Abstract
Neisseria meningitidis serogroup B infections are among the major causes of fulminant septicemia and meningitis, especially severe in young children, and no broad vaccine is available yet. Because of poor immunogenicity of the serogroup B capsule, many efforts are now devoted to the identification of protective protein antigens. Among those are PorA and, more recently, transferrin-binding protein B (TbpB). In this study, TbpB of N. meningitidis was genetically fused to the N-terminal domain of the Bordetella pertussis filamentous hemagglutinin (FHA), and the fha-tbpB hybrid gene was expressed in B. pertussis either as a plasmid-borne gene or as a single copy inserted into the chromosome. The hybrid protein was efficiently secreted by the recombinant strains, despite its large size, and was recognized by both anti-FHA and anti-TbpB antibodies. A single intranasal administration of recombinant virulent or pertussis-toxin-deficient, attenuated B. pertussis to mice resulted in the production of antigen-specific systemic immunoglobulin G (IgG), as well as local IgG and IgA. The anti-TbpB serum antibodies were of the IgG1, IgG2a, and IgG2b isotypes and were found to express complement-mediated bactericidal activity against N. meningitidis. These observations indicate that recombinant B. pertussis may be a promising vector for the development of a mucosal vaccine against serogroup B meningococci.
Serogroup B Neisseria meningitidis is responsible for fulminant septicemia and is a common cause of pyogenic meningitis. In addition to sporadic outbreaks, large epidemics of serogroup B meningococcal disease occur in many parts of the world (7). Therefore, the development of a vaccine against N. meningitidis serogroup B remains a high priority worldwide. Unlike other serogroups for which the capsular polysaccharides constitute efficacious vaccines, the serogroup B capsule is poorly immunogenic in humans (2). Therefore, attention has been turned to protein antigens in an attempt to use them for serogroup B meningococcal vaccine development. Among them, the transferrin-binding proteins (Tbp), especially TbpB, appear to be particularly promising. TbpA and TbpB constitute the major components of meningococcal receptors for human transferrin (hTf) (for a review, see reference 6). TbpB is a surface-exposed lipoprotein expressed during iron starvation and plays a critical role in N. meningitidis iron uptake from iron-loaded hTF (5). It has been shown previously to be protective in animals after passive or active immunization (1, 9, 24), and humans mount bactericidal antibody responses to TbpB upon meningococcal infections (3). Complement-mediated bactericidal activity of immune sera is considered to be a major mechanism of protection by preventing the multiplication of the microorganism after its passage through the upper respiratory tract epithelium and invasion of the bloodstream (34).
In modern vaccine development, strong emphasis is laid on mucosal delivery systems (25). In particular, the intranasal (i.n.) route holds promise for the induction of protective immune responses, since it is able to elicit both strong local and strong systemic immune responses. i.n. vaccination may therefore be of particular interest against respiratory tract infections, such as those caused by N. meningitidis (11, 14). However, efficacious mucosal vaccination generally requires multiple high-dose regimens (47). We have recently developed recombinant Bordetella pertussis strains able to induce mucosal (38) and systemic (33) antibody responses against heterologous antigens after a single i.n. administration. Interestingly, and in contrast to other live bacterial vectors, attenuated B. pertussis strains induced stronger systemic antibody responses than did their virulent parent strains (32, 33).
In this study, we have thus used recombinant B. pertussis to present TbpB at its surface, utilizing the highly efficient secretion mechanism of the B. pertussis filamentous hemagglutinin (FHA) (for a review, see reference 26). The recombinant strain was found to induce anti-TbpB antibody responses, both in the bronchoalveolar lavage fluids (BALF) and in the sera of mice inoculated with a single i.n. dose. In addition, the serum antibodies expressed bactericidal activity in an in vitro complement-mediated bactericidal activity assay.
MATERIALS AND METHODS
Strains, plasmids, culture conditions, and DNA manipulations.
The B. pertussis strains and plasmids used in this study are listed in Table 1. B. pertussis growth conditions were as described previously (15). The Bordetella strains were transformed by electroporation at 12.5 kV/cm, 600 Ω, and 25 μF. N. meningitidis B16B6 (B:2a:P1.2) and M982 (B:9:P1.9) were obtained from C. Frasch and grown in Mueller-Hinton medium at 37°C in a 10% CO2-enriched atmosphere. To induce the production of TbpB, the meningococcal strains were grown for 4 h in the presence of 30 μM ethylene diaminodiorthohydroxyphenyl acetic acid (Sigma, St. Louis, Mo.). The anti-N. meningitidis bactericidal activities of the mouse sera were tested as described previously (9). Bactericidal titers are expressed as the highest dilution of serum which killed ≥50% of the bacteria, compared to the complement-only control. All DNA manipulations were performed as described previously (43). Southern blot analyses were performed using nonradioactive DNA probes labeled with digoxigenin-dUTP (Boehringer Mannheim Corp., Mannheim, Germany) according to the instructions of the supplier.
TABLE 1.
