Abstract
Buruli ulcer, caused by Mycobacterium ulcerans, is characterized by deep and necrotizing skin lesions, mostly on the arms and legs. Together with tuberculosis and leprosy, this mycobacterial disease has become a major health problem in tropical and subtropical regions, particularly in central and western Africa. No specific vaccine is available for Buruli ulcer. There is, however, evidence in the literature that suggests a cross-reactive protective role of the tuberculosis vaccine M. bovis BCG. To identify potential mechanisms for this cross-protection, we identified and characterized the M. ulcerans homologue of the important protective mycobacterial antigen 85 (Ag85A) from BCG. The homologue is well conserved in M. ulcerans, showing 84.1% amino acid sequence identity and 91% conserved residues compared to the sequence from BCG. This antigen was sufficiently conserved to allow cross-reactive protection, as demonstrated by the ability of M. ulcerans- infected mice to exhibit strong cellular immune responses to both BCG and its purified Ag85 complex. To further address the mechanism of cross-reactive protection, we demonstrate here that prior vaccination with either BCG or plasmid DNA encoding BCG Ag85A is capable of significantly reducing the bacterial load in the footpads of M. ulcerans- infected mice, as determined by Ziehl-Neelsen staining and by actual counting of CFU on 7H11 Middlebrook agar. Together, the results reported here support the potential of a cross-protective Ag85-based future vaccine against tuberculosis, Buruli ulcer, and leprosy.
Buruli ulcer, caused by Mycobacterium ulcerans, is an emerging mycobacteriosis characterized by deep and necrotizing skin lesions, mostly of the upper and lower limbs. Together with tuberculosis (TB) and leprosy, this mycobacterial disease has become a major health problem in developing countries (20). Buruli ulcer affects mostly children in tropical and subtropical regions of central and western Africa. Other foci of endemicity are present in some regions of Australia, Southeast Asia, and a few scattered parts of Latin America (44). There is no evidence for direct person-to-person transmission, and it has been suggested that contaminated water of rivers, swamps, and lakes is the most likely reservoir for the disease. A sensitive and specific PCR method, based on the insertion sequences IS2404 and IS2606, has enabled the detection of M. ulcerans in water samples from a swamp and a golf course irrigation system, but direct culture of this mycobacterium from environmental samples has been unsuccessful (34, 35). A very close phylogenetic relationship exists between M. ulcerans and M. marinum, another mycobacterial species causing cutaneous problems (38).
Buruli ulcer can manifest itself in different forms, ranging from a small skin nodule to a deep and enlarging skin ulcer ultimately necessitating chirurgical care. Treatment with antimycobacterial agents has generally been disappointing, especially in patients with extensive ulcers. Wide surgical excision and skin grafting or even amputation of the affected limbs is often the only treatment possible. Buruli ulcer is generally found on lower-temperature parts of the body, such as the arms and legs, and the disease is accompanied by remarkably few systemic symptoms and is rarely fatal (44). The infection remains confined to the subcutaneous tissue and overlying skin. The absence of a positive skin test response in most patients may indicate that cellular immune responses are not or are only weakly induced. Interestingly, spontaneous healing is often accompanied by skin test conversion (44), again suggesting a pivotal role of the immune system in the control of the disease. Clearly, a better understanding of the immune response generated following M. ulcerans infection is essential in this respect. No specific vaccine is available for Buruli ulcer, but evidence in the literature suggests a cross-reactive protective role of the M. bovis BCG vaccine used against TB (37).
For TB, it has been shown convincingly in mouse and guinea pig experimental models that important protective antigens can be found among the secreted or exported proteins of the bacillus, which are present in large amounts in mycobacterial culture filtrates (CF) (1, 13, 14). A major protein component of these CF is the antigen 85 (Ag85) complex, a 30- to 32-kDa family of three proteins, Ag85A, Ag85B, and Ag85C, which are encoded by three distinct but highly homologous genes (47). The bacteriostatic drug isoniazid enhances the expression of the Ag85 complex in M. tuberculosis CF (11), and its role in cell wall synthesis through its mycolyl-transferase activity is now well documented (4, 33). Homologues of the Ag85 complex have been described in all of the mycobacterial species tested so far and in another member of the actinomycete family, i.e., Corynebacterium glutamicum (21). The Ag85 complex induces strong T-cell proliferation and gamma interferon (IFN-γ) production in most healthy individuals infected with M. tuberculosis and in BCG-vaccinated mice and humans (15, 19). On the other hand, TB patients show decreased cellular immune responses but increased antibody production in response to Ag85 (19, 45). In leprosy, strong cross-reactive cellular and humoral immune responses against purified Ag85 from M. bovis BCG can also be detected in, respectively, healthy contacts and leprosy patients (23–25). Ag85 plays an important role in protective immunity against TB, and we and others have previously demonstrated that vaccination with plasmid DNA encoding Ag85A and Ag85B can protect mice against an experimental aerosol and intravenous challenge with M. tuberculosis (3, 16, 22, 40).
In order to analyze the possible role of the Ag85 complex in the cross-reactive protection conferred by BCG against M. ulcerans infection, we have first determined the complete nucleotide sequence of Ag85A from M. ulcerans and we show that the gene is 84% identical to its homologue in M. bovis BCG. Furthermore, mice infected in the footpad with M. ulcerans can mount a cross-reactive cellular immune response, restricted to the draining lymph nodes (LN), against M. bovis BCG CF and its major Ag85 complex. Finally, prior vaccination with BCG or with plasmid DNA encoding Ag85A from M. bovis BCG can partially protect mice against a subsequent footpad challenge with M. ulcerans, resulting in a more-than-20-fold reduction in the number of mycobacteria in the infected footpad.
MATERIALS AND METHODS
Mycobacterial strains.
M. ulcerans type 1 strain 5150 from the Democratic Republic of Congo was isolated at the Institute of Tropical Medicine of Antwerp (30). Bacteria were grown on Löwenstein-Jensen or Middlebrook 7H9 medium at 32 to 33°C for approximately 1 month. M. bovis BCG strain GL2 was grown at the Pasteur Institute in Brussels for 2 weeks as a surface pellicle at 37°C in synthetic Sauton medium.
Genomic DNA isolation.
A 500-mg pellet of M. ulcerans obtained from 50 ml of a 6-week 7H9 liquid culture was resuspended in 0.1 M NaCl–1 mM EDTA–50 mM Tris-HCl buffer (buffer I) and heated for 20 min at 80°C. DNA was extracted by using lysozyme, pronase, and sodium dodecyl sulfate (SDS) treatment. For the extraction, 1 volume of chloroform-isoamyl alcohol (24:1) was added to mycobacteria in buffer I. The aqueous phase was then extracted three times with 1 volume of neutralized phenol. After several cycles of freeze-thawing, the aqueous phase was recovered and added to 1 volume of ether. RNase A (10 μg/ml) was added to the DNA, incubated for 1 h at 37°C, and removed by three extractions with phenol-chloroform-isoamyl alcohol. After precipitation of the DNA with 2 volumes of ethanol, the pellet was resuspended in Tris-EDTA buffer and analyzed by electrophoresis on agarose gel. Purity was evaluated by calculation of the ratio of optical density at 260 nm (OD260) to OD280 and the ratio of OD260 to OD230.
