Abstract
Background and Objectives: Mortality and illness due to COVID-19 have been linked to a condition known as cytokine release syndrome (CRS) that is characterized by excessive production of inflammatory cytokines, particularly interleukin-6 (IL-6). Tocilizumab (TCZ), a recent IL-6 antagonist, has been redeployed as adjunctive treatment for CRS remission in COVID-19 patients. This study aimed to determine the efficacy of Tocilizumab on patients’ survival and the length of stay in hospitalized COVID-19 patients admitted to the intensive care unit. Methods: Between January 2021 and June 2021, a multicenter retrospective cohort study was carried out in six tertiary care hospitals in Egypt’s governorate of Giza. Based on the use of TCZ during ICU stay, eligible patients were divided into two groups (control vs. TCZ). In-hospital mortality was the main outcome. Results: A total of 740 patient data records were included in the analysis, where 630 patients followed the routine COVID-19 protocol, while 110 patients received TCZ, need to different respiratory support after hospitalization, and inflammatory mediators such as C-reactive protein (CRP), ferritin, and Lactate dehydrogenase (LDH) showed a statistically significant difference between the TCZ group and the control group. Regarding the primary outcome (discharged alive or death) and neither the secondary outcome (length of hospital stay), there is no statistically significant difference between patients treated with TCZ and the control group. Conclusions: Our cohort of patients with moderate to severe COVID-19 did not assert a reduction in the risk of mortality or the length of stay (LOS) after TCZ administration.
Keywords: COVID-19, Tocilizumab, cytokine release syndrome, in-hospital mortality
1. Introduction
The coronavirus SARS-CoV-2 has been at the center of a worldwide pandemic that has plagued the world since December 2019 [1,2]. Patients infected with COVID-19 have a 75% chance of completing their recovery without major complications, but a 25% probability that they may become critically ill and require intensive care unit (ICU) treatment or even ending with death [3]. COVID-19 can cause a wide variety of symptoms and signs, from no symptoms at all to life-threatening pneumonia and respiratory failure causing acute respiratory distress syndrome (ARDS), leading to a high need for supplemental oxygen. It is possible for the disease to progress from its first phase of viral replication into a second phase driven by the host inflammatory response, resulting in the rapid onset of severe respiratory failure. Typical radiological data implies that infection with coronavirus 2 (SARS-CoV-2) may trigger a hyperimmune response associated with acute respiratory distress syndrome. Patients in the worst shape can experience something called a “cytokine release storm” [4].
Cytokine release syndrome (CRS) has been reported to cause COVID-19 related morbidity and mortality. CRS is due to the excess release of proinflammatory cytokines, especially Interleukin-6 (IL-6) [5]. IL-6 causes respiratory dysfunction including severe alveolar damage, alveolar-capillary leakage, and impaired blood–gas exchange (especially oxygen diffusion) [6]. Recent IL-6 antagonists, especially tocilizumab, have been repurposed for the remission of CRS in COVID-19 patients as adjunctive therapy where multiple case series have suggested a potential role for tocilizumab.
Tocilizumab (TCZ) is a monoclonal antibody against the IL-6 receptor that has found widespread application in the treatment of rheumatic illnesses and severe Chimeric Antigen Receptor (CAR) T cell-induced CRS around the world. Treatment of critically ill patients with COVID-19 may benefit from TCZ because of its potent influence on the inflammatory response brought on by the virus. However, it is important to use TCZ at the right points in the treatment plan [7].
There was large variation in the results of the previous studies that range from effect on mortality, time to clinical improvement, and prevention of progression to a more severe form of the disease [8]. This indicates that the standardization of the tocilizumab dose or the stage of the disease at which the drug is administered have not been well established, as well as the type of patients that will benefit from this drug. Other factors that may contribute in the pathophysiology of the disease are the patient’s characteristics and the presence of different SARS-CoV-2 strains [9]. By providing an estimate of the immunomodulatory agent’s possible effects, and for the high cost of tocilizumab, which in turn may be a burden on the healthcare system, observational studies may aid in the design of randomized clinical trials for the remission of critically-ill COVID-19 patients [10]. Thus, we aim to assess the efficacy of tocilizumab in the remission of COVID-19 patients hospitalized in the ICU. We also aim to assess the effect of tocilizumab on the length of the hospital stays and the result of co-administration of tocilizumab with other treatments.
