Abstract
Extracellular proteases belong to the main virulence factors of pathogenic fungi. Their proteolytic activities plays a crucial role in the acquisition of nutrients from the external environment, destroying host barriers and defenses, and disrupting homeostasis in the human body, e.g., by affecting the functions of plasma proteolytic cascades, and playing sophisticated regulatory roles in various processes. Interestingly, some proteases belong to the group of moonlighting proteins, i.e., they have additional functions that contribute to successful host colonization and infection development, but they are not directly related to proteolysis. In this review, we describe examples of such multitasking of extracellular proteases that have been reported for medically important pathogenic fungi of the Candida, Aspergillus, Penicillium, Cryptococcus, Rhizopus, and Pneumocystis genera, as well as dermatophytes and selected endemic species. Additional functions of proteinases include supporting binding to host proteins, and adhesion to host cells. They also mediate self-aggregation and biofilm formation. In addition, fungal proteases affect the host immune cells and allergenicity, understood as the ability to stimulate a non-standard immune response. Finally, they play a role in the proper maintenance of cellular homeostasis. Knowledge about the multifunctionality of proteases, in addition to their canonical roles, greatly contributes to an understanding of the mechanisms of fungal pathogenicity.
Keywords: Candida, Aspergillus, dermatophytes, Sap, yapsins, proteinases, allergens
1. Fungal Proteases Are Important Virulence Factors
Among the wide range of virulence factors found in pathogenic fungi, hydrolytic enzymes are a key component contributing to the invasiveness of these microorganisms. Phospholipases, lipases, proteases, hemolysins, phosphatases, ureases, and other hydrolases are an effective fungal arsenal for successful penetration, survival, and dissemination within the host organism [1,2,3]. Extracellular proteases are primarily important for the acquisition of nutrients from the environment, and they are necessary for the proper growth and development of fungal cells. Furthermore, they are also strongly involved in host tissue damage and overcoming host defense barriers, including the epithelium, basement membrane, interstitial matrix, and endothelial cell layer, facilitating fungal spread and progression of the infection. Proteolytic activity also allows for the effective evasion of the host immune system through the degradation of host defense proteins and peptides, and the dysregulation of plasma proteolytic cascades [4]. Thus, these enzymes can be considered as actually multitasking. However, these are functionalities that directly and naturally result from the degradative potential of fungal proteases, which, however, depends on the substrate specificity of individual enzymes. For some proteases, it may be broad, making their action multifaceted, while other proteolytic enzymes are more specifically targeted at selected substrates and may participate in a refined manner in the regulatory actions of various processes [5].
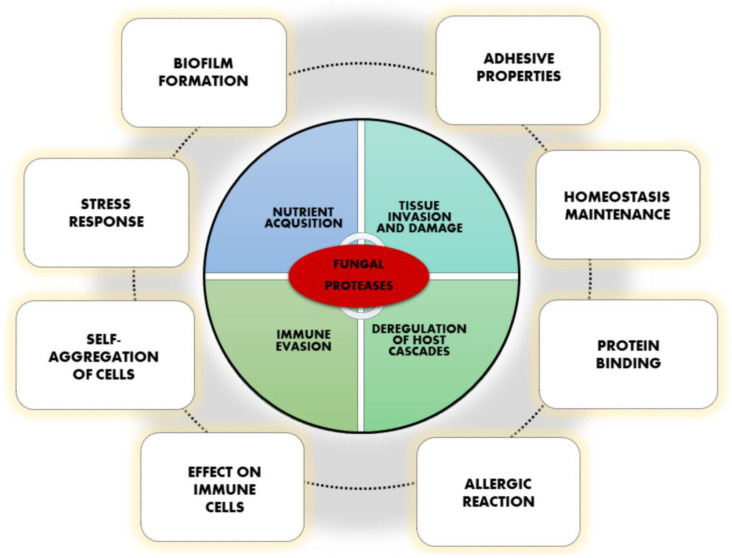
Recently, several reports have revealed new additional functions of fungal proteases that are important during infection, albeit not directly related to their proteolytic activity and destructive abilities (Figure 1) [4]. Although numerous different proteins could be designated as being bifunctional or multifunctional, a quite specific group called moonlighting proteins stands out among them and could be distinguished on the basis of performing an additional function, essentially distinct from the primary, evolutionary conserved activity, and often in a diverse cellular localization [6,7]. Moonlighting proteins are not pleiotropic proteins, splice variants, or effects of gene fusion or products of proteolytic fragmentation, and they often do not occur repeatedly between taxonomically related organisms [6,8]. Unraveling the novel functions of fungal proteases may place these enzymes not only within a diverse group of multifunctional proteins, but may even assign the moonlighting protein attributes to selected representatives, primarily on the basis of their proteolytic-independent roles. A similar phenomenon has also been demonstrated for bacterial proteases, for which, in addition to proteolysis, participation in adhesion, protein folding, or cell signaling are frequently reported [9].
Figure 1.
Various functions of fungal proteases important in virulence.
The canonical catalytic activity of proteases is limited to a relatively small area of the molecule; other remaining surfaces may be involved in regulatory functions, extracellular secretion, and protease deposition in a specific cellular location, as well as in interactions with the hydrolyzed substrate, and they might be also convenient for creating alternative functionalities [10]. Presumably, the selective pressure on the establishment of novel beneficial functions by evolutionarily old proteins and the tendency to limit the energy costs by cells, related to the use of existing molecules for divergent purposes, might also contribute to the emergence of new, nonclassical functions of fungal proteases [10,11]. The involvement of protein multifunctionality in the virulence process may be particularly useful for microbial pathogens, which can thus achieve even more benefits during host invasion by using not only the available arsenal of classic virulence factors with their canonical activities, but also by exploiting the additional potential of produced proteins. In this review, we focus mainly on the non-canonical functions of fungal proteases, distinct from their evident proteolytic activities, and that are important in the pathogeneses of fungal infections, which further determine the classification of certain proteases as moonlighting proteins. In addition, we also refer to the proteolytic activity of fungal enzymes that are not directly related to the degradation of different substrates, but that are based on the more subtle modulation of various mechanisms contributing to the enhancement of fungal virulence.
Currently, several hundred species of fungi are considered as being important infectious agents that endanger the human population, being responsible for various types of infections, including burdensome superficial mycoses and difficult-to-treat invasive fungal infections that are associated with high mortality rates [12,13]. Among the medically important fungi are the Candida species, including the most widely distributed C. albicans, as well as the emerging non-albicans Candida yeasts; furthermore, the ubiquitous fungi Aspergillus fumigatus, Cryptococcus neoformans, Pneumocystis jiroveci, and dermatophytes; and notable endemic fungal species from the genera Paracoccidioides, Blastomyces, Histoplasma, Coccidioides, and Sporothrix [14]. These microorganisms produce a wide variety of proteases belonging to different classes, distinguished on the basis of the catalytic mechanism (Table 1). For some particular fungal proteolytic enzymes, multifunctionality has been frequently demonstrated, as well as its significant contribution to fungal virulence. The best documented examples of such additional functionalities for extracellular proteases produced by selected fungal species are discussed below.
Table 1.
Examples of extracellular proteases produced by selected fungi that are pathogenic for humans.
| Fungal Species | Identified Proteases | Protease Classification |
|---|---|---|
| Candida spp. | ||
| C. albicans | Sap1-Sap10 | aspartic proteases |
| C. glabrata | yapsins (CgYps1-11) |
aspartic proteases |
| C. parapsilosis | Sapp1, Sapp2 | aspartic proteases |
| Aspergillus spp. | ||
| secreted subtilisins (i.e., Alp1/Asp f 13) | serine proteases | |
| elastinolytic proteases Mep, fungalysins (i.e., Asp f 5) |
metalloproteases | |
| secreted aspartyl endopeptidase (i.e., aspergillopepsin-1/ Asp f 10) |
aspartic proteases | |
| protease PnmB | serine protease | |
| Penicillium spp. | ||
| secreted subtilisin-like proteases (i.e., Pen c 13, Pen ch 13) |
serine proteases | |
| Cryptococcus neoformans | ||
| major aspartyl peptidase 1 May1 | aspartic protease | |
| endopeptidase Prb1 | serine protease | |
| secreted metalloprotease Mpr1 | metalloprotease | |
| Pneumocystis jiroveci | kexin (Kex1) | serine protease |
| Rhizopus oryzae | rhizopuspepsin, Rhi o 1 | aspartic protease |
| Malassezia furfur | secretory aspartyl protease Mfsap1 | aspartic protease |
| Dermatophytes | ||
| secreted subtilisins (Sub2–Sub7) |
serine proteases | |
| fungalysins (Mep1, Mep3, Mep4) |
metalloproteases | |
| dipeptidyl peptidases (DppIV, DppV) | serine exoproteases | |
| Endemic fungi | ||
| Paracoccidioides brasiliensis | extracellular subtilisin-like protease (PbSP) | serine protease |
| Histoplasma capsulatum | N-acetylated α-linked acidic dipeptidase (NAALADase) | metallocarboxypeptidase |
| Sporothrix schenckii | proteinase I | serine protease |
| proteinase II | cysteine protease |
2. Involvement in Binding, Adhesion, Self-Aggregation, and Biofilm Formation
The ability of fungi to adhere to the surface of host cells and tissues, as well as to bind to host proteins and to produce biofilms on various surfaces, is crucial for the development of fungal infection. Fungal pathogens have exploited a number of different mechanisms that facilitate adhesion, based primarily on the production of specialized adhesive proteins exposed on the surfaces of fungal cells—mainly typical adhesins belonging to glycosylphosphatidylinositol (GPI)-anchored cell wall proteins, and also the involvement of other cell wall components, including polysaccharides, as well as general cell surface hydrophobicity, and electrostatic and van der Waals forces [15]. Furthermore, the cooperation of surface moonlighting proteins, many of which are cytoplasmic enzymes by origin, such as enolase, triosephosphate isomerase, or aldolase, also contributes to enhanced adhesion, as has been repeatedly demonstrated for different fungi [7,16]. In addition, it has been shown that fungal proteases may also reveal extraordinary functions that are related to adhesion.
To date, at least two proposed explanations are considered on how fungal proteases can support adhesion to host cells. First, that secreted proteases have the ability to proteolytically modify the surfaces of host cells or fungal cells to reveal proteins that are more suitable ligands for fungal binding or more efficient pathogen adhesins, respectively. Otherwise, fungal cell surface proteases themselves may serve as ligands that are involved in the binding of host cells or proteins, independently of their proteolytic activities [2,17]. The latter hypothesis allows for the assignment of moonlighting functions to particular proteases, while the former is based on the canonical functions of these proteins, which is proteolytic activity, although directed at the modulation of the properties of fungal cells related to their virulence, and interactions with the host and the environment. Furthermore, the overlapping mechanisms of the participation of proteases in the adhesion phenomenon cannot be excluded either.
