Abstract
A safe, more sensitive, nonradioactive, neutral red uptake assay was adopted to replace the traditional 51Cr release assay for detection of Brucella-specific cytotoxic T lymphocyte (CTL) activity. Our studies indicated that Brucella abortus strain RB51 vaccination of mice induced specific CTLs against both strain RB51- and strain 2308-infected J774.A1 macrophages but not against Listeria monocytogenes-infected J774.A1 cells. The antigen-specific cytotoxic activity was exerted by T lymphocytes but not by NK cells. CD3+ CD4+ T cells secreted the highest level of gamma interferon (IFN-γ) and were able to exert a low but significant level of specific lysis of Brucella-infected macrophages. They also exerted a low level of nonspecific lysis of noninfected macrophages. In contrast, CD3+ CD8+ T cells secreted low levels of IFN-γ but demonstrated high levels of specific lysis of Brucella-infected macrophages with no nonspecific lysis. These findings indicate that B. abortus strain RB51 vaccination of mice induces specific CTLs and suggest that CD3+ CD4+ and CD3+ CD8+ T cells play a synergistic role in the anti-Brucella activity.
Brucella abortus is a gram-negative, facultative intracellular bacterial pathogen that causes brucellosis in humans and cattle (4). In the infected host, B. abortus multiplies within the phagosomes of phagocytic cells (e.g., macrophages) by inhibiting the phagosome-lysosome fusion (4). Similar to most of the intracellular bacterial infections, cell-mediated immunity seems to play a critical role in protection against virulent Brucella infection, although antibodies specific for the O polysaccharide of the lipopolysaccharide can confer a certain level of protection in some host species (1, 2, 17, 31). Passive transfer assays with mice indicated that both CD4+ and CD8+ T-cell subsets are involved in the protective immunity to brucellosis (1, 2). One mechanism by which immune T cells confer protection from B. abortus infection is by secreting molecules such as gamma interferon (IFN-γ), which stimulates the antimicrobial activity of macrophages, allowing intracellular bacterial killing (16, 35). The crucial role of IFN-γ in resistance to Brucella infection has been demonstrated for mice by in vivo antibody neutralization experiments (35). Another mechanism of T-cell-mediated immunity is the lysis of infected cells by the specific cytotoxic T lymphocytes (CTLs) (23). Present knowledge about the role of CTLs in the acquired resistance to brucellosis is limited. The development of Brucella-specific CTLs in vaccinated animals and the phenotypic and functional characterization of such CTL's have not been studied in detail. The classic CTL assay is based on determining the level of 51Cr released from lysed target cells. Unfortunately, this assay is not very sensitive (23, 24), and the use of radioactive 51Cr in a biosafety level 3 environment restricts the usefulness of this assay in Brucella research even further. Therefore, we have developed a highly sensitive, nonradioactive assay for Brucella-specific CTLs and utilized this assay to study the development of specific CTLs in mice immunized with various vaccine strains of Brucella spp.
B. abortus RB51 is an attenuated stable rough strain currently used in the United States and many other countries as the vaccine of choice against bovine brucellosis. Strain RB51 induces immune protection against challenge with virulent B. abortus solely by cell-mediated immunity. This is indicated by the fact that strain RB51-induced immunity can only passively transferred only by immune T cells and not by antibodies in the mouse model (17). Recent studies carried out in our laboratory indicated that protection induced by strain RB51 vaccination is preceded by the preferential development of a Th1-type immune response (30–32). Since a Th1-type immune response usually evokes CTLs, it is reasonable to hypothesize that in the mouse model strain RB51 vaccination induces antigen-specific CTLs, which should be able to lyse Brucella-infected macrophages. In the present study, we demonstrated that strain RB51 vaccination of mice resulted in the development of a strong cytotoxic T-cell response. The specific anti-Brucella cytolytic activity was mainly exerted by CD3+ CD8+ T cells. CD3+ CD4+ T cells also developed after immunization, secreted high levels of IFN-γ, and exhibited certain levels of specific and nonspecific lytic activity against Brucella-infected target cells. NK cells appeared not to contribute significantly to the observed Brucella-specific cytotoxic activity.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. abortus strains 2308 and RB51 were from our culture collection. Listeria monocytogenes strain EGD was obtained from P. Elzer (Department of Veterinary Sciences, Lousiana State University, Baton Rouge). All bacteria were grown either in tryptic soy broth or on tryptic soy agar plates.
Vaccination of mice.
Six-week-old BALB/c female mice were inoculated intraperitoneally with 4 × 108 CFU of B. abortus strain RB51 or the same volume of saline (negative control). The mice were euthanatized at 6 weeks postimmunization by CO2 inhalation. These mice served as splenocyte sources for the CTL assays.
Cell lines.
The J774.A1 macrophage cell line was purchased from the American Type Culture Collection (ATCC; Manassas, Va.). The cells were cultured in complete tissue culture medium (c-DMEM) consisting of DMEM (Dulbecco's modified Eagle's medium; ATCC) supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Norcross, Ga.). The A/Sn mouse lymphoma YAC-1 cell line was also purchased from the ATCC (ATCC TIB-160). This cell line is sensitive to the cytotoxic activity of NK cells of mice (10, 34). Therefore, YAC-1 cells were used as target cells in the NK cytotoxicity assay. YAC-1 cells were cultured in complete RPMI medium (c-RPMI) consisting of RPMI 1640 (GIBCO BRL) supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals), 2 mM l-glutamine (Media Tech, Herndon, Va.), and 10 mM HEPES buffer (Sigma, St. Louis, Mo.).
Cytotoxicity assay.
In general, the assay was carried out by coculturing nylon wool-enriched splenocytes derived from RB51-immunized or nonimmunized mice with stimulator cells (RB51-infected macrophages treated with mitomycin C) allowing us to obtain effector T cells. Effector T cells were then confronted with target cells (RB51- or strain 2308-infected or noninfected macrophages) which had been labeled with 51Cr before, or were stained with neutral red after, the cocultivation with the effector T cells. Experiments using effector T cells separated by CD4+- and CD8+-specific magnetic beads before exposure to target cells were also carried out.
