Abstract
Cannabinoids modulate dopamine (DA) transmission and DA-related behavior, which has been thought to be mediated initially by activation of cannabinoid CB1 receptors (CB1Rs) on GABA neurons. However, there is no behavioral evidence supporting it. In contrast, here we report that CB1Rs are also expressed in a subset of DA neurons and functionally underlie cannabinoid action in male and female mice. RNAscope in situ hybridization (ISH) assays demonstrated CB1 mRNA in tyrosine hydroxylase (TH)-positive DA neurons in the ventral tegmental area (VTA) and glutamate decarboxylase 1 (GAD1)-positive GABA neurons. The CB1R-expressing DA neurons were located mainly in the middle portion of the VTA with the number of CB1-TH colocalization progressively decreasing from the medial to the lateral VTA. Triple-staining assays indicated CB1R mRNA colocalization with both TH and vesicular glutamate transporter 2 (VgluT2, a glutamate neuronal marker) in the medial VTA close to the midline of the brain. Optogenetic activation of this population of DA neurons was rewarding as assessed by optical intracranial self-stimulation. Δ9-tetrahydrocannabinol (Δ9-THC) or ACEA (a selective CB1R agonist) dose-dependently inhibited optical intracranial self-stimulation in DAT-Cre control mice, but not in conditional knockout mice with the CB1R gene absent in DA neurons. In addition, deletion of CB1Rs from DA neurons attenuated Δ9-THC-induced reduction in DA release in the NAc, locomotion, and anxiety. Together, these findings indicate that CB1Rs are expressed in a subset of DA neurons that corelease DA and glutamate, and functionally underlie cannabinoid modulation of DA release and DA-related behavior.
SIGNIFICANCE STATEMENT Cannabinoids produce a series of psychoactive effects, such as aversion, anxiety, and locomotor inhibition in rodents. However, the cellular and receptor mechanisms underlying these actions are not fully understood. Here we report that CB1 receptors are expressed not only in GABA neurons but also in a subset of dopamine neurons, which are located mainly in the medial VTA close to the midline of the midbrain and corelease dopamine and glutamate. Optogenetic activation of these dopamine neurons is rewarding, which is dose-dependently inhibited by cannabinoids. Selective deletion of CB1 receptor from dopamine neurons blocked cannabinoid-induced aversion, hypoactivity, and anxiolytic effects. These findings demonstrate that dopaminergic CB1 receptors play an important role in mediating cannabinoid action.
Keywords: 9-tetrahydrocannabinol, cannabinoid, CB1 receptor, dopamine neurons, aversion, anxiety, locomotion
Introduction
Cannabis is the most commonly used illicit drug worldwide (World Health Organization, 2016). In the United States, legalization efforts are progressing and initiation of cannabis use is on the rise (Substance Abuse and Mental Health Services Administration, 2021). Cannabinoids produce diverse physiological and behavioral changes in experimental animals, including the characteristic tetrad effects (analgesia, catalepsy, hypothermia, immobility), a change in affective state (reward vs aversion, anxiolytic vs anxiogenic effects), and deleterious effects on cognition (Panagis et al., 2008; Wang et al., 2020; Hempel and Xi, 2022). However, the neural mechanisms underlying these actions are not fully understood.
Cannabinoid CB1 receptors (CB1Rs) are highly expressed in the brain (Herkenham et al., 1990; Herkenham, 1992; Hempel and Xi, 2022), particularly in the basal ganglia and mesolimbic dopamine (DA) system; therefore, it has been thought that CB1R mechanisms may underlie the motivational and motor effects of cannabinoids by modulating DA transmission in these brain regions (Di Marzo et al., 2000; Matyas et al., 2006). This hypothesis is supported by findings that cannabinoids, such as Δ9-tetrahydrocannabinol (Δ9-THC), may increase DA neuron firing in the VTA and SNc (French et al., 1997; Cheer et al., 2003) as well as DA release in the NAc (Tanda et al., 1997; Cheer et al., 2004). These effects have been thought to be mediated by activation of CB1Rs on GABAergic neurons that project to DA neurons (Matyas et al., 2008; Fitzgerald et al., 2012; Covey et al., 2017; Davis et al., 2018) as CB1R activation has been shown to inhibit presynaptic GABA release in midbrain slices (Cheer et al., 2000; Szabo et al., 2002; Riegel and Lupica, 2004; Melis et al., 2013; Wang et al., 2015). However, other work is not consistent with the above findings. For example, electrophysiological studies indicate that the cannabinoid agonist HU210 not only activates but also inhibits different subpopulations of VTA DA neurons (Cheer et al., 2003). Fast-cyclic voltammetry assays in brain slices demonstrate that WIN55212-2 or CP55940 failed to alter (Castañeda et al., 1991; Szabo et al., 1999) or produced a reduction in electrical stimulation-induced DA release in the dorsal striatum in guinea pigs, rats, and mice (Pillolla et al., 2007; Sidlo et al., 2008). In vivo microdialysis experiments indicate that Δ9-THC produced a dose-dependent reduction in NAc DA release in mice (Li et al., 2021). These findings parallel accumulating behavioral evidence demonstrating that cannabinoids are not rewarding, but aversive in rodents (Vlachou et al., 2007; Panagis et al., 2008; Vlachou and Panagis, 2014; Han et al., 2017; Humburg et al., 2021; Hempel and Xi, 2022).
We have recently reported that CB1Rs expressed in VTA glutamate neurons at least in part underlie cannabinoid-induced aversion and locomotion inhibition (Han et al., 2017; Humburg et al., 2021). Unexpectedly, in these studies, we also observed CB1 mRNA expression in some TH-positive DA neurons. This inspired us to determine whether CB1Rs are consistently expressed on midbrain DA neurons, and therefore, playing a role in cannabinoid action.
The literature on CB1R expression in DA neurons is mixed: some studies failed to detect CB1R immunostaining in SNc DA neurons (Julian et al., 2003; Fitzgerald et al., 2012; Davis et al., 2018), whereas others detected CB1R immunostaining in cultured DA neurons (Hernandez et al., 2000; Lau et al., 2017) or DA neurons in midbrain tissues and retina (Wenger et al., 2003; Kim et al., 2008; da Silva Sampaio et al., 2018). We also extensively examined CB1R immunostaining in the VTA. We found that CB1R immunostaining was detected mainly on cell membrane and nerve fibers, but not in the cell bodies of neurons (Han et al., 2017), suggesting that immunohistochemistry may not be an ideal technique to determine the phenotype(s) of neurons expressing CB1Rs, as the fibers from multiple types of CB1R-expressing neurons are intertwined.
In the present study, we addressed this question using a combination of advanced approaches, including RNAscope ISH, optogenetics, and transgenic mice. We found that CB1R mRNA is expressed on a subset of DA neurons that corelease DA and glutamate; and activation of CB1Rs on DA neurons underlies cannabinoid-induced aversion, hypoactivity, and anxiolytic behavior.
Materials and Methods
Animals
Adult male and female heterozygous DAT-Cre (DAT-Cre+/−), CB1-flox (CB1flox/flox), and homozygous DA-CB1-KO (CB1flox/flox;DAT-Cre+/–) with C57 genetic background were used in the experiments. Male and female mice, aged 4–8 weeks, were used in RNAscope ISH, locomotion, in vivo brain microdialysis, and elevated plus maze (EPM), while male mice with the age of 6-24 weeks were used in optical intracranial self-stimulation (oICSS) due to the nature of the experiment, which was involved in time-consuming AAV-ChR2-GFP expression, oICSS training, and the following drug tests. Heterozygous DAT-Cre (B6.SJL-Slc6a3tm1.1(Cre)Bkmn/J; stock #006660) knock-in mice were purchased from The Jackson Laboratory. All of the transgenic mice used in this study were bred at the National Institute on Drug Abuse and maintained on a reverse 12 h light–dark cycle (lights off 7:00 A.M./lights on 7:00 P.M.) with food and water available ad libitum. Mouse genotyping was performed by Transnetyx using qRT-PCR on tail snips. All experimental procedures were conducted in accordance with the Guide for the care and use of laboratory animals of the U.S. National Research Council and were approved by the National Institute on Drug Abuse Animal Care and Use Committee.
RNAscope in situ hybridization (ISH)
RNAscope ISH was used to detect cell type-specific CB1 mRNA expression in DA-CB1-KO mice and their littermate control mice (DAT-Cre). Mice were deeply anesthetized, and the whole brain was removed and rapidly frozen on dry ice. Fresh-frozen tissue sections (14 μm thick) were mounted on positively charged microscopic glass slides (Fisher Scientific) and stored at –80°C until RNAscope ISH assays could be performed. Multiple target gene-specific RNAscope probes were used to observe the cellular distributions of CB1 mRNA in TH or VgluT2-expressing DA neurons: CB1 RNAscope probe (catalog #420721, targeting 530-1458 bp of the mouse Cnr1 mRNA sequence, NM_007726.3), TH RNAscope probe (catalog #317621-C2, targeting 483-1603 bp of the Mus musculus TH mRNA sequence, NM_009377.1), and VgluT2 RNAscope probe (catalog #319171-C3, targeting 1986-2998 bp of the Mus musculus VgluT2 (Slc17a6) mRNA sequence, NM_080853.3). All these probes were designed and provided by Advanced Cell Diagnostics. The RNAscope mRNA-staining steps were performed following the manufacturer's protocols. Stained slides were coverslipped with fluorescent mounting medium (ProLong Gold Anti-fade Reagent P36930; Invitrogen) and scanned into digital images with an Olympus FluoView FV1000 confocal microscope at 40× or 60× magnification using manufacturer-provided software. The CB1+ DA neurons were counted in 3-5 sections per brain from 4 mice under ×40 magnification (Han et al., 2017).
Chemicals
Cocaine HCl and Δ9-THC were provided by National Institute on Drug Abuse Intramural Research Program. The stock Δ9-THC solution is 50 mg/ml (w/v) solution in 100% ethanol. Arachidonyl-2′-chloroethylamide (ACEA), JWH133, SCH22390, and L-741626 were obtained from Tocris Bioscience. The vehicle used to dilute Δ9-THC or other cannabinoids is 5% Cremophore (C5135, Sigma-Aldrich).
