Abstract
The Plasmodium vivax merozoite surface protein 1 (MSP-1) 42-kDa fragment (PvMSP-1 p42) is a promising vaccine candidate antigen against the blood stage of the malarial parasite. We have developed a process for the production of this vaccine target, keeping in mind its use in human volunteers. A novel strain, Origami(DE3), of Escherichia coli with mutations in the glutathione and thioredoxin reductase genes yielded 60% more soluble PvMSP-1 p42 than the conventional E. coli BL21(DE3) strain. Recombinant PvMSP-1 p42 was purified to ≥99% purity with a rapid two-step protocol designed for easy scaling up. The final product had a low endotoxin content and was stable in its lyophilized form. PvMSP-1 p42 was found to have the predicted primary and tertiary structures and consisted of a single conformer containing one free cysteine, as predicted. The product was recognized by conformational monoclonal antibodies against P. vivax MSP-1. Immunogenicity studies of PvMSP-1 p42 were carried out with two strains of mice and the adjuvants Montanide ISA51 and Montanide ISA720. Both formulations were found to induce high levels of immunoglobulin G1 (IgG1), IgG2b, and IgG2a antibodies along with low levels of IgG3. Lymphocytes from animals in all the PvMSP-1 p42-immunized groups showed proliferative responses upon stimulation with PvMSP-1 p42; the cytokines interleukin 2 (IL-2), gamma interferon, IL-4, and IL-10 were detected in the culture supernatants. These results indicate that PvMSP-1 p42 in combination with both of the adjuvants elicited cellular and humoral responses in mice.
Plasmodium vivax is one of the two major human malaria parasites and alone is responsible for 40 to 50% of all malaria cases in Latin America and southeastern Asia. The emergence of drug-resistant P. vivax strains (1) has emphasized the need for a vaccine. Progress toward a vaccine to prevent P. vivax infection is severely constrained by the availability of recombinant P. vivax antigens suitable for efficacy trials in humans. The choice of an expression system for the production of any recombinant protein is critical, particularly if the protein contains conformational epitopes stabilized by multiple disulfide bonds. Conventionally, Escherichia coli is considered unsuitable for the expression of such structured antigens because of its reducing cytoplasmic environment (18). For that reason, many P. vivax antigens containing complex tertiary-domain structures have been expressed in eukaryotic systems, such as yeast (14, 15, 16) and baculovirus (9, 22). Efforts have been under way to develop an E. coli strain with an oxidative internal environment. One such modified E. coli strain (Origami) was recently reported to allow disulfide bond formation of recombinant proteins expressed in its cytoplasm (2). Using this strain of E. coli, we report the production of a soluble P. vivax merozoite surface protein 1 (MSP-1) 42-kDa fragment (PvMSP-1 p42), a malaria vaccine candidate that requires the formation of multiple disulfide bonds for correct folding.
MSP-1 is found on the surface of merozoites throughout the genus Plasmodium. For P. falciparum it has been shown that MSP-1 is synthesized as a 195-kDa precursor that is processed by several proteolytic steps during schizont rupture and merozoite invasion. The 195-kDa protein is cleaved to an 83-kDa fragment (p83) and a 42-kDa fragment (p42); the latter is further cleaved to an 11-kDa C-terminal fragment (p19) and a 33-kDa fragment (p33) (reviewed in reference 7). The p19 region contains conserved cysteines that are cross-linked by multiple disulfide linkages forming two epidermal growth factor-like domains (5). It has been shown in rodent models of malaria that the presence of the two epidermal growth factor-like domains in the p19 region is critical for the induction of MSP-1-based protective immunity (19, 20). In addition, it has been shown that immunization with recombinant P. vivax MSP-1 p19 made in baculovirus-infected insect cells can protect monkeys against parasite challenge (6, 30). Although the p33 region has not been shown to be critical for protection, several immunodominant B- and T-cell epitopes have been mapped to it; these epitopes are highly immunogenic during natural malaria infection in humans (10). A baculovirus-expressed P. cynomolgi MSP-1 p42 construct protected rhesus monkeys against homologous challenge (24). Given the close evolutionary relationship between the two species, we have chosen to express the P. vivax equivalent of this P. cynomolgi p42 construct in E. coli.
Initial attempts to express PvMSP-1 p42 in a conventional E. coli expression host, such as BL21, resulted in the majority of the product being insoluble; however, we found that a “redox-modified” E. coli strain (Origami) expressed the same protein almost completely in the soluble fraction. We describe here the expression conditions and purification methodology used to obtain a PvMSP-1 p42 product of high purity and low endotoxin content. In addition, we examine the humoral and cellular immune responses of mice to this vaccine candidate protein using two adjuvants approved for human use, Montanide ISA51 (M51) and Montanide ISA720 (M720).
MATERIALS AND METHODS
Cloning of the PvMSP-1 p42 gene.
Genomic DNA of the P. vivax Sal I strain (kindly provided by William E. Collins, Centers for Disease Control and Prevention, Atlanta, Ga.) was prepared using a Qiaamp blood kit (Qiagen, Valencia, Calif.). Genomic DNA was used as a template for the amplification of the PvMSP-1 p42 gene with the following set of PCR primers: forward, 5′CGTGAATTCATGGACCAAGTAACAACGGGAGAG3′; and reverse, 5′ACGTCTGCAGATTAAACGTCCATGCACAGGA3′). The PCR product was cloned into a sequencing plasmid, sequenced, and used as a template for the amplification of the expression construct with the following set of primers: forward, 5′CATGCCATGGCAGACCAAGAACAACGGGA3′; and reverse, 5′AATAGTTTAGCGGCCGCTTAGCTACAGAAAAC3′). The PCR product was ligated to the NcoI-NotI sites of vector pETAT(NK2) (kindly provided by Evelina Angov, Walter Reed Army Institute of Research, Silver Spring, Md.).