Preparation of stimulator cells.
One 75-cm2 tissue culture flask with confluent J774.A1 macrophage growth was exposed to live B. abortus strain RB51 at a ratio of 1:100 (cells to RB51) for 4 to 5 h. The medium was then discarded, and extracellular bacteria were rinsed away with c-DMEM containing 50 μg of gentamicin per ml. Another flask of normal macrophages without Brucella was prepared as a control. The macrophages were scraped off with a sterile rubber policeman and centrifuged at 200 × g for 5 min. The pulsed macrophages were suspended in 5 ml of c-DMEM with 35 μg of mitomycin C per ml in a 37°C water bath for 45 min. The macrophages were then washed by centrifugation four times with DMEM supplemented with 5% heat-inactivated fetal bovine serum. Trypan blue exclusion was used to count macrophage cell numbers and to determine viability (3).
Preparation of target cells.
The protocol used to prepare RB51-infected target cells was the same as that for stimulator cells, but the mitomycin C step was omitted. Strain 2308-infected J774.A1 cells were sometimes used as target cells mainly for comparison of RB51-induced CTL activities against RB51- and strain 2308-infected target cells. The preparation of strain 2308-infected target cells followed the same protocol used for RB51-infected target cells.
Preparation of effector cells.
Mice were killed by CO2 asphyxiation at 6 weeks postimmunization. The spleens were removed under aseptic conditions. Single spleen cell suspensions were prepared from the spleens according to standard procedures (3). Red blood cells were lysed with ACK solution (150 mM NH4Cl, 1 mM KHCO3, 0.1 mM Na2EDTA [pH 7.3]). Splenocytes from strain RB51-infected or saline-inoculated mice were resuspended in 2 ml of c-RPMI and passed through nylon wool columns to enrich for T lymphocytes as described previously (3) with modifications. Briefly, 1.2 g of fluffy nylon wool (DuPont Biotechnology System Division, Boston, Mass.) was inserted into a 10-ml syringe tube with a three-way stopcock, and the whole package was autoclaved. The sterilized nylon wool column with a 23-gauge needle was clamped to a ring stand, and the nylon wool was soaked with 12 ml of c-RPMI and equilibrated by standing at room temperature for at least 1 h. The splenocytes were suspended in 2 ml of medium and laid on top of the nylon wool with the stopcock open. After the cell suspension entered into the column, the stopcock was closed and 2 ml more of medium was added on top of the nylon wool. This step was repeated four more times, and 20 min was allowed to let cells bind to nylon wool between steps. Finally, an 18-gauge needle was used to replace the 23-gauge needle and 40 ml of medium was used to elute nonadherent T cells into a graduated 50-ml conical tube. The cell number and viability were determined by the trypan blue exclusion test. The enriched T cells were resuspended in c-RPMI containing 50 μg of gentamicin per ml and distributed to wells of 24-well cell culture plates (Corning, Corning, N.Y.) at a concentration of 4 million viable cells/well. Stimulator cells were also added to the wells at a concentration of 0.4 million cells/well. The mix of enriched T cells and stimulator cells was then incubated for 5 days at 37°C with 5% CO2. After the 5-day incubation, cells were collected, and the live effector cells were obtained by removing dead cells by Histopaque (1083) centrifugation as described previously (9).
Flow cytometric analysis.
Lymphocytes were incubated with both fluorescein isothiocyanate-conjugated anti-CD3 (clone 145-2C11; isotype, Armenian hamster immunoglobulin G1 [IgG1] κ) and phycoerythrin-conjugated anti-CD4 (clone RM4-5; isotype, rat [DA] IgG2a, κ) or anti-CD8 (clone 53-6.7; isotype, rat [Lou/Ws1/M] IgG2a, κ) or anti-Nkpan (clone DX5; isotype, rat IgM, κ) monoclonal antibodies on ice in the dark for 30 min. Control experiments consisted of staining with fluorescein isothiocyanate-conjugated rat IgG1 isotype control immunoglobulin (clone R3-34) and phycoerythrin-conjugated rat IgG2b, κ isotype control immunoglobulin (clone R35-38) at the same concentrations. All the monoclonal antibodies were purchased from PharMingen (San Diego, Calif.). Cells were then washed with phosphate-buffered saline (PBS) and fixed with PBS containing 2% paraformaldehyde. Dual-color immunophenotyping of cell samples was performed on a Coulter Epics XL/MXL flow cytometer (Hialeah, Fla.). A gated lymphocyte population was derived from a bivariate histogram display of forward and side scatter, and immunofluorescence data were analyzed with the Immuno-4 software program (7, 27).
Magnetic cell sorting.
CD4+ and CD8+ T cells were purified by immunomagnetic methods (20). Briefly, live T cells isolated by Histopaque column purification were incubated with MACS magnetic MicroBeads to which monoclonal antibodies against CD4 molecule (clone GK1.5; isotype, rat IgG2b) or CD8 molecule (clone 53-6.7; isotype, rat IgG2a) had been coupled (Miltenyi Biotec, Auburn, Calif.) at the concentration of 10 μl of MicroBeads per 107 total cells for 15 min in a refrigerator at 4°C (see Fig. 3). The cells were then washed with PBS supplemented with 2 mM EDTA and 0.5% bovine serum albumin. Following passage of the cells through a steel wool column in a magnetic field, the positive selected CD4+ or CD8+ T cells were eluted out. The purity of the selected CD4+ or CD8+ T-cell population was above 92% as determined by flow cytometry using the appropriate specific monoclonal antibodies as described above.
FIG. 3.