Extensively studied examples of multifunctional proteases are secreted aspartyl proteases (Saps) produced by Candida species, which are involved in numerous roles related to the regulation of cell adhesion and biofilm formation, also in complex environments. The results presented for C. albicans have indicated that fungal biofilm formation was positively associated with the secretion of proteases [18], and individual proteases from the Sap family were often involved in adhesion and invasion on epithelial cells [19,20]. C. albicans Sap9 and Sap10, which are GPI-anchored glycosylated proteins located in the cell membrane and/or the cell wall, unlike other members of the Sap family, were indicated to play an important role in the maintenance of fungal cell wall integrity, since mutants lacking these proteinases exhibited impaired growth in the presence of substances that affect the cell wall, including hygromycin B, calcofluor, Congo red, or itraconazole, and they were characterized by the increased level of cell wall chitin [21]. Additionally, these mutants demonstrated altered binding and invasion of epithelial cells; since SAP9-deficient cells showed increased adhesion, SAP10-deficient cells presented a lower level of adhesion compared to the wild-type strain, while the mutant lacking both proteases also had reduced adhesion; however, both single mutants presented a reduced capacity to invade and to damage reconstituted human epithelium in the model of oral infection [21]. Although Albrecht et al. [21] did not exclude the action of Sap10 as a typical adhesin, the influence of both proteinases on the adhesion of fungal cells was attributed mainly to their enzymatic activity and the proteolytic modulation of fungal surface proteins, which was particularly significant in the light of further reports on the abilities of Sap9 and Sap10 to hydrolyze a wide variety of covalently linked GPI-modified surface proteins, including chitinase Cht2, transglucosidase Pga4, or Als2 adhesin from the agglutinin-like sequence (Als) family [22]. Therefore, the results from the work of Schild et al. [22], describing the influences of Sap9 and Sap10 on the maintenance of chitinase activity, corroborated previous findings describing the increase in the amount of chitin in the cell wall in mutants lacking both proteases [21]. Furthermore, a C. albicans mutant strain lacking SAP9 displayed a reduction in yeast-to-hyphae transformation under serum hypha-induced conditions, and a noticeable downregulation of the transcription factor Efg1, essential in inducing the process of morphological transition [23,24]. As a consequence, this resulted in a significant decrease in the endocytosis of the sap9Δ/Δ mutant strain by host oral epithelial cells [24]. Sap9 was also indicated as one of the factors that are involved in protein–receptor interactions between fungal cells, fungal biofilm formation, and in the interactions of C. albicans with other microorganisms in mixed species biofilms [25]. Mono-species biofilms formed by the C. albicans mutant strain deprived of SAP9 were characterized as being more compacted, and with a larger number of condensed hyphae, when compared to a wild type strain, for which biofilms were more spatially developed. Similarly, such an observation was also reported for mixed biofilms formed with the bacteria of Streptococcus species; again, the biofilm architecture for the mutant strain was somewhat different, with more bacterial cells being accumulated on the saliva-coated cover slips, than was observed for the mixed biofilm formed by the wild type C. albicans strain and bacteria. Thus, the obtained results implied that Sap9 might be advantageous in facilitating C. albicans competition with streptococci for salivary pellicle colonization [25].
For other members of the C. albicans Sap family, it has been shown that Sap7 facilitated fungal adhesion to the monolayers of colon adenocarcinoma-derived cells in the initial stages of invasion [26]. Additionally, Sap1 was demonstrated to be involved in the colonization of skin in a cutaneous mouse model, and the adhesion step was independent of Sap1 proteolytic activity, contrary to the stage of cavitation on the skin surface, while both mechanisms were required for skin invasion [27,28]. Furthermore, for C. albicans, Sap4-Sap6 proteinase adhesive properties were repeatedly reported, as these proteinases are equipped with amino acid sequences RGD/KGD that are identified as integrin-binding fragments. Sap4 has one RGD motif and Sap5 contains the RGDKGD sequence, while Sap6 has two sequential RGD motifs, all of which are located at the surface-exposed tip of the arm 1 loop situated close to the cavity with the enzyme active site [29]. These motifs were found as being responsible for the binding of Sap4-Sap6 to integrins localized on the surfaces of oral epithelial cells. After this interaction, proteinases were internalized to endosome and lysosome, and then they permeabilized the lysosomal membrane, leading to the apoptosis of host cells [29]. Additionally, Kumar et al. [17] reported that in a murine model of oral candidiasis, the C. albicans mutant strain overexpressing SAP6 formed thicker fungal plaques than the mutant strains lacking Sap6 enzyme. Moreover, Sap6, independently of its proteolytic activity, enabled the aggregation of C. albicans cells—the effect that was assigned to RGDRGD sequence binding to germinated fungal cells [17]. Furthermore, for Sap6, the ability to bind zinc ions was also revealed, as well as the presence of four amyloid-forming regions, which, in the presence of zinc, were additionally responsible for fungal cell aggregation [30]. Hence, Sap6 can be considered as the truly multifunctional, and even moonlighting protein responsible for the assembly of cells in the biofilm, regulating adhesion and the uptake of zinc [30].
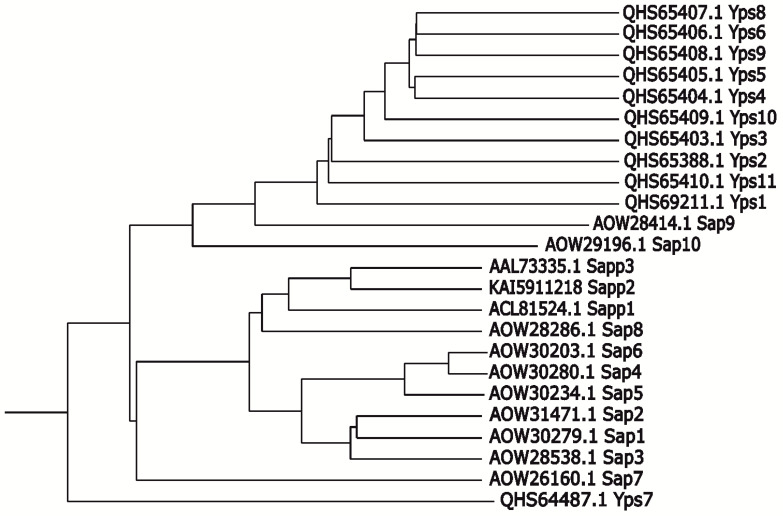
For C. parapsilosis, with the use of mutant strains with the expression of only a single aspartic proteinase—Sapp1, Sapp2, or Sapp3, the participation of Sapp1 and Sapp2, but not Sapp3, in the binding of C. parapsilosis to the TR146 oral epithelial cell line was demonstrated; however, the mechanisms of these interactions require further elucidation [31]. Of these secreted enzymes, Sapp1 was indicated as being temporarily embedded within the C. parapsilosis cell wall, and capable of operating in the immediate vicinity of the fungal cells; therefore, its functionality may be similar to those described for Sap9 and Sap10 of C. albicans; nonetheless, this issue requires further research [32]. For C. glabrata, a family of eleven GPI-linked aspartyl proteases, referred to as yapsins (CgYps) were identified, and their similarity to C. albicans Sap9 and Sap10 proteases was also proposed (Figure 2) [33,34,35]. On the basis of analyses performed using C. glabrata mutant strains with the deletion of genes encoding representatives of CgYps, especially CgYPS1 and CgYPS7, it has been shown that CgYps play a key role in the colonization, dissemination, and long-term maintenance of C. glabrata in the kidney, liver, and spleen, in a mouse model of systemic infection [33,36]. Furthermore, CgYps participated in the colonization of brain tissues [36]. Additionally, the Cgyps1-11Δ mutant was shown to be impaired in biofilm formation on abiotic surfaces, while CgYPS1 expression fully complemented this defect [36]. Thus, it seems that this proteinase plays an indirect role in biofilm formation by acting as a modulator of β-glucan exposition on the yeast cell wall [36].
Figure 2.
Dendrogram presenting the relationship of Candida spp. extracellular proteases. Prepared using the T-REX tool (http://www.trex.uqam.ca/) [37].
For fungal species other than Candida, there have also been a few reports on the involvement of proteases in adhesion, but their mechanisms of interaction were not always identified, and it was not determined whether they are dependent on or independent of proteolytic activity. For Microsporum canis, a dermatophyte capable of infecting cats, dogs, and humans, the involvement of the secreted subtilisin Sub3 in fungal adhesion to the feline epidermis was demonstrated, although it was shown not to be crucial for invasion on host cells [38,39]. Nevertheless, the exact mechanisms of Sub3 adhesion have not yet been identified, and it is not clear whether it is based on the ability to perform the proteolytic modeling of other surface molecules, or whether Sub3 itself acts as a ligand for binding [40]. With the use of immunoproteomics, Aspergillus fumigatus alkaline protease 1 (Alp1/Asp f 13/oryzin) was identified as one of the fibrinogen-binding proteins, involved in the interactions of fungi with the human extracellular matrix, and thus promoting fungal growth, the adherence of fungal conidia, and infection development [41]. For Cryptococcus neoformans—an encapsulated fungus responsible for pneumonia and meningitis in immunocompromised individuals—the activity of serine protease, as well as secreted metalloprotease Mpr1, was demonstrated as being pivotal for interactions with microvascular endothelial cells and the disruption of the blood–brain barrier, facilitating subsequent fungal traversal to the central nervous system [42,43]. Moreover, Mjokane et al. [44,45] speculated recently that the activity of uncharacterized serine protease from C. neoformans may activate the SARS-CoV-2 spike (S) protein, with consequent membrane fusion and the endocytosis of virial particles by host cells, thus facilitating the development of the coronavirus disease COVID-19 in patients affected with concomitant fungal infection [44,45].