The ligation mixture was transformed into DH5α cells, and recombinant clones were selected on ampicillin. The cloned insert was sequenced and transformed into BL21(DE3) and Origami(DE3) E. coli expression hosts (Novagen, Madison, Wis.). The transformants were selected on ampicillin plates, and expression was checked by use of isopropyl-β-d-galactopyranoside (IPTG)-induced cultures. Glycerol stocks of a clone expressing PvMSP-1 p42 were made and stored at −70°C. The deduced amino acid sequence of the protein obtained from this clone has 18 vector-encoded residues on the N terminus (MAHHHHHHPGGSGSGTMA) linked to amino acids 1350 (Asp) to 1729 (Ser) of native PvMSP-1.
Expression of PvMSP-1 p42.
Expression from both host strains was carried out with a 10-liter bioreactor (New Brunswick Scientific, New Brunswick, N.J.). Terrific Broth containing ampicillin at 100 μg ml−1 or tetracycline at 12.5 μg ml−1 was inoculated with 100 ml of overnight-grown seed culture; the temperature was maintained at 37°C, the pH was 7.2, and agitation was done at 800 rpm. The optical density (OD) at 600 nm (OD600) was monitored, and the temperature was rapidly (<20 min) reduced to 25oC at an OD600 of 7. IPTG was added to a final concentration of 0.1 mM. Induction was carried at 25oC for 2 h, and cells were harvested by centrifugation. Cell paste was weighed and routinely stored at −70oC.
Purification of PvMSP-1 p42.
All buffers used during purification were endotoxin free and were kept chilled while the purification was carried out at room temperature. The E. coli cell paste was thawed (1:8, wt/vol) in chilled resuspension buffer (20 mM sodium phosphate [NaP], 500 mM NaCl [pH 7.4]). Bacteria were lysed by microfluidization (model 1109 apparatus; Microfluidic Corp., Newton, Mass.), and the soluble fraction was obtained after centrifugation at 15,000 × g for 40 min at 4oC. The supernatant was further cleared by filtration through a 0.45-μm-pore-size filter and was passed through a Ni-nitrilotriacetic acid (NTA) Superflow column (Qiagen) (7 ml of matrix per 40 g of paste) in a 600-E liquid chromatography system (Waters, Milford, Mass.) at a flow rate of 2.5 ml min−1. The column was washed with 40 mM imidazole in resuspension buffer until the OD280 of the eluate was stabilized. The column was equilibrated with 20 mM NaP buffer (pH 8.0), and PvMSP-1 p42 was eluted with 20 mM NaP buffer containing 500 mM imidazole (pH 8.0) (elution buffer). Fractions containing the protein peak were pooled and diluted fivefold in elution buffer minus imidazole. This sample was loaded on a Q-Sepharose FASTflow column (Amersham Pharmacia Biotech, Piscataway, N.J.) (4 ml of matrix per 40 g of paste). The column was washed with 20 mM NaP buffer (pH 8.0) and then with the same buffer containing 100 mM NaCl until the OD280 was stabilized. Pure PvMSP-1 p42 was eluted with 200 mM NaCl (pH 8.0) in 20 mM NaP buffer. Samples were dialyzed overnight against phosphate-buffered saline (PBS) at 4oC, and the amount of protein was estimated with a Micro BCA protein assay reagent kit (Pierce, Rockford, Ill.). The protein concentration was adjusted to 350 μg ml−1, and the protein was stored in 150-μl aliquots (∼50 μg per vial) at −70°C.
Purified PvMSP-1 p42 was evaluated for homogeneity by densitometric analysis by reducing sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (4 to 12% NuPAGE; Invitrogen, Carlsbad, Calif.) with up to 20 μg of protein loaded in a single well and stained with Coomassie blue. The absence of host E. coli-specific proteins was confirmed by Western blotting with anti-E. coli polyclonal antibodies (Dako Corp., Carpinteria, Calif.).
Lyophilization and stability.
Frozen aliquots of PvMSP-1 p42 were lyophilized for 24 h (Flex-Dry MP; FTS Systems, Stone Ridge, N.Y.). The stability of the lyophilized material was checked by incubating freeze-dried aliquots at −70, −3, 4, 25, and 37°C for up to 4 weeks. Samples at 24, 48, and 72 h and at 1 and 4 weeks were analyzed by nonreducing SDS-PAGE and staining with Coomassie blue.
N-terminal sequencing, MS, and disulfide analysis.
Purified PvMSP-1 p42 was resolved by SDS-PAGE, transferred to a polyvinylidene difluoride (PVDF) membrane, and sequenced using the Edman degradation method. Protein samples were analyzed by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (MS) (Voyager Biospectrometry RP system; Applied Biosystems) using Sinapinic acid matrix. PvMSP-1 p42 was reduced in the presence of 10 mM dithiothreitol (Bio-Rad, Richmond, Calif.) and 6 M guanidine HCl (Fisher Scientific, Fair Lawn, N.J.) at 50°C for 60 min. Alkylation was carried out in the presence of 6 M guanidine HCl and 100 mM iodoacetamide (Sigma, St Louis, Mo.) for 1 h at 37oC in the dark. Guanidine HCl was removed from samples by ethanol precipitation before analysis on Coomassie blue-stained nonreducing SDS-polyacrylamide gels for comparative mobility and on immunoblots for monoclonal antibody reactivity. Monoclonal antibodies raised against p42 and p19 of P. vivax MSP-1 were kindly provided by Shirley Longacre (Pasteur Institute, Paris, France). Free sulfhydryl groups were estimated by use of Ellman's reagent (5,5′-dithio-bis-3 nitrobenzoic acid) (11). l-Cysteine was used to plot the standard curve.
Immunoblotting and IFA.
Immunoblotting was carried out using standard protocols, and immunoblots were developed with a SuperSignal chemiluminescence kit (Pierce) or BM Blue POD substrate (Roche, Indianapolis, Ind.). Rabbit anti-PvMSP-1 p42 antibodies were affinity purified using PvMSP-1 p42-coupled tosyl-activated magnetic beads (Dynal, Oslo, Norway). The indirect immunofluorescence assay (IFA) was done by standard protocols with a methanol-fixed blood smear of P. vivax Sal I strain-infected Aotus monkey blood and fluorescein-conjugated antibodies.