Optical intracranial self-stimulation (oICSS)
Optical stimulation experiments were conducted in standard operant conditioning chambers (Med Associates, Fairfax, VT). Each chamber was equipped with two wall-mounted levers, two cue lamps, a house lamp, an audio stimulus generator, and four pairs of infrared detectors. Mice were gently connected to a cable that was in turn connected to a 473 nm laser tuned for channelrhodopsin-2 (ChR2) stimulation via an optical swivel. Computer software controlled a pulse generator that controlled the lasers.
Animal surgeries
Male and female mice (∼4 weeks of age) were anesthetized with ketamine and xylazine (100 + 10 mg/kg, i.p.) and placed in a stereotaxic frame (David Kopf Instruments). For intra-VTA microinjection of virus, a custom-made 30-guage stainless injector was used to infuse Cre-inducible recombinant adeno-associated virus (AAV) that encodes ChR2 and enhanced green fluorescent protein (eGFP; i.e., AAV- EF1α-DIO-ChR2-eGFP, 300 nl, ∼2 × 1012 genomes/ml, University of North Carolina Gene Therapy Center) unilaterally into the VTA (AP –3.2; ML 0.1; DV –4.2 mm relative to bregma) using a micropump (WPI 2000 UltraMicroPump, Sarasota, FL) with a speed of 50 nl/min. For optical brain stimulation, a custom-built optrode (200 μm multimode optical fiber, Thorlabs, Newton, NJ) tethered to an intracranial ceramic ferrule (MMFER2007C-2300, Precision Fiber Products, Chula Vista, CA) was implanted into the VTA (AP −0.32; ML 0.1, DV −3.7 mm relative to bregma) at the AAV injection site. Dental cement was used to fix the optrode assembly to the skull. Following AAV vector injection and optrode implantation, mice were allowed to recover for at least 2 weeks before optical self-stimulation experiments began.
oICSS procedure
The general procedures for oICSS were the same as we reported previously (Han et al., 2017; Humburg et al., 2021). After 2 weeks of recovery from surgery, mice were placed into operant chambers containing two operant levers, an active lever and an inactive lever, respectively (ENV-307W-CT, Med Associates). The optrode implanted into the mouse brain (VTA) was connected to a 473 nm laser (OEM Laser Systems, Scottsdale, AZ) via an optical swivel (Doric Lenses, Quebec QC, Canada). Animals were initially trained on a fixed-ratio 1 reinforcement schedule; each active lever response led to delivery of a 1 s pulse train of light stimulation (473 nm, 20 mW, 5 ms duration, 25 Hz) accompanied by a 1 s illumination of cue light above the lever. While inactive lever presses were counted, they had no programmed consequence. Each daily training session lasted 60 min.
Rate-frequency oICSS procedure
Following establishment of lever-pressing for oICSS, animals were presented with a series of 6 different stimulation frequencies (100, 50, 25, 10, 5, 1 Hz) in descending order to obtain rate frequency response curves. Animals were allowed to respond for 10 min per stimulation frequency. The animals were then divided into three groups (5-12 mice per group) to observe the effects of DA receptor antagonists (SCH23390 or L-741626, i.p., 15 min prior to testing), cocaine, or the cannabinoids (Δ9-THC, ACEA, JWH133), respectively, on oICSS maintained by optogenetic stimulation of VTA DA neurons in DAT-Cre mice. Each animal received 3-5 drug injections during the oICSS experiments. After each test, animals received an additional 3–7 days of oICSS restabilization until a new baseline of lever responding was established. The order of testing for the various doses of the drugs was counterbalanced. The effects of DA receptor antagonists, cocaine, or cannabinoids (Δ9-THC, ACEA, JWH133) on oICSS were evaluated by comparing drug-induced changes in active lever presses in DAT-Cre mice and DA-CB1-KO mice.
In vivo microdialysis with HPLC assays
DAT-Cre (n = 7) and DA-CB1-KO (n = 8) mice were anesthetized using a cocktail of ketamine and xylazine (100 + 10 mg/mg) prior to insertion of an intracranial guide cannulae (MAB 4.15.IC, SciPro) into the NAc (stereotaxic coordinates: AP 1.4 mm, ML ±1.5 mm, DV −3.8 mm with an angle of 8° from vertical). Dental acrylic was applied to secure the guide cannulae to the skull. Standard aseptic surgical and stereotaxic procedures were followed. Subjects were given 7 d to recover preceding the experimental procedure. A probe (MAB 4.15.2.PES, SciPro, Sanborn, NY) was inserted into the NAc 12 h before sample collection to reduce the occurrence of damage induced neurotransmitter release. A syringe pump (Bioanalytical Systems, West Lafayette, IN) infused dialysate buffer at least 2 h before sample collection. Baseline microdialysate samples were taken in 20 min intervals for 1 h, followed by an intraperitoneal Δ9-THC injection (1 or 3 mg/kg) and further sampling for 3 h. Samples were frozen at −80°C until analysis could be performed using an ESA electrochemical detection system as we reported previously (Xi et al., 2011; Li et al., 2021).
Locomotor activity
This experiment was designed to compare the effects of Δ9-THC on basal locomotion between DAT-Cre (or CB1-flox) and DA-CB1−KO mice. Three groups of naive mice were placed in open-field locomotor chambers (Accuscan) and habituated for 1 h. After 3 d of habituation, two groups of mice were used to determine the effects of Δ9-THC on basal levels of locomotion, in which each animal randomly received vehicle or one dose of Δ9-THC (1, 3, 10 mg/kg, i.p.) with 2-4 d of intervals.
EPM test
The EPM (elevated plus maze) apparatus has four arms (5 × 30 cm) at right angles to each other, elevated 30 cm from the floor. Two arms have 16 cm black plastic walls (closed arms), and two arms have 16 cm arms without wall (open arms). Adult mice typically spend ∼25% of their time on the open arms. Mice were placed on the center of the maze, and behavior was videorecorded for 5 min. Increased time (seconds) in both the open arms indicates increased anxiolytic effects after drug administration.
Statistical analysis
All data are presented as mean ± SEM. Data analysis was performed with Prism 5 (GraphPad Software). One-way or two-way ANOVAs for repeated measures over drug dose and stimulation frequency were used to analyze the significance of the effects after each drug treatment. Post hoc individual group comparisons were made using the Student–Newman–Keuls method.
Results
CB1 mRNA expression in midbrain DA neurons
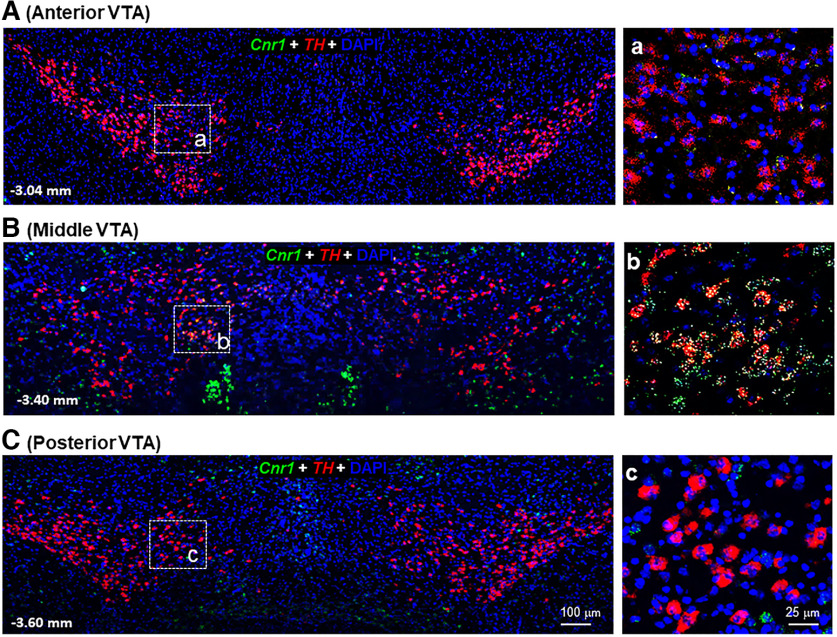
We first used RNAscope ISH assays to characterize CB1R gene expression in DA neurons from the anterior (rostral) to the posterior (causal) VTA in mice (Fig. 1). We found that CB1 mRNA was barely detectable in the anterior (Fig. 1A) or posterior VTA (Fig. 1C) but highly expressed in some TH-positive DA neurons in the middle portion of the VTA (Fig. 1B).
Figure 1.
Representative TH (red) and CB1 (green) mRNA RNAscope images in the anterior, middle, and posterior VTA. A–C, Large-scale images under 20× magnification, illustrating TH-positive DA neurons and CB1 mRNA staining in the VTA and SNc. a–c, High-magnification images (40×) from white dashed-line boxes in the left figure panels, illustrating TH and CB1 mRNA colocalization observed mainly in the middle, but not the anterior or posterior, VTA. Cnr1, the gene expresses CB1 receptor; DAPI, a blue dye labels nuclei.
