Abstract
Immunization of BALB/c mice with a plasmid containing the gene for Trypanosoma cruzi trans-sialidase (TS) induced antibodies that inhibited TS enzymatic activity, CD4+ Th1 and CD8+ Tc1 cells, and protective immunity against infection. We used this model to obtain basic information on the requirement of CD4 or CD8 or B-cell epitopes for an effective DNA-induced immunity against T. cruzi infection. For that purpose, mice were immunized with plasmids containing DNA sequences encoding (i) the entire TS protein, (ii) the TS enzymatic domain, (iii) the TS CD4+ T-cell epitopes, (iv) the TS CD8+ T-cell epitope, or (v) TS CD4+ and CD8+ T-cell epitopes. Plasmids expressing the entire TS or its enzymatic domain elicited similar levels of TS-inhibitory antibodies, γ interferon (IFN-γ)-producing T cells, and protective immunity against infection. Although the plasmid expressing TS CD4 epitopes was immunogenic, its protective efficacy against experimental infection was limited. The plasmid expressing the CD8 epitope was poorly immunogenic and provided little protective immunity. The reason for the limited priming of CD8+ T cells was due to a requirement for CD4+ T cells. To circumvent this problem, a plasmid expressing both CD4+ and CD8+ T-cell epitopes was produced. This plasmid generated levels of IFN-γ-producing T cells and protective immunity comparable to that of the plasmid expressing the entire catalytic domain of TS. Our observations suggest that plasmids expressing epitopes recognized by CD4+ and CD8+ T cells may have a better protective potential against infection with T. cruzi.
Recently, independent groups studied the immunogenic properties of plasmids containing genes encoding distinct antigens expressed on the surface of infective forms of Trypanosoma cruzi. This protozoan parasite causes Chagas' disease, an acute and chronic illness that afflicts between 16 and 18 million people in Latin America. Immunization with plasmids containing T. cruzi genes generate immune responses mediated by antibodies and CD4+ and CD8+ T cells. Most relevant, DNA-vaccinated mice display remarkable protective immunity, surviving lethal infection with T. cruzi (6, 28, 35). These observations argued that, in the short term, genetic vaccination might be used as a valuable tool for the identification of antigens that can elicit protective immune responses in humans against this protozoan parasite. Also, in the long run, genetic vaccination can be explored as a possible strategy for the development of immunoprophylactic or therapeutic measures to fight this illness.
During Chagas' disease, mice and humans develop parasite-specific major histocompatibility complex (MHC) class I- and MHC class II-restricted T cells (3, 7, 32, 37). These subpopulations of T cells seem to complement each other to provide optimal host resistance against infection. Genetically modified knockout (KO) mice that do not express either MHC class I or MHC class II antigens are highly susceptible to infection compared to wild-type mice (31). CD4 or CD8 KO mice were also highly susceptible to infection, emphasizing the importance of both T-cell populations during naturally acquired immune responses (26).
Similarly to T. cruzi infection, we found that BALB/c mice immunized with a plasmid containing a gene encoding the catalytic domain of T. cruzi trans-sialidase (TS) and that had been shown to be protected against a lethal challenge with infective forms of the parasite developed immune responses mediated by CD4+ and CD8+ T cells. From mice immunized with the TS gene, we isolated CD4+ Th1 and CD8+ Tc1 clones. These clones displayed remarkable antiparasitic activities in vitro (23, 24).
Based on the observation that DNA immunization with the TS gene could elicit distinct immunological mechanisms, we considered that a detailed comparison of the immunogenicity of plasmids containing either the entire TS gene or DNA sequences encoding its immunogenic portions would be important. From this type of study, we expected to obtain basic information on the requirement of CD4 or CD8 or B-cell epitopes for an effective DNA-induced immunity against T. cruzi. For this purpose, we compared the levels of antibody response, gamma interferon (IFN-γ) secretion, and protective immunity against experimental infection in mice immunized with plasmids containing DNA sequences encoding (i) the entire TS protein, (ii) the TS enzymatic domain, (iii) TS CD4+ T-cell epitopes, (iv) the TS CD8+ T-cell epitope, and (v) TS CD4+ and CD8+ T-cell epitopes.
MATERIALS AND METHODS
Plasmids.
p154/13 contains the nucleotide sequence coding for amino acids (aa) 1 to 678 of TS inserted into a commercially available plasmid, pcDNA3 (6). This region contains the signal peptide (aa 1 to 33) and the entire catalytic domain of TS (aa 34 to 678; Table 1). pΔ154/13, pΔ154/13-CD8, and pcDNA3-TS were generated by modifying p154/13. This plasmid was initially cut with Xhol. This treatment removed a fragment of 1,209 bp located in the 3′ region of the TS gene. After separation in agarose gel, the higher-molecular-weight band was excised from the gel and DNA purified with the aid of a Nucleiclean kit (Sigma). This DNA was used to generate the other three plasmids. pΔ154/13 was obtained by ligation of the DNA in the presence of T4 ligase and transformation into competent Escherichia coli DH5α. This plasmid contains 825 bp coding for the first 275 aa of TS. It includes the TS signal peptide (aa 1 to 33) and 242 aa of the N-terminal region of the catalytic domain of TS (Table 1).
TABLE 1.
Characteristics of the plasmids used for DNA immunization
| Plasmid | Base pairsa | Amino acids | TS region(s) |
|---|---|---|---|
| pcDNA3-TS | 1–3180 | 1–1060 | Entire protein |
| p154/13 | 1–2034 | 1–678 | Signal peptide, catalytic domain |
| pΔ154/13 | 1–825 | 1–275 | Signal peptide, part of the catalytic domain (CD4 epitopes) |
| pCD8-epitope | 1077–1101 | 359–367 | CD8 epitope |
| pΔ154/13-CD8 | 1–825 | 1–275 | Signal peptide, part of the catalytic domain and CD8 epitope |
| 1077–1101 | 359–367 | ||
| pcDNA3 | None | None | None |
Based on the sequence of TS 154 gene (31).
pΔ154/13-CD8 was generated by ligation of Xhol-treated DNA in the presence of oligonucleotides 5′-TCGA ATT TAT AAC GTT GGG CAA GTA TCC ATT TAA-3′ (forward) and 5′-TCGA TTA AAT GGA TAC TTG CCC AAC GTT ATA AAT-3′ (reverse). (Underlined nucleotides represent the XhoI restriction site.) After transformation, several colonies were screened by hybridization with forward oligonucleotide labeled with [γ32-P]ATP using T4 polynucleotide kinase. The presence of a nucleotide sequence encoding the CD8 epitope was further confirmed by direct sequencing analysis with the Thermosequenase cycle sequencing kit (Amersham) using the T7 primer label with [γ-32P]ATP. This plasmid contains 825 bp coding for the first 275 aa of TS and 27 bp coding for the CD8 epitope of TS (Table 1).
The DNA containing the pcDNA3 and the 5′ region of the TS gene was ligated to an Xhol fragment obtained from the original TS 154 gene (34). This Xhol fragment contained 2,358 bp encoding part of the TS catalytic domain, the C-terminal repeats, and amino acids that are exchanged by the glycophosphatidylinositol anchor. After transformation, a colony was selected with a plasmid containing the insert in the correct orientation. This plasmid contained the entire coding region of the originally cloned TS 154 gene and was designated pcDNA3-TS (Table 1).
We also generated a plasmid containing the sequence encoding the TS CD8 epitope preceded by an initiation code (MIYNVGOVSI). pcDNA3 was cut with EcoRI and BamHI. After agarose gel separation, purified DNA was ligated in the presence of the oligonucleotides 5′-AATT ATG ATT TAT AAC GTT GGG CAA GTA TCC ATT TAA-3′ (forward) and 5′-GATC TTA AAT GGA TAC TTG CCC AAC GTT ATA AAT CAT-3′ (reverse). After transformation, several colonies were screened as described above by hybridization with labeled forward oligonucleotide. The presence of a nucleotide sequence encoding the CD8 epitope was further confirmed by direct sequencing (Table 1).