CTL activity against strain 2308- and RB51-infected target cells. The preparation of B. abortus strain 2308-infected target cells followed the same protocol used for RB51-pulsed target cells. The T cells isolated from mice vaccinated with strain RB51 were stimulated in vitro as described in Materials and Methods. The data are means for triplicate estimations, and standard deviations did not exceed 20% of the means. Noninfected target macrophages, RB51-pulsed target macrophages, and strain 2308-infected target cells were incubated with T cells derived from normal mice (solid rectangles, solid squares, and open diamonds, respectively) and RB51-immunized mice (solid triangles, solid circles, and solid diamonds, respectively).
Colorimetric CTL assay.
A previously described procedure (24) was followed with some modifications. Effector and target cells were mixed at various ratios and incubated for 16 h at 37°C. A working solution of neutral red was prepared by diluting a 1% (wt/vol) stock solution to an 0.036% working solution in warm PBS (pH 7.1 to 7.2) just prior to use. After one wash with warm PBS (pH 7.2 to 7.4), 200 μl of 0.036% neutral red solution in PBS was added to stain unlysed target cells. After 30 min, the cells were thoroughly washed and then lysed with 0.22 ml of 0.05 M acetic acid–0.05% sodium dodecyl sulfate solution. The amount of dye released was measured by taking optical density (OD) readings at 570 nm. As a control for nonlysis and maximal uptake of neutral red stain, target cells were cultured alone without effector cells. The percentage of specific lysis was established by applying the formula specific lysis = (OD of control − OD of experimental group)/OD of control × 100.
51Cr release CTL assay.
Standard procedures were followed (3) with slight modifications. Briefly, target cells were mixed with 0.1 mCi of 51Cr (specific activity, 571 mCi/mg of Cr; catalog no. 62015; ICN Pharmaceuticals, Costa Mesa, Calif.) in 100 μl of fetal bovine serum and incubated in a 37°C water bath for 1 h. The labeled target cells were washed thereafter. Then effector and target cells were mixed at various ratios and incubated for 16 h at 37°C in a 5% CO2 incubator, the 51Cr released into supernatants was measured in a gamma counter, and specific lysis was established through the formula specific lysis = (cpmexperiment − cpmspontaneous)/(cpmtotal − cpmspontaneous) × 100, where cpm is counts per minute.
IFN-γ and IL-4 detection.
Sandwich enzyme-linked immunosorbent assays (ELISAs) were used to determine IFN-γ and interleukin 4 (IL-4) levels in the culture supernatants of the effector cell and target cell cocultures. The culture supernatants were collected just before adding neutral red solution to determine lysis levels (see Fig. 4). For the ELISA detection of IFN-γ and IL-4, rat monoclonal antibodies to IFN-γ or IL-4 (ATCC) were first absorbed into wells of polystyrene ELISA plates (Nunc Maxisorp) at a concentration of 0.1 μg/well in 50 μl of coating buffer (1× PBS, pH 7.4). After overnight incubation at room temperature (RT), the plates were blocked with 2% bovine serum albumin in 1× PBS (pH 7.4) for 1 h at RT. The plates were washed three times with 50 mM Tris-HCl containing 0.2% Tween 20 (pH 7.0 to 7.4). The culture supernatants and standard recombinant mouse IFN-γ or IL-4 (PharMingen) were added and incubated for 3 h at RT. After the plates were washed three times, 100 μl of biotinylated rat anti-mouse IFN-γ or IL-4 (PharMingen) was added at the concentration of 0.25 μg/ml. After the 1-h incubation at RT, the plates were washed, 100 μl of 1:4,000-diluted horseradish peroxidase conjugated with streptavidin (Vector Laboratories, Burlingame, Calif.) was added to the wells, and the plates were incubated at RT for half an hour. After washing, 100 μl of the TMB Microwell peroxidase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was added and plates were incubated in the dark for 30 min. The reaction was stopped by adding 100 μl of 0.18 M sulfuric acid per well, and the plates were read for absorbance at 450 nm with a microplate reader (Molecular Devices, Sunnyvale, Calif.). The assays were performed in triplicate. The concentration of IFN-γ or IL-4 in the culture supernatants was calculated by using a linear regression equation obtained from the absorbance values of the standards as indicated by the manufacturer.
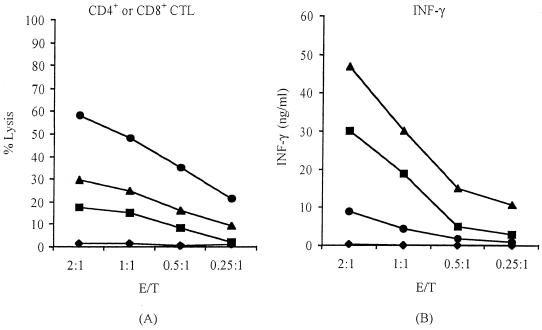
FIG. 4.
Different roles of CD4+ and CD8+ T cells in the cytotoxic lysis and release of IFN-γ. The two T-cell populations were isolated by the MACS magnetic kit and showed more than 92% purity by flow cytometry. The individual T-cell populations were cocultured at various concentrations with either strain RB51-infected or normal J774.A1 cells. The cytotoxic activity (A) and the amount of IFN-γ released into the supernatants (B) were measured. The data are means for triplicate estimations, and standard deviations did not exceed 20% of the means. Cocultures of CD4+ T cells with noninfected target macrophages (solid squares) and RB51-pulsed target macrophages (solid triangles) and CD8+ T cells with noninfected target cells (solid diamonds) and RB51-pulsed target cells (solid circles) were tested.
Statistical analysis.
The data for the CTL lysis and cytokine secretion were subjected to the analysis of variance, and the means were compared by using Tukey's honest significant difference procedure (SAS system for mixed models; SAS Institute Inc., Cary, N.C.). The linear relationship between target cell number and neutral red uptake was detected by R2 value in the context of linear correlation (21).
RESULTS
Linear relationship between target cell number and neutral red uptake.
