Abstract
The Neisseria meningitidis FAM20 strain secretes two proteins of unknown function, FrpA and FrpC, which contain typical RTX domains found in cytotoxins from other gram-negative pathogens. To evaluate whether the Frp proteins could be involved in meningococcal virulence, 65 isolates of all serogroups were screened by PCR for the presence of both frp genes. The frpA allele was, however, poorly conserved. A single strain harbored an frpA allele of the previously described size, while large insertions were detected in the frpA loci of 22 isolates (34%), and the 42 remaining isolates (65%) did not contain frpA at all. In contrast, frpC alleles, albeit of variable length, were detected in all invasive and most carrier strains. This suggests that meningococci may produce a family of FrpC proteins of various molecular masses. High levels of both immunoglobulin G (IgG) and IgA class antibodies recognizing recombinant FrpC were indeed detected in convalescent-phase sera of most patients at 2 and 4 to 5 weeks after the first symptoms of meningococcal disease. These results show that FrpC-like proteins are produced and may play a role in invasive meningococcal infections.
Neisseria meningitidis colonizes the nasopharynges and oropharynges of about 10% of healthy individuals. In a small proportion of infected subjects, meningococci can invade the bloodstream and cross the blood-brain barrier, causing septicemia and/or meningitis. Eventually, the bacterium causes sporadic outbreaks and epidemics of invasive meningococcal disease with high mortality and morbidity rates (2, 12). Definition of the factors determining the development of meningococcal disease is, however, difficult, because the available animal models do not adequately reproduce the natural route of infection and human pathology.
The antigenic hypervariability, polysaccharide capsule production, adhesion, and signaling mechanisms of meningococci have all been thoroughly studied and are thought to play an important role in meningococcal carriage and disease (2, 21). Unlike a number of other gram-negative bacterial pathogens, however, no proteinaceous exotoxins have so far been implicated in meningococcal disease. Recently, three iron-regulated frp alleles of N. meningitidis were sequenced (frpA, frpC, and frpC-like). These frp genes encode large secreted proteins of unknown biological activity (17, 19, 20) which possess the characteristic carboxy-proximal RTX (repeat-in-toxin) repetitions of a nonapeptide motif, L-X-G-G-X-G-(D/N)-D-X. Various numbers of such repeats are found in the RTX domains of several cytotoxins involved in the virulence of other gram-negative genera, such as Escherichia, Proteus, Actinobacillus, Morganella, Pasteurella, Bordetella, and Vibrio (1, 9, 22, 23).
The assignment of the Frp proteins to the RTX protein family suggests that they might play a role in meningococcal carriage and/or disease. However, no intact frp gene was found in the sequenced genome of the serogroup A isolate Z2491 (13), which contains only fragments of frp genes scattered around the chromosome. In contrast, two different Frp proteins are expressed and secreted under iron-limited conditions by the serogroup C isolate FAM20 (18–20). These share large portions of identical sequence, but only 13 nonapeptide repeats are found in the 122-kDa FrpA, while 43 repeats are present in the 198-kDa FrpC protein (19, 20). The N-terminal 293 amino acid residues of FrpA and the 407 N-terminal residues of FrpC, however, do not exhibit any sequence homology to each other or to any known protein. This part of the FrpC protein harbors an Arg-Gly-Asp (RGD) sequence, which for a number of other proteins and bacterial virulence factors has been implicated in binding to integrins of mammalian cell membranes (5, 11, 12). A third type, a 141-kDa FrpC-like protein, is encoded in the genome of the serogroup B isolate MC58 (17). It corresponds to a truncated variant of FrpC, missing residues 251 to 377 from the amino-terminal portion and residues 1319 to 1718 from the repeats. The genome of MC58, however, also contains a gene for a longer FrpC protein nearly identical to that of FAM20 (17).
In a limited previous study, production of Frp proteins was detected in five out of eight meningococcal strains tested (19). In this study, we have detected the presence of frp alleles in a set of 65 isolates of N. meningitidis. It is shown for the first time that convalescent-phase sera of a number of patients after invasive meningococcal disease contain high levels of antibodies recognizing the FrpC protein. This suggests that FrpC may be involved in the pathogenesis of meningococcal disease.
MATERIALS AND METHODS
Bacterial strains, growth conditions and plasmids.
Antigenically and phenotypically characterized isolates of N. meningitidis from patients with invasive meningococcal disease and isolates from healthy carriers were from a collection of strains of the National Reference Laboratory for Meningococcal Infections at the National Institute of Public Health in Prague, Czech Republic. The strains, however, were not matching pairs of isolates from patients and their corresponding individual contacts, since such pairs were not available for this study. The isolates were grown on Mueller-Hinton Agar (Bio-Merieux) supplemented with defibrinated sheep blood (chocolate modification) in an atmosphere of 5% CO2 at 37°C for 24 h. The genomic DNA was isolated from plated pooled bacteria as described elsewhere (7). The Escherichia coli strain XL1-Blue (Stratagene) was used throughout this work for DNA manipulation and was grown at 37°C in Luria-Bertani (LB) medium supplemented with 150 μg of ampicillin/ml for plasmid-containing strains. The BL21(λDE3) E. coli strain (Novagen) was used for expression of the FrpC protein and was grown at 30°C in LB medium containing 150 μg of ampicillin/ml. Plasmid pTZ19RMluI is a construct prepared by ligation of PstI-digested pTZ19R (Pharmacia) with a double-stranded adapter, 5′-TACGCGTATGCA, introducing a new MluI site. Plasmid pT7-7ΔBsaAI was constructed by digestion of pT7-7 (16) with BsaAI and religation of the vector with a double-stranded synthetic hexanucleotide 5′-GGATCC. pTYB2 (the intein-mediated purification with an affinity chitin-binding tag protocol [IMPACT] T7 system) was from New England Biolabs.
PCR amplification.
The reaction mixtures contained 10 mM Tris-HCl (pH 8.8), 50 mM KCl, 0.1% Triton X-100, MgCl2 (Table 1), 200 μM (each) deoxynucleoside triphosphate, 1 μM (each) primer (Table 1 and Fig. 1), 0.5 μg of genomic DNA, and deionized water up to the final volume of 100 μl. After 5 min of DNA denaturation at 95°C, PCR was initiated by the addition of 2.5 U of Taq DNA polymerase (Top-Bio, Prague, Czech Republic) for amplification of fragments below 2 kb and 1 U of the LA DNA polymerase mix (Top-Bio) for amplification of longer fragments. Thirty cycles were performed under the conditions specified in Table 1 for each primer pair. Amplification of the conserved 1,779-bp pilAB intergenic region (7) was used as a positive control for the amplification reaction itself and for the quality of template DNA preparations.
TABLE 1.
