Abstract
Pseudomonas aeruginosa exoenzyme S (ExoS) is an ADP-ribosyltransferase that modifies low-molecular-weight GTPases. Here we studied the effect of Rab5 ADP-ribosylation by ExoS on its cellular function, i.e., regulation of early endocytic events. Coculture of CHO cells with P. aeruginosa induced a marked decrease in horseradish peroxidase (HRP) uptake compared to noninfected cells, while coculture with a P. aeruginosa mutant strain that fails to produce ExoS did not lead to any change in HRP uptake. Microinjection of recombinant ExoS into Xenopus oocytes induced strong inhibition of basal HRP uptake by oocytes. Moreover, coinjection of recombinant ExoS with Rab5 abolished the typical stimulation of HRP uptake obtained after GTPase microinjection. Cytosols prepared from injected oocytes were used in an endosome-endosome fusion assay. Cytosol from ExoS-microinjected oocytes was ineffective in promoting endosome-endosome fusion. However, in these conditions, the addition of Rab5 to the assay led to fusion recovery. Finally, we found that the interaction of Rab5 with EEA1 was markedly diminished after Rab5 ADP-ribosylation by ExoS.
Pseudomonas aeruginosa is an opportunistic gram-negative pathogen that can cause infections in patients with cystic fibrosis, neutropenia, or leukemia and in burn wound victims (5). P. aeruginosa is able to infect and survive within the host as a result of a number of virulence determinants, including exoenzyme S (ExoS), which has been shown to increase tissue damage and bacterial dissemination (23). ExoS is an extracellular ADP-ribosyltransferase whose secretion into target cells is achieved by a type III secretion pathway (28). Once in the cytosol, ExoS enzymatic activity is stimulated by a critical eukaryotic factor identified as the 14-3-3 protein (11), leading to ADP-ribosylation of a number of target proteins, including Ras (22). The fact that the14-3-3 protein has been shown to be part of a multiprotein complex translocated to the plasma membrane in the presence of Ras-GTP is probably related to Ras modification by ExoS. Indeed, it was recently shown that ExoS interacts with residues of 14-3-3 proteins located in the Raf-binding groove (21, 30). Ras was shown to be ADP-ribosylated in vitro on several Arg residues (12). More recently, it was demonstrated that modification of Arg41 disrupts Ras signal transduction by inhibiting interactions between Ras and Cdc25, its guanine nucleotide exchange factor (13). Arg41 is adjacent to the switch I domain of Ras, a domain interacting with Ras effectors. The fact that Ras is modified at different sites by ExoS could partly explain the ADP-ribosylation of several other proteins of the Ras-GTPase superfamily (8). Accordingly, we previously reported in vitro ADP-ribosylation of different members of the Rab protein subfamily (4). Moreover, sequencing radiolabeled peptides obtained after trypsin hydrolysis of ADP-ribosylated Rab4 and separation by reverse-phase high-pressure liquid chromatography on a DEAE-C18 column had indicated more than one ADP-ribosylation site (J. d'Alayer, Pasteur Institute; M. Vidal, data not shown).
We demonstrated ADP-ribosylation of Rab4 by incubation of endocytic vesicles purified from reticulocytes with recombinant ExoS, indicating vesicular colocalization of 14-3-3 proteins, the obligate ExoS activator, and Rab GTPases. In agreement with this, the involvement of 14-3-3 proteins in vesicular transport has been suggested in different systems, such as clathrin-mediated endocytosis in Saccharomyces cerevisiae (14), regulated exocytosis in chromaffin cells (25), retrograde transport from the Golgi to the endoplasmic reticulum (10). As noted for the Ras pathway, the localization of 14-3-3 proteins in the vicinity of GTPases may facilitate Rab modification by ExoS. Using streptolysin O-permeabilized reticulocytes, we demonstrated that ExoS slowed down recycling of internalized radiolabeled transferrin back to the plasma membrane (4). Since membrane recycling from the endocytic compartment is regulated by Rab4, this highly suggested that ADP-ribosylation of Rab4 by ExoS affects its functioning.