Probes.
Three different probes were used to clone a fragment including the complete gene coding for Ag85A of M. ulcerans.
(i) BB1-BB3 probe.
Since the BB1 and BB3 sequences are highly conserved among Ag85-encoding genes from several mycobacterial species, this 162-bp fragment of the Ag85-encoding gene was amplified by PCR on M. ulcerans genomic DNA by using primers ATCAACACCCCGGCCGTCGAG (BB1 sense) and CGGCAGCTCGCTGGTCAGGA (BB3 antisense) (10).
(ii) Probes A and B.
Probes A and B were obtained by digestion with PstI and XhoI of plasmid 5.1, which contains the gene encoding Ag85B from M. bovis BCG (7, 27). Probe A (700 bp) encodes the N-terminal region of the protein, whereas probe B (300 bp) encodes the C-terminal region of the protein. All probes were labeled with [32P]dCTP by using the multiprime labeling kit (Amersham, Amersham, United Kingdom).
Southern blot hybridization.
Purified genomic DNA from M. ulcerans (5 μg) was digested overnight by BamHI (Fermentas) and separated on a 1% Seakem GTG agarose gel (FMC). Before overnight transfer onto a nylon Hybond N membrane (Amersham), the gel was denatured in a 0.5 M NaOH–1.5 M NaCl solution and neutralized in a 3 M Na acetate solution. The DNA transferred onto the membrane was fixed by short-wavelength UV exposure. Hybridization with the three different Ag85 probes was then realized sequentially. Each filter was prehybridized in Rapid-Hyb buffer (Amersham) at 65°C and then hybridized overnight with the denatured probe at 2 × 106 cpm/ml. The filter was washed two times in 2 × SSPE (1× SSPE is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% SDS for 10 min at room temperature and once in 1× SSPE–0.1% SDS for 15 min at 65°C. For BB1–BB3, a more stringent procedure was used that included a wash in 0.7× SSPE–0.1% SDS. After air drying, each filter was autoradiographed on Kodak X-Omat film. Complete stripping of each probe was obtained by immersion of the membrane for 30 min in 0.1% SDS at 95°C.
Cloning of a 1,400-bp fragment encoding Ag85A from M. ulcerans.
Purified DNA of M. ulcerans (20 μg) was digested with BamHI, and a fragment of about 1,400 bp was cut and extracted from the agarose gel by GenElute agarose spin columns (Sigma). Ligation was made in pBlueScript II-SK+ vector (pBSK+) (Stratagene), previously digested with BamHI, using the T4 DNA ligase (Roche). Transformation was made in electrocompetent DH5α cells (Gibco BRL) by electroporation. Cultures were incubated overnight on ampicillin-containing agar plates. Addition of isopropyl-β-d-thiogalactopylanoside (IPTG) and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) allowed us to distinguish between colonies containing the insert (white) and colonies without the insert (blue). Each positive individual colony was streaked onto a grid Hybond N membrane laid on the surface of an agar plate. After overnight growth, filters were denaturated and neutralized on Whatman 3MM paper, washed in 2× SSC, and then fixed by short-wavelength UV exposure. After treatment with proteinase K (100 μg/ml, 1 h at 37°C), filters were hybridized with the 162-bp [32P]dCTP-labeled BB1–BB3 M. ulcerans DNA probe.
DNA sequencing.
DNA prepared from clone 71 was obtained by using a Qiagen plasmid midi protocol (Qiagen). DNA sequencing was carried out by Texas red dye primer cycle sequencing with an automatic Vistra 725 sequencer using a DYEnamic Direct cycle sequencing kit (Amersham). External oligonucleotides T3 and T7 were used initially, followed by six other internal oligonucleotides, allowing complete sequencing of the clone 71 insert in both orientations.
Plasmid construction.
The gene encoding Ag85A from M. tuberculosis (which is 100% identical to that of M. bovis BCG) was amplified by PCR without its mycobacterial signal sequence by using primers containing BglII restriction sites and inserted into the V1Jns.tPA vector, driven by the IE1 cytomegalovirus promoter and encoding the signal sequence for human tissue plasminogen activator (16).
Mice.
C57BL/10 (B10) and C57BL/6 (B6 [H-2b]) mice were bred in the animal facilities of the Pasteur Institute of Brussels. Only male mice, 6 to 8 weeks old at the start of the experiment, were used.
M. ulcerans infection.
An M. ulcerans suspension was prepared from cultures grown for 1 month on Löwenstein-Jensen medium. Cultures (1 mg/ml) were mixed with 2-mm-diameter glass beads (Vel, Leuven, Belgium) and vortexed vigorously, and the number of acid-fast bacilli (AFB) was determined by Ziehl-Neelsen staining. Mice were injected in the right footpad with 0.03 ml of the M. ulcerans suspension in Dubos medium (Difco) containing 3 × 104 or 105 AFB.
Vaccination.
B6 mice were anesthesized by intraperitoneal injection of ketamine (100 mg/ml) -xylazine (10 mg/ml) and injected three times, at 3-week intervals, in both quadriceps with 2 × 50 μg of plasmid DNA encoding Ag85A or the empty V1Jns.tPA vector (control DNA) using a 0.3-cm3 insulin syringe (Becton Dickinson). Mice were infected with M. ulcerans 5 weeks after the third DNA vaccination. For BCG vaccination, mice were injected intravenously with 0.5 mg (±2 × 106 CFU) of live M. bovis BCG at the time of the first DNA injection (11 weeks before challenge).
Antigens.
CF was prepared from M. bovis BCG cultures by ammonium sulfate precipitation as described before (15). Purified protein derivative (PPD) was prepared from 8-week-old CF of M. bovis strain Vallée. The Ag85 complex (Ag85A plus Ag85B plus Ag85C) was purified from M. bovis BCG CF by sequential chromatography on phenyl Sepharose, DEAE-Sephacel, and Sephadex G75 (8). Pokeweed mitogen (PWM; Gibco Life Sciences), a T-cell-dependent B-cell mitogen, was used as a control to measure polyclonal T-cell stimulation. Recombinant catalase-peroxidase (KatG) and malate synthase (GlcB) proteins from M. tuberculosis were a kind gift of J. T. Belisle (Colorado State University).
Cytokine production.
Mice were killed 7 weeks after infection with 3 × 104 AFB, and spleen and inguinal LN were removed aseptically. Spleens and LN from five mice were pooled, but LN draining the infected (right) and uninfected (left) limbs were analyzed separately. A suspension of 4 × 106 leukocytes/ml was incubated in the presence of PPD (25 μg/ml), CF (25 μg/ml), or Ag85 (5 μg/ml) in round-bottom microwell plates in RPMI medium supplemented with 10% fetal calf serum, antibiotics, and 5 × 10−5 M 2-mercaptoethanol. Cells were incubated for 1 or 3 days at 37°C in 5% CO2. Supernatants from three separate wells were pooled for each antigen.