Parasites and animals.
Female, 5-to-8-week-old BALB/c mice used in this study were purchased from the University of São Paulo. Bloodstream trypomastigotes of the Y strain were obtained from 7-day infected mice. The blood was collected from the axillary vein and transferred to a tube containing heparin. After centrifugation, the parasites were collected in plasma, centrifuged, and washed twice in phosphate-buffered saline (PBS). The concentration of parasites was estimated and adjusted to 32,500 per ml. Each mouse was inoculated intraperitoneally (i.p.) with 0.2 ml (6,500 trypomastigotes). Parasite development was monitored in the blood according to the standard method (14).
DNA immunization.
Plasmids were produced in E. coli DH5α and purified on cesium chloride density gradients as described earlier (6). DNA concentration was estimated at 260 nm and confirmed by agarose gel stained with ethidium bromide. Each plasmid DNA was diluted in sterile PBS to a concentration of 1 mg/ml. BALB/c mice were immunized according to a protocol described earlier (6). Both tibialis anterioris muscles were injected with 3.5 μg of cardiotoxin (Sigma). Five days later, 50 μg of plasmid DNA was injected intramuscularly (i.m.) at the same sites as for cardiotoxin injection (a total of 100 μg of plasmid DNA per mouse). The subsequent doses consisted of the same amount of plasmid DNA injected 3, 5, and 7 weeks after the first dose. Experiments of DNA immunization and infection with T. cruzi were reproduced at least three times with similar results.
Statistical analysis.
The Student's and alternate t tests were used to compare the possible differences in the mean values of peak parasitemia. Fisher's exact test was used to compare the frequencies of mice that survived T. cruzi infection. The differences were considered significant when the P value was <0.05.
Recombinant protein and detection of antibodies to TS.
The recombinant TS catalytic domain (TS-cat) was produced in E. coli transformed with plasmid TS-cat7 as described earlier in detail (22). This protein contains the entire catalytic domain of the enzyme including aa 34 to 678. The purity of recombinant TS-cat was determined by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis. A single band of 70 kDa was visualized in the gel. Protein concentration was estimated by the Bradford procedure (Bio-Rad).
Anti-TS antibodies were detected by enzyme-linked immunosorbent assay (ELISA) using polystyrene flat-bottom microtiter plates coated with recombinant TS-cat. Each well was incubated overnight at 4°C with 200 ng of protein dissolved in 0.05 ml of 0.1 M NaHCO3, pH 8.5. Unbound antigen was removed by washing with PBS (pH 7.4) containing 0.05% Tween 20 (PBS-Tween). Wells were treated with 2% bovine serum albumin (BSA) and 5% dry nonfat milk in PBS (PBS-BSA). After 2 h, 50 μl of the sera from immunized and control mice at the indicated dilutions were incubated for 60 min at 37°C. After five washes with PBS-Tween, wells were incubated for 30 min at 37°C with anti-mouse immunoglobulin G (IgG) (heavy and light chain) conjugated to peroxidase diluted 1:4,000, and bound immunocomplexes were detected with o-phenylenediamine. Plates were read at 492 nm on an ELISA reader.
The presence of antibodies that inhibit TS activity was detected essentially as described earlier (6). The final concentration of serum was 1:10. TS activity was determined by the transfer of sialic acid from sialyllactose to d-glucose-1-[14C]lactose (Amersham) and detection of the radioactive sialylated products by chromatography on QAE-Sephadex A-25. The results were obtained as counts per minute of [14C]-sialyllactose formed and are presented as the percent inhibition of enzymatic activity calculated as follows: percent inhibition = [(X − Blank)/(A − Blank) − 1] × 100, where X is the radioactivity of the enzyme when incubated in the presence of sera from immunized mice, Blank is the radioactivity in the absence of the enzyme, and A is the radioactivity of the reaction obtained in the presence of the enzyme without any sera.
Synthetic peptide.
The synthetic peptide IYNVGQVSI (TS359–367) was purchased from Neosystem (Strasbourg, France). As estimated by high-performance liquid chromatography analysis, it was more than 90% pure. This peptide represents aa 359 to 367 encoded by the TS 154 gene (34).
Cell-mediated immune response assays. (i) Cell cultures.
As culture medium we used RPMI 1640 supplemented with 10 mM HEPES, 2 mM l-glutamine, and 100 U of penicillin and streptomycin (Sigma) per ml. For stimulation of the spleen cells, we added to the medium 5 × 10−5 M 2-mercaptoethanol, 1 mM sodium pyruvate, 1% nonessential amino acid solution, a 1% dilution of a vitamin solution, 10% (vol/vol) fetal calf serum (Hyclone), and 30 U of recombinant human interleukin-2 (kindly provided by Hoffmann-LaRoche) per ml. Cultures were maintained at 37°C in an atmosphere containing 5% CO2.
(ii) IFN-γ secretion by spleen cells stimulated with transfected or peptide-coated A20J cells.
Responder cells were obtained from spleens of DNA-immunized mice 2 to 6 weeks after the last immunization. Stimulator cells were mouse lymphoma A20J cells that express MHC class I and II molecules. These cells were kindly provided by M. Tsuji from New York University. Transfected A20J-TS cells were generated and maintained as described earlier (23). Spleen cells (4 × 107 cells per 10 ml) were expanded in vitro in the presence of 4 × 106 irradiated A20J-TS cells. After 6 days in culture, cells were extensively washed and counted and their concentration was adjusted to 1.25 × 106 cells/ml. One hundred microliters of this suspension containing 1.25 × 105 cells was incubated with 105 irradiated A20J cells. These cells were (i) control A20J cells transfected with pcDNA3 (A20J-pcDNA3), (ii) A20J-TS, (iii) control A20J cells, and (iv) A20J cells coated with a 1 μM concentration of peptide TS359–367. These cells were cultured in triplicate using 96-well flat-bottom plates in a final volume of 0.2 ml. After 18 h, the supernatants were collected and IFN-γ was estimated by capture ELISA.
The capture and detection monoclonal antibodies (MAbs) were R46A2 and biotinylated XMG1.2, respectively (both were purchased from Pharmingen, San Diego, Calif.). High-binding microtiter plates were coated with 0.05 ml of capture MAb (5 μg/ml) diluted in PBS and incubated overnight at 4°C. After washes with PBS-Tween, wells were blocked with PBS-BSA for 2 h at room temperature. After removing the blocking solution, 0.05 ml of T-cell supernatant was added per well. Supernatants were diluted twice or up to 10 times in order to estimate precisely the cytokine concentration. Each determination was performed in triplicate. After overnight incubation at 4°C, plates were washed and biotinylated MAb was added at a final concentration of 5 μg/ml in PBS-BSA. After washes, 0.05 ml of avidin-peroxidase diluted in PBS-BSA was added to each well at a final concentration of 2 μg/ml. After a 2-h incubation at room temperature, excess labeled avidin-peroxidase was removed during washing and the reaction was developed with o-phenylenediamine. The concentration of cytokine in each sample was determined from standard curves executed in parallel with a known concentration of recombinant IFN-γ (Pharmingen). The detection limit of the assays was 0.2 ng/ml.
In vivo depletion of CD4+ and CD8+ T cells.