2. Methods
2.1. Study Design and Seting
From January 2021 to June 2021, a multi-center retrospective cohort study was conducted on the intensive care units of six hospitals in Giza Governorate, Egypt. Among these, Al Tahrir, Om Elmasryeen, and Al Hawamdyia are the three General Hospitals, and the Central Hospitals include Al Warraq, Six of October, and Elsheikh Zayed. The Declaration of Helsinki and World Health Organization recommendations were all adhered to throughout the current study. The research protocol has been reviewed and approved by the “Research Ethics Committee” of the Central Directorate for Research and Health Development in MOHP (REC No. 23-2021/14).
2.2. Subjects
The study included all patients with a diagnosis of moderate to severe COVID-19 pneumonia who were admitted to the intensive care units of Giza hospitals. The study excluded patients with mild COVID-19, patients under the age of 18, patients who died within 48 h of being admitted, pregnant women, patients who were breastfeeding, and patients with mild COVID-19 symptoms.
2.3. Sampling Technique and Method of Selection
Consecutive sampling techniques were applied. Certain hospitals in Giza Governorate were selected to collect data from the patient records. All patients of each hospital who fulfilled the inclusion criteria were chosen. Data of 112 tocilizumab administrated patients were collected, and 2 of them were excluded for not fulfilling the criteria. On the other hand, 1257 data records were obtained from patients who were taking the routine protocol. Only 630 were included in the study, which was due to duplication of entry, entry errors, not fulfilling inclusion criteria, and incomplete data records.
2.4. Data Collection Tools
We used a predesigned structured questionnaire to collect the following data from the hospitals. The questionnaire was organized as follows: (Form A) covered patient identifiers, age, sex, coexisting conditions, investigations performed at the time of admission, treatments received in the hospitals and patients’ outcomes. (Form B) covered TCZ-related conditions: Criteria of severity of COVID-19 associated cytokine storm syndrome, contraindication, and side effects of TCZ.
2.5. Treatment Protocol and Outcomes
COVID-19 patients were tested using a COVID-19 laboratory panel consisting of C-reactive protein (CRP), ferritin, d-dimer, lactate dehydrogenase, and troponin I, as per institutional procedure. Tocilizumab treatment was given to patients who met the following requirements: symptoms of respiratory compromise including tachypnea, dyspnea, OR peripheral capillary oxygen saturation (SpO2) 90% on at least 4 L of oxygen OR rising oxygen requirements over 24 h, PLUS 2 or more of the following predictors for severe disease: C-reactive protein level higher than 35 mg/L, a ferritin level over 500 ng/mL, a D-dimer level over 1 mcg/L, a neutrophil-lymphocyte ratio over 4, or a lactate dehydrogenase level over 200 U/L.
All patients received a single IV infusion of 400 mg of tocilizumab as part of the therapy protocol. After tocilizumab delivery, antimicrobial prophylaxis was not consistently administered [11]. The patients’ clinical status was evaluated as the main outcomes, including being alive at discharge and death rate, and the secondary outcome including time to hospital discharge and predictors of mortality were also analyzed in the study.
2.6. Statistical Analysis
Data management and statistical analysis were performed using the Statistical Package for Social Sciences (SPSS) version 24(SPSS Inc, Chicago, IL). After being checked for accuracy, the obtained data were recoded, entered into a database, and statistically evaluated using the proper statistical tools and tests. Means, standard deviations, or medians and ranges were used to summarize numerical data. The Shapiro–Wilk test and Kolmogorov–Smirnov test were used to determine the data normality. Percentages were used to summarize categorical data. In quantitative variables, comparisons between patient groups were made using the independent t-test. Fisher’s exact tests and χ2 (chi square) tests were used to assess differences for categorical variables. A multivariate logistic regression was used to evaluate factors affecting primary outcomes. Kaplan–Meier curve and log rank test were used for survival analysis. p-values are always two-sided. p-values below 0.05 were deemed significant, and the Bonferroni technique was used to account for multiplicity.
3. Results
3.1. Baseline Characteristics of the Study Population
Out of the 740 patients recruited in the study, 630 patients followed the routine COVID-19 protocol while 110 patients received TCZ. The TCZ group had a median age of 58.5, whereas the control group’s median age was 63. Male patients accounted for a slightly larger proportion of the patient number in both groups, at 68 (61.8%) and 283 (44.9%). Regarding the severity of the disease, the proportions of patients with moderate severity were 69 (62.7%), 441 (70%) and severe illness were 41 (37.3%), 189 (30%) in the TCZ and control, respectively, without any statistically significant difference.