3. Effect on Cells of the Host Immune System
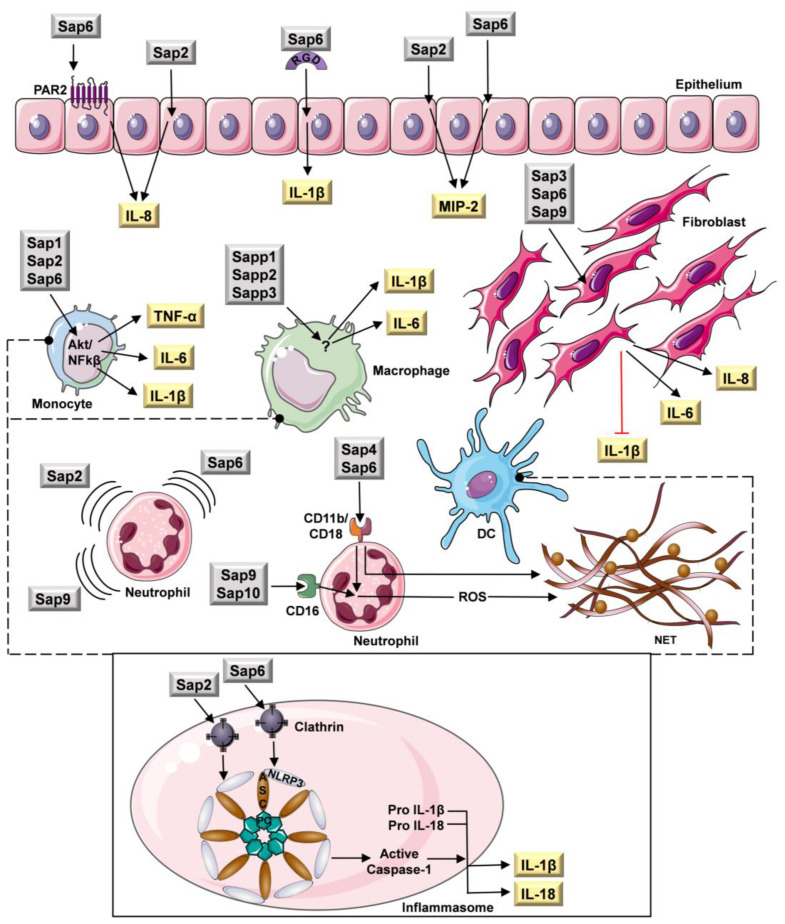
During the development of infection, pathogenic fungi are constantly exposed to varied activities of the host immune system, including the action of numerous molecules involved in the immune response and various types of host defense cells, aimed at destroying the microbial invaders. The human immunity directed at fungi comprises innate immunity and adaptive immunity; the former includes the complex antimicrobial activities of phagocytic cells—neutrophils, mononuclear leukocytes (monocytes and macrophages), and dendritic cells (DC). There is also the involvement of natural killer cells, γδ T cells, epithelium and endothelium, the action of cellular pattern recognition receptors, and the antigen presentation and production of cytokines and chemokines (reviewed in detail in [46]). Several mechanisms used by fungi to evade the host immune system are directly related to the proteolytic degradation of host defense proteins, i.e., immunoglobulins, lactoferrin, lactoperoxidase, components, and the regulators of the complement system and antifungal peptides, resulting in their inactivation [4]. However, it has been demonstrated that fungal proteases may also be involved in more refined interactions with the host immune system, affecting host cells and modulating their functionalities, as well as modifying the effectiveness of cytokines, based not only on proteolysis, but also independently of enzymatic activity. For instance, fungal proteases are able to regulate the host inflammatory cytokine response, not only via proteolytic activation of the interleukin 1β (IL-1β) precursor [47] or the degradation of various cytokines [48], but also by stimulating different cells to secrete these molecules, or by inhibiting their production (Figure 3). For example, fungal proteases may activate dedicated receptors present at the surfaces of different types of host cells—protease-activated receptors (PARs)—triggering the intracellular response through nuclear factor-κB (NF-κB), and the production of a variety of cytokines and chemokines that are capable of further stimulation of dendritic cells [49,50,51]. PARs are transmembrane proteins belonging to the G-protein-coupled receptor (GPCR) family, the activation of which consists of the targeted proteolysis of the extracellular N-terminus, followed by the exposition of an intramolecular tethered ligand and further intracellular signal transduction [52].
Figure 3.
Multitasking of Candida spp. extracellular proteases in relation to the host immune cells. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license. ASC—apoptosis-associated speck-like protein containing a CARD, DC—dendritic cells, IL—interleukin, NET—neutrophil extracellular traps, NLRP3—intracellular NOD-like receptor 3, PAR2—proteinase-activated receptor 2, PC—pro-caspase-1, ROS—reactive oxygen species, Sap—secreted aspartyl proteinases, TNF-α—tumor necrosis factor α, MIP-2—macrophage inflammatory protein 2.
However, it was also demonstrated that C. albicans Sap1-Sap3 and Sap6, independent of their proteolytic activity, pH optima, and PAR activation, possessed the ability to induce human monocytes to the secretion of the proinflammatory cytokines IL-1β and TNF-α, via Akt/NF-κB activation, and that they were also capable of inducing Ca2+ influx. In the same studies, it was also shown that similarly, Sap1, Sap2, and Sap6 stimulated these host cells toward the production of interleukin 6 (IL-6) [53]. IL-1β was also secreted by oral epithelial cells after contact with Sap6, independent of its enzymatic activity. The interaction of host cells with the RGD integrin-binding domain of the Sap6 molecule was considered as being essential to initiate this process [54,55]. In the case of fibroblasts, Sap3, Sap6, and Sap9 stimulated the secretion of IL-6, but restrained the production of IL-1β; however, the mechanism of interactions was not indicated [56]. For C. parapsilosis, there is also the indirect evidence of the stimulation of cytokine production in response to secreted proteinases. Singh et al. [31] demonstrated that macrophages stimulated with mutants deprived of SAPP1-SAPP3 produced significantly less IL-1β and IL-6, and moderately, although not significantly, less IL-8 than after stimulation with the wild-type strain. Fungal proteinases can also stimulate host cells to the production of chemokines, which induce neutrophil influx to the site of invasion. Gabrielli et al. [57] demonstrated that C. albicans Sap2 and Sap6 induced the secretion of IL-8 and macrophage inflammatory protein 2 (MIP-2) by vaginal epithelial cells. This phenomenon was partially reduced in the presence of pepstatin A, which is an inhibitor of aspartic proteases. This suggests an at least partial contribution of the proteolytic activities of Sap2 and Sap6 to this stimulation [57]. Moreover, Kumar et al. [55] showed that Sap6 also stimulated the oral epithelial cells to IL-8 production, albeit probably via a mechanism dependent on its protease activity and p38/c-Fos signaling, which was initiated by the proteolytic activation of PAR-2 localized on the cell surface. The enhanced secretion of IL-8 was also observed after the contact of fibroblast with Sap3, Sap6, and Sap9 [56]. In addition, there are several reports of fungal proteinases possessing chemoattractant properties, similar to chemokines [58]. Hornbach et al. [59] demonstrated that Sap9 directly stimulated neutrophils to chemotaxis toward C. albicans [59].
In a murine vaginitis model, the same activity was demonstrated also for Sap2, when neutrophils were found to migrate to the infection site in response to this proteinase [60]. Additionally, Gabrielli et al. [57] demonstrated that Sap2 and Sap6 might also act as direct chemoattractants in vitro, and that their enzymatic activities were not necessary for the Sap-induced migration of neutrophils, which allows them to be referred to as moonlighting proteins.
Inflammasome NLRP3 is proinflammatory multiprotein complex consisting of intracellular NOD-like receptor 3 (NLRP3), and apoptosis-associated speck-like protein containing a CARD (ASC) and pro-caspase-1 (PC). After activation, caspase-1 converts the precursors of IL-1β and interleukin 18 (IL-18) to the active forms. The complex is activated by diverse stimuli, including ionic flux, mitochondrial dysfunction, the generation of reactive oxygen species (ROS), and lysosomal damage [61]. Pietrella et al. [62] demonstrated that the NLRP3 inflammasome may be directly activated by Sap2 and Sap6. Both enzymes activated NLRP3 and caspase-1, after internalization mediated by clathrin, with the consequent secretion of maturated IL-1β and IL-18. This phenomenon was observed in monocytes, macrophages, and dendritic cells, regardless of pepstatin A presence, indicating that it proceeded independently of the proteolytic activities of the fungal enzymes [62]. A non-canonical inflammasome activation pathway through type I interferon production and the activation of caspase-11 that activates caspase-1 was also reported for Sap2 and Sap6 in murine macrophage cell lines. This indirect stimulation also required the internalization of proteinases [63]. As reported, macrophages expressed caspase-11 in response to both Sap2 and Sap6, and the response was more extended for Sap2. Importantly, the activation of caspase-11 did not affect Sap-induced NLRP3 expression, but it also potentiated caspase-1 activation, and as a result, the secretion of proinflammatory cytokines [63].
Hornbach et al. [59] reported that the deletion of the SAP9 gene reduces the C. albicans-mediated apoptosis of human polymorphonuclear neutrophils (PMNs) via the inhibition of ROS release, while the process of programmed cell death is fundamental in the regulation of neutrophil homeostasis and balancing infection [59]. It has also been shown that the contact of neutrophils with proteinases Sap4, Sap6, Sap9, and Sap10 elicited an effective cell response to microbial stimuli and neutrophil extracellular traps (NETs) generation, which are fibrils composed of decondensed chromatin and granular proteins possessing strong antimicrobial activity. NET production was stimulated independently of Sap proteolytic activity, and at least partially through a ROS-dependent mechanism [64]. Neutrophil activation was proceeded by the CD11b (macrophage-1 antigen, Mac-1) receptor, and additionally, the CD11a (lymphocyte function-associated antigen 1, LFA-1), CD18, CD14, CD16, and TLR2 receptors. The collaboration of different types of neutrophil receptors in response to Saps resulted in signal transduction via the activation of tyrosine kinases Src and Syk, followed by the activation of pathways involving phosphoinositide 3-kinase (PI3K) and extracellular signal-regulated kinases ERK1/2 [64].
The main role of the family of CgYps has been repeatedly attributed to their functions in maintaining the proper structure and composition of the fungal cell wall [33,36,65,66,67]. The CgYPS-deficient mutant CgYPS1-11Δ under the conditions of the logarithmic growth phase presented significantly lower levels of β-glucan and mannan in the yeast cell wall, with a simultaneous increase in the chitin content [36]. Furthermore, the expression under its own promoter and ectopic expression confirmed that CgYps1 is required to maintain an appropriate β-glucan content [36,65], while CgYps1 with CgYps7 play a key role in maintaining levels of chitin and mannan [36]. Since components of the yeast cell wall are known to regulate the innate immune response, and given that C. glabrata exhibits a remarkable ability to survive and proliferate inside macrophages [68], it could be speculated that the preservation of an appropriate composition of the cell wall by CgYps1 could be aimed at attracting to the site of infection of the immune cells, capable of recognizing C. glabrata β-glucan via the Dectin-1 receptor [69,70]. Contrary, the absence of Sap9 and Sap10 proteases in the cell wall of C. albicans did not affect the surface exposition of β-1,3-glucan, and thus, interactions with macrophages [22]. The consequence of the internalization of C. glabrata cells inside host immune cells was the up-regulation of pathogen CgYPS-encoding genes, including CgYPS2, CgYPS4, CgYPS5, and CgYPS8-11 for fungi residing in macrophages; and CgYPS1, CgYPS2, CgYPS4-6, and CgYPS8-11 for fungal internalization by neutrophils [33,71]. Site-directed mutagenesis identified the putative catalytic aspartate residue of CgYps1 at position 91 involved in cell wall metabolism as being crucial for the intracellular survival and proliferation of yeasts [36]. Interestingly, in this context, Sapp1 and Sapp2, but not Sapp3, inhibit phagosome–lysosome fusion, and may promote the intracellular survival of C. parapsilosis in human macrophages [31].
