Abstract
A concise review covering updated presence and role of 2-phenethylamines in medicinal chemistry is presented. Open-chain, flexible alicyclic amine derivatives of this motif are enumerated in key therapeutic targets, listing medicinal chemistry hits and appealing screening compounds. Latest reports in discovering new bioactive 2-phenethylamines by research groups are covered too.
Keywords: 2-phenethylamine, medicinal chemistry, ligands, adrenoceptors, carbonyl anhydrase, dopamine receptor, DAT, 5-HT, MAO, PPAR, sigma receptors, TAAR1
1. Introduction
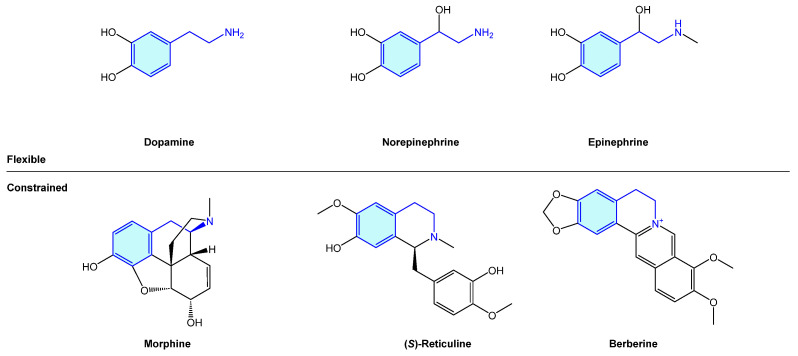
The 2-phenethylamine motif is widely present in nature, from simple, open-chain structures to more complex polycyclic molecular arrangements. The importance of this moiety is probably best exemplified by the endogenous catecholamines dopamine, norepinephrine and epinephrine (an example of open-chain 2-phenethylamines), exhibiting a central role in dopaminergic neurons, which play a critical role in voluntary movement, stress or mood [1]. Several naturally occurring alkaloids, i.e., morphine, (S)-reticuline or berberine, embedded in the 2-phenethylamine unit form more complex cyclic frameworks derived from its natural biosynthetic pathways (Figure 1).
Figure 1.
Examples of naturally occurring biologically active compounds displaying a 2-phenethylamine scaffold.
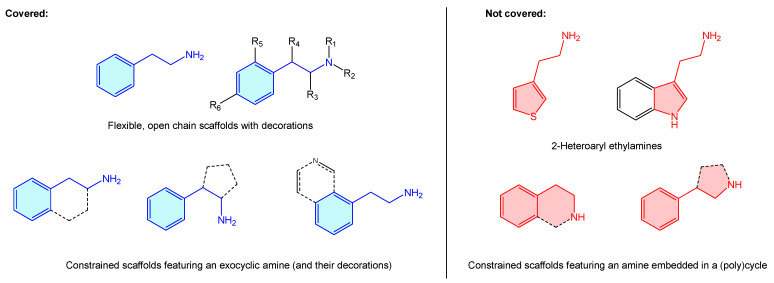
Additionally, in addition to their prominent therapeutic applications, it is worth mentioning the recreational use of a long list of alkaloids incorporating the aforementioned moiety (“designer drugs”) [2], responsible for drug abuse-related conditions [3,4,5,6,7,8]. Surprisingly, the literature lacks a comprehensive summary that bundles up 2-phenethylamine-based structures and known therapeutic targets, including basic hits or advanced leads. Pairing these will present an appealing opportunity to both new and experienced researchers to summarize 2-phenethylamines target binding and therapeutic scope, as well as selectivity/antitarget issues. Considering all these, a review covering the medicinal chemistry landscape is presented here as a brief, central resource linking up 2-phenethylamine hits and receptors. From the structural point of view, 2-phenethylamines present a vast therapeutic chemical space, not just as is, but considering different substitutions, functional group decorations, ring enclosures or heteroaromatic analogues. Describing such a massive quantity of scaffolds with the phenethylamine resemblance is beyond the scope of this review and more, such asly requires a dedicated book. For this reason, the present review covers only structures where there is an alicyclic amine (Figure 2, blue examples), clustering them all under the 2-phenethylamine label. The authors believe that such cases as 2-heteroaryl ethylamines or cyclic amines, despite presenting 2-phenethylamine resemblance, represent other categorized entities (i.e., tetrahydroisoquinolines or 3-phenyl-pirrolidines examples) on their own, worth independent review (Figure 2, red examples). This review also covers those 2-phenethylamines where the phenyl ring is condensed with a heteroaryl ring, as the primary motif ring is a phenyl one.
Figure 2.
Description of 2-phenethylamine scope of the present review.
This review is divided in sections covering those molecular targets where 2-phenethylamines were found to be biologically relevant. Medicinal chemistry leads and state-of-the-art research on novel molecules are described here.
2. 2-Phenethylamine Targets of Biological Importance
2.1. Adenosine Receptors
Adenosine receptors family are G-protein-coupled receptors (GPCR) widely distributed in human body tissue. They have four members, named A1, A2A, A2B and A3, with well-reported activities in mediating inflammation, cardiovascular vasodilation or central and peripheral nervous system pathological responses [9,10,11,12].
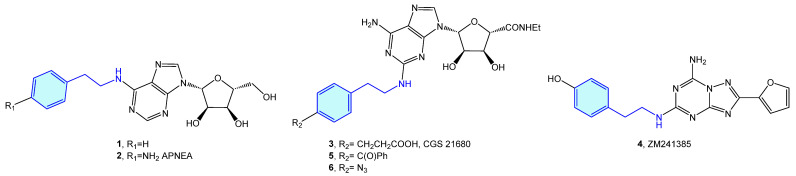
The 2-phenethylamine moiety may be found in a range of AR (adenosine receptors) ligands, such as N6-(2-phenylethyl)adenosine (1) [13], APNEA (N6-[2-(4-ainophenyl)ethyl]adenosine) (2) [14,15], CGS 21680 (3) [16,17,18,19] or ZM241385 (4) [20,21,22]. Murai et al. synthesized photoreactive CGS 21680 derivatives comprising photophores, such as benzophenone 5 or phenylazide 6 for photoaffinity labeling (Figure 3). This allows elucidation of the functional analysis of adenosine receptor A2A through competitive binding assays against [3H]-NECA (N-ethyladenosine-5′-uronamide) [23].
Figure 3.
2-Phenylethyl-based AR ligands.
2.2. α-Adrenergic Receptors
Another class of GPCR targeted by 2-phenethylamines are constituted by α-adrenergic receptors (or α-adrenoceptors). There are two main groups of α-adrenergic receptors, α1 and α2, with several subtypes within (α1A, α1B, α1D, α2A, α2B, α2C). Group α1 is distributed in cardiovascular, intestinal, CNS and urinary systems, while group α2 is located in pancreas, CNS, and cardiovascular regions as well [24].
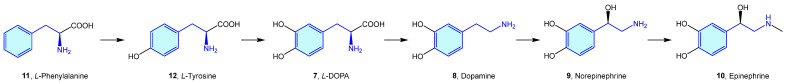
Probably the best representatives of the 2-phenethylamine chemical space are the endogenous catecholamines L-DOPA (7), dopamine (8), norepinephrine (9) (noradrenaline) and epinephrine (10) (adrenaline), biosynthetically produced in cascade from phenylalanine (11)/tyrosine (12) naturally occurring amino acids (Figure 4).
Figure 4.
Catecholamine biosynthetic pathways.
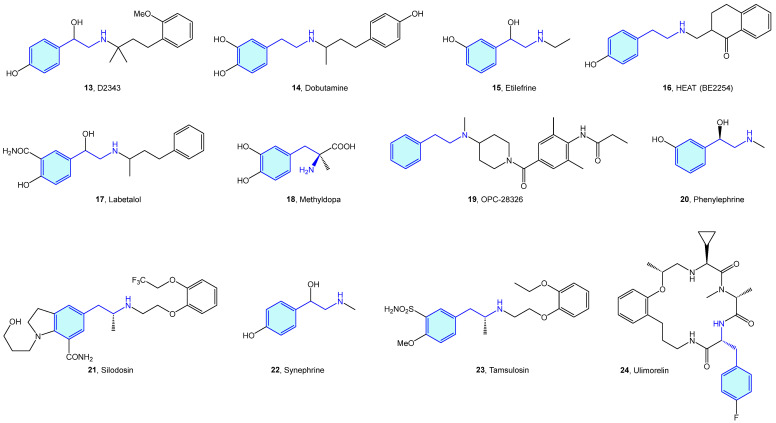
Based on these catecholamines, several studies were performed to investigate the effect of chirality, further functionalization, and activity on different derivatives [25,26,27,28,29,30,31]. This later triggered the elaboration of many derivatives conserving the 2-phenylethyl moiety, which have been frequently used in the context of medicinal chemistry as tool compounds (Figure 5, Table 1).
Figure 5.
2-Phenylethyl-based α-adrenergic medicinal chemistry leads.
Table 1.
α-Adrenergic medicinal chemistry leads.
| Molecule | Name | Primary Targets | Secondary Targets | References |
|---|---|---|---|---|
| 13 | D2343 | α1 | β2-adrenoceptor | [32] |
| 14 | Dobutamine | α1 | β1, β2-adrenoceptors | [33,34,35] |
| 15 | Etilefrine | α1 | AMP-activated protein kinase (AMPK) | [36] |
| 16 | HEAT (BE2254) | α1 | [37,38,39] | |
| 17 | Labetalol | α1 | β-adrenoceptors | [40] |
| 18 | Methyldopa | α2 | [41,42] | |
| 19 | OPC-28326 | α2 | [43,44] | |
| 20 | Phenylephrine | α1 | [45,46] | |
| 21 | Silodosin | α1 | [47,48] | |
| 22 | Synephrine | α1 | β-adrenoceptors | [49,50] |
| 23 | Tamsulosin | α1 | [51] | |
| 24 | Ulimorelin | α1 | Ghrelin receptor (GRLN) | [52] |
2.3. β-Adrenergic Receptors
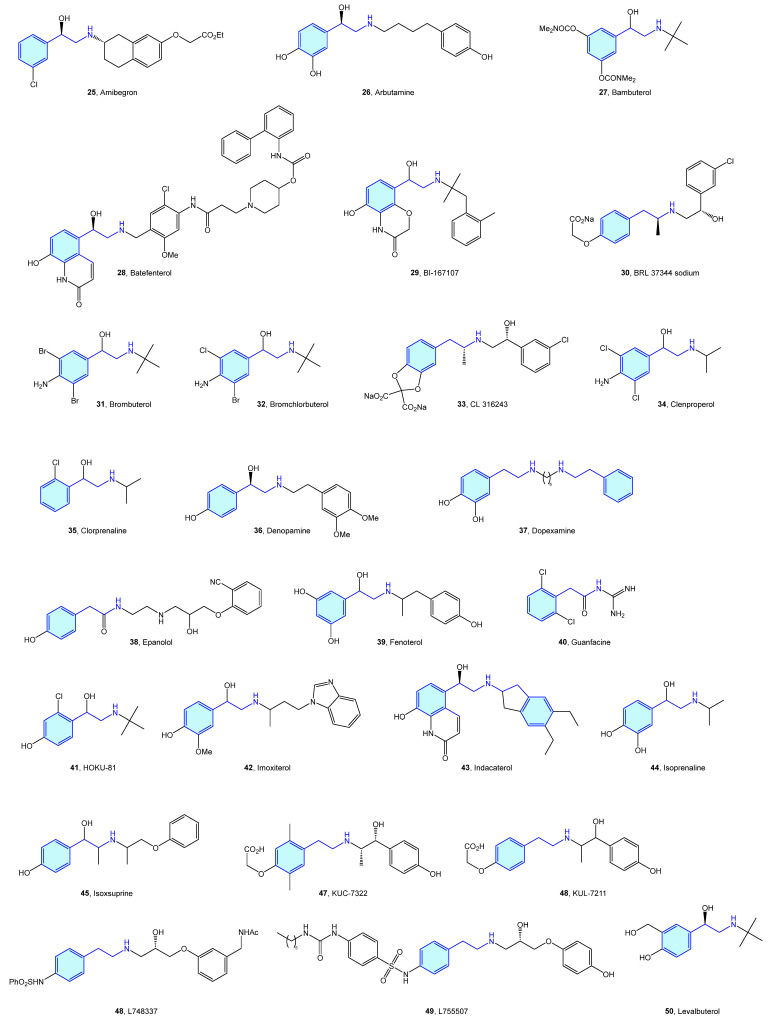
Close to the previously described α-type, the β-adrenergic receptors are also activated by catecholamines norepinephrine and epinephrine. There are three receptor subtypes (β1, β2 and β3) that are implicated in diverse cardiovascular and pulmonary functions [53,54], leading to a vast ligand chemical space (Figure 6, Table 2) to treat cardiogenic shock, heart failure, asthma, overactive bladder (agonists), arrhythmias, hypertension (antagonists commonly known as beta blockers) [55].
Figure 6.
2-Phenylethyl-based β-adrenergic medicinal chemistry leads.
Table 2.
β-Adrenergic medicinal chemistry leads.
| Molecule | Name | Primary Targets | Secondary Targets | References |
|---|---|---|---|---|
| 25 | Amibegron | β3 | [56,57] | |
| 26 | Arbutamine | β | [58,59,60] | |
| 27 | Bambuterol | β | [61] | |
| 28 | Batefenterol | β2 | Muscarinic M2, M3 | [62,63] |
| 29 | BI-167107 | β2 | [64] | |
| 30 | BRL 37344 sodium | β3 | [65] | |
| 31 | Brombuterol | β | [66] | |
| 32 | Bromchlorbuterol | β | [67] | |
| 33 | CL 316243 | β3 | [68,69] | |
| 34 | Clenproperol | β2 | [70] | |
| 35 | Clorprenaline | β2 | [71] | |
| 36 | Denopamine | β1 | [72] | |
| 37 | Dopexamine | β2 | [73] | |
| 38 | Epanolol | β | [74] | |
| 39 | Fenoterol | β2 | [75,76] | |
| 40 | Guanfacine | β1 | α2-adrenoceptors | [77] |
| 41 | HOKU-81 | β2 | [78] | |
| 42 | Imoxiterol | β | [79] | |
| 43 | Indacaterol | β | [80] | |
| 44 | Isoprenaline | β | [81,82,83] | |
| 45 | Isoxsuprine | β | N-methyl-D-aspartate (NMDA) | [84] |
| 46 | KUC-7322 | β3 | [85] | |
| 47 | KUL-7211 | β | [86] | |
| 48 | L748337 | β3 | [87] | |
| 49 | L755507 | β3 | [88] | |
| 50 | Levalbuterol | β2 | [89] | |
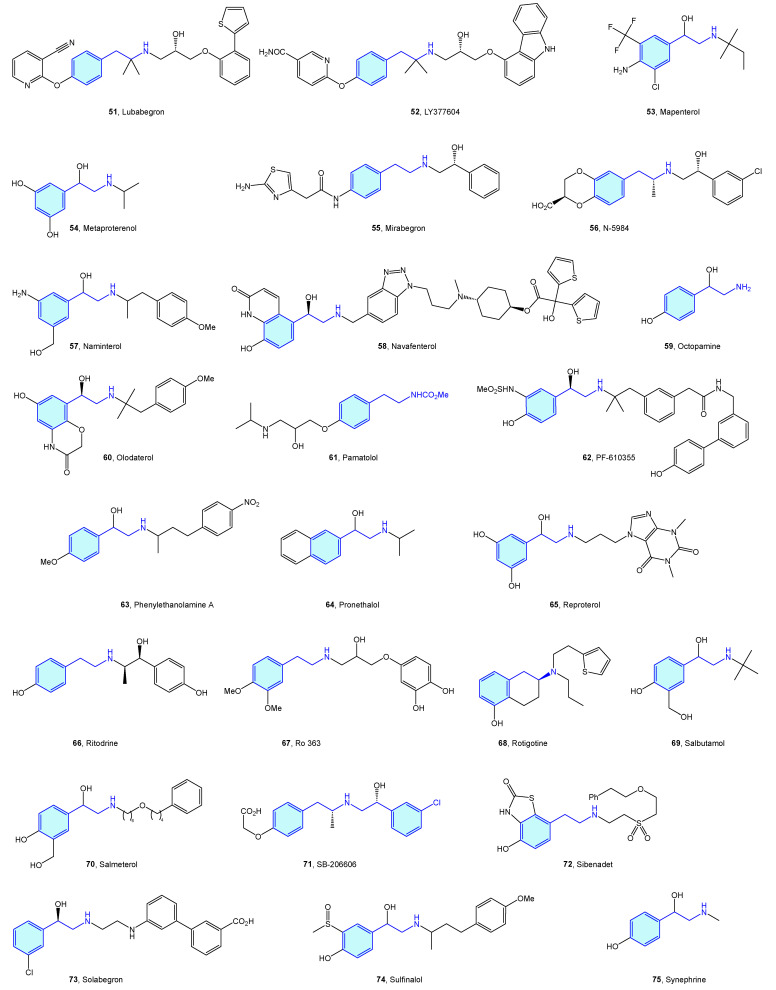
| 51 | Lubabegron | β | [90] | |
| 52 | LY377604 | β3 | [91] | |
| 53 | Mapenterol | β2 | [92] | |
| 54 | Metaproterenol | β2 | [93,94] | |
| 55 | Mirabegron | β3 | [95] | |
| 56 | N-5984 | β3 | [96] | |
| 57 | Naminterol | β2 | [97] | |
| 58 | Navafenterol | β2 | Muscarinic M3 | [98] |
| 59 | Octopamine | β | [99,100] | |
| 60 | Olodaterol | β2 | [101,102] | |
| 61 | Pamatolol | β | [103] | |
| 62 | PF-610355 | β2 | [104,105] | |
| 63 | Phenylethanolamine A | β | [106] | |
| 64 | Pronethalol | β | [107] | |
| 65 | Reproterol | β2 | Phosphodiesterase (PDE) | [108] |
| 66 | Ritodrine | β2 | [109] | |
| 67 | Ro 363 | β1 | [110] | |
| 68 | Rotigotine | β | α2-adrenoceptor, 5-HT1A, Dopamine D2, D3, D4, D5 | [111] |
| 69 | Salbutamol | β2 | [112] | |
| 70 | Salmeterol | β2 | [113] | |
| 71 | SB-206606 | β3 | [114] | |
| 72 | Sibenadet | β | Dopamine D2 | [115] |
| 73 | Solabegron | β3 | [116] | |
| 74 | Sulfinalol | β | [117] | |
| 75 | Synephrine | β | α-adrenoceptor | [118] |
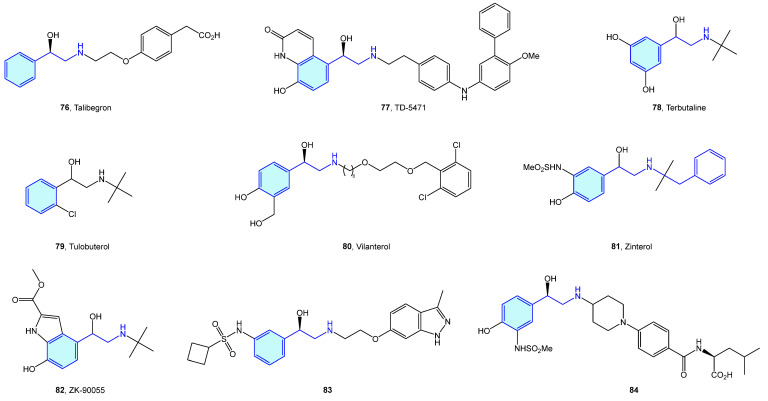
| 76 | Talibegron | β3 | [119] | |
| 77 | TD-5471 | β2 | [120] | |
| 78 | Terbutaline | β2 | [121,122] | |
| 79 | Tulobuterol | β2 | [123] | |
| 80 | Vilanterol | β | [124,125] | |
| 81 | Zinterol | β2 | [126] | |
| 82 | ZK-90055 | β2 | [127] | |
| 83 | - | β3 | [128] | |
| 84 | - | β3 | [129] |
2.4. Aldose Reductase
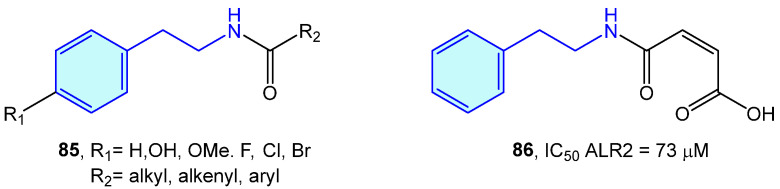
ALR2 aldose reductase is an enzyme of the polyol pathway responsible for the transformation of glucose into sorbitol, with relevant involvement in long-term diabetic complications. A series of modified 2-phenethylamines 85 comprising the insertion of aliphatic chains, aromatic rings or carboxylic acids were elaborated by Sun et al. [130]. This resulted in the obtention of a small library of derivatives with low inhibition effects towards in vitro pig kidney ALR2 (Figure 7).
