Abstract
Hepatocellular carcinoma (HCC) represents a worldwide health matter with a major care burden, high prevalence, and poor prognosis. Its pathogenesis mainly varies depending on the underlying etiological factors, although it develops from liver cirrhosis in the majority of cases. This review summarizes the role of the most interesting soluble factors as biomarkers for early diagnosis and as recommended targets for treatment in accordance with the new challenges in precision medicine. In the premalignant environment, inflammatory cells release a wide range of cytokines, chemokines, growth factors, prostaglandins, and proangiogenic factors, making the liver environment more suitable for hepatocyte tumor progression that starts from acquired genetic mutations. A complex interaction of pro-inflammatory (IL-6, TNF-α) and anti-inflammatory cytokines (TGF-α and -β), pro-angiogenic molecules (including the Angiopoietins, HGF, PECAM-1, HIF-1α, VEGF), different transcription factors (NF-kB, STAT-3), and their signaling pathways are involved in the development of HCC. Since cytokines are expressed and released during the different stages of HCC progression, their measurement, by different available methods, can provide in-depth information on the identification and management of HCC.
Keywords: biomarkers, cytokines, hepatocellular carcinoma, personalized medicine
1. Introduction
Hepatocellular carcinoma (HCC) is the most frequent type of cancer affecting the liver, and its incidence almost exceeds mortality. All those risk factors (chronic HBV or HCV infection, alcohol, aflatoxin B1, NAFLD/NASH) that concur with liver cirrhosis may be involved in HCC pathogenesis [1].
Cirrhotic liver tissue is characterized by low levels of hepatocyte cell proliferation in favor of a greater abundance of inflammatory mediators, fibrosis, and activation of the extracellular matrix environment. Therefore, a hepatocyte clone with a deregulated proliferative rate finds more suitable conditions for expansion, unlike in a normal and proliferating liver [2].
Following a viral infection or toxic tissue damage, a tightly regulated and coordinated multistep process may start in the liver, characterized by activation and local infiltration of immune cells and subsequent engagement in tissue repair. In this refined orchestration of events, the release of a wide range of soluble factors takes place [3].
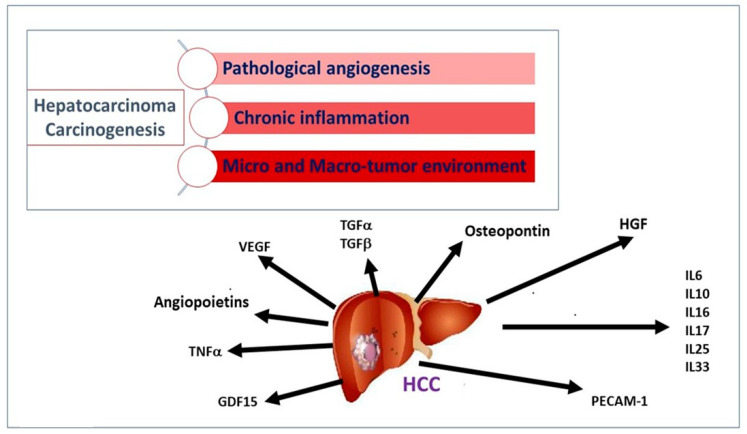
In liver cirrhosis, a wide proliferation of stellate cells has been described, generating an abundance of extracellular matrix proteins, cytokines, growth factors, and oxidative stress products. The unbalanced expression of these factors and the initial unresolved inflammatory response produce a suitable microenvironment for developing neoplasm. Cytokines released by the tumor, neighboring non-tumor cells, and immune cells can act as a promoter of tumor survival [4] (Figure 1). Carcinogenetic events of HCC involve angiogenesis, chronic inflammation, and tumor micro and macro-environment (Figure 1).
Figure 1.
Major pathogenetic events and cytokine network involved in hepatocellular carcinogenesis. Following persistent liver damage, locally activated chronic inflammation favors the release of soluble factors sustaining the proliferation and survival of tumor cells. Angiogenic factors (angiopoietins, VEGF), growth factors for normal and transformed hepatocytes (HGF), adhesion molecules and cytokines for recruitment and activation of leukocytes (PECAM, IL-6), and stellate cells upregulates TGF in the liver and, consequently, regeneration, proliferation, and hepatocyte dysplasia, and, ultimately, the development of HCC.
Since cytokines production regulates HCC evolution and worsening progression, their evaluation can provide useful information on the identification and management of HCC.
2. Cytokines and Growth Factors
2.1. Stimulators of Angiogenesis and Tumor Invasiveness
The progression of liver disease takes into account pathological angiogenesis, a prerequisite that facilitates the development of HCC. Angiogenesis is the result of a multiphase process and is the limiting step of tumor growth. In normal conditions, there is a balance between angiogenic inducers and inhibitors that keeps the angiogenic process under control and prevents inappropriate tissue vascularization. Angiogenesis inhibitors often derive from circulating extracellular matrix proteins (because of injury to the matrix), e.g., fibronectin, prolactin, collagen XVIII (endostatin), Hepatocyte Growth Factor fragment NK1, and angiostatin. Although tumors initially engage the pre-existing vascularity, an angiogenetic “switch” consisting of the production of factors inducing angiogenesis crucially modifies the tumor phenotype [5].
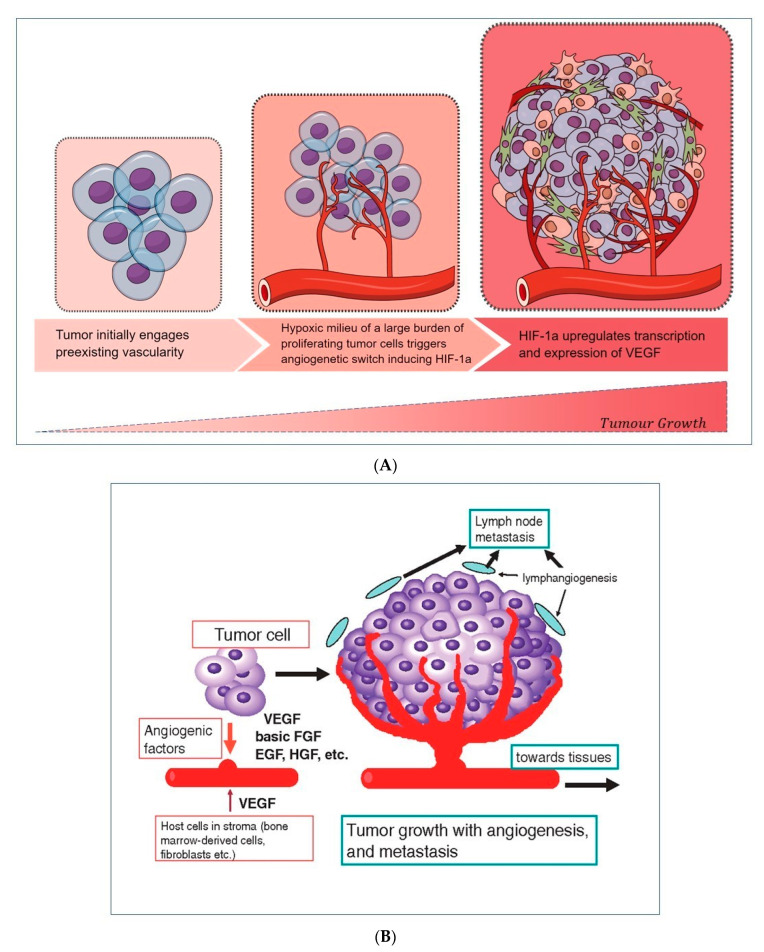
Vascular endothelial growth factor (VEGF) is the most powerful stimulator of normal and pathological angiogenesis (Table 1). Circulating VEGF may be derived mainly from the large burden of tumor cells released under hypoxic conditions. Its expression is regulated by the hypoxia-inducible factor 1α (HIF-1α), which, induced during the hypoxic conditions, triggers the transcription of VEGF that stimulates the formation of new vessels (Figure 2A) [6]. This indicates that VEGF participates in the initial phase of angiogenesis. As a result, the transition of endothelial cells from an inactive to an active state can occur along with their proliferation, migration, and formation of new vessels, which can act as new gates for the recruitment of inflammatory cells, releasing cytokines and inducing further inflammation (Figure 2B). Different reports analyzed serum levels of VEGF in HCC patients in comparison to patients with or without HCV-related cirrhosis, often with opposite results [7,8]. Mukozu et al. showed that VEGF was higher in HCC patients compared to controls [7], while the results from Abden–Ramahal et al. displayed significantly higher serum levels of VEGF in HCC in comparison to cirrhotic patients, but no significant differences in healthy controls [8]. However, altogether these data highlight the important role of VEGF as a biomarker of vascular invasion in disease progression from liver cirrhosis to HCC.