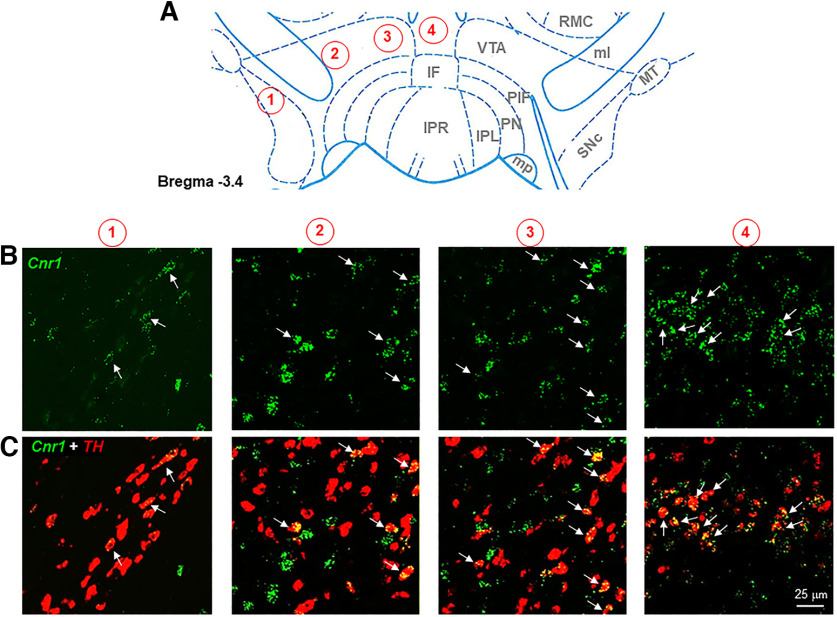
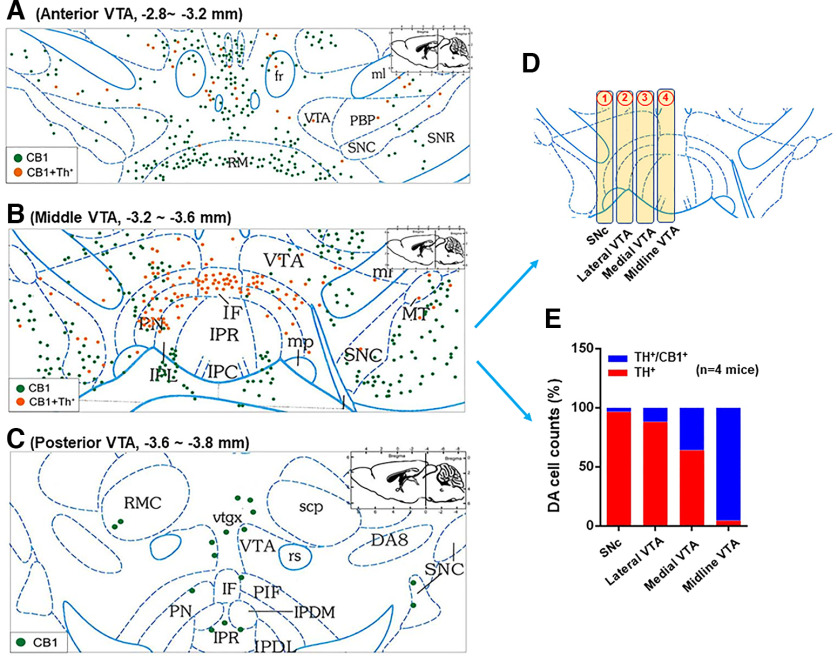
We then examined the subregional distribution of CB1-expressing DA neurons from the lateral SNc/VTA to the medial VTA (Fig. 2). We found that the majority of CB1-expressing DA neurons are located in the medial VTA, an area close to the midline of the midbrain, with the number of colocalizations progressively decreasing from the medial VTA to the lateral SNc/VTA. Quantitative cell counting data indicate that >90% TH+ DA neurons express CB1 mRNA in the midline VTA with the number of CB1-TH colocalization cells decreasing to ∼70% in the medial VTA and ∼20% in the lateral VTA (Fig. 3). Notably, CB1-TH colocalizations are barely detectable in the SNc, which is consistent with previous reports (Julian et al., 2003; Davis et al., 2018).
Figure 2.
Representative TH (red) and CB1 (green) mRNA RNAscope images from the lateral VTA and SNc to the medial VTA in the middle portion of VTA (A, from 1 to 4). B, CB1 mRNA staining. C, CB1-TH double staining, illustrating TH-CB1 locolization (yellow) observed mainly in the medial VTA close to the midline of the midbrain with the number of colocalization progressively decreasing toward the lateral VTA and SNc.
Figure 3.
Spatial distributions of CB1-expressing DA neurons in the midbrain from the anterior (A) to middle (B) and posterior (C) VTA. The images showed the representative data from a single mouse brain section. D, E, Quantitative cell counting data in the middle VTA, illustrating that TH-CB1 colocalization (orange) was observed mainly in the medial VTA with the number of CB1-TH colocalization progressively decreased from the medial toward the lateral VTA and SNc.
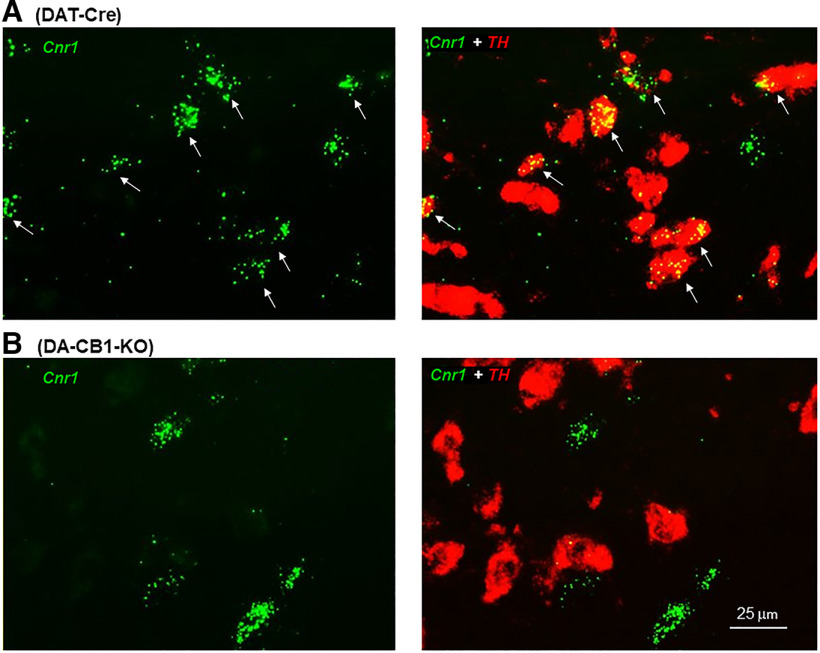
To confirm the CB1 mRNA signal specificity, we used DA-CB1-KO mice as negative controls. We found colocalization of CB1 and TH mRNA in DAT-Cre mice (Fig. 4A), but not DA-CB1-KO mice (Fig. 4B). Notably, the CB1 mRNA signal was still detectable in other non–TH-positive neurons, indicating selective CB1R deletion in DA neurons of DA-CB1-KO mice (Fig. 4B).
Figure 4.
Representative RNAscope images, illustrating TH (red) and CB1 (green) mRNA colocalization (marked by white arrows) in a subset of VTA DA neurons in DAT-Cre mice (A), but not in DA-CB1-KO mice (B).
CB1 mRNA expression in VTA GABA neurons
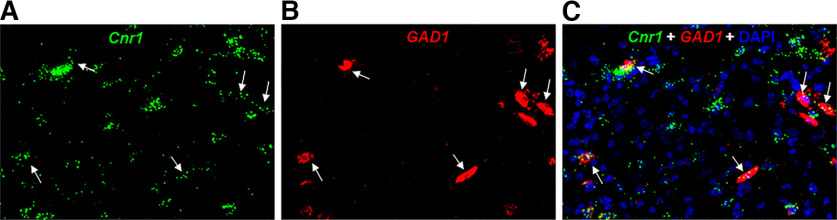
As stated above, electrophysiological evidence indicates functional CB1R expression in VTA GABA neurons (Szabo et al., 2002; Riegel and Lupica, 2004; Melis and Pistis, 2012). Indeed, RNAscope ISH assays detected CB1 mRNA expression in ∼60% GABA neurons in the lateral VTA in mice (Han et al., 2017; Humburg et al., 2021). In this study, we further examined CB1 mRNA expression in the medial VTA. We found that CB1 mRNA signal is much weaker in GAD1-positive GABA neurons than in other non-GABA neurons in this brain region (Fig. 5).
Figure 5.
Representative RNSacope images, illustrating that low-density CB1 mRNA is colocalized with GAD1, a GABAergic neuronal marker, in the medial VTA, suggesting CB1R expression in GABA neurons. A, CB1 mRNA-staining; B, GAD1 mRNA-staining; C, CB1 and GAD1 colocalization in DAPI-labeled cells.
CB1 mRNA expression in DA neurons that corelease DA and glutamate
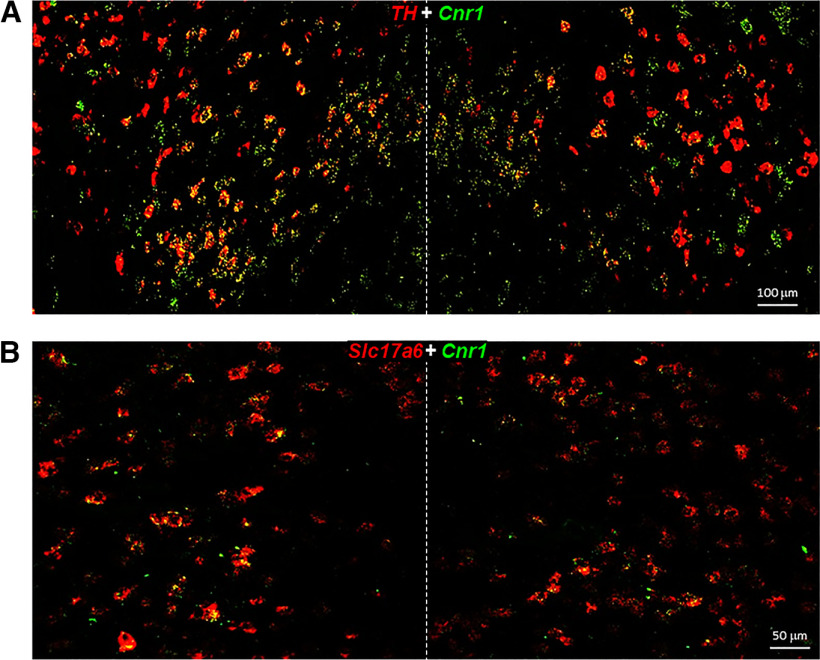
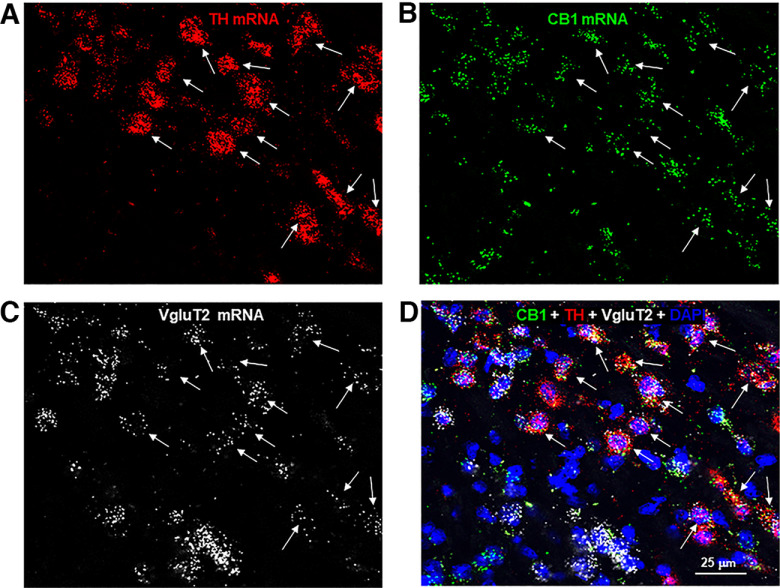
Next, we determined whether those CB1R-expressing non-GABAergic neurons are glutamatergic neurons or a dual phenotype of DA-glutamate neurons as high-density glutamatergic neurons are detected in the medial VTA (Morales and Root, 2014; Han et al., 2017). To test this hypothesis, we first examined colocalization of CB1-TH and CB1-VgluT2 (a glutamatergic neuronal marker in the subcortical brain regions) in this region. Figure 6 shows large-scale representative images in the medial VTA, illustrating that the majority of CB1+ neurons are TH+ or VgluT2+. We then used triple-label RNAscope ISH assays to examine CB1-TH-VgluT2 colocalization. We found that indeed the majority of DA neurons (91.4 ± 6.7%) in this brain region showed TH-CB1-VgluT2 colocalization (Fig. 7). In addition, CB1 mRNA was also detected in other VgluT2+ only glutamate neurons (Fig. 7), suggesting that CB1R is not only expressed in dual DA-glutamate neurons, but also expressed in other glutamate neurons that do not express TH.
