Abstract
Systemic sclerosis (SSc) is a multisystem life-threatening fibrosing disorder that lacks effective treatment. The link between the inflammation observed in organs such as the skin and profibrotic mechanisms is not well understood. The plasmacytoid dendritic cell (pDC) is a key cell type mediating Toll-like receptor (TLR)–induced inflammation in autoimmune disease patients, including lupus and skin diseases with interface dermatitis. However, the role of pDCs in fibrosis is less clear. We show that pDCs infiltrate the skin of SSc patients and are chronically activated, leading to secretion of interferon-α (IFN-α) and CXCL4, which are both hallmarks of the disease. We demonstrate that the secretion of CXCL4 is under the control of phosphatidylinositol 3-kinase δ and is due to the aberrant presence of TLR8 on pDCs of SSc patients, which is not seen in healthy donors or in lupus pDCs, and that CXCL4 primarily acts by potentiating TLR8- but also TLR9-induced IFN production by pDCs. Depleting pDCs prevented disease in a mouse model of scleroderma and could revert fibrosis in mice with established disease. In contrast, the disease was exacerbated in mice transgenic for TLR8 with recruitment of pDCs to the fibrotic skin, whereas TLR7 only partially contributed to the inflammatory response, indicating that TLR8 is the key RNA-sensing TLR involved in the establishment of fibrosis. We conclude that the pDC is an essential cell type involved in the pathogenesis of SSc and its removal using depleting antibodies or attenuating pDC function could be a novel approach to treat SSc patients.
INTRODUCTION
Systemic sclerosis (SSc) is a multisystem, fibrosing disorder in which vasculopathy, autoimmunity, and inflammation lead to diverse life-altering and life-threatening clinical manifestations (1). SSc has the highest degree of morbidity and mortality of the rheumatic diseases with a 10-year mortality rate of 23 to 45% (2, 3). The female predominance is about 4:1, and the usual age of onset is 35 to 55 years. The pathophysiology of SSc is not completely understood, but substantial evidence shows interplay between immunologic derangement, endothelial dysfunction, and profibrotic mechanisms. In particular, the contribution of B cells has been well studied, and abnormalities of B cell function have been demonstrated both in animal models of SSc and in SSc patients (4, 5). A clear understanding of SSc pathogenesis has been impeded by the heterogeneous nature of the disease—which is seen clinically, serologically, and at the level of gene expression in the skin and peripheral blood (6). The management of most of the complications of SSc is not standardized, with important discrepancies between experts in drug choices, especially after first-line agents (7). The modified Rodnan skin score, which can reproducibly assess skin involvement, correlates with prognosis and disease progression in other organ systems as well. Studying the skin in SSc patients is thus of great interest because the involvement of the skin often reflects similar pathological processes in the bowel and lungs.
The past decade has seen a rebirth of interest in innate immunity, catalyzed in large part by studies of the Toll-like receptors (TLRs), one of the largest and best-studied families of pattern recognition receptors (8-10). Endogenous ligands released from damaged tissues or apoptotic cells have been identified for most human TLRs, and the sensing of self nucleic acids through TLRs can play an important part in sterile inflammation and autoimmunity (11, 12). To prevent their unwanted activation by self nucleic acids, the nucleic acid–sensing TLRs are contained in endosomes rather than on the cell surface, and their expression is tightly regulated. Indeed, the increased expression of either TLR7 or huTLR8 in transgenic mice is enough to induce autoimmunity even in the absence of any other genetic alterations (13-15). Furthermore, the biological consequence of TLR signaling is highly dependent on the pattern of expression of individual TLRs on hematopoietic cells. For example, although human TLR7 and TLR8 share similar recognition of single-stranded RNA (ssRNA), we and others have shown that they have a distinct expression pattern in human blood cells (15-18), which leads to different quantitative and qualitative immune responses. In particular, TLR8 is absent in plasmacytoid dendritic cells (pDCs), which is reflected by the absence of interferon-α (IFN-α) induction when human peripheral blood mononuclear cells (PBMCs) are activated with a TLR8 agonist, in contrast to what is seen with TLR7 (15). In addition, understanding the function of TLR8 has proven difficult because the mouse TLR8, although a potentially functional receptor (19, 20), differs from its human ortholog due to the lack of five amino acids that are key for RNA recognition (21).
In at least two instances, the pDC is the key cell type mediating TLR-induced inflammation in autoimmune disease patients. In lupus, we and others have shown that pDCs produce large amounts of type I IFN due to TLR7 and TLR9 recognition of endogenous RNA and DNA in the form of immune complexes (22-24). We also showed that pDC activation prevents optimal response to corticosteroid treatment by lupus patients (25), and two recent studies identified pDCs as the key cell type promoting lupus in mouse models of the disease (26, 27). The importance of pDCs has also been observed in a series of related cutaneous autoimmune diseases such as dermatomyositis, lichen sclerosus, cutaneous graft-versus-host disease, or cutaneous lupus that share a common pathological inflammatory feature described as “interface dermatitis” (28). In these patients, pDCs massively infiltrate the skin and produce IFN-α, which plays a major role in the development of cutaneous lesions. In this setting, using a skin injury model, we and others have demonstrated that pDCs are a dominant player in the autoimmune inflammatory damage (29, 30).
Recent reports have indicated that pDCs may also be associated with the pathogenesis of SSc. First, it was recently shown that pDCs traffic to the skin in a mouse model of the stiff skin syndrome (31). It was also shown that pDCs from SSc patients can secrete CXCL4 and that serum levels of CXCL4 correlated with skin and pulmonary disease in these patients, although it was not shown whether pDCs are the only cells contributing to CXCL4 increased levels (32). CXCL4, also known as platelet factor 4, is released at high concentrations from activated platelets (33), and although not well defined, the function of CXCL4 seems to be associated with coagulation and inflammation.
Although it is well described that pDCs infiltrate the skin following injury or in certain diseases, little is known about what is controlling pDC trafficking and subsequent activation in the skin or whether pDCs can play a role in promoting/sustaining inflammation-related fibrosis. The nature of pDC activation in SSc patients, the role of TLRs, and the impact of CXCL4 on pDCs response are also unknown.
RESULTS
pDCs infiltrate the skin of SSc patients and are chronically activated with spontaneous secretion of CXCL4 and IFN-α
Activated pDCs have been observed to accumulate in the skin of SSc patients (32) in a similar way to what has been observed in skin lesions in diseases characterized by interface dermatitis (28). We observed the presence of pDCs in skin biopsies of SSc patients but not of healthy donor (HD) (Fig. 1, A to C), highlighting the role played by these cells in patients and confirming the importance of better understanding what controls their activation in the skin of patients. Similar to what has been shown in lupus patients (34, 35), we noted a significant (P < 0.001) decrease in the number of pDCs circulating in the blood of SSc patients as compared to HDs (Fig. 1, D and E). This decrease is an indicator of in vivo activation of pDCs and their migration into peripheral lymphoid tissues and to the skin (Fig. 1, A to C). No significant difference was observed in total B cells or conventional dendritic cells (cDCs), whereas monocytes were increased (Fig. 1, D and E). When cultured in the absence of additional stimuli, purified pDCs from the blood of patients spontaneously secreted IFN-α but also CXCL4 (Fig. 1F), a chemokine recently identified as a biomarker for the disease (32). In some cases, the quantity of IFN was below the detection level of our assay. The kinetics of IFN-α production by pDCs is rapid, which may explain the low levels of IFN, as the cell preparation likely contains a mix of cells recently activated along with cells activated more than 24 hours. There was no difference in the secretion of any other chemokine analyzed, namely, CCL3, CCL4, and CCL5, between SSc and HD pDCs nor did we observe secretion of tumor necrosis factor (TNF), IFN-γ, or interleukin-12p40 (IL-12p40) from either SSc or HD pDCs. We have showed that pDCs, when activated, can up-regulate costimulatory molecules such as CD80, CD83, or CD86 and then mature into antigen-presenting cells (36, 37). We observed that pDCs from SSc patients did not express these costimulatory molecules (Fig. 1G), demonstrating that the chronic activation of pDCs in these patients likely involved the IFN pathway without the involvement of nuclear factor κB (NF-κB)–dependent signals, which are required for the induction of these costimulation molecules (37).
Fig. 1. pDCs infiltrate the skin of SSc patients and spontaneously secrete IFN-α and CXCL4.
