Abstract
The purpose of this review is to provide verified data on the current knowledge acquired in preclinical and clinical studies regarding topically used herbal products and their active constituents (formulations and dressings) with diabetic wound healing activity. Moreover, herbal products and their active constituents used for diabetic wound infections, and various cellular and molecular mechanisms of their actions will also be described. The electronic databases were searched for articles published from 2012 to 2022. Publications with oral or systemic administration of herbal products in diabetic wound healing, published before 2012, available only as an abstract, or in languages other than English were excluded from the study. The 59 articles comparing topically used herbal products in diabetic wound healing treatment versus control treatments (placebo or active therapy) were selected. Herbal products through different mechanisms of action, including antimicrobial, anti-inflammatory, antioxidant activity, stimulation of angiogenesis, production of cytokines and growth factors, keratinocytes, and fibroblast migration and proliferation may be considered as an important support during conventional therapy or even as a substitute for synthetic drugs used for diabetic wound treatment.
Keywords: herbal products, diabetic wounds, foot ulcer wounds, bacterial diabetic wound infections, diabetic wound dressing
1. Introduction
Diabetes is a metabolic disorder associated with the endocrine system that resulted in hyperglycemic conditions. Prolonged and untreated hyperglycemia or uncontrolled glucose levels leads to serious diabetic complications, such as nephropathy, neuropathy, retinopathy, hypertension, hyperlipidemia, increased risk of cardiovascular disease, and heart attacks [1]. The most costly and devastating complication of diabetes is delayed wound healing processes which can lead to serious complications such as a high risk of bacterial infections, gangrene, limb amputations, sepsis, and even death [2]. About 15% of diabetic patients have diabetic foot ulcers (DFU), and 14–24% of these patients subsequently experience a lower extremity amputation, with the mortality rate from amputation approaching 50–59% five years post-amputation [3]. Therefore, acceleration of wound healing should be a priority in preventing diabetes complications.
Wound healing difficulties in diabetes patients are multidirectional. The hyperglycemic condition in the wound site leads to chronic inflammation, impaired vascularization and tissue regeneration, reduced production of growth factors, excessive protease activity, and oxidative stress [4]. Moreover, open wounds are particularly prone to infection, especially by bacteria, and provide an entry point for systemic infections. Aerobic or facultative pathogens such as Staphylococcus aureus, Pseudomonas aeruginosa, and β-hemolytic streptococci are the primary causes of delayed healing and infection in both acute and chronic wounds [5,6]. Therefore, an important part of the standard treatment of diabetic wounds, in addition to wound debridement, revascularization, and acceleration of the healing process, is the control of bacterial infections. Topical antimicrobial agents (silver nitrate, povidone-iodine) or systemic administration of antibiotics (silver sulfadiazine, mafenide, mupirocin, bacitracin) do not always help to reduce the risk of progressive infection, especially if the bacteria are antibiotic resistant [7]. The reduction in wound healing time is crucial for diabetes especially to lower the chance of infection and decrease complications and costs. Herbal products and their active compounds may inhibit bacterial growth and may have a significant clinical value in the treatment of resistant microbial strains [8]. Moreover, some herbal products affect wound healing activities through anti-inflammatory and antioxidant activities, cell proliferation, and angiogenesis [9,10]. The purpose of this review is to provide verified data on the current knowledge acquired in preclinical and clinical studies regarding topically used herbal products (formulations and dressings) with diabetic wound healing activity. Moreover, herbal products and their active constituents used for microbial diabetic wound infections, and the various cellular and molecular mechanisms of their actions will also be described.
2. Methods
2.1. Search Strategy
This review was conducted and narrated in accordance with the Preferred Reported Items for Systematic Review and Meta-analysis (PRISMA) guidelines [11]. The PubMed, Scopus, and Google Scholar databases were searched for articles published from 2012 to 2022. Search terms included “herbal products and diabetic wound healing”, “herbal products and diabetic foot ulcers”, “plant extracts used in diabetic wounds”, “essential oils used in diabetic wounds”, and “herbal constituent used in diabetic wounds”. The references from reviews about herbal products and topical treatment of diabetic wounds were searched for additional articles and case reports. A manual search was also conducted based on citations in the published literature.
2.2. Inclusion and Exclusion Criteria
The results of animal- and human-based studies on topically used herbal products in diabetic wound healing comparing herbal products treatment versus control treatments (placebo or active therapy) were selected. Routes of administration other than topical (e.g., oral, systemic) of herbal products in diabetic wound healing were excluded from the study. Moreover, publications published before 2012, available only as an abstract, or in languages other than English were excluded.
2.3. Study Selection
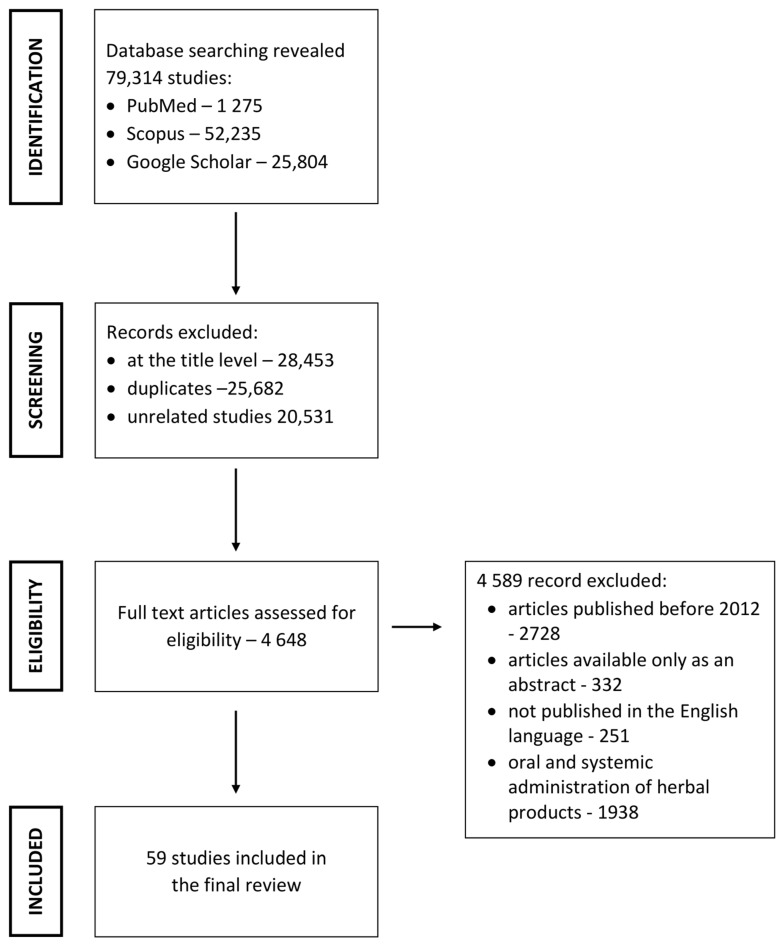
Overall, 79,314 articles were found in the databases. Of these, 74,666 articles were excluded at the title level; among them were also duplicates and unrelated articles. Furthermore, 4589 articles were excluded as not meeting the inclusion criteria. Finally, 59 articles were used for the review (Figure 1).
Figure 1.
Search strategy used to identify relevant articles.
3. Herbal Products and Their Active Constituents Used for Diabetic Wound Healing
3.1. Animal-Based Studies
Animal-based studies are important in the research on the use of herbal products and their active constituents for diabetic wound healing (Table 1, Table 2 and Table 3). The most described animal studies are based on diabetes induced by streptozotocin (STZ) and alloxan monohydrate. Alloxan monohydrate and STZ are the most popular diabetogenic agents used for assessing the anti-diabetic or hypoglycemic capacity of test compounds. These compounds are cytotoxic glucose analogs that preferentially accumulate in pancreatic β cells via the GLUT2 glucose transporter [12]. STZ is a glucosamine–nitrosourea compound, a cytostatic antibiotic produced by Streptomyces achromogenes, used clinically as a chemotherapeutic agent in the treatment of pancreatic β-cell carcinoma. STZ damages pancreatic β cells, resulting in hypoinsulinemia and hyperglycemia [13]. Moreover, STZ induces type 1 and 2 diabetes in rodents [14]. Alloxan monohydrate, a urea derivative, selectively inhibits glucose-induced insulin secretion through its ability to inhibit the β cell glucose sensor glucokinase [12]. Inhibition of glucokinase reduces glucose oxidation and ATP generation, thereby suppressing the ATP signal that triggers insulin secretion [12]. Alloxan monohydrate can induce type 1 diabetes [15]. In a few reported studies, induction of diabetes was based on the combination of STZ with a high-fat diet (STZ/HFD) [16] or carried out on genetically modified animals with diabetes (leptin receptor-deficient (Leprdb/JNju, db/db) mice) [17]. Due to single-gene mutations that lead to the lack of action by the satiety factor leptin or its cognate receptor, these rodents spontaneously develop severe hyperphagia leading to obesity and manifest some type 2 diabetes mellitus (T2DM)-like characteristics [18].
The wound healing activity of herbal products and their active constituents conducted on animals with induced diabetes are mainly based on the excision wound model and one study on the punch wound model [19]. The excision wound model is induced by the removal of some part of the skin at the depth of the epidermis and upper dermis (a partial thickness (or split-thickness) wound) or both epidermis and dermis up to the fascia or subcutaneous tissue (a full-thickness wound) [20]. Excision wounds well illustrate the skin defects that can be observed in diabetic wounds and allow the evaluation of re-epithelialization and wound closure. All herbal products and their active constituents (Table 1, Table 2 and Table 3) showed wound contraction in a shorter time, increased wound breaking strength, increased re-epithelialization, and better granulation compared to that of standard drugs (5% and 10% povidone-iodine, 1% silver nitrate, 1% silver sulfadiazine, 2% mupirocin, 8.5% mafenide, bacitracin) and commercially available wound dressings (Comfeel—hydrocolloid dressing, Kaltostat—alginate dressing) as positive controls.
3.2. Human-Based Studies
In comparison to many animal-based studies, there are only a few clinical trials describing the influence of herbal products on diabetic wound healing (Table 4). Unfortunately, there are no human-based studies showing the effect of active compounds isolated from herbal products on diabetic wound healing. This imbalance between animal-based studies and human-based studies arises from the fact that clinical trials designed to prove herbal products efficacy and safety are expensive, long-lasting, and require unique regulatory pathways and special permission from regulatory bodies. Despite these limitations, some scientific studies are being performed.
Table 1.
Herbal products used for diabetic wound healing, animal-based studies.