IL-2 assay.
Interleukin-2 (IL-2) activity was determined in duplicate on 24-h culture supernatants (pool of five mice) using a bio-assay with IL-2-dependent CTLL-2 cells as described before (15). IL-2 levels are expressed as mean counts per minute ± the standard deviation (SD). The detection limit is around 10 pg/ml.
IFN-γ assay.
IFN activity was quantified in duplicate on 72-h spleen cell culture supernatants (pool of five mice) using a mouse IFN-γ enzyme-linked immunosorbent assay as described before (46). Coating antibody R4-6A2 and biotinylated detection antibody XMG1.2 were obtained from Pharmingen. The standard murine recombinant IFN-γ used was obtained from Gibco. Titers are expressed as the mean number of picograms per milliliter. The detection limit of the assay is 20 pg/ml.
Lymphoproliferation.
Pooled inguinal LN cells (LN draining infected hind legs separated from LN draining uninfected hind legs) from five M. ulcerans-infected mice were incubated in vitro at 106 leukocytes/ml in the presence of control medium, bovine PPD (5 μg/ml), Ag85 from BCG (1 μg/ml), or PWM (1:50 dilution) in round-bottom microwell plates in RPMI 1640 medium supplemented with 10% Fetal calf serum, antibiotics, and 5 × 10−5 M 2-mercaptoethanol. Cells were incubated for 3 or 4 days at 37°C in 5% CO2, and [3H]thymidine (Amersham) was added at 0.4 μCi/well during the last 24 h. Cells were harvested onto glass fiber filter mats using a Titertek cell harvester, and radioactivity was measured in a Betaplate liquid scintillation counter (Wallace). The lymphoproliferation level was expressed as mean counts per minute ± SD from quadruplicate cultures.
Western blot analysis.
Naive B6 and B10 mice or B6 mice vaccinated with BCG 11 weeks before were infected with 3 × 104 M. ulcerans AFB or 2 × 106 CFU of M. bovis BCG. Sera were collected at different time points, diluted 1:20, and tested against CF from M. bovis BCG as described before (17). The specificities of BCG-specific monoclonal antibodies (MAbs) directed against the 30- to 32-kDa Ag85 complex (MAb 17-4), the 40-kDa PstS-3 protein (MAb 2C1-5), the 65-kDa heat shock protein (MAb IA1), and an 80-kDa CF protein (MAb 5D9) have been described before (17, 18, 41). MAbs IT42 and IT57, directed against the 80-kDa catalase-peroxidase (KatG) protein from M. tuberculosis CF, were kindly provided by J. Belisle (Colorado State University).
Enumeration of AFB and CFU in the footpad.
In a first experiment, B10 mice were injected in the footpad with 105 AFB and growth was evaluated for up to 8 weeks after the initial infection. In the vaccination experiments, infected footpads of B6 mice were analyzed 5 or 7 weeks after M. ulcerans inoculation with 105 or 3 × 104 AFB, respectively. The skin and bones were removed carefully. The tissues were homogenized in a Dounce homogenizer and resuspended in 2 ml of Dubos broth medium (Difco) containing 0.4 g of glass beads 2 mm in diameter (Vel). Bacilli were counted as described previously by Shepard and McRae (36). Briefly, 1 volume of tissue homogenate was diluted in 1 volume of 10% milk–0.15% formaldehyde medium. A volume of 5 μl was fixed on a glass slide (in triplicate) by heating at 60 to 70°C for 15 min. The slide was then stained with Ziehl-Neelsen stain, and the AFB in 20 fields were counted under a microscope. For enumeration of actual CFU, serial dilutions of tissue homogenate were plated on 7H11 Middlebrook agar, supplemented with oleic acid-albumin-dextrose-catalase enrichment medium. Petri dishes were sealed in plastic bags, and yellow colonies were counted after 8 weeks of incubation at 32 to 33°C. Numbers of AFB (viable and nonviable organisms) or CFU (viable organisms) were determined on individual mice (four to seven mice per group) and converted to log10 values, and statistical analysis was performed on mean log10 ± SD values by using Student's t test.
Nucleotide sequence accession number.
The nucleotide sequence of the gene coding for Ag85A from M. ulcerans has been assigned GenBank accession number AJ300576.
RESULTS
Cloning of a 1,400-bp DNA fragment containing the Ag85A-encoding gene from M. ulcerans.
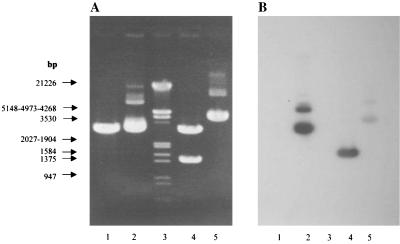
After BamHI digestion of the complete genomic DNA from M. ulcerans, sequential Southern blot hybridization with probes A and B (from Ag85B of M. bovis BCG) and probe BB1–BB3 from M. ulcerans revealed two fragments with estimated sizes of 1,400 and 7,300 bp. These two BamHI fragments hybridized with all three probes (results not shown). The 1,400-bp fragment was isolated, purified, and ligated into pBSK+. The transformation in DH5α cells yielded 139 individual colonies. Among these, only one (clone 71) hybridized with the M. ulcerans DNA BB1–BB3 probe. Southern blot analysis of plasmid DNA from clone 71 confirmed that the hybridization of this DNA to probe BB1–BB3 is entirely due to the 1,400-bp insert (and not to the pBSK+ vector) (Fig. 1).
FIG. 1.
Agarose gel electrophoresis (A) and Southern blot analysis (B) after hybridization under stringent conditions with the 162-bp probe BB1-BB3 from M. ulcerans. Lanes: 1, pBSK+ (1 μg); 2, plasmid DNA from clone 71 (1 μg); 3, molecular weight marker III (Roche); 4, plasmid DNA from clone 71 after BamHI digestion (1 μg); 5, V1Jns.tPA-85A from M. bovis BCG (1 μg).
Nucleotide sequence analysis.
