Abstract
The radiation-attenuated Schistosoma mansoni vaccine is highly effective in rodents and primates but has never been tested in humans, primarily for safety reasons. To strengthen its status as a paradigm for a human recombinant antigen vaccine, we have undertaken a small-scale vaccination and challenge experiment in chimpanzees (Pan troglodytes). Immunological, clinical, and parasitological parameters were measured in three animals after multiple vaccinations, together with three controls, during the acute and chronic stages of challenge infection up to chemotherapeutic cure. Vaccination induced a strong in vitro proliferative response and early gamma interferon production, but type 2 cytokines were dominant by the time of challenge. The controls showed little response to challenge infection before the acute stage of the disease, initiated by egg deposition. In contrast, the responses of vaccinated animals were muted throughout the challenge period. Vaccination also induced parasite-specific immunoglobulin M (IgM) and IgG, which reached high levels at the time of challenge, while in control animals levels did not rise markedly before egg deposition. The protective effects of vaccination were manifested as an amelioration of acute disease and overall morbidity, revealed by differences in gamma-glutamyl transferase level, leukocytosis, eosinophilia, and hematocrit. Moreover, vaccinated chimpanzees had a 46% lower level of circulating cathodic antigen and a 38% reduction in fecal egg output, compared to controls, during the chronic phase of infection.
Despite decades of intense efforts to control human schistosomiasis, the disease is still one of the major health problems in Africa and parts of South America and Asia. Although education, improved sanitation, eradication of the snail vector, and chemotherapeutic cure of infected patients are important for reducing the prevalence and morbidity in areas of endemicity, only an effective vaccine can provide protection against reinfection (7, 11, 68). Evidence in human populations for natural resistance (63, 64) and acquired immunity against schistosome infection (8, 20, 27) suggests that the development of a vaccine is a realistic aim. As the parasites do not replicate in the definitive host, even a partial reduction in worm burden would be beneficial in reducing morbidity and transmission (14). However, identification of relevant protective immune mechanisms in humans is hampered by their continual exposure to schistosome cercariae, uncertainties about the worm burden acquired, the behavior and nutritional status of the patients, and superinfection with other parasites (9, 25, 44, 61). For these reasons, the approach has yet to provide a clear lead for vaccine development.
More progress has been made using laboratory animal models, including nonhuman primates. The most effective and reproducible protocol to date is vaccination with radiation-attenuated (RA) cercariae (15), which results in a 50 to 80% reduction in challenge worm burden. Unlike the analogous attenuated-sporozoite malaria vaccine (52), this schistosome vaccine has not been tested in human volunteers, primarily for reasons of safety (15), and such vaccines have generally proved impractical for field use because of their brief shelf life. However, the RA vaccine could form a basis for a recombinant vaccine, if the relevant immune mechanisms and protective antigens were known (21). Thus, it is important to establish the strongest possible probability that it would be effective in humans. Chimpanzees, which are genetically and physiologically closest to humans, can become infected with schistosomes in the wild (1, 51) and develop pathology indistinguishable from that in human patients (35, 55, 56, 66), unlike rodents or other primates. We have therefore undertaken a small-scale vaccination and challenge experiment to test RA vaccine efficacy in chimpanzees, taking advantage of the full range of immunological assays now available. The study has added value in permitting a longitudinal investigation, in the control animals, of the way in which immune responses to schistosomes evolve through the acute to the chronic stage of infection. This is impermissible in human subjects, even if the point of infection were known, because of the ethical requirement to treat infected individuals with chemotherapy upon diagnosis. We demonstrate that chimpanzees can be protected against challenge infection with Schistosoma mansoni, manifested as an amelioration of the acute disease as well as the overall morbidity, and a reduction in circulating cathodic antigen (CCA) levels and egg burden compared to the control chimpanzees.
MATERIALS AND METHODS
Chimpanzees.
The study was carried out using six unrelated healthy 5- to 6-year-old male chimpanzees (Pan troglodytes), selected randomly from the breeding colony at the Biomedical Primate Research Center (BPRC). During the entire experiment, the chimpanzees were housed in a social group of nine animals. All protocols were approved by independent Scientific and Ethical Committees at BPRC and the University of York. The study was performed in compliance with the relevant laws relating to the conducting of animal experiments and according to the recommendations and guidelines of the Primate Vaccine Evaluation Network of the European Commission. The animals were regularly monitored for abnormalities in the feces; changes in appetite and behavior; and alterations in body temperature, weight, and general health status. Sampling procedures were restricted to those which could be reasonably carried out on human patients and were performed under intramuscular (i.m.) ketamine sedation (except fecal sampling). At the end of this study, the chimpanzees were cured and retired within the facilities at the BPRC.
Vaccination schedule.
Cercariae of a Puerto Rican isolate of S. mansoni (Department of Parasitology, Leiden University Medical Center) were irradiated at a dose of 300 Gy using a 60Co source at the BPRC. The whole experimental system was validated at the BPRC by a successful vaccination-challenge study in C57BL/6 mice (data not shown). Three chimpanzees (V1, V2, and V3) were anesthetized i.m. with an appropriate dose of tiletamine-zolazepam (Zoletil; Virbac, Barneveld, The Netherlands). The vaccination and challenge regimen was based on previous work in vervets and baboons, where three exposures elicited approximately 50% protection (69, 70). The chimpanzees were exposed to 9,000 attenuated cercariae in 10 ml of water, applied to the shaved abdominal skin in stainless steel rings (diameter, 52 mm; height, 15 mm), for 30 min. The vaccination procedure was repeated twice, 5 weeks and 10 weeks later, using a different exposure site on the abdomen on each occasion. Three weeks after the last immunization, the three vaccinated chimpanzees as well as three control chimpanzees (C1, C2, and C3) were challenged on an equally carefully placed site on the abdomen with 2,000 normal, i.e., nonattenuated cercariae, again under tiletamine-zolazepam anesthesia. The dose of 2,000 challenge parasites was chosen to provoke measurable secondary responses in the vaccinated group but not excessive immunopathology in the infected control group, based upon observations in humans with high-intensity infections in areas of endemicity (16). Between week 28 and 36 postchallenge (p.c.), at different time points for the individual chimpanzees based on clinical status, each was given an i.m. injection of 40 mg of praziquantel (Droncit; Bayer, Mijdrecht, The Netherlands) per kg of body weight on two successive days, in order to terminate the infection. In each case, 1 mg of methylprednisolone (Solu-Medrol; Brocacef, Maarssen, The Netherlands) per kg was administered i.m. 1.5 h prior to treatment in order to prevent possible side effects such as abdominal cramping.
Preparation of antigens.
Soluble adult worm antigens (SWAP), soluble larval antigens (SLAP), and antigens released by schistosomula within the first 3 h after transformation (0–3hRAP) were prepared as published elsewhere (43). Soluble egg antigens (SEA) were obtained after homogenization of eggs by sonication and pelleting the debris at 100,000 × g for 60 min. Egg-secreted proteins (ESP) were concentrated from the supernatant of eggs cultured in RPMI-1640 (Life Technologies, Paisley, United Kingdom) supplemented with penicillin (300 U/ml), streptomycin (300 μg/ml), and gentamicin (500 μg/ml) for 72 h at 37°C in 5% CO2 (6). Recombinant His-tagged SmE16 (33, 42) was purified using TALON affinity matrix (Clontech Laboratories, Basingstoke, United Kingdom) (21).
Fecal egg counts.
Fresh fecal samples (ca. 10 g each) were collected at 1- to 2-week intervals, by placing a collecting tray under the individual night cages. The number of eggs per gram of feces was determined from each of three distinct samples per animal, using the Percoll separation technique described recently (M. Eberl, P. al-Sherbiny, S. Hagan, A. W. Ljubojevic, A. W. Thomas, and R. A. Wilson, submitted for publication). This method, developed to allow accurate measurements of fecal egg numbers, was far more sensitive than the conventional Kato-Katz smear technique (34) and capable of detecting very low numbers of eggs per gram of feces. It proved necessary to take account of the variation in fecal consistency resulting from the occurrence of schistosome-induced diarrhea in some animals, which diluted the concentration of eggs. From week 15 to 29 p.c., at approximately 2-week intervals, the chimpanzees were retained in their individual night cages for 19 h to determine the total fecal output from each; this allowed an estimation of the total egg output per day per chimpanzee. Levels of vaccine-induced protection were calculated as the reduction in eggs per day in vaccinated chimpanzees compared to control animals.
Blood sampling and isolation of PBMC.
