Abstract
Clostridium sordellii lethal toxin (TcsL) is a large clostridial toxin (LCT) that glucosylates Ras, Rac, and Ral. TcsL differs from other LCTs because it modifies Ras, which does not cycle from cytosol to membrane. By using a suite of inhibitors, steps in cell entry by TcsL were dissected, and entry appears to be dependent on endosomal acidification. However, in contrast to TcdB, TcsL was substantially slower in its time course of entry. TcsL cytopathic effects (CPE) were blocked by bafilomycin A1 and neutralized by antiserum up to 2 h following treatment of cells with the toxin. The slow time course of intoxication and relatively high cytopathic dose were alleviated by exposing TcsL to acid pH, resulting in a time course similar to that of TcdB. The optimal pH range for activation was 4.0 to 5.0, which increased the rate of intoxication over 5-fold, lowered the minimal intoxicating dose by over 100-fold, and allowed complete substrate modification within 2 h, as shown by differential glucosylation. Fluorescence analysis of TcsL with 2-(p-toluidinyl) naphthalene-6-sulfonic acid as a probe suggested the acid pH stimulated a hydrophobic transition in the protein, a likely prelude to membrane insertion. Finally, acid entry by TcsL caused TcdB-like morphological changes in CHO cells, which suggestings that acid activation may impact substrate recognition profiles for TcsL.
Clostridium sordellii, is an important veterinary pathogen, causing abosomal bloat, hemorrhage, ulcers, liver disease, myositis, and sudden death in sheep (14, 21). C. sordellii has also been reported in human maternal deaths with a disease presenting as a toxic shock-like syndrome (4, 15). Unfortunately, the etiology of C. sordellii disease remains poorly understood. Myonecrosis is apparently rather infrequent, and death may be due to septicemia, as well as the expression of several exotoxins. Like many disease-causing clostridia, a single major virulence factor from C. sordellii has not been proposed, and most of the C. sordellii toxins—including phospholipase C, neuraminidase, and hemolysin—are largely uncharacterized (20). In contrast, two other C. sordellii extracellular toxins, hemorrhagic toxin (TcsH) and lethal toxin (TcsL), have garnished interest in recent years (6). Intrigue with TcsH and TcsL stems from both their unique mechanisms of action and their potential use as tools in cell biology (8, 16).
TcsH and TcsL are members of the large clostridial toxins (LCTs), which represent a novel group of exceptionally large (270 to 304 kDa) bacterial virulence factors with the capacity to inactivate multiple target substrates. Along with TcsH and TcsL, LCTs include Clostridium difficile toxins A and B (TcdA and -B) and Clostridium novyi alpha toxin (Tcnα). (For recent reviews of LCTs, see references 1, 5, and 6.) LCTs target members of the Ras superfamily of small GTP-binding proteins and, using UDP-glucose as a cosubstrate, TcsL, TcsH, TcdA, and TcdB glucosylate Ras proteins with various substrate specificities. TcsH, TcdA, and TcdB glucosylate Ras superfamily members Rho, Rac, and Cdc42, whereas TcsL glucosylates Ras, Rac, and Ral. Unlike other LCTs, Tcnα utilizes UDP-n-acetylglucosamine, rather than UDP-glucose, as a cosubstrate for glycosylation of Rho, Rac, and Cdc42. In all cases, the LCT glycosylates a threonine (T37 or T35) residue in loop 1 of the effector-binding region, thus blocking important downstream signaling events. For Rho, Rac, and Cdc42, this inactivation may prevent effective membrane-to-cytosol cycling, regulation of actin polymerization, vesicular trafficking, and transcriptional activation, which ultimately causes extensive actin condensation, cell rounding, and cell death. Glucosylation of Ras prevents effective downstream signaling, such as phosphorylation of ERK1 and ERK2 mitogen-activated protein kinase (17).
As intracellular bacterial toxins, LCTs must enter or contact the cytosol to modify their target substrates. TcdB's mechanism of cell entry is the best characterized among LCTs and appears to require endosomal acidification and translocation from endocytic vesicles (10, 18). TcdB appears to translocate to the cytosol within 30 min following cell treatment, and at low pH the toxin exposes hydrophobic domains and forms ion-conducting channels (3, 18). Still, very little is known about the steps in cell entry for these remarkably large toxins or whether these steps in entry play any part in localization with particular substrates. The substrate targets of most LCTs cycle from the cytosol, where they are maintained in the GDP-bound form, to the plasma membrane, where the process of nucleotide exchange and activation can occur (11). In contrast, Ras, a target of TcsL, remains anchored at the cell membrane and does not cycle to the cytosol (2). Whether cellular localization plays a role in recognition of GTPase substrates by LCTs is not known. In addition, it is not clear if the cellular glucosylation of Ras by TcsL is driven solely by substrate specificity or also involves a mechanism of cell entry different from those of other LCTs. In this study, we investigated the mechanisms of cell entry by TcsL and report that TcsL cytopathic effects (CPE) are dramatically enhanced by an extracellular acid pH and blocked by lysosomotropic inhibitors. The acid-pulsed entry increased the rate of TcsL intoxication, lowered the cytopathic dose, allowed complete substrate modification, and caused ultrastructural effects similar to those of TcdB.