Depletion of CD4+ or CD8+ cells was performed essentially as described previously (15). The hybridomas producing rat IgG anti-CD4 (GK1.5) or anti-CD8 (2.43) were purchased from the American Type Culture Collection. Ascites were produced in BALB/c nude mice and precipitated with 35% (wt/vol) ammonium sulfate. After centrifugation, the pellet was resuspended in PBS extensively dialyzed against this buffer. The concentration of rat IgG was estimated by radioimmunoassay using mouse-absorbed anti-rat IgG (Kirkegaard & Perry Laboratories). For three consecutive days, each mouse received daily doses of 1 mg of anti-CD4 or anti-CD8 MAb. DNA immunization was performed 2 days after the last dose. The efficacy of the depletion was estimated by flow cytometry analysis using anti-CD4 or anti-CD8 antibody followed by fluorescein-conjugated anti-rat IgG. The amount of CD4+ cells was reduced by ∼97% in mice treated with anti-CD4 MAb. Treatment with anti-CD8 MAb eliminated 95% of CD8+ cells.
RESULTS
Comparison of the immunogenicity and protective immunity elicited by plasmids containing the entire TS gene or the sequence encoding its enzymatic domain.
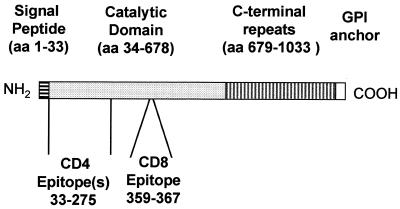
In earlier studies, we reported that protective immunity against experimental T. cruzi infection in BALB/c mice could be generated by immunization with TS plasmid 154/13 (p154/13). This plasmid contains the coding region for the catalytic domain of the enzyme preceded by amino acids representing the signal peptide of TS. In addition to the enzymatic domain, TS expressed in trypomastigotes of T. cruzi has a C-terminal repeat domain and is linked to the membrane by a glycophosphatidylinositol anchor (27). A schematic description of the amino acid deduced primary structure of TS is shown in Fig. 1.
FIG. 1.
Schematic view of the primary structure of T. cruzi TS.
To determine whether immunization with the entire TS gene could modify (improve or reduce) immunity to the functional catalytic domain of the enzyme, we immunized mice with a plasmid containing the entire TS gene (pcDNA3-TS; Table 1). In the sera of BALB/c mice immunized with p154/13 and pcDNA3-TS, we detected similar antibody titers to recombinant TS-cat (Fig. 2A). Comparison of antibody titers after each plasmid immunization also failed to reveal significant differences between mice immunized with p154/13 or pcDNA3-TS (data not shown).
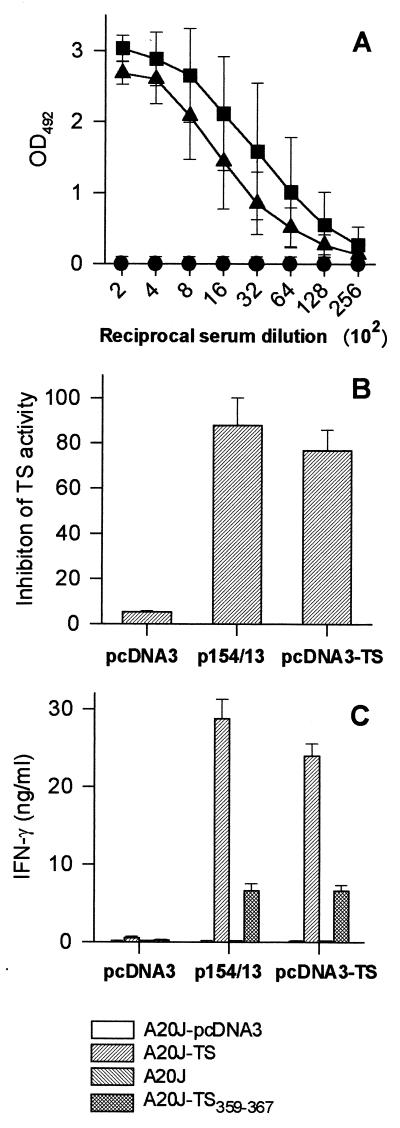
FIG. 2.
Immune responses of mice immunized with pcDNA3-TS and p154/13. BALB/c mice were immunized as described in detail in Materials and Methods with pcDNA3-TS (▴), p154/13 (■), or pcDNA3 (●). Fourteen days after the last immunization, blood samples were collected and the sera were assayed for the presence of antibodies to TS by ELISA using polystyrene wells coated with recombinant TS-cat (A), or for the presence of TS-inhibitory antibodies (B). The results represent the mean values obtained from eight mice ± the standard deviations (SD). Pooled spleen cells obtained from three mice immunized with pcDNA3-TS, p154/13, or pcDNA3 were expanded for 6 days in the presence of irradiated A20J-TS cells. (C) The expanded cells were restimulated in the presence of A20J-pcDNA3, A20J-TS, A20J cells, or A20J cells coated with 1 μM TS359-367 peptide (A20J-TS359–367). IFN-γ was estimated in supernatants collected after 18 h. Results are expressed as averages of triplicate cultures ± SD.
In addition to inducing antibodies that recognize recombinant TS-cat by ELISA, immunization with these plasmids elicited antibodies that significantly inhibited TS enzymatic activity in vitro (Fig. 2B). Titration curves performed with pooled sera collected from mice immunized with each plasmid also failed to reveal a difference in the concentration of TS-inhibitory antibodies (data not shown). In contrast, control mice immunized with pcDNA3 did not present anti-TS antibodies as determined by ELISA or inhibition of TS enzymatic activity (Fig. 2A and B, respectively).
The presence of IFN-γ-producing cells in the spleens of DNA-immunized mice was determined after in vitro expansion of these cells by stimulation with A20J-TS cells. After 6 days of expansion, spleen cells were restimulated in vitro with A20J-TS cells or control A20J-pcDNA3 cells. Using this assay, we established that CD4+ and CD8+ T cells were responsible for IFN-γ secretion (23). In parallel, spleen cells were restimulated in vitro with A20J cells coated with peptide TS359–367, which represents the TS CD8 epitope (23).
After restimulation with A20J-TS cells, spleen cells from mice immunized with p154/13 or pcDNA3-TS produced comparable amounts of IFN-γ (Fig. 2C). Upon restimulation in vitro with A20J cells coated with peptide TS359–367, spleen cells from mice immunized with p154/13 or pcDNA3-TS secreted nearly identical amounts of IFN-γ (Fig. 2C). This pattern was observed in several independent experiments.
Protective immunity elicited by immunization with these two plasmids was evaluated after a challenge with 6,500 bloodstream trypomastigotes. The course of parasitemia and survival of mice immunized with p154/13 or pcDNA3-TS were very similar (Fig. 3A and B). These mice displayed a significantly lower parasitemia than animals injected with pcDNA3 (P < 0.001 at day 7; Fig. 3A). Also, almost all mice immunized with TS plasmids survived infection. In contrast, a significant fraction of animals injected with pcDNA3 died after challenge with T. cruzi (P < 0.05; Fig. 3B).
FIG. 3.
Trypomastigote-induced parasitemia and mortality in mice immunized with pcDNA3-TS, p154/13, and pcDNA3. BALB/c mice were immunized with pcDNA3-TS, p154/13, or pcDNA3. Three weeks after the last immunization, mice were challenged i.p. with 6,500 bloodstream trypomastigotes. (A) Course of parasitemia, estimated as described in Materials and Methods. The results represent the mean values obtained from eight mice ± the standard deviations. At the peak of infection (day 7), the parasitemia of mice immunized with pcDNA3-TS or p154/13 was significantly lower than the parasitemia of control animals injected with pcDNA3 (p<0.001). (B) Kaplan-Meier curves for survival of eight mice immunized with the indicated plasmid(s). Statistically significant survival was observed in mice immunized with pcDNA3-TS or p154/13 compared to animals that received pcDNA3 (P < 0.05).