| Herbs | Model of the Study | Pharmacological Data | Effect | Mechanism of Action | Antimicrobial Activity | Ref. |
|---|---|---|---|---|---|---|
| Aloe vera | STZ-induced diabetic Wistar rat, excision wound model |
A. vera gel; control 1: untreated group; control 2: untreated diabetic group; treatment: once a day for 9 days |
significantly increases level of GAGs and breaking strength on day 9 | - | - | [21] |
| Aloe Vera, Adiantum capillus veneris, Commiphora molmol, henna | STZ- induced diabetic Wistar rats; excision wound model |
ointment with herbal powder mixed in equal parts with Vaseline; control 1: untreated non-diabetic group; control 2: untreated diabetic group; control 3: Vaseline; treatment: once a day for 21 days |
better wound closure; expression of the Mmp9 gene decreased significantly in diabetic group after 14 days |
- | - | [22] |
|
Aloe vera,
Nigella sativa |
AM-induced diabetic Wistar rats; excision wound model |
N. sativa oil gel (NSO); Aloe vera gel (AV); control: untreated group; treatment: 100 mL of gel and transparent film dressing |
significantly smaller wound area in AV than NSO group; necrotic tissue and inflammation decreased in AV group compared with NSO group; re-epithelialization was better in AV than NSO group |
- | - | [23] |
|
Aloe vera,
Teucrium polium |
STZ-induced diabetic BALB/c mice; excision wound model | 5% and 10% T. polium hydroethanolic extract in ointment; 5% and 10% A. vera gel in ointment; combination of 5% T. polium extract and 5% A. vera gel in ointment; positive control: mupirocin; treatment: once a daily for 14 days |
mixed herbal ointment shortened the inflammatory phase and reduced the levels of tissue MDA, TNF-α, and IL-1β compared to mupirocin; fibroblast proliferation, collagen deposition, and expression of VEGF, IGF-1, GLUT-1, and FGF-2 were significantly increased by all herbal ointments |
anti-inflammatory activity | - | [24] |
|
Agrimonia pilosa;
Nelumbo nucifera; Boswellia carteri; Pollen typhae |
STZ-induced diabetic C57BL/6 mice; excision wound model | mixed powder of A. pilosa, N. nucifera, B. carteri, P. typhae (ANBP); control: untreated group; treatment: once a day for 21 days |
accelerated wound healing, promoted vascularization, and inhibited inflammation | angiogenic activity; anti-inflammatory activity |
- | [25] |
| Annonas quamosa | STZ-induced diabetic Wistar rats; excision wound model |
A. squamosa ethanolic extract; control 1: untreated non-diabetic group; control 2: untreated diabetic group; treatment: 200 μL, once daily |
better wound healing through increased levels of enzymatic and non-enzymatic antioxidants in wound tissues | antioxidant activity | - | [26] |
| Arnebia euchroma, Pistacia atlantica | AM-induced diabetic Wistar rats; excision wound model |
10% A. euchroma extract in Eucerin; 5% A. euchroma extract and 5% P. atlantica EO in natural cow oil; 10% A. euchroma extract and 10% P. atlantica EO in natural cow oil; 10% A. euchroma extract and 10% P. atlantica EO in Eucerin; positive control: honey; negative control: Eucerin; treatment: once a day |
the most effective in wound healing was 5% A. euochroma and gum mixture of animal oils | - | - | [27] |
| Azadirachta indica, Glycyrrhiza glabra, Ficus infectoria, Shorea robusta, Curcuma longa, Berberis aristata, Rubia cordifolia, Pongamia glabra, Ficus religiosa, Ficus bengalensis, Centella asiatica | AM-induced diabetic Wistar rats; incision and excision wound models |
Cream A (extracts of G. glabra, F. infectoria, S. robusta, C. longa, B. aristata, R. cordifolia, A. indica, P. glabra, Yashad Bhasma as Ayurvedic preparation); Cream B (extracts of F. religiosa, F. bengalensis, C. asiatica, S. robusta, G. glabra, A. indica, P. glabra, Jatyadi Oil, and Yashad Bhasma); positive control: framycetin sulfate cream; treatment: once a day for 10 days (incision method) and 16 days (excision method) |
Cream B was found to be more an effective wound healing agent than cream A and framycetin | - | - | [28] |
|
Blepharis
maderaspatensis |
STZ-induced diabetic Wistar rats; excision wound model |
paste formula of 10 g, 15 g, 20 g of extract, 60 g black powder mixed with egg white and 2 or 3 drops of lime juice q.s.; negative control: untreated group; positive control: 1% framycetin sulphate; treatment: twice every day until the wound healed completely |
paste with 20% extract completely healed wounds by 18th day of treatment | - | - | [29] |
| Butea monosperma | AM-induced diabetic Wistar rats; excision wound model |
20% w/w methanolic flower extract in white petroleum jelly; control: Vaseline; positive control: soframycin ointment; treatment: 11 days |
wound contraction | - | - | [30] |
| Camellia sinensis | AM-induced diabetic Wistar rats; incision and excision wound models | 0.6% green tea methanolic extract; control 1: nontreated diabetic group; control 2: untreated non-diabetic group; control 3: Vaseline; positive control: 5% w/w povidone iodine; treatment: twice daily until completely healed |
faster wound contraction; increased collagen and fibronectin deposition with higher expression of NO; promoted angiogenesis process via molecular control of circulating hypoxia-responsive microRNAs: miR-424, miR-210, miR-199a, and miR-21 |
angiogenic activity | - | [31] |
|
Cassia auriculata, Mangifera indica, Ficus banghalensis, Cinnamomum tamala, Trichosynthis diocia |
STZ-induced diabetic Wistar rats; incision, excision, dead space models | aqueous extracts mixed in equal proportions in polyherbal formulation; control 1 and 2: untreated non-diabetic and diabetic groups; positive control: 5% glibenclamide ointment; treatment: once a day for 18 days (excision model) or until wound was healed (incision model) |
significant increase in wound breaking strength, epithelialization, and level of hydroxyproline | antioxidant activity | - | [32] |
| Cotinus coggygria | STZ-induced diabetic Wistar rats; excision wound model | 5% (w/w) ethanol extract of C. coggygria ointment; control: untreated group; treatment: 0.5–1 g, once a day for 14 days |
significantly increased hydroxyproline content and elevation in GSH, statistically significant decrease in MDA level in the treated group vs. control group | antioxidant activity, anti-inflammatory activity |
- | [33] |
| Cymbopogon nardus | STZ-induced diabetic Swiss albino mice; excision wound model |
C. nardus EO dispersed in 100 mL of olive oil; control 1: saline-treated diabetic group; control 2: C. albicans-infected diabetic group; positive control: clotrimazole (1 mg/day) dispersed in 100 mL of olive oil; treatment: 25 mg once a day for 21 days |
attenuated the growth of the fungus on diabetic wounds and simultaneously reduced the inflammation which leads to acceleration of the wound healing process | anti-inflammatory activity, antifungal activity |
C. albicans,
C. glabrata, C. tropicalis |
[34] |
| Euphorbia hirta | AM-induced diabetic Swiss albino rats; excision wound model | 5% and 10% ethanolic extract of E. hirta ointment; positive control: 5% povidone iodine ointment; treatment: once a day for 16 days |
significant wound closure | - | - | [35] |
|
Hypericum
perforatum |
STZ-diabetic Sprague–Dawley rats; incision and excision wound model |
H. perforatum in olive oil; control 1: untreated non-diabetic group; control 2: untreated diabetic group; control 3: olive oil; treatment: once a day for 21 days |
faster inflammatory response and better healing; significantly higher tensile strength, tissue hydroxyproline concentration, and collagen density |
anti-inflammatory activity | - | [36] |
|
Hypericum
perforatum |
STZ-induced diabetic Wistar rats; excision wound model |
5% and 10% H. perforatum gel; control 1: untreated group; control 2: gel base; treatment: once a day for 15 days |
faster wound closure rate, improved tissue regeneration by enhancing fibroblast proliferation, collagen bundle synthesis, and revascularization |
angiogenic activity | - | [37] |
| Lantana camara | AM-induced diabetic rats; excision wound model | 10% ethanolic extract of L. camara emulgel; control 1: untreated non-diabetic group; control 2: untreated diabetic group; positive control: soframycin ointment; treatment: twice daily for 12 days |
faster wound closure and reduced epithelization period | - | - | [38] |
| Lycium depressum | STZ-induced diabetic Wistar rats; incision and excision wound model |
1 g, 2 g, 4 g powder of methanolic L. depressum extracts in ointment; control 1: untreated group; control 2: base formulation; treatment: once a day |
enhanced wound contraction, decreased epithelialization time, increased hydroxyproline content | - | - | [39] |
|
Momordica
charantia |
STZ-induced diabetic Sprague–Dawley rats;excision wound model |
M. charantia fruit extract powder and ointment; control 1 and 2: untreated non-diabetic and diabetic group; control 3: ointment base; positive control: povidone iodine ointment; treatment: once a day for 10 days |
faster wound closure rate; intense TGF-β expression |
angiogenic activity | - | [40] |
| Moringa oleifera | STZ-induced diabetic Wistar rats; excision wound model |
0.5%, 1%, and 2% w/w aqueous fraction of M. oleifera; control 1: non-diabetic group; control 2: diabetic group; positive control: 1% w/w silver sulfadiazine; treatment: once a day for 21 days |
decreased wound size, improved wound contraction, tissue regeneration; downregulation of inflammatory mediators, such as TNF-α, IL-1β, IL-6, iNOS synthase, COX-2, and upregulation of VEGF |
angiogenic activity; anti-inflammatory activity |
S. aureus, P. aeruginosa, E. coli |
[41] |
| Nigella sativa | STZ-induced diabetic Wistar rats; excision wound model |
20% and 40% hydroethanolic N. sativa extracts ointment; control 1: untreated non-diabetic group; control 2: Eucerin-treated non-diabetic group; control 3: phenytoin (1%)-treated non-diabetic group; control 4: untreated diabetic group; control 5: phenytoin (1%)-treated diabetic group; treatment: 21 days |
the shortest duration of wound healing in diabetic N. sativa extract (40%)-treated group (15 days) followed by diabetic N. sativa (20%)-treated group (18 days) | anti-inflammatory activity | - | [42] |
|
Pelargonium
graveolens, Olive riadecombens |
STZ-induced diabetic Wistar rats; excision wound model | formulations with 1% EOs alone; mixture with 1% P. graveolens and 1% O. decombens; control 1: basic formula; control 2: saline; treatment: once a day for 30 days |
reduction of wound size; highest tissue repair in EO mixture group |
- | - | [43] |
| Piper betel | STZ-induced diabetic Sprague-Dawley rats; excision wound model |
paste with powder of P. betel and 0.9% saline; control 1: untreated non-diabetic rats; control 2: untreated diabetic rats; positive control: 1% silver nitrate cream; treatment: once a day for 7 days |
significant increase in hydroxyproline content and SOD; decreased MDA level; decrease in 11b-HSD-1 expressions |
antioxidant activity | - | [44] |
|
Plantago lanceolata, Arnica montana, Tagetes patula, Symphytum officinale, Calendula officinalis, Geum urbanum |
STZ-induced diabetic Wistar rats; excision wound model |
mixture of alcoholic herbal extract-loaded chitosan formulation; control 1: chitosan formulation; positive control: Betadine ointment; treatment: once daily for 14 days |
wound contraction and accelerated wound healing process; more complete re-epithelialization and denser collagen deposition |
antioxidant activity | - | [45] |
| Prosopis farcta | STZ-induced diabetic Wistar rats; excision wound model | fruit powder and root extract of P. farcta; control 1: untreated non-diabetic group; control 2: untreated diabetic group; treatment: twice a day for 15 days |
fruit powder and root extract accelerated wound healing | - | - | [46] |
| Psidium guajava | AM-induced diabetic Wistar rats; excision wound model | gel with 5% and 10% (w/w) of tannin-enriched fraction of P. guajava leaves; control 1: saline; control 2: gel without tannin fraction; positive control: Aloe vera gel 90% w/w; treatment: daily for 12 days |
wound contraction | - | - | [47] |
| Salvia kronenburgii; Salvia euphratica | STZ-induced diabetic Wistar rats; incision and excision wound models |
0.5% and 1% (w/w) ethanol extracts ointment; control 1: untreated group; control 2: ointment base; positive control: Fitocream with 15% (w/w) Triticum vulgare L. aqueous extract; treatment: 0.5 g ointments, topically once daily for 14 days |
wound contraction; increased re-epithelialization and angiogenesis, decreased dermal inflammation; oxidative damage to DNA was reduced on day 7 for S. euphratica ointment and on day 14 for S. kronenburgii ointment |
angiogenic activity; antioxidant activity |
S. aureus, E. coli, A. baumannii, A. hydrophila, M. tuberculosis, C. glabrata, C. parapsilosis, C. tropicalis |
[48] |
|
Solanum
xanthocarpum |
STZ-induced diabetic Wistar rats; inclusion and exclusion wound model | 5% and 10% extract of S. xanthocarpumgel; control 1: non-diabetic group with gel base; control 2: diabetic group with gel base; positive control: A. vera cream and juice; treatment: once a day for 14 days |
significant increase in collagen, hexosamine, hyaluronic acid, lipid peroxidation, NO; reduced levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α); enhanced level of VEGF |
anti-inflammatory activity | - | [49] |
| Stryphnodendronadstringens | STZ-induced diabetic Wistar rat, excision wound model |
1% crude extract gel; control 1: base gel; treatment: once a day for 14 days |
stimulation of the production of collagen fibers at the wound site; increased upregulation of COX-2 and VEGF |
anti-inflammatory activity | - | [50] |
| Quercus infectoria | STZ-induced diabetic Wistar rats, excision wound model | 30% w/v Q. infectoria formulation; control: saline; treatment: 15 mL once a day until wound closure |
enhanced the wound healing process with abundant cellular infiltration, collagen deposition, and re-epithelialization | antioxidant activity; antimicrobial activity |
MRSA | [51] |
Legends: 11b-HSD-1—11β-Hydroxysteroid dehydrogenase type 1; AM—alloxan monohydrate; COX-2—cyclooxygenase-2; EO—essential oil; FGF-2—fibroblast growth factor-2; GAG—glycosaminoglycan; GLUT-1—glucose transporter-1; GSH—glutathione, IGF-1—insulin-like growth factor 1; IL-1β—interleukin-1β; IL-6—interleukin 6; iNOS—inducible nitric oxide synthase; MDA—malondialdehyde; MRSA—methicillin-resistant Staphylococcus aureus; NO—nitric oxide; SOD—superoxide dismutase; STZ—streptozotocin; TGF-β—transforming growth factor-β; TNF-α—tumor necrosis factor-α; VEGF—vascular endothelial growth factor.
Table 2.