Oligonucleotide primers and PCR amplification conditions
| Primer pair | Sequence 5′→3′a | Positionb | PCR parametersc
|
||||
|---|---|---|---|---|---|---|---|
| MgCl2 (mM) | Denaturation | Annealing | Extension | ||||
| Primers and conditions used to amplify frpA and frpC loci | |||||||
| frpA | TTAACACCCTATTCACCACTCTTG | 193 | 216 | 1.6 | 94°C; 15 s | 54°C; 30 s | 68°C; 4 min |
| TTATGCAGATGGCGTTACC | 3495 | 3477 | |||||
| frpA1 | TTAACACCCTATTCACCACTCTTG | 193 | 216 | 1.5 | 94°C; 1 min | 55°C; 1 min | 72°C; 1 min |
| AGCCATCGCCTCTGTTTG | 558 | 541 | |||||
| frpA2 | ATGGAAACGAGAGGCTTGGCG | 683 | 703 | 1.0 | 94°C; 1 min | 60°C; 1 min | 72°C; 1 min |
| AACCACTCTCTTGCCCATTCAG | 1004 | 983 | |||||
| frpC | ATGAATGAGGGTGAAGTTGT | 1964 | 1983 | 1.4 | 94°C; 15 s | 54°C; 30 s | 68°C; 5 min |
| TTATGCAGATGGCGTTACC | 7453 | 7435 | |||||
| frpC-non-rtx | ATGAATGAGGGTGAAGTTGT | 1964 | 1983 | 1.4 | 94°C; 15 s | 56°C; 30 s | 68°C; 2 min |
| TTATTATGACCAAAGCCTAC | 4581 | 4562 | |||||
| frpC-rtx | AGAAAGTGTTGGGTCAGG | 4485 | 4502 | 1.2 | 94°C; 15 s | 56°C; 30 s | 68°C; 2 min |
| TTATGCAGATGGCGTTACC | 7453 | 7435 | |||||
| orf1-frpC | ATGAGACCATATGCTACTACC | 1754 | 1774 | 1.5 | 94°C; 1 min | 55°C; 1 min | 72°C; 3 min |
| GGTAACGGCTTTATAGTAACT | 2939 | 2919 | |||||
| Primers used to amplify the 5′ and 3′ ends of frpC | |||||||
| frpC5′ | cgcgcaTATGAATGAGGGTGAAGTTGTT | 2585 | 2606 | 1.5 | 94°C; 1 min | 60°C; 1 min | 72°C; 1 min |
| cccgaaTTCTATCCCCAAACCTAATG | 3038 | 3019 | |||||
| frpC3′ | cgcggaaTTCTGAACAAGACAACGTAC | 7835 | 7854 | 1.5 | 94°C; 1 min | 60°C; 1 min | 72°C; 1 min |
| cccggaTCCTTATGCAGATGGCGTTAC | 8078 | 8058 | |||||
| frpC3′-TYB | GTTCCGGAAGTGATTTGG | 7807 | 7824 | 1.5 | 94°C; 1 min | 52°C; 1 min | 72°C; 1 min |
| TGCAGATGGCGTTACCAA | 8072 | 8055 | |||||
Lowercase letters indicate the nucleotides that were added for cloning (restriction sites are underlined).
Nucleotide positions of frpA and frpC primers according to sequences published for the FAM20 isolate (accession No. L06299 and L06302, respectively).
Optimal concentration of magnesium and PCR conditions with a given pair of primers.
FIG. 1.
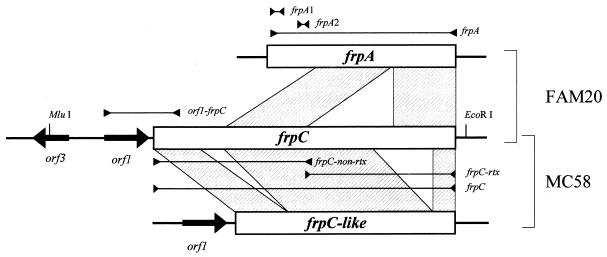
Schematic representation of the frpA, frpC, and frpC-like alleles of FAM20 and MC58 isolates. The fragments amplified by PCR are indicated by bars with arrowheads pointing in and labeled with the names of the PCR primer pairs used (Table 1). The regions of similarity between the frp alleles are indicated by hatched boxes. Large arrows indicate putative open reading frames.
Southern hybridization analysis.
MluI- and HincII-digested genomic DNAs were fractionated on 0.6% agarose gels, transferred to nylon membranes (Hybond N+; Amersham Pharmacia Biotech), and fixed at 80°C for 2 h. The blots were hybridized with digoxigenin-labeled probes (DIG High Prime DNA labeling kit; Roche Molecular Biochemicals) corresponding to the frpA1 and frpA2 regions of frpA (Fig. 1 and Table 1). Hybridization took place at 38°C overnight in DIG Easy Hyb solution (Roche Molecular Biochemicals). The blot was washed twice with low-stringency buffer (2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]–0.1% sodium dodecyl sulfate [SDS]) at room temperature for 5 min and twice in high-stringency buffer (0.5× SSC–0.1% SDS) at 65°C for 15 min. The probe was visualized by immunodetection with anti-digoxigenin–alkaline phosphatase-conjugated antibody using the chemiluminescence substrate CSPD (Roche Molecular Biochemicals).
DNA sequencing.
Nine of the 1,186-bp and one 2.7-kb PCR products amplified with the orf1-frpC primer pair were cloned and entirely sequenced. The DNA sequences were edited manually, and all ambiguous parts were also resequenced from the complementary strand. Comparison of DNA and deduced protein sequences was performed with the BLAST and MegAlign Lasergene software (DNAStar, Inc., Madison, Wis.).
Cloning of the orf1-frpC locus of N. meningitidis.
Based on the nucleotide sequence of strain FAM20, the EcoRI and MluI restriction sites were used to clone the entire orf1-frpC locus from the N. meningitidis isolate 10/96 (C:2a:P1-2,5). The 6- to 10-kb-long MluI-EcoRI fragments of chromosomal DNA were purified on 0.4% agarose gels and ligated with MluI-EcoRI-digested pTZ19RMluI to obtain a partial genomic library. Ten pools of 36 individual clones each were formed and screened by PCR using the orf1-frpC primer pair (Table 1) to identify the recombinants with the cloned orf1-frpC locus. The detection was refined in two subsequent rounds of PCR screening, progressively reducing the pools to six clones each until eight individual genomic clones of the orf1-frpC locus were found. One of them was chosen for further work and was called pTZ19RfrpC locus.
Construction of expression vectors.
To construct a vector for high-level expression, the open reading frame of frpC was subcloned into the expression vector pT7-7ΔBsaAI under the control of the transcription and translation initiation signals of gene 10 from bacteriophage T7 (16). First, the 5′ end of frpC was amplified with the primer pair frpC5′ (Table 1). The 466-bp PCR product was cloned as an NdeI-EcoRI fragment into pT7-7ΔBsaAI, yielding the pT7-7NdeIEcoRI construct. Next, the 3′ end of frpC was amplified by PCR using the second pair of primers, frpC3′ (Table 1), and the 257-bp PCR fragment was inserted as an EcoRI-BamHI fragment into pT7-7NdeIEcoRI, yielding pT7-7NdeIBamHI. The absence of undesired mutations in the subcloned PCR products was verified by DNA sequencing and comparison to the published sequence of frpC from the FAM20 strain (19). Next, the entire frpC gene was reconstituted by insertion of a 5,220-bp BsaAI fragment, encoding the remaining part of frpC, between the two BsaAI sites within the subcloned 5′ and 3′ ends of frpC on pT7-7NdeIBamHI to yield the plasmid pT7-7frpC. The integrity of the subcloned frpC gene was confirmed by restriction analysis and partial DNA sequencing. Expression of the 198-kDa FrpC protein was assessed by comparative SDS-polyacrylamide gel electrophoresis of extracts from induced cultures of strains carrying pT7-7frpC and mock-transformed E. coli.
Starting from pT7-7frpC, the pTYB2frpC plasmid was constructed. The 3′ end of frpC was amplified by PCR from pT7-7frpC using the primer pair frpC3′-TYB (Table 1) and fused in frame with the gene for intein-chitin binding domain (CBD) by cloning it into the SmaI site of pTYB2 (IMPACT T7 system). The reading frame and fusion point were verified by DNA sequencing, and an XbaI-Eco47III fragment of pT7-7frpC harboring the remaining part of the frpC gene was added to restore the full-length frpC reading frame in pTYB2frpC. The resulting plasmid encoded an in-frame fusion of the FrpC protein with the self-excisable intein and CBD. Detailed schemes of the constructs will be provided upon request.
Expression and purification of FrpC.
For a typical purification of recombinant FrpC, 500 ml of LB medium supplemented with 150 μg of ampicillin/ml was inoculated 1:100 with an overnight culture of E. coli BL21(λDE3) harboring the expression vector pTYB2frpC. The culture was grown with shaking at 30°C to an optical density at 600 nm of 0.6, and isopropyl-β-d-thiogalactopyranoside (1 mM final concentration) was added to induce the synthesis of the FrpC–intein-CBD fusion protein. After an additional 3 h of growth, the cells were collected by centrifugation, washed, and resuspended in cold 50 mM Tris-HCl–100 mM NaCl–1 mM EDTA (pH 7.4). The cells were then disrupted by sonication at 4°C, and the extract was cleared at 20,000 × g for 30 min and used for purification of the soluble form of FrpC.