Comparison of the immunogenicity and protective immunity elicited by plasmids containing DNA sequences encoding the TS enzymatic domain or its immunogenic epitopes.
In our earlier studies, we isolated CD4+ Th1 and CD8+ Tc1 clones from mice immunized with p154/13. These CD4 and CD8 clones displayed remarkable antiparasitic activities in vitro, inhibiting almost completely parasite replication in infected macrophages or fibroblast cells, respectively (23, 24). Using CD8+ T-cell clones and synthetic peptides, it was possible to precisely map the single CD8 epitope (TS359–367) recognized by these clones (Fig. 1 and reference 23). The epitope recognized by the CD4+ clones was partially mapped using recombinant TS-cat protein and A20J cells transfected with pΔ154/13. The recombinant TS-cat protein and pΔ154/13 express aa 34 to 678 and aa 1 to 275 of TS, respectively (reference 22 and Table 1). CD4+ Th1 clones 2F1 and 2F3 secreted IFN-γ when stimulated with recombinant TS-cat protein or A20J cells transfected with pΔ154/13 (reference 23 and unpublished results, respectively). Based on these experiments, we concluded that there is one or more CD4 epitopes located between aa 34 and 275 of TS (Fig. 1). The identification of these epitopes allowed us to determine the immunogenicity and protective efficacy of plasmids containing sequences encoding CD4 or CD8 epitopes of T. cruzi TS.
To evaluate the immunogenic properties of a plasmid containing the sequence encoding the CD4 epitope(s) of TS, mice were immunized with pΔ154/13. In parallel, we immunized mice with p154/13, which expresses the catalytic domain of TS. In the sera of mice immunized with pΔ154/13 or p154/13, we detected similar antibody titers to recombinant TS-cat (Fig. 4A). Although immunization with pΔ154/13 elicited antibodies that recognized very well the recombinant TS-cat by ELISA, these antibodies did not inhibit TS enzymatic activity in vitro (Fig. 4B). Increasing the serum concentration to 50% of the final volume also failed to inhibit TS enzymatic activity (data not shown).
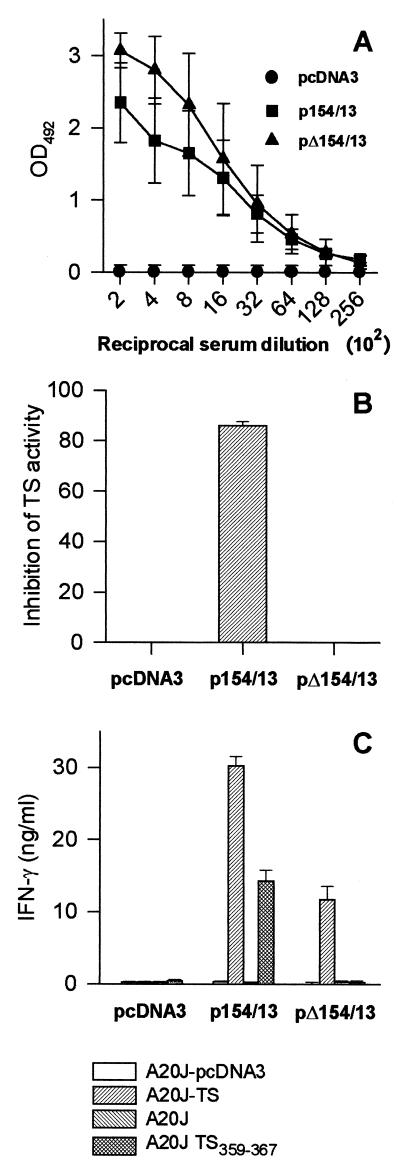
FIG. 4.
Immune responses of mice immunized with p154/13 and pΔ154/13. BALB/c mice were immunized with p154/13, pΔ154/13, or pcDNA3. Fourteen days after the last immunization, blood samples were collected and the sera were assayed for the presence of antibodies to recombinant TS-cat by ELISA (A) or for the presence of TS-inhibitory antibodies (B). The results represent the mean value obtained from eight mice ± the standard deviations (SD). Pooled spleen cells obtained from three mice immunized with p154/13, pΔ154/13, or pcDNA3 were expanded for 6 days in the presence of irradiated A20J-TS cells. (C) The expanded cells were restimulated in the presence of A20J-pcDNA3, A20J-TS, A20J cells, or A20J cells coated with 1 μM TS359-367 peptide (A20J-TS359-367). IFN-γ was estimated in supernatants collected after 18 h. Results are expressed as averages of triplicate cultures ± SD.
The presence of IFN-γ-producing cells in the spleens of DNA-immunized mice was determined as described above. In several independent experiments, upon restimulation with A20J-TS cells, spleen cells from mice immunized with p154/13 produced higher amounts of IFN-γ than cells from animals immunized with pΔ154/13 (Fig. 4C). Also relevant was the fact that spleen cells from mice that received pΔ154/13 did not secrete IFN-γ when restimulated in vitro with A20J cells coated with peptide TS359–367 (Fig. 4C). This was expected because this plasmid does not contain the sequence encoding the TS CD8 epitope (Table 1).
In vitro experiments of T-cell depletion using anti-CD4 and anti-CD8 MAbs in the presence of complement confirmed that only IFN-γ-producing CD4+ T cells were present in animals immunized with pΔ154/13. On the other hand, immunization with p154/13 induced IFN-γ-producing CD4+ and CD8+ T cells (data not shown and reference 23).
After challenge with T. cruzi trypomastigotes, the peak parasitemia of mice immunized with pΔ154/13 was extremely variable in all three experiments performed. Nevertheless, the course of parasitemia and number of mice immunized with pΔ154/13 or pcDNA3 that survived the infection were not statistically different (Fig. 5A and B, respectively). In contrast, mice immunized with p154/13 had lower levels of parasitemia than mice injected with pΔ154/13 or pcDNA3 (P = 0.011 or P < 0.001, respectively; Fig. 5A). Also, all p154/13-immunized animals survived T. cruzi infection (Fig. 5B).
FIG. 5.
Trypomastigote-induced parasitemia and mortality in mice immunized with p154/13, pΔ154/13, and pcDNA3. BALB/c mice were immunized with p154/13, pΔ154/13, or pcDNA3. Three weeks after the last immunization, mice were challenged i.p., with 6,500 bloodstream trypomastigotes. (A) Course of infection, with the mean values obtained from eight mice ± the standard deviations. At the peak of infection (day 8), the parasitemia of mice immunized with each plasmid were compared. The results were as follows: (i) p154/13 versus pcDNA3, P < 0.001; (ii) p154/13 versus pΔ154/13, P = 0.011; (iii) pΔ154/13 versus pcDNA3, P > 0.05. (B) Kaplan-Meier curves for survival of eight mice immunized with the indicated plasmid. Statistically significant survival was observed in mice immunized with p154/13 compared to animals that received pΔ154/13 or pcDNA3 (P < 0.05). The number of mice immunized with pΔ154/13 or pcDNA3 that survived infection was not significantly different (P > 0.05).
The immunogenicity and protective immunity generated by a plasmid containing only the sequence encoding the TS CD8 epitope was determined in mice immunized with a plasmid designated as pCD8-epitope (Table 1). IFN-γ secretion by spleen cells of mice immunized with pCD8-epitope was evaluated in vitro upon restimulation with A20J cells coated with peptide TS359–367. IFN-γ secretion was detected on several occasions but was significantly lower than the concentration detected in the supernatants of spleen cells from animals immunized with p154/13 (Fig. 6A).
FIG. 6.