At regular time points, 5 ml of peripheral blood was obtained from each chimpanzee for the preparation of serum, which was aliquoted and stored at −80°C until use. At most sampling times, an additional 70 ml of blood was obtained for isolation of peripheral blood mononuclear cells (PBMC). Cells were recovered by centrifugation over LSM medium (ICN Biomedicals, Zoetermeer, The Netherlands) for 30 min at 900 × g. The lymphocytes were washed twice with RPMI-1640 medium supplemented with 2 mM l-glutamine and gentamicin (50 μg/ml). Purified PBMC were adjusted to a concentration of 107/ml, frozen in medium containing 10% dimethyl sulfoxide–20% heat-inactivated human serum (Bloedbank Leiden-Haaglanden, Sanguin Foundation, Leiden, The Netherlands), and stored at −140°C until use. Blood chemistry markers, hematocrit, leukocytosis, and peripheral blood eosinophilia were analyzed using routine methods at the diagnostic laboratory of a regional hospital (Diagnostic Center, Samenwerkingsverband Sociale Zekerheid Delft, Delft, The Netherlands).
Circulating antigen levels.
Serum samples were pretreated with trichloroacetic acid to precipitate and remove interfering proteins and dissociate immune complexes; soluble circulating anodic antigens (CAA) and CCA were then detected by sandwich enzyme-linked immunosorbent assay (ELISA) using specific monoclonal antibodies (17, 18).
PBMC stimulation assays.
All PBMC samples were assayed simultaneously, to allow direct comparison between individual chimpanzees and between different sampling time points. PBMC were thawed and washed twice in RPMI-1640 supplemented with 2 mM l-glutamine, gentamicin (50 μg/ml), and 10% fetal calf serum (Life Technologies). Cultures were set up in triplicate in 96-well plates at a density of 2 × 105 PBMC per well, in a final volume of 100 μl for proliferation assays, or 200 μl for cytokine analysis. Cells were incubated at 37°C in 5% CO2 in medium alone or in the presence of a 10-μg/ml concentration of each antigen preparation. Supernatants were removed after 72 h, and gamma interferon (IFN-γ), interleukin-4 (IL-4), IL-5, and IL-10 were detected by corresponding two-site human cytokine ELISA kits, validated for determination of chimpanzee cytokines (U-CyTech bv, Utrecht University, Utrecht, The Netherlands). Plates for proliferation assays were pulsed after 96 h with 92.5 kBq of [3H]thymidine per well (Amersham Pharmacia, Roosendaal, The Netherlands) for an additional 18 h and subsequently harvested on filters. The filters were baked for 1 h at 80°C, and tritium incorporation was measured on a Packard Matrix 9600 helium counter.
Antibody levels.
Microtiter plates were coated overnight at 4°C with SWAP (2.5 μg/ml), SEA (0.5 μg/ml), or SmE16 (0.2 μg/ml), diluted in phosphate-buffered saline (23). The plates were washed five times with 10 mM Tris-HCl (pH 8.0)–150 mM NaCl–0.05% Tween 20 (TBST) and blocked with 3% bovine serum albumin (BSA) (Sigma) in TBST for 1 h at 37°C. Sera were diluted 1:500 in 0.5% BSA–TBST for detection of SmE16-specific antibodies, 1:1,500 for SWAP, and 1:20,000 for SEA, and the plates were incubated for 2 h at 37°C. After five washes, the wells were probed for 1 h at 37°C with alkaline phosphatase-labeled goat anti-human immunoglobulin G (IgG) or goat anti-human IgM (Biosource, Etten-Leur, The Netherlands) diluted 1:5,000 in 0.5% BSA–TBST. After five further washes, BluePhos Microwell substrate solution (Kirkegaard & Perry Laboratories, Dynex Technologies, Billingshurst, United Kingdom) was added to each well. Absorbance was quantified at 630 nm using a Dynatech MR500 ELISA reader.
Statistical analysis.
A hierarchical analysis of variance (ANOVA) (59) was used to test for significant variation in egg output at the following levels: (i) average variation among fecal samples from the same animal, (ii) average variation among animals within each group, and (iii) differences between group means. The error used for each level of variance was the mean square for the next significant level down the hierarchy. Significance of differences in CCA levels between the two groups was tested by two-tailed Student's t tests for unequal variance.
RESULTS
Vaccination results in a lower fecal egg output.
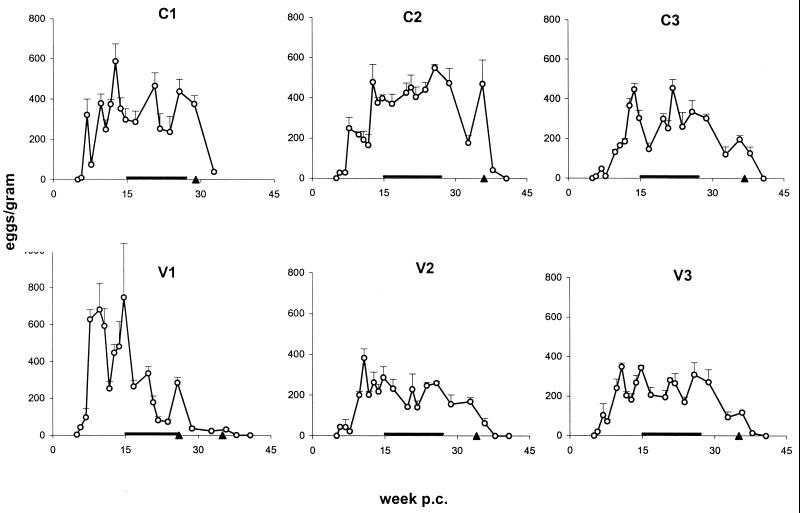
In order to monitor the time course of infection and to provide an estimate of the parasite burden in the chimpanzees, fecal samples were analyzed starting at week 4 p.c. The first schistosome eggs were detectable at day 35 p.c. in one chimpanzee (V1) and at day 40 p.c. in the remaining animals (Fig. 1). Fecal egg counts reached their maximum at week 10 to 15 p.c., paradoxically somewhat earlier in the vaccinated animals than in the control animals, and remained relatively stable throughout the infection, with the exception of V1. After chemotherapy, the egg counts dropped immediately, and two to three sampling points later no eggs were detectable, proving successful elimination of parasites in all chimpanzees.
FIG. 1.
Fecal egg burdens. Data shown represent means and SEM (error bars) from three fecal samples taken per time point. Symbols: triangles, date of treatment with praziquantel; horizontal bars, period over which the protection level was calculated from daily egg output (Table 1). Abbreviations: C, control animals; V, vaccinated animals.
When egg output was determined on a per-day basis (i.e., over 19 h), a lower level was observed in vaccinated animals than in the control animals (Table 1). The observed fluctuations in numbers of eggs detected at the different sampling times most likely reflect variations in the daily feces production (data not shown), rather than showing temporary changes in worm fecundity or excretion efficiency. Since the egg output during chronic infection with S. mansoni is considered reasonably stable in human patients (2) and in chimpanzees (reference 55 and Fig. 1), it was subjected to an ANOVA using all data available between week 15 p.c. (i.e., after the acute phase) and the time of chemotherapy. Table 2 shows that there was, on average, significant variation among the fecal samples from the same animal, over and above the average level of variation among the three replicate subsamples of each fecal sample. There was no significant animal-to-animal variation within a group above that attributable to the variation among the fecal samples. However, there was variation between the two groups over and above that at the levels of fecal samples and animals within groups; the average egg output of the vaccinated group was 38.4% less than that of the control group.
TABLE 1.
Analysis of total fecal egg output per daya
| Wk p.c. | Avg egg output ± SEM
|
|
|---|---|---|
| Control groupb | Vaccinated groupb | |
| 15 | 126,398 ± 28,644 | 91,806 ± 28,995 |
| 17 | 66,644 ± 9,591 | 59,803 ± 14,646 |
| 21 | 94,634 ± 7,477 | 21,563 ± 1,217 |
| 22 | 88,657 ± 33,044 | 36,624 ± 4,618 |
| 24 | 126,490 ± 26,350 | 69,091 ± 38,669 |
| 26 | 136,202 ± 9,220 | 114,803 ± 3,840 |
| Øc | 106,504 ± 15,759 | 65,615 ± 19,887 |
For statistical analysis, see Table 2.
Average total egg output for each group ± SEM from three fecal samples analyzed per animal.
Average fecal egg output per group between week 15 and week 26 p.c. (protection level, 38.4%).
TABLE 2.