B. pertussis strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| BPSM | Smr Nalr Tohama I derivative | 31 |
| BPGR4 | BPSM derivative lacking fhaB | 27 |
| BPRA | BPSM derivative lacking ptx | 4 |
| BPIC1 | BPSM derivative producing Fha44-TbpB/M982 | This study |
| BPIC3 | BPRA derivative producing Fha44-TbpB/B16B6 | This study |
| BPIC4 | BPSM derivative expressing Fha44-TbpB/B16B6 | This study |
| Plasmids | ||
| pTG4710 | pARA13 derivative containing tbpB/B16B6 | 45 |
| pTG4714 | pARA13 derivative containing tbpB/M982 | 45 |
| pTG6705 | pTG4710 derivative with tbpB mutations (R185–200) | 45 |
| pTG6706 | pTG4710 derivative with tbpB mutations (R306–321) | 45 |
| pBG4 | pBBR122 derivative with the 5′ end of fhaB (fha44) | 39 |
| pRIT13202 | pUC8 derivative containing fhaB | 10 |
| pIC-20H | ColE1-based cloning vector | 28 |
| pUC18 | ColE1-based cloning vector | 48 |
| pNFNT | pBG4 derivative with 5′ end of tbpB/B16B6 | This study |
| pNFCT | pBG4 derivative with 3′ end of tbpB/B16B6 | This study |
| pNFT/B16B6 | pBG4 derivative with tbpB/B16B6 | This study |
| pNFT/M982 | pBG4 derivative with tbpB/M982 | This study |
| pIC5 | pIC-20H derivative with fha44-tbpB/M982 | This study |
| pIC6 | pIC5 derivative with 2.4-kb BamHI fhaB fragment | This study |
| pIC7 | pUC18 derivative with 2.4-kb BamHI fhaB fragment | This study |
| pIC8 | pIC7 derivative with fha44-tbpB/B16B6 | This study |
Construction of plasmids and recombinant Bordetella.
The TbpB-encoding sequences of N. meningitidis strains B16B6 and M982 were amplified by PCR from clones pTG4710, pTG6705, pTG6706, and pTG4714 (45), using the oligonucleotides AAAGATCTCTGGGTGGCGGCGGCAGTTCG and AAAGATCTTCGCACCGAATACCACCGATGCTT (for strain B16B6) and TCAGATCTCTGGGCGGCGGCGGCAGTTTCGAT and GCAGATCTGCTGTTGGCGTTTCGCACCGAATACC (for strain M982), respectively, each containing a BglII site (underlined). The amplified DNA encompassed nucleotides 151 to 1864 for strain B16B6 and 151 to 2232 for strain M982 (21).
The PCR products were digested by BglII and cloned into the BamHI site of pBG4. The resulting plasmids, named pNFNT, pNFCT, pNFT/B16B6, and pNFT/M982, were introduced into B. pertussis BPGR4 (27). pNFT/B16B6 and pNFT/M982 encode the full-length TbpB from strains B16B6 and M982, respectively, whereas pNFNT and pNFCT encode the N-terminal and C-terminal portions of TbpB from strain B16B6, respectively. The latter two plasmids contain the 5′ BglII fragment amplified from pTG6706 and the 3′ BglII fragment amplified from pTG6705, respectively.
To insert the B16B6 tbpB gene into the B. pertussis chromosome at the fhaB locus, the 2.4-kb BamHI fragment from pRIT13202 was first introduced into the BamHI site of pUC18, yielding pIC7. This plasmid was further digested by EcoRI to introduce the 4.5-kb EcoRI fragment from pNFT/B16B6, generating pIC8. This plasmid was then introduced into B. pertussis BPRA and BPSM to generate strains BPIC3 and BPIC4, respectively, by allelic exchange. To insert the M982 tbpB gene into the fhaB gene on the B. pertussis chromosome, the 4.9-kb EcoRI fragment from pNFT/M982 was first inserted into the EcoRI site of pIC-20H, generating pIC5. The 2.4-kb BamHI fragment from pRIT13202 was then inserted into the BamHI site of pIC5, yielding pIC6. This plasmid was inserted into the B. pertussis BPSM chromosome by allelic exchange at the fhaB locus, yielding strain BPIC1. The proper insertion of the fha-tbpB hybrid genes was analyzed by Southern blotting.
Protein analysis, immunodetection, and transferrin binding assays.
For the detection of the Fha44-TbpB hybrid proteins, the culture supernatants or whole-cell lysates of the various Bordetella strains were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 8% polyacrylamide gels (20). The proteins were stained with Coomassie blue or electrotransferred onto polyvinylidene difluoride membranes and stained with rat (37) or chicken (13) anti-Fha44 antibodies or rabbit polyclonal (45) or mouse monoclonal (511F8; Aventis Pasteur) anti-TbpB antibodies in phosphate-buffered saline (PBS) containing 0.1% Tween and 1% bovine serum albumin (BSA), followed by alkaline phosphatase (AP)-conjugated anti-immunoglobulin G (IgG; Sigma Chemical Co.) and AP substrate.
For transferrin binding assays, the polyvinylidene difluoride membrane was incubated for 1 h at 37°C in blocking buffer (50 mM Tris-HCl, 2 mM CaCl2, 90 mM NaCl, pH 7.4) containing 50 mg of BSA/ml and then incubated at room temperature for 1 h in the presence of 100 μg of hTf (Sigma)/ml. After being washed in blocking buffer containing 5 mg of BSA/ml, the membrane was incubated for 1 h at 37°C with polyclonal goat anti-hTf antibodies (Sigma) diluted 1/1,000, washed with blocking buffer, and incubated with AP-conjugated anti-goat IgG-AP (Sigma) diluted 1/5,000 and then with AP substrate. Because of a slight anti-TbpB reactivity, the anti-hTf antiserum was incubated with purified TbpB before use.
Purified TbpB was provided by Transgène (Strasbourg, France). FHA and Fha44 were purified from B. pertussis culture supernatants by heparin-Sepharose chromatography (30).
Antibody responses.
