Abstract
Backgrounds: Concomitant abdominal aortic aneurysms (AAA) and gastrointestinal malignancies are uncommon. Endovascular repair (EVAR) is widely used to treat AAA. However, no consensus exists on the optimal strategy for treating AAA when associated with pancreatic adenocarcinoma. In addition, only few reports of pancreaticoduodenectomy (PD) after EVAR exist. Presentation of case: A pancreatic tumor was detected during follow-up after EVAR for AAA in an 83-year-old female patient. The diagnosis was high-grade intraepithelial neoplasia. Modified pylorus-preserving pancreaticoduodenectomy was safely performed. The patient recovered moderately and was discharged two weeks after surgery. The pathological diagnosis was middle-grade pancreatic ductal adenocarcinoma. The patient survived for 24 months with no recurrence or cardiovascular complications. Conclusions: Conducting periodic follow-ups after AAA surgery is helpful for the early discovery of gastrointestinal tumors. EVAR surgery is safe and feasible and thus recommended for AAA patients with pancreatic cancer, although it may increase the risk of cancer. The stage of malignancy and post-EVAR medical history can be valuable in evaluating the benefits of pancreatic surgery for such cases.
Keywords: pancreatic adenocarcinoma, abdominal aortic aneurysm, endovascular repair, pancreaticoduodenectomy
1. Introduction
Pancreatic cancer is one of the most malignant gastrointestinal tumors with insidious onset and rapid progression. This cancer is characterized by high aggressiveness, metastasis, and recurrence rate. According to the latest data from the National Cancer Institute, the five-year survival rate of pancreatic cancer is only 11.5% [1]; only 20% of cases are resectable at the time of discovery [2].
Case reports of abdominal aortic aneurysms (AAAs) complicated with pancreatic cancer are rare in the medical literature. Previously, surgical resection was the recommended treatment for abdominal aorta. However, a simultaneous resection of AAA and tumors of the digestive system can enormously increase the trauma and risks of artificial vessel infection. In addition, staged resection of AAA and tumor lesions can result in adhesion in the area of the first operation, leading to postoperative complications that increase the difficulty of the second operation. This problem is very significant in the case of pancreatic lesions, considering the anatomy of the pancreas and how close it is to the aorta.
With the widespread use of endovascular repair (EVAR), perioperative mortality is significantly reduced, and postoperative recovery time is shortened accordingly [3,4]. In 2006, Sheen et al. reported the first case of an AAA patient treated with pancreaticoduodenectomy (PD) nine days after EVAR [5]. Based on CT scans, the patient had no anastomotic fistula or stent displacement one month after pancreatic surgery. However, a long-term follow-up was not performed and no information regarding further progression of the patient were available [5]. In 2013, Masahiko et al. [6] investigated a patient with AAA who underwent EVAR and reported a pancreatic mass two years after EVAR. Thus, a laparoscopic pancreaticoduodenectomy (LPD) was carried. The mass was pathologically diagnosed as intraductal papillary mucinous adenoma with small foci of carcinoma in situ. This was the first report on the combined treatment of EVAR and LPD. Although the patient developed grade B pancreatic fistula after surgery, it was cured after drug treatment and no tumor recurrence or cardiovascular complications were observed for 18 months. Here, we report a case of PD after endovascular repair for AAA.
Presentation of Case
An 83-year-old female with a medical history of hypertension, hyperlipemia, and diabetes mellitus was referred to our hospital due to a pancreatic lesion identified as a solid tumor of the pancreas head by contrast-enhanced computed tomography (Figure 1). Her height, weight, and body mass index (BMI) were 172 cm, 65.3 kg, and 22.1 kg/m2, respectively. She had undergone EVAR due to AAA at the age of 73 years old. After that, she had regular follow-up visits. The AAA was located on the infrarenal aorta (Figure 1c).
Figure 1.