For all comorbidities, there was no significant difference between the TCZ and the control group except for cardiovascular diseases (p = 0.009), as shown in Table 1. Indeed, D dimer, creatinine, complete blood count, lymphopenia, lymphocytosis, thrombocytopenia, thrombocytosis, leukopenia, and leukocytosis all showed no statistically significant differences between the TCZ group and the control group, as illustrated in Table 2. In addition, all the recruited patients received standard treatments during hospitalization according to the Egyptian protocol for COVID-19 management, The different treatment groups were illustrated in Table 3. Additionally, at hospital admission, the two groups’ CT scan results, including ground glass opacity, infiltrations, and patchy shadowing, were compared, as indicated in Table 4.
Table 1.
Baseline clinical variables of the study population.
| Clinical Variables | Tocilizumab (n = 110) (1) | Control (n = 630) (1) | p Value (2) | |
|---|---|---|---|---|
| Sex | Male | 68 (61.8%) | 283 (44.9%) | 0.01 |
| Female | 42 (38.2%) | 347 (55.1%) | ||
| Age | 58.5 (17.5) | 63(17) | 0.127 | |
| COVID Severity | Moderate | 69 (62.7%) | 441 (70%) | 0.128 |
| Severe | 41 (37.3%) | 189 (30%) | ||
| Comorbidities | Diabetes Mellitus | 60 (54.5%) | 316 (50.2%) | 0.396 |
| Cardiovascular Diseases | 17 (15.5%) | 171 (27.1%) | 0.009 | |
| Hypertension | 54 (49.1%) | 336 (53.3%) | 0.411 | |
| Chronic liver Diseases | 6 (5.5%) | 36 (5.7%) | 0.913 | |
| Chronic Kidney Diseases | 3 (2.7%) | 50 (7.9%) | 0.051 | |
| Asthma | 14 (12.7%) | 80 (12.7%) | 0.993 | |
| Smoking | 6 (5.5%) | 29 (4.6%) | 0.0698 | |
(1) Median (IQR); n (%); (2) Mann Whitney test; Pearson’s Chi-squared test.
Table 2.
Baseline laboratory variables of the study population.
| Laboratory Variables | Tocilizumab (n = 110) (1) | Control (n = 630) (1) | p Value (2) |
|---|---|---|---|
| C-reactive protein (CRP) | 70 (41–74) | 44.5 (40–48.9) | <0.001 |
| Ferritin | 882 (535–882) | 681 (625–681) | <0.001 |
| D Dimer | 151 (77–249) | 151 (95–204) | 0.705 |
| Lactate dehydrogenase (LDH) | 668.3 (621–759) | 707.5 (694–720) | <0.001 |
| Aspartate transaminase (AST) | 43.5 (39–44) | 47.5 (33–48) | 0.001 |
| Alanine transaminase (ALT) | 39.5 (30–40) | 52 (28–52) | <0.001 |
| Urea | 50.5 (34.75–63) | 64 (44–76) | 0.001 |
| Creatinine | 1.2 (−1–2) | 1.2 (1–2) | 0.073 |
| Complete blood count (CBC) Normal | 23 (20.9%) | 124 (19.7%) | 0.766 |
| Monocytosis | 29 (26.4%) | 95 (15.1%) | 0.003 |
| Lymphopenia | 69 (62.7%) | 334 (53%) | 0.059 |
| lymphocytosis | 8 (7.3%) | 32 (5.1%) | 0.348 |
| Neutrophilia | 51 (46.4%) | 214 (34%) | 0.012 |
| Neutropenia | 8 (7.3%) | 12 (1.9%) | 0.005 F |
| Thrombocytopenia | 18 (16.4%) | 107(17%) | 0.873 |
| Thrombocytosis | 4 (3.6%) | 30 (4.8%) | 0.603 |
| Leukopenia | 6 (5.5%) | 24 (3.8%) | 0.603 F |
| Leukocytosis | 48 (43.6%) | 229 (36.3%) | 0.145 |
(1) Median (IQR); n (%); (2) Mann–Whitney test; Pearson’s Chi-squared test; F = Fisher’s exact test.
Table 3.
