Abstract
T-cell molecular mimicry between streptococcal and heart proteins has been proposed as the triggering factor leading to autoimmunity in rheumatic heart disease (RHD). We searched for immunodominant T-cell M5 epitopes among RHD patients with defined clinical outcomes and compared the T-cell reactivities of peripheral blood and intralesional T cells from patients with severe RHD. The role of HLA class II molecules in the presentation of M5 peptides was also evaluated. We studied the T-cell reactivity against M5 peptides and heart proteins on peripheral blood mononuclear cells (PBMC) from 74 RHD patients grouped according to the severity of disease, along with intralesional and peripheral T-cell clones from RHD patients. Peptides encompassing residues 1 to 25, 81 to 103, 125 to 139, and 163 to 177 were more frequently recognized by PBMC from RHD patients than by those from controls. The M5 peptide encompassing residues 81 to 96 [M5(81–96) peptide] was most frequently recognized by PBMC from HLA-DR7+ DR53+ patients with severe RHD, and 46.9% (15 of 32) and 43% (3 of 7) of heart-infiltrating and PBMC-derived peptide-reactive T-cell clones, respectively, recognized the M5(81–103) region. Heart proteins were recognized more frequently by PBMC from patients with severe RHD than by those from patients with mild RHD. The similar pattern of T-cell reactivity found with both peripheral blood and heart-infiltrating T cells is consistent with the migration of M-protein-sensitized T cells to the heart tissue. Conversely, the presence of heart-reactive T cells in the PBMC of patients with severe RHD also suggests a spillover of sensitized T cells from the heart lesion.
Rheumatic fever (RF) is a sequel of group A streptococcal throat infection and remains an important health problem in developing countries. About 30% of RF patients develop rheumatic heart disease (RHD), with high morbidity and cost to the public health system.
Molecular mimicry between streptococcal antigens, mainly the M protein, and heart tissue proteins is proposed as an important factor leading to the heart lesions found in RHD patients. Several studies have been performed with human peripheral blood mononuclear cells (PBMC) showing reactivity against the streptococcal cell wall and tissue antigens (20, 25). CD4+ T cells are the predominant population at the site of heart lesions (23, 16).
Yoshinaga et al. (30) reported that T-cell lines derived from heart valve specimens and PBMC from RF patients react with cell wall and membrane streptococcal antigens. These lymphocytes did not cross-react with M protein or mammalian cytoskeletal proteins. Autoreactivity to heart antigens caused by streptococcal infections was also suggested by results of immunization in which peripheral T lymphocytes from RHD patients stimulated in vitro with streptococci were able to recognize a 50- to 54-kDa myocardial protein fraction (7). Our group previously reported intralesional T-cell clones, from surgical fragments of patients with severe RHD, capable of recognizing immunodominant M5 peptides and heart tissue proteins in a cross-reactive way (13). Our results allowed us to establish the significance of T-cell molecular mimicry between beta-hemolytic streptococci and heart tissue and its implication in the pathogenesis of RHD. Even though the identification of cross-reactive T cells infiltrating the hearts of patients with severe RHD is taken as significant evidence for autoimmunity in pathogenesis (26), further comparison of recognition repertoires in RHD patients from different clinical groups with healthy controls may allow the identification of preferential antigenic targets for T cells from RHD patients.
In the present study, we analyzed the reactivity of intralesional T lymphocytes and PBMC from RHD patients and healthy individuals against synthetic M5 peptides and human heart tissue protein fractions. Given the previously reported association of the HLA-DR7, DR53 haplotype with RF-RHD (12, 29), we also assessed the ability of RHD-associated HLA molecules DR7 and DR53 to bind and present immunodominant M5 peptides.
MATERIALS AND METHODS
Patients.
We studied 74 patients with RHD, from the Heart Institute, Children's Institute, University of São Paulo, selected according to Jones's modified criteria (5). The average length of patient follow-up was 5 years. RHD patients were divided into two groups: 41 patients with severe RHD and 33 patients with mild RHD. Patients with severe RHD presented severe mitral and aortic valve regurgitation; some presented congestive cardiac failure. Patients with mild RHD presented mild mitral valve regurgitation. Two patients with severe RHD presented chorea, while 19 patients with mild RHD presented concomitant carditis and chorea. The mean age was 13.0 (standard deviation [SD], 2.7) years and 13.5 (SD, 3.5) years for patients with severe and mild RHD, respectively. There were no significant differences in gender distribution among these groups. Eight patients with severe RHD and one patient with mild RHD had recovered from disease reactivation 3 to 6 months before the study. The blood samples were taken in absence of immunosuppressive drugs. Thirty-five healthy adult individuals made up our control group for peripheral blood reactivity studies, with a mean age of 32.5 (SD, 8.8) years and without previous history of RF or recently documented streptococcal throat infection. Blood samples and surgical fragment collection procedures were cleared by the Committee of Ethics of the Heart Institute, HC-FMUSP.
T-cell lines and T-cell clones.