Active constituents isolated from herbal products used for diabetic wound healing, animal-based studies.
| Herbal Products | Model of the Study | Pharmacological Data | Effect | Mechanism of Action | Ref. |
|---|---|---|---|---|---|
| 20(S)-protopanaxadiol from Panax notoginseng |
leptin receptor-deficient (Leprdb/JNju, db/db) mice; excision wound model |
20(S)-protopanaxadiol (PPD); control: PBS; treatment: 15 μL of PPD (0.6, 6, and 60 mg/mL) or PBS every other day for 14 days |
PPD accelerated wound closure and epithelial gaps, elevated VEGF expression and capillary formation; PPD stimulated angiogenesis via HIF-1α-mediated VEGF expression by activating p70S6K through PI3K/Akt/mTOR and Raf/MEK/ERK signaling cascades |
angiogenic activity | [17] |
| arnebin-1 from Arnebia euchroma (Zicao) | AM-induced diabetic Sprague–Dawley rats; punch wound model |
0.1% arnebin-1 ointment; control 1: non-diabetic untreated group; control 2: untreated diabetic group; control 3: diabetic group with vehicle ointment; treatment: once a day for 7 days |
significantly increased wound closure rate; reduced number of macrophages, increased number of fibroblasts, remarkable degree of neovascularization and epithelization; synergetic effect with VEGF |
angiogenic activity | [19] |
| kaempferol | STZ-induced diabetic Wistar rats; incision and excision wound model | 0.5% and 1% (w/w) kaempferol (KM) ointments; control 1: untreated non-diabetic group; control 2: untreated diabetic group; control 3: ointment base; treatment: 0.5 g ointment, once a day for 14 days |
the best wound healing effect using 1% KM ointment; increased hydroxyproline and collagen; improved wound resistance (tensile strength), wound closure, and accelerated re-epithelialization |
antioxidant activity; anti-inflammatory activity |
[52] |
| kirenol from Siegesbeckia orientalis | STZ-induced diabetic Wistar rats; excision wound model |
diabetic and non-diabetic rats treated with 15% and 30% kirenol; treatment: once a day for 14 days |
wound closure, enhanced granule-forming tissue with noticeable propagation of fibroblasts, amplified vascular initiation, and sediment of collagen fibers; decreased NF-κB, COX-2, iNOS, MMP-2, and MMP-9 levels |
angiogenic activity; anti-inflammatory activity |
[53] |
| luteolin | STZ-induced diabetic Wistar rats; incision and excision wound models | 0.5% and 1% (w/w) luteolin ointments; control 1: untreated non-diabetic group; control 2; untreated diabetic group; control 2: ointment base; treatment: once daily for 14 days |
the best wound healing activity was observed in incision and excision wounds treated with 0.5% (w/w) luteolin ointment | - | [54] |
| luteolin; flavonoids fraction from Martynia annua |
STZ-induced diabeticWistar rats; excision wound model | 0.2% and 0.5% w/w of luteolin and flavonoid fraction ointment; control 1: untreated group; control 2: ointment base; positive control: 5% povidone iodine; treatment: twice daily |
enhanced wound healing through free radical-scavenging activity | antioxidant activity | [55] |
| neferine from Nelumbo nucifera (lotus) | STZ-induced diabetic Wistar rats; excision wound model | 10% neferine; control 1: untreated non-diabetic group; control 2: untreated diabetic group; control 3: untreated diabetic group with excision wound treatment: once a day for 14 days |
significant wound closure rate, decrease in the period of re-epithelialization, higher amount of collagen and protein content; mRNA level of Nrf-2, collagen-1, TGF-β, and α-SMA were decreased, and Kaep-1 was significantly increased; downregulation of inflammatory mediators (NF-κβ, TNF-α, IL-1β, IL-8, iNOS, and COX-2) and upregulation of GFs |
anti-inflammatory activity | [56] |
| pongamol; flavonoid-rich fraction from Tephrosia purpurea | STZ-induced diabetic rats, excision wound model | ointments with 5% (w/w) flavonoid-rich fraction and 0.2 and 0.5% (w/w) pongamol (PONG); positive control: povidone iodine; treatment: once a day for 20 days |
100% wound contraction; increased hydroxyproline and enzyme levels (SOD, CAT, and GSH), matured collagen fibers and fibroblasts with better angiogenesis |
antioxidant activity, angiogenic activity |
[57] |
| quercetin | STZ- induced diabetic Wistar rats; excision wound model |
quercetin ointment (1 g quercetin mixed with 99 g of petroleum jelly); control: petroleum jelly; positive control: 5% povidone ointment; treatment: once a day for 21 days |
increased wound healing | - | [58] |
Legends: α-SMA—Smooth muscle alpha-actin; AM—alloxan monohydrate; CAT—catalase; COX-2—cyclooxygenase-2; GF—growth factor; GSH—glutathione; HIF-1α—hypoxia-inducible factor 1α; IL-1β—interleukin-1β; iNOS—inducible nitric oxide synthase; Kaep-1—Kelch-like ECH-associated protein 1; MMP—matrix metalloproteinase; NF-κB—nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf-2—nuclear factor erythroid 2-related factor 2; SOD—superoxide dismutase; STZ—streptozotocin; TNF-α—tumor necrosis factor-α; TGF-β—transforming growth factor-β; VEGF—vascular endothelial growth factor.
Table 3.
Herbal products and their active constituents loaded on dressings used for diabetic wound healing, animal-based studies.
| Herbs | Model of the Study | Pharmacological Data | Effect | Mechanism of Action | Antimicrobial Activity | Ref. |
|---|---|---|---|---|---|---|
|
Aloe vera,
Hypericum perforatum |
STZ-induced diabetic Wistar rats; excision wound model | 15% A. vera gel with poly ε-caprolactone/gelatin (PCL/Ge) in nanofiber dressings; 15% H. perforatum oil with PCL/Ge in nanofiber dressings; control 1: PCL/Ge; control 2: A. veragel/H. perforatum oil; positive control: 10% povidone-iodine; treatment: wound was covered with dressings after 7th day of STZ induction |
H. perforatum oil gel-based nanofibers was better than A. vera gel-based nanofibers for wound healing | - | - | [59] |
| apigenin from Morus alba | STZ-induced diabetic Wistar rats; excision wound model |
apigenin (APN)-loaded hydrogels (HGs) with gellan gum–chitosan (GGCH) and PEG as a cross-linker (APN-loaded GGCH-HGs); control 1: vehicle (GGCH-HGs); positive control: Betadine; treatment: 18 days |
APN GGCH-HGs effectively stimulated wound contraction with significant antioxidant activity and increased collagen content; increased level of SOD and CAT in granuloma tissue of APN-treated group |
antioxidant activity | - | [60] |
| Blechnum orientale | STZ-induced diabetic rats; excision wound model |
hydrogel (sodium carboxymethyl-cellulose) with 4% w/w B. orientale extract; treatment: once a day for 14 days |
wound closure at 12 days; re-epithelialization, higher fibroblast proliferation, collagen synthesis, and angiogenesis |
antioxidant activity; antibacterial activity; angiogenic activity |
MRSA | [61] |
| curcumin | STZ-induced diabetic Wistar rats; excision wound model |
nanohybrid scaffold incorporating curcumin-loaded chitosan nanoparticles (CUR-CSNPs) impregnated into collagen–alginate (COL/ALG); control 1: sterile gauze; control 2: COL/ALG scaffold without CUR-CSNPs; treatment: once a day for 15 days |
faster wound closure, complete epithelialization with thick granulation tissue formation; lack of compact collagen deposition in placebo scaffold group; presence of inflammatory cells in control group |
- | - | [62] |
| curcumin from Curcuma longa |
STZ-induced diabetic Sprague– Dawley rats; excision wound model |
curcumin-loaded gum tragacanth/poly(ε-caprolactone) electrospun nanofibers (GT/PCL/Cur nanofibers); control: untreated diabetic group; treatment: wounds were wrapped with GT/PCL/Cur nanofibers for 15 days |
wound closure with well-formed granulation tissue dominated by fibroblast proliferation, collagen deposition, complete early regeneration of epithelial layer; formation of sweat glands and hair follicle tissue; increased amount of angiogenesis, granulation tissue area, and fibroblast numbers, and decreased epithelial gap |
angiogenic activity; antibacterial activity | MRSA, ESBL Gram-negative bacteria | [63] |
| curcumin, Lithospermi radix |
STZ-induced diabetic Sprague–Dawley rats; excision wound model | 1 μg/mL curcumin and 625 μg/mL L. radix extract loaded in GC/L/C bilayer nanofibrous scaffolds (gelatin/PVA solution with curcumin and extract was electrospun onto the chitosan scaffolds); control 1: gauze; GC membrane, GC/L membrane, GC/C membrane, GC/L/C scaffold; positive control: Comfeel®; treatment: once a day for 14 days |
decreased levels of pro-inflammatory markers (IL-6, TNF-α) provided evidence for the anti-inflammatory effects of GC/L/C treatment; increase in recovery rate of wound on day 7 |
anti-inflammatory activity | - | [64] |
| hydroxysafflor yellow A from Carthamus tinctorius |
STZ-induced diabetic Sprague–Dawley rats; excision wound model |
hydroxysafflor yellow A and deferoxamine (HSYA/DFO) loaded in chitosan/gelatin hydrogels in ratio of 5:5; control 1: PBS; control 2: hydrogel base; control 3: HSYA and DFO solution; treatment: once a day for solutions/once every 2 days for hydrogel for 16 days |
HSYA/DFO exerted synergistic effect on enhancing angiogenesis by up regulation of HIF-1α expression | angiogenic activity | - | [65] |
| Malva sylvestris | STZ-induced diabetic Wistar rats; excision wound model |
nanofibers of polyurethane and carboxymethyl cellulose (PU/CMC) with 15% w/w M. sylvestris extract; control group: gauze bandage and PU/CMC; treatment: once a day for 14 days |
higher collagen deposition and neovascularization; increased macrophage infiltration and fibroblast proliferation on day 7; enhanced collagenization and epithelium regeneration on day 14 |
anti-inflammatory activity, angiogenic activity |
S. aureus;
E. coli |
[66] |
| Moringa oleifera | STZ/HFD-induced diabetic Sprague–Dawley rats; excision wound model | 0.1, 0.5, and 1% M. oleifera leaves (MOL) aqueous extract loaded in hydrocolloid film dressing; control 1: untreated non-diabetic group; control 2: untreated diabetic group; positive control: Kaltostat; treatment: once a day for 21 days |
0.5% film significantly enhanced the wound closure at day 7; high collagen deposition and complete re-epithelialization after treatment with 0.5 and 1% MOL hydrocolloid film dressing |
- | - | [16] |
| polysaccharide from Astragali Radix | STZ-induced diabetic Sprague–Dawley rats; excision wound model |
Astragalus polysaccharide (APS)-loaded tissue engineering scaffolds (TES); control 1: untreated healthy group; control 2: TES alone; treatment: 5 mg each, once a day for 12 days |
APS in TES mimics structure of extracellular matrices and restored skin microcirculation; faster collagen synthesis, wound closure, and appendage and epidermal differentiation |
angiogenic activity | - | [67] |
| polysaccharide from Curcuma zedoaria | STZ-induced diabetic Sprague–Dawley rats; excision wound model | polysaccharide (ZWP) in chitosan/silk hydrogel sponge loaded with platelet-rich plasma (PRP) exosomes (PRP-Exos/ZWP); control 1: gauze containing 100 μL PBS; control 2: chitosan/silk hydrogel group; control 3: chitosan/silk hydrogel sponge loaded with PRP exosomes (PRP-Exos); treatment: wound dressings changed every 3 days for 15 days |
wound closure, up regulation of collagen synthesis and deposition, and angiogenesis at the wound site were observed for PRP-Exos/ZWP | angiogenic activity | - | [68] |
| polysaccharide from Periplaneta americana | STZ-induced diabetic Wistar rats; excision wound model | hydrogel (carbomer 940, carboxymethyl cellulose) with polysaccharide P. americana; control 1: saline-treated non-diabetic group; control 2: saline-treated diabetic group; control 3: hydrogel base; positive control: Kangfuxin solution; treatment: once daily for 15 days |
polysaccharide hydrogel effectively accelerated wound healing; increased inflammation alleviation, angiogenesis, and macrophage polarization |
anti-inflammatory activity; angiogenic activity |
- | [69] |
| resveratrol | STZ-induced diabetic Wistar rats; excision wound model |
resveratrol solution; resveratrol-loaded microparticles; resveratrol loaded microparticle impregnated dermal matrix (DM-MP-RSV) control 1: untreated non-diabetic group; control 2: untreated diabetic group; control 3: untreated diabetic wound group; control 4: dermal matrix (DM); treatment: once a day for 14 days |
the highest healing score in the DM-MP-RSV group with an increased antioxidant activity | antioxidant activity | - | [70] |
| vicenin-2 | STZ-induced diabetic Sprague–Dawley rats; punch wound model |
12.5, 25, and 50 μM Vicenin-2 hydrocolloid film (sodium alginate); control 1: non-diabetic, blank film-treated; control 2: diabetic; blank film-treated; positive control: 316 μM allantoin film; treatment: 0.8 cm2 film dressing and adhesive-permeable bandage wrapping; every day for 14 days |
enhanced diabetic wound healing; reduced pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α), mediators (iNOS and COX-2), and NO via the NF-κB pathway; enhanced cell proliferation, migration, and wound contraction via the VEGF and TGF-β pathways |
anti-inflammatory activity | - | [71] |
Legends: CAT—catalase; COX-2—cyclooxygenase; ESBL—extended spectrum beta-lactamase; HIF-1α—hypoxia-inducible factor 1α; IL-1β—interleukin 1β; IL-6—interleukin 6; iNOS—inducible nitric oxide synthase; MRSA—methicillin-resistant Staphylococcus aureus; NF-κB—nuclear factor kappa-light-chain-enhancer of activated B cells; NO—nitric oxide; SOD—superoxide dismutase; STZ—streptozotocin; TGF-β—transforming growth factor-β; TNF-α—tumor necrosis factor-α; VEGF—vascular endothelial growth factor.
Table 4.
