Abstract
Most isolates of Streptococcus pneumoniae are mixed populations of transparent (T) and opaque (O) colony phenotypes. Differences in the production of capsular polysaccharide (CPS) between O and T variants were accentuated by changes in the environmental concentration of oxygen. O variants demonstrated a 5.2- to 10.6-fold increase in amounts of CPS under anaerobic compared to atmospheric growth conditions, while CPS production remained low under all conditions for T variants. Increased amounts of CPS in O compared to T pneumococci were associated with increased expression of cps-encoded proteins. The inhibitory effect of oxygen on expression of CPS in O variants correlated with decreased tyrosine phosphorylation of CpsD, a tyrosine kinase and regulator of CPS synthesis. Modulation of CpsD expression and its activity by tyrosine phosphorylation may allow the pneumococcus to adapt to the requirements of both colonization, where decreased CPS allows for adherence, and bacteremia, where increased CPS may be required to escape from opsonic clearance. In patients with invasive infection, paired isolates from the same patient were shown to have predominately a T colony phenotype without phosphotyrosine on CpsD when cultured from the nasopharynx, and an O phenotype that phosphorylates CpsD in response to oxygen when cultured from the blood. Differences in the availability of oxygen, therefore, may be a key factor in allowing for the selection of distinct phenotypes in these two host environments.
Streptococcus pneumoniae (the pneumococcus) colonizes the mucosal surface of the human nasopharynx. Unlike the many other species of streptococci carried in the human respiratory tract, the pneumococcus is also a common cause of disease. Infection generally occurs when host factors allow the organism access to the normally sterile parts of the upper (resulting in otitis media and sinusitis) and lower respiratory tract (resulting in pneumonia). From these sites it may also pass through tissue barriers into the bloodstream, resulting in the most serious forms of pneumococcal disease, sepsis and meningitis.
The characteristic of the pneumococcus most clearly associated with its ability to cause disease and that distinguishes it from closely related but non pathogenic oral streptococci is its expression of a polysaccharide capsule (40). S. pneumoniae is capable of synthesizing at least 90 structurally unique capsular polysaccharide (CPS), which form the basis of serotyping. The expression of CPS renders the organism resistant to the major mechanism of clearance, opsonophagocytosis, in hosts lacking type-specific antibody of sufficient quantity or avidity (13, 24). Expression of anti phagocytic CPS, however, has been shown to inhibit adherence of pneumococci to host cells, a critical step in carriage and possibly later aspects in the pathogenesis of disease (23). Similar findings in other encapsulated bacterial pathogens, including Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pyogenes and Streptococcus agalactiae, have led to an appreciation that the amounts of CPS must be varied at different stages in the pathogenesis of invasive infection to allow both for adhesive interactions with host cells and for resistance to humoral clearance mechanisms (7, 10, 15, 28, 29, 31).
Previous reports from this laboratory have demonstrated both intra- and interstrain variability in the quantity of CPS produced by the pneumococcus, although the regulatory mechanisms controlling its expression remain incompletely understood (13, 16). The majority of clinical isolates consist of heterogeneous populations of at least two phenotypes distinguished by their differences in colony opacity (37, 38). Opaque (O) and transparent (T) colony variants of the same strain differ in the amounts of CPS produced, as well as in other characteristics (13). It is also well established that relatively minor differences in amounts of CPS may have a major impact on virulence (16). The 1.2- to 5.6-fold-higher quantities of CPS in the O form compared to the T form of the same strain correlates with increased resistance to opsonophagocytosis following exposure to human serum containing type-specific antibody (14). In addition, only the O variant of several strains was able to cause sepsis in a murine model of systemic infection (13). In contrast, the T variants express greater amounts of the other major cell surface polysaccharide, the cell wall teichoic acid. The pneumococcal cell wall teichoic acid to which CPS is covalently linked contains an unusual host-like constituent, phosphorylcholine, that contributes to pneumococcal adherence to epithelial cells through binding of the receptor for platelet-activating factor (3, 4, 13, 30). The T form also displays an altered distribution of cell surface choline-binding proteins, including CbpA, which functions in adherence and colonization (25). During carriage in an infant rat model, there is a selection for variants of the T phenotype, which expresses increased amounts of these adhesins and diminished quantities of adherence-inhibiting CPS (38). Taken together, these observations suggest that the pneumococcus varies between a form with cell surface characteristics optimized for adherence and carriage (T pneumococci) and a form that adheres poorly but is better adapted for survival during inflammation or invasive infection, when the amounts of antibody, complement, and phagocytic cells might otherwise limit the viability of the organism (O pneumococci). This transition from one form to the other is seen in the predominant phenotype isolated during the course of experimental otitis media following intranasal challenge in chinchillas. Initially, there is a preponderance of the T variant but, as inflammation progresses over time, there is an increasing selection for the O form (34). The relationship between the opacity phenotype and pneumococcal infection in humans has yet to be described.
In this report we examine the hypothesis that differences in ambient oxygen concentration affect the regulation of CPS synthesis. The results of this study suggest that (i) the oxygen availability on the airway surface suppresses CPS production and, therefore, may facilitate adherence of pneumococci during the commensal state, and that (ii) the less-aerobic microenvironment encountered, for example, in the dense consolidation of the alveolar spaces during pneumonia or the inflammatory exudate in the middle ear space during otitis media may enhance virulence by triggering increased expression of CPS (8, 9, 26). Phenotypic differences in clinical isolates, furthermore, support the concept that different host environments select for distinct subpopulations of pneumococci in natural infection.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The clinical isolates of S. pneumoniae P303, P324, P68, P10, and D39 used in this study were previously described (14). Broth cultures were plated onto tryptic soy plates solidified with 1% agar onto which 5,000 U of catalase (Worthington Biochemical, Freehold, N.J.) was spread and incubated at 37°C in a candle extinction jar unless otherwise specified. The O and T phenotypes of each isolate were separated and studied as uniform populations (>99.9% the desired phenotype). Colony morphology was determined on transparent medium under magnification and oblique, transmitted illumination as previously described (38). Bacteria were grown to mid-log phase (A620 = 0.3) or for 16 h (stationary phase) with gentle shaking at 100 rpm for atmospheric conditions (aerobic growth) or without shaking for microaerophilic and anaerobic conditions in unsealed shallow vessels at 37°C in a semisynthetic (C+Y medium; pH 6.8) or tryptic soy medium as specified (33). Different concentrations of environmental carbon dioxide in aerobic conditions were provided in an incubator with adjustable levels of CO2. Strict anaerobic growth conditions were obtained with the BBL GasPak System (Becton Dickinson, Cockeysville, Md.) either with or without the sodium bicarbonate component for generating an atmosphere with or without 10% carbon dioxide, respectively, according to the manufacturer's specifications. After harvesting of the bacteria, the pH of the growth medium was measured to ensure that there was no differential effect of different culture conditions. Unless otherwise stated, chemicals and reagents were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Cloning and nucleotide sequencing of cpsD.