Intralesional T-cell lines were derived from in vitro culture of surgical fragments of mitral valve, aortic valve, papillar muscle, or left atrium of eight patients with severe RHD who submitted to surgery for valve correction. Tissue was finely minced with injection needles, placed in flat-bottom 96-well plates (Becton Dickinson Co.), with Dulbecco's modified Eagle medium (Sigma Chemical Co.) supplemented with 2 mM l-glutamine (Sigma), 10% pooled normal human serum, 10 mM HEPES (Sigma), antibiotics (gentamicin, 40 mg/ml; Peflacyn, 20 mg/ml) and human recombinant interleukin 2 (IL-2) (40 U/ml; Biosource Inc.), on an HLA-DR-matched feeder layer of PBMC at 105 cells/well, irradiated at 5,000 rads (28). Peripheral T-cell lines were derived from one patient with severe RHD (patient 8) and two patients with mild RHD (patients 9 and 10) by PBMC stimulation with a pool of the N-terminal M5 peptides (total final concentration, 10 μg/ml) in the presence of IL-2 followed by phytohemagglutinin (PHA)-P stimulation. T-cell clones were obtained by limiting dilution of intralesional and peripheral T-cell lines using 0.3 cell/well in the presence of PHA-P (5 μg/ml) and 105 irradiated PBMC/well in an IL-2-enriched growth medium as described (13). Plates that had more than 15% positive wells were discarded. T-cell clones were restimulated every 21 days and tested after up to three restimulations.
Peptide synthesis and preparation of human heart tissue protein fractions and of human cardiac myosin.
Peptides based on the published M5 protein sequence (19) were synthesized by the “tea bag” method by tert-butoxycarbonyl chemistry and checked by mass spectrometry and high-performance liquid chromatography. Sequences for the following M5 peptides were as indicated: M5 peptide encompassing residues 1 to 20 [M5(1–20) peptide], TVTRGTISDPQRAKEALDKY; M5(11–25), QRAKEALDKYELENH; M5(62–82), LERKTAELTSEKKEHEAENDK; M5(81–96), DKLKQQRDTLSTQKET; M5(83–103), KQQRDTLSTQKETLEREVQN; M5(91–103), STQKETLEREVQN; M5(125–139), TRQELANKQQESKEN; M5(141–154), KALNELLEKTVKDK; M5(163–177), ETIGTLKKILDETVK. Tissue fractions from human myocardium, aortic valve, purified human ventricular cardiac myosin were obtained from lysates of postmortem normal tissue samples, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto nitrocellulose membranes from which nitrocellulose suspensions containing heart protein fractions were obtained as described (13). For molecular weight values, see Fig. 1.
FIG. 1.
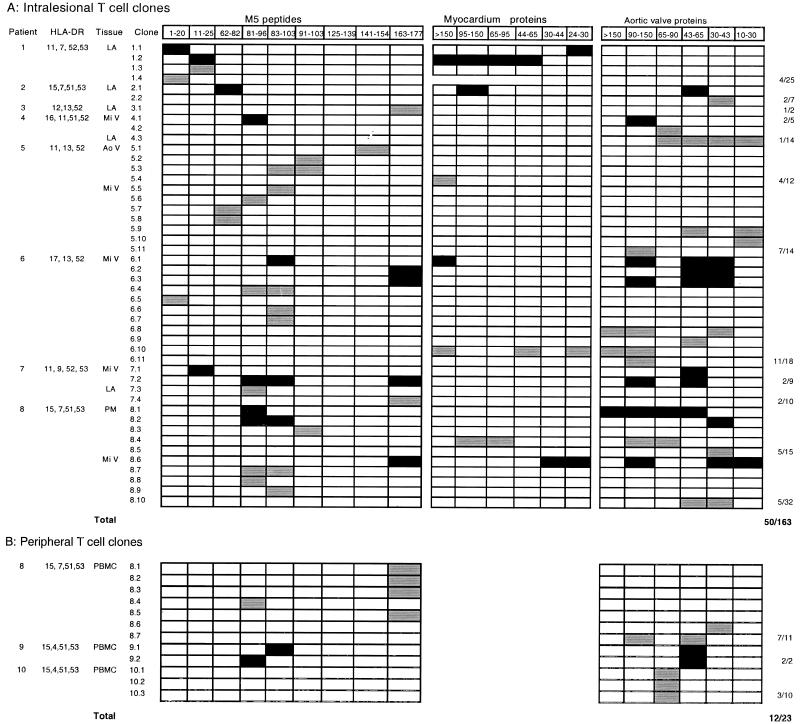
Reactivity against N-terminal M5 peptides and heart tissue proteins. (A) Intralesional T-cell clones from patients with severe RHD; (B) peripheral T-cell clones from RHD patients. PBMC-derived T-cell clones were not tested with myocardium protein fractions. Abbreviations: LA, left atrium; Mi V, mitral valve; Ao V, aortic valve; PM, papillar muscle. Black squares, M5 peptide-heart tissue protein cross-reactive T-cell clones; gray squares, non-cross-reactive T-cell clones recognizing either M5 peptide or heart tissue proteins.