Statistical analysis of total fecal egg output per daya
| Source | df | SS | MS (error) | Fb |
|---|---|---|---|---|
| Groups | 1 | 44,479.5694 | 44,479.5694 (3) | 8.68∗∗ |
| Animals in groups | 4 | 26,516.7487 | 6,629.1872 (3) | 1.29 NS |
| Fecal samples from animals | 30 | 153,753.6108 | 5,125.1204 (4) | 4.25∗∗∗ |
| Replicates in fecal samples | 72 | 86,832.4025 | 1,206.0056 | |
| Total | 107 | 311,579.3312 |
df, degrees of freedom; SS, sum of squares; MS, mean square; F, variance ratio.
ANOVA for data from weeks 15 and 26 p.c.: ∗∗, P < 0.01; ∗∗∗, P < 0.001; NS, P > 0.05.
Vaccination results in lower CCA levels.
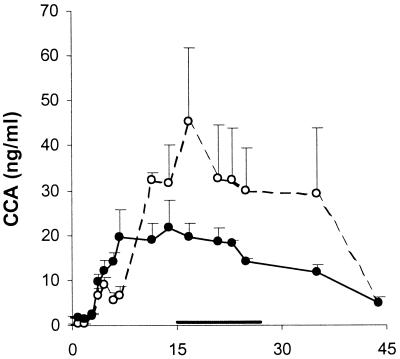
As a second correlate of worm burden, we tested serum samples for the presence of circulating schistosome antigens. Both CAA and CCA became apparent by week 3 p.c., coincident with the acquisition of blood feeding by the developing larvae. In four animals, there was a good correlation between CAA levels and fecal egg counts, with maximum values of ca. 250 to 500 ng/ml. However, V1 had higher CAA levels and C3 had very much lower CAA levels than anticipated from their respective egg counts (data not shown). As a consequence, the mean CAA levels in the two groups were not markedly different. In contrast, from week 12 p.c. onwards, CCA levels in the controls were about twice those in the vaccinated chimpanzees (Fig. 2). Calculation of average CCA levels over the same period during which total fecal egg output was estimated gave values (means ± standard errors of the means [SEM]) of 34.3 ± 2.8 ng/ml for the controls and 18.4 ± 1.3 ng/ml for the vaccinated group. This corresponds to a reduction of 46.3% (P < 0.01) and is remarkably similar to the reduction in fecal egg output (Table 1). Importantly, CAA and CCA values returned to baseline by week 45 p.c., due to rapid clearance after chemotherapy.
FIG. 2.
CCAs. Sera were obtained at regular intervals and assayed for CCA. Data shown represent means and SEM (error bars) from three individuals. Symbols: horizontal bar, period over which the protection level was calculated; open circles, control group; closed circles, vaccinated group.
Vaccinated chimpanzees experience diminished clinical signs of disease.
Repeated vaccination did not result in any detectable changes of the general health status. However, after challenge infection, general malaise was observed in both groups, and loss of appetite was observed mainly in the controls around weeks 7 and 8 p.c. (at the peak acute stage); no fever was detected in either group. After week 7 to 11 p.c., chimpanzees C1, V1, and V2 showed signs of diarrhea and developed bloody feces over the following weeks. The other three chimpanzees, C2, C3, and V3, experienced only occasional mild diarrhea. During the chronic infection there were no problems with appetite, but general malaise was evident during periods of heavy diarrhea. In order to ease the symptoms, C1 and V1 were treated once with an antispasmodic drug, butylscopolaminebromide (Buscopan; Boehringer Ingelheim, Alkmaar, The Netherlands). No differences in intestinal pathology were observed between the two groups; a detailed examination will be published elsewhere (J. A. M. Langermans et al., unpublished data). After chemotherapy with praziquantel, the chimpanzees showed a complete recovery. From infection until treatment, all individuals showed increases in body weight that were similar to data from naïve male animals of comparable age in the colony, over the same period (data not shown).
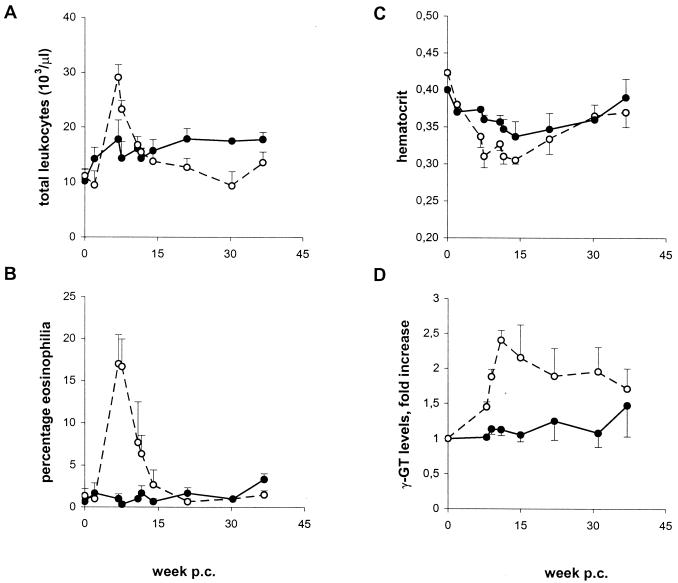
Hematological tests included determination of total leukocyte counts, percentage eosinophilia, and hematocrit. The mean values for both groups were similar in the naïve animals prior to vaccination or challenge. However, at the height of acute infection (week 7 to 8 p.c.), vaccinated animals had virtually unchanged leukocyte and eosinophil levels, whereas in control animals, the total leukocyte number was significantly elevated (Fig. 3A), and a pronounced eosinophilia was present (Fig. 3B). Both control leukocyte numbers and eosinophil counts returned to almost normal levels by week 14 p.c. Surprisingly, from that time point on, vaccinated chimpanzees had slightly elevated leukocyte numbers throughout the chronic stage, compared to controls. Hematocrit dropped in both groups after challenge infection and stayed below the naïve level at least until week 30 p.c., with controls having lower values than vaccinated animals (Fig. 3C). Blood chemistry profiles were determined for alkaline phosphatase, lactate dehydrogenase, bilirubin, and gamma-glutamyl transferase (γ-GT). No differences between the two groups were found for the first three markers, which stayed within the normal range for chimpanzees throughout the experiment (data not shown). However, in control animals the values of γ-GT showed an increase from week 9 p.c., up to 2.5 times the normal level, and remained elevated thereafter, while no significant increase was observed in the vaccinated group (Fig. 3D). In contrast, Doppler ultrasonographic examination of individual chimpanzees did not reveal any detectable differences in liver pathology between the two groups (data not shown).
FIG. 3.
Clinical parameters. Total leukocyte numbers (A), eosinophil counts (B), hematocrits (C), and levels of serum γ-GT (D) were determined in the naïve chimpanzees before any treatment (depicted as week 0) and at regular time points after challenge. Data shown represent means and SEM (error bars) from three individuals; data for γ-GT are calculated as fold increase above the values for naïve chimpanzees (range, 22 to 46 U/ml). Symbols: open circles, control group; closed circles, vaccinated group.
Vaccination induces a strong cellular immune response, which is down-regulated after challenge infection.
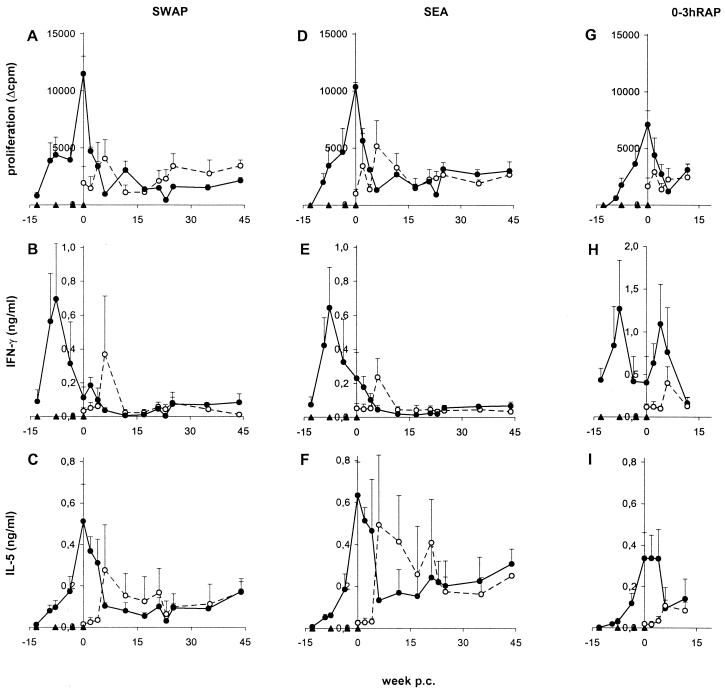
After the first exposure to attenuated parasites, PBMC from the vaccinated chimpanzees showed a detectable proliferative response to SWAP and to SEA, which was subsequently boosted by further immunizations (Fig. 4A, D). The maximum cellular reactivity coincided with the time of challenge infection, after which the antigen-specific cell proliferation dropped rapidly and then remained at a low but constant level. Unexpectedly, the vaccinated chimpanzees exhibited no prominent memory response after challenge. In the infected control animals, PBMC proliferation values peaked at week 6 p.c., at the start of the acute phase of infection. These maximum levels were lower than the peak response in the vaccinated chimpanzees, which most likely reflected the differences in the preceding antigenic loads in the two groups (3 × 9,000 attenuated cercariae versus 2,000 normal cercariae).