Figure 6.
Large-scale RNAscope images under lower magnification (20×) in the medial VTA, illustrating CB1-TH mRNA colocalization in most of TH+ DA neurons (A) and CB1-VgluT2 colocalization in a majority of VgluT2+ glutamate neurons (B). Slc17a6, the gene expressing VgluT2; Cnr1, the gene expressing CB1R.
Figure 7.
Representative triple-labeled images, illustrating CB1-TH-VgluT2 colocalization in most of TH+ DA neurons in the medial VTA. A, TH mRNA-staining; B, CB1 mRNA-staining; C, VgluT2 mRNA-staining; D, CB1-TH-VgluT2 co-localization in DAPI-labeled cells. Notably, CB1 mRNA was also detected in VgluT2+ only glutamate neurons.
Optogenetic stimulation of DA neurons in the medial VTA is rewarding
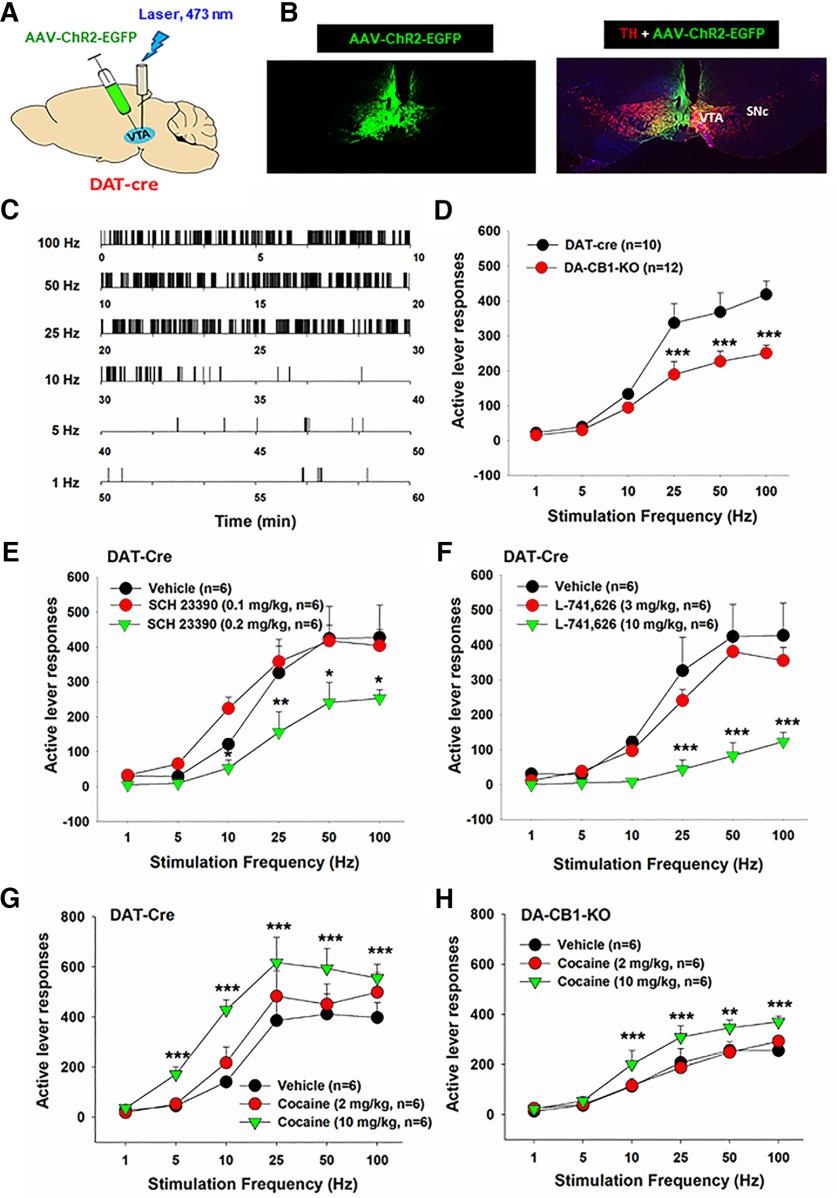
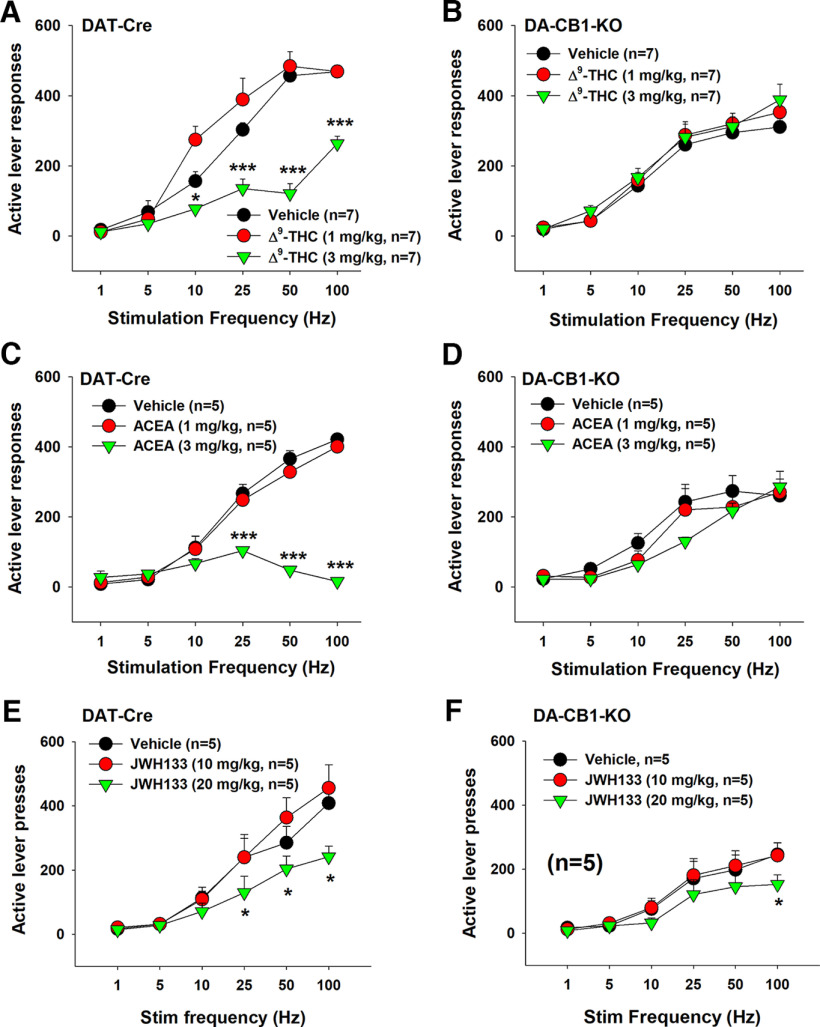
To determine the functional role of these dopaminergic CB1Rs in cannabinoid action, we measured the effects of Δ9-THC and ACEC (a selective CB1R agonist) on oICSS maintained by optical activation of VTA DA neurons located in the medial VTA in both DAT-Cre and DA-CB1-KO mice. Figure 8A shows the experimental procedures, illustrating that the AAV-DIO-ChR2-eGFP vector that expresses light-sensitive ChR2 and fluorescent eGFP was microinjected into the medial VTA in DAT-Cre mice, followed by an optical fiber implanted into the same brain region (1 mm above the medial VTA). Figure 8B shows representative fluorescent eGFP and TH immunostaining, illustrating DAT promoter-driven ChR2-eGFP expression within the medial VTA. Figure 8C shows representative active lever responses observed within a session from a single animal under different stimulation frequencies (from high to low). Response-contingent photoactivation of VTA DA neurons induced robust active lever pressing in a stimulation frequency-dependent manner: the higher the stimulation frequency, the greater the number of active lever presses, and vice versa. These findings indicate that optical stimulation of DA neurons in the medial VTA is rewarding. Unexpectedly, we found that genetic deletion of CB1Rs from DA neurons induced a significant reduction in active lever pressing for VTA photostimulation (Fig. 8D, two-way ANOVA, genotype main effect, F(1,20) = 9.09, p < 0.01; stimulation frequency main effect, F(5,100) = 73.66, p < 0.001; treatment × frequency interaction, F(5,100) = 5.36, p < 0.001). This finding suggests that dopaminergic CB1R expression tonically modulates the mesolimbic DA system and brain reward function. To confirm whether the oICSS responding is DA-dependent, DA D1 and D2 receptor antagonists were administered in DAT-Cre mice. We found that pretreatment with SCH23390 (a selective D1R antagonist; Fig. 8E) or L-741626 (a selective D2R antagonist; Fig. 8F) dose-dependently blocked oICSS in DAT-Cre mice. A two-way repeated-measures ANOVA revealed a significant SCH23390 treatment main effect (Fig. 8E, F(2,10) = 5.16, p < 0.05), frequency main effect (F(5,25) = 35.96, p < 0.001), and treatment × frequency interaction (F(10,50) = 2.26, p < 0.05). A similar assay also revealed a significant L741,626 treatment main effect (Fig. 8F, F(2,10) = 9.69, p < 0.01), frequency main effect (F(5,25) = 49.33, p < 0.001), and treatment × frequency interaction (F(10,50) = 5.09, p < 0.001). In contrast, enhancing DA via cocaine injections (2, 10 mg/kg, i.p.) produced a significant increase in oICSS and shifted the rate-frequency response curve leftward and upward in DAT-Cre mice (Fig. 8G, cocaine treatment main effect, F(2,10) = 67.22, p < 0.001; frequency main effect, F(5,25) = 24.38, p < 0.001; treatment × frequency interaction, F(10,50) = 11.13, p < 0.001) and DA-CB1-KO mice (Fig. 8H, cocaine treatment main effect, F(2,10) = 17.65, p < 0.001; frequency main effect, F(5,25) = 49.59, p < 0.001; treatment × frequency interaction, F(10,50) = 2.94, p < 0.01), suggesting that in the presence of cocaine, less stimulation strength (Hz) is required to maintain the optical brain-stimulation behavior.