Proliferation assays
Proliferation assays were performed in Falcon flat-bottom 96-well plates using 105 mononuclear cells/well isolated from peripheral blood by centrifugation on a 1,077 density gradient for 120 h at 37°C in a humidified 5% CO2 incubator. Either of streptococcal M5 peptides (5 μg/ml), heart tissue fractions (20 μl/well), or purified human myosin was added. Negative controls were Dulbecco's modified Eagle medium for peptide experiments and 20 μl of a protein-free nitrocellulose suspension for heart tissue fraction and myosin experiments. PHA-P (5 μg/ml) was used for positive control of proliferative responses. Triplicate wells were pulse-labeled with 0.5 μCi of tritiated thymidine (Amersham Pharmacia Biotech) per well for the final 18 h of culture; cells were harvested and analyzed in an automated gas phase beta counter (Matrix 96: Packard Co.). For T-cell clones 2 × 104 T cells/well were incubated with 105 HLA-DR-matched irradiated PBMC (5,000 rads) for 96 h. The proliferative response of PBMC and T-cell clones was considered positive when the stimulation index (SI) (SI = mean experimental cpm/negative control cpm) was ≥2.5.
HLA class II antigens.
Patients and controls were typed for HLA-DR by PCR amplification with sequence-specific primers (HLA-DR1 to DR18, HLA-DR51, DR52 and DR53) and for HLA-DQ by PCR amplification with sequence-specific oligonucleotides (12 and 22 different oligonucleotides for DQA1 and DQB1, respectively). DNA was isolated from proteinase K-treated peripheral blood leukocytes by salting-out extraction (21).
HLA binding assay.
The binding assay was performed for HLA-DR7 and DR53 molecules purified and then incubated with 125I-radiolabeled peptides as described (18). The data were then plotted, and the dose yielding a 50% inhibitory concentration (IC50) was measured. Each peptide was tested in two to four completely independent experiments.
Statistical analysis.
Differences in the frequency of positive proliferative responses of PBMC from patients in different clinical groups and healthy individuals were evaluated using Fisher's exact test. A P value of ≤0.05 was considered significant.
RESULTS
Presentation and T-cell response to streptococcal M5 peptides, heart tissue fractions and human myosin.
PBMC reactivity against N-terminal M5 peptides was found in 54 and 57% of patients with severe and mild RHD, respectively, and 26% of healthy individuals. Six peptides were more frequently recognized by PBMC from RHD patients than by those from controls (Table 1). PBMC from severe RHD patients preferentially recognized peptides in the regions of residues 1 to 20 and 81 to 103. Peptide M5(81–96) was recognized by PBMC from 46% of patients with severe RHD versus those from 8.6% of controls (P = 0.0005). This peptide was also twice as frequently recognized by PBMC from severe than those from patients with mild RHD (46 versus 27%, P = 0.08). The overlapping peptides M5(91–103) and M5(83–103) were recognized by 24.3% of patients with severe RHD and 3.0% of controls (P = 0.01) (Table 1) and by 27% of patients with severe RHD and 11.4% of controls (P not significant), respectively. Peptide M5(1–20) was recognized by PBMC from 35.1% of patients with severe RHD and those from 8.6% of controls, (P = 0.01) (Table 1). Peptides M5(11–25) and M5(125–139) were predominantly recognized by PBMC from patients with mild RHD compared with controls (P = 0.008 and P = 0.01, respectively) (Table 1). Although 54.5% of patients with mild RHD and 5.4% of patients with severe RHD presented Sydenham chorea (P < 0.001) we could not identify any specific antipeptide reactivity among these patients. Rates of recognition of the M5(163–177) peptide (Table 1) were comparable in patients with mild and severe disease.
TABLE 1.
Reactivity of PBMC from RHD patients and controls against relevant M5 peptides
| Peptide | Result for PBMC from:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients with severe RHD (n = 37)
|
Patients with mild RHD (n = 33)
|
Health controls (n = 35)
|
|||||||
| % Reactivity | SIa | SE | % Reactivity | SI | SE | % Reactivity | SI | SE | |
| M5(1–20) | 35.1b | 4.8 | 0.67 | 27.3 | 3.4 | 0.26 | 8.6 | 2.8 | 0.15 |
| M5(11–25) | 21.6 | 5.4 | 1.01 | 36.4c | 4.0 | 0.60 | 8.6 | 2.8 | 0.06 |
| M5(81–96) | 46.0d | 4.4 | 0.45 | 27.3 | 4.3 | 0.49 | 8.6 | 3.3 | 0.29 |
| M5(81–103) | 27.0 | 4.2 | 1.20 | 24.2 | 4.4 | 1.30 | 11.4 | 3.3 | 0.40 |
| M5(91–103) | 24.3b | 3.8 | 0.48 | 9.1 | 4.1 | 0.35 | 3.0 | 2.9f | |
| M5(125–139) | 16.2 | 3.9 | 0.81 | 24.2b | 3.6 | 0.39 | 3.0 | 2.6f | |
| M5(163–177) | 24.3e | 5.5 | 1.26 | 24.2e | 3.6 | 0.37 | 5.7 | 3.5 | 0.26 |
SI, mean of positive SI values.