FIG. 4.
PBMC response to schistosome antigens. PBMC were cultured in the presence of SWAP (A to C), SEA (D to F), and 0–3hRAP (G to I) and assayed for thymidine incorporation (A, D, and G), secretion of IFN-γ (B, E, and H), and IL-5 (C, F, and I). Data shown represent means and SEM (error bars) from three individuals. Symbols: open circles, control group; closed circles, vaccinated group; triangles, vaccinations and challenge. Due to limitations of the material available, 0–3hRAP was only tested until week 14 p.c.
A type 1 cytokine response is induced by the first vaccination, and a type 2 response is induced by further vaccinations.
The vaccinated animals mounted a type 1 cytokine response after the first vaccination, as judged by the rapid increase in IFN-γ in SWAP- and SEA-stimulated PBMC cultures; production was down-regulated after subsequent vaccinations and was hardly detectable after challenge infection (Fig. 4B and E). PBMC from the control chimpanzees produced IFN-γ in the presence of SWAP and SEA only at week 6 p.c. and negligible amounts at later time points, after progression to the chronic disease state. In comparison with the pattern of IFN-γ, the levels of the type 2 cytokine IL-5 increased gradually with each vaccination and peaked at the time of challenge (Fig. 4C and F). They decreased within a few weeks p.c. but remained elevated throughout the whole course of the challenge infection, with higher values for SEA than SWAP stimulation. Thus, IL-5 but not IFN-γ production mirrored the pattern of PBMC proliferation. In the control group, IL-5 remained elevated after a dramatic rise at week 6 p.c., with higher levels after culture in the presence of SEA than SWAP; after week 25 p.c., the profiles were similar in both groups. Levels of IL-4 were lower but generally followed the pattern of IL-5 (data not shown).
Only antigens released by larvae induce a secondary type 1 cytokine response after challenge infection.
Examination of other antigen preparations revealed that the overall PBMC response after stimulation with lung-stage antigens (soluble larval antigens) was almost identical to the data obtained with SWAP (data not shown). In addition, the response to antigens secreted by live eggs (ESP) matched closely the proliferation and cytokine pattern observed in the presence of SEA (data not shown). In contrast, antigens released by freshly transformed schistosomula (0–3hRAP) induced a prominent p.c. IFN-γ response by PBMC from the vaccinated chimpanzees (Fig. 4H). This secondary IFN-γ secretion was at an earlier time point than the first appearance of IFN-γ in the control group, peaking at week 4 p.c. In contrast to IFN-γ, neither the PBMC proliferation nor the IL-5 response in the presence of 0–3hRAP showed any obvious difference to the other antigen preparations (Fig. 4G and I).
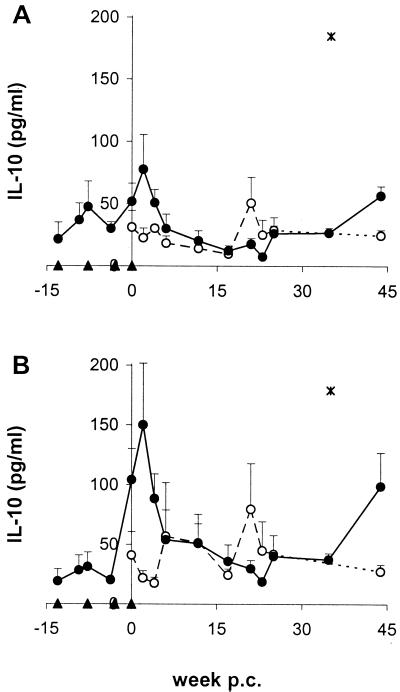
Vaccination elicits production of the regulatory cytokine IL-10.
In the vaccinated group, stimulation of PBMC with SWAP and SEA led to an early increase of IL-10, in parallel with the elevated amounts of IFN-γ, but showed a peak at much higher levels shortly after the maximum IL-5 response (Fig. 5). In both groups, SEA induced more IL-10 than SWAP. Chemotherapy also seemingly had an influence on the IL-10 secretion of PBMC, since the levels after treatment were significantly elevated in the three vaccinated chimpanzees (week 44 p.c.) as well as in one control animal (C1; week 35 p.c.).
FIG. 5.
IL-10 response to schistosome antigens. PBMC were cultured in the presence of SWAP (A) and SEA (B) and were assayed for IL-10. Data shown represent means and SEM (error bars) from three individuals. Symbols: open circles, control group; closed circles, vaccinated group; asterisks, IL-10 values for one control (C1) at week 35 p.c., which were almost 10-fold higher than for the other animals, and therefore excluded from the calculation of average IL-10 levels. Data at week 45 p.c. are from two animals.
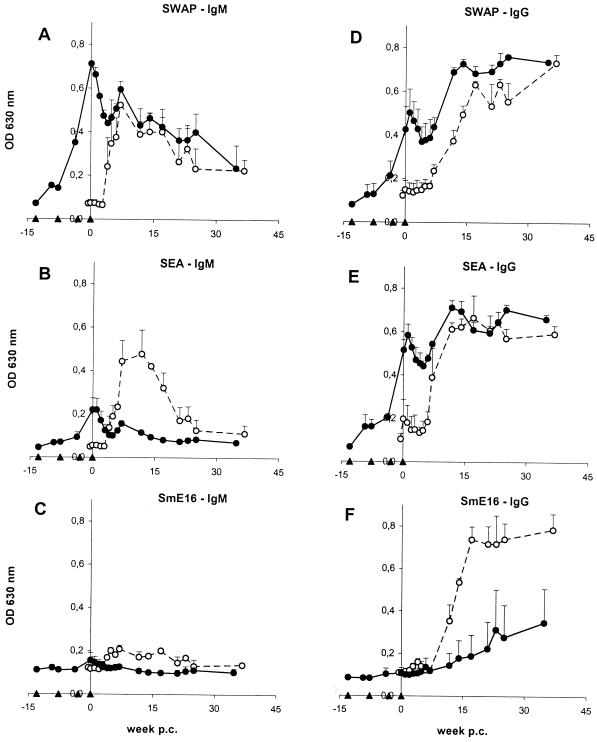
Vaccination induces high titers of IgM and IgG antibodies against parasite antigens.
In the vaccinated group, SWAP- and SEA-reactive IgM antibody titers increased after vaccination and were at a peak at the time of challenge infection (Fig. 6A and B); IgG titers peaked very shortly afterwards (Fig. 6D and E). However, analogous to the cellular response, IgM and IgG antibody titers declined after challenge infection and only recovered after the onset of the acute stage of the infection. SWAP- and SEA-reactive IgM titers decayed with time, whereas IgG remained at constant levels throughout the chronic stage of the infection. In the controls, IgM antibodies against SWAP and SEA were detectable from week 4 to 5 p.c. onwards, followed by IgG antibodies after week 5 to 6 p.c. IgG titers finally reached a plateau at the same levels as in the vaccinated group, but with SEA-reactive antibodies showing a steeper rise. IgM titers against both worm and egg antigens declined gradually, as seen in the vaccinated chimpanzees. For IgM, comparison of the two groups revealed that SWAP-reactive levels were similar, but those for SEA were much greater in the controls.
FIG. 6.
Antibody response to schistosome antigens. Sera were obtained at regular intervals and assayed for IgM (A to C) and IgG (D to F) antibodies directed against SWAP (A and D), SEA (B and E), and SmE16 (C and F). Data shown represent means and SEM (error bars) from three individuals. Symbols: open circles, control group; closed circles, vaccinated group.
Vaccinated chimpanzees have lower antibody titers against the egg-specific antigen SmE16.