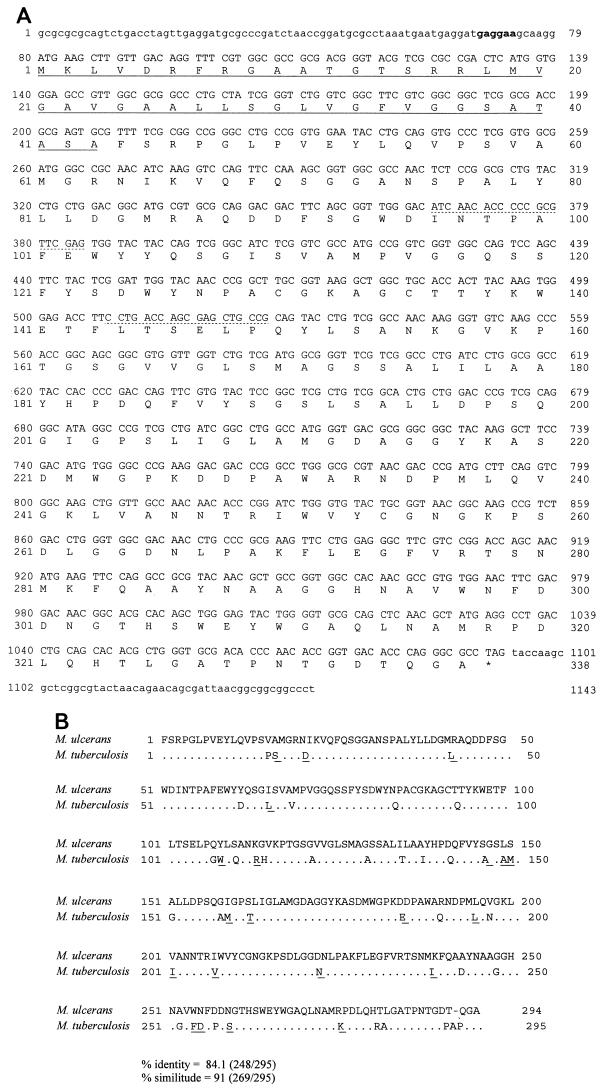
Sequencing the 1,400-bp purified DNA from clone 71, we found a 1,014-bp open reading frame corresponding to a gene coding for an Ag85 homolog from M. ulcerans (Fig. 2A). As for all other known Ag85 sequences, the conserved mature protein starts with an FSRPGL sequence. The gene encoding the mature protein is preceded by a signal peptide of 129 bp (43 amino acids [aa]) that is necessary for its the secretion. Alignment of the Ag85 sequence of M. ulcerans with those encoding Ag85A, Ag85B, and Ag85C from M. tuberculosis revealed a high similarity with, respectively, 84, 73.7, and 59.7% identical amino acids. We therefore concluded that DNA from clone 71 encodes the Ag85A homolog of M. ulcerans (Fig. 2B).
FIG. 2.
(A) Nucleotide and deduced amino acid sequences of Ag85A from M. ulcerans strain 5150 type I. Uppercase letters represent the complete open reading frame sequence. The signal peptide with an ATG as the initial codon is underlined. The conserved BB1 and BB3 primers used to select a probe in M. ulcerans are underlined with a dotted line. The putative Shine-Dalgarno or ribosome-binding sequence is indicated in bold. (B) Sequence comparison of mature Ag85A protein of M. ulcerans and M. tuberculosis (which is 100% identical to that of M. bovis BCG). Dots indicate identical amino acids; conserved amino acid differences (determined according to the Needleman-Wunsh criterion) are underlined.
Multiplication of M. ulcerans in the footpads of B10 mice.
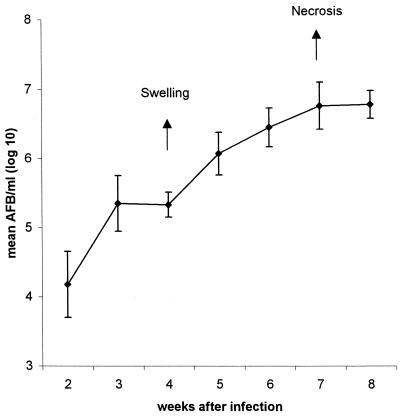
Injection of 105 M. ulcerans AFB into the footpads of B10 mice resulted in significant multiplication of the mycobacteria in the injected leg. No AFB could be detected in the other leg. The number of AFB increased more than 100-fold over the 8-week period (Fig. 3). Footpad swelling was observed after 4 weeks of infection. Necrosis of the footpad and extension of the infection appeared after 7 to 8 weeks. For ethical reasons, mice were euthanatized at that time point. For the subsequent vaccination experiments described in this study, mice were sacrificed at 5 weeks after infection with 105 AFB and at 7 weeks after infection with 3 × 104 AFB, when all of the control mice demonstrated visible swelling of the infected footpad.
FIG. 3.
Mycobacterial replication of M. ulcerans in footpads of B10 mice infected with 105 AFB. Results represent the mean ± SD log10 AFB per milliliter (six mice were tested individually for each time point). Arrows point out different steps during mycobacterial replication in the footpad, i.e., visible swelling or footpad enlargement after 4 weeks and skin ulceration with extensive necrosis of subcutaneous fat after 7 weeks.
Cross-reactive T-cell response against M. bovis BCG antigens in mice infected with M. ulcerans.
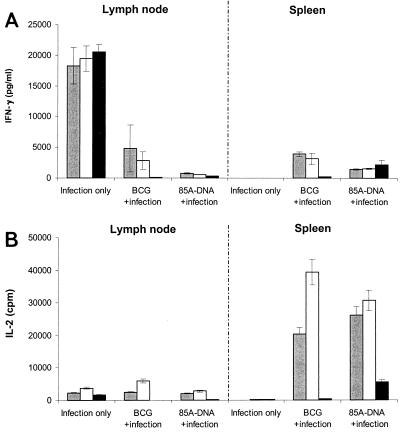
IFN-γ and IL-2 production was examined in inguinal LN cell and spleen cell cultures from B6 mice infected with 3 × 104 M. ulcerans AFB only and in B6 mice infected with M. ulcerans following vaccination with BCG or with DNA encoding Ag85A from BCG. A significant cross-reactive IFN-γ and IL-2 response to M. bovis PPD, CF from M. bovis BCG, and its purified 30- to 32-kDa Ag85 complex could be detected in inguinal LN cell cultures from B6 mice infected in the footpad with M. ulcerans 7 weeks before (Fig. 4). Interestingly, cross-reactive IL-2 or IFN-γ responses were observed only in cell cultures from LN draining the infected hind leg and not in cell cultures from LN draining the uninfected hind leg (data not shown) or in spleen cell cultures from the same mice, clearly demonstrating the localized aspect of the M. ulcerans infection 7 weeks after inoculation. Previous vaccination with BCG or with plasmid DNA encoding Ag85A from M. tuberculosis before infection resulted in significantly lower IL-2 and IFN-γ production in the draining LN, probably because vaccination reduced the multiplication of M. ulcerans (see Table 1). Confirming previous results (14, 15), spleen cell Th1 responses were readily detected in mice vaccinated with BCG or Ag85A DNA upon in vitro stimulation with PPD, BCG CF, and Ag85 (Fig. 4).
FIG. 4.
IFN-γ (A) and IL-2 (B) production in LN cell and spleen cell cultures from unvaccinated and vaccinated B6 mice infected with 3 × 104 M. ulcerans strain 5150 AFB. Cells were restimulated for 3 days in vitro with bovine PPD (grey bars), BCG CF (white bars), or purified Ag85 (black bars). Data are expressed as mean numbers of picograms per milliliter ± SD (IFN-γ) and as mean counts per minute ± SD (IL-2) in culture supernatants (tested in duplicate) from pools of five mice.
TABLE 1.
