu reaction buffer, and 2.5 U of Pfu DNA polymerase (Stratagene). The mixtures were subjected to one denaturing cycle of 2 min at 95°C and then 18 cycles under the following conditions: a 30-s denaturing cycle at 95°C, followed by a 30-s annealing cycle at 60°C and a 12-min extension cycle at 72°C, and completed by a final extension cycle of 60 min at 72°C. The plasmid containing the mutated clone was electroporated into Escherichia coli DH5α electrocompetent cells (2). The transformed cells were selected for ampicillin resistance (75 μg/ml). Mutations were initially screened using restriction digest sites introduced into the design of the primers. The exoS mutant genes were subsequently sequenced to confirm the specific mutation and the absence of additional sequence aberrations.
The pUCP vectors encoding mutant forms of ExoS were transformed into the P. aeruginosa strain PA103ΔUT by electroporation using the protocol of Dennis and Sokol (7). Briefly, PA103ΔUT were grown to late-logarithmic phase and then electroporated with 150 ng of the mutated plasmid. Following a 1-h recovery in SOC medium (containing, in 500 ml, 10 g of Bacto-tryptone and 2.5 g of Bacto-yeast extract [Difco, Detroit, Mich.], 290 mg of NaCl, 93 mg of KCl, 1 g of MgCl2 · 6H2O, 600 mg of MgSO4, and 3.5 ml of 50% glucose), bacteria were plated onto carbenicillin selection medium (400 μg/ml). To confirm that colonies of the P. aeruginosa contained the correct ExoS mutant, plasmids were isolated from P. aeruginosa and electroporated back into DH5α, and diagnostic restriction digests were performed on reisolated plasmids.
Eukaryotic cell culture.
HT-29 colon carcinoma cells obtained from the American Type Culture Collection (ATCC HTB 38, Rockville, Md.) were maintained in McCoy's 5A medium, supplemented with 10% fetal bovine serum (Gibco-BRL) (McCoys-FBS) at 37°C in 5% CO2–95% air. In preparation for culture with bacteria, HT-29 cells were detached from the culture flask with 0.25% trypsin–1 mM EDTA (trypsin-EDTA) (Gibco-BRL), resuspended in McCoys-FBS, counted, and seeded at 105 cells/ml in 48- or 6-well culture plates or 100-mm dishes (Costar, Cambridge, Mass.). After 48 h, the cell culture medium was removed and replaced with McCoys-BSA containing 107 CFU of the indicated bacterial strain per ml, or no bacteria, and then cultured for 4 h and assayed for changes in cell function as described below.
Examination of the effects of ExoS on cell function. (i) Quantification of DNA synthesis.
Following a 4-h exposure to bacteria, HT-29 cells were washed with phosphate-buffered saline (PBS) and pulsed for 20 h with 1 μCi of [methyl-3H]thymidine (25 Ci/mmol; Amersham Life Sciences, Arlington Heights, Ill.) per ml in McCoys-FBS containing 200 μg of gentamicin (Sigma) and 100 μg of ciprofloxacin (Bayer, West Haven, Conn.) per ml (McCoys-FBS-GC), which was found to inhibit residual bacterial growth. DNA synthesis was quantified as previously described (35) and is reported as the percent [3H]thymidine incorporation relative to nonbacterial treated control cells.
(ii) Examination of cell morphology.
Transient (reversible) and long-term (irreversible) effects of bacterially translocated ExoS on HT-29 cell morphology were assessed by phase-contrast microscopy using a Zeiss Axioplan microscope, and images were obtained with a Spot 2e Digital Camera (Diagnostic Instruments, Inc., Sterling Heights, Mich.). Transient effects on morphology were examined immediately after the 4-h coculture period. Long-term effects on morphology were examined by removal of bacteria following a 4-h coculture period, washing the cells with McCoys-FBS-GC, and allowing cells to recover for 20 h in McCoys-FBS-GC.
Confirmation of ExoS mutant production and secretion.
To examine ExoS production by PA103ΔUT strains containing mutant forms of ExoS, a 1-ml aliquot of bacteria grown in TSBD-N for coculture studies was centrifuged at 7,500 × g for 10 min. Secreted proteins, in 4 μl of culture supernatant, were resolved by sodium dodecyl sulfate (SDS)–7.5% polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, Mass.) by the methods of Laemmli (29) and Towbin et al. (41), respectively. ExoS immunoblots were developed using a rat antiserum produced in our laboratory against the native form of ExoS (35), followed by peroxidase-conjugated goat anti-rat antibody (Sigma), and visualized by enhanced chemiluminescence (ECL; Amersham, Arlington Heights, Ill.). ExoS was quantified by densitometric analysis of immunoblot images within the linear concentration range, and values obtained using the NIH image version 1.6 program were related to a previously quantified ExoS standard. The ADPRT activity of ExoS mutants was determined in TSBD-N culture supernatants of PA103ΔUT containing the respective ExoS construct using the artificial substrate, soybean trypsin inhibitor (SBTI; Sigma) as the ADP-ribose acceptor, as previously described (26), and quantified relative to an ExoS standard. The specific ADPRT activity of ExoS constructs was calculated as the ratio of ExoS ADPRT activity to densitometric calculations of ExoS protein concentration and is expressed as femtomoles of ADP-ribose transferred per minute per nanogram of ExoS protein in culture supernatants.
Analysis of ADP-ribosylation of Ras by ExoS mutants in vitro and in vivo. (i) In vitro.
ADP-ribosylation of H-Ras was examined by using the pXCR plasmid as the source of the H-Ras gene coding sequence, kindly provided by Larry Feig (Tufts University School of Medicine, Boston, Mass.) (9). H-Ras was expressed and purified according to previously published procedures (8, 43). The ADP-ribosyltransferase reactions were run at 20°C for 1 h in 0.2 M Tris-acetate (pH 6.0)–1 mM MgCl2–10 mM NAD–0.2 μM 14-3-3ξ (Upstate Biotechnology, Lake Placid, N.Y.), using 0.5 μM H-Ras as a substrate and 30 μl of culture supernatant from PA103ΔUT strains producing the indicated ExoS construct. Reactions were stopped with 4× Laemmli sample buffer and heating at 95°C for 5 min, resolved by SDS–12% PAGE, and blotted onto PVDF membranes. To detect Ras, membranes were probed with mouse anti-H-Ras LA069 and mouse anti-pan Ras LA045 antibodies (Quality Biotech, Camden, N.J.), followed by horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin G (IgG; Jackson ImmunoResearch, West Grove, Pa.), and visualized by ECL.
(ii) In vivo.
ADP-ribosylation of cellular Ras by bacterially translocated ExoS was assessed by SDS-PAGE and two-dimensional electrophoresis (2DE) based on the altered mobility of Ras after exposure of HT-29 cells to the indicated bacterial strains for a 4- or 5-h coculture period, as previously described (32, 43). Briefly, following the coculture period, Ras was immunoprecipitated from cell extracts using monoclonal Y13-259 Ras antibody (ATCC) and protein G-agarose (Sigma). For SDS-PAGE analyses, immunoprecipitates were resuspended in Laemmli sample buffer, resolved by SDS–12% PAGE, and transferred to PVDF membranes. For 2DE analyses, immunoprecipitates were solubilized in 2DE rehydration buffer (8 M urea, 2% Triton X-100, 0.3% dithiothreitol [DTT], 1.5% Pharmalytes [Pharmacia, Piscataway, N.J.]). Proteins were separated by pI on Immobiline pH 4 to 7 gradient gel strips (Pharmacia) by focusing for 2 h at 100 V, followed by 3 h at 1,000 V, and then 16 h at 3,500 V. Strips were equilibrated for 10 min with SDS equilibration buffer (50 mM Tris-Cl, pH 6.8; 6 M urea; 30% glycerol; 2% SDS) containing 20 mg of DTT per ml, followed by 10 min with SDS equilibration buffer containing 25 mg of iodoacetamine (Sigma) per ml. Proteins were resolved in the second dimension on SDS–12% polyacrylamide gels and transferred to PVDF membranes. Immunoblotted Ras in SDS-PAGE and 2DE analyses was detected by using mouse anti-H-Ras LA069 and mouse anti-pan Ras LA045 antibodies, as described above.