Skin biopsies from (A) control subjects or (B) systemic sclerosis (SSc) patients were immunostained for CD123 (red signal; original magnification, ×200; inset, ×400), and (C) quantification of the number of CD123+ counted in five high-power microscopic fields (based on five bioptic samples of patients with SSc and of five controls) is shown. (D) Representative dot plots of plasmacytoid dendritic cells (pDCs), B cells, conventional DCs (cDCs), and monocytes from peripheral blood mononuclear cells (PBMCs) from healthy donors (HDs) or SSc patients analyzed by flow cytometry. (E) Percentages of pDCs, monocytes, B cells, and cDCs within PBMC of either HDs (open circle, n = 10 to 15) or SSc patients (n = 14 to 33) defined as early diffuse (closed circle), late diffuse (closed square), or limited (closed triangle) were quantified by flow cytometry. ns, not significant. (F) Purified pDCs from HDs (n = 8) or SSc patients (n = 20) were cultured for 24 hours without stimuli. CXCL4 and interferon-α (IFN-α) were quantified by enzyme-linked immunosorbent assay (ELISA). Results were normalized to 10,000 cells to account for the differences between donors in the number of cells put in culture. (G) The mean fluorescence intensity (MFI) of the costimulatory molecules CD83 and CD86 expressed on pDCs (n = 10 for HDs; n = 33 for SSc patients) was quantified by flow cytometry. Statistical significance was evaluated using a Mann-Whitney U test. *P < 0.05, **P < 0.01, ***P < 0.001.
pDCs from SSc patients display an aberrant expression profile of TLRs with the presence of TLR8
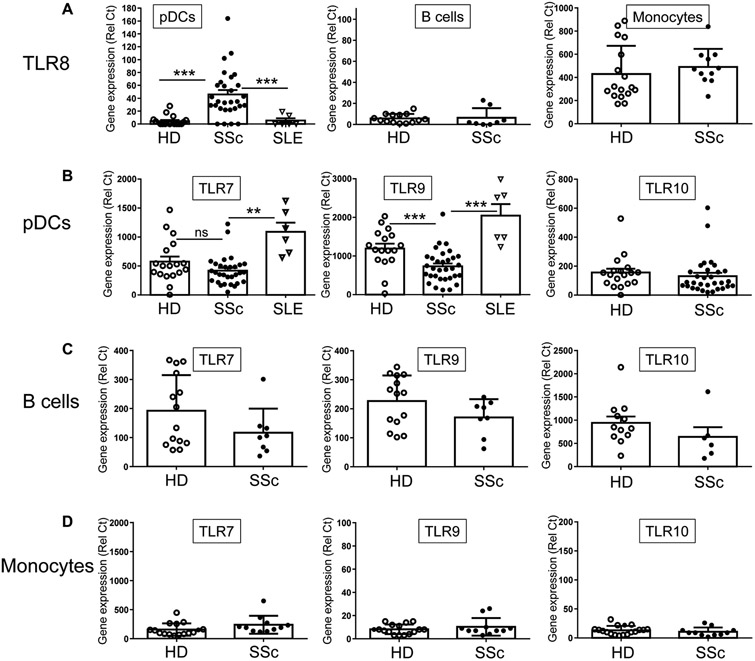
The sensing of nucleic acids by TLRs leads to substantial production of IFN-α by pDCs, suggesting that the chronic activation of pDCs in SSc patients may involve TLRs. We thus quantified the mRNA expression levels of the nucleic acid–sensing TLR7, TLR8, and TLR9, as well as TLR10 on purified pDCs from SSc patients, systemic lupus erythematosus (SLE) patients or HDs. Similar to HDs, pDCs from SSc patients expressed both BDCA2 and BDCA4 (fig. S1), and we confirmed that monocytes from SSc patients did not have an aberrant expression of BDCA4 (fig. S2). The expression levels of TLR7, TLR9, and TLR10 were similar in PBMCs prepared from SSc patients or HDs and were equally reduced in pDC-depleted PBMCs, which is expected because these three TLRs are normally expressed on pDCs (fig. S3A). However, we observed that TLR8 expression was increased in PBMCs of SSc patients and surprisingly that the levels of expression of TLR8 in patients were normalized to those of HDs when pDCs were depleted from PBMCs (fig. S3A). In these experiments, monocytes were not affected by the depletion protocol (fig. S4), which is consistent with their lack of BDCA4 expression (fig. S2).
Furthermore, although pDCs isolated from HDs did not express TLR8 as has repeatedly been shown (15-18), we observed aberrant expression of TLR8 in pDCs isolated from the blood of SSc patients (Fig. 2A). The presence of TLR8 on pDCs was seen in 25 of the 29 patients tested, including patients with early or late diffuse SSc but also in patients with limited SSc. We also observed that TLR8 was not expressed on pDCs isolated from lupus patients (Fig. 2A), another disease where pDCs have been involved. Although our gating strategy excluded cell types known to express TLR8 (see Materials and Methods), we confirmed that we could not detect markers for non-pDC cells (in particular neutrophils that express abundant TLR8), which excludes a contribution of contaminating cells to our results. Similar findings were obtained using pDCs from HDs or SLE patients. These data thus suggest that the pDCs from SSc patients do express TLR8, which appears to be a feature of SSc. We tested various inflammatory cocktails of cytokines, including IFN-α and IFN-γ, IL-12, IL-6, IL-10, TNF, or TGF-β (transforming growth factor–β), to determine what is regulating TLR8 expression on pDCs of SSc patients, but none of these molecules could induce TLR8 on pDCs of HDs (fig. S3B). In particular, CXCL4 or IFN-γ–induced protein 10 (IP-10) (two CXCR3B agonists) had no impact on TLR8 nor TLR7 and TLR9 expression levels in pDCs (fig. S3C). This dysregulation of TLR8 expression was not observed in other cell subsets including monocytes, which naturally expressed TLR8 with similar expression between HDs and SSc patients (Fig. 2A). Similar to pDCs, purified B cells from HDs do not express TLR8, and we observed that TLR8 was not present in B cells from SSc patients (Fig. 2A), suggesting that the dysregulation of TLR8 is a distinct feature of pDCs of SSc patients. TLR7 and TLR10 expression was similar in SSc and HD pDCs, whereas TLR9 expression was slightly decreased in SSc pDCs, as is often the case when pDCs are activated (Fig. 2B). Of note, expression of TLR7 and TLR9 was significantly greater (P < 0.01 and P < 0.001, respectively) in pDCs of SLE patients as compared to SSc patients (Fig. 2B). No difference was observed in B cells or monocytes for TLR7, TLR9, or TLR10 (Fig. 2, C and D). These data suggest that in addition to being chronically activated, pDCs of SSc patients have an abnormal profile of TLRs characterized by the expression of TLR8.
Fig. 2. TLR8 is aberrantly expressed on pDCs from SSc patients.
(A) TLR8 expression levels [relative Ct (Rel Ct)] were quantified in purified pDCs [n = 18 HDs; n = 29 SSc patients; n = 6 systemic lupus erythematosus (SLE) patients], B cells (n = 14 HDs; n = 8 SSc patients), or monocytes (n = 17 HDs; n = 11 SSc patients) from PBMCs as indicated. (B to D) Gene expression level of TLR7, TLR9, and TLR10 was quantified in (B) purified pDCs (n = 17 to 18 HDs; n = 29 to 31 SSc patients; n = 6 SLE patients), (C) purified B cells (n = 12 to 14 HDs; n = 6 to 8 SSc patients), or (D) purified monocytes (n = 16 to 18 HDs; n = 11 SSc patients) by quantitative polymerase chain reaction (qPCR). All results are represented as means ± SEM. Statistical significance was evaluated using a Mann-Whitney U test, and only comparisons that are significant are shown. *P < 0.05, **P < 0.01, ***P < 0.001.
TLR8 signaling in pDCs of SSc patients induces CXCL4, whereas TLR7 and TLR9 do not
The chemokine CXCL4 can be secreted by many cells within the PBMCs including pDCs (32) and was more abundant in PBMCs from SSc patients as compared to HDs [Fig. 3A and (32)]. However, when pDCs were depleted from PBMCs, the increased secretion of CXCL4 observed in SSc PBMCs was normalized to HD PBMC levels (Fig. 3A), demonstrating that the excess of CXCL4 observed in PBMCs of SSc patients is solely due to the activation of pDCs and not to the contribution of other cell types.