Mycobacterial multiplication in unvaccinated and vaccinated B6 mice infected by 3 × 104 or 105 M. ulcerans strain 5150 AFB
| Vaccine | Infection with 3 × 104M. ulcerans AFB
|
Infection with 105M. ulcerans AFB
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. of AFB/mla | ΔLog10b | P valuec | No. of AFB/mla | ΔLog10b | No. of CFU/mla | ΔLog10b | P valuec | |
| None | NDd | 6.16 ± 0.28 (7) | 5.99 ± 0.26 (7) | |||||
| Control DNA | 5.95 ± 0.55 (4) | 5.85 ± 0.25 (6) | 5.63 ± 0.45 (6) | |||||
| M. bovis BCG | 4.60 ± 0.35 (6) | 1.35 | <0.005 | 4.00 ± 0.02 (7) | 1.85 | 4.21 ± 0.14 (7) | 1.42 | <0.005 |
| Ag85A DNA | 4.29 ± 0.42 (5) | 1.66 | <0.005 | 4.71 ± 0.67 (7) | 1.14 | 4.52 ± 0.30 (7) | 1.11 | <0.005 |
Mean ± SD of CFU or AFB per milliliter, expressed in log10. The number of mice per group is in parentheses.
ΔLog10, difference compared to mice vaccinated with control DNA.
Each P value shown reflects a comparison with the mean log10 value for control DNA-treated mice infected with M. ulcerans.
ND, not done.
In line with the cytokine results, inguinal LN cells from M. ulcerans-infected mice showed a positive lymphoproliferative response following in vitro stimulation with PPD or purified Ag85 (Fig. 5). Again, antigen-specific T-cell responses were only observed in cell cultures from LN draining the infected footpad and not in those from LN draining the uninfected one. Proliferative responses to PWM could be found in both infected and uninfected LN cell cultures but were clearly higher in the former, probably because of the presence of a higher percentage of activated CD4+ T cells in the infected leg.
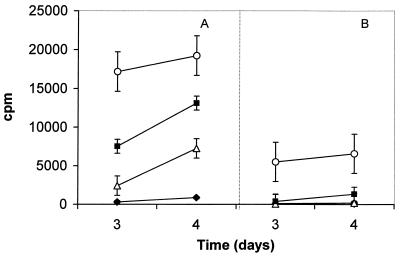
FIG. 5.
Lymphoproliferative response of pooled inguinal LN cells from a pool of five B6 mice infected with 3 × 104 M. ulcerans AFB 7 weeks before. (A) LN draining infected hind legs. (B) LN draining uninfected hind legs. Cells were restimulated for 3 or 4 days in vitro with medium alone (closed diamonds), bovine PPD (black squares), purified Ag85 (open triangles), or PWM (open circles). Data are expressed as mean counts per minute ± SD of triplicate cultures.
Cross-reactive B-cell response against M. bovis BCG antigens in mice infected with M. ulcerans.
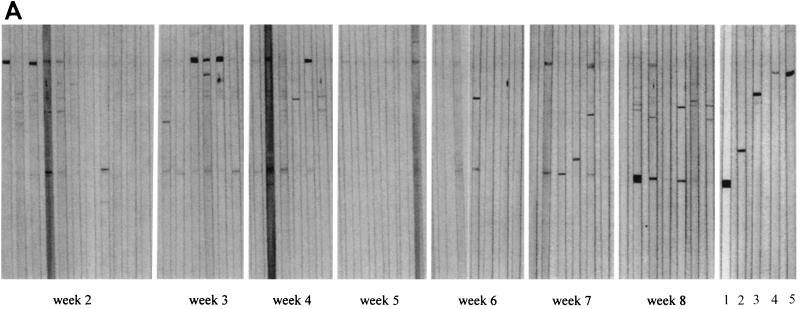
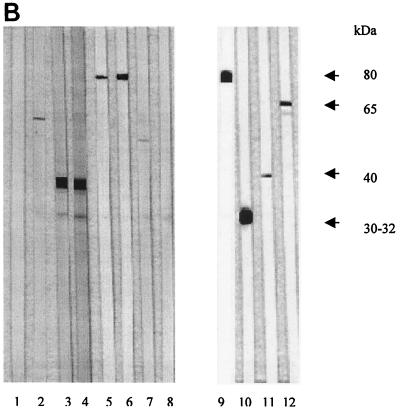
Western blot analysis of individual sera from M. ulcerans-infected B10 mice demonstrated a cross-reactive antibody response against a small number of BCG CF antigens. Whereas early following infection (weeks 2 to 4), this response was directed almost exclusively against a BCG CF protein with an estimated molecular mass of 80 kDa, later in infection (weeks 7 and 8), antibodies were mostly directed against the 30- to 32-kDa Ag85 complex proteins (Fig. 6A). By using recombinant catalase-peroxidase and malate synthase proteins from M. tuberculosis, we demonstrated that the antibody response against the 80-kDa protein in sera from M. ulcerans-infected mice was directed against a homologue of the KatG protein (data not shown). Interestingly, sera from none of the five B6 mice previously vaccinated with BCG and subsequently infected with M. ulcerans produced antibodies against the KatG homologue (Fig. 6B, data shown for two mice). Confirming previous results, sera from BCG-vaccinated B6 mice reacted very weakly against BCG CF after one immunization and strongly against the 40-kDa PstS-3 lipoprotein following two BCG immunizations (17) (Fig. 6B).
FIG. 6.
(A) Western blot analysis of CF antigens from M. bovis BCG separated by SDS–15% polyacrylamide gel electrophoresis of sera (diluted 1:20) from B10 mice infected with 105 AFB of M. ulcerans and tested over an 8-week period. Reactivity of BCG-specific MAbs is shown. Lanes: 1, MAb 17-4, directed against the 30- to 32-kDa Ag85 complex; 2, MAb 2C1-5, directed against the 40-kDa PstS-3 protein; 3, MAb IA1, directed against the 65-kDa hsp65 protein; 4 and 5, IT42 and IT57, directed against the 80-kDa KatG protein. (B) Western blot analysis against CF antigens from M. bovis BCG of sera from B6 mice vaccinated with BCG once (lane 1 and 2) or twice (lane 3 and 4) and from mice infected with 3 × 104 AFB of M. ulcerans only (lane 5 and 6) or after prior BCG vaccination (lane 7 and 8). Reactivity of BCG-specific MAbs is shown. Lanes: 9, MAb 5D9, directed against the 80-kDa KatG protein; 10, MAb 17-4, directed against 30- to 32-kDa Ag85 complex; 11, MAb 2C1-5, directed against the 40-kDa PstS-3 protein; 12, MAb IA1, directed against the 65-kDa hsp65 protein.
Protective effect of vaccination with M. bovis BCG or with plasmid DNA encoding Ag85A against M. ulcerans multiplication.