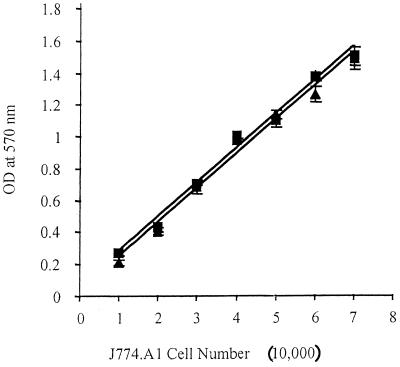
The rationale of the colorimetric CTL assay is based on the fact that live target cells (macrophages), but not T lymphocytes, take up neutral red dye (24). In addition, a linear relationship between the amount of neutral red uptake and the number of infected or noninfected target cells per well (24) must exist. As shown in Fig. 1, there is a linear relationship (R2 value of nearly 1) between the number of normal or infected J774.A1 cells and the neutral red uptake, indicating that infection with B. abortus did not alter the ability of these cells to take up the dye. Similar to the previous observations (24), our studies also indicated that T lymphocytes that were used as effector cells in the CTL assays did not take up neutral red dye (data not shown).
FIG. 1.
Linear relationship between macrophage cell number and uptake of neutral red dye by J774.A1 macrophages. Increasing numbers of target cells per well were added to wells of a 96-well plate. The normal J774.A1 macrophages (solid triangles) and the strain RB51-pulsed J774.A1 macrophages (solid squares) were allowed to adhere for 4 h before addition of neutral red dye for 45 min, at which time cells were lysed with lysis buffer and OD values were measured with an ELISA microplate reader. Each point represents the mean ± standard deviation of four determinations. The linear relationships are indicated by the R2 values of 0.9881 for RB51-pulsed J774.A1 macrophages and 0.9867 for normal J774.A1 macrophages.
Specific phenotype profiles of effector cells.
Flow cytometry data showed that the nylon wool treatment of splenocytes resulted in the enrichment of CD3+ CD4+ and CD3+ CD8+ T cells (Table 1). Both CD3+ CD4+ and CD3+ CD8+ T-cell percentages increased, and their combined cell population increased to approximately 88%. The ratio of CD4+ to CD8+ T cells ranged from 2.3 to 2.7 (Table 1), and this ratio remained unaltered after nylon wool enrichment. Moreover, the CD3+ CD4+ and CD3+ CD8+ T-cell percentages and their ratio did not change significantly after 5-day coculturing of the enriched T cells with the stimulator cells (Table 1). Nylon wool treatment did not enrich NK cells, and the NK cell proportion did not increase after the 5-day cocultures (Table 1).
TABLE 1.
Specific phenotype analysis of effector cells by flow cytometrya
| Cell population analyzed | Result for cell type:
|
||||
|---|---|---|---|---|---|
| CD3+ CD4+ (%) | CD3+ CD8+ (%) | CD3+ CD4+ and CD3+ CD8+ total (%) | CD3+ CD4+/ CD3+ CD8+ ratio | CD3+ NKpan+ (%) | |
| Splenocytes from mouse type before or after nylon wool enrichment | |||||
| Naive | |||||
| Before | 31.2 | 13.0 | 44.2 | 2.4 | 4.6 |
| After | 64.7 | 23.6 | 88.3 | 2.7 | 4.4 |
| RB51 | |||||
| Before | 28.7 | 12.7 | 41.4 | 2.3 | 3.4 |
| After | 62.9 | 25.4 | 88.3 | 2.5 | 2.4 |
| Live cells from coculture of T cells from mouse type with RB51-pulsed macrophages | |||||
| Naive | 63.5 | 25.1 | 88.6 | 2.5 | 2.6 |
| RB51 | 62.3 | 25.9 | 88.2 | 2.4 | 2.8 |
Spleens from three saline-inoculated (naive) or RB51-immunized mice were collected after 6 weeks of immunization. The spleens were pooled in each group, and splenocytes were isolated and processed through a nylon wool column to enrich T lymphocytes. The enriched T cells were further cocultured with RB51-pulsed, mitomycin C-treated J774 macrophages for 5 days, and then the live cells were separated by Histopaque centrifugation. Phenotype profiles of the cell populations at different stages of effector cell preparation were analyzed by flow cytometry. The experiment was repeated three times with similar results.
The colorimetric CTL assay is more sensitive than the 51Cr release assay.
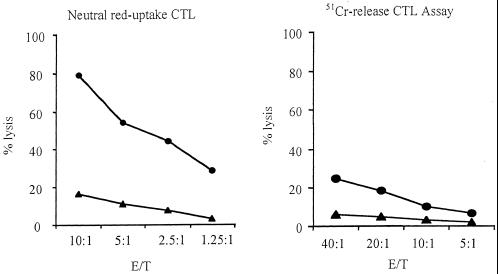
As depicted in Fig. 2, nylon wool-purified T cells derived from strain RB51-immunized mice specifically lysed strain RB51-infected J774.A1 macrophages using both the neutral red and the 51Cr release assay. Using the neutral red uptake assay, the lysis level reached approximately 80% when the effector/target cell ratio (E/T ratio) was 10:1 (Fig. 2A). The same cell population also demonstrated a low level of nonspecific lysis of normal J774.A1 macrophages, reaching about 20% at E/T ratios of 10:1. The effector T cells derived from saline-injected mice did not exert any obvious lysis against either normal or strain RB51-infected macrophages (data not shown), suggesting that the cytolytic T-cell activity was specific and was induced by strain RB51 vaccination of the mice.
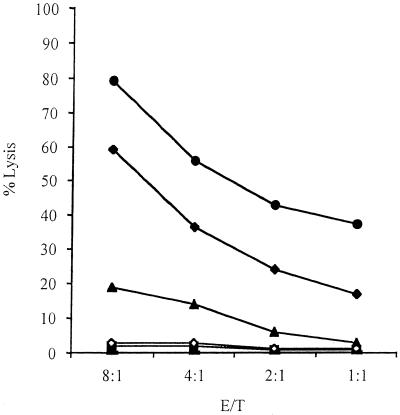
FIG. 2.