Cellular fractionation and analysis of bacterial translocation of ExoS mutant proteins.
HT-29 cells, plated in 100-mm dishes, were cocultured with PA103ΔUT-ExoS mutant strains for 4.5 h and fractionated into cytosolic and membrane components using a modification of the methods of Kenny and Finlay (24). Briefly, bacteria were removed following the coculture period, and HT-29 cells were rinsed twice with 1 ml of PBS, scraped into 1 ml of PBS, and then washed twice with 1 ml of PBS, with centrifuging at 400 × g for 5 min between washes. Cells were resuspended in 100 μl of saponin buffer (0.2% saponin, 100 mM NaCl, 250 mM sucrose, 5 mM EDTA, 100 μg of ciprofloxacin and the protease inhibitors per ml, phenylmethylsulfonyl fluoride [1 mM], and 10 μg of leupeptin and 10 μg aprotinin per ml [Sigma]) and incubated on ice for 10 min. Cells were then passed through a 28-gauge needle on a 1-ml syringe seven times and centrifuged at 16,000 × g for 20 min, and the supernatant (cytosolic fraction) was removed. The pellet was resuspended in 100 μl of 1% Triton X-100–100 mM NaCl–5 mM EDTA, plus ciprofloxacin and protease inhibitors, and vortexed for 15 s every 5 min for 20 min to solubilize the membrane proteins (TX-100 fraction). The Triton X-100 insoluble pellet that remained (pellet fraction) contained adherent bacteria and host cell cytoskeleton and nuclei. For analysis of ExoS protein in the cytosolic or TX-100 fractions, 50 μl of each sample was mixed with 4× Laemmli sample buffer and heated at 95°C for 5 min. The pellet fraction was resuspended in 100 μl of 1× Laemmli sample buffer and heated as described above. A 50-μl volume of each fraction was resolved on SDS–15% polyacrylamide gels and immunoblotted for ExoS as described above. To directly compare ExoS protein to functional substrate modification, ExoS blots were reprobed for Ral, another in vivo target of ExoS ADPRT activity, using a monoclonal anti-RalA monoclonal antibody (Transduction Laboratories, Lexington, Ky.), followed by HRP-conjugated anti-mouse IgG, and then visualized by ECL. ADPRT activity in remaining portions of cytosolic and TX-100 fractions was quantified in vitro using SBTI as a substrate, as described above.
RESULTS
Role of ADPRT activity in the effects of ExoS on HT-29 cell function.
The identification of ExoS as a bifunctional toxin (38) adds another degree of complexity to an understanding of the cellular mechanism of action of ExoS. To determine the role of ExoS ADPRT activity in previously identified cellular effects of bacterially translocated ExoS, five strains of P. aeruginosa were compared for their effects on HT-29 cell function. These strains included (i) the prototype ExoS-producing strain, 388, and (ii) its non-ExoS-producing, isogenic mutant, 388ΔS, previously used to define ExoS-specific effects on cell function (34, 35); (iii) a derivative of strain PA103, PA103ΔUT, that does not produce type III effector proteins ExoT, ExoU, ExoY, or ExoS, to which the ExoS structural gene was introduced on the pUCP plasmid (PA103ΔUT-ExoS); (iv) PA103ΔUT containing the pUCP plasmid encoding the E381A mutant form of ExoS that has 0.02% of wild-type ADPRT activity (31) (PA103ΔUT-E381A); and (v) PA103ΔUT containing the pUCP vector control (PA103ΔUT-pUCP). The phenotypes of these strains and ExoS ADPRT activity in culture supernatants following growth in ExoS induction medium are presented in Tables 1 and 2 (see also Fig. 1).
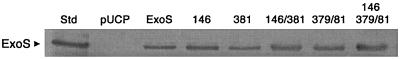
FIG. 1.
ExoS cross-reactive protein produced by plasmid constructs. An ExoS immunoblot of culture supernatants from strains expressing the indicated construct is shown. (See also Tables 1 and 2).
(i) Proliferation.
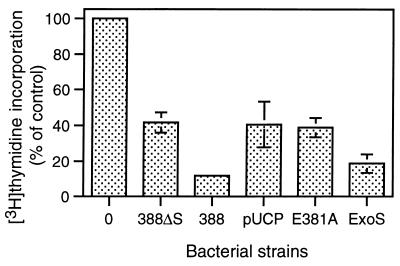
Previous studies examining ExoS-specific effects on eukaryotic cell function found the ExoS-producing strain 388 to cause a greater inhibition of DNA synthesis than strain 388ΔS (35). This suggested that bacterial translocation of ExoS caused an alteration in signal transduction pathways affecting cell proliferation. To examine the role of the ADPRT activity of ExoS in its effects on cell proliferation, strain PA103ΔUT expressing ADPRT-active ExoS or E381A ADPRT-defective ExoS was cocultured with HT-29 epithelial cells and compared with strains 388 and 388ΔS for their differential effects on DNA synthesis. As shown in Fig. 2, [3H]thymidine incorporation in cells cultured with strain PA103ΔUT-ExoS was inhibited by 81.3% ± 5.3%, which was similar to the 88.3% ± 1.3% inhibition of DNA synthesis caused by strain 388. In comparison, bacterial translocation of the E381A ADPRT mutant ExoS caused a 61.3% ± 5.3% decrease in DNA synthesis, similar to the 58.4% ± 5.7% and 59.4% ± 12.6% decreases caused by strains 388ΔS and the PA103ΔUT-pUCP control, respectively. Previous studies indicated that the effects of non-ExoS-producing strains on DNA synthesis related to the production of non-type III-secreted factors by P. aeruginosa (34). Relative to the effects of ExoS on cell function, these results indicate that ExoS ADPRT activity is required for the inhibition of DNA synthesis caused by bacterially translocated ExoS.
FIG. 2.
Inhibition of DNA synthesis associated with ExoS ADP-ribosyltransferase activity. HT-29 cells were seeded at 105 cells/ml and grown for 48 h to ∼40% confluency. Cell culture medium was then removed and replaced with McCoys plus 0.6% BSA medium containing no bacteria (“0”) or 107 CFU of strain 388 (388), non-ExoS-producing strain 388ΔS (388ΔS), or of PA103ΔUT strains containing the pUCP control (pUCP), the pUCP-ExoS E381A ADPRT mutant (E381A), or the pUCP-ExoS (ExoS) vectors. Following a 4-h coculture period, bacteria were removed and replaced with medium containing [3H]thymidine and antibiotics to inhibit further bacterial growth and then assayed for DNA synthesis after 20 h. The results are expressed as the percent [3H]thymidine incorporation relative to nonbacterially treated control cells, and the means and standard errors of the assays performed in triplicate from three independent studies are shown.
(ii) Morphology.
Since transfection studies found the amino-terminal GAP activity of ExoS to alter cytoskeletal structure (38), we next examined how bacterially translocated ExoS that maintained GAP activity but had E381A mutant ADPRT activity would affect HT-29 cell morphology. Severe cell rounding was observed following a 4-h exposure to the ExoS-producing strains, PA103ΔUT-ExoS and 388, compared to cells treated with no bacteria or the PA103ΔUT-pUCP control (Fig. 3, 4 h). Slightly less cell rounding was observed following exposure to strains 338ΔS and PA103ΔUT-E381A, both of which retain GAP activity for Rho, Rac, and Cdc42. Strain 338ΔS maintains GAP function through its expression of ExoT (27), while PA103ΔUT-E381A retains GAP function in the E381A mutant form of ExoS.