All purification steps were performed at 4°C. The extract was loaded on a chitin bead column (New England Biolabs) equilibrated in 50 mM Tris-HCl–100 mM NaCl–1 mM EDTA (pH 7.4), and the column was washed extensively with 10 bed volumes of the same buffer. Buffered 50 mM dithiothreitol solution was loaded on the column, and the flow was stopped for overnight incubation at 4°C in order to allow self-excision of the intein-CBD domain from the fusion protein. The free FrpC was then eluted by restoring buffer flow through the column. Fractions containing FrpC were loaded on a DEAE-Sepharose column equilibrated with 50 mM Tris-HCl–100 mM NaCl–1 mM EDTA (pH 7.4), and the column was washed with 10 bed volumes of the same buffer. FrpC was eluted with 0.5 M NaCl in column buffer and stored frozen at −20°C. The identity of the purified protein was verified by amino-terminal sequencing and Western blotting with the RTX-specific monoclonal antibody 9D4 (10). The yield after the two purification steps was ≈2 mg of FrpC per liter of culture.
Serum samples.
Human sera were obtained from 19 individuals who suffered from invasive meningococcal disease (septicemia and/or meningitis). Serum samples were drawn from the patients immediately after their admission to the hospital (serum 1), 2 weeks later (serum 2), and 4 to 5 weeks later (serum 3). Furthermore, serum samples were obtained from 11 healthy individuals who were in close contact with patients during the first stages of invasive disease. While isolation of meningococci from blood or fluids of most these patients was successful, isolation of meningococci from nasopharynx swabs of all but one of the respective contact individuals failed. All contacts were also free of any clinical symptoms of the meningococcal disease. The serum samples were assayed for the presence of bactericidal antibodies against meningococci by standard methods. In corresponding cerebrospinal fluid or blood samples, the ethiological agent of the disease, N. meningitidis, was identified by cultivation, latex agglutination, and PCR methods. A control group of human sera was obtained from 18 healthy adult volunteers who had no contact with invasive meningococcal disease.
ELISA of human sera.
Wells of the PolySorp enzyme-linked immunosorbent assay (ELISA) plates (Nunc, Roskilde, Denmark) were coated overnight at 4°C with 100 μl of the purified FrpC protein solution at 1 μg/ml in 0.05 M sodium carbonate buffer (pH 9.6). After being washed with phosphate-buffered saline (PBS), pH 7.4, the plates were blocked for 2 h at 37°C with PBS containing 1% bovine serum albumin (Sigma, Steinheim, Germany) and 0.05% Tween-20 in PBS (PBST) and washed three times with PBS. The human sera were initially diluted 1:100 and then diluted threefold for seven dilutions (from 1:100 to 1:72,900) in 1% bovine serum albumin-PBST. Duplicate 100-μl samples of the diluted sera were incubated in antigen-coated wells at 37°C for 2 h. After the plates were washed with PBST, 100 μl of either horseradish peroxidase-conjugated swine anti-human immunoglobulin G (IgG) (SwAHu IgG-Px; diluted 1:5,000) or horseradish peroxidase-conjugated swine anti-human IgA (SwAHu IgA-Px; diluted 1:1,000) (SEVAC, Prague, Czech Republic) per well was added in 10% normal swine serum-PBS and incubated for 45 and 60 min, respectively. After the plates were rinsed with PBST, o-phenylenediamine peroxidase substrate was added, and the plates were incubated at room temperature in the dark for 10 (SwAHu IgG-Px) or 40 (SwAHu IgA-Px) min. The reaction was stopped by the addition of 50 μl of 2 M H2SO4, and absorbance at 492 nm was measured. The cutoff value for determination of both anti-FrpC IgG and anti-FrpC IgA antibody titers was determined as the mean plus 2 standard deviations from the test results of negative sera from healthy volunteers at 1:100 dilution. The titers of FrpC-specific antibodies were then defined as the reciprocal of the last dilution yielding an A492 greater than the cutoff value.
Nucleotide sequence accession numbers.
The GenBank accession numbers of the frpA, frpC, and frpC-like alleles of the FAM20 and MC58 isolates are L06302, L06299, and NMB0585, respectively.
RESULTS
In order to examine whether Frp proteins might be involved in the virulence of N. meningitidis, we analyzed the presence of the frp alleles in a set of 65 meningococcal isolates of all serogroups. This comprised 38 invasive isolates recovered from blood or cerebrospinal fluids of patients with invasive meningococcal disease and 27 carrier isolates recovered from the nasopharynges of healthy individuals.
The frpA allele is not present in most meningococcal isolates.
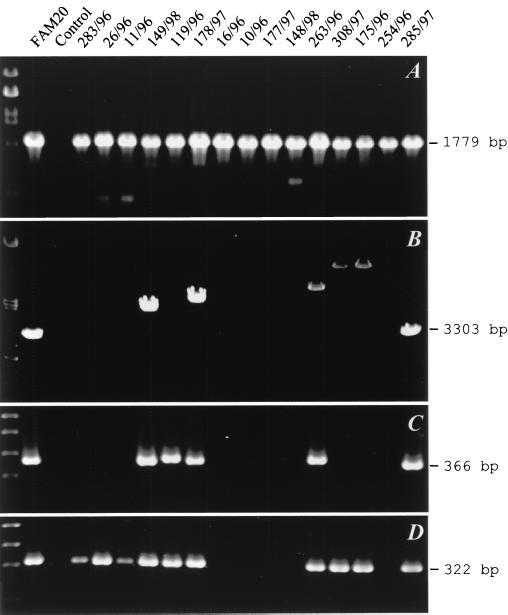
The complete genome sequencing of the Z2491 and MC58 isolates revealed that in parallel with intact frp alleles, numerous fragments of the frp genes can also be scattered around the meningococcal chromosome (13, 17). To limit their detection, specific PCR amplification of entire frpA and frpC alleles (Fig. 1 and Table 1) had to be employed instead of Southern DNA hybridization. As shown in Fig. 2A, in control PCR the 1,779-bp conserved pilAB intergenic region was unambiguously amplified from the DNA of all isolates. In contrast, with primers matching the ends of the frpA open reading frame, the amplification of an frpA allele of the expected size of 3,303 bp was obtained with only 1 of the 65 isolates (Tables 2 and 3). Moreover, 1- to 8-kb-longer frpA fragments were amplified from 22 of the isolates (34%), showing that large DNA insertions occurred within their frpA alleles (Fig. 2B and Tables 2 and 3). However, no frpA-specific PCR product was obtained for the rest of the isolates (65%), suggesting that the frpA allele was poorly conserved. To check whether the entire frpA allele was absent in most isolates or whether a diversity of its 5′- and/or 3′-terminal sequences prevented PCR, we performed selective amplification of two different portions of frpA. With the frpA1 primer pair (Fig. 1 and Table 1), an expected 366-bp fragment of the 5′-proximal part of the frpA allele was amplified from only 7 out of 38 invasive isolates (Fig. 2C and Table 2) and 12 out of 27 carrier isolates (Table 3). With the frpA2 primer pair (Fig. 1), a 322-bp PCR product corresponding to DNA downstream of the frpA1 target was amplified for 16 out of 38 invasive isolates (Fig. 2D and Table 2). Moreover, a FAM20-derived frpA-specific probe, encompassing both frpA1 and frpA2 primer sites, failed to hybridize with genomic DNA of the PCR-negative isolates (not shown). Hence, an frpA allele was absent in most tested meningococcal isolates.
FIG. 2.
PCR detection of frpA alleles. The positions of the primer pairs used are listed in Table 1 and indicated in Fig. 1. (A) Amplification products of the pilAB locus specific for Neisseria spp. (7), which served as a positive control for template DNA quality and functionality of the amplification reaction. (B) Products of amplification of the entire frpA alleles. (C and D) frpA fragments amplified by primer pairs frpA1 (C) and frpA2 (D). The codes of the N. meningitidis isolates from which DNA was extracted are indicated above the lanes. The DNA of isolate FAM20 was used as a positive control. λ DNA digested with PstI (A and B) and a 100-bp DNA ladder (C and D) were used as size standards in the first lane.