Endotoxin assay.
Endotoxin levels were measured with a Limulus amebocyte lysate kit (Pyrochrome; Cape Cod Inc., Falmouth, Mass.) using the end-point chromogenic method.
Mouse immunization.
BALB/c (H-2d) and C57BL/6 (H-2b) female mice, 6 to 8 weeks old, were immunized with formulations containing PvMSP-1 p42 along with either M51 or M720 (Seppic Inc., Paris, France) as an adjuvant. Each formulation included 25 μg of protein and either 50% M51 or 70% (by volume) M720 adjuvant. Mice were immunized subcutaneously with 100 μl of the formulation three times, with a 2-week interval between immunizations. Mice were euthanatized 14 days after the last immunization; serum samples and spleens were collected. Control groups were immunized with the same amount of adjuvant in saline.
ELISA.
Antibody responses against PvMSP-1 p42 were evaluated by an enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well microplates (Dynax, Chantilly, Va.) were coated with 100 ng of either reduced and alkylated or nonreduced PvMSP-1 p42 per well, kept overnight at 4°C, and then blocked for 1 h with PBS containing 0.05% Tween 20 and 5% casein (Sigma). Plates were washed three times and incubated for 2 h at room temperature with individual and pooled mouse sera. Plates were washed again with PBS containing 0.05% Tween 20; 1:4,000-diluted secondary anti-mouse immunoglobulin G (IgG), IgG1, IgG2a, IgG2b, or IgG3 antibodies labeled with horseradish peroxidase (Southern Biotechnologies Associates, Birmingham, Ala.) were added; and plates were incubated for 1 h. Plates were washed and developed with 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate (6)] (ABTS)–peroxidase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) and examined at 405 nm. For determination of each IgG subclass, individual sera were tested in duplicate using fourfold serial dilutions starting at 1:100.
Lymphoproliferative cellular responses.
Spleens were surgically removed from euthanatized mice, and a cell suspension was obtained by organ grinding in Hanks balanced salt solution (Invitrogen). Leukocytes (pooled in each group) were resuspended at 5 × 106 cells ml−1 in Iscove's modified Dulbecco's medium (BioWhittaker, Walkersville, Md.) supplemented with 0.5% normal mouse serum, 2 mM l-glutamine, 55 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 100 U of penicillin-streptomycin (Invitrogen) ml−1. Aliquots (100 μl) of the cell preparation were added to wells of a round-bottom 96-well plate. Cells were grown in the absence or presence of 0.1, 0.2, 0.5, 1.0, and 2.5 μM PvMSP-1 p42 or a control protein, E. coli recombinant P. falciparum thrombospondin-related adhesive protein (PfTRAP; unpublished data), at 0.5 μM. Positive control cultures were included on each plate and were stimulated with 2 μg of concanavalin A ml−1 after 48 h. Cultures at a final volume of 200 μl per well were grown for 5 days at 37°C under a humidified atmosphere with 5% CO2. Splenocytes were pulse-labeled during the final 16 h with 1 μCi of tritiated thymidine (Amersham Pharmacia Biotech) per well and were harvested onto glass-fiber filters for liquid scintillation counting (counts per minute). Stimulation indexes were calculated as the counts per minute for the test antigen divided by the counts per minute for the control.
Cytokine production.
Pooled cells were cultured at 37°C under a humidified atmosphere with 5% CO2 in the presence of 0.5 μM PvMSP-1 p42 or PfTRAP antigen in 24-well plates at 2.5 × 106 cells per well. Supernatants were collected after 48, 72, and 96 h and screened for the presence of interleukin 2 (IL-2), IL-4, IL-10, and gamma interferon (IFN-γ) using the respective Quantikine M sandwich EIA kit (R&D Systems Inc., Minneapolis, Minn.). Control and sample values were deduced from the standard curve.
Statistical analysis.
Data were processed using Microsoft Excel 2000 software. Linear regression analysis was used to calculate the serum dilution needed to give an OD equal to the mean plus three standard deviations (SD) of the negative controls. An analysis of variance was used to determine the level of significance of the differences observed between groups.
RESULTS
The Origami(DE3) strain of E. coli enhances the expression of soluble PvMSP-1 p42.
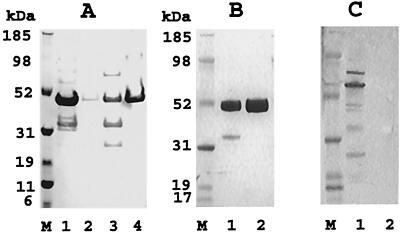
The PvMSP-1 p42 gene encoding 380 amino acids (1350 to 1729) of the published sequence (12) was cloned in vector pETAT(NK2). The insert was sequenced on both DNA strands, and no amino acid differences were found compared to the published sequence. Two E. coli host strains, BL21(DE3) and Origami(DE3), were tested for the production of soluble PvMSP-1 p42. Both host strains were transformed with the same recombinant plasmid and grown and induced under identical fermentation conditions, and PvMSP-1 p42 expressed in the soluble and insoluble fractions was partially purified under identical conditions (Fig. 1A). Recombinant PvMSP-1 p42 produced in E. coli had an apparent molecular mass of ∼50 kDa under reducing conditions. Densitometric analysis of the ∼50-kDa band from both purifications showed that total PvMSP-1 p42 production was 15% better in Origami(DE3) (Fig. 1, compare lanes 1 and 2 with lanes 3 and 4). In addition, Origami(DE3) cells contained 60% more protein in the soluble fraction than BL21(DE3) cells (Fig. 1, compare lanes 1 and 3). The soluble/insoluble PvMSP-1 p42 ratio for Origami(DE3) was 8:1, whereas it was 0.4:1 for BL21(DE3). Therefore, the Origami(DE3) strain was chosen for process development of PvMSP-1 p42 fermentation and purification.
FIG. 1.