Medication administered during hospitalization to the study population.
| Medication | Tocilizumab (n = 110) (1) | Control (n = 630) | p Value (2) |
|---|---|---|---|
| Antivirals | 34 (30.9%) | 245 (38.9%) | 0.111 C |
| Hydroxychloroquine | 38 (34.5%) | 48 (7.2%) | 0.001 C |
| Ivermectin | 77 (70%) | 368 (58%) | 0.022 C |
| Anticoagulants | 110 (100%) | 609 (96%) | 0.058 F |
| Corticosteroids | 110 (100%) | 588 (93%). | 0.002 F |
| Convalescent Plasma | 3 (2.7%) | 3 (0.5%) | 0.046 F |
| Antibiotics | 107 (97.3%) | 621 (98%) | 0.401 F |
| Antifungals | 0 (0%) | 35 (5.6%) | 0.006 F |
(1) n (%); (2) F = Fisher’s exact test; C = Pearson’s Chi-squared test.
Table 4.
Clinical investigations at hospital admission of the study population.
| Clinical Investigations | Tocilizumab (n = 110) (1) |
Control (n = 630) (1) |
p Value (2) | |
|---|---|---|---|---|
| CT Scan Findings | Ground glass opacity | 53 (48.2%) | 352 (55.9%) | 0.001 F |
| Infiltrations | 2 (1%) | 19 (3%) | ||
| Major abnormality | 14 (12.7%) | 80 (12.7%) | ||
| Mixed ground glass opacity and Infiltrations | 37 (33.6%) | 66 (10.5%) | ||
| Patchy shadowing | 4 (3.6%) | 113 (17.9%) |
(1) n (%); (2) F = Fisher’s exact test.
3.2. Effect on the Clinical Outcomes
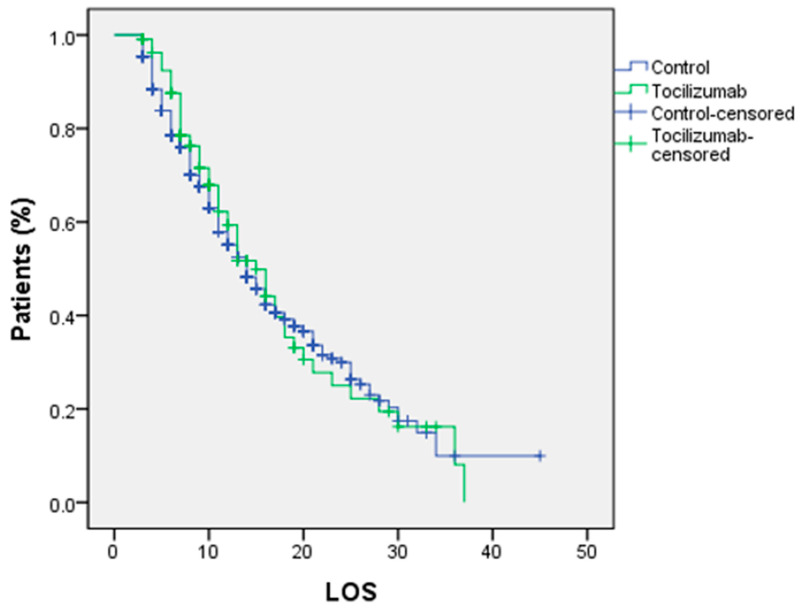
The primary outcome (death rate) shows a higher rate in the TCZ group without any statistically significant difference with the control group (p = 0.297), while the secondary outcome (length of hospital stay) shows a significant difference in favor of the control group (p = 0.045) as shown in Table 5. Furthermore, a comparison of LOS between the two groups using a Kaplan–Meier curve and a log rank test shows no statistically significant difference (p = 0.6690), as shown in Figure 1. Regarding the correlation between patients who required respiratory support and who were either discharged alive or died, it was shown that invasive and noninvasive ventilation had a greater death rate than other respiratory support systems, as indicated in Table 6.
Table 5.
Clinical outcomes evaluation between the two groups.
| Outcome | Tocilizumab (n = 110) (1) |
Control (n = 630) (1) |
p Value (2) |
|---|---|---|---|
| Primary Outcome | |||
| Death (Mortality) | 59 (53.6%) | 304 (48.3%) | 0.297 |
| Discharged alive | 51 (46.4%) | 326 (51.7%) | |
| Secondary Outcome | |||
| Length of stay (Days) (3) | 10 (7–15) | 8 (5–14) | 0.045 |
(1) n (%); (2) Pearson’s Chi-squared test, (3) Median (IQR); Mann–Whitney test.
Figure 1.
Kaplan–Meier curve of the survival rate among patients of the study groups (log rank test p < 0.669).
Table 6.