Rab proteins regulate vesicular traffic during discrete transport steps. Rab5, one of the most studied Rab proteins in recent years, is involved in early steps of the endocytic process. Development of endosome-endosome fusion assays allowed characterization of several other proteins involved in this Rab5-regulated docking and fusion step. Rabex-5 catalyzes the GDP-GTP exchange and is complexed with rabaptin-5, which could stabilize Rab5 in the GTP state. These proteins are recruited from the cytosol by Rab5 on the endosomal membrane (16). Activated (i.e., GTP bound) Rab5 can then recruit early endosome antigen 1 (EEA1), another cytosolic protein, to early endosomes (6). Vesicle binding of EEA1 may be stabilized by interaction with PIP3 through its FYVE finger. Accordingly, two phosphatidylinositol-3-OH kinases were recently demonstrated to interact with the active form of Rab5 (7). Rabaptin-5, EEA1, and phosphatidylinositol-3-OH kinase activity are required for endosome-endosome fusion (19, 26). In the present report, we use three different approaches to show that ExoS affects endocytosis through Rab5 ADP-ribosylation: (i) cell coculture with bacterial mutants, (ii) oocyte microinjection, and (iii) in vitro fusion assay. We also found that Rab5 interaction with EEA1 was markedly diminished after GTPase ADP-ribosylation by ExoS.
MATERIALS AND METHODS
Expression and purification of recombinant proteins.
The glutathione S-transferase (GST)-fused proteins used in this study, including 14-3-3 protein (generously provided by Andrey Shaw, Washington University, St. Louis, Mo.), Rab5WT, Rab5-Q79L, and Rab5-S34N-, were expressed and affinity purified with glutathione-Sepharose (3). His-ExoS was produced as described (17) in Escherichia coli BL21, using the construct kindly provided by Dara Frank, Medical College of Wisconsin, Milwaukee.
Bacterial strains and CHO cell coculture.
P. aeruginosa 388 and 388ΔS, described previously (18), were kindly provided by Dara Frank. Monolayers of CHO cells and CHO cells stably transfected with rab5WT (2) were grown in 35-mm dishes (∼5 × 105 cells/dish). The level of Rab5 expression in transfected cells was about four to five times the expression level in wild-type CHO cells. Cells were cocultured with bacteria (multiplicity of infection, ∼20:1) for 10 h and washed three times with serum-free alpha minimal essential medium (α-MEM), and horseradish peroxidase (HRP) endocytosis was initiated by the addition of 1 ml of α-MEM containing 2 mg of HRP per ml and 0.2% (wt/vol) bovine serum albumin at 37°C for 30 min. HRP uptake was estimated as previously described (20).
Xenopus oocyte microinjection and HRP uptake.
Stage V-VI Xenopus oocytes were isolated by partial ovariectomy under tricaine anesthesia and then defolliculated by treatment with 1 mg/ml collagenase (type 1A; Sigma) in 0 mM Ca2+ ND96 (below) for 1 h. From 6 h after defolliculation, oocytes were pressure injected with ∼50 nl of His-ExoS (200 ng) and/or Rab5WT (100 ng). Oocytes were maintained at room temperature in ND96 solution (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM Na-HEPES, pH 7.5) containing 2 mM Ca2+ and supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml) for 8 to 12 h prior to analysis. HRP uptake by oocytes was then assayed as described (24). For in vitro fusion assays, cytosols were prepared by centrifugation after lysis in HEPES buffer (0.25 M sucrose, 10 mM KCl, 0.5 mM EGTA, 20 mM HEPES, pH 7.4) of microinjected oocytes.
In vitro endosome-endosome fusion assay.
Early endosomes prepared from J774 E-clone macrophages were loaded with anti-2,4-dinitrophenol (anti-DNP) mouse monoclonal antibody or DNP–β-glucuronidase as described (9). In vitro reconstitution of fusion was performed as described (9) using oocyte cytosol. In some experiments, in vitro-prenylated Rab5WT (2) was added to the assay.
ADP-ribosylation assay.
ADP-ribosylation was carried out as previously described (4) using GST-Rab5 proteins (1 μg), His-ExoS (100 ng), GST–14-3-3 (0.8 μg), or oocyte cytosol (8 μg of protein) and 2 μCi of [α-32P]NAD (1,000 Ci/mmol) (Amersham). Biotinylation of NAD used for oocyte microinjection was carried out using 8-([N-biotinyl(6-aminohexyl)]amino)NAD (Sigma) and normal human serum-biotin (Pierce), and biotinylated NAD was purified on a C18 column using an ammonium bicarbonate-methanol gradient as described (29). Biotinylated Rab5 was detected by overlay using streptavidin-HRP (Amersham) and by Western blotting using 4F11 monoclonal anti-Rab5 antibody.
EEA1 binding assay to bead-immobilized Rab5.