(A) Coomassie blue-stained reducing SDS-polyacrylamide gel of PvMSP-1 p42 partially purified on a 0.5-ml Ni-NTA-agarose column under identical conditions from the soluble and insoluble fractions of strains Origami(DE3) and BL21(DE3) (1 g of paste for each). PvMSP-1 p42 was eluted from each of the four columns in equal volumes (2 ml), and 20 μl of each sample was loaded into the wells. Lanes: 1, soluble Origami(DE3); 2, insoluble Origami(DE3); 3, soluble BL21(DE3); 4 insoluble BL21(DE3); M, molecular mass markers. (B) Coomassie blue-stained reducing SDS-polyacrylamide gel (20 μg of protein per well). Lanes: 1, Ni-NTA elution; 2, Q-Sepharose elution. (C) Anti-E. coli immunoblot of the gel shown in panel B (2 μg of protein per well).
The production and purification protocol is rapid and scalable.
The fermentation conditions described above were found optimal for soluble protein production. On average, 150 g of wet cell mass was harvested from a 10-liter fermentation culture. Purification was initiated by lysis and separation of the soluble fraction by centrifugation. The soluble fraction was loaded on a Ni2+ column for initial purification. The column was washed with resuspension buffer containing 40 mM imidazole, and PvMSP-1 p42 protein eluted from the Ni2+ column was >80% pure (Fig. 1B, lane 1). Fractions containing the protein were pooled and diluted fivefold to reduce the imidazole concentration before being loaded onto a Q-Sepharose anion exchanger. Impurities either flowed through the Q-Sepharose column or were removed in the 100 mM NaCl wash. Purified PvMSP-1 p42 was eluted in 200 mM NaCl (pH 8.0) at a final yield of 80 to 100 mg of PvMSP-1 p42 per 10-liter fermentation. Densitometric analysis of the final products from independent purification experiments showed >99% pure full-length product on a Coomassie blue-stained reducing SDS-polyacrylamide gel (Fig. 1B, lane 2). The host E. coli protein content in 1,000 μg of pure PvMSP-1 p42 preparation ml−1 was routinely below 1 μg ml−1 (minimum detection limit), as measured by immunoblotting (Fig. 1C, lane 2). Purity evaluation with high-pressure liquid chromatography gel filtration and reversed-phase columns detected a single symmetrical peak in the final PvMSP-1 p42 preparation (data not shown).
Purified recombinant PvMSP-1 p42 has a low endotoxin content.
The final product was analyzed by the Limulus amebocyte lysate assay for the presence of endotoxins. The final preparation of PvMSP-1 p42 contained between 30 and 50 endotoxin units per 50 μg of protein (estimated single human dose).
Purified recombinant PvMSP-1 p42 is stable.
The stability of PvMSP-1 p42 was estimated by incubating lyophilized protein under different temperature conditions and analyzing the protein by SDS-PAGE over a 4-week period. PvMSP-1 p42 in lyophilized form was found to be stable at 37, 25, 4, −30, and −70°C, with no signs of breakdown or aggregation (data not shown). Dimers and multimers were observed upon storage in PBS solutions at 4°C for more than 1 week.
Recombinant PvMSP-1 p42 has the correct primary and tertiary structures.
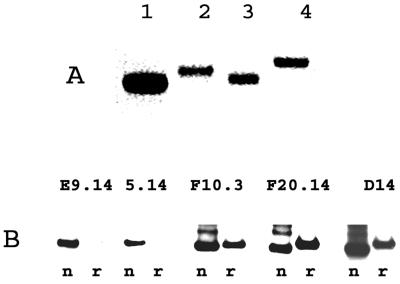
N-terminal sequencing of the final product using Edman degradation revealed the first 23 amino acids to be Ala His His His His His His Pro Gly Gly Ser Gly Ser Gly Thr Met Ala Asp Gln Val Thr Thr Gly (the first 17 amino acids are encoded by the vector; the 6 PvMSP-1 p42-specific residues are shown in bold). MALDI-TOF MS showed a peak at 45,031 Da. The theoretical molecular weight of full-length PvMSP-1 p42 is 45,035. Coomassie blue staining with nonreducing SDS-PAGE of freshly purified PvMSP-1p42 revealed a tight homogenous band (Fig. 2A, lane 1), indicating that it is largely composed of a single conformer. Due to the presence of the odd number of 11 cysteines in PvMSP-1 p42, it is most likely that at least one cysteine is not involved in disulfide bond formation. This idea was further evidenced by a slight decrease in the mobility of the alkylated protein (Fig. 2A, lane 2). Alkylation of the native protein resulted in a mass increment of 58 atomic mass units, as measured by MALDI-TOF MS; this value corresponds to the addition of a single alkyl-amide group to the protein. Reduction of PvMSP-1 p42 with dithiothreitol (Fig. 2A, lane 3) and its reduction and alkylation (Fig. 2A, lane 4) caused decreased mobility on SDS-PAGE, indicating that other alkylation sites were accessible only upon breakage of the disulfide bonds. Ellman's test for free sulfhydryl groups performed on pure PvMSP-1 p42 revealed 1.07 μmol of free—SH per μmol of PvMSP-1 p42.
FIG. 2.
(A) Coomassie blue-stained nonreducing SDS-polyacrylamide gel of purified PvMSP-1 p42 (∼2 μg of protein per well). Lanes: 1, nonreduced; 2, alkylated; 3, reduced; 4, reduced and alkylated. (B) Immunoblot with anti-PvMSP-1 monoclonal antibodies (shown above the lanes). Lanes: n, nonreduced protein; r, reduced and alkylated protein.