Figure 2.
(A) Hypoxic environment stimulates neoangiogenesis, thanks to a circuit involving HIF-1α -induced VEGF release by tumor cells. (B) A wide range of cellular released growth factors, derived from a tumor, stroma, and leukocytes, ensures the formation of new vascularity and the sustainment of cell growth, allowing the worsening progression of the initial tumor burden.
Patients with HCC showed a significant increase in VEGF after anticancer therapy compared to the values reported at the time of diagnosis, as well as to the levels of lymphocytes [9]. This may be partly explained by the rebound effect of VEGF, induced by hypoxia following locoregional treatments, often associated with treatment failure and low survival rates in patients [10]. Levels of VEGF are increased in patients who later experienced progression of HCC compared to those who remained stable [11]. Additionally, higher VEGF levels prior to sorafenib treatment (a multikinase inhibitor employed in several locally recurrent or metastatic solid tumors, including HCC) are associated with shorter survival [12,13].
Table 1.
Role of the main cytokines and growth factors in hepatocarcinogenesis.
| Biomarkers | Abbreviation | Role in HCC | Study |
|---|---|---|---|
| Vascular endothelial growth factor | VEGF |
|
Tammela, T. et.al., 2005 [6] |
| Angiopoietin/Tie system |
|
Naldini, A. et al., 2005 [14] | |
| Tyrosine kinase with Ig and EGF-homology domains-1 and 2 | Tie1, Tie2 | ||
| Angiopoietin ligands 1–4 | Ang1, Ang2, Ang3, Ang4 | ||
| Hepatocyte growth factor | HGF |
|
García-Vilas JA, et al., 2018 [15] |
| Platelet endothelial cell adhesion molecule-1 | PECAM-1 |
|
DeLisser, H.M. et al., 1997 [16] |
| Interleukin 6 | IL-6 |
|
He, G. et al., 2013 [17] |
| Transforming growth factor alpha | TGF-α |
|
Shao, Y. et al., 2017 [18] |
| Transforming growth factor beta | TGF-β |
|
Shao, Y. et al., 2017 [18] |
| Interleukin 10 | IL-10 |
|
Shakiba, E.et al., 2018 [19] |
| Interleukin 16–33, -17, -25 | IL 16-IL-33, IL-17, IL-25 |
|
Cruikshank, W. et al., 2000 [20] Askoura M. et al., 2022 [21] |
| Growth differentiation factor 15 | GDF15 |
|
Myojin, Y. et al., 2022 [22] |
| Tumor necrosis factor | TNF |
|
Tiegs, G.et al., 2022 [23] |
| Osteopontin |
|
Zhao, H. et al. 2018, [24] | |
The Tyrosine kinase proteins with Ig and EGF-homology domains 1 and 2 (Tie1 and Tie2) and their angiopoietin ligands 1–4 (Ang1, 2, 3, and 4) play a key role during the late phase of angiogenesis and are responsible for the maturation of newly established vascular structures (Table 1). Ang1 and Ang2 have been deeply studied and characterized. The activity of the Angiopoietin/Tie system determines the stabilization of new vessels [25]. There is growing evidence that the angiopoietin/Tie signal can modify ongoing inflammation [14]. Ang1 appears to be a powerful activator of Tie2, as well as a regulator of blood vessel formation and development. Experimental studies showed that Ang1 acts as an anti-inflammatory molecule [26] but can induce pulmonary hypertension as a complication [27]. Ang1 also neutralizes tissue factor activity that is relevant for the induction of coagulation, thrombosis, and inflammatory response. Furthermore, Ang1 reduces the adhesion of VEGF-related leukocytes to the endothelium [28,29]. Conversely, Ang2 acts as a competitive antagonist of Ang1, deregulates the signal pathway of Tie2 [25], and plays a pro-inflammatory role [30,31]. Additionally, significantly high Ang2 serum levels have been observed in patients during liver carcinogenesis [32]. An increase in Ang2 levels has been observed in correlation with the liver disease progression [9,33], while Ang1 negatively correlates with the model for end-stage liver disease (MELD) and the hepatic fibrosis index. All these data confirm the potential diagnostic utility of Ang1 and Ang2 levels as developing new quantitative biomarkers for staging cirrhosis. Serum Ang2 concentrations decrease significantly after treatment with Direct Antiviral Drugs (DAAs), and, consequently, the Ang2/Ang1 ratio also drops. Ang2 is potentially useful in monitoring antiviral therapy [9]. In a recent study, we reported that patients who died from HCC had significantly lower Ang1 levels than those who did not die, placing Ang1 as a potential prognostic index [9].
It has been shown that the Hepatocyte growth factor (HGF) is over-expressed in HCC compared to the normal and cirrhotic liver without signs of neoplasia (Table 1) [9,15,34]. Expression of HGF and its receptor supports the existence of both autocrine and paracrine mechanisms of HGF action in HCC if compared to the unique paracrine mechanism found in normal liver tissue (in the absence of cancer), suggesting that it also plays a role in tumor development and progression [15,34,35]. Stellate cells and myofibroblasts are induced to secrete HGF from tumor cell products, and HGF, in turn, stimulates tumor cell invasiveness. Recent reports show that higher serum HGF levels negatively correlate with patient survival time [36] and positively with tumor size [37].
Furthermore, the comparison of cirrhotic patients with and without HCC suggests that HGF levels are potentially useful for monitoring the onset of HCC after a diagnosis of cirrhosis [9]. Interestingly, patients with lower HGF levels prior to treatment display major benefits from sorafenib therapy in terms of overall survival and time to progression [12].
Platelet endothelial cell adhesion molecule-1 (PECAM-1), also known as CD31, is normally found on the surface of endothelial cells, platelets, leukocyte subpopulations, and Kupffer cells [38] (Table 1). This molecule is highly expressed within the vascular compartment but largely concentrated at junctions between adjacent cells, and its receptors mediate these interactions that play a crucial role during angiogenesis. In this context, PECAM-1 can mediate both homophilic and heterophilic adhesion [16]. PECAM-1 has been found to positively correlate with MELD, and its identification may aid in assessing the degree of tumor angiogenesis, which may indicate a rapidly growing tumor [39]. Furthermore, PECAM-1 promotes the formation of metastases by inducing the epithelium-mesenchymal transition in HCC by increasing the regulation of β1 integrin through the FAK (focal adhesion kinase) /Akt signaling pathway [40].
2.2. Stimulators of Chronic Inflammation, Liver Fibrosis, and Proliferation
Interleukin (IL)-6 acts as an important inducer of the acute phase response and infection defense in the liver [41]. IL-6 binds to the signal-transducing subunit gp130 on target cells either in complex with the membrane-bound or with the soluble IL-6 receptor to activate intracellular signaling. By the latter ‘trans-signaling’ mechanism, IL-6 can target monocyte chemotaxis and maintain sustained chronic inflammation towards any injured tissue [42,43]. Increased serum levels of IL-6 have been found in patients with advanced HCC compared to those with the early stage [44] (Table 1). Additionally, elevated serum IL-6 levels in HCC patients undergoing hepatectomy are associated with lower overall survival and are prone to early relapses [45]. Research results from mouse models of HCC have shown that isolated HCC progenitor cells can give rise to cancer in the presence of ongoing liver damage and that these cells promote their own growth and progress to malignancy via autocrine IL-6 signaling [17]. A clinical study analyzing 128 HCC patients treated with sorafenib evaluated the prognostic value of serum IL-6 levels before treatment. Elevated pretreatment IL-6 concentrations have been found to be an independent predictor of poor overall survival, although there is no association with the efficacy of sorafenib [18].