Figure 7.
2-Phenylethyl-based ALR2 ligands by Sun et al [130].
2.5. Carbonic Anhydrase
Carbonic anhydrases (CAs) are Zn-based (also Fe-based) metalloenzymes present across all living organisms of the different life kingdoms. Divided in eight different families (α, β, γ, δ, ε, ζ, η, θ, and t types), they catalyze the hydration of carbon dioxide to bicarbonate, with the purpose of transporting CO2 to HCO3- between tissue types, contributing to pH homeostasis, bone calcification and electrolyte transport, and ultimately participate in biogenic routes/processes, such as lipogenesis, ureagenesis or gluconeogenesis [131,132,133].
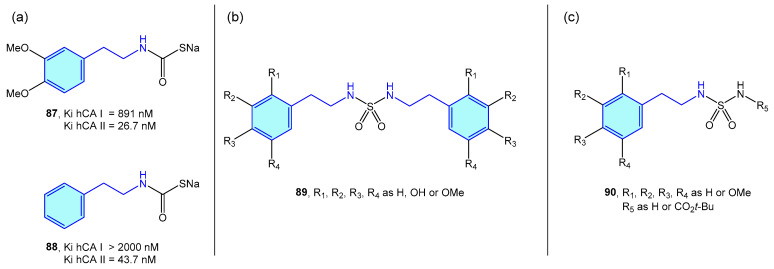
Several groups have independently used 2-phenethylamine based sulfamides and monothiocarbamates (Figure 8) as CA inhibitors, as frequently targeting a specific CA is related to a certain syndrome or disease, such as glaucoma [134], obesity [135], or certain types of cancer [136].
Figure 8.
2-Phenylethyl-based CA ligands.
Nocentini et al. tested phenethylamine monothiocarbamates 87 and 88 as well as other cyclic derivatives against human CA I/II (hCA), with 26–43 nM activities in type II (Figure 8a) [137]. Symmetric sulfamides were employed by Topal et al. (Figure 8b) with hCAI/II inhibition demonstrated at nanomolar level [138]. Branched sulfamides integrating the 1-phenyl-2-phenethylamine scaffold, elaborated by Akıncıoğlu et al. [139] were found to be single-digit nanomolar inhibitors of both type I/II hCA (Figure 8c).
2.6. Dopamine β-Hydroxylase
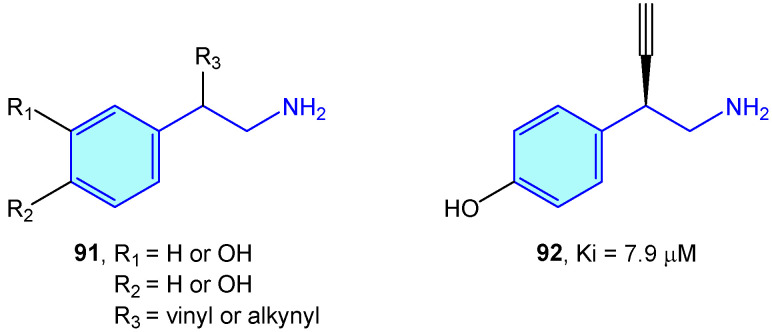
Dopamine β-hdroxylase (DBH) is a Cu-based oxidoreductase that controls dopamine transformation into norepinephrine (Figure 2) in several neuron types (like adrenergic or noradrenergic ones) [140]. Limited studies have been developed on the use of modified 2-phenethylamines to target DBH. Kruse et al. [141] developed 2-vinyl- and 2-alkynyl-based 2-phenethylamines with moderate vitro activities (Figure 9).
Figure 9.
2-Phenylethyl-based DBH ligands by Kruse et al [141].
More advanced DBH inhibitors are constituted by imidazolethione amines, such as etamicastat [142,143], nepicastat [144] or zamicastat [145], with low resemblance to dopaminergic amines.
2.7. Dipeptidyl Peptidases (DPP)
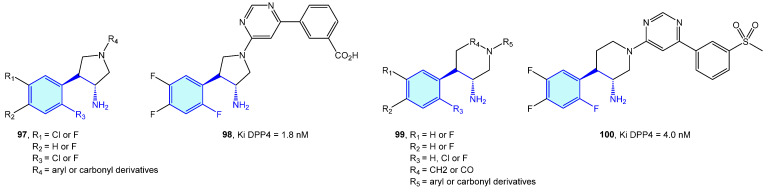
Dipeptidyl peptidases are exopeptidases responsible for proteolytic transformations, specifically cleaving the peptide bond after the penultimate proline residue. DPP4 is a serine protease displaying a critical role in cell adhesion, inflammation processes and immune regulation by deactivating GLP-1 and hence lowering blood glucose levels [146]. Type 2 diabetes is the main therapeutic area were DPP4 ligands have been discovered (Figure 10 and Figure 11, Table 3).
Figure 10.
2-Phenylethyl-based DPP4 medicinal chemistry leads.
Figure 11.
2-Phenylethyl-based DPP4 receptor ligands.
Table 3.
DPP4 medicinal chemistry leads.
Backes et al. [154] and and Pei et al. [155] developed pyrrolidine-constrained and piperidine-constrained phenethylamines selectively targeting DPP4 with interesting PK profiles (Figure 11).
2.8. Dopamine Receptors (DX)
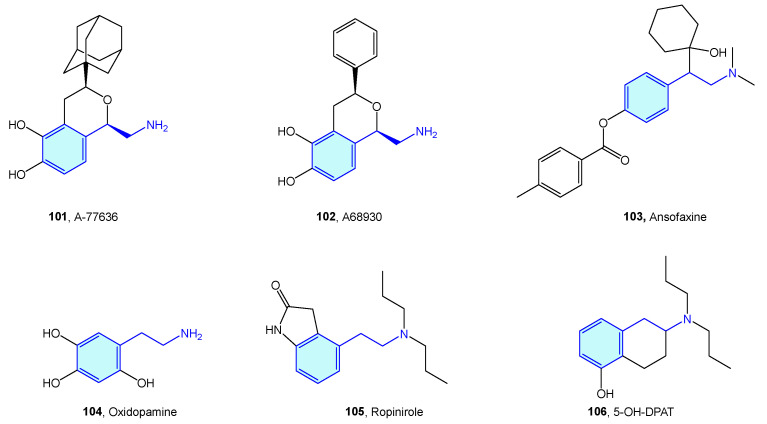
Dopamine receptors are a class of GPCR widely distributed in the brain, with key functionalities related to cognition, motivation and muscular drive. Pharmacologically, they are grouped into two families: D1-type (D1 and D5 receptors) and D2-type (D2S, D2L, D3 and D4) [156,157]. Medicinal chemistry of D1/D2-type receptor ligands addresses mainly the treatment of schizophrenia. From the chemical point of view concerning this review, a few small molecules presenting a basic 2-phenethylamine structure are reported in the literature (Figure 12, Table 4).
Figure 12.
2-Phenylethyl-based dopamine receptor medicinal chemistry leads.
Table 4.
Dopamine receptor medicinal chemistry leads.
2.9. Dopamine Transporter (DAT)
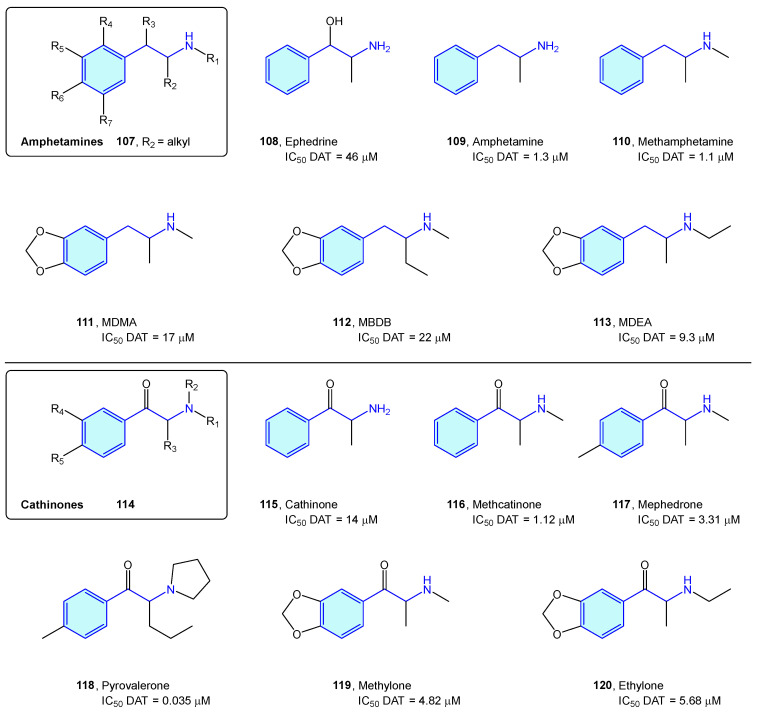
The dopamine transporter receptor modulates the availability of released dopamine in the synaptic space by relocating it back into the presynaptic cell. It serves as a main target for recreational drugs as well as psychostimulant and antidepressant drugs. Classically, DAT ligands are classified in amphetamine-type and cocaine-type structures [164]. While cocaine-type molecules exert inhibitory binding to DAT, amphetamine-type ones are substrates that are effectively transported to the presynaptic neuron, stimulating efflux of cytosolic dopamine [165].
Amphetamine-type DAT ligands can be grouped in two families: amphetamine derivatives 107 and cathinone derivatives 114. Amphetamines are 1-alkyl-2-phenethylamine derivatives 107 summarized below (Figure 13, Table 5). SAR attributes [166] are well known in this series, with diversification of the biological response with aryl substitutions, pharmacokinetic parameter shift upon alkylation of the amino group and decrease in dopaminergic pathways influenced by elongation of the alkyl chain at position 1. Due to being controlled substances in most countries, the number of compounds is constantly increasing, trying to avoid the introduction of compounds in the corresponding prohibition lists of each state [164,167]. Major concerns of this series are neurotoxicity, myocardial infarction, aneurysms, pulmonary hypertension, and tooth decay [168].
Figure 13.
Amphetamine/cathinone-type families.
Table 5.
Dopamine transporter classical binders.
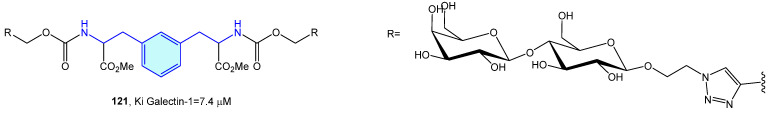
2.10. Galectin-1 Receptor
Galectins are a family of soluble carbohydrate binding proteins with several roles in inflammation, immune response, autophagy or signaling. Comprising 16 members, only 12 are expressed in humans [171]. Tejler et al. [172] described the synthesis of lactose derivatives, such as 121 with the phenethylamine moiety by 1,3-dipolar cycloadditions, with selective galectin-1 inhibition (Figure 14).
Figure 14.
2-Phenylethyl-based galectin-1 receptor ligands.
2.11. HIV-1 Reverse Transcriptase Receptor
Human immunodeficiency virus (HIV), origin of acquired immunodeficiency syndrome (AIDS), is a single-stranded (ss) RNA virus whose infection and propagation mechanisms requires reverse transcription into double-stranded (ds) DNA as a critical stage [173]. HIV-1 reverse transcriptase receptor (HIV-1 RT) is a well-explored target for antiretroviral therapies [174].
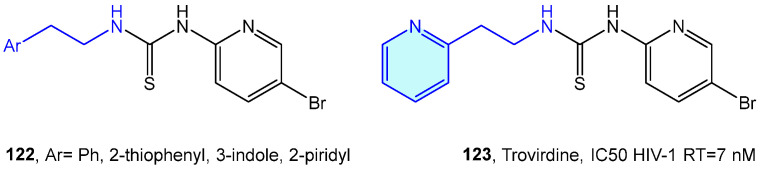
Venkatachalam et al. [175] designed a library of phenethyl thiourea compounds with good potency against HIV-1 RT inhibition without any evidence of cytotoxicity [175,176,177]. Eventually (Figure 15), these derivatives evolve in the phenethylthiazolylthiourea (PETT) family, with trovirdine as its prominent inhibitor [178,179,180].
Figure 15.
2-Phenylethyl-based HIV-1 RT receptor ligands.
2.12. 5-Hydroxytriptamine (5-HT) Receptors
5-Hydroxytryptamine (5-HT) receptors are one of the most extensively studied receptor families, with seven subtypes (5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7) identified [181]. Therapeutic indications of 5-HT ligands and advanced leads (Figure 16, Table 6) cover different conditions, such as migraine, depression, social phobia, obsessive–compulsive disorder, anxiety, schizophrenia, eating disorders, panic-disorders, hypertension, pulmonary hypertension, emesis, vomiting, and irritable bowel syndrome (IBS) [182].
Figure 16.
2-Phenylethyl-based 5-HT medicinal chemistry leads.
Table 6.