CT images of (a) intra-aortic endovascular stent in transverse arterial phase; (b) a solid tumor of 31 mm diameter in the pancreas head with dilatation of the central pancreatic duct in transverse venous phase; (c) sagittal plane showing an infrarenal AAA and the endovascular stent in coronal arterial phase; (d) location of pancreatic cancer and endovascular repair of AAA in sagittal venous phase.
The CT scan showed a solid tumor of 31 mm diameter in the pancreas head, with dilatation of the main pancreatic duct (Figure 1b). Laboratory tests revealed elevated levels of CA199 (>1000 U/mL, normal <27 U/mL) and CA125 (>43.87 U/mL, normal <35 U/mL). Endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) indicated that a few highly dysplastic glands were seen in the inflammatory exudate without definite interstitial infiltration.
On 2 November 2020, modified PPPD was successfully performed under general anesthesia. During the operation, AAA was found to be located behind the horizontal part of the duodenum. The complex mass of the uncinate process of the pancreas was palpable (approximately 3 × 4 cm in size). The left and right hepatic arteries had normal blood flow and originated from the proper hepatic artery without vascular variation. Modified PPPD procedure was then performed along with regional lymph node dissection (0/12), including no. 8a, 8p, 12a, 12p, 12b, 12c, 13a, and 13b. An intraoperative histological analysis revealed a negative pancreatic transection margin (R0). The patient recovered uneventfully without complications and was discharged from the hospital 12 days after surgery. Postoperative pathological analysis reported moderately differentiated ductal adenocarcinoma of the pancreas (T2N0M0), 3.5 × 3.3 × 3.3 cm in size. No regional lymph node metastases or perineural infiltrations were observed. The patient survived for 24 months with no recurrence or cardiovascular complications.
2. Discussion
It is controversial to simultaneously treat concomitant AAA and malignancies in the adjacent region, especially pancreatic tumors. We systematically searched online literature databases (PubMed, Wanfang, and CNKI) for articles containing “gastrointestinal tumor”, “pancreatic cancer”, and “abdominal aortic aneurysm.” We identified a total of 20 cases of pancreatic lesions with AAA and summarized the patients’ age, gender, aneurysm size, treatment method, interval time, pathology, tumor stage, and prognosis (Table 1) [5,6,7,8,9,10,11,12,13,14,15,16]. Fifteen cases were males, one was female and four were undefined. Such ratio is consistent with previous reports identifying the gender as a risk factor for AAA. Mean age of patients was 67.8 y. Patients included 16 cases of malignant pancreatic tumors, three benign tumors and one with chronic pancreatitis. Additionally, an analysis of stage 4 pancreatic cancer patients (6 cases) showed that cases provided with two types of treatments had a better prognosis.
Table 1.
Summary of AAA with pancreatic diseases in the literature.
| First Author, Year | Age (Years) | Sex | Size of AAA (cm) | Approach | Interval | Biopsy | Stage | Prognosis (Months) |
|---|---|---|---|---|---|---|---|---|
| Szilagy, 1967 [7] | 65 | M | - | Cholecystojejunostomy | NA | pancreatic adenocarcinoma | 4 | 3 |