GST-Rab5 affinity chromatography was done as described (6) with cytosol prepared using rat brains. Briefly, recombinant GST-Rab5, GST-Rab5-Q79L, or GST-Rab5-S34N was immobilized on glutathione-Sepharose beads and loaded or not with the indicated nucleotides. Rat brain cytosol was then incubated for 4 h at room temperature with the beads in the absence or presence of His-ExoS. After several washes, beads were resuspended in Laemmli buffer, and proteins were separated by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS-PAGE). Pulled-down EEA1 was analyzed by Western blot. Rab5 Western blots were also carried out on the same membrane to verify that equal amounts of immobilized Rab proteins were present in the bead pellets.
EEA1 association with endosomal vesicles.
Endocytic vesicles were purified from rat reticulocytes (4) incubated with cytosol supplemented with NAD (2 mM) and ZnCl2 (1 mM), with or without His-ExoS (800 ng) or wortmannin (500 nM), in a total volume of 300 μl for 4 h at room temperature. The samples were analyzed in two ways. A rapid method consisted in pelleting the vesicles by Airfuge (Beckman) ultracentrifugation (30 min, 30 lb/in2) and analyzing the presence of EEA1 in the vesicle pellets by SDS-PAGE and Western blot using mouse monoclonal anti-EEA1 (Transduction Laboratories) and peroxidase-conjugated donkey anti-mouse immunoglobulin G (Jackson ImmunoResearch Laboratories) using the ECL detection system (Amersham). This method was refined by layering the incubation samples on top of a 5 to 35% sucrose density gradient. Fractions (500 μl) collected after centrifugation at 35,000 rpm for 1 h at 4°C in an SW50 Beckman rotor were acetone precipitated and analyzed for the presence of EEA1 by Western blotting. Quantification of Western blots was carried out using ImageQuant software (Molecular Dynamics).
RESULTS AND DISCUSSION
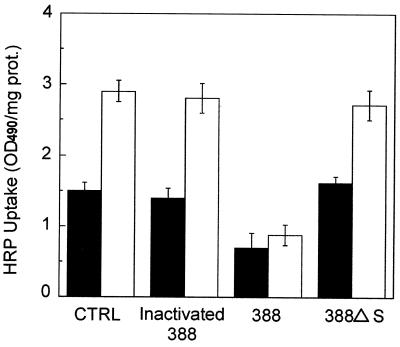
To study the involvement of ExoS in endocytosis, we first compared the fluid-phase uptake of HRP by cells infected with P. aeruginosa 388 parental strain or P. aeruginosa 388 ΔS, a mutant strain that fails to produce ExoS (18). As shown in Fig. 1, coculture of CHO cells with P. aeruginosa 388 parental strain led to a marked decrease (about 50%) in HRP uptake compared to noninfected control cells (CTRL). In contrast, endocytosis of CHO cells cocultured with heat-killed P. aeruginosa 388 parental strain or with P. aeruginosa 388ΔS was only marginally affected, indicating that secretion of ExoS by live P. aeruginosa is related to the endocytosis inhibition. Note that similar results were obtained using CHO cells overexpressing Rab5WT, although the amount of HRP taken up was about twofold higher in transfected CHO cells. Since Rab5 is a key Rab GTPase for endocytosis regulation, we considered Rab5 a possible target candidate for the ExoS effect. To more closely study ExoS involvement in the inhibition of fluid-phase uptake, we conducted two assays to monitor Rab GTPase regulation of the endocytic process: Xenopus oocyte microinjection and in vitro endosome-endosome fusion.
FIG. 1.
ExoS inhibits HRP uptake by CHO cells. CHO cells (▪) and CHO cells stably transfected with Rab5WT (□) were cocultured or not (control, CTRL) with 388 parental (heat inactivated or not) and 388ΔS mutant P. aeruginosa strains as described in Materials and Methods, washed, and assayed for HRP uptake as described by Li and Stahl (24). One of two independent experiments is shown. Each experiment was done in triplicate. Bars show standard deviation (SD). CTRL, cells cultured in absence of bacteria.