Herbal products used for diabetic wound healing, human-based studies.
| Herbs | Model of the Study | Pharmacological Data | Effect | Ref. |
|---|---|---|---|---|
|
Actindia deliciosa (kiwifruit) |
randomized clinical trial; 37 patients with neuropathic diabetic foot ulcer;
|
pure extract of kiwifruit; treatment: twice daily for 21 days |
reduction in surface area of foot ulcer; significantly higher amounts of collagen and granulation tissues; significantly higher levels of angiogenesis; no significant antibacterial activity |
[72] |
| Aloe vera | random clinical trial; 60 patients with type 2 diabetes:
|
2% A. vera ointment; positive control: Betadine; treatment: once a day for 14 days |
accelerated wound healing | [73] |
|
Centella asiatica,
Plectranthus amboinicus |
single-center, randomized, controlled, open-label study; 24 diabetic foot ulcer patients:
|
1.25% WH-1 cream (fraction of PA-F4 from P. amboinicus and S1 from C. asiatica in 1:4 ratio); treatment: twice daily for 14 days |
no statistically significant differences in wound size after WH-1 cream application | [74] |
| olive oil | double-blind, randomized clinical trial; 34 patients with Wagner’s ulcer grade 1 or 2:
|
treatment: once a day for 4 weeks; routine care: ulcers cleaned with 1000 mL sterile 0.9% saline solution every day, after drying, wound was dressed with sterile gauze and latex-free tape |
complete ulcer healing in olive oil group | [75] |
| Securinega leucopyrus | case study, one patient with chronic diabetic wound | paste of S. leucopyrusin sesame oil; treatment: once daily for 15 days |
complete healing after one-month treatment | [76] |
The human studies are based on the diabetic foot ulcer (DFU) model. The DFU model is closely associated with peripheral vascular disease, neuropathy, and the chronic non-healing nature of wounds [77]. High glucose levels subsequently destruct the nerve fibers [78] and induce capillary size reduction, accelerating atherosclerosis and vasoconstriction, which collectively leads to occlusive arterial disease and DFU formation [79]. Moreover, DFUs, especially when they become infected, are a leading cause of morbidity and may lead to severe consequences, such as amputation. Optimal treatment of these diabetic foot problems usually requires a multidisciplinary approach, typically including wound debridement, pressure off-loading, glycemic control, negative pressure therapy, and surgical interventions [80]. These procedures allow the cleaning of the wound bed of excess exudate and microorganisms, and at the same time provides optimal conditions for tissue regeneration [80]. Topical application of Aloe vera [73], olive oil [75], kiwifruit [72], and Securinega leucopyrus [76] showed a reduction in surface area or complete foot ulcer healing. In turn, topical application of a cream with the active fraction isolated from Plectranthus amboinicus and Centella asiatica showed no statistically significant differences in wound size compared to hydrocolloid fiber dressings as a control [74].
4. Herbal Products and Their Active Constituents Loaded in Dressings Used for Diabetic Wound Healing
There is quite a lot of research on medicinal plant-based dressings for wound healing applications [81,82,83]. Some of them refer to diabetic wounds healing, including gauze, foams, drug-impregnated dressings (iodine, silver, polysaccharides), natural polymer-based dressing (hydrocolloids and hydrogel-based alginate, chitosan, collagen, cellulose), synthetic polymer-based dressings (poly (lactide-co-glycolide), polyurethanes, polyetheneglycols), and electrospun scaffolds [84]. Unfortunately, some types of traditional dressings can protect the wounds from the external environment but do not respond well to the wound-healing process. The most popular dressings for diabetic wounds based on herbal products and their active constituents are hydrogels, hydrocolloids, foams, and different nanofiber-based scaffolds (Table 3).
Hydrogels consist of natural or synthetic polymers and up to 70% water. These structures maintain a moist wound environment, which significantly accelerates the regeneration of the epidermis, prevents the risk of necrotic tissue formation, stimulates the process of autolytic wound cleansing, and inhibits the development of pathogens, which reduces the risk of wound infection, allergic reactions, and pain in the wound [85]. The advantages of hydrogels translate into the popularity of their use in the treatment of diabetic wounds. It was shown that polysaccharides isolated from Periplaneta americana and loaded onto a hydrogel (carbomer 940, carboxymethyl cellulose) [69], Blechnum orientale extract in a hydrogel (sodium carboxymethyl-cellulose) [61], apigenin-loaded hydrogel (gellan gum–chitosan with polyethylene glycol as a cross-linker) [60], and hydroxysafflor yellow A and deferoxamine loaded into hydrogels (chitosan/gelatin) [65] effectively stimulated wound contraction.
Hydrocolloids can absorb minimal to moderate amounts of wound fluids, and they can prevent water, bacteria, and oxygen from entering into the wound, as well as reduce the pH of the wound, inhibiting bacteria growth [84]. Unfortunately, hydrocolloid dressings are not appropriate for deeper and infected wounds that need oxygen to increase the healing rate of the wound. Moringa oleifera aqueous leaf extract (0.5%)-loaded hydrocolloid film dressings had proven to be the most promising approach to accelerate the diabetic wound healing process in both full-thickness excisions and partial thickness abrasion wounds in the HFD/STZ-induced diabetic type 2 model with comparable activity to commercial Kaltostat dressings [16]. In addition, vicenin-2 hydrocolloid film (sodium alginate) enhanced diabetic wound healing through increased cell proliferation, migration, and wound contraction [71].
Foam dressings consist of a porous structure that is excellent for absorbing large amounts of exudates, providing occlusion, protecting against bacteria and other infectious agents, promoting autolysis debridement, permeability to gases and water vapors, and are easy to remove [86]. Gastrodia elata extract and tea tree EO loaded in foam dressing containing silk fibroin protein accelerated wound recovery and achieved full closure of the wound within 21 days [87]. Moreover, histological analysis of regenerated skin tissues indicated that foam dressings enhanced the generation of thicker, denser, and more abundant collagen fibers in the dermis layer in comparison with the positive and negative control groups.
Nanofiber-based scaffolds offer a large surface area-to-volume ratio to allow cell adhesion and increase their exudate-absorbing capacity, antibacterial properties, and encapsulation of drugs for the desired period which helps in achieving their controlled release [88]. This release-controlling property is not provided by any of the dressings mentioned above nor by existing novel drug delivery systems (e.g., liposomes, nanostructured lipid carriers, nanoparticles, and dendrimers) used for topical applications [84]. Recently, wound dressings based on electrospun nanofiber scaffolds have attracted researchers’ attention since they replicate the characteristics of skin, have a high surface area-to-volume ratio, and tunable porous structure for easy nutrient infiltration and gas exchange [89]. Moreover, bilayer nanofibrous scaffolds reduce the frequency of dressing changes and minimize patients’ discomfort. Curcumin-loaded poly (ε-caprolactone) nanofibers as diabetic wound dressing increased the rate of wound closure and sustained release of curcumin for 72 h [90]. In addition, curcumin loaded in chitosan nanoparticles impregnated into collagen–alginate scaffolds [62] and bilayer nanofibrous scaffolds containing curcumin and Lithospermi radix extract (gelatin/poly(vinyl alcohol) solution with curcumin electrospun onto chitosan scaffolds) [64] showed faster diabetic wound closure. Polyurethane-based nanofiber wound dressings containing Malva sylvestris extract improved diabetic wound healing better than gauze bandage-treated wounds [66]. H. perforatum oil gel-based electrospun nanofibers showed better wound healing activity than Aloe vera gel-based electrospun nanofibers [59]. It was also shown that Astragalus polysaccharide-loaded tissue engineering scaffolds mimicked the structure of extracellular matrices and restored skin microcirculation, and increased collagen synthesis, wound closure, and appendage and epidermal differentiation [67]. The use of the antimicrobial activities and wound healing properties of herbal products and their active constituents loaded in dressing is a promising approach in diabetic wound healing and requires further research.
5. Herbal Products and Their Active Constituents Used for Diabetic Wound Infections
Prolonged diabetic wound infections are a factor in the delayed wound healing process. Moreover, if infected diabetic wounds are not treated properly, they could lead to systemic infection, sepsis, and even death. Furthermore, diabetic foot infections are the main cause of leg amputation. Diabetic wounds are more prone to microbial infections than normal wounds due to the high levels of blood glucose in the wound fluids that allow microbes to grow rapidly [91]. It was shown that an infected diabetic wound is more difficult and longer to heal compared to an uninfected diabetic wound. Kandimalla et al. [34] observed that fungal-infected wounds were not healed for up to 21 days whereas in non-infected diabetic wounds were healed by this period. Histopathology of the wound showed a wide area of necrosis with no signs of wound healing in infected diabetic wounds compared to normal diabetic wounds. Therefore, it is very important to implement a strict program for the prevention and treatment of diabetic foot ulcers, as well as proper management of microbial infections.
The most common bacteria detected in DFUs are superficial Gram-negative bacteria (P. aeruginosa, β-hemolytic Streptococcus, Proteus spp.) and Gram-positive bacteria (methicillin-susceptible S. aureus, methicillin-resistant S. aureus, β-hemolytic Streptococcus), and deeply penetrating anaerobes (Peptostreptococcus spp., Bacteroides spp., Prevotella spp., Clostridium spp.) [92,93,94,95]. Moreover, diabetic foot infections caused by bacteria such as P. aeruginosa, Escherichia coli, Citrobacter spp., Acinetobacter spp., and Staphylococcus aureus can develop into non-healing chronic wounds. Furthermore, the antibiotic susceptibility assay data of DFU isolates have also confirmed the distribution of multiantibiotic-resistant bacteria in the wound site of diabetic patients [96]. It was shown that all the Gram-positive isolates displayed resistance against penicillin and vancomycin, whereas P. aeruginosa had increased resistance against the most efficient antimicrobials such as ciprofloxacin (77%) and gentamycin (69%) [97].
Topical antimicrobial therapy is one of the most important methods of diabetic wound care. Herbal products and their active constituents are known to possess antimicrobial activity, even against resistant strains, making them a reliable source to combat diabetic wound infections [8,98]. Some researchers found that herbal products and their active constituents have strong antimicrobial activity against microorganism isolated from diabetic wounds or applied on infected diabetic wounds. It was found that quercetin and its esterified complex with 4-formyl phenyl boronic acid (4FPBA−Q) showed a remarkable effect against bacterial suspensions (1 × 105 CFU/mL) containing Gram-positive (S. aureus) and Gram-negative (P. aeruginosa, S. typhi) bacteria isolated from diabetic foot ulcers [99]. Malva sylvestris extract loaded in polyurethane/carboxymethylcellulose nanofibers showed antibacterial against S. aureus and E. coli [66]. The aqueous fraction of Moringa oleifera was found to be active against S. aureus, P. aeruginosa, and E. coli [41]. Hydrogels with water extracts of Blechnum orientale [61] and Quercus infectoria [100] was active against the MRSA strain. Curcumin-loaded electrospun nanofibers showed antibacterial activity against MRSA and ESBL Gram-negative bacteria [63]. An herbal ointment with ethanol extracts of Salvia kronenburgii and Salvia euphratica showed antibacterial activity against S. aureus, E. coli, A. baumannii, A. hydrophila, and M. tuberculosis, as well as antifungal activity against C. glabrata, C. parapsilosis, and C. tropicalis [48]. Besides bacterial infections, diabetic wounds are complicated by fungal infections. Candida species are the most common yeast that infects diabetic wounds which leads to delays in the wound healing process [101]. Cymbopogon nardus EO dispersed in olive oil attenuated the growth of fungi (C. albicans, C. glabrata, C. tropicalis) on chronic diabetic wounds and simultaneously reduced the inflammation which led to acceleration of the wound healing process [34].
6. Mechanism of Action of Herbal Products and Their Active Constituents Used for Diabetic Wound Healing
Wound healing involves a complex sequence of events involving cellular and biochemical processes including four overlapping phases; (1) homeostasis (coagulation which controls excessive blood loss from the damaged vessels) (few minutes); (2) inflammatory (influx of macrophages and proteases to clean up debris and pathogens, secretion of growth factors and pro-inflammatory cytokines) (0–3 days); (3) proliferative (fibroblast migration, extracellular matrix formation, granulation, re-epithelialization, neoangiogenesis) (3–21 days), and (4) maturation or remodeling that occurs within the dermis (collagen crosslinking and reorganization, increase in the tensile strength of the extracellular matrix, scar formation) (21 day–2 years) [4,102]. These overlapping phases of wound healing as well as their length in diabetes patients are disturbed. Diabetic patients have a reduced ability to metabolize glucose resulting in hyperglycemic conditions which further complicate the wound healing process [103]. Hypoxia due to glycation of hemoglobin, leads to the alteration of red blood cell membranes and the narrowing of blood vessels, which further leads to a deficient supply of nutrients and oxygen to the tissue [104]. This local ischemia due to microvascular complications in diabetes considerably delays the wound healing processes. Serum glucose concentrations of more than 150 mL/dL were considered indicative of immune system dysfunction and leads to long-term inflammatory disease [105]. Microvascular complications, irregular inflammatory responses, impaired angiogenesis, tissue oxidative stress, impaired production of cytokines and growth factors, reduction of nitric oxide, impaired keratinocytes and fibroblast migration and proliferation, and abnormal levels of matrix metalloproteinases are the main factors that disturb the diabetic wound healing process [4]. Herbal products and their active constituents, through different mechanisms of action, affect the cellular and biochemical processes occurring in the different phases of wound healing (Figure 2).
Figure 2.
Influence of herbal products and their active constituents on the phases of diabetic wound healing.