5-HT medicinal chemistry leads.
| Molecule | Name | Primary Targets | Secondary Targets | References |
|---|---|---|---|---|
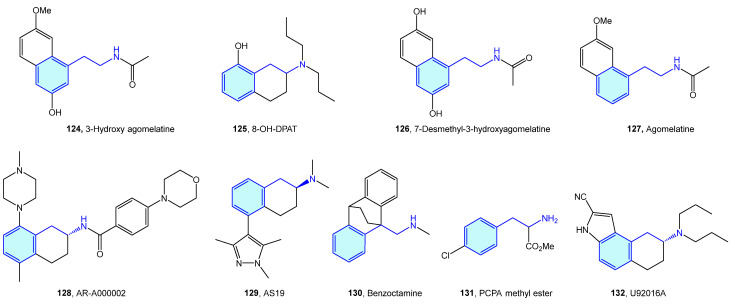
| 124 | 3-Hydroxy agomelatine | 5-HT2C | [183] | |
| 125 | 8-OH-DPAT | 5-HT1A | [184,185] | |
| 126 | 7-Desmethyl-3-hydroxyagomelatine | 5-HT2C | Melatonin MT1, MT2 | [186] |
| 127 | Agomelatine | 5-HT | Melatonin MT1, MT2 | [187] |
| 128 | AR-A000002 | 5-HT1B | [188] | |
| 129 | AS19 | 5-HT7 | [189,190] | |
| 130 | Benzoctamine | 5-HT | [191] | |
| 131 | PCPA methyl ester | 5-HT | [192] | |
| 132 | U92016A | 5-HT1A | [193,194] |
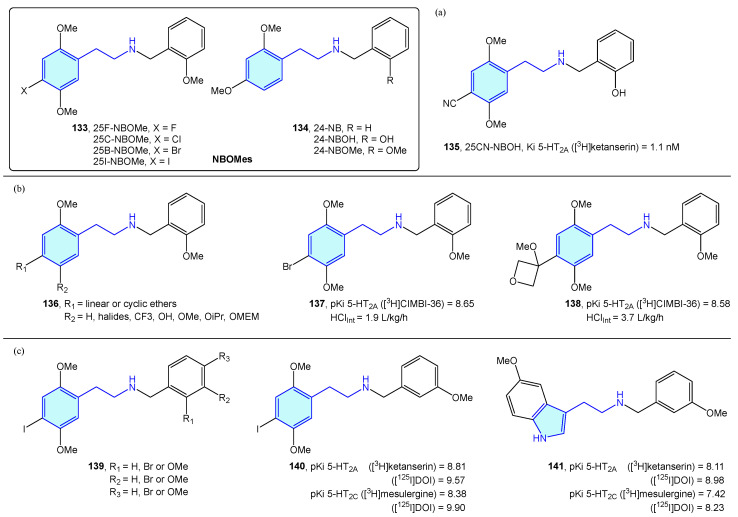
A novel class of 2-phenethylamines with hallucinogenic/psychedelic effects are N-benzylphenethylamines or NBOMes (133, 134) (Figure 17) [195]. These agents have a selective binding profile towards 5-HT2 receptor subtypes (5-HT2A, 5-HT2B, 5-HT2C), making them promising therapeutic compounds. Traditionally, the assumption of converting the primary amine into a secondary one was associated with a prominent loss in 5-HT2A activity. N-benzyl substitution was found to be the exception, increasing affinity and potency at the receptor [196]. SAR exploration of the NBOMes scaffold led to defining avoidable regions for SAR expansion, while mapping tolerated substitutions seeking potency/selectivity [197]. From the original 25X-NBOMe halide derivatives [198], different derivatives have evolved.
Figure 17.
NBOMes with 5-HT receptor activity.
Jensen et al. developed 25CN-NBOH (135) (Figure 17a) as a result of halide substitution by the cyano moiety, displaying high-picomolar/low-nanomolar binding affinities (competition binding assays with [3H]ketanserin) and functional potencies at 5-HT2A receptor. Leth-Petersen et al. [199] designed a library of 25B-NBOMe analogues 136, such as 137 or 138 in the search of decreasing intrinsic clearance (Figure 17b). Despite the authors’ efforts to decrease intrinsic clearance by lipophilicity reduction, no correlation was found, although several 5-HT2 potent compounds were synthesized. Nichols et al. [200] explored the impact of methoxy and bromo scanning along the benzyl ring of 25I-NBOMe (139). Ortho or meta positions enhanced activity, whereas the para substitution reduced it. One of the best derivatives was 140, which was compared with its tryptamine congener 141, less potent overall in the 5-HT2 assays performed (Figure 17c).
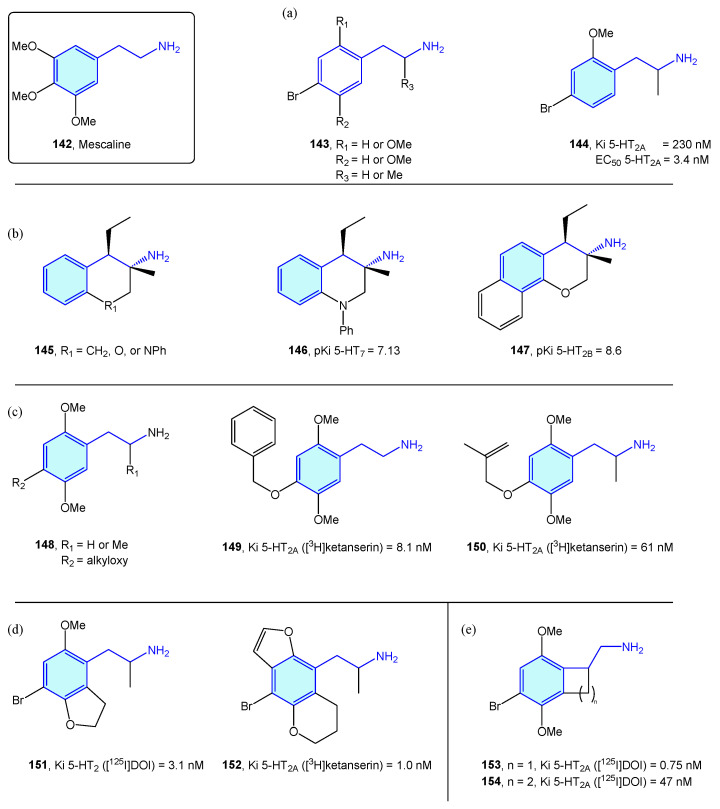
NBOMes and polyalkoxylated phenethylamines could be envisaged as mescaline (Figure 16, 142) evolving structures. In this sense, significant efforts have been made to derive rational SAR maps together with attractive bioactive chemical matter [201]. Marcher-Rørsted et al. [202] reported the insertion of 2,5-dimethoxy motif in phenethylamine-like 5-HT2A agonists. They demonstrated that this motif is relevant for in vivo potency, but without observed correlations in affinity or potency in competition binding assays (Figure 18a). Oxygen-to-sulfur exchange reduces hallucinogenic-associated activity [203], while removal of one of the 2- or 5-position methoxy groups decreased in vivo activity [166]. Porter et al. [204] have derived 3-amino-chromanes and tetrahydroquinolines as selective 5-HT2B/5-HT7 ligands (Figure 18b). 5-HT2B is not considered an optimal target, due to valvular heart disease and myofibroblast proliferation by long-term consumption of selective agonists [205,206]. 5-HT7 is implicated in sleep, mood and circadian rhythm functions [207]. Kolaczynska et al. [208] analyzed the impact in 5-HT activity of 4-alkoxy exploration of 2,5-dimethoxyphenethylamines and amphetamines (Figure 18c). Both derivatives were found to interact strongly and selectively with 5-HT2A, demonstrating that size and lipophilicity increase in this region favors 5-HT2A/C affinity. Schultz et al. [209] and Nichols et al. [210] explored the fusion of furane/pyrane rings with the aromatic ring, as probes of the binding pocket size of 5-HT2A receptor subtype and lone pair suitable orientation of the 2,5-oxy substituents, furnishing nanomolar-range receptor affinities (Figure 18d). McLean et al. [211] portrayed the conformationally restriction of 2-phenethylamines via 1-aminomethylbenzocycloalkanes syntheses (Figure 18e). Benzocyclobutene derivative 153’s strong potency against 5-HT2A showed the hypothesis that the side chain of the phenethylamine binds in an out-of-the-plane conformation. Some of these conformationally restricted phenethylamines exhibit affinity for muscarinic receptors [212].
Figure 18.
2-Phenethylamines with 5-HT receptor activity.
2.13. Monoamine Oxidase (MAO) Receptors
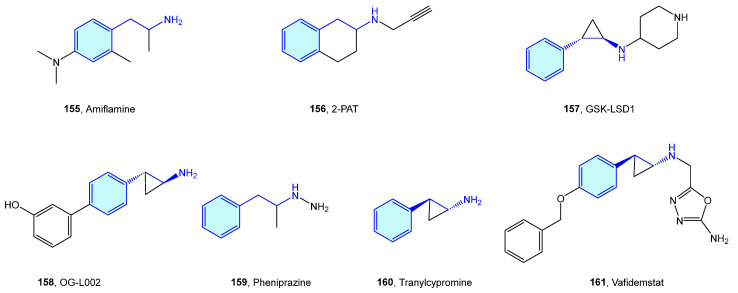
Monoamine oxidases (MAO) are flavin-containing enzymes that catalyze the oxidative deamination of monoamines, which are bound to the outer membrane of mitochondria. Common MAO substrates are 5-hydroxy-tryptamine and catecholamines (dopamine, norepinephrine and epinephrine). MAO A and MAO B are the two enzyme isoforms, sharing a 70% sequence identity and differentiating each other in the substrate scope [213]. Theoretical approaches to rationalize isoform selection by substrates have been described [214,215]. As these two oxidases are responsible for neurotransmitter inactivation by oxidation, their dysfunction drives several neurological disorders, hence the therapeutic attractiveness (Figure 19, Table 7).
Figure 19.
2-Phenethylamine MAO medicinal chemistry leads.
Table 7.
MAO medicinal chemistry leads.
| Molecule | Name | Primary Targets | Secondary Targets | References |
|---|---|---|---|---|
| 155 | Amiflamine | MAO A | [216] | |
| 156 | 2-PAT | MAO A, B | [217] | |
| 157 | GSK-LSD1 | MAO | Lysine specific demethylase 1 (LSD1) | [218] |
| 158 | OG-L002 | MAO A, B | [219] | |
| 159 | Pheniprazine | MAO | [220] | |
| 160 | Tranylcypromine | MAO | [221] | |
| 161 | Vafidemstat | MAO B | Lysine specific demethylase 1 (LSD1) | [222] |
2.14. Opioid Receptors
Opioid receptors are a class of GPCR proteins consisting of three receptor types, mainly µ-, δ-, and κ- types, with a variety of functional roles in the nervous system, such as pain signaling, growth, respiration, and immunological response [223,224]. Opioid ligands modulate neuronal inhibition and ultimately analgesia. Relevant side effects are well known, such as constipation or drug dependence/abuse.
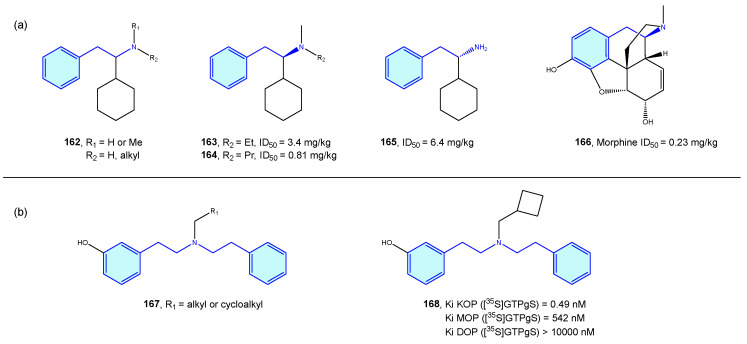
Takahashi et al. [225,226,227,228,229,230] derived a small series of flexible 2-phenethylamines with analgesic activities, with moderate potency effects when compared with pentazocine or morphine (Figure 20a). Manchado et al. [231] developed quick asymmetric routes furnishing this type of derivative. Spetea et al. [232] developed selective diphenethylamine-based tertiary amines as κ-opioid receptors with 100-fold and 1000-fold selectivity difference compared with its congeners (Figure 20b).
Figure 20.
2-Phenethylamines with 5-HT receptor activity.
2.15. Peroxisome Proliferator-Activated (PPAR) Receptors
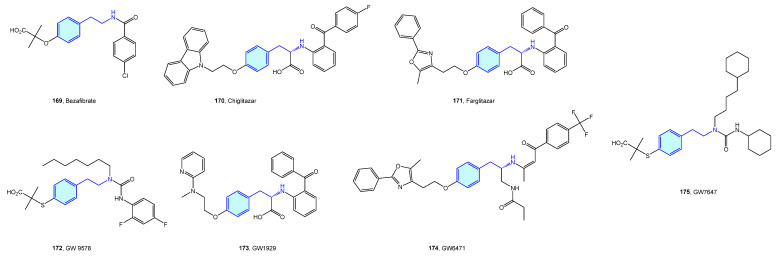
Peroxisome proliferator-activated (PPAR) receptors are peroxisome receptors and subcellular organelles performing several tasks related to cholesterol and fatty acid metabolism. Three members, named PPAR-α, PPAR-δ, and PPAR-γ, form this family, with different levels of expression and functionalities in diverse tissues, from energy storage in endothelial and vascular smooth muscle cells (type γ) to energy expenditure across all bodies (type δ). Several agents have been developed in relation to PPAR to address obesity, inflammation or neurodegenerative disorders (Figure 21, Table 8) [233,234].
Figure 21.
2-Phenethylamine PPAR medicinal chemistry leads.
Table 8.
PPAR medicinal chemistry leads.
2.16. Sigma Receptors
Initially described as opioid receptors, sigma receptors conform their own family, unrelated to other receptors. Both members σ1R and σ2R are primarily found at the endoplasmic reticulum, and participate in diverse conditions, such as cancer, pain, neurodegenerative diseases or depression [243]. BD-1047 (Figure 22) is an open-chain, flexible 2-phenethylamine acting as antagonist of σ1R [244].
Figure 22.
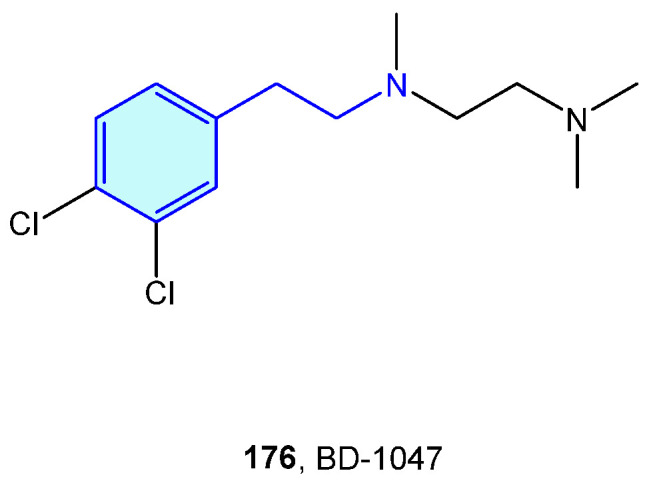
A 2-Phenethylamine Sigma-1 receptor hit.
2.17. Trace Amine-Associated Receptors (TAAR)
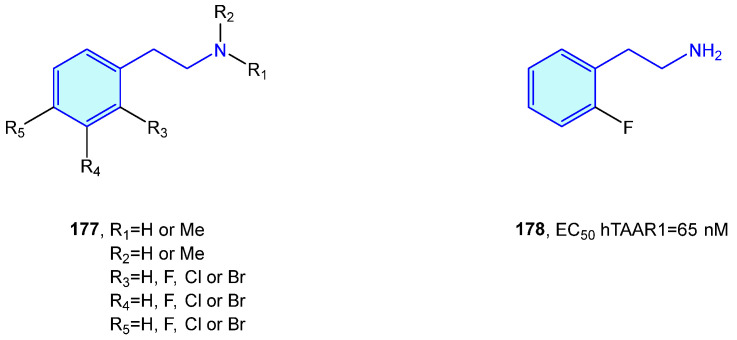
Relatively recently discovered, trace amine-associated receptors (TAAR) are a GPCR family composed of nine members (TAAR1 to 9) with prospective therapeutic applications in the field of schizophrenia and metabolic disorders [245]. Lewin et al. [246] carried out SAR explorations with simple 2-phenethylamines to envisage improved pharmacological hits (Figure 23).
Figure 23.
2-Phenethylamines with hTAAR1 bioactivity.
3. Methods
All described compounds, targets and activities were retrieved using “2-phenethylamine” as title or keyword term in the chemical databases SciFinder [247] and Scopus [248]. Additionally, a SciFinder and Scopus structure search, with the scope described early in this review (Figure 2), was employed.
4. Conclusions
This review represents a concise, central summary of relevant 2-phenethylamine-based leads and research hits, which spans receptors and their corresponding therapeutic indications. This report serves as a guide to researchers interested in medicinal chemistry to identify suitable ligand–target associations displaying the aforementioned motif, as well as help to identify prospective targets of brand-new molecules with the 2-phenethylamine core embedded.
Future directions will include both a complementary report covering synthetic strategies to access 2-phenethylamine derivatives and a second, satellite review of 2-heteroaryl-ethylamines in medicinal chemistry.
