Abstract
Pneumococcal surface protein A (PspA) is a pneumococcal virulence factor capable of eliciting protection against pneumococcal infection in mice. Previous studies have demonstrated that the protection is antibody mediated. Here we examined the ability of pspA to elicit a protective immune response following genetic immunization of mice. Mice were immunized by intramuscular injections with a eukaryotic expression vector encoding the α-helical domain of PspA/Rx1. Immunization induced a PspA-specific serum antibody response, and immunized mice survived pneumococcal challenge. Survival and antibody responses occurred in a dose-dependent manner, the highest survival rates being seen with doses of 10 μg or greater. The ability of genetic immunization to elicit cross-protection was demonstrated by the survival of immunized mice challenged with pneumococcal strains differing in capsule and PspA types. Also, immunized mice were protected from intravenous and intratracheal challenges with pneumococci. Similar to the results seen with immunization with PspA, the survival of mice genetically immunized with pspA was antibody mediated. There was no decline in the level of protection 7 months after immunization. These results support the use of genetic immunization to elicit protective immune responses against extracellular pathogens.
Much attention is being focused on the promising new technology of DNA vaccination or genetic immunization. Genetic immunization offers advantages over immunization with purified protein. DNA vaccines are capable of eliciting both humoral and cell-mediated immune responses (38). Plasmid DNA containing unmethylated CpG motifs and used in immunizations has been found to modulate immune responses (21, 33). The presence of these motifs induces cytokine production and a generalized activation of antigen-presenting cells, giving the DNA an adjuvant effect (2, 41, 42). The cytokine profile induced leads to a predominantly T-helper (Th) 1 (Th-1) response that reduces the likelihood of allergic responses that may occur when proteins are used (21, 31). Another advantage is the significantly lower cost of production, since DNA is more easily produced and purified than proteins. Considering that the greatest morbidity and mortality from pneumococcal diseases are seen in the populations of developing countries, such factors are extremely important. DNA vaccines are more heat stable than protein vaccines, a fact which increases the efficacy of their use in developing countries, where storage and transportation capabilities may be lacking. The economic and physical characteristics of DNA vaccines make them good candidates for global vaccination programs.
As the prevalence of multidrug-resistant pneumococcal strains increases, the development of an effective vaccine becomes the primary focus in preventing pneumococcal diseases. Although the capsular polysaccharide (PS) of the pneumococcus is considered the major antigenic determinant conferring immunity following infection (22), the current 23-valent PS vaccine has had little impact on global morbidity and mortality (9, 12, 15, 34). More importantly, PS is poorly immunogenic in the most important risk group, children under 3 years old (13, 17, 18, 32). Therefore, efforts in developing a pneumococcal vaccine capable of eliciting a T-cell-dependent immune response have become a priority. The pneumococcal conjugate vaccine recently approved for human use overcomes the T-cell-independent nature of PS antigens, thereby making them more immunogenic in children (16, 30, 35). Although this strategy is an effective one, conjugate vaccines have negative aspects of their own. In addition to their high cost, which reduces their availability, conjugate vaccines are further limited in the number of different PSs which can be incorporated, a problem which reduces the potential range of protection. These concerns have led researchers to look for pneumococcal proteins capable of eliciting protective immunity.
Previous studies have established pneumococcal surface protein A (PspA) as a virulence factor found on all pneumococcal isolates (8, 28). PspA consists of four major domains (25). The N-terminal half of the molecule comprises an α-helical domain. Following the α helix are two highly conserved domains: the proline-rich domain and the choline binding domain. Seventeen amino acids on the C-terminal end form the cytoplasmic tail. Based on immunization studies with full-length and truncated fragments of PspA, the α-helical domain was determined to contain protection-eliciting epitopes (8, 26, 27). Therefore, this domain was of particular interest in our study. Immunization studies using purified PspA have also demonstrated the ability of PspA to elicit protective immune responses that are cross-reactive among pneumococci with different capsule and PspA types (5, 25, 27). These characteristics offer the possibility of inducing broad protection by immunizing with one or just a few PspA types (7).
It was previously demonstrated that genetic immunization with full-length pspA was able to elicit protection against pneumococcal challenge (24). However, the level of protection was below that obtained with immunization with purified PspA, and there was an apparent lack of correlation between antibodies against PspA and protection. In this study, we examined the possibility that a fragment of pspA, when used for genetic immunization, is able to elicit protection similar to that seen with immunization with purified PspA. Our results demonstrate that genetic immunization can elicit effective protection against an extracellular bacterial pathogen. Moreover, our model system should be useful in optimizing the expression of bacterial genes for genetic immunization.
MATERIALS AND METHODS
Plasmid construction and preparation.