Immune response, trypomastigote-induced parasitemia, and mortality of mice immunized with p154/13, pCD8-epitope, and pcDNA3. BALB/c mice were immunized with p154/13, pCD8-epitope, or pcDNA3. Fourteen days after the last immunization, pooled spleen cells obtained from three mice immunized with each plasmid were expanded for 6 days in the presence of irradiated A20J-TS cells. (A) The expanded cells were restimulated in the presence of A20J-pcDNA3, A20J-TS, A20J cells, or A20J cells coated with 1 μM peptide TS359-367 (A20J-TS359-367). IFN-γ was estimated in supernatants collected after 18 h. Results are expressed as averages of triplicate cultures ± the standards of deviation (SD). Three weeks after the last immunization, mice were challenged i.p. with 6,500 bloodstream trypomastigotes. (B) Course of infection, with mean values obtained from eight mice ± SD. At the peak of infection (day 7), the parasitemia of mice immunized with each plasmid was compared. The results were as follows: (i) p154/13 versus pcDNA3, P < 0.001; (ii) p154/13 versus pCD8-epitope, P < 0.01; (iii) pCD8-epitope versus pcDNA3, P < 0.01. (C) Kaplan-Meier curves for survival of eight mice immunized with the indicated plasmid. Statistically significant survival was observed in mice immunized with p154/13 compared to animals that received pCD8-epitope or pcDNA3 (P < 0.05). The number of mice immunized with pCD8-epitope or pcDNA3 that survived infection was not significantly different (P > 0.05).
The course of parasitemia and survival of mice immunized with pCD8-epitope or p154/13 were also significantly different. The peak parasitemia of pCD8-epitope-immunized mice was higher than that of animals injected with p154/13 (P < 0.01; Fig. 6B). Still, the peak parasitemia was slightly lower than in control animals injected with pcDNA3 (P < 0.01). In spite of the lower peak parasitemia, the mortality rates of mice injected with pCD8-epitope or pcDNA3 were almost identical (Fig. 6C). In contrast, a significantly higher proportion of animals immunized with p154/13 survived T. cruzi infection (P < 0.05; Fig. 6C).
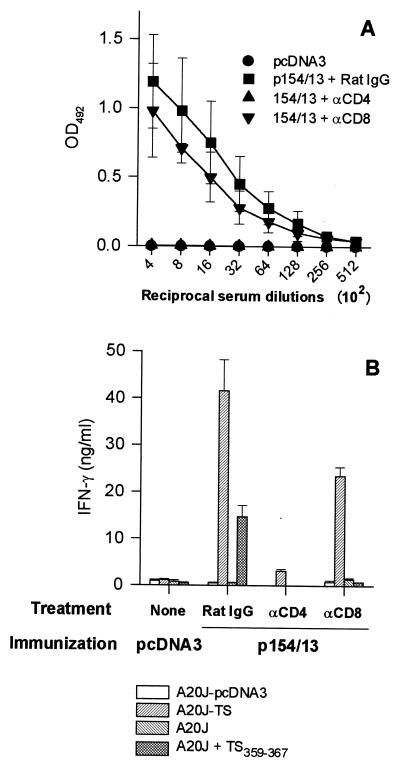
The limited priming and/or expansion of CD8+ T cells by immunization with CD8-epitope could be explained if these cells required a concomitant activation of CD4+ T cells. To test this hypothesis, we treated mice with anti-CD4 or anti-CD8 MAb before immunization with p154/13. In mice treated with anti-CD4 MAb, we were unable to detect serum IgG antibodies to recombinant TS-cat (Fig. 7A). In both experiments, IFN-γ secretion by CD4+ or CD8+ spleen cells was also severely impaired (Fig. 7B). Treatment with anti-CD8 MAb reduced IFN-γ secretion after restimulation with A20J-TS (Fig. 7B) and completely inhibited IFN-γ secretion by spleen cells specific for the TS359–367 peptide (Fig. 7B). These results suggest that priming of B and CD8+ T cells after immunization with p154/13 was dependent on the presence of CD4+ T cells.
FIG. 7.
Depletion of CD4+ T cells significantly reduces TS-specific antibodies and IFN-γ-secreting CD4+ and CD8+ T cells induced by immunization with p154/13. BALB/c mice were treated for three consecutive days with anti-CD4, anti-CD8, or rat-IgG. One and 22 days later, these mice were immunized i.m. with 100 μg of p154/13. In parallel, control mice received 100 μg of pcDNA3. (A) Fourteen days after the last immunization, blood samples were collected and the sera were assayed for the presence of antibodies to recombinant TS-cat by ELISA The results represent the mean values obtained from four mice ± the standard deviations (SD). (B) Pooled spleen cells obtained from two mice immunized with p154/13 and treated with anti-CD4, anti-CD8, or rat-IgG were expanded for 6 days in the presence of irradiated A20J-TS cells. The expanded cells were restimulated in the presence of A20J-pcDNA3, A20J-TS, A20J cells, or A20J cells coated with 1 μM peptide TS359-367 (A20J-TS359-367). IFN-γ was estimated in supernatants collected after 18 h. Results are expressed as the average of triplicate cultures ± SD.
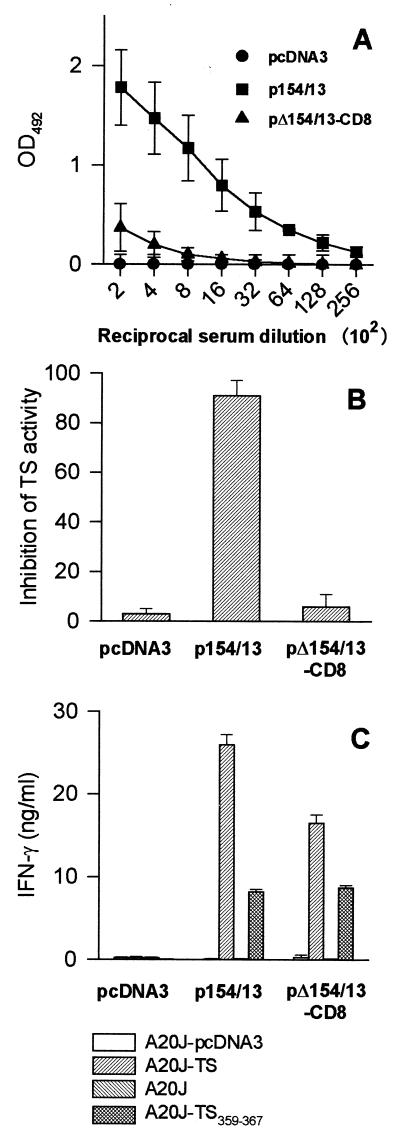
To confirm that both CD4 and CD8 epitopes are required for efficient DNA-induced immunity against T. cruzi, we immunized mice with pΔ154/13-CD8, which contains the sequence encoding both the CD4 and CD8 epitopes of TS (Table 1). In several experiments, we observed much lower antibody titers to recombinant TS-cat in the sera of mice immunized with pΔ154/13-CD8 than in mice injected with p154/13 (Fig. 8A). Also, these antibodies did not inhibit TS enzymatic activity in vitro (Fig. 8B).
FIG. 8.
Immune responses of mice immunized with p154/13 and pΔ154/13-CD8. BALB/c mice were immunized with p154/13, pΔ154/13-CD8, or pcDNA3. Fourteen days after the last immunization, blood samples were collected and the sera were assayed for the presence of antibodies to recombinant TS-cat by ELISA (A) or for the presence of TS-inhibitory antibodies (B). Results represent the mean values obtained from eight mice ± the standard deviations (SD). (C) Pooled spleen cells obtained from three mice immunized with p154/13, pΔ154/13-CD8, or pcDNA3 were expanded for 6 days in the presence of irradiated A20J-TS cells. The expanded cells were restimulated in the presence of A20J-pcDNA3, A20J-TS, A20J cells, or A20J cells coated with 1 μM TS359-367 peptide (A20J-TS359-367). IFN-γ was estimated in supernatants collected after 18 h. Results are expressed as the average of triplicate cultures ± SD.