The complete sequence of cpsD from the O and T variants of P303 was obtained by inverse PCR by using primers based on a conserved 5′ portion of the gene in order to obtain the sequence at the more variable 3′ region. Chromosomal DNA digested with DraI was ligated to itself and amplified with primers 5′-TACAGACCTATCACAAGGGC-3′ and 5′-CCTGTAATCTTATCCCTTGC-3′. The PCR product was cloned into the pCR2.1 TOPO vector (Invitrogen Co., Carlsbad, Calif.) for sequencing of the insert. This region was then extended in the 5′ direction with primers based on the more-conserved 5′ portion of gene to obtain the entire cpsD sequence from both the O variant and the T variant. The accession number is AF359247.
Generation of antisera to CpsD.
The entire cpsD gene from P303 was amplified by PCR using primers 5′-CATATGCCAACATTAGAAATCTCAC-3′ and 5′-CTCGAGTTTTTTATTTTTCCCGTAATCT-3′ and digested with NdeI and XhoI for cloning into these sites in pET29b(+) (Novagen, Madison, Wis.). Production of the protein was induced in Escherichia coli host strain BL21(DE3)/pLysS by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside) at a final concentration 2 mM. The protein was isolated from these cells and purified with a Ni2+ column (His-Bind Resin) according to the manufacturer's standard protocol for denaturing conditions at pH 7.2 (Novagen). After the purity of the protein was confirmed in Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, it was used to immunize rabbits to generate immune serum at a commercial facility according to its standard protocol (Cocalico Biologicals, Reamstown, Pa.).
Analysis and quantification of CPS.
The expression of CPS was confirmed by the quellung reaction. The presence and size of refractile zones were determined after an equal volume of cells grown in liquid culture to mid-log phase and type-specific antisera (Statens Seruminstitut, Copenhagen, Demark) in 1% methylene blue were mixed on a glass slide and agitated for 1 min.
Amounts of CPS were measured in O and T variants grown in semisynthetic medium as described above. Cells were harvested at 2,000 × g and washed in phosphate-buffered saline (PBS), and the cell fraction was sonicated for three 10-s intervals on ice prior to storage at −20°C. A capture enzyme-linked immunosorbent assay (ELISA) technique was used to determine quantities of CPS present in variants grown under different conditions (13). Type-specific rabbit anti serum (Statens Seruminstitut) at a dilution of 1:5,000 in 0.05 M Na2CO3 (pH 9.6) was fixed overnight at room temperature on microtiter plates. Between each incubation step, the plate was washed five times with Tris buffer (10 mM Tris, 150 mM NaCl, 0.05% Brij, 0.02% sodium azide). Purified type 6A, 6B, 9V, or 18C CPS at a known concentration purchased from the American Type Culture Collection (Rockville, Md.) was used as a standard. CPS in cell sonicate fractions was detected with Monoclonal antibodies (MAbs) HASP 4 (against group 6 CPS), HASP 22 (against type 18C CPS), and HASP 33 (against type 9V CPS) obtained from Uffe B. Skov-Sørenson and used at a concentration determined in pilot experiments. Binding of MAbs was detected with an antiserum to mouse immunoglobulin M conjugated to alkaline phosphatase and developed as previously described (13). The total cellular protein determination was carried out on sonicated cells with the Micro Bicinchoninic Acid Kit according to the manufacturer's directions (Pierce Chemical Co., Rockford, Ill.). The amount of CPS in the supernatant fraction was normalized to the protein concentration in the corresponding cell sonicate fraction. A similar capture ELISA was used to compare amounts of total teichoic acid in cell sonicates as previously described (13). All experiments were performed in duplicate at least three times and expressed as mean values per total cellular protein concentration.
Western analysis.
Bacteria were grown in semisynthetic medium to mid-log phase under atmospheric conditions or under anaerobic conditions with or without supplemental carbon dioxide as described above. The bacteria were maintained at 4°C, collected at 1,500 × g, washed in an equal volume of PBS (pH 7.2) and resuspended in 2.5% of the original culture volume in PBS. For cells to be treated with alkaline phosphatase, 50 mM Tris-Cl (pH 7.2), 100 mM NaCl, and 1 mM dithiothreitol were used instead of PBS. The washed, concentrated cells were then sonicated as described above. When specified, the sonicates were treated with or without calf intestinal alkaline phosphatase at 25 U per 25 μl of sonicate for 10 min at 30°C. An aliquot of the sonicate was used to measure the total protein content as described above. Equal amounts of sample based on the protein concentration were added to gel loading buffer and heated to 100°C for 5 min before separation on 12.5 or 15% SDS-PAGE gels prior to transfer to Immobilon-P membranes as previously described (36). Equal loading of sonicates was confirmed by Ponseau S staining of membranes prior to immunoblotting. Antisera to CpsD from P303 was used at a dilution of 1 in 1,000, and binding of the antibody was detected with antisera to rabbit immunoglobulins conjugated to alkaline phosphatase. Immunoblotting was carried out with antiphosphotyrosine MAb (P-Tyr-102; Cell Signaling Technology, Inc., Beverly, Mass.) according to the manufacturer's specifications for blocking, binding, and developing using the ECL Kit (Amersham, Arlington, Ill.). In some experiments, after the membranes were developed the antibody was stripped off according to protocol provided in the ECL Kit (Amersham), and equal loading of specimens was confirmed by reimmunobotting membranes with antiserum raised in rabbits to pneumolysin and then detected using an antisera to rabbit immunoglobulins conjugated to alkaline phosphatase.
Analysis of clinical isolates.
We were initially unsuccessful in isolating pneumococcus from the nasopharynx of bacteremic patients because of prior antibiotic treatment. It was necessary to obtain paired isolates from the same patient for this comparison because of marked strain-to-strain heterogeneity in colony morphology that was unrelated to opacity. Cultures of the nasopharynx and blood, therefore, had to be obtained at the same time and before the initiation of antimicrobial therapy. This limitation required that the study be carried out in a location with a high rate of pneumococcal bacteremia to allow a sufficient numbers of paired isolates to be obtained. Paired blood-cerebrospinal fluid and nasopharyngeal isolates were obtained from adult patients at the Queen Elizabeth Central Hospital in Blantyre, Malawi. These isolates were minimally passaged, avoiding selection of single colonies to minimize selection of a variant population. Isolates were only considered as paired isolates if subsequently shown to be of the same type by the quellung reaction and by identical patterns of restriction fragment length polymorphisms following digestion of chromosomal DNA with EcoRI and ClaI. Many of the patients were also positive for the human immunodeficiency virus (HIV), thus accounting for the high incidence of invasive pneumococcal disease.
RESULTS
Effect of oxygen on CPS expression.