The plasmid used in this study was constructed by the ligation of a KpnI/XbaI-digested pNGVL3 expression vector (National Gene Vector Laboratory, Ann Arbor, Mich.) and a KpnI/XbaI-digested PCR-amplified fragment from the region encoding the α-helical domain of Streptococcus pneumoniae PspA/Rx1. The pNGVL3 vector is driven by a cytomegalovirus immediate-early enhancer and a promoter upstream of a multiple cloning site. Downstream of the multiple cloning site is a rabbit beta-globulin poly(A) signal for proper expression in eukaryotic cells. The region encoding the α-helical domain of PspA was amplified as previously described (26) from S. pneumoniae Rx1 genomic DNA using the following primers: LSM180 (upstream primer), 5′ GGGCGGTACCATTATGGCCAGTCAGTCTAAGCT 3′, and LSM181 (downstream primer), 5′ GGCTCTACCCCTAAGCTCTTAAGGTCAGC 3′. The upstream primer contains a KpnI site and a translational start site. The downstream primer contains an XbaI site and a stop codon. Vector and insert DNAs were gel purified, ligated, and transformed into Escherichia coli DH5α. The derived construct was designated pJB100 and was sequenced to confirm the insert. Plasmid DNA was prepared using an EndoFree Plasmid Giga kit (Qiagen, Santa Clarita, Calif.) and stored at −20°C until used. Plasmid DNA was diluted in lactated Ringer's (LR) solution for immunizations.
In vitro expression.
Expression studies were carried out using HeLa cells. The HeLa cells were transfected with two constructs: pJB10, which contains the Rx1 PspA α-helix-encoding insert in pcDNA3.1 (Invitrogen, Valencia, Calif.), and pJB100, which contains the same insert in a pNGVL3 vector. Cells that received no DNA served as a negative control. In preparation for transfection, HeLa cells were grown in Dulbecco modified Eagle medium with 6% fetal bovine serum to approximately 40% confluence in a 25-cm2 flask. The HeLa cells were then transfected using 10 μg of plasmid DNA complexed with 60 μl of Superfect transfection reagent (Qiagen). Cells were incubated with the transfection complex for 2 to 3 h at 37°C. Cells were washed twice with phosphate-buffered saline, and medium was added. After 48 h of growth, cells were removed from the flask by treatment with 1 ml of 0.25% trypsin–0.1% EDTA and counted. Cells were then suspended in sodium dodecyl sulfate loading buffer with 100 μg of phenylmethylsulfonyl fluoride/ml, and the suspension was placed in a boiling water bath for 5 min. Immunoblot analysis with anti-PspA monoclonal antibody XiR278 was performed as previously described (27).
Immunization studies.
Immunization studies were carried out using CBA/N (CBA/CAHN-BTK XID/J) mice (Jackson Laboratory, Bar Harbor, Maine). Mice received intramuscular injections of plasmids on days 0, 14, and 28. The immunizations consisted of plasmids diluted in a 50-μl delivery volume of LR solution and were given as lingual injections (24). Mice were challenged 1 week after the final boost (day 35), with the exception of the mice in the long-term protection group, which were challenged 7 months after the final boost. Mice were challenged by intravenous (i.v.) injections of 0.2 ml of a pneumococcal strain diluted in LR solution. Additional mice were challenged by intratracheal (i.t.) inoculation with 20 μl of pneumococcal strain A66 diluted in LR solution. All mice were observed for greater than 21 days postchallenge.
Anti-PspA antibody assays.
Serum antibody titers were determined by enzyme-linked immunosorbent assays (ELISAs). Mice were bled on days 0, 14, 28, and 35 for the collection of serum. Plates were coated with 0.3 μg of pneumococcal strain D39 PspA/ml and blocked with 1% bovine serum albumin in phosphate-buffered saline. After the plates were washed, serum samples were added and serially diluted. The plates were incubated overnight at 4°C and washed before the addition of a biotinylated goat anti-mouse immunoglobulin-specific antibody (Southern Biotechnology Associates, Birmingham, Ala.). Following incubation and washing, streptavidin-alkaline phosphatase was added to each well, and the plates were allowed to incubate for 1 h at 37°C. After a final wash, 1 mg of p-nitrophenyl phosphate disodium (Sigma, St. Louis, Mo.)/ml was added. Plates were examined at 405 nm, and antibody concentrations were calculated based on a standard curve generated using an anti-PspA antiserum of known concentration. Isotype determination of the anti-PspA antiserum was performed in the same manner using biotinylated goat anti-immunoglobulin G1 (IgG1) or biotinylated goat anti-IgG2a as the secondary antibody. Samples were diluted 1:3, and the results of the isotype-specific assays were expressed as the highest dilution giving a reading greater than 0.1 unit of optical density above the background.
Passive protection assays.
Passive protection assays were performed as previously described (40). Briefly, naive CBA/N mice received 0.2-ml intraperitoneal injections of pooled serum collected from mice immunized with 10 μg of pJB100 or PspA. The immune serum from protein-immunized mice was diluted to a concentration equivalent to that of the pJB100 immune serum (8 μg of antigen-specific antibody/ml). Mice were given a 1:2, 1:20, or 1:200 dilution of serum in LR solution. Another group of mice received injections of LR solution to serve as an additional control. One hour after receiving the immune serum, mice were challenged with i.v. injections of 500 CFU of pneumococcal strain WU2.