Upon restimulation with A20J-TS cells, spleen cells from mice immunized with pΔp154/13 produced slightly lower amounts of IFN-γ than cells from animals immunized with 154/13-CD8 (Fig. 8C). This difference was observed in several independent experiments. Lymphocytes from mice immunized with pΔ154/13-CD8 and restimulated in vitro with A20J cells coated with TS359–367 peptide secreted similar amounts of IFN-γ as cells from mice injected with p154/13 (Fig. 8C).
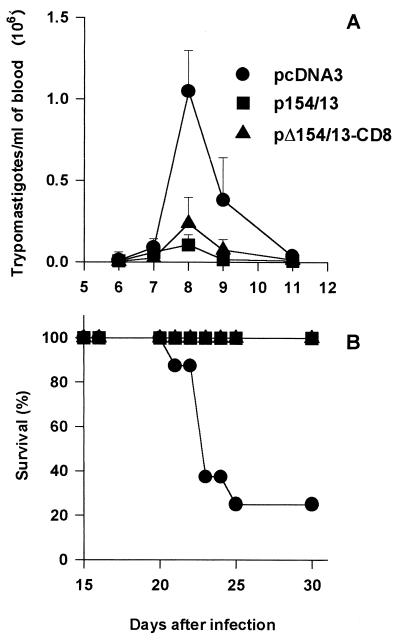
After challenge with T. cruzi trypomastigotes, the peak parasitemia of mice immunized with pΔ154/13-CD8 or p154/13 did not differ significantly from one another (P > 0.05; Fig. 9A) and were significantly lower than the parasitemia of animals injected with pcDNA3 (P < 0.001; Fig. 9A). A similar observation was made for mortality rate. While the majority of mice injected with pcDNA3 died, all animals immunized with pΔ154/13-CD8 or p154/13 survived a challenge with T. cruzi trypomastigotes (P < 0.05; Fig. 9B).
FIG. 9.
Trypomastigote-induced parasitemia and mortality in mice immunized with p154/13, pΔ154/13-CD8, and pcDNA3. BALB/c mice were immunized with p154/13, pΔ154/13-CD8, or pcDNA3. Three weeks after the last immunization, mice were challenged i.p. with 6,500 bloodstream trypomastigotes. (A) Course of infection and the mean values obtained from eight mice ± standard deviations. At the peak of infection (day 8). the parasitemia of mice immunized with each plasmid was compared. The results were as follows: (i) p154/13 versus pcDNA3, P < 0.001; (ii) p154/13 versus pΔ154/13-CD8, P > 0.05; (iii) pΔ154/13-CD8 versus pcDNA3, P < 0.001. (B) Kaplan-Meier curves for survival of eight mice immunized with the indicated plasmid. Statistically significant survival was observed in mice immunized with p154/13 or pΔ154/13-CD8 compared to animals that received pcDNA3 (P < 0.01).
DISCUSSION
The purpose of the present study was to compare the effectiveness of immunization using various plasmids containing the entire TS gene or sequences encoding its immunogenic portions. We found that three distinct plasmids containing DNA sequences encoding TS CD4+ and CD8+ T-cell epitopes could provide a degree of immunity sufficient to reduce the parasitemia and mortality of DNA-immunized animals caused by a challenge with T. cruzi trypomastigotes. In contrast, plasmids expressing either CD4+ or CD8+ T-cell epitopes of TS were unable to provide a similar degree of protective immunity against infection.
The fact that acquired resistance to T. cruzi infection was only effectively achieved by DNA immunization with plasmids capable of generating both CD4+ and CD8+ T cells corroborates the findings of earlier study that used native paraflagellar rod proteins for immunization (18). In this study, a high degree of protective immunity against T. cruzi infection was achieved after immunization of wild-type and B-cell-deficient mice. On the other hand, CD4+ T-cell-depleted or β2-microglobulin KO mice failed to control infection, indicating that CD4+ and MHC class I-restricted cells were important for protective immunity.
TS-specific CD4+ T cells appear to play multiple roles in immunity elicited by DNA immunization. In our system, it is plausible that TS-specific CD4+ T cells participate as an effector mechanism of protection. In mice immunized with p154/13, CD4+ T cells seem to be a major source of IFN-γ, accounting for ∼65% of the IFN-γ secreted by spleen cells in vitro (23). From DNA-vaccinated mice, we isolated CD4+ Th1 clones that efficiently activated macrophages to eliminate intracellular forms of T. cruzi in vitro. The antiparasitic activity of macrophages activated by CD4+ T cells was dependent on IFN-γ and nitric oxide production (24). Whether similar mechanisms operate in vivo remains to be determined.
CD4+ T cells can also participate in protective immunity by providing help for antibody production. Mice immunized with pcDNA3-TS or p154/13 produced antibodies that recognized the catalytic domain of TS and drastically inhibited the activity of this enzyme in vitro (Fig. 2A and B). The production of antibodies specific for recombinant TS-cat was strictly dependent on the activation of CD4+ T cells because in mice treated with anti-CD4 MAb, little or no specific antibodies were detected (Fig. 7A). TS-inhibitory antibodies have the ability to significantly reduce parasite sialylation in vitro (21). In vitro studies also have suggested that the process of sialylation is important for parasite survival in the extracellular host environment and during invasion of nonphagocytic cells (19). Most relevant, an earlier study showed that passive transfer of antibodies specific for the TS catalytic domain reduced mouse infection with T. cruzi (4).
Although TS-inhibitory antibodies may participate in protective immune responses, immunity against T. cruzi infection could be achieved in mice that had no TS-inhibitory antibodies. Immunization with pΔ154/13-CD8 failed to induce TS-inhibitory antibodies (Fig. 8B). Nevertheless, mice immunized with this plasmid had a reduced parasitemia and mortality after challenge with T. cruzi trypomastigotes (Fig. 9A and B). Therefore, anti-TS antibodies may help but are not crucial for protective immunity generated by DNA immunization.
In spite of the activation of IFN-γ-producing CD4+ T cells, in several experiments immunization of BALB/c mice with pΔ154/13 failed to confer a significant degree of protective immunity against T. cruzi infection. Two not-mutually-excluding possibilities could explain the lower efficacy of pΔ154/13. First, CD4+ T-cell activation could be reduced due to the loss of CD4 epitopes present in the region spanning aa 276 to 678 of TS. Alternatively, activation of CD8+ T cells could be important for efficient protective immunity against a lethal challenge with T. cruzi. To address the question of whether activation of CD8+ T cells could restore the protective efficacy of pΔ154/13, we generated pΔ154/13-CD8. Protective immunity elicited by immunization with pΔ154/13-CD8 was similar to that with p154/13, suggesting that activation of CD8+ T cells was crucial for protective immunity (Fig. 9A and B).
CD4+ T cells induced by plasmid immunization seem to be crucial for priming and expansion of specific CD8+ T cells. Immunization with pCD8-epitope was unable to efficiently prime TS-specific CD8+ T cells (Fig. 6A). In contrast, in mice immunized with three distinct plasmids containing DNA sequences encoding both CD4+ and CD8+ T-cell epitopes, IFN-γ secretion by cells specific for peptide TS359-367 was significantly higher. The importance of CD4+ T cells in the priming of CD8+ T cells was corroborated by the fact that in mice treated with anti-CD4 MAb prior to immunization with p154/13, IFN-γ secretion by TS-specific CD8+ T cells was undetectable (Fig. 7B).