The presence of reduction-sensitive epitopes was confirmed by reactivity with previously characterized monoclonal antibodies raised against PvMSP-1 p42 and PvMSP-1 p19 expressed by baculovirus. Monoclonal antibodies E9.14 and F10.3 recognize conformational disulfide bond-dependent epitopes in the p19 region. Monoclonal antibody 5.14 also recognizes a putatively conformational epitope on p42. E. coli-produced soluble PvMSP-1 p42 was recognized by all three of these monoclonal antibodies on nonreduced Western blots (Fig. 2B). The reactivity with monoclonal antibodies 5.14 and E9.14 was decreased after reduction and alkylation (Fig. 2B, compare lanes n and r). Monoclonal antibody F10.3 showed decreased reactivity with reduced PvMSP-1 p42. An P. falciparum MSP-1-specific monoclonal antibody was used as a negative control and showed no reactivity with PvMSP-1 p42 (data not shown). Reactivity was also seen with monoclonal antibodies F20.14 and D14, both of which recognize linear epitopes in p42, and with polyclonal sera from a rabbit immunized with p19 (data not shown).
PvMSP-1 p42 induces specific antibody responses in immunized mice.
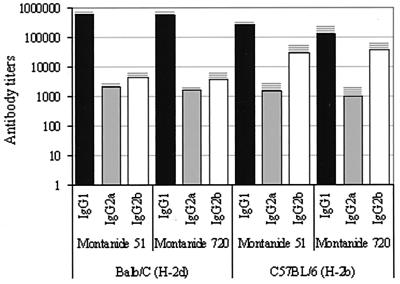
Vaccination with PvMSP-1 p42 elicited strong antibody and T-cell responses and was well tolerated in mice, with no apparent signs of lesion formation. Individual sera from four animals in each group were tested for anti-PvMSP-1 p42 IgG, IgG1, IgG2a, IgG2b, and IgG3 by an ELISA. The mean OD plus 3 SD for the controls (using both strains and all anti-IgG subclasses at a 1:100 dilution) was 0.080 (mean = 0.030, SD = 0.016). An OD cutoff of 0.1 was selected for antibody titer determinations. The dilution that gave an OD of 0.1 was determined using regression analysis of the linear portion of the curve for each serum. Mean end-point titers for each immunized group are shown in Fig. 3. All four PvMSP-1 p42-immunized groups showed high IgG titers, with the IgG1 titer being the highest (above 2 × 105), followed by the IgG2b, IgG2a, and IgG3 titers (data not shown), in that order. For each mouse strain, both adjuvants gave similar antibody responses, with some differences in the levels of IgG1 and IgG2b between the two strains. Regardless of the adjuvant used, BALB/c mice produced about 3.5 times more IgG1 antibodies than C57BL/6 mice. Conversely, C57BL/6 mice produced about six times more IgG2b antibodies. These differences were statistically significant (F > 16.7, P < 0.001). Total IgG titers were also determined using reduced and alkylated PvMSP-1 p42 as a coating antigen. ODs were 30 to 80% lower for all groups with reduced and alkylated protein.
FIG. 3.
IgG subclass antibody responses in mice immunized with PvMSP-1 p42. Antibody titers are shown as the logarithmic values of the mean dilution plus SD (bars and horizontal lines) that gave an OD of 0.1.
PvMSP-1 p42 induces T-cell responses in immunized mice.
Table 1 summarizes the cellular responses found for PvMSP-1 p42-immunized groups. Like the antibody responses, the T-cell stimulation indexes were similar in immunized groups of the same strain, regardless of the adjuvant used (F < 1.4, P > 0.26). The stimulation indexes for the BALB/c group were, however, higher than those for the C57BL/6 group (F = 94.5, P < 0.0001), regardless of the adjuvant used. Cytokine levels in culture supernatants from splenocytes after 48 and 72 h of stimulation with PvMSP-1 p42 were measured. In general, BALB/c mice showed higher cytokine levels, except for IFN-γ production in C57BL/6 mice. Nanogram levels of IFN-γ were observed in all groups, with higher levels being seen in the M720-immunized group (Table 1). Cells stimulated with the control protein (PfTRAP) showed undetectable levels of IL-2, IL-4, and IFN-γ and 135 ± 31 pg of IL-10 ml−1.
TABLE 1.
Lymphoproliferative and cytokine responses in mice immunized with PvMSP-1 p42
| Mice | Adjuvant | Stimulation indexa | Concn (pg/ml of supernatant)b of:
|
|||
|---|---|---|---|---|---|---|
| IL-2 | IL-4 | IL-10 | IFN-γ | |||
| H-2d | M 51 | 5.9 ± 0.6 | 211 ± 11 | 59 ± 8 | 607 ± 51 | 2,826 ± 1,034 |
| H-2d | M 720 | 6.2 ± 0.5 | 137 ± 22 | 46 ± 2 | 832 ± 31 | 3,895 ± 914 |
| H-2b | M 51 | 3.3 ± 0.2 | 37 ± 4 | 29 ± 0 | 658 ± 10 | 1,104 ± 180 |
| H-2b | M 720 | 3.8 ± 0.4 | 43 ± 8 | 17 ± 8 | 761 ± 44 | 5,135 ± 1,016 |
Data are reported as the mean and SD for cells stimulated with 0.2, 0.5, 1.0, and 2.5 μM PvMSP-1 p42. Background average counts for cells stimulated with the control antigen, PfTRAP, were 4,365 ± 1,504 cpm for BALB/c mice (H-2d) and 4,903 ± 1,875 cpm for C57BL/6 mice (H-2b). Similar scintillation counts were observed for cells from adjuvant control groups stimulated with PvMSP-1 p42 (data not shown). Stimulation with concanavalin A gave counts above 60,000 cpm.
Peaks of cytokine production detected in cell culture supernatants after 48 and 72 h of in vitro stimulation with 0.5 μM PvMSP-1 p42. Data are reported as the mean and SD.
Recombinant PvMSP-1 p42 resembles the native parasite protein.
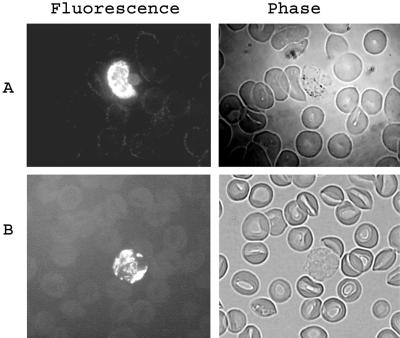
Affinity-purified rabbit anti-PvMSP-1 p42 antibodies tested positive in an IFA against blood stages of the P. vivax Sal I strain. Figure 4A shows an early schizont with bright fluorescence. Polyclonal antibodies in all PvMSP-1 p42-immunized mice also tested positive in the IFA. Figure 4B shows a late trophozoite stained by pooled serum from BALB/c mice immunized with PvMSP-1 p42 and M51. No recognition was found using control sera.