Figure 8.
Optical intracranial self-stimulation (oICSS) experiment in mice. A, Schematic diagrams illustrating that AAV-ChR2-eGFP vectors were microinjected into the medial VTA and intracranial optical fibers (i.e., optrodes) were implanted in the same brain region. B, Representative images of TH and AAV-ChR2-eGFP expression in the medial VTA. C, Representative oICSS records in a single session from a single mouse under descending stimulation frequency (from high to low, 10 min per frequency), indicating that optogenetic activation of VTA DA neurons induced robust oICSS behavior (lever presses) in DAT-Cre mice in a stimulation frequency-dependent manner. D, Comparison of oICSS between DAT-Cre and DA-CB1-KO mice, illustrating that DA-CB1-KO mice displayed significantly lower oICSS response to laser stimulation than DAT-Cre mice. E, F, Pretreatment with SCH23390, a selective D1 receptor antagonist (15 min prior to testing), or L-741,626, a selective D2 receptor antagonist (15 min prior to testing), dose-dependently inhibited the oICSS in DAT-Cre mice. G, H, Pretreatment with cocaine produced a dose-dependent increase in oICSS and shifted the oICSS curve upward in DAT-Cre mice (G) and DA-CB1-KO mice (H). *p < 0.05, **p < 0.01, ***p < 0.001, compared with the vehicle control group.
Cannabinoids inhibit oICSS in DAT-Cre mice, not in DA-CB1-KO mice
Next, we examined the effects of cannabinoids on oICSS mediated by optical stimulation of DA neurons in the medial VTA. Figure 9 shows that Δ9-THC dose-dependently shifted the rate-frequency function curve downward in DAT-Cre mice (Fig. 9A, two-way repeated-measures ANOVA, Δ9-THC treatment main effect, F(2,12) = 69.45, p < 0.001; stimulation frequency main effect, F(5,30) = 106.19, p < 0.001, treatment × frequency interaction, F(10,60) = 15.78, p < 0.001). A similar two-way repeated-measures ANOVA for the data in DA-CB1-KO (Fig. 9B) revealed a significant Δ9-THC treatment main effect (F(2,12) = 3.49, p < 0.05) and stimulation frequency main effect (F(5,30) = 49.89, p < 0.001), but a nonsignificant treatment × frequency interaction (F(10,60) = 0.866, p > 0.05). We also examined the effects of ACEA, a selective CB1R agonist, on oICSS. ACEA produced a dose-dependent inhibition in oICSS in DAT-Cre littermates (Fig. 9C, ACEA treatment main effect, F(2,8) = 136.93, p < 0.001; frequency main effect, F(5,20) = 61.24, p < 0.001; treatment × frequency interaction, F(10,40) = 53.05, p < 0.001), but not in DA-CB1-KO mice (Fig. 9D). The same statistical assays revealed a significant frequency main effect only (F(5,20) = 31.35, p < 0.001) without a significant ACEA treatment main effect (F(2,8) = 3.03, p > 0.05) or treatment × frequency interaction (F(10,40) = 1.85, p > 0.05). In other words, in the presence of Δ9-THC or ACEA, higher stimulation strength (Hz) was required to maintain oICSS behavior, suggesting that both cannabinoids produce reward-attenuating or aversive effects. However, deletion of the CB1R from DA neurons eliminated the reward-attenuating effect of Δ9-THC and ACEA.
Figure 9.
Effects of cannabinoids on oICSS maintained by stimulation of medial VTA DA neurons. A, B, Systemic administration of Δ9-THC (1, 3 mg/kg, i.p., 15 min prior to testing) dose-dependently shifted the rate-frequency function curve downward in DAT-Cre mice (A), but not in DA-CB1-KO mice (B). C, D, Systemic administration of ACEA (1, 3 mg/kg, i.p., 15 min prior to testing) dose-dependently shifted the rate-frequency function curve downward in DAT-Cre (C), but not in DA-CB1-KO mice (D). E, F, Systemic administration of JWH133 (10, 20 mg/kg, i.p., 15 min prior to testing) dose-dependently shifted the rate-frequency function curve to the right in DAT-Cre (E) and DA-CB1-KO (B) mice. *p < 0.05, ***p < 0.001, compared with the vehicle control group.
In contrast to Δ9-THC or ACEA, systemic administration of JWH133, a selective CB2R agonist, inhibited oICSS in both DAT-Cre mice and DA-CB1-KO mice (Fig. 9E,F). A two-way repeated-measures ANOVA revealed a JWH133 treatment main effect (Fig. 9E, F(2,12) = 3.58, p = 0.06), a frequency main effect (F(5,30) = 57.07, p < 0.001), and treatment × frequency interaction (F(10,60) = 3.03, p < 0.01) in DAT-Cre mice. Similarly, a two-way repeated-measures ANOVA revealed a JWH133 treatment main effect (Fig. 9F, F(2,10) = 1.30, p > 0.05), a frequency main effect (F(5,25) = 53.81, p < 0.001), and treatment × frequency interaction (F(10,50) = 2.93, p < 0.05) in DA-CB1-KO mice. Post hoc group comparisons indicate a significant reduction in oICSS at 100 Hz in DA-CB1-KO mice after 20 mg/kg JWH133 administration. These findings confirm that the above effects with Δ9-THC or ACEA are CB1R-dependent, while the effect of JWH133 is not. The latter finding with JWH133 is consistent with our previous reports that cannabinoid CB2R modulates the mesolimbic DA system and brain reward function (Xi et al., 2011; Zhang et al., 2014).
Effects of Δ9-THC on extracellular DA in the NAc
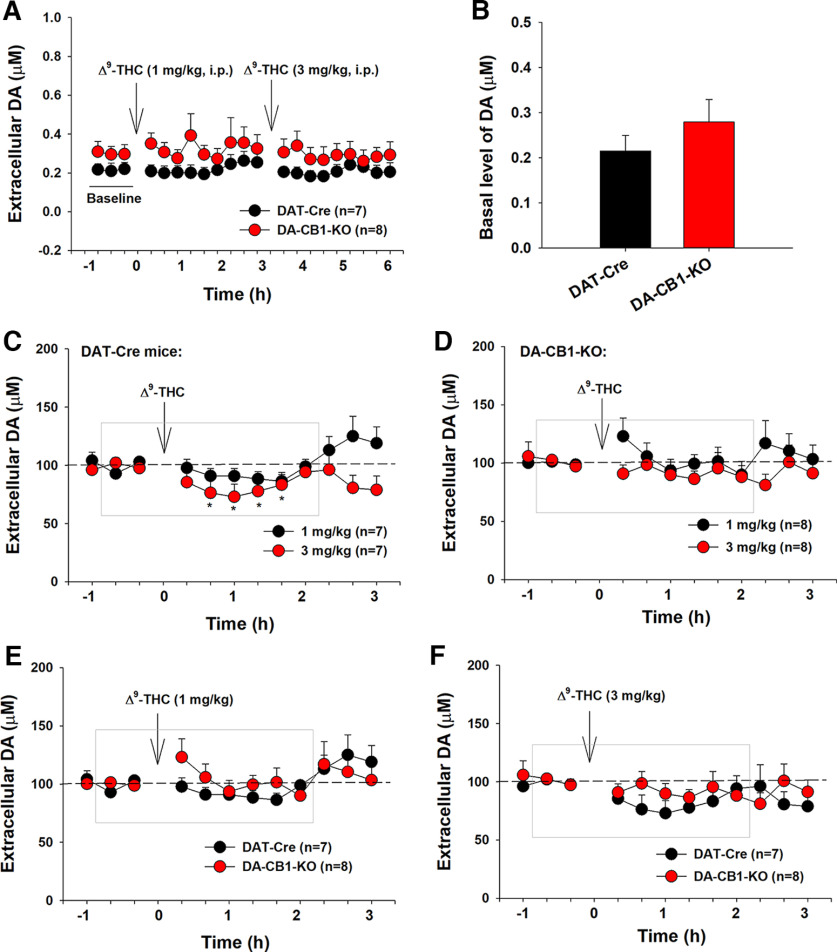
We have previously reported that Δ9-THC (1, 3 mg/kg, i.p.) produced a significant reduction in extracellular DA in the NAc of WT mice (Li et al., 2021). In this study, we further examined whether dopaminergic CB1R mechanisms contribute to Δ9-THC-induced reduction in DA release. Figure 10 shows the DA response to Δ9-THC in both genotypes of mice. DA-CB1-KO mice displayed an overall higher basal level of extracellular DA than DAT-Cre mice (Fig. 10A,B). A two-way repeated-measures ANOVA revealed a significant time main effect (Fig. 10A; F(20,260) = 2.14, p < 0.01), but a nonsignificant genotype main effect (F(1,14) = 1.25, p > 0.05) or time × genotype interaction (F(20,260) = 0.41, p > 0.05). Figure 10B shows the mean values of three baseline samples, indicating a trend toward an increase in basal level of extracellular DA in DA-CB1-KO mice compared with DAT-Cre control mice (t = 1.71, p = 0.058), suggesting that dopaminergic CB1Rs tonically regulate (depress) basal DA release. Inconsistent with our finding in WT mice (Li et al., 2021), systemic administration of Δ9-THC produced a mild but significant reduction in extracellular DA in DAT-Cre mice (Fig. 10C), but not in DA-CB1-KO mice (Fig. 10D). A two-way repeated-measures ANOVA for the data shown in the gray box in Figure 10C revealed a significant time main effect (F(7,42) = 3.09, p = 0.01), but a nonsignificant genotype main effect (F(1,6) = 4.68, p > 0.05). Post hoc group comparisons indicated a significant reduction in extracellular DA after 3 mg/kg Δ9-THC administration in DAT-Cre mice compared with the baselines. However, the same assays did not reveal time (or Δ9-THC treatment) main effect (Fig. 10D, F(7,49) = 0.98, p > 0.05) or genotype main effect (Fig. 10D, F(1,7) = 2.72, p > 0.05) in DA-CB1-KO mice. We also compared the DA response to Δ9-THC between the two groups of mice (Fig. 10E,F). A two-way repeated-measures ANOVA did not reveal a significant genotype main effect (Fig. 10E, F(1,13) = 0.63, p > 0.05; Fig. 10F, F(1,13) = 0.58, p > 0.05), time main effect (Fig. 10E, F(11,143) = 1.25, p > 0.05; Fig. 10F, F(11,143) = 1.49, p > 0.05) or genotype × time interaction (Fig. 10E, F(11,143) = 1.09, p > 0.05; Fig. 10F, F(11,143) = 0.87, p > 0.05). The nonsignificant difference between the DAT-Cre control and DA-CB1-KO mice may be related to the mild DA response to Δ9-THC and poor temporal and spatial resolutions of in vivo microdialysis in detecting subtle changes in extracellular DA after drug administration.