Statistics.
The results were analyzed statistically by the two-tailed Fisher exact test.
RESULTS
Expression of the α-helical domain of Rx1 PspA in eukaryotic cells.
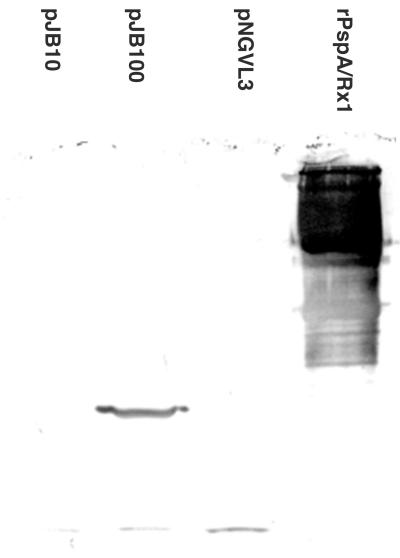
HeLa cells were used to examine the expression of PspA in a eukaryotic cell line (Fig. 1). Cell lysates containing equivalent numbers of transfected cells were used in immunoblot assays to detect the presence of PspA/Rx1 and to determine the relative levels of expression for pJB10 and pJB100. Cells transfected with pJB100 expressed a protein with an apparent molecular mass of 43 kDa. However, in cells transfected with pJB10, this protein was detected as a faint band visible only in the original blot. Based on the reactivity of XiR278 in the Western blotting, levels of expression were higher with pJB100 than with pJB10. Therefore, pJB100 was chosen for further evaluation in the immunization studies.
FIG. 1.
Immunoblot of HeLa cell lysates tested with anti-PspA monoclonal antibody XiR278. The indicated cell lysates or purified full-length PspA/Rx1 (rightmost lane) were reacted with XiR278. The pJB100 lane shows a band of the expected size of about 43 kDa. A 43 kDa band in the pJB10 lane was visible only in the original blot.
Immunization with pJB100.
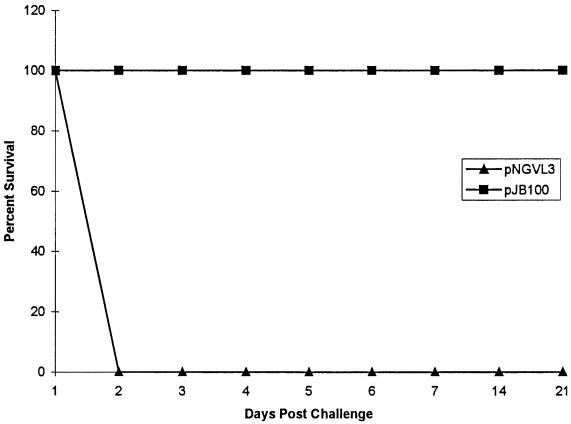
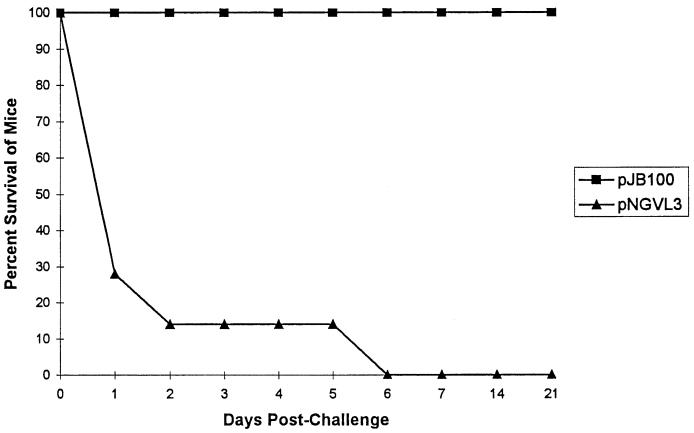
Two groups of mice, each receiving 50 μg of plasmid DNA, were immunized in the initial experiment (Fig. 2). One group of mice received pNGVL3, the vector without any pspA insert, as a control. A second group of mice received pJB100. Following immunization, the mice were challenged i.v. with approximately 800 CFU of pneumococcal strain WU2. This dose is greater than 50 times the 50% lethal dose (LD50) for WU2 (6). Mice were observed for greater than 21 days postchallenge. All of the mice that received pJB100 survived the challenge. The mean number of days to death in the control group was 2 days.
FIG. 2.
Immunization with pJB100. CBA/N mice were immunized with 50 μg of either pJB100 or pNGVL3 (control). Mice were challenged i.v. with pneumococcal strain WU2. Data represent the results for five or more mice per group.
Dose dependence.