Comparison of neutral red uptake CTL assay with 51Cr release assay. The effector cells were prepared as described in Materials and Methods by in vitro stimulation of nylon wool-enriched T cells obtained from mice at 6 weeks after vaccination with strain RB51. The target cells were noninfected (solid triangles) or RB51-infected (solid circles) J774.A1 macrophages. The data are means for triplicate estimations, and standard deviations did not exceed 20% of the means.
Comparison of the neutral red assay with the 51Cr release assay indicated that the lysis patterns were similar: the higher the E/T ratio, the more lysis (Fig. 2). Nevertheless, the lysis level detected was much higher with the neutral red assay: i.e., with a 10:1 E/T ratio, neutral red gave 80% lysis (Fig. 2A) while the 51Cr release assay gave less than 20% lysis (Fig. 2B). The 20% lysis level was detected in the 51Cr release assay with a 40:1 E/T ratio (Fig. 2B). These results strongly suggest that the neutral red assay is more sensitive than is the classic 51Cr release assay. It should be mentioned that, even with the neutral red assay, prior in vitro stimulation of the effector cells was needed to detect significant levels of CTL activity.
Specificity of the induced CTLs.
The ability of CTLs derived from strain RB51-vaccinated mice to lyse strain 2308-infected J774.A1 macrophages was assayed and compared to the ability to lyse strain RB51-pulsed J774.A1 macrophages (Fig. 3). The CTLs derived from strain RB51-vaccinated mice specifically lysed strain 2308-infected J774 macrophages, but the lysis levels were approximately 20% lower than the lysis levels achieved against strain RB51-pulsed target cells.
To analyze the specificity of the CTL assays, L. monocytogenes-infected J774.A1 macrophages were used as target cells. The effector cells derived from strain RB51-vaccinated mice did not lyse any L. monocytogenes-infected macrophages (data not shown), indicating that it was a Brucella-specific assay without cross-reaction.
Differences in cytotoxic and IFN-γ responses of CD3+ CD4+ and CD3+ CD8+ T cells.
In order to determine the contribution of specific T-cell populations to the observed CTL activity, after the 5-day coculturing of stimulator cells and nylon wool-enriched T cells derived from strain RB51-immunized mice, the CD3+ CD4+ and CD3+ CD8+ cells were separated by a magnetic cell sorting method and tested for their individual abilities to lyse target cells and secrete IFN-γ and IL-4 during the lysis process. The purity of isolated CD3+ CD4+ or CD3+ CD8+ T cells was typically more than 92%. The isolated CD3+ CD8+ cells never had more than 0.5% CD3+ CD4+ contaminating T cells or more than 0.5% NK cells. The same was true for isolated CD3+ CD4+ T cells. The CD3+ CD8+ T cells achieved high levels of lytic ability against strain RB51-pulsed macrophages but did not lyse any normal macrophages (Fig. 4A). These CD3+ CD8+ T cells also secreted low levels of IFN-γ when cocultured with strain RB51-pulsed target cells and did not secrete any IFN-γ when incubated with normal macrophages (Fig. 4B). In contrast to the CD3+ CD8+ T cells, the CD3+ CD4+ T cells lysed normal macrophages and lysed significantly fewer strain RB51-pulsed target cells than did the CD3+ CD8+ T cells (Fig. 4A). The same CD3+ CD4+ T cells secreted high levels of IFN-γ whenever the target cells were normal or strain RB51-pulsed macrophages, although IFN-γ levels tended to be lower with the noninfected target cells (Fig. 4B). IL-4 was never detected (data not shown), indicating an exclusive induction of the Th1 response.
Lack of a significant role for NK cells in the cytotoxic activity.
Flow cytometric analysis indicated that the number of NK cells was less than 5% of the splenocyte population, and this percentage decreased even further after nylon wool separation. Although the number of NK cells did not increase after the incubation of the enriched T cells with the stimulator cells, the NK cells might be able to undergo activation during this process and lyse target cells. The possibility of whether these NK cells became highly active and contributed to the observed cytotoxic activity was investigated by using YAC-1 cells as target cells. YAC-1 cells are very sensitive to lysis by activated NK cells and therefore are frequently used to detect NK cell activity (10). Our studies indicated that the effector cells derived from strain RB51-immunized mice did not lyse any YAC-1 target cells (data not shown).
DISCUSSION
An important finding of this study is that T cells from the spleens of RB51-immunized mice will lyse Brucella-infected macrophages. In this study, a nonradioactive, colorimetric assay was adopted for assaying T-cell cytotoxicity against Brucella-infected J774.A1 cells as target cells. This colorimetric assay was more sensitive than was the 51Cr release assay, at least while using RB51-infected target cells. The increased sensitivity of the colorimetric, neutral red uptake assay compared to that of the traditional 51Cr release assay was also reported previously for several other systems (5, 24). In virus systems, the sensitivity of the neutral red assay is 6- to over 25-fold higher than that of the 51Cr release assay (24). By comparing the cytolytic activities of T cells from L. monocytogenes-infected mice against specific target cells using the 51Cr release assay and the neutral red assay, it was found that the E/T ratios of 1, 0.5, 0.25, and 0.12 in the neutral red assay had sensitivities similar to those of the E/T ratios of 100, 50, 25, and 12 in the 51Cr release assay (5). These numbers compare well with our findings. The ability of J774.A1 macrophage cells to ingest Brucella spp. was previously demonstrated by light and electron microscopy studies (16, 33). Studies presented in this paper demonstrate that J774A.1 cells can also process and present Brucella antigens and, hence, as previously reported (12), can be used as credible Brucella antigen-presenting cells in CTL assays. The utilization of this cell line for preparing target cells combined with the nonradioactive means of assaying the cytotoxic activity should allow further studies in characterizing the specific CTLs involved in host protective immunity against Brucella infections.