Distribution of respiratory support at the time of hospitalization regarding the primary outcome.
| Respiratory Support | Death (n = 363) (1) |
Discharged Alive (n = 377) (1) |
p Value (2) |
|---|---|---|---|
| Ordinary simple nasal cannula | 6 (1.7%) | 36 (9.5%) | <0.001 |
| Simple mask | 30 (8.3%) | 151 (40.1%) | |
| Mask with reservoir (non-rebreathing mask) | 115 (31.7%) | 93 (24.7%) | |
| High flow nasal oxygen | 17 (4.7%) | 44 (11.7%) | |
| Noninvasive ventilation, Continuous positive airway pressure (CPAP) | 125 (34.4%) | 47 (12.5%) | |
| Invasive ventilation, Mechanical ventilation (MV) | 70 (19.3%) | 6 (1.6%) |
(1) n (%); (2) Pearson’s Chi-squared test.
A multivariant logistic regression analysis revealed that the only factors associated with mortality were COVID-19 severity (OR = 1.536, C.I. = 1.022–2.309, p = 0.039), CT scan findings (OR = 1.562, C.I. = 0.434–5.627, p = 0.045), Thrombocytosis (OR = 2.833, C.I. = 1.053–7.627, p = 0.039), Ivermectin (OR = 1.605, C.I. = 1.093–2.356, p = 0.016) Convalescent plasma (OR = 14.838, C.I. = 1.256–17.298, p = 0.032), Antifungals (OR = 0.242, C.I. = 0.090–0.650, p = 0.005), and Respiratory support (OR = 0.422, C.I. = 0.360–0.495, p = 0.001), as shown in Table 7.
Table 7.
Multivariant logistic regression analysis for factors affecting mortality.
| Risk Factor | Odd Ratio | 95% CI | p-Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Sex | 1.358 | 0.926 | 1.990 | 0.117 |
| Smoking | 0.725 | 0.311 | 1.690 | 0.456 |
| Diabetes | 1.065 | 0.726 | 1.561 | 0.749 |
| Cardiovascular diseases | 0.928 | 0.601 | 1.434 | 0.737 |
| Hypertension | 1.500 | 0.997 | 2.257 | 0.052 |
| Chronic liver disease | 1.183 | 0.559 | 2.505 | 0.661 |
| Creatine kinase (CK) | 0.570 | 0.254 | 1.281 | 0.174 |
| Asthma | 0.896 | 0.516 | 1.557 | 0.698 |
| COVID Severity | 1.536 | 1.022 | 2.309 | 0.039 * |
| CT scan findings | 1.562 | 0.434 | 5.627 | 0.045 * |
| CBC Normal | 1.625 | 0.985 | 2.682 | 0.058 |
| Monocytosis | 0.841 | 0.507 | 1.395 | 0.502 |
| Lymphopenia | 0.832 | 0.541 | 1.280 | 0.403 |
| Lymphocytosis | 0.502 | 0.213 | 1.181 | 0.114 |
| Neutrophilia | 1.120 | 0.721 | 1.739 | 0.614 |
| Neutropenia | 0.958 | 0.323 | 2.839 | 0.938 |
| Thrombocytopenia | 1.210 | 0.745 | 1.967 | 0.441 |
| Thrombocytosis | 2.833 | 1.053 | 7.627 | 0.039 * |
| Leukopenia | 0.791 | 0.319 | 1.962 | 0.613 |
| Leukocytosis | 0.659 | 0.426 | 1.019 | 0.061 |
| Antivirals | 0.739 | 0.501 | 1.089 | 0.126 |
| Hydroxychloroquine | 0.940 | 0.514 | 1.719 | 0.841 |
| Ivermectin | 1.605 | 1.093 | 2.356 | 0.016 * |
| Anticoagulants | 0.542 | 0.172 | 1.710 | 0.297 |
| Corticosteroids | 1.826 | 0.808 | 4.129 | 0.148 |
| Convalescent plasma | 14.838 | 1.256 | 17.298 | 0.032 * |
| Antibiotics | 0.687 | 0.161 | 2.935 | 0.612 |
| Antifungals | 0.242 | 0.090 | 0.650 | 0.005 * |
| Respiratory support | 0.422 | 0.360 | 0.495 | 0.000 * |
| Tocilizumab | 0.764 | 0.446 | 1.308 | 0.326 |
* p-Value ≤ 0.05 is statistically significant.
4. Discussion
Cytokine storm was thought to be a significant factor in COVID-19 exacerbation and even death, since it can cause immunological dysregulation, lung tissue damage, hypoxia, and even respiratory failure [12,13]. Tocilizumab was reported to regulate the cytokine storm and inflammation in COVID-19 [14,15].