Author Contributions
Conceptualization, C.T.N., A.M., L.B., D.D. and N.M.G.; investigation, C.T.N., A.M. and L.B.; data curation, C.T.N., A.M. and L.B.; writing—original draft preparation, C.T.N.; writing—review and editing, C.T.N., A.M., L.B., D.D. and N.M.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors gratefully acknowledge the financial support of this work provided by Ministerio de Ciencia e Innovación (PID2020-118303GB-I00/MCIN/AEI/10.13039/501100011033) and Junta de Castilla y Leon (SA0076P20). C.T.N. thanks Junta de Castilla y Leon for a postdoctoral contract (SA0076P20).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chinta S.J., Andersen J.K. Dopaminergic neurons. Int. J. Biochem. Cell Biol. 2005;37:942–946. doi: 10.1016/j.biocel.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Dean B.V., Stellpflug S.J., Burnett A.M., Engebretsen K.M. 2C or Not 2C: Phenethylamine Designer Drug Review. J. Med. Toxicol. 2013;9:172–178. doi: 10.1007/s13181-013-0295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kronstrand R., Guerrieri D., Vikingsson S., Wohlfarth A., Gréen H. Fatal Poisonings Associated with New Psychoactive Substances. In: Maurer H.H., Brandt S.D., editors. New Psychoactive Substances: Pharmacology, Clinical, Forensic and Analytical Toxicology. Springer International Publishing; Cham, Switzerland: 2018. pp. 495–541. [DOI] [PubMed] [Google Scholar]
- 4.Grecco G.G., Sprague J.E. Impact of Functional Group Modifications on Designer Phenethylamine Induced Hyperthermia. Chem. Res. Toxicol. 2016;29:871–878. doi: 10.1021/acs.chemrestox.6b00030. [DOI] [PubMed] [Google Scholar]
- 5.Inan F., Brunt T.M., Contrucci R.R., Hondebrink L., Franssen E.J.F. Novel Phenethylamines and Their Potential Interactions With Prescription Drugs: A Systematic Critical Review. Ther. Drug. Monit. 2020;42:271–281. doi: 10.1097/FTD.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 6.Cocchi V., Gasperini S., Hrelia P., Tirri M., Marti M., Lenzi M. Novel Psychoactive Phenethylamines: Impact on Genetic Material. Int. J. Mol. Sci. 2020;21:9616. doi: 10.3390/ijms21249616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sogos V., Caria P., Porcedda C., Mostallino R., Piras F., Miliano C., De Luca M.A., Castelli M.P. Human Neuronal Cell Lines as An In Vitro Toxicological Tool for the Evaluation of Novel Psychoactive Substances. Int. J. Mol. Sci. 2021;22:6785. doi: 10.3390/ijms22136785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagai F., Nonaka R., Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur. J. Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson K.A., Gao Z.-G. Reference Module in Neuroscience and Biobehavioral Psychology. Elsevier; Amsterdam, The Netherlands: 2017. Adenosine☆. [Google Scholar]
- 10.Ballesteros-Yáñez I., Castillo C.A., Merighi S., Gessi S. The Role of Adenosine Receptors in Psychostimulant Addiction. Front. Pharmacol. 2018;8:985. doi: 10.3389/fphar.2017.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masino S.A., Kawamura M., Ruskin D.N. Chapter Eleven—Adenosine Receptors and Epilepsy: Current Evidence and Future Potential. In: Mori A., editor. International Review of Neurobiology. Academic Press; New York, NY, USA: 2014. pp. 233–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Calker D., Biber K., Domschke K., Serchov T. The role of adenosine receptors in mood and anxiety disorders. J. Neurochem. 2019;151:11–27. doi: 10.1111/jnc.14841. [DOI] [PubMed] [Google Scholar]
- 13.Tchilibon S., Kim S.-K., Gao Z.-G., Harris B.A., Blaustein J.B., Gross A.S., Duong H.T., Melman N., Jacobson K.A. Exploring distal regions of the A3 adenosine receptor binding site: Sterically constrained N6-(2-phenylethyl)adenosine derivatives as potent ligands. Biorg. Med. Chem. 2004;12:2021–2034. doi: 10.1016/j.bmc.2004.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borowicz K.K., Kleinrok Z., Czuczwar S.J. N6-2-(4-Aminophenyl)ethyl-adenosine enhances the anticonvulsive action of conventional antiepileptic drugs in the kindling model of epilepsy in rats. Eur. Neuropsychopharmacol. 2000;10:237–243. doi: 10.1016/S0924-977X(00)00081-X. [DOI] [PubMed] [Google Scholar]
- 15.Borowicz K.K., Kleinrok Z., Czuczwar S.J. N6-2-(4-Aminophenyl)ethyl-adenosine enhances the anticonvulsive activity of antiepileptic drugs. Eur. J. Pharmacol. 1997;327:125–133. doi: 10.1016/S0014-2999(97)89651-3. [DOI] [PubMed] [Google Scholar]
- 16.Bao R., Shui X., Hou J., Li J., Deng X., Zhu X., Yang T. Adenosine and the adenosine A2A receptor agonist, CGS21680, upregulate CD39 and CD73 expression through E2F-1 and CREB in regulatory T cells isolated from septic mice. Int. J. Mol. Med. 2016;38:969–975. doi: 10.3892/ijmm.2016.2679. [DOI] [PubMed] [Google Scholar]
- 17.Komaki S., Ishikawa K., Arakawa Y. Trk and cAMP-dependent survival activity of adenosine A2A agonist CGS21680 on rat motoneurons in culture. Neurosci. Lett. 2012;522:21–24. doi: 10.1016/j.neulet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Melani A., Corti F., Cellai L., Giuliana Vannucchi M., Pedata F. Low doses of the selective adenosine A2A receptor agonist CGS21680 are protective in a rat model of transient cerebral ischemia. Brain Res. 2014;1551:59–72. doi: 10.1016/j.brainres.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M., Li X.-L., Li H., Wang S., Wang C.-C., Yue L.-T., Xu H., Zhang P., Chen H., Yang B., et al. Activation of the adenosine A2A receptor exacerbates experimental autoimmune neuritis in Lewis rats in association with enhanced humoral immunity. J. Neuroimmunol. 2016;293:129–136. doi: 10.1016/j.jneuroim.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Ludwig S., Hong C.-S., Razzo B.M., Fabian K.P.L., Chelvanambi M., Lang S., Storkus W.J., Whiteside T.L. Impact of combination immunochemotherapies on progression of 4NQO-induced murine oral squamous cell carcinoma. Cancer Immunol. Immunother. 2019;68:1133–1141. doi: 10.1007/s00262-019-02348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poucher S.M., Keddie J.R., Singh P., Stoggall S.M., Caulkett P.W.R., Jones G., Collis M.G. The in vitro pharmacology of ZM 241385, a potent, non-xanthine, A2a selective adenosine receptor antagonist. Br. J. Pharmacol. 1995;115:1096–1102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z., Che P.-L., Du J., Ha B., Yarema K.J. Static Magnetic Field Exposure Reproduces Cellular Effects of the Parkinson’s Disease Drug Candidate ZM241385. PLoS ONE. 2010;5:e13883. doi: 10.1371/journal.pone.0013883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murai Y., Masuda K., Ogasawara Y., Wang L., Hashidoko Y., Hatanaka Y., Iwata S., Kobayashi T., Hashimoto M. Synthesis of Photoreactive 2-Phenethylamine Derivatives—Synthesis of Adenosine Derivatives Enabling Functional Analysis of Adenosine Receptors by Photoaffinity Labeling. Eur. J. Org. Chem. 2013;2013:2428–2433. doi: 10.1002/ejoc.201201657. [DOI] [Google Scholar]
- 24.Friedhoff A.J., Silva R. Catecholamines. In: Ramachandran V.S., editor. Encyclopedia of the Human Brain. Academic Press; New York, NY, USA: 2002. pp. 595–602. [Google Scholar]
- 25.Vago T., Meroni R., Dagani R., Baldi G., Pagani M., Bevilacqua M., Norbiato G. Characterization of α2-adrenoceptor binding properties of imidazoline-like drugs, azoloazepine derivatives and β-phenethylamine-like drugs in human platelet membranes. J. Pharm. Pharmacol. 1985;37:593–596. doi: 10.1111/j.2042-7158.1985.tb03079.x. [DOI] [PubMed] [Google Scholar]
- 26.Mottram D.R., Thakar Y. The action and interaction of β-phenethylamines and imidazolines on prejunctional α2-adrenoceptors of guinea-pig ileum in the presence of the non-competitive antagonist benextramine. J. Pharm. Pharmacol. 1984;36:668–672. doi: 10.1111/j.2042-7158.1984.tb04840.x. [DOI] [PubMed] [Google Scholar]
- 27.Smith H.E., Burrows E.P., Mobley P.L., Robinson S.E., Sulser F. Agonist effects of.beta.-phenethylamines on the noradrenergic cyclic adenosine 3′,5′-monophosphate generating system in rat limbic forebrain. Stereoisomers of p-hydroxynorephedrine. J. Med. Chem. 1977;20:978–981. doi: 10.1021/jm00217a028. [DOI] [PubMed] [Google Scholar]
- 28.Ghouri M.S.K., Haley T.J. Alpha-Adrenergic Receptor-Blocking Properties of Six Phenethylamine Derivatives In Vitro. J. Pharm. Sci. 1969;58:760–761. doi: 10.1002/jps.2600580626. [DOI] [PubMed] [Google Scholar]
- 29.Akinaga J., Lima V., Kiguti L.R.d.A., Hebeler-Barbosa F., Alcántara-Hernández R., García-Sáinz J.A., Pupo A.S. Differential Phosphorylation, Desensitization, and Internalization of α1A−Adrenoceptors Activated by Norepinephrine and Oxymetazoline. Mol. Pharmacol. 2013;83:870–881. doi: 10.1124/mol.112.082313. [DOI] [PubMed] [Google Scholar]
- 30.Esbenshade T.A., Hamada A., Miller D.D., Feller D.R. Effects of the stereochemical orientation of phenethylamines and imidazolines on α-adrenergic receptor-mediated dna synthesis in primary cultured rat hepatocytes. Chirality. 1992;4:279–285. doi: 10.1002/chir.530040504. [DOI] [PubMed] [Google Scholar]
- 31.Katerinopoulos H.E., Tagmatarchis N., Zaponakis G., Kefalakis N., Kordatos K., Spyrakis M., Thermos K. β-Alkoxy-substituted phenethylamines: A family of compounds potentially active at the dopamine and α-adrenergic receptors. Eur. J. Med. Chem. 1995;30:949–954. doi: 10.1016/0223-5234(96)88314-0. [DOI] [Google Scholar]
- 32.Olsson O.A.T., Ahlquist B., Gustafsson B., Persson G. The Effects in Myometrial Preparations of D2343, a β2-Adrenoceptor Agonist Possessing α1-Receptor Blocking Qualities. Acta Pharmacol. Toxicol. 1984;55:391–397. doi: 10.1111/j.1600-0773.1984.tb02000.x. [DOI] [PubMed] [Google Scholar]
- 33.Tuttle R.R., Mills J. Dobutamine: Development of a new catecholamine to selectively increase cardiac contractility. Circ. Res. 1975;36:185–196. doi: 10.1161/01.RES.36.1.185. [DOI] [PubMed] [Google Scholar]
- 34.Tyrankiewicz U., Skorka T., Jablonska M., Petkow-Dimitrow P., Chlopicki S. Characterization of the cardiac response to a low and high dose of dobutamine in the mouse model of dilated cardiomyopathy by MRI in vivo. J. Magn. Reson. Imaging. 2013;37:669–677. doi: 10.1002/jmri.23854. [DOI] [PubMed] [Google Scholar]
- 35.Vallet B., Dupuis B., Chopin C. Dobutamine: Mechanisms of action and use in acute cardiovascular pathology. [(accessed on 10 January 2023)];Ann. Cardiol. Angeiol. 1991 40:397–402. Available online: https://pubmed.ncbi.nlm.nih.gov/1859148/ [PubMed] [Google Scholar]
- 36.Xu C., Ai D., Shi D., Suo S., Chen X., Yan Y., Cao Y., Zhang R., Sun N., Chen W., et al. Accurate Drug Repositioning through Non-tissue-Specific Core Signatures from Cancer Transcriptomes. Cell Rep. 2018;25:523–535.e5. doi: 10.1016/j.celrep.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 37.Tsujimoto G., Timmins P.V., Hoffman B.B. Alpha adrenergic receptors in the rabbit bladder base smooth muscle: Alpha-1 adrenergic receptors mediate contractile responses. [(accessed on 10 January 2023)];J. Pharmacol. Exp. Ther. 1986 236:384. Available online: https://jpet.aspetjournals.org/content/236/2/384.long. [PubMed] [Google Scholar]
- 38.McPherson G.A., Summers R.J. A Study Of α1-adrenoceptors In Rat Renal Cortex: Comparison Of [3H]-prazosin Binding With The α1-adrenoceptor Modulating Gluconeogenesis Under Physiological Conditions. Br. J. Pharmacol. 1982;77:177–184. doi: 10.1111/j.1476-5381.1982.tb09284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu G., Gluchowski C., Forray C. Design and Synthesis of an α1a-Adrenergic Receptor Subtype-Selective Antagonist from BE2254. Chem. Biol. Drug Des. 2006;67:437–439. doi: 10.1111/j.1747-0285.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- 40.Greenslade F.C., Tobia A.J., Madison S.M., Krider K.M., Newquist K.L. Labetalol binding to specific alpha- and beta-adrenergic sites in vitro and its antagonism of adrenergic responses in vivo. J. Mol. Cell. Cardiol. 1979;11:803–811. doi: 10.1016/0022-2828(79)90405-X. [DOI] [PubMed] [Google Scholar]
- 41.Fox G.R., Virgo B.B. The effects of phenobarbital, atropine, l-α-methyldopa, and DL-propranolol on dieldrin-induced hyperglycemia in the adult rat. Toxicol. Appl. Pharmacol. 1985;78:342–350. doi: 10.1016/0041-008X(85)90239-X. [DOI] [PubMed] [Google Scholar]
- 42.Sweet C.S. New centrally acting antihypertensive drugs related to methyldopa and clonidine. Hypertension. 1984;6:II51. doi: 10.1161/01.HYP.6.5_Pt_2.II51. [DOI] [PubMed] [Google Scholar]
- 43.Orito K., Kishi M., Imaizumi T., Nakazawa T., Hashimoto A., Mori T., Kambe T. α2-adrenoceptor antagonist properties of OPC-28326, a novel selective peripheral vasodilator. Br. J. Pharmacol. 2001;134:763–770. doi: 10.1038/sj.bjp.0704309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sumi M., Sata M., Hashimoto A., Imaizumi T., Yanaga K., Ohki T., Mori T., Nagai R. OPC-28326, a selective femoral arterial vasodilator, augments ischemia induced angiogenesis. Biomed. Pharmacother. 2007;61:209–215. doi: 10.1016/j.biopha.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Minneman K.P., Theroux T.L., Hollinger S., Han C., Esbenshade T.A. Selectivity of agonists for cloned alpha 1-adrenergic receptor subtypes. [(accessed on 10 January 2023)];Mol. Pharmacol. 1994 46:929. Available online: https://molpharm.aspetjournals.org/content/46/5/929/tab-article-info. [PubMed] [Google Scholar]
- 46.Ford A.P.D.W., Daniels D.V., Chang D.J., Gever J.R., Jasper J.R., Lesnick J.D., Clarke D.E. Pharmacological pleiotropism of the human recombinant α1A-adrenoceptor: Implications for α1-adrenoceptor classification. Br. J. Pharmacol. 1997;121:1127–1135. doi: 10.1038/sj.bjp.0701207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossi M., Roumeguère T. Silodosin in the treatment of benign prostatic hyperplasia. Drug Des. Dev. Ther. 2010;4:291–297. doi: 10.2147/DDDT.S10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villa L., Buono R., Fossati N., Rigatti P., Montorsi F., Benigni F., Hedlund P. Effects by silodosin on the partially obstructed rat ureter in vivo and on human and rat isolated ureters. Br. J. Pharmacol. 2013;169:230–238. doi: 10.1111/bph.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Y.-T., Lin H.-C., Chang Y.-Y., Yang Y.-Y., Lee S.-D., Hong C.-Y., Hong C.-Y. Hemodynamic Effects of Synephrine Treatment in Portal Hypertensive Rats. Jpn. J. Pharmacol. 2001;85:183–188. doi: 10.1254/jjp.85.183. [DOI] [PubMed] [Google Scholar]