Transforming growth factors (TGF)-α and TGF-β are closely related to the hepatocarcinogenesis process (Table 1). Normal hepatocytes show low TGF-α expression compared to tumor cells. In fact, following chronic inflammation due to persistent liver damage, the secreted cytokine pool upregulates TGF-α in the liver and, consequently, regeneration, proliferation, hepatocyte dysplasia, and, ultimately, the development of HCC [46]. TGF-β is a key regulator of the late phase of inflammatory processes, not only promoting tissue repair but also inhibiting leukocyte activation and infiltration, acting at least in part using control of adhesion molecules on parenchymal cells [47]. In this way, TGF-β counteracts the effects of proinflammatory cytokines, leading to the inhibition of cellular processes, such as proliferation, differentiation, and survival. Paradoxically, cancer cells may exploit these microenvironment modifications to their advantage [48]. During carcinogenesis, malignant cells can often attenuate the suppressive TGF-β signaling by altering the expression of its receptors but also hijacking the signaling cascade. HCC cell lines with metastatic potential have been described to downregulate TGF-βR2. Interestingly, a reduced expression of TGF-βR2 in HCC correlates with larger tumor size and various metastatic features, such as poor differentiation, portal vein invasion, and intrahepatic metastases [49,50]. In the early stages of cancer, TGF-β acts as a tumor suppressor by inducing cytostasis and apoptosis, while in the later stages, it promotes pro-tumorigenic events, such as the transition of epithelial cells to mesenchymal, invasion, metastasis, and angiogenesis [51]. The simultaneous exposure (or addition) of TGF-β and IL-6 to human HCC cell cultures (Huh) highlighted an attenuation of the pro-proliferative effects induced by IL-6 by TGF-β. This explains a decrease in the transcription levels of the IL-6 receptor (IL-6R), in the expression of STAT-3 (signal transducer and activator of transcription) induced by IL-6, its nuclear localization, and, finally, a reduced activation of p65 compared to the unperturbed activation of the pathway. SMAD (small mother against decapentaplegic)-dependent TGF-β, resulting in the transition from epithelial to mesenchymal cells and, thus, loss of cell polarity and cell adhesion, as well as the acquisition of invasive and migratory properties, coupled with cell growth arrest [52].
IL-10 is a potent anti-inflammatory cytokine (Table 1). Its role in HCC is less documented than in viral infections. A recent meta-analysis showed that IL-10 levels in HCC patients increased compared to cirrhotic patients and healthy controls but not compared to viral hepatitis patients [53]. Individuals with resectable HCC and IL-10 levels > 12 pg/mL display worse postoperative outcomes [54]. A study showed that in unresectable HCC, the serum levels of IL-10 acted as a negative prognostic factor [19].
Tumor necrosis factor (TNF) (Table 1) is a cytokine produced by proteolytic cleavage from a transmembrane protein precursor (mTNF) into a soluble TNF (sTNF). sTNF binds TNF receptors (TNFR1) (constitutively expressed in most tissues) and TNFR2 (expressed only in hematopoietic and endothelial cells), while mTNF only the type 2 receptor. TNF induces numerous biological responses in the liver, such as apoptosis, necrosis hepatocytes, hepatic inflammation, and regeneration, as well as the progression of HCC [23]. Studies on mouse models provided the role of TNF in the immunopathogenesis of HCC by focusing attention on the transcription factor NF-kB (nuclear factor kappa–light–chain–enhancer of activated B cells) involved in the regulation of the pathway activated by the binding of TNF to the TNFR receptor [23]. In fact, a deficiency of the TNFR-dependent anti-apoptotic NF-kB signaling pathway seems to be essential for the induction of compensatory proliferation of alive hepatocytes in response to hepatocyte death, which results in the development of HCC [55]. Mice lacking the kB regulatory subunit of the kinase-function protein complex (IKK), particularly in hepatocytes, spontaneously develop a chronic liver disease that evolves into HCC [56]. Furthermore, specific deletion of hepatocyte IKKβ protein exacerbated chemically induced liver cancer in mice, possibly worsening carcinogen-induced hepatocyte death and induction of compensatory hepatocyte proliferation [57]. The role of TNFR2 receptors has not been widely analyzed; therefore, preclinical studies in chronic liver injury models are widely desired. The few studies on TNFR2 suggest that they may facilitate TNFR1-induced liver cell death. New-generation drugs against TNFR2 could be relevant for suppressing regulatory T-cell activity and, consequently, improving the efficacy of cancer immunotherapy [58]. A recent study has shown the correlation among polymorphisms of TNF-α and IL-10 genes as an increased risk of developing HCC in patients with chronic HCV infection, suggesting that gene variants are associated with more severe inflammation of the liver [59]. IL-10 is a cytokine with strong anti-inflammatory properties, which plays an important role in limiting the host’s immune response, thereby reducing damage and keeping the tissue in normal balance [60].
A study on HCV patients who have completed antiviral treatment with DAAs has shown the correlation between the genotyping of IL-10 (polymorphism IL-10 rs1800871) and the incidence of complications, such as HCC. This explains that the IL-10 genotype can help select the safest and most accurate drug regimen also based on the follow-up of the resistance of the genotype [59].
Jing et al. found that TNF-α overexpression promotes HCC through the activation of hepatic progenitor cells, while TNF-α deficiency inhibits the activation and proliferation of these cells, reducing tumor incidence. This confirmed that TNF-α plays a significant role in liver damage and prognosis [61].
Expression of IL-6 and TNF-α during chronic liver injury activates the transduction pathway downstream of the transcription factor STAT3, which drives neoplastic transformation in the hepatic microenvironment [62]. IL-6 mediates its pro-proliferative effects through activation and direct interaction with the p65 subunit of NF-kB, activation of which is associated with a frequent and early event in liver fibrosis and HCC, regardless of etiology [63,64].
2.3. Liver Tumor Inducers
IL-16 is a pleiotropic cytokine whose activity influences both the chemical attraction and the modulation of the activation of T lymphocytes (Table 1) [20]. It has been identified as an important over-expressed cytokine in human liver tissue of HCC in both non-tumor and tumor regions compared to benign tumors and non-cancerous liver levels. Furthermore, IL-16 production can activate the ERK (extracellular signal-regulated kinase)/cyclin D1 signaling pathway, leading to tumor growth [65].
Different research groups evaluate osteopontin as an early marker of HCC. Produced by Kupffer cells, stellate cells, and hepatocytes, this cytokine is highly expressed at sites of inflammation and tissue remodeling [66]. Osteopontin mediates a wide range of biological functions in the immune and vascular systems and has been extensively studied in numerous cancers [24]. An increase in serum osteopontin levels was found in individuals with HCC compared to liver cirrhosis alone or chronic liver disease. The specific diagnostic efficacy of osteopontin in detecting early-stage HCC by differentiating them from non-HCC patients varies considerably among studies. Two studies report that osteopontin levels within two years of diagnosis have a reasonable predictive value of HCC with an AUC (area under the curve) of 0.82 [67,68].