Repeated exposure to attenuated cercariae led to a rapid development of both SWAP- and SEA-reactive IgG and IgM antibodies in the absence of parasite maturation or oviposition, implying marked cross-reactivity between larval, adult worm, and egg antigenic epitopes. In contrast, in both groups, IgG antibodies against the egg-specific calcium-binding protein SmE16 were not detectable before week 6 to 7 p.c., confirming the stage specificity of the protein (Fig. 6F). Most importantly, the control chimpanzees produced much higher levels of SmE16-specific IgG antibodies than the vaccinated individuals. SmE16-specific IgM levels were generally low, but also appeared to be higher in the controls (Fig. 6C).
DISCUSSION
Our study is the first to report the successful use of an attenuated vaccine to protect chimpanzees against a humanopathogenic helminth, as earlier attempts with Schistosoma japonicum (30) or Onchocerca volvulus vaccines failed (50). The beneficial effect of prior exposure to the RA vaccine on the outcome of subsequent S. mansoni challenge infection can be deduced from parasitological, immunological, and clinical data. We observed a significant reduction in serum CCA levels and in the egg output of the vaccinated chimpanzees and significantly lower titers of egg antigen-specific antibodies, compared to the controls. Additionally, previous vaccination ameliorated the signs of the acute immune response in the vaccinated animals and reduced morbidity during the acute and early chronic infection, as judged by serum γ-GT levels, leukocytosis, eosinophilia, and hematocrit. This is also the first longitudinal study to monitor the development of the immune response from infection through the acute and chronic phases of the disease, using up-to-date assays, in the host most closely related to humans. All clinical data available on human immune responses and pathology are from cross-sectional studies, due to the requirement of administering chemotherapy upon diagnosis.
Exposure of chimpanzees to the RA vaccine induced a strong cellular proliferative response to parasite antigens, the intensity of which was enhanced by each additional vaccination, as previously observed in baboons (70). The initial type 1 bias, evidenced by IFN-γ production, was not boosted by the repeat vaccinations that augmented IL-4 and IL-5 production, indicative of a type 2 response. This is consistent with analogous data obtained with multiply vaccinated mice (13). Although a Th1-Th2 switch observed in schistosome-infected mice has been associated with the onset of egg production (47), our data confirm that multiple exposures to larval antigens are clearly a sufficient trigger (13). Indeed, a marked degree of cross-reactivity is evident between the several antigen preparations used for lymphocyte stimulation, suggesting that it is not the parasite stage but rather repeated exposures to common antigenic epitopes that are responsible. Challenge of vaccinated chimpanzees coincided with the peak proliferative response, which unexpectedly decayed rapidly thereafter, giving little evidence for a subsequent memory reaction. Down-modulation of cellular immune responses after cercarial challenge has also been reported in mice (48) and baboons (70) exposed to the RA vaccine; in the former study IL-10 induction was proposed as the causative factor. In the present investigation, we observed IL-10 production rising gradually and peaking shortly after challenge, making this cytokine a good candidate for down-regulation of the proliferative and type 2 response (60) but not the early type 1 response (unless IFN-γ production is inhibited at much lower IL-10 concentrations than those at which IL-5 is inhibited). This begs the question as to whether other cytokines, such as transforming growth factor β (19, 45), or cellular processes, such as apoptosis (12), might be involved. It needs to be emphasized that this down-modulation of immune responses occurred in vaccinated chimpanzees before oviposition. Nevertheless, in humans with intestinal schistosomiasis, IL-10 is secreted by PBMC, particularly in response to egg antigens (5, 37, 67), which were also the most efficient inducers in our own study. A major task is to identify the cross-reactive components responsible for driving IL-10 production, with glycan epitopes being likely candidates (62).
Exposure of the control chimpanzees to normal challenge cercariae elicited only low proliferative responses and negligible cytokine production by antigen-stimulated PBMC in the ensuing weeks. It is unclear to what extent this reflects the lower parasite dose (2,000 cercariae) or immunogenicity of normal larvae, compared to the vaccinating situation. Only after oviposition was there a marked elevation in cellular reactivity, which signaled the start of the acute stage, with the simultaneous secretion of IFN-γ and IL-5 by PBMC; the egg antigen-driven type 2 response quickly came to dominate. There are parallels here with human schistosomiasis, in which PBMC sampled from patients at the acute stage of the disease show strong proliferative responses and IFN-γ secretion (37, 41). In contrast, PBMC from patients with chronic intestinal schistosomiasis secrete hardly any IFN-γ (63) but secrete significant amounts of IL-5 (5, 39).
The down-modulatory transition from the acute to the chronic stage appeared to be complete by week 12 to 14 postexposure, depending on the immunological parameter selected. Thereafter, the proliferative and type 2 cytokine responses remained at a low but detectable level. Termination of the chronic phase by chemotherapy, bringing about a massive release of somatic antigens, boosted production of type 2 but not type 1 cytokines. The vaccinated animals showed a strikingly different response with little or no evidence of an acute stage after the start of egg production. In that respect, the vaccine was clearly host protective. Measurement of biochemical and hematological parameters over the acute and early chronic stages provided further evidence for this protective effect. A drop in hematocrit is used as an indicator of blood loss and overall morbidity in schistosomiasis patients (24). Higher hematocrits of vaccinated chimpanzees compared to controls may be a consequence of a lower worm burden and/or less intestinal inflammation. Furthermore, the vaccinated chimpanzees did not exhibit leukocytosis or eosinophilia, which are hallmarks of acute schistosomiasis in humans (54). These clinical features were very evident in the control group and have been reported in earlier chimpanzee studies (55, 65). Serum γ-GT is a marker for hepatocellular injury, with increased levels observed in human patients with severe periportal fibrosis, hepatosplenomegaly, and ascites (32, 38). However, we observed elevated levels in control animals from the acute stage onwards, but not in vaccinated chimpanzees. Thus, our observation may reflect early liver tissue damage (e.g., from egg-secreted proteases [6]) due in part to the greater egg deposition in the control group but also due to the apparent lack of an acute inflammatory phase of infection in the vaccinated group. To our knowledge, presence of γ-GT during acute schistosomiasis has only been reported before in calves experimentally infected with Schistosoma bovis (36). It can be assumed that due to the experimental challenge conditions in this and in our own study, synchronized egg deposition occurred, as opposed to the more gradual incremental egg deposition resulting from trickle infections. Alkaline phosphatase as a marker for biliary obstruction and lactate dehydrogenase and bilirubin as markers for general liver pathology may become more pronounced later during chronic disease (4). In this respect, the lack of any ultrasonographically detectable differences between the two groups was not surprising given the limited duration of the present study, which was too short to allow for development of significant pipe stem fibrosis or portal hypertension (55, 65).
The RA vaccine also elicited a strong humoral immune response in the chimpanzees. Egg antigens were the most potent inducers of antibodies compared to other preparations (note the lower antigen concentrations and higher serum dilutions used in the ELISA protocol). The recognition of SEA by sera before challenge cannot be attributed to breakthrough of vaccinating parasites since eggs were not detectable in the feces before week 5 to 6 p.c., nor IgG antibodies against the stage-specific egg antigen SmE16 in the serum before week 6 to 7 p.c. Moreover, levels of circulating antigens were not elevated before challenge. The most likely explanation is that antigenic epitopes, such as common housekeeping and structural proteins, or glycan epitopes are shared between larvae, adult worms, and eggs. Glycan epitopes have already been demonstrated on cercariae and eggs (23, 46). As with cellular immunity, challenge of vaccinated chimpanzees coincided with a peak in both IgM and IgG titers, which then declined for 5 to 6 weeks, indicating that the 2,000 normal larvae were not providing a significant boost to the humoral response. This trend was reversed by oviposition, transiently for IgM but more permanently for IgG, indicating the importance of eggs in the maintenance of high antischistosome titers, and the relatively weaker response to adult worm antigens (23); similar observations on egg-specific antibodies have been made in chronically infected humans (10). The profile of antibody levels in the control chimpanzees also revealed little or no reactivity until after oviposition. The most notable differences between the two groups were in titers of SEA-specific IgM and SmE16-specific IgM and IgG. This may imply a disparity in the antigenic stimulus between control and vaccinated animals due to a difference in worm burden or fecundity, and hence a difference in egg production. In the case of SmE16, the difference cannot be ascribed to desensitization by vaccination, as the antigen is egg specific (42).