As shown in Table 1, a more-than-20-fold reduction in the number of M. ulcerans AFB was observed in mice previously vaccinated with BCG or with DNA encoding Ag85A compared to that in mice vaccinated with the empty control plasmid or in naive mice. This highly significant protection was observed in two independent experiments following challenge with increasing doses of M. ulcerans, i.e., 3 × 104 and 105 AFB. Furthermore, the number of actual CFU of M. ulcerans was also determined in the experiment with the 105-AFB challenge. Here again, the reduction in the number of CFU in Ag85A DNA-vaccinated mice compared to that in mice injected with the empty vector was highly significant. Numbers of AFB or CFU per milliliter in naive mice infected with M. ulcerans were not significantly different from numbers in mice vaccinated with control DNA.
DISCUSSION
Following tuberculosis and leprosy, Buruli ulcer disease is the third most common mycobacterial disease (44). The first International Conference on Buruli Ulcer Control and Research, held in 1998 in Côte d'Ivoire, expressed concern that little is known about this disease and called on the international community to support control and research efforts. It is clear that prevention of infection through vaccination of populations at high risk could ultimately be the best strategy for controlling this emerging disease.
The currently available tuberculosis vaccine, i.e., the M. bovis BCG vaccine, is very effective in preventing disseminated TB in infants and young children (31) but does not have a significant impact on pulmonary TB in young adults (6). Interestingly, this same BCG vaccine was reported to confer protection against leprosy in Papua New Guinea (2), central Brazil (32), and northern Malawi (29) and against Buruli ulcer in two controlled clinical trials in Uganda (5, 37), indicating that immune responses against common mycobacterial antigens present in the BCG vaccine may be important for the control of mycobacterial diseases. Obviously, the toxin produced by M. ulcerans may be another key antigen relevant for vaccine development, but it is unknown whether protective immune responses can be generated against this polyketide-like structure, which moreover was reported to have immunosuppressive activities (12).
It is now generally accepted that a major protective antigen present in the BCG vaccine is the so-called Ag85 complex, a protein family involved in cell wall synthesis and triggering strong cell-mediated immune responses in situations of controlled, primary M. tuberculosis infection. In this paper, we have reported on the cloning and sequencing of a gene of M. ulcerans strain 5150 encoding an Ag85 homologue. M. ulcerans and M. marinum are known to be genetically very similar (42), and while this work was in progress, sequencing of a 417-bp fragment of M. ulcerans revealed a similarity of more than 99% with the Ag85A gene of M. marinum (38). Comparison of our M. ulcerans sequence with this partial sequence and the complete sequence of M. bovis BCG confirmed our blast analysis, indicating that our gene indeed encodes the Ag85A component from M. ulcerans.
Mice experimentally infected in the hind footpad with M. ulcerans type 1 strain 5150 from the Democratic Republic of Congo demonstrated a strong cross-reactive Th1-type immune response against M. bovis BCG and the 30- to 32-kDa Ag85 complex purified from BCG CF. Significant IL-2 and IFN-γ secretion, as well as lymphoproliferative responses, could be demonstrated in inguinal LN cells draining the infected footpad but not in cells from LN draining the uninfected footpad or in the spleen. The 84% sequence identity and 91% similarity between the mature M. bovis BCG and M. ulcerans Ag85A proteins could effectively explain this cross-reactive Th1 immune response. Interestingly, one of the two Ag85 peptide regions previously described as immunodominant in BCG and Ag85A DNA-vaccinated B6 mice (aa 261 to 280) was almost completely identical (one conserved change at aa 275) (18, 39). In line with the strong but localized cellular immune response, systemic antibody responses against BCG CF antigens were very weak in M. ulcerans-infected mice. However, antibodies directed against an 80-kDa protein antigen from BCG CF were found in 17 (35%) of 48 M. ulcerans-infected mice and, interestingly, prior BCG vaccination inhibited the appearance of these antibodies. At least two protein antigens with similar sizes have been described in BCG CF, i.e., a peroxidase-catalase (KatG) and a malate synthase (GlcB) (Suman Laal, New York University School of Medicine, personal communication). By using recombinant KatG and GlcB from M. tuberculosis, we found that the anti-80-kDa antibodies in sera from M. ulcerans-infected mice were directed against the former protein, demonstrating that M. ulcerans expresses a catalase-peroxidase homologue as well. Recently, Dobos et al. reported on the serologic response to CF antigens of M. ulcerans in 70% of patients suffering from Buruli ulcer. Three M. ulcerans antigens with estimated molecular masses of 70, 36 to 38, and 5 kDa were commonly recognized by Buruli ulcer patients' sera, and this antibody response was consistent throughout the early to late disease stages (9). Apart from the weak skin test reactions to tuberculin or burulin and the paper of Dobos et al. on serology, little is known concerning the cellular and humoral immune responses of patients with Buruli ulcer. Our results indicate that analysis of the T-cell response to CF antigens and Ag85, in particular, could give us more insight into the immune mechanisms involved in control of the infection. Also, it would be interesting to analyze the antibody response against whole BCG CF in infected subjects in order to find out whether the KatG protein is a dominant B-cell antigen in infected humans, as it is in infected B6 (and BALB/c [data not shown]) mice. M. ulcerans can be grown in liquid protein-free media (28), and we plan to start a comparative study of immune responses to M. bovis BCG and M. ulcerans CF in the near future in Benin (E. Lozes, personal communication).
Vaccination with BCG or with plasmid DNA encoding Ag85A from M. bovis BCG was very effective in reducing the bacterial replication of M. ulcerans in the footpad. Both the number of AFB determined by classical Ziehl-Neelsen staining and the number of CFU determined by plating on specific 7H11 agar were about 20-fold lower in vaccinated mice than in mice vaccinated with the empty control vector or in naive, unvaccinated mice. Although our results have to be interpreted with caution, they are promising for future Buruli ulcer vaccine development. Plasmid DNA vaccination appears to be a powerful and easy method for the definition of protective antigens for this disease. These initial experiments now have to be repeated with other M. ulcerans isolates and with plasmids encoding other antigens, additional mouse strains, and eventually other experimental animal species, and with longer resting periods between vaccination and challenge. Also, a direct comparison of the immunogenicity and protective efficacy of plasmid DNA encoding Ag85A from M. ulcerans and of M. bovis BCG is in progress. Despite the extensive homology between both Ag85A proteins, minor differences do exist and use of the Ag85A-encoding gene from M. ulcerans could provide a more potent vaccine. Finally, immunotherapeutic plasmid vaccination, which has been reported to be effective for TB with plasmid DNA encoding hsp65 (26) but not Ag85A (43), can now be examined for M. ulcerans infection.
In conclusion and in view of the more than 90% amino acid sequence similarity between Ag85A from M. ulcerans and Ag85A from M. bovis BCG, these results hold promise for future TB, Buruli ulcer, and possibly leprosy vaccines based on common antigenic components.