6.1. Free Radicals and Oxidative Stress
Oxidative stress is caused by an increase in free radicals, reactive oxygen species (ROS), and/or reactive nitrogen species (RNS) in the body, which leads to intercellular biochemical dysregulation of the redox status [106]. The antioxidant system includes the major ROS-scavenging enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH), and prevents damaging effects of oxidative stress [107]. An imbalance of free radicals and antioxidants in the body results in the overproduction of ROS which leads to cell/tissue damage, inflammation, neuropathy, ischemic lesion, and topical infection, delaying diabetic wound healing [108]. Therefore, decreasing ROS levels through antioxidative systems may improve diabetic wound healing.
Herbal products and their active constituents are well known for their antioxidant activity [109,110]. Annona squamosal ethanolic extract promoted increased levels of enzymatic and non-enzymatic antioxidants in wound tissues, thus detoxifying free radicals to promote better wound healing in normal and diabetic rats [26]. An ointment containing luteolin (0.5% w/w) and the flavonoid fraction (0.5% w/w) isolated from Martynia annua [55], a resveratrol solution, resveratrol-loaded microparticles, and resveratrol-loaded microparticles impregnated in a dermal matrix [70] enhancing diabetic wound healing through free radical scavenging. Piper betel paste significantly decreased the oxidative stress markers such as SOD and expression of 11β-hydroxysteroid dehydrogenase type 1 (11b-HSD-1) in diabetic wounds compared to untreated diabetic wounds [44]. Apigenin from Morus alba loaded into a hydrogel effectively stimulated diabetic wound contraction with significant antioxidant activity through increased levels of SOD and CAT in granuloma tissue [60]. Pongamol and flavonoid-rich fractions from Tephrosia purpurea ointment stimulated diabetic wound healing through antioxidant activity by increasing SOD, CAT, and GSH levels [57]. Hydrogels with 4% w/w Blechnum orientale extract exhibited stronger antioxidant activity compared to standards (ascorbic acid, α-tocopherol, BHT as butylohydroksytoluen, and Trolox-C as an analog of vitamin E) and effectively treated diabetic ulcer wounds [61]. An herbal formulation of Cassia auriculata, Mangifera indica, Ficus banghalensis, Cinnamomum tamala, and Trichosynthis diocia) [32], a mixture of alcoholic herbal extracts (Plantago lanceolata, Arnica montana, Tagetes patula, Symphytum officinale, Calendula officinalis, Geum urbanum) loaded onto chitosan [45], ethanol extracts of Salvia kronenburgii and Salvia euphratica ointment [48], Cotinus coggygria ointments [33], Quercus infectoria formulations [51], and kaempferol ointments [52] showed significant antioxidant activity and improved diabetic wound closure.
6.2. Impaired Inflammatory Cell Response
An increase in glucose levels and free fatty acids promotes the activation of macrophage-mediated inflammation in diabetes, contributing to the elevated production of pro-inflammatory cytokines [111]. M1-like macrophages with pro-inflammatory activity produce cytokines (IL-12, IL-1β, IL-6, TNFα, iNOS), while M2-like macrophages with anti-inflammatory activity are dominant in the proliferative phase of diabetic wound healing [112,113]. These recruit macrophages and immune cells that serve a pivotal role in orchestrating the appropriate healing of diabetic wound [114]. It was shown that macrophage dysregulation [115] and macrophage-derived IL-1β [116,117] lead to a prolonged inflammatory phase and impaired diabetic wound healing.
Herbal products and their active constituents have well-known anti-inflammatory activities which may be useful for the treatment of diabetic wound healing and its complications [118]. A combination of Aloe vera gel and Teucrium polium hydroethanolic extract in an ointment shortened the inflammatory phase and reduced the levels of tissue malondialdehyde (MDA), TNF-α, and IL-1β compared to the mupirocin positive control [24]. Moringa oleifera improved wound contraction through the downregulation of inflammatory mediators, such as TNF-α, IL-1β, IL-6, iNOS synthase, and COX-2 and the upregulation of VEGF [41]. Solanum xanthocarpum gel reduced levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) and enhanced levels of VEGF [49]. Kirenol from Siegesbeckia orientalis affect wound closure by decreasing NF-κB, COX-2, iNOS, MMP-2, and MMP-9 levels [53]. Curcumin and Lithospermi radix extract loaded in bilayer nanofibrous scaffolds decreased levels of pro-inflammatory markers (IL-6 and TNF-α) and provided evidence for the anti-inflammatory effects of this treatment [64]. Neferine from Nelumbo nucifera (lotus) acts by down regulating inflammatory mediators (NF-κβ, TNF-α, IL-1β, IL-8, iNOS, and COX-2) and upregulating of GFs [56]. Vicenin-2 hydrocolloid films reduced levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) and mediators (iNOS and COX-2), and NO via the NF-κB pathway [71]. It was also shown that macrophages/monocytes isolated from the wounds of diabetic mice treated with Quercus infectoria extract exhibited lower expression of the inflammatory cytokines IL-1β and TNF-α [100]. Polysaccharides from Periplaneta americana in a hydrogel [69] and Malva sylvestris extract in nanofibers [66] effectively accelerated wound healing through alleviation of inflammation and macrophage polarization. Hypericum perforatum oil extract [36], Cotinus coggygria ointment [33], hydroethanolic Nigella sativa extract ointment [42], Cymbopogon nardus essential oil dispersed in olive oil [34], Salvia kronenburgii and Salvia euphratica ointment [48], a mixed powder of Agrimonia Pilosa, Nelumbo nucifera, Boswellia carteri, and Pollen typhae [119], and a kaempferol ointment [52] reduced inflammation which led to the acceleration of the diabetic wound healing process.
6.3. Impaired Growth Factors Production
Growth factors play a critical role in initiating and sustaining the different phases of wound healing [120]. Several growth factors that are released at the wound site are presumed to be necessary for wound healing such as transforming growth factor (TGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), insulin-like growth factor (IGF), keratinocyte growth factor (KGF), platelet-derived growth factor (PDGF). and vascular endothelial growth factor (VEGF) [120]. The down-regulation of growth factor receptors and rapid degradation of growth factors leads to delayed wound healing in diabetics [121]. TGF-β recruits and promotes the stimulation of inflammatory cells including neutrophils, macrophages, and lymphocytes, as well as keratinocytes, fibroblasts, and induces the production of growth factors [122]. The reduced concentration of TGF-β has been reported in diabetic wounds [123]. EGF is associated with the systemic attenuation of pro-inflammatory markers and antioxidant effects in diabetic foot ulcer patients [124]. Moreover, EGF stimulates fibroblast replication, collagen formation, and re-epithelialization which promote diabetic wound healing [125]. Lack of FGF-7 [126] and KGF [127] inhibited cell proliferation and delayed diabetic wound healing.
Some herbal products and their active constituents enhance cell proliferation, migration, and diabetic wound contraction via the stimulation of growth factors. Momordica charantia accelerated wound closure rate through intense TGF-β expression [40]. Aloe veragel and Teucrium polium extract in an ointment significantly increased expression of VEGF, IGF-1, GLUT-1, and FGF-2 [24]. Stryphnodendron adstringens gel [50], arnebin-1 from Arnebia euchroma [19], and 20(S)-protopanaxadiol from Panax notoginseng [17] accelerated wound closure and elevated VEGF expression. Vicenin-2 facilitated healing in hyperglycemic conditions by increasing the release of growth factors such as VEGF and TGF-β to enhance cell proliferation, migration, and wound contraction via the VEGF and TGF-β pathways [71].
6.4. Impaired Keratinocyte and Fibroblast Proliferation and Migration
During the proliferative phase of wound healing, keratinocytes (epidermal skin cells), endothelial cells (the primary vascular cell type), and fibroblasts (the primary cell type in connective tissues) proliferate, migrate, and differentiate, which enables the formation of granulation tissue, reconstitution of the dermal matrix, restoration of surface integrity, and promotion of wound closure [128]. These processes may be supported by herbal products and their active constituents. Ointments containing Aloe vera or Teucrium polium alone and in combination triggered diabetic wound healing through fibroblast proliferation and collagen deposition [24]. Topical application of Hypericum perforatum in olive oil showed significantly higher tensile strength, tissue hydroxyproline concentration, and collagen density compared to the control group [36]. Hypericum perforatum gel improved tissue regeneration by enhancing fibroblast proliferation and collagen synthesis [37]. Camellia sinensis extract increased collagen and fibronectin deposition [31]. Blechnum orientale hydrogel exhibited re-epithelialization and higher fibroblast proliferation and collagen synthesis [61]. Curcumin from Curcuma longa loaded into electrospun nanofibers stimulated wound closure with well-formed granulation tissue areas dominated by fibroblast proliferation, collagen deposition, rapidly regenerated epithelial layer, and formation of sweat glands and hair follicle tissues [63]. Polysaccharides from Astragali Radix loaded into tissue engineering scaffolds increased collagen synthesis, wound closure, and appendage and epidermal differentiation [67].
6.5. Impaired Angiogenesis
Angiogenesis (or neovascularization) is an essential part of the wound healing process consisting of the formation of a new capillary network (microvasculature) in response to hypoxia or other stimuli [129]. The hypoxic conditions in diabetes induce macrophages to secrete pro-angiogenic growth factors such as FGF, VEGF, and PDGF and cytokines, such as TGF-β and IL-1 that are involved in the control of various aspects of angiogenesis [130,131]. VEGF is one of the most important angiogenic factors in wounds and its production lies downstream of hypoxia and hyperglycemia. Hypoxia following injury activates hypoxia-inducible factor-1 (HIF-1), a transcriptional activator that promotes angiogenesis by upregulating target genes such VEGF-A [132], while hyperglycemia induces indirect VEGF overexpression mediated by TGF-β [133]. In addition, the upregulation of FGF and PDGF is associated with angiogenesis in diabetes and stimulates wound healing in diabetic mice [134].
It is well known that chronic, non-healing diabetic wounds are closely linked to poor vascular networks. Herbal products and their active constituents are a rich source of novel angio-modulators that may affect the angiogenesis process in diabetic wound healing [135]. Camellia sinensis extract promotes the angiogenesis process and vascular remodeling via molecular control of circulating hypoxia-responsive microRNAs: miR-424, miR-210, miR-199a, and miR-21 in diabetic and non-diabetic wounds [31]. Topical application of the aqueous fraction of Moringa oleifera enhanced wound healing in diabetic rats through upregulation of VEGF and accelerating the angiogenesis process [41]. Blechnum orientale hydrogels [61], Hypericum perforatum gels [37], Malva sylvestris extract nanofibers [66], Salvia kronenburgii and Salvia euphratica ointments [48], a mixture of Agrimonia eupatoria, Nelumbo nucifera, Boswellia carteri, and pollen from Typhae angustifoliae [119] improved tissue regeneration by revascularization. 20(S)-protopanaxadiol from Panax notoginseng accelerated wound closure through elevation of VEGF expression and capillary formation, and stimulation of angiogenesis via HIF-1α-mediated VEGF expression by activating p70S6K through PI3K/Akt/mTOR and Raf/MEK/ERK signaling cascades [17]. Arnebin-1 from Arnebia euchroma in an ointment promoted wound healing by a remarkable degree due to neovascularization through the synergetic effects of arnebin-1 and VEGF [19]. Hydroxysafflor yellow A from Carthamus tinctorius and deferoxamine loaded in chitosan/gelatin hydrogels exerted a synergistic effect on enhancing angiogenesis by upregulation of HIF-1α expression [65]. Polysaccharides from Astragali Radix loaded onto tissue engineering scaffolds restored skin microcirculation [67], while polysaccharides from Periplaneta americana loaded in hydrogels effectively accelerated wound healing through inflammation alleviation, angiogenesis, and macrophage polarization [69]. Kirenol from Siegesbeckia orientalis [53], pongamol and the flavonoid-rich fraction from Tephrosia purpurea in an ointment [57], and curcumin from Curcuma longa loaded onto electrospun nanofibers [63] affected vascularization and angiogenesis in diabetic wounds.
7. Conclusions
There are numerous animal-based studies and only a few clinical trials confirming the activity of herbal products and their active constituents in the stimulation of diabetic wound healing. Topical applications of herbal products and their active constituents in formulation or loaded in various dressings seem to be a good alternative for the treatment of diabetic wounds. The new dressings offer several beneficial properties, such as absorbing excess discharge from the wound, maintaining a moist environment conducive to healing, creating a protective barrier against bacterial penetration, being suitable for wounds with necrosis, and affecting the release of active ingredients over time, which can positively affect diabetic wound healing. Herbal products and their active constituents through different mechanisms of action, including antimicrobial, anti-inflammatory, and antioxidant activities, stimulation of angiogenesis and keratinocytes, production of cytokines and growth factors, and promotion of fibroblast migration and proliferation, may be considered as an important support during conventional therapy or even as a substitute for synthetic drugs used for diabetic wounds treatment.