For estimating fecal egg output, a novel gradient technique was developed that proved to have a high sensitivity and was excellent for dealing with loose stools (Eberl et al., submitted). The significantly lower daily egg release from vaccinated chimpanzees is a convincing result, given the small sample size and absence of precedents for experimental design; this is supported by the close match of the estimates of egg burdens with the CCA levels throughout the whole course of the study (49). The nearest comparisons are investigations involving triple exposures of vervets and baboons to the RA vaccine, in which protection levels of approximately 50% have been reported, based on perfusion data (3, 69, 70). In both these simian hosts, a good correlation was obtained between anti-worm IgG titers at the end of the vaccination period and the worm burden of individual animals. Our data from chimpanzees, with a dominant type 2 response and high antibody titers at the time of challenge, also point to an antibody-mediated effector mechanism. Nevertheless, the operation of an additional type 1-mediated mechanism was indicated by the secondary production of IFN-γ by PBMC in response to antigens released by larvae. Cell-mediated protection appears to be most effective following a single exposure of mice to the RA vaccine, where IFN-γ is dominant (57, 58). However, in the murine host, multiple vaccinations fail to boost cell-mediated immunity but progressively enhance the humoral arm of the protective response (13, 31). Further evidence for the importance of type 2-mediated effector mechanisms comes from human patients, where a correlation exists between IL-4 and IL-5 production by PBMC and resistance to reinfection after drug treatment (40, 53). It has to be noted that in humans, IgE and IgA antibodies have been postulated to participate in protective immunity (20, 26, 27). Unfortunately, due to limitations of material, assays for parasite-specific IgA or IgE could not be performed.
This intensive chimpanzee study also underlines the potential difficulties facing the evaluation of any schistosome vaccine in a human population, not least in the indirect estimation of worm burden by fecal egg output. A longitudinal sampling regimen was required to establish the validity of a 38% protection level in the chimpanzees. Thus, a vaccine would need to generate a strong protection, with a large differential in egg output between test and placebo groups, to demonstrate efficacy. Conversely, CCA levels and the various clinical parameters of protection were easier to evaluate and merit serious consideration for inclusion in future vaccine trials. Moreover, the differences in antibody responses between test and control groups, illustrated by the egg antigen data, deserve attention. Our study highlights a need for a range of stage-specific recombinant antigen markers in addition to SmE16 in eggs. Despite the manifold problems facing the development and testing of a schistosome vaccine, we are cautiously optimistic. That the RA vaccine generates a significant protection in prepubescent chimpanzees gives us confidence to continue the task of converting it to a recombinant antigen formulation (21, 22, 28, 29), as the human target group for vaccination programs will mainly be children.
ACKNOWLEDGMENTS
This work was supported by EC contract CT97-0212, INCO-DC Program “Health Research with Developing Countries,” and EC contract CT97-9104, INCO-DC Primate Reference Program supported by DGXII.
We are especially grateful to the veterinarians and the animal caretakers at BPRC. We thank Ewald Beck for providing the plasmid pDS-SmE16, Srdjan Ljubojevic and Peter Ashton for providing SEA and ESP and for their help with some fecal samples, Terry Crawford for statistical advice, and Adrian Mountford and Rodrigo Corrêa-Oliveira for their stimulating discussion.
M.E. and J.A.M.L. contributed equally to this work.
REFERENCES
- 1.Abe K, Kagei N, Teramura Y, Ejima H. Hepatocellular carcinoma associated with chronic Schistosoma mansoni infection in a chimpanzee. J Med Primatol. 1993;22:237–239. [PubMed] [Google Scholar]
- 2.Agnew A, Fulford A J, Mwanje M T, Gachuhi K, Gutsmann V, Krijger F W, Sturrock R F, Vennervald B J, Ouma J H, Butterworth A E, Deelder A M. Age-dependent reduction of schistosome fecundity in Schistosoma haematobium but not Schistosoma mansoni infections in humans. Am J Trop Med Hyg. 1996;55:338–343. doi: 10.4269/ajtmh.1996.55.338. [DOI] [PubMed] [Google Scholar]
- 3.Amory Soisson L, Reid G D F, Farah I O, Nyindo M, Strand M. Protective immunity in baboons vaccinated with a recombinant antigen or radiation-attenuated cercariae of Schistosoma mansoni is antibody-dependent. J Immunol. 1993;151:4782–4789. [PubMed] [Google Scholar]
- 4.Aranda-Michel J, Sherman K E. Tests of the liver: use and misuse. Gastroenterologist. 1998;6:34–43. [PubMed] [Google Scholar]
- 5.Araújo M I, Ribeiro de Jesus A, Bacellar O, Sabin E, Pearce E, Carvalho E M. Evidence of a T helper type 2 activation in human schistosomiasis. Eur J Immunol. 1996;26:1399–1403. doi: 10.1002/eji.1830260633. [DOI] [PubMed] [Google Scholar]
- 6.Ashton P D, Harrop R, Shah B, Wilson R A. The schistosome egg: development and secretions. Parasitology. 2001;122:329–338. doi: 10.1017/s0031182001007351. [DOI] [PubMed] [Google Scholar]
- 7.Bergquist N R, Colley D G. Schistosomiasis vaccines: research to development. Parasitol Today. 1998;14:99–104. doi: 10.1016/s0169-4758(97)01207-6. [DOI] [PubMed] [Google Scholar]
- 8.Butterworth A E, Fulford A J, Dunne D W, Ouma J H, Sturrock R F. Longitudinal studies on human schistosomiasis. Philos Trans R Soc Lond B Biol Sci. 1988;321:495–511. doi: 10.1098/rstb.1988.0105. [DOI] [PubMed] [Google Scholar]
- 9.Butterworth A E, Curry A J, Dunne D W, Fulford A J, Kimani G, Kariuki H C, Klumpp R, Koech D, Mbugua G, Ouma J H, Roberts M, Thiongo F W, Capron A, Sturrock R F. Immunity and morbidity in human schistosomiasis mansoni. Trop Geogr Med. 1994;46:197–208. [PubMed] [Google Scholar]
- 10.Caldas I R, Corrêa-Oliveira R, Colosimo E, Carvalho O S, Massara C L, Colley D G, Gazzinelli G. Susceptibility and resistance to Schistosoma mansoni reinfection: parallel cellular and isotypic immunologic assessment. Am J Trop Med Hyg. 2000;62:57–64. doi: 10.4269/ajtmh.2000.62.57. [DOI] [PubMed] [Google Scholar]
- 11.Capron A. Schistosomiasis: forty years' war on the worm. Parasitol Today. 1998;14:379–384. doi: 10.1016/s0169-4758(98)01322-2. [DOI] [PubMed] [Google Scholar]
- 12.Carneiro-Santos P, Martins-Filho O, Alves-Oliveira L F, Silveira A M S, Coura-Filho P, Viana I R C, Wilson R A, Corrêa-Oliveira R. Apoptosis: a mechanism of immunoregulation during human schistosomiasis mansoni. Parasite Immunol. 2000;22:267–277. doi: 10.1046/j.1365-3024.2000.00294.x. [DOI] [PubMed] [Google Scholar]
- 13.Caulada-Benedetti Z, al-Zamel F, Sher A, James S. Comparison of Th1- and Th2-associated immune reactivities stimulated by single versus multiple vaccination of mice with irradiated Schistosoma mansoni cercariae. J Immunol. 1991;146:1655–1660. [PubMed] [Google Scholar]
- 14.Chan M S, Woolhouse M E, Bundy D A. Human schistosomiasis: potential long-term consequences of vaccination programmes. Vaccine. 1997;15:1545–1550. doi: 10.1016/s0264-410x(97)00071-6. [DOI] [PubMed] [Google Scholar]
- 15.Coulson P S. The radiation-attenuated vaccine against schistosomes in animal models: paradigm for a human vaccine? Adv Parasitol. 1997;39:271–336. doi: 10.1016/s0065-308x(08)60048-2. [DOI] [PubMed] [Google Scholar]