Figure 10.
Effects of Δ9-THC on extracellular DA in the NAc in DAT-Cre and DA-CB1-KO mice. A, Extracellular DA concentration (μM) before and after Δ9-THC administration, illustrating an overall higher extracellular DA level in DA-CB1-KO mice than DAT-Cre control mice during the 7 h of microdialysis experiments. B, The mean values of three baseline samples, indicating a slightly higher basal level of extracellular DA in DA-CB1-KO mice than in DAT-Cre mice. C, D, Δ9-THC produced a dose-dependent decrease in extracellular DA in DAT-Cre mice (C), but not in DA-CB1-KO mice (D). E, F, Comparison of NAc DA response to 1 mg/kg THC (E) or 3 mg/kg Δ9-THC (F) between DAT-Cre mice and DA-CB1-KO mice. *p < 0.05, compared with baseline.
Δ9-THC inhibits open-field locomotion in DAT-Cre mice and DA-CB1-KO mice
We also examined whether deletion of CB1R from DA neurons altered locomotor response to Δ9-THC. We found that DA-CB1-KO mice displayed a higher basal level of activity (before Δ9-THC administration) (Fig. 11C) than CB1-flox (Fig. 11A) or DAT-Cre mice (Fig. 11B). Figure 11D shows the mean values of six baseline samples, indicating a significant increase in basal level of locomotion in DA-CB1-KO mice compared with DAT-Cre mice (F(2,21) = 10.31, p < 0.001), suggesting that dopaminergic CB1Rs tonically modulate basal locomotor activity. Systemic administration of Δ9-THC (1, 3, 10 mg/kg, i.p.) dose-dependently decreased locomotion in CB1-flox mice (Fig. 11E, F(3,18) = 17.02, p < 0.001) and DAT-Cre control mice (Fig. 11F, F(3,21) = 7.41, p = 0.001) as well as DA-CB1-KO mice (Fig. 11G, F(3,21) = 39.58, p < 0.001). However, deletion of CB1Rs from DA neurons blocked locomotor inhibition produced by 3 mg/kg, but not 10 mg/kg, Δ9-THC in DA-CB1-KO mice (Fig. 11G).
Figure 11.
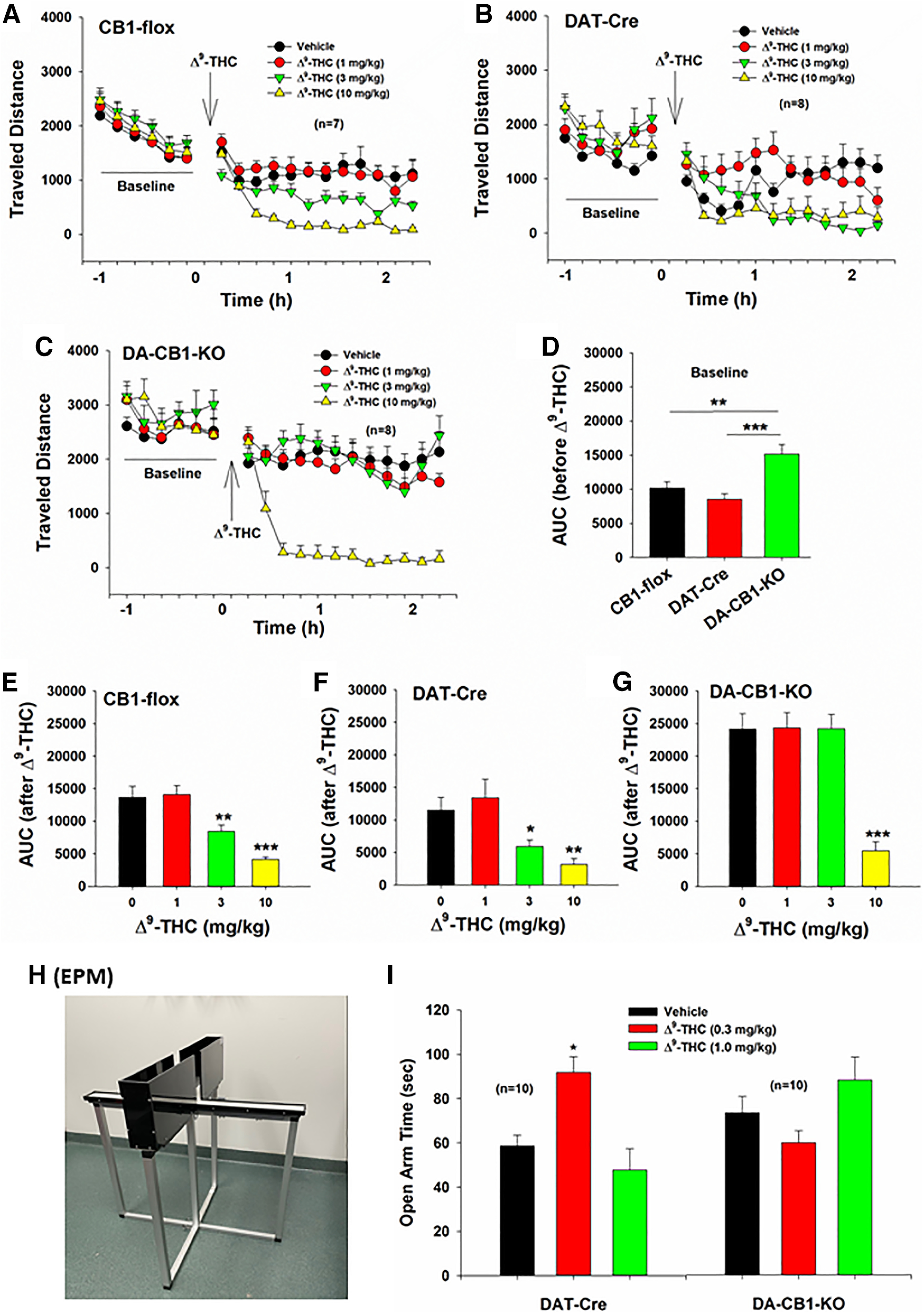
Effects of Δ9-THC on open-field locomotion and anxiety-like behavior. A–C, Systemic administration of Δ9-THC (1, 3, 10 mg/kg, i.p., 15 min prior to testing) dose-dependently inhibited open-field locomotion in CB1-flox mice, DAT-Cre mice, and DA-CB1-KO mice. D, Calculation of the area under curve (AUC) of locomotor activity indicates that DA-CB1-KO mice displayed a significantly higher basal level of locomotion (before Δ9-THC administration) than CB1-flox mice or DAT-Cre mice. E–G, Calculated AUC data indicating the dose-dependent locomotor effects of Δ9-THC in CB1-flox mice, DAT-Cre mice, and DA-CB1-KO mice. Deletion of CB1R from DA neurons blocked lower doses (3 mg/kg) of Δ9-THC-induced reduction in locomotion (G). H, An image of EPM device we used in this study. I, Δ9-THC, at low doses (0.3 mg/kg), produced anxiolytic effects, manifested as increased time in the open arms of the EPM device. Deletion of CB1R from DA neurons reversed Δ9-THC's anxiolytic effects, producing an opposite anxiogenic effect as assessed by decreased time spent in the open arms of the EPM device.
Δ9-THC produces anxiolytic effects in DAT-Cre mice
Last, we evaluated the anxiolytic effects of Δ9-THC in DAT-Cre and DA-CB1-KO mice using the EPM (Fig. 11H). We found that 0.3 mg/kg Δ9-THC produced a significant anxiolytic effect in DAT-Cre mice, manifested as increased time spent in the open arms of the maze (Fig. 11I). This effect was blocked by deletion of CB1Rs from DA neurons (Fig. 11I). A one-way repeated-measures ANOVA over drug dose revealed a significant Δ9-THC treatment main effect in DAT-Cre mice (F(2,18) = 12.96, p < 0.001) and in DA-CB1-KO mice (F(2,18) = 1.69, p > 0.05). Post hoc group comparisons revealed a significant difference between the vehicle and 0.3 mg/kg Δ9-THC groups in DAT-Cre mice (p < 0.05).
Discussion
There are several major findings in the present study, including: (1) CB1R mRNA was detected in a subset of DA neurons located mainly in the middle portion of the VTA. The number of CB1R-expressing DA neurons progressively decreased from the medial VTA to the lateral VTA/SNc. (2) CB1 mRNA was colocalized with TH and VgluT2, suggesting that dual DA-glutamate neurons express CB1Rs. (3) Optical activation of DA neurons in the medial VTA is rewarding. Selective deletion of CB1Rs from DA neurons reduced oICSS response to laser stimulation, suggesting that dopaminergic CB1Rs tonically modulate DA neuron excitability and brain reward function. (4) Systemic administration of Δ9-THC or ACEA dose-dependently inhibited oICSS in DAT-Cre mice, but not in DA-CB1-KO mice, suggesting an effect that is mediated by activation of CB1Rs in VTA DA neurons. Last, (5) deletion of CB1Rs from DA neurons blocked Δ9-THC-induced reduction in NAc DA release, hypoactivity, and anxiolytic effects. Together, these findings, for the first time, demonstrate that dopaminergic CB1Rs are functionally involved in multiple behavioral effects of cannabinoids.