Author Contributions
Conceptualization, A.H.; methodology, A.H. and A.P.H.; writing—original draft preparation, visualization, A.H.; writing—review and editing, A.P.H.; supervision, A.P.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Brownlee M. The Pathobiology of Diabetic Complications. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 2.Kalan L.R., Meisel J.S., Loesche M., Horwinski J., Soaita I., Chen X., Uberoi A., Gardner S.E., Grice E.A. Strain- and Species-Level Variation in the Microbiome of Diabetic Wounds Is Associated with Clinical Outcomes and Therapeutic Efficacy. Cell Host Microbe. 2019;25:641–655.e5. doi: 10.1016/j.chom.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okonkwo U.A., DiPietro L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017;18:1419. doi: 10.3390/ijms18071419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel S., Srivastava S., Singh M.R., Singh D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019;112:108615. doi: 10.1016/j.biopha.2019.108615. [DOI] [PubMed] [Google Scholar]
- 5.Louie T.J., Bartlett J.G., Tally F.P., Gorbach S.L. Aerobic and Anaerobic Bacteria in Diabetic Foot Ulcers. Ann. Intern. Med. 1976;85:461–463. doi: 10.7326/0003-4819-85-4-461. [DOI] [PubMed] [Google Scholar]
- 6.Lipsky B.A., Brendt A.R., Deery H.G., Embil W.S., Joseph A.W., Karchmer B.A. Diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 2004;39:885–910. doi: 10.1086/424846. [DOI] [PubMed] [Google Scholar]
- 7.Dang C.N., Prasad Y.D.M., Boulton A.J.M., Jude E.B. Methicillin-resistant Staphylococcus aureus in the diabetic foot clinic: A worsening problem. Diabet. Med. 2003;20:159–161. doi: 10.1046/j.1464-5491.2003.00860.x. [DOI] [PubMed] [Google Scholar]
- 8.Herman A., Herman A.P. Herbal products and their active constituents used alone and in combination with antibiotics against multidrug-resistant bacteria. Planta Med. 2022. ahead of print . [DOI] [PubMed]
- 9.Pazyar N., Yaghoobi R., Rafiee E., Mehrabian A., Feily A. Skin Wound Healing and Phytomedicine: A Review. Ski. Pharmacol. Physiol. 2014;27:303–310. doi: 10.1159/000357477. [DOI] [PubMed] [Google Scholar]
- 10.Maver T., Maver U., Kleinschek K.S., Smrke D.M., Kreft S. A review of herbal medicines in wound healing. Int. J. Dermatol. 2015;54:740–751. doi: 10.1111/ijd.12766. [DOI] [PubMed] [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2007;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 13.Leiter E.H. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: Influence of inbred background, sex, and thymus. Proc. Natl. Acad. Sci. USA. 1982;79:630–634. doi: 10.1073/pnas.79.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akinlade O.M., Owoyele B.V., Soladoye A.O. Streptozotocin-induced type 1 and 2 diabetes in rodents: A model for studying diabetic cardiac autonomic neuropathy. Afr. Health Sci. 2021;21:719–727. doi: 10.4314/ahs.v21i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Queiroz L.A., Assis J.B., Guimarães J., Sousa E.S., Milhomem A.C., Sunahara K.K., Sa-Nunes A., Martins J.O. Endangered lymphocytes: The effects of alloxan and streptozotocin on immune cells in type 1 induced diabetes. Mediators Inflamm. 2021;2021:9940009. doi: 10.1155/2021/9940009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chin C.-Y., Ng P.-Y., Ng S.-F. Moringa oleifera standardised aqueous leaf extract-loaded hydrocolloid film dressing: In vivo dermal safety and wound healing evaluation in STZ/HFD diabetic rat model. Drug Deliv. Transl. Res. 2018;9:453–468. doi: 10.1007/s13346-018-0510-z. [DOI] [PubMed] [Google Scholar]
- 17.Zhang E.-Y., Gao B., Shi H.-L., Huang L.-F., Yang L., Wu X.-J., Wang Z.-T. 20(S)-Protopanaxadiol enhances angiogenesis via HIF-1α-mediated VEGF secretion by activating p70S6 kinase and benefits wound healing in genetically diabetic mice. Exp. Mol. Med. 2017;49:e387. doi: 10.1038/emm.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B., Chandrasekera P.C., Pippin J.J. Leptin- and Leptin Receptor-Deficient Rodent Models: Relevance for Human Type 2 Diabetes. Curr. Diabetes Rev. 2014;10:131–145. doi: 10.2174/1573399810666140508121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng Z., Zhu B.-H. Arnebin-1 promotes the angiogenesis of human umbilical vein endothelial cells and accelerates the wound healing process in diabetic rats. J. Ethnopharmacol. 2014;154:653–662. doi: 10.1016/j.jep.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 20.Kumar V.I.N.A.Y., Khan A.A., Nagarajan K. Animal models for the evaluation of wound healing activity. Int. Bul. Drug Res. 2013;3:93–107. [Google Scholar]
- 21.Daburkar M., Rathore A.S., Tangadpaliwar S., Bhutada P., Lohar V. An in vivo and in vitro investigation of the effect of Aloe vera gel ethanolic extract using animal model with diabetic foot ulcer. J. Pharm. Bioallied Sci. 2014;6:205–212. doi: 10.4103/0975-7406.135248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galehdari H., Negahdari S., Kesmati M., Rezaie A., Shariati G. Effect of the herbal mixture composed of Aloe Vera, Henna, Adiantum capillus-veneris, and Myrrha on wound healing in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2016;16:386. doi: 10.1186/s12906-016-1359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sari Y., Purnawan I., Kurniawan D.W., Sutrisna E. A Comparative Study of the Effects of Nigella sativa Oil Gel and Aloe Vera Gel on Wound Healing in Diabetic Rats. J. Evid-Based Integr. Med. 2018;23:2515690X18772804. doi: 10.1177/2515690X18772804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gharaboghaz M.N.Z., Farahpour M.R., Saghaie S. Topical co-administration of Teucrium polium hydroethanolic ex-tract and Aloe vera gel triggered wound healing by accelerating cell proliferation in diabetic mouse model. Biomed. Pharmacother. 2020;127:110189. doi: 10.1016/j.biopha.2020.110189. [DOI] [PubMed] [Google Scholar]
- 25.Hou Q., He W.-J., Chen L., Hao H.-J., Liu J.-J., Dong L., Tong C., Li M.-R., Zhou Z.-Z., Han W.-D., et al. Effects of the Four-Herb Compound ANBP on Wound Healing Promotion in Diabetic Mice. Int. J. Low. Extrem. Wounds. 2015;14:335–342. doi: 10.1177/1534734615575244. [DOI] [PubMed] [Google Scholar]
- 26.Ponrasu T., Subamekala M.K., Ganeshkuma M., Suguna L. Role of Annona squamosa on antioxidants during wound healing in streptozotocin-nicotinamide induced diabetic rats. J. Pharmacogn. Phytochem. 2013;2:77–84. [Google Scholar]
- 27.Sharifi A., Shafiei E., Hoseinzadeh M. The Study of the Effectiveness of a Mixture of Arnebia Euochroma and Gum Extract in Animal Oils and Comparing It with Honey in Diabetic Foot Ulcer. J. Chem. Health Risks. 2019;9:167–172. doi: 10.22034/JCHR.2019.666336. [DOI] [Google Scholar]
- 28.Nehete M.N., Nipanikar S., Kanjilal A.S., Kanjilal S., Tatke P.A. Comparative efficacy of two polyherbal creams with framycetin sulfate on diabetic wound model in rats. J. Ayurveda Integr. Med. 2016;7:83–87. doi: 10.1016/j.jaim.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacob J., Aleykutty N.A., Harindran J. Evaluation of wound healing activity in streptozotocin induced diabetic rats by ethanolic extract of Blepharisma deraspatensis (L.) B. Heyne ex Roth. Int. J. Herb Med. 2017;5:45–47. [Google Scholar]
- 30.Panwar S., Jain N.K., Gupta M.K. Wound healing potential of methanolic extract of flowers of Butea monosperma Lin. in diabetic animals. J. Drug Deliv. Therap. 2018;8:306–310. doi: 10.22270/jddt.v8i5-s.1979. [DOI] [Google Scholar]
- 31.Al-Rawaf H.A., Gabr S.A., Alghadir A.H. Circulating Hypoxia Responsive microRNAs (HRMs) and Wound Healing Potentials of Green Tea in Diabetic and Nondiabetic Rat Models. Evid.-Based Complement. Altern. Med. 2019;2019:9019253. doi: 10.1155/2019/9019253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majumder P., Paridhavi M. A novel poly-herbal formulation hastens diabetic wound healing with potent antioxidant po-tential: Acomprehensive pharmacological investigation. Pharmacognosy J. 2019;11:324–331. doi: 10.5530/pj.2019.11.48. [DOI] [Google Scholar]
- 33.Aksoy H., Sen A., Sancar M., Sekerler T., Akakin D., Bitis L., Uras F., Kultur S., Izzettin F.V. Ethanol extract of Cotinus coggygria leaves accelerates wound healing process in diabetic rats. Pharmaceut. Biol. 2016;54:2732–2736. doi: 10.1080/13880209.2016.1181660. [DOI] [PubMed] [Google Scholar]
- 34.Kandimalla R., Kalita S., Choudhury B., Dash S., Kalita K., Kotoky J. Chemical Composition and Anti-Candidiasis Mediated Wound Healing Property of Cymbopogon nardus Essential Oil on Chronic Diabetic Wounds. Front. Pharmacol. 2016;7:198. doi: 10.3389/fphar.2016.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuhin R.H., Begum M.M., Rahman S., Karim R., Begum T., Ahmed S.U., Mostofa R., Hossain A., Abdel-Daim M., Begum R. Wound healing effect of Euphorbia hirta linn. (Euphorbiaceae) in alloxan induced diabetic rats. BMC Complement. Altern. Med. 2017;17:423. doi: 10.1186/s12906-017-1930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altıparmak M., Eskitaşçıoğlu T. Comparison of Systemic and Topical Hypericum Perforatum on Diabetic Surgical Wounds. J. Investig. Surg. 2016;31:29–37. doi: 10.1080/08941939.2016.1272654. [DOI] [PubMed] [Google Scholar]
- 37.Yadollah-Damavandi S., Chavoshi-Nejad M., Jangholi E., Nekouyian N., Hosseini S., Seifaee A., Rafiee S., Karimi H., Ashkani-Esfahani S., Parsa Y., et al. Topical Hypericum perforatum Improves Tissue Regeneration in Full-Thickness Excisional Wounds in Diabetic Rat Model. Evid.-Based Complement. Altern. Med. 2015;2015:245328. doi: 10.1155/2015/245328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sultana S.S., Swapna G., Lakshmi G.S.S., Swathi S., Jyothi G.N., Devi A.S. Formulation and evaluation of herbal emulgel of Lantana camara leaves extract for wound healing activity in diabetic rats. Indo. Am. J. Pharmac. Res. 2016;6:6404–6417. [Google Scholar]
- 39.Naji S., Zarei L., Pourjabali M., Mohammadi R. The extract of Lyciumde pressum stocks enhances wound healing in streptozotocin-induced diabetic rats. Int. J. Low. Extrem. Wounds. 2017;16:85–93. doi: 10.1177/1534734617700538. [DOI] [PubMed] [Google Scholar]
- 40.Hussan F., Teoh S.L., Muhamad N., Mazlan M., Latiff A. Momordica charantia ointment accelerates diabetic wound healing and enhances transforming growth factor-β expression. J. Wound Care. 2014;23:400–407. doi: 10.12968/jowc.2014.23.8.400. [DOI] [PubMed] [Google Scholar]
- 41.Muhammad A.A., Fakurazi S., Arulselvan P., See C.P., Abas F. Evaluation of wound healing properties of bioactive aqueous fraction from Moringa oleifera Lam on experimentally induced diabetic animal model. Drug Des. Dev. Ther. 2016;10:1715–1730. doi: 10.2147/DDDT.S96968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mirazi N., Nourbar E., Yari S., Rafieian-Kopaei M., Nasri H. Effect of hydroethanolic extract of Nigella sativa L. on skin wound healing process in diabetic male rats. Int. J. Prev. Med. 2019;10:18. doi: 10.4103/ijpvm.IJPVM_276_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahboubi M., Taghizadeh M., Khamechian T., Tamtaji O.R., Mokhtari R., Talaei S.A. The Wound Healing Effects of Herbal Cream Containing Oliveria Decumbens and Pelargonium Graveolens Essential Oils in Diabetic Foot Ulcer Model. World J. Plast. Surg. 2018;7:45–50. [PMC free article] [PubMed] [Google Scholar]
- 44.Ghazali N.A., Elmy A., Yuen L.C., Sani N.Z., Das S., Suhaimi F., Yusof R., Yusoff N.H., Thent Z.C. Piper betel leaves induces wound healing activity via proliferation of fibroblasts and reducing 11b hydroxysteriod dehydrogenase-1 expression in diabetic rat. J. Ayurveda Integr. Med. 2016;30:1e11. doi: 10.1016/j.jaim.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colobatiu L., Gavan A., Potârniche A.V., Rus V., Diaconeasa Z., Mocan A., Tomuta I., Mirel S., Mihaiu M. Evaluation of bioactive compounds-loaded chitosan films as a novel and potential diabetic wound dressing material. React. Funct. Polym. 2019;145:104369. doi: 10.1016/j.reactfunctpolym.2019.104369. [DOI] [Google Scholar]