In addition, the important role of CgYPS in suppressing the host innate immune response has been demonstrated. The infection of human THP-1 macrophages with the C. glabrata Cgyps1-11Δ mutant strain caused an increased production of the pro-inflammatory cytokine IL-1β, dependent on the activation of spleen tyrosine kinase (Syk) signaling, compared to the wild-type strain [36]. Therefore, the inability of the Cgyps1-11Δ mutant to survive and proliferate within mouse and human macrophages might be correlated with the activation of the NLRP3 inflammasome [36,72]. Furthermore, recent studies have shown that CgYPS plays an important role in the regulation of glucose homeostasis in fungal cells [67], while maintaining an adequate level of glucose metabolism is crucial for both fungi and host immune cells during Candida invasion, supporting host survival in life-threatening systemic candidal infection [73]. Although the interaction of macrophages with C. glabrata cells has not been thoroughly investigated so far, the authors of the study speculated that C. glabrata, similar to C. albicans, alters glucose homeostasis by inducing the glycolysis pathway, and yeasts may use the resulting glucose-depleted environment to induce macrophage death [33,67,73,74]. Furthermore, it has been shown that C. glabrata, after macrophage phagocytosis, inhibits the glycolysis pathway, and induces gluconeogenesis and the glyoxylate cycle [33,74]. Combining the conclusions of these reports may provide a complementary explanation for the lethality of the Cgyps1-11Δ mutant after its internalization by the host cells [36]. As studies using the C. albicans sap6Δ strain have also shown, the lack of Sap6 can alter the integrity of the cell wall, as well as the resistance of the strain confronted by phagocytosis by the THP-1 human monocytes [75].
4. Fungal Proteases as Allergens
For numerous fungal proteases, immunogenicity, defined as the ability to induce a humoral and cellular immune response, has been repeatedly indicated. Additionally, antigenicity, defined as the ability to specifically associate with the resultant effector factors of the immune response, including secreted antibodies, the major histocompatibility complex (MHC), and surface receptors present on T lymphocytes, has also been attributed to these enzymes. Furthermore, some fungal proteases are also interrelated with allergenicity, understood as the capability to stimulate a non-standard immune response, resulting in altered an physiology of the organism, the emergence of tissue destruction symptoms, and other reactions that are harmful and bothersome for the allergy-stricken individual [76].
Representatives of the Aspergillus, Penicillium, Cladosporium, and Alternaria genera are the fungi that are most frequently identified as being involved in the development of inhalant allergy and asthma in humans [77,78,79]. The initial contact of an individual with a potentially allergenic substance of fungal origin is related to sensitization–interaction, and processing by dendritic cells and antigen presentation via the MHC Class II complex, followed by the induction of T helper 2 (Th2) lymphocytes in the presence of early interleukin 4 (IL-4), and subsequent immunoglobulin E (IgE) production by B lymphocytes [80,81]. An allergic reaction, as a complex response of the immune system to re-contact with an allergen after sensitization, is associated with the binding of the foreign molecule with specific IgE on the surfaces of mast cells, followed by the release of various bioactive substances and inflammatory mediators, including histamines, leukotrienes, prostaglandins, cytokines, and serine proteases, which are all involved in the manifestation of allergy symptoms such as vasodilation, increased vascular permeability, enhanced mucus production, and the contraction of bronchial smooth muscle. Furthermore, mast cell degranulation also leads to the further activation of other immune cells, and subsequently affects epithelial cells, fibroblasts, and smooth muscle cells [80,81,82]. A serious consequence of chronic airway inflammation related to an allergic reaction may be the development of asthma—a complex disease that currently affects millions of people around the world—related to the narrowing and remodeling of the airways, and resulting in problems with breathing [83,84,85].
During the development of inhalant allergy, contact of the allergen with DC in the airway lumen, followed by the migration of immune cells through the epithelium for antigen presentation to naïve T lymphocytes, is not the only possible sensitization route for allergens that are proteases, as their enzymatic activities could greatly facilitate the direct penetration of the antigen through the epithelial layer of the respiratory tract, followed by its contact with submucosal DC [50,81,86,87,88]. Different proteases produced by fungi may alter the airway epithelium, demonstrating also the proinflammatory effect with the accompanying activation of epithelial cells, possibly through a mechanism mediated primarily by the PAR2 receptor, and the consequent calcium signaling and production of cytokines, including IL-6 and IL-8 [89,90,91,92]. The World Health Organization and International Union of Immunological Societies (WHO/IUIS) Allergen Nomenclature Database lists numerous well-documented examples of fungal proteases that are recognized as airborne allergens [93,94]. These proteases often belong to the pan-fungal allergen group, taking into account the observed IgE cross-reactivity [90]. Although most of them are alkaline and vacuolar serine proteases, some allergens also derive from the family of metalloproteases, (i.e., Asp f 5 from A. fumigatus), or aspartate proteases (i.e., Asp f 10 of the same species or Rhi o 1 from Rhizopus oryzae); additionally, even if some proteases recognized as allergens are intracellular proteins by origin, they are also repeatedly identified as being secreted extracellularly or present on the surfaces of fungal cells, and their allergenic properties are generally related to the proteolytic activity [77,95,96,97].
An interesting example of the potential of fungal proteases as the allergens is the activity of alkaline serine protease Alp1 (Asp f 13) secreted from swollen conidia of A. fumigatus. Alp1 is not only an important fungal virulence factor, but it is also a well-known trigger for airway hyperresponsiveness, although the complex mechanisms of its action are still being investigated [96,98]. Alp1 may induce pathophysiological RhoA-dependent Ca2+ sensitivity and bronchoconstriction after penetration of the bronchial submucosa and the degradation of the extracellular matrix proteins, i.e., collagens and fibronectin, leading to the destruction of their interactions with airway smooth muscle cells [99]. Moreover, Alp1 has the ability to disrupt the integrity of bronchial epithelium cells via the degradation of the junctional protein E-cadherin, followed by the activation of the mechanosensitive calcium channel TRPV4, calcium flux signaled through calcineurin, and subsequently, airway inflammation and allergic sensitization [100]. Nevertheless, the interactions of Alp1 with PAR2 or other PARs during the responses of epithelial cells remain vague [101]. Furthermore, alkaline serine protease Pen c 13 produced by Penicillium citrinum demonstrated the capability to activate PAR1 and PAR2 receptors on epithelial cells, increasing IL-8 production [102]. Furthermore, Pen c 13 also caused a direct disruption of junctional proteins between epithelial cells, followed by the increased penetration and production of Th2 cytokines IL-4, interleukin 5 (IL-5), and interleukin 13 (IL-13) [87]. Penicillium chrysogenum alkaline serine protease Pen ch 13 was demonstrated to stimulate the release of histamine by basophils, and to induce, in a protease-dependent manner, the expression of prostaglandin E2, IL-8, transforming growth factor TGF-β1, and cyclooxygenase COX-2 in epithelial cells [103,104]. Pen ch 13 also directly degraded the tight junction protein occludin, contributing to the destruction of the pulmonary epithelial barrier [104]. In the case of Alternaria alternata, serine proteases have been shown to be at least partially involved in the development of allergic asthma [91]; however, for this fungus, both the PAR2-dependent mechanism and the PAR2-independent route based on epidermal growth factor receptor (EGFR) activation were recently shown to be responsible for the immune response and cytokine production by epithelial cells; albeit, all fungal factors involved in this process still need to be characterized in detail [105,106,107]. In the case of Rhi o 1 aspartic endopeptidase produced by R. oryzae, the ability to bind IgE and to stimulate histamine release from peripheral blood mononuclear cells sensitized earlier with serum with anti-Rhi o 1 IgE antibodies was also demonstrated. This observation allowed for the classification of Rhi o 1 as an inhalant allergen [108].
Proteases from other fungi, including dermatophytes and some endemic species, also demonstrate the ability to induce an immune response, although only a few are currently confirmed allergens. They can, however, be a useful diagnostic tool, or they can also be used to prevent fungal infection by vaccination. Subtilisins and fungalysins produced by dermatophyte species possess antigenic properties and are capable of triggering immune reactions [109]. In the case of Trichophyton dermatophytes, proteases Sub6 and DppV may elicit an IgE-mediated immediate-hypersensitivity or delayed-type hypersensitivity responses, acting as contact allergens, as they bind IgG and IgE antibodies and stimulate cytokine production [110]. For Microsporum canis Sub3 subtilisin, a cell-mediated immune response was demonstrated in the experimentally infected guinea pig model, and for the extracellular metalloproteinase Mep3 of the same species, an antibody response was observed, although neither protease was protective in the vaccination trials [111,112,113,114]. In addition, the secretory aspartyl protease Mfsap1 from an opportunistic cutaneous pathogen Malassezia furfur has been recently indicated as strongly contributing to the development of inflammation in barrier-compromised murine skin, and the mechanisms may involve a direct interaction of protease with the host, or the Mfsap1-dependent modification of fungal cell surface hydrophobicity [115]. An endemic fungus Paracoccidioides brasiliensis has also been reported to produce serine and cysteine proteases that trigger IL-6 and IL-8 secretion by lung epithelial cells in PAR1- and PAR2-dependent manners [116], and representatives of the former group of Paracoccidioides proteases have also been demonstrated to have antigenic properties [117]. Of other endemic fungi, Histoplasma capsulatum produces an N-acetylated α-linked acidic dipeptidase (NAALADase) known as the most important antigen during histoplasmosis, which is advantageous for the diagnosis of this serious disease affecting mainly the lungs [118], while Sporothrix schenckii secretes serine and cysteine proteinases that are capable of interacting with and cleaving immunoglobulin G (IgG) [119]. The Kexin-like serine protease Kex1 produced by Pneumocystis jirovecii, the worldwide causative agent of pneumonia in immunocompromised individuals, has been indicated as a promising target for immunization to prevent this disease [120], and as a convenient diagnostic marker [121]. It was found that a higher risk of chronic Pneumocystis infections and the intensification of asthma symptoms in children with severe asthma correlated with the lowered level of protective anti-Kex1 antibodies; therefore, plasma Kex1 IgG concentration could be a supporting predictive factor for pediatric patients [122].
In general, for C. albicans, proteases appear to play only a minor role in Th2 and Th17 cell-driven allergic airway disease [123]. However, some Saps show immunogenic properties: for instance, the intravaginal immunization of rats with recombinant C. albicans Sap2 resulted in the local production of anti-Sap2 IgG and immunoglobulin A (IgA), and provided a protective effect against candidial infection by stimulating the humoral and cell-mediated immune responses against C. albicans infection [124,125]. Interestingly, mice immunization with C. parapsilosis Sap2 demonstrated enhanced survival during systemic C. tropicalis infection through a mixed cellular and humoral response resulting in the enhanced killing of fungal cells by neutrophils and the inhibition of biofilm formation, indicating that the protective anti-Sap2 antibodies cross-reacted with different Candida species [126].
5. Multiple Roles in Cellular Homeostasis
An effective adaptation to changing conditions in the different niches of the host organism, where changes in pH, oxidative stress, or nutrient deficiency may occur, is a prerequisite for the survival of pathogenic microorganisms. The canonical functions of extracellular proteases, based on their proteolytic activity, play a key role in the proper growth and development of fungi [4]. Interestingly, the additional moonlighting functions of these proteases have also been reported to maintain proper cellular homeostasis [66,127,128,129].