In a recent study, Askoura et al. described the role of interleukins IL-33, IL-17, and IL-25 in patients with HCV, the progression of the disease from chronicity to HCC, as well as the importance of using them as biomarkers of disease progression (Table 1). They measured serum levels of interleukins in HCV-related chronic hepatitis patients, HCC, and healthy controls. Their amounts were correlated with the degree of liver fibrosis and viral load. In contrast to serum levels of IL-25, which increased only in HCC patients, serum levels of IL-33 and IL-17 significantly increased in HCV and HCC patients. Furthermore, increasing serum levels of IL-33 seem to parallel the progression of liver fibrosis and viral load. The results indicate a significant role of IL-33 in liver inflammation and fibrosis progression in HCV infection, while IL-17 and IL-25 were featured as biomarkers for developing HCC [21].
After HCV eradication, patients undergo follow-up for the risk of developing HCC. Growth differentiation factor 15 (GDF15) is a cytokine, induced by mitochondrial dysfunction or oxidative stress (Table 1). In one study, serum levels of GDF15 were measured from patients with chronic HCV infection without a history of HCC who had achieved a sustained virological response with DAAs. Serum levels of GDF15 were higher in patients with HCC onset after treatment with DAAs than in untreated patients. Furthermore, the score obtained using an algorithm composed of the GDF15, AFP (alpha-fetoprotein), and the FIB-4 index stratifies the risk of developing HCC de novo after the elimination of HCV [22].
3. Detection and Measurement of Cytokines
The demand for increased testing, particularly for early events of hepatocellular carcinogenesis, for its recurrence or detection of minimal residual disease in those asymptomatic patients requiring alternative approaches. Different methods for measurement of cytokines are currently available, including immunoassays for the detection of single molecules (ELISA, western blot), multiplex assays (chemiluminescent, bead-based (Luminex), and planar antibody arrays), and mass spectrometry [69].
3.1. Enzyme Immuno Assays: EIA
As well as for diagnostics and clinical research, immunoassays represent the most often employed tests for detecting cytokines in biological fluids or cell culture media. The ELISA tests developed in 1971 are the most employed for their high specificity and sensitivity given by pairs of optimized antibodies and protein concentrations equal to 1–100 pg/mL (~10 times lower than the concentration of the most abundant plasma proteins), respectively [70].
The non-competitive ELISA, which uses a capture monoclonal antibody (primary), a biotinylated-detection monoclonal antibody (secondary), and a substrate complex of the streptavidin enzyme, is among the most widely used tests [71,72] and, although not as sensitive as biological tests, are more specific and have a quick and easy execution. Competitive EIA, unlike the non-competitive assay, is based on employing polyclonal capture antibodies and biotin-labeled ligands that compete for binding sites of the antibody with the sample ligand. Compared to the non-competitive assay, EIA is more sensitive, with high discriminating power in detecting free and protein-bound cytokines or soluble cytokine receptors [70,71,72].
3.2. Western Blot
Unlike ELISA, which determines the quantification of proteins in solution, Western Blotting is suitable for qualitative and semi-quantitative detection of cytokines by denaturing and separating them on the polyacrylamide gel, transferring to a nitrocellulose or polyvinylidene membrane difluoride, and, finally, quantifying using specific antibodies [73]. Although not as sensitive as ELISA, it adds data to the molecular weight of proteins and, therefore, can be used to determine splice variants or degradation of cytokine molecules. In addition, it may distinguish inactive precursors of cytokines from active forms characterizing the neoplastic environment [74]. It also allows the study of the phosphorylation sites of receptor proteins, e.g., the effects of VEGF-A and its inhibitor (bevacizumab) on cell proliferation, migration, invasion, and production of other cytokines in melanoma cell lines [75].
3.3. Electrochemiluminescence Immunoassays
Electrochemiluminescence (ECL), whose principle converts electrical energy into light emission, is based on the multi-array technology replaced by the ruthenium complex of the nineties [76]. ECL technology allows the simultaneous quantification of one to ten analytes on 96 or 384-well plates. Anti-cytokine antibodies are adherent to the surface of carbon electrodes, located on each well of the plate, and after the addition of the sample, the detection of cytokines occurs thanks to antibodies conjugated to an electro-chemiluminescent label (sulfo-tag). The passage of current through the carbon electrodes excites the sulfo-tags, and the light intensity will be directly proportional to the analytes in the sample. The ECL technique used both in clinical practice and scientific research, is based on the emission of a specific signal with an almost equal sensitivity compared to cytometry [77].
3.4. Luminex
The evolution of the ELISA is represented by multiplex assays that allow measuring multiple cytokines (for better disease monitoring) in the same reaction well, saving significant quantities of sample, time, and costs. The technique is based on antibody arrays using microspheres, such as Luminex assays, or planar arrays (antibody arrays and antibody microarrays) [78,79].
Both methods have high reproducibility, but recent studies have shown that the mean kit quality control coefficient of variation (CV) ranges from 1.9 to 18.2% for Luminex and 2.4 to 13.9% for Planar Antibody Array and that the latter has a lower limit of quantification (LLoQ) than Luminex [80].
Such assays can measure up to 100 analytes in parallel, but the possibility of cross-reactivity with antibodies has reduced the dosage to 30 analytes. Luminex assays provide high throughput, precision, and sensitivity, reaching pg/mL concentrations and reproducibility achieved with 25–50 µL of the sample [81].
3.5. Planar Antibody Array
The sandwich-based Planar antibody array methodology uses pairs of antibodies tested to eliminate cross-reactivity to any other antigen / antibody in the array. Therefore, high sensitivity, specificity, and productivity of the technique allows to detect between 10 and 80 analytes up to the quantification of 1000 secreted proteins including cytokines and chemokines [78].
Antibodies immobilized on nitrocellulose membranes or on the glass slides allow easy semi-quantitative and quantitative measurements with fluorescent detection.
The methodology finds application above helpful in the search for possible diagnostic and prognostic biomarkers based on the development of the tumorigenesis process and tumor progression [82].
3.6. Mass Spectrometry (MS)
This proteomics technique involves several steps, including the isolation of proteins, digestion by proteases into smaller peptides, concentration and removal of salts, separation by high-performance liquid chromatography (HPLC), and ionization based on the mass-to-charge ratio (m/z) of peptides [83].
The high specificity of the methodology in the identification and quantification of peptides/proteins of MS is contrasted by the low sensitivity due to the pretreatment of the sample by the cytokines with the lowest molecular weight that negatively influence the laborious and difficult ionization process, such as to provide a low yield of peptides and, consequently, poor detection of these cytokines. Therefore, MS offers a higher multiplexing capacity but lower sensitivity than immunoassays [83].
3.7. Challenging Frontiers of Analytic Methods
Thanks to advanced technologies, ultra-sensitive methods that improve the sensitivity of the analytic range down to the order of femtoliters and reduce the effects of the sample matrix and minimization of sample volumes are currently available. Starting from traditional colorimetric, chemiluminescent, and fluorescent detection, laboratory diagnostics are now moving towards electrochemical, optical, mechanical, or surface plasmon resonance measurement biosensing [84,85,86].
Extracellular vesicles are plentifully released into the systemic circulation, where they harbor molecules that provide biochemical information about their cells of origin [87].
Extracellular vesicles represent a challenging source of circulating analytes for cancer liquid biopsy. A promising diagnostic alternative in precision medicine is noninvasive and can be done more frequently than a tissue biopsy.
4. Conclusive Remarks
HCC is a major healthcare burden with a high prevalence and poor prognosis. The identification of molecules as biomarkers for early cancer detection and therapeutic targets for cancer treatment is an important issue in precision medicine.
In clinical practice, screening high-risk patients with ultrasound and/or alpha-fetoprotein dosage is widespread. Now, the reduction in mortality is related to viral infection control measures.
In this scenario, it is crucial for clinicians to provide benefits for HCC therapies. Targeting the hallmarks of cancer represents one of the approaches to anchoring this problem. For HCC, hallmarks include maintenance of proliferative signaling, avoidance of growth suppressors, escape immune destruction, replicative immortality, promotion of inflammation, activation of invasions and metastases, inducing angiogenesis, mediating genome instability and mutation, resisting cell death, and deregulating cellular energy [88]. This means that more hallmarks, pathways, and cytokines are involved.