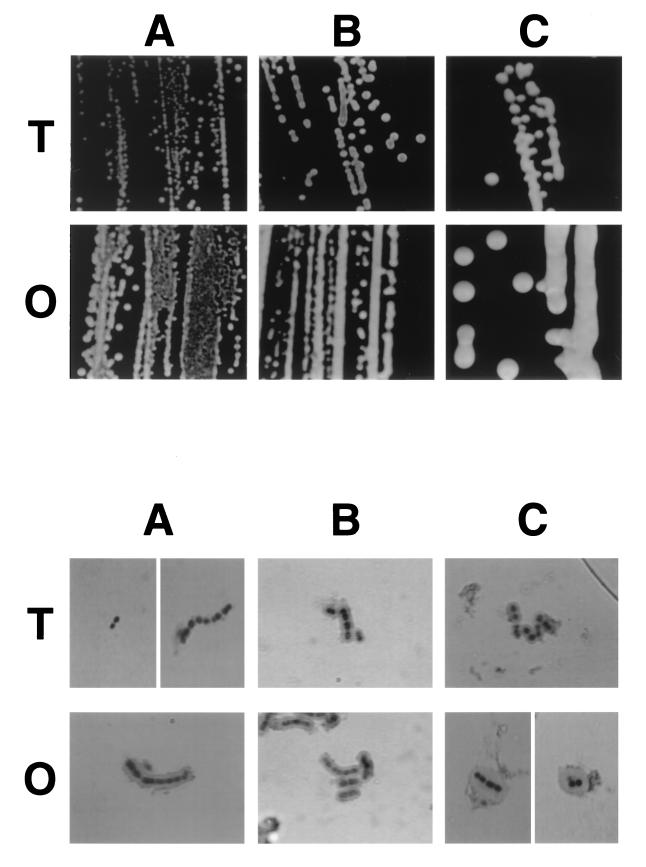
Initial studies addressed whether differences in the environmental concentration of oxygen or carbon dioxide affected the rate of spontaneous switching between the O and T forms. Single colonies of the O or T variants of type 6A clinical isolate, P303, were passed and grown under atmospheric and anaerobic conditions with or without supplemental carbon dioxide (5 to 10%). None of the conditions compared had a significant effect on the previously described background rate (10−3 to 10−4/generation) of spontaneous switching from the T to the O phenotype or from the O to the T phenotype (38). Growth under anaerobic or microaerophilic conditions, however, was associated with a shift to a larger and more mucoid colony phenotype with the greatest effect on the O variant (Fig. 1, upper panels).
FIG. 1.
The effect of environmental oxygen and carbon dioxide on colony morphology and capsule size. Opaque (O) and transparent (T) variants of a type 6A isolate were grown in nutrient broth at 37°C to mid-log phase under the following conditions: atmospheric (A), microaerophilic (B), or anaerobically with supplemental carbon dioxide (C). The upper panels shows colony size and opacity with oblique, transmitted illumination (magnification, ×45). The lower panels show the refractile zone occupied by the capsule as visualized using the quellung reaction with group 6 antiserum (magnification, ×3,200).
Since organisms of the O phenotype have been shown to express an increased amount of CPS compared to the those of the T form, the possibility that the greater colony size resulted from increased production of this material was examined (13). In initial studies, the refractile zone of CPS surrounding the bacterium was visualized by using the quellung reaction with group 6 antisera (Fig. 1, lower panels). This refractile zone was larger for the O variant grown under anaerobic or microaerophilic conditions (in 10% CO2) compared to growth of the same phenotype in atmospheric conditions or compared to that of the T variant under any of the conditions tested, including the absence of oxygen. Under atmospheric conditions, capsular material could not be visualized on T pneumococci when we used this technique. This result confirmed the ability of the O variant to synthesize increased quantities of cell-associated CPS and indicated that reduced oxygen and/or higher carbon dioxide tension may accentuate these differences.
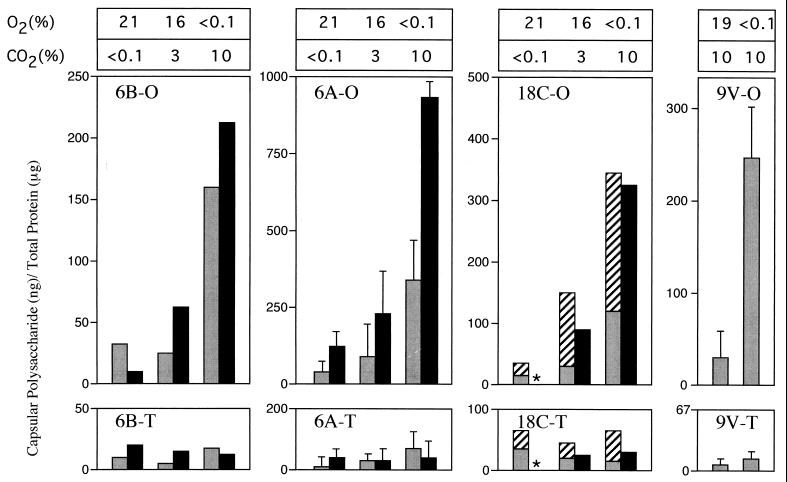
The effect of the environmental oxygen and/or the carbon dioxide concentration was then assessed in a quantitative assay developed for measuring amounts of CPS (Fig. 2) (13). The amounts of cell-associated CPS expressed relative to total cellular protein content was measured using a capture ELISA. For P303, the quantity of CPS correlated with colony surface area and with capsular volumes calculated on the basis of refractile zones in quellung experiments (Table 1). As expected, P303-O pneumococci grown to either mid-log phase or stationary phase under all conditions tested showed increased amounts of cell-associated CPS in comparison to P303-T. The growth of P303-O under anaerobic conditions (in 10% CO2) compared to atmospheric conditions was associated with a 7.9-fold rise in the quantity of CPS expressed. In contrast to P303-O, there was little effect of the different conditions tested on the low level of expression of CPS in P303-T. When the T and O variants of P303 were compared under anaerobic conditions (in 10% CO2), there was 29-fold more CPS synthesized by the O form. Growth to mid-log phase compared to growth to the stationary phase changed the absolute amounts of CPS (with CPS amounts generally higher in organisms at mid-log-phase) but did not affect the relative differences between variants or growth conditions. The effect of environmental conditions for P303 was similar for spontaneous O and T variants of two unrelated clinical isolates, P324 and P68, of types 6B and 18C, respectively. In addition, the amounts of CPS in the culture supernatant of the type 18C strain were measured after growth to stationary phase. The higher amounts of CPS in the culture supernatant of the O variant as the concentration of oxygen was lowered and carbon dioxide raised showed that the environmental effect of could not be accounted for by decreased release from the cell. A type 9V isolate, P10, was used to determine whether the increased synthesis of CPS was caused by decreased oxygen or increased carbon dioxide. The O variant of the type 9V isolate grown to stationary phase in an environment of 10% carbon dioxide expressed 12-fold more CPS in the absence of oxygen compared to that expressed in the presence of oxygen. This suggested that changes in the availability of oxygen rather than carbon dioxide are sufficient to explain the observed effect. There was no effect of different environmental conditions on the amount of teichoic acid as measured in similar capture ELISAs.