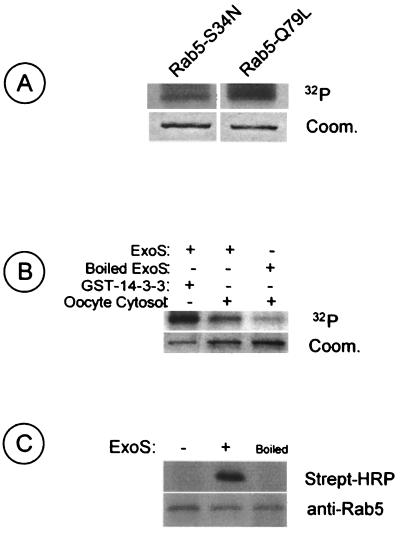
ADP-ribosylation activity of the bacterial enzyme absolutely requires a eukaryotic cytosolic factor named FAS (factor-activating exoenzyme S) and characterized as 14-3-3 ζ protein (11). We had previously shown in vitro ADP-ribosylation of Rab5 using recombinant Rab5WT and a cytosolic protein fraction enriched with FAS (4). Recombinant Rab5 was preloaded with either GTP-γS or GDP before ExoS ADP-ribosylation to determine whether the nucleotide state of Rab5 has some influence on ADP-ribosylation efficiency. Rab5 was similarly ADP-ribosylated irrespective of the nucleotide bound to Rab5 (not shown), and this was confirmed using the two Rab5 mutants Rab5-Q79L and Rab5-S34N, mimicking the Rab5 GTP- and GDP-bound states, respectively. As noted in Fig. 2A, both mutants were ADP-ribosylated in vitro. However, it seems that the Rab5:Q79L mutant (GTP-bound form) was more efficiently ADP-ribosylated than the Rab5:S34N mutant (GDP-bound form). Further work will be necessary to confirm this observation, since these two mutants represent two different nucleotide status. It is possible that those mutants show a distinctive three-dimensional conformation, where the potential site for ADP-ribosylation is more accessible for Rab5-Q79L than for the Rab5-S34N mutant. The 14-3-3 ζ protein is involved in multiple functions in many cell types, including Xenopus oocytes. To determine whether the Xenopus homologue of 14-3-3 ζ functions as a cofactor for ExoS, oocyte cytosol was used as a source of FAS in the in vitro ADP-ribosylation assay. GST-Rab5 was ADP-ribosylated, although less efficiently, when oocyte cytosol was added to the assay instead of recombinant 14-3-3 (Fig. 2B). However, the GTPase was virtually not modified when ExoS was heat inactivated prior to addition.
FIG. 2.
ADP-ribosylation of Rab5 protein by ExoS. (A) GST-Rab5-S34N and GST-Rab5-Q79L were used as substrates in in vitro ADP-ribosylation using [α-32P]NAD by recombinant His-ExoS as described in Materials and Methods. After SDS-PAGE separation, [32P]ADP-ribosylated Rab proteins were detected by autoradiography (upper panel) and Coomassie staining (lower panel). (B) In vitro [32P]ADP-ribosylation of GST-Rab5WT using heat-inactivated or active recombinant His-ExoS and GST–14-3-3 or Xenopus oocyte cytosol as a source of FAS. Autoradiography (upper panel) and Coomassie staining (lower panel) of Rab proteins are presented. (C) Oocytes were injected with GST-Rab5WT and NAD-biotin with or without His-ExoS (heat inactivated or not). GST-Rab5 was purified from oocyte extracts using glutathione-Sepharose beads and analyzed by streptavidin-HRP overlay (upper panel) and immunoblotting (lower panel).
The Xenopus oocyte is an excellent model system to study Rab GTPase regulation of HRP endocytosis. Microinjection of recombinant Rab5WT and mutant Rab5-Q79L has been shown to increase HRP uptake, and conversely, injection of mutant Rab5-S34N decreased HRP uptake compared to Rab5WT (24). The oocyte system was thus used to assess the effect of Rab5 ADP-ribosylation by ExoS on the endocytic pathway. We first verified Rab5 ADP-ribosylation in oocytes by coinjecting GST-Rab5, ExoS, and biotinylated NAD. Rab5 was then purified from the oocyte homogenate using the GST moiety, and ADP-ribosylation was revealed after SDS-PAGE separation and transfer on membranes using streptavidin-HRP (Fig. 2C).