ACKNOWLEDGMENTS
The excellent technical skills of C. Fissette (ITM), A. Van Aerde (ITM), V. Motte, F. Jurion, K. Palfliet, N. De Smet, and A. Vanonckelen are gratefully acknowledged. Special thanks go to M. Decock (Hôpital Erasme ULB, Brussels, Belgium) for help with the Western blots and to V. Rosseels for help with the scans. We are grateful to I. Feck for technical assistance in DNA sequencing, to P. Gilot for helping with the cloning procedure, and particularly to J. Ooms for enthusiastic technical assistance throughout this study. We thank M. A. Liu (previously at Merck Research Laboratories, West Point, Pa.) for giving us the V1Jns.tPA vector and J. Belisle (Colorado State University, Fort Collins) for MAbs IT42 and IT57 and the recombinant KatG and GlcB proteins (material produced through funds from NIH, NIAID, contract NO1-AI-75320, entitled “Tuberculosis Research Materials and Vaccine Testing”).
This work was supported by grants from the Damiaanaktie-Belgium and by grant G.0266.00 from the Fonds voor Wetenschappelijk Onderzoek Vlaanderen.
REFERENCES
- 1.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagshawe A, Scott G C, Russell D A, Wigley S C, Merianos A, Berry G. BCG vaccination in leprosy: final results of the trial in Karimui, Papua New Guinea, 1963–79. Bull W H O. 1989;67:389–399. [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin S L, D'Souza C D, Orme I M, Liu M A, Huygen K, Denis O, Tang A, Zhu L, Montgomery D, Ulmer J B. Immunogenicity and protective efficacy of DNA vaccines encoding secreted and non-secreted forms of Mycobacterium tuberculosis Ag85A. Tubercle Lung Dis. 1999;79:251–259. doi: 10.1054/tuld.1998.0196. [DOI] [PubMed] [Google Scholar]
- 4.Belisle J T, Vissa V D, Sievert T, Takayama K, Brennan P J, Besra G S. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 5.Bradley D J, Hutt M S R, Kiryabwire J W M, Kisuule A, Lloyd A, Mafigiri J, Morrow R H, Passon W, Phillips I, Pike M C, Pike P A, Revill D L. B.C.G. vaccination against Mycobacterium ulcerans infection (Buruli Ulcer). First results of a trial in Uganda. Lancet. 1969;i:111–115. [PubMed] [Google Scholar]
- 6.Colditz G A, Brewer T F, Berkey C S, Wilson M E, Burdick E. Efficacy of BCG vaccine in the prevention of tuberculosis: meta-analysis of the published literature. JAMA. 1994;271:689–702. [PubMed] [Google Scholar]
- 7.Content J, de la Cuvellerie A, de Wit L, Vincent-Levy-Frébault V, Ooms J, de Bruyn J. The genes coding for the antigen 85 complexes of Mycobacterium tuberculosis and Mycobacterium bovis BCG are members of a gene family: cloning, sequence determination, and genomic organization of the gene coding for antigen 85-C of M. tuberculosis. Infect Immun. 1991;59:3205–3212. doi: 10.1128/iai.59.9.3205-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Bruyn J, Huygen K, Bosmans R, Fauville M, Lippens R, Van Vooren J-P, Falmagne P, Weckx M, Wiker H G, Harboe M, Turneer M. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb Pathog. 1987;2:351–366. doi: 10.1016/0882-4010(87)90077-5. [DOI] [PubMed] [Google Scholar]
- 9.Dobos K M, Spotts E A, Marston B J, Horsburgh C R, Jr, King C H. Serologic response to culture filtrate antigens of Mycobacterium ulcerans during Buruli ulcer disease. Emerg Infect Dis. 2000;6:158–164. doi: 10.3201/eid0602.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fauville-Dufaux M, Vanfleteren B, De Wit L, Vincke J P, Van Vooren J P, Yates M D, Serruys E, Content J. Rapid detection of tuberculous and non-tuberculous mycobacteria by polymerase chain reaction amplification of a 162 bp DNA fragment from antigen 85. Eur J Clin Microbiol Infect Dis. 1992;11:797–803. doi: 10.1007/BF01960878. [DOI] [PubMed] [Google Scholar]
- 11.Garbe T R, Hibler N S, Deretic V. Isoniazid induces expression of the antigen 85 complex in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1996;40:1754–1756. doi: 10.1128/aac.40.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George K M, Chatterjee D, Gunawardana G, Welty D, Hayman J, Lee R, Small P. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science. 1999;283:854–857. doi: 10.1126/science.283.5403.854. [DOI] [PubMed] [Google Scholar]
- 13.Horwitz M A, Lee B W E, Dillon B J, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubbard R D, Flory C M, Collins F M. Immunization of mice with mycobacterial culture filtrate proteins. Clin Exp Immunol. 1992;87:94–98. doi: 10.1111/j.1365-2249.1992.tb06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huygen K, Abramowicz D, Vandenbussche P, Jacobs F, De Bruyn J, Kentos A, Drowart A, Van Vooren J-P, Goldman M. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992;60:2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J-P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 17.Huygen K, Drowart A, Harboe M, ten Berg R, Cogniaux J, Van Vooren J P. Influence of genes from the major histocompatibility complex on the antibody repertoire against culture filtrate antigens in mice infected with live Mycobacterium bovis BCG. Infect Immun. 1993;61:2687–2693. doi: 10.1128/iai.61.6.2687-2693.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huygen K, Lozes E, Gilles B, Drowart A, Palfliet K, Jurion F, Roland I, Art M, Dufaux M, Nyabenda J, De Bruyn J, Van Vooren J-P, Deleys R. Mapping of Th1 helper T-cell epitopes on major secreted mycobacterial antigen 85A in mice infected with live Mycobacterium bovis BCG. Infect Immun. 1994;62:363–370. doi: 10.1128/iai.62.2.363-370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huygen K, Van Vooren J-P, Turneer M, Bosmans R, Dierckx P, De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988;27:187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 20.Johnson R, Stinear T P, Hayman J A. Mycobacterium ulcerans—a mini-review. J Med Microbiol. 1999;48:511–513. doi: 10.1099/00222615-48-6-511. [DOI] [PubMed] [Google Scholar]
- 21.Joliff G, Mathieu L, Hahn V, Bayan N, Duchiron F, Renaud M, Shechter E, Leblon G. Cloning and nucleotide sequence of the csp1 gene encoding PS1, one of the two major secreted proteins of Corynebacterium glutamicum: the deduced N-terminal region of PS1 is similar to the Mycobacterium antigen 85 complex. Mol Microbiol. 1992;6:2349–2362. doi: 10.1111/j.1365-2958.1992.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 22.Kamath A T, Feng C G, MacDonald M, Briscoe H, Britton W J. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun. 1999;67:1702–1707. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Launois P, DeLeys R, N'Diaye Niang M, Drowart A, Andrien M, Dierckx P, Cartel J-L, Sarthou J-L, Van Vooren J-P, Huygen K. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect Immun. 1994;62:3679–3687. doi: 10.1128/iai.62.9.3679-3687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Launois P, Huygen K, De Bruyn J, N'Diaye M, Diouf B, Sarthou J L, Grimaud J, Millan J. T cell response to purified filtrate antigen 85 from Mycobacterium bovis bacillus Calmette-Guérin (BCG) in leprosy patients. Clin Exp Immunol. 1991;86:286–290. doi: 10.1111/j.1365-2249.1991.tb05811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Launois P, Niang M N, De Bruyn J, Sarthou J L, Rivier F, Drowart A, Van Vooren J-P, Millan J, Huygen K. The major secreted antigen complex (Ag 85) from Mycobacterium bovis bacille Calmette-Guérin is associated with protective T cells in leprosy: a follow-up study of 45 household contacts. J Infect Dis. 1993;167:1160–1167. doi: 10.1093/infdis/167.5.1160. [DOI] [PubMed] [Google Scholar]
