Abstract
Vaccination against the SARS-CoV-2 virus (COVID-19) has proven to be the most effective measure to prevent the spread and reduce infection severity. A case report of de novo membranous nephropathy (MN) following immunization with inactivated virus vaccine (CoronaVac®, Sinovac Biotech) is presented here. A 53-year-old man presented with a sudden onset of leg edema a week after receiving an inactivated virus vaccine and a relapse of nephrotic syndrome (NS) with acute kidney injury (AKI) after a booster dose. Screening for serum anti-phospholipase A2 receptor antibody and secondary causes of MN were negative. Kidney biopsy revealed an early MN pattern with focal spike formation, whilst numerous subepithelial electron-dense deposits and a few small deposits in the mesangial area were observed through electron microscopy. A short course of steroids and oral cyclophosphamide was prescribed, resulting in the complete remission of NS and AKI. MN following SARS-CoV-2 vaccination should call for medical importance. Awareness of the association between vaccination and MN should be kept in mind to avoid unnecessary treatment with long-term immunosuppressive agents.
Keywords: acute kidney injury, inactivated SARS-CoV-2 virus vaccination, membranous nephropathy, nephrotic syndrome
1. Background
Since the outbreak of the SARS-CoV-2 pandemic, the need for the SARS-CoV-2 vaccine has increased to alleviate the number of infections and reduce the severity of the disease [1]. Many studies have demonstrated the efficacy and safety of SARS-CoV-2 vaccines [2]. Four types of COVID-19 vaccines are being used worldwide including messenger RNA (mRNA), viral vector, protein subunit, and whole virus vaccines (known as inactivated virus vaccines). The inactivated virus vaccine elicits weaker immunogenicity and lowers clinical protection than the mRNA-based vaccine [3,4,5,6]. The inactivated viral vaccine (CoronaVac®, Sinovac Biotech) is produced by beta-propiolactone-inactivation of the CN2 strain of SARS-CoV-2 isolated from the patient’s bronchoalveolar lavage, which is closely linked to the 2019-nCoV-BetaCoV Wuhan/WIV04/2019 [1]. Results of phase 3 trials have shown that the inactivated viral vaccine has a high efficacy rate of 51–84% and a good safety profile [7,8,9]. Glomerular diseases following SARS-CoV-2 vaccines have been periodically reported including minimal change disease (MCD) and IgA nephropathy [10,11]. However, only a few cases of de novo or relapsed MN following inactivated viral vaccination have been documented [12,13]. A de novo MN with possible relapse stimulated by the second vaccination is reported here.
2. Case Presentation
A 53-year-old male patient presented with intermittent lower extremity edema and foamy urine for 2 weeks, which was spontaneously resolved. He received his first dose of the inactivated SARS-CoV-2 (CoronaVac®, Sinovac Biotech) vaccine a week before the onset of symptoms. He then had his second immunization after 4 weeks from the first dose. He experienced a sudden onset of leg and scrotal edema and puffy eyelids the next day after completing his primary vaccination series. He had abdominal discomfort and gained 5 kg of weight in one week. He denied any symptoms of gross hematuria, headache, or oliguria. Prior to admission, the SARS-CoV-2 virus was not detected by RT-PCR from the patient’s nasopharyngeal swab sample.
On physical examination, the vital signs were as follows: blood pressure 150/90 mmHg and heart rate 68 beats/min. His body mass index was 28.3 kg/m2. He had puffy eyelids without paleness and jaundice. His abdomen was distended with positive shifting dullness. Bilateral leg pitting edema and scrotal edema were also observed. His laboratory tests yielded serum creatinine 1.5 mg/dL, serum urea nitrogen 29 mg/dL, albumin 2.3 g/dL, cholesterol 507 mg/dL, and triglyceride 255 mg/dL. His urinary protein and erythrocytes levels were 3+ and 2+, respectively, and urine sediment depicted 3–5 per high-power field of red blood cells. His urine protein to creatinine ratio was 13.4 g protein per gram of creatinine. Tests for treponemal, HBsAg, anti-HCV, anti-HIV, and antinuclear antibodies were negative. Complement components C3 and C4 were within the normal values. Ultrasonography of the entire abdomen revealed that both kidneys were normal in shape and echogenicity.