Although several studies have reported immune responses mediated by CD8+ T cells after DNA immunization, only a few studies have addressed the requirements for their priming. In three studies, priming of specific CD8+ T cells by DNA immunization was compared in mice immunized with plasmids containing minigenes encoding the CD8 epitope alone or in the presence of sequences encoding a CD4 epitope. The expression of CD4 epitopes either restored or significantly improved CD8+ T-cell immune responses (8, 11, 17). These results suggested that in these cases CD8+ T-cell priming required the activation of CD4+ T cells. In one of these studies, as in our case, depletion of CD4+ T cells drastically reduced CD8+ T-cell priming (17).
In other cases, however, priming of specific CD8+ T cells following DNA immunization with plasmids containing minigenes could also be achieved in the absence of CD4+ T-cell activation (2, 8, 11, 12, 25). These results are probably not conflicting; rather, they may reflect different requirements for CD8+ T-cell priming observed after immunization with distinct epitopes. Very recent evidence has suggested that CD8 epitopes with very high affinities for MHC class I molecules can efficiently prime CD8+ T cells in MHC class II-deficient mice (9). In contrast, priming of CD8+ T cells with epitopes with lower affinities for MHC class I molecules required the coadministration of either a CD4 epitope or an anti-CD40 MAb (9).
In circumstances where priming of specific CD8+ T cells was observed with plasmids containing only minigenes encoding CD8 epitopes, protective immunity against viral infection could not be obtained (2, 8, 25). In one case, immunity was observed against a bacterial infection (Listeria monocytogenes) when using a plasmid expressing the CD8 epitope of listeriolysin (33). However, the degree of protective immunity was not compared to that with plasmids containing the entire listeriolysin gene (5). In general, these observations suggested that CD4+ T cells induced by DNA immunization were important either to provide optimal activation of CD8+ T cells or as an effector mechanism of protection, or both.
Although many studies have provided evidence that CD8+ T cells participate in the protective immunity against experimental T. cruzi infection, the precise mechanism used by these cells has not been clearly defined. The fact that CD8+ T cells secrete IFN-γ may suggest that this is a mechanism leading to the elimination of intracellular forms of the parasite. It is well established that IFN-γ is an important mediator of naturally acquired immunity against the infection (10, 30). However, as in the case of CD4+ T cells, a direct link between the IFN-γ secretion by CD8+ T cells and the in vivo antiparasitic activity of these cells has not been provided.
In addition to producing IFN-γ, CD8+ T cells may exert their antiparasitic effect by direct lysis of target cells infected with T. cruzi or by secreting other potentially active mediators such as tumor necrosis factor α, granulisin, or a number of different chemokines (1, 20, 29). In fact, it has been described that CD8+ T cells specific for amastigote or trypomastigote antigens are capable of lysing nonphagocytic cells infected with T. cruzi in vitro (16, 36). However, it is unclear whether cytolysis of infected target cells by CD8+ T cells is an effective mechanism to restrain T. cruzi infection in vivo. For example, genetically modified mice that do not express perforin or granzyme B are not more susceptible to infection than wild-type animals (13). These observations argue against a crucial role for perforin- or granzyme B-mediated lysis in resistance. The elucidation of the antiparasitic mechanisms mediated by CD8 T cells will certainly require further investigation using more accurate experimental models.
In summary, host acquired resistance to T. cruzi infection was only effectively achieved by DNA immunization with plasmids of the TS gene capable of generating both CD4+ Th1 and CD8+ Tc1 cells. It will be important to determine whether activation of these two T-cell populations is also important during protective immunity elicited by DNA immunization with other T. cruzi genes (28, 35).
ACKNOWLEDGMENTS
This work was supported by grants from FAPESP, CNPq, PRONEX, and FINEP (Brazil). A.E.F. and S.S.K. are recipients of fellowships from FAPESP.
REFERENCES
- 1.Aliberti J C, Machado F S, Souto J T, Campanelli A P, Teixeira M M, Gazzinelli R T, Silva J S. Beta-chemokines enhance parasite uptake and promote nitric oxide-dependent microbiostatic activity in murine inflammatory macrophages infected with Trypanosoma cruzi. Infect Immun. 1999;67:4819–4826. doi: 10.1128/iai.67.9.4819-4826.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An L L, Rodriguez F, Harkins S, Zhang J, Whitton J L. Quantitative and qualitative analyses of the immune responses induced by a multivalent minigene DNA vaccine. Vaccine. 2000;18:2132–2141. doi: 10.1016/s0264-410x(99)00546-0. [DOI] [PubMed] [Google Scholar]
- 3.Arnholdt A C V, Piuvezam M R, Russo D M, Lima A P C, Pedrosa R C, Reed S G, Scharfstein J. Analysis and partial epitope mapping of human T cell responses to Trypanosoma cruzi cysteinil proteinase. J Immunol. 1993;151:3171–3179. [PubMed] [Google Scholar]
- 4.Chuenkova M, Pereira M E A. Trypanosoma cruzi trans-sialidase: enhancement of virulence in a murine model of Chagas' disease. J Exp Med. 1995;181:1693–1703. doi: 10.1084/jem.181.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornell K A, Bouwer H G, Hinrichs D J, Barry R A. Genetic immunization of mice against Listeria monocytogenes using plasmid DNA encoding listeriolysin O. J Immunol. 1999;163:322–329. [PubMed] [Google Scholar]
- 6.Costa F, Franchin G, Pereira-Chioccola V L, Ribeirão M, Schenkman S, Rodrigues M M. Immunization with a plasmid DNA containing the gene of trans-sialidase reduces Trypanosoma cruzi infection in mice. Vaccine. 1998;16:768–774. doi: 10.1016/s0264-410x(97)00277-6. [DOI] [PubMed] [Google Scholar]
- 7.Dos Reis G A. Cell-mediated immunity in experimental Trypanosoma cruzi infection. Parasitol Today. 1997;13:335–342. doi: 10.1016/s0169-4758(97)01073-9. [DOI] [PubMed] [Google Scholar]
- 8.Fomsgaard A, Nielsen H V, Kirkby N, Bryder K, Corbet S, Nielsen C, Hinkula J, Buus S. Induction of cytotoxic T-cell responses by gene gun DNA vaccination with minigenes encoding influenza A virus HA and NP CTL-epitopes. Vaccine. 1999;18:681–691. doi: 10.1016/s0264-410x(99)00279-0. [DOI] [PubMed] [Google Scholar]
- 9.Franco A, Tilly D A, Gramaglia I, Croft M, Cipolla L, Meldal M, Grey H M. Epitope affinity for MHC class I determines helper requirement for CTL priming. Nat Immunol. 2000;1:145–149. doi: 10.1038/77827. [DOI] [PubMed] [Google Scholar]
- 10.Hölscher C, Kölher G, Müller U, Mossman H, Schaub G A, Brombacher F. Defective nitric oxide effector functions lead to extreme susceptibility of Trypanosoma cruzi-infected mice deficient in gamma interferon receptor or inducible nitric oxide synthase. Infect Immun. 1998;66:1208–1215. doi: 10.1128/iai.66.3.1208-1215.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishioka G Y, Fikes J, Hermanson G, Livingston B, Crimi C, Qin M, del Guercio M F, Oseroff C, Dahlberg C, Alexander J, Chesnut R W, Sette A. Utilization of MHC class I transgenic mice for development of minigene DNA vaccines encoding multiple HLA-restricted CTL epitopes. J Immunol. 1999;162:3915–3925. [PubMed] [Google Scholar]