TABLE 2.
PCR detection of pilAB, frpA, and frpC in N. meningitidis strains isolated from patients with invasive meningococcal disease
| Straina | Phenotypeb | Detection with primer pairc
|
|||||
|---|---|---|---|---|---|---|---|
| pilAB | frpA | frpA1 | frpA2 | frpCe | orf1-frpC | ||
| FAM20 | C | + | + | + | + | VIII | + |
| 283/96 | A:4,21:P1-10 | + | − | − | + | IV | +f |
| 26/96 | B:4:P1-10 | + | − | − | + | II | + (E) |
| 150/98d | B:4:P1-15 | + | − | + | + | III | + |
| 22/96 | B:4:P1-16 | + | + (L) | − | + | II | + |
| 11/96 | B:15:P1-7,16 | + | − | − | + | III | +f |
| 149/98d | B:15:P1-7,16 | + | + (L) | + | + | XI | + |
| 119/96 | B:22:NST | + | − | + | + | VII | +f |
| 178/97 | B:NT:P1-5 | + | + (L) | + | + | VII | + |
| 220/96 | B:NT:NST | + | − | + | + | IV | + |
| 16/96 | C:2a:P1-2 | + | − | − | − | VI | +f |
| 88/96 | C:2a:P1-2 | + | − | − | − | VI | + |
| 154/96 | C:2a:P1-2 | + | − | − | − | VI | + |
| 180/96 | C:2a:P1-2 | + | − | − | − | V | + |
| 179/97d | C:2a:P1-2 | + | − | − | − | IX | + |
| 180/97 | C:2a:P1-2 | + | − | − | − | VI | + |
| 183/97d | C:2a:P1-2 | + | − | − | − | VI | + |
| 10/96 | C:2a:P1-2,5 | + | − | − | − | VI | +f |
| 14/96 | C:2a:P1-2,5 | + | − | − | − | VI | + |
| 86/96 | C:2a:P1-2,5 | + | − | − | − | VI | + |
| 177/97d | C:2a:P1-2,5 | + | − | − | − | V | + |
| 182/97d | C:2a:P1-2,5 | + | − | − | − | VI | + |
| 184/97 | C:2a:P1-2,5 | + | − | − | − | VI | + |
| 185/97 | C:2a:P1-2,5 | + | − | − | − | VI | + |
| 188/97 | C:2a:P1-2,5 | + | − | − | − | VI | + |
| 311/97d | C:2a:P1-2,5 | + | − | − | + | VI | + |
| 329/97d | C:2a:P1-2,5 | + | − | − | + | VI | + |
| 148/98d | C:2a:P1-2,5 | + | − | − | − | VI | + |
| 152/98d | C:2a:P1-2,5 | + | − | − | − | VI | + |
| 176/97 | C:2a:NST | + | − | − | − | VI | + |
| 186/97 | C:2a:NST | + | − | − | − | VI | + |
| 263/96 | C:2b:P1-2,5 | + | + (L) | + | + | X | +f (E) |
| 308/97d | C:4,21:P1-6 | + | + (L) | − | + | II | + (E) |
| 175/96 | C:4,21:NST | + | + (L) | − | + | II | +f (E)f |
| 181/97d | C:NT:P1-2 | + | − | − | − | VI | + |
| 98/96 | C:NT:P1-3 | + | + (L) | − | + | III | + |
| 254/96 | C:NT:NST | + | − | − | − | IV | +f |
| 187/97 | C:NT:NST | + | − | − | − | VI | + |
| 285/97 | Y/W:2a:P1-2,5 | + | + | + | + | VI | +f |
Meningococcal strain FAM20 (20) was used as a positive PCR control. All additional meningococcal strains were isolated from cerebrospinal fluid or blood of patients with invasive meningococcal disease in the Czech Republic between 1996 and 1998.
Phenotype of each meningococcal strain characterized by serogroup, serotype, and serosubtype.
Sequences of the respective pairs of oligonucleotide primers used for PCR are given in Table 1. The letters in parenthesis indicate that in respect to FAM20 (20), a longer (L) than predicted, or an additional (E) product was amplified. +, specific PCR product amplified; −, no specific PCR product amplified.
Sera of the patient from which this strain was isolated were tested for the presence of FrpC-specific antibodies by ELISA.
The detected frpC alleles were classified into 11 categories according to the size of the respective PCR product. The size correspondences of amplified fragments were as follows: I, 3.3 kb; II, 3.7 kb; III, 3.9 kb; IV, 4.5 kb; V, 4.9 kb; VI, 5.5 kb; VII, 5.7 kb; VIII, 6.1 kb; IX, 6.5 kb; X, 6.7 kb; XI, 7.0 kb.
The amplified PCR product was cloned and sequenced.
TABLE 3.
PCR detection of pilAB, frpA, and frpC in N. meningitidis strains isolated from infected nondiseased carriers
| Straina | Phenotypeb | Detection with primer pairc
|
||||
|---|---|---|---|---|---|---|
| pilAB | frpA | frpA1 | frpCe | orf1-frpC | ||
| 67/97 | B:1:P1-5 | + | + (L) | + | X | + |
| 12/97 | B:4:P1-1,7 | + | + (L) | − | II | + |
| 314/97d | B:4:P1-16 | + | + (L) | − | I | + |
| 78/97 | B:4:NST | + | + (L) | + | I | + |
| 228/97 | B:15:P1-7 | + | − | − | V | + |
| 263/97 | B:21:P1-7 | + | + (L) | − | I | + |
| 64/97 | B:22:P1-9 | + | + (L) | + | − | − |
| 106/97 | B:23:P1-16 | + | + (L) | + | X | + |
| 75/97 | B:NT:P1-5 | + | + (L) | + | VII | + |
| 76/97 | B:NT:P1-5 | + | + (L) | + | VII | + |
| 77/97 | B:NT:P1-5 | + | + (L) | + | IV | + |
| 225/97 | B:NT:P1-5 | + | + (L) | + | I | + |
| 2/97 | B:NT:P1-14 | + | − | + | VII | + |
| 107/97 | B:NT:NST | + | − | + | V | + |
| 47/97 | C:2a:P1-2,5 | + | − | − | VI | + |
| 207/97 | C:2a:P1-2,5 | + | − | − | VI | + |
| 211/97 | C:2a:P1-2,5 | + | − | − | VI | + |
| 150/96 | C:4,21:NST | + | + (L) | − | II | + (E) |
| 116/97 | C:NT:P1-2,5 | + | − | − | VI | + |
| 117/97 | C:NT:P1-2,5 | + | − | − | VI | + |
| 123/97 | PA:4:P1-15 | + | − | − | − | − |
| 65/97 | PA:15:P1-7 | + | + (L) | − | VI | + |
| 77/96 | PA:NT:NST | + | + (L) | + | IV | + |
| 112/96 | PA:NT:NST | + | − | − | VII | + |
| 139/96 | PA:NT:NST | + | − | + | IV | + |
| 156/96 | PA:NT:NST | + | + (L) | − | − | − |
| 356/95 | NA:NT:NST | + | − | − | VIII | + |
Meningococcal strains were isolated from nasopharynxes of healthy individuals from the Czech Republic who were in contact with patients with invasive meningococcal disease.
b,c,d,e See legend to Table 2.
frpC-like alleles of variable size are present in all invasive and most carrier isolates.