Significant results compared healthy controls (P = 0.01).
Significant in comparison with healthy controls (P = 0.008).
Significant in comparison with healthy controls (P = 0.0005).
Significant in comparison with healthy controls (P = 0.04).
Positive SI values of an individual.
PBMC from 61% of patients with severe RHD were able to recognize myocardial and heart valve fractions, compared to those from 15% of patients with mild RHD and 20% of controls (severe versus mild RHD, P = 0.0002; severe RHD versus controls, P = 0.0006) (Table 2). Valve-derived protein fractions were also more frequently recognized than myocardial protein fractions by PBMC from patients with severe RHD. The myocardial fraction of >150 kDa was the single most frequently recognized heart tissue fraction by PBMC from patients with severe RHD (26.5% versus no recognition by patients with mild RHD, P = 0.002, and versus 2.8% of controls, P = 0.006) (Table 2). For only two of nine patients did the PBMC responsive to the myocardial fraction of >150 kDa also recognize purified human cardiac myosin (data not shown). The aortic valve fraction 65 to 90 kDa was recognized by PBMC from 21.2% of patients with severe RHD and those from 3% of patients with mild RHD (P = 0.03), and the aortic valve fraction of 30 to 43 kDa was recognized by PBMC from 16.7% of patients with severe RHD, while it was not recognized by PBMC from patients with mild RHD (P = 0.03) (Table 2).
TABLE 2.
PBMC recognition of relevant heart tissue protein fractions and global reactivity by RHD patients and controls
| Subject group | Result for PBMC from heart tissue protein fraction
|
% Global reactivityd | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ia
|
IIIb
|
Vc
|
||||||||
| % Reactivity | SIe | SE | % Reactivity | SI | SE | % Reactivity | SI | SE | ||
| Severe RHD (n = 36) | 26.5f | 5.5 | 0.52 | 21.2g | 7.6 | 2.9 | 16.7g | 4.3 | 0.69 | 61.0h |
| Mild RHD (n = 33) | 0 | 0 | 0 | 3.0 | 3.0i | 0 | 0 | 0 | 15.0 | |
| Healthy control (n = 35) | 2.8 | 2.5i | 5.7 | 2.7 | 0.20 | 11.4 | 3.3 | 0.45 | 20.0 | |
Myocardium protein (>150 kDa).
Aortic valve protein (65 to 90 kDa).
Aortic valve protein (30 to 43 kDa).
Myocardium plus aortic valve proteins.
SI, mean of positive SI values.
Significant in comparison with healthy controls (P = 0.006) and patients with mild RHD (P = 0.002).
Significant in comparison with patients with mild RHD (P = 0.03).
Significant in comparison with healthy controls (P = 0.0006) and patients with mild RHD (P = 0.0002).
Positive SI of an individual.
Twelve out of 23 (40%) peptide-stimulated peripheral T-cell clones derived from three RHD patients were able to recognize M5 peptides and heart tissue proteins. Interestingly, peptides M5(81–96), M5(83–103), and M5(163–177) were predominantly recognized. The aortic valve fractions of 90 to 150 kDa, 43 to 65 kDa, and 30 to 43 kDa were also recognized by five peripheral T-cell clones (Fig. 1B). Two clones recognizing the overlapping peptides M5(81–96) and M5(83–103) cross-reactively recognized the aortic valve fraction of 43 to 65 kDa (Fig. 1B).
Thirty-two out of 163 heart-infiltrating T-cell clones derived from patients with severe RHD (19.6%) recognized M5 peptides. These T-cell clones predominantly recognized M5 peptides encompassing residues 81 to 103 (15 of 32 [46.9%]). Peptides M5(1–20), M5(11–25), and M5(163–177) were recognized by 3 of 32 (9.4%), 3 of 32 (9.4%), and 6 of 36 (16.7%) peptide-reactive heart-infiltrating T-cell clones, respectively (Fig. 1A). Fourteen out of 163 heart infiltrating T-cell clones (8.6%) derived from eight patients with severe RHD only recognized aortic valve protein fractions of 90 to 150 kDa, 43 to 65 kDa, and 30 to 43 kDa. Twelve out of 163 intralesional T-cell clones (7.4%) displayed cross-reactivity with M5 peptides located in regions of residues 1 to 25, 81 to 103, and 166 to 177 and mainly with the valvular tissue fractions mentioned above, as previously reported (13) (Fig. 1A). The mentioned valve fractions were recognized by 50 to 60% of the cross-reactive T-cell clones (Fig. 1A) The presence of recent episodes of disease activity had no correlation with a specific pattern of reactivity against heart tissue protein fractions and streptococcal M5 peptides.