Fig. 3. TLR8 signaling induces CXCL4 and IFN-α secretion by SSc PDCs.
(A) Gene expression levels of CXCL4 were quantified in total PBMC or pDC–depleted (dep) PBMC prepared from either HDs (n = 6) or SSc patients (n = 23) by qPCR. Statistical significance was evaluated using the unpaired Mann-Whitney test between HD and SSc PBMC and with a paired t test between PBMC and pDC-depleted PBMC from SSc patients. (B) Purified pDCs from HDs (n = 4 to 10) were cultured either with media (Med) alone as a control or in the presence of a TLR7 agonist [heat-inactivated VR95 influenza virus at a multiplicity of infection (MOI) of 2], a TLR8 agonist (ORN-8L at 200 μg/ml), or a TLR9 agonist (CpG-C274 at 0.5 μM), and 24 hours later, CXCL4 in the supernatants was measured by ELISA. Results were normalized to the value of media alone and represented as means ± SEM. (C and D) pDCs purified from SSc patients (n = 15 and 12, respectively) were cultured for 24 hours either in media alone or with either (C) ORN-8L or (D) CpG-C274, and CXCL4 production was analyzed by ELISA. Results were normalized to control and represented as means ± SEM. (E) HD (n = 10) and SSc pDCs (n = 20) were stimulated with ORN-8L for 24 hours, and supernatants were collected for measurement of IFN-α, IP-10, IL-6, and TNF by multiplex bead assay. Results were normalized to 10,000 cells. (F) Purified pDCs from SSc patients (n = 12) were cultured either with media alone or in the presence of a TLR9 agonist for 24 hours, and supernatants were collected for measurement of IFN-α, IP-10, IL-6, and TNF by multiplex bead assay. Results were normalized to 10,000 cells. All results are represented as means ± SEM. For (B) to (E), statistical significance was evaluated using a Mann-Whitney U test. *P < 0.05, **P < 0.01, ***P < 0.001.
Culturing pDCs purified from HDs with agonists of either TLR7 or TLR9 did not lead to CXCL4 production (Fig. 3B). In contrast, we observed that TLR8 but not TLR9 signaling could further induce CXCL4 secretion by pDCs from SSc patients (Fig. 3, C and D). The triggering of TLR8 in pDCs of SSc patients also induced the production of IFN-α, IP-10, IL-6, and TNF, whereas it had no effect on HD pDCs (Fig. 3E). Although TLR9 signaling did not induce CXCL4, the response by pDCs from SSc patients was otherwise normal with the induction of IFN-α, IP-10, IL-6, and TNF (Fig. 3F). Together, these data demonstrate that the aberrant expression of TLR8 on the pDCs of these patients can change the nature of the response of those cells to nucleic acids and can induce the secretion of CXCL4. This is a unique situation where the atypical expression of a TLR on a cell type can affect the nature of its response, with a clear association with a disease.
CXCL4 exacerbates the TLR-mediated IFN-α response in SSc pDCs, and its production is under the control of the P13Kδ pathway
Very little is known on how CXCL4 can affect immune function and whether this chemokine has any effect on the direct induction of fibrosis in patients. In contrast, the contribution of type I IFN and pDCs has been well described in many autoimmune diseases and although its effect on fibrosis is more controversial, the presence of an IFN-related gene signature has been described in SSc patients (38-40). This raises the question as to whether the impact of CXCL4 in SSc patients is solely to potentiate the pathogenic IFN response or to promote an inflammatory response independently of the IFN pathway. When purified pDCs from HDs or SSc patients were cultured with CXCL4 alone, there was no effect on IFN production. However, we observed that adding CXCL4 significantly increased (P < 0.001) the production of IFN-α and of IP-10 in response to TLR8 by purified pDCs from SSc patients (Fig. 4A), whereas it had no effect on IL-6 or TNF production (Fig. 4B). This observation indicates that in addition to its potential direct effect on other immune functions, CXCL4 has a critical role in potentiating the TLR-induced IFN production in SSc patients.
Fig. 4. CXCL4 potentiates TLR8-mediated activation of SSc pDCs.
(A and B) Purified pDCs from SSc patients (n = 8) were cultured in media alone (control), with ORN-8L (200 μg/ml), or with ORN-8L and CXCL4 (10 μg/ml) for 24 hours, and (A) IFN-α and IP-10 or (B) IL-6 and TNF were quantified in the supernatants by ELISA. Values were normalized to ORN-8L alone and represented as means ± SEM. (C to E) Purified pDCs from HDs were cultured alone, and purified pDCs from SSc patients were cultured for 24 hours either alone or in the presence of the PI3Kδ inhibitor CAL-101 (10 μM); (C) CXCL4, (D) IFN-α, or (E) IL-6 levels were quantified by ELISA. Results are normalized to 10,000 cells. All results are represented as means ± SEM, and statistical significance was evaluated using a Mann-Whitney U test. *P < 0.05, **P < 0.01, ***P < 0.001.
Although purified pDCs from SSc patients can spontaneously secrete IFN-α and CXCL4 (Fig. 1F), we did not observe an increase of costimulatory molecules on these cells (Fig. 1G), suggesting that the chronic activation of pDCs in these patients likely involves the transcription factor IRF7 (IFN regulatory factor 7) (41). We have previously shown that IRF7 translocation to the nucleus of pDCs and subsequent production of IFN-α are under the strict control of phosphatidylinositol 3-kinase δ (PI3Kδ) (41) and thus decided to test the involvement of PI3Kδ in controlling pDC activation in SSc patients. When cultured in the presence of the specific inhibitor of PI3Kδ CAL-101, the secretion of both CXCL4 and IFN-α was inhibited and normalized to that of HD pDCs (Fig. 4, C and D). CAL-101 was used at a concentration that can effectively block TLR9-induced IFN production by pDCs from HDs without affecting IL-6 production or cell viability (fig. S5, A and B). Blocking PI3Kδ had no effect on the secretion of IL-6 by pDCs of SSc patients (Fig. 4E), which indicates that inhibiting PI3Kδ could block the chronic activation of pDCs in SSc patients without globally affecting the ability of these cells to be activated in response to pathogens.
CXCL4 potentiates TLR9-induced IFN-α but has minimal effect on TLR7-mediated activation of pDCs purified from either HDs or SSc patients
We also measured the effect of CXCL4 on TLR7- and TLR9-induced response by human pDCs from both HDs and SSc patients. These two TLRs can induce high levels of IFN-α by human pDCs, and recent findings suggested that TLR9-induced IFN-α production was reduced by blocking CXCL4 (32). To evaluate the impact of CXCL4 on TLR-induced pDCs response, we used agonists of TLR7 and TLR9 at sub-optimal doses and used both a CpG-C and a CpG-B oligodeoxynucleotide (ODN). We observed that CXCL4 drastically affected the TLR9 response irrespective of the CpG used (Fig. 5, A and B) with little to no effect on the TLR7 response (Fig. 5C). This effect was similar both in HDs (Fig. 5, A to C) and SSc patients (Fig. 5, D and E), which is consistent with the fact that CXCR3B is expressed at similar level in purified pDCs of HDs and SSc patients (fig. S6) and CXCL4 itself did not induce IFN-α. These data thus suggest that the effect of CXCL4 is not restricted to TLR8 in patients and can affect TLR9 as well.
Fig. 5. CXCL4 potentiates TLR9-mediated activation but has minimal effect on TLR7-mediated activation of pDCs purified from SSc or HDs.
Purified pDCs from HDs (A to C) or SSc patients (D and E) were cultured in media alone (control), either (A and D) with a CpG-C TLR9 agonist (0.1 μM) or with CpG-C and CXCL4 (10 μg/ml), or (B) with a CpG-B TLR9 agonist (0.25 μM) or with CpG-B and CXCL4, and (C and E) with a TLR7 agonist [heat-inactivated VR95 influenza virus at a MOI of 0.5 (FLU)] or with FLU and CXCL4 for 24 hours (n = 6 to 18 as indicated). IFN-α was quantified in the supernatants by ELISA. Values were normalized to 10,000 cells. All results are represented as means ± SEM, and statistical significance was evaluated using a Mann-Whitney U test. *P < 0.05, **P < 0.01, ***P < 0.001.
pDCs are required for the establishment and maintenance of skin fibrosis in a mouse model of scleroderma
The display of an IFN signature in SSc patients (38-40), the presence of pDCs in the skin of localized scleroderma (morphea) and of SSc patients (Fig. 1A) (32, 42), and our new data showing that pDCs are chronically activated in SSc patients suggest that pDCs may participate in the pathogenesis of the disease. However, whether pDCs directly contributes to the fibrosis in the skin is unclear.