T lymphocytes from strain RB51-immunized mice were able to kill not only RB51-infected macrophages but also strain 2308-infected macrophages (Fig. 3). Considering the fact that the practical reason to immunize animals with strain RB51 is to achieve protection against virulent strains such as strain 2308, this observation is highly important. If strain RB51 immunization had induced CTLs against strain 2308-infected target cells, the significance of such CTLs in protective immunity could be questioned. On the other hand, lack of CTL activity against strain 2308-infected target cells would eliminate the CTL assay as a potential in vitro correlate of protective immunity. Comparable lysis results were obtained using strain 2308- or strain RB51-infected cells (Fig. 3). This was expected, since strain RB51 was derived from strain 2308 and, therefore, antigenically is very closely related to strain 2308 (26). Since it is safer to work with the highly attenuated strain RB51 than with the virulent strain 2308, the majority of the experiments reported in this paper were carried out with strain RB51-infected macrophages as target cells instead of strain 2308-infected macrophages.
One additional advantage of using the neutral red CTL assay is that the supernatants from the coculture of effector cells and target cells can be easily collected and analyzed for cytokine secretion analysis without worrying about any presence of radioactive material. In the supernatants from the coculture of effector cells and target cells, no detectable level of IL-4 secretion was observed at any time (data not shown). This observation is consistent with relevant studies indicating that strain RB51 preferentially induces a Th1 type of immune response (29, 30, 32). Live B. abortus vaccines induce IFN-γ but not IL-4 production in vitro and in vivo in mouse models (36). It was previously reported by our group that splenocytes from strain RB51-immunized mice could not secrete detectable levels of IL-4 upon in vitro stimulation with heat-inactivated strain RB51, strain RB51 antigen extracts, or any specific recombinant Brucella antigens (29, 30, 32). In contrast, IFN-γ production could be readily detected in those studies. In the present study, the effector T cells derived from strain RB51-immunized mice secreted a high level of IFN-γ when cocultured with strain RB51-pulsed target cells and these IFN-γ levels correlated positively with the lysis levels of strain RB51-pulsed target cells (data not shown). This suggests that both IFN-γ production and development of CTL activity are necessary for optimal protection against brucellosis. When the CD3+ CD4+ and CD3+ CD8+ T cells were separated and analyzed individually for their secretion of IFN-γ, it was found that the CD3+ CD4+ T cells secreted the majority of IFN-γ while the CD3+ CD8+ T cells secreted low levels of IFN-γ when cocultured with strain RB51-pulsed target cells (Fig. 4B). Consistent with these results, it was previously reported that CD8+ T cells derived from B. abortus strain 19-immunized mice could produce IFN-γ (11, 37) and that the CD4+ Th1 cells were the main sources of IFN-γ production (11). It appears, therefore, that after strain RB51 immunization the CD3+ CD4+ T cells are the main source of IFN-γ.
This study clearly found that a certain level of cytotoxic activity by antigen-specific CD3+ CD4+ T cells is induced after strain RB51 immunization, although their cytolytic ability was much less than that of CD3+ CD8+ T cells (Fig. 4A). Similar observations have been made for other systems. For example, it was reported elsewhere that major histocompatibility complex (MHC) class II-restricted CD4+ T cells specifically lysed L. monocytogenes-infected macrophages (18) and that these killer cells belong to the Th1 subset and are capable of expressing Fas ligand (FasL) to induce apoptosis in Fas-positive target cells (14). The major target cells for these CD4+ CTLs are cells from the immune system such as T and B cells and macrophages that express Fas upon activation.
It is possible for T cells to exert antigen-specific and nonspecific lysis of target cells. In this study, when we examined specific and nonspecific lysis by the CD3+ CD4+ T cells and CD3+ CD8+ T cells, only CD3+ CD8+ T cells demonstrated antigen-specific responses with no nonspecific lysis (Fig. 4A). Some non-antigen-specific lysis of normal macrophages by the effector T cells derived from strain RB51-immunized mice was detected (Fig. 2 to 4) and more precisely by the CD3+ CD4+ T cells derived from strain RB51-immunized mice (Fig. 4A). As discussed above, CD3+ CD4+ T cells secreted much higher IFN-γ levels than did CD3+ CD8+ T cells. It is possible that the high level of IFN-γ induced the lysis of normal macrophages, probably by apoptosis (6, 22, 28). Lysis did not occur when T cells derived from normal mice were used because these T cells did not secrete any IFN-γ. Another possibility is that minor MHC differences existing between J774.A1 and native BALB/c mouse macrophages triggered the non-antigen-specific lysis. Since the colorimetric assay was a very sensitive assay, it made this effect appear obvious but not necessarily unique. The difference in the nonspecific lysis observed between CD4+ and CD8+ T cells has also been shown in other systems. For example, the CD4+ T cells from human immunodeficiency virus (HIV)-infected patients exerted nonspecific cytolytic activity against target cells expressing HIV envelope glycoprotein (15). This was described as a non-MHC-restricted, calcium-independent cytotoxic effect probably based on cell-to-cell fusion. This nonspecific lysis did not occur with target cells expressing another HIV antigen (Gag/p55 protein). However, CD8+ T cells did not show any of the CD4+ T-cell-mediated nonspecific lysis and presented only classical antigen-specific lysis. This nonspecific lysis was also shown in the 51Cr release assay using strain 19-induced CTLs (23) and was also shown in L. monocytogenes-related CTL studies with 51Cr release or neutral red uptake assays (18). The findings were mostly ignored due to the significant difference between nonspecific and specific CTL lyses. Our present study did not include experiments to determine the MHC restriction of the CD4+ and CD8+ CTLs. Such information is needed to further understand the antigen recognition mechanisms of these CTLs.