MATERIALS AND METHODS
Cell culture and media.
CHO-K1 cells, human epitheloid carcinoma (HeLa) cells, and mouse macrophage RAW cells were all obtained from the American Type Culture Collection (ATCC), Manassas, Va. These cell lines were maintained in RP-10 medium supplemented with 10% fetal bovine serum. Cultures were grown at 37°C in the presence of 6% CO2. Unless otherwise noted, all reagents and chemicals were purchased from Sigma Chemical Co., St. Louis, Mo.
Purification of TcsL and TcdB.
Cells of C. sordellii strain 9714 (ATCC) or C. difficile strain 10463 (ATCC) were grown in cellulose ester dialysis tubing with a 10,000- to 12,000-molecular-weight cutoff (Spectrum Medical Industries, Houston, Tex.), suspended in 1 liter of 0.5× brain heart infusion broth (Becton Dickinson, Sparks, Md.). Following growth at 37°C for 72 h, the culture was centrifuged at 12,000 × g for 30 min, and the supernatant was collected. TcsL and TcdB were subsequently purified at 4°C by sequential steps of high-resolution liquid chromatography as previously described (18). Each step in the purification was followed by cytotoxicity assays with CHO cells, Western blot analysis with TcdB polyclonal antiserum (a generous gift from Rodney Tweten), and visualization by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Additionally, the activity of purified TcsL was confirmed based on its substrate recognition profile (glucosylation of Rac, but not Rho and Cdc42). Following the final step of purification, the protein concentration was determined by Bradford assay (Bio-Rad Laboratories, Hercules, Calif.), and the sample was frozen at −80°C in 100-μl aliquots. Prior to use, samples of each toxin were thawed on ice and used immediately. Since repeated freeze-thawing caused increased degradation, aliquots of TcsL and TcdB were thawed once, used in the appropriate experiment, and discarded.
Inhibitor experiments.
For inhibitor assays, CHO, HeLa, or RAW cells from a confluent monolayer were plated at 5 × 104 cells/well in a 96-well plate and incubated for 18 h. Cells were then preincubated for 30 min with 1 × 10−2 M ammonium chloride, 1 × 10−5 M monensin, or 1 × 10−7 M bafilomycin A1 at 37°C. One picomole of TcsL or TcdB was added to the inhibitor-treated cells, and the CPE were monitored by visualization. For the time course assay, CHO cells from a confluent monolayer were plated at 5 × 104 cells/well in a 96-well plate and incubated for 18 h, at which point, 1 pmol of TcsL was added to the cells. At the indicated time points, bafilomycin A1 was added to the treated cells to a final concentration of 5 × 10−7 M. Each sample was monitored for 16 h, and CPE were determined by visualization.
Acid pulse experiments.
CHO, HeLa, or RAW cells from confluent monolayers were plated at 5 × 104 cells/well in a 96-well plate, incubated for 18 h, and then treated with either 5 × 10−7 M bafilomycin A1 or 100 mM ammonium chloride for 30 min. Following treatment with the lysosomotropic agents, TcsL was added to cells across 10-fold dilutions in amounts ranging from 10 pmol to 1 fmol in a total volume of 100 μl. One hour following toxin treatment at 37°C, an acid pulse was performed by exposing the cells to acidified medium (pH 4.0) for 10 min and then replacing it with neutralized medium (pH 7.5). To determine the optimal pH for activation, cells were incubated with TcsL for 1 h at 37°C, and buffered medium was added to cells for 10 min and then removed and replaced with neutralized medium (pH 7.5). The buffers and pHs are as follows: 100 mM ammonium acetate for pHs 4.0, 4.5, 5.0, and 5.5; 100 mM morpholineethanesulfonic acid (MES) for pHs 6.0 and 6.5; and 100 mM Tris for pHs 7.0 and 7.5. The treated cells were monitored for 11 h at 37°C, and CPE was determined by visualization.
Fluorescent analysis of TcsL.