Currently, the molecular mechanisms underlying HCC remain partly enigmatic. Studies based on the so-called “omics” sciences (e.g., transcriptomics, proteomics, metabolomics) facilitate the learning of global changes in molecules in a given disease in a high-throughput way and, therefore, are suitable for understanding the complex changes that lead to the onset and evolution of HCC.
In the premalignant environment, inflammatory cells release a multitude of cytokines, chemokines, growth factors, prostaglandins, and proangiogenic factors, making the liver environment a favorable zone for hepatocyte transformation from an accumulation of genetic mutations. Survival of transformed hepatocytes is possible through the activation of anti-apoptotic pathways and suppression of immune surveillance [89], as shown in Figure 2A,B. VEGF, stimulated by HIF-1α induced in the hypoxic environment, plays a central role [6].
The risk of developing HCC increases with the severity of liver inflammation and fibrosis. Chronic inflammation is sustained by a range of inflammatory mediators also identified as a cause of carcinogenesis [90]. A complex interaction of several pro-inflammatory cytokines (IL-6, TNF-α) and anti-inflammatory cytokines (TGF-α and β), different transcription factors (NF-kB, STAT-3), and their signaling pathways are involved in the development of HCC [68,91].
We recently found that angiogenic markers, with emphasis on Ang1/2, may contribute to developing quantitative tools for liver disease staging and therapy monitoring [9]. A comparison of cirrhotic patients with and without HCC suggests that HGF levels are potentially useful for monitoring the onset of HCC after a diagnosis of cirrhosis. Elevated Ang1 levels in HCC patients appear to have a protective role, as well as prognostic significance [9].
Characterization of the expression profile of tumor-associated inflammatory cytokines in HCC will require novel diagnostic and therapeutic strategies, such as a better understanding of cytokine regulatory mechanisms in the hepatic microenvironment.
Early diagnosis of HCC remains the goal of precision medicine. The laboratory participates in the follow-up of the patient at risk of a neoformation or recurrence, after any surgical resection, by measuring the cytokines that activate gene transcription of the cell to produce an adequate response to that stimulus. Therefore, the measurement of biomarkers can represent an accurate diagnostic tool for the oncologist to be used alongside imaging procedures.
Abbreviations
AFP (alpha-fetoprotein); Ang (angiopoietin); AUC (area under the curve); DAAs (direct antiviral drugs); ECL (electrochemiluminescence); EIA (enzyme immunoassay); ELISA (enzyme-linked immunosorbent assay); ERK (extracellular signal-regulated kinase); FAK (focal adhesion kinase); GDF15 (growth differentiation factor 15); HCC (hepatocellular carcinoma); HGF (hepatocyte growth factor); HIF-1α (hypoxia-inducible factor 1a); HPLC (high-performance liquid chromatography); IKK (kinase-function protein complex); LLoQ (lower limit of quantification); MELD (model for end stage liver disease); MS (mass spectrometry); NAFLD (non-alcoholic fatty liver disease); NF-kb (nuclear factor kappa-light-chain-enhancer of activated B cells); NASH (non-alcoholic steatohepatitis); PECAM-1 (platelet endothelial cell adhesion molecule-1); SMAD (small mother against decapentaplegic; STAT-3 (signal transducer and activator of transcription); TGF (tumor growth factor); Tie 1 and 2 (tyrosine kinase with Ig and EGF-omology domains-1 receptors); TNF (tumor necrosis factor); Vascular endothelial growth factor (VEGF).
Author Contributions
Conceptualization: K.P., U.B., M.M. Methodology: A.S., C.N., F.D. Data Curation: A.S., R.D.S., G.C. Resources: R.S., C.A.M.C., F.G. Original Draft Preparation: K.P., A.S., V.B. Review & Editing: K.P., F.E., U.B., M.M. Funding acquisition: M.M. Project Administration and supervision: U.B., M.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This review and its publication have been funded by Università Cattolica del Sacro Cuore Fondazione Policlinico Universitario “A. Gemelli” IRCCS as a part of its programs on promotion and dissemination of scientific research (Linea D1 to M.M.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.AIOM. AIRTUM I Numeri Del Cancro in Italia, XI Edizione 2021. [(accessed on 1 January 2021)]. Available online: https://www.aiom.it/i-numeri-del-cancro-in-italia/
- 2.Yang Y., Kim S., Seki E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Semin. Liver Dis. 2019;39:026–042. doi: 10.1055/s-0038-1676806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and Chemokines: At the Crossroads of Cell Signalling and Inflammatory Disease. Biochim. Biophys. Acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Cabillic F., Corlu A. Regulation of Transdifferentiation and Retrodifferentiation by Inflammatory Cytokines in Hepatocellular Carcinoma. Gastroenterology. 2016;151:607–615. doi: 10.1053/j.gastro.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 5.Medina J., Arroyo A.G., Sánchez-Madrid F., Moreno-Otero R. Angiogenesis in Chronic Inflammatory Liver Disease. Hepatology. 2004;39:1185–1195. doi: 10.1002/hep.20193. [DOI] [PubMed] [Google Scholar]
- 6.Tammela T., Enholm B., Alitalo K., Paavonen K. The Biology of Vascular Endothelial Growth Factors. Cardiovasc. Res. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Mukozu T., Nagai H., Matsui D., Kanekawa T., Sumino Y. Serum VEGF as a Tumor Marker in Patients with HCV-Related Liver Cirrhosis and Hepatocellular Carcinoma. Anticancer. Res. 2013;33:1013–1021. doi: 10.1016/S0016-5085(12)63799-8. [DOI] [PubMed] [Google Scholar]
- 8.Zekri A.-R.N., Bahnassy A.A., Alam El-Din H.M., Morsy H.M., Shaarawy S., Moharram N.Z., Daoud S.S. Serum Levels of β-Catenin as a Potential Marker for Genotype 4/Hepatitis C-Associated Hepatocellular Carcinoma. Oncol. Rep. 2011;26:825–831. doi: 10.3892/or.2011.1355. [DOI] [PubMed] [Google Scholar]
- 9.Pocino K., Napodano C., Marino M., Di Santo R., Miele L., De Matthaeis N., Gulli F., Saporito R., Rapaccini G.L., Ciasca G., et al. A Comparative Study of Serum Angiogenic Biomarkers in Cirrhosis and Hepatocellular Carcinoma. Cancers. 2021;14:11. doi: 10.3390/cancers14010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong G., Lin X.-H., Liu H.-H., Gao D.-M., Cui J.-F., Ren Z.-G., Chen R.-X. Intermittent Hypoxia Alleviates Increased VEGF and Pro-Angiogenic Potential in Liver Cancer Cells. Oncol. Lett. 2019;18:1831–1839. doi: 10.3892/ol.2019.10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyahara K., Nouso K., Morimoto Y., Takeuchi Y., Hagihara H., Kuwaki K., Onishi H., Ikeda F., Miyake Y., Nakamura S., et al. Pro-Angiogenic Cytokines for Prediction of Outcomes in Patients with Advanced Hepatocellular Carcinoma. Br. J. Cancer. 2013;109:2072–2078. doi: 10.1038/bjc.2013.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llovet J.M., Peña C.E.A., Lathia C.D., Shan M., Meinhardt G., Bruix J. SHARP Investigators Study Group Plasma Biomarkers as Predictors of Outcome in Patients with Advanced Hepatocellular Carcinoma. Clin. Cancer Res. 2012;18:2290–2300. doi: 10.1158/1078-0432.CCR-11-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong L., Giacomini M.M., Giacomini C., Maitland M.L., Altman R.B., Klein T.E. PharmGKB Summary: Sorafenib Pathways. Pharm. Genom. 2017;27:240–246. doi: 10.1097/FPC.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naldini A., Carraro F. Role of Inflammatory Mediators in Angiogenesis. Curr. Drug Targets. Inflamm. Allergy. 2005;4:3–8. doi: 10.2174/1568010053622830. [DOI] [PubMed] [Google Scholar]