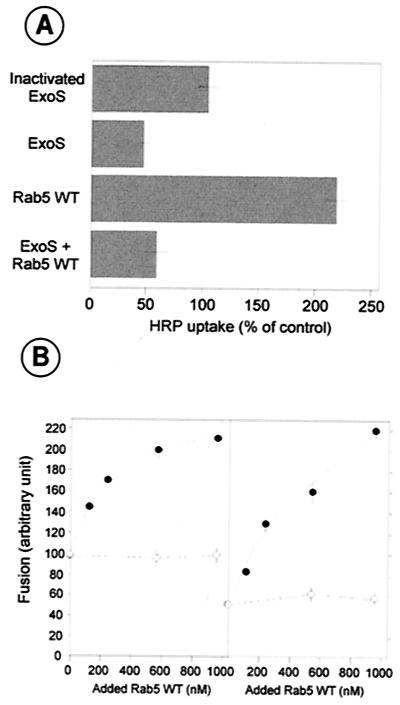
Once we established that the oocytes have a 14-3-3-like activity, we decided to investigate the effect of microinjection of ExoS on the endocytosis of HRP. Recombinant ExoS (active or heat inactivated) and Rab5WT were thus microinjected in Xenopus oocytes, and HRP uptake by oocytes was assayed. As shown in Fig. 3A, ExoS strongly decreased basal HRP uptake in control oocytes, but had no effect when first heat inactivated. As previously demonstrated, HRP uptake was highly stimulated by Rab5WT injection, whereas HRP uptake was not increased when ExoS was coinjected with Rab5WT, reaching a level near that obtained with ExoS injected alone. This suggests, in agreement with the results presented in Fig. 2C, that coinjected Rab5 is efficiently modified by ExoS in oocytes. This also supports data of CHO cell infection by P. aeruginosa, where overexpression of Rab5 did not affect proportionally the effect of ExoS on HRP uptake (Fig. 1).
FIG. 3.
HRP uptake by microinjected oocytes and the use of cytosol from microinjected oocytes in endosome-endosome fusion assay. (A) Xenopus oocytes were microinjected with heat-inactivated or active His-ExoS, GST-Rab5WT, or GST-Rab5WT plus His-ExoS and assayed for HRP uptake as described (24). Control oocytes were microinjected with buffer. Values are means of determinations from at least four oocytes. Horizontal lines indicate SD for each sample. (B) In vitro endosome-endosome fusion experiment using 1.2 mg of cytosol protein prepared from Xenopus oocytes microinjected with active ExoS (right panel) or heat-inactivated recombinant ExoS (left panel) per ml. Indicated concentrations of prenylated Rab5WT (●) were added to the fusion assay, and heat-inactivated prenylated Rab5WT was used as a control (○).
The vesicle fusion process has been reconstituted in vitro and shown to require ATP and cytosol (9). Cytosols prepared from oocytes injected as above were then tested in an endosome-endosome fusion assay. The results obtained were in agreement with the results of HRP uptake experiments using injected oocytes. Cytosol from buffer-microinjected oocytes was able to promote endosome-endosome fusion (set at 100%). In contrast, cytosol from oocytes injected with ExoS was not effective (38.2 ± 14.5%, n = 3), whereas cytosol from oocytes injected with heat-inactivated ExoS exhibited the typical fusion activity (96.3 ± 11.8%, n = 3). Cytosol injected with Rab5 stimulated fusion (157.3 ± 16.8%, n = 3), which was abolished when ExoS was coinjected with Rab5 (55.4 ± 10.2%, n = 3). Interestingly, the inhibition of endosome-endosome fusion by cytosol from oocytes injected with ExoS could be reversed by adding increasing concentrations of Rab5WT to the assay (Fig. 3B). When Rab5 was heat inactivated before addition to the assay, no fusion recovery could be obtained. This is consistent with the hypothesis that ExoS inhibition of endocytosis of HRP and endosome fusion occurred through modification of Rab5 function.
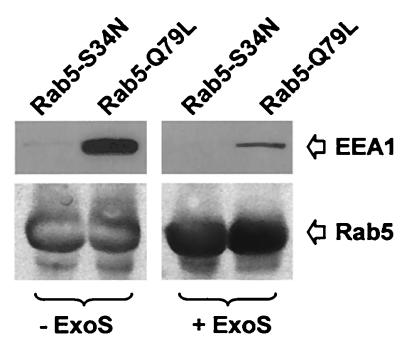
To further investigate the effect of Rab5 ADP-ribosylation by ExoS, we studied the effect of Rab5 modification on the interaction with a downstream effector, EEA1 (6). EEA1 from rat brain cytosol bound to GST-Rab5WT immobilized on glutathione-Sepharose beads (not shown). This assay was used to study ExoS effect on Rab5-EEA1 interaction. Note that in this case, 14-3-3 protein contained in cytosol was sufficient to activate ExoS as previously observed (4). Interaction of EEA1 with Rab5 was observed with the GTP-locked GTPase by preloading GST-Rab5WT with GTP-γS (not shown) or by using the GTPase-defective mutant GST-Rab5-Q79L (Fig. 4). In contrast, no binding was observed when Rab5 was loaded with GDP (not shown) or when GST-Rab5-S34N was used (Fig. 4). The addition of ExoS during cytosol incubation with immobilized Rab5 led to a marked decrease in the amount of EEA1 pelleted by Rab5-Q79L-Sepharose (Fig. 4).