FIG. 4.
(A) Fluorescence and phase images of a methanol-fixed P. vivax Sal I parasite (early schizont) on a Aotus monkey blood smear immunostained with affinity-purified PvMSP-1 p42 antibodies raised in rabbits. (B) Fluorescence and phase images of a late trophozoite of P. vivax Sal I immunostained with pooled anti-PvMSP-1 p42 sera from the BALB/c mice in the M51 group.
DISCUSSION
MSP-1 is one of the most important vaccine candidates from the blood stage of the malarial parasite. There is evidence of the protective role of MSP-1 in rodent and simian models of malaria. Advanced evaluation of such a promising antigen in human volunteers requires process development for the production of pharmaceutical-grade recombinant MSP-1. Here we present a process that can be used for the large-scale production of recombinant PvMSP-1 p42 in E. coli. Although vaccination with the p19 region of MSP-1 has been shown to protect animals, we have chosen to express the entire p42 domain, as the p33 portion contains some important B- and T-cell determinants (10). Moreover, we believe that the p33 portion provides a hydrophilic scaffolding for the correct folding of p19, just as glutathione S-transferase–p19 fusion constructs have been previously shown to fold correctly in E. coli (3).
Prokaryotic expression systems such as E. coli produce large quantities of protein with a relatively simple fermentation protocol. This system shares an important feature with Plasmodium in that it lacks N glycosylation (8). However, one of the major drawbacks of E. coli is the reducing nature of its cytoplasm, which inhibits the formation of disulfide bridges and which may result in incorrect folding of complex proteins or the formation of protein aggregates. MSP-1 contains a cysteine-rich (p19) domain with five or six disulfide bonds (depending on the species). The presence of correctly formed disulfide bonds in the p19 region has been shown to be critical for the induction of a protective immune response against the parasite in animal models (19, 20). Although PvMSP-1 p42 could be expressed in the conventional E. coli host BL21(DE3), a large portion of the product was insoluble. Attempts to improve solubility by various fermentation conditions were unsuccessful. We then focused on expressing the gene in redox-modified hosts, such as AD494 (Novagen), a thioredoxin reductase (trxB) mutant host. The protein solubility, however, showed no substantial improvement. Recently, E. coli host strain Origami, a thioredoxin and glutathione reductase (gor) gene mutant (2), was shown to promote disulfide bond formation within the cell cytoplasm. Using this host strain, we achieved significant enhancement in the yield of soluble PvMSP-1 p42. Furthermore, a combination of low IPTG concentration and low-temperature induction was found to favor the expression of soluble PvMSP-1 p42, probably because of the reduction in the rate of protein synthesis (18). The above strategy might be useful in improving the yield of other vaccine antigens expressed in E. coli.
The E. coli expression vector used here, pETAT(NK2), is a derivative of vector pET32 and has been especially engineered for the production of vaccine candidate antigens in E. coli. The plasmid was constructed to have a tetr gene for selection during fermentation, because ampicillin is not a preferred antibiotic for use in the manufacturing of products for human use. This property raised an important issue when the switch to Origami cells was made, as this strain is tetracycline resistant. Using a series of fermentation experiments and colony counts, we confirmed that there was no significant plasmid loss or decline in protein yield when tetracycline was used during fermentation rather than ampicillin.
The purification scheme described here is rapid, and the whole process from cell lysis to elution of the final product can be carried out within 2 days. Purification can be carried out at room temperature and is designed for easy scaling up. The two-step purification comprises stepwise increments in eluent concentrations during wash and elution instead of continuous gradients; this was done to make the process robust and to facilitate reproducibility. The process gives greater than 99% pure PvMSP-1 p42 with an endotoxin content within permissible levels for an injectable pharmaceutical.
We used multiple techniques to determine the purity of the product. Reducing SDS-PAGE analysis with overloaded protein (up to 20 μg per well) and immunoblotting with an anti- E. coli antibody confirmed the high level of purity of the product. In addition, the protein was also found to be homogenous by high-pressure liquid chromatography analysis on reversed-phase and gel filtration columns, with no signs of aggregation (data not shown). PvMSP-1 p42 was found to be stable at room temperature in its lyophilized form. The primary structure was confirmed by N-terminal sequencing and MS analysis. The N-terminal methionine could not be identified during sequencing; however, the 23 subsequent amino acids, including six PvMSP-1-specific residues, were confirmed by N-terminal sequencing. The molecular mass of PvMSP-1 p42 was found to be within 4 atomic mass units of the predicted mass. We confirmed the presence of a predicted free cysteine in the final product. The protein was also recognized by monoclonal antibodies against conformational and linear epitopes on baculovirus-expressed PvMSP-1 (22).
The immunogenicity of PvMSP-1 p42 in mice was examined with two metabolizable oil-based adjuvants: M51 and M720. The two adjuvants differ in surfactant content, with M720 forming thinner emulsions. Both adjuvants are generally considered safe for human use and have been applied to malaria vaccine trials with monkeys and humans (17, 25, 26). Vaccination with both adjuvants induced IgG1, IgG2a, and IgG2b antibodies along with the production of cytokines IL-4, IL-10, IL-2, and IFN-γ. Cytokines IL-2 and IFN-γ are associated with the production of IgG2a and are indicators of T-helper 1 (Th1) cell activation and of predominantly cell-mediated responses; in contrast, cytokines IL-4 and IL-10, secreted by T-helper 2 (Th2) cells, are associated with the production of IgG1 and indicate antibody-mediated responses (13). The results indicated that both Th1 and Th2 subsets of T-helper cells are elicited by PvMSP-1 vaccination. The activation of both subsets of T-helper cells, sometimes with one response dominating the other, has been shown to correlate with protection against blood-stage challenge in murine models (4, 23). An ideal blood-stage vaccine candidate would be one that can activate both Th1 and Th2 responses (21, 29).