The mouse colonization assay was performed as described previously (38). After i.n. administration of 5 × 106 CFU of the various B. pertussis strains, IgA and IgG responses in the BALF and sera were analyzed using enzyme-linked immunosorbent assays (ELISAs) at the indicated time points. Immulon 3 flat-bottomed 96-well plates (Dynatech Laboratories, Chantilly, Va.) were coated with 50 μl of PBS containing 10 μg of purified FHA, Fha44, or TbpB/ml. After blocking with PBS containing 0.1% Tween, 1% BSA, and 4% fetal calf serum, 50 μl of BALF or sera was added in twofold serial dilutions. The plates were then incubated overnight at 4°C, and biotinylated goat anti-IgA, anti-IgG, anti-IgG1, anti-IgG2a, or anti-IgG2b antibodies (Amersham France, Les Ulis, France) were added for 90 min at 37°C at a 1/500, 1/1,000, 1/5,000, 1/12,000 or 1/12,000 dilution, respectively. Anti-IgA and anti-IgG antibodies were detected by the addition of peroxidase-conjugated streptavidin (Amersham) at a 1/1,000 dilution and 1 mg of o-phenylenediamine (Sigma)/ml in 0.1 M citrate buffer (pH 5.5) containing 0.03% H2O2. The reaction was stopped by the addition of 2 M HCl, and the optical density was measured at 492 nm. Results are expressed as titers, defined as the highest dilution of BALF and sera yielding an optical density two and three times, respectively, above the values obtained for the mice infected with BPSM or BPRA.
RESULTS
Production of chimeric TbpB-FHA proteins in Bordetella.
Previous studies have shown that the highly efficient secretion mechanism of FHA can be used to present heterologous antigens by B. pertussis (38). Subsequently, we have found that a truncated derivative of FHA, named Fha44, that contains the N-proximal secretion determinants (16) was secreted even more efficiently than was full-length FHA (37). We therefore used Fha44 as a carrier for TbpB. Since TbpBs are relatively large proteins (65 to 90 kDa) and adopt tight folding (45) and because a globular conformation may not be compatible with FHA-mediated secretion (13), we constructed hybrid proteins that contained either the full-length TbpB from N. meningitidis B16B6 or N-terminal or C-terminal portions thereof. The full-length TbpB ranged from residue 2 of the mature protein to residue 569, whereas the N-terminal and C-terminal portions comprised amino acids 2 to 133 and 217 to 569, respectively. These proteins were named Fha44-TbpB, Fha44-TbpBN, and Fha44-TbpBC, respectively.
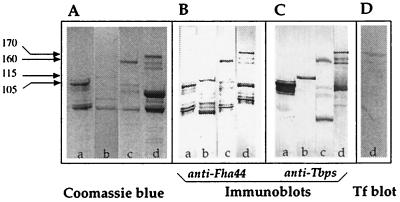
The various TbpB-encoding sequences were amplified by PCR and fused to the Fha44-encoding sequence present in pBG4, a plasmid able to replicate in B. pertussis. The pBG4 derivatives were then introduced into B. pertussis BPGR4, a strain which carries a chromosomal deletion of the FHA structural gene fhaB (27). Unconcentrated culture supernatants of the recombinant bacteria grown to late exponential phase were then analyzed by SDS-PAGE and immunoblotting. As shown in Fig. 1A, polypeptides of the expected sizes (105 kDa for Fha44-TbpBN, 115 kDa for Fha44-TbpBC, and 160 kDa for Fha44-TbpB), as well as smaller polypeptides, were readily detected. Immunoblot analyses using anti-Fha44 (Fig. 1B) or anti-TbpB antiserum (Fig. 1C) confirmed that these proteins contain both Fha44 and TbpB epitopes. Most of the smaller polypeptides reacted only with the anti-Fha44 antibodies, indicating that they lack the TbpB domain and therefore most likely represent N-terminal proteolytic breakdown products of the chimeras. These results indicate that the Fha44 carrier protein is able to mediate highly efficient secretion of large passenger proteins, such as the N-terminal or C-terminal portions of TbpB, and even the full-length TbpB. In addition, the hybrid proteins could also be detected at the bacterial cell surface (data not shown).
FIG. 1.
Production and secretion of Fha44-TbpB hybrid proteins by B. pertussis. Fifty-microliter samples of unconcentrated culture supernatants from B. pertussis BPGR4 containing pNFNT (lanes a), pNFCT (lanes b), pNFT/B16B6 (lanes c), or pNFT/M982 (lanes d) were analyzed by SDS-PAGE and Coomassie blue staining (A), by immunoblotting using rat anti-Fha44 (B) or rabbit anti-TbpB (C) antibodies directed against TbpB from either strain B16B6 (lanes a to c, panel C) or strain M982 (lane d, panel C), and by hTf binding overlay assays (D). The molecular masses of the chimeras are given in kilodaltons in the left margin.
To determine whether the TbpB secretion was specific for the B16B6 strain, we also fused full-length TbpB from N. meningitidis M982 to Fha44. This protein is considered to represent a quite distinct antigenic group of TbpB proteins (40). As shown in Fig. 1, lanes d, TbpB from strain M982 was secreted as efficiently as that from strain B16B6. In addition, the M982 hybrid protein was able to bind to hTf (Fig. 1D), indicating that at least its N-terminal domain is properly folded in the B. pertussis culture supernatant, since hTf binding requires correct folding of this domain (39, 45).
Construction of B. pertussis producing chromosomally encoded Fha44-TbpB.
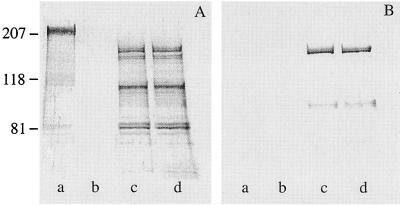
Unfortunately, the plasmid-borne genes were readily lost during colonization of the mouse respiratory tract by the recombinant B. pertussis. We therefore inserted the fha44-tbpB/B16B6 hybrid gene into the B. pertussis chromosome. It was inserted by allelic exchange into the fhaB locus of virulent B. pertussis BPSM and of attenuated B. pertussis BPRA, resulting in strains BPIC4 and BPIC3, respectively. BPRA is a strain from which the genes encoding pertussis toxin (PTX), the major B. pertussis virulence determinant, have been deleted (4). This strain is strongly attenuated in the mouse respiratory infection model (18, 33). Immunoblot analyses of unconcentrated culture supernatants of these strains revealed the presence of polypeptides immunoreactive with both the anti-Fha44 and anti-TbpB antibodies that correspond to the Fha44-TbpB hybrid molecules of the expected size (Fig. 2). These results indicate that high levels of production and secretion of the hybrid protein can be obtained, even when only a single copy of the gene is inserted in the bacterial chromosome.