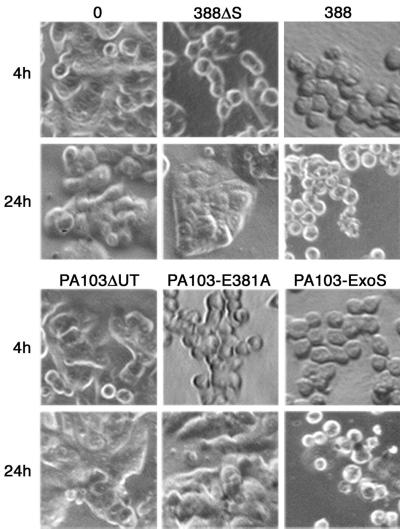
FIG. 3.
Effects of ExoS ADP-ribosyltransferase activity on transient and long-term alterations in cell morphology. HT-29 cells were grown and cocultured as described in Fig. 2 with no bacteria (“0”) strains 388 and 388ΔS, or PA103ΔUT strains containing the indicated vector. Cells were examined for alterations in morphology by phase-contrast microscopy following removal of bacteria after a 4-h coculture period (4h). Long-term effects on morphology were assessed by culturing cells for an additional 20 h after removal of bacteria in medium containing antibiotics (24h). Transient cell rounding was detected following coculture with strains 388ΔS and PA103ΔUT-E381A, while severe cell rounding persisted for 24 h in cells cocultured with ADPRT-active ExoS produced by strains 388 and PA103ΔUT-ExoS. The images shown are representative of three or more independent studies.
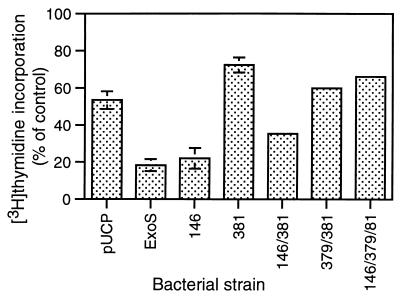
FIG. 4.
Inhibition of HT-29 cell DNA synthesis caused by ExoS GAP and ADPRT mutants. HT-29 cells were seeded and cocultured with the indicated bacterial strains for 4 h and assayed for DNA synthesis as described in Fig. 2. Strains examined in parallel in these studies include PA103ΔUT containing the pUCP vector control (pUCP), pUCP-ExoS (ExoS), pUCP-ExoS with an R146A GAP mutation (146), pUCP-ExoS with an E381A ADPRT mutation (381), pUCP-ExoS with both an R146A GAP and an E381A ADPRT mutation (146/381), pUCP-ExoS with an E379A-E381A ADPRT double mutation (379/381), and pUCP-ExoS with an R146A GAP and E379A-E381A double ADPRT mutation (146/379/381). Results are expressed as the percentage of [3H]thymidine incorporation relative to nonbacterially treated control cells, and the means and standard deviations of the assays performed in triplicate from a representative experiment of three independent studies are shown.
While ExoS-producing strain 388, as well as non-ExoS-producing strain 388ΔS, can cause cytoskeletal alterations, ExoS-associated morphological alterations were previously found to be irreversible, while those caused by strain 388ΔS were reversible following removal of bacteria (34). To assess the role of ExoS ADPRT activity in irreversible (long-term) alterations in HT-29 cell morphology, cells were allowed to recover from a 4-h exposure to bacteria by culturing them in medium containing antibiotics for an additional 20 h. As shown in Fig. 3 (24 h), cells exposed to strains 388ΔS and PA103ΔUT-E381A were able to regain normal morphology upon the removal of bacteria. However, a high percentage of cells exposed to strain 388 and PA103ΔUT-ExoS remained rounded after the 20-h recovery period. These studies support the idea that long-term cell rounding caused by bacterially translocated ExoS requires ExoS ADPRT activity. Alternatively, reversible (transient) cell rounding observed following a 4-h exposure to strains 388ΔS and PA103ΔUT-E381A, which lack ExoS ADPRT activity, was presumed to relate to the GAP activity expressed by these strains.
Role of the GAP activity in the effects of ExoS on HT-29 cell function.
Transient-transfection studies of Pederson et al. (38) confirmed the ability of N-terminal residues 1 to 234 of ExoS to cause morphological alterations, independent of ADPRT activity. A GAP activity for Rho, Rac, and Cdc42 was subsequently localized to residues 90 to 234 of ExoS, and Arg146 within this region was found to be integral to GAP activity in vitro and to GAP-associated CHO cell rounding in transfection studies (18, 37). Based on these studies, we constructed an R146A mutant of ExoS, with or without ADPRT mutations, to examine the independent and coordinated role of GAP activity in the effects of bacterially translocated ExoS on HT-29 cell function. These ExoS constructs and the mean specific activity of each construct (ADPRT activity per nanogram of ExoS cross-reactive protein) are shown in Table 2. The ExoS cross-reactive protein secreted by each of the mutants when cultured in vitro is shown in Fig. 1.
(i) Proliferation.
When strains expressing the R146A and double R146A-E381A mutant forms of ExoS were examined in parallel for their effects on HT-29 cell DNA synthesis, the R146A mutation appeared to have minimal impact on ExoS inhibition of DNA synthesis. PA103ΔUT expressing the R146A mutant caused a 77.9% ± 5.6% inhibition of DNA synthesis which compared closely to the 81.6% ± 3.3% inhibition caused by PA103ΔUT-ExoS (Fig. 4). Unexpectedly, when an R146A GAP mutation was coupled with the E381A ADPRT mutation, a consistently greater inhibition of DNA synthesis was detected than that caused by the single E381A mutation. This difference is represented in Fig. 4 by a 64.5% ± 1.2% and 27.5% ± 4.0% inhibition in DNA synthesis caused by the R146A-E381A and E381A mutants, respectively. The increased inhibition did not appear to relate to different rates of production of the ExoS mutants, as supported by their comparable levels in bacterial culture supernatants (Fig. 1). The results from these studies indicate that the GAP function of ExoS, as defined by the R146A mutation, is not required for inhibitory effects of ExoS on DNA synthesis. However, these studies also provide evidence that coupling a GAP mutation with an E381A ADPRT mutation somehow modulates inhibitory effects of the E381A mutation on DNA synthesis.
(ii) Morphology.
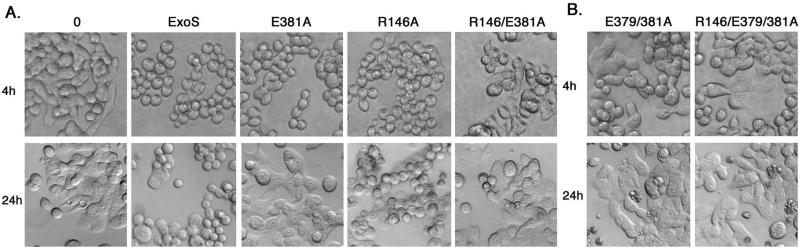
Transient and long-term effects of the GAP-ADPRT ExoS mutants on HT-29 cell morphology were examined in parallel and are shown in Fig. 5A. Cell rounding was apparent following a 4-h exposure to PA103ΔUT expressing ExoS, E381A, and R146A forms of ExoS, but rounding was lessened following exposure to the R146A-E381A ExoS mutant. While the short-term cell rounding caused by the E381A mutant was predicted to relate to its intact GAP function, the finding that this rounding was also evident in R146A mutant treated cells indicates that early effects on cell morphology do not require GAP function. In examining long-term effects of ExoS GAP mutants on cell morphology, 20 h after the removal of bacteria, cell rounding persisted to a greater extent in cells treated with ExoS and the R146A mutant, with cell rounding remaining minimal in cells treated with the E381A or R146A-E381A mutant. The studies indicate that ExoS ADPRT activity, but not GAP activity, is required for long-term effects of ExoS on cell morphology. In addition, the finding that some rounding was evident following a 4-h exposure to the double R146A-E381A mutant implies that non-GAP, non-E381 related functions of ExoS are also affecting cell morphology.
FIG. 5.