FIG. 4.
ExoS inhibits EEA1 interaction with Rab5. GST-Rab5-S34N and GST-Rab5-Q79L (20 μg of protein) were incubated with glutathione-Sepharose (150-μl packed gel) at 4°C. Immobilized Rab proteins (75-μl packed gel) were then incubated with 100 μl of rat brain cytosol (4 h at room temperature) in the presence or absence of His-ExoS (1 μg/ml) in a final volume of 300 μl. Sepharose beads were then washed several times, resuspended in Laemmli buffer, and loaded on SDS–10% PAGE. Proteins were transferred to a polyvinylidene difluoride membrane, and EEA1 binding was analyzed by immunoblotting. Rab5 immunoblotting was carried out on the same membrane to verify the amount of immobilized Rab5. The results shown are representative of three independent experiments.
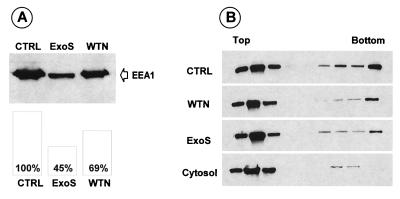
Once established that the ADP-ribosylation of Rab5 inhibited its interaction with EEA1, we decided to investigate the binding of EEA1 to endocytic vesicles (EVs). EVs from rat reticulocytes which contain Rab5 (27) were used to study EEA1 binding to early endosomes under different conditions. Purified EVs preincubated with cytosol in the absence or presence of ExoS or wortmannin were pelleted and analyzed for the presence of EEA1 by immunoblotting (Fig. 5A). Incubation in the presence of ExoS markedly decreased the amount of EEA1 copelleting with EVs (45 versus 100%), while the effect of wortmannin was less pronounced (69%). No EEA1 was found associated with purified EVs when the vesicles were not preincubated with cytosol (not shown). To confirm these data, sucrose gradient analysis was performed. As shown in Fig. 5B, when cytosol alone was loaded on the sucrose density gradient, no EEA1 was recovered in the bottom fraction, while incubation of the cytosol with purified EVs induced a partial pelleting of EEA1 with the vesicles. Quantification of the blots indicated that 8% of the total signal was present in the four bottom fractions for the cytosol loaded alone on the gradient, while the signal in the same four bottom fractions represented 38% of the total signal when EVs were incubated with cytosol (control). The presence of ExoS during incubation of purified EVs with cytosol decreased EEA1 binding to the vesicles (compare the bottom fraction of the control and ExoS lanes). Note that incubation of cytosol with EVs in the presence of wortmannin gave a similar extent of EEA1 copelleting with vesicles. Blot quantification indicated that 18 and 21% of EEA1 was detected in the four bottom fractions for ExoS and wortmannin, respectively.
FIG. 5.
EEA1 association with endosomal vesicles. Rat reticulocyte endocytic vesicles were incubated with rat brain cytosol in the absence (control, CTRL) or presence of 500 nM wortmannin (WTN) or 2.6 μg of His-ExoS (ExoS) per ml. (A) The endocytic vesicles were pelleted by Airfuge centrifugation (30 lb/in2, 30 min), and the same amount of protein was loaded on SDS-PAGE followed by immunoblotting with anti-EEA1 antibodies (upper part). Quantification of the Western blots (lower part) was carried out using ImageQuant software. The results shown are representative of two independent experiments. (B) The incubation mixtures were loaded on a sucrose gradient (5 to 35%), and collected fractions were analyzed for EEA1 by immunoblotting as described in Materials and Methods. Cytosol alone was loaded as a control for vesicle-unbound EEA1 migration in the sucrose gradient.