Even though our study did not find any advantages of tocilizumab over the conventional treatment in terms of mortality or length of hospital stay, other observational studies have shown better outcomes. The two largest observational studies to date on this topic have demonstrated an association between tocilizumab use and decreased mortality [16,17]. Guaraldi et al. evaluated the impact of tocilizumab regardless of the time of administration on a cohort of 1351 COVID-19 patients [16]. However, invasive ventilation or death was the composite endpoint, and patients admitted to the intensive care unit were not included. In the STOP-COVID tocilizumab study, which comprised 3924 severely ill COVID-19 patients admitted to ICUs, patients who received tocilizumab within the first two days of admission had a lower mortality risk than those who did not get tocilizumab within the first two days of admission [17]. In contrast to these two trials, the largest cohort study of 544 COVID-19 patients, carried out by Cardona-Pascual et al., supports our findings that tocilizumab treatment did not lower the risk of mortality in patients with moderate to severe COVID-19 [18]. However, ICU stay in tocilizumab group was found to be shorter than in the control group. In the observational studies stated above, different dosages (single or double) and subpopulations were investigated (moderate or severe or critically ill patients).
According to our knowledge, this study is the first large multicenter cohort observational study in Egypt to examine the role of tocilizumab in COVID-19 infection. We enrolled 740 COVID-19 patients, and the findings showed that there was no statistically significant difference in mortality between the tocilizumab group and the control group.
In particular, recent RCTs have not demonstrated a decrease in mortality in COVID-19 patients receiving tocilizumab [19,20,21]. Regardless of the time of administration, the number of tocilizumab doses administered, or the subset of critically ill patients admitted to the ICU, our results are consistent with those from the RCTs and demonstrate no benefit in the mortality in COVID-19 patients treated with tocilizumab. Our findings may also be consistent with earlier RCT and observational studies. A total of 51.7% of patients in the control group and 46.4% of those who received tocilizumab had been discharged from the hospital alive. Our discharge rate was similar to the study conducted by Somers et al. [22]. Indeed, we reported a high mortality rate of 53.6% compared to the control group, which is consistent with a prior study that assessed the association between tocilizumab and mortality in COVID-19 patients conducted by Campochiaro et al., who found no appreciable change in the mortality in tocilizumab-treated patients [23]. On the other hand, tocilizumab has been associated with a reduced risk of death and hospital-related mortality, according to a number of other studies [16,17,24,25,26,27,28,29].
Overall, results from different studies on the mortality rate associated with tocilizumab treatment in COVID-19 patients are contradictory, most likely as a result of disparate study designs and clinical severity classification heterogeneity. It should be noted that comparisons between observational studies are challenging due to variations in baseline disease classification heterogeneity, participant clinical severity heterogeneity, non-standardized timing, dosage, and administration of tocilizumab, low statistical power resulting from a small participant pool, lack of standardized care in the control groups, and a lack of corticosteroid effect evaluation [30]. Indeed, we contrasted the respiratory support that we offered to hospitalized patients at all participating hospitals between those who were discharged and those who sadly died. It was observed that non-rebreathing oxygen support devices, CPAP, and mechanical ventilation (MV) had the highest mortality rates compared to simple oxygen masks and nasal canula.
The incidence of serious infections is the greatest concern with tocilizumab therapy [31]. In this study, patients in the tocilizumab group required a median hospital stay of 10 days compared to 8 days in the control group. This difference can be attributed to the secondary bacterial infections, which necessitate additional antibiotic medication and decrease patient oxygenation.
The multicenter design of this trial, careful data quality monitoring, and a homogeneous target population of patients with moderate and severe pneumonia are the strengths of the study.
5. Conclusions
Our cohort of patients with moderate and severe COVID-19 did not assert a reduction in the risk of mortality or the length of stay (LOS). To evaluate the potential role of tocilizumab in selected patients such as ICU patients, additional RCTs are required.
6. Limitations
Even though we imputed missing data using modern techniques, the laboratory variables had some incomplete data. IL-6 serum concentrations were not frequently available; thus, this study did not evaluate their potential utility or how they might have predicted a patient’s response to tocilizumab.
Additionally, in the retrospective design of the research there were some possible confounders from the concomitant application of multiple interventions at the same time of TCZ administration. So, the results need confirmation by randomized controlled trials.