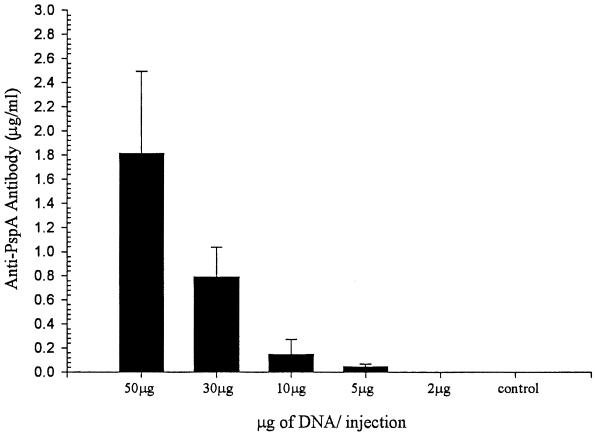
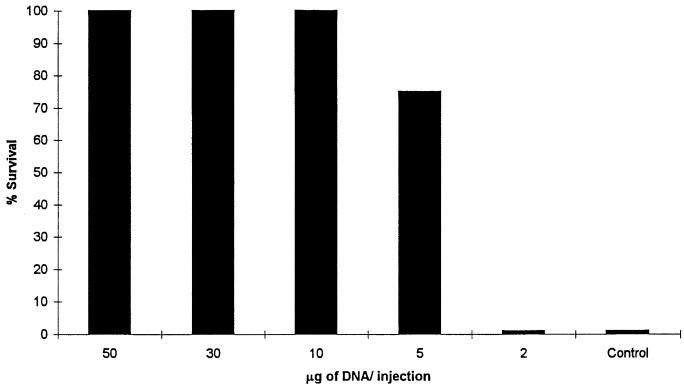
To determine the optimum immunization dose, groups of mice were immunized with different doses of pJB100. Groups receiving doses of 50, 30, 10, 5, and 2 μg of pJB100 were evaluated for anti-PspA antibody responses (Fig. 3) and for protection against a fatal challenge (Fig. 4). An additional group receiving pNGVL3 served as the control. Serum samples collected on day 28 were analyzed by ELISA to determine the antibody responses. To determine the levels of protection provided at the different doses, mice were challenged i.v. with approximately 80 CFU of WU2 on day 35. Doses of 50, 30, and 10 μg provided 100% protection. The level of protection was reduced in the group receiving 5 μg, and protection was completely absent in the group receiving 2 μg. Since the 10-μg dose was the minimal dose that provided complete protection, it was chosen as the minimal dose for all subsequent immunization studies. The reduced levels of protection correlated with what would be expected of dose-dependent responses.
FIG. 3.
Dose-dependent antibody responses. CBA/N mice were immunized with the indicated amounts of pJB100 or 50 μg of pNGVL3 (control). Serum samples collected on day 28 were analyzed by ELISA to determine the antibody responses. Antibody concentrations were calculated based on a standard curve generated using an anti-PspA antiserum of known concentration. Data represent the results for four mice per group. Error bars show standard errors of the means.
FIG. 4.
Dose-dependent survival. CBA/N mice were immunized with the indicated amounts of pJB100 or 50 μg of pNGVL3 (control). Mice were challenged on day 35 with pneumococcal strain WU2. Data represent the results for four mice per group.
Anti-PspA antibody responses.
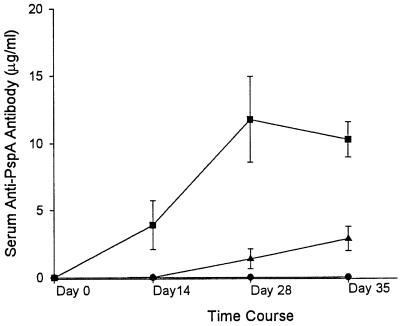
Antibody responses to pneumococcal surface antigens have been generally regarded as the primary mechanism of protection against pneumococcal infection. To evaluate the humoral responses to pJB100, serum anti-PspA antibody concentrations were measured by ELISA. Serum was collected on days 0, 14, 28, and 35 (Fig. 5). Anti-PspA antibody concentrations rose quickly in the group immunized with 50 μg of pJB100 and reached a plateau at day 28. The rate of responses to the 10-μg dose was reduced, and the antibody levels at day 35 were significantly lower than those in the group receiving the 50-μg dose. Anti-PspA antibodies were not detected in control mice receiving pNGVL3.
FIG. 5.
Anti-PspA antibody responses following immunization with pJB100. CBA/N mice were immunized on days 0, 14, and 28 with pJB100 (10 [triangles] or 50 [squares] μg) or pNGVL3 (50 μg) (circles). Data represent the results for seven or more mice per group. Error bars show standard errors of the means.
IgG isotype specificity for determination of Th subtype.
We used the IgG isotype of the anti-PspA antibodies as a surrogate marker to determine the Th subtype. Previous studies have shown that DNA vaccines given as intramuscular injections generate predominantly Th-1 responses (11, 21, 23, 37). Since Th-2 responses are generally accepted as the Th responses involved in protection during a natural infection (10, 40), it was important to evaluate the Th subtype following immunization with pJB100. The predominant Th subtype was determined by the ratio of IgG1 to IgG2a. Immunization results are given in Table 1.