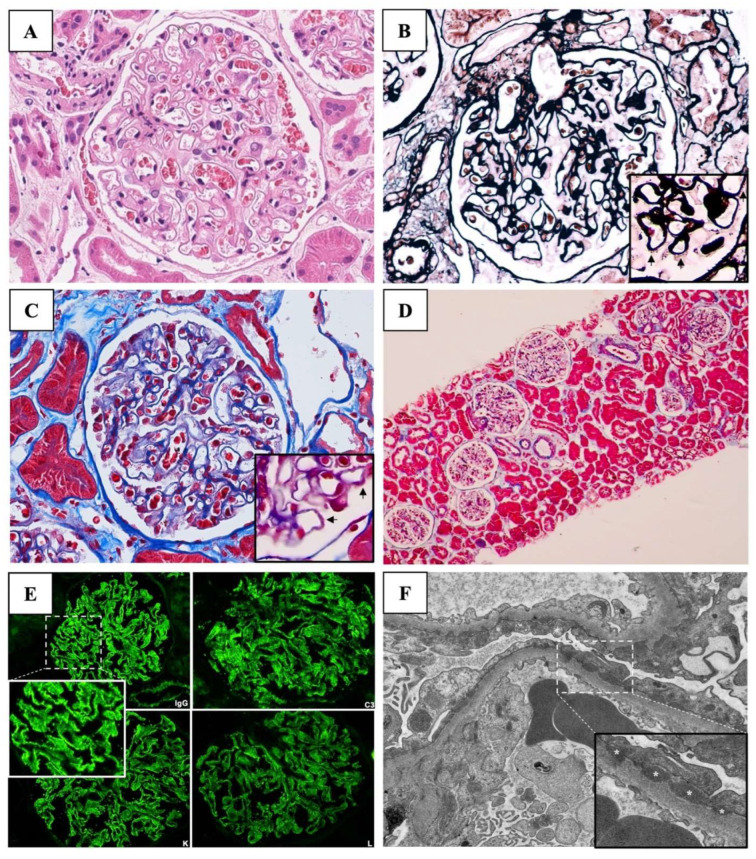
He was clinically diagnosed with an acute onset of nephrotic syndrome (NS) associated with acute kidney injury (AKI). A kidney biopsy was performed, followed by a prescription of daily oral prednisolone at a dosage of 70 mg per day. The kidney biopsy finding showed 20 glomeruli with a normal glomerular basement membrane thickness. No glomerular proliferation and sclerosis were observed. Focal spike formation and scant subepithelial fuchsinophilic granules were detected in the Jones silver and Masson Trichrome stains, respectively, indicating an early stage of membranous pattern on light microscopy (LM). Diffuse interstitial edema was evidenced without accompanying tubular injury and interstitial inflammation. Immunofluorescence staining of 10 glomeruli showed diffuse granular deposition of the IgG (3+), C3 (3+), Kappa, and Lambda light chains (2+) along the capillary wall. MN was diagnosed (Figure 1).
Figure 1.
Kidney biopsy findings. (A) Light microscopy of the glomerulus with Hematoxylin and Eosin (original magnification, ×200) revealed normal glomeruli. (B) Jones silver stain (original magnification, ×200) showed focal spike formation on the glomerular basement membranes (arrows). (C) Masson trichrome stain (original magnification, ×200) depicts the subepithelial fuchsinophilic granules (arrows). (D) Masson trichrome stain (original magnification, ×4) revealed diffuse interstitial edema without accompanying tubular injury and interstitial inflammation. (E) Direct immunofluorescent studies demonstrate diffuse granular deposition with strongly positive (3+) stains for IgG, C3, kappa, and lambda with negative stains of IgA, IgM, and C1q. (F) Electron microscopy (original magnification, ×5000) revealed numerous subepithelial electron-dense deposits (asterisks) and a few small deposits in mesangial, intramembranous, and subendothelial area with podocyte foot process effacement (magnification, ×10,000). * denotes electron-dense deposits under electron microscopy.
Since the patient was classified as very high risk according to the KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases (accompanying AKI not otherwise explained) [14], 3-day intravenous methylprednisolone (1 gm/day) was administered, followed by a 1.5-month course of oral prednisolone (0.5 mg/kg/day) and cyclophosphamide (2 mg/kg/day). In week 15, the electron microscopy (EM) report was issued denoting numerous subepithelial electron-dense deposits (EDDs) and a few small deposits in the mesangial, intramembranous, and subendothelial areas, suggesting secondary MN.