Acknowledgments
We would like to extend our cordial appreciation to all medical staff, who were the first line of defense in this pandemic, and the clinical research teams in all hospitals for their hard and sincere work in collecting the data. We would like to express our deep gratitude to the Central Directorate for Research and Health Development in MOHP (the Ministry of Health and Population) for their support during the entirety of this work.
Author Contributions
H.Y.I. designed the research; D.H.A. analyzed the data; R.M.Y., M.H.A., H.A.G., R.R.M. and M.A.Y.N.E. performed the research; A.E.A.W. and R.A.F. wrote and revised the research; F.O.A., A.I.A. and A.S.A. funding acquisition; N.M.E. and A.S.R. performed the supervision, drafted of the article, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All procedures involved in the present study were carried out in conjunction with good clinical practice, the Declaration of Helsinki, and World Health Organization Guidelines. The “Research Ethics Committee” in the Central Directorate for Research and Health Development in MOHP (REC No. 23-2021/14) reviewed and accepted the research protocol.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study also Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Applicable upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the Deanship of Scientific Research at Jouf University under grant No. DSR-2021-01-031871.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Farahat R.A., Baklola M., Umar T.P. Omicron B.1.1.529 subvariant: Brief evidence and future prospects. Ann. Med. Surg. 2022;83:104808. doi: 10.1016/j.amsu.2022.104808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaalan M., Abou Warda A.E., Osman S.M., Fathy S., Sarhan R.M., Boshra M.S., Sarhan N., Gaber S., Ali A.M.A. The Impact of Sociodemographic, Nutritional, and Health Factors on the Incidence and Complications of COVID-19 in Egypt: A Cross-Sectional Study. Viruses. 2022;14:448. doi: 10.3390/v14030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe COVID-19. N. Engl. J.Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 6.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price C.C., Altice F.L., Shyr Y., Koff A., Pischel L., Goshua G., Azar M.M., Mcmanus D., Chen S.-C., Gleeson S.E., et al. Tocilizumab Treatment for Cytokine Release Syndrome in Hospitalized Patients With Coronavirus Disease 2019: Survival and Clinical Outcomes. Chest. 2020;158:1397–1408. doi: 10.1016/j.chest.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durán-Méndez A., Aguilar-Arroyo A.D., Vivanco-Gómez E., Nieto-Ortega E., Pérez-Ortega D., Jiménez-Pérez C., Hernández-Skewes K.Y., Montiel-Bravo G., Roque-Reyes O.J., Romero-Lechuga F., et al. Tocilizumab reduces COVID-19? J. Transl. Med. 2020;18:1–5. [Google Scholar]
- 9.DeMerle K., Angus D.C., Seymour C.W. Precision medicine for COVID-19: Phenotype anarchy or promise realized? JAMA. 2021;325:2041–2042. doi: 10.1001/jama.2021.5248. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Baño J., Pachón J., Carratalà J., Ryan P., Jarrín I., Yllescas M., Arribas J.R., Berenguer J., Muñoz E.A., Gil Divasson P., et al. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: A multicentre cohort study (SAM-COVID-19) Clin. Microbiol. Infect. 2021;27:244–252. doi: 10.1016/j.cmi.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang E., Isonaka S., Yang H., Salce E., Rosales E., Jordan S.C. Tocilizumab treatment in critically ill patients with COVID-19: A retrospective observational study. Int. J. Infect. Dis. 2021;105:245–251. doi: 10.1016/j.ijid.2021.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seguin A., Galicier L., Boutboul D., Lemiale V., Azoulay E. Pulmonary involvement in patients with hemophagocytic lymphohistiocytosis. Chest. 2016;149:1294–1301. doi: 10.1016/j.chest.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Crisafulli S., Isgrò V., La Corte L., Atzeni F., Trifirò G. Potential role of anti-interleukin (IL)-6 drugs in the treatment of COVID-19: Rationale, clinical evidence and risks. BioDrugs. 