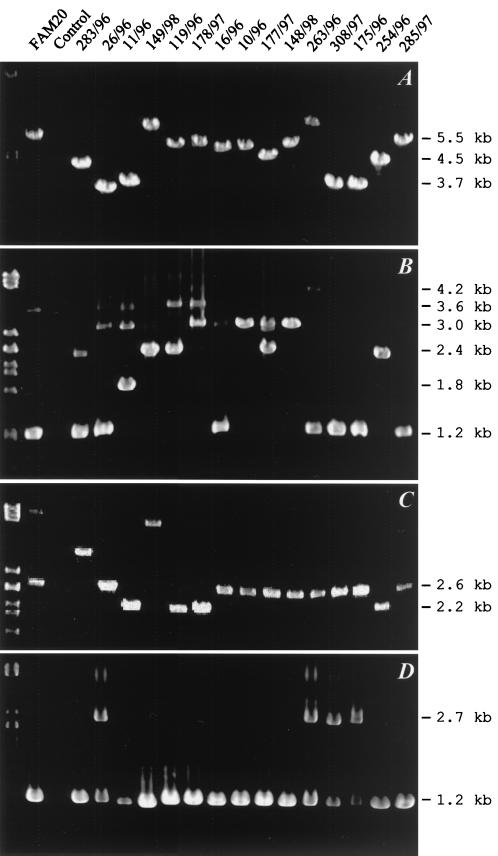
In contrast to frpA, the frpC alleles could be amplified from all 38 invasive meningococcal isolates and from 24 out of 27 carrier isolates when a pair of primers specific for the full-length frpC allele of FAM20 was used (Fig. 1 and Table 1). However, as illustrated in Fig. 3A and summarized in Tables 2 and 3, the sizes of the frpC-specific products varied considerably. Therefore, conservation of two different frpC portions was investigated.
FIG. 3.
PCR detection of the frpC loci. The positions of the primer pairs used are listed in Table 1 and indicated in Fig. 1. (A) Amplification products of the entire frpC alleles. (B) Amplification products of the repetitive portions obtained with the primer pair frpC-rtx. (C) Products of amplification of the nonrepetitive portions obtained with the frpC-non-rtx primer pair. (D) PCR products obtained with the orf1-frpC primer pair. The codes of the N. meningitidis isolates are indicated above the lanes. DNA of strain FAM20 was used as a positive control. Fragments of λ DNA digested with PstI were used as size standards loaded in lane 1.
As shown in Fig. 3B, products of various sizes were obtained with the PCR specific for the frpC repeats. Not surprisingly, additional repeat-specific fragments were amplified in parallel with the major PCR product for most of the strains. The presence of more than one copy of frp repeats per chromosome was, indeed, previously observed for the FAM20 and MC58 isolates (Fig. 1). Interestingly, the size differences between the obtained PCR products appeared to be very close to integral multiples of 600 bp, and fragments of approximately 1.2, 1.8, 2.4, 3.0, 3.6, and 4.2 kb were obtained. This suggests that a DNA block encoding an approximately 600-bp repeat was present in variable numbers of copies in the different frpC alleles. However, only a limited correlation was observed between the size pattern of the amplified repetitive sequences and entire frpC alleles (cf. Fig. 3A and B).
As documented in Fig. 3C, the size variation of frpC alleles was to some extent due also to variations in the nonrepetitive portion encoding the first 872 residues of FrpC. Amplification of this part of frpC yielded a 2.6-kb product for most isolates, which was expected for the sequenced frpC alleles from FAM20 and MC58. However, a 2.2-kb product was amplified from other isolates, corresponding in size to the expected product of the shorter frpC-like allele of MC58. Moreover, larger products of approximately 3.6 and 4.7 kb were also amplified from some isolates. This shows that deletions as well as DNA insertions within the nonrepetitive portions of frpC contributed to the size variation of the frpC alleles.
The 5′-terminal sequences of frpC are highly conserved among isolates.
It was important to assess how conserved are the upstream and 5′-terminal sequences of frpC, which contain the transcriptional as well as the translational initiation signals determining the level of expression of the gene. Therefore, an orf1-frpC primer pair was used to amplify a 1,186-bp fragment from the 5′ end of the orf1-frpC locus (Fig. 1 and Table 1). As shown in Fig. 3D, a PCR product of the expected length was obtained for all 38 invasive isolates tested (Table 2) and for 24 out of the 27 noninvasive isolates (Table 3).
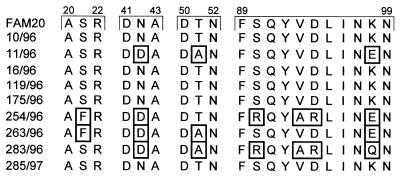
Nine PCR products from isolates representing all serogroups were cloned and sequenced. A complete sequence conservation at the nucleotide level was found in the orf1-frpC intergenic region harboring the translation initiation signals of frpC. More importantly, between 95 and 100% identity was also found for the deduced sequences containing the first 118 amino acid residues of FrpC from the nine isolates. Five sequences were identical to that of FAM20 (Table 4), and the remaining four had a limited set of amino acid substitutions at conserved positions (Fig. 4).
TABLE 4.
Similarity and divergence among deduced amino-terminal sequences of FrpC
| Strain | Similarity (%)a
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FAM20 | 10/96 | 11/96 | 16/96 | 119/96 | 175/96 | 254/96 | 263/96 | 283/96 | 285/97 | |
| FAM20 | 100.0 | 97.5 | 100.0 | 100.0 | 100.0 | 95.0 | 96.6 | 95.0 | 100.0 | |
| 10/96 | 0.0 | 97.5 | 100.0 | 100.0 | 100.0 | 95.0 | 96.6 | 95.0 | 100.0 | |
| 11/96 | 2.6 | 2.6 | 97.5 | 97.5 | 97.5 | 95.8 | 99.2 | 96.6 | 97.5 | |
| 16/96 | 0.0 | 0.0 | 2.6 | 100.0 | 100.0 | 95.0 | 96.6 | 95.0 | 100.0 | |
| 119/96 | 0.0 | 0.0 | 2.6 | 0.0 | 100.0 | 95.0 | 96.6 | 95.0 | 100.0 | |
| 175/96 | 0.0 | 0.0 | 2.6 | 0.0 | 0.0 | 95.0 | 96.6 | 95.0 | 100.0 | |
| 254/96 | 5.2 | 5.2 | 4.3 | 5.2 | 5.2 | 5.2 | 96.6 | 97.5 | 95.0 | |
| 263/96 | 3.4 | 3.4 | 0.8 | 3.4 | 3.4 | 3.4 | 3.4 | 95.8 | 96.6 | |
| 283/96 | 5.2 | 5.2 | 3.4 | 5.2 | 5.2 | 5.2 | 2.6 | 4.3 | 95.0 | |
| 285/97 | 0.0 | 0.0 | 2.6 | 0.0 | 0.0 | 0.0 | 5.2 | 3.4 | 5.2 | |
Percentages of similarity are given above and percentages of divergence below the diagonal of the table. The first 118-amino-acid-residue-long sequences of FrpC deduced from 1,186-bp orf1-frpC-specific PCR fragments were used for comparison by the MegAlign program. The percent divergence was calculated by comparing sequence pairs in relation to the phylogeny reconstructed by MegAlign software. Percent similarity compares sequences directly, without accounting for phylogenetic relationships.
FIG. 4.
Locations (boxed) of the sequence differences in the amino-terminal 118-residue portions of FrpC from nine clinical isolates and FAM20. The sequences were aligned using MegAlign software. The residue positions are indicated above the sequence blocks. Only sequence portions containing substitutions are shown.
In parallel with the expected 1,186-bp product, a fragment of about 2.7 kb was also amplified in four invasive isolates and one noninvasive isolate (Fig. 3D and Tables 2 and 3). Sequencing of this product from isolate 175/96 revealed the presence of two copies of orf1 separated by a truncated open reading frame for an IS1016-like transposase and followed by the 5′-terminal portion of frpC. Amplification of the 1,186-bp PCR product on the template of the longer fragment, however, was precluded by sequence differences in the respective orf1 copies. The results show that a second copy of the orf1-frpC locus, or at least its 5′-terminal part, was present within the chromosome of 5 out of 65 isolates tested. The same conclusion was also reached by Southern blot analysis (not shown).
Cloning of the orf1-frpC locus and expression and purification of recombinant FrpC.