- 15.García-Vilas J.A., Medina M.Á. Updates on the hepatocyte growth factor/c-Met axis in hepatocellular carcinoma and its therapeutic implications. World J. Gastroenterol. 2018;24:3695–3708. doi: 10.3748/wjg.v24.i33.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLisser H.M., Christofidou-Solomidou M., Strieter R.M., Burdick M.D., Robinson C.S., Wexler R.S., Kerr J.S., Garlanda C., Merwin J.R., Madri J.A., et al. Involvement of Endothelial PECAM-1/CD31 in Angiogenesis. Am. J. Pathol. 1997;151:671–677. [PMC free article] [PubMed] [Google Scholar]
- 17.He G., Dhar D., Nakagawa H., Font-Burgada J., Ogata H., Jiang Y., Shalapour S., Seki E., Yost S.E., Jepsen K., et al. Identification of Liver Cancer Progenitors Whose Malignant Progression Depends on Autocrine IL-6 Signaling. Cell. 2013;155:384–396. doi: 10.1016/j.cell.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao Y.-Y., Lin H., Li Y.-S., Lee Y.-H., Chen H.-M., Cheng A.-L., Hsu C.-H. High Plasma Interleukin-6 Levels Associated with Poor Prognosis of Patients with Advanced Hepatocellular Carcinoma. Jpn. J. Clin. Oncol. 2017;47:949–953. doi: 10.1093/jjco/hyx103. [DOI] [PubMed] [Google Scholar]
- 19.Shakiba E., Ramezani M., Sadeghi M. Evaluation of serum interleukin-10 levels in hepatocellular carcinoma patients: A systematic review and meta-analysis. Clin. Exp. Hepatol. 2018;4:35–40. doi: 10.5114/ceh.2018.73484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruikshank W.W., Kornfeld H., Center D.M. Interleukin-16. J. Leukoc. Biol. 2000;67:757–766. doi: 10.1002/jlb.67.6.757. [DOI] [PubMed] [Google Scholar]
- 21.Askoura M., Abbas H.A., Al Sadoun H., Abdulaal W.H., Abu Lila A.S., Almansour K., Alshammari F., Khafagy E.-S., Ibrahim T.S., Hegazy W.A.H. Elevated Levels of IL-33, IL-17 and IL-25 Indicate the Progression from Chronicity to Hepatocellular Carcinoma in Hepatitis C Virus Patients. Pathogens. 2022;11:57. doi: 10.3390/pathogens11010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myojin Y., Hikita H., Tahata Y., Doi A., Kato S., Sasaki Y., Shirai K., Sakane S., Yamada R., Kodama T., et al. Serum Growth Differentiation Factor 15 Predicts Hepatocellular Carcinoma Occurrence after Hepatitis C Virus Elimination. Aliment. Pharmacol. Ther. 2022;55:422–433. doi: 10.1111/apt.16691. [DOI] [PubMed] [Google Scholar]
- 23.Tiegs G., Horst A.K. Seminars in Immunopathology. Springer; Berlin/Heidelberg, Germany: 2022. TNF in the Liver: Targeting a Central Player in Inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H., Chen Q., Alam A., Cui J., Suen K.C., Soo A.P., Eguchi S., Gu J., Ma D. The Role of Osteopontin in the Progression of Solid Organ Tumour. Cell Death Dis. 2018;9:356. doi: 10.1038/s41419-018-0391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Augustin H.G., Koh G.Y., Thurston G., Alitalo K. Control of Vascular Morphogenesis and Homeostasis through the Angiopoietin-Tie System. Nat. Rev. Mol. Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 26.Jeon B.H., Khanday F., Deshpande S., Haile A., Ozaki M., Irani K. Tie-Ing the Antiinflammatory Effect of Angiopoietin-1 to Inhibition of NF-KappaB. Circ. Res. 2003;92:586–588. doi: 10.1161/01.RES.0000066881.04116.45. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan C.C., Du L., Chu D., Cho A.J., Kido M., Wolf P.L., Jamieson S.W., Thistlethwaite P.A. Induction of Pulmonary Hypertension by an Angiopoietin 1/TIE2/Serotonin Pathway. Proc. Natl. Acad. Sci. USA. 2003;100:12331–12336. doi: 10.1073/pnas.1933740100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koh G.Y. Orchestral Actions of Angiopoietin-1 in Vascular Regeneration. Trends Mol. Med. 2013;19:31–39. doi: 10.1016/j.molmed.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Fiedler U., Augustin H.G. Angiopoietins: A Link between Angiogenesis and Inflammation. Trends Immunol. 2006;27:552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Fiedler U., Reiss Y., Scharpfenecker M., Grunow V., Koidl S., Thurston G., Gale N.W., Witzenrath M., Rosseau S., Suttorp N., et al. Angiopoietin-2 Sensitizes Endothelial Cells to TNF-Alpha and Has a Crucial Role in the Induction of Inflammation. Nat. Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 31.Scharpfenecker M., Fiedler U., Reiss Y., Augustin H.G. The Tie-2 Ligand Angiopoietin-2 Destabilizes Quiescent Endothelium through an Internal Autocrine Loop Mechanism. J. Cell Sci. 2005;118:771–780. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- 32.Sugimachi K., Tanaka S., Taguchi K., Aishima S., Shimada M., Tsuneyoshi M. Angiopoietin Switching Regulates Angiogenesis and Progression of Human Hepatocellular Carcinoma. J. Clin. Pathol. 2003;56:854–860. doi: 10.1136/jcp.56.11.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scholz A., Rehm V.A., Rieke S., Derkow K., Schulz P., Neumann K., Koch I., Pascu M., Wiedenmann B., Berg T., et al. Angiopoietin-2 Serum Levels Are Elevated in Patients with Liver Cirrhosis and Hepatocellular Carcinoma. Am. J. Gastroenterol. 2007;102:2471–2481. doi: 10.1111/j.1572-0241.2007.01377.x. [DOI] [PubMed] [Google Scholar]
- 34.Noguchi O., Enomoto N., Ikeda T., Kobayashi F., Marumo F., Sato C. Gene Expressions of C-Met and Hepatocyte Growth Factor in Chronic Liver Disease and Hepatocellular Carcinoma. J. Hepatol. 1996;24:286–292. doi: 10.1016/S0168-8278(96)80006-7. [DOI] [PubMed] [Google Scholar]
- 35.Ljubimova J.Y., Petrovic L.M., Wilson S.E., Geller S.A., Demetriou A.A. Expression of HGF, Its Receptor c-Met, c-Myc, and Albumin in Cirrhotic and Neoplastic Human Liver Tissue. J. Histochem. Cytochem. 1997;45:79–87. doi: 10.1177/002215549704500111. [DOI] [PubMed] [Google Scholar]
- 36.Vejchapipat P., Tangkijvanich P., Theamboonlers A., Chongsrisawat V., Chittmittrapap S., Poovorawan Y. Association between Serum Hepatocyte Growth Factor and Survival in Untreated Hepatocellular Carcinoma. J. Gastroenterol. 2004;39:1182–1188. doi: 10.1007/s00535-004-1469-8. [DOI] [PubMed] [Google Scholar]
- 37.Breuhahn K., Longerich T., Schirmacher P. Dysregulation of Growth Factor Signaling in Human Hepatocellular Carcinoma. Oncogene. 2006;25:3787–3800. doi: 10.1038/sj.onc.1209556. [DOI] [PubMed] [Google Scholar]