Fluorescent analyis of TcsL was performed as previously described for TcdB (18). Briefly, 2-(p-toluidinyl) naphthalene-6-sulfonic acid, sodium salt (TNS) (Molecular Probes, Eugene, Oreg.) solutions were prepared in the appropriate buffers for each of the pHs to be analyzed and used at a final concentration of 150 μM. Twenty picomoles of TcsL was added to each buffer in a final volume of 2 ml and incubated at 37°C for 20 min. Each sample was analyzed on an SLM 8100 photon counting fluorimeter (Spectronic Instruments, Rochester, N.Y.) with an excitation of 366 nm and an emission scan of 380 to 500 nm with a slit width of 2.0. For the pH shift experiments, 20 pmol of TcsL was incubated with a mixture of 150 μM TNS, 50 mM Tris, 1 mM EDTA, and 100 mM NaCl (pH 7.5) for 20 min, and fluorescence was determined. The pH was adjusted to 4.0 by the gradual addition of 1 N HCl, and the emission spectrum was generated. The pH was then adjusted back to 7.5 by gradual addition of 1 N NaOH, and the fluorescence spectrum was determined again.
TcsL neutralization assays.
CHO cells and HeLa cells from a confluent monolayer were plated at 5 × 104 cells/well in a 96-well plate and incubated for 18 h, at which point, TcsL or TcdB was added to cells at a final concentration of 2 × 10−9 M. At the indicated times, anti-TcdB antibody was added to the TcsL- and TcdB-treated cells, or cells were washed four times with 100 μl of phosphate-buffered saline (PBS). Cells were then monitored for 11 h, and CPE were determined by visualization.
SEM.
Scanning electron microscopy (SEM) analysis was carried out at the Samuel Noble electron microscopy facility (The University of Oklahoma). CHO cells from a confluent monolayer were plated at 105 cells/well in a 24-well plate and incubated for 18 h, at which point, cells were treated with TcsL, acid-pulsed TcsL, or TcdB. After 11 h, cells were incubated with 2.5% gluteraldehyde–0.1 M cacodylate buffer at pH 7.3 for 30 min. Cells were then incubated with 0.1 M cacodylate buffer–1% osmium tetroxide (pH 7.3) for 1 h. The cells were then washed three times with 1 ml of cacodylate buffer per well at pH 7.3. Samples were then dehydrated in a graded series of ethanol ranging from 30% to 100%. Critical point drying with an Autosamdri-814 device (Tousimis Research Corporation, Rockville, Md.) followed, at which point, samples were coated with gold up to a thickness of 400 Å in a Hammer VI sputter coating unit (Anatech, Ltd., Alexandria, Va.). Observations were performed in a JEOL JSM-880 SEM.
Glucosylation assay.
Extracts from treated CHO cells were used as a source of substrate for TcsL glucosylation. To prepare these extracts, CHO cells were grown in 75-cm2 tissue culture flasks until confluent at a density of ∼1 × 107 cells/flask. The cells were treated with 20 pmol of TcsL in the presence of bafilomycin A1, with or without an acid pulse. Two hours after treatment, the cells were then washed three times in ice-cold PBS, followed by mechanical removal in the presence of lysis buffer (1 mM MgCl2, 1 mM MnCl2, 0.1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml, 25 mM triethanolamine-HCl [pH 7.5]), similar to a previously described method (13). Cells were sonicated on ice five times for 30-s intervals, and the resulting extract was centrifuged at 40,000 × g for 8 h. The supernatant was removed and concentrated in a Centricon concentrator (Millipore, Bedford, Mass.) with a 10-kDa molecular mass cutoff until the extract reached a final volume of 0.5 ml.
For the glucosylation assay, CHO extracts (2 mg/ml) were added to a glucosylation mix containing 50 mM HEPES, 100 mM KCl, 1 mM MnCl2, 1 mM MgCl2, 100 μg of bovine serum albumin per ml, 35 μM [14C]UDP-glucose (308 Ci/mol; ICN Pharmaceuticals, Inc., Irvine, Calif.), and 10 μg of TcsL per ml in a final reaction volume of 20 μl. The reaction mixture was incubated for 2 h at 37°C and resolved by SDS-PAGE on a 15% acrylamide gel and imaged on a Packard electronic autoradiograph instant imager (Packard Instrument Company, Meriden, Conn.) similar to previously described methods (12).
RESULTS
Effects of lysosomotropic agents and time course of cell entry by TcsL.
The steps in cell entry for TcdA and TcdB have been documented by others and our group (9, 10, 18). While TcsL is genetically similar to TcdA and TcdB, the toxin targets different substrates, and the extent to which cell entry contributes to this difference in target recognition is not known. Since TcsL is known to interact with cytosolic targets, the toxin may exploit vesicular trafficking to gain access to substrates. In the first set of experiments, we directly compared the impact inhibitors of endosomal acidification or inhibiting conditions had on TcsL and TcdB entry. Using the CHO, HeLa and RAW cell lines, we tested monensin, bafilomycin A1, and ammonium chloride for the ability to block TcsL CPE and found, similar to previous reports (3, 17), that TcsL-induced CPE were blocked by the inhibitors of endosomal and lysosomal acidification. Similar to earlier reports on TcdB, cellular intoxication was blocked by monensin, bafilomycin A1, and ammonium chloride (data not shown).