Effects of ExoS GAP and ADPRT mutations on transient and long-term alterations in cell morphology. HT-29 cells were grown and cocultured with no bacteria (“0”) or strain PA103ΔUT containing the indicated ExoS construct as described in Fig. 4 and assayed for transient (4h) or long-term (24h) effects on cell morphology, as described in Fig. 3. In (A) Transient cell rounding was detected following coculture with strains containing ExoS, E381A, and R146A, with less rounding caused by the R146A-E381A GAP-ADPRT mutant. Cell rounding persisted at 24 h in cells cocultured with ADPRT-active ExoS and the R146A mutant. (B) Comparisons of morphological alterations caused by the E379A-E381A double ADPRT and R146A-E379A-E381A GAP-double ADPRT mutants found minimal transient or long-term cell rounding. The images shown are from analyses performed in parallel and are representative of multiple independent studies.
Comparison of the E381A and E379A-E381A ADPRT double mutant on effects of ExoS on HT-29 cell function.
Studies of Radke et al. (39) showed two glutamic acid residues, E379 and E381, to contribute to the ExoS ADPRT reaction. The E381 residue functions as the primary catalytic residue, while the E379 residue contributes to the transfer of ADP-ribose to the target protein. To assess how the E379 residue might contribute to the toxicity caused by the R146A-E381A mutant, an E379A-E381A double ADPRT ExoS mutant was constructed, with or without the R146A mutation, and analyzed for its effects on HT-29 cell function. DNA synthesis assays found that the introduction of an E379A-E381A ADPRT mutation in combination with the R146A mutation abrogated the inhibitory effects of the R146A/E381A mutation on DNA synthesis. The R146A-E379A-E381A triple mutant caused a 33.7% ± 1.6% inhibition of DNA synthesis, closely approaching the 27.5% ± 4.0% inhibition caused by the E381A mutant (Fig. 4). Examination of transient (4-h) and long-term (24-h) effects on HT-29 cell morphology found the E379A-E381A ADPRT mutant and the R146A-E379A-E381A GAP-ADPRT mutant to have the least effect on cell rounding (Fig. 5B). Notable in these analyses was the greater rounding caused by the E381A mutant after 4 h compared to that caused by the E379A-E381A mutant. This suggests that the activity of the E379 residue contributes, either directly or indirectly, to transient cell rounding caused by the E381A mutant. Together, the results redirect attention to the role of the E379 residue in the observed effects of the R146A-E381A mutant on cell morphology and inhibition of DNA synthesis.
Examining the mechanism for the effects of ExoS mutants on cell function. (i) Comparison of the in vitro and in vivo ADPRT activity of GAP and ADPRT ExoS mutants.
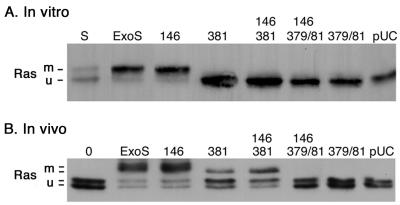
While the studies described above confirmed a requirement of ExoS ADPRT activity for severe effects of ExoS on DNA synthesis and morphology, constructs containing both GAP and ADPRT mutations provided evidence that these two domains might be able to modulate the function of each other. To gain further understanding of how the R146A mutation in the GAP domain of ExoS might be affecting its ADPRT activity, ExoS mutant constructs were compared for their ability to modify Ras, a cellular substrate of ExoS ADPRT activity, both in vitro and in vivo following bacterial translocation. As shown in Table 2, ExoS constructs having a single E381A or double E379A-E381A ADPRT mutation showed undetectable levels of ADPRT activity in in vitro assays using SBTI as an ADP-ribose acceptor. Similarly, as shown in Fig. 6A, when bacterial culture supernatants containing the mutant forms of ExoS were assayed for their ability to ADP-ribosylate Ras in vitro, as detected by a shift in the molecular mass of Ras (5), ExoS and the R146A mutant were able to ADP-ribosylate Ras, whereas ADPRT mutant forms of ExoS were not. In vivo, as in vitro, cellular Ras has been found to exhibit a shift in molecular mass upon ADP-ribosylation by bacterially translocated ExoS (32). When bacterially translocated ExoS with GAP and/or ADPRT mutations was examined for its ability to ADP-ribosylate cellular Ras, Ras was predictably modified following coculture with strains expressing ADPRT active ExoS and the R146A mutant (Fig. 6B). However, a shift in the mass of Ras was also observed following exposure to strains expressing the E381A and E381A-R146A mutant forms of ExoS. This shift was not detected following coculture with strains in which both the E379 and the E381 ADPRT residues were mutated nor upon culture with the pUCP vector control. The data support the idea that the E381A mutant retains residual ADPRT activity that is able to modify Ras in an in vivo setting but unable to do this under in vitro assay conditions.
FIG. 6.
ADP-ribosylation of Ras by ExoS GAP and ADPRT mutants in vitro and in vivo. (A) In vitro. ADP-ribosylation of purified recombinant H-Ras was examined in reaction mixtures containing 0.5 μM H-Ras, 10 mM NAD, 0.2 μM 14-3-3ξ, and 30 μl of culture supernatant from PA103ΔUT strains producing the indicated ExoS construct. Reactions were allowed to proceed for 1 h at room temperature, stopped with 4× Laemmli buffer and heating at 95°C, resolved by SDS–12% PAGE, blotted onto PVDF membrane, probed with mouse anti-H-Ras LA069 and anti-pan Ras LA045 antibodies, and visualized by ECL. A reaction containing purified ExoS (S) was included as a positive control. (B) In vivo. ADP-ribosylation of cellular Ras was examined following a 5-h coculture of HT-29 cells with PA103ΔUT containing the indicated ExoS plasmid construct. Following removal of bacteria, cells were lysed in TBS-TDS (10 mM Tris [pH 7.4], 140 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS), and Ras was immunoprecipitated from cell extracts using Y13-259 Ras antibody and protein G-agarose. Immunoprecipitates were resolved by SDS–12% PAGE, transferred to PVDF membranes, and Ras was detected and visualized as in panel A. The mobility of modified (m) and unmodified (u) Ras is indicated. The two bands observed for unmodified Ras in vivo represent the different Ras species, while the two bands observed for modified Ras in vivo reflect differences in the efficiency of Ras modification.
(ii) Analysis of Ras modification by ExoS mutants in vivo.
In repeated analyses, using coculture periods varying from 4 to 6 h, ExoS and the R146A mutant were found to consistently ADP-ribosylate Ras more efficiently than the E381A and R146A-E381A mutants. This is evident in Fig. 6B by the increased intensity of the modified Ras bands caused by ExoS and the R146A mutant and by the slightly faster mobility of Ras modified by the E381A and R146A-E381A mutants. Also notable in Fig. 6B is the increased intensity of the modified Ras band in cells exposed to the R146A-E381A mutant compared to the E318A mutant, which is consistent with the former being able to modify Ras more efficiently than the latter. Quantification of the intensity of modified Ras bands in four independent studies found the modification of the R146A-E381A mutant to be (1.8 ± 0.4)-fold more efficient than that of E381A.