In the case of C. glabrata, an approach based on the use of mutants with the deletion of single or multiple CgYPS-encoding genes, demonstrated that these proteinases are additionally involved in thermal stress survival, the regulation of pH homeostasis, and vacuole homeostasis [66,127,128]. Under heat-induced conditions, nine YPS genes were overexpressed, namely CgYPS1, CgYPS2, CgYPS4, and CgYPS6-11; however, only CgYPS1 was shown to be regulated primarily by the calcineurin-Crz1 pathway, and partially by the Slt2 MAPK pathway [127]. The Cgyps1Δ mutant cultured at elevated temperatures demonstrated a drastic reduction in growth, with the observed effect being restored, both after the complementation of the YPS1 gene, and in the presence of sorbitol as an osmotic stabilizer. Together, these observations suggest that among the C. glabrata yapsins, predominantly Yps1 is involved in the maintenance of the cell wall integrity, preventing yeast cell lysis under heat stress [127]. Additionally, studies by Bairwa et al. demonstrated a unique role for CgYps1 in yeast survival under conditions of low external pH [66]. In the Cgyps1Δ mutant, intracellular acidification was noted, which consequently interfered with the regular course of physiological processes, and was partly caused by the disruption of membrane proton pump activity. In addition, the Cgyps1Δ mutant exhibited an increased production of ROS [66]. Although the acidic environment also increased the expression of six additional genes encoding yapsins, that is, CgYPS2, CgYPS4, CgYPS6, CgYPS8, CgYPS10, and CgYPS11, their exact functionality in regulating pH homeostasis has not been described so far [66]. As well, the role of CgYPS in the regulation of vacuole pH homeostasis has been revealed, and studies using the CgYPS-deficient mutant showed the pleiotropic effects of proteases deficiency, including the disruption of the size and internal pH of the vacuole, as well as a decrease in the activity of vacuolar membrane V-ATPase and vacuolar carboxypeptidase Y [128].
The pepsin-like aspartyl peptidase May1 (major aspartyl peptidase 1) produced by C. neoformans was identified as the major endopeptidase secreted under the conditions of low pH, and its activity was found to be important for fungal tolerance to acidic environments; thus, it was supposed to play a role in the C. neoformans ability to reside inside the phagolysosomes of macrophages [129,130]. Moreover, in the case of the C. neoformans deletion strain deprived of serine endopeptidase Prb1, a hypomelanization phenotype was observed, indicating the potential role of this protease in the process of melanization, which is crucial for the virulence of this fungus [129].
An interesting example of a protease that is a moonlighting protein is the serine endopeptidase PnmB produced by Aspergillus nidulans. This fungal species is characterized by a low pathogenicity, and it can cause serious diseases only in people with primary immunodeficiency disorder—chronic granulomatous disease [131]. PnmB plays an important role in nutrient acquisition and nitrogen metabolism. It may be equipped with the signal peptide directing protease to the outside of the cell, where it degrades available proteins, thus providing a nitrogen source for the fungus. In addition, PnmB may exhibit a more sophisticated, regulatory role intracellularly, where the truncated form of the protein functions. Under conditions of nitrogen starvation, PnmB is involved in controlling the activity of GATA transcription factor AreA through the proteolytic degradation of its co-repressor NmrA, leading to the subsequent activation of genes that are involved in nutrient acquisition and metabolism, and in a positive regulatory feedback mechanism, enhancing PnmB transcription by AreA [132]. Interestingly, the extracellular and intracellular forms of PnmB differ not only in the presence of a signal peptide for secretion, but also in substrate specificity, with a broad spectrum of activity being exhibited by the secreted form, and a high specificity that is limited to NmrA, but the underlying mechanisms have not been fully recognized [132,133].
The problem of resistance to antimicrobial drugs is particularly important now in the case of fungal infections. Several reports have shown an association between a decreased susceptibility to antifungal drugs and an increased degree of Sap production by Candida species [134]. Resistance to antifungal drugs was correlated with biofilm formation and the secretion of Saps, particularly Sap2, Sap9, and Sap10; however, the exact mechanisms are still unknown [18,135,136].
6. Conclusions
Since the very beginning of research on the virulence factors of human pathogenic fungi, an important role has been attributed to fungal proteases, emphasizing the role of their proteolytic activity in the acquisition of nutrients and the degradation of host protective barriers during the development of infection. So far, many interesting examples have been documented that relate the mechanisms of fungal pathogenesis to the proteolytic degradation of host bioactive peptides, and structural and regulatory proteins. However, in addition to the canonical function of these fungal enzymes, functionalities that are independent of proteolytic activity have also been indicated for selected representatives of fungal proteases, some of which are related to the impairment of host defense mechanisms and the modulation of immune response, as well as the induction of an exaggerated immune reaction, followed by the development of an allergy. These additional roles often reveal atypical protease functions that are often independent of hydrolytic degradation, and thus, they may place the protease in the group of moonlighting proteins. Moreover, once released into the external environment, proteases can act at remote sites in the host organism. Therefore, it may complicate insight into the participation of proteases in the pathogenesis of fungal infection, and hinder the inhibition of some processes during antifungal treatment, based on the elimination of proteolytic activity. Therefore, knowledge of the multitasking proteases is essential for understanding and for adequately controlling fungal diseases.
Author Contributions
J.K.-K., D.S. and G.B.—Writing—original draft; M.R.-K. and A.K.—Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was financially supported by the National Science Centre, Poland (grant number 2021/43/D/NZ6/01464 awarded to J.K.-K. and 2020/39/D/NZ6/00854 awarded to D.S.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hogan L.H., Klein B.S., Levitz S.M. Virulence factors of medically important fungi. Clin. Microbiol. Rev. 1996;9:469–488. doi: 10.1128/CMR.9.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naglik J.R., Challacombe S.J., Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 2003;67:400–428. doi: 10.1128/MMBR.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutherford J.C. The emerging role of urease as a general microbial virulence factor. PLoS Pathog. 2014;10:e1004062. doi: 10.1371/journal.ppat.1004062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapala-Kozik M., Bochenska O., Zajac D., Karkowska-Kuleta J., Gogol M., Zawrotniak M., Kozik A. Extracellular proteinases of Candida species pathogenic yeasts. Mol. Oral Microbiol. 2018;33:113–124. doi: 10.1111/omi.12206. [DOI] [PubMed] [Google Scholar]
- 5.Aoki W., Kitahara N., Miura N., Morisaka H., Yamamoto Y., Kuroda K., Ueda M. Comprehensive characterization of secreted aspartic proteases encoded by a virulence gene family in Candida albicans. J. Biochem. 2011;150:431–438. doi: 10.1093/jb/mvr073. [DOI] [PubMed] [Google Scholar]
- 6.Jeffery C.J. Moonlighting proteins. Trends Biochem. Sci. 1999;24:8–11. doi: 10.1016/S0968-0004(98)01335-8. [DOI] [PubMed] [Google Scholar]
- 7.Satala D., Karkowska-Kuleta J., Zelazna A., Rapala-Kozik M., Kozik A. Moonlighting Proteins at the Candidal Cell Surface. Microorganisms. 2020;8:1046. doi: 10.3390/microorganisms8071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mani M., Chen C., Amblee V., Liu H., Mathur T., Zwicke G., Zabad S., Patel B., Thakkar J., Jeffery C.J. MoonProt: A database for proteins that are known to moonlight. Nucleic Acids Res. 2015;43:D277–D282. doi: 10.1093/nar/gku954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarocki V.M., Tacchi J.L., Djordjevic S.P. Non-proteolytic functions of microbial proteases increase pathological complexity. Proteomics. 2015;15:1075–1088. doi: 10.1002/pmic.201400386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.López-Otín C., Bond J.S. Proteases: Multifunctional enzymes in life and disease. J. Biol. Chem. 2008;283:30433–30437. doi: 10.1074/jbc.R800035200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copley S.D. Moonlighting is mainstream: Paradigm adjustment required. Bioessays. 2012;34:578–588. doi: 10.1002/bies.201100191. [DOI] [PubMed] [Google Scholar]
- 12.Rayens E., Norris K.A., Cordero J.C.D.S.F. Mortality Trends in Risk Conditions and Invasive Mycotic Disease in the United States, 1999-2018. Clin. Infect. Dis. 2022;74:309–318. doi: 10.1093/cid/ciab336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Köhler J.R., Casadevall A., Perfect J. The spectrum of fungi that infects humans. Cold Spring Harb. Perspect. Med. 2014;5:a019273. doi: 10.1101/cshperspect.a019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockhart S.R., Toda M., Benedict K., Caceres D.H., Litvintseva A.P. Endemic and Other Dimorphic Mycoses in The Americas. J. Fungi. 2021;7:151. doi: 10.3390/jof7020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modrzewska B., Kurnatowski P. Adherence of Candida sp. to host tissues and cells as one of its pathogenicity featurese. Ann. Parasitol. 2015;61:3–9. [PubMed] [Google Scholar]