FIG. 2.
Production and secretion of the hybrid protein Fha44-TbpB/B16B6 by B. pertussis. Twenty microliters of unconcentrated culture supernatants from B. pertussis BPSM (lanes a) or BPGR4 (lanes b) and from recombinant strains BPIC3 (lanes c) or BPIC4 (lanes d) was analyzed by immunoblotting using anti-Fha44 IgY (A) or monoclonal anti-TbpB/B16B6 (B) antibody 511F8. The numbers at left are molecular masses in kilodaltons.
Anti-TbpB antibody responses.
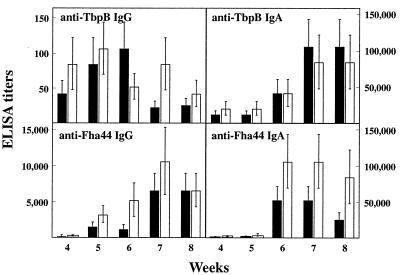
To evaluate the anti-TbpB antibody responses elicited by i.n. administration of the recombinant B. pertussis strains, OF1 mice received a single i.n. administration of 5 × 106 CFU of BPIC3 or BPIC4. The colonization kinetics of the recombinant strains, as measured by the numbers of CFU in the lungs, roughly followed those of the parental strains. The PTX-deficient strains (BPRA and BPIC3) were able to colonize but were cleared slightly faster than were the virulent strains BPSM and BPIC4, consistent with previously reported studies (18, 33). Anti-TbpB and anti-Fha44 IgA and IgG responses were analyzed by ELISA in both BALF and sera at different time points. Both anti-TbpB IgG and anti-TbpB IgA, as well as anti-Fha44 IgG and IgA, were readily detected in BALF after a single i.n. administration of BPIC3 or BPIC4 (Fig. 3). Anti-TbpB IgG was detected as early as 4 weeks after administration and declined at week 7, whereas anti-TbpB IgA and the anti-Fha44 antibodies (IgG and IgA) were mostly seen 6 weeks after infection. Attenuation of the B. pertussis strain through the deletion of the PTX genes did not appear to influence significantly the antibody responses in BALF.
FIG. 3.
Anti-TbpB and anti-Fha44 antibody responses in BALF after i.n. administration of B. pertussis BPIC3 or BPIC4. Groups of three 4-week-old female OF1 mice were infected i.n. with 5 × 106 CFU of B. pertussis BPIC3 (black bars) or BPIC4 (white bars). Infections with similar amounts of B. pertussis BPRA or BPSM served as negative controls. At the indicated time points, the mice were sacrificed, BALF were harvested, and anti-TbpB and anti-Fha44 IgG and IgA titers were estimated by ELISA. Results are expressed as means ± standard deviations.
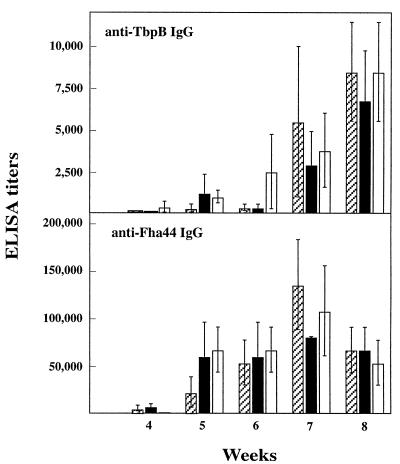
When serum antibody responses against TbpB and Fha44 were measured, anti-TbpB and anti-Fha44 serum IgG were found to be induced 5 to 7 weeks after a single i.n. administration of either recombinant strain (Fig. 4), whereas no detectable anti-Fha44 or anti-TbpB IgA was found (data not shown).
FIG. 4.
Anti-TbpB and anti-Fha44 serum antibody responses after i.n. administration of B. pertussis BPIC1, BPIC3, or BPIC4. Groups of three 4-week-old female OF1 mice were infected i.n. with 5 × 106 CFU of B. pertussis BPIC1 (hatched bars), BPIC3 (black bars), or BPIC4 (white bars). Infections with similar amounts of B. pertussis BPRA or BPSM served as negative controls. At the indicated time points, the mice were bled by retro-orbital plexus puncture, and anti-TbpB and anti-Fha44 serum IgG titers were estimated by ELISA. Results are expressed as means ± standard deviations.
Bactericidal antibodies against N. meningitidis induced by recombinant B. pertussis.
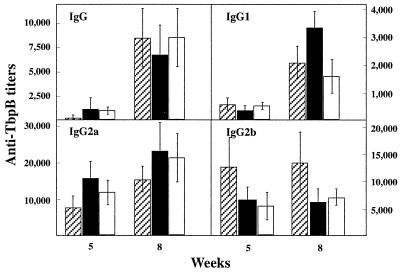
Complement-mediated bactericidal activity of antibodies is a major defense mechanism against N. meningitidis in humans (34). In mice, this activity is associated with antigen-specific IgG2a. We therefore analyzed the isotypic profiles of the anti-TbpB serum antibodies after i.n. administration of BPIC3 and BPIC4. As illustrated in Fig. 5, anti-TbpB IgG1, IgG2a, and IgG2b were induced after infection with either strain. The TbpB-specific IgG2a and IgG2b titers were quite high as soon as 5 weeks after infection, whereas the specific IgG1 titers were low after 5 weeks but increased four- to sevenfold at week 8. Bactericidal titers for antisera were as follows: preimmune serum, <8; anti-TbpB/B16B6 antiserum (hyperimmune serum obtained from rabbits against TbpB isolated from strain B16B6), 512; anti-B. pertussis BPSM antiserum, <8; and anti-B. pertussis BPIC4 antiserum (collected 8 weeks after i.n. administration of B. pertussis BPIC4), 256. These data show that the mouse antisera containing high levels of anti-TbpB/B16B6 IgG2a can kill N. meningitidis B16B6 through complement-mediated lysis.