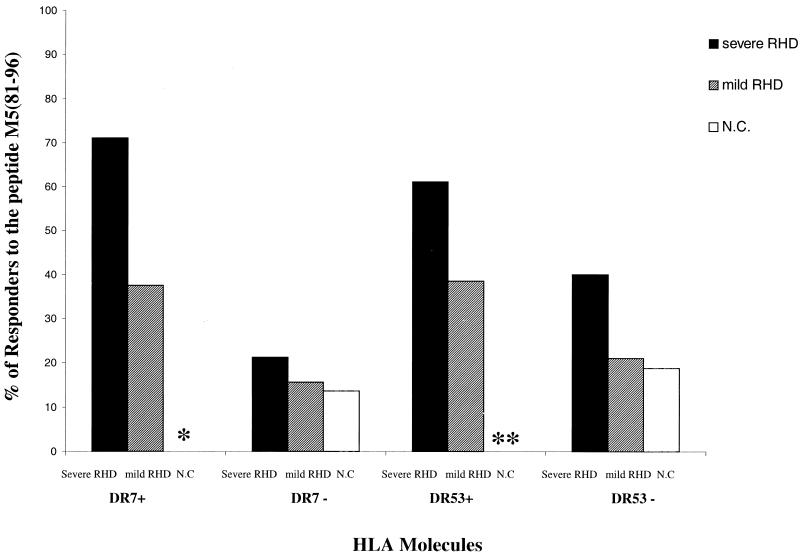
Our previous results have shown an association between RF and the HLA-DR7, DR53 haplotype (12, 29) in the Brazilian population. Since HLA-DR53 is in linkage disequilibrium with DR4, DR7, and DR9 antigens, not all HLA-DR53 molecules carry HLA-DR7. Among the peripheral reactivity of patients with severe RHD the ability to respond to the M5(81–96) peptide seemed to be related to the presence of HLA-DR7 and HLA-DR53 molecules. Seventy-one percent of the HLA-DR7+ DR53+ (10 out of 14 DR7+ patients) recognized this peptide. Considering all DR53+ patients, we found an additional DR9+ DR53+ responder patient; thus, we found 61% (11 out of 18 DR53+ patients) able to respond to the M5(81–96) peptide (Fig. 2). On the other hand, none of the DR53+ or DR7+ DR53+ normal individuals recognized this peptide (Fig. 2). We found that intralesional T-cell clones recognized this peptide in the context of several HLA-DR molecules. Among the responders to this peptide only two patients (patients 7 and 8) are DR53+ (Fig. 1A).
FIG. 2.
Frequency of M5(81–96) peptide recognition by PBMC from HLA-DR7+ DR53+ patients with severe RHD. The proportion of responders to the M5(81–96) peptide was measured by proliferation assay. DR7+ individuals are HLA-DR53+; DR53+ individuals are DR7+, DR4+, or DR9+; DR7− individuals can be DR53+ (if they are DR4+ or DR9+); HLA-DR53− individuals are DR7−, DR4−, or DR9−. ∗, P = 0.008 for HLA-DR7+ patients with severe RHD versus HLA-DR7+ controls (N.C); ∗∗, P < 0.001 for HLA-DR53+ patients with severe RHD with HLA-DR53+ controls.
Given the present results and our previous reports on HLA class II antigens associated with RF as above cited, we analyzed the capacity of HLA-DR7 and -DR53 molecules to bind streptococcal M5 peptides. None of the 9 N-terminal M5 peptides bound to HLA-DR7 molecules (IC50 ≥ 35,000 nM). However, binding with intermediate affinity (IC50 in the 100 to 1,000 nM range) was detected for M5(81–96) peptide with the HLA-DR53 molecule (IC50 = 670 nM) (data not shown).
DISCUSSION
We have found that peripheral T cells from more than 50% of RHD patients and 26% of healthy individuals are able to recognize N-terminal streptococcal M5 epitopes. This indicates that PBMC from RF-RHD patients display a vigorous recall response to previous streptococcal infection. The ability of normal adult individuals to respond to streptococcal peptides is in all probability related to previous streptococcal throat infection.
We have identified five immunodominant M5 peptides comprised in the N-terminal M5 protein regions that are not only preferentially recognized by PBMC from patients with severe or mild RHD but also by intralesional and peripheral T-cell clones. The M5(81–103) region was recognized by several intralesional T-cell clones obtained from five of eight patients with severe RHD, along with peripheral T-cell clones obtained from one patient with severe RHD (Fig. 1). It has been shown that immunized mice display strong T-cell proliferative responses against the M5(81–103) region (4), further supporting the argument for immunodominance of this region. The trend towards increased recognition of M5(81–103) by peripheral T cells from severe rather than mild cases of RHD (Table 2) is in line with its frequent recognition by heart-infiltrating T-cell clones (Fig. 1A). Together, our data support a role for the M5(81–103) region containing an immunodominant epitope of the N-terminal portion of streptococcal M protein and suggest a role for differential T-cell recognition in the development of severe carditis.