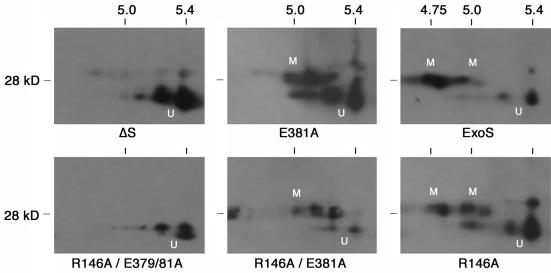
To gain further understanding of ADP-ribosylation events leading to the differences in Ras modification detected by SDS-PAGE, Ras was analyzed by 2DE following bacterial translocation of the ExoS mutants. Unmodified Ras focuses as two major isoforms by 2DE, representing its two major posttranslationally modified isoforms. This is shown in Fig. 7 in the ΔS and R146A-E379A-E381A panels and is the same as that observed in untreated, control cells (not shown). Exposure to the R146A-E381A and E381 mutants resulted in a ∼3-kDa shift in the mass of Ras which was associated with a single pI shift of ∼0.25 U. This combined shift in mass and pI of Ras is consistent with the transfer of a single ADP-ribose moiety to Arg41 on Ras (13, 43). Differences in the efficiency of Arg41 modification by the R146A-E381A and E381A mutant were also evident in the relative increase in intensity of modified Ras versus that of unmodified Ras, when Ras modification by the two mutants was compared. In examining Ras modification by PA103ΔUT expressing ExoS or the R146A mutant, the same 3-kDa–0.25-pI shift in Ras was detected as for the R146A-E381A and E381A mutants, but this was accompanied by a second ∼0.25-pI shift in Ras associated with a minimal change in mass. This double modification of Ras is consistent with that previously reported for the transfer of ADP-ribose moieties to both Arg41 and Arg128 on Ras (14, 43). These studies indicate that the less-efficient Ras modification caused by bacterially translocated E381A or R146A-E381A mutants compared to ExoS relates to their ability to transfer an ADP-ribose moiety to the preferred Arg41 site of ADP-ribosylation on H-Ras but not to the secondary, Arg128 site of ADP-ribosylation. The finding that this initial transfer is more efficient in the R146A-E381A than E381A mutant implicates the potential for the GAP region of ExoS to modulate this preferred ADPRT activity of ExoS. Conversely, these studies support the idea that both the E379 and the E381 residues are required for the double ADP-ribosylation of Ras by ExoS and that the combined loss of E379 and E381 activity results in abrogation of Ras modification.
FIG. 7.
2DE analysis of the in vivo ADP-ribosylation of Ras by ExoS GAP and ADPRT mutants. Following a 5-h coculture of HT-29 cells with strain PA103ΔUT producing the indicated ExoS protein, Ras was immunoprecipitated from cell extracts, as in Fig. 6B. Ras immunoprecipitates were solubilized in 2DE rehydration buffer, as described in Materials and Methods, and focused on Immobiline pH 4 to 7 gradient gel strips. Strips were equilibrated in SDS equilibration buffer, proteins were resolved in the second dimension on SDS–12% polyacrylamide gels and transferred to PVDF membranes, and Ras was detected as in Fig. 6. The mobility of unmodified (U) and modified (M) Ras is indicated. Multiple spots represent the different Ras isoforms. pI and molecular mass markers (in kilodaltons [kD], are indicated above and to the left of each panel, respectively.
(iii) Analyses of ExoS mutant translocation.
To gain further understanding of cellular processes that might contribute to differences in substrate modification by ExoS mutants, the translocation and localization of ExoS mutant constructs in HT-29 cells was examined in cell fractionation studies. In previous studies examining the translocation of ExoS into HT-29 cells by ExoS producing strain 388, we were unable to detect ExoS protein in S-100 cytosolic and P-100 plasma membrane fractions by immunoblot analysis, although ExoS ADPRT activity was detected in both fractions (unpublished data). Several laboratories, including those studying Pseudomonas sp. (16, 37), have now been able to detect type III effector proteins in the cytosol of eukaryotic cells following bacterial translocation. We therefore used methodologies from these laboratories to examine whether differences in the efficiency of membrane translocation of ExoS GAP and ADPRT mutants might contribute to their differences in substrate modification.
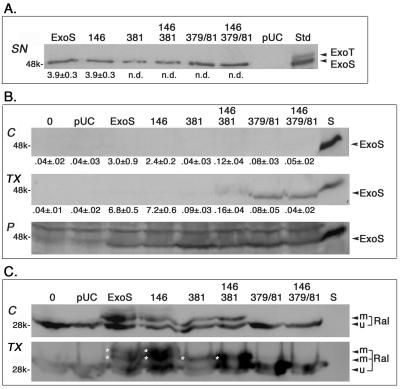
Multiple fractionation approaches involving mechanical (17, 37), saponin or digitonin (24, 25), and Triton X-100 (16) cell-permeabilizing procedures were used to examine ExoS translocation to the cytosol. Essentially the same results were obtained in all studies, which are represented in Fig. 8. Here, HT-29 cells were treated with 0.2% saponin following coculture with bacteria separated into soluble (cytosolic) and pellet fractions by centrifugation at 16,000 × g, and membrane proteins were solubilized from the pellet using 1% Triton X-100. This resulted in cytosolic, TX-100 membrane-soluble and -insoluble pellet fractions. Figure 8A shows the levels ExoS products and in vitro ADPRT activity of bacterial culture supernatants, prior to the processing of bacteria and addition to HT-29 cells. While all bacteria were able to produce ExoS product, Fig. 8B shows (i) the lack of detection of any of the ExoS products in the cytosolic fraction, although ExoS ADPRT activity was detected; (ii) the detection of ExoS ADPRT-inactive products, particularly those with a double E379A-E381A mutation, in the Triton X-100 membrane-soluble fraction, and (iii) the detection of all ExoS products in the insoluble pellet fraction. To assess the direct relationship between the ExoS product and ExoS function, the ExoS blot (Fig. 8B) was reprobed for the in vivo ExoS substrate, Ral (Fig. 8C). Evident in the Ral blot is the overloading of samples in an effort to detect ExoS and the relative levels of Ral and efficiency of Ral modification by the respective ExoS constructs in the cytosolic and TX-100 membrane soluble fractions. The conclusions drawn from these studies are as follows. (i) Although ADPRT-active ExoS and evidence of its functional modification of Ral were detected in the cytosolic faction, the ADPRT-active protein was undetectable. (ii) ADPRT-inactive mutant forms of ExoS were detectable in the membrane soluble fraction, implying that mutations in ADPRT activity resulted in the stabilization or retention of ExoS in the cell membrane. (iii) The detection of ExoS product in the insoluble pellet fraction of all constructs, which includes adherent bacteria (25), confirms that ExoS product is being produced by all strains in association with HT-29 cells. (iv) In examining Ral modification, we detected more-efficient Ral modification by ADPRT-active ExoS or R146A constructs then with the E381A or R146A-E381A constructs (two versus one modified forms), and more-efficient Ral modification was detected in the TX-100 membrane fraction.
FIG. 8.
Monitoring the translocation ExoS mutant protein and ADPRT activity in HT-29 cells. HT-29 cells, plated in 100-mm dishes, as described in Fig. 2, were cocultured with PA103ΔUT-ExoS mutant strains for 4.5 h, permeabilized with 0.2% saponin, and then fractionated into cytosolic and membrane components using a modification of the method of Kenny and Finlay (24), as detailed in Materials and Methods. The three fractions obtained include (i) the cytosolic fraction (C), which contains cytosolic, microsomal, and plasma membrane proteins; (ii) the TX-100 fraction (TX), which contains membrane-solubilized proteins; and (iii) the insoluble pellet fraction (P), which includes adherent bacteria and host cell cytoskeleton and nuclei. (A) Analysis of ExoS in bacterial culture supernatants. A 10-μl volume of TSBD-N culture supernatants of the indicated bacterial strain was resolved on SDS–15% polyacrylamide gels and immunoblotted for ExoS or assayed for ExoS ADPRT activity, as in Table 1, prior to the processing of bacteria and coculture with HT-29 cells. An ExoS-ExoT standard (Std), quantified to have 50 ng of ExoS protein, was included in the immunoblot. (B) Analysis of ExoS in HT-29 cell fractions. A 50-μl volume of cytosolic (C), TX-100 (TX), or pellet (P) fractions of HT-29 cells, following coculture with the indicated bacterial strains, was resolved on SDS-PAGE and immunoblotted for ExoS. A 10-μl volume of the respective fraction was assayed for ExoS ADPRT in parallel with culture supernatants assayed in panel A. A 20-μl volume of PA103-ExoS culture supernatant (S) was included in immunoblot analyses as a positive control. (C) Analysis of Ral modification in HT-29 cell fractions. To directly compare ExoS protein levels to functional substrate modification, ExoS blots were reprobed for Ral, using a monoclonal anti-RalA antibody (Transduction Laboratories), followed by HRP-conjugated anti-mouse IgG, and then visualized by ECL. Asterisks identify modified Ral bands. The mean ± the standard deviation of the ExoS ADPRT activity of the respective samples is indicated below the immunoblots in panels A and B and is expressed as femtomoles of ADP-ribose transferred per minute per microliter of sample. ExoS, ExoT, and the mobility of modified (m) and unmodified (u) Ral are labeled, as are the molecular mass references.