FIG. 5.
Anti-TbpB serum IgG isotype profiles elicited after i.n. administration of B. pertussis BPIC1, BPIC3, or BPIC4. Groups of three 4-week-old female OF1 mice were infected i.n. with 5 × 106 CFU of B. pertussis BPIC1 (hatched bars), BPIC3 (black bars), or BPIC4 (white bars). Five and eight weeks later, the sera were analyzed by ELISA for the presence of specific anti-TbpB IgG, IgG1, IgG2a, and IgG2b isotypes. Results are expressed as means ± standard deviations.
Lack of cross-reactivity among two different TbpBs of different isotypes.
TbpB proteins display molecular heterogeneity (22, 29, 41), and on the basis of TbpB cross-reactivity, major isotypes have been identified previously (40). The two main isotypes are represented by strains B16B6 and M982. To assess the specificity of the anti-TbpB antibodies induced after i.n. administration of recombinant B. pertussis, the anti-TbpB/B16B6 antibodies were tested by ELISA on TbpB from strain M982. No reactivity was detected. In addition, when mice were infected with B. pertussis BPIC1, a strain that produces the N. meningitidis M982 TbpB fused to Fha44, anti-TbpB/M982 antibodies were induced with kinetics similar to those induced by BPIC3 and BPIC4 (Fig. 4). However, these antibodies did not recognize TbpB from strain B16B6, demonstrating the specificity of the anti-TbpB antisera.
DISCUSSION
The development of efficacious single-dose vaccines to be delivered mucosally constitutes one of the priorities of many health institutions worldwide, including the World Health Organization. Most efforts have so far been devoted to oral delivery of vaccine formulations. However, this route usually requires large quantities of antigen and several administrations to achieve protective immunity, probably due to poor uptake and substantial degradation of the antigens in the proteolytic and acidic environment of the stomach before they can reach the induction sites. The i.n. route may therefore constitute an attractive alternative, perhaps particularly well adapted to induce protective immunity against respiratory pathogens. In this study, we have used genetically engineered B. pertussis for the presentation of the protective antigen TbpB of N. meningitidis serogroup B to the respiratory mucosa. N. meningitidis serogroup B represents one of the major causes of severe childhood meningitis and septicemia, often with a fatal outcome.
TbpB was fused to the N-terminal 80-kDa fragment of FHA, named Fha44, and efficiently secreted into the culture supernatant of recombinant B. pertussis. Previous studies have indicated that Fha44 contains the secretion determinants of FHA, and here we demonstrate that it is sufficient for high-level secretion of even quite large (68 to 87 kDa for the two TbpB proteins used here) passenger proteins. In addition, the recombinant Fha44-TbpB hybrid protein was able to bind hTf in an overlay assay, indicating that the TbpB portion is highly structured, which is essential for its interaction with hTf. We have previously shown that disulfide bond formation and the resulting globular fold of passenger proteins may be incompatible with secretion via FHA through the outer membrane of B. pertussis (13). If this were a general rule, it would imply that the recombinant TbpB in this study acquires its three-dimensional structure only after it has reached the outer surface of the microorganism. The secretion of FHA requires the presence of an outer membrane accessory protein named FhaC (46). Recent evidence strongly suggests that this protein forms pores in the outer membrane, the size of which may be compatible with the passage of proteins in an extended conformation (17).
A single i.n. administration of the recombinant B. pertussis strain elicited the production of anti-TbpB antibodies both in BALF and in the sera of mice. This is in contrast to previous studies with recombinant B. pertussis producing the Schistosoma mansoni glutathione S-transferase (Sm28GST) fused to full-length FHA (38). In these studies, anti-Sm28GST antibodies were found only in the BALF of mice after a single i.n. administration. Anti-Sm28GST serum antibodies could be found only after subsequent boosting with the purified protein (32). However, anti-Sm28GST serum antibodies were readily induced after a single i.n. administration when the hybrid protein was produced by an attenuated B. pertussis strain, deficient for PTX production (33), suggesting that the attenuation increased the immunogenicity of the bacteria producing the hybrid protein. In contrast to these observations, we found here that the absence of PTX had no significant effect on the levels of anti-TbpB serum antibodies induced by B. pertussis that produce the Fha44-TbpB chimera.
Several reasons might account for this difference. The amounts of Fha44-TbpB produced by B. pertussis were substantially higher than those of FHA-Sm28GST. High levels of Fha44-TbpB were readily found in unconcentrated culture supernatants, whereas only minute amounts of FHA-Sm28GST could be found even in highly concentrated culture supernatants (38). In the latter case, the recombinant protein remained essentially cell associated. This may be due to the intrinsic properties of the passenger proteins. Sm28GST is an intracellular protein in S. mansoni and has been generally found to be difficult to secrete in heterologous hosts (19), whereas TbpB crosses two membranes in its natural meningococcal environment. In addition, Fha44, the carrier protein to which TbpB was fused, is more efficiently secreted by B. pertussis than is FHA, the Sm28GST carrier (37), and it may be worthwhile to compare the two carrier proteins fused to the same passenger protein.