- 16.Marcos C.M., de Oliveira H.C., da Silva J.d.F., Assato P.A., Fusco-Almeida A.M., Mendes-Giannini M.J.S. The multifaceted roles of metabolic enzymes in the Paracoccidioides species complex. Front. Microbiol. 2014;5:719. doi: 10.3389/fmicb.2014.00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar R., Saraswat D., Tati S., Edgerton M. Novel Aggregation Properties of Candida albicans Secreted Aspartyl Proteinase Sap6 Mediate Virulence in Oral Candidiasis. Infect. Immun. 2015;83:2614–2626. doi: 10.1128/IAI.00282-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajendran R., Robertson D.P., Hodge P.J., Lappin D.F., Ramage G. Hydrolytic enzyme production is associated with Candida albicans biofilm formation from patients with type 1 diabetes. Mycopathologia. 2010;170:229–235. doi: 10.1007/s11046-010-9319-0. [DOI] [PubMed] [Google Scholar]
- 19.Schaller M., Schackert C., Korting H.C., Januschke E., Hube B. Invasion of Candida albicans correlates with expression of secreted aspartic proteinases during experimental infection of human epidermis. J. Investig. Dermatol. 2000;114:712–717. doi: 10.1046/j.1523-1747.2000.00935.x. [DOI] [PubMed] [Google Scholar]
- 20.Korting H.C., Hube B., Oberbauer S., Januschke E., Hamm G., Albrecht A., Borelli C., Schaller M. Reduced expression of the hyphal-independent Candida albicans proteinase genes SAP1 and SAP3 in the efg1 mutant is associated with attenuated virulence during infection of oral epithelium. J. Med. Microbiol. 2003;52:623–632. doi: 10.1099/jmm.0.05125-0. [DOI] [PubMed] [Google Scholar]
- 21.Albrecht A., Felk A., Pichova I., Naglik J.R., Schaller M., De Groot P., MacCallum D., Odds F.C., Schäfer W., Klis F., et al. Glycosylphosphatidylinositol-anchored proteases of Candida albicans target proteins necessary for both cellular processes and host-pathogen interactions. J. Biol. Chem. 2006;281:688–694. doi: 10.1074/jbc.M509297200. [DOI] [PubMed] [Google Scholar]
- 22.Schild L., Heyken A., de Groot P.W.J., Hiller E., Mock M., de Koster C., Horn U., Rupp S., Hube B. Proteolytic cleavage of covalently linked cell wall proteins by Candida albicans Sap9 and Sap10. Eukaryot. Cell. 2011;10:98–109. doi: 10.1128/EC.00210-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoldt V.R., Sonneborn A., Leuker C.E., Ernst J.F. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982–1991. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang H., Tsang P.C.S., Pow E.H.N., Lam O.L.T., Tsang P.W.K. Potential role of Candida albicans secreted aspartic protease 9 in serum induced-hyphal formation and interaction with oral epithelial cells. Microb. Pathog. 2020;139:103896. doi: 10.1016/j.micpath.2019.103896. [DOI] [PubMed] [Google Scholar]
- 25.Dutton L.C., Jenkinson H.F., Lamont R.J., Nobbs A.H. Role of Candida albicans secreted aspartyl protease Sap9 in interkingdom biofilm formation. Pathog. Dis. 2016;74:ftw005. doi: 10.1093/femspd/ftw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staniszewska M., Bondaryk M., Zukowski K., Chudy M. Role of SAP7-10 and Morphological Regulators (EFG1, CPH1) in Candida albicans’ Hypha Formation and Adhesion to Colorectal Carcinoma Caco-2. Polish J. Microbiol. 2015;64:203–210. doi: 10.5604/01.3001.0009.2115. [DOI] [PubMed] [Google Scholar]
- 27.Ray T.L., Payne C.D. Scanning electron microscopy of epidermal adherence and cavitation in murine candidiasis: A role for Candida acid proteinase. Infect. Immun. 1988;56:1942–1949. doi: 10.1128/iai.56.8.1942-1949.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kvaal C., Lachke S.A., Srikantha T., Daniels K., Mccoy J., Soll D.R. Misexpression of the opaque-phase-specific gene PEP1 (SAP1) in the white phase of Candida albicans confers increased virulence in a mouse model of cutaneous infection. Infect. Immun. 1999;67:6652–6662. doi: 10.1128/IAI.67.12.6652-6662.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H., Downs D., Ghosh K., Ghosh A.K., Staib P., Monod M., Tang J. Candida albicans secreted aspartic proteases 4-6 induce apoptosis of epithelial cells by a novel Trojan horse mechanism. FASEB J. 2013;27:2132–2144. doi: 10.1096/fj.12-214353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar R., Breindel C., Saraswat D., Cullen P.J., Edgerton M. Candida albicans Sap6 amyloid regions function in cellular aggregation and zinc binding, and contribute to zinc acquisition. Sci. Rep. 2017;7:2908. doi: 10.1038/s41598-017-03082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh D.K., Németh T., Papp A., Tóth R., Lukácsi S., Heidingsfeld O., Dostal J., Vágvölgyi C., Bajtay Z., Józsi M., et al. Functional Characterization of Secreted Aspartyl Proteases in Candida parapsilosis. mSphere. 2019;4:e00484-19. doi: 10.1128/mSphere.00484-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinterová Z., Šanda M., Dostál J., Hrušková-Heidingsfeldová O., Pichová I. Evidence for the presence of proteolytically active secreted aspartic proteinase 1 of Candida parapsilosis in the cell wall. Protein Sci. 2011;20:2004–2012. doi: 10.1002/pro.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaur R., Ma B., Cormack B.P. A family of glycosylphosphatidylinositol-linked aspartyl proteases is required for virulence of Candida glabrata. Proc. Natl. Acad. Sci. USA. 2007;104:7628–7633. doi: 10.1073/pnas.0611195104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monod M., Hube B., Hess D., Sanglard D. Differential regulation of SAP8 and SAP9, which encode two new members of the secreted aspartic proteinase family in Candida albicans. Microbiology. 1998;144:2731–2737. doi: 10.1099/00221287-144-10-2731. [DOI] [PubMed] [Google Scholar]
- 35.Gagnon-Arsenault I., Tremblay J., Bourbonnais Y. Fungal yapsins and cell wall: A unique family of aspartic peptidases for a distinctive cellular function. FEMS Yeast Res. 2006;6:966–978. doi: 10.1111/j.1567-1364.2006.00129.x. [DOI] [PubMed] [Google Scholar]
- 36.Rasheed M., Battu A., Kaur R. Aspartyl proteases in Candida glabrata are required for suppression of the host innate immune response. J. Biol. Chem. 2018;293:6410–6433. doi: 10.1074/jbc.M117.813741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boc A., Diallo A.B., Makarenkov V. T-REX: A web server for inferring, validating and visualizing phylogenetic trees and networks. Nucleic Acids Res. 2012;40:W573–W579. doi: 10.1093/nar/gks485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baldo A., Tabart J., Vermout S., Mathy A., Collard A., Losson B., Mignon B. Secreted subtilisins of Microsporum canis are involved in adherence of arthroconidia to feline corneocytes. J. Med. Microbiol. 2008;57:1152–1156. doi: 10.1099/jmm.0.47827-0. [DOI] [PubMed] [Google Scholar]
- 39.Baldo A., Mathy A., Tabart J., Camponova P., Vermout S., Massart L., Maréchal F., Galleni M., Mignon B. Secreted subtilisin Sub3 from Microsporum canis is required for adherence to but not for invasion of the epidermis. Br. J. Dermatol. 2010;162:990–997. doi: 10.1111/j.1365-2133.2009.09608.x. [DOI] [PubMed] [Google Scholar]
- 40.Baldo A., Monod M., Mathy A., Cambier L., Bagut E.T., Defaweux V., Symoens F., Antoine N., Mignon B. Mechanisms of skin adherence and invasion by dermatophytes. Mycoses. 2012;55:218–223. doi: 10.1111/j.1439-0507.2011.02081.x. [DOI] [PubMed] [Google Scholar]
- 41.Upadhyay S.K., Gautam P., Pandit H., Singh Y., Basir S.F., Madan T. Identification of fibrinogen-binding proteins of Aspergillus fumigatus using proteomic approach. Mycopathologia. 2012;173:73–82. doi: 10.1007/s11046-011-9465-z. [DOI] [PubMed] [Google Scholar]
- 42.Xu C.Y., Zhu H.M., Wu J.H., Wen H., Liu C.J. Increased permeability of blood-brain barrier is mediated by serine protease during Cryptococcus meningitis. J. Int. Med. Res. 2014;42:85–92. doi: 10.1177/0300060513504365. [DOI] [PubMed] [Google Scholar]
- 43.Na Pombejra S., Jamklang M., Uhrig J.P., Vu K., Gelli A. The structure-function analysis of the Mpr1 metalloprotease determinants of activity during migration of fungal cells across the blood-brain barrier. PLoS ONE. 2018;13:e0203020. doi: 10.1371/journal.pone.0203020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mjokane N., Folorunso O.S., Ogundeji A.O., Sebolai O.M. The Possible Role of Microbial Proteases in Facilitating SARS-CoV-2 Brain Invasion. Biology. 2021;10:966. doi: 10.3390/biology10100966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mjokane N., Maliehe M., Folorunso O.S., Ogundeji A.O., Gcilitshana O.M.N., Albertyn J., Pohl C.H., Sebolai O.M. Cryptococcal Protease(s) and the Activation of SARS-CoV-2 Spike (S) Protein. Cells. 2022;11:437. doi: 10.3390/cells11030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romani L. Immunity to fungal infections. Nat. Rev. Immunol. 2004;4:11–23. doi: 10.1038/nri1255. [DOI] [PubMed] [Google Scholar]
- 47.Beauséjour A., Grenier D., Goulet J.P., Deslauriers N. Proteolytic Activation of the Interleukin-1β Precursor by Candida albicans. Infect. Immun. 1998;66:676. doi: 10.1128/IAI.66.2.676-681.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaller M., Borelli C., Korting H.C., Hube B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses. 2005;48:365–377. doi: 10.1111/j.1439-0507.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- 49.Jeong S.K., Kim H.J., Youm J.K., Ahn S.K., Choi E.H., Sohn M.H., Kim K.E., Hong J.H., Shin D.M., Lee S.H. Mite and cockroach allergens activate protease-activated receptor 2 and delay epidermal permeability barrier recovery. J. Investig. Dermatol. 2008;128:1930–1939. doi: 10.1038/jid.2008.13. [DOI] [PubMed] [Google Scholar]