- 50.Thomas J.E., Munir J.A., McIntyre P.Z., Ferguson M.A. STEMI in a 24-year-old man after use of a synephrine-containing dietary supplement: A case report and review of the literature. [(accessed on 10 January 2023)];Tex. Heart Inst. J. 2009 36:586–590. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2801940/ [PMC free article] [PubMed] [Google Scholar]
- 51.Chapple C., Andersson K.-E. Tamsulosin: An overview. World J. Urol. 2002;19:397–404. doi: 10.1007/s00345-002-0246-7. [DOI] [PubMed] [Google Scholar]
- 52.Broad J., Callaghan B., Sanger G.J., Brock J.A., Furness J.B. Analysis of the ghrelin receptor-independent vascular actions of ulimorelin. Eur. J. Pharmacol. 2015;752:34–39. doi: 10.1016/j.ejphar.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Bylund D.B. Beta Adrenoceptors*. In: Enna S.J., Bylund D.B., editors. xPharm: The Comprehensive Pharmacology Reference. Elsevier; New York, NY, USA: 2007. pp. 1–7. [Google Scholar]
- 54.Hayward L.F., Mueller P.J., Hasser E.M. Adrenergic Receptors. In: Martini L., editor. Encyclopedia of Endocrine Diseases. Elsevier; New York, NY, USA: 2004. pp. 112–115. [Google Scholar]
- 55.Wu Y., Zeng L., Zhao S. Ligands of Adrenergic Receptors: A Structural Point of View. Biomolecules. 2021;11:936. doi: 10.3390/biom11070936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bianchetti A., Manara L. In vitro inhibition of intestinal motility by phenylethanolaminotetralines: Evidence of atypical β-adrenoceptors in rat colon. Br. J. Pharmacol. 1990;100:831–839. doi: 10.1111/j.1476-5381.1990.tb14100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simiand J., Keane P.E., Guitard J., Langlois X., Gonalons N., Martin P., Bianchetti A., Le Fur G., Soubrié P. Antidepressant profile in rodents of SR 58611A, a new selective agonist for atypical β-adrenoceptors. Eur. J. Pharmacol. 1992;219:193–201. doi: 10.1016/0014-2999(92)90296-G. [DOI] [PubMed] [Google Scholar]
- 58.Abou-Mohamed G., Nagarajan R., Ibrahim T.M., Caldwell R.W. Characterization of the adrenergic activity of arbutamine, a novel agent for pharmacological stress testing. Cardiovasc. Drugs Ther. 1996;10:39–47. doi: 10.1007/BF00051129. [DOI] [PubMed] [Google Scholar]
- 59.Nagarajan R., Abou-Mohamed G., Myers T., Caldwell R.W. A novel catecholamine, arbutamine, for a pharmacological cardiac stress agent. Cardiovasc. Drug Ther. 1996;10:31–38. doi: 10.1007/BF00051128. [DOI] [PubMed] [Google Scholar]
- 60.Ruiz M., Takehana K., Petruzella F.D., Watson D.D., Beller G.A., Glover D.K. Arbutamine stress perfusion imaging in dogs with critical coronary artery stenoses: (99m)Tc-sestamibi versus (201)Tl. [(accessed on 10 January 2023)];J. Nucl. Med. 2002 43:664. Available online: https://jnm.snmjournals.org/content/43/5/664.long. [PubMed] [Google Scholar]
- 61.Sitar D.S. Clinical Pharmacokinetics of Bambuterol. Clin. Pharmacokinet. 1996;31:246–256. doi: 10.2165/00003088-199631040-00002. [DOI] [PubMed] [Google Scholar]
- 62.Hughes A.D., Chen Y., Hegde S.S., Jasper J.R., Jaw-Tsai S., Lee T.-W., McNamara A., Pulido-Rios M.T., Steinfeld T., Mammen M. Discovery of (R)-1-(3-((2-Chloro-4-(((2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydroquinolin-5-yl)ethyl)amino)methyl)-5-methoxyphenyl)amino)-3-oxopropyl)piperidin-4-yl [1,1′-Biphenyl]-2-ylcarbamate (TD-5959, GSK961081, Batefenterol): First-in-Class Dual Pharmacology Multivalent Muscarinic Antagonist and β2 Agonist (MABA) for the Treatment of Chronic Obstructive Pulmonary Disease (COPD) J. Med. Chem. 2015;58:2609–2622. doi: 10.1021/jm501915g. [DOI] [PubMed] [Google Scholar]
- 63.Hegde S.S., Hughes A.D., Chen Y., Steinfeld T., Jasper J.R., Lee T.-W., McNamara A., Martin W.J., Pulido-Rios M.T., Mammen M. Pharmacologic Characterization of GSK-961081 (TD-5959), a First-in-Class Inhaled Bifunctional Bronchodilator Possessing Muscarinic Receptor Antagonist and <em>β</em><sub>2</sub>-Adrenoceptor Agonist Properties. J. Pharmacol. Exp. Ther. 2014;351:190. doi: 10.1124/jpet.114.216861. [DOI] [PubMed] [Google Scholar]
- 64.Rasmussen S.G.F., Choi H.-J., Fung J.J., Pardon E., Casarosa P., Chae P.S., DeVree B.T., Rosenbaum D.M., Thian F.S., Kobilka T.S., et al. Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature. 2011;469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu C., Cheng W., Sun Y., Dang Y., Gong F., Zhu H., Li N., Li F., Zhu Z. Activating Brown Adipose Tissue for Weight Loss and Lowering of Blood Glucose Levels: A MicroPET Study Using Obese and Diabetic Model Mice. PLoS ONE. 2014;9:e113742. doi: 10.1371/journal.pone.0113742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sangiorgi E., Curatolo M. Application of a sequential analytical procedure for the detection of the β-agonist brombuterol in bovine urine samples. J. Chromatogr. Biomed. 1997;693:468–478. doi: 10.1016/S0378-4347(97)00056-X. [DOI] [PubMed] [Google Scholar]
- 67.Wang R., Zhang W., Wang P., Su X. A paper-based competitive lateral flow immunoassay for multi β-agonist residues by using a single monoclonal antibody labelled with red fluorescent nanoparticles. Microchim. Acta. 2018;185:191. doi: 10.1007/s00604-018-2730-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bloom J.D., Dutia M.D., Johnson B.D., Wissner A., Burns M.G., Largis E.E., Dolan J.A., Claus T.H. Disodium (R,R)-5-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]-1,3-benzodioxole-2,2-dicarboxylate (CL 316,243). A potent.beta.-adrenergic agonist virtually specific for.beta.3 receptors. A promising antidiabetic and antiobesity agent. J. Med. Chem. 1992;35:3081–3084. doi: 10.1021/jm00094a025. [DOI] [PubMed] [Google Scholar]
- 69.Shin W., Okamatsu-Ogura Y., Matsuoka S., Tsubota A., Kimura K. Impaired adrenergic agonist-dependent beige adipocyte induction in obese mice. J. Vet. Med. Sci. 2019;81:799–807. doi: 10.1292/jvms.19-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.González-Antuña A., Rodríguez-González P., Centineo G., García Alonso J.I. Simultaneous determination of seven β2-agonists in human and bovine urine by isotope dilution liquid chromatography–tandem mass spectrometry using compound-specific minimally 13C-labelled analogues. J. Chromatogr. A. 2014;1372:63–71. doi: 10.1016/j.chroma.2014.10.065. [DOI] [PubMed] [Google Scholar]
- 71.Peng T.A.O., Zhang F.S., Yang W.C., Li D.X., Chen Y., Xiong Y.H., Wei H.U.A., Lai W.H. Lateral-Flow Assay for Rapid Quantitative Detection of Clorprenaline Residue in Swine Urine. J. Food Prot. 2014;77:1824–1829. doi: 10.4315/0362-028X.JFP-14-103. [DOI] [PubMed] [Google Scholar]
- 72.Nishio R., Matsumori A., Shioi T., Wang W., Yamada T., Ono K., Sasayama S. Denopamine, a β1-adrenergic agonist, prolongs survival in a murine model of congestive heart failure induced by viral myocarditis: Suppression of tumor necrosis factor-α production in the heart. J. Am. Coll. Cardiol. 1998;32:808–815. doi: 10.1016/S0735-1097(98)00314-3. [DOI] [PubMed] [Google Scholar]
- 73.Bangash M.N., Patel N.S.A., Benetti E., Collino M., Hinds C.J., Thiemermann C., Pearse R.M. Dopexamine can attenuate the inflammatory response and protect against organ injury in the absence of significant effects on hemodynamics or regional microvascular flow. Crit. Care. 2013;17:R57. doi: 10.1186/cc12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hosie J., Scott A.K., Petrie J.C., Cockshott I.D. Pharmacokinetics of epanolol after acute and chronic oral dosing in elderly patients with stable angina pectoris. Br. J. Clin. Pharmacol. 1990;29:333–337. doi: 10.1111/j.1365-2125.1990.tb03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang W., Chen J., Li X.G., Xu J. Anti-inflammatory activities of fenoterol through β-arrestin-2 and inhibition of AMPK and NF-κB activation in AICAR-induced THP-1 cells. Biomed. Pharmacother. 2016;84:185–190. doi: 10.1016/j.biopha.2016.09.044. [DOI] [PubMed] [Google Scholar]
- 76.Heel R.C., Brogden R.N., Speight T.M., Avery G.S. Fenoterol: A Review of its Pharmacological Properties and Therapeutic Efficacy in Asthma. Drugs. 1978;15:3–32. doi: 10.2165/00003495-197815010-00002. [DOI] [PubMed] [Google Scholar]
- 77.Arnsten A.F., Jin L.E. Guanfacine for the treatment of cognitive disorders: A century of discoveries at Yale. [(accessed on 10 January 2023)];Yale J. Biol. Med. 2012 85:45–58. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3313539/ [PMC free article] [PubMed] [Google Scholar]
- 78.Gomi Y., Shirahase H., Funato H. Effects of 1-(2-chloro-4-hydroxyphenyl)-t-butyl-aminoethanol (HOKU-81), A New Bronchodilator, on Isolated Trachea and Atria of Guinea Pig. Jpn. J. Pharmacol. 1979;29:515–524. doi: 10.1254/jjp.29.515. [DOI] [PubMed] [Google Scholar]
- 79.Underwood S.L., Lewis S.A., Raeburn D. RP 58802B, a long-acting β2-adrenoceptor agonist: Assessment of antiasthma activity in the guinea-pig in vivo. Pulm. Pharmacol. 1992;5:203–212. doi: 10.1016/0952-0600(92)90042-F. [DOI] [PubMed] [Google Scholar]
- 80.Su Ui L., Kyung-Seop A., Min Hee S., Ji-Won P., Hyung Won R., Hyun-Jun L., Sung-Tae H., Sei-Ryang O. Indacaterol Inhibits Tumor Cell Invasiveness and MMP-9 Expression by Suppressing IKK/NF-κB Activation. Mol. Cells. 2014;37:585–591. doi: 10.14348/molcells.2014.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delpy E., Coste H., de Gouville A.-C.L.M. Effects of cyclic GMP elevation on isoprenaline-induced increase in cyclic AMP and relaxation in rat aortic smooth muscle: Role of phosphodiesterase 3. Br. J. Pharmacol. 1996;119:471–478. doi: 10.1111/j.1476-5381.1996.tb15696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Müller F.U., Boheler K.R., Eschenhagen T., Schmitz W., Scholz H. Isoprenaline stimulates gene transcription of the inhibitory G protein alpha-subunit Gi alpha-2 in rat heart. Circ. Res. 1993;72:696–700. doi: 10.1161/01.RES.72.3.696. [DOI] [PubMed] [Google Scholar]
- 83.Vannucci S.J., Nishimura H., Satoh S., Cushman S.W., Holman G.D., Simpson I.A. Cell surface accessibility of GLUT4 glucose transporters in insulin-stimulated rat adipose cells. Modulation by isoprenaline and adenosine. Biochem. J. 1992;288:325–330. doi: 10.1042/bj2880325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Falkay G., Kovács L. Affinity of tocolytic agents on human placental and myometrial beta-adrenergic receptors. J. Perinat. Med. 1986;14:109–113. doi: 10.1515/jpme.1986.14.2.109. [DOI] [PubMed] [Google Scholar]
- 85.Igawa Y., Schneider T., Yamazaki Y., Tatemichi S., Homma Y., Nishizawa O., Michel M.C. Functional investigation of β-adrenoceptors in human isolated detrusor focusing on the novel selective β3-adrenoceptor agonist KUC-7322. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012;385:759–767. doi: 10.1007/s00210-012-0763-x. [DOI] [PubMed] [Google Scholar]
- 86.Tomiyama Y., Murakami M., Hayakawa K., Akiyama K., Yamazaki Y., Kojima M., Shibata N., Akahane M. Pharmacological Profile of KUL-7211, a Selective β-Adrenoceptor Agonist, in Isolated Ureteral Smooth Muscle. J. Pharmacol. Sci. 2003;92:411–419. doi: 10.1254/jphs.92.411. [DOI] [PubMed] [Google Scholar]
- 87.Neidhold S., Eichhorn B., Kasper M., Ravens U., Kaumann A.J. The function of α- and β-adrenoceptors of the saphenous artery in caveolin-1 knockout and wild-type mice. Br. J. Pharmacol. 2007;150:261–270. doi: 10.1038/sj.bjp.0706980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sato M., Hutchinson D.S., Evans B.A., Summers R.J. The β3-Adrenoceptor Agonist 4-[[(Hexylamino)carbonyl]amino]-N-[4-[2-[[(2S)-2-hydroxy-3-(4-hydroxyphenoxy)propyl]amino]ethyl]-phenyl]-benzenesulfonamide (L755507) and Antagonist (S)-N-[4-[2-[[3-[3-(Acetamidomethyl)phenoxy]-2-hydroxypropyl]amino]-ethyl]phenyl]benzenesulfonamide (L748337) Activate Different Signaling Pathways in Chinese Hamster Ovary-K1 Cells Stably Expressing the Human β3-Adrenoceptor. Mol. Pharmacol. 2008;74:1417–1428. doi: 10.1124/mol.108.046979. [DOI] [PubMed] [Google Scholar]
- 89.Randall M.J., Kostin S.F., Burgess E.J., Hoyt L.R., Ather J.L., Lundblad L.K., Poynter M.E. Anti-Inflammatory Effects of Levalbuterol-Induced 11β-Hydroxysteroid Dehydrogenase Type 1 Activity in Airway Epithelial Cells. Front. Endocrinol. 2015;5:236. doi: 10.3389/fendo.2014.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kube J.C., Holland B.P., Word A.B., Allen J.B., Calvo-Lorenzo M., McKenna D., Vogel G. Effects of various doses of lubabegron on calculated ammonia gas emissions, growth performance, and carcass characteristics of beef cattle during the last 56 days of the feeding period. Transl. Anim. Sci. 2021;5:txab137. doi: 10.1093/tas/txab137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jesudason C.D., Baker J.E., Bryant R.D., Fisher J.W., O’Farrell L.S., Gaich G.A., He M.M., Kahl S.D., Kriauciunas A.V., Heiman M.L., et al. Combination of a Beta Adrenoceptor Modulator and a Norepinephrine-Serotonin Uptake Inhibitor for the Treatment of Obesity. ACS Med. Chem. Lett. 2011;2:583–586. doi: 10.1021/ml200071k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bi S., Zhao T., Wang Y., Zhou H. Study on the interactions of mapenterol with serum albumins using multi-spectroscopy and molecular docking. Luminescence. 2016;31:372–379. doi: 10.1002/bio.2969. [DOI] [PubMed] [Google Scholar]
- 93.Ibrahim F.A., Fathy M.E., Elmansi H.M. Highly sensitive spectrofluorimetric method for rapid determination of orciprenaline in biological fluids and pharmaceuticals. Luminescence. 2019;34:77–83. doi: 10.1002/bio.3579. [DOI] [PubMed] [Google Scholar]
- 94.Noh H., Yu M.R., Kim H.J., Lee J.H., Park B.-W., Wu I.H., Matsumoto M., King G.L. Beta 2-adrenergic receptor agonists are novel regulators of macrophage activation in diabetic renal and cardiovascular complications. Kidney Int. 2017;92:101–113. doi: 10.1016/j.kint.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takasu T., Ukai M., Sato S., Matsui T., Nagase I., Maruyama T., Sasamata M., Miyata K., Uchida H., Yamaguchi O. Effect of (R)-2-(2-Aminothiazol-4-yl)-4′-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl} Acetanilide (YM178), a Novel Selective β3-Adrenoceptor Agonist, on Bladder Function. J. Pharmacol. Exp. Ther. 2007;321:642–647. doi: 10.1124/jpet.106.115840. [DOI] [PubMed] [Google Scholar]
- 96.Yanagisawa T., Sato T., Yamada H., Sukegawa J., Nunoki K. Selectivity and Potency of Agonists for the Three Subtypes of Cloned Human β-Adrenoceptors Expressed in Chinese Hamster Ovary Cells. Tohoku J. Exp. Med. 2000;192:181–193. doi: 10.1620/tjem.192.181. [DOI] [PubMed] [Google Scholar]