DISCUSSION
Bacterially translocated ExoS has been found to exert complex and diverse effects on HT-29 epithelial cell function (34), implying that multiple signal transduction pathways are being affected by ExoS. While the precise cellular mechanism of action of ExoS remains unknown, its complex effects on cell function are likely influenced by its bifunctional mechanism of action, with ExoS including a GAP activity within its amino terminus (18), and an ADPRT activity within its carboxy terminus (26). We were able to explore the independent and coordinate functions of ExoS GAP and ADPRT activities in the effects of bacterially translocated ExoS on epithelial cell function relying on previous studies that identified Arg146 as integral to the GAP function of ExoS (18, 37) and Glu381 as integral to ExoS ADPRT function (31). The effects of mutations in GAP and/or ADPRT activity on the effects of ExoS on cell function were then assessed and differentiated from each other and other P. aeruginosa type III effector proteins by using strain PA103ΔUT to translocate ExoS into HT-29 cells.
When bacterially translocated E381A ADPRT mutant ExoS (having an in vitro specific ADPRT activity of <0.01 fmol min−1 ng−1) was evaluated relative to previously defined effects of ExoS on DNA synthesis and morphology, the cellular effects observed were much like those caused by the non-ExoS-producing strain, 388ΔS. The primary cellular alteration attributed to the PA103ΔUT-E381A mutant was transient cell rounding, from which the cells were able to recover following the removal of bacteria. Similar transient morphological alterations caused by strains PA103ΔUT-E381A and 388ΔS were predicted to relate to their type III-mediated translocation of functionally similar GAP activities which target the cytoskeletal regulatory proteins, Rho, Rac, and Cdc42 (18, 27). The role of GAP activity in transient effects on cell morphology was subsequently directly tested in coculture studies that examined the effects of a bacterially translocated R146A GAP mutant form of ExoS. Transient, reversible cell rounding caused by the R146A mutant form of ExoS was found to be as severe as that caused by ExoS, indicating that the loss of GAP activity had no obvious impact on the effect of ExoS on transient cell rounding. However, effects on transient cell rounding were lessened when HT-29 cells were cocultured with PA103ΔUT expressing an R146A-GAP–E381A-ADPRT mutant form of ExoS, implying that effects of ExoS GAP activity on cell morphology could be recognized when examined in conjunction with an ADPRT-inactive form of ExoS. This premise was subsequently challenged when an E379A-E381A double ADPRT mutant was constructed which abrogated transient effects on cell rounding in the presence or absence of an R146A mutation. These results would be consistent with the E379 residue of ExoS being involved either directly or indirectly in transient rounding caused by the E381A mutant, which draws into question the role of GAP function in transient effects of ExoS on cell morphology.
The lack of detectable effects of bacterially translocated ExoS GAP activity on cell morphology is notable relative to previously detectable effects of the N-terminal GAP domain on cell morphology in transfection studies (38). Recent three-dimensional structure analysis of the complex between the amino-terminal GAP domain of ExoS and Rac also support the idea that ExoS, like other GAPs, can downregulate Rac using an arginine finger, Arg146, to stabilize the transition state of the GTPase reaction (44). One explanation for our inability to detect an alteration in cell morphology by ExoS GAP when bacterially translocated is that the cellular targeting specificity dictated by this mode of internalization may limit its direct effect on the cell structure. It should also be noted, relative to an understanding of the effects of ExoS GAP activity on cell function, that our studies were limited to HT-29 epithelial cells and did not explore previously identified effects of ExoS on macrophage phagocytosis (12) or bacterial invasion (6).
It became apparent from comparisons of the effects of bacterially translocated ExoS and E381A mutant ExoS on cell function that ExoS ADPRT activity was required for what were previously identified as ExoS-specific effects on cell function (34). The integral role of ExoS ADPRT activity in these effects implies that the cellular mechanism of action of ExoS relates to the proteins targeted by ExoS ADPRT activity in vivo. Ras and Ral, but not Rap-1, have been identified as cellular targets of bacterially translocated ExoS ADPRT activity (32) (Fraylick et al., Abstr. 100th Gen. Meet. Am. Soc. Microbiol. 2000). Ras is integral to signal transduction pathways affecting DNA synthesis and cytoskeletal structure. Hence, the interruption of Ras signaling upon ADP-ribosylation by ExoS provides a potential explanation for the diverse effects of ExoS on cell function. While the cellular function of Ral is less well understood, Ral is known to be involved in signaling processes that affect cell proliferation and the GTPase regulation of Rac and Cdc42 (1, 19, 23, 36), which is consistent with the ADP-ribosylation of Ral by ExoS also playing a role in the cellular mechanism of action of ExoS. Experimental difficulties in identifying cellular proteins ADP-ribosylated in vivo by bacterially translocated ExoS currently limit a complete understanding of the cellular mechanism of action of ExoS ADPRT activity. Knowing that ExoS exhibits diverse substrate specificity in vitro (3–5) and can ADP-ribosylate more than one protein in vivo leads to predictions that the effects of ExoS ADPRT activity on cell function will relate to its selective targeting of multiple proteins.
An unexpected finding in comparisons of the effects of ExoS GAP and ADPRT mutations on cell function was evidence that the two domains might be modulating the function of each other. This modulatory effect was first noticed in DNA synthesis studies when the effects of the R146A-E381A GAP-ADPRT mutant and the E381A ADPRT mutant were compared. In these studies, the abrogation of ExoS function by the E381A mutation was to some degree reversed when combined with a GAP mutation, with the R146A-E381A double mutant causing about a 50% greater inhibition of DNA synthesis than the E381A mutant. The cellular mechanism for this modulatory effect was further explored by assaying the ability of the GAP and ADPRT mutants to ADP-ribosylate Ras both in vitro and in vivo. While neither the E381A or R146A-E381A mutant were able to ADP-ribosylate Ras in vitro, exposure of HT-29 cells to strains expressing both mutants resulted in a shift in the molecular mass of cellular Ras, a result consistent with these mutant forms of ExoS having ADPRT activity when bacterially translocated. Comparisons of Ras modification by SDS-PAGE analyses indicated that Ras was being ADP-ribosylated more efficiently by PA103ΔUT-ExoS and the R146A mutant than by the E381A and R146A-E381A mutants. Subsequent 2DE analyses supported that bacterially translocated E381A and R146A-E381A were able to ADP-ribosylate H-Ras at the preferred site of modification, Arg41, but not the secondary site of ADP-ribosylation, which is modified by ExoS and the R146A mutant. Also, consistent with the ability of the R146A-E381A mutant to cause a greater inhibition of DNA synthesis than E381A, the R146A-E381A mutant was able to modify Ras more efficiently, implicating the ability of the GAP activity of ExoS to functionally modulate its ADPRT activity. A candidate residue being targeted in the modulation of ADPRT activity by the R146A-E381A mutant is Glu379 (39), which is supported by the finding that the introduction of an E379A-E381A double ADPRT mutation, in combination with R146A, abrogates these modulatory effects.