| 79 | M | - | None | NA | pancreatic adenocarcinoma | 4 | 3 | |
| Gibbs, 1973 [8] | 48 | F | 6 | One stage | NA | pancreatic cystadenoma | - | 15+ |
| Komori, 1993 [9] | - | - | - | Only resection of pancreatic tumor | NA | pancreatic adenocarcinoma | - | - |
| - | - | - | Only resection of the aneurysm | NA | pancreatic adenocarcinoma | - | - | |
| - | - | - | None | NA | pancreatic adenocarcinoma | - | - | |
| Deiparine, 1995 [10] | 62 | M | 9.0 | PD first, resection of the aneurysm | 2 weeks | Pancreatic islet cell tumor | - | 25+ |
| 71 | M | 6.0 | PD first, resection of the aneurysm | 2 months | chronic pancreatitis | - | 36+ | |
| Konno, 1998 [11] | 73 | M | 5.0 | AAA first, total PD | 2 weeks | NA | 4 | 14 |
| Palm, 2000 [12] | 73 | M | 5.9 | Only chemotherapy | NA | NA | 4 | 10 |
| Piecuch, 2003 [13] | 73 | - | 5.5 | One stage (Resection of aneurysm + PD) | NA | pancreatic adenocarcinoma | - | - |
| Ryan, 2005 [14] | 58 | M | 3.8 | Chemotherapy +PD | NA | pancreatic adenocarcinoma | 4 | 3 |
| Sheen, 2006 [5] | 60 | M | 6.0 | EVAR first, PD | 9 days | NA | 2 | - |
| Veraldi, 2008 [15] | 64 | M | 5.6 | One stage (EVAR + PD) | NA | pancreatic adenocarcinoma | 1 | 28 |
| 73 | M | 6.2 | EVAR first, PD | 3 days | pancreatic adenocarcinoma | 1 | 37 | |
| 71 | M | 5.5 | One stage (Resection of aneurysm + PD) | NA | pancreatic mucinous cystic adenoma | - | 10+ | |
| Kawguchi, 2013 [6] | 70 | M | 3.1 | LPD | NA | IPMN + small foci of carcinoma in situ | 1 | 18 |
| Tremont, 2021 [16] | 62 | M | 8.6 | Chemotherapy | NA | pancreatic adenocarcinoma | 4 | 0.3 |
| 66 | M | 3.5 | None | NA | benign cystadenoma | - | 134 | |
| 85 | M | 2.8 | Chemotherapy + surgery | NA | pancreatic adenocarcinoma | 4 | 45 |
Note: AAA: abdominal aortic aneurysm; Interval: Interval between AAA repair and cancer treatment; M: male; F: female; PD: pancreaticoduodenectomy; LPD: Laparoscopic pancreaticoduodenectomy; EVAR: endovascular repair.
It is widely recognized that risk profiles of AAA and pancreatic neoplasia are similar, which may influence the prevalence and workup of pancreatic tumor in patients with AAA [16]. Patients diagnosed with AAA need to be continuously monitored for aneurysms before and after surgical repair; thus, a computed tomography (CT) scan can help detect gastrointestinal tumor lesions at early stages [17].
As the number of middle-aged and elderly residents increases, the incidence of AAA and pancreatic cancer is consistently on the rise. Interestingly, risk factors for AAA and pancreatic cancer overlap, such as smoking history, prevalence in males, and advanced age [18,19]. Therefore, pancreatic cancer complicated with AAA is not uncommon. However, the surgical strategies for such cases are very challenging for both general and vascular surgeons due to the surgical sequence, interval and expectation [20,21,22]. Many reports on AAA complicated with the gastrointestinal already exist; however, reports of pancreatic cancer are limited [5,6,23]. Abdullah et al. [24] supported the necessity of simultaneous treatment of malignant tumors and AAA in life-threatening cases. EVAR (when possible) followed by resection of malignant lesions were adopted by the majority of centers.