FIG. 2.
Effect of environmental oxygen and carbon dioxide and the opacity phenotype on the amounts of CPS measured by a capture ELISA. O and T variants of isolates of the four pneumococcal types indicated were grown to mid-log phase (solid bars) or stationary phase (stippled bars) in the concentrations of oxygen and carbon dioxide shown above the bars. Amounts of CPS were determined in sonicated cell pellets (solid and stippled bars) or in culture supernatants (hatched bars) and are expressed relative to the quantity of total cellular protein in sonicates. An asterisk indicates that this variant did not grow under this condition. Values represent the mean of at least two separate experiments performed in duplicate ± the standard deviation (when n ≥ 3).
TABLE 1.
Characteristics of pneumococci grown under various conditions.
| Characteristic | Colony morphologya of:
|
|||||
|---|---|---|---|---|---|---|
| O variant under (%O2, %CO2):
|
T variant under (%O2, %CO2):
|
|||||
| 21, <0.1 | 16, 3 | <0.1, 10 | 21, <0.1 | 16, 3 | <0.1, 10 | |
| Avg colony area (mm2)b | 3.5 | 7.4 | 170 | 1.0 | 5.2 | 12 |
| Vol occupied by a single cell with capsule zone (μm3)c | 3.6 | 6.8 | 63 | ND | 4.6 | 11 |
| CPS (ng/μg of total protein)d | 130 | 220 | 990 | 35 | 19 | 34 |
For O and T variants of the same type 6A clinical isolate, P303. ND, zone of capsular material was undetectable.
Based on the colony diameter after 16 h of growth at 37°C on nutrient agar. Values represent the average of determinations on five single-well isolated colonies.
Based on the size of the refractile zone visualized with the quellung reaction and the formula for the volume of a spherical ellipsoid: V = (π/6)LW2. The values represent the average of determinations for three diplococcal forms divided by two to provide an estimate for a single cell.
Based on a capture ELISA of organisms grown in liquid culture to mid-log phase. The values are the mean of two separate determinations performed in duplicate.
Association of CpsD and CPS expression.
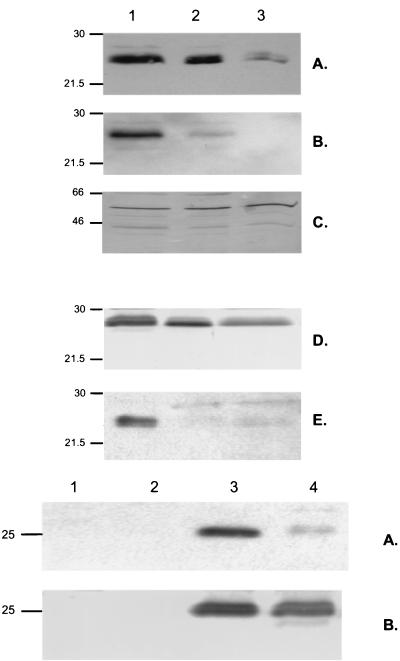
The next phase of the study examined the control of CPS synthesis. In order to examine the transcription of the cps locus, which is required for expression of the capule by S. pneumoniae, a probe based on cps6A was generated and used in Northern blots. The probe detected a large transcript (>9.5 kb) that could represent the entire cps region (data not shown). The lack of stability of this mRNA species, however, prevented accurate, consistent comparisons of the level of transcription between O and T variants, as well as under different growth conditions. Since it was not possible to assess levels of transcription, the expression of proteins in this locus was compared. CpsD, expressed by the last gene in the region common to cps loci of different types, from P303 was generated in E. coli and purified, and antiserum was raised to detect and compare the levels of expression in the pneumococcus (12). In Fig. 3 (upper panels), the level of expression of CpsD as detected in whole-cell sonicates of P303-O in Western blots was not affected by ambient levels of oxygen or carbon dioxide (atmospheric versus anaerobic with 10% CO2). In contrast, when T and O variants of the same strain were compared under the same growth conditions, the expression of CpsD was uniformily diminished in the T pneumococci. A similar correlation of CpsD expression and opacity phenotype was observed in the unrelated type 9V isolate, P10. Since the T variants produce decreased amounts, of CPS, this result was consistent with the hypothesis that T pneumococci synthesis less CPS due to lowered expression of cps gene products.
FIG. 3.
Western analysis showing expression and tyrosine phosphorylation of CpsD in S. pneumoniae. (Upper panels) The opaque (lanes 1 and 2) and transparent (lane 3) colony forms of type 6A isolate P303 (A to C) and type 9V isolate P10 (D and E) were grown to mid-log phase under anaerobic (lane 1) or atmospheric (lanes 2 and 3) growth conditions. Equal amounts of cell sonicates based on total protein content were separated by SDS-PAGE, transferred to a membrane, and analyzed in serial immunoblots with antisera raised against CpsD (A and D), an MAb to phosphotyrosine (B and E), and antiserum to pneumolysin to demonstrate equivalent loading (C). Size markers are in kilodaltons. (Lower panels) Prototype pneumococcal strain D39 (lanes 3 and 4) or the nonencapsulated mutant of this strain, R6 (lanes 1 and 2), lacking a region of the cps locus, including cpsD, were grown under anaerobic (lanes 1 and 3) or atmospheric conditions (lanes 2 and 4). Equivalent amounts of whole-cell sonicates were separated by SDS-PAGE and, following transfer to membranes, immunoblotted with an MAb to phosphotyrosine (A) or antiserum to CpsD (B). The marker indicates the predicted size of the P303 CpsD based on its sequence.
Environmental oxygen affects tyrosine phosphorylation of CpsD.
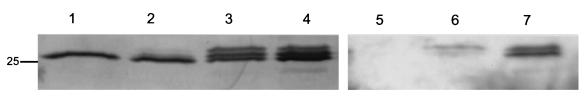
In the course of these studies, it was reported that CpsD which resembles the autophosphorylating protein kinase Wzc involved in chain length regulation of the exopolysaccharide colonic acid of E. coli may also be phosphorylated on tyrosine residues (17, 35). Based on these findings, we determined whether opacity phenotype or differences in environmental oxygen and carbon dioxide affect tyrosine phosphorylation of CpsD. An MAb to phosphotyrosine recognized a single broad band of the predicted size for CpsD in Western blots of whole-cell sonicates of the prototype strain D39 (Fig. 3, lower panels). Additional evidence that this band was CpsD included the observations that (i) this band was not present in strain R6, a mutant of D39 with a 7-kb deletion spanning cpsD, and (ii) the antisera to CpsD recognized a similar band of the equivalent size. In fact, as shown in Fig. 4, the increased resolution of a larger gel format revealed that the antiserum to CpsD recognized at least a triplet of bands of approximately 25 kDa in P303-O grown under anaerobic conditions (with 10% CO2). Only the two higher-molecular-weight bands also reacted with the MAb to phosphotyrosine in serial immunoblots of the same membrane. The elimination of the two higher-molecular-weight bands and the phosphotyrosine epitopes after the cell sonicates were pretreated with alkaline phosphatase prior to loading confirmed that these molecular species represented distinct tyrosine phosphorylated forms of CpsD and that the lower band was the unphosphorylated protein.