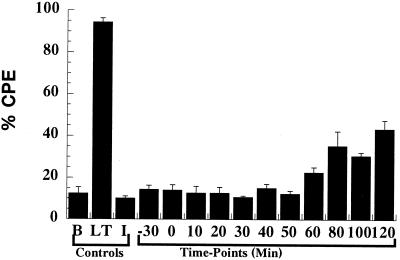
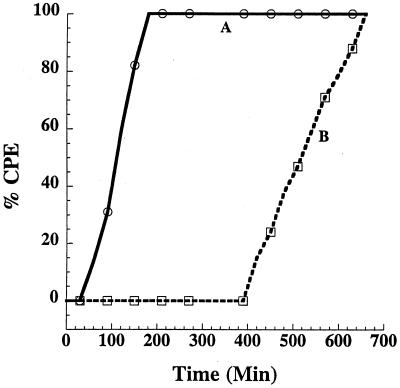
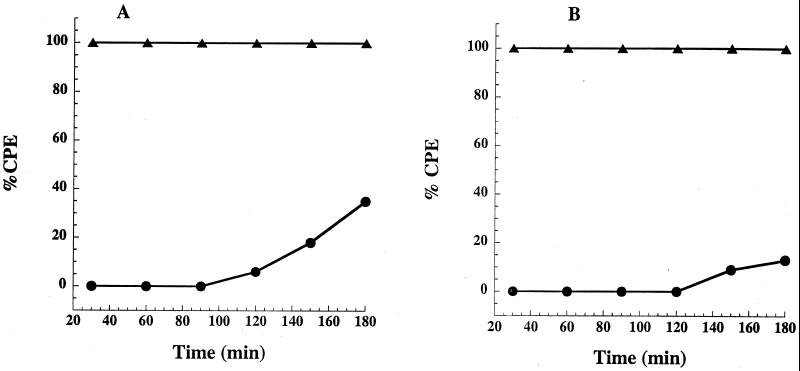
Using bafilomycin A1, we were also able to estimate the time course of cytosolic entry by TcsL and make comparison with the known time course of entry for TcdB. In this experiment, target cells (CHO, HeLa, and RAW) were treated with bafilomycin A1 at various times prior to and following toxin treatment. If the toxin enters the cytosol, addition of bafilomycin A1 should no longer block the CPE. As shown in Fig. 1, unlike previous work with TcdB, in which we reported that entry was almost complete within 30 min, we found that addition of bafilomycin A1 up to 2 h following TcsL treatment blocked more than 60% of TcsL cytotoxicity. These results suggested TcsL entry into the cytosol was significantly slower than had been reported for TcdB and might account for differences in the time course of intoxication between the two toxins.
FIG. 1.
Time course of TcsL cytosolic entry. In a 96-well plate, CHO cells (5 × 104 cells/well) were incubated with TcsL (1 pmol) in a final volume of 100 μl, and bafilomycin A1 (1 × 10−7 M) was added at 10-min intervals from 0 to 180 min. Each sample was tested in triplicate, and CPE were determined at 16 h. The error bars mark the standard deviation from the mean. Similar levels of inhibition were found in two subsequent repetitions of the same experiment. B, PBS control; LT, TcsL; I, bafilomycin.
Extracellular pH pulse bypasses the bafilomycin A1 block.
To determine whether the blocking effects of bafilomycin A1 could be bypassed, cells pretreated with bafilomycin A1 were treated with TcsL for 1 h at 37°C and then acid pulsed (pH 4.0) for 10 min. Interestingly, as shown in Fig. 2 and 3, the acid pulse entry of TcsL not only relieved the bafilomycin A1 block, but also dramatically increased the rate of intoxication, as well as significantly reducing the minimal cytopathic dose. Treatment with acid-pulsed TcsL resulted in toxic effects within less than 1 h and reached 100% within 2 h. In contrast, the first CPE from TcsL treatment at neutral pH were not evident until 6 h and did not reach their maximum until after 10 h. At neutral pH, the amount of TcsL yielding a 50% cytopathic dose was 500 fmol, whereas the acid pulse 50% cytopathic dose was less than 1 fmol. The CPE was not a reflection of cell necrosis, since at 2 h, all of the cells appeared viable by trypan blue exclusion assay (data not shown).
FIG. 2.
pH range of acid pulse-induced entry of TcsL. In a 96-well plate, CHO cells (5 × 104 cells/well) were incubated with bafilomycin A1 (5 × 10−7 M) in a final volume of 100 μl for 30 min. The cells were then treated with TcsL (1 pmol) for 1 h at 37°C, subjected to a 10-min pH pulse across a range of pHs, and finally returned to pH 7.8 by addition of neutralized medium. Samples were observed for 11 h, and CPE were determined by visualization. Similar effects were determined for CHO, HeLa, and RAW cells. Curves: A, pH 4.0; B, pH 4.5; C, pH 5.0; D, TcsL (no pulse); E, pH 5.5; F, pH 6.0; G, pH 6.5; H, pH 7.0; I, pH 7.5.