2020;34:415–422. doi: 10.1007/s40259-020-00430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19 mortality and pathology in a dose and timing-dependent fashion: A multi-centric study. Sci. Rep. 2021;11:19728. doi: 10.1038/s41598-021-99291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., Franceschini E., Cuomo G., Orlando G., Borghi V., et al. Tocilizumab in patients with severe COVID-19: A retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta S., Wang W., Hayek S.S., Chan L., Mathews K.S., Melamed M.L., Brenner S.K., Leonberg-Yoo A., Schenck E.J., Radbel J., et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern. Med. 2021;181:41–51. doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardona-Pascual I., Berlana D., Martinez-Valle F., Campany-Herrero D., Montoro-Ronsano J.B. Effect of tocilizumab versus standard of care in adults hospitalized with moderate-severe COVID-19 pneumonia. Med. Clínica. 2022;158:301–307. doi: 10.1016/j.medcli.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermine O., Mariette X., Tharaux P.L., Resche-Rigon M., Porcher R., Ravaud P., CORIMUNO-19 Collaborative Group Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: A randomized clinical trial. JAMA Intern. Med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L., Bruzzi P., Boni F., Braglia L., Turrà C., et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: A randomized clinical trial. JAMA Intern. Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., Horick N.K., Healy B.C., Shah R., Bensaci A.M., et al. Efficacy of tocilizumab in patients hospitalized with COVID-19. N. Engl. J. Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Somers E.C., Eschenauer G.A., Troost J.P., Golob J.L., Gandhi T.N., Wang L., Zhou N., Petty L.A., Baang J.H., Dillman N.O., et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin. Infect. Dis. 2021;73:e445–e454. doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campochiaro C., Della-Torre E., Cavalli G., De Luca G., Ripa M., Boffini N., Tomelleri A., Baldissera E., Rovere-Querini P., Ruggeri A., et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: A single-centre retrospective cohort study. Eur. J. Intern. Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biran N., Ip A., Ahn J., Go R.C., Wang S., Mathura S., Sinclaire B.A., Bednarz U., Marafelias M., Hansen E., et al. Tocilizumab among patients with COVID-19 in the intensive care unit: A multicentre observational study. Lancet Rheumatol. 2020;2:e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martínez-Sanz J., Muriel A., Ron R., Herrera S., Pérez-Molina J.A., Moreno S., Serrano-Villar S. Effects of tocilizumab on mortality in hospitalized patients with COVID-19: A multicentre cohort study. Clin. Microbiol. Infect. 2021;27:238–243. doi: 10.1016/j.cmi.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarhan R.M., Madney Y.M., Abou Warda A.E., Boshra M.S. Therapeutic efficacy, mechanical ventilation, length of hospital stay, and mortality rate in severe COVID-19 patients treated with tocilizumab. Int. J. Clin. Pract. 2021;75:14079. doi: 10.1111/ijcp.14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russo G., Solimini A., Zuccalà P., Zingaropoli M.A., Carraro A., Pasculli P., Perri V., Marocco R., Kertusha B., Del Borgo C., et al. Real-life use of tocilizumab with or without corticosteroid in hospitalized patients with moderate-to-severe COVID-19 pneumonia: A retrospective cohort study. PLoS ONE. 2021;16:e0257376. doi: 10.1371/journal.pone.0257376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pawar A., Rishi J., Daniel H., Adrian J.S., Gale S., Bao M., Sarsour K., Schneeweiss S., Kim S.C. Risk of serious infections in tocilizumab versus other biologic drugs in patients with rheumatoid arthritis: A multidatabase cohort study. Ann. Rheum. Dis. 2019;78:456–464. doi: 10.1136/annrheumdis-2018-214367. [DOI] [PubMed] [Google Scholar]
- 28.Sarhan R.M., Harb H.S., Warda A.E.A., Salem-Bekhit M.M., Shakeel F., Alzahrani S.A., Madney Y.M., Boshra M.S. Efficacy of the early treatment with tocilizumab-hydroxychloroquine and tocilizumab-remdesivir in severe COVID-19 Patients. J. Infect. Public Health. 2022;15:116–122. doi: 10.1016/j.jiph.2021.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabbani R., Hasan J., Anam A.M., Huq S.M.R. Impact of three consecutive intravenous doses of tocilizumab in severe COVID-19 pneumonia: A retrospective cohort study. Microbes Infect. Chemother. 2021;1:e1251. doi: 10.54034/mic.e1251. [DOI] [Google Scholar]
- 30.Jones G., Ding C. Chapter 71—Tocilizumab (Actemra®) In: Dübel S., Reichert J.M., editors. Handbook of Therapeutic Antibodies. Wiley; New York, NY, USA: 2014. [DOI] [Google Scholar]
- 31.Akdeniz S., Sen A. Effect of tocilizumab treatment for COVID-19-induced acute respiratory distress syndrome (ARDS) on renal function of patients. Trop. J. Pharm. Res. 2022;21:629–634. doi: 10.4314/tjpr.v21i3.24. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Applicable upon request.