It was of interest to produce a recombinant and highly purified FrpC antigen, which would allow determination of the levels of FrpC-specific antibodies in convalescent-phase sera. Therefore, the orf1-frpC locus was cloned from an N. meningitidis 10/96 (C:2a:P1-2,5) strain, which is representative of the most common type of invasive isolate in the Czech Republic.
In the sequence of FAM20, the entire orf1-frpC locus is contained on an approximately 8-kb MluI-EcoRI fragment. Therefore, a partial genomic library was constructed with size-separated MluI-EcoRI fragments of 10/96 DNA. The library was screened by several rounds of direct colony PCR detection with the orf1-frpC primers. Among the 360 recombinants obtained, eight independent clones carrying the entire orf1-frpC locus were identified. One of them, harboring the expected 8-kb insert, was characterized by restriction analysis and partial DNA sequencing and used in further work.
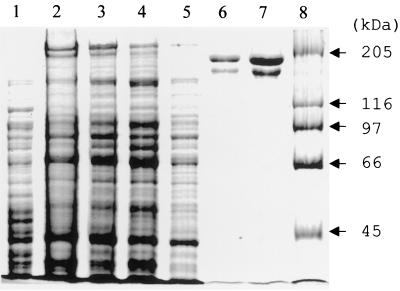
To achieve high production of recombinant FrpC in E. coli, the frpC open reading frame was fused to the strong translation initiation signals of gene 10 of bacteriophage T7 and placed under the control of an inducible T7 promoter. Next, an intein-CBD was fused in frame to the carboxy-terminal end of FrpC. This allowed affinity purification of FrpC from the soluble cytosolic fraction of recombinant E. coli on chitin agarose by the IMPACT T7 system. As shown in Fig. 5, upon self-excision of the intein-CBD from the fusion and subsequent anion-exchange chromatography, a highly purified FrpC protein was recovered. It should be noted, however, that in parallel with the expected full-length 198-kDa FrpC form, a 150-kDa protein was copurified by this procedure (Fig. 5). N-terminal sequencing of this protein species revealed that it was a proteolytic fragment of FrpC which had the proline 415 of FrpC as the amino-terminal residue (R. Osicka, K. Prochazkova, and P. Šebo, unpublished data). However, as estimated from the SDS-polyacrylamide gel electrophoresis analyses (Fig. 5), the full-length and processed forms of FrpC constituted over 95% of the total protein in the preparation.
FIG. 5.
Purification of FrpC from E. coli BL21(λDE3)/pTYB2frpC using a combination of affinity and ion-exchange chromatographies. Lanes: 1, crude extract from uninduced cells; 2, crude extract from cells induced for production of the FrpC–intein-CBD fusion protein; 3, clarified crude extract from induced cells; 4, chitin column flowthrough; 5, chitin column wash; 6, fraction of eluted 198-kDa FrpC after intein-mediated excision of intein-CBD; 7, FrpC after ion-exchange chromatography on a DEAE-Sepharose column; 8, high-molecular-mass standards. The samples were analyzed on a 7.5% polyacrylamide gel and stained with Coomassie blue.
FrpC-related proteins induce specific serum antibodies during invasive meningococcal disease.
Positive serological evidence would indicate that FrpC-related proteins are expressed during natural infections. Therefore, a specific ELISA protocol was developed, using the purified recombinant FrpC as a coating antigen. In addition to standard negative controls, a mock extract of E. coli BL21, processed by the same chromatographic procedures as FrpC, was also used for coating. This allowed us to control for the reaction of sera with any residual E. coli components within the FrpC preparations.
Three groups of sera were examined for the presence of FrpC-specific antibodies, as described in Table 5. The first group consisted of sera from 12 patients who suffered from a characteristic invasive meningococcal disease and from whom an N. meningitidis strain could be isolated as the etiological agent of the disease. The second group consisted of sera from seven patients who developed characteristic symptoms of the invasive meningococcal disease but for whom the isolation of the causative pathogenic agent failed. For most of these patients, three serum samples were available. These were drawn on the day of admission to the hospital, 2 weeks later, and 4 to 5 weeks later, respectively. The third group comprised sera of 11 characterized healthy primary contacts of diseased individuals, of which 7 were direct contacts of the five patients from the first two groups. N. meningitidis could, however, be isolated from only one of these contacts. Finally, sera of 18 healthy adult volunteers who did not have any known contact with a person with meningococcal disease served as controls for determination of the cutoff antibody levels.
TABLE 5.
Detection of FrpC-specific IgG and IgA antibodies in human sera by enzyme-linked immunosorbent assay
| Individual codea | N. meningitidis straina | frpC allele typeb | Serum codec
|
IgG ELISA titerd
|
IgA ELISA titerd
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | ||||
| Patients | P01P | 177/97 | V | 1 | NC | 2 | Neg | 100 | ||||
| P07P | 179/97 | IX | 7 | 8 | 9 | Neg | 900 | 900 | Neg | Neg | Neg | |
| P15P | 181/97 | VI | 16 | 17 | 18 | Neg | 100 | 100 | ||||
| P19P | 182/97 | VI | 22 | 23 | 24 | 100 | 900 | 900 | 100 | 900 | 900 | |
| P21P | 183/97 | VI | 25 | 26 | 27 | 300 | 300 | 300 | ||||
| O47P | 308/97 | II | 228 | 229 | 230 | Neg | Neg | Neg | ||||
| O65P | 311/97 | VI | 244 | 411 | 412 | 100 | 300 | 300 | ||||
| B11P | 329/97 | VI | 296 | 422 | 421 | 100 | 900 | 900 | Neg | 300 | 100 | |
| P35P | 148/98 | VI | 469 | 470 | 471 | 300 | 8,100 | 8,100 | Neg | 900 | 300 | |
| P37P | 149/98 | XI | 472 | 473 | 474 | Neg | 100 | 100 | ||||
| P39P | 150/98 | III | 475 | 476 | NC | Neg | 100 | |||||
| P41P | 152/98 | VI | 477 | 478 | 479 | Neg | 100 | 100 | ||||
| O49P | NI | ND | 231 | 232 | 233 | Neg | 100 | 100 | ||||
| H23P | NI | ND | 288 | 289 | NC | Neg | Neg | |||||
| B07P | NI | ND | 290 | 291 | 292 | 900 | 72,900 | 72,900 | 900 | 2,700 | 8,100 | |
| B13P | NI | ND | 423 | 424 | 425 | Neg | 100 | 100 | ||||
| B15P | NI | ND | 426 | 427 | 428 | Neg | Neg | Neg | ||||
| B17P | NI | ND | 429 | 430 | 431 | Neg | 8,100 | 2,700 | Neg | 900 | 300 | |
| H37P | NI | ND | NC | 450 | NC | Neg | ||||||
| Contacts | P04K | NI | ND | 4 | 100 | |||||||
| P08K | NI | ND | 29 | 300 | ||||||||
| P12K | NI | ND | 31 | 900 | 300 | |||||||
| O02K | NI | ND | 74 | Neg | ||||||||
| O20K | NI | ND | 203 | Neg | ||||||||
| O24K | NI | ND | 205 | Neg | ||||||||
| O34K | 314/97 | I | 210 | 100 | ||||||||
| B06K | NI | ND | 297 | Neg | ||||||||
| B10K | NI | ND | 299 | 300 | Neg | |||||||
| B18K | NI | ND | 303 | 300 | ||||||||
| B20K | NI | ND | 304 | 300 | ||||||||
Sera were collected from 19 patients with invasive meningococcal disease. N. meningitidis was isolated from cerebrospinal fluid or blood of 12 patients; the isolation of a pathogenic agent failed in 7 cases. Sera were also obtained from 11 healthy primary contacts of diseased individuals, and N. meningitidis was isolated from the nasopharynx of one of them. NI, N. meningitidis was not isolated.
frpC allele of each meningococcal strain is characterized by its length (see Table 2). ND, not determined.