FIG. 3.
pH-enhanced rate of CHO cell intoxication by TcsL. In a 96-well plate, CHO cells (5 × 104 cells/well) were treated with either 1 pmol of TcsL or acid-pulsed TcsL (pH 4.0) in the presence of bafilomycin A1 (5 × 10−7 M) in a final volume of 100 μl and then observed for 11 h. CPE were determined by visualization. Curves: A, acid-pulsed TcsL; B, TcsL. Similar rates were obtained for HeLa and RAW cells.
We also investigated whether the acid-pulsed TcsL effect could be initiated by preincubating the toxin at pH 4.0 prior to treating cells. When TcsL was preincubated for 30 min at pH 4.0 and then added to cells in neutral-pH medium, there was no enhanced CPE; however, when TcsL pretreated at acid pH was added to cells with acidified medium, the CPE were similar to those of acid-pulsed TcsL (data not shown).
The optimal pH for TcsL activation was determined by pH pulsing of the toxin in the presence of cells at pHs ranging from 8.0 to 4.0. As can be seen in Fig. 2, pH 5.5 appears to be the highest pH at which the rate of intoxication increases dramatically.
Acid pH stimulates hydrophobic transitions in TcsL.
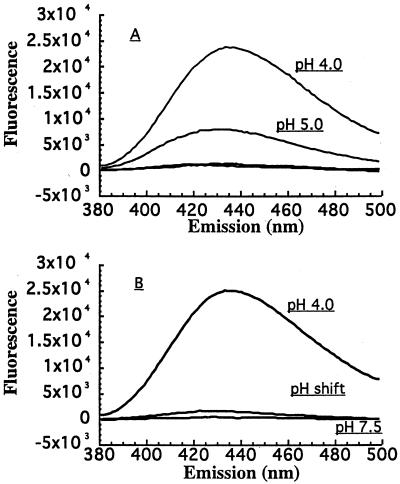
TNS is a convenient probe for determining the exposure or sequestering of hydrophobic domains under various conditions. In an earlier report, we had shown that TcdB goes through low-pH-induced structural changes that stimulate an increase in TNS fluorescence (18). To determine whether acid pH was having a similar impact on TcsL structure, we carried out a set of TNS fluorescence experiments. As shown in Fig. 4, and in line with our previous report on TcdB, we found that as TcsL is exposed to lower pH, the protein begins to expose more hydrophobic regions. Furthermore, and also similar to TcdB, this hydrophobic transition is reversible, since raising the pH back to neutrality results in a decrease in TNS fluorescence.
FIG. 4.
TNS fluorescent analysis of TcsL. The analysis of pH-induced conformational changes in TcsL was carried out with the fluorescent probe TNS. Each spectrum represents the experimental sample with background (TNS and buffer alone) subtracted. For the pH shift condition, the toxin was treated with TNS at pH 7.5. The solution was then titrated to pH 4.0 by gradual addition of 1 N HCl and returned to neutrality by addition of 1 N NaOH. (A) TcsL hydrophobicity across a range of pHs. TcsL was incubated with TNS at pHs 4.0, 5.0, 6.0, 7.0, and 8.0, and the fluorescent spectrum of each sample was obtained. TNS fluorescence for samples above pH 5.0 were not above background levels. Emission from pHs 6.0, 7.0, and 8.0 were not detectable above background levels. (B) TNS analysis of TcsL hydrophobicity following pH shift.
Neutralization of extracellular TcsL.
While TcdB and TcsL appear to be similar in some aspects of cell entry and acid-induced structural changes, the two proteins are set apart by the long delay in cytosolic entry by TcsL at neutral pH. This slow entry might be explained by a slow rate of effective cell binding or relatively slow entry under test conditions. To address these possibilities, we used a washing procedure and antibody neutralization assay to determine at which time point TcsL has been endocytosed under neutral-pH conditions. In one experiment, cells were treated with TcsL and washed three times vigorously at specific time points to remove accessible toxin, then monitored for 18 h to determine CPE. As shown in panel A of Fig. 5, TcsL CPE could be depleted by washing the cells up to 3 h after treatment, indicating a majority of TcsL was still accessible at the cell surface and apparently not tightly bound. For comparison, TcdB was subjected to the same wash experiment, and, as shown in Fig. 5, by 20 min, the CPE could not be reduced by washing the cells.
FIG. 5.