FIG. 4.
Western analysis showing the effect of growth conditions and alkaline phosphatase treatment on tyrosine phosphorylation of CpsD. The O variant of the isolate P303 was grown under atmospheric conditions (lane 2 and 6) or anaerobic conditions without supplemental CO2 (lane 4) and anaerobic conditions with 10% CO2 (lanes 1, 3, 5, and 7), whole-cell sonicates were separated using a larger gel format, transferred to a membrane, and serially immunoblotted with antiserum to CpsD (lanes 1 to 4) and an MAb to phosphotyrosine (lanes 5 to 7). In lanes 1 and 5 the cell sonicates were treated with alkaline phosphatase prior to loading. The marker indicates the predicted size (in kilodaltons) of the P303 CpsD based on its sequence.
For the unrelated isolates D39, P10-O, and P303-O growth in anaerobic conditions (in 10% CO2) was associated with increased expression of the tyrosine phosphorylated forms of CpsD compared to the same strain grown under atmospheric growth conditions (Fig. 3). For P303-O there was no difference in expression of the tyrosine phosphorylated forms when grown under anaerobic conditions with or without supplemental carbon dioxide. This observation was consistent with ELISA data showing that O2 rather than CO2 is the major determinant of the level CPS production. For the T variants of each of these isolates, there was little to no apparent tyrosine phosphorylation of CpsD. The nucleotide sequences of the O and T variants of cpsD in P303 were identical and, therefore, differences in amino acid sequence were unlikely to explain the lack of tyrosine phosphorylation in the T variants (data not shown). The lack of phosphotyrosine could be a consequence of the diminished expression of CpsD in T pneumococci. Together, these observations suggested an association between ambient levels of oxygen and tyrosine phosphorylation of CpsD, a putative regulatory protein controlling the amount of CPS produced by the pneumococcus.
Characteristics of S. pneumoniae in human carriage and infection.
In order to test whether or not there is a selection for phenotypes producing different amounts of CPS in carriage and bacteremia in humans, paired isolates of the same type were obtained from the nasopharynx and blood-cerebrospinal fluid of patients presenting with signs of sepsis. Of the 19 paired isolates analyzed, 17 (89%) were of the T phenotype in the nasopharynx, whereas 12 (63%) were of the O phenotype in the blood (Table 2). This pattern was similar in the groups with or without known HIV infection. Of the 10 paired isolates in which the phenotype from the two sites were discordant, all showed more of the T form in the nasopharynx (P < 0.001, Student t test). Therefore, it was possible based solely on colony morphology to detect phenotypic differences in most of the paired isolates.
TABLE 2.
O phenotype of isolates from human carriage and invasive infection
| Colony phenotype (source)a | No. of patients | Type(s) (n)b | HIV statusc
|
||
|---|---|---|---|---|---|
| + | − | NA | |||
| T/T | 7 | 1 (4), 4, 18, 19 | 5 | 1 | 1 |
| O/O | 2 | 1 (2) | 1 | 0 | 1 |
| O/T | 0 | ||||
| T/O | 10 | 1 (4), 4 (2), 6, 12, 15, 22 | 5 | 1 | 4 |
Presented as nasopharyngeal fluid/blood-cerebrospinal fluid. A total of 4 of 19 invasive isolates were obtained from the cerebrospinal fluid rather than from blood.
n = 1 except as noted.
NA, clinical status not available.
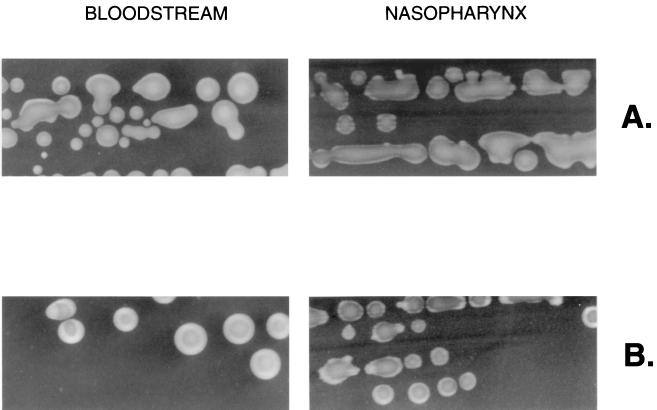
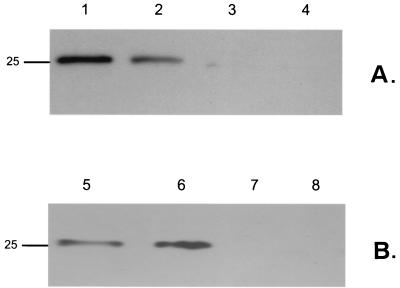
Some these isolates reacted with the antibodies used in this study. This allowed for two of the paired isolates (types 1 and 4) with discordant colony phenotypes to be analyzed in Western blots as an additional and more objective assessment of whether survival in the nasopharynx versus the bloodstream selected for different phenotypes (Fig. 5). For these two sets of paired isolates, tyrosine phosphorylation of CpsD was detected in the isolate obtained from the bloodstream but not in the isolate of the same type cultured at the same time from the nasopharynx. In the two isolates obtained from the bloodstream, tyrosine phosphorylation was most prominent when grown in the absence of oxygen, as predicted. The results, therefore, confirmed that there may be differences in the isolates from two sites in the same patient and that, based on both colony morphology and Western analysis, there may be selection for the O variant, the form in which tyrosine phosphorylation of CpsD is detected, in invasive infection.
FIG. 5.
S. pneumoniae of the same type was cultured from both the bloodstream and the nasopharynx of two individuals: patient A (type 1 isolate) and patient B (type 4 isolate). (Upper panels) Colony morphology of the paired isolates grown under microaerophilic conditions and viewed with oblique, transmitted illumination (magnification, ×45). (Lower panels) Western analysis showing tyrosine phosphorylation of CpsD in paired clinical isolates from the nasopharynx and bloodstream. Isolates obtained from blood culture (lanes 1, 2, 5, and 6) and nasal swabs (lanes 3, 4, 7, and 8) were grown under atmospheric (lanes 2, 4, 5, and 7) or anaerobic (lanes 1, 3, 6, and 8) conditions. The marker indicates the predicted size (in kilodaltons) of the P303 CpsD based on its sequence.