In addition to the levels of heterologous antigen secretion, the nature of the carrier protein (Fha44 or full-length FHA) may also conceivably affect the immune responses with respect to the influence of PTX. FHA is the major adhesin of B. pertussis (for a review, see reference 26). It contains at least three distinct binding sites, two of which are missing in Fha44. A carbohydrate-binding site is located in the C-terminal half of FHA and is believed to interact with glycoprotein ligands at the surface of ciliated epithelial cells (35). Full-length FHA, but not Fha44, also contains an Arg-Gly-Asp site known to be involved in the interaction of B. pertussis with alveolar macrophages via binding to the β2 integrin CR3 (36). Using CR3 as the port of entry into macrophages results in increased survival of B. pertussis within these cells, which very likely influences antigen presentation by macrophages. Recent observations indicate that the action of PTX has an impact on antigen presentation by professional phagocytes (unpublished observations). PTX catalyzes ADP-ribosylation of Giα proteins (44) involved in signal transduction, some of which play a major role in antigen presentation by macrophages. A link between the interaction of FHA with CR3 via the Arg-Gly-Asp site and the PTX-mediated ADP-ribosylation of macrophage Giα may perhaps explain the differences in immune responses observed between passenger proteins fused to Fha44 and those fused to FHA. These hypotheses warrant further investigation.
The anti-TbpB serum antibody isotypes induced by the recombinant B. pertussis strains were mixed, including IgG1, IgG2a, and IgG2b. These antibodies also expressed bactericidal activity against N. meningitidis, suggesting that i.n. administration with the TbpB-producing B. pertussis may result in protective immunity, since the major antimeningococcal protective mechanism in children is based on the presence of bactericidal antibodies (34).
TbpB is present in all clinical isolates of all meningococcal serogroups. It is even present in other neisserial species, such as Neisseria gonorrhoeae (8). Therefore, anti-TbpB vaccines may be widely cross-protective among all the serogroups. However, considerable antigenic and sequence heterogeneity among meningococcal TbpBs has been described elsewhere (22, 23, 29, 41, 42), and different TbpBs possess both strain-specific and cross-reactive epitopes. Based on the presence of mutually exclusive epitopes, the TbpB proteins can be divided into two major isotypes, I and II, represented by the B16B6 TbpB and the M982 TbpB, respectively. Polyclonal anti-TbpB antibodies cross-react with TbpB proteins within each family but not between the two families (40). As shown here, this is also the case when the two proteins are administered i.n. by recombinant B. pertussis. To provide protection against the full array of meningococcal strains, at least two B. pertussis strains may therefore have to be administered simultaneously, or preferably, they may have to be produced within the same strain. However, the strain specificity and the cross-reactivity of TbpB epitopes appear to be species dependent. Although mice generate essentially strain-specific anti-TbpB antibodies, patients recovering from natural infection produce cross-reactive anti-TbpB antibodies, as evidenced by immunoblot analyses (3, 12). It will therefore be interesting to investigate whether recombinant B. pertussis producing only one TbpB protein induces cross-reactive immunity against all N. meningitidis serogroups after a single i.n. administration in humans. However, before human studies can be initiated, the recombinant B. pertussis vectors have to be properly attenuated. Although the deletion of the ptx genes has been shown to strongly attenuate B. pertussis in the mouse model (18, 33), work to further attenuate B. pertussis is ongoing.
ACKNOWLEDGMENTS
We are indebted to C. Frasch for the N. meningitidis strains, L. Lissolo for rabbit anti-TbpB hyperimmune sera, M. Mignon and C. Chabanel for technical assistance in bactericidal assays, M.-J. Quentin-Millet for critically reading the manuscript, and Transgène for purified TbpB.
F.J.-D. is a researcher of the CNRS, and S.A. holds a postdoctoral fellowship of Aventis Pasteur. This work was supported by INSERM, Institut Pasteur de Lille, and Région Nord-Pas de Calais.
REFERENCES
- 1.Ala'Aldeen D A A, Borriello S P. The meningococcal transferrin-binding proteins 1 and 2 are both surface exposed and generate bactericidal antibodies capable of killing homologous and heterologous strains. Vaccine. 1996;14:49–53. doi: 10.1016/0264-410x(95)00136-o. [DOI] [PubMed] [Google Scholar]
- 2.Ala'Aldeen D A A, Cartwright K A V. Neisseria meningitidis vaccines and vaccine candidates. J Infect. 1996;33:153–157. doi: 10.1016/s0163-4453(96)92081-2. [DOI] [PubMed] [Google Scholar]
- 3.Ala'Aldeen D A A, Stevenson P, Griffiths E, Gorringe A R, Irons L I, Robinson A, Hyde S, Borriello S P. Immune responses in humans and animals to meningococcal transferrin-binding proteins: implications for vaccine design. Infect Immun. 1994;62:2984–2990. doi: 10.1128/iai.62.7.2984-2990.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoine R, Locht C. The role of the disulfide bond and the carboxy-terminal region of the S1 subunit in the assembly and biosynthesis of pertussis toxin. Infect Immun. 1990;58:1518–1526. doi: 10.1128/iai.58.6.1518-1526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulton I C, Gorringe A R, Allison N, Robinson A, Gorinsky B, Joannou C L, Evans R W. Transferrin-binding protein B isolated from Neisseria meningitidis discriminates between apo and diferric human transferrin. Biochem J. 1998;334:269–273. doi: 10.1042/bj3340269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulton I C, Gorringe A R, Shergill J K, Joannou C L, Evans R W. A dynamic model of the meningococcal transferrin receptor. J Theor Biol. 1999;198:497–505. doi: 10.1006/jtbi.1999.0928. [DOI] [PubMed] [Google Scholar]