Time course of TcsL extracellular neutralization. In a 96-well plate, CHO cells (5 × 104 cells/well) were treated with 1 pmol of TcdB, TcsL, or acid-pulsed TcsL in a final volume of 100 μl. At the indicated time points, cells were then subjected to neutralizing antiserum or wash treatments. (A) Treated cells were washed vigorously with PBS four times at the indicated time points. (B) Neutralizing antiserum (10 μl) was added to cells at the indicated time points. In both experiments, cells were then observed for 11 h, and CPE were determined by visualization. Curves: solid triangles, TcdB; solid circles, TcsL.
Neutralizing antibody had a similar effect on treated cells. The addition of anti-TcdB polyclonal serum, which cross-neutralizes TcsL, also blocked the CPE of TcsL 3 h following the addition of TcsL to target cells (Fig. 5B). When cells were acid pulsed with TcsL, the effects of washing and antibody neutralization were similar to those of TcdB. The delay in TcsL activity did not appear to be due to extracellular activation, since preincubation of TcsL with culture medium did not increase the rate of intoxication (data not shown).
SEM analysis of CHO cells treated with acid-pulsed TcsL.
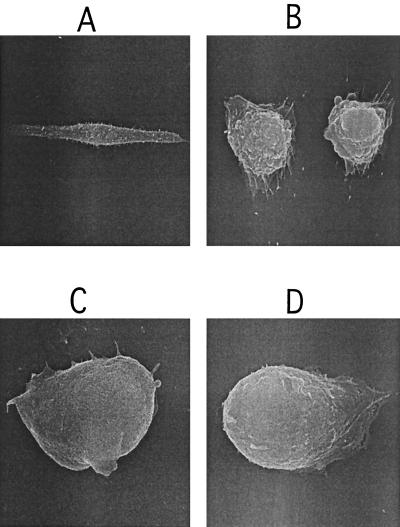
In addition to adopting a time course of intoxication similar to that of TcdB, acid-pulsed TcsL appeared to confer morphological changes similar to those of TcdB. When examined under an inverted light microscope, cells treated with acid-pulsed TcsL demonstrated a different morphology from cells treated with TcsL at neutral pH (data not shown). Furthermore, the cells treated with acid-pulsed TcsL looked remarkably similar to TcdB-treated cells (data not shown). To better characterize these changes in morphology, we analyzed the treated cells by SEM. Since the time courses of intoxication between acid-pulsed TcsL and TcsL at neutral pH are different, we attempted to normalize the effect by examining the cells at time points at which rounding was complete, but cell death, assayed by trypan blue exclusion, had not occurred. For TcdB and acid-pulsed TcsL, this time point was 2 h, and for TcsL at neutral pH, this time point was 11 h. As shown in Fig. 6, the results of SEM analysis of CHO cells treated with acid-pulsed TcsL appeared similar to those with TcdB-treated cells, with rounding and smooth cell surfaces. This morphology was clearly different from that of cells treated with TcsL at neutral pH, in which rounding and extensive cell surface blebbing had occurred.
FIG. 6.
SEM analysis of TcdB-, TcsL-, and acid-pulsed TcsL-treated CHO cells. CHO cells were grown on coverslips and treated with 1 pmol of TcdB, TcsL, or acid-pulsed TcsL in a final volume of 100 μl. After detection of changes in morphology, cells were fixed, dried, and mounted for SEM analysis and visualization. (A) PBS control (magnification, ×1,600). (B) TcsL (magnification, ×3,300). (C) TcdB (magnification, ×7,500). (D) Acid-pulsed TcsL (magnification, ×7,500).
Differential glucosylation in extracts from cells treated with acid-pulsed TcsL.
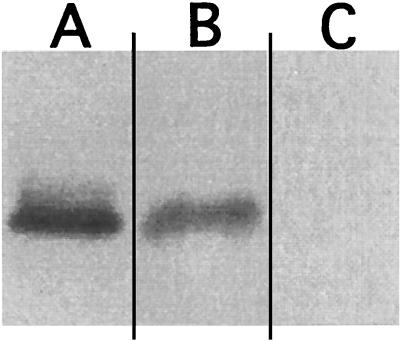
Recently, Barth et al. (3) reported on the pH-induced channel-forming activity of TcdB and TcsL. Therefore, it was possible that the CPE of cells treated with acid-pulsed TcsL were due to the formation of channels at the cell surface and not related to cytosolic entry. To address this possibility, we examined lysates from cells treated with TcsL at neutral pH or with an acid pulse to determine the relative amount of glucosylated substrate. CHO cells were treated with TcsL under the two conditions and incubated for 2 h, at which point, the cells were collected and lysates were prepared. The lysates from these samples were then used in glucosylation assays to determine whether acid-pulsed entry allowed TcsL access to the substrate targets. As can be seen in Fig. 7, lysates from cells treated with acid-pulsed TcsL did not have accessible substrate, whereas cells treated with TcsL at neutral pH were still able to present substrate for glucosylation. These results suggest that while channels may be formed during the acid pulse entry, the observed CPE are more likely a result of substrate modification.