NK cells are capable of lysing microbe-infected cells, mostly due to the production of IFN-γ (13). It was reported elsewhere that early production of IFN-γ by NK cells and not by T cells was essential in resistance to listeriosis (8) and that the early production of IFN-γ by NK cells is also important to induce Th1 immune responses (19, 25). It was also shown elsewhere that an aqueous ether extract residue of B. abortus strain 456 stimulated NK cell cytolytic activity against YAC-1 cells in the mouse model (34). These studies suggest a probable important role of NK cells in our assay system. However, we could not detect any NK cell-mediated lytic activity in our assays. This lack of activity may be explained by the low number of NK cells in the system (Table 1), which meant that the cells did not secrete detectable IFN-γ levels. Another explanation is that the NK cells did not get activated, as indicated by their inability to lyse YAC-1 cells. Previous studies also indicated no role of NK cells in the early control of B. abortus 2308 infections by using the YAC-1 cell cytotoxicity assay and the lack of effect after depletion of NK cells in BALB/c and C57BL/10 mouse models (10). Unlike L. monocytogenes, B. abortus causes only chronic infections instead of acute infections. Thus, the early nonspecific production of IFN-γ is probably minor compared to the IFN-γ produced by antigen-specific CD4+ T cells (10).
Many studies have been undertaken regarding the roles of macrophages and cytokines secreted mainly by CD4+ T cells against Brucella infection. However, research on CTLs is very limited partly due to the complexity of the assays involved and the use of radioactive 51Cr. There is only one 51Cr release assay report so far in Brucella research; it demonstrated that B. abortus strain 19-stimulated MHC class I-restricted T cells might play an important role in controlling virulent B. abortus infection in C57BL/6 mice (23). Our studies clearly indicate that strain RB51 immunization leads to the development of a CD3+ CD4+ T-helper-cell population which produces high levels of IFN-γ and a CD3+ CD8+ T-cell population which has a highly antigen-specific cytotoxic activity. NK cells, on the other hand, seem not to play an important role in the cytotoxic phenomenon. Considering that both CD4+ and CD8+ T cells play important roles in protective immunity as demonstrated by Araya and Winter (2) in active T-lymphocyte transfer experiments, it is our opinion that optimal protection against brucellosis requires both IFN-γ-secreting T cells and antigen-specific CTLs. It would be interesting to examine if a correlation can be established between the strength of the in vitro CTL activity and the in vivo protection levels against brucellosis. If such a correlation exists, the CTL assay may allow the prediction of the level of immunity inducible by Brucella vaccine candidates.
ACKNOWLEDGMENTS
This project was supported by the U.S. Army Medical Research, Development, Acquisition and Logistics Command (Prov.) under contract no. DAMD 17-94-C-4042; by U.S. Department of Agriculture grant 97-35204-4483; and by a graduate assistantship to Y.H. from the Virginia-Maryland Regional College of Veterinary Medicine.
REFERENCES
- 1.Araya L N, Elzer P H, Rowe G E, Enright F M, Winter A J. Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J Immunol. 1989;143:3330–3337. [PubMed] [Google Scholar]
- 2.Araya L N, Winter A J. Comparative protection of mice against virulent and attenuated strains of Brucella abortus by passive transfer of immune T cells or serum. Infect Immun. 1990;58:254–256. doi: 10.1128/iai.58.1.254-256.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc; 1994. [Google Scholar]
- 4.Corbel M J. Brucellosis: an overview. Emerg Infect Dis. 1997;3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Libero G, Kaufmann S H. Antigen-specific Lyt-2+ cytolytic T lymphocytes from mice infected with the intracellular bacterium Listeria monocytogenes. J Immunol. 1986;137:2688–2694. [PubMed] [Google Scholar]
- 6.Di Virgilio F, Ferrari D, Falzoni S, Chiozzi P, Munerati M, Steinberg T H, Baricordi O R. P2 purinoceptors in the immune system. Ciba Found Symp. 1996;198:290–305. doi: 10.1002/9780470514900.ch17. [DOI] [PubMed] [Google Scholar]
- 7.Donner K J, Becker K M, Hissong B D, Ahmed S A. Comparison of multiple assays for kinetic detection of apoptosis in thymocytes exposed to dexamethasone or diethylstilbesterol. Cytometry. 1999;35:80–90. doi: 10.1002/(sici)1097-0320(19990101)35:1<80::aid-cyto11>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Dunn P L, North R J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991;59:2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esquifino A L, Szary A, Brown-Borg H M, Bartke A. Age-related effects of ectopic pituitary transplants on the activation of Ames dwarf mouse lymphocytes in vitro. Proc Soc Exp Biol Med. 1996;211:87–93. doi: 10.3181/00379727-211-43956. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes D M, Benson R, Baldwin C L. Lack of a role for natural killer cells in early control of Brucella abortus 2308 infections in mice. Infect Immun. 1995;63:4029–4033. doi: 10.1128/iai.63.10.4029-4033.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes D M, Jiang X, Jung J H, Baldwin C L. Comparison of T cell cytokines in resistant and susceptible mice infected with virulent Brucella abortus strain 2308. FEMS Immunol Med Microbiol. 1996;16:193–203. doi: 10.1111/j.1574-695X.1996.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 12.Fujii H, Mannen K, Takita-Sonoda Y, Hirai K, Cruz-Abrenica M S, Kawano Y, Nishizono A, Mifune K. Target cells of cytotoxic T lymphocytes directed to the individual structural proteins of rabies virus. Microbiol Immunol. 1994;38:721–726. doi: 10.1111/j.1348-0421.1994.tb01847.x. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Penarrubia P, Koster F T, Kelley R O, McDowell T D, Bankhurst A D. Antibacterial activity of human natural killer cells. J Exp Med. 1989;169:99–113. doi: 10.1084/jem.169.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn S, Erb P. The immunomodulatory role of CD4-positive cytotoxic T-lymphocytes in health and disease. Int Rev Immunol. 1999;18:449–464. doi: 10.3109/08830189909088493. [DOI] [PubMed] [Google Scholar]