To further examine cellular processes that might contribute to the differences in substrate modification by the GAP and ADPRT mutant forms of ExoS, HT-29 cell fractionation studies were performed following exposure to bacteria, and the translocation of the different ExoS products was monitored. While other laboratories studying type III-mediated translocation by E. coli, Yersinia spp., and P. aeruginosa (16, 24, 30, 37) have been able to detect type III effector proteins in the cytosol of host cells, we were consistently unable to detect ExoS protein in the cytosolic fraction of HT-29 cells, but we were able to detect ExoS ADPRT-inactive protein in fractions that included soluble membrane proteins. In an effort to detect cytosolic ExoS protein, multiple fractionation methods were employed (16, 17, 24, 25, 37), and highly concentrated cytosolic extracts (representing ∼2.5 × 108 cells) were examined using an immunoblot procedure calculated to have a sensitivity of <1 ng of ExoS protein. Immunoblots were also probed with multiple ExoS antibodies, known to recognize different ExoS epitopes (10), to rule out that cell processing events might be deleting or masking antibody recognition sites. Contrary to the inability to detect ExoS protein in the cytosolic fraction, functional evidence of ExoS ADPRT activity in in vitro assays and in the ADP-ribosylation of Ral was apparent in the cytosol. While we do not yet have a complete understanding for the discrepancy in the detection of ExoS protein and ADPRT activity within the cell, our analysis of ADPRT mutant forms of ExoS has allowed the development of a model which helps explain what is observed and what might be happening as ExoS ADPRT activity is being translocated into the host cell.
In developing this model we first noted that, consistent with previous reports (16, 37), ExoS protein with defects in ADPRT activity, but not ExoS with wild-type activity, was detected in cellular fractions that included solubilized membrane proteins. This provides evidence that a defect in ADPRT activity stabilizes the membrane-associated form of ExoS. While this stabilization was evident in fractionation studies with E381A mutants (data not shown), it was more evident in the double E379A-E381A mutants and links ExoS ADPRT activity with the efficiency of ExoS membrane translocation. Second, it was apparent based on the levels of ADPRT-active protein detected in ExoS-producing bacterial culture supernatants that the high levels of ADPRT activity in the host cytosolic fraction following exposure to ExoS-producing bacteria (∼3 fmol min−1 μl−1) should be detectable in ExoS protein analyzes. In this regard, however, we have found that the addition of cytosolic extracts to ADPRT activity assays greatly enhances (>10-fold) the detectable levels of ADPRT activity. Evidence of the enhancement of ADPRT activity by cellular components, coupled with the observed more efficient ADPRT substrate modification detected within the membrane fraction, provides a glimpse of the potential of the eukaryotic cell microenvironment to enhance the efficiency of ExoS ADPRT activity beyond that apparent in in vitro analyses. In our proposed model relating substrate modification by ExoS to membrane translocation, our data support that mutants with defects in ADPRT activity retain a membrane association and, within this microenvironment, residual ADPRT activity of the E381A and R146A-E381A ExoS mutants is facilitated. This would explain the detection of ADPRT activity by these mutants in vivo but not in vitro. The lack of detection of ADPRT-active ExoS protein in the membrane soluble and cytoplasmic fractions is predicted to relate to the facilitated translocation of the ADPRT active form of ExoS through the membrane, which, when coupled with its highly efficient ADP-ribosylation of cellular substrates, results in a shutdown of cellular activity and further internalization of ExoS. Although not directly addressed in our studies, another factor that might play an important role in the inability to detect ExoS protein in the host cell once internalized is the efficient clearance of ExoS by eukaryotic proteases. Consistent with this possibility, ExoS has been reported to be highly sensitive to proteolysis (28). Thus, in comparisons of ExoS protein internalization with that of other type III effectors, our data support that the ADPRT activity of ExoS effectively limits its accumulation within the host cell and that this then precludes its detection by methodologies capable of detecting other type III effector proteins.
In summary, while trying to clarify the functional contributions of the GAP and ADPRT activities of ExoS in its cellular mechanism of action, we have uncovered additional complexities in the effects of ExoS on cell function. Our studies support the idea that ExoS ADPRT activity is the key player in the previously identified effects of ExoS on DNA synthesis and long-term cell rounding. Conversely, while we were unable to clearly distinguish a role of GAP function, as defined by the R146A mutation, in the effects of ExoS on DNA synthesis and cell morphology, we were able to detect the potential of ExoS GAP activity to modulate its ADPRT activity. Mutations in ExoS ADPRT activity appear to alter its efficiency of membrane translocation, but differences detected in substrate modification by these mutants relate more to inherent defects in protein function, rather than to altered membrane translocation. Together, these studies draw attention to the coordinate role of ExoS ADPRT and GAP activities in the effects of ExoS on eukaryotic cell function and the integral relationship of the host cell in these effects.
ACKNOWLEDGMENTS
We thank Dara Frank for providing bacterial strains used in this study. We also acknowledge the contribution of Monja Dishmon and Claudia Rocha to studies included in this project.
This work was supported by Public Health Service grants AI41694 and AI45569 from the National Institute of Allergy and Infectious Diseases and by the Medical University of South Carolina Institutional Research Funds.
REFERENCES
- 1.Albright C F, Giddings B W, Liu J, Vito M, Wienberg R A. Characterization of a guanine nucleotide dissociation stimulator for a ras-related GTPase. EMBO J. 1993;12:339–347. doi: 10.1002/j.1460-2075.1993.tb05662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chuang S E, Chen A L, Chao C C. Growth of Escherichia coli and how temperature dramatically increases the transformation frequency by electroporation. Nucleic Acid Res. 1995;23:1641. doi: 10.1093/nar/23.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coburn J, Dillon S T, Iglewski B H, Gill D M. Exoenzyme S of Pseudomonas aeruginosa ADP-ribosylates the intermediate filament protein vimentin. Infect Immun. 1989;57:996–998. doi: 10.1128/iai.57.3.996-998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coburn J, Gill D M. ADP-ribosylation of p21ras and related proteins by Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1991;59:4259–4262. doi: 10.1128/iai.59.11.4259-4262.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coburn J, Wyatt R T, Iglewski B H, Gill D M. Several GTP-binding proteins, including p21c-H-ras, are preferred substrates of Pseudomonas aeruginosa exoenzyme S. J Biol Chem. 1989;264:9004–9008. [PubMed] [Google Scholar]
- 6.Cowell B A, Chen D Y, Frank D W, Vallis A J, Fleiszig S M J. ExoT of cytotoxic Pseudomonas aeruginosa prevents uptake by corneal epithelial cells. Infect Immun. 2000;68:403–406. doi: 10.1128/iai.68.1.403-406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennis J J, Sokol P A. Electrotransformation of Pseudomonas. In: Nickoloff J A, editor. Methods in molecular biology. Totowa, N.J: Humana Press; 1995. pp. 125–133. [DOI] [PubMed] [Google Scholar]
- 8.Farnsworth C L, Marshall M S, Gibbs J B, Stacey D W, Feig L A. Preferential inhibition of the oncogenic form of RasH by mutations in the GAP binding effector domain. Cell. 1991;64:625–633. doi: 10.1016/0092-8674(91)90246-u. [DOI] [PubMed] [Google Scholar]