- 50.Lambrecht B.N., Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Carey R.M., Freund J.R., Hariri B.M., Adappa N.D., Palmer J.N., Lee R.J. Polarization of protease-activated receptor 2 (PAR-2) signaling is altered during airway epithelial remodeling and deciliation. J. Biol. Chem. 2020;295:6721–6740. doi: 10.1074/jbc.RA120.012710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reed C.E., Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J. Allergy Clin. Immunol. 2004;114:997–1008. doi: 10.1016/j.jaci.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 53.Pietrella D., Rachini A., Pandey N., Schild L., Netea M., Bistoni F., Hube B., Vecchiarelli A. The Inflammatory response induced by aspartic proteases of Candida albicans is independent of proteolytic activity. Infect. Immun. 2010;78:4754–4762. doi: 10.1128/IAI.00789-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruoslahti E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 55.Kumar R., Rojas I.G., Edgerton M. Candida albicans Sap6 Initiates Oral Mucosal Inflammation via the Protease Activated Receptor PAR2. Front. Immunol. 2022;13:912748. doi: 10.3389/fimmu.2022.912748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bartnicka D., Gonzalez-Gonzalez M., Sykut J., Koziel J., Ciaston I., Adamowicz K., Bras G., Zawrotniak M., Karkowska-Kuleta J., Satala D., et al. Candida albicans Shields the Periodontal Killer Porphyromonas gingivalis from Recognition by the Host Immune System and Supports the Bacterial Infection of Gingival Tissue. Int. J. Mol. Sci. 2020;21:1984. doi: 10.3390/ijms21061984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabrielli E., Sabbatini S., Roselletti E., Kasper L., Perito S., Hube B., Cassone A., Vecchiarelli A., Pericolini E. In vivo induction of neutrophil chemotaxis by secretory aspartyl proteinases of Candida albicans. Virulence. 2016;7:819–825. doi: 10.1080/21505594.2016.1184385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ran Y., Iwabuchi K., Yamazaki M., Tsuboi R., Ogawa H. Secreted aspartic proteinase from Candida albicans acts as a chemoattractant for peripheral neutrophils. J. Dermatol. Sci. 2013;72:191–193. doi: 10.1016/j.jdermsci.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Hornbach A., Heyken A., Schild L., Hube B., Löffler J., Kurzai O. The glycosylphosphatidylinositol-anchored protease Sap9 modulates the interaction of Candida albicans with human neutrophils. Infect. Immun. 2009;77:5216–5224. doi: 10.1128/IAI.00723-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pericolini E., Gabrielli E., Amacker M., Kasper L., Roselletti E., Luciano E., Sabbatini S., Kaeser M., Moser C., Hube B., et al. Secretory Aspartyl Proteinases Cause Vaginitis and Can Mediate Vaginitis Caused by Candida albicans in Mice. MBio. 2015;6:e00724. doi: 10.1128/mBio.00724-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Y., Wang H., Kouadir M., Song H., Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128. doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pietrella D., Pandey N., Gabrielli E., Pericolini E., Perito S., Kasper L., Bistoni F., Cassone A., Hube B., Vecchiarelli A. Secreted aspartic proteases of Candida albicans activate the NLRP3 inflammasome. Eur. J. Immunol. 2013;43:679–692. doi: 10.1002/eji.201242691. [DOI] [PubMed] [Google Scholar]
- 63.Gabrielli E., Pericolini E., Luciano E., Sabbatini S., Roselletti E., Perito S., Kasper L., Hube B., Vecchiarelli A. Induction of caspase-11 by aspartyl proteinases of Candida albicans and implication in promoting inflammatory response. Infect. Immun. 2015;83:1940–1948. doi: 10.1128/IAI.02895-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zawrotniak M., Bochenska O., Karkowska-Kuleta J., Seweryn-Ozog K., Aoki W., Ueda M., Kozik A., Rapala-Kozik M. Aspartic Proteases and Major Cell Wall Components in Candida albicans Trigger the Release of Neutrophil Extracellular Traps. Front. Cell. Infect. Microbiol. 2017;7:414. doi: 10.3389/fcimb.2017.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krysan D.J., Ting E.L., Abeijon C., Kroos L., Fuller R.S. Yapsins are a family of aspartyl proteases required for cell wall integrity in Saccharomyces cerevisiae. Eukaryot. Cell. 2005;4:1364–1374. doi: 10.1128/EC.4.8.1364-1374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bairwa G., Kaur R. A novel role for a glycosylphosphatidylinositol-anchored aspartyl protease, CgYps1, in the regulation of pH homeostasis in Candida glabrata. Mol. Microbiol. 2011;79:900–913. doi: 10.1111/j.1365-2958.2010.07496.x. [DOI] [PubMed] [Google Scholar]
- 67.Askari F., Rasheed M., Kaur R. The yapsin family of aspartyl proteases regulate glucose homeostasis in Candida glabrata. J. Biol. Chem. 2022;298:101593. doi: 10.1016/j.jbc.2022.101593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kasper L., Seider K., Hube B. Intracellular survival of Candida glabrata in macrophages: Immune evasion and persistence. FEMS Yeast Res. 2015;15:fov042. doi: 10.1093/femsyr/fov042. [DOI] [PubMed] [Google Scholar]
- 69.Brown G.D., Taylor P.R., Reid D.M., Willment J.A., Williams D.L., Martinez-Pomares L., Wong S.Y.C., Gordon S. Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 2002;196:407–412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen S.M., Shen H., Zhang T., Huang X., Liu X.Q., Guo S.Y., Zhao J.J., Wang C.F., Yan L., Xu G.T., et al. Dectin-1 plays an important role in host defense against systemic Candida glabrata infection. Virulence. 2017;8:1643–1656. doi: 10.1080/21505594.2017.1346756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fukuda Y., Tsai H.F., Myers T.G., Bennett J.E. Transcriptional profiling of Candida glabrata during phagocytosis by neutrophils and in the infected mouse spleen. Infect. Immun. 2013;81:1325–1333. doi: 10.1128/IAI.00851-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gross O., Poeck H., Bscheider M., Dostert C., Hannesschläger N., Endres S., Hartmann G., Tardivel A., Schweighoffer E., Tybulewicz V., et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 73.Tucey T.M., Verma J., Harrison P.F., Snelgrove S.L., Lo T.L., Scherer A.K., Barugahare A.A., Powell D.R., Wheeler R.T., Hickey M.J., et al. Glucose Homeostasis Is Important for Immune Cell Viability during Candida Challenge and Host Survival of Systemic Fungal Infection. Cell Metab. 2018;27:988–1006.e7. doi: 10.1016/j.cmet.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar K., Askari F., Sahu M.S., Kaur R. Candida glabrata: A Lot More Than Meets the Eye. Microorganisms. 2019;7:39. doi: 10.3390/microorganisms7020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buu L.M., Chen Y.C. Sap6, a secreted aspartyl proteinase, participates in maintenance the cell surface integrity of Candida albicans. J. Biomed. Sci. 2013;20:101. doi: 10.1186/1423-0127-20-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang J., Tao A. Antigenicity, Immunogenicity, Allergenicity. Allergy Bioinforma. 2015;8:175. doi: 10.1007/978-94-017-7444-4_11. [DOI] [Google Scholar]
- 77.Yike I. Fungal proteases and their pathophysiological effects. Mycopathologia. 2011;171:299–323. doi: 10.1007/s11046-010-9386-2. [DOI] [PubMed] [Google Scholar]
- 78.Rick E.M., Woolnough K., Pashley C.H., Wardlaw A.J. Allergic Fungal Airway Disease. J. Investig. Allergol. Clin. Immunol. 2016;26:344–354. doi: 10.18176/jiaci.0122. [DOI] [PubMed] [Google Scholar]
- 79.Furlong-Silva J., Cook P.C. Fungal-mediated lung allergic airway disease: The critical role of macrophages and dendritic cells. PLoS Pathog. 2022;18:e1010608. doi: 10.1371/journal.ppat.1010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kay A.B. Allergy and allergic diseases. First of two parts. N. Engl. J. Med. 2001;344:30–37. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 81.Galli S.J., Tsai M., Piliponsky A.M. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hammad H., Lambrecht B.N. Dendritic cells and airway epithelial cells at the interface between innate and adaptive immune responses. Allergy. 2011;66:579–587. doi: 10.1111/j.1398-9995.2010.02528.x. [DOI] [PubMed] [Google Scholar]
- 83.Busse W.W., Lemanske R.F. Asthma. N. Engl. J. Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 84.Bush R.K. Indoor allergens, environmental avoidance, and allergic respiratory disease. Allergy Asthma Proc. 2008;29:575–579. doi: 10.2500/aap.2008.29.3172. [DOI] [PubMed] [Google Scholar]
- 85.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. Erratum: Department of Error (The Lancet (2020) 396(10258) (1204–1222), (S0140673620309259), (10.1016/S0140-6736(20)30925-9)). Lancet 2020, 396, 1562. https://doi.org/10.1016/S0140-6736(20)32226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robinson B.W.S., Venaille T.J., Mendis A.H.W., McAleer R. Allergens as proteases: An Aspergillus fumigatus proteinase directly induces human epithelial cell detachment. J. Allergy Clin. Immunol. 1990;86:726–731. doi: 10.1016/S0091-6749(05)80176-9. [DOI] [PubMed] [Google Scholar]
- 87.Chen J.C., Chuang J.G., Su Y.Y., Chiang B.L., Lin Y.S., Chow L.P. The protease allergen Pen c 13 induces allergic airway inflammation and changes in epithelial barrier integrity and function in a murine model. J. Biol. Chem. 2011;286:26667–26679. doi: 10.1074/jbc.M110.193987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matsumura Y. Role of Allergen Source-Derived Proteases in Sensitization via Airway Epithelial Cells. J. Allergy. 2012;2012:903659. doi: 10.1155/2012/903659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kauffman H.F., Christomee J.F., Van De Riet M.A., Timmerman A.J.B., Borger P. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J. Allergy Clin. Immunol. 2000;105:1185–1193. doi: 10.1067/mai.2000.106210. [DOI] [PubMed] [Google Scholar]
- 90.Shen H.D., Tam M.F., Tang R.B., Chou H. Aspergillus and Penicillium allergens: Focus on proteases. Curr. Allergy Asthma Rep. 2007;7:351–356. doi: 10.1007/s11882-007-0053-8. [DOI] [PubMed] [Google Scholar]
- 91.Boitano S., Flynn A.N., Sherwood C.L., Schulz S.M., Hoffman J., Gruzinova I., Daines M.O. Alternaria alternata serine proteases induce lung inflammation and airway epithelial cell activation via PAR2. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011;300:L605–L614. doi: 10.1152/ajplung.00359.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tiwary M., Samarasinghe A.E. Initiation and Pathogenesis of Severe Asthma with Fungal Sensitization. Cells. 2021;10:913. doi: 10.3390/cells10040913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pomés A., Davies J.M., Gadermaier G., Hilger C., Holzhauser T., Lidholm J., Lopata A.L., Mueller G.A., Nandy A., Radauer C., et al. WHO/IUIS Allergen Nomenclature: Providing a common language. Mol. Immunol. 2018;100:3–13. doi: 10.1016/j.molimm.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.WHO/IUIS Allergen Nomenclature Home Page. [(accessed on 27 December 2022)]. Available online: http://www.allergen.org/index.php.