To address this question, skin fibrosis was induced in C57/BL6 mice by injection of bleomycin (BLM), a cancer drug that has long been known to cause fibrosis. In mice, BLM treatment constitutes a well-characterized mouse model for SSc and can induce skin fibrosis with a prevalence of almost 100% (43, 44), leading to increased skin thickness and loss of the subcutaneous fat in favor of sclerotic tissue (45). The fibrotic outcome of BLM treatment encompasses collagen deposition in the skin and the induction of α–smooth muscle actin (α-SMA)–positive stromal cells. We first confirmed that pDCs infiltrate the skin in this model after 4 weeks of BLM injection by measuring the presence of Siglec-H+ cells in the skin (Fig. 6, A to D). The immunohistochemistry staining was confirmed using in situ RNA hybridization for the siglech transcript in the skin of phosphate-buffered saline (PBS)– or BLM-treated mice (fig. S7, A and B). To evaluate the direct contribution of pDCs in skin fibrosis, these cells were depleted using CLEC4C-DTR (C-type lectin domain family 4, member C2-diphtheria toxin receptor) transgenic mice by injection of diphtheria toxin (DT) starting 24 hours before the first BLM injection and continuing through the entire experiment. As previously described (27), DT injection in CLEC4C-DTR mice is very efficient in specifically deleting pDCs (fig. S7, C and D) and does not affect other cell types in mice (fig. S7E), and we observed a significant reduction (P < 0.01) of pDC infiltration in the skin of the mice (Fig. 6, A to D). The loss of pDCs led to a significant reduction (P < 0.001) in the skin thickness of BLM-injected mice with an almost preserved subcutaneous fat (Fig. 6, E and F) and a normal content of collagen in the skin (Fig. 6G). Dermal myofibroblasts have the capacity to synthesize and deposit extracellular matrix components (46) and can be identified by expression of α-SMA. The depletion of pDCs resulted in a significantly decreased number of these cells (P < 0.001) as compared to pDC-sufficient mice (Fig. 6, H and I, and fig. S7F). The expression of TGF-β, a well-known marker of fibrosis for its impact on the synthesis and deposition of extracellular matrix by fibroblasts (47), was also reduced in pDC-depleted mice (Fig. 6J).
Fig. 6. pDCs infiltrate the skin of BLM-treated mice, and their depletion attenuates skin fibrosis.
pDC depletion was achieved as described in Materials and Methods. (A to C) Representative images of Siglec-H+ cells (red signal; original magnification, ×400) and (D) average (value based on five skin sections with n = 6 to 13 mice per group) in wild-type (WT) mice injected with (A) PBS and (B) with BLM and of (C) CLEC4C-DTR mice injected with DT and BLM. (E) Representative images of Masson’s trichrome–stained skin sections at a ×10 magnification, arrow indicates the area used to quantify skin thickness, and (F) average of skin thickness of WT mice receiving PBS [left panel in (E); open circle in (F)], WT mice injected with DT and treated with BLM [middle panel in (E); closed circle in (F)], and CLEC4C-DTR mice injected with DT and BLM [right panel in (E); closed square in (F)] is shown. (G) Collagen content in the skin expressed as a ratio of collagen per total protein (tot prot.) is shown. (H and I) Representative images of α–smooth muscle actin (α-SMA) immunohistochemistry of the dermis at a ×40 magnification and averages of the number of α-SMA–positive cells per surface area in dermis are shown. (J to L) Relative gene expression of the indicated genes using the NanoString technology. All values represent means ± SEM; n = 6 to 14 mice for each group. Data are cumulative of three independent experiments, and statistical significance was evaluated using a Mann-Whitney U test. *P < 0.05, **P < 0.01, ***P < 0.001.
We have shown that pDCs can induce a strong IFN response in the skin following injury (29), and the same was observed in this fibrosis model, with the presence of multiple IFN-regulated genes in BLM-treated animals, genes that were not expressed in pDC-depleted BLM-treated animals (Fig. 6K). We also observed a blockade of proinflammatory cytokines/chemokines such as TNF and monocyte chemoattractant protein–1 (MCP-1) (Fig. 6L) that have been associated with fibrosis (48, 49) in pDC-depleted mice, which was accompanied by a reduction in the overall immune cell infiltration in the skin. These data demonstrate that the pDC is a critical cell type directly involved in skin fibrosis because its depletion affects not only the inflammatory response that is induced by BLM but also the actual fibrotic outcome.
The role of pDCs in the maintenance of the disease was evaluated by depleting pDCs after 3 weeks of BLM treatment followed by the continuation of the BLM injections for an extra 2 weeks. After 3 weeks of BLM treatment, we observed an increase of the skin thickness in the mice and increased infiltration of pDCs, and this was even more apparent after 5 weeks (Fig. 7, A and B). When pDCs were depleted at 3 weeks in mice that had already established disease, at 5 weeks, we observed a reduction of the skin thickness as compared to pDC-competent mice. Furthermore, mice that received BLM for 5 weeks with pDCs depleted at 3 weeks had reduced disease even compared to the mice treated with BLM for just 3 weeks (Fig. 7A). This suggests that pDCs are critical for the maintenance of fibrosis in the skin and that their depletion leads to reversion of disease. Using in situ RNA hybridization for the cxcl4 transcript, we also show that the presence of pDCs in the skin correlated with the presence of CXCL4 (Fig. 7, C and D), which links both pDCs and CXCL4 to the promotion of skin fibrosis, although it is unclear whether CXCL4 is produced by the pDCs themselves, as it is in the human situation, or whether CXCL4 is produced by other cells that are recruited to the skin in a pDC-dependent manner because of the presence of multiple chemokines.
Fig. 7. pDCs are critical for the maintenance of skin fibrosis and for the presence of CXCL4 in the skin.
Fibrosis was induced by injection of BLM in WT mice for either 3 or 5 weeks (wks) or in CLEC4C-DTR mice for 5 weeks as described in Materials and Methods. At 3 weeks of BLM treatment, pDC depletion was achieved in CLEC4C-DTR mice by DT injection (in red) while fibrosis induction was sustained for 2 more weeks. (A) Average of skin thickness and (B) of the number of Siglec-H+ cells in the skin. (C) Representative images of the cxcl4 transcript by RNA in situ hybridization (brown signal; original magnification, ×400; inset, ×630) and (D) average of the number of CXCL4+ cells. Experiment was performed twice (n = 4 to 10 per group). All results are represented as means ± SEM, and statistical significance was evaluated using a Mann-Whitney U test. *P < 0.05, **P < 0.01, ***P < 0.001.
TLR8 exacerbates disease in the BLM-induced fibrosis model
We show herein that the aberrant expression of TLR8 in SSc pDCs leads to the production of both CXCL4 and IFN-α, suggesting that this receptor has a key role in the disease. To better understand whether TLR8 can promote fibrosis in a relevant in vivo model, skin fibrosis was induced in mice that carry the human TLR8 gene that we have recently described (15). These mice, called huTLR8Tg or Tg8, have a single copy of the huTLR8 gene under the control of huTLR8 genomic regulatory regions, and TLR8 cellular distribution is similar to the human situation (15). In addition, we have evaluated the respective contribution of TLR7 and TLR8 to disease because both receptors recognize RNA and seem to regulate each other’s response (15, 19). We used genetically modified mice and performed BLM experiments using huTLR8Tg (have both functional TLR8 and TLR7), huTLR8Tg × TLR7ko (have functional TLR8 but no TLR7), TLR7ko (deficient for both TLR7 and TLR8), and C57/BL6 mice, which are de facto TLR8-deficient but have TLR7. Upon BLM treatment, we observed aggravated fibrosis and an increase of skin thickness in huTLR8Tg mice over wild-type (WT) mice (Fig. 8, A and B). In addition, the infiltration of pDCs present in BLM-treated B6 mice was significantly increased in the TLR8Tg mice (Fig. 8, C and D) and did not require the presence of TLR7. The distribution of pDCs in the skin seemed altered in Tg8 mice with cells present in all the skin layers. Mice deficient for TLR7 were not protected from BLM-induced fibrosis (Fig. 8, A and B), and huTLR8Tg × TLR7ko mice also showed exacerbated response to BLM as compared to WT or TLR7ko mice (Fig. 8, A and B). Furthermore, we did not see significant differences (P < 0.01) in disease between the huTLR8Tg and huTLR8Tg × TLR7ko mice, pointing to a dominant role of TLR8 in this disease (Fig. 8, A and B). However, some of the inflammation observed in the skin could be partially attributed to TLR7.