- 46.Ranjbar-Heidari A., Khaiatzadeh J., Mahdavishahri N., Tehranipoor M. The effect of fruit pod powder and aquatic extract of Prosopisfarcta on healing cutaneous wounds in diabetic rat. Zahedan J. Res. Med. Sci. 2012;14:e93444. [Google Scholar]
- 47.Jayakumari S. Formulation and evaluation of herbal gel from tannin-enriched fraction of Psidium guajava Linn. leaves for diabetic wound healing. Int. J. Green Pharm. 2018;12:S490–S496. [Google Scholar]
- 48.Güzel S., Özay Y., Kumaş M., Uzun C., Özkorkmaz E.G., Yıldırım Z., Ulger M., Guler G., Celik A., Camlica Y., et al. Wound healing properties, antimicrobial and antioxidant activities of Salvia kronenburgii Rech. f. and Salvia euphratica Montbret, Aucher&Rech. f. var. euphratica on excision and incision wound models in diabetic rats. Biomed. Pharmacother. 2019;111:1260–1276. doi: 10.1016/j.biopha.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 49.Parmar K.M., Shende P.R., Katare N., Dhobi M., Prasad S.K. Wound healing potential of Solanum xanthocarpum in streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2018;70:1389–1400. doi: 10.1111/jphp.12975. [DOI] [PubMed] [Google Scholar]
- 50.Pinto S.C.G., Bueno F.G., Panizzon G.P., Morais G., dos Santos P.V.P., Baesso M.L., de Souza Leite-Mello E.V., de Mello J.C.P. Stryphnodendron adstringens: Clarifying Wound Healing in Streptozotocin-Induced Diabetic Rats. Planta Med. 2015;81:1090–1096. doi: 10.1055/s-0035-1546209. [DOI] [PubMed] [Google Scholar]
- 51.Chokpaisarn J., Chusri S., Amnuaikit T., Udomuksorn W., Voravuthikunchai S.P. Potential wound healing activity of Quercus infectoria formulation in diabetic rats. Peerj. 2017;5:e3608. doi: 10.7717/peerj.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Özay Y., Güzel S., Yumrutaş Ö., Pehlivanoğlu B., Erdoğdu İ.H., Yildirim Z., Turk B.A., Darcan S. Wound healing effect of kaempferol in diabetic and nondiabetic rats. J. Surg. Res. 2019;233:284–296. doi: 10.1016/j.jss.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Ren J., Yang M., Chen J., Ma S., Wang N. Anti-inflammatory and wound healing potential of kirenol in diabetic rats through the suppression of inflammatory markers and matrix metalloproteinase expressions. Biomed. Pharmacother. 2020;129:110475. doi: 10.1016/j.biopha.2020.110475. [DOI] [PubMed] [Google Scholar]
- 54.Özay Y., Güzel S., Erdoğdu I.H., Yıldırım Z., Pehlivanoglu B., Türk B.A., Darcan S. Evaluation of the Wound Healing Properties of Luteolin Ointments on Excision and Incision Wound Models in Diabetic and Non-Diabetic Rats. Rec. Nat. Prod. 2018;12:350–366. doi: 10.25135/rnp.38.17.08.135. [DOI] [Google Scholar]
- 55.Lodhi S., Singhai A.K. Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia annua Linn. on streptozotocin induced diabetic rats. Asian Pac. J. Trop. Med. 2013;6:253–259. doi: 10.1016/S1995-7645(13)60053-X. [DOI] [PubMed] [Google Scholar]
- 56.Li J., Chou H., Li L., Li H., Cui Z. Wound healing activity of neferine in experimental diabetic rats through the inhibition of inflammatory cytokines and nrf-2 pathway. Artif. Cells Nanomed. Biotechnol. 2019;48:96–106. doi: 10.1080/21691401.2019.1699814. [DOI] [PubMed] [Google Scholar]
- 57.Lodhi S., Jain A.P., Sharma V.K., Singhai A.K. Wound-healing effect of flavonoid-rich fraction from Tephrosia purpurea Linn. on streptozotocin-induced diabetic rats. J. Herbs Spices Med. Plants. 2013;19:191–205. doi: 10.1080/10496475.2013.779620. [DOI] [Google Scholar]
- 58.Verma P.K., Ahmad M., Sultana M., Raina R., Pankaj N.K., Prawez S. Hypoglycemic, hypolipidemic, and wound healing potential of quercetin in streptozotocin-induced diabetic rats. Pharmacogn. Mag. 2017;13:S633–S639. doi: 10.4103/pm.pm_108_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guleken Z., Depciuch J., Ege H., Ilbay G., Kalkandelen C., Ozbeyli D., Bulut H., Sener G., Tarhan N., Kuruca S.E. Spectrochemical and biochemical assay comparison study of the healing effect of the Aloe vera and Hypericum perforatum loaded nanofiber dressings on diabetic wound. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021;254:119639. doi: 10.1016/j.saa.2021.119639. [DOI] [PubMed] [Google Scholar]
- 60.Shukla R., Kashaw S.K., Jain A.P., Lodhi S. Fabrication of Apigenin loaded gellan gum–chitosan hydrogels (GGCH-HGs) for effective diabetic wound healing. Int. J. Biol. Macromol. 2016;91:1110–1119. doi: 10.1016/j.ijbiomac.2016.06.075. [DOI] [PubMed] [Google Scholar]
- 61.Lai J.C.Y., Lai H.Y., Rao N.K., Ng S.F. Treatment for diabetic ulcer wounds using a fern tannin optimized hydrogel for-mulation with antibacterial and antioxidative properties. J. Ethnopharmacol. 2016;189:277–289. doi: 10.1016/j.jep.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 62.Karri V.V.S.R., Kuppusamy G., Talluri S.V., Mannemala S.S., Kollipara R., Wadhwani A.D., Mulukutla S., Raju K.R.S., Malayandi R. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int. J. Biol. Macromol. 2016;93:1519–1529. doi: 10.1016/j.ijbiomac.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 63.Ranjbar-Mohammadi M., Rabbani S., Bahrami S.H., Joghataei M., Moayer F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly(ε-caprolactone) electrospun nanofibers. Mater. Sci. Eng. C. 2016;69:1183–1191. doi: 10.1016/j.msec.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 64.Yang B.-Y., Hu C.-H., Huang W.-C., Ho C.-Y., Yao C.-H., Huang C.-H. Effects of Bilayer Nanofibrous Scaffolds Containing Curcumin/Lithospermi Radix Extract on Wound Healing in Streptozotocin-Induced Diabetic Rats. Polymers. 2019;11:1745. doi: 10.3390/polym11111745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao S.-Q., Chang C., Li J.-J., Li Y., Niu X.-Q., Zhang D.-P., Li L.-J., Gao J.-Q. Co-delivery of deferoxamine and hydroxysafflor yellow A to accelerate diabetic wound healing via enhanced angiogenesis. Drug Deliv. 2018;25:1779–1789. doi: 10.1080/10717544.2018.1513608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Almasian A., Najafi F., Eftekhari M., Ardekani M.R.S., Sharifzadeh M., Khanavi M. Polyurethane/carboxymethylcellulose nanofibers containing Malvasylvestris extract for healing diabetic wounds: Preparation, characterization, in vitro and in vivo studies. Material. Sci. Eng. C. 2020;114:111039. doi: 10.1016/j.msec.2020.111039. [DOI] [PubMed] [Google Scholar]
- 67.Yang Y., Wang F., Yin D., Fang Z., Huang L. Astragulus polysaccharide-loaded fibrous mats promote the restoration of microcirculation in/around skin wounds to accelerate wound healing in a diabetic rat model. Colloids Surf. B Biointerfaces. 2015;136:111–118. doi: 10.1016/j.colsurfb.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 68.Xu N., Wang L., Guan J., Tang C., He N., Zhang W., Fu S. Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int. J. Biol. Macromol. 2018;117:102–107. doi: 10.1016/j.ijbiomac.2018.05.066. [DOI] [PubMed] [Google Scholar]
- 69.Wang T., Liao Q., Wu Y., Wang X., Fu C., Geng F., Qu Y., Zhang J. A composite hydrogel loading natural polysaccharides derived from Periplaneta americana herbal residue for diabetic wound healing. Int. J. Biol. Macromol. 2020;164:3846–3857. doi: 10.1016/j.ijbiomac.2020.08.156. [DOI] [PubMed] [Google Scholar]
- 70.Gokce E.H., Tanrıverdi S.T., Eroglu I., Tsapis N., Gokce G., Tekmen I., Fattal E., Ozer O. Wound healing effects of collagenlaminin dermal matrix impregnated with resveratrol loaded hyaluronic acid-DPPC microparticles in diabetic rats. Eur. J. Pharmac. Biopharmac. 2017;119:17–27. doi: 10.1016/j.ejpb.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 71.Tan W.S., Arulselvan P., Ng S., Norma C., Taib M., Sarian M.N. Improvement of diabetic wound healing by topical ap-plication of Vicenin-2 hydrocolloid film on Sprague Dawley rats. BMC Compl. Altern. Med. 2019;19:20. doi: 10.1186/s12906-018-2427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohajeri G., Safaee M., Sanei M.H. Effects of topical Kiwifruit on healing of neuropathic diabetic foot ulcer. J. Res. Med Sci. 2014;19:520–524. [PMC free article] [PubMed] [Google Scholar]
- 73.Ghesmati F., Firoozi M., Varaeii S. Studying the effect of Aloe vera ointment on wound healing of CABG surgery in diabetic patients. J. Res. Med. Dent. Sci. 2018;6:256–260. [Google Scholar]
- 74.Kuo Y.S., Chien H.F., Lu W. Plectranthus amboinicus and Centella asiatica cream for the treatment of diabetic foot ulcers. Evid. BasedCompl. Altern. Med. 2012;2012:418679. doi: 10.1155/2012/418679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nasiri M., Fayazi S., Jahani S., Yazdanpanah L., Haghighizadeh M.H. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: A double-blind randomized clinical trial study in Iran. J. Diabetes Metab. Disord. 2015;14:38. doi: 10.1186/s40200-015-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ajmeer A.S., Dudhamal T.S., Gupta S.K., Mahanta V. Katupila (Securinega leucopyrus) as a potential option for diabetic wound management. J. Ayurveda Integr. Med. 2014;5:60. doi: 10.4103/0975-9476.128872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Phang S.J., Arumugam B., Kuppusamy U.R., Fauzi M.B., Looi M.L. A review of diabetic wound models—Novel insights into diabetic foot ulcer. J. Tissue Eng. Regen. Med. 2021;15:1051–1068. doi: 10.1002/term.3246. [DOI] [PubMed] [Google Scholar]
- 78.Pop-Busui R., Boulton A.J., Feldman E.L., Bril V., Freeman R., Malik R.A., Sosenko J.M., Ziegler D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care. 2016;40:136–154. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bandyk D.F. The diabetic foot: Pathophysiology, evaluation, and treatment. Seminars Vascul. Surg. 2018;31:43–48. doi: 10.1053/j.semvascsurg.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 80.Abbas M., Uçkay I., Lipsky B.A. In diabetic foot infections antibiotics are to treat infection, not to heal wounds. Expert Opin. Pharmacother. 2015;16:821–832. doi: 10.1517/14656566.2015.1021780. [DOI] [PubMed] [Google Scholar]
- 81.Adamu B.F., Gao J., Jhatial A.K., Kumelachew D.M. A review of medicinal plant-based bioactive electrospun nano fibrous wound dressings. Mater. Des. 2021;209:109942. doi: 10.1016/j.matdes.2021.109942. [DOI] [Google Scholar]
- 82.Gaspar-Pintiliescu A., Stanciuc A.-M., Craciunescu O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019;138:854–865. doi: 10.1016/j.ijbiomac.2019.07.155. [DOI] [PubMed] [Google Scholar]
- 83.Naomi R., Idrus R.B.H., Fauzi M. Plant- vs. Bacterial-Derived Cellulose for Wound Healing: A Review. Int. J. Environ. Res. Public Health. 2020;17:6803. doi: 10.3390/ijerph17186803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Awasthi A., Gulati M., Kumar B., Kaur J., Vishwas S., Khursheed R., Porwal O., Alam A., Kr A., Corrie L., et al. Recent Progress in Development of Dressings Used for Diabetic Wounds with Special Emphasis on Scaffolds. BioMed Res. Int. 2022;2022:1659338. doi: 10.1155/2022/1659338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghomi E.R., Khalili S., Nouri Khorasani S., Esmaeely Neisiany R., Ramakrishna S. Wound dressings: Current ad-vances and future directions. J. Appl. Polym. Sci. 2019;136:47738. doi: 10.1002/app.47738. [DOI] [Google Scholar]
- 86.Nielsen J., Fogh K. Clinical utility of foam dressings in wound management: A review. Chronic Wound Care Manag. Res. 2015;2:31. doi: 10.2147/cwcmr.s50832. [DOI] [Google Scholar]
- 87.Bai M.-Y., Chen M.-C., Yu W.-C., Lin J.-Y. Foam dressing incorporating herbal extract: An all-natural dressing for potential use in wound healing. J. Bioact. Compat. Polym. 2016;32:293–308. doi: 10.1177/0883911516672240. [DOI] [Google Scholar]