- 26.Lowrie D B, Tascon R E, Bonato V L D, Lima V M F, Faccioli L H, Stavropoulos E, Colston M J, Hewinson R G, Moelling K, Silva C L. Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400:269–271. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- 27.Matsuo K, Yamaguchi R, Yamazaki A, Tasaka H, Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular α antigen. J Bacteriol. 1988;170:3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mve-Obiang A, Remacle J, Palomino J C, Houbion A, Portaels F. Growth and cytotoxic activity by Mycobacterium ulcerans in protein-free media. FEMS Microbiol Lett. 1999;181:153–157. doi: 10.1111/j.1574-6968.1999.tb08838.x. [DOI] [PubMed] [Google Scholar]
- 29.Pönnighaus J M, Fine P E M, Sterne J A C, Wilson R J, Msosa E, Gruer P J K, Jenkins P A, Lucas S B, Liomba N G, Bliss L. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet. 1992;339:636–639. doi: 10.1016/0140-6736(92)90794-4. [DOI] [PubMed] [Google Scholar]
- 30.Portaels F, Fonteyne P-A, De Beenhouwer H, De Rijk P, Guédénon A, Hayman J, Meyers W M. Variability in 3′ end of 16S rRNA sequence of Mycobacterium ulcerans is related to geographic origin of isolates. J Clin Microbiol. 1996;34:962–965. doi: 10.1128/jcm.34.4.962-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodrigues L C, Diwan V K, Wheeler J G. Protective effect of BCG against tuberculosis meningitis and military tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22:1154–1158. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues M L O, Silva S A, Neto J C A, de Andrade A L S S, Martelli C M T, Zicker F. Protective effect of intradermal BCG against leprosy; a case-control study in central Brazil. Int J Leprosy. 1992;60:335–339. [PubMed] [Google Scholar]
- 33.Ronning D R, Klabunde T, Besra G S, Vissa V D, Belisle J, Sacchettini J C. Crystal structure of the secreted form of antigen 85C reveals potential targets for mycobacterial drugs and vaccines. Nat Struct Biol. 2000;7:141–146. doi: 10.1038/72413. [DOI] [PubMed] [Google Scholar]
- 34.Ross B C, Johnson R, Oppedisano F, Marino L, Sievers A, Stinear T, Hayman J A, Veitch K, Robins-Browne R M. Detection of Mycobacterium ulcerans in environmental samples during an outbreak of ulcerative disease. Appl Environ Microbiol. 1997;63:4135–4138. doi: 10.1128/aem.63.10.4135-4138.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross B C, Marino L, Oppediasano F, Edwards R, Robins-Browne R M, Johnson P D R. Development of a PCR for rapid diagnosis of Mycobacterium ulcerans infection. J Clin Microbiol. 1997;35:1696–1700. doi: 10.1128/jcm.35.7.1696-1700.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shepard C C, McRae D H. A method for counting acid-fast bacteria. Int J Leprosy. 1968;36:78–82. [PubMed] [Google Scholar]
- 37.Smith P G, Revill W D L, Lukwago E, Rykushin Y P. The protective effect of BCG against Mycobacterium ulcerans disease: a controlled trial in an endemic area of Uganda. Trans R Soc Trop Med Hyg. 1976;70:449–457. doi: 10.1016/0035-9203(76)90128-0. [DOI] [PubMed] [Google Scholar]
- 38.Stinear T P, Jenkin G A, Johnson P D R, Davies J K. Comparative genetic analysis of Mycobacterium ulcerans and Mycobacterium marinum reveals evidence of recent divergence. J Bacteriol. 2000;182:6322–6330. doi: 10.1128/jb.182.22.6322-6330.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanghe A, D'Souza S, Rosseels V, Denis O, Ottenhoff T H M, Dalemans W, Wheeler C, Huygen K. Increased immunogenicity and protective efficacy of a tuberculosis DNA vaccine encoding Ag85 following protein boost. Infect Immun. 2001;69:3041–3047. doi: 10.1128/IAI.69.5.3041-3047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanghe A, Denis O, Lambrecht B, Motte V, van den Berg T, Huygen K. Tuberculosis DNA vaccine encoding Ag85A is immunogenic and protective when administered by intramuscular needle injection, but not by epidermal gene gun bombardment. Infect Immun. 2000;68:3854–3860. doi: 10.1128/iai.68.7.3854-3860.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thole J E R, Van Schooten W C A, Keulen W J, Hermans P W M, Janson A A M, de Vries R R P, Kolk A H J, van Embden J D A. Use of recombinant antigens expressed in Escherichia coli K-12 to map B-cell and T-cell epitopes on the immunodominant 65-kilodalton protein of Mycobacterium bovis BCG. Infect Immun. 1988;56:1633–1640. doi: 10.1128/iai.56.6.1633-1640.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tonjum T, Welty D B, Jantzen E, Small P L. Differentiation of Mycobacterium ulcerans, M. marinum, and M. haemophilum: mapping of their relationships to M. tuberculosis by fatty acid profile analysis, DNA-DNA hybridization, and 16S rRNA gene sequence analysis. J Clin Microbiol. 1998;36:918–925. doi: 10.1128/jcm.36.4.918-925.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner J, Rhoades E R, Keen M, Belisle J T, Frank A A, Orme I M. Effective preexposure tuberculosis vaccines fail to protect when they are given in an immunotherapeutic mode. Infect Immun. 2000;68:1706–1709. doi: 10.1128/iai.68.3.1706-1709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Werf T S, van der Graaf W T A, Tappero J W, Asiedu K. Mycobacterium ulcerans infection. Lancet. 1999;354:1013–1018. doi: 10.1016/S0140-6736(99)01156-3. [DOI] [PubMed] [Google Scholar]
- 45.Van Vooren J-P, Drowart A, De Bruyn J, Launois P, Millan J, Delaporte E, Develoux M, Yernault J C, Huygen K. Humoral responses against the 85A and 85B antigens of Mycobacterium bovis BCG in patients with leprosy and tuberculosis. J Clin Microbiol. 1992;30:1608–1610. doi: 10.1128/jcm.30.6.1608-1610.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vercammen M, Scorza T, Huygen K, De Braekeleer J, Diet R, Jacobs D, Saman E, Verschueren H. DNA vaccination with genes encoding Toxoplasma gondii antigens GRA1, GRA7, and ROP2 induces partially protective immunity against lethal challenge in mice. Infect Immun. 2000;68:38–45. doi: 10.1128/iai.68.1.38-45.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiker H G, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]