Serum samples were drawn from patients on admission to the hospital (1), 2 weeks later (2), and 4 to 5 weeks later (3). Sera of healthy primary contact individuals were obtained within 14 days after the first disease symptoms of the given patients. NC, serum was not collected.
Titers were defined as the reciprocal of the last dilution with an A492 greater than the cutoff value. Neg, negative.
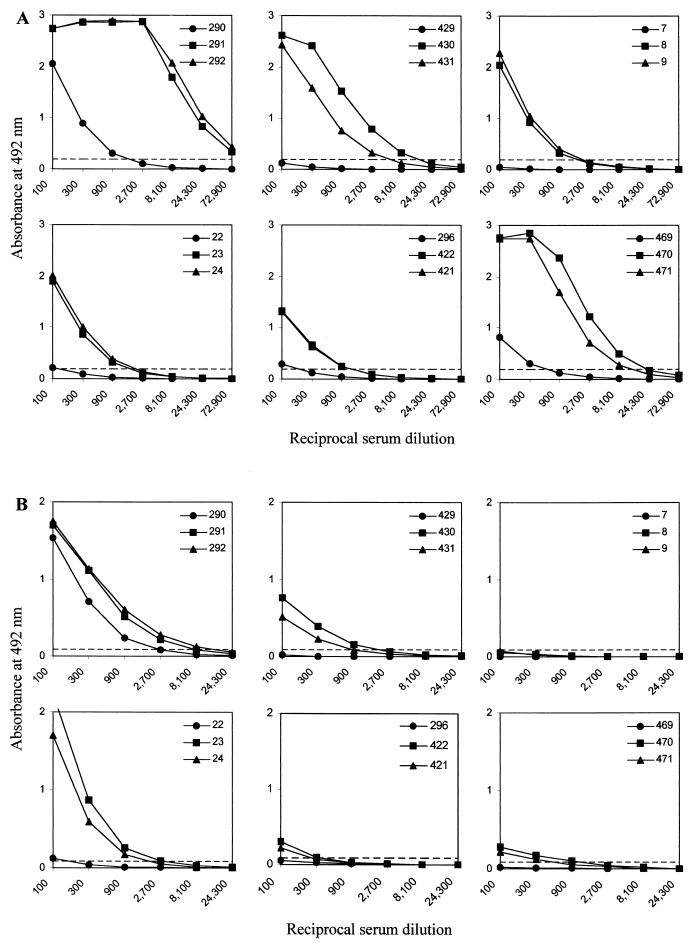
As shown in Table 5, the sera of 4 out of 19 patients with diagnosed meningococcal disease (the first two groups) had no significant levels of FrpC-specific IgG antibodies. The sera of nine other patients exhibited increased levels of FrpC-specific antibodies, with end point titer values between 100 and 300. High levels of antibodies reacting with FrpC were detected in the sera of 6 out of 19 patients, with titers ranging from 900 up to the extremely high titer of 72,900 for one patient (Table 5). When sera taken from these six patients at the time of admission to the hospital, 2 weeks later, and 4 to 5 weeks later were compared, a 10- to 100-fold rise in the titer of anti-FrpC antibodies was consistently observed (Fig. 6A). Moreover, specific IgA class antibodies reacting with FrpC were also detected in the sera of five out of six of these patients. The IgA titers ranged from 300 to 8,100 and exhibited a similar rise over time (Fig. 6B). These results demonstrate that an FrpC-like antigen was produced during systemic infection by most invasive meningococcal strains.
FIG. 6.
Titration of sera of patients after invasive meningococcal disease. Results for six patients are given. The sera were taken at the time of admission of the patient to the hospital (•) and 2 (▪) and 4 to 5 (▴) weeks later. Detection of FrpC-specific IgG (A) and IgA (B) antibodies is shown. The dashed line indicates the cutoff value calculated as the mean plus 2 standard deviations of the test results of negative sera from healthy volunteers diluted 1:100. The three serum numbers for each patient are given in the upper right corner of each panel.
It is worth mentioning that in the five available matching pairs of sera, where the patients had high levels of FrpC-specific antibodies (e.g., B07P, B11P, and P07P), the sera of their corresponding close contacts (B10K, B18K plus B20K, and P08K, respectively), also contained detectable antibodies to FrpC. In contrast, in pairs O49P-O24K and O47P-O20K, where the patient had low or no antibodies against FrpC, the respective contact sera were also negative for reaction with FrpC.
DISCUSSION
We show here that the sera of a majority of patients who suffered from invasive meningococcal disease contained antibodies specifically recognizing the FrpC RTX protein of N. meningitidis. Indeed, in some of these patients, the levels of FrpC-specific antibodies were already rather high 2 weeks after diagnosis of the disease. This demonstrates that an FrpC-related antigen was expressed by most of the invasive meningococcal clones and was immunogenic during a natural infection. It also raises the possibility that FrpC-related proteins contribute to meningococcal pathology.
Interestingly, 7 out of the 11 healthy contacts of diseased patients also had increased levels of FrpC-specific antibodies. Moreover, in the group of 18 healthy individuals with no reported contact with meningococcal disease, three sera were also positive well above the cutoff values. The circulation of meningococci in healthy populations and the probability of infection are, indeed, very high, with about 10% of individuals being colonized at a given time (3). Therefore, a plausible interpretation of the results is that FrpC may also be produced during the unrecognized-carrier state, when meningococci are just colonizing the nasopharynx. Experiments are under way to determine whether antibodies against FrpC are bactericidal.
Sera of four patients diagnosed with a characteristic meningococcal disease were seronegative for FrpC. Individual immune responses against a given antigen, however, are known to differ significantly within an outbred human population, depending also on the individual course of infection and applied treatment. Therefore, it could not be concluded whether the seronegative patients were infected by strains which did not produce an FrpC-like protein. The frpC alleles could be amplified from all of the invasive isolates. It was not systematically determined whether these alleles of various sizes were functional genes or had disrupted reading frames due to deletions and/or insertions. Twelve matching pairs of patient sera and the corresponding isolates, however, could be analyzed in this study. While only one of the 12 patients was seronegative for FrpC, a range of different sizes was observed for the frpC alleles detected in the group of corresponding meningococcal isolates (cf. Table 5). This suggests that these size-variable frpC alleles in fact encode a family of immunogenic FrpC-like proteins of various molecular masses. This conclusion tends to be supported also by Western blot analysis of iron-limited cultures of a set of meningococcal strains, where FrpC-like proteins of various molecular masses were detected (data not shown).
The variability of frpC alleles was at least partly due to differences in the sizes and/or numbers of the highly redundant RTX repeat blocks. The observed size differences of amplified repetitive domains suggest that these might consist of integral multiples of a 600-bp DNA block. Such a block is, indeed, present once in the frpA alle of FAM20, twice in the frpC-like allele of MC58, and four times in the full-length frpC gene of both the FAM20 and MC58 strains (17, 19, 20). It is worth noting that similar variation in the size of the RTX domain was recently also observed for a related RTX protein, ApxIVA from Actinobacillus (14). For frpC, however, not all size variation could be attributed to the repetitive sequences. Deletions as well as insertions were also observed in the nonrepetitive portion of the gene encoding the first 872 residues of FrpC. This further documents the genomic fluidity and high recombination frequency of meningococci (4, 6, 8, 13, 17). Moreover, there was no correlation between the detected size of the frpC allele and the serological characteristics of the isolates. This was also to be expected, since the serotype and subtype characteristics were previously found to reflect only poorly, if at all, the clonal lineages in meningococci (4, 6, 8).
Interestingly, a rather high sequence conservation of the 5′ end of the frpC gene was found in the portion encoding the 118 amino-terminal residues of FrpC from nine local isolates. Five of the sequences were fully conserved in respect to that of the FAM20 strain, while the remaining four sequences had a limited set of amino acid substitutions at conserved positions. The sequence encoding the RGD tripeptide, shown to be involved in binding of other proteins to cellular integrins, was, however, intact in all of the frpC alleles examined. These results suggest that the N-terminal portion of the FrpC protein may be rather conserved and subject to selective pressure for conservation of function.