DISCUSSION
We present evidence that the ambient oxygen concentration may be an important factor in the ability of S. pneumoniae, an acrotolerant streptococcus, to regulate the characteristics of its cell surface. Our approach took advantage of observations that the pneumococcus varies between two phenotypes previously shown to differ in their amounts of CPS (13). Findings in the current study show that oxygen levels affect the O and T phase variants differently. There is greatly enhanced production of CPS in O variants in conditions of reduced oxygen, whereas synthesis of CPS in T variants remains comparatively low under both aerobic and anaerobic conditions. The effect of oxygen on CPS expression was shown by two different methods, quellung, which allows visualization of the capsule size, and a capture ELISA, which enables amounts of CPS to be quantified. Both of these methods rely on the use of type-specific antiserum to detect CPS. Results from experiments using either technique show that microaerophilic growth conditions (O2 levels at 17% or lower) are sufficient to induce a significant increase in CPS expression when compared to atmospheric growth conditions. A maximal effect, however, is observed in strict anaerobiasis.
Although it is not clear when and if this species would be exposed to strict anaerobic conditions, ambient oxygen levels would be diminished whenever the organism is not in its commensal state on the airway surface. In particular, the availability of oxygen is decreased in the common manifestations of pneumococcal disease such as pneumonia, empyema, and otitis media where the exudative inflammatory response occludes air spaces and limits gas exchange. Oxygen levels in the uninflamed middle ear space, for example, resemble that of venous blood, are less than a third that of the airway, and may be further reduced by the presence of effusion (8, 9, 26). If an effect similar to that described in this study occurs in vivo, the reduced-availability of oxygen would select for an upregulation in CPS expression. Since even-relatively small difference in the amounts of CPS can be critical to the expression of virulence, the effect of oxygen might provide a survival advantage during infection in situations whenever O variants are represented (13).
The analysis of paired isolates from human carriage and bacteremia is consistent with previous animal studies and supportive of the hypothesis that environments with lower oxygen content promote selection for the more virulent O form. It was found that in bacteremic infection of humans, which is generally a complication of pneumococcal pneumonia, most isolates from the bloodstream (or cerebrospinal fluid) are as opaque or more compared to the isolate from the same patient cultured from the nasopharynx (22). This difference in colony morphology is indicative of increased amounts of CPS in these blood isolates. In contrast, the T variant found in 89% of the colonizing isolates in the natural host produces relatively little CPS regardless of the ambient oxygen concentration. T variants may predominate in this setting since smaller amounts of CPS may be sufficient to inhibit phagocytosis on the airway surface where there is relatively little complement and antibody, while still allowing for efficient adherence to host cells. Another consideration is that because it was necessary to carry out this study in a largely HIV-positive population due to their high rates of pneumococcal infection, these findings may or may not be relevant to immunocompetent hosts. Results from this limited study of human isolates, however, add support to the hypothesis that phenotypic variation confers on the pneumococcus the flexibility to alter its surface characteristics for the differing requirements of colonization and infection.
Since the expression of a large capsule may be detrimental to the needs of carriage but necessary to evade opsonsophagocytic clearance during infection, the amounts of CPS may need to be precisely regulated. At least two factors are described as contributing to the differences in CPS levels: the variation in opacity phenotype noted in the majority of clinical isolates and the availability of oxygen in the environment. For a pathogen such as the pneumococcus residing on the surface of the airway, the concentration of oxygen in its environment would seem to be an effective means of sensing changes in its host environment that might require adaptation. There has been little specific evidence to suggest that environmental signals, which are important, for instance, in the induction of competence, may also contribute to the pathogenesis of pneumococcal disease (19). A recent study from our laboratory demonstrated that the expression of pyruvate oxidase (SpxB), the major factor in production of unusually high concentrations of hydrogen peroxide by the pneumococcus, is differentially regulated in O and T variants by ambient oxygen levels (20, 21). The generation of H2O2 by the pneumococcus under aerobic conditions has cytotoxic effects on host cells as well as on other bacterial species that inhabit and potentially compete for the same niche in the upper respiratory tract (5, 21). Oxygen has also been implicated as a factor in the regulation of natural transformation (2). In another streptococcal species (Streptococcus pyogenes), it is carbon dioxide rather than oxygen tension that mediates changes in the expression of virulence factors. Our findings for the pneumococcus do not suggest that carbon dioxide concentration affects the regulation of its major virulence factor, CPS. In group A streptococci, the amounts of the hyaluronic capsule are also regulated but not through signaling involving carbon dioxide concentration (18). Instead, the amounts of the hyaluronic capsule are regulated through an unknown environmental signal transmitted through differential phosphorylation of a two-component signal transduction system (15). In the case of the pneumococcus it is oxygen that triggers these changes and there is no evidence for a substantial effect when mutants in 11 of the 12 known two-component signal transduction systems were tested (32; data not shown). This leaves it unclear how signals related to oxygen content in the environment are transmitted so as to affect gene expression in CPS synthesis.
One implication of this study is further evidence that the amounts of CPS are not uniform for a given strain. We have previously documented that genetic transformation tends to cause a selection bias for T variants, since the lower amount of CPS in this form appears to allow for more efficient uptake of DNA (39). Virulence studies that depend on the analysis of mutants generated by transformation of encapsulated strains would, therefore, tend to demonstrate decreased virulence regardless of the specific mutation. In addition to the contribution of the opacity phenotype, differences in growth conditions and, in particular, oxygenation have the potential to bias studies that depend on encapsulation. Vaccine efficacy studies, for example, will increasingly depend on in vitro correlates of protection, since there is now an effective product for use in childhood that will restrict further clinical trials. The opsonophagocyctic titer of anti-CPS antibody, one of few in vitro assays that is predictive of vaccine efficacy, has been shown to be highly sensitive to variations in the amount of CPS (14). These assays, as well as virulence studies, could be affected by differences in opacity phenotype and oxygenation during growth of test organisms.
Although the genes involved in the expression of the pneumococcal capsule have been described, the mechanism controlling the levels of CPS expression remain largely undefined. In this report, we focused on cpsD, a gene common to the capsulation loci of all serotypes thus far examined which appears to be a negative regulator of CPS synthesis in S. pneumoniae and other encapsulated bacteria. Homologues of CpsD are present in many other pathogens that express surface polysaccharides, such as Streptococcus agalactiae, Staphylococcus aureus, Klebsiella pneumoniae, and Acinetobacter johnsonii and A. lwoffii (1, 6, 27, 41). This family comprises one of the few known examples of bacterial proteins that are phosphorylated on tyrosine residues (11). One of the common features of this group of bacterial tyrosine kinases is a C terminus with a tyrosine-rich repeat which has been proposed as the target of autophosphoryltation activity. For the pneumococcus, changing the tyrosine residues in the C-terminal region (YGX)4 in Cps 19fD to phenylalanine residues resulted in a loss of immunodetectable phosphotyrosine and a mutant with a mucoid colony phenotype under standard aerobic growth conditions. This led to the proposal that tyrosine phoshorylation of CpsD involving this motif increases the negative regulatory activity of the protein on CPS biosynthesis. A similar C terminus containing multiple tyrosine residues (215-CGSYGNYGDYGKNKK-229) was found in CpsD of the type 6A strain analyzed in this study. An unexpected observation was that anaerobic conditions which resulted in the highest level of CPS production in this and several other isolates also had the most immunodetectable phosphotyrosine on CpsD. This suggests that although the levels of tyrosine phosphorylation correlate with differences in the amounts of CPS, its effect may not be associated with increased negative regulatory activity and lower expression of CPS. Alternatively, if tyrosine phosphorylation acts to enhance the negative regulation of CpsD as proposed, there must be other unknown upstream or downstream factors affecting the degree of phosphorylation and mediating the oxygen effect. In E. coli the autophosphorylating enzyme Wzc is linked to a phosphotyrosine-protein phosphatase Wzb, which has no significant homologue in the pneumococcal genome. Rather, it has been suggested that CpsB functions in dephosphorylating CpsD and could be an additional factor affecting tyrosine phosphorylation (17).