TABLE 1.
IgG isotype specificity of serum anti-PspA antibody responsesa
| Antigen | End-point titer of:
|
IgG1/IgG2a ratio | |
|---|---|---|---|
| IgG1 | IgG2a | ||
| pJB100 (10 μg) | 17,600 ± 10,857 | 17,600 ± 10,857 | 1:1 |
| pJB100 (50 μg) | 7,200 ± 3,118 | 56,700 ± 30,666 | 1:8 |
| PspA | 387,900 ± 131,000 | 96,000 ± 30,604 | 4:1 |
| D39 | 200,100 ± 113,463 | 48,300 ± 28,947 | 4:1 |
ELISA were performed to determine the presence of isotype-specific anti-PspA antibodies. Results are expressed as average end-point titers ± standard errors of the means for groups of four or more mice and were used to calculate the IgG1/IgG2a ratio.
Antigen-specific IgG1 antibody served as a Th-2 marker, and antigen-specific IgG2a served as a Th-1 marker (1, 29, 36). An IgG1/IgG2a ratio of >1 denotes a Th-2 response, and a ratio of <1 suggests a Th-1 response. Mice immunized with protein or infected with D39 both responded in a Th-2 manner. This result was expected, since the route of administration would lead to antigen processing in the major histocompatibility complex class II pathway that leads to a Th-2 response. Mice receiving the 50-μg dose of pJB100 responded in a Th-1 fashion. However, mice receiving 10 μg of pJB100 exhibited mixed Th responses.
Cross-protection.
The ability of Rx1 PspA to elicit cross-protection in protein immunization studies is well documented (7, 8, 25–27). However, it is important to determine if this holds true for genetic immunization with Rx1 pspA. To evaluate the ability of pJB100 to elicit cross-protection, mice were immunized with 10 μg of plasmid as described above and challenged with different pneumococcal strains (Table 2). The pneumococcal strains chosen represent three different capsule types and two different PspA clades (5). In each case, survival of immunized mice was observed.
TABLE 2.
Cross protection of CBA/N mice immunized with pJB100a
| Challenge strain
|
Median no. of days to death after immunization with:
|
Alive/dead mouse ratio after immunization with:
|
P | ||||
|---|---|---|---|---|---|---|---|
| Name | Capsular serotype | PspA clade | pJB100 | pNGVL3 | pJB100 | pNGVL3 | |
| WU2 | 3 | 2 | >21 | 2.5 | 30:0 | 0:28 | <0.0001 |
| A66 | 3 | 1 or 2 | >21 | 2.0 | 4:0 | 0:4 | <0.02 |
| BDL6A | 6A | 1 | >21 | 6.0 | 10:0 | 1:9 | <0.0001 |
| EF6796 | 6A | 2 | >21 | 9.0 | 6:0 | 1:5 | <0.007 |
| BG9163 | 6B | 2 | >20 | 10 | 13:1 | 3:11 | <0.0003 |
Mice were immunized with 10 μg of pJB100 or pNGVL3 (control) and challenged with the indicated strain by i.v. injection on day 35.
Protection against i.t. challenge.
Since the pneumococcus is primarily a respiratory pathogen, we examined the ability of pJB100 to protect against challenge via the respiratory tract. Mice were immunized with 10 μg of plasmid on the same immunization schedule as that used in the other experiments. On day 35, mice were challenged i.t. with approximately 80 CFU of A66. The LD50 for A66 by this route was determined to be less than 20 CFU (data not shown). All mice in the immunized group survived challenge by this route of infection (Fig. 6).
FIG. 6.
i.t. challenge of immunized mice. Mice were immunized with 10 μg of pJB100 or pNGVL3 (control) and challenged on day 35 with pneumococcal strain A66. Data represent the results for six mice per group.
Passive immunization assays.
Protection against pneumococcal infection is considered antibody mediated. Therefore, we evaluated the ability of immune serum generated in pJB100-immunized mice to provide protection in naive mice. As a positive control, we used immune serum from PspA/Rx1 immunized mice (Table 3). Mice that received pooled pJB100 immune serum at a dilution of at least 1:20 were protected. Therefore, as little as 0.08 μg of anti-PspA antibody per mouse was sufficient for protection.
TABLE 3.
Passive immunization of naive mice with PspA immune serum from pJB100-immunized micea
| Antigen | Serum dilution | Alive/dead mouse ratio |
|---|---|---|
| pJB100 | 1:2 | 4:0 |
| 1:20 | 4:0 | |
| 1:200 | 1:3 | |
| PspA | 1:2 | 4:0 |
| 1:20 | 2:2 | |
| 1:200 | 0:4 | |
| LR solution | 0:4 |
Naive CBA/N mice received intraperitoneal injections of pooled immune serum from mice immunized with pJB100 or PspA. Mice were challenged i.v. with pneumococcal strain WU2 1 h after receiving the pooled serum. The PspA immune serum was diluted to a concentration equivalent to that of the pJB100 immune serum (8 μg of antigen-specific antibody/ml) prior to preparation of serial dilutions for passive immunization.