- 16.de Clercq D, Vercruysse J, Picquet M, Shaw D J, Diop M, Ly A, Gryseels B. The epidemiology of a recent focus of mixed Schistosoma haematobium and Schistosoma mansoni infections around the ‘Lac de Guiers’ in the Senegal River Basin, Senegal. Trop Med Int Health. 1999;4:544–550. doi: 10.1046/j.1365-3156.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- 17.Deelder A M, de Jonge N, Boerman O C, Fillie Y E, Hilberath G W, Rotmans J P, Gerritse M J, Schut D W. Sensitive determination of circulating anodic antigen in Schistosoma mansoni infected individuals by an enzyme-linked immunosorbent assay using monoclonal antibodies. Am J Trop Med Hyg. 1989;40:268–272. doi: 10.4269/ajtmh.1989.40.268. [DOI] [PubMed] [Google Scholar]
- 18.de Jonge N, Kremsner P G, Krijger F W, Schommer G, Fillie Y E, Kornelis D, van Zeyl R J, van Dam G J, Feldmeier H, Deelder A M. Detection of the schistosome circulating cathodic antigen by enzyme immunoassay using biotinylated monoclonal antibodies. Trans R Soc Trop Med Hyg. 1990;84:815–818. doi: 10.1016/0035-9203(90)90094-u. [DOI] [PubMed] [Google Scholar]
- 19.Doetze A, Satoguina J, Burchard G, Rau T, Löliger C, Fleischer B, Hoerauf A. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by Th3/Tr1-type cytokines IL-10 and transforming growth factor-beta but not by a Th1 to Th2 shift. Int Immunol. 2000;12:623–630. doi: 10.1093/intimm/12.5.623. [DOI] [PubMed] [Google Scholar]
- 20.Dunne D W, Butterworth A E, Fulford A J, Kariuki H C, Langley J G, Ouma J H, Capron A, Pierce R J, Sturrock R F. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992;22:1483–1494. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- 21.Eberl M, Mountford A P, Jankovic D, Beck E. Isolation of T cell antigens by using a recombinant protein library and its application to the identification of novel vaccine candidates against schistosomiasis. Infect Immun. 1999;67:3383–3389. doi: 10.1128/iai.67.7.3383-3389.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eberl M, Beck E, Coulson P S, Okamura H, Wilson R A, Mountford A P. IL-18 potentiates the adjuvant properties of IL-12 in the induction of a strong Th1 type immune response against a recombinant antigen. Vaccine. 2000;18:2002–2008. doi: 10.1016/s0264-410x(99)00532-0. [DOI] [PubMed] [Google Scholar]
- 23.Eberl M, Langermans J A M, Vervenne R A, Nyame A K, Cummings R D, Thomas A W, Coulson P S, Wilson R A. Antibodies to glycans dominate the host response to schistosome larvae and eggs: is their role protective or subversive? J Infect Dis. 2001;183:1238–1247. doi: 10.1086/319691. [DOI] [PubMed] [Google Scholar]
- 24.Gryseels B, Polderman A M. The morbidity of schistosomiasis mansoni in Maniema (Zaire) Trans R Soc Trop Med Hyg. 1987;81:202–209. doi: 10.1016/0035-9203(87)90215-x. [DOI] [PubMed] [Google Scholar]
- 25.Gryseels B. Schistosomiasis vaccines: a devils' advocate view. Parasitol Today. 2000;16:46–48. doi: 10.1016/s0169-4758(99)01597-5. [DOI] [PubMed] [Google Scholar]
- 26.Grzych J M, Grezel D, Xu C B, Neyrinck J L, Capron M, Ouma J H, Butterworth A E, Capron A. IgA antibodies to a protective antigen in human Schistosomiasis mansoni. J Immunol. 1993;150:527–535. [PubMed] [Google Scholar]
- 27.Hagan P, Blumenthal U J, Dunn D, Simpson A J, Wilkins H A. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 28.Harrop R, Coulson P S, Wilson R A. Characterization, cloning and immunogenicity of antigens released by lung-stage larvae of Schistosoma mansoni. Parasitology. 1999;118:583–594. doi: 10.1017/s003118209900431x. [DOI] [PubMed] [Google Scholar]
- 29.Harrop R, Jennings N, Mountford A P, Coulson P S, Wilson R A. Characterization, cloning and immunogenicity of antigens released by transforming cercariae of Schistosoma mansoni. Parasitology. 2000;121:385–394. doi: 10.1017/s003118209900640x. [DOI] [PubMed] [Google Scholar]
- 30.Hsü S Y. Immunization against Schistosoma japonicum in chimpanzees by administration of x-irradiated cercariae. Trans R Soc Trop Med Hyg. 1970;64:597–600. doi: 10.1016/0035-9203(70)90083-0. [DOI] [PubMed] [Google Scholar]
- 31.Jankovic D, Wynn T A, Kullberg M C, Hieny S, Caspar P, James S, Cheever A W, Sher A. Optimal vaccination against Schistosoma mansoni requires the induction of both B cell- and IFN-gamma-dependent effector mechanisms. J Immunol. 1999;162:345–351. [PubMed] [Google Scholar]
- 32.Kardorff R, Gabone R M, Mugashe C, Obiga D, Ramarokoto C E, Mahlert C, Spannbrucker N, Lang A, Gunzler V, Gryseels B, Ehrich J H, Doehring E. Schistosoma mansoni-related morbidity on Ukerewe Island, Tanzania: clinical, ultrasonographical and biochemical parameters. Trop Med Int Health. 1997;2:230–239. doi: 10.1046/j.1365-3156.1997.d01-269.x. [DOI] [PubMed] [Google Scholar]
- 33.Kasper G, Brown A, Eberl M, Vallar L, Kieffer N, Berry C, Girdwood K, Eggleton P, Quinnell R, Pritchard D I. A calreticulin-like molecule from the human hookworm Necator americanus interacts with C1q and the cytoplasmic signalling domains of some integrins. Parasite Immunol. 2001;23:141–152. doi: 10.1046/j.1365-3024.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- 34.Katz N, Coelho P M Z, Pellegrino J. Evaluation of Kato's quantitative method through the recovery of Schistosoma mansoni eggs added to human feces. J Parasitol. 1970;56:1032–1033. [PubMed] [Google Scholar]
- 35.Kuntz R E, McCullough B, Moore J A, Huang T C. Experimental infection with Schistosoma intercalatum (Fisher, 1934) in the chimpanzee (Pan troglodytes) and the gibbon (Hylobates lar) Am J Trop Med Hyg. 1978;27:632–634. doi: 10.4269/ajtmh.1978.27.632. [DOI] [PubMed] [Google Scholar]
- 36.Mahmoud O M, Elsamani F, Gameel A A, Taylor M G. Serum enzyme changes in calves experimentally infected with Schistosoma bovis. J Comp Pathol. 1987;97:335–339. doi: 10.1016/0021-9975(87)90098-3. [DOI] [PubMed] [Google Scholar]
- 37.Malaquias L C C, Falcão P L, Silveira A M S, Gazzinelli G, Prata A, Coffman R L, Pizziolo V, Souza C P, Colley D G, Corrêa-Oliveira R. Cytokine regulation of human immune response to Schistosoma mansoni: analysis of the role of IL-4, IL-5 and IL-10 on peripheral blood mononuclear cell responses. Scand J Immunol. 1997;46:393–398. doi: 10.1046/j.1365-3083.1997.d01-136.x. [DOI] [PubMed] [Google Scholar]
- 38.Mansour M M, Farid Z, Bassily S, Salah L H, Watten R H. Serum enzyme tests in hepatosplenic schistosomiasis. Trans R Soc Trop Med Hyg. 1982;76:109–111. doi: 10.1016/0035-9203(82)90032-3. [DOI] [PubMed] [Google Scholar]
- 39.Marguerite M, Gallissot M C, Diagne M, Moreau C, Diakkhate M M, Roberts M, Remoue F, Thiam A, Decam C, Rogerie F, Cottrez F, Neyrinck J L, Butterworth A E, Sturrock R F, Piau J P, Daff B, Niang M, Wolowczuk I, Riveau G, Auriault C, Capron A. Cellular immune responses of a Senegalese community recently exposed to Schistosoma mansoni: correlations of infection level with age and inflammatory cytokine production by soluble egg antigen-specific cells. Trop Med Int Health. 1999;4:530–543. doi: 10.1046/j.1365-3156.1999.00443.x. [DOI] [PubMed] [Google Scholar]
- 40.Medhat A, Shehata M, Bucci K, Mohamed S, Dief A D, Badary S, Galal H, Nafeh M, King C L. Increased interleukin-4 and interleukin-5 production in response to Schistosoma haematobium adult worm antigens correlates with lack of reinfection after treatment. J Infect Dis. 1998;178:512–519. doi: 10.1086/515630. [DOI] [PubMed] [Google Scholar]
- 41.Montenegro S M L, Miranda P, Mahanty S, Abath F G C, Teixeira K M, Coutinho E M, Brinkman J, Gonçalves I, Domingues L A, Domingues A L, Sher A, Wynn T A. Cytokine production in acute versus chronic human schistosomiasis mansoni: the cross-regulatory role of interferon-γ and interleukin-10 in the responses of peripheral blood mononuclear cells and splenocytes to parasite antigens. J Infect Dis. 1999;179:1502–1514. doi: 10.1086/314748. [DOI] [PubMed] [Google Scholar]