- 97.Two novel beta agonists compare favourably with salbutamol. Inpharma Wkly. 1992;820:6. doi: 10.2165/00128413-199208200-00014. [DOI] [Google Scholar]
- 98.Aparici M., Carcasona C., Ramos I., Montero J.L., Otal R., Ortiz J.L., Cortijo J., Puig C., Vilella D., De Alba J., et al. Pharmacological Profile of AZD8871 (LAS191351), a Novel Inhaled Dual M3 Receptor Antagonist β2-Adrenoceptor Agonist Molecule with Long-Lasting Effects and Favorable Safety Profile. J. Pharmacol. Exp. Ther. 2019;370:127–136. doi: 10.1124/jpet.118.255620. [DOI] [PubMed] [Google Scholar]
- 99.Farooqui T. Octopamine-Mediated Neuromodulation of Insect Senses. Neurochem. Res. 2007;32:1511–1529. doi: 10.1007/s11064-007-9344-7. [DOI] [PubMed] [Google Scholar]
- 100.Axelrod J., Saavedra J.M. Octopamine. Nature. 1977;265:501–504. doi: 10.1038/265501a0. [DOI] [PubMed] [Google Scholar]
- 101.Bouyssou T., Casarosa P., Naline E., Pestel S., Konetzki I., Devillier P., Schnapp A. Pharmacological Characterization of Olodaterol, a Novel Inhaled β2-Adrenoceptor Agonist Exerting a 24-Hour-Long Duration of Action in Preclinical Models. J. Pharmacol. Exp. Ther. 2010;334:53–62. doi: 10.1124/jpet.110.167007. [DOI] [PubMed] [Google Scholar]
- 102.Yi C., Xing G., Wang S., Li X., Liu Y., Li J., Lin B., Woo A.Y.-H., Zhang Y., Pan L., et al. Design, synthesis and biological evaluation of 8-(2-amino-1-hydroxyethyl)-6-hydroxy-1,4-benzoxazine-3(4H)-one derivatives as potent β2-adrenoceptor agonists. Biorg. Med. Chem. 2020;28:115178. doi: 10.1016/j.bmc.2019.115178. [DOI] [PubMed] [Google Scholar]
- 103.Hoffmann K.J., Skånberg I., Borg K.O. Species differences in the metabolism of pamatolol, a cardioselective beta—Adrenoceptor antagonist. Eur. J. Drug Metab. Pharmacokinet. 1979;4:163–173. doi: 10.1007/BF03189420. [DOI] [PubMed] [Google Scholar]
- 104.Glossop P.A., Lane C.A.L., Price D.A., Bunnage M.E., Lewthwaite R.A., James K., Brown A.D., Yeadon M., Perros-Huguet C., Trevethick M.A., et al. Inhalation by Design: Novel Ultra-Long-Acting β2-Adrenoreceptor Agonists for Inhaled Once-Daily Treatment of Asthma and Chronic Obstructive Pulmonary Disease That Utilize a Sulfonamide Agonist Headgroup. J. Med. Chem. 2010;53:6640–6652. doi: 10.1021/jm1005989. [DOI] [PubMed] [Google Scholar]
- 105.Fuso L., Mores N., Valente S., Malerba M., Montuschi P. Long-Acting Beta-Agonists and their Association with Inhaled Corticosteroids in COPD. Curr. Med. Chem. 2013;20:1477–1495. doi: 10.2174/0929867311320120003. [DOI] [PubMed] [Google Scholar]
- 106.Li M., Yang H., Li S., Zhao K., Li J., Jiang D., Sun L., Deng A. Ultrasensitive and Quantitative Detection of a New β-Agonist Phenylethanolamine A by a Novel Immunochromatographic Assay Based on Surface-Enhanced Raman Scattering (SERS) J. Agr. Food Chem. 2014;62:10896–10902. doi: 10.1021/jf503599x. [DOI] [PubMed] [Google Scholar]
- 107.Seifert R. Pronethalol. In: Enna S.J., Bylund D.B., editors. xPharm: The Comprehensive Pharmacology Reference. Elsevier; New York, NY, USA: 2007. pp. 1–3. [Google Scholar]
- 108.Seifert R. Reproterol. In: Enna S.J., Bylund D.B., editors. xPharm: The Comprehensive Pharmacology Reference. Elsevier; New York, NY, USA: 2007. pp. 1–5. [Google Scholar]
- 109.Canadian Preterm Labor Investigators Group Treatment of Preterm Labor with the Beta-Adrenergic Agonist Ritodrine. N. Engl. J. Med. 1992;327:308–312. doi: 10.1056/NEJM199207303270503. [DOI] [PubMed] [Google Scholar]
- 110.Iakovidis D., Malta E., McPherson G.A., Raper C. In Vitro Activity Of Ro363, a β1-adrenoceptor Selective Agonist. Br. J. Pharmacol. 1980;68:677–685. doi: 10.1111/j.1476-5381.1980.tb10860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wood M., Dubois V., Scheller D., Gillard M. Rotigotine is a potent agonist at dopamine D1 receptors as well as at dopamine D2 and D3 receptors. Br. J. Pharmacol. 2015;172:1124–1135. doi: 10.1111/bph.12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McMacken G., Cox D., Roos A., Müller J., Whittaker R., Lochmüller H. The beta-adrenergic agonist salbutamol modulates neuromuscular junction formation in zebrafish models of human myasthenic syndromes. Hum. Mol. Genet. 2018;27:1556–1564. doi: 10.1093/hmg/ddy062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Johnson M. Salmeterol. Med. Chem. Rev. 1995;15:225–257. doi: 10.1002/med.2610150303. [DOI] [PubMed] [Google Scholar]
- 114.Muzzin P., Boss O., Mathis N., Revelli J.P., Giacobino J.P., Willcocks K., Badman G.T., Cantello B.C., Hindley R.M., Cawthorne M.A. Characterization of a new, highly specific, beta 3-adrenergic receptor radioligand, [3H]SB 206606. [(accessed on 10 January 2023)];Mol. Pharmacol. 1994 46:357–363. Available online: https://molpharm.aspetjournals.org/content/46/2/357.long. [PubMed] [Google Scholar]
- 115.Ind P.W., Laitinen L., Laursen L., Wenzel S., Wouters E., Deamer L., Nystrom P. Early clinical investigation of Viozan™ (sibenadet HCI), a novel D2 dopamine receptor, β2-adrenoceptor agonist for the treatment of chronic obstructive pulmonary disease symptoms. Respir. Med. 2003;97:S9–S21. doi: 10.1016/S0954-6111(03)80011-2. [DOI] [PubMed] [Google Scholar]
- 116.Hicks A., McCafferty G.P., Riedel E., Aiyar N., Pullen M., Evans C., Luce T.D., Coatney R.W., Rivera G.C., Westfall T.D., et al. GW427353 (Solabegron), a Novel, Selective β3-Adrenergic Receptor Agonist, Evokes Bladder Relaxation and Increases Micturition Reflex Threshold in the Dog. J. Pharmacol. Exp. Ther. 2007;323:202–209. doi: 10.1124/jpet.107.125757. [DOI] [PubMed] [Google Scholar]
- 117.Sybertz E.J., Baum T., Pula K.K., Nelson S., Eynon E., Sabin C. Studies on the Mechanism of the Acute Antihypertensive and Vasodilator Actions of Several β-Adrenoceptor Antagonists. J. Cardiovasc. Pharmacol. 1982;4:749–758. doi: 10.1097/00005344-198209000-00009. [DOI] [PubMed] [Google Scholar]
- 118.Stohs S.J., Preuss H.G., Shara M. A Review of the Receptor-Binding Properties of p-Synephrine as Related to Its Pharmacological Effects. Oxidative Med. Cell. Longev. 2011;2011:482973. doi: 10.1155/2011/482973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kozłowska H., Szymska U., Schlicker E., Malinowska B. Atypical β-adrenoceptors, different from β3-adrenoceptors and probably from the low-affinity state of β1-adrenoceptors, relax the rat isolated mesenteric artery. Br. J. Pharmacol. 2003;140:3–12. doi: 10.1038/sj.bjp.0705421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jacobsen J.R., Aggen J.B., Church T.J., Klein U., Pfeiffer J.W., Pulido-Rios T.M., Thomas G.R., Yu C., Moran E.J. Multivalent design of long-acting β2-adrenoceptor agonists incorporating biarylamines. Bioorg. Med. Chem. Lett. 2014;24:2625–2630. doi: 10.1016/j.bmcl.2014.04.069. [DOI] [PubMed] [Google Scholar]
- 121.Mohamed Ismail N.A., Ibrahim M., Mohd Naim N., Mahdy Z.A., Jamil M.A., Mohd Razi Z.R. Nifedipine versus terbutaline for tocolysis in external cephalic version. Int. J. Gynecol. Obstet. 2008;102:263–266. doi: 10.1016/j.ijgo.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 122.Loureiro J.A., Andrade S., Ramalho M.J., Oliveira N., Pereira M.C. The interaction of a β2 adrenoceptor agonist drug with biomimetic cell membrane models: The case of terbutaline sulphate. Life Sci. 2021;285:119992. doi: 10.1016/j.lfs.2021.119992. [DOI] [PubMed] [Google Scholar]
- 123.Yamaya M., Nishimura H., Nadine L., Kubo H., Ryoichi N. Tulobuterol inhibits rhinovirus infection in primary cultures of human tracheal epithelial cells. Physiol. Rep. 2013;1 doi: 10.1002/phy2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kempsford R., Norris V., Siederer S. Vilanterol trifenatate, a novel inhaled long-acting beta2 adrenoceptor agonist, is well tolerated in healthy subjects and demonstrates prolonged bronchodilation in subjects with asthma and COPD. Pulm. Pharmacol. Ther. 2013;26:256–264. doi: 10.1016/j.pupt.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 125.Slack R.J., Barrett V.J., Morrison V.S., Sturton R.G., Emmons A.J., Ford A.J., Knowles R.G. In Vitro Pharmacological Characterization of Vilanterol, a Novel Long-Acting β2-Adrenoceptor Agonist with 24-Hour Duration of Action. J. Pharmacol. Exp. Ther. 2013;344:218–230. doi: 10.1124/jpet.112.198481. [DOI] [PubMed] [Google Scholar]
- 126.Gwee M.C.E., Nott M.W., Raper C., Rodger I.W. Pharmacological actions of a new β-adrenoceptor agonist, MJ-9184–1, in anaesthetized cats. Br. J. Pharmacol. 1972;46:375–385. doi: 10.1111/j.1476-5381.1972.tb08134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Waldeck B. β-Adrenoceptor Agonists after Terbutaline. Pharmacol. Toxicol. 1995;77:25–29. doi: 10.1111/j.1600-0773.1995.tb01937.x. [DOI] [PubMed] [Google Scholar]
- 128.Wada Y., Nakano S., Morimoto A., Kasahara K.-I., Hayashi T., Takada Y., Suzuki H., Niwa-Sakai M., Ohashi S., Mori M., et al. Discovery of Novel Indazole Derivatives as Orally Available β3-Adrenergic Receptor Agonists Lacking Off-Target-Based Cardiovascular Side Effects. J. Med. Chem. 2017;60:3252–3265. doi: 10.1021/acs.jmedchem.6b01197. [DOI] [PubMed] [Google Scholar]
- 129.Hu B., Ellingboe J., Han S., Largis E., Mulvey R., Oliphant A., Sum F.-W., Tillett J. (4-Piperidin-1-yl)phenyl Amides: Potent and Selective Human β3 Agonists. J. Med. Chem. 2001;44:1456–1466. doi: 10.1021/jm000544b. [DOI] [PubMed] [Google Scholar]
- 130.Sun W.S., Park Y.S., Yoo J., Park K.D., Kim S.H., Kim J.-H., Park H.-J. Rational Design of an Indolebutanoic Acid Derivative as a Novel Aldose Reductase Inhibitor Based on Docking and 3D QSAR Studies of Phenethylamine Derivatives. J. Med. Chem. 2003;46:5619–5627. doi: 10.1021/jm0205346. [DOI] [PubMed] [Google Scholar]
- 131.Genç H., Kalin R., Köksal Z., Sadeghian N., Kocyigit U.M., Zengin M., Gülçin İ., Özdemir H. Discovery of Potent Carbonic Anhydrase and Acetylcholinesterase Inhibitors: 2-Aminoindan β-Lactam Derivatives. Int. J. Mol. Sci. 2016;17:1736. doi: 10.3390/ijms17101736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kose L.P., Gulcin İ., Yıldırım A., Atmaca U., Çelik M., Alwasel S.H., Supuran C.T. The human carbonic anhydrase isoenzymes I and II inhibitory effects of some hydroperoxides, alcohols, and acetates. J. Enzym. Inhib. Med. Chem. 2016;31:1248–1253. doi: 10.3109/14756366.2015.1120723. [DOI] [PubMed] [Google Scholar]
- 133.Artunç T., Çetinkaya Y., Göçer H., Gülçin İ., Menzek A., Şahin E., Supuran C.T. Synthesis of 4-[2-(3,4-dimethoxybenzyl)cyclopentyl]-1,2-dimethoxybenzene Derivatives and Evaluations of Their Carbonic Anhydrase Isoenzymes Inhibitory Effects. Chem. Biol. Drug Des. 2016;87:594–607. doi: 10.1111/cbdd.12695. [DOI] [PubMed] [Google Scholar]
- 134.Scozzafava A., Supuran C.T. Glaucoma and the Applications of Carbonic Anhydrase Inhibitors. In: Frost S.C., McKenna R., editors. Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications. Springer; Dordrecht, The Netherlands: 2014. pp. 349–359. [DOI] [PubMed] [Google Scholar]
- 135.Queen A., Khan P., Azam A., Hassan I.M. Understanding the Role and Mechanism of Carbonic Anhydrase V in Obesity and its Therapeutic Implications. Curr. Protein Pept. Sci. 2018;19:909–923. doi: 10.2174/1389203718666170810145436. [DOI] [PubMed] [Google Scholar]
- 136.Chen F., Licarete E., Wu X., Petrusca D., Maguire C., Jacobsen M., Colter A., Sandusky G.E., Czader M., Capitano M.L., et al. Pharmacological inhibition of Carbonic Anhydrase IX and XII to enhance targeting of acute myeloid leukaemia cells under hypoxic conditions. J. Cell. Mol. Med. 2021;25:11039–11052. doi: 10.1111/jcmm.17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nocentini A., Vullo D., Del Prete S., Osman S.M., Alasmary F.A.S., AlOthman Z., Capasso C., Carta F., Gratteri P., Supuran C.T. Inhibition of the β-carbonic anhydrase from the dandruff-producing fungus Malassezia globosa with monothiocarbamates. J. Enzym. Inhib. Med. Chem. 2017;32:1064–1070. doi: 10.1080/14756366.2017.1355307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Topal F., Aksu K., Gulcin I., Tümer F., Goksu S. Inhibition Profiles of Some Symmetric Sulfamides Derived from Phenethylamines on Human Carbonic Anhydrase I, and II Isoenzymes. Chem. Biodivers. 2021;18:e2100422. doi: 10.1002/cbdv.202100422. [DOI] [PubMed] [Google Scholar]
- 139.Akıncıoğlu A., Kocaman E., Akıncıoğlu H., Salmas R.E., Durdagi S., Gülçin İ., Supuran C.T., Göksu S. The synthesis of novel sulfamides derived from β-benzylphenethylamines as acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase enzymes inhibitors. Bioorg. Chem. 2017;74:238–250. doi: 10.1016/j.bioorg.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 140.Weinshenker D. Dopamine Beta-Hydroxylase. In: Enna S.J., Bylund D.B., editors. xPharm: The Comprehensive Pharmacology Reference. Elsevier; New York, NY, USA: 2007. pp. 1–15. [Google Scholar]
- 141.Kruse L.I., Kaiser C., DeWolf W.E., Jr., Chambers P.A., Goodhart P.J., Ezekiel M., Ohlstein E.H. Beta.-Substituted phenethylamines as high-affinity mechanism-based inhibitors of dopamine.beta.-hydroxylase. J. Med. Chem. 1988;31:704–706. doi: 10.1021/jm00399a002. [DOI] [PubMed] [Google Scholar]
- 142.Vaz-da-Silva M., Rocha J.-F., Lacroix P., Falcão A., Almeida L., Soares-da-Silva P. Cardiac safety profile of etamicastat, a novel peripheral selective dopamine-β-hydroxylase inhibitor in non-human primates, human young and elderly healthy volunteers and hypertensive patients. IJC Metab. Endocr. 2015;7:10–24. doi: 10.1016/j.ijcme.2015.03.002. [DOI] [Google Scholar]
- 143.Loureiro A.I., João Bonifácio M., Fernandes-Lopes C., Igreja B., Wright L.C., Soares-da-Silva P. Etamicastat, a new dopamine-ß-hydroxylase inhibitor, pharmacodynamics and metabolism in rat. Eur. J. Pharmacol. 2014;740:285–294. doi: 10.1016/j.ejphar.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 144.Beliaev A., Learmonth D.A., Soares-da-Silva P. Synthesis and Biological Evaluation of Novel, Peripherally Selective Chromanyl Imidazolethione-Based Inhibitors of Dopamine β-Hydroxylase. J. Med. Chem. 2006;49:1191–1197. doi: 10.1021/jm051051f. [DOI] [PubMed] [Google Scholar]