- 15.Heinkelein M, Euler-Konig I, Klinker H, Ruckle-Lanz H, Jassoy C. Lysis of human immunodeficiency virus type 1 antigen-expressing cells by CD4 and CD8 T cells ex vivo. I Infect Dis. 1996;174:209–213. doi: 10.1093/infdis/174.1.209. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X, Baldwin C L. Effects of cytokines on intracellular growth of Brucella abortus. Infect Immun. 1993;61:124–134. doi: 10.1128/iai.61.1.124-134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jimenez de Bagues M P, Elzer P H, Jones S M, Blasco J M, Enright F M, Schurig G G, Winter A J. Vaccination with Brucella abortus rough mutant RB51 protects BALB/c mice against virulent strains of Brucella abortus, Brucella melitensis, and Brucella ovis. Infect Immun. 1994;62:4990–4996. doi: 10.1128/iai.62.11.4990-4996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufmann S H, Hug E, Vath U, De Libero G. Specific lysis of Listeria monocytogenes-infected macrophages by class II-restricted L3T4+ T cells. Eur J Immunol. 1987;17:237–246. doi: 10.1002/eji.1830170214. [DOI] [PubMed] [Google Scholar]
- 19.Laskay T, Rollinghoff M, Solbach W. Natural killer cells participate in the early defense against Leishmania major infection in mice. Eur J Immunol. 1993;23:2237–2241. doi: 10.1002/eji.1830230928. [DOI] [PubMed] [Google Scholar]
- 20.Martinez C, Delgado M, Gomariz R P, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide-38 inhibit IL-10 production in murine T lymphocytes. J Immunol. 1996;156:4128–4136. [PubMed] [Google Scholar]
- 21.Motulsky H. Intuitive biostatistics. New York, N.Y: Oxford University Press; 1995. [Google Scholar]
- 22.Nagafuji K, Takenaka K, Shibuya T, Harada M, Niho Y. Fas antigen (CD95) and hematopoietic progenitor cells. Leuk Lymphoma. 1996;24:43–56. doi: 10.3109/10428199609045713. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira S C, Splitter G A. CD8+ type 1 CD44hi CD45 RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur J Immunol. 1995;25:2551–2557. doi: 10.1002/eji.1830250922. [DOI] [PubMed] [Google Scholar]
- 24.Parish C R, Mullbacher A. Automated colorimetric assay for T cell cytotoxicity. J Immunol Methods. 1983;58:225–237. doi: 10.1016/0022-1759(83)90277-6. [DOI] [PubMed] [Google Scholar]
- 25.Scharton T M, Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schurig G G, Roop II R M, Bagchi T, Boyle S, Buhrman D, Sriranganathan N. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet Microbiol. 1991;28:171–188. doi: 10.1016/0378-1135(91)90091-s. [DOI] [PubMed] [Google Scholar]
- 27.Smith-Norowitz T A, Sobel R A, Mokhtarian F. B cells and antibodies in the pathogenesis of myelin injury in Semliki Forest virus encephalomyelitis. Cell. 2000;200:27–35. doi: 10.1006/cimm.2000.1613. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi T, Harada H, Lamphier M. Regulation of the interferon system and cell growth by the IRF transcription factors. J Cancer Res Clin Oncol. 1995;121:516–520. doi: 10.1007/BF01197763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vemulapalli R, Duncan A J, Boyle S M, Sriranganathan N, Toth T E, Schurig G G. Cloning and sequencing of yajC and secD homologs of Brucella abortus and demonstration of immune responses to YajC in mice vaccinated with B. abortus RB51. Infect Immun. 1998;66:5684–5691. doi: 10.1128/iai.66.12.5684-5691.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vemulapalli R, He Y, Boyle S M, Sriranganathan N, Schurig G G. Brucella abortus strain RB51 as a vector for heterologous protein expression and induction of specific Th1 type immune responses. Infect Immun. 2000;68:3290–3296. doi: 10.1128/iai.68.6.3290-3296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vemulapalli R, He Y, Buccolo L S, Boyle S M, Sriranganathan N, Schurig G G. Complementation of Brucella abortus RB51 with a functional wboA gene results in O-antigen synthesis and enhanced vaccine efficacy but no change in rough phenotype and attenuation. Infect Immun. 2000;68:3927–3932. doi: 10.1128/iai.68.7.3927-3932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vemulapalli R, He Y, Cravero S, Sriranganathan N, Boyle S M, Schurig G G. Overexpression of protective antigen as a novel approach to enhance vaccine efficacy of Brucella abortus strain RB51. Infect Immun. 2000;68:3286–3289. doi: 10.1128/iai.68.6.3286-3289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wise D J. Ph.D. dissertation. Blacksburg, Va: Virginia Polytechnic Institute and State University; 1995. Intracellular growth of Brucella abortus and B. melitensis in murine macrophage-like cell lines and partial characterization of a biologically active extract from B. abortus strain RB51; pp. 15–60. [Google Scholar]
- 34.Yabu K, Youngner J S, Feingold D S, Keleti G, Gorelik E. Augmentation of natural killer cell activity in mice by Bru-Pel. J Immunother. 1991;10:307–312. doi: 10.1097/00002371-199110000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Zhan Y, Cheers C. Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect Immun. 1993;61:4899–4901. doi: 10.1128/iai.61.11.4899-4901.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhan Y, Kelso A, Cheers C. Differential activation of Brucella-reactive CD4+ T cells by Brucella infection or immunization with antigenic extracts. Infect Immun. 1995;63:969–975. doi: 10.1128/iai.63.3.969-975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhan Y, Yang J, Cheers C. Cytokine response of T-cell subsets from Brucella abortus-infected mice to soluble Brucella proteins. Infect Immun. 1993;61:2841–2847. doi: 10.1128/iai.61.7.2841-2847.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]