- 9.Feig L A, Cooper G M. Relationship among guanine nucleotide exchange, GTP hydrolysis, and transforming potential of mutated ras proteins. Mol Cell Biol. 1988;8:2472–2478. doi: 10.1128/mcb.8.6.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson M, Maxwell J A, da Silva J, Vincent T S, Olson J C. Comparison of the exoS gene and protein expression in soil and clinical isolates of Pseudomonas aeruginosa soil isolates. Infect Immun. 2001;69:2198–2210. doi: 10.1128/IAI.69.4.2198-2210.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleiszig S M J, Evans D J, Do N, Shin S, Mostov K E. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect Immun. 1997;65:2861–2867. doi: 10.1128/iai.65.7.2861-2867.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frithz-Lindsten E, Du Y, Rosqvist R, Forsberg A. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol Microbiol. 1997;25:1125–1139. doi: 10.1046/j.1365-2958.1997.5411905.x. [DOI] [PubMed] [Google Scholar]
- 13.Ganesan A K, Frank D W, Misra R P, Schmidt G, Barbieri J T. Pseudomonas aeruginosa exoenzyme S ADP-ribosylates Ras at multiple sites. J Biol Chem. 1998;273:7332–7337. doi: 10.1074/jbc.273.13.7332. [DOI] [PubMed] [Google Scholar]
- 14.Ganesan A K, Mende-Mueller L, Selzer J, Barbieri J T. Pseudomonas aeruginosa exoenzyme S, a double ADP-ribosyltransferase, resembles vertebrate mono-ADP-ribosyltransferases. J Biol Chem. 1999;274:9503–9508. doi: 10.1074/jbc.274.14.9503. [DOI] [PubMed] [Google Scholar]
- 15.Ganesan A K, Vincent T S, Olson J C, Barbieri J T. Pseudomonas aeruginosa exoenzyme S disrupts Ras-mediated signal transduction by inhibiting guanine nucleotide exchange factor catalyzed nucleotide exchange. J Biol Chem. 1999;274:21823–21829. doi: 10.1074/jbc.274.31.21823. [DOI] [PubMed] [Google Scholar]
- 16.Garrity-Ryan L, Kazamierczak B, Kowal R, Commolli J, Hauser A, Engel J N. The arginine finger domain of ExoT contributes to actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect Immun. 2000;68:7100–7113. doi: 10.1128/iai.68.12.7100-7113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gauthier A, de Grado M, Finlay B B. Mechanical fractionation reveals structural requirements for enteropathogenic Escherichia coli Tir insertion into host membranes. Infect Immun. 2000;68:4344–4348. doi: 10.1128/iai.68.7.4344-4348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goehring U-M, Schmidt G, Pederson K J, Aktories K, Barbieri J T. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase activating protein for Rho-GTPases. J Biol Chem. 1999;274:36369–36372. doi: 10.1074/jbc.274.51.36369. [DOI] [PubMed] [Google Scholar]
- 19.Goi T, Rasanescu G, Urano T, Feig L. Ral-specific guanine nucleotide exchange factor activity opposes other Ras effectors in PC12 cells by inhibiting neurite outgrowth. Mol Cell Biol. 1999;19:1731–1741. doi: 10.1128/mcb.19.3.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henriksson M L, Rosqvist R, Telepnev M, Wolf-Watz H, Hallberg B. Ras effector pathway activation by epidermal growth factor is inhibited in vivo by exoenzyme S ADP-ribosylation of Ras. Biochem J. 2000;347:217–222. [PMC free article] [PubMed] [Google Scholar]
- 21.Iglewski B H. Pseudomonas toxins. In: Hardegree M C, Tu A T, editors. Handbook of toxins. Vol. 4. New York, N.Y: Marcel Dekker; 1988. pp. 249–265. [Google Scholar]
- 22.Iglewski B H, Sadoff J, Bjorn M J, Maxwell E S. Pseudomonas aeruginosa exoenzyme S: an adenosine diphosphate ribosyltransferase distinct from toxin A. Proc Natl Acad Sci USA. 1978;75:3211–3215. doi: 10.1073/pnas.75.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jullien-Flores V, Dorseuil O, Romero F, Letourneur F, Saragosti S, Berger R, Tavitian A, Gacon G, Camonis J H. Bridging Ral GTPase to Rho pathways. J Biol Chem. 1995;270:22473–22477. doi: 10.1074/jbc.270.38.22473. [DOI] [PubMed] [Google Scholar]
- 24.Kenny B, Finlay B B. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-γ1. Infect Immun. 1997;65:2528–2536. doi: 10.1128/iai.65.7.2528-2536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenny B, Warawa J. Enteropathogenic Escherichia coli (EPEC) Tir receptor molecule does not undergo full modification when introduced into host cells by EPEC-independent mechanisms. Infect Immun. 2001;69:1444–1453. doi: 10.1128/IAI.69.3.1444-1453.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight D A, Finck-Barbancon V, Kulich S M, Barbieri J T. Functional domains of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1995;63:3182–3186. doi: 10.1128/iai.63.8.3182-3186.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krall R, Goehring U M, Aktories K, Barbieri J T. Pseudomonas aeruginosa ExoT is a RhoGTP-ase activating protein. Infect Immun. 2000;68:6066–6068. doi: 10.1128/iai.68.10.6066-6068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulich S M, Frank D W, Barbieri J T. Expression of recombinant exoenzyme S of Pseudomonas aeruginosa. Infect Immun. 1995;63:1–8. doi: 10.1128/iai.63.1.1-8.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Lee V T, Anderson D M, Schneewind O. Targeting of Yersinia Yop proteins to the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol Microbiol. 1998;28:593–601. doi: 10.1046/j.1365-2958.1998.00822.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Kulich S M, Barbieri J T. Identification of glutamic acid 381 as a candidate active site residue of Pseudomonas aeruginosa exoenzyme S. Biochemistry. 1996;35:2754–2758. doi: 10.1021/bi952340g. [DOI] [PubMed] [Google Scholar]
- 32.McGuffie E M, Frank D W, Vincent T S, Olson J C. Modification of Ras in eukaryotic cells by Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1998;66:2607–2613. doi: 10.1128/iai.66.6.2607-2613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGuffie E M, Fraylick J E, Vincent T S, Olson J C. Differential sensitivity of human epithelial cells to Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1999;67:3494–3503. doi: 10.1128/iai.67.7.3494-3503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson J C, Fraylick J E, McGuffie E M, Dolan K M, Yahr T L, Frank D W, Vincent T S. Interruption of multiple cellular processes in HT-29 epithelial cells by Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1999;67:2847–2854. doi: 10.1128/iai.67.6.2847-2854.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olson J C, McGuffie E M, Frank D W. Effects of differential expression of the 49-kilodalton exoenzyme S by Pseudomonas aeruginosa on cultured eukaryotic cells. Infect Immun. 1997;65:248–256. doi: 10.1128/iai.65.1.248-256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park S H, Weinberg R A. A putative effector of Ras has homology to Rho/Rac GTPase activating proteins. Oncogene. 1995;11:2349–2355. [PubMed] [Google Scholar]
- 37.Pederson K J, Pal S, Vallis A J, Frank D W, Barbieri J T. Intracellular localization and processing of Pseudomonas aeruginosa ExoS in eukaryotic cells. Mol Microbiol. 2000;37:287–299. doi: 10.1046/j.1365-2958.2000.01990.x. [DOI] [PubMed] [Google Scholar]
- 38.Pederson K J, Vallis A J, Aktories K, Frank D W, Barbieri J T. The amino-terminal domain of Pseudomonas aeruginosa ExoS disrupts actin filaments via small-molecular-weight GTP-binding proteins. Mol Microbiol. 1999;32:393–401. doi: 10.1046/j.1365-2958.1999.01359.x. [DOI] [PubMed] [Google Scholar]
- 39.Radke J, Pederson K J, Barbieri J T. Pseudomonas aeruginosa exoenzyme S is a biglutamic acid ADP-ribosyltransferase. Infect Immun. 1999;67:1508–1510. doi: 10.1128/iai.67.3.1508-1510.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schweizer H P. Escherichia-Pseudomonas shuttle vector derived from pUC18/19. Gene. 1991;97:109–112. doi: 10.1016/0378-1119(91)90016-5. [DOI] [PubMed] [Google Scholar]
- 41.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vallis A J, Finck-Barbancon V, Yahr T L, Frank D W. Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect Immun. 1999;67:2040–2044. doi: 10.1128/iai.67.4.2040-2044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincent T S, Fraylick J E, McGuffie E M, Olson J C. ADP-ribosylation of oncogenic Ras proteins by Pseudomonas aeruginosa exoenzyme S in vivo. Mol Microbiol. 1999;32:1043–1053. doi: 10.1046/j.1365-2958.1999.01420.x. [DOI] [PubMed] [Google Scholar]
- 44.Wurtele M, Wolf E, Pederson K J, Buchwald G, Ahmadian M R, Barbieri J T, Wittinghofer A. How the Pseudomonas aeruginosa ExoS toxin downregulates Rac. Nat Struct Biol. 2001;8:23–26. doi: 10.1038/83007. [DOI] [PubMed] [Google Scholar]
- 45.Yahr T L, Goranson J, Frank D W. Exoenzymes S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol Microbiol. 1996;22:991–1003. doi: 10.1046/j.1365-2958.1996.01554.x. [DOI] [PubMed] [Google Scholar]