ExoS of P. aeruginosa is a bifunctional cytotoxin. The N-terminal part of ExoS was demonstrated to be a GTPase-activating protein for Rho GTPases (15), inducing disruption of actin microfilaments. ADP-ribosylation activity located at the C terminus of ExoS is not an absolute requirement for this effect, although the presence of this domain strongly enhances both cytotoxicity and resistance to phagocytosis. ADP-ribosylation of Ras was shown to inhibit nucleotide exchange by disrupting Ras-Cdc25 interactions (13). ExoS could thus inhibit phagocytosis by affecting both the cytoskeleton-based engulfment process and phagosome maturation. Indeed, from the results presented in this report, we propose that the ExoS protein is also able to block Rab5 function, which is known to be involved in phagosome maturation (1). More generally, vesicular transport in general may be affected by ADP-ribosylation of different Rab proteins, as we previously suggested in the case of Rab4 (4). Our data indicate that the impairment of Rab5 function is due directly to ExoS modification of the Rab5 protein. The exact mechanism by which ExoS is able to affect the interaction of Rab5 and EEA1 protein is presently unclear. It is conceivable that the decrease in the Rab5-EEA1 interaction is due to steric hindrance caused by ADP-ribosylation of Rab5 itself, although further investigations will be required to determine the mechanisms involved. However, our results show for the first time that Rab5-EEA1 interactions are specifically inhibited by ExoS, which correlates with the observed ADP-ribosylation of the Rab5 protein in vivo. It is now clear that ExoS is not only able to modify Rab5 but also capable of affecting Rab5 function, as shown in this work in vitro by endosome-endosome fusion assay and in vivo by oocyte microinjection. Taken together, our results show that Rab5, like Ras, could be an in vivo target for ExoS of P. aeruginosa and that ExoS is able to inhibit Rab5 function, although it cannot be excluded that other factors involved in the fusion process might be ADP-ribosylated.
ACKNOWLEDGMENTS
We are greatly indebted to D. Frank for the generous gift of P. aeruginosa strains and E. coli expressing His-ExoS and A. Shaw for E. coli expressing GST-14-3-3 protein. We thank M. I. Colombo for critical reading of the manuscript and Dee Owyoung for editing assistance.
This work was supported by a collaborative grant from CNRS/NSF (98N92/0262) and by grants from the University Montpellier II and the NIH.
REFERENCES
- 1.Alvarez-Dominguez C, Stahl P D. Increased expression of Rab5a correlates directly with accelerated maturation of Listeria monocytogenes phagosomes. J Biol Chem. 1999;274:11459–11462. doi: 10.1074/jbc.274.17.11459. [DOI] [PubMed] [Google Scholar]
- 2.Barbieri M A, Hoffenberg S, Roberts R, Mukhopadhyay A, Pomrehn A, Dickey B F, Stahl P D. Evidence for a symmetrical requirement for Rab5-GTP in in vitro endosome-endosome fusion. J Biol Chem. 1998;273:25850–25855. doi: 10.1074/jbc.273.40.25850. [DOI] [PubMed] [Google Scholar]
- 3.Barbieri M A, Li G, Colombo M I, Stahl P D. Rab5, an early acting endosomal GTPase, supports in vitro endosome fusion without GTP hydrolysis. J Biol Chem. 1994;269:18720–18722. [PubMed] [Google Scholar]
- 4.Bette-Bobillo P, Giro P, Sainte-Marie J, Vidal M. Exoenzyme S from P. aeruginosa ADP-ribosylates rab4 and inhibits transferrin recycling in SLO-permeabilized reticulocytes. Biochem Biophys Res Commun. 1998;244:336–341. doi: 10.1006/bbrc.1998.8263. [DOI] [PubMed] [Google Scholar]
- 5.Bodey G P, Bolivar R, Fainstein V, Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983;5:279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- 6.Christoforidis S, McBride H M, Burgoyne R D, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- 7.Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip S C, Waterfield M D, Backer J M, Zerial M. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol. 1999;1:249–252. doi: 10.1038/12075. [DOI] [PubMed] [Google Scholar]
- 8.Coburn J, Gill D M. ADP-ribosylation of p21ras and related proteins by Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1991;59:4259–4262. doi: 10.1128/iai.59.11.4259-4262.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz R, Mayorga L S, Weidman P J, Rothman J E, Stahl P D. Vesicle fusion following receptor-mediated endocytosis requires a protein active in Golgi transport. Nature. 1989;339:398–400. doi: 10.1038/339398a0. [DOI] [PubMed] [Google Scholar]
- 10.Dorner C, Ullrich A, Haring H U, Lammers R. The kinesin-like motor protein KIF1C occurs in intact cells as a dimer and associates with proteins of the 14-3-3 family. J Biol Chem. 1999;274:33654–33660. doi: 10.1074/jbc.274.47.33654. [DOI] [PubMed] [Google Scholar]