The fact that heart tissue fractions were recognized by PBMC from 61% of patients with severe RHD and only 15 to 20% of patients with mild RHD or controls (Table 2) suggests either that (i) recognition is nonspecific and secondary to heart tissue damage or (ii) recognition of specific heart antigens is pathogenetically relevant. The finding of preferential recognition of selected myocardial along with aortic valve protein fractions (Table 2), by PBMC and several intralesional T-cell clones from patients with severe RHD (Fig. 1), supports the second hypothesis. These data are in line with our previous results showing the presence of streptococcal and heart tissue protein cross-reactive T-cell clones in the heart lesions (13). It has been suggested that antiheart responses found in peripheral blood may be secondary to molecular mimicry with streptococcal M protein (22). We have previously shown that oligoclonal T-cell expansions are more frequently observed in the heart lesions than in the PBMC of patients with severe RHD. The frequency of these oligoclonal infiltrating T cells was variable in the analyzed patients. Our findings could suggest a differential epitope recognition at the two lesional heart sites after a common initial bacterial challenge (14).
The lack of significant recognition of cardiac myosin by PBMC from RHD patients is in line with reports on idiopathic dilated cardiomyopathy (3) and may indicate that cardiac myosin is not a relevant heart antigen in the context of RHD. This contrasts with several reports showing the presence of cardiac myosin and streptococcal antigen cross-reactive antibodies in sera of patients (10, 17) and from animal models as well as cross-reactivity by murine and human monoclonal antibodies (6, 9, 15).
HLA class II DR7 or DR53 antigens have been linked with genetic susceptibility to RF (12, 29, 2, 1, 24). Recently, it was shown that DRB1*0701 and DQA1*0201 are associated with mitral valve disease in Egypt (11). In our group of patients with severe RHD only a few patients presented the DR7, DQA1*0201 haplotype (data not shown). The immunodominant M5(81–96) peptide was preferentially recognized by peripheral T cells from RHD a patients coexpressing DR7,DR53 molecules (Fig. 2), in line with the previously reported association. The fact that intralesional T-cell clones from some DR7+ DR53+ patients failed to recognize peptide M5(81–96) may be due to the limited number of T-cell clones analyzed per individual. On the other hand, recognition of the peptide M5(81–96) by T-cell clones from DR7− DR53− patients indicates that other HLA molecules are also able to present this peptide. Interestingly, none of the nine N-terminal peptides studied bound to HLA-DR7, and only the M5(81–96) peptide was able to bind to HLA-DR53 molecules within the range of affinity associated with T-cell recognition in the context of HLA-DR molecules (27). This may imply that HLA-DR53 preferentially presents immunodominant peptide M5(81–96) to T cells from RHD patients. However, given the recent demonstration that certain self-peptides with undetectable MHC binding values can induce experimental T-cell autoimmunity (8), one cannot exclude the possibility that HLA-DR7 and other HLA-DR molecules can in fact present M5 epitopes. On the other hand, no PBMC from healthy individuals carrying HLA-DR7 or HLA-DR53 molecules recognized this peptide (Fig. 2). Healthy individuals may or may not have had previous contact with streptococci, but if they had they certainly did not develop tissue-damaging T cells to trigger lesions in their hearts. Thus, it is possible that DR7−, DR53− restricted M5(81–96)-reactive T cells are indeed related to disease progression. The lower number of M5(81–96) peptide-responder individuals carrying HLA-DR7 or -DR53 among patients with mild RHD in comparison to those in the group with severe RHD may indicate that recognition of the M5(81–96) peptide in the context of the mentioned HLA-DR alleles is related to heart tissue damage. It is likely, however, that individuals lacking response to M5(81–96) may target other pathogenic epitopes in the streptococcal M protein (13).
Together with the finding of streptococcal M5 peptide-heart protein cross-reactive T-cell clones in the heart lesions of RHD patients (13), the present data lend support to the idea that T cells sensitized in the periphery by M5 protein during streptococcal infection, in particular the M5(81–96) peptide, in HLA-DR7+ DR53+ RHD patients, migrate to the heart and initiate heart tissue damage after activation due to cross-reactive recognition of the relevant heart antigen. The identification of several M5(81–96) peptide-reactive T-cell clones recognizing valve fractions of 90 to 150 kDa and 30 to 65 kDa (Fig. 1A) further indicates the role of cross-reactivity between streptococcal and valve proteins in the pathogenesis of RHD. The nature of these target heart proteins is presently under investigation using bidimensional T-cell Western blotting and a proteomics approach. The identification of antigenic and/or pathogenic epitopes in streptococcal M protein may lead to the development of safe synthetic peptide-based vaccines and novel immunotherapeutic strategies aiming to control heart damage.
ACKNOWLEDGMENTS
This work was supported by grants 620087/94.3 from PADCT-CNPq and 75197-555101 from HHMI.