We also observed an absence of phosphotyrosine on CpsD in the T variants of the several strains analyzed. Although it was not technically feasible to show that this was due to decreased transcription of the cps locus in this phenotype, the lack of immunodetectable phosphotyrosine correlates with decreased expression of CpsD. These data support the conclusion that there is a downregulation of cps expression in the T variant accounting for the low levels of CPS found in T pneumococci. Thus, there appear to be at least two separate levels of regulation of CPS synthesis: one associated with the phosphorylation of two or more tyrosine residues in CpsD and the other affecting the expression of protein(s) in this locus, including CpsD. The analysis of two sets of paired clinical isolates showed that pneumococci recovered from the nasal swabs did not have the phosphotyrosine epitope regardless of the growth conditions. These data could be explained by selection for a phenotype with diminished expression of the capsulation locus when resident in the nasopharynx, a result that, together with observations on colony morphology and capsule synthesis, suggests that in contrast to invasive infection pneumococci may express relatively little CPS during human carriage.
ACKNOWLEDGMENTS
We thank R. Austrian (University of Pennsylvania) for critical review and for providing clinical isolates and assistance with quellung experiments. We gratefully acknowledge the support of the patients and staff of the Queen Elizabeth Central Hospital, Blantyre, Malawi, and the support of E. E. Zijlstra, Head of the Department of Medicine at the University of Malawi College of Medicine. Antiserum to pneumolysin was generously provided by T. Mitchell.
Stephen Gordon is a Wellcome Trust Fellow in Clinical Tropical Medicine. Henry Epino holds a Fulbright Scholarship. This work was supported by grants from the U.S. Public Health Service (AI38436 and AI44231 to J.N.W.).
REFERENCES
- 1.Arakawa Y, Wacharotayankun R, Nagatsuka T, Ito H, Kato N, Ohta M. Genomic organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain Chedid. J Bacteriol. 1995;177:1788–1796. doi: 10.1128/jb.177.7.1788-1796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auzat I, Chapuy-Regaud S, Le Bras G, Dos Santos D, Ogunniyi A, Le Thomas I, Garel J, Paton J, Trombe M. The NADH oxidase of Streptococcus pneumoniae: its involvement in competence and virulence. Mol Microbiol. 1999;34:1018–1028. doi: 10.1046/j.1365-2958.1999.01663.x. [DOI] [PubMed] [Google Scholar]
- 3.Cundell D R, Gerard N P, Gerard C, Idanpaan-Heikkila I, Tuomanen E I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 4.Cundell D R, Weiser J N, Shen J, Young A, Tuomanen E I. Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect Immun. 1995;63:757–761. doi: 10.1128/iai.63.3.757-761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duane P G, Rubins J B, Wiesel H R, Janoff E N. Identification of hydrogen peroxide as a Streptococcus pneumoniae toxin for rat alveolar epithelial cells. Infect Immun. 1993;61:4392–4397. doi: 10.1128/iai.61.10.4392-4397.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grangeasse C, Doublet P, Vaganay E, Vincent C, Deleage G, Duclos B, Cozzone A J. Characterization of a bacterial gene encoding an autophosphorylating protein tyrosine kinase. Gene. 1997;204:259–265. doi: 10.1016/s0378-1119(97)00554-4. [DOI] [PubMed] [Google Scholar]
- 7.Hammerschmidt S, Muller A, Sillmann H, Muhlenhoff M, Borrow R, et al. Capsule phase variation in Neisseria meningitidis serogroup B by slipped-strand mispairing in the polysialyltransferase gene (siaD): correlation with bacterial invasion and the outbreak of meningococcal disease. Mol Microbiol. 1996;20:1211–1220. doi: 10.1111/j.1365-2958.1996.tb02641.x. [DOI] [PubMed] [Google Scholar]
- 8.Harell M, Mover-Lev H, Levy D, Sade J. Gas composition of the human nose and nasopharyngeal space. Acta Otolaryngol. 1996;116:82–84. doi: 10.3109/00016489609137718. [DOI] [PubMed] [Google Scholar]
- 9.Hergils L, Magnuson B. Middle ear gas composition in pathologic conditions: mass spectroscopy in otitis media with effusion and atelectasis. Ann Otol Rhinol Laryngol. 1997;106:743–745. doi: 10.1177/000348949710600905. [DOI] [PubMed] [Google Scholar]
- 10.Hulse M, Smith S, Chi E, Pham A, Rubens C. Effect of type III group B streptococcal capsular polysaccharide on invasion of respiratory epithelial cells. Infect Immun. 1993;61:4835–4841. doi: 10.1128/iai.61.11.4835-4841.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilan O, Bloch Y, Frankel G, Ullrich H, Geider K, Rosenshine I. Protein tyrosine kinases in bacterial pathogens are associated with virulence and production of exopolysaccharide. EMBO J. 1999;18:3241–3248. doi: 10.1093/emboj/18.12.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang S, Wang L, Reeves P. Molecular characterization of Streptococcus pneumoniae type 4, 6B, 8, and 18C capsular polysaccharide gene clusters. Infect Immun. 2001;69:1244–1255. doi: 10.1128/IAI.69.3.1244-1255.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Weiser J. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis. 1998;177:368–377. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 14.Kim J O, Romero-Steiner S, Sørensen U, Blom J, Carvalho M, Barnardi S, Carlone G, Weiser J N. Relationship between cell-surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect Immun. 1999;67:2327–2333. doi: 10.1128/iai.67.5.2327-2333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin J C, Wessels M R. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A streptococcus. Mol Microbiol. 1998;30:209–219. doi: 10.1046/j.1365-2958.1998.01057.x. [DOI] [PubMed] [Google Scholar]
- 16.MacLeod C M, Krauss M R. Relation of virulence of pneumococcal strains for mice to the quantity of capsular polysaccharide formed in vitro. J Exp Med. 1950;92:1–9. doi: 10.1084/jem.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morona J, Paton J, Miller D, Morona R. Tyrosine phosphorylation of CpsD negatively regulates capsular polysaccharide biosynthesis in Streptococcus pneumoniae. Mol Microbiol. 2000;35:1431–1442. doi: 10.1046/j.1365-2958.2000.01808.x. [DOI] [PubMed] [Google Scholar]