Fig. 8. TLR8 exacerbates disease in the BLM-induced fibrosis model.
(A) Representative images of Masson’s trichrome–stained skin sections at a magnification of ×10 of WT mice injected with PBS or WT mice, Tg8 mice, TLR7KO mice, and Tg8 × TLR7KO (Tg8 × 7KO) mice injected with BLM. (B) Average of skin thickness (n = 11 to 18 per group). (C) Representative images of Siglec-H+ cells in the skin of WT mice injected with PBS or with BLM and of Tg8 mice, TLR7KO mice, and Tg8 × TLR7KO mice injected with BLM. (D) Histograms represent number of Siglec-H+ cells and are cumulative of at least 10 mice from three independent experiments. (E and F) Relative expression of indicated genes regulated by TLR8 and/or TLR7 using the NanoString technology. (G and H) Gene expression levels of huTLR8 by qPCR for (G) macrophages (F4/80+, CD11b+) or (H) pDCs (F4/80−, CD11b− and B220+, Siglec-H+, BST2+) purified from WT or Tg8 mice (n = 3 to 4) that received PBS or 4 weeks of BLM as indicated (see Materials and Methods). All results are represented as means ± SEM from three to six independent experiments for each group, and statistical significance was evaluated using a Mann-Whitney U test. *P < 0.05, **P < 0.01, ***P < 0.001.
First, we observed that the infiltration of pDCs was reduced in TLR7ko mice (Fig. 8, C and D). Second, the inflammatory response observed in the skin was dependent on both TLR7 and TLR8 with key differences between the genes of interest (Fig. 8, E and F). The expression of genes such as CXCL9 (MIG), CXCL10 (IP-10), RANTES, or TNF seemed to be regulated by TLR8 signaling because their expression was increased in TLR8Tg mice irrespective of the presence of TLR7 (Fig. 8E). Other genes, mostly regulated by IFN-α, were reduced in TLR7ko mice (with sometimes a contribution of TLR8) (Fig. 8, E and F), which may be explained by the reduced skin infiltration of pDCs observed in TLR7ko mice (Fig. 8, C and D). To better understand how the BLM model relates to the human situation with respect to TLR8 activation of pDCs, we quantified the expression levels of TLR8 in purified splenic macrophages, which we have shown to express huTLR8 (Fig. 8G) (15) or in splenic pDCs (Fig. 8H) in mice treated for 4 weeks either with PBS or BLM. We observed that in mice injected with PBS, pDCs do not express TLR8 but that this receptor is up-regulated in pDCs following the induction of fibrosis with BLM (Fig. 8H). Together, these data indicate that TLR8 is the key RNA-sensing TLR involved in the establishment of fibrosis and can promote not only an increase in the inflammatory response but also skin fibrosis.
DISCUSSION
In the past decade, substantial evidence has pointed to the involvement of self–nucleic acid recognition in inflammatory and autoimmune diseases. This is particularly clear for TLR7 and TLR9, which can induce type I IFN from pDCs but also promote the activation of self-reactive B cells. Their involvement in diseases such as lupus or skin diseases has been demonstrated in multiple preclinical studies, both in human and mouse models (11, 12). The role of TLR8 is less clear, although we have shown that mice overexpressing TLR8 develop a multiorgan inflammatory response leading to autoimmune pancreatitis and spontaneous arthritis (15). The contribution of TLRs to autoimmunity has been highlighted in multiple disease models and has been supported by two critical sets of evidence. First, it has been shown that TLRs, and in particular the nucleic acid–sensing TLRs, can recognize self-ligands, leading to an unwanted inflammatory response. The clearest example is the role played by TLR9 and TLR7 in lupus following the accumulation of self-DNA/RNA via immune complexes (22-24, 50). Another example is the importance of self–antimicrobial peptides in promoting autoimmunity (51). Second, it has been shown that the overexpression of TLRs alone is sufficient to induce autoimmunity in otherwise WT animals, as shown for TLR7 or TLR8 (13-15).
One of the main unresolved questions is to define which cell types are mediating the inflammatory response following nucleic acid sensing, a challenge that is augmented by the significant species differences between rodents and humans in TLR expression patterns. This is particularly true for the RNA-sensing TLR7 and TLR8 because of the lack of an appropriate animal model to study their respective functions in vivo. Our understanding of the contribution of TLR8 to inflammation has been mostly based on findings using mouse TLR7, which, similar to human TLR8, can recognize ssRNA in the endosome but has drastically different cellular distribution and ligand specificity (17).
We used genetically modified mice to address these challenges and to demonstrate the relevance of our findings to SSc patients. pDCs were depleted in CLEC4C-DTR mice using similar treatment to that recently described in a mouse model of lupus (27), which allowed us to test the direct impact of pDCs in both the establishment and maintenance of skin fibrosis in vivo. We also used huTLR8CL8 transgenic mice to characterize the role of TLR8 in vivo because these mice have a single copy of the TLR8 gene and no spontaneous inflammatory phenotype or abnormalities but respond to agonists of human TLR8 in vivo (15). The role of both RNA-sensing TLRs was further tested by using mice deficient for TLR7, TLR8, neither, or both. Using these mice and samples from well-characterized SSc patients, we made the following observations. We observed that pDCs infiltrate the skin of SSc patients, as has been observed in other skin diseases (28), and we noted that the number of circulating pDCs is reduced in the blood of SSc patients, which indicates that the cells are activated, as has been shown in lupus and other skin diseases. This was not the case for total B cells, although it is possible that subsets of B cells or plasma cells may be affected in these patients and a number of clinical strategies are focusing on these cells. We show that pDCs purified from patients spontaneously secrete IFN-α and CXCL4 but do not bear expression of costimulatory molecules, suggesting that the cells are activated but have not matured into fully differentiated DCs. At a mechanistic level, we demonstrate that this chronic activation of pDCs and subsequent production of CXCL4 and type I IFNs are due to the expression of TLR8 and its signaling on the pDCs of patients but not of HDs or of patients with SLE. When pDCs from HDs were cultured with agonists of TLR7 or TLR9, with IFN-α, CXCL4, or other inflammatory cytokines, none of these culture conditions affected TLR8 expression, and it is thus unclear what is controlling TLR8 expression in the pDCs of these patients. We also show that CXCL4 potentiates the TLR8-induced production of IFN by pDCs of SSc patients and that the production of CXCL4 and IFN is under the strict control of PI3Kδ. The effect of CXCL4 was also very pronounced on TLR9 signaling but did not affect TLR7, both in HDs and SSc patients. Our results are consistent with the experiments described by van Bon et al. (32) where CXCL4 inhibition seemed to affect CpG-induced IFN response, although levels of the IFN in response for TLR9 in that study was modest (few hundreds pg/ml) as compared to what has been repeatedly described for CpG-activated pDCs. It is well established in other indications such as lupus that TLR7 and TLR9 exert very distinct roles in the pathogenesis of disease. Lupus-prone mice deficient for TLR9 have exacerbated disease, even though these mice have reduced amount of antinucleosome autoantibodies, whereas similar mice deficient for TLR7 display milder autoimmunity. The mechanism underlying this difference is still unclear, and our observation that CXCL4 drastically affects TLR9-induced IFN-α, whereas it has no effect on TLR7, may provide a first line of evidence that the signaling of these two receptors can be differentially regulated by the inflammatory situation and highlights a potential role for TLR9 in SSc.