REFERENCES
- 1.Anastasiou-Nana M, Anderson J L, Carlquist J F, Nana J N. HLA DR typing and lymphocyte subset evaluation in rheumatic heart disease: a search for immune response factors. Am Heart J. 1986;112:992–997. doi: 10.1016/0002-8703(86)90311-x. [DOI] [PubMed] [Google Scholar]
- 2.Ayoub E M, Barret D J, MacLaren N K, Krischen J P. Association of class II histocompatibility leukocyte antigens with rheumatic fever. J Clin Investig. 1986;77:2019–2026. doi: 10.1172/JCI112531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bromelow K V, Souberbielle B, Alavi A, Goldman J H, Libera L D, Dalgleish A G, McKenna W J. Lack of T cell response to cardiac myosin and a reduced response to PPD in patients with idiopathic dilated cardiomyopathy. J Autoimmun. 1997;10:219–227. doi: 10.1006/jaut.1996.0119. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham M W, Antone S M, Smart M, Liu R, Kosanke S. Molecular analysis of human cardiac myosin-cross-reactive B- and T-cell epitopes of the group A streptococcal M5 protein. Infect Immun. 1997;65:3913–3923. doi: 10.1128/iai.65.9.3913-3923.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dajani A S, Ayoub E, Bierman F Z, Bisno A L, Deny F W, Durack D T, Ferrieri P, Freed M, Gerber M, Kaplan E L, Karchmer A W, Markowitz M, Rahimtoola, Shulman S, Stollerman G, Takahashi M, Taranta A, Taubert K A, Wilson W. Guidelines for diagnosis of rheumatic fever: Jones criteria, updated 1992. Circulation. 1993;87:302–307. [Google Scholar]
- 6.Dale J B, Beachey E H. Epitopes of streptococcal M proteins shared with cardiac myosin. J Exp Med. 1885;162:583–591. doi: 10.1084/jem.162.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Demellawy M, El-Ridi R, Guirguis N I, Alim M A, Kotby A, Kotb M. Preferential recognition of myocardial antigens by T lymphocytes from rheumatic heart disease patients. Infect Immun. 1997;65:2197–2205. doi: 10.1128/iai.65.6.2197-2205.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fairchild P J, Wraith D C. Lowering the tone: mechanisms of immunodominance among epitopes with low affinity for MHC. Immunol Today. 1996;17:80–84. doi: 10.1016/0167-5699(96)80584-6. [DOI] [PubMed] [Google Scholar]
- 9.Fenderson P G, Fischetti V A, Cunningham M W. Tropomyosin shares immunologic epitopes with group A streptococcal M proteins. J Immunol. 1989;142:2475–2481. [PubMed] [Google Scholar]
- 10.Froude J, Gibofisk A, Buskirk D R, Khanna A, Zabriskie J B. Cross-reactivity between streptococcus and human tissue: a model of molecular mimicry and autoimmunity. Curr Top Microbiol Immunol. 1989;145:5–26. doi: 10.1007/978-3-642-74594-2_2. [DOI] [PubMed] [Google Scholar]
- 11.Guédez Y, Kotby A, El-Demellaway M, Galal A, Thomson G, Zaher S, Kassem S, Kotb M. HLA class II associations with rheumatic heart disease are more evident and consistent among clinically homogeneous patients. Circulation. 1999;99:2784–2790. doi: 10.1161/01.cir.99.21.2784. [DOI] [PubMed] [Google Scholar]
- 12.Guilherme L, Weidebach W, Kiss M H, Snitcowsky R, Kalil J. Association of human leukocyte class II antigens with rheumatic fever/rheumatic heart disease in a Brazilian population. Circulation. 1991;83:1995–1998. doi: 10.1161/01.cir.83.6.1995. [DOI] [PubMed] [Google Scholar]
- 13.Guilherme L, Cunha-Neto E, Coelho V, Snitcowsky R, Pomerantzeff P M, Assis R V, Pedra F, Neumann J, Goldberg A, Patarroyo M E, Pileggi F, Kalil J. Human heart-infiltrating T-cell clones from rheumatic heart disease patients recognize both streptococcal and cardiac proteins. Circulation. 1995;92:415–420. doi: 10.1161/01.cir.92.3.415. [DOI] [PubMed] [Google Scholar]
- 14.Guilherme L, Dulphy N, Douay C, Coelho V, Cunha-Neto E, Oshiro S E, Assis R V, Tanaka A C, Pomerantzeff P M A, Charron D, Toubert A, Kalil J. Molecular evidence for antigen-driven immune responses in cardiac lesions of rheumatic heart disease patients. Int Immunol. 2000;12:1064–1073. doi: 10.1093/intimm/12.7.1063. [DOI] [PubMed] [Google Scholar]
- 15.Gulizia J M, Cunningham M W, McManus B M. Immunoreactivity of anti-streptococcal monoclonal antibodies to human heart valves. Evidence for multiple cross-reactive epitopes. Am J Pathol. 1991;138:285–301. [PMC free article] [PubMed] [Google Scholar]