Cannabinoids modulate DA transmission
Cannabinoids and endocannabinoids regulate DA transmission, which is thought to be mediated by activation of CB1Rs on GABAergic interneurons and glutamatergic afferent inputs onto VTA DA neurons (Melis et al., 2004; Riegel and Lupica, 2004; Fitzgerald et al., 2012; Covey et al., 2017). As midbrain DA neurons receive extensive GABAergic input from numerous brain regions (Jhou et al., 2009; Matsui et al., 2014; Paladini, 2017), removing this inhibitory constraint increases tonic firing and bursting rates of DA neurons (Lobo et al., 2010). Therefore, it is generally believed that this GABA-mediated disinhibition of DA neurons may underlie cannabinoid reward. However, so far, there is no behavioral evidence supporting this hypothesis. In contrast, growing evidence indicates that cannabinoids are not rewarding, but aversive, in rodents, particularly in mice (Panagis et al., 2008; Vlachou and Panagis, 2014; Hempel and Xi, 2022), which cannot be explained by the above GABAergic CB1R hypothesis. To address this question, we have recently used advanced RNAscope ISH assays, combined with conditional CB1-KO mice, to examine CB1 mRNA in the midbrain. We found that CB1 mRNAs are indeed expressed not only in VTA GABA neurons, but also in glutamate neurons (Han et al., 2017; Humburg et al., 2021). Activation of CB1Rs on glutamate neurons attenuated Δ9-THC-induced aversion and hypoactivity in VgluT2-Cre mice, but not in glutamate-CB1-KO mice, suggesting that a glutamate CB1R mechanism, at least in part, underlies cannabinoid-induced negative affective and locomotor effects (Han et al., 2017).
In this report, we found that CB1R mRNAs are also expressed in a subset of DA neurons located in the medial VTA where much weaker CB1 mRNA signal was detected in GABA neurons. Rostral (anterior) and caudal (posterior) portions of the VTA did not show appreciable CB1R expression in DA neurons. In the lateral VTA and SNc, we detected TH-CB1 mRNA colocalization only in a very small population (<20%) of DA neurons. This unique restricted distribution of CB1R-expressing DA neurons within the VTA may well explain why previous immunostaining and electrophysiological assays failed to detect CB1 signaling in DA neurons in the lateral VTA or SNc (Julian et al., 2003; Davis et al., 2018). Furthermore, selective deletion of CB1Rs from DA neurons abolished the CB1 mRNA signal in DA neurons in DA-CB1-KO mice, confirming that the detected mRNA signal is CB1R-specific.
Given that high-density glutamatergic neurons are located in the medial VTA (Morales and Root, 2014; Han et al., 2017), we further examined CB1-TH-VgluT2 colocalization and found that CB1Rs are highly expressed in a subset of DA neurons that corelease DA and glutamate.
Dopaminergic CB1Rs underlie cannabinoid-induced aversion
To determine whether CB1Rs on DA neurons are functional, we used optogenetics to selectively stimulate DA neurons in the medial VTA where high-density CB1R-expressing DA neurons are located. We found that optogenetic stimulation of DA neurons maintained oICSS, which was enhanced by cocaine, but attenuated by DA receptor antagonists. Systemic administration of Δ9-THC or ACEA dose-dependently inhibited oICSS in DAT-Cre mice, but not in DA-CB1-KO mice, indicating that dopaminergic CB1Rs are involved in cannabinoid-induced aversion or reward attenuation. This finding is consistent with previous reports that cannabinoids (Δ9-THC, WIN55212-2, AM2201) or endocannabinoid enhancers (e.g., fatty acid amide hydrolase or monoacylglycerol lipase inhibitors) dose-dependently inhibit ICSS maintained by electrical stimulation of the medial forebrain bundle (Wiebelhaus et al., 2015; Spiller et al., 2019) or optical stimulation of DA neuron in the lateral VTA or glutamate neurons in the medial VTA (Han et al., 2017; Humburg et al., 2021).
We note that DA-CB1-KO mice displayed lower oICSS performance and blunted oICSS response to cocaine compared with DAT-Cre mice. These differences are unlikely due to the difference in AAV-ChR2 expression or optical fiber placement between both genotypes of mice as the postexperimental histological examinations failed to detect any difference. A reasonable explanation is that deletion of CB1Rs from DA neurons may cause DA neuron disinhibition, leading to an increase in basal DA neuronal firing, which subsequently decreases neuronal responses to optical stimulation or psychostimulants due to a maximal ceiling firing effect. This interpretation is supported by the finding that DA-CB1-KO mice displayed higher basal levels of extracellular DA in the NAc and higher basal locomotor activity possibly due to the removal of the inhibitory CB1Rs from DA neurons. Thus, these findings provide additional evidence supporting CB1R expression in VTA DA neurons.
Dopaminergic CB1R underlies low-dose Δ9-THC-induced hypoactivity
It is well known that the cannabinoids produce characteristic locomotor effects, such as a reduction in open-field locomotion, catalepsy, and immobility by activation of CB1Rs (Wiebelhaus et al., 2015; Han et al., 2017; Wang et al., 2020; Li et al., 2021). We have recently reported that glutamatergic CB1Rs partially underlie Δ9-THC-induced locomotor inhibition in VgluT2-Cre mice (Han et al., 2017). In the present study, we found that that selective deletion of CB1Rs from DA neurons caused an increase in basal levels of open-field locomotion and blocked lower-dose Δ9-THC-induced hypoactivity, suggesting that dopaminergic CB1Rs also play an important role in cannabinoid modulation of locomotion.
Dopaminergic CB1Rs underlie Δ9-THC-induced anxiolytic effects
It was reported that Δ9-THC produces anxiolytic effects at low doses when administered systemically or locally into the amygdala and PFC, which is effectively blocked by CB1R antagonism (Berrendero and Maldonado, 2002; Rubino et al., 2007). CP55940 also produces an anxiolytic-like response at a low dose, which is absent in mice lacking CB1Rs in glutamatergic neurons (Rey et al., 2012). In the present study, we found that deletion of CB1Rs from midbrain DA neurons also blocked low-dose Δ9-THC-induced anxiolytic effects. Together, all these findings suggest that the anxiolytic effects of cannabinoids may be mediated by activation of CB1Rs in both VTA DA neurons and glutamate neurons in the PFC and amygdala.
Notably the same low-dose Δ9-THC produced mild anxiogenic effects in DA-CB1-KO mice compared with DAT-Cre control mice, suggesting that two different mechanisms may control anxiety-like behavior; that is, CB1R activation on DA neurons produces anxiolytic effects, while CB1R activation on other types of cells produces anxiogenic effects. Thus, in the absence of dopaminergic CB1R, cannabinoids may produce net anxiogenic effects. This observation is supported by the finding that mice lacking CB1Rs on GABAergic failed to demonstrate anxiety in response to CP55940 (Rey et al., 2012). We should point out that the present findings from a single behavioral test (EPM) could not be fully convincing. Thus, further research is needed to confirm the above findings using other anxiety-related behavioral models, such as light dark box test and locomotor performance in the center versus peripheral areas in an open-field chamber.
In conclusion, the present study demonstrates that, in addition to their expression in midbrain GABA neurons and glutamate neurons (Han et al., 2017; Humburg et al., 2021), CB1Rs are also expressed in a subset of DA neurons and functionally involved in cannabinoid action. Given that VTA DA neurons receive both excitatory glutamatergic and inhibitory GABAergic inputs (Morales and Root, 2014), we hypothesized that the psychoactive (reward vs aversive, anxiolytic vs anxiogenic) and locomotor effects of cannabinoids may depend on the final net effect of cannabinoids on CB1Rs expressed in different phenotypes of neurons. Activation of CB1Rs on VTA DA neurons and glutamate terminals produces aversive, anxiolytic, and locomotor inhibitory effects, while activation of CB1R on VTA GABA neurons or GABAergic afferent terminals may produce rewarding, anxiogenic, and locomotor-stimulating effects, although supporting evidence is still needed. If more CB1Rs are expressed in VTA GABAergic neurons or their afferents, cannabis will be rewarding. In contrast, if more CB1Rs are expressed in VTA DA neurons or glutamatergic afferents, cannabis will be aversive and anxiolytic. Congruently, if CB1R expression or functional activity is equivalent on GABA versus DA and glutamate neurons, cannabis may have little net effect on brain reward function. The present findings provide a new dopaminergic CB1R mechanism to explain why and how cannabinoids produce unpleasant subjective effects, anxiety, and locomotor inhibition.