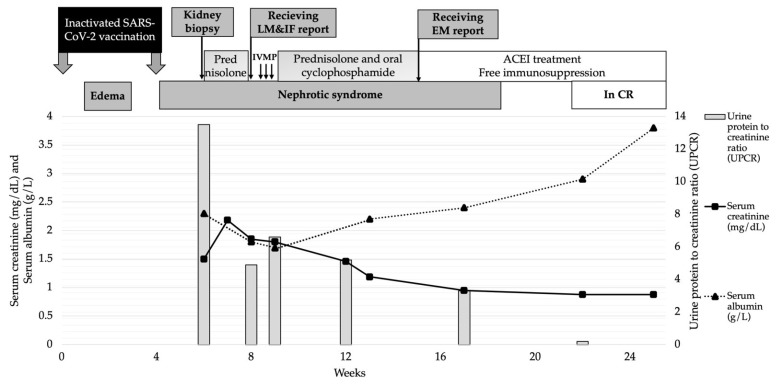
The serum anti-phospholipase A2 receptor (PLA2R) antibody (Ab) was negative. Other chronic infections and age-related malignancies were excluded. The suspicion of SARS-CoV-2 vaccine-induced secondary MN was raised; therefore, the steroid and immunosuppressive agent were replaced with angiotensin-converting enzyme inhibitors (ACEi). Complete remissions of proteinuria and abnormal kidney function were achieved after 2 weeks of discontinuation of the immunosuppression without subsequent relapses (Figure 2).
Figure 2.
Patient’s clinical course and treatment. Abbreviations: IVMP, intravenous methyl prednisolone; MKD, mg/kg/day; CR, complete remission.
3. Discussion
A case of de novo MN with a possible disease flare or worsening of the clinical expression stimulated by the second vaccination was presented here with a favorable outcome, albeit presenting with AKI. Secondary MN was suspected because of the negative serum PLA2R Ab and the presence of mesangial EDDs. Chronic infections and malignancies had been ruled out. The patient fully recovered after a short course of steroid and immunosuppressive therapy.
Several glomerular diseases have been reported following SARS-CoV-2 vaccination [10,11]. The most commonly reported glomerulonephritis (GN) is MCD [10,11,15]. In a recent systematic review of vaccine-induced kidney adverse reactions from 90 case report articles, 134 cases of de novo GN were identified including MCD (52/134, 39%), IgA nephropathy (48/134, 36%), ANCA-associated GN (16/134, 12%), and MN (8/134, 6%) [16].
The mechanism of vaccine-induced MN remains inconclusive [17]. There is a case report of de novo MN and a case series of MN flares/relapses in patients with autoimmune disease following influenza H1N1 vaccinations [17,18]. An immunological response to the vaccination is postulated as the pathogenesis of the MN [17]. MN has been reported in association with all categories of the SARS-CoV-2 vaccine [10,11,18]. However, the mRNA-based vaccine has the highest number of case reports with vaccine-associated GN compared to the other vaccines, which is consistent with the finding of the highest immunogenicity of the vaccine [19,20]. In the systematic review of vaccine-induced kidney adverse reactions, mRNA vaccines contributed to 84% of the vaccine-associated GN cases, followed by viral vector vaccines (13%) and whole virus vaccines (3%) [16].
Recent case reports of de novo MN following vaccination with BNT162b2 (Pfizer-BioNTech) and exacerbation of MN after mRNA-1273 (Moderna) appear to support that vaccination with SARS-CoV-2 triggers MN [21]. Although the mechanism underlying the association has not yet been well-understood, two possible mechanisms have been proposed to explain how the vaccine may contribute to MN including vaccine-triggering genetically prone primary MN and vaccine-induced cross-immune response (molecular mimicry) [17,22,23]. In addition, numerous autoantibody-driven temporal pathogenesis in SARS-CoV-2 vaccine-associated MN have been postulated including autoantibodies to PLA2R [10,11,12,24], neural epithelial growth factor like-1 (NELL-1) [10], and intracellular proteins exostosin 1 (EXT1) [11]. However, Caza et al. presented cases with negatives of all of these antibodies [11]. As in the presented case, anti-PLA2R Ab was negative.