FIG. 7.
TcsL differential glucosylation of extracts from TcsL- and acid-pulsed TcsL-treated cells. Extracts from CHO cells that had been treated with 1 pmol of TcsL or acid-pulsed TcsL were used in the glucosylation assay to determine if pretreatment under these conditions blocks substrate. Glucosylation assays were carried out with [14C]UDP-glucose and TcsL with the extracts as target substrates. Incorporation of the radiolabeled glucose was determined by autoradiography. (A) Glucosylation of extracts from PBS-treated CHO cells. (B) Glucosylation of extracts from TcsL-treated CHO cells. (C) Glucosylation of extracts from TcsL-treated (acid pulsed; pH 4.0) CHO cells.
DISCUSSION
Although TcsL and TcdB are genetically similar (83% identity), these toxins target different sets of substrates (5). Interestingly, TcsL targets Ras, which, unlike other LCT substrates, remains anchored at the inner plasma membrane, and, following farnesylation, does not cycle from this site to the cytosol. Whether TcsL also differs from TcdB in its mechanism of entry has not been investigated. Based on the results from our inhibitor assays, TcsL entry appeared to be similar to TcdB entry. Furthermore, acid pH appears to have a similar effect on TcdB and TcsL structure, stimulating the exposure of hydrophobic regions. The possibility that these two toxins are temporally different in their entry pathways became evident to us from the bafilomycin A1 time course studies. In a previous study, we found that treatment with bafilomycin A1 was ineffective at blocking TcdB activity when bafilomycin A1 was added to cells 30 min after toxin treatment (18). In the current study of TcsL, results from the washing and antibody neutralization experiments suggest the lag in cell entry could be accounted for, at least in part, by a slow initiation of endocytosis or low affinity for the tested cells at neutral pH. All of these effects—slow entry, low rate of intoxication, and high cytopathic dose—could be alleviated by providing a brief acid pulse at the cell surface.
The increase in cytopathic activity for acid-pulsed TcsL differs from findings in our previous report on TcdB (18). In the earlier TcdB experiments, the blocking effects of lysosomotropic agents could be bypassed by using an extracellular acid pulse; however, acid pulse entry did not dramatically reduce the cytopathic dose. While different from TcdB, acid-pulsed TcsL entry does appear to be similar to the activity reported for diphtheria toxin almost 20 years ago (19). In the case of diphtheria toxin, while presumably not the natural mode of entry, bypassing time-consuming steps in vesicular trafficking with an acid pulse increases the rate of inhibition of protein synthesis. As a further comparison, Helicobacter pylori VacA requires extracellular acid pH for activation and is optimally active under these conditions (7). For VacA, the acid-enhanced activity appears to be part of the natural mode of action for this toxin, since H. pylori may face an acidified environment. In the case of TcsL, whether the acid pH conditions reflect a natural mechanism of activation or simulation of the endocytic vesicle is unclear.
Finally, there is only limited information on C. sordellii disease, and very little is known about the tissue or cell type that this organism targets. In this study, we selected three cell lines (CHO, HeLa, and RAW) to use as targets in our assays, and each of these cell lines gave similar data. Since the receptor for TcsL is not known, we cannot dismiss the possibility that low receptor number might account for the slow entry, as well as accounting for the results from the wash and antibody neutralization experiments. Low receptor number would not account, however, for the blocking activity of lysosomotropic inhibitors and the acid pulse effects. Taken together, these data certainly suggest endosomal trafficking and acid pH are important to the entry and cytopathic activity of TcsL.
ACKNOWLEDGMENT
This study was supported by the Oklahoma Center for the Advancement of Science and Technology.