- 11.Fu H J, Coburn J, Collier R J. The eukaryotic host factor that activates exoenzyme S of Pseudomonas aeruginosa is a member of the 14-3-3 protein family. Proc Natl Acad Sci USA. 1993;90:2320–2324. doi: 10.1073/pnas.90.6.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganesan A K, Frank D W, Misra R P, Schmidt G, Barbieri J T. Pseudomonas aeruginosa Exoenzyme S ADP-ribosylates Ras at multiple sites. J Biol Chem. 1998;273:7332–7337. doi: 10.1074/jbc.273.13.7332. [DOI] [PubMed] [Google Scholar]
- 13.Ganesan A K, Vincent T S, Olson J C, Barbieri J T. Pseudomonas aeruginosa exoenzyme S disrupts Ras-mediated signal transduction by inhibiting guanine nucleotide exchange factor-catalyzed exchange. J Biol Chem. 1999;274:21823–21829. doi: 10.1074/jbc.274.31.21823. [DOI] [PubMed] [Google Scholar]
- 14.Gelperin D, Weigle J, Nelson K, Roseboom P, Irie K, Matsumoto K, Lemmon S. 14-3-3 proteins: potential roles in vesicular transport and ras signaling in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1995;92:11539–11543. doi: 10.1073/pnas.92.25.11539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goehring U M, Schmidt G, Pederson K J, Aktories K, Barbieri J T. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J Biol Chem. 1997;274:36369–36372. doi: 10.1074/jbc.274.51.36369. [DOI] [PubMed] [Google Scholar]
- 16.Horiuchi H, Lippe R, McBride H M, Rubino M, Woodman P, Stenmark H, Rybin V, Wilm M, Ashman K, Mann M, Zerial M. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- 17.Knight D A, Finck-Barbançon V, Kulich S M, Barbieri J T. Functional domains of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1995;63:3182–3186. doi: 10.1128/iai.63.8.3182-3186.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulich S M, Frank D W, Barbieri J T. Expression of recombinant exoenzyme S of Pseudomonas aeruginosa. Infect Immun. 1995;63:1–8. doi: 10.1128/iai.63.1.1-8.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G, D'Souza-Schorey C, Barbieri M A, Roberts R L, Klippel A, Williams L T, Stahl P D. Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proc Natl Acad Sci USA. 1995;92:10207–10211. doi: 10.1073/pnas.92.22.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li G, Stahl P D. Structure-function relationship of the small GTPase rab5. J Biol Chem. 1993;268:24475–24480. [PubMed] [Google Scholar]
- 21.Masters S C, Pederson K J, Zhang L, Barbieri J T, Fu H. Interaction of 14-3-3 with a nonphosphorylated protein ligand, exoenzyme S of Pseudomonas aeruginosa. Biochemistry. 1999;38:5216–5221. doi: 10.1021/bi982492m. [DOI] [PubMed] [Google Scholar]
- 22.McGuffie E M, Frank D W, Vincent T S, Olson J C. Modification of Ras in eukaryotic cells by Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1998;66:2607–2613. doi: 10.1128/iai.66.6.2607-2613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGuffie E M, Fraylick J E, Hazen-Martin D J, Vincent T S, Olson J C. Differential sensitivity of human epithelial cells to Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1999;67:3494–3503. doi: 10.1128/iai.67.7.3494-3503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukhopadhyay A, Barbieri A, Funato K, Roberts R, Stahl P D. Sequential actions of rab5 and rab7 regulate endocytosis in the Xenopus oocyte. J Cell Biol. 1997;136:1227–1237. doi: 10.1083/jcb.136.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth D, Morgan A, Martin H, Jones D, Martens G J M, Aitken A, Burgoyne R D. Characterization of 14-3-3 proteins in adrenal chromaffin cells and demonstration of isoform-specific phospholipid binding. Biochem J. 1994;301:305–310. doi: 10.1042/bj3010305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonsen A, Lippe R, Christoforidis S, Gaullier J M, Brech A, Callaghan J, Toh B H, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 27.Vidal M J, Stahl P D. The small GTP-binding proteins Rab4 and ARF are associated with vesicles released during reticulocyte maturation. Eur J Cell Biol. 1993;60:261–267. [PubMed] [Google Scholar]
- 28.Yahr T, Goranson J, Frank D. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol Microbiol. 1996;22:991–1003. doi: 10.1046/j.1365-2958.1996.01554.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J. Use of biotinylated NAD to label and purify ADP-ribosylated proteins. Methods Enzymol. 1997;280:255–265. doi: 10.1016/s0076-6879(97)80117-6. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Wang H, Masters S C, Wang B, Barbieri J T, Fu H. Residues of 14-3-3ζ required for activation of Exoenzyme S of Pseudomonas aeruginosa. Biochemistry. 1999;38:12159–12164. doi: 10.1021/bi991019l. [DOI] [PubMed] [Google Scholar]