Table 1 demonstrates the pooled clinical spectra of SARS-CoV-2 vaccine-associated MN from the published literature. MN can occur after the first or boosted vaccination in both native [10,11,12,13,21,22,24,25,26,27] and kidney transplant recipients [28]. Clinical manifestation of MN following SARS-CoV-2 virus vaccination is usually full-blown NS with abrupt onset varying from 1 day to 4 weeks (Table 1).
Table 1.
Clinical spectra and outcomes of membranous nephropathy following SARS-CoV-2 vaccination.
| No. | Study | Age | G | Vaccine Type | Dose | Onset | Serum Albumin (g/L) |
Serum Creatinine (mg/dL) |
Hematuria (/HPF) |
Proteinuria (g/g Creatinine) |
De Novo/ Relapse GN |
Type of MN | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | This case | 53 | M | Sinovac | 2nd | 1 d | 2.3 | 1.5 | 3–5 | 13.4 | De novo | Neg only PLA2R | GC, CY 3 mo. | Response |
| 2. | Aydin [12], 2021 |
66 | F | Sinovac | 1st | 2 wk | 2.6 | 2.78 | N/A | 9.42 | Relapse | PLA2R | N/A | N/A |
| 3. | Caza [11], 2021 | 54 | M | Moderna | 2nd | 1 d | 3.4 | 1.3 | Pos | 3+ | De novo | 1 PLA2R, 1 EXT, 1 neg PLA2R/ EXT |
GC Rituximab |
No response |
| 4. | 68 | M | J + J | 1st | <4 wk | 3.2 | 3.3 | Neg | 0.6 | De novo | Conservative | Partial response | ||
| 5. | 47 | M | Moderna | 2nd | 6 d | 2.3 | 0.7 | Pos | 2.7 | De novo | None | Partial response | ||
| 6. | Klomjit [10], 2021 |
50 | F | Pfizer | 2nd | 4 wk | 3.5 | 0.7 | 3–10 | 6.5 | De novo | NELL-1 | Conservative | Response |
| 7. | 39 | M | Pfizer | 2nd | 1 wk | 2 | 1.13 | 3–10 | 8.7 | Relapse | PLA2R | TAC | Response | |
| 8. | 70 | M | Moderna | 2nd | 4 wk | 2.7 | 2.1 | <3 | 16.6 | Relapse | PLA2R | Obinutuzumab | N/A | |
| 9. | Gueguen [25], 2021 | 76 | M | Pfizer Moderna |
1st 2nd |
4 d N/A |
1.6 2.2 |
0.86 1.15 |
Pos N/A |
6.5 3.8 |
De novo Relapse |
PLA2R N/A |
Conservative/Rituximab | Partial response |
| 10. | Da [31], 2021 | 70 | M | Pfizer | 1st | 1 wk | 1.7 | 1.29 | N/A | 4.4 | De novo | THSD7A | Conservative | No response |
| 11. | Liang [32], 2021 | 62 | F | Moderna | 2nd | 1 mo | N/A | 1.6 | N/A | 11.2 | Relapse | PLA2R | Conservative/Rituximab | N/A |
| 12. | Chavarot [28], 2022 |
66 | M | Pfizer | 2nd | 8 wk | N/A | 1.36 | N/A | Neg | De novo post-KT | PLA2R | Conservative | N/A |
| 13. | Psyllaki [24], 2022 |
68 | M | Pfizer | 1st | 7 d | 2.9 | GFR 70 mL/min/1.73 m2 | N/A | 19 | De novo | PLA2R | Rituximab | Partial response |
| 14. | Fenoglio [22], 2022 |
82 | F | Pfizer | 2nd | 88 d | N/A | N/A | N/A | NS | De novo | Neg PLA2R, THSD7A | GC | N/A |
| 15. | 67 | F | Pfizer | 2nd | 89 d | N/A | N/A | N/A | NS | De novo | Neg PLA2R, THSD7A | Rituximab | N/A | |