- 7.Caugant D A, Froholm L O, Bovre K, Holten E, Frasch C E, Mocca L F, Zollinger W D, Selander R K. Intercontinental spread of a genetically distinctive complex of clones of Neisseria meningitidis causing epidemic disease. Proc Natl Acad Sci USA. 1986;83:4927–4931. doi: 10.1073/pnas.83.13.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelissen C N, Anderson J E, Sparling P F. Characterization of the diversity and the transferrin-binding domain of gonococcal transferrin-binding protein 2. Infect Immun. 1997;65:822–828. doi: 10.1128/iai.65.2.822-828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danve B, Lissolo L, Mignon M, Dumas P, Colombani S, Schryvers A B, Quentin-Millet M-J. Transferrin-binding proteins isolated from Neisseria meningitidis elicit protective and bactericidal antibodies in laboratory animals. Vaccine. 1993;11:1214–1220. doi: 10.1016/0264-410x(93)90045-y. [DOI] [PubMed] [Google Scholar]
- 10.Delisse-Gathoye A-M, Locht C, Jacob F, Raaschou-Nielsen M, Heron I, Ruelle J-L, De Wilde M, Cabezon T. Cloning, sequence and expression analysis of the filamentous hemagglutinin gene of Bordetella pertussis in Escherichia coli, and the antigenic structure of the protein. Infect Immun. 1990;58:2895–2905. doi: 10.1128/iai.58.9.2895-2905.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drabick J J, Brandt B L, Moran E E, Saunders N B, Shoemaker D R, Zollinger W D. Safety and immunogenicity testing of an intranasal group B meningococcal native outer membrane vesicle vaccine in healthy volunteers. Vaccine. 1999;18:160–172. doi: 10.1016/s0264-410x(99)00216-9. [DOI] [PubMed] [Google Scholar]
- 12.Gorringe A R, Borrow R, Fox A J, Robinson A. Human antibody response to meningococcal transferrin binding proteins: evidence for vaccine potential. Vaccine. 1995;13:1207–1212. doi: 10.1016/0264-410x(95)00055-6. [DOI] [PubMed] [Google Scholar]
- 13.Guédin S, Willery E, Locht C, Jacob-Dubuisson F. Evidence that a globular conformation is not compatible with FhaC-mediated secretion of the Bordetella pertussis haemagglutinin. Mol Microbiol. 1998;29:763–774. doi: 10.1046/j.1365-2958.1998.00970.x. [DOI] [PubMed] [Google Scholar]
- 14.Haneberg B, Dalseg R, Wedege E, Hoiby E A, Haugen I L, Oftung F, Andersen S R, Naess L M, Aase A, Michaelsen T E, Holst K J. Intranasal administration of a meningococcal outer membrane vesicle vaccine induces persistent local mucosal antibodies and serum antibodies with strong bactericidal activity in humans. Infect Immun. 1998;66:1334–1341. doi: 10.1128/iai.66.4.1334-1341.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannah J H, Menozzi F D, Renauld G, Locht C, Brennan M J. Sulfated glycoconjugate receptors for the Bordetella pertussis adhesin filamentous hemagglutinin (FHA) and mapping of the heparin-binding domain on FHA. Infect Immun. 1994;62:5010–5019. doi: 10.1128/iai.62.11.5010-5019.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacob-Dubuisson F, Buisine C, Mielcarek N, Clément E, Menozzi F D, Locht C. Amino-terminal maturation of the Bordetella pertussis filamentous hemagglutinin. Mol Microbiol. 1996;19:65–78. doi: 10.1046/j.1365-2958.1996.349883.x. [DOI] [PubMed] [Google Scholar]
- 17.Jacob-Dubuisson F, El-Hamel C, Saint N, Guédin S, Willery E, Molle G, Locht C. Channel formation by FhaC, the outer membrane protein involved in the secretion of the Bordetella pertussis filamentous hemagglutinin. J Biol Chem. 1999;274:37731–37735. doi: 10.1074/jbc.274.53.37731. [DOI] [PubMed] [Google Scholar]
- 18.Khelef N, Bachelet C-M, Vargaftig B B, Guiso N. Characterization of murine lung inflammation after infection with parental Bordetella pertussis and mutants deficient in adhesins or toxins. Infect Immun. 1994;62:2893–2900. doi: 10.1128/iai.62.7.2893-2900.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kremer L, Riveau G, Baulard A, Capron A, Locht C. Neutralizing antibodies elicited in mice immunized with recombinant BCG producing the Schistosoma mansoni glutathion S-transferase. J Immunol. 1996;156:4309–4317. [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Legrain M, Mazarin V, Irwin S W, Bouchon B, Quentin-Millet M-J, Jacobs E, Schryvers A B. Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene. 1993;130:73–80. doi: 10.1016/0378-1119(93)90348-7. [DOI] [PubMed] [Google Scholar]
- 22.Legrain M, Rokbi B, Villeval D, Jacobs E. Characterization of genetic exchanges between various highly divergent tbpBs, having occurred in Neisseria meningitidis. Gene. 1998;208:51–59. doi: 10.1016/s0378-1119(97)00646-x. [DOI] [PubMed] [Google Scholar]
- 23.Linz B, Schenker M, Zhu P, Achtman M. Frequent interspecific genetic exchange between commensal Neisseriae and Neisseria meningitidis. Mol Microbiol. 2000;36:1049–1058. doi: 10.1046/j.1365-2958.2000.01932.x. [DOI] [PubMed] [Google Scholar]
- 24.Lissolo L, Maitre-Wilmotte G, Dumas P, Mignon M, Danve B, Quentin-Millet M-J. Evaluation of transferrin-binding protein 2 within the transferrin-binding protein complex as a potential antigen for future meningococcal vaccines. Infect Immun. 1995;63:884–890. doi: 10.1128/iai.63.3.884-890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locht C. Live bacterial vectors for intranasal delivery of protective antigens. Pharm Sci Technol Today. 2000;3:121–128. doi: 10.1016/s1461-5347(00)00256-x. [DOI] [PubMed] [Google Scholar]
- 26.Locht C, Bertin P, Menozzi F D, Renauld G. The filamentous hemagglutinin, a multifaceted adhesin produced by virulent Bordetella spp. Mol Microbiol. 1993;9:653–660. doi: 10.1111/j.1365-2958.1993.tb01725.x. [DOI] [PubMed] [Google Scholar]