Long-term protection.
Providing protection over a significant portion of an immunized individual's life span is a major goal in vaccine development. Here we assess the ability of pJB100 to elicit protection over an extended period of time. CBA/N mice were immunized with 10 μg of pJB100 or control (pNGVL3) on the same immunization schedule as that used in the other experiments. Serum was collected on days 0, 14, 28, and 35 and at 11 weeks. Data for days 0 to 35 were used in Fig. 5 for the control group and the 10-μg group. Anti-PspA antibody levels not only persisted but actually increased between day 35 and week 11 in the immunized group (Fig. 5); the antibody concentrations rose from 2.850 ± 0.908 μg/ml (mean ± standard error of the mean) on day 35 to 7.72 ± 1.74 μg/ml at week 11 (the level of antibodies in the control group was <0.005 μg/ml). The mice were challenged with WU2 7 months after the initial immunization. All 10 mice receiving pJB100 survived the challenge, demonstrating the ability of pJB100 to elicit persistent antibody responses and long-term protection; only 1 of 9 mice in the control group survived. The difference in survival between the immunized and control mice was statistically significant (P ≤ 0.0001).
DISCUSSION
The pneumococcus is an important antibiotic-resistant pathogen. The mortality rate for invasive pneumococcal infections remains at 15 to 20% (3, 14). With increasing reports of multiply-resistant pneumococcal isolates (4, 19, 20), the mortality rate is likely to rise in the future. There is an urgent need for the development of cost-effective pneumococcal vaccines.
The survival of immunized mice after challenge established the effectiveness of pJB100. The overall survival rates for mice receiving pJB100 were greater than 99%. Protection was confirmed by a greater than 3-log increase in the LD50 for the immunized mice (data not shown). The results presented in this study demonstrate three major characteristics of the effectiveness of pJB100. First, pJB100 elicited cross-protective immunity similar to that seen with immunization with PspA. However, DNA vaccines offer the ability to express different PspA molecules from a single construct. This factor could potentially broaden the range of protection to cover all known clinical isolates. It also makes the standardization and production of a multivalent DNA vaccine much easier than those of a multivalent protein vaccine. A second demonstrated characteristic was the ability of pJB100 to protect against both systemic and respiratory challenges. This finding is significant because systemic and lower respiratory tract infections are the most devastating forms of pneumococcal disease. Last, the ability of pJB100 to provide long-term protection was shown. Long-lasting immunity is the ultimate goal in the development of any vaccine.
Since protection against pneumococcal infections is considered to be primarily antibody mediated, it was important to evaluate the humoral responses to immunization with pJB100. Immunization with pJB100 proved capable of eliciting a significant anti-PspA immune response. Dose experiments revealed a correlation between anti-PspA antibody concentration and protection. This correlation was not seen in earlier genetic immunization studies with full-length pspA. Therefore, it was important to establish the relationship between antibodies and protection in order to evaluate immune responses to pJB100. The passive immunization experiments illustrated the functionality of anti-PspA antibodies and their relationship to protection. The survival of mice receiving pJB100 immune serum demonstrated the direct correlation between anti-PspA antibodies and protection. In addition, the passive immunization experiments demonstrated that genetic immunization with pJB100 yields an antibody response that is qualitatively equivalent to that seen with immunization with protein. A comparison of IgG isotype profiles among genetically immunized, protein-immunized, and infected mice served as an indicator of relative Th function. The data confirm other reports that the Th-1 responses elicited by DNA vaccines are dependent on the quantities of DNA administered (21, 31, 37). Since protection is seen with both Th-1 and Th-2 IgG isotypes, no conclusions can be made about the importance of Th subtype in this situation. However, these observations are important for evaluating future experiments designed to modulate immune responses.
The development of a pneumococcal DNA vaccine not only offers the promise of a potential human vaccine but also offers insight into the efficacy of DNA vaccines for use against extracellular pathogens. Because of the ability of DNA vaccines to elicit cell-mediated immunity, much of the work done on DNA vaccines has focused on intracellular pathogens, with less attention being given to extracellular pathogens. Genetic immunization is thought to elicit immune responses that closely resemble those seen with natural infections with intracellular pathogens (37, 39). Despite the fact that antigen is expressed intracellularly, pJB100 elicited an effective humoral response. The mechanism by which this occurs has not been fully elucidated. Investigations into Th responses and costimulatory requirements could lead to the development of more effective vaccines. The key to examining these elements lies in the use of an established model system, such as the pneumococcal challenge model in mice. This model system allows the effective characterization of DNA vaccines against extracellular pathogens. Now that we have incorporated genetic immunization into the pneumococcal challenge model, we can effectively explore variables involved in immune responses to DNA vaccines.
ACKNOWLEDGMENTS
This study was supported by NIH grant AI43653.