Footnotes
References
- Berrendero F, Maldonado R (2002) Involvement of the opioid system in the anxiolytic-like effects induced by delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl) 163:111–117. 10.1007/s00213-002-1144-9 [DOI] [PubMed] [Google Scholar]
- Castañeda EM, Oddie SD, Whishaw Q (1991) THC does not affect striatal dopamine release: microdialysis in freely moving rats. Pharmacol Biochem Behav 40:587–591. 10.1016/0091-3057(91)90367-B [DOI] [PubMed] [Google Scholar]
- Cheer JF, Kendall DA, Marsden CA (2000) Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology (Berl) 151:25–30. 10.1007/s002130000481 [DOI] [PubMed] [Google Scholar]
- Cheer JF, Kendall DA, Mason R, Marsden CA (2003) Differential cannabinoid-induced electrophysiological effects in rat ventral tegmentum. Neuropharmacology 44:633–641. 10.1016/s0028-3908(03)00029-7 [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM (2004) Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci 24:4393–4400. 10.1523/JNEUROSCI.0529-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DP, Mateo Y, Sulzer D, Cheer JF, Lovinger DM (2017) Endocannabinoid modulation of dopamine neurotransmission. Neuropharmacology 124:52–61. 10.1016/j.neuropharm.2017.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Sampaio L, Kubrusly RC, Colli YP, Trindade PP, Ribeiro-Resende VT, Einicker-Lamas M, Paes-de-Carvalho R, Gardino PF, de Mello FG, De Melo Reis RA (2018) Cannabinoid receptor type 1 expression in the developing avian retina: morphological and functional correlation with the dopaminergic system. Front Cell Neurosci 12:58. 10.3389/fncel.2018.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MI, Crittenden JR, Feng AY, Kupferschmidt DA, Naydenov A, Stella N, Graybiel AM, Lovinger DM (2018) The cannabinoid-1 receptor is abundantly expressed in striatal striosomes and striosome-dendron bouquets of the substantia nigra. PLoS One 13:e0191436. 10.1371/journal.pone.0191436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Hill MP, Bisogno T, Crossman AR, Brotchie JM (2000) Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson's disease. FASEB J 14:1432–1438. 10.1096/fasebj.14.10.1432 [DOI] [PubMed] [Google Scholar]
- Fitzgerald ML, Shobin E, Pickel VM (2012) Cannabinoid modulation of the dopaminergic circuitry: implications for limbic and striatal output. Prog Neuropsychopharmacol Biol Psychiatry 38:21–29. 10.1016/j.pnpbp.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French ED, Dillon K, Wu X (1997) Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport 8:649–652. 10.1097/00001756-199702100-00014 [DOI] [PubMed] [Google Scholar]
- Han X, He Y, Bi GH, Zhang HY, Song R, Liu QR, Egan JM, Gardner EL, Li J, Xi ZX (2017) CB1 receptor activation on VgluT2-expressing glutamatergic neurons underlies delta(9)-tetrahydrocannabinol (delta(9)-THC)-induced aversive effects in mice. Sci Rep 7:12315. 10.1038/s41598-017-12399-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel B, Xi ZX (2022) Receptor mechanisms underlying the CNS effects of cannabinoids: CB1 receptor and beyond. Adv Pharmacol 93:275–333. 10.1016/bs.apha.2021.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M (1992) Cannabinoid receptor localization in brain: relationship to motor and reward systems. Ann NY Acad Sci 654:19–32. 10.1111/j.1749-6632.1992.tb25953.x [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC (1990) Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA 87:1932–1936. 10.1073/pnas.87.5.1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez M, Berrendero F, Suarez I, Garcia-Gil L, Cebeira M, Mackie K, Ramos JA, Fernandez-Ruiz J (2000) Cannabinoid CB(1) receptors colocalize with tyrosine hydroxylase in cultured fetal mesencephalic neurons and their activation increases the levels of this enzyme. Brain Res 857:56–65. 10.1016/s0006-8993(99)02322-7 [DOI] [PubMed] [Google Scholar]
- Humburg BA, Jordan CJ, Zhang HY, Shen H, Han X, Bi GH, Hempel B, Galaj E, Baumann MH, Xi ZX (2021) Optogenetic brain-stimulation reward: a new procedure to re-evaluate the rewarding versus aversive effects of cannabinoids in dopamine transporter-Cre mice. Addict Biol 26:e13005. 10.1111/adb.13005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC (2009) The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 61:786–800. 10.1016/j.neuron.2009.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian MD, Martin AB, Cuellar B, Rodriguez De Fonseca F, Navarro M, Moratalla R, Garcia-Segura LM (2003) Neuroanatomical relationship between type 1 cannabinoid receptors and dopaminergic systems in the rat basal ganglia. Neuroscience 119:309–318. 10.1016/s0306-4522(03)00070-8 [DOI] [PubMed] [Google Scholar]
- Kim SR, Bok E, Chung YC, Chung ES, Jin BK (2008) Interactions between CB(1) receptors and TRPV1 channels mediated by 12-HPETE are cytotoxic to mesencephalic dopaminergic neurons. Br J Pharmacol 155:253–264. 10.1038/bjp.2008.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BK, Cota D, Cristino L, Borgland SL (2017) Endocannabinoid modulation of homeostatic and non-homeostatic feeding circuits. Neuropharmacology 124:38–51. 10.1016/j.neuropharm.2017.05.033 [DOI] [PubMed] [Google Scholar]
- Li X, Hempel BJ, Yang HJ, Han X, Bi GH, Gardner EL, Xi ZX (2021) Dissecting the role of CB1 and CB2 receptors in cannabinoid reward versus aversion using transgenic CB1- and CB2-knockout mice. Eur Neuropsychopharmacol 43:38–51. 10.1016/j.euroneuro.2020.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ (2010) Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330:385–390. 10.1126/science.1188472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Jarvie BC, Robinson BG, Hentges ST, Williams JT (2014) Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal. Neuron 82:1346–1356. 10.1016/j.neuron.2014.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF (2006) Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience 137:337–361. 10.1016/j.neuroscience.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Matyas F, Urban GM, Watanabe M, Mackie K, Zimmer A, Freund TF, Katona I (2008) Identification of the sites of 2-arachidonoylglycerol synthesis and action imply retrograde endocannabinoid signaling at both GABAergic and glutamatergic synapses in the ventral tegmental area. Neuropharmacology 54:95–107. 10.1016/j.neuropharm.2007.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Pistis M (2012) Hub and switches: endocannabinoid signalling in midbrain dopamine neurons. Philos Trans R Soc Lond B Biol Sci 367:3276–3285. 10.1098/rstb.2011.0383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL (2004) Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci 24:53–62. 10.1523/JNEUROSCI.4503-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, De Felice M, Lecca S, Fattore L, Pistis M (2013) Sex-specific tonic 2-arachidonoylglycerol signaling at inhibitory inputs onto dopamine neurons of Lister Hooded rats. Front Integr Neurosci 7:93. 10.3389/fnint.2013.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Root DH (2014) Glutamate neurons within the midbrain dopamine regions. Neuroscience 282:60–68. 10.1016/j.neuroscience.2014.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini JM (2017) Neurophysiology of substantia nigra dopamine neurons: modulation by GABA and glutamate. Handb Basal Ganglia Struct Funct 335–360. [Google Scholar]
- Panagis G, Vlachou S, Nomikos GG (2008) Behavioral pharmacology of cannabinoids with a focus on preclinical models for studying reinforcing and dependence-producing properties. Curr Drug Abuse Rev 1:350–374. 10.2174/1874473710801030350 [DOI] [PubMed] [Google Scholar]
- Pillolla G, Melis M, Perra S, Muntoni AL, Gessa GL, Pistis M (2007) Medial forebrain bundle stimulation evokes endocannabinoid-mediated modulation of ventral tegmental area dopamine neuron firing in vivo. Psychopharmacology (Berl) 191:843–853. 10.1007/s00213-007-0733-z [DOI] [PubMed] [Google Scholar]
- Rey AA, Purrio M, Viveros MP, Lutz B (2012) Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA(B) receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology 37:2624–2634. 10.1038/npp.2012.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR (2004) Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci 24:11070–11078. 10.1523/JNEUROSCI.3695-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Sala M, Vigano D, Braida D, Castiglioni C, Limonta V, Guidali C, Realini N, Parolaro D (2007) Cellular mechanisms underlying the anxiolytic effect of low doses of peripheral Delta9-tetrahydrocannabinol in rats. Neuropsychopharmacology 32:2036–2045. 10.1038/sj.npp.1301330 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2021) Key substance use and mental health indicators in the United States: results from the 2020 National Survey on Drug Use and Health. HHS Publication No. PEP21-07-01-003, NSDUH Series H-56. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Retrieved from https://www.samhsa.gov/data/. [Google Scholar]
- Sidlo Z, Reggio PH, Rice ME (2008) Inhibition of striatal dopamine release by CB1 receptor activation requires nonsynaptic communication involving GABA, H2O2, and KATP channels. Neurochem Int 52:80–88. 10.1016/j.neuint.2007.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller KJ, Bi GH, He Y, Galaj E, Gardner EL, Xi ZX (2019) Cannabinoid CB1 and CB2 receptor mechanisms underlie cannabis reward and aversion in rats. Br J Pharmacol 176:1268–1281. 10.1111/bph.14625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Muller T, Koch H (1999) Effects of cannabinoids on dopamine release in the corpus striatum and the nucleus accumbens in vitro. J Neurochem 73:1084–1089. 10.1046/j.1471-4159.1999.0731084.x [DOI] [PubMed] [Google Scholar]
- Szabo B, Siemes S, Wallmichrath I (2002) Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci 15:2057–2061. 10.1046/j.1460-9568.2002.02041.x [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G (1997) Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276:2048–2050. 10.1126/science.276.5321.2048 [DOI] [PubMed] [Google Scholar]
- Vlachou S, Panagis G (2014) Regulation of brain reward by the endocannabinoid system: a critical review of behavioral studies in animals. Curr Pharm Des 20:2072–2088. 10.2174/13816128113199990433 [DOI] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Stephens DN, Panagis G (2007) Lack of evidence for appetitive effects of Delta 9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav Pharmacol 18:311–319. 10.1097/FBP.0b013e3282186cf2 [DOI] [PubMed] [Google Scholar]
- Wang H, Treadway T, Covey DP, Cheer JF, Lupica CR (2015) Cocaine-induced endocannabinoid mobilization in the ventral tegmental area. Cell Rep 12:1997–2008. 10.1016/j.celrep.2015.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XF, Galaj E, Bi GH, Zhang C, He Y, Zhan J, Bauman MH, Gardner EL, Xi ZX (2020) Different receptor mechanisms underlying phytocannabinoid- versus synthetic cannabinoid-induced tetrad effects: opposite roles of CB1/CB2 versus GPR55 receptors. Br J Pharmacol 177:1865–1880. 10.1111/bph.14958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger T, Moldrich G, Furst S (2003) Neuromorphological background of cannabis addiction. Brain Res Bull 61:125–128. 10.1016/s0361-9230(03)00081-9 [DOI] [PubMed] [Google Scholar]
- Wiebelhaus JM, Grim TW, Owens RA, Lazenka MF, Sim-Selley LJ, Abdullah RA, Niphakis MJ, Vann RE, Cravatt BF, Wiley JL, Negus SS, Lichtman AH (2015) Delta9-tetrahydrocannabinol and endocannabinoid degradative enzyme inhibitors attenuate intracranial self-stimulation in mice. J Pharmacol Exp Ther 352:195–207. 10.1124/jpet.114.218677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2016) The health and social effects of nonmedical cannabis use. Available at https://www.who.int/publications/i/item/9789241510240.
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL (2011) Brain cannabinoid CB(2) receptors modulate cocaine's actions in mice. Nat Neurosci 14:1160–1166. 10.1038/nn.2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ, Gardner EL, Wu J, Xi ZX (2014) Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci USA 111:E5007–E5015. [DOI] [PMC free article] [PubMed] [Google Scholar]