- 38.Fodor D., Jung I., Turdean S., Satala C., Gurzu S. Angiogenesis of hepatocellular carcinoma: An immunohistochemistry study. World J. Hepatol. 2019;11:294–304. doi: 10.4254/wjh.v11.i3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCormick B.A., Zetter B.R. Adhesive Interactions in Angiogenesis and Metastasis. Pharmacol. Ther. 1992;53:239–260. doi: 10.1016/0163-7258(92)90011-N. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y.-Y., Kong L.-Q., Zhu X.-D., Cai H., Wang C.-H., Shi W.-K., Cao M.-Q., Li X.-L., Li K.-S., Zhang S.-Z., et al. CD31 Regulates Metastasis by Inducing Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma via the ITGB1-FAK-Akt Signaling Pathway. Cancer Lett. 2018;429:29–40. doi: 10.1016/j.canlet.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt-Arras D., Rose-John S. IL-6 Pathway in the Liver: From Physiopathology to Therapy. J. Hepatol. 2016;64:1403–1415. doi: 10.1016/j.jhep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Bartoccioni E., Scuderi F., Marino M., Provenzano C. IL-6, Monocyte Infiltration and Parenchymal Cells. Trends Immunol. 2003;24:299–300. doi: 10.1016/S1471-4906(03)00112-1. author reply 300–301. [DOI] [PubMed] [Google Scholar]
- 43.Marino M., Scuderi F., Ponte E., Maiuri M.T., De Cristofaro R., Provenzano C., Rose-John S., Cittadini A., Bartoccioni E. Novel Path to IL-6 Trans-Signaling through Thrombin-Induced Soluble IL-6 Receptor Release by Platelets. J. Biol. Regul. Homeost. Agents. 2013;27:841–852. [PubMed] [Google Scholar]
- 44.Kao J.-T., Lai H.-C., Tsai S.-M., Lin P.-C., Chuang P.-H., Yu C.-J., Cheng K.-S., Su W.-P., Hsu P.-N., Peng C.-Y., et al. Rather than Interleukin-27, Interleukin-6 Expresses Positive Correlation with Liver Severity in Naïve Hepatitis B Infection Patients. Liver Int. 2012;32:928–936. doi: 10.1111/j.1478-3231.2011.02742.x. [DOI] [PubMed] [Google Scholar]
- 45.Lai S.-C., Su Y.-T., Chi C.-C., Kuo Y.-C., Lee K.-F., Wu Y.-C., Lan P.-C., Yang M.-H., Chang T.-S., Huang Y.-H. DNMT3b/OCT4 Expression Confers Sorafenib Resistance and Poor Prognosis of Hepatocellular Carcinoma through IL-6/STAT3 Regulation. J. Exp. Clin. Cancer Res. 2019;38:474. doi: 10.1186/s13046-019-1442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J., Wang W.-L., Li Q., Qiao Q. Expression of Transforming Growth Factor-Alpha and Hepatitis B Surface Antigen in Human Hepatocellular Carcinoma Tissues and Its Significance. World J. Gastroenterol. 2004;10:830–833. doi: 10.3748/wjg.v10.i6.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marino M., Scuderi F., Mannella F., Bartoccioni E. TGF-Β1 and IL-10 Modulate IL-1β-Induced Membrane and Soluble ICAM-1 in Human Myoblasts. J. Neuroimmunol. 2003;134:151–157. doi: 10.1016/S0165-5728(02)00399-5. [DOI] [PubMed] [Google Scholar]
- 48.Massagué J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furuta K., Misao S., Takahashi K., Tagaya T., Fukuzawa Y., Ishikawa T., Yoshioka K., Kakumu S. Gene Mutation of Transforming Growth Factor Beta1 Type II Receptor in Hepatocellular Carcinoma. Int. J. Cancer. 1999;81:851–853. doi: 10.1002/(SICI)1097-0215(19990611)81:6<851::AID-IJC2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 50.Alqahtani A., Khan Z., Alloghbi A., Said Ahmed T.S., Ashraf M., Hammouda D.M. Hepatocellular Carcinoma: Molecular Mechanisms and Targeted Therapies. Medicina. 2019;55:E526. doi: 10.3390/medicina55090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonzalez-Sanchez E., Vaquero J., Férnandez-Barrena M.G., Lasarte J.J., Avila M.A., Sarobe P., Reig M., Calvo M., Fabregat I. The TGF-β Pathway: A Pharmacological Target in Hepatocellular Carcinoma? Cancers. 2021;13:3248. doi: 10.3390/cancers13133248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srivastava A., Sharma H., Khanna S., Sadhu Balasundaram T., Chowdhury S., Chowdhury R., Mukherjee S. Interleukin-6 Induced Proliferation Is Attenuated by Transforming Growth Factor-β-Induced Signaling in Human Hepatocellular Carcinoma Cells. Front. Oncol. 2021;11:811941. doi: 10.3389/fonc.2021.811941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shakiba E., Ramezani M., Sadeghi M. Evaluation of Serum Interleukin-6 Levels in Hepatocellular Carcinoma Patients: A Systematic Review and Meta-Analysis. Clin. Exp. Hepatol. 2018;4:182–190. doi: 10.5114/ceh.2018.78122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chau G.Y., Wu C.W., Lui W.Y., Chang T.J., Kao H.L., Wu L.H., King K.L., Loong C.C., Hsia C.Y., Chi C.W. Serum Interleukin-10 but Not Interleukin-6 Is Related to Clinical Outcome in Patients with Resectable Hepatocellular Carcinoma. Ann. Surg. 2000;231:552–558. doi: 10.1097/00000658-200004000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grivennikov S.I., Karin M. Inflammatory Cytokines in Cancer: Tumour Necrosis Factor and Interleukin 6 Take the Stage. Ann. Rheum. Dis. 2011;70((Suppl. 1)):i104–i108. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 56.Luedde T., Beraza N., Kotsikoris V., van Loo G., Nenci A., De Vos R., Roskams T., Trautwein C., Pasparakis M. Deletion of NEMO/IKKgamma in Liver Parenchymal Cells Causes Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 57.Maeda S., Kamata H., Luo J.-L., Leffert H., Karin M. IKKbeta Couples Hepatocyte Death to Cytokine-Driven Compensatory Proliferation That Promotes Chemical Hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 58.Nie Y., He J., Shirota H., Trivett A.L., Yang D., Klinman D.M., Oppenheim J.J., Chen X. Blockade of TNFR2 Signaling Enhances the Immunotherapeutic Effect of CpG ODN in a Mouse Model of Colon Cancer. Sci. Signal. 2018;11:eaan0790. doi: 10.1126/scisignal.aan0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghanm S.E., Shebl N.A.-F., El Sayed I.E.T., Abdel-Bary H.M., Saad B.F., Othman Saad W. Direct Relationship between Interleukin-10 Gene Polymorphism and Hepatocellular Carcinoma Complicated by Direct Acting Antiviral Treatment of Hepatitis C Virus. Asian Pac. J. Cancer Prev. 2021;22:3203–3210. doi: 10.31557/APJCP.2021.22.10.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El-Serag H.B. Epidemiology of Viral Hepatitis and Hepatocellular Carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jing Y., Sun K., Liu W., Sheng D., Zhao S., Gao L., Wei L. Tumor Necrosis Factor-α Promotes Hepatocellular Carcinogenesis through the Activation of Hepatic Progenitor Cells. Cancer Lett. 2018;434:22–32. doi: 10.1016/j.canlet.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Villanueva A., Luedde T. The Transition from Inflammation to Cancer in the Liver. Clin. Liver Dis. 2016;8:89–93. doi: 10.1002/cld.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moran D.M., Mattocks M.A., Cahill P.A., Koniaris L.G., McKillop I.H. Interleukin-6 Mediates G(0)/G(1) Growth Arrest in Hepatocellular Carcinoma through a STAT 3-Dependent Pathway. J. Surg. Res. 2008;147:23–33. doi: 10.1016/j.jss.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu J., Lin H., Wu G., Zhu M., Li M. IL-6/STAT3 Is a Promising Therapeutic Target for Hepatocellular Carcinoma. Front. Oncol. 2021;11:760971. doi: 10.3389/fonc.2021.760971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takeba Y., Ohta Y., Ootaki M., Kobayashi T., Kida K., Watanabe M., Koizumi S., Otsubo T., Iiri T., Matsumoto N. Identification of Interleukin-16 Production on Tumor Aggravation in Hepatocellular Carcinoma by a Proteomics Approach. Tumour Biol. 2021;43:309–325. doi: 10.3233/TUB-211507. [DOI] [PubMed] [Google Scholar]