Immunization of two strains of mice with the two adjuvants resulted in comparable B- and T-cell responses, with M720 leading to higher levels of IFN-γ in both strains of mice. We plan to go forward with M720 in a future study of immune responses in rhesus monkeys. The difference in cytokine responses and IgG profiles observed between the two strains indicates some genetic restriction of the immune response against PvMSP-1 p42, as has been seen for several other Plasmodium antigens (27).
The reactivity of sera from all the groups was 30 to 80% lower with reduced PvMSP-1 p42 than with the native protein. A similar observation was made with human immune sera against P. vivax, where titers were, on average, 50% lower against reduced p19 (28). This result indicates a large contribution of conformational epitopes to the overall antibody response against PvMSP1 p42. The generation of such conformational anti-MSP-1 antibodies is critical to raising a protective response against the parasite (19, 20). Rabbit and mouse antibodies raised against PvMSP-1 p42 reacted with native MSP-1 on the parasite in an IFA, further establishing the nearly native structure of PvMSP-1 p42. Recombinant PvMSP-1 p42 also reacted positively in Western blotting and ELISA analyses with sera collected from an area in which P. vivax is endemic (data not shown).
The process development efforts described here are a critical part of the development of a subunit vaccine and address some of the issues facing protein chemists involved in the production of protein-based pharmaceuticals. The availability of a process to reproducibly make clinical-grade PvMSP-1 p42 will help in establishing the efficacy of this antigen as a human malaria vaccine. The same, well-characterized protein can serve as a valuable reagent in immunological or functional studies.
ACKNOWLEDGMENTS
We are pleased to acknowledge Shirley Longacre at Pasteur Institute for the monoclonal antibodies; Greg E. Garcia and Deborah R. Moorad at WRAIR for N-terminal sequencing; Bader Fileta and members of the Department of Clinical Investigation at WRAMC for mass spectroscopy; Svetlana Kitov at WRAIR for endotoxin assays; W. E. Collins at CDC and Patrick E. Duffy at WRAIR for the P. vivax parasites; and David Miles at WRAIR for photography.
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). This work was performed while S.D. held an NRC Research Associate award at WRAIR.
REFERENCES
- 1.Barat L M, Bloland P B. Drug resistance among malaria and other parasites. Infect Dis Clin North Am. 1997;11:969–987. doi: 10.1016/s0891-5520(05)70400-1. [DOI] [PubMed] [Google Scholar]
- 2.Bessette P H, Aslund F, Beckwith J, Georgiou G. Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc Natl Acad Sci USA. 1999;96:13703–13708. doi: 10.1073/pnas.96.24.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burghaus P A, Holder A A. Expression of the 19-kilodalton carboxy-terminal fragment of the Plasmodium falciparum merozoite surface protein-1 in Escherichia coli as a correctly folded protein. Mol Biochem Parasitol. 1994;64:165–169. doi: 10.1016/0166-6851(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 4.Burns J M, Dunn P D, Russo D M. Protective immunity against Plasmodium yoelii malaria induced by immunization with particulate blood-stage antigens. Infect Immun. 1997;65:3138–3145. doi: 10.1128/iai.65.8.3138-3145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chitarra V, Holm I, Bentley G A, Petres S, Longacre S. The crystal structure of C-terminal merozoite surface protein 1 at 1.8 A resolution, a highly protective malaria vaccine candidate. Mol Cell. 1999;3:457–464. doi: 10.1016/s1097-2765(00)80473-6. [DOI] [PubMed] [Google Scholar]
- 6.Collins W E, Kaslow D C, Sullivan J S, Morris C L, Galland G, Yang C, Saekhou A M, Xiao L, Lal A A. Testing the efficacy of a recombinant merozoite surface protein (MSP-119) of Plasmodium vivax in Saimiri boliviensis monkeys. Am J Trop Med Hyg. 1999;60:350–356. doi: 10.4269/ajtmh.1999.60.350. [DOI] [PubMed] [Google Scholar]
- 7.Cooper J A. Merozoite surface antigen-1 of Plasmodium. Parasitol Today. 1993;9:50–54. doi: 10.1016/0169-4758(93)90031-a. [DOI] [PubMed] [Google Scholar]
- 8.Dieckmann-Schuppert A, Bender S, Odenthal-Schnittler M, Bause E, Schwarz R T. Apparent lack of N-glycosylation in the asexual intraerythrocytic stage of Plasmodium falciparum. Eur J Biochem. 1992;205:815–825. doi: 10.1111/j.1432-1033.1992.tb16846.x. [DOI] [PubMed] [Google Scholar]
- 9.Dutta S, Daugherty J R, Ware L A, Lanar D E, Ockenhouse C F. Expression, purification and characterization of a functional region of the Plasmodium vivax Duffy binding protein. Mol Biochem Parasitol. 2000;109:179–184. doi: 10.1016/s0166-6851(00)00244-9. [DOI] [PubMed] [Google Scholar]
- 10.Egan A, Waterfall M, Pinder M, Holder A, Riley E. Characterization of human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1: evidence for poor T-cell recognition of polypeptides with numerous disulfide bonds. Infect Immun. 1997;65:3024–3031. doi: 10.1128/iai.65.8.3024-3031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellman G L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 12.Gibson H L, Tucker J E, Kaslow D C, Krettli A U, Collins W E, Kiefer M C, Bathurst I C, Barr P J. Structure and expression of the gene for Pv200, a major blood-stage surface antigen of Plasmodium vivax. Mol Biochem Parasitol. 1992;50:325–333. doi: 10.1016/0166-6851(92)90230-h. [DOI] [PubMed] [Google Scholar]