- 27.Locht C, Geoffroy M-C, Renauld G. Common accessory genes for the Bordetella pertussis filamentous hemagglutinin and fimbriae share sequence similarities with the papC and papD gene families. EMBO J. 1992;11:3175–3183. doi: 10.1002/j.1460-2075.1992.tb05394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsh J L, Erfle M, Wykes E J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984;32:481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- 29.Mazarin V, Rokbi B, Quentin-Millet M-J. Diversity of the transferrin-binding protein Tbp2 of Neisseria meningitidis. Gene. 1995;158:145–146. doi: 10.1016/0378-1119(95)00151-u. [DOI] [PubMed] [Google Scholar]
- 30.Menozzi F D, Gantiez C, Locht C. Interaction of the Bordetella pertussis filamentous hemagglutinin with heparin. FEMS Microbiol Lett. 1991;78:59–64. doi: 10.1111/j.1574-6968.1991.tb04417.x. [DOI] [PubMed] [Google Scholar]
- 31.Menozzi F D, Mutombo R, Renauld G, Gantiez C, Hannah J, Leininger E, Brennan M J, Locht C. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect Immun. 1994;62:769–778. doi: 10.1128/iai.62.3.769-778.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mielcarek N, Cornette J, Schacht A-M, Pierce R J, Locht C, Capron A, Riveau G. Intranasal priming with recombinant Bordetella pertussis for the induction of a systemic immune response against a heterologous antigen. Infect Immun. 1997;65:544–550. doi: 10.1128/iai.65.2.544-550.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mielcarek N, Riveau G, Remoué F, Antoine R, Capron A, Locht C. Homologous and heterologous protection after single intranasal administration of live attenuated recombinant Bordetella pertussis. Nat Biotechnol. 1998;16:454–457. doi: 10.1038/nbt0598-454. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson A, Lepow I H. Host defense against Neisseria meningitidis requires a complement-dependent bactericidal activity. Science. 1979;205:298–299. doi: 10.1126/science.451601. [DOI] [PubMed] [Google Scholar]
- 35.Prasad S M, Yin Y, Rodzinski E, Tuomanen E I, Masure H R. Identification of a carbohydrate recognition domain in filamentous hemagglutinin from Bordetella pertussis. Infect Immun. 1993;61:2780–2785. doi: 10.1128/iai.61.7.2780-2785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Relman D, Tuomanen E, Falkow S, Golenbock D T, Saukkonen K, Wright S D. Recognition of a bacterial adhesin by an integrin: macrophage CR3 (αMβ2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990;61:1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- 37.Renauld-Mongénie G, Cornette J, Mielcarek N, Menozzi F D, Locht C. Distinct roles of the N-terminal and C-terminal precursor domains in the biogenesis of the Bordetella pertussis filamentous hemagglutinin. J Bacteriol. 1996;178:1053–1060. doi: 10.1128/jb.178.4.1053-1060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renauld-Mongénie G, Mielcarek N, Cornette J, Schacht A-M, Capron A, Riveau G, Locht C. Induction of mucosal immune responses against a heterologous antigen fused to filamentous hemagglutinin after intranasal immunization with recombinant Bordetella pertussis. Proc Natl Acad Sci USA. 1996;93:7944–7949. doi: 10.1073/pnas.93.15.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renauld-Mongénie G, Poncet D, von Ollescik-Elbheim L, Cournez T, Mignon M, Schmidt M A, Quentin-Millet M-J. Identification of human transferrin-binding sites within meningococcal transferrin-binding protein B. J Bacteriol. 1997;179:6400–6407. doi: 10.1128/jb.179.20.6400-6407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rokbi B, Mazarin V, Maitre-Wilmotte G, Quentin-Millet M-J. Identification of two major families of transferrin receptors among Neisseria meningitidis strains based on antigenic and genomic features. FEMS Microbiol Lett. 1993;110:51–58. doi: 10.1111/j.1574-6968.1993.tb06294.x. [DOI] [PubMed] [Google Scholar]
- 41.Rokbi B, Mignon M, Caugant D A, Quentin-Millet M-J. Heterogeneity of tbpB, the transferrin-binding protein B gene, among serogroup B Neisseria meningitidis strains of the ET-5 complex. Clin Diagn Lab Immunol. 1997;4:522–529. doi: 10.1128/cdli.4.5.522-529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rokbi B, Renauld-Mongenie G, Mignon M, Danve B, Poncet D, Chabanel C, Caugant D A, Quentin-Millet M-J. Allelic diversity of the two transferrin binding protein B gene isotypes among a collection of Neisseria meningitidis strains representative of serogroup B disease: implication for the composition of a recombinant TbpB-based vaccine. Infect Immun. 2000;68:4938–4947. doi: 10.1128/iai.68.9.4938-4947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 44.Tamura M, Nogimori K, Murai S, Yajima M, Ito K, Katada T, Ui M, Ishii S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982;21:5516–5522. doi: 10.1021/bi00265a021. [DOI] [PubMed] [Google Scholar]
- 45.Vonder Haar R A, Legrain M, Kolbe H V J, Jacobs E. Characterization of a highly structured domain in Tbp2 from Neisseria meningitidis involved in binding to human transferrin. J Bacteriol. 1994;176:6207–6213. doi: 10.1128/jb.176.20.6207-6213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willems R J L, Geuijen C, van der Heide H G J, Renauld G, Bertin P, van der Akker W M R, Locht C, Mooi F R. Mutational analysis of the Bordetella pertussis fim/fha gene cluster: identification of a gene with sequence similarities to haemolysin accessory genes involved in export of FHA. Mol Microbiol. 1994;11:337–347. doi: 10.1111/j.1365-2958.1994.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 47.Wu H-Y, Russell M W. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol Res. 1997;16:187–201. doi: 10.1007/BF02786362. [DOI] [PubMed] [Google Scholar]
- 48.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]