- 12.Iwasaki A, Dela Cruz C S, Young A R, Barber B H. Epitope-specific cytotoxic T lymphocyte induction by minigene DNA immunization. Vaccine. 1999;17:2081–2088. doi: 10.1016/s0264-410x(98)00411-3. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Tarleton R L. The relative contribution of antibody production and CD8+ T cell function to immune control of Trypanosoma cruzi. Parasite Immunol. 1998;20:207–216. doi: 10.1046/j.1365-3024.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- 14.Krettli A U, Brener Z. Protective effects of specific antibodies in Trypanosoma cruzi infections. J Immunol. 1976;116:755–760. [PubMed] [Google Scholar]
- 15.Li S, Rodrigues M, Rodriguez D, Rodriguez J R, Esteban M, Palese P, Nussenzweig R S, Zavala F. Priming with recombinant influenza virus followed by administration of recombinant vaccinia virus induces CD8+ T-cell-mediated protective immunity against malaria. Proc Natl Acad Sci USA. 1993;90:5214–5218. doi: 10.1073/pnas.90.11.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Low H P, Santos M A, Wizel B, Tarleton R L. Amastigote surface proteins of Trypanosoma cruzi are targets for CD8+ CTL. J Immunol. 1998;160:1817–1823. [PubMed] [Google Scholar]
- 17.Maecker H T, Umetsu D T, DeKruyff R H, Levy S. Cytotoxic T cell responses to DNA vaccination: dependence on antigen presentation via class II MHC. J Immunol. 1998;161:6532–6536. [PubMed] [Google Scholar]
- 18.Miller M J, Wrightsman R A, Stryker G A, Manning J E. Protection of mice against Trypanosoma cruzi by immunization with paraflagellar rod proteins requires T cell, but not B cell, function. J Immunol. 1997;158:5330–5337. [PubMed] [Google Scholar]
- 19.Ming M, Chuenkova M, Ortega-Barria E, Pereira M E A. Mediation of Trypanosoma cruzi invasion by sialic acid on the host cell and trans-sialidase on the trypanosome. Mol Biochem Parasitol. 1993;59:243–252. doi: 10.1016/0166-6851(93)90222-j. [DOI] [PubMed] [Google Scholar]
- 20.Munoz-Fernandez M A, Fernandez M A, Fresno M. Synergism between tumor necrosis factor-alpha and interferon-gamma on macrophage activation for the killing of intracellular Trypanosoma cruzi through a nitric oxide-dependent mechanism. Eur J Immunol. 1992;22:301–307. doi: 10.1002/eji.1830220203. [DOI] [PubMed] [Google Scholar]
- 21.Pereira-Chioccola V L, Acosta-Serrano A, Almeida I C, Ferguson M A J, Souto-Padron T, Rodrigues M M, Travassos L R, Schenkman S. Mucin-like molecules form a negatively charged coat that protects Trypanosoma cruzi trypomastigotes from killing by human anti-α-galactosyl antibodies. J Cell Sci. 2000;113:1299–1307. doi: 10.1242/jcs.113.7.1299. [DOI] [PubMed] [Google Scholar]
- 22.Ribeirão M, Pereira-Chioccola V L, Eichinger D, Rodrigues M M, Schenkman S. Temperature differences for trans-glycosilation and hydrolysis reaction reveal an acceptor binding site in the catalytic mechanism of Trypanosoma cruzi trans-sialidase. Glycobiology. 1997;7:1237–1246. doi: 10.1093/glycob/7.8.1237. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues M M, Ribeirão M, Pereira-Chioccola V, Renia L, Costa F. Predominance of CD4 Th1 and CD8 Tc1 cells revealed by characterization of the cellular immune response generated by immunization with a DNA vaccine containing a Trypanosoma cruzi gene. Infect Immun. 1999;67:3855–3863. doi: 10.1128/iai.67.8.3855-3863.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigues M M, Ribeirão M, Boscardin S B. CD4 Th1 but not Th2 clones efficiently activate macrophages to eliminate Trypanosoma cruzi through a nitric oxide dependent mechanism. Immunol Lett. 2000;73:43–50. doi: 10.1016/s0165-2478(00)00205-4. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez F, An L L, Harkins S, Zhang J, Yokoyama M, Widera G, Fuller J T, Kincaid C, Campbell I L, Whitton J L. DNA immunization with minigenes: low frequency of memory cytotoxic T lymphocytes and inefficient antiviral protection are rectified by ubiquitination. J Virol. 1998;72:5174–5181. doi: 10.1128/jvi.72.6.5174-5181.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rottenberg M E, Bakhiet M, Olsson T, Kristensson K, Mak T, Wigzell H, Orn A. Differential susceptibilities of mice genomically deleted of CD4 and CD8 to infections with Trypanosoma cruzi or Trypanosoma brucei. Infect Immun. 1993;61:5129–5133. doi: 10.1128/iai.61.12.5129-5133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schenkman S, Eichinger D, Pereira M E, Nussenzweig V. Structural and functional properties of Trypanosoma trans-sialidase. Annu Rev Microbiol. 1994;48:499–523. doi: 10.1146/annurev.mi.48.100194.002435. [DOI] [PubMed] [Google Scholar]
- 28.Sepulveda P, Hontebeyrie M, Liegeard P, Mascilli A, Norris K A. DNA-based immunization with Trypanosoma cruzi complement regulatory protein elicits complement lytic antibodies and confers protection against Trypanosoma cruzi infection. Infect Immun. 2000;68:4986–4991. doi: 10.1128/iai.68.9.4986-4991.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stenger S, Rosat J P, Bloom B R, Krensky A M, Modlin R L. Granulysin: a lethal weapon of cytolytic T cells. Immunol Today. 1999;20:390–394. doi: 10.1016/s0167-5699(99)01449-8. [DOI] [PubMed] [Google Scholar]
- 30.Tarleton R L, Grusby M J, Zhang L. Increased susceptibility of Stat4-deficient and enhanced resistance in Stat6-deficient mice to infection with Trypanosoma cruzi. J Immunol. 2000;165:1520–1525. doi: 10.4049/jimmunol.165.3.1520. [DOI] [PubMed] [Google Scholar]
- 31.Tarleton R L, Sun J, Zhang L, Postan M, Glimcher L. Trypanosoma cruzi infection in MHC-deficient mice: further evidence for the role of both class I- and class II-restricted T cells in immune resistance and disease. Int Immunol. 1996;8:13–22. doi: 10.1093/intimm/8.1.13. [DOI] [PubMed] [Google Scholar]
- 32.Tarleton R L, Zhang L. Chagas disease etiology: autoimmunity or parasite persistence? Parasitol Today. 1999;15:94–99. doi: 10.1016/s0169-4758(99)01398-8. [DOI] [PubMed] [Google Scholar]
- 33.Uchijima M, Yoshida A, Nagata T, Koide Y. Optimization of codon usage of plasmid DNA vaccine is required for the effective MHC class I-restricted T cell responses against an intracellular bacterium. J Immunol. 1998;161:5594–5599. [PubMed] [Google Scholar]
- 34.Uemura H, Schenkman S, Nussenzweig V, Eichinger D. Only some members of a gene family in Trypanosoma cruzi encode proteins that express both trans-sialidase and neuraminidase activities. EMBO J. 1992;11:3837–3844. doi: 10.1002/j.1460-2075.1992.tb05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wizel B, Garg N, Tarleton R. Vaccination with trypomastigote surface antigen1-encoding plasmid DNA confers protection against lethal Trypanosoma cruzi infection. Infect Immun. 1998;66:5073–5081. doi: 10.1128/iai.66.11.5073-5081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wizel B, Nunes M, Tarleton R L. Identification of Trypanosoma cruzi trans-sialidase family members as targets of protective CD8+ TC1 responses. J Immunol. 1997;159:6120–6130. [PubMed] [Google Scholar]
- 37.Wizel B, Palmieri M, Mendoza C, Arana B, Sidney J, Sette A, Tarleton R L. Human infection with Trypanosoma cruzi induces parasite antigen-specific cytotoxic T lymphocyte responses. J Clin Investig. 1998;102:1062–1071. doi: 10.1172/JCI3835. [DOI] [PMC free article] [PubMed] [Google Scholar]