Characterization of the biological activities of the better-conserved FrpC proteins is now intensely pursued. Except for the repeat domain, which is highly homologous in all RTX proteins, the FrpC protein exhibits significant sequence similarity only to the newly identified RTX protein ApxIVA from Actinobacillus pleuropneumoniae (15). The area of similarity is located in the central 300 amino acids of ApxIVA and between residues 300 and 600 of the prototype FrpC protein of FAM20, with 42% identical and 50% identical plus similar amino acid residues, respectively. The biological function of ApxIVA also remains unknown. It appears to exhibit a weak hemolytic activity suggestive of a channel-forming capacity (15). However, no hemolytic activity of FrpC could be detected in our experiments (data not shown).
In contrast to frpC, the entire frpA allele was conserved in only 1 out of 65 isolates tested. It is unlikely that the additional 22 frpA alleles detected, which contain large DNA insertions, represent functional frpA genes. Some of these isolates apparently harbored only a part of the frpA-specific DNA, which could be amplified by only one primer pair (frpA2) and not by another matching primer pair immediately upstream (frpA1). This suggests that fragments of frpA were present in the chromosomes of this portion of the isolates tested. Moreover, the combination of Southern blot and PCR analyses showed that an frpA allele was absent in most of the strains tested. Indeed, a complete sequence of frpA was also not found in the two meningococcal strains whose genomes have been sequenced. Collectively these data suggest that a complete frpA gene may not be present in many strains.
Finally, when the observed size variation of frpC alleles is considered, it appears inappropriate to continue the differentiation of the meningococcal RTX proteins into FrpA and FrpC-like merely on the basis of size. Since large portions of identical sequence are shared by FrpA and FrpC, we suggest considering FrpA an insertion variant of FrpC and calling all the RTX proteins of meningococci FrpC. These could then be further classified according to their sizes.
ACKNOWLEDGMENTS
Stimulating discussions and the gift of purified FAM20 genomic DNA by Xavier Nassif and the gift of the 9D4 monoclonal antibody by E. L. Hewlett are gratefully acknowledged.
This work was supported by grants 310/96/K102 of the Grant Agency of the Czech Republic and ME167 of the Ministry of Education, Youth and Sports of the Czech Republic and by the French-Czech research collaboration award DRI/637 MAE PECO.
REFERENCES
- 1.Binet R, Letoffe S, Ghigo J M, Delepelaire P, Wandersman C. Protein secretion by Gram-negative bacterial ABC exporters—a review. Gene. 1997;192:7–11. doi: 10.1016/s0378-1119(96)00829-3. [DOI] [PubMed] [Google Scholar]
- 2.Booy R, Kroll J S. Bacterial meningitis and meningococcal infection. Curr Opin Pediatr. 1998;10:13–18. doi: 10.1097/00008480-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Caugant D A. Population genetics and molecular epidemiology of Neisseria meningitidis. APMIS. 1998;106:505–525. [PubMed] [Google Scholar]
- 4.Caugant D A, Mocca L F, Frasch C E, Froholm L O, Zollinger W D, Selander R K. Genetic structure of Neisseria meningitidis populations in relation to serogroup, serotype, and outer membrane protein pattern. J Bacteriol. 1987;169:2781–2792. doi: 10.1128/jb.169.6.2781-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Souza S E, Ginsberg M H, Plow E F. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. Trends Biochem Sci. 1991;16:246–250. doi: 10.1016/0968-0004(91)90096-e. [DOI] [PubMed] [Google Scholar]
- 6.Feil E J, Maiden M C, Achtman M, Spratt B G. The relative contributions of recombination and mutation to the divergence of clones of Neisseria meningitidis. Mol Biol Evol. 1999;16:1496–1502. doi: 10.1093/oxfordjournals.molbev.a026061. [DOI] [PubMed] [Google Scholar]
- 7.Giorgini D, Taha M K. Molecular typing of Neisseria meningitidis serogroup A using the polymerase chain reaction and restriction endonuclease pattern analysis. Mol Cell Probes. 1995;9:297–306. doi: 10.1016/s0890-8508(95)91540-0. [DOI] [PubMed] [Google Scholar]
- 8.Holmes E C, Urwin R, Maiden M C. The influence of recombination on the population structure and evolution of the human pathogen Neisseria meningitidis. Mol Biol Evol. 1999;16:741–749. doi: 10.1093/oxfordjournals.molbev.a026159. [DOI] [PubMed] [Google Scholar]
- 9.Lally E T, Hill R B, Kieba I R, Korostoff J. The interaction between RTX toxins and target cells. Trends Microbiol. 1999;7:356–361. doi: 10.1016/s0966-842x(99)01530-9. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Gray M, Guo L, Sebo P, Hewlett E. Epitope mapping of monoclonal antibodies against Bordetella pertussis adenylate cyclase toxin. Infect Immun. 1999;67:2090–2095. doi: 10.1128/iai.67.5.2090-2095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leininger E, Roberts M, Kenimer J G, Charles I G, Fairweather N, Novotny P, Brennan M J. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc Natl Acad Sci USA. 1991;88:345–349. doi: 10.1073/pnas.88.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leong J M, Morrissey P E, Marra A, Isberg R R. An aspartate residue of the Yersinia pseudotuberculosis invasin protein that is critical for integrin binding. EMBO J. 1995;14:422–431. doi: 10.1002/j.1460-2075.1995.tb07018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parkhill J, Achtman M, et al. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature. 2000;404:502–506. doi: 10.1038/35006655. [DOI] [PubMed] [Google Scholar]
- 14.Schaller A, Djordjevic S, Eamens G, Forbes W, Kuhn R, Kuhnert P, Gottschalk M, Nicolet J, Frey J. Identification and detection of Actinobacillus pleuropneumoniae by PCR based on the gene apxIVA. Vet Microbiol. 2001;79:47–62. doi: 10.1016/s0378-1135(00)00345-x. [DOI] [PubMed] [Google Scholar]
- 15.Schaller A, Kuhn R, Kuhnert P, Nicolet J, Anderson T J, MacInnes J I, Segers R P, Frey J. Characterization of apxIVA, a new RTX determinant of Actinobacillus pleuropneumoniae. Microbiology. 1999;145:2105–2116. doi: 10.1099/13500872-145-8-2105. [DOI] [PubMed] [Google Scholar]
- 16.Tabor S, Richardson C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tettelin H, Saunders N J, et al. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287:1809–1815. doi: 10.1126/science.287.5459.1809. [DOI] [PubMed] [Google Scholar]
- 18.Thompson S A, Sparling P F. The RTX cytotoxin-related FrpA protein of Neisseria meningitidis is secreted extracellularly by meningococci and by HlyBD+Escherichia coli. Infect Immun. 1993;61:2906–2911. doi: 10.1128/iai.61.7.2906-2911.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson S A, Wang L L, Sparling P F. Cloning and nucleotide sequence of frpC, a second gene from Neisseria meningitidis encoding a protein similar to RTX cytotoxins. Mol Microbiol. 1993;9:85–96. doi: 10.1111/j.1365-2958.1993.tb01671.x. [DOI] [PubMed] [Google Scholar]
- 20.Thompson S A, Wang L L, West A, Sparling P F. Neisseria meningitidis produces iron-regulated proteins related to the RTX family of exoproteins. J Bacteriol. 1993;175:811–818. doi: 10.1128/jb.175.3.811-818.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tzeng Y L, Stephens D S. Epidemiology and pathogenesis of Neisseria meningitidis. Microbes Infect. 2000;2:687–700. doi: 10.1016/s1286-4579(00)00356-7. [DOI] [PubMed] [Google Scholar]
- 22.Welch R A. Pore-forming cytolysins of gram-negative bacteria. Mol Microbiol. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 23.Welch R A, Bauer M E, Kent A D, Leeds J A, Moayeri M, Regassa L B, Swenson D L. Battling against host phagocytes: The wherefore of the RTX family of toxins? Infect Agents Dis. 1995;4:254–272. [PubMed] [Google Scholar]