| 16. | 82 | M | Pfizer | 2nd | 29 d | N/A | N/A | N/A | NS | De novo | PLA2R | Rituximab | N/A | |
| 17. | Rashid [27], 2022 |
56 | M | Moderna | 1st | 4 wk | 2.2 | 13.96 | Blood 2+ | 12.2 | De novo | PLA2R | Hemodialysis Rituximab |
Response |
| 18. | Visch [21], 2022 |
80 | M | Pfizer | 2nd | 4 wk | 2.6 | 1.32 | N/A | 5 | Relapse | PLA2R | Rituximab | No response |
| 19. | 60 | M | Pfizer | 2nd | 6 wk | 1.7 | 1.92 | N/A | 5 | Relapse | PLA2R | Rituximab/CY/ GC |
Response | |
| 20. | 77 | F | Pfizer | 1st | 4 wk | 2.2 | 0.7 | N/A | 12.5 | Relapse | PLA2R | Tacrolimus | Response | |
| 21. | 78 | M | Pfizer | 2nd | 1 wk | 3.4 | 1.87 | N/A | 4.9 | Relapse | N/A | GC | Response | |
| 22. | 48 | M | Pfizer | 2nd | 3 wk | 3.1 | 1.41 | N/A | 1.7 | Relapse | PLA2R | Conservative | No response | |
| 23. | 56 | M | Pfizer | 2nd | 2 wk | 3.2 | 1.47 | N/A | 3.4 | Relapse | PLA2R | Conservative | No response | |
| 24. | 84 | M | Pfizer | 2nd | 10 wk | 3.3 | 1.55 | N/A | 3 | Relapse | PLA2R | Tacrolimus/GC→ Rituximab | Response | |
| 25. | 39 | M | Pfizer | 2nd | 4 wk | 1.8 | 1.38 | N/A | 3.7 | De novo Worsening |
PLA2R | Rituximab/CY/ GC |
Response | |
| 26. | 75 | M | Pfizer | 2nd | 2 wk | 2.1 | 0.88 | N/A | 8 | De novo Worsening |
PLA2R | Rituximab/CY/ GC |
N/A | |
| 27. | 48 | M | Pfizer | 1st | 2 wk | 2.5 | 1.26 | N/A | 2.22 | De novo Worsening |
PLA2R | Rituximab/CY/ GC |
Response | |
| 28. | 58 | M | Pfizer | 2nd | 3 wk | 2.4 | 1.02 | N/A | 8 | De novo Worsening |
PLA2R | Tacrolimus | Response | |
| 29. | Zhao [13], 2022 | 57 | W | Sinovac | 1st 2nd |
1 d 1 d |
N/A 2.85 |
N/A 0.42 |
N/A 1+ |
N/A 1.6 |
De novo Relapse |
PLA2R | Conservative | Partial response |
| 30. | Paxton [26], 2022 | 22 | M | Pfizer | 2nd | 4 wk | 8 | 0.72 | N/A | 7 | De novo | PLA2R | Rituximab | Partial response |
| 31. | Saigal [33], 2022 | 32 | M | Astra Zeneca | N/A | 14 d | N/A | N/A | N/A | N/A | De novo | N/A | GC/CY | Partial response |
| 32. | 47 | M | Astra Zeneca | N/A | 11 d | N/A | N/A | N/A | N/A | De novo | N/A | Conservative | Response | |
| 32. | Pitre [34], 2022 | 65 | F | J + J | 1st | 5 mo | N/A | 1.7 | N/A | 1.7 | De novo | PLA2R | GC | Partial response |
| 33. | Ma [19], 2022 | 42 | F | Astra Zeneca | 1st | 2 wk | 1.6 | 0.8 | N/A | 16 | N/A | N/A | GC/MMF | Response |
Abbreviations: G, gender; M, male; F, female; GC, glucocorticoids; CY, cyclophosphamide; MMF, mycophenolate mofetil; PLA2R, M-type phospholipase A2 receptor antibody; NELL-1, neural epithelial growth factor like-1; EXT1, intracellular proteins exostosin; THSD7A, thrombospondin type 1 domain containing 7A; NS, Nephrotic syndrome; J + J, Johnson & Johnson SARS-CoV-2 vaccine; N/A, not applicable.