Our conclusions from patient samples were supported by our in vivo findings. We demonstrate that when pDCs are depleted (using CLEC4C-DTR mice), mice are protected from disease using a scleroderma mouse model and that removing pDCs in already established disease led to reduced fibrosis, stressing the critical role played by pDCs in the disease. In addition to observing a reduction in the IFN response in the skin, the depletion of pDCs also led to a significant amelioration of skin fibrosis as measured histologically or through the presence of profibrotic genes highlighting the importance of pDCs in promoting fibrosis. Using TLR8TgCL8 mice and when compared to WT littermate controls, we observed an exacerbation of disease in the BLM mouse model of scleroderma, confirming in the mouse the key role played by TLR8. We also describe that TLR7 participates in the infiltration of pDCs in the skin of the sick mice and the subsequent IFN response but that TLR8 emerges as the dominant receptor driving fibrosis. We thus propose a model where the aberrant expression of TLR8 on pDCs from SSc patients induces the secretion of CXCL4, which then can favor TLR responses, ultimately promoting autoimmunity and fibrosis leading to disease. It is worth noting though that the experiments where pDCs were depleted have been conducted in mice that do not express TLR8, suggesting that although TLR8 seems to be a key player in the pathogenesis of SSc, other TLR8-independent pathways are also involved in the disease. SSc is a complex and heterogeneous disease, and this suggests the existence of multiple mechanisms involved in the pathogenesis of the disease. Furthermore, platelets are the main source for CXCL4, which is an important chemoattractant for neutrophils, monocytes, and fibroblasts. Our data argue for a role for platelets, via the production of CXCL4 (33), in potentiating TLR responses at the site of inflammation or in response to tissue injury both in normal and pathological situations.
We understand that there are inherent limitations in conducting such translational studies using patient’s samples. Most of our findings were gathered from blood samples, which only give a partial view of the inflammatory situation in tissues. Studies in vivo using mouse models did complement our findings in SSc patients, but there are limitations associated with the relevance of disease models in mice and in particular of the well documented differences in TLR biology between mice and humans.
There is no effective treatment for SSc, and there is a clear need to better understand the key factors that promote disease pathogenesis to develop new therapeutic strategies. The involvement of the skin is a key component of the disease in scleroderma, and symptoms can be affected by environmental exposure. Our findings that pDCs are a key player in promoting the disease and the definition of the role played by TLR8 and PI3Kδ could open new ways to treat these patients. Our findings could not readily explain the female predominance observed in SSc because similar overexpression of TLR8 was seen in male and female patients. It is worth noting however that TLR8 is expressed on the X chromosome, but it is unclear whether this predominance can be associated with TLR8 response. pDCs are the main type I IFN producers in human blood and participate in antiviral immunity, but these cells have been associated with autoimmunity in various contexts, in particular in a number of cutaneous autoimmune diseases. Although it is well described that pDCs infiltrate the skin following injury, we know surprisingly little about what is controlling pDC trafficking and subsequent activation in the skin. Our data demonstrate that pDCs can also directly promote fibrosis since their deletion can prevent skin fibrosis even in already established disease. We also show that the pDC-driven inflammation in the skin seems to be associated with the actual skin fibrosis, and one hypothesis would be that the fibrotic response may start as part of a regulatory feedback to the inflammatory spur. Whether pDCs only take part in the inflammatory response or whether they have a direct effect on the production of profibrotic mediators will be key to our understanding of how these cells contribute to disease. There are novel approaches to directly target pDCs using, for example, CD123-depleting antibodies or antibodies specific for BDCA2, which are currently in clinical development (https://clinicaltrials.gov). In addition, many inhibitors of PI3Kδ are available, including CAL-101, which was recently approved (under the name Zydelig) for three cancer indications, as well as other molecules that are being tested in autoimmune diseases. None of these molecules or approaches has been tested in patients with SSc, but our data now provide some rationale to explore those and other approaches such as direct inhibition of TLR8, type I IFN, or CXCL4-induced signaling for the treatment of this devastating disease.
MATERIALS AND METHODS
Study design
The research objective of our study was to determine how pDCs, through TLR activation, might contribute to the pathogenesis of SSc. SSc patients between 21 and 79 years of age, SLE patients between 25 and 54 years of age, and healthy donors between 27 and 60 years of age were included in the human study. Patients were treated with a variety of therapies. However, they were excluded from the B cell analysis if they received rituximab, and they were excluded from the TLR assays if they were treated with hydroxychloroquine. No blinding or randomization was performed for the human studies. For in vivo studies, 8- to 12-week-old female mice were used. Immunohistochemistry and immunostaining analyses were performed in a blinded manner. Primary data are located in table S3.
Mice and BLM model
C57BL/6 and CLEC4C-DTR transgenic mice (27) and TLR7ko mice were obtained from The Jackson Laboratory, and the TLR8Tg mice were generated as described (15). Skin fibrosis was induced using the BLM-induced model as originally described by Yamamoto (43, 44) and adapted for better consistency (45). BLM treatment can induce skin fibrosis in a small (about 1 cm) area at the site of injection with a prevalence of almost 100%. Mice (8- to 12-week-old female) received daily subcutaneous injections for 3 to 5 weeks in three adjacent spots on the shaved lower back either with 60 μg (100 μl) of filter-sterilized BLM or PBS. To deplete pDCs, CLEC4C-DTR mice were injected twice weekly by intraperitoneal injection with 400 ng of DT. Injections either started 1 day before and during the 4 weeks of experimental fibrosis or started at 3 weeks after the start of the BLM injections and continued for the remaining 2 weeks (BLM treatment was not interrupted). The depletion efficiency was confirmed by flow cytometry analysis of pDCs (B220+PDCA-1+) in the spleen (fig. S7, C and D). All animal procedures were performed in accordance with the regulations of the Institutional Animal Care and Use Committee of the Hospital for Special Surgery and Weill Cornell Medical College.
Details on the evaluation of skin fibrosis and gene expression analysis, in situ mRNA hybridization analysis, and immunohistochemical analysis and on how mouse macrophages and pDCs were isolated are provided in the Supplementary Materials.
Patients
Participants were recruited from the institutional review board–approved Hospital for Special Surgery Scleroderma or Systemic Lupus Erythematosus Registries. Patients and healthy volunteers provided written informed consent before enrollment. All SSc patients fulfilled the 2013 ACR/EULAR classification criteria for SSc (52). Patients were categorized as having limited cutaneous or diffuse cutaneous subtype of SSc according to LeRoy and Medsger Jr. (53). Disease duration was defined as the time from the first SSc-related symptom apart from the Raynaud phenomenon and was classified as early if the disease duration was ≤2 years. The clinical and demographic characteristics of the SSc patients are described in Table 1 and table S1.
Table 1.
Clinical and demographic characteristics of the SSc patients.
| Age—years, mean (SD) | 51.32 (12.82) |
| Sex—n, % female | 61, 77.2% |
| 55, 69.6% Caucasian | |
| Race—n, % | 18, 22.8% African American |
| 5, 6.3% Asian | |
| Disease duration—years, mean (SD) | 5.6 (4.9) |
| Scleroderma subtype | |
| n, % diffuse | 62, 78.5% |
| n, % limited | 13, 16.5% |
| MRSS—mean (SD) | 16.4 (11) |
| Autoantibody | |
| n, % Scl70 | 28, 35.4% Scl70 |
| n, % RNA polymerase 3 | 21, 26.6% POL3 |
| n, % centromere | 11, 13.9% CENB |
| n, % RNP | 4, 5.06% RNP |
| Interstitial lung disease present—n, % | 43, 54.4% |
| Pulmonary hypertension present—n, % | 8, 10.1% |
All SLE patients fulfilled the 1997 ACR criteria (54) and its complementary criteria, the 2012 Systemic Lupus International Collaborating Clinics criteria (55) for SLE patients. The clinical and demographic characteristics of the SLE patients are described in Table 2 and table S2.
Table 2.
Clinical and demographic characteristics of the SLE patients.
| Age—years, mean (SD) | 40.67 (10.98) |
| Sex—n, % female | 5, 83.33% |
| 2, 33.3% Caucasian | |
| Race—n, % | 2, 33.3% African American |
| 2, 33.3% Asian | |
| Disease duration—years, mean (SD) | 15 (3.29) |
| ACR criteria—n, % | |
| Malar rash | 3, 50% |
| Discoid rash | 0, 0% |
| Photosensitivity | 3, 50% |
| Oral ulcers | 3, 50% |
| Arthritis | 5, 83% |
| Serositis | 3, 50% |
| Renal disorder | 2, 33% |
| Neurologic disorder | 0, 0% |
| Hematologic disorder | 6, 100% |
| Immunologic disorder | 5, 83% |
| Positive ANA | 6, 100% |
| Positive titer of anti-dsDNA—n, % total | 3, 50% |
| 1+ positive | 1 |
| 2+ positive | 1 |
| 3+ positive | 1 |
| 4+ positive | 0 |
| History of positive anti-RBP—n, % total | 4, 66.67% |
| Anti-Ro | 3 |
| Anti-La | 1 |
| Anti-Sm | 0 |
| Anti-RNP | 3 |
| SLEDAI—mean (SD) | 4.5 (2.43) |
Details on how cells were purified from the blood of HDs or patients, on the flow cytometry staining and analysis, on real-time quantitative polymerase chain reaction analysis, and on measurement of chemokines and cytokines are provided in the Supplementary Materials.