- 145.Bicker J., Alves G., Fortuna A., Soares-da-Silva P., Falcão A. In vitro assessment of the interactions of dopamine β-hydroxylase inhibitors with human P-glycoprotein and Breast Cancer Resistance Protein. Eur. J. Pharm. Sci. 2018;117:35–40. doi: 10.1016/j.ejps.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 146.Gallwitz B. Clinical Use of DPP-4 Inhibitors. Front. Endocrinol. 2019;10:389. doi: 10.3389/fendo.2019.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yoon H., Sung J.H., Song M.J. Effects of the Antidiabetic Drugs Evogliptin and Sitagliptin on the Immune Function of CD26/DPP4 in Th1 Cells. Biomol. Ther. 2021;29:154–165. doi: 10.4062/biomolther.2020.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tan X., Hu J. Evogliptin: A new dipeptidyl peptidase inhibitor for the treatment of type 2 diabetes. Expert Opin. Pharmaco. 2016;17:1285–1293. doi: 10.1080/14656566.2016.1183645. [DOI] [PubMed] [Google Scholar]
- 149.Kim T.-H., Kim M.-K., Cheong Y.-H., Chae Y.-N., Lee Y., Ka S.-O., Jung I.-H., Shin C.-Y., Bae E.J., Son M.-H. Hepatic role in an early glucose-lowering effect by a novel dipeptidyl peptidase 4 inhibitor, evogliptin, in a rodent model of type 2 diabetes. Eur. J. Pharmacol. 2016;771:65–76. doi: 10.1016/j.ejphar.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 150.Duffy N.A., Green B.D., Irwin N., Gault V.A., McKillop A.M., O’Harte F.P.M., Flatt P.R. Effects of antidiabetic drugs on dipeptidyl peptidase IV activity: Nateglinide is an inhibitor of DPP IV and augments the antidiabetic activity of glucagon-like peptide-1. Eur. J. Pharmacol. 2007;568:278–286. doi: 10.1016/j.ejphar.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 151.Cahn A., Cernea S., Raz I. An update on DPP-4 inhibitors in the management of type 2 diabetes. Expert Opin. Emerg. Drugs. 2016;21:409–419. doi: 10.1080/14728214.2016.1257608. [DOI] [PubMed] [Google Scholar]
- 152.Thomas L., Eckhardt M., Langkopf E., Tadayyon M., Himmelsbach F., Mark M. (R)-8-(3-Amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a Novel Xanthine-Based Dipeptidyl Peptidase 4 Inhibitor, Has a Superior Potency and Longer Duration of Action Compared with Other Dipeptidyl Peptidase-4 Inhibitors. J. Pharmacol. Exp. Ther. 2008;325:175–182. doi: 10.1124/jpet.107.135723. [DOI] [PubMed] [Google Scholar]
- 153.Kim S.-J., Nian C., Doudet D.J., McIntosh C.H.S. Inhibition of Dipeptidyl Peptidase IV With Sitagliptin (MK0431) Prolongs Islet Graft Survival in Streptozotocin-Induced Diabetic Mice. Diabetes. 2008;57:1331–1339. doi: 10.2337/db07-1639. [DOI] [PubMed] [Google Scholar]
- 154.Backes B.J., Longenecker K., Hamilton G.L., Stewart K., Lai C., Kopecka H., von Geldern T.W., Madar D.J., Pei Z., Lubben T.H., et al. Pyrrolidine-constrained phenethylamines: The design of potent, selective, and pharmacologically efficacious dipeptidyl peptidase IV (DPP4) inhibitors from a lead-like screening hit. Bioorg. Med. Chem. Lett. 2007;17:2005–2012. doi: 10.1016/j.bmcl.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 155.Pei Z., Li X., von Geldern T.W., Longenecker K., Pireh D., Stewart K.D., Backes B.J., Lai C., Lubben T.H., Ballaron S.J., et al. Discovery and Structure−Activity Relationships of Piperidinone- and Piperidine-Constrained Phenethylamines as Novel, Potent, and Selective Dipeptidyl Peptidase IV Inhibitors. J. Med. Chem. 2007;50:1983–1987. doi: 10.1021/jm061436d. [DOI] [PubMed] [Google Scholar]
- 156.Sibley D.R., Neve K. D2 Dopamine Receptor*. In: Enna S.J., Bylund D.B., editors. xPharm: The Comprehensive Pharmacology Reference. Elsevier; New York, NY, USA: 2007. pp. 1–13. [Google Scholar]
- 157.Martel J.C., Gatti McArthur S. Dopamine Receptor Subtypes, Physiology and Pharmacology: New Ligands and Concepts in Schizophrenia. Front. Pharmacol. 2020;11:1003. doi: 10.3389/fphar.2020.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kebabian J.W., Britton D.R., DeNinno M.P., Perner R., Smith L., Jenner P., Schoenleber R., Williams M. A-77636: A potent and selective dopamine D1 receptor agonist with antiparkinsonian activity in marmosets. Eur. J. Pharmacol. 1992;229:203–209. doi: 10.1016/0014-2999(92)90556-J. [DOI] [PubMed] [Google Scholar]
- 159.Matsuyama N., Shibata S., Matoba A., Kudo T.-A., Danielsson J., Kohjitani A., Masaki E., Emala C.W., Mizuta K. The dopamine D1 receptor is expressed and induces CREB phosphorylation and MUC5AC expression in human airway epithelium. Respir. Res. 2018;19:53. doi: 10.1186/s12931-018-0757-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang R., Li X., Shi Y., Shao Y., Sun K., Wang A., Sun F., Liu W., Wang D., Jin J., et al. The Effects of LPM570065, a Novel Triple Reuptake Inhibitor, on Extracellular Serotonin, Dopamine and Norepinephrine Levels in Rats. PLoS ONE. 2014;9:e91775. doi: 10.1371/journal.pone.0091775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sviglerova J., Kuncova J., Stengl M. Chapter 7—Cardiovascular Models: Heart Secondarily Affected by Disease (Diabetes Mellitus, Renal Failure, Dysfunctional Sympathetic Innervation) In: Conn P.M., editor. Animal Models for the Study of Human Disease. 2nd ed. Academic Press; New York, NY, USA: 2017. pp. 175–203. [Google Scholar]
- 162.Eden R.J., Costall B., Domeney A.M., Gerrard P.A., Harvey C.A., Kelly M.E., Naylor R.J., Owen D.A.A., Wright A. Preclinical pharmacology of ropinirole (SK&F 101468-A) a novel dopamine D2 agonist. Pharmacol. Biochem. Behav. 1991;38:147–154. doi: 10.1016/0091-3057(91)90603-Y. [DOI] [PubMed] [Google Scholar]
- 163.Ågren R., Stepniewski T.M., Zeberg H., Selent J., Sahlholm K. Dopamine D2 Receptor Agonist Binding Kinetics—Role of a Conserved Serine Residue. Int. J. Mol. Sci. 2021;22:4078. doi: 10.3390/ijms22084078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jîtcă G., Ősz B.E., Tero-Vescan A., Vari C.E. Psychoactive Drugs—From Chemical Structure to Oxidative Stress Related to Dopaminergic Neurotransmission. A Review. Antioxidants. 2021;10:381. doi: 10.3390/antiox10030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vaughan R.A., Foster J.D. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci. 2013;34:489–496. doi: 10.1016/j.tips.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shulgin A.T., Sargent T., Naranjo C. Structure–Activity Relationships of One-Ring Psychotomimetics. Nature. 1969;221:537–541. doi: 10.1038/221537a0. [DOI] [PubMed] [Google Scholar]
- 167.Abbruscato T.J., Trippier P.C. DARK Classics in Chemical Neuroscience: Methamphetamine. ACS Chem. Neurosci. 2018;9:2373–2378. doi: 10.1021/acschemneuro.8b00123. [DOI] [PubMed] [Google Scholar]
- 168.Yu S., Zhu L., Shen Q., Bai X., Di X. Recent Advances in Methamphetamine Neurotoxicity Mechanisms and Its Molecular Pathophysiology. Behav. Neurol. 2015;2015:103969. doi: 10.1155/2015/103969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rickli A., Hoener M.C., Liechti M.E. Monoamine transporter and receptor interaction profiles of novel psychoactive substances: Para-halogenated amphetamines and pyrovalerone cathinones. Eur. Neuropsychopharm. 2015;25:365–376. doi: 10.1016/j.euroneuro.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 170.Simmler L., Buser T., Donzelli M., Schramm Y., Dieu L.-H., Huwyler J., Chaboz S., Hoener M., Liechti M. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Johannes L., Jacob R., Leffler H. Galectins at a glance. J. Cell Sci. 2018;131:jcs208884. doi: 10.1242/jcs.208884. [DOI] [PubMed] [Google Scholar]
- 172.Tejler J., Tullberg E., Frejd T., Leffler H., Nilsson U.J. Synthesis of multivalent lactose derivatives by 1,3-dipolar cycloadditions: Selective galectin-1 inhibition. Carbohydr. Res. 2006;341:1353–1362. doi: 10.1016/j.carres.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 173.Hu W.-S., Hughes S.H. HIV-1 Reverse Transcription. CSH Perspect. Med. 2012;2:a006882. doi: 10.1101/cshperspect.a006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ha B., Larsen K.P., Zhang J., Fu Z., Montabana E., Jackson L.N., Chen D.-H., Puglisi E.V. High-resolution view of HIV-1 reverse transcriptase initiation complexes and inhibition by NNRTI drugs. Nat. Commun. 2021;12:2500. doi: 10.1038/s41467-021-22628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Venkatachalam T.K., Uckun F.M. Synthesis of Symmetrical and Asymmetrical Phenethyl Thiourea Compounds as Nonnucleoside Inhibitors of HIV--1 Reverse Transcriptase. Synth. Commun. 2005;35:2039–2056. doi: 10.1081/SCC-200066677. [DOI] [Google Scholar]
- 176.Venkatachalam T.K., Sudbeck E.A., Uckun F.M. Regiospecific synthesis, X-ray crystal structure and biological activities of 5-bromothiophenethyl thioureas. Tetrahedron Lett. 2001;42:6629–6632. doi: 10.1016/S0040-4039(01)01290-4. [DOI] [Google Scholar]
- 177.Mao C., Sudbeck E.A., Venkatachalam T.K., Uckun F.M. Structure-based drug design of non-nucleoside inhibitors for wild-type and drug-resistant HIV reverse transcriptase. Biochem. Pharmacol. 2000;60:1251–1265. doi: 10.1016/S0006-2952(00)00408-1. [DOI] [PubMed] [Google Scholar]
- 178.Zhang H., Vrang L., Bäckbro K., Lind P., Sahlberg C., Unge T., Öberg B. Inhibition of human immunodeficiency virus type 1 wild-type and mutant reverse transcriptases by the phenyl ethyl thiazolyl thiourea derivatives trovirdine and MSC-127. Antivir. Res. 1995;28:331–342. doi: 10.1016/0166-3542(95)00056-9. [DOI] [PubMed] [Google Scholar]
- 179.Bell F.W., Cantrell A.S., Hoegberg M., Jaskunas S.R., Johansson N.G., Jordan C.L., Kinnick M.D., Lind P., Morin J.M., Jr. Phenethylthiazolethiourea (PETT) Compounds, a New Class of HIV-1 Reverse Transcriptase Inhibitors. 1. Synthesis and Basic Structure-Activity Relationship Studies of PETT Analogs. J. Med. Chem. 1995;38:4929–4936. doi: 10.1021/jm00025a010. [DOI] [PubMed] [Google Scholar]
- 180.Cantrell A.S., Engelhardt P., Högberg M., Jaskunas S.R., Johansson N.G., Jordan C.L., Kangasmetsä J., Kinnick M.D., Lind P., Morin J.M., et al. Phenethylthiazolylthiourea (PETT) Compounds as a New Class of HIV-1 Reverse Transcriptase Inhibitors. 2. Synthesis and Further Structure−Activity Relationship Studies of PETT Analogs. J. Med. Chem. 1996;39:4261–4274. doi: 10.1021/jm950639r. [DOI] [PubMed] [Google Scholar]
- 181.Pithadia A.B., Jain S.M. 5-Hydroxytryptamine Receptor Subtypes and their Modulators with Therapeutic Potentials. J. Clin. Med. Res. 2009;1:72. doi: 10.4021/jocmr2009.05.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hoyer D. 5-Hydroxytryptamine Receptors. In: Enna S.J., Bylund D.B., editors. xPharm: The Comprehensive Pharmacology Reference. Elsevier; New York, NY, USA: 2007. pp. 1–7. [Google Scholar]
- 183.Chagraoui A., Protais P., Filloux T., Mocaër E. Agomelatine(S 20098) antagonizes the penile erections induced by the stimulation of 5-HT2C receptors in Wistar rats. Psychopharmacology. 2003;170:17–22. doi: 10.1007/s00213-003-1465-3. [DOI] [PubMed] [Google Scholar]
- 184.Naiman N., Lyon R.A., Bullock A.E., Rydelek L.T., Titeler M., Glennon R.A. 2-(Alkylamino)tetralin derivatives: Interaction with 5-HT1A serotonin binding sites. J. Med. Chem. 1989;32:253–256. doi: 10.1021/jm00121a045. [DOI] [PubMed] [Google Scholar]
- 185.Mori T., Uzawa N., Iwase Y., Masukawa D., Rahmadi M., Hirayama S., Hokazono M., Higashiyama K., Shioda S., Suzuki T. Narcolepsy-like sleep disturbance in orexin knockout mice are normalized by the 5-HT1A receptor agonist 8-OH-DPAT. Psychopharmacology. 2016;233:2343–2353. doi: 10.1007/s00213-016-4282-1. [DOI] [PubMed] [Google Scholar]
- 186.Li M., Tang F., Xie F., Lv Y., Yu P., Liu Z., Cheng Z. Development and validation a LC–MS/MS method for the simultaneous determination of agomelatine and its metabolites, 7-desmethyl-agomelatine and 3-hydroxy-agomelatine in human plasma: Application to a bioequivalence study. J. Chromatogr. B. 2015;1003:60–66. doi: 10.1016/j.jchromb.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 187.Millan M.J., Gobert A., Lejeune F., Dekeyne A., Newman-Tancredi A., Pasteau V., Rivet J.-M., Cussac D. The Novel Melatonin Agonist Agomelatine (S20098) Is an Antagonist at 5-Hydroxytryptamine 2C Receptors, Blockade of Which Enhances the Activity of Frontocortical Dopaminergic and Adrenergic Pathways. J. Pharmacol. Exp. Ther. 2003;306:954–964. doi: 10.1124/jpet.103.051797. [DOI] [PubMed] [Google Scholar]
- 188.Ahlgren C., Eriksson A., Tellefors P., Ross S.B., Stenfors C., Malmberg Å. In vitro characterization of AR-A000002, a novel 5-hydroxytryptamine1B autoreceptor antagonist. Eur. J. Pharmacol. 2004;499:67–75. doi: 10.1016/j.ejphar.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 189.Perez-García G.S., Meneses A. Effects of the potential 5-HT7 receptor agonist AS 19 in an autoshaping learning task. Behav. Brain Res. 2005;163:136–140. doi: 10.1016/j.bbr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 190.Brenchat A., Romero L., García M., Pujol M., Burgueño J., Torrens A., Hamon M., Baeyens J.M., Buschmann H., Zamanillo D., et al. 5-HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice. PAIN. 2009;141:239–247. doi: 10.1016/j.pain.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 191.Lippmann W., Pugsley T.A. Effects Of Benzoctamine And Chlordiazepoxide On Turnover And Uptake Of 5-hydroxytryptamine In The Brain. Br. J. Pharmacol. 1974;51:571–575. doi: 10.1111/j.1476-5381.1974.tb09676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Oh D.-R., Kim Y., Jo A., Choi E.J., Oh K.-N., Kim J., Kang H., Kim Y.R., Choi C.Y. Sedative and hypnotic effects of Vaccinium bracteatum Thunb. through the regulation of serotonegic and GABAA-ergic systems: Involvement of 5-HT1A receptor agonistic activity. Biomed. Pharmacother. 2019;109:2218–2227. doi: 10.1016/j.biopha.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 193.McCall R.B., Romero A.G., Bienkowski M.J., Harris D.W., McGuire J.C., Piercey M.F., Shuck M.E., Smith M.W., Svensson K.A., Schreur P.J. Characterization of U-92016A as a selective, orally active, high intrinsic activity 5-hydroxytryptamine1A agonist. [(accessed on 10 January 2023)];J. Pharmacol. Exp. Ther. 1994 271:875–883. Available online: https://jpet.aspetjournals.org/content/271/2/875.long. [PubMed] [Google Scholar]
- 194.Romero A.G., Leiby J.A., McCall R.B., Piercey M.F., Smith M.W., Han F. Novel 2-substituted tetrahydro-3H-benz[e]indolamines: Highly potent and selective agonists acting at the 5-HT1A receptor as possible anxiolytics and antidepressants. J. Med. Chem. 1993;36:2066–2074. doi: 10.1021/jm00067a003. [DOI] [PubMed] [Google Scholar]
- 195.Braden M.R., Parrish J.C., Naylor J.C., Nichols D.E. Molecular Interaction of Serotonin 5-HT2A Receptor Residues Phe339(6.51) and Phe340(6.52) with Superpotent N-Benzyl Phenethylamine Agonists. Mol. Pharmacol. 2006;70:1956. doi: 10.1124/mol.106.028720. [DOI] [PubMed] [Google Scholar]