- 88.Do A.-V., Khorsand B., Geary S.M., Salem A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015;4:1742–1762. doi: 10.1002/adhm.201500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu M., Duan X.P., Li Y.M., Yang D.P., Long Y.Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C. 2017;76:1413–1423. doi: 10.1016/j.msec.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 90.Merrell J.G., McLaughlin S.W., Tie L., Laurencin C.T., Chen A.F., Nair L.S. Curcumin-loaded poly(ε-caprolactone) nanofibres: Diabetic wound dressing with anti-oxidant and anti-inflammatory properties. Clin. Exp. Pharmacol. Physiol. 2009;36:1149–1156. doi: 10.1111/j.1440-1681.2009.05216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hirsch T., Spielmann M., Zuhaili B., Koehler T., Fossum M., Steinau H.-U., Yao F., Steinstraesser L., Onderdonk A.B., Eriksson E. Enhanced susceptibility to infections in a diabetic wound healing model. BMC Surg. 2008;8:5. doi: 10.1186/1471-2482-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baig M.S., Banu A., Zehravi M., Rana R., Burle S.S., Khan S.L., Islam F., Siddiqui F.A., Massoud E.E.S., Rahman H., et al. An overview of diabetic foot ulcers and associated problems with special emphasis on treatments with antimi-crobials. Life. 2022;12:1054. doi: 10.3390/life12071054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perim M.C., Borges J.D.C., Celeste S.R.C., Orsolin E.D.F., Mendes R.R., Mendes G.O., Ferreira R.L., Carreiro S.C., Pranchevicius M.C.D.S. Aerobic bacterial profile and antibiotic resistance in patients with diabetic foot infections. Rev. Soc. Bras. Med. Trop. 2015;48:546–554. doi: 10.1590/0037-8682-0146-2015. [DOI] [PubMed] [Google Scholar]
- 94.Jneid J., Lavigne J., La Scola B., Cassir N. The diabetic foot microbiota: A review. Hum. Microbiome J. 2017;5-6:1–6. doi: 10.1016/j.humic.2017.09.002. [DOI] [Google Scholar]
- 95.Ramirez-Acuña J.M., A Cardenas-Cadena S., A Marquez-Salas P., Garza-Veloz I., Perez-Favila A., A Cid-Baez M., Flores-Morales V., Martinez-Fierro M.L. Diabetic Foot Ulcers: Current Advances in Antimicrobial Therapies and Emerging Treatments. Antibiotics. 2019;8:193. doi: 10.3390/antibiotics8040193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Appapalam S.T., Muniyan A., Mohan K.V., Panchamoorthy R. A Study on Isolation, Characterization, and Exploration of Multiantibiotic-Resistant Bacteria in the Wound Site of Diabetic Foot Ulcer Patients. Int. J. Low. Extrem. Wounds. 2019;20:6–14. doi: 10.1177/1534734619884430. [DOI] [PubMed] [Google Scholar]
- 97.Jaju K., Pichare A., Davane M., Nagoba B. Profile and antibiotic susceptibility of bacterial pathogens associated with dia-betic foot ulcers from a rural area. Wounds. 2019;31:158–162. [PubMed] [Google Scholar]
- 98.Herman A., Herman A.P. Herbal products and their active constituents used alone and in combination with antifungal drugs against drug-resistant Candida sp. Antibiotics. 2021;10:655. doi: 10.3390/antibiotics10060655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abid H.M.U., Hanif M., Mahmood K., Aziz M., Abbas G., Latif H. Wound-Healing and Antibacterial Activity of the Quercetin–4-Formyl Phenyl Boronic Acid Complex against Bacterial Pathogens of Diabetic Foot Ulcer. ACS Omega. 2022;7:24415–24422. doi: 10.1021/acsomega.2c01819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chokpaisarn J., Urao N., Voravuthikunchai S.P., Koh T.J. Quercus infectoria inhibits Set7/NF-κB inflammatory pathway in macrophages exposed to a diabetic environment. Cytokine. 2017;94:29–36. doi: 10.1016/j.cyto.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mlinaric-Missoni E., Kalenic S., Vukelic M., de Syo D., Belicza M., Vazic-Babic V. Candida infections of diabetic foot ulcers. Diabetol. Croat. 2005;34:29–35. [Google Scholar]
- 102.Gantwerker E.A., Hom D.B. Skin: Histology and Physiology of Wound Healing. Facial Plast. Surg. Clin. N. Am. 2011;19:441–453. doi: 10.1016/j.fsc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 103.Velander P., Theopold C.F.P., Hirsch T., Bleiziffer O., Zuhaili B., Fossum M., Hoeller D., Gheerardyn R., Chen M., Visovatti S., et al. Impaired wound healing in an acute diabetic pig model and the effects of local hyperglycemia. Wound Repair Regen. 2008;16:288–293. doi: 10.1111/j.1524-475X.2008.00367.x. [DOI] [PubMed] [Google Scholar]
- 104.Shaikh-Kader A., Houreld N.N., Rajendran N.K., Abrahamse H. The link between advanced glycation end products and apoptosis in delayed wound healing. Cell Biochem. Funct. 2019;37:432–442. doi: 10.1002/cbf.3424. [DOI] [PubMed] [Google Scholar]
- 105.Rosyid F.N. Etiology, pathophysiology, diagnosis and management of diabetics’ foot ulcer. Int. J. Res. Med Sci. 2017;5:4206. doi: 10.18203/2320-6012.ijrms20174548. [DOI] [Google Scholar]
- 106.Deng L., Du C., Song P., Chen T., Rui S., Armstrong D.G., Deng W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxidative Med. Cell. Longev. 2021;2021:8852759. doi: 10.1155/2021/8852759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ahmadinejad F., Geir Møller S., Hashemzadeh-Chaleshtori M., Bidkhori G., Jami M.-S. Molecular Mechanisms behind Free Radical Scavengers Function against Oxidative Stress. Antioxidants. 2017;6:51. doi: 10.3390/antiox6030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sanchez M.C., Lancel S., Boulanger E., Neviere R. Targeting oxidative stress and mitochondrial dysfunction in the treat-ment of impaired wound healing: A systematic review. Antioxidants. 2018;7:98. doi: 10.3390/antiox7080098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schinella G.R., Tournier H.A., Prieto J.M., De Buschiazzo P.M., Ríos J.L. Antioxidant activity of anti-inflammatory plant extracts. Life Sci. 2002;70:1023–1033. doi: 10.1016/S0024-3205(01)01482-5. [DOI] [PubMed] [Google Scholar]
- 110.Savych A., Mazur O. Antioxidant activity in vitro of antidiabetic herbal mixtures. Pharmacol. OnLine. 2021;2:17–24. [Google Scholar]
- 111.Wolf S.J., Melvin W.J., Gallagher K. Macrophage-mediated inflammation in diabetic wound repair. Semin. Cell Dev. Biol. 2021;119:111–118. doi: 10.1016/j.semcdb.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grosick R., Alvarado-Vazquez P.A., Messersmith A.R., Romero-Sandoval E.A. High glucose induces a priming effect in macrophages and exacerbates the production of pro-inflammatory cytokines after a challenge. J. Pain Res. 2018;11:1769–1778. doi: 10.2147/JPR.S164493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boniakowski A.E., Kimball A.S., Jacobs B.N., Kunkel S.L., Gallagher K.A. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J. Immunol. 2017;199:17–24. doi: 10.4049/jimmunol.1700223. [DOI] [PubMed] [Google Scholar]
- 114.Ganesh G.V., Ramkumar K.M. Macrophage mediation in normal and diabetic wound healing responses. Inflamm. Res. 2020;69:347–363. doi: 10.1007/s00011-020-01328-y. [DOI] [PubMed] [Google Scholar]
- 115.Barman P.K., Koh T.J. Macrophage Dysregulation and Impaired Skin Wound Healing in Diabetes. Front. Cell Dev. Biol. 2020;8:528. doi: 10.3389/fcell.2020.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mirza R.E., Fang M.M., Ennis W.J., Koh T.J. Blocking Interleukin-1β Induces a Healing-Associated Wound Macrophage Phenotype and Improves Healing in Type 2 Diabetes. Diabetes. 2013;62:2579–2587. doi: 10.2337/db12-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dai J., Shen J., Chai Y., Chen H. IL-1β Impaired Diabetic Wound Healing by Regulating MMP-2 and MMP-9 through the p38 Pathway. Mediat. Inflamm. 2021;2021:6645766. doi: 10.1155/2021/6645766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kong M., Xie K., Lv M., Li J., Yao J., Yan K., Wu X., Xu Y., Ye D. Anti-inflammatory phytochemicals for the treatment of diabetes and its complications: Lessons learned and future promise. Biomed. Pharmacother. 2020;133:110975. doi: 10.1016/j.biopha.2020.110975. [DOI] [PubMed] [Google Scholar]
- 119.Hou Q., He W.-J., Hao H.-J., Han Q.-W., Chen L., Dong L., Liu J.-J., Li X., Zhang Y.-J., Ma Y.-Z., et al. The Four-Herb Chinese Medicine ANBP Enhances Wound Healing and Inhibits Scar Formation via Bidirectional Regulation of Transformation Growth Factor Pathway. PLoS ONE. 2014;9:e112274. doi: 10.1371/journal.pone.0112274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grazul-Bilska A.T., Johnson M.L., Bilski J.J., A Redmer D., Reynolds L.P., Abdullah A., Abdullah K.M. Wound healing: The role of growth factors. Drugs Today. 2003;39:787–800. doi: 10.1358/dot.2003.39.10.799472. [DOI] [PubMed] [Google Scholar]
- 121.Zubair M., Ahmad J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev. Endocr. Metab. Disord. 2019;20:207–217. doi: 10.1007/s11154-019-09492-1. [DOI] [PubMed] [Google Scholar]
- 122.Roberts A.B. Transforming growth factor-β: Activity and efficacy in animal models of wound healing. Wound Repair Regen. 1995;3:408–418. doi: 10.1046/j.1524-475X.1995.30405.x. [DOI] [PubMed] [Google Scholar]
- 123.Bitar M.S., Labbad Z.N. Transforming growth factor-β and insulin-like growth factor I in relation to diabetes-induced im-pairment of wound healing. J. Surg. Res. 1996;6:113–119. doi: 10.1006/jsre.1996.0090. [DOI] [PubMed] [Google Scholar]
- 124.García-Ojalvo A., Berlanga Acosta J., Figueroa-Martínez A., Béquet-Romero M., Mendoza-Marí Y., Fernández-Mayola M., Fabelo-Martinez A., Guillén-Nieto G. Systemic translation of locally infiltrated epidermal growth factor in diabetic lower extremity wounds. Int. Wound J. 2019;16:1294–1303. doi: 10.1111/iwj.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dogan S., Demirer S.E.H.E.R., Kepenekci I., Erkek B., Kiziltay A.Y.S.E.L., Hasirci N.E.S.R.İ.N., Müftüoglu S., Nazikoglu A., Renda N., Dincer U.D., et al. Epidermal growth factor-containing wound closure en-hances wound healing in non-diabetic and diabetic rats. Int. Wound J. 2009;6:107–115. doi: 10.1111/j.1742-481X.2009.00584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peng C., Chen B., Kao H.-K., Murphy G., Orgill D.P., Guo L. Lack of FGF-7 Further Delays Cutaneous Wound Healing in Diabetic Mice. Plast. Reconstr. Surg. 2011;128:673e–684e. doi: 10.1097/PRS.0b013e318230c521. [DOI] [PubMed] [Google Scholar]
- 127.Werner S., Breeden M., Hübner G., Greenhalgh D.G., Longaker M.T. Induction of Keratinocyte Growth Factor Expression Is reduced and Delayed During Wound Healing in the Genetically Diabetic Mouse. J. Investig. Dermatol. 1994;103:469–473. doi: 10.1111/1523-1747.ep12395564. [DOI] [PubMed] [Google Scholar]
- 128.Wojtowicz A.M., Oliveira S., Carlson M.W., Zawadzka A., Rousseau C.F., Baksh D. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair Regen. 2014;22:246–255. doi: 10.1111/wrr.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Soares R. Angiogenesis in diabetes. Unraveling the angiogenicparadox. Open Circ.Vasc. J. 2010;3:3–9. doi: 10.2174/1877382601003020003. [DOI] [Google Scholar]
- 130.Nishikori Y., Shiota N., Okunishi H. The role of mast cells in cutaneous wound healing in streptozotocin-induced diabetic mice. Arch. Dermatol. Res. 2014;306:823–835. doi: 10.1007/s00403-014-1496-0. [DOI] [PubMed] [Google Scholar]
- 131.Tellechea A., Leal E.C., Kafanas A., Auster M.E., Kuchibhotla S., Ostrovsky Y., Tecilazich F., Baltzis D., Zheng Y., Carvalho E., et al. Mast Cells Regulate Wound Healing in Diabetes. Diabetes. 2016;65:2006–2019. doi: 10.2337/db15-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu L., Marti G.P., Wei X., Zhang X., Zhang H., Liu Y.V., Semenza G.L., Harmon J.W. Age-dependent impairment of HIF-1α̣ expression in diabetic mice: Correction with electroporation-facilitated gene therapy increases wound healing, an-giogenesis, and circulating angiogenic cells. J. Cell Physiol. 2008;217:319–327. doi: 10.1002/jcp.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sharma K., Ziyadeh F.N. Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediator. Diabetes. 1995;44:1139–1146. doi: 10.2337/diab.44.10.1139. [DOI] [PubMed] [Google Scholar]
- 134.Greenhalgh D.G., Sprugel K.H., Murray M.J., Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am. J. Pathol. 1990;136:1235–1246. [PMC free article] [PubMed] [Google Scholar]
- 135.Li J., Li R., Wu X., Zheng C., Shiu P.H.-T., Rangsinth P., Lee S.M.-Y., Leung G.P.-H. An Update on the Potential Application of Herbal Medicine in Promoting Angiogenesis. Front. Pharmacol. 2022;13:928817. doi: 10.3389/fphar.2022.928817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this article.