Statistical analysis
Data were analyzed using a Mann-Whitney U test (t test using non-parametric criteria for independent samples). Comparisons between PBMC and pDC-depleted PBMC were tested using a parametric paired t test. All analyses were performed using Prism software (GraphPad Software). Differences were considered significant at a P level less than 0.05 with *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Material
Table S3. Primary data.
Fig. S1. BDCA4-positive cells express BDCA2, and both are specific markers for pDC.
Fig. S2. Monocytes of SSc do not express BDCA4.
Fig. S3. Reduction of the expression levels of TLRs in pDC-depleted PBMC.
Fig. S4. Monocytes are not depleted in pDC-depleted PBMC.
Fig. S5. CAL-101 specifically inhibits IFN-α but not IL-6 production by TLR9-activated pDCs.
Fig. S6. pDCs purified from HDs or SSc patients have similar expression of CXCR3B.
Fig. S7. Injection of DT leads to the specific depletion of pDCs in CLEC4C-DTR mice.
Table S1. Clinical and demographic characteristics of the 79 individual SSc patients as well as the overall description of the patient population are shown.
Table S2. Clinical and demographic characteristics of the six individual SLE patients as well as the overall description of the patient population are shown.
Acknowledgments:
We would like to thank the patients for their participation in the study and our colleagues at the Hospital for Special Surgery for their critical reading of the manuscript. We thank K. Merritt, A. Morquette, D. Lerner, A. Fernandez, and E. Pelrine for their help with coordinating the patients’ samples.
Funding:
F.J.B. is supported by the Hospital for Special Surgery and by the Michael R. Bloomberg Chair. M.D.A.K. is supported by the Charles L. Christian Research Fellowship. C.T. is supported by the Italian Association for Cancer Research (IG 15999) and the University of Palermo.
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/10/423/eaam8458/DC1
Competing interests: F.J.B. is the inventor on the patent application (#62/492,562) submitted by the Hospital for Special Surgery that covers the effect of CXCL4 during innate responses. All other authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Varga J, Abraham D, Systemic sclerosis: A prototypic multisystem fibrotic disorder. J.Clin. Invest 117, 557–567 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferri C, Valentini G, Cozzi F, Sebastiani M, Michelassi C, La Montagna G, Bullo A, Cazzato M, Tirri E, Storino F, Giuggioli D, Cuomo G, Rosada M, Bombardieri S, Todesco S, Tirri G; Systemic Sclerosis Study Group of the Italian Society of Rheumatology (SIR-GSSSc), Systemic sclerosis: Demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 81, 139–153 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Mayes MD, Lacey JV Jr., Beebe-Dimmer J, Gillespie BW, Cooper B, Laing TJ, Schottenfeld D, Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 48, 2246–2255 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa M, Hamaguchi Y, Yanaba K, Bouaziz J-D, Uchida J, Fujimoto M, Matsushita T, Matsushita Y, Horikawa M, Komura K, Takehara K, Sato S, Tedder TF, B-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosis. Am. J. Pathol 169, 954–966 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lafyatis R, Kissin E, York M, Farina G, Viger K, Fritzler MJ, Merkel PA, Simms RW, B cell depletion with rituximab in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 60, 578–583 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sargent JL, Whitfield ML, Capturing the heterogeneity in systemic sclerosis with genome-wide expression profiling. Expert Rev. Clin. Immunol 7, 463–473 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker KM, Pope J; Participating members of the Scleroderma Clinical Trials Consortium (SCTC); Canadian Scleroderma Research Group (CSRG), Treatment of systemic sclerosis complications: What to use when first-line treatment fails—A consensus of systemic sclerosis experts. Semin. Arthritis Rheum 42, 42–55 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Janeway CA Jr., Medzhitov R, Innate immune recognition. Annu. Rev. Immunol 20, 197–216 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Brubaker SW, Bonham KS, Zanoni I, Kagan JC, Innate immune pattern recognition:A cell biological perspective. Annu. Rev. Immunol 33, 257–290 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao X, Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat. Rev. Immunol 16, 35–50 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Barrat FJ, Elkon KB, Fitzgerald KA, Importance of nucleic acid recognition in inflammation and autoimmunity. Annu. Rev. Med 67, 323–336 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Pelka K, Shibata T, Miyake K, Latz E, Nucleic acid-sensing TLRs and autoimmunity: Novel insights from structural and cell biology. Immunol. Rev 269, 60–75 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Walsh ER, Pisitkun P, Voynova E, Deane JA, Scott BL, Caspi RR, Bolland S, Dual signaling by innate and adaptive immune receptors is required for TLR7-induced B-cell-mediated autoimmunity. Proc. Natl. Acad. Sci. U.S.A 109, 16276–16281 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S, Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 27, 801–810 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guiducci C, Gong M, Cepika A-M, Xu Z, Tripodo C, Bennett L, Crain C, Quartier P, Cush JJ, Pascual V, Coffman RL, Barrat FJ, RNA recognition by human TLR8 can lead to autoimmune inflammation. J. Exp. Med 210, 2903–2919 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hattermann K, Picard S, Borgeat M, Leclerc P, Pouliot M, Borgeat P, The Toll-like receptor 7/8-ligand resiquimod (R-848) primes human neutrophils for leukotriene B4, prostaglandin E2 and platelet-activating factor biosynthesis. FASEB J. 21, 1575–1585 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Forsbach A, Nemorin J-G, Montino C, Müller C, Samulowitz U, Vicari AP, Jurk M, Mutwiri GK, Krieg AM, Lipford GB, Vollmer J, Identification of RNA sequence motifs stimulating sequence-specific TLR8-dependent immune responses. J. Immunol 180, 3729–3738 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Janke M, Poth J, Wimmenauer V, Giese T, Coch C, Barchet W, Schlee M, Hartmann G, Selective and direct activation of human neutrophils but not eosinophils by Toll-like receptor 8. J. Allergy Clin. Immunol 123, 1026–1033 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Demaria O, Pagni PP, Traub S, de Gassart A, Branzk N, Murphy AJ, Valenzuela DM, Yancopoulos GD, Flavell RA, Alexopoulou L, TLR8 deficiency leads to autoimmunity in mice. J. Clin. Invest 120, 3651–3662 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umiker BR, Andersson S, Fernandez L, Korgaokar P, Larbi A, Pilichowska M, Weinkauf CC, Wortis HH, Kearney JF, Imanishi-Kari T, Dosage of X-linked Toll-like receptor 8 determines gender differences in the development of systemic lupus erythematosus. Eur. J. Immunol 44, 1503–1516 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Xu C, Hsu L-C, Luo Y, Xiang R, Chuang T-H, A five-amino-acid motif in the undefined region of the TLR8 ectodomain is required for species-specific ligand recognition. Mol. Immunol 47, 1083–1090 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Båve U, Magnusson M, Eloranta M-L, Perers A, Alm GV, Rönnblom L, FcγRIIa is expressed on natural IFN-α-producing cells (plasmacytoid dendritic cells) and is required for the IFN-α production induced by apoptotic cells combined with lupus IgG. J. Immunol 171, 3296–3302 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, Chang B, Duramad O, Coffman RL, Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med 202, 1131–1139 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD, Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Invest 115, 407–417 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T, Chan JH, Wright T, Punaro M, Bolland S, Soumelis V, Banchereau J, Coffman RL, Pascual V, Barrat FJ, TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature 465, 937–941 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sisirak V, Ganguly D, Lewis KL, Couillault C, Tanaka L, Bolland S, D’Agati V, Elkon KB, Reizis B, Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J. Exp. Med 211, 1969–1976 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowland SL, Riggs JM, Gilfillan S, Bugatti