REFERENCES
- 1.Aktories K, Schmidt G, Just I. Rho GTPases as targets of bacterial protein toxins. Biol Chem. 2000;381:421–426. doi: 10.1515/BC.2000.054. [DOI] [PubMed] [Google Scholar]
- 2.Bar-Sagi D, Hall A. Ras and Rho GTPases: a family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 3.Barth H, Pfeifer G, Hofmann F, Maier E, Benz R, Aktories K. Low pH-induced formation of ion channels by Clostridium difficile toxin B in target cells. J Biol Chem. 2001;276:10670–10676. doi: 10.1074/jbc.M009445200. [DOI] [PubMed] [Google Scholar]
- 4.Bitti A, Mastrantonio P, Spigaglia P, Urru G, Spano A I, Moretti G, Cherchi G B. A fatal postpartum Clostridium sordellii associated toxic shock syndrome. J Clin Pathol. 1997;50:259–260. doi: 10.1136/jcp.50.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boquet P. Bacterial toxins inhibiting or activating small GTP-binding proteins. Ann N Y Acad Sci. 1999;886:83–90. doi: 10.1111/j.1749-6632.1999.tb09403.x. [DOI] [PubMed] [Google Scholar]
- 6.Boquet P, Munro P, Fiorentini C, Just I. Toxins from anaerobic bacteria: specificity and molecular mechanisms of action. Curr Opin Microbiol. 1998;1:66–74. doi: 10.1016/s1369-5274(98)80144-6. [DOI] [PubMed] [Google Scholar]
- 7.de Bernard M, Papini E, de Filippis V, Gottardi E, Telford J, Manetti R, Fontana A, Rappuoli R, Montecucco C. Low pH activates the vacuolating toxin of Helicobacter pylori, which becomes acid and pepsin resistant. J Biol Chem. 1995;270:23937–23940. doi: 10.1074/jbc.270.41.23937. [DOI] [PubMed] [Google Scholar]
- 8.Doussau F, Gasman S, Humeau Y, Vitiello F, Popoff M, Boquet P, Bader M F, Poulain B. A Rho-related GTPase is involved in Ca(2+)-dependent neurotransmitter exocytosis. J Biol Chem. 2000;275:7764–7770. doi: 10.1074/jbc.275.11.7764. [DOI] [PubMed] [Google Scholar]
- 9.Fiorentini C, Thelestam M. Clostridium difficile toxin A and its effects on cells. Toxicon. 1991;29:543–567. doi: 10.1016/0041-0101(91)90050-2. [DOI] [PubMed] [Google Scholar]
- 10.Florin I, Thelestam M. Lysosomal involvement in cellular intoxication with Clostridium difficile toxin B. Microb Pathog. 1986;1:373–385. doi: 10.1016/0882-4010(86)90069-0. [DOI] [PubMed] [Google Scholar]
- 11.Hall A, Nobes C D. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos Trans R Soc Lond B Biol Sci. 2000;355:965–970. doi: 10.1098/rstb.2000.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofmann F, Busch C, Aktories K. Chimeric clostridial cytotoxins: identification of the N-terminal region involved in protein substrate recognition. Infect Immun. 1998;66:1076–1081. doi: 10.1128/iai.66.3.1076-1081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Just I, Fritz G, Aktories K, Giry M, Popoff M R, Boquet P, Hegenbarth S, von Eichel-Streiber C. Clostridium difficile toxin B acts on the GTP-binding protein Rho. J Biol Chem. 1994;269:10706–10712. [PubMed] [Google Scholar]
- 14.Lewis C J, Naylor R D. Sudden death in sheep associated with Clostridium sordellii. Vet Rec. 1998;142:417–421. doi: 10.1136/vr.142.16.417. [DOI] [PubMed] [Google Scholar]
- 15.McGregor J A, Soper D E, Lovell G, Todd J K. Maternal deaths associated with Clostridium sordellii infection. Am J Obstet Gynecol. 1989;161:987–995. doi: 10.1016/0002-9378(89)90768-0. [DOI] [PubMed] [Google Scholar]
- 16.Palsson E M, Popoff M, Thelestam M, O'Neill L A. Divergent roles for Ras and Rap in the activation of p38 mitogen-activated protein kinase by interleukin-1. J Biol Chem. 2000;275:7818–7825. doi: 10.1074/jbc.275.11.7818. [DOI] [PubMed] [Google Scholar]
- 17.Popoff M R, Chaves-Olarte E, Lemichez E, von Eichel-Streiber C, Thelestam M, Chardin P, Cussac D, Antonny B, Chavrier P, Flatau G, Giry M, de Gunzburg J, Boquet P. Ras, Rap, and Rac small GTP-binding proteins are targets for Clostridium sordellii lethal toxin glucosylation. J Biol Chem. 1996;271:10217–10224. doi: 10.1074/jbc.271.17.10217. [DOI] [PubMed] [Google Scholar]
- 18.Qa'Dan M, Spyres L M, Ballard J D. pH-induced conformational changes in Clostridium difficile toxin B. Infect Immun. 2000;68:2470–2474. doi: 10.1128/iai.68.5.2470-2474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandvig K, Olsnes S. Rapid entry of nicked diphtheria toxin into cells at low pH. Characterization of the entry process and effects of low pH on the toxin molecule. J Biol Chem. 1981;256:9068–9076. [PubMed] [Google Scholar]
- 20.Smith L, editor. The pathogenic anaerobic bacteria. Springfield, Ill: CC Thomas; 1975. pp. 291–298. [Google Scholar]
- 21.Vatn S, Tranulis M A, Hofshagen M. Sarcina-like bacteria, Clostridium fallax and Clostridium sordellii in lambs with abomasal bloat, haemorrhage and ulcers. J Comp Pathol. 2000;122:193–200. doi: 10.1053/jcpa.1999.0363. [DOI] [PubMed] [Google Scholar]