As in this case, we reported acute onset of 1 week to develop symptoms following the first dose and a few days following the second dose of inactivated SARS-CoV-2 vaccines. The second episode might have resulted from a disease flare stimulated by the second vaccination. The early onset of NS has been mentioned in the passive Hayman nephritis, a classic model of human MN [29]. The MN is induced by a single injection of heterologous antisera to the rat renal tubular antigen extract in susceptible rat strains [23]. Massive proteinuria occurs in almost all animals within 5 days, followed by low-grade proteinuria lasting 60–150 days [23]. The onset of massive proteinuria is diminished by 2–3 days if the Ab is boosted [23]. Zhao et al. also demonstrated the occurrence of de novo MN immediately following immunization with the inactivated SARS-CoV-2 vaccine, and the NS entered partial remission after receiving angiotensin II receptor blocker treatment [13]. In the presented case, the abrupt onset of the NS was consistent with the early stage of membranous lesions through LM (focal spike formation) and EM (no membrane reaction).
The theory of vaccination triggering MN flare is supported by a retrospective study of 245 patients with biopsy-proven MN from a single center. The relapse rate of MN occurred at 5% during the SARS-CoV-2 pandemic era compared to 2% prior to the era [21]. However, the causal relationship of these findings needs further examination [11,30].
Treatment of secondary MN following SARS-CoV-2 vaccination is controversial. Based on the evidence of primary MN, conservative treatment and immunosuppressive medication are therapeutic options. Rituximab, obinutuzumab, mycophenolate mofetil, and oral cyclophosphamide have been used to reach partial response outcomes [10,19,21,24,25,26,27]. The patient achieved complete remission without subsequent second relapse after the short course of immunosuppressive agents, suggesting a causal relationship between the vaccination and secondary MN. Close monitoring of GN relapse after a booster dose of SARS-CoV-2 vaccination is warranted since the same type of vaccine might exacerbate the immunologic response [13,25].
In conclusion, secondary MN following SARS-CoV-2 vaccination should call for medical importance. Not only is it spontaneous remission or remission despite a short course of immunosuppressant, but also a high index of suspicion of the association might avoid unnecessary treatment with long-term steroid and immunosuppressive agents. Therefore, further investigation into the pathogenesis is warranted.
Author Contributions
Conceptualization, T.T., W.P. and T.K.; Resources, L.C., S.W. and V.B.; Writing—original draft preparation, T.T., W.P. and T.K.; Writing—review and editing, T.T, T.K., S.S., S.P. and W.P.; Supervision, T.K., S.P. and W.P.; Funding acquisition, S.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Naresuan University Institutional Review Board 3rd Floor Sirindhorn Building, Naresuan University Hospital, Phitsanulok, 65000 Thailand, IRB No. P3-0142/2564.
Informed Consent Statement
Informed consent was obtained from the patient.
Data Availability Statement
Not applicable.
Conflicts of Interest
T.K. has received consultancy fees from VISTERRA, ELEDON, Otsuka OLE, and Otsuka VISIONARY as a country investigator, is a current recipient of the National Research Council of Thailand, and has received speaking honoraria from Astra Zeneca and Baxter Healthcare. The other authors declare that they have no conflicts of interest.
Funding Statement
S.P. received grant support from the National Science, Research and Innovation Fund (NSRF, no R2566B001) and the National Research Council of Thailand (NRCT) and Naresuan University: N42A650331). T.K. received funding support from the Thailand Science Research and Innovation Fund Chulalongkorn University (CU_FRB65_hea (19)_026_30_07).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kyriakidis N.C., López-Cortés A., González E.V., Grimaldos A.B., Prado E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6:28. doi: 10.1038/s41541-021-00292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng H., Peng Z., Luo W., Si S., Mo M., Zhou H., Xin X., Liu H., Yu Y. Efficacy and Safety of COVID-19 Vaccines in Phase III Trials: A Meta-Analysis. Vaccines. 