- 18.Okada N, Geist R T, Caparon M G. Positive transcriptional control of mry regulates virulence in the group A streptococcus. Mol Microbiol. 1993;7:893–903. doi: 10.1111/j.1365-2958.1993.tb01180.x. [DOI] [PubMed] [Google Scholar]
- 19.Orihuela C, Janssen R, Robb C, Watson D, Niesel D. Peritoneal culture alters Streptococcus pneumoniae protein profiles and virulence properties. Infect Immun. 2000;68:6082–6086. doi: 10.1128/iai.68.10.6082-6086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Overweg K, Pericone C D, Verhoef G G, Weiser J N, Meiring H D, De Jong A P, De Groot R, Hermans P W. Differential protein expression in phenotypic variants of Streptococcus pneumoniae. Infect Immun. 2000;68:4604–4610. doi: 10.1128/iai.68.8.4604-4610.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pericone C, Weiser J. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect Immun. 2000;68:3990–3997. doi: 10.1128/iai.68.7.3990-3997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raz R, Elhanan G, Shimoni Z, Kitzes R, Rudnicki C, Igra Y, Yinnon A. Pneumococcal bacteremia in hospitalized Israeli adults: epidemiology and resistance to penicillin. Israeli Adult Pneumococcal Bacteremia Group. Clin Infect Dis. 1997;24:1164–1168. doi: 10.1086/513635. [DOI] [PubMed] [Google Scholar]
- 23.Ring A, Weiser J N, Tuomanen E I. Pneumococcal penetration of the blood-brain barrier: molecular analysis of a novel re-entry path. J Clin Investig. 1998;102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero-Steiner S, Musher D, Cetron M, Pais L, Groover J, Fiore A, Plikaytis B, Carlone G. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis. 1999;29:281–288. doi: 10.1086/520200. [DOI] [PubMed] [Google Scholar]
- 25.Rosenow C, Ryan P, Weiser J N, Johnson S, Fontan P, Ortqvist A, Masure H R. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol. 1997;25:819–829. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 26.Sade J, Luntz M, Levy D. Middle ear gas composition and middle ear aeration. Ann Otol Rhinol Laryngol. 1995;104:369–373. doi: 10.1177/000348949510400506. [DOI] [PubMed] [Google Scholar]
- 27.Sau S, Bhasin N, Wann E R, Lee J C, Foster T J, Lee C Y. The Staphylococcus aureus allelic genetic loci for serotype 5 and 8 capsule expression contain the type-specific genes flanked by common genes. Microbiology. 1997;143:2395–2405. doi: 10.1099/00221287-143-7-2395. [DOI] [PubMed] [Google Scholar]
- 28.Schrager H M, Rheinwald J G, Wessels M R. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J Clin Investig. 1996;98:1954–1958. doi: 10.1172/JCI118998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sellin M, Hakansson S, Norgren M. Phase-shift of polysaccharide capsule expression in group B streptococci, type III. Microb Pathog. 1995;18:401–415. doi: 10.1006/mpat.1995.0036. [DOI] [PubMed] [Google Scholar]
- 30.Sorenson U B S, Henrichsen J, Chen H-C, Szu S C. Covalent linkage between the capsular polysaccharide and cell wall peptidoglycan of Streptococcus pneumoniae revealed by immunochemical methods. Microb Pathog. 1990;8:325–334. doi: 10.1016/0882-4010(90)90091-4. [DOI] [PubMed] [Google Scholar]
- 31.St. Geme J, III, Falkow S. Loss of capsule expression by Haemophilus influenzae type b results in enhanced adherence to and invasion of human cells. Infect Immun. 1991;59:1325–1333. doi: 10.1128/iai.59.4.1325-1333.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Throup J, Koretke K, Bryant A, Ingraham K, Chalker A, Ge Y, Marra A, Wallis N, Brown J, Holmes D, Rosenberg M, Burnham M. A genomic analysis of two-component signal transduction in Streptococcus pneumoniae. Mol Microbiol. 2000;35:566–576. doi: 10.1046/j.1365-2958.2000.01725.x. [DOI] [PubMed] [Google Scholar]
- 33.Tomasz A. A chemically defined medium for Streptococcus pneumoniae. Bacteriol Proc. 1964;64:29. [Google Scholar]
- 34.Tong H, Weiser J, James M, DeMaria T. Effect of influenza A virus infection on nasopharngeal colonization and otitis media induced by transparent or opaque phenotypic variants of Streptococcus pneumoniae in the chinchilla model. Infect Immun. 2001;69:602–606. doi: 10.1128/IAI.69.1.602-606.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vincent C, Doublet P, Grangeasse C, Vaganay E, Cozzone A, Duclos B. Cells of Escherichia coli contain a protein-tyrosine kinase, Wzc, and a phosphotyrosine-protein phosphatase, Wzb. J Bacteriol. 1999;181:3472–3477. doi: 10.1128/jb.181.11.3472-3477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wani J, Gilbert J, Plaut A, Weiser J. Identification, cloning and sequencing of the immunoglobulin A1 protease gene of Streptococcus pneumoniae. Infect Immun. 1996;64:3967–3974. doi: 10.1128/iai.64.10.3967-3974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiser J N. Phase variation in colony opacity. Microb Drug Resist. 1998;4:129–145. doi: 10.1089/mdr.1998.4.129. [DOI] [PubMed] [Google Scholar]
- 38.Weiser J N, Austrian R, Sreenivasan P K, Masure H R. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun. 1994;62:2582–2589. doi: 10.1128/iai.62.6.2582-2589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiser J N, Kapoor M. Effect of intrastrain variation in amount of capsular polysaccharide on the genetic transformation of Streptococcus pneumoniae: implications for virulence studies of encapsulated strains. Infect Immun. 1999;67:3690–3692. doi: 10.1128/iai.67.7.3690-3692.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whatmore A M, Efstratiou A, Pickerill A P, Broughton K, Woodard G, Sturgeon D, George R, Dowson C G. Genetic relationships between clinical isolates of Streptococcus pneumoniae, Streptococcus oralis, and Streptococcus mitis: characterization of “a typical” pneumococci and organisms allied to S. mitis harboring S. pneumoniae virulence factor-encoding genes. Infect Immun. 2000;68:1374–1382. doi: 10.1128/iai.68.3.1374-1382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto S, Miyake K, Koike Y, Watanabe M, Machida Y, Ohta M, Iijima S. Molecular characterization of type-specific capsular polysaccharide biosynthesis genes of Streptococcus agalactiae type Ia. J Bacteriol. 1999;181:5176–5184. doi: 10.1128/jb.181.17.5176-5184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]