M, Vermi W, Kolbeck R, Unanue ER, Sanjuan MA, Colonna M, Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J. Exp. Med 211, 1977–1991 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenzel J, Tüting T, An IFN-associated cytotoxic cellular immune response against viral, self-, or tumor antigens is a common pathogenetic feature in “interface dermatitis.” J. Invest. Dermatol 128, 2392–2402 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Guiducci C, Tripodo C, Gong M, Sangaletti S, Colombo MP, Coffman RL, Barrat FJ, Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J. Exp. Med 207, 2931–2942 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregorio J, Meller S, Conrad C, Di Nardo A, Homey B, Lauerma A, Arai N, Gallo RL, DiGiovanni J, Gilliet M, Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J. Exp. Med 207, 2921–2930 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerber EE, Gallo EM, Fontana SC, Davis EC, Wigley FM, Huso DL, Dietz HC, Integrin-modulating therapy prevents fibrosis and autoimmunity in mouse models of scleroderma. Nature 503, 126–130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Bon L, Affandi AJ, Broen J, Christmann RB, Marijnissen RJ, Stawski L, Farina GA, Stifano G, Mathes AL, Cossu M, York M, Collins C, Wenink M, Huijbens R, Hesselstrand R, Saxne T, DiMarzio M, Wuttge D, Agarwal SK, Reveille JD, Assassi S, Mayes M, Deng Y, Drenth JPH, de Graaf J, den Heijer M, Kallenberg CGM, Bijl M, Loof A, van den Berg WB, Joosten LAB, Smith V, de Keyser F, Scorza R, Lunardi C, van Riel PLCM, Vonk M, van Heerde W, Meller S, Homey B, Beretta L, Roest M, Trojanowska M, Lafyatis R, Radstake TRDJ, Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N. Engl. J. Med 370, 433–443 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Files JC, Malpass TW, Yee EK, Ritchie JL, Harker LA, Studies of human plate alpha-granule release in vivo. Blood 58, 607–618 (1981). [PubMed] [Google Scholar]
- 34.Cederblad B, Blomberg S, Vallin H, Perers A, Alm GV, Rönnblom L, Patients with systemic lupus erythematosus have reduced numbers of circulating natural interferon-α- producing cells. J. Autoimmun 11, 465–470 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J, Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science 294, 1540–1543 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Duramad O, Fearon KL, Chan JH, Kanzler H, Marshall JD, Coffman RL, Barrat FJ, IL-10 regulates plasmacytoid dendritic cell response to CpG-containing immunostimulatory sequences. Blood 102, 4487–4492 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Guiducci C, Ott G, Chan JH, Damon E, Calacsan C, Matray T, Lee K-D, Coffman RL, Barrat FJ, Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J. Exp. Med 203, 1999–2008 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rice LM, Ziemek J, Stratton EA, McLaughlin SR, Padilla CM, Mathes AL, Christmann RB, Stifano G, Browning JL, Whitfield ML, Spiera RF, Gordon JK, Simms RW, Zhang Y, Lafyatis R, A longitudinal biomarker for the extent of skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 67, 3004–3015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan FK, Zhou X, Mayes MD, Gourh P, Guo X, Marcum C, Jin L, Arnett FC Jr., Signatures of differentially regulated interferon gene expression and vasculotrophism in the peripheral blood cells of systemic sclerosis patients. Rheumatology (Oxford) 45, 694–702 (2006). [DOI] [PubMed] [Google Scholar]
- 40.York MR, Nagai T, Mangini AJ, Lemaire R, van Seventer JM, Lafyatis R, A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 56, 1010–1020 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Guiducci C, Ghirelli C, Marloie-Provost M-A, Matray T, Coffman RL, Liu Y-J, Barrat FJ, Soumelis V, PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J. Exp. Med 205, 315–322 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghoreishi M, Vera Kellet C, Dutz JP, Type 1 IFN-induced protein MxA and plasmacytoid dendritic cells in lesions of morphea. Exp. Dermatol 21, 417–419 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto T, Takagawa S, Katayama I, Yamazaki K, Hamazaki Y, Shinkai H, Nishioka K, Animal model of sclerotic skin. I: Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J. Invest. Dermatol 112, 456–462 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Batteux F, Kavian N, Servettaz A, New insights on chemically induced animal models of systemic sclerosis. Curr. Opin. Rheumatol 23, 511–518 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Chia JJ, Zhu T, Chyou S, Dasoveanu DC, Carballo C, Tian S, Magro CM, Rodeo S, Spiera RF, Ruddle NH, McGraw TE, Browning JL, Lafyatis R, Gordon JK, Lu TT, Dendritic cells maintain dermal adipose-derived stromal cells in skin fibrosis. J. Clin. Invest 126, 4331–4345 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang J, Beyer C, Palumbo-Zerr K, Zhang Y, Ramming A, Distler A, Gelse K, Distler O, Schett G, Wollin L, Distler JHW, Nintedanib inhibits fibroblast activation and ameliorates fibrosis in preclinical models of systemic sclerosis. Ann. Rheum. Dis 75, 883–890 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Palumbo-Zerr K, Zerr P, Distler A, Fliehr J, Mancuso R, Huang J, Mielenz D, Tomcik M, Fürnrohr BG, Scholtysek C, Dees C, Beyer C, Krönke G, Metzger D, Distler O, Schett G, Distler JHW, Orphan nuclear receptor NR4A1 regulates transforming growth factor-β signaling and fibrosis. Nat. Med 21, 150–158 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Koca SS, Isik A, Ozercan IH, Ustundag B, Evren B, Metin K, Effectiveness of etanercept in bleomycin-induced experimental scleroderma. Rheumatology (Oxford) 47, 172–175 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto T, Nishioka K, Role of monocyte chemoattractant protein-1 and its receptor,CCR-2, in the pathogenesis of bleomycin-induced scleroderma. J. Invest. Dermatol 121, 510–516 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Hagberg N, Rönnblom L, Systemic lupus erythematosus—A disease with a dysregulated type I interferon system. Scand. J. Immunol 82, 199–207 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang Y-H, Homey B, Cao W, Wang Y-H, Su B, Nestle FO, Zal T, Mellman I, Schröder J-M, Liu Y-J, Gilliet M, Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569 (2007). [DOI] [PubMed] [Google Scholar]
- 52.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, Matucci-Cerinic M, Naden RP, Medsger TA Jr., Carreira PE, Riemekasten G, Clements PJ, Denton CP, Distler O, Allanore Y, Furst DE, Gabrielli A, Mayes MD, van Laar JM, Seibold JR, Czirjak L, Steen VD, Inanc M, Kowal-Bielecka O, Müller-Ladner U, Valentini G, Veale DJ, Vonk MC, Walker UA, Chung L, Collier DH, Csuka ME, Fessler BJ, Guiducci S, Herrick A, Hsu VM, Jimenez S, Kahaleh B, Merkel PA, Sierakowski S, Silver RM, Simms RW, Varga J, Pope JE, 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum. 65, 2737–2747 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LeRoy EC, Medsger TA Jr., Criteria for the classification of early systemic sclerosis. J. Rheumatol 28, 1573–1576 (2001). [PubMed] [Google Scholar]
- 54.Hochberg MC, Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 40, 1725 (1997). [DOI] [PubMed] [Google Scholar]
- 55.Petri M, Orbai A-M, Alarcón GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae S-C, Hanly JG, Sánchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG Jr., Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G Jr., Magder LS, Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64, 2677–2686 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S3. Primary data.
Fig. S1. BDCA4-positive cells express BDCA2, and both are specific markers for pDC.
Fig. S2. Monocytes of SSc do not express BDCA4.
Fig. S3. Reduction of the expression levels of TLRs in pDC-depleted PBMC.
Fig. S4. Monocytes are not depleted in pDC-depleted PBMC.
Fig. S5. CAL-101 specifically inhibits IFN-α but not IL-6 production by TLR9-activated pDCs.
Fig. S6. pDCs purified from HDs or SSc patients have similar expression of CXCR3B.
Fig. S7. Injection of DT leads to the specific depletion of pDCs in CLEC4C-DTR mice.
Table S1. Clinical and demographic characteristics of the 79 individual SSc patients as well as the overall description of the patient population are shown.
Table S2. Clinical and demographic characteristics of the six individual SLE patients as well as the overall description of the patient population are shown.