- 95.Redes J.L., Basu S., Ram-Mohan S., Ghosh C.C., Chan E.C., Sek A.C., Zhao M., Krishnan R., Rosenberg H.F., Druey K.M. Aspergillus fumigatus–Secreted Alkaline Protease 1 Mediates Airways Hyperresponsiveness in Severe Asthma. ImmunoHorizons. 2019;3:368–377. doi: 10.4049/immunohorizons.1900046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blango M.G., Pschibul A., Rivieccio F., Krüger T., Rafiq M., Jia L.J., Zheng T., Goldmann M., Voltersen V., Li J., et al. Dynamic Surface Proteomes of Allergenic Fungal Conidia. J. Proteome Res. 2020;19:2092–2104. doi: 10.1021/acs.jproteome.0c00013. [DOI] [PubMed] [Google Scholar]
- 97.Rowley J., Namvar S., Gago S., Labram B., Bowyer P., Richardson M.D., Herrick S.E. Differential Proinflammatory Responses to Aspergillus fumigatus by Airway Epithelial Cells In Vitro Are Protease Dependent. J. Fungi. 2021;7:468. doi: 10.3390/jof7060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Behnsen J., Lessing F., Schindler S., Wartenberg D., Jacobsen I.D., Thoen M., Zipfel P.F., Brakhage A.A. Secreted Aspergillus fumigatus protease Alp1 degrades human complement proteins C3, C4, and C5. Infect. Immun. 2010;78:3585–3594. doi: 10.1128/IAI.01353-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Balenga N.A., Klichinsky M., Xie Z., Chan E.C., Zhao M., Jude J., Laviolette M., Panettieri R.A., Druey K.M. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat. Commun. 2015;6:6763. doi: 10.1038/ncomms7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wiesner D.L., Merkhofer R.M., Ober C., Kujoth G.C., Niu M., Keller N.P., Gern J.E., Brockman-Schneider R.A., Evans M.D., Jackson D.J., et al. Club Cell TRPV4 Serves as a Damage Sensor Driving Lung Allergic Inflammation. Cell Host Microbe. 2020;27:614–628.e6. doi: 10.1016/j.chom.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Druey K.M., McCullough M., Krishnan R. Aspergillus fumigatus Protease Alkaline Protease 1 (Alp1): A New Therapeutic Target for Fungal Asthma. J. Fungi. 2020;6:88. doi: 10.3390/jof6020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chiu L.-L., Perng D.-W., Yu C.-H., Su S.-N., Chow L.-P. Mold allergen, pen C 13, induces IL-8 expression in human airway epithelial cells by activating protease-activated receptor 1 and 2. J. Immunol. 2007;178:5237–5244. doi: 10.4049/jimmunol.178.8.5237. [DOI] [PubMed] [Google Scholar]
- 103.Chou H., Lai H.Y., Tam M.F., Chou M.Y., Wang S.R., Han S.H., Shen H. Der cDNA cloning, biological and immunological characterization of the alkaline serine protease major allergen from Penicillium chrysogenum. Int. Arch. Allergy Immunol. 2002;127:15–26. doi: 10.1159/000048165. [DOI] [PubMed] [Google Scholar]
- 104.Tai H.Y., Tam M.F., Chou H., Peng H.J., Su S.N., Perng D.W., Shen H.D. Pen ch 13 allergen induces secretion of mediators and degradation of occludin protein of human lung epithelial cells. Allergy. 2006;61:382–388. doi: 10.1111/j.1398-9995.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 105.Denis O., Vincent M., Havaux X., De Prins S., Treutens G., Huygen K. Induction of the specific allergic immune response is independent of proteases from the fungus Alternaria alternata. Eur. J. Immunol. 2013;43:907–917. doi: 10.1002/eji.201242630. [DOI] [PubMed] [Google Scholar]
- 106.Daines M., Zhu L., Pereira R., Zhou X., Bondy C., Pryor B.M., Zhou J., Chen Y. Alternaria induces airway epithelial cytokine expression independent of protease-activated receptor. Respirology. 2020;25:502–510. doi: 10.1111/resp.13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rivas C.M., Schiff H.V., Moutal A., Khanna R., Kiela P.R., Dussor G., Price T.J., Vagner J., DeFea K.A., Boitano S. Alternaria alternata-induced airway epithelial signaling and inflammatory responses via protease-activated receptor-2 expression. Biochem. Biophys. Res. Commun. 2022;591:13–19. doi: 10.1016/j.bbrc.2021.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sircar G., Saha B., Mandal R.S., Pandey N., Saha S., Bhattacharya S.G. Purification, Cloning and Immuno-Biochemical Characterization of a Fungal Aspartic Protease Allergen Rhi o 1 from the Airborne Mold Rhizopus oryzae. PLoS ONE. 2015;10:e0144547. doi: 10.1371/journal.pone.0144547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Monod M. Secreted proteases from dermatophytes. Mycopathologia. 2008;166:285–294. doi: 10.1007/s11046-008-9105-4. [DOI] [PubMed] [Google Scholar]
- 110.Woodfolk J.A. Allergy and dermatophytes. Clin. Microbiol. Rev. 2005;18:30–43. doi: 10.1128/CMR.18.1.30-43.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Descamps F.F., Brouta F., Vermout S.M., Willame C., Losson B.J., Mignon B.R. A recombinant 31.5 kDa keratinase and a crude exo-antigen from Microsporum canis fail to protect against a homologous experimental infection in guinea pigs. Vet. Dermatol. 2003;14:305–312. doi: 10.1111/j.1365-3164.2003.00342.x. [DOI] [PubMed] [Google Scholar]
- 112.Descamps F., Brouta F., Vermout S., Monod M., Losson B., Mignon B. Recombinant expression and antigenic properties of a 31.5-kDa keratinolytic subtilisin-like serine protease from Microsporum canis. FEMS Immunol. Med. Microbiol. 2003;38:29–34. doi: 10.1016/S0928-8244(03)00101-9. [DOI] [PubMed] [Google Scholar]
- 113.Brouta F., Descamps F., Vermout S., Monod M., Losson B., Mignon B. Humoral and cellular immune response to a Microsporum canis recombinant keratinolytic metalloprotease (r-MEP3) in experimentally infected guinea pigs. Med. Mycol. 2003;41:495–501. doi: 10.1080/13693780310001615385. [DOI] [PubMed] [Google Scholar]
- 114.Vermout S.M., Brouta F.D., Descamps F.F., Losson B.J., Mignon B.R. Evaluation of immunogenicity and protective efficacy of a Microsporum canis metalloprotease subunit vaccine in guinea pigs. FEMS Immunol. Med. Microbiol. 2004;40:75–80. doi: 10.1016/S0928-8244(03)00296-7. [DOI] [PubMed] [Google Scholar]
- 115.Goh J.P.Z., Ruchti F., Poh S.E., Koh W.L.C., Tan K.Y., Lim Y.T., Thng S.T.G., Sobota R.M., Hoon S.S., Liu C., et al. The human pathobiont Malassezia furfur secreted protease Mfsap1 regulates cell dispersal and exacerbates skin inflammation. Proc. Natl. Acad. Sci. USA. 2022;119:e2212533119. doi: 10.1073/pnas.2212533119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.de Oliveira P., Juliano M.A., Tanaka A.S., Carmona A.K., dos Santos S.M.B., de Barros B.C.S.C., Maza P.K., Puccia R., Suzuki E. Paracoccidioides brasiliensis induces cytokine secretion in epithelial cells in a protease-activated receptor-dependent (PAR) manner. Med. Microbiol. Immunol. 2017;206:149–156. doi: 10.1007/s00430-016-0490-x. [DOI] [PubMed] [Google Scholar]
- 117.Moreira A.L.E., Oliveira M.A.P., Silva L.O.S., Inácio M.M., Bailão A.M., Parente-Rocha J.A., Cruz-Leite V.R.M., Paccez J.D., de Almeida Soares C.M., Weber S.S., et al. Immunoproteomic Approach of Extracellular Antigens From Paracoccidioides Species Reveals Exclusive B-Cell Epitopes. Front. Microbiol. 2020;10:2968. doi: 10.3389/fmicb.2019.02968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Toyotome T., Watanabe A., Ochiai E., Kamei K. N-acetylated α-linked acidic dipeptidase is identified as an antigen of Histoplasma capsulatum. Biochem. Biophys. Res. Commun. 2015;458:483–487. doi: 10.1016/j.bbrc.2015.01.129. [DOI] [PubMed] [Google Scholar]
- 119.Rosa D.D.A., Gezuele E., Calegari L., Goñi F. Excretion-secretion products and proteases from live Sporothrix schenckii yeast phase: Immunological detection and cleavage of human IgG. Rev. Inst. Med. Trop. Sao Paulo. 2009;51:1–7. doi: 10.1590/S0036-46652009000100001. [DOI] [PubMed] [Google Scholar]
- 120.Kling H.M., Norris K.A. Vaccine-Induced Immunogenicity and Protection Against Pneumocystis Pneumonia in a Nonhuman Primate Model of HIV and Pneumocystis Coinfection. J. Infect. Dis. 2016;213:1586–1595. doi: 10.1093/infdis/jiw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tomás A.L., Cardoso F., de Sousa B., Matos O. Detection of anti-Pneumocystis jirovecii antibodies in human serum using a recombinant synthetic multi-epitope kexin-based antigen. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:2205–2209. doi: 10.1007/s10096-020-03936-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rayens E., Noble B., Vicencio A., Goldman D.L., Bunyavanich S., Norris K.A. Relationship of Pneumocystis antibody responses to paediatric asthma severity. BMJ open Respir. Res. 2021;8:e000842. doi: 10.1136/bmjresp-2020-000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu Y., Zeng Z., Guo Y., Song L., Weatherhead J.E., Huang X., Zeng Y., Bimler L., Chang C.Y., Knight J.M., et al. Candida albicans elicits protective allergic responses via platelet mediated T helper 2 and T helper 17 cell polarization. Immunity. 2021;54:2595–2610.e7. doi: 10.1016/j.immuni.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sandini S., La Valle R., Deaglio S., Malavasi F., Cassone A., De Bernardis F. A highly immunogenic recombinant and truncated protein of the secreted aspartic proteases family (rSap2t) of Candida albicans as a mucosal anticandidal vaccine. FEMS Immunol. Med. Microbiol. 2011;62:215–224. doi: 10.1111/j.1574-695X.2011.00802.x. [DOI] [PubMed] [Google Scholar]
- 125.Shukla M., Chandley P., Rohatgi S. The Role of B-Cells and Antibodies against Candida Vaccine Antigens in Invasive Candidiasis. Vaccines. 2021;9:1159. doi: 10.3390/vaccines9101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shukla M., Rohatgi S. Vaccination with Secreted Aspartyl Proteinase 2 Protein from Candida parapsilosis Can Enhance Survival of Mice during C. tropicalis-Mediated Systemic Candidiasis. Infect. Immun. 2020;88:e00312-20. doi: 10.1128/IAI.00312-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Miyazaki T., Izumikawa K., Yamauchi S., Inamine T., Nagayoshi Y., Saijo T., Seki M., Kakeya H., Yamamoto Y., Yanagihara K., et al. The glycosylphosphatidylinositol-linked aspartyl protease Yps1 is transcriptionally regulated by the calcineurin-Crz1 and Slt2 MAPK pathways in Candida glabrata. FEMS Yeast Res. 2011;11:449–456.e7. doi: 10.1111/j.1567-1364.2011.00734.x. [DOI] [PubMed] [Google Scholar]
- 128.Bairwa G., Rasheed M., Taigwal R., Sahoo R., Kaur R. GPI (glycosylphosphatidylinositol)-linked aspartyl proteases regulate vacuole homoeostasis in Candida glabrata. Biochem. J. 2014;458:323–334. doi: 10.1042/BJ20130757. [DOI] [PubMed] [Google Scholar]
- 129.Clarke S.C., Dumesic P.A., Homer C.M., O’Donoghue A.J., La Greca F., Pallova L., Majer P., Madhani H.D., Craik C.S. Integrated Activity and Genetic Profiling of Secreted Peptidases in Cryptococcus neoformans Reveals an Aspartyl Peptidase Required for Low pH Survival and Virulence. PLoS Pathog. 2016;12:e1006051. doi: 10.1371/journal.ppat.1006051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Levitz S.M., Nong S.H., Seetoo K.F., Harrison T.S., Speizer R.A., Simons E.R. Cryptococcus neoformans resides in an acidic phagolysosome of human macrophages. Infect. Immun. 1999;67:885–890. doi: 10.1128/IAI.67.2.885-890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.King J., Henriet S.S.V., Warris A. Aspergillosis in Chronic Granulomatous Disease. J. Fungi. 2016;2:15. doi: 10.3390/jof2020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li A., Parsania C., Tan K., Todd R.B., Wong K.H. Co-option of an extracellular protease for transcriptional control of nutrient degradation in the fungus Aspergillus nidulans. Commun. Biol. 2021;4:1409. doi: 10.1038/s42003-021-02925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao X., Hume S.L., Johnson C., Thompson P., Huang J., Gray J., Lamb H.K., Hawkins A.R. The transcription repressor NmrA is subject to proteolysis by three Aspergillus nidulans proteases. Protein Sci. 2010;19:1405–1419. doi: 10.1002/pro.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Silva R.C., Padovan A.C.B., Pimenta D.C., Ferreira R.C., da Silva C.V., Briones M.R.S. Extracellular enolase of Candida albicans is involved in colonization of mammalian intestinal epithelium. Front. Cell. Infect. Microbiol. 2014;4:66. doi: 10.3389/fcimb.2014.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Feng W., Yang J., Pan Y., Xi Z., Qiao Z., Ma Y. The correlation of virulence, pathogenicity, and itraconazole resistance with SAP activity in Candida albicans strains. Can. J. Microbiol. 2016;62:173–178. doi: 10.1139/cjm-2015-0457. [DOI] [PubMed] [Google Scholar]
- 136.Kadry A.A., El-Ganiny A.M., El-Baz A.M. Relationship between Sap prevalence and biofilm formation among resistant clinical isolates of Candida albicans. Afr. Health Sci. 2018;18:1166–1174. doi: 10.4314/ahs.v18i4.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.