- 196.Poulie C.B.M., Jensen A.A., Halberstadt A.L., Kristensen J.L. DARK Classics in Chemical Neuroscience: NBOMes. ACS Chem. Neurosci. 2020;11:3860–3869. doi: 10.1021/acschemneuro.9b00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nichols D.E. Structure-Activity Relationships of Phenethylamine Hallucinogens. J. Pharm. Sci. 1981;70:839–849. doi: 10.1002/jps.2600700802. [DOI] [PubMed] [Google Scholar]
- 198.Elbardisy H.M., Foster C.W., Marron J., Mewis R.E., Sutcliffe O.B., Belal T.S., Talaat W., Daabees H.G., Banks C.E. Quick Test for Determination of N-Bombs (Phenethylamine Derivatives, NBOMe) Using High-Performance Liquid Chromatography: A Comparison between Photodiode Array and Amperometric Detection. ACS Omega. 2019;4:14439–14450. doi: 10.1021/acsomega.9b01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Leth-Petersen S., Petersen I.N., Jensen A.A., Bundgaard C., Bæk M., Kehler J., Kristensen J.L. 5-HT2A/5-HT2C Receptor Pharmacology and Intrinsic Clearance of N-Benzylphenethylamines Modified at the Primary Site of Metabolism. ACS Chem. Neurosci. 2016;7:1614–1619. doi: 10.1021/acschemneuro.6b00265. [DOI] [PubMed] [Google Scholar]
- 200.Nichols D.E., Sassano M.F., Halberstadt A.L., Klein L.M., Brandt S.D., Elliott S.P., Fiedler W.J. N-Benzyl-5-methoxytryptamines as Potent Serotonin 5-HT2 Receptor Family Agonists and Comparison with a Series of Phenethylamine Analogues. ACS Chem. Neurosci. 2015;6:1165–1175. doi: 10.1021/cn500292d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nichols D.E. Structure–activity relationships of serotonin 5-HT2A agonists. WIRES Mem. Trans. 2012;1:559–579. doi: 10.1002/wmts.42. [DOI] [Google Scholar]
- 202.Marcher-Rørsted E., Halberstadt A.L., Klein A.K., Chatha M., Jademyr S., Jensen A.A., Kristensen J.L. Investigation of the 2,5-Dimethoxy Motif in Phenethylamine Serotonin 2A Receptor Agonists. ACS Chem. Neurosci. 2020;11:1238–1244. doi: 10.1021/acschemneuro.0c00129. [DOI] [PubMed] [Google Scholar]
- 203.Jacob P., III, Shulgin A.T. Sulfur analogs of psychotomimetic agents. 2. Analogs of (2,5-dimethoxy-4-methylphenyl)- and of (2,5-dimethoxy-4-ethylphenyl)isopropylamine. J. Med. Chem. 1983;26:746–752. doi: 10.1021/jm00359a021. [DOI] [PubMed] [Google Scholar]
- 204.Porter M.R., Xiao H., Wang J., Smith S.B., Topczewski J.J. 3-Amino-chromanes and Tetrahydroquinolines as Selective 5-HT2B, 5-HT7, or σ1 Receptor Ligands. ACS Med. Chem. Lett. 2019;10:1436–1442. doi: 10.1021/acsmedchemlett.9b00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Roth B.L. Drugs and Valvular Heart Disease. N. Engl. J. Med. 2007;356:6–9. doi: 10.1056/NEJMp068265. [DOI] [PubMed] [Google Scholar]
- 206.Connolly H.M., Crary J.L., McGoon M.D., Hensrud D.D., Edwards B.S., Edwards W.D., Schaff H.V. Valvular Heart Disease Associated with Fenfluramine–Phentermine. N. Engl. J. Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 207.Nichols D.E., Nichols C.D. Serotonin Receptors. Chem. Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 208.Kolaczynska K.E., Luethi D., Trachsel D., Hoener M.C., Liechti M.E. Receptor Interaction Profiles of 4-Alkoxy-Substituted 2,5-Dimethoxyphenethylamines and Related Amphetamines. Front. Pharmacol. 2019;10:1423. doi: 10.3389/fphar.2019.01423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Schultz D.M., Prescher J.A., Kidd S., Marona-Lewicka D., Nichols D.E., Monte A. ‘Hybrid’ benzofuran–benzopyran congeners as rigid analogs of hallucinogenic phenethylamines. Biorg. Med. Chem. 2008;16:6242–6251. doi: 10.1016/j.bmc.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nichols D.E., Snyder S.E., Oberlender R., Johnson M.P., Huang X. 2,3-Dihydrobenzofuran analogs of hallucinogenic phenethylamines. J. Med. Chem. 1991;34:276–281. doi: 10.1021/jm00105a043. [DOI] [PubMed] [Google Scholar]
- 211.McLean T.H., Parrish J.C., Braden M.R., Marona-Lewicka D., Gallardo-Godoy A., Nichols D.E. 1-Aminomethylbenzocycloalkanes: Conformationally Restricted Hallucinogenic Phenethylamine Analogues as Functionally Selective 5-HT2A Receptor Agonists. J. Med. Chem. 2006;49:5794–5803. doi: 10.1021/jm060656o. [DOI] [PubMed] [Google Scholar]
- 212.Monte A.P., Marona-Lewicka D., Lewis M.M., Mailman R.B., Wainscott D.B., Nelson D.L., Nichols D.E. Substituted Naphthofurans as Hallucinogenic Phenethylamine−Ergoline Hybrid Molecules with Unexpected Muscarinic Antagonist Activity. J. Med. Chem. 1998;41:2134–2145. doi: 10.1021/jm980076u. [DOI] [PubMed] [Google Scholar]
- 213.Gaweska H., Fitzpatrick P.F. Structures and mechanism of the monoamine oxidase family. Biomol. Concepts. 2011;2:365–377. doi: 10.1515/BMC.2011.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hudspith L., Shmam F., Dalton C.F., Princivalle A., Turega S.M. Neurotransmitter selection by monoamine oxidase isoforms, dissected in terms of functional groups by mixed double mutant cycles. Org. Biomol. Chem. 2019;17:8871–8877. doi: 10.1039/C9OB01558B. [DOI] [PubMed] [Google Scholar]
- 215.Heuson E., Storgaard M., Huynh T.H.V., Charmantray F., Gefflaut T., Bunch L. Profiling substrate specificity of two series of phenethylamine analogs at monoamine oxidase A and B. Org. Biomol. Chem. 2014;12:8689–8695. doi: 10.1039/C4OB01377H. [DOI] [PubMed] [Google Scholar]
- 216.Vallejos G., Rezende M.C., Cassels B.K. Charge-transfer interactions in the inhibition of MAO-A by phenylisopropylamines—A QSAR study. J. Comput. Aid. Mol. Des. 2002;16:95–103. doi: 10.1023/A:1016344030772. [DOI] [PubMed] [Google Scholar]
- 217.Meiring L., Petzer J.P., Legoabe L.J., Petzer A. The evaluation of N-propargylamine-2-aminotetralin as an inhibitor of monoamine oxidase. Bioorg. Med. Chem. Lett. 2022;67:128746. doi: 10.1016/j.bmcl.2022.128746. [DOI] [PubMed] [Google Scholar]
- 218.Purich D.L. The Inhibitor Index: A Desk Reference on Enzyme Inhibitors, Receptor Antagonists, Drugs, Toxins, Poisons and Therapeutic Leads. CRC Press; Boca Raton, FL, USA: 2017. [Google Scholar]
- 219.Liang Y., Quenelle D., Vogel J.L., Mascaro C., Ortega A., Kristie T.M. A Novel Selective LSD1/KDM1A Inhibitor Epigenetically Blocks Herpes Simplex Virus Lytic Replication and Reactivation from Latency. mBio. 2013;4:e00558-12. doi: 10.1128/mBio.00558-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Horita A. Beta-phenylisopropylhydrazine, A Potent And Long Acting Monoamine Oxidase Inhibitor. [(accessed on 10 January 2023)];J. Pharmacol. Exp. Ther. 1958 122:176–181. Available online: https://jpet.aspetjournals.org/content/122/2/176. [PubMed] [Google Scholar]
- 221.Shimamoto K., Matsuo T., Hattori K., Honjo T. Effects of Skf-385 And Reserpine on The Tissue Catecholamine Content in Rabbits. Jpn. J. Pharmacol. 1964;14:425–433. doi: 10.1254/jjp.14.425. [DOI] [PubMed] [Google Scholar]
- 222.Antonijoan R.M., Ferrero-Cafiero J.M., Coimbra J., Puntes M., Martínez-Colomer J., Arévalo M.I., Mascaró C., Molinero C., Buesa C., Maes T. First-in-Human Randomized Trial to Assess Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of the KDM1A Inhibitor Vafidemstat. CNS Drugs. 2021;35:331–344. doi: 10.1007/s40263-021-00797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Stein C. Opioid Receptors. Annu. Rev. Med. 2016;67:433–451. doi: 10.1146/annurev-med-062613-093100. [DOI] [PubMed] [Google Scholar]
- 224.Sobczak M., Sałaga M., Storr M.A., Fichna J. Physiology, signaling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: Current concepts and future perspectives. J. Gastroenterol. 2014;49:24–45. doi: 10.1007/s00535-013-0753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Takahashi H., Chida Y., Yoshii T., Suzuki T., Yanaura S. Asymmetric α-Substituted Phenethylamines. VI.: Synthesis and Analgesic Activity of Optically Pure (R)-and (S)-N-Akyl-1-cyclohexyl-2-phenylethylamines. Chem. Pharm. Bull. 1986;34:2071–2077. doi: 10.1248/cpb.34.2071. [DOI] [PubMed] [Google Scholar]
- 226.Takahashi H., Chida Y., Higashiyama K., Onishi H. Asymmetric α-Substituted Phenethylamines. V. Synthesis of Chiral 1-Alkyl-2-phenylethylamines via Grignard Reaction of 4-Phenyl-1, 3-oxazolidines. Chem. Pharm. Bull. 1985;33:4662–4670. doi: 10.1248/cpb.33.4662. [DOI] [Google Scholar]
- 227.Takahashi H., Chida Y., Suzuki T., Onishi H., Yanaura S. Asymmetric α-Substituted Phenethylamines. IV. The Synthesis and Analgesic Activity of Optically Pure (S) and (R)-1-Cyclohexyl-and 1-Cyclohexenyl-2-phenylethylamines. Chem. Pharm. Bull. 1984;32:2714–2723. doi: 10.1248/cpb.32.2714. [DOI] [PubMed] [Google Scholar]
- 228.Takahashi H., Chida Y., Suzuki T., Yanaura S., Suzuki Y., Masuda C. Asymmetric α-Substituted Phenethylamines. III. The Synthesis and Analgesic Activity of optically Pure (S)- and (R)-1-Aryl-2-phenylethylamines. Chem. Pharm. Bull. 1983;31:1659–1665. doi: 10.1248/cpb.31.1659. [DOI] [PubMed] [Google Scholar]
- 229.Suzuki Y., Takahashi H. Asymmetric α-Substituted Phenethylamines. II. The Enantioselective Synthesis of 1-Aryl-2-phenylethylamines from L- and D-Amino Acids by Means of 1, 3-Asymmetric Induction. Chem. Pharm. Bull. 1983;31:31–40. doi: 10.1248/cpb.31.31. [DOI] [PubMed] [Google Scholar]
- 230.Takahashi H., Suzuki Y., Inagaki H. Asymmetric α-Substituted Phenethylamines. I. Synthesis of Optically Pure 1-Aryl-N-(2′-hydroxy-1′-isopropylethyl)-2-phenylethylamines. Chem. Pharm. Bull. 1982;30:3160–3166. doi: 10.1248/cpb.30.3160. [DOI] [Google Scholar]
- 231.Manchado A., García M., Salgado M.M., Díez D., Garrido N.M. A novel Barton decarboxylation produces a 1,4-phenyl radical rearrangement domino reaction. Tetrahedron. 2018;74:5240–5247. doi: 10.1016/j.tet.2018.05.043. [DOI] [Google Scholar]
- 232.Spetea M., Berzetei-Gurske I.P., Guerrieri E., Schmidhammer H. Discovery and Pharmacological Evaluation of a Diphenethylamine Derivative (HS665), a Highly Potent and Selective κ Opioid Receptor Agonist. J. Med. Chem. 2012;55:10302–10306. doi: 10.1021/jm301258w. [DOI] [PubMed] [Google Scholar]
- 233.Tyagi S., Gupta P., Saini A., Kaushal C., Sharma S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharma. Technol. Res. 2011;2:236–240. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Willson T.M., Brown P.J., Sternbach D.D., Henke B.R. The PPARs: From Orphan Receptors to Drug Discovery. J. Med. Chem. 2000;43:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 235.Usui-Ouchi A., Ouchi Y., Ebihara N. The peroxisome proliferator-activated receptor pan-agonist bezafibrate suppresses microvascular inflammatory responses of retinal endothelial cells and vascular endothelial growth factor production in retinal pigmented epithelial cells. Int. Immunopharmacol. 2017;52:70–76. doi: 10.1016/j.intimp.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 236.Franko A., Neschen S., Rozman J., Rathkolb B., Aichler M., Feuchtinger A., Brachthäuser L., Neff F., Kovarova M., Wolf E., et al. Bezafibrate ameliorates diabetes via reduced steatosis and improved hepatic insulin sensitivity in diabetic TallyHo mice. Mol. Metab. 2017;6:256–266. doi: 10.1016/j.molmet.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.He B.K., Ning Z.Q., Li Z.B., Shan S., Pan D.S., Ko B.C.B., Li P.P., Shen Z.F., Dou G.F., Zhang B.L., et al. In Vitro and In Vivo Characterizations of Chiglitazar, a Newly Identified PPAR Pan-Agonist. PPAR Res. 2012;2012:546548. doi: 10.1155/2012/546548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chen L., Yang B., McNulty J.A., Clifton L.G., Binz J.G., Grimes A.M., Strum J.C., Harrington W.W., Chen Z., Balon T.W., et al. GI262570, a Peroxisome Proliferator-Activated Receptor γ Agonist, Changes Electrolytes and Water Reabsorption from the Distal Nephron in Rats. J. Pharmacol. Exp. Ther. 2005;312:718–725. doi: 10.1124/jpet.104.074088. [DOI] [PubMed] [Google Scholar]
- 239.Brown P.J., Winegar D.A., Plunket K.D., Moore L.B., Lewis M.C., Wilson J.G., Sundseth S.S., Koble C.S., Wu Z., Chapman J.M., et al. A Ureido-Thioisobutyric Acid (GW9578) Is a Subtype-Selective PPARα Agonist with Potent Lipid-Lowering Activity. J. Med. Chem. 1999;42:3785–3788. doi: 10.1021/jm9903601. [DOI] [PubMed] [Google Scholar]
- 240.Brown K.K., Henke B.R., Blanchard S.G., Cobb J.E., Mook R., Kaldor I., Kliewer S.A., Lehmann J.M., Lenhard J.M., Harrington W.W., et al. A novel N-aryl tyrosine activator of peroxisome proliferator-activated receptor-gamma reverses the diabetic phenotype of the Zucker diabetic fatty rat. Diabetes. 1999;48:1415–1424. doi: 10.2337/diabetes.48.7.1415. [DOI] [PubMed] [Google Scholar]
- 241.Xu H.E., Stanley T.B., Montana V.G., Lambert M.H., Shearer B.G., Cobb J.E., McKee D.D., Galardi C.M., Plunket K.D., Nolte R.T., et al. Structural basis for antagonist-mediated recruitment of nuclear co-repressors by PPARα. Nature. 2002;415:813–817. doi: 10.1038/415813a. [DOI] [PubMed] [Google Scholar]
- 242.Luo M., Yeruva S., Liu Y., Chodisetti G., Riederer B., Menon M.B., Tachibana K., Doi T., Seidler U.E. IL-1β-Induced Downregulation of the Multifunctional PDZ Adaptor PDZK1 Is Attenuated by ERK Inhibition, RXRα, or PPARα Stimulation in Enterocytes. Fronti. Physiol. 2017;8:61. doi: 10.3389/fphys.2017.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Aydar E., Cobos E.J., Maurice T., Murell-Lagnado R.D., Safrany S.T. Editorial: Sigma Receptors. Front. Pharmacol. 2020;11:590519. doi: 10.3389/fphar.2020.590519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Skuza G., Rogóz Z. Effect of BD 1047, a sigma1 receptor antagonist, in the animal models predictive of antipsychotic activity. [(accessed on 10 January 2023)];Pharmacol. Rep. 2006 58:626–635. Available online: http://www.if-pan.krakow.pl/pjp/pdf/2006/5_626.pdf. [PubMed] [Google Scholar]
- 245.Christian S.L., Berry M.D. Trace Amine-Associated Receptors as Novel Therapeutic Targets for Immunomodulatory Disorders. Front. Pharmacol. 2018;9:680. doi: 10.3389/fphar.2018.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lewin A.H., Navarro H.A., Wayne Mascarella S. Structure–activity correlations for β-phenethylamines at human trace amine receptor 1. Biorg. Med. Chem. 2008;16:7415–7423. doi: 10.1016/j.bmc.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.CAS SciFinder-n. 2023. [(accessed on 10 January 2023)]. Available online: https://www.cas.org/solutions/cas-scifinder-discovery-platform/cas-scifinder.
- 248.Elsevier B.V. Scopus. 2023. [(accessed on 10 January 2023)]. Available online: https://www.elsevier.com/solutions/scopus.