- 66.Brown L.F., Berse B., Van de Water L., Papadopoulos-Sergiou A., Perruzzi C.A., Manseau E.J., Dvorak H.F., Senger D.R. Expression and Distribution of Osteopontin in Human Tissues: Widespread Association with Luminal Epithelial Surfaces. Mol. Biol. Cell. 1992;3:1169–1180. doi: 10.1091/mbc.3.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Da Costa A.N., Plymoth A., Santos-Silva D., Ortiz-Cuaran S., Camey S., Guilloreau P., Sangrajrang S., Khuhaprema T., Mendy M., Lesi O.A., et al. Osteopontin and Latent-TGF β Binding-Protein 2 as Potential Diagnostic Markers for HBV-Related Hepatocellular Carcinoma. Int. J. Cancer. 2015;136:172–181. doi: 10.1002/ijc.28953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duarte-Salles T., Misra S., Stepien M., Plymoth A., Muller D., Overvad K., Olsen A., Tjønneland A., Baglietto L., Severi G., et al. Circulating Osteopontin and Prediction of Hepatocellular Carcinoma Development in a Large European Population. Cancer Prev. Res. 2016;9:758–765. doi: 10.1158/1940-6207.CAPR-15-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kupcova Skalnikova H., Cizkova J., Cervenka J., Vodicka P. Advances in Proteomic Techniques for Cytokine Analysis: Focus on Melanoma Research. Int. J. Mol. Sci. 2017;18:E2697. doi: 10.3390/ijms18122697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hornbeck P.V. Enzyme-Linked Immunosorbent Assays. Curr. Protoc. Immunol. 2015;110:2.1.1–2.1.23. doi: 10.1002/0471142735.im0201s110. [DOI] [PubMed] [Google Scholar]
- 71.Alhajj M., Farhana A. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Enzyme Linked Immunosorbent Assay. [PubMed] [Google Scholar]
- 72.Whiteside T.L. Cytokines and Cytokine Measurements in a Clinical Laboratory. Clin. Diagn. Lab. Immunol. 1994;1:257–260. doi: 10.1128/cdli.1.3.257-260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Towbin H., Staehelin T., Gordon J. Electrophoretic Transfer of Proteins from Polyacrylamide Gels to Nitrocellulose Sheets: Procedure and Some Applications. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Apte R.N., Dotan S., Elkabets M., White M.R., Reich E., Carmi Y., Song X., Dvozkin T., Krelin Y., Voronov E. The Involvement of IL-1 in Tumorigenesis, Tumor Invasiveness, Metastasis and Tumor-Host Interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 75.Logan P., Burnier J., Burnier M.N. Vascular Endothelial Growth Factor Expression and Inhibition in Uveal Melanoma Cell Lines. Ecancermedicalscience. 2013;7:336. doi: 10.3332/ecancer.2013.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei H., Wang E. Electrochemiluminescence of Tris(2,2′-Bipyridyl)Ruthenium and Its Applications in Bioanalysis: A Review. Luminescence. 2011;26:77–85. doi: 10.1002/bio.1279. [DOI] [PubMed] [Google Scholar]
- 77.Dabitao D., Margolick J.B., Lopez J., Bream J.H. Multiplex Measurement of Proinflammatory Cytokines in Human Serum: Comparison of the Meso Scale Discovery Electrochemiluminescence Assay and the Cytometric Bead Array. J. Immunol. Methods. 2011;372:71–77. doi: 10.1016/j.jim.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilson J.J., Burgess R., Mao Y.-Q., Luo S., Tang H., Jones V.S., Weisheng B., Huang R.-Y., Chen X., Huang R.-P. Antibody Arrays in Biomarker Discovery. Adv. Clin. Chem. 2015;69:255–324. doi: 10.1016/bs.acc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 79.Valekova I., Skalnikova H.K., Jarkovska K., Motlik J., Kovarova H. Multiplex Immunoassays for Quantification of Cytokines, Growth Factors, and Other Proteins in Stem Cell Communication. Methods Mol. Biol. 2015;1212:39–63. doi: 10.1007/7651_2014_94. [DOI] [PubMed] [Google Scholar]
- 80.Günther A., Becker M., Göpfert J., Joos T., Schneiderhan-Marra N. Comparison of Bead-Based Fluorescence Versus Planar Electrochemiluminescence Multiplex Immunoassays for Measuring Cytokines in Human Plasma. Front. Immunol. 2020;11:572634. doi: 10.3389/fimmu.2020.572634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kupcova Skalnikova H., Vodickova Kepkova K., Vodicka P. Luminex XMAP Assay to Quantify Cytokines in Cancer Patient Serum. Methods Mol. Biol. 2020;2108:65–88. doi: 10.1007/978-1-0716-0247-8_6. [DOI] [PubMed] [Google Scholar]
- 82.Sanchez-Carbayo M. Antibody Array-Based Technologies for Cancer Protein Profiling and Functional Proteomic Analyses Using Serum and Tissue Specimens. Tumour. Biol. 2010;31:103–112. doi: 10.1007/s13277-009-0014-z. [DOI] [PubMed] [Google Scholar]
- 83.Nilsson T., Mann M., Aebersold R., Yates J.R., Bairoch A., Bergeron J.J.M. Mass Spectrometry in High-Throughput Proteomics: Ready for the Big Time. Nat. Methods. 2010;7:681–685. doi: 10.1038/nmeth0910-681. [DOI] [PubMed] [Google Scholar]
- 84.Simon S., Ezan E. Ultrasensitive Bioanalysis: Current Status and Future Trends. Bioanalysis. 2017;9:753–764. doi: 10.4155/bio-2017-0018. [DOI] [PubMed] [Google Scholar]
- 85.Singh M., Truong J., Reeves W.B., Hahm J.-I. Emerging Cytokine Biosensors with Optical Detection Modalities and Nanomaterial-Enabled Signal Enhancement. Sensors. 2017;17:E428. doi: 10.3390/s17020428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang X., Tang Y., Alt R.R., Xie X., Li F. Emerging Techniques for Ultrasensitive Protein Analysis. Analyst. 2016;141:3473–3481. doi: 10.1039/C6AN00059B. [DOI] [PubMed] [Google Scholar]
- 87.Di Santo R., Vaccaro M., Romanò S., Di Giacinto F., Papi M., Rapaccini G.L., De Spirito M., Miele L., Basile U., Ciasca G. Machine Learning-Assisted FTIR Analysis of Circulating Extracellular Vesicles for Cancer Liquid Biopsy. JPM. 2022;12:949. doi: 10.3390/jpm12060949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hanahan D., Weinberg R.A. Hallmarks of Cancer: The next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 89.Yu L.-X., Ling Y., Wang H.-Y. Role of Nonresolving Inflammation in Hepatocellular Carcinoma Development and Progression. NPJ Precision Onc. 2018;2:6. doi: 10.1038/s41698-018-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dallio M., Sangineto M., Romeo M., Villani R., Romano A.D., Loguercio C., Serviddio G., Federico A. Immunity as Cornerstone of Non-Alcoholic Fatty Liver Disease: The Contribution of Oxidative Stress in the Disease Progression. Int. J. Mol. Sci. 2021;22:E436. doi: 10.3390/ijms22010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zegeye M.M., Lindkvist M., Fälker K., Kumawat A.K., Paramel G., Grenegård M., Sirsjö A., Ljungberg L.U. Activation of the JAK/STAT3 and PI3K/AKT Pathways Are Crucial for IL-6 Trans-Signaling-Mediated pro-Inflammatory Response in Human Vascular Endothelial Cells. Cell Commun. Signal. 2018;16:55. doi: 10.1186/s12964-018-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.