2021;9:582. doi: 10.3390/vaccines9060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres R., Toro L., Sanhueza M.E., Lorca E., Ortiz M., Pefaur J., Clavero R., Machuca E., Gonzalez F., Herrera P., et al. Clinical Efficacy of SARS-CoV-2 Vaccination in Hemodialysis Patients. Kidney Int. Rep. 2022;7:2176–2185. doi: 10.1016/j.ekir.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashby D.R., Caplin B., Corbett R.W., Asgari E., Kumar N., Sarnowski A., Hull R., Makanjuola D., Cole N., Chen J., et al. Severity of COVID-19 after Vaccination among Hemodialysis Patients: An Observational Cohort Study. Clin. J. Am. Soc. Nephrol. 2022;17:843–850. doi: 10.2215/CJN.16621221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boongird S., Chuengsaman P., Setthaudom C., Nongnuch A., Assanatham M., Phanprasert S., Kitpermkiat R., Kiertiburanakul S., Malathum K., Phuphuakrat A., et al. Short-Term Immunogenicity Profiles and Predictors for Suboptimal Immune Responses in Patients with End-Stage Kidney Disease Immunized with Inactivated SARS-CoV-2 Vaccine. Infect. Dis. Ther. 2022;11:351–365. doi: 10.1007/s40121-021-00574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr E.J., Wu M., Harvey R., Wall E.C., Kelly G., Hussain S., Howell M., Kassiotis G., Swanton C., Gandhi S., et al. Neutralising antibodies after COVID-19 vaccination in UK haemodialysis patients. Lancet. 2021;398:1038–1041. doi: 10.1016/S0140-6736(21)01854-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanriover M.D., Doğanay H.L., Akova M., Güner H.R., Azap A., Akhan S., Köse Ş., Erdinç F., Akalın E.H., Tabak Ö.F., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jara A., Undurraga E.A., González C., Paredes F., Fontecilla T., Jara G., Pizarro A., Acevedo J., Leo K., Leon F., et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021;385:875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palacios R., Patiño E.G., de Oliveira Piorelli R., Conde M., Batista A.P., Zeng G., Xin Q., Kallas E.G., Flores J., Ockenhouse C.F., et al. Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac—PROFISCOV: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:853. doi: 10.1186/s13063-020-04775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klomjit N., Alexander M.P., Fervenza F.C., Zoghby Z., Garg A., Hogan M.C., Nasr S.H., Minshar M.A., Zand L. COVID-19 Vaccination and Glomerulonephritis. Kidney Int. Rep. 2021;6:2969–2978. doi: 10.1016/j.ekir.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caza T.N., Cassol C.A., Messias N., Hannoudi A., Haun R.S., Walker P.D., May R.M., Seipp R.M., Betchick E.J., Amin H., et al. Glomerular Disease in Temporal Association with SARS-CoV-2 Vaccination: A Series of 29 Cases. Kidney360. 2021;2:1770–1780. doi: 10.34067/KID.0005372021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aydın M.F., Yıldız A., Oruç A., Sezen M., Dilek K., Güllülü M., Yavuz M., Ersoy A. Relapse of primary membranous nephropathy after inactivated SARS-CoV-2 virus vaccination. Kidney Int. 2021;100:464–465. doi: 10.1016/j.kint.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y.Z.L., Wang G., Guan J., Pai P. The Development of De novo Acute Tubulointerstitial Nephritis and Membranous Nephropathy Following Inactivated COVID-19 Vaccine: Causal or Casual? Clin. Case Rep. Int. 2022;6:1344. [Google Scholar]
- 14.KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100:S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Carr E.J., Kronbichler A., Graham-Brown M., Abra G., Argyropoulos C., Harper L., Lerma E.V., Suri R.S., Topf J., Willicombe M., et al. Systematic Review of Early Immune Response to SARS-CoV-2 Vaccination Among Patients with Chronic Kidney Disease. Kidney Int. Rep. 2021;6:2292–2304. doi: 10.1016/j.ekir.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J., Cao J., Ye Q. Renal Side Effects of COVID-19 Vaccination. Vaccines. 2022;10:1783. doi: 10.3390/vaccines10111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel C., Shah H.H. Membranous nephropathy and severe acute kidney injury following influenza vaccination. Saudi J. Kidney Dis. Transpl. 2015;26:1289–1293. doi: 10.4103/1319-2442.168676. [DOI] [PubMed] [Google Scholar]