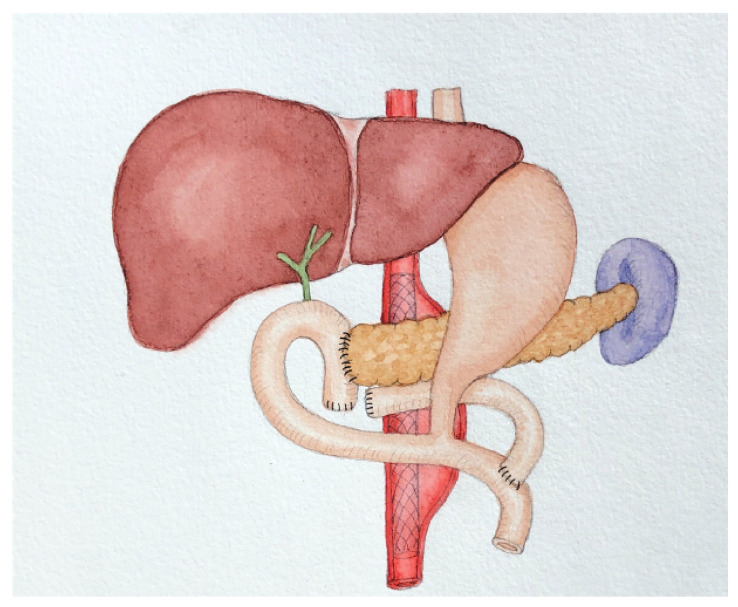
Previously, researchers believed that when pancreatic cancer and AAA co-occurred, PD should be performed first followed by AAA resection. Deiparine et al. [10] reported two cases of pancreatic lesions complicated with AAA (one with pancreatic cancer and one with chronic pancreatitis) achieving good results, suggesting that carrying pancreatic surgery first could provide definite pathological results. The evaluation of cell type and malignant tumor stage would help determining whether the aneurysm should be repaired. It is easier to repair AAA after PD through a retroperitoneal approach than a peritoneal one. The posterior peritoneal approach avoids the destruction associated with PD or Roux-en-Y reconstruction [10]. However, with the development of interventional technologies, EVAR has gradually become the preferable choice for AAA treatment. Compared to AAA resection, EVAR surgery significantly reduces perioperative mortality and shortens the time between the two operations. Because it is minimally invasive, it reduces the interference with other tumors’ treatment [3,4]. Subsequently, researchers have used EVAR treatment followed by pancreatic surgery [5,6]. Because the lesions of the abdominal aorta and pancreas are close to each other, operative field interferes significantly and the risk of concurrent operation is high. Deiparine et al. [10] advocated retroperitoneal incision: PD through the right surgical approach, and left abdominal aortic bypass. EVAR has not been associated with such concerns. However, concerns regarding the elevated mortality rate from EVAR have been raised, which may be attributed in part to an increased cancer burden at a later stage after exposure to external radiation from surgery or monitoring the stent with CT [25]. It may explain the phenomenon mentioned by Portelli et al., that incidental pancreatic tumors are common during the monitoring of aortic aneurysms [16]. Moreover, previous trials have shown a higher rate of local vascular or device-related complications and 30-day reintervention after EVAR compared to surgical resection [26,27,28]. Overall, we believe that EVAR remains the first option for AAA associated with pancreatic cancer, given its ability to reduce early mortality and shorten surgical intervals. For the severity of the postoperative complications of AAA, the open repair of AAA surgery needs artificial blood vessels. It is highly invasive to perform AAA resection and radical resection of digestive system tumors simultaneously due to the increased risk of artificial blood vessel infection [29]. If performed in stages, adhesions in the area of the first surgery may increase the difficulty in the second surgery and the possibility of postoperative complications. In particular, the pancreas is anatomically close to the aorta. Evaluating the likelihood of postoperative pancreatic fistula and early intervention may be beneficial. Regarding the choice of pancreatic surgery methods, Felix et al. [30] conducted a systematic review to compare LPD and PD and pointed out that LPD was safe and feasible, yet obtained apparent advantages compared to PD [30,31,32]. In the previous case report, Masahiko et al. [6] believed that residual abdominal aortic aneurysm had little interference with the operation. Laparoscopic surgery could magnify the surgical field, and so LPD was thought be to an indication for patients with AAA and benign pancreatic tumors [6]. However, due to the long history of AAA and the large aneurysm, we believe that the choice of open surgery is safer and more feasible. Out of consideration of AAA location, we adopted a modified pylorus-preserving pancreaticoduodenectomy (PPPD) (Figure 2), a surgical approach that preserves the horizontal part of the duodenum and the upper part of the jejunum yet reduces the impact of PD on AAA, while ensuring clean surgical margins. This has separated us from other medical institutions.
Figure 2.
Modified PPPD. PPPD: pylorus-preserving pancreaticoduodenectomy.