We are grateful to Kristie Sidie for excellent technical assistance in collecting ELISA data.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Akbari O, Panjwani N, Garcia S, Tascon R, Lowrie D, Stockinger B. DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J Exp Med. 1999;189:169–177. doi: 10.1084/jem.189.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold K E, Leggiadro R J, Breiman R F, Lipman H B, Schwartz B, Appleton M A, Cleveland K O, Szeto H C, Hill B C, Tenover F C, Elliott J A, Facklam R R. Risk factors for carriage of drug-resistant Streptococcus pneumoniae among children in Memphis, Tennessee. J Pediatr. 1996;128:757–764. doi: 10.1016/s0022-3476(96)70326-8. [DOI] [PubMed] [Google Scholar]
- 4.Breiman R F, Butler J C, Tenover F C, Elliott J A, Facklam R R. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 5.Briles D E, Hollingshead S K, Swiatlo E, Brooks-Walter A, Szalai A, Virolainen A, McDaniel L S, Benton K A, White K, Prellner K, Hermansson A, Aerts P C, van Dijk H, Crain M J. PspA and PspC: their potential for use as pneumococcal vaccines. Microb Drug Resist. 1997;3:401–408. doi: 10.1089/mdr.1997.3.401. [DOI] [PubMed] [Google Scholar]
- 6.Briles D E, Horowitz J, McDaniel L S, Benjamin W H, Jr, Claflin J L, Booker C L, Scott G, Forman C. Genetic control of susceptibility to pneumococcal infection. Curr Top Microbiol Immunol. 1986;124:103–120. doi: 10.1007/978-3-642-70986-9_7. [DOI] [PubMed] [Google Scholar]
- 7.Briles D E, King J D, Gray M A, McDaniel L S, Swiatlo E, Benton K A. PspA, a protection-eliciting pneumococcal protein: immunogenicity of isolated native PspA in mice. Vaccine. 1996;14:858–867. doi: 10.1016/0264-410x(96)82948-3. [DOI] [PubMed] [Google Scholar]
- 8.Briles D E, Tart R C, Swiatlo E, Dillard J P, Smith P, Benton K A, Ralph B A, Brooks-Walter A, Crain M J, Hollingshead S K, McDaniel L S. Pneumococcal diversity: considerations for new vaccine strategies with an emphasis on pneumococcal surface protein A (PspA) Clin Microbiol Rev. 1998;11:645–657. doi: 10.1128/cmr.11.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruyn G A W, van Furth R. Pneumococcal polysaccharide vaccines: indications, efficacy and recommendations. Eur J Clin Microbiol Infect Dis. 1991;10:897–910. doi: 10.1007/BF02005442. [DOI] [PubMed] [Google Scholar]
- 10.Bruyn G A W, Zegers B J M, van Furth R. Mechanisms of host defence against infection with Streptococcus pneumoniae. Clin Infect Dis. 1992;14:251–262. doi: 10.1093/clinids/14.1.251. [DOI] [PubMed] [Google Scholar]
- 11.Chow Y-H, Chiang B-L, Lee Y-L, Chi W-K, Lin W-C, Chen Y-T, Tao M-H. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol. 1998;160:1320–1329. [PubMed] [Google Scholar]
- 12.Control C F D. Pneumococcal polysaccharide vaccines: recommendations of the Immunization Practices Advisory Committee. Morb Mortal Wkly Rep. 1989;38:64–68. [Google Scholar]
- 13.Cowan M J, Ammann A J, Wara D W, Howie V M, Schultz L, Doyle N, Kaplan M. Pneumococcal polysaccharide immunization in infants and children. Pediatrics. 1978;62:721–727. [PubMed] [Google Scholar]
- 14.Duchin J S, Breiman R F, Diamond A, Lipman H B, Block S L, Hedrick J A, Finger R, Elliott J A. High prevalence of multidrug-resistant Streptococcus pneumoniae among children in a rural Kentucky community. Pediatr Infect Dis J. 1995;14:745–750. doi: 10.1097/00006454-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Fedson D S. Pneumococcal vaccination in the United States and 20 other developed countries, 1981–1996. Clin Infect Dis. 1998;26:1117–1123. doi: 10.1086/520272. [DOI] [PubMed] [Google Scholar]
- 16.Gillespie S H. Aspects of pneumococcal infection including bacterial virulence, host response and vaccination. J Med Microbiol. 1989;28:237–248. doi: 10.1099/00222615-28-4-237. [DOI] [PubMed] [Google Scholar]
- 17.Gotschlich E C, Goldschneider I, Lepow M L, Gold R. The immune response to bacterial polysaccharides in man. In: Haber E, Krauss R, editors. Antibodies in human diagnosis and therapy. New York, N.Y: Raven Press; 1977. pp. 391–402. [Google Scholar]
- 18.Gray B M, Dillion H C, Briles D E. Epidemiological studies of Streptococcus pneumoniae in infants: development of antibody to phosphocholine. J Clin Microbiol. 1983;18:1102–1107. doi: 10.1128/jcm.18.5.1102-1107.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs M R. Treatment and diagnosis of infections caused by drug-resistant Streptococcus pneumoniae. Clin Infect Dis. 1992;15:119–127. doi: 10.1093/clinids/15.1.119. [DOI] [PubMed] [Google Scholar]
- 20.Klugman K P. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990;3:171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leclerc C, Deriaud E, Rojas M, Whalen R G. The preferential induction of a Th1 immune response by DNA-based immunization is mediated by the immunostimulatory effect of plasmid DNA. Cell Immunol. 1997;179:97–106. doi: 10.1006/cimm.1997.1161. [DOI] [PubMed] [Google Scholar]