- 42.Moser D, Doenhoff M J, Klinkert M Q. A stage-specific calcium-binding protein expressed in eggs of Schistosoma mansoni. Mol Biochem Parasitol. 1992;512:229–238. doi: 10.1016/0166-6851(92)90073-s. [DOI] [PubMed] [Google Scholar]
- 43.Mountford A P, Harrop R, Wilson R A. Antigens derived from lung-stage larvae of Schistosoma mansoni are efficient stimulators of proliferation and gamma interferon secretion by lymphocytes from mice vaccinated with irradiated cercariae. Infect Immun. 1995;63:1980–1986. doi: 10.1128/iai.63.5.1980-1986.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen A, Magnussen P, Ouma J H, Andreassen J, Friis H. The contribution of hookworm and other parasitic infections to haemoglobin and iron status among children and adults in western Kenya. Trans R Soc Trop Med Hyg. 1998;92:643–649. doi: 10.1016/s0035-9203(98)90795-7. [DOI] [PubMed] [Google Scholar]
- 45.Omer F M, Kurtzhals J A, Riley E M. Maintaining the immunological balance in parasitic infections: a role for TGF-β? Parasitol Today. 2000;16:18–23. doi: 10.1016/s0169-4758(99)01562-8. [DOI] [PubMed] [Google Scholar]
- 46.Omer-Ali P, Mansour M, Woody J N, Smithers S R, Simpson A J. Antibody to carbohydrate and polypeptide epitopes on the surface of schistosomula of Schistosoma mansoni in Egyptian patients with acute and chronic schistosomiasis. Parasitology. 1989;98:417–424. doi: 10.1017/s0031182000061503. [DOI] [PubMed] [Google Scholar]
- 47.Pearce E J, Caspar P, Grzych J M, Lewis F A, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth. Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pemberton R M, Wilson R A. T-helper type-1-dominated lymph node responses induced in C57BL/6 mice by optimally irradiated cercariae of Schistosoma mansoni are down-regulated after challenge infection. Immunology. 1995;84:310–316. [PMC free article] [PubMed] [Google Scholar]
- 49.Polman K, de Vlas S J, Gryseels B, Deelder A M. Relating serum circulating anodic antigens to faecal egg counts in Schistosoma mansoni infections: a modelling approach. Parasitology. 2000;121:601–610. doi: 10.1017/s0031182000006843. [DOI] [PubMed] [Google Scholar]
- 50.Prince A M, Brotman B, Johnson E H, Jr, Smith A, Pascual D, Lustigman S. Onchocerca volvulus: immunization of chimpanzees with X-irradiated third-stage (L3) larvae. Exp Parasitol. 1992;74:239–250. doi: 10.1016/0014-4894(92)90147-3. [DOI] [PubMed] [Google Scholar]
- 51.Renquist D M, Johnson A J, Lewis J C, Johnson D J. A natural case of Schistosoma mansoni in the chimpanzee (Pan troglodytes versus) Lab Anim Sci. 1975;25:763–768. [PubMed] [Google Scholar]
- 52.Rieckmann K H, Beaudoin R L, Cassells J S, Sell K W. Use of attenuated sporozoites in the immunization of human volunteers against falciparum malaria. Bull W H O. 1979;57(Suppl. 1):261–265. [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts M, Butterworth A E, Kimani G, Kamau T, Fulford A J, Dunne D W, Ouma J H, Sturrock R F. Immunity after treatment of human schistosomiasis: association between cellular responses and resistance to reinfection. Infect Immun. 1993;61:4984–4993. doi: 10.1128/iai.61.12.4984-4993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rocha M O, Pedroso E R, Neves J, Rocha R S, Greco D B, Lambertucci J R, Rocha R L, Katz N. Characterization of the non-apparent clinical form in the initial phase of schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1993;35:247–251. doi: 10.1590/s0036-46651993000300005. [DOI] [PubMed] [Google Scholar]
- 55.Sadun E H, von Lichtenberg F, Hickman R L, Bruce J I, Smith J H, Schoenbechler M J. Schistosomiasis mansoni in the chimpanzee: parasitologic, clinical, serologic, pathologic and radiologic observations. Am J Trop Med Hyg. 1966;15:496–506. doi: 10.4269/ajtmh.1966.15.496. [DOI] [PubMed] [Google Scholar]
- 56.Sadun E H, von Lichtenberg F, Cheever A W, Erickson D G, Hickman R L. Experimental infection with Schistosoma haematobium in chimpanzees. Am J Trop Med Hyg. 1970;19:427–458. doi: 10.4269/ajtmh.1970.19.427. [DOI] [PubMed] [Google Scholar]
- 57.Sher A, Coffman R L, Hieny S, Cheever A W. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J Immunol. 1990;145:3911–3916. [PubMed] [Google Scholar]
- 58.Smythies L E, Coulson P S, Wilson R A. Monoclonal antibody to IFN-γ modifies pulmonary inflammatory responses and abrogates immunity to Schistosoma mansoni in mice vaccinated with attenuated cercariae. J Immunol. 1992;149:3654–3658. [PubMed] [Google Scholar]
- 59.Sokal R R, Rohlf F J. Biometry. 2nd ed. San Francisco, Calif: W. H. Freeman & Co.; 1981. [Google Scholar]
- 60.Sundstedt A, Hoiden I, Rosendahl A, Kalland T, van Rooijen N, Dohlsten M. Immunoregulatory role of IL-10 during superantigen-induced hyporesponsiveness in vivo. J Immunol. 1997;158:180–186. [PubMed] [Google Scholar]
- 61.Tanner M, Burnier E, Mayombana C, Betschart B, de Savigny D, Marti H P, Suter R, Aellen M, Ludin E, Degremont A A. Longitudinal study on the health status of children in a rural Tanzanian community: parasitoses and nutrition following control measures against intestinal parasites. Acta Trop. 1987;44:137–174. [PubMed] [Google Scholar]
- 62.Velupillai P, dos Reis E A, dos Reis M G, Harn D A. LewisX-containing oligosaccharide attenuates schistosome egg antigen-induced immune depression in human schistosomiasis. Hum Immunol. 2000;61:225–232. doi: 10.1016/s0198-8859(99)00136-6. [DOI] [PubMed] [Google Scholar]
- 63.Viana I R, Sher A, Carvalho O S, Massara C L, Eloi-Santos S M, Pearce E J, Colley D G, Gazzinelli G, Corrêa-Oliveira R. Interferon-gamma production by peripheral blood mononuclear cells from residents of an area endemic for Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1994;88:466–470. doi: 10.1016/0035-9203(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 64.Viana I R, Corrêa-Oliveira R, Carvalho O S, Massara C L, Colosimo E, Colley D G, Gazzinelli G. Comparison of antibody isotype responses to Schistosoma mansoni antigens by infected and putative resistant individuals living in an endemic area. Parasite Immunol. 1995;17:297–304. doi: 10.1111/j.1365-3024.1995.tb00895.x. [DOI] [PubMed] [Google Scholar]
- 65.von Lichtenberg F, Sadun E H. Experimental production of bilharzial pipe-stem fibrosis in the chimpanzee. Exp Parasitol. 1968;22:264–278. doi: 10.1016/0014-4894(68)90102-1. [DOI] [PubMed] [Google Scholar]
- 66.von Lichtenberg F, Sadun E H, Cheever A W, Erickson D G, Johnson A J, Boyce H W. Experimental infection with Schistosoma japonicum in chimpanzees. Am J Trop Med Hyg. 1971;20:850–893. doi: 10.4269/ajtmh.1971.20.850. [DOI] [PubMed] [Google Scholar]
- 67.Williams M E, Montenegro S, Domingues A L, Wynn T A, Teixeira K, Mahanty S, Coutinho A, Sher A. Leukocytes of patients with Schistosoma mansoni respond with a Th2 pattern of cytokine production to mitogen or egg antigens but with a Th0 pattern to worm antigens. J Infect Dis. 1994;170:946–954. doi: 10.1093/infdis/170.4.946. [DOI] [PubMed] [Google Scholar]
- 68.Wilson R A, Coulson P S. Why don't we have a schistosomiasis vaccine? Parasitol Today. 1998;14:97–99. doi: 10.1016/s0169-4758(97)01198-8. [DOI] [PubMed] [Google Scholar]
- 69.Yole D S, Reid G D, Wilson R A. Protection against Schistosoma mansoni and associated immune responses induced in the vervet monkey Cercopithecus aethiops by the irradiated cercaria vaccine. Am J Trop Med Hyg. 1996;54:265–270. doi: 10.4269/ajtmh.1996.54.265. [DOI] [PubMed] [Google Scholar]
- 70.Yole D S, Pemberton R, Reid G D, Wilson R A. Protective immunity to Schistosoma mansoni induced in the olive baboon Papio anubis by the irradiated cercaria vaccine. Parasitology. 1996;112:37–46. doi: 10.1017/s0031182000065057. [DOI] [PubMed] [Google Scholar]