- 16.Kemeny E, Grieve T, Marcus R, Sareli P, Zabriskie B. Identification of mononuclear cells and T cell subsets in rheumatic valvulitis. Clin Immunol Immunopathol. 1989;52:225–237. doi: 10.1016/0090-1229(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 17.Khanna A K, Nomura Y, Fischetti V A, Zabriskie J B. Antibodies in the sera of acute rheumatic fever patients bind to human cardiac tropomyosin. J Autoimmun. 1997;10:99–106. doi: 10.1006/jaut.1996.0107. [DOI] [PubMed] [Google Scholar]
- 18.Krieger J I, Karr R W, Grey H M, Yu W Y, O'Sullivan D, Batovsky L, Zhong-Li Z, Colón S M, Gaeta F C A, Sidney J, Albertson M, Del Guercio M F, Chestnut R W, Sette A. Single amino acid changes in DR and antigen define residues critical for peptide-MHC binding and T cell recognition. J Immunol. 1991;146:2331–2340. [PubMed] [Google Scholar]
- 19.Manjula B N, Acharya A S, Mische M S, Fairwell T, Fischetti V A. The complete amino acid sequence of a biologically active 197-residue fragment of M protein isolated from type 5 group A streptococci. J Biol Chem. 1984;259:3686–3693. [PubMed] [Google Scholar]
- 20.McLaughin J F, Paterson P Y, Hartz R S, Embury S H. Rheumatic carditis: in vitro responses of peripheral blood leukocytes to heart and streptococcal antigens. Arthritis Rheum. 1972;15:600–608. doi: 10.1002/art.1780150606. [DOI] [PubMed] [Google Scholar]
- 21.Olerup O, Zetterquist H. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens. 1992;39:225–235. doi: 10.1111/j.1399-0039.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 22.Pruksakorn S, Currie B, Brandt E, Phornphutkul C, Hunsakunachal S, Manmontri A, Robinson J H, Kehoe M A, Galbraith A, Good M F. Identification of T cell autoepitopes that cross-react with C-terminal segment of the M protein of group A streptococci. Int Immunol. 1994;6:1235–1244. doi: 10.1093/intimm/6.8.1235. [DOI] [PubMed] [Google Scholar]
- 23.Raizada V, Williams R C, Jr, Chopra P, Gopinath N, Prakash K, Sharma K B, Cherian K M, Panday S, Arora R, Nigam M, Zabriskie J B, Husby G. Tissue distribution of lymphocytes in rheumatic heart valves as defined by monoclonal anti-T cell antibodies. Am J Med. 1983;74:90–96. doi: 10.1016/0002-9343(83)91124-5. [DOI] [PubMed] [Google Scholar]
- 24.Rajapakse C N A, Halim K, Al-Orainey L, Ael-Nozha M, Aska A K. A genetic marker for rheumatic heart disease. Br Heart J. 1987;58:659–662. doi: 10.1136/hrt.58.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Read S E, Fischetti V A, Utermohlen V, Falk R E, Zabriskie J Br. Cellular reactivity studies to streptococcal antigens. Migration inhibition studies in patients with streptococcal infections and rheumatic fever. J Clin Investig. 1974;54:439–450. doi: 10.1172/JCI107780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose N R, Bona C. Defining criteria for autoimmune diseases (Witebsky's postulates revisited) Immunol Today. 1993;14:426–430. doi: 10.1016/0167-5699(93)90244-F. [DOI] [PubMed] [Google Scholar]
- 27.Southwood S, Sidney J, Kondo A, Del Guercio M F, Appella E, Hoffman S, Kubo R T, Chesnut R W, Grey H M, Sette A. Several common HLA-DR types share largely overlapping peptide binding repertoires. J Immunol. 1998;160:3363–3373. [PubMed] [Google Scholar]
- 28.Weber T, Kaufman C, Zeevi A, Zerbi T R, Hardesty R J, Kormos R H, Griffith B P, Duquesnoy R J. Lymphocyte growth from cardiac allograft biopsy specimens with no or minimal cellular infiltrates: association with subsequent rejection episode. Heart Transplant. 1989;8:233–240. [PubMed] [Google Scholar]
- 29.Weidebach W, Goldberg A C, Chiarella J, Guilherme L, Snitcowsky R, Pileggi F, Kalil J. HLA class II antigens in rheumatic fever: analysis of DR locus by restriction fragment length polymorphism and oligotyping. Hum Immunol. 1994;40:253–258. doi: 10.1016/0198-8859(94)90024-8. . . [DOI] [PubMed] [Google Scholar]
- 30.Yoshinaga M, Figueiroa F, Wahid M R, Marcus R H, Suh E, Zabriskie J B. Antigenic specificity of lymphocytes isolated from valvular specimens of rheumatic fever patients. J Autoimmun. 1995;8:601–613. doi: 10.1016/0896-8411(95)90011-x. [DOI] [PubMed] [Google Scholar]