- 13.Golding B, Zaitseva M, Golding H. The potential for recruiting immune responses toward type 1 or type 2 T cell help. Am J Trop Med Hyg. 1994;50(Suppl.):33–40. doi: 10.4269/ajtmh.1994.50.33. [DOI] [PubMed] [Google Scholar]
- 14.Hisaeda H, Stowers A W, Tsuboi T, Collins W E, Sattabongkot J S, Suwanabun N, Torii M, Kaslow D C. Antibodies to malaria vaccine candidates pvs25 and pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect Immun. 2000;68:6618–6623. doi: 10.1128/iai.68.12.6618-6623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaslow D C, Kumar S. Expression and immunogenicity of the C terminus of a major blood-stage surface protein of Plasmodium vivax, Pv200(19), secreted from Saccharomyces cerevisiae. Immunol Lett. 1996;51:187–189. doi: 10.1016/0165-2478(96)02570-9. [DOI] [PubMed] [Google Scholar]
- 16.Kocken C H, Dubbeld M A, Van Der Wal A M, Pronk J T, Waters A P, Langermans J A, Thomas A W. High-level expression of Plasmodium vivax apical membrane antigen 1 (AMA-1) in Pichia pastoris: strong immunogenicity in Macaca mulatta immunized with P. vivax AMA-1 and adjuvant SBAS2. Infect Immun. 1999;67:43–49. doi: 10.1128/iai.67.1.43-49.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawrence G, Cheng Q Q, Reed C, Taylor D, Stowers A, Cloonan N, Rzepczyk C, Smillie A, Anderson K, Pombo D, Allworth A, Eisen D, Anders R, Saul A. Effect of vaccination with 3 recombinant asexual-stage malaria antigens on initial growth rates of Plasmodium falciparum in non-immune volunteers. Vaccine. 2000;18:1925–1931. doi: 10.1016/s0264-410x(99)00444-2. [DOI] [PubMed] [Google Scholar]
- 18.Lilie H, Schwarz E, Rudolf R. Advances in refolding of proteins produced in E. coli. Curr Opin Biotechnol. 1998;9:497–501. doi: 10.1016/s0958-1669(98)80035-9. [DOI] [PubMed] [Google Scholar]
- 19.Ling I T, Ogun S A, Holder A A. Immunization against malaria with a recombinant protein. Parasite Immunol. 1994;16:63–67. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 20.Ling I T, Ogun S A, Holder A A. The combined epidermal growth factor-like modules of Plasmodium yoelii merozoite surface protein-1 are required for a protective immune response to the parasite. Parasite Immunol. 1995;17:425–433. doi: 10.1111/j.1365-3024.1995.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 21.Long C A, Daly T M, Kima P, Srivastava I. Immunity to erythrocytic stages of malarial parasites. Am J Trop Med Hyg. 1994;50(Suppl.):27–32. doi: 10.4269/ajtmh.1994.50.27. [DOI] [PubMed] [Google Scholar]
- 22.Longacre S, Mendis K N, David P H. Plasmodium vivax merozoite surface protein 1 C-terminal recombinant proteins in baculovirus. Mol Biochem Parasitol. 1994;64:191–205. doi: 10.1016/0166-6851(94)00002-6. [DOI] [PubMed] [Google Scholar]
- 23.Patterson P S, Bosshardt S C, Udhayukumar V, Xiao L, Kidd M, Hunter R L, Lal A A. Prolonged expression of IFN-gamma induced by protective blood-stage immunization against Plasmodium yoelii malaria. Vaccine. 1999;18:173–180. doi: 10.1016/s0264-410x(99)00217-0. [DOI] [PubMed] [Google Scholar]
- 24.Perera K L, Handunnetti S M, Holm I, Longacre S, Mendis K. Baculovirus merozoite surface protein 1 C-terminal recombinant antigens are highly protective in a natural primate model for human Plasmodium vivax malaria. Infect Immun. 1998;66:1500–1506. doi: 10.1128/iai.66.4.1500-1506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perlaza B L, Arevalo-Herrera M, Brahimi K, Quintero G, Palomino J C, Gras-Masse H, Tartar A, Druilhe P, Herrera S. Immunogenicity of four Plasmodium falciparum preerythrocytic antigens in Aotus lemurinus monkeys. Infect Immun. 1998;66:3423–3428. doi: 10.1128/iai.66.7.3423-3428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saul A, Lawrence G, Smillie A, Rzepczyk C M, Reed C, Taylor D, Anderson K, Stowers A, Kemp R, Allworth A, Anders R F, Brown G V, Pye D, Schoofs P, Irving D O, Dyer S L, Woodrow G C, Briggs W R, Reber R, Sturchler D. Human phase I vaccine trials of 3 recombinant asexual stage malaria antigens with Montanide ISA720 adjuvant. Vaccine. 1999;17:3145–3159. doi: 10.1016/s0264-410x(99)00175-9. [DOI] [PubMed] [Google Scholar]
- 27.Sjolander A, Andersson R, Hansson M, Berzins K, Perlmann P. Genetic restriction and specificity of the immune response in mice to fusion proteins containing repeated sequences of the Plasmodium falciparum antigen Pf155/RESA. Immunology. 1995;84:360–366. [PMC free article] [PubMed] [Google Scholar]
- 28.Soares I S, Levitus G, Souza J M, Del Portillo H A, Rodrigues M M. Acquired immune responses to the N- and C-terminal regions of Plasmodium vivax merozoite surface protein 1 in individuals exposed to malaria. Infect Immun. 1997;65:1606–1614. doi: 10.1128/iai.65.5.1606-1614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor-Robinson A W, Phillips R S, Severn A, Moncada S, Liew F Y. The role of TH1 and TH2 cells in a rodent malaria infection. Science. 1993;260:1931–1934. doi: 10.1126/science.8100366. [DOI] [PubMed] [Google Scholar]
- 30.Yang C, Collins W E, Sullivan J S, Kaslow D C, Xiao L, Lal A A. Partial protection against Plasmodium vivax blood-stage infection in Saimiri monkeys by immunization with a recombinant C-terminal fragment of merozoite surface protein 1 in block copolymer adjuvant. Infect Immun. 1999;67:342–349. doi: 10.1128/iai.67.1.342-349.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]