- 18.Kostianovsky A., Charles P., Alves J.F., Goulet M., Pagnoux C., Le Guern V., Mouthon L., Krivine A., Villiger P., Launay O., et al. Immunogenicity and safety of seasonal and 2009 pandemic A/H1N1 influenza vaccines for patients with autoimmune diseases: A prospective, monocentre trial on 199 patients. Clin. Exp. Rheumatol. 2012;30:S83–S89. [PubMed] [Google Scholar]
- 19.Ma Q., Li X., Xu G. New-Onset and Relapsed Membranous Nephropathy post SARS-CoV-2 and COVID-19 Vaccination. Viruses. 2022;14:2143. doi: 10.3390/v14102143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald I., Murray S.M., Reynolds C.J., Altmann D.M., Boyton R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines. 2021;6:74. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visch R., Wetzels J., Vink C., van de Logt A.E. COVID-19 Vaccination in Patients with Membranous Nephropathy. Kidney Int. Rep. 2022;7:1922–1923. doi: 10.1016/j.ekir.2022.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fenoglio R., Lalloni S., Marchisio M., Oddone V., De Simone E., Del Vecchio G., Sciascia S., Roccatello D. New Onset Biopsy-Proven Nephropathies after COVID Vaccination. Am. J. Nephrol. 2022;53:325–330. doi: 10.1159/000523962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Natori Y., Shindo N., Natori Y. Proteinuria induced by anti-dipeptidyl peptidase IV (gp108); role of circulating and glomerular antigen. Clin. Exp. Immunol. 1994;95:327–332. doi: 10.1111/j.1365-2249.1994.tb06532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Psyllaki A., Stavrakaki I., Androvitsanea A., Gakiopoulou H., Petrakis I., Stylianou K. Two cases of glomerular involvement after vaccination against COVID-19: Epiphenomenon or causality? Clin. Kidney J. 2022;15:574–575. doi: 10.1093/ckj/sfab252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gueguen L., Loheac C., Saidani N., Khatchatourian L. Membranous nephropathy following anti-COVID-19 mRNA vaccination. Kidney Int. 2021;100:1140–1141. doi: 10.1016/j.kint.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paxton L., McMahon L., Wong L. De novo PLA2R positive membranous nephropathy following BNT162b2 mRNA COVID-19 vaccine. Intern. Med. J. 2022. online ahead of print . [DOI] [PMC free article] [PubMed]
- 27.Rashid W., Mousa H., Khan J., Ijaz F., Ezell G.D. A Case of Membranous Nephropathy Hypothesized to be Associated With COVID-19 Vaccine. Cureus. 2022;14:e24245. doi: 10.7759/cureus.24245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chavarot N., Padden M., Amrouche L., Malard S., Scemla A., Sberro-Soussan R., Léon J., Legendre C., Duong J.P., Zuber J., et al. De novo posttransplant membranous nephropathy following BNT162b2 mRNA COVID-19 vaccine in a kidney transplant recipient. Am. J. Transplant. 2022;22:3188–3189. doi: 10.1111/ajt.17166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salant D.J., Quigg R.J., Cybulsky A.V. Heymann nephritis: Mechanisms of renal injury. Kidney Int. 1989;35:976–984. doi: 10.1038/ki.1989.81. [DOI] [PubMed] [Google Scholar]
- 30.Izzedine H., Bonilla M., Jhaveri K.D. Nephrotic syndrome and vasculitis following SARS-CoV-2 Vaccine: True association or circumstantial? Nephrol. Dial. Transplant. 2021;36:1565–1569. doi: 10.1093/ndt/gfab215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Da Y., Goh G.H., Khatri P. A Case of Membranous Nephropathy Following Pfizer-BioNTech mRNA vaccine against Coronavirus 2019. Kidney Int. 2021;100:938–939. doi: 10.1016/j.kint.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang K.V., Kurtz E.C., Pittappilly M., Ahmad S., Minervini M.I. Primary membranous nephropathy flare after COVID-19 vaccination. J. Am. Soc. Nephrol. 2021;32:485. [Google Scholar]
- 33.Saigal M., Taduri G., Gudditi S., Herur S., Alaparthi P., Kinjarapu S. POS-872 Post covid vaccination- new onset glomerulonephritis—A mere co incidence or a impending reality? Kidney Int. Rep. 2022;7:S377. doi: 10.1016/j.ekir.2022.01.910. [DOI] [Google Scholar]
- 34.Martinez-Pitre P.J., Fogo A.B., Alqudsi M. 337 Relapse of Membranous Nephropathy and Renal Sarcoidosis Following Exposure to SARS-CoV-2 Vaccine (Johnson & Johnson) Am. J. Kidney Dis. 2022;79:S102–S103. doi: 10.1053/j.ajkd.2022.01.342. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.