With the growing research interest in pancreatic cancer, more and more surgeons are realizing the importance of the meso-pancreas [33,34,35,36], which refers to the complete resection of perineural and lymphatic tissues, in addition to structures surrounding the pancreatic head/uncinate. This approach may decrease the recurrence rate and improve survival due to the complete clearance of the region [37]. However, we did not perform middle pancreatectomy and lymph node dissection in this area, because the location of the aortic aneurysm was adjacent to the middle pancreas, which increased the risk of surgery.
Previous literature has reported that the circumferential resection margin (CRM) consists of the ventral and dorsal pancreatic surfaces, as well as the medial pancreatic margin [38,39]. Adding CRM status to the standard pathological assessment can improve the accuracy of marginal excision [38] and correlate with median survival [37]. Unfortunately, such an approach is not adopted by the pathology department of our hospital.
The effects of chemotherapy on aneurysms should also be considered. Russwurm et al. [12] reported a case of AAA and hepatic metastasis in the pancreas. who received chemotherapy (gemcitabine) as a treatment. During the first four weeks, the patient was given prednisone (10 mg/d). When monitoring the size of AAA, an aneurysm expansion was observed, but when the chemotherapeutic agents were withdrawn, the expansion was stagnated. They hence speculated that the use of chemotherapeutic agents might accelerate the growth of aneurysm [12]. Recently, Maxwell et al. [40] examined the effects of malignant tumors and chemotherapy on AAA and found that aneurysms behaved similarly to those in patients without malignancy. In this case, the patient was older and had poor tolerance to chemotherapy, with a history of cardiovascular and cerebrovascular diseases. Since chemotherapy may increase the risk for cardiovascular, cerebrovascular embolization, and stent embolization, it was not adopted as treatment plan. Blood pressure was controlled before and after the surgery. Fortunately, no post-surgical complications occurred and the patient was discharged smoothly. Enhanced CT scans were performed every six months, and no evident tumor recurrence was found. The patient survived for 24 months.
3. Conclusions
Regular follow-ups after AAA surgery can help in the early detection of gastrointestinal tumors. Although EVAR may increase the burden of cancer at a later stage (due to exposure to external radiation from surgery or monitoring the stent with CT), it is recommended for patients with complicated AAA pancreatic cancer. It is also necessary to evaluate whether patients can benefit from pancreatic surgery after EVAR by considering the stage of malignant tumor and their medical history. For patients with advanced pancreatic cancer, most patients receiving two types of treatments usually have a better prognosis, and those receiving only one kind of treatment also have a better prognosis than those without treatment. However, some treatments, such as chemotherapy, may accelerate the growth of AAA and thus should be carefully evaluated.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by National Key Clinical Specialty Discipline Construction Program of China (Award Number: [2021-QTL-004]) to Zhiying Yang.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Versteijne E., Suker M., Groothuis K., Akkermans-Vogelaar J.M., Besselink M.G., Bonsing B.A., Buijsen J., Busch O.R., Creemers G.M., van Dam R.M., et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020;38:1763–1773. doi: 10.1200/JCO.19.02274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleeff J., Korc M., Apte M., La Vecchia C., Johnson C.D., Biankin A.V., Neale R.E., Tempero M., Tuveson D.A., Hruban R.H., et al. Pancreatic cancer. Nat. Rev. Dis. Primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 3.Greenhalgh R.M., Brown L.C., Powell J.T., Thompson S.G., Epstein D., Sculpher M.J. Endovascular versus open repair of abdominal aortic aneurysm. N. Engl. J. Med. 2010;362:1863–1871. doi: 10.1056/NEJMoa0909305. [DOI] [PubMed] [Google Scholar]
- 4.Kumar R., Dattani N., Asaad O., Bown M.J., Sayers R.D., Saratzis A. Meta-analysis of Outcomes Following Aneurysm Repair in Patients with Synchronous Intra-abdominal Malignancy. Eur. J. Vasc. Endovasc. Surg. 2016;52:747–756. doi: 10.1016/j.ejvs.2016.07.084. [DOI] [PubMed] [Google Scholar]
- 5.Sheen A.J., Baguneid M., Ellenbogen S., Walker M.G., Siriwardena A.K. Sequential endovascular repair and pancreaticoduodenectomy for abdominal aortic aneurysm copresenting with periampullary cancer. Ann. Vasc. Surg. 2006;20:114–116. doi: 10.1007/s10016-005-9406-8. [DOI] [PubMed] [Google Scholar]
- 6.Kawaguchi M., Ishikawa N., Shimada M., Nishida Y., Moriyama H., Ohtake H., Watanabe G. Laparoscopic pancreaticoduodenectomy after endovascular repair for abdominal aortic aneurysm. Int. J. Surg. Case. Rep. 2013;4:1117–1119. doi: 10.1016/j.ijscr.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szilagyi D.E., Elliott J.P., Berguer R. Coincidental malignancy and abdominal aortic aneurysm. Problems of management. Arch. Surg. 1967;95:402–412. doi: 10.1001/archsurg.1967.01330150078012. [DOI] [PubMed] [Google Scholar]
- 8.Gibbs B.F., Jr., James D.R., Ketron G.D., Milnes R.F. High abdominal aortic aneurysm, hepatic artery aneurysm, and cystadenoma of pancreas treated surgically in a young woman. Ann. Surg. 1973;178:186–189. doi: 10.1097/00000658-197308000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komori K., Okadome K., Itoh H., Funahashi S., Sugimachi K. Management of concomitant abdominal aortic aneurysm and gastrointestinal malignancy. Am. J. Surg. 1993;166:108–111. doi: 10.1016/S0002-9610(05)81039-6. [DOI] [PubMed] [Google Scholar]
- 10.Deiparine M.K., Prian G.W., Koep L.J. Abdominal aortic aneurysm repair after pancreaticoduodenectomy. J. Vasc. Surg. 1995;21:537–539. doi: 10.1016/S0741-5214(95)70299-7. [DOI] [PubMed] [Google Scholar]
- 11.Konno H., Kaneko H., Hachiya T., Maruo Y., Tanaka T., Suzuki S., Nakamura S., Baba S. Surgical management for a malignancy of the digestive organs accompanied with an abdominal aortic aneurysm. Surg. Today. 1998;28:988–991. doi: 10.1007/s005950050269. [DOI] [PubMed] [Google Scholar]
- 12.Palm S.J., Russwurm G.P., Chang D., Rozenblit A.M., Ohki T., Veith F.J. Acute enlargement and subsequent rupture of an abdominal aortic aneurysm in a patient receiving chemotherapy for pancreatic carcinoma. J. Vasc. Surg. 2000;32:197–200. doi: 10.1067/mva.2000.105665. [DOI] [PubMed] [Google Scholar]
- 13.Piecuch J., Arendt J., Sosada K., Mikusek W., Stepień T. Simultaneous operation on the tumor of the pancreas head and abdominal aortic aneurysm-case study. Wiad Lek. 2003;56:199–201. [PubMed] [Google Scholar]
- 14.Ryan D.P., Fernandez-del Castillo C., Willett C.G., Brugge W.R., Sahani D., Brachtel E.F. Case records of the Massachusetts General Hospital. Case 20-2005. A 58-year-old man with locally advanced pancreatic cancer. N. Engl. J. Med. 2005;352:2734–2741. doi: 10.1056/NEJMcpc059014. [DOI] [PubMed] [Google Scholar]
- 15.Veraldi G.F., Minicozzi A.M., Bernini M., Genco B., Tedeschi U. Treatment of abdominal aortic aneurysms associated with pancreatic tumors: Personal experience and review of the literature (1967–2006) Int. Angiol. 2008;27:539–542. [PubMed] [Google Scholar]
- 16.Portelli Tremont J.N., Jadi J., Pham V., Kim H.J., Maduekwe U.N. The prevalence of pancreatic incidentalomas in patients undergoing surveillance for abdominal aortic aneurysms. Am. J. Surg. 2021;222:892–896. doi: 10.1016/j.amjsurg.2021.07.049. [DOI] [PubMed] [Google Scholar]
- 17.Kouvelos G.N., Patelis N., Antoniou G.A., Lazaris A., Bali C., Matsagkas M. Management of concomitant abdominal aortic aneurysm and colorectal cancer. J. Vasc. Surg. 2016;63:1384–1393. doi: 10.1016/j.jvs.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Forsdahl S.H., Singh K., Solberg S., Jacobsen B.K. Risk factors for abdominal aortic aneurysms: A 7-year prospective study: The Tromsø Study, 1994–2001. Circulation. 2009;119:2202–2208. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- 19.Olson S.H., Kurtz R.C. Epidemiology of pancreatic cancer and the role of family history. J. Surg. Oncol. 2013;107:1–7. doi: 10.1002/jso.23149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Treska V., Molacek J., Certik B., Houdek K., Hosek P., Soukupova V., Stogerova C., Svejdova A. Management of Concomitant Abdominal Aortic Aneurysm and Intra-abdominal, Retroperitoneal Malignancy. In Vivo. 2021;35:517–523. doi: 10.21873/invivo.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang B., Wu K., Liu Y., Lai H., Zeng Z. Synchronous Gastrointestinal Tumor and Abdominal Aortic Aneurysm or Dissection Treated with Endovascular Aneurysm Repair Followed by Tumor Resection. Gastroenterol. Res. Pract. 2019;2019:8087256. doi: 10.1155/2019/8087256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohandas S., Malik H.T., Syed I. Concomitant abdominal aortic aneurysm and gastrointestinal malignancy: Evolution of treatment paradigm in the endovascular era—review article. Int. J. Surg. 2013;11:112–115. doi: 10.1016/j.ijsu.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Winter J.M., Cameron J.L., Lillemoe K.D., Campbell K.A., Chang D., Riall T.S., Coleman J., Sauter P.K., Canto M., Hruban R.H., et al. Periampullary and pancreatic incidentaloma: A single institution’s experience with an increasingly common diagnosis. Ann. Surg. 2006;243:673–680; discussion 680–673. doi: 10.1097/01.sla.0000216763.27673.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jibawi A., Ahmed I., El-Sakka K., Yusuf S.W. Management of concomitant cancer and abdominal aortic aneurysm. Cardiol. Res. Pract. 2011;2011:516146. doi: 10.4061/2011/516146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markar S.R., Vidal-Diez A., Sounderajah V., Mackenzie H., Hanna G.B., Thompson M., Holt P., Lagergren J., Karthikesalingam A. A population-based cohort study examining the risk of abdominal cancer after endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 2019;69:1776–1785. doi: 10.1016/j.jvs.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 26.Greenhalgh R.M., Brown L.C., Kwong G.P., Powell J.T., Thompson S.G. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: Randomised controlled trial. Lancet. 2004;364:843–848. doi: 10.1016/S0140-6736(04)16979-1. [DOI] [PubMed] [Google Scholar]
- 27.Prinssen M., Verhoeven E.L., Buth J., Cuypers P.W., van Sambeek M.R., Balm R., Buskens E., Grobbee D.E., Blankensteijn J.D. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N. Engl. J. Med. 2004;351:1607–1618. doi: 10.1056/NEJMoa042002. [DOI] [PubMed] [Google Scholar]
- 28.Becquemin J.P. The ACE trial: A randomized comparison of open versus endovascular repair in good risk patients with abdominal aortic aneurysm. J. Vasc. Surg. 2009;50:222–224; discussion 224. doi: 10.1016/j.jvs.2009.04.074. [DOI] [PubMed] [Google Scholar]
- 29.Porcellini M., Nastro P., Bracale U., Brearley S., Giordano P. Endovascular versus open surgical repair of abdominal aortic aneurysm with concomitant malignancy. J. Vasc. Surg. 2007;46:16–23. doi: 10.1016/j.jvs.2006.09.070. [DOI] [PubMed] [Google Scholar]
- 30.Nickel F., Haney C.M., Kowalewski K.F., Probst P., Limen E.F., Kalkum E., Diener M.K., Strobel O., Müller-Stich B.P., Hackert T. Laparoscopic Versus Open Pancreaticoduodenectomy: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann. Surg. 2020;271:54–66. doi: 10.1097/SLA.0000000000003309. [DOI] [PubMed] [Google Scholar]
- 31.Qin R., Kendrick M.L., Wolfgang C.L., Edil B.H., Palanivelu C., Parks R.W., Yang Y., He J., Zhang T., Mou Y., et al. International expert consensus on laparoscopic pancreaticoduodenectomy. Hepatobiliary Surg. Nutr. 2020;9:464–483. doi: 10.21037/hbsn-20-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang M., Peng B., Liu J., Yin X., Tan Z., Liu R., Hong D., Zhao W., Wu H., Chen R., et al. Practice Patterns and Perioperative Outcomes of Laparoscopic Pancreaticoduodenectomy in China: A Retrospective Multicenter Analysis of 1029 Patients. Ann. Surg. 2021;273:145–153. doi: 10.1097/SLA.0000000000003190. [DOI] [PubMed] [Google Scholar]
- 33.Fernandes E.S.M., Strobel O., Girão C., Moraes-Junior J.M.A., Torres O.J.M. What do surgeons need to know about the mesopancreas. Langenbecks Arch. Surg. 2021;406:2621–2632. doi: 10.1007/s00423-021-02211-y. [DOI] [PubMed] [Google Scholar]
- 34.Gockel I., Domeyer M., Wolloscheck T., Konerding M.A., Junginger T. Resection of the mesopancreas (RMP): A new surgical classification of a known anatomical space. World J. Surg. Oncol. 2007;5:44. doi: 10.1186/1477-7819-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J., Tian X., Chen Y., Ma Y., Liu C., Tian L., Wang J., Dong J., Cui D., Wang Y., et al. Total mesopancreas excision for the treatment of pancreatic head cancer. J. Cancer. 2017;8:3575–3584. doi: 10.7150/jca.21341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalisvaart M., Broadhurst D., Marcon F., Pande R., Schlegel A., Sutcliffe R., Marudanayagam R., Mirza D., Chatzizacharias N., Abradelo M., et al. Recurrence patterns of pancreatic cancer after pancreatoduodenectomy: Systematic review and a single-centre retrospective study. HPB. 2020;22:1240–1249. doi: 10.1016/j.hpb.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Safi S.A., Haeberle L., Fluegen G., Lehwald-Tywuschik N., Krieg A., Keitel V., Luedde T., Esposito I., Rehders A., Knoefel W.T. Mesopancreatic excision for pancreatic ductal adenocarcinoma improves local disease control and survival. Pancreatology. 2021;21:787–795. doi: 10.1016/j.pan.2021.02.024. [DOI] [PubMed] [Google Scholar]
- 38.Esposito I., Kleeff J., Bergmann F., Reiser C., Herpel E., Friess H., Schirmacher P., Büchler M.W. Most pancreatic cancer resections are R1 resections. Ann. Surg. Oncol. 2008;15:1651–1660. doi: 10.1245/s10434-008-9839-8. [DOI] [PubMed] [Google Scholar]
- 39.Häberle L., Esposito I. Circumferential resection margin (CRM) in pancreatic cancer. Surg. Pract. Sci. 2020;1:100006. doi: 10.1016/j.sipas.2020.100006. [DOI] [Google Scholar]
- 40.Maxwell D.W., Kenney L., Sarmiento J.M., Rajani R.R. Aortic Aneurysm Natural Progression is Not Influenced by Concomitant Malignancy and Chemotherapy. Ann. Vasc. Surg. 2021;71:29–39. doi: 10.1016/j.avsg.2020.08.137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this article.