- 22.MacLeod C M, Hodges R G, Heidelberger M, Bernhard W G. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1945;82:445–465. [PMC free article] [PubMed] [Google Scholar]
- 23.Manickan E, Rouse R J D, Yu Z, Wire W S, Rouse B T. Genetic immunization against herpes simplex virus: protection is mediated by CD4+ T lymphocytes. J Immunol. 1995;155:259–265. [PubMed] [Google Scholar]
- 24.McDaniel L S, Loechel F, Benedict C, Greenway T, Briles D E, Conry R M, Curiel D T. Immunization with a plasmid expressing pneumococcal surface protein A (PspA) can elicit protection against fatal infection with Streptococcus pneumoniae. Gene Ther. 1997;4:375–377. doi: 10.1038/sj.gt.3300401. [DOI] [PubMed] [Google Scholar]
- 25.McDaniel L S, McDaniel D O, Hollingshead S K, Briles D E. Comparison of the PspA sequence from Streptococcus pneumoniae EF5668 to the previously identified PspA sequence from strain Rx1 and ability of PspA from EF5668 to elicit protection against pneumococci of different capsular types. Infect Immun. 1998;66:4748–4754. doi: 10.1128/iai.66.10.4748-4754.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDaniel L S, Ralph B A, McDaniel D O, Briles D E. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb Pathog. 1994;17:323–337. doi: 10.1006/mpat.1994.1078. [DOI] [PubMed] [Google Scholar]
- 27.McDaniel L S, Sheffield J S, Delucchi P, Briles D E. PspA, a surface protein of Streptococcus pneumoniae, is capable of eliciting protection against pneumococci of more than one capsular type. Infect Immun. 1991;59:222–228. doi: 10.1128/iai.59.1.222-228.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDaniel L S, Yother J, Vijayakumar M, McGarry L, Guild W R, Briles D E. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA) J Exp Med. 1987;165:381–394. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosmann T R, Coffman R L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien K L, Steinhoff M C, Edwards K, Keyserling H, Thoms M L, Madore D. Immunologic priming of young children by pneumococcal glycoprotein conjugate, but not polysaccharide, vaccines. Pediatr Infect Dis J. 1996;15:425–430. doi: 10.1097/00006454-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Raz E, Tighe H, Sato Y, Corr M, Dudler J A, Roman M, Swain S L, Spiegelberg H L, Carson D A. Preferential induction of a Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization. Proc Natl Acad Sci USA. 1996;93:5141–5145. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rijkers G T, Sanders E A M, Breukels M A, Zegers B J M. Responsiveness of infants to capsular polysaccharides: implications for vaccine development. Rev Med Microbiol. 1996;7:3–12. [Google Scholar]
- 33.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen M-D, Silverman G J, Lotz M, Carson D A, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro E D, Berg A T, Austrian R, Schroeder D, Parcells V, Margolis A, Adair R K, Clemmens J D. Protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 35.Shelly M A, Jacoby H, Riley G J, Graves B T, Pichichero M, Treanor J J. Comparison of pneumococcal polysaccharide and CRM197-conjugated pneumococcal oligosaccharide vaccines in young and elderly adults. Infect Immun. 1997;65:242–247. doi: 10.1128/iai.65.1.242-247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Street N E, Mosmann T R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991;5:171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- 37.Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89–97. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 38.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 39.Vogel F R, Sarver N. Nucleic acid vaccines. Clin Microbiol Rev. 1995;8:406–410. doi: 10.1128/cmr.8.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wortham C, Grinberg L, Kaslow D C, Briles D E, McDaniel L S, Lees A, Flora M, Snapper C M, Mond J J. Enhanced protective antibody responses to PspA after intranasal or subcutaneous injections of PspA genetically fused to granulocyte-macrophage colony-stimulating factor or interleukin-2. Infect Immun. 1998;66:1513–1520. doi: 10.1128/iai.66.4.1513-1520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yi A-K, Chace J H, Cowdery J S, Krieg A M. INF-γ promotes IL-6 and IgM secretion in response to CpG motifs in bacterial DNA and oligodeoxynucleotides. J Immunol. 1996;156:558–564. [PubMed] [Google Scholar]
- 42.Yi A-K, Tuetken R, Redford T, Waldschmidt M, Kirsch J, Krieg A M. CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species. J Immunol. 1